Extracellular vesicles in bone aging: therapeutic strategies and applications
Abstract
As global aging intensifies, the issue of bone aging has become increasingly prominent. Osteoporosis and osteoarthritis, which are common complications of bone aging, significantly impair patients’ quality of life due to their high prevalence and disability rates, thereby presenting a major public health challenge. Extracellular vesicles (EVs), nanoscale particles released by cells, are regarded as an ideal platform for bone aging due to their high biocompatibility, ease of modification, and significant therapeutic efficacy. This review provides a comprehensive summary of the latest advancements in mammalian-, bacterial-, and plant-derived EVs, particularly in the context of bone aging. Furthermore, organoids, as lab-grown models replicating organ physiology, produce organoid-derived EVs that represent an especially promising avenue for therapeutic application. This review focuses on exploring potential therapeutic strategies that capitalize on the unique advantages of each EV type for treating bone aging. It is anticipated that a thorough comprehension of these EV types will unveil new avenues for bone aging treatment.
Keywords
INTRODUCTION
As individuals age, bone aging becomes a natural physiological process, characterized by the deterioration of bone function and a reduction in bone density[1]. These changes are a consequence of bone metabolism, and an imbalance in this process can readily lead to the onset of bone diseases, particularly osteoporosis (OP) and osteoarthritis (OA)[2]. A survey by the World Health Organization projects that the global population aged 60 and above will increase from 900 million in 2015 to 2 billion by 2050[3]. Therefore, bone aging not only affects the physical health of the elderly but also significantly affects their quality of life, exerting substantial pressure on global healthcare systems[4]. Various therapeutic drugs have been developed to regulate bone homeostasis, including molecular-targeted drugs, selective estrogen receptor modulators, synthetic parathyroid hormone, calcitonin, and bisphosphonates[5,6]. Although drugs such as bisphosphonates and denosumab are widely used and effective in managing bone loss, they may carry risks such as atypical fractures, osteonecrosis of the jaw, and rebound bone loss upon discontinuation, which limit their long-term application in certain patient populations. Consequently, the development of novel therapeutic strategies to address bone aging-related diseases has become an urgent priority.
According to the International Society for Extracellular Vesicles, extracellular vesicles (EVs) are defined as membrane-bound particles that bud from cells spontaneously and lack the ability to replicate[7]. Earlier investigations have revealed that these vesicles encapsulate multiple biologically active components, including RNA and proteins. They are crucial in regulating cellular properties and play key roles in angiogenesis, immunomodulation, cell proliferation, and intercellular communication[8,9]. EVs have garnered significant attention among researchers, particularly those dedicated to the exploration of autoimmune diseases, cancer, and degenerative disorders[10-13]. Meanwhile, researchers have identified great potential for them to modulate bone metabolism[5].
This review provides a comprehensive and systematic exploration of the generation, composition, structural characteristics, internalization mechanisms, and isolation techniques associated with the four distinct classes of EVs. Building on this foundational understanding, we delve into advanced engineering strategies for the functional modification of EVs, highlighting their potential to enhance therapeutic efficacy. Subsequently, we present an in-depth analysis of the latest research advancements in the application of EVs for the treatment of bone aging-related pathologies [Figure 1]. By elucidating the unique properties and functional roles of these four EV subtypes, this review aims to pave the way for the development of novel and targeted therapeutic interventions to address the multifaceted challenges of bone aging.
Figure 1. Both natural and engineered EVs offer significant promise in the treatment of bone aging. MEVs, BEVs, and PEVs can efficiently regulate bone metabolism, thus offering a promising therapeutic approach for related diseases. Based on existing research, we propose a novel therapeutic strategy utilizing OEVs. Created in BioRender. Bigbone B. (2025) https://BioRender.com/ldwwnlx.00. EVs: Extracellular vesicles; MEVs: mammalian extracellular vesicles; PEVs: plant-derived extracellular vesicles; BEVs: bacterial extracellular vesicles; OEVs: organoid-derived extracellular vesicles.
OVERVIEW OF EVs
EVs serve as a designation for nanovesicles secreted by cells, characterized by a phospholipid bilayer structure[14,15]. In this section, we furnish an exhaustive overview detailing the mechanisms governing the generation, structure, composition, isolation, and internalization processes associated with diverse types of EVs. We will present their generation mechanisms separately [Figure 2].
Figure 2. The biogenesis of BEVs, MEVs, and PEVs varies among different organisms. Gram-positive and Gram-negative bacteria produce EVs through distinct mechanisms. In Gram-positive bacteria, EVs are generated through a process known as bubbling cell death, specifically referred to as CMVs. Gram-negative bacteria employ two main mechanisms to produce BEVs: outer membrane blebbing, which results in OMVs, and explosive cell lysis, which leads to the formation of both OIMVs and EOMVs. MEVs are primarily produced via microvesicles, apoptotic vesicles, and the MVBs pathway, while PEVs are mainly produced through the EXPO pathway, vesicular pathway, and MVBs pathway. Created in BioRender. Bigbone B. (2025) https://BioRender.com/gxc0cps. BEVs: Bacterial extracellular vesicles; MEVs: mammalian extracellular vesicles; PEVs: plant-derived extracellular vesicles; CMVs: cytoplasmic membrane vesicles; OMVs: outer membrane vesicles; OIMVs: outer-inner membrane vesicles; EOMVs: explosive outer membrane vesicles; MVBs: multivesicular bodies; EXPO: exocyst-positive organelle; EE: early endosome.
Generation mechanism of MEVs
Based on the process of formation and biological properties, we classified mammalian-derived EVs (MEVs) into three categories, namely microvesicles, apoptotic vesicles, and exosomes[16]. Microvesicles form directly through cell membrane outgrowth, whereas apoptotic vesicles originate from cell fragmentation during apoptosis. The formation of exosomes is relatively complex and has received great attention due to their unique biological functions[17]. Here, we use MEVs to represent exosomes. The formation of MEVs can be roughly divided into three stages. Initially, the plasma membrane undergoes invagination, giving rise to endocytosed vesicles, which then undergo fusion to generate early endosomes. Following this, endocytosed vesicles undergo invagination to encapsulate intracellular cargo, forming multiple intraluminal vesicles (ILVs). These ILVs are subsequently transformed into multivesicular bodies (MVBs), which then fuse with the plasma membrane, releasing their contents into the extracellular space[18]. In previous studies, researchers explored and conclusively identified the involvement of RAB proteins, cytoskeletal components (actin and microtubule proteins), synaptic binding proteins, and Soluble NSF Attachment Protein REceptor (SNARE) proteins in the secretion process of EVs[19-21]. Furthermore, recent investigations have revealed that phosphatidylinositol plays a regulatory role in the secretion of EVs[22].
Generation mechanism of BEVs
Bacterial-derived EVs (BEVs) were initially identified as a result of outer membrane blistering in Gram-negative bacteria[23]. For an extended period, there was a prevailing belief among researchers that these vesicles were restricted to Gram-negative bacteria. Nevertheless, notably, BEVs from Gram-positive bacteria, particularly Staphylococcus aureus, have been successfully isolated from culture supernatants[24]. Subsequently, BEVs have also been observed in Streptomyces coelicolor and Bacillus subtilis using transmission and scanning electron microscopy[25,26].
Gram-positive bacteria
In previous studies, three hypotheses have been proposed regarding the generation of EVs by Gram-positive bacteria. The first is that the cell wall is partially degraded by proteases, resulting in cell wall laxity and a larger pore size for the release of EVs[24,27]. The second suggests that EVs utilize protein channels in the peptidoglycan layer to pass through the extracellular environment[28,29]. The third involves the generation of a specific pressure by EVs during their formation by the cell membrane. This pressure facilitates the traversal of EVs through the aperture of the cell wall. Concurrently, the size of the cell wall aperture exerts a measure of control over the dimensions of EVs[28,29]. Nevertheless, contemporary studies propose that Gram-positive bacteria are generated through bubbling cell death[30]. The general principle is that the peptidoglycan layer is weakened or degraded for various reasons, thereby allowing the cytoplasmic membrane vesicles (CMVs) produced by the cytoplasmic membrane to be readily released. This phenomenon has been exemplified, as seen with endolysin and β-lactam antibiotics, which weaken the peptidoglycan layer[30,31]. We postulate that other enzymes capable of disrupting the cell wall may similarly induce bubbling cell death, thereby contributing to the production of EVs.
Gram-negative bacteria
Contemporary research indicates that Gram-negative bacteria utilize two primary production mechanisms: outer membrane blebbing and explosive cell lysis[30]. The introduction of hydrophobic molecules or involvement in peptidoglycan biosynthesis within the outer membrane induces destabilization, resulting in the formation of classical outer membrane vesicles (OMVs)[23,30]. The production of BEVs is profoundly influenced by genetic background and growth conditions. The literature indicates that administration of either gentamicin or polymyxin disrupts lipid homeostasis, thereby inducing extracellular membrane stress and promoting OMV formation through membrane blebbing[32,33]. In addition, the occurrence of explosive cell lysis is associated with the attenuation of endolysin activity, causing degradation of the peptidoglycan layer. Consequently, this weakening causes the inner membrane to protrude into the periplasm, triggering vesicle production. Within this mechanism, both outer-inner membrane vesicles (OIMVs) and explosive OMVs (EOMVs) may be generated[30]. Under the same production mechanism, OIMVs possess two membranes while EOMVs have only one. A growing body of evidence suggests that antibiotics can trigger the SOS response in cells, initiating the expression of endolysin and provoking explosive cell lysis[34,35].
Generation mechanism of PEVs
Compared with other EVs, the generation mechanism of plant-derived EVs (PEVs) has received limited attention in the research domain. A more comprehensive understanding of this mechanism necessitates further exploration by researchers. Three primary mechanisms have been proposed: the vesicle pathway, the MVB pathway, and the exocyst-positive organelle (EXPO) pathway[36]. It has been reported that organelles in southern mustard cells fuse with the plasma membrane, resulting in the production of single-membrane vesicles[37]. Additionally, vesicles released via the vacuole pathway usually contain defensive substances[38]. The MVB pathway is the primary route for PEV production, and it has been reported that MVBs can fuse with the plasma membrane to release vesicles in carrot cells[39]. Many subsequent studies have also shown that this phenomenon occurs in various plant cells, confirming the MVB pathway as the main mode of PEV production[40].
Structure and composition of EVs
MEVs
Microvesicles, apoptotic vesicles, and exosomes measure approximately 200-1,000 nm, 500-2,000 nm, and 40-160 nm in diameter, respectively[41]. MEVs primarily contain a diverse array of RNA and nucleic acids, with membranes rich in sphingolipids, cholesterol, and ceramides[42,43]. The intricacy of cargo sorting is noteworthy. Current findings suggest the involvement of crucial proteins, including CD81, CD63, tumor susceptibility gene 101 protein (TSG101), apoptosis-linked gene 2-interacting protein X (Alix), and the endosomal sorting complexes required for transport (ESCRT)[41]. TSG101, Alix, CD9, CD81, CD63, SNARE, and RAB GTPases are commonly used as markers to characterize successful EV extraction and to indicate the presence of EVs[44].
BEVs
Structurally, Gram-positive bacteria have an inner layer consisting of a single lipid membrane and an outer layer containing peptidoglycan and phosphatidic acid[45]. The robust outer cell wall likely hinders the release of EVs. The first Staphylococcus aureus EVs were detected with diameters ranging from 20 to 100 nm[24]. Due to the diversity of bacterial species and distinct production mechanisms, we observed significant variation in the diameters of EVs produced by different bacteria[25,46-49]. EVs produced by Gram-positive bacteria contain various cargoes, such as endolysins, cytoplasmic membrane proteins, DNA, RNA, and virulence factors[50]. These distinct molecules may be responsible for eliciting varied biological functions. It is of significance that EVs from Gram-positive bacteria exhibit an absence of lipopolysaccharides. This distinction can be attributed to the inherent characteristic of lipopolysaccharides being a constituent of the cell wall in Gram-negative bacteria.
The structural composition of Gram-negative bacteria includes membrane structures such as an outer membrane, a cytoplasmic membrane, and a periplasmic space[51]. The structural makeup of the outer membrane consists of an outer leaflet made of lipopolysaccharides and an inner leaflet composed of phospholipids. In contrast, the cytoplasmic membrane is typically characterized by phospholipid bilayers. Concurrently, the periplasmic space encompasses layers of peptidoglycan, lipoproteins, and periplasmic proteins[52]. The reticular peptidoglycan layer within the periplasm bestows bacteria with their distinctive morphology and serves as a protective shield against absolute pressure and osmotic variations. Evidently, Gram-negative bacteria have undergone more thorough investigation in comparison to Gram-positive counterparts. The EVs produced under different mechanisms are not quite the same due to different production mechanisms. Abundant studies have substantiated the existence of DNA, RNA, periplasmic proteins, and cytoplasm encapsulated within OMVs[53,54]. In contrast, both OIMVs and EOMVs demonstrate a somewhat greater cargo capacity, including a diverse array of cytoplasmic components such as DNA, RNA, phages, hydrophobic molecules, cytoplasmic membrane proteins, outer membrane proteins, endolysins, and virulence factors[55,56].
PEVs
PEVs are bilayer vesicles ranging from 30 to 500 nm and contain a variety of nucleic acids, proteins, and metabolites[57]. Although related studies are limited, it is known that water channel proteins, membrane junctional proteins, and heat shock proteins play a role in the biogenesis of PEVs[58]. PEVs are rich in lipids such as phosphorylcholine, phosphatidic acid, and phosphatidylethanolamine, which are important for various biological functions and intercellular interactions[59]. Additionally, PEVs sourced from distinct sources exhibit a diverse array of metabolites. For instance, those derived from fruits may encompass vitamin C[60]. Ginger-derived PEVs have been reported to encapsulate bioactive constituents, including 6-gingerol and 10-gingerol, which exert anti-inflammatory and anti-cancer effects through the modulation of the nuclear factor-kappa B (NF-κB) and phosphatidylinositol 3’-kinase/Akt (PI3K/Akt) signaling pathways[61]. Furthermore, accumulating evidence indicates that PEVs can transport plant-derived microRNAs (miRNAs), such as miR-168, into mammalian cells, thereby regulating gene expression associated with inflammation and tumor progression[62].
Isolation of EVs
To better advance related research, a fast and effective method for extracting EVs is essential. MEVs are widely present in body fluids; BEVs can be extracted from culture medium, and PEVs are often obtained from plant sap. However, implementing these methods in practice presents significant challenges.
MEVs
We summarize the current methods for isolating EVs, including precipitation, density gradient centrifugation (DGC), affinity separation, ultracentrifugation (UC), ultrafiltration (UF), and size exclusion chromatography (SEC). Various separation methods present distinct advantages and drawbacks. According to the first global survey of commonly used techniques, more than 80% of researchers favored UC, including differential centrifugation, as their primary method for isolating MEVs[63]. In a recent study,
Figure 3. Overview of EV Isolation. (A) Isolation of MEVs. The process involves ultracentrifugation of pretreated samples, preceded by low-speed centrifugation to remove impurities such as cells and cellular debris; (B) Isolation of PEVs. After pretreatment, plant juice undergoes a sequential centrifugation process at low, medium, and high speeds. PEVs are then obtained from the liquid layer using the discontinuous sucrose gradient method, as shown in the figure; (C) Isolation of BEVs. Samples are first centrifuged at low speed and filtered to remove bacteria. The filtered supernatant is then concentrated using ultrafiltration membranes. BEVs are isolated by two rounds of ultracentrifugation and finally resuspended in PBS. Created in BioRender. Bigbone B. (2025) https://BioRender.com/zavrv7g. EVs: Extracellular vesicles; MEVs: mammalian extracellular vesicles; BEVs: bacterial extracellular vesicles; PEVs: plant-derived extracellular vesicles; UC: ultracentrifugation; DGC: density gradient centrifugation; UF: ultrafiltration; PBS: phosphate-buffered saline.
BEVs
Choosing different separation methods based on the characteristics of bacteria and culture media can enable strategic integration, leading to optimal yield and concentration. For example, in the cultivation process aimed at obtaining Akkermansia muciniphila (AKK), the fermentation medium for this strain is enriched with porcine mucin. Nevertheless, the surplus mucin presents challenges, notably heightened protein viscosity, thus introducing complexities into the isolation and extraction procedures of BEVs[66]. Liu et al. successfully isolated AKK-derived BEVs using a combination of UC, UF, and DGC techniques[67]. Furthermore, the growth requirements of specific bacteria, such as Helicobacter pylori, demand more stringent conditions. Although we were able to isolate the BEVs using the above methods, the microvesicles contained therein were difficult to isolate completely[41]. To address the complex culture conditions required by certain bacteria, we have developed a protocol suitable for a wide range of bacterial species. By applying this isolation protocol, BEVs were successfully isolated from strains such as E. coli Nissle 1917 and AKK [Figure 3B].
PEVs
Standard separation methods for PEVs are still not established, and to obtain high-quality PEVs, most current protocols are based on those for MEVs. At present, the combination of multiple separation methods to separate PEVs remains the best solution [Figure 3C]. Zhan et al. obtained PEVs of Pueraria lobata origin using a combination of UC and UF[68]. Interestingly, Hwang et al. also successfully isolated PEVs of yam origin using UC at different speeds[69]. It is worth mentioning that Yang et al. developed a method that combines electrophoresis with dialysis and successfully isolated PEVs from lemon juice[70]. In this strategy, separation is achieved by driving RNAs and proteins toward the cathode and anode under an electric field, while PEVs were confined in a dialysis bag. Most importantly, the number of PEVs isolated by this method is comparable to that obtained by UC and does not require expensive equipment. Without a doubt, the amalgamation of electrophoresis and dialysis emerges as an exceedingly promising method for the separation of PEVs.
Internalization of EVs
A wealth of compelling evidence suggests that internalization of MEVs, BEVs, and PEVs generally does not induce cytotoxicity[58,60,71,72]. The composition and structural features of diverse EVs demonstrate resemblances, so it is reasonable to speculate that the internalization process of EVs is roughly the same. Based on previous findings, we conclude that there are three main modes of internalization of EVs, including endocytosis, membrane fusion, and receptor-mediated signaling [Figure 4][73]. Recent evidence indicates that phagocytosis constitutes an additional route of EV internalization, occurring predominantly in phagocytic cells, including macrophages, neutrophils, and dendritic cells (DCs)[74]. The pathway by which EVs are internalized depends on both their cargo and surface structure. Within this array of mechanisms, receptor-mediated signaling stands out as a primary mode of cellular communication. It involves interactions with cells harboring specific receptors, facilitating the transmission of information, elicitation of responses, and regulation of signaling pathways. The pivotal receptors in this context are predominantly toll-like receptors (TLRs)[75]. The membrane fusion arises from the fundamental characteristic of EVs as phospholipid bilayer structures. This distinctive attribute empowers EVs to undergo direct membrane fusion with cells sharing a comparable structural composition, thereby facilitating their seamless entry into the target cells. Finally, the intricate process of endocytosis is broadly categorized into specific and non-specific types. Diverse modalities within this mechanism engage various biomolecules. In specific endocytosis, lattice proteins, vesicle proteins, dynamin, and cholesterol play a facilitating role[76,77]. In this approach, actin-based cellular protrusions, called filopodia, act as "endocytosis hotspots" for EV uptake. EVs move along retractile fibers and filopodia toward the target cell before being endocytosed[78]. In this manner, endocytosis can span a period of up to 20 min[79]. Non-specific endocytosis is broadly categorized into cytosolic drinking, heparan sulphate proteoglycan (HSPG)-mediated endocytosis, and lipid raft-mediated endocytosis[80]. The primary form of endocytosis entails the degradation of foreign cargo through fusion with lysosomes. Therefore, this approach is acknowledged as a mechanism employed by immune cells to break down non-specific antigens[81]. The latter two pathways, although classified as non-specific uptake mechanisms, reveal a certain degree of selectivity influenced by the varying abundance of HSPG and lipid rafts on specific cells in comparison to others[82,83].
Figure 4. Overview of internalization of MEVs, BEVs and PEVs. Cells uptake them through three principal mechanisms: membrane fusion, endocytosis, and receptor-mediated signaling. Subsequently, once inside the cell, they release their contents, fulfilling their designated biological functions. Created in BioRender. Bigbone B. (2025) https://BioRender.com/e93q0ii. EVs: Extracellular vesicles; MEVs: mammalian extracellular vesicles; BEVs: bacterial extracellular vesicles; PEVs: plant-derived extracellular vesicles.
ENGINEERING OF EVs
EVs have potential applications in bone disease therapy; however, their mass production is challenging due to various limitations, such as the low yield of MEVs. To address this, we focused on BEVs. To obtain better immunogenicity, we focused on PEVs. However, EVs still face some common limitations, the most obvious being a lack of targeting ability, low therapeutic efficiency, and poor pharmacokinetics. These challenges can be addressed through engineering modifications [Figure 5]. In addition, we propose using artificial intelligence (AI) to assist in EV modification.
Figure 5. Overview of engineering modifications to EVs. It is divided into modification of parental strains, physical modification, chemical modification, and biological modification. Created in BioRender. Bigbone B. (2025) https://BioRender.com/sy9pesy. EVs: Extracellular vesicles; CRISPR-Cas9: clustered regularly interspaced short palindromic repeats-associated protein 9.
Engineering parental strain
Due to the distinctive biogenesis mechanisms associated with BEVs, including explosive cell lysis and bubbling cell death, there is the potential for BEVs to access the contents within the parent bacteria. In recent years, Tang et al. introduced the concept of materials synthetic biology, a conceptual framework that amalgamates the engineering principles of materials science and synthetic biology. This integration systematically transforms living systems into dynamic and responsive materials with emergent and programmable functions[84]. Combined with the unique advantages of BEVs, this integration makes BEVs full of possibilities in enhancing therapeutic OA capabilities. Chen et al., Li et al. and Cheng et al. have applied synthetic biology strategies to apply BEVs in the antitumor field and have achieved success[71,85,86]. In the engineering of parental strains, two main strategies are overexpression and knockout[72]. Among the overexpression strategies, the focus is primarily on either bone therapeutic effectors or bone-targeting effectors.
Currently, specific effectors can be overexpressed or knocked down based on clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 and plasmid-based modification techniques. CRISPR/Cas9 genome editing enables producer cells to stably express bone-targeting ligands (SDSSD, E7, or bisphosphonate peptides) or therapeutic proteins on EV membranes via lysosome-associated membrane protein 2b (LAMP2B) or CD63 fusion constructs[87]. EVs can also be engineered to deliver transient CRISPR complexes for in vivo editing, with rigorous off-target evaluation imperative[88]. Although bone morphogenetic protein-2 (BMP-2) is a potent osteoinductive agent, its clinical use has been linked to ectopic ossification, inflammatory responses, and osteolytic reactions. To mitigate these risks in EV-based delivery platforms, potential strategies include localized controlled release systems (EV-loaded hydrogels), bone-homing ligand modification to reduce systemic exposure, membrane-anchored BMP-2 display to restrict diffusion, using inducible or transient expression systems in producer cells, and substituting BMP-2 with pro-osteogenic miRNAs or small molecules to reduce ectopic ossification risk. Vascular endothelial growth factor (VEGF) and BMP-2 are typical bone therapeutic effectors. BMP-2 significantly promotes osteoblast differentiation in bone marrow mesenchymal stem cells (BMSCs)[89]. Hay et al. orchestrated the growth of stem cells and the process of osteogenesis by strategically overexpressing BMP-2 within engineered Lactococcus lactis[90]. Likewise, VEGF serves as a potent factor in vascular growth, making a substantial contribution to the differentiation of osteoblasts[91]. Lu et al. also applied Lactococcus lactis overexpressing VEGF to regulate angiogenesis[92]. The CXC chemokine receptor 4 (CXCR4) possesses bone-targeting capabilities, with its ligand being stromal cell-derived factor 1 (SDF1) expressed by BMSCs[93]. Cheng et al. devised a display system utilizing Cly A, enabling the presentation of multiple proteins on the membrane surface of BEVs[86]. Thus, receptors or ligands similar to CXCR4 and SDF1 will be displayed by the Cly A display system, potentially providing access to bone-targeting-competent BEVs. The CRISPR-Cas9 and λ-red recombination systems are commonly used tools in the knockout strategy. It has been confirmed that deleting specific genes (msbA, msbB, lpxM, and lpxL1) also results in the production of low-toxicity BEVs[72]. Thomas et al. formulated a strain wherein the nlpL gene was deliberately removed, significantly amplifying the production of BEVs[94].
Analogous strategies have been applied to parental plant strains, where editing tools are used to eliminate or introduce plant-specific effectors[95]. Researchers anticipate that transgenic plants could produce PEVs with tailored functions. However, challenges such as low editing rates, protracted growth periods, off-target effects, and the robustness of plant cell walls have temporarily hindered the realization of this groundbreaking concept[96].
Biological engineering
At present, the genetic engineering of EVs can be comprehensively classified into three primary methodologies. First, the application of fluorescent or radioactive tracer technology facilitates accurate
Lai et al. used a combination of Gaussia luciferase and metabolic biotinylation to create an EV for multimodal imaging, which was able to demonstrate the distribution and metabolism of EVs in vivo, providing a new approach for subsequent pharmacological studies[97]. Zhang et al. obtained lentiviral-transfected EVs with high Syndecan-1 expression, and EVs played a good therapeutic role in a lipopolysaccharide-stimulated acute lung injury model[102]. The overexpression strategy is also very practical in BEVs, as mentioned above. Endowing EVs with targeting ability has been a hot topic of research, and LAMP2B is the most commonly used related protein. Due to the adaptability of the N-terminus of LAMP2B in accepting diverse targeting sequences, researchers have documented a range of outcomes. Notably, fusing human epidermal growth factor receptor 2 with the N-terminus of LAMP2B demonstrated targeted binding for colorectal tumor applications[103]. The cardiac-targeting peptide, upon binding to the N-terminus of LAMP2B, intensifies the targeting of cardiac tissue. Simultaneously, the attachment of cartilage affinity peptides to the N-terminus of LAMP2B enhances its ability for cartilage targeting[100,104]. Notably, the transmembrane protein CD63 has also been a hotspot for researchers. Liang et al. engineered MEVs with hepatocellular carcinoma-targeting ability by expressing a gene fusion between the transmembrane protein CD63 and the Apo-A1 sequence in 293T cells[105].
Chemical engineering
In the realm of chemical engineering, both covalent and non-covalent reactions are widely employed methodologies. Non-covalent interactions primarily include receptor-ligand binding, multivalent electrostatic interactions, and hydrophobic insertion. Meanwhile, covalent reaction methods provide key strategies, including bioconjugation, aldehyde-amine reactions, and click chemistry.
Receptor-ligand interactions rely on the specific recognition between receptors and their corresponding ligands, enabling targeted binding and signaling. Liu et al. designed a technique using dip-pen nanolithography to generate lipid membrane microarrays loaded with specific antibodies, including biotin antibodies and streptavidin[106]. This approach enables the selective and efficient separation of EVs. Yang et al. devised a pH-responsive superparamagnetic nanoparticle cluster, capitalizing on the specific recognition between transferrin and the transferrin receptor. This specificity facilitated the successful isolation of transferrin receptor-positive EVs from blood[107]. The mechanism of multivalent electrostatic interactions hinges on the interplay between positive and negative electrostatic attractions[108]. Due to the inherently negatively charged nature of EV membranes, Tamura et al. used a simple mixture of cationic branched-chain starch with EVs. Their investigations unveiled that these EVs exhibited an expedited internalization into HepG2 cells and an enhanced affinity for the liver[108].
Bioconjugation is generally based on the covalent binding of two molecules. Gao et al. reported that CD63, a membrane protein in MEVs, was able to bind to COP5, thereby achieving efficient capture of EVs[111]. Aldehyde-amine condensation can be used for EVs binding to aptamers, which can be used for precision medicine due to the extremely high affinity and specificity of aptamers[112]. Primary amines, sulfhydryl groups, and carboxyl groups in the membrane surface proteins of EVs offer possibilities for click chemistry. Different functional groups can be covalently linked for various purposes. According to reports, cerium oxide successfully binds to EVs via a thiol-maleimide reaction and modulates both innate and adaptive immunity in a collagen-induced arthritis model[113].
Physical engineering
The most commonly used strategies in physical engineering are membrane coating, membrane fusion, and loading drugs. Membrane coating can effectively increase the extensibility and functionality of EVs[114].
Membrane fusion commonly refers to the merging of EVs and liposomes. Prior to this fusion, liposomal membranes are subject to functionalization, bestowing diverse abilities upon the EVs[116]. Their fusion can be achieved by several routes, including polyethylene glycol (PEG)-mediated fusion, co-incubation, and extrusion[93,117,118]. Hu et al. reported that a nanoparticle with bone-targeting ability and strong therapeutic effect was constructed by fusing bone-targeting effector-containing MEVs with bone therapeutic effector-containing liposomes via extrusion[93]. Piffoux et al. also found the fusion of PEG-triggered EVs with functionalized liposomes[117]. Lin et al. developed EV-liposome hybrids by simple incubation and successfully encapsulated a CRISPR-Cas9 expression vector[118].
Drugs can be loaded into EVs in various ways, such as incorporation into nanomaterials for direct fusion with EVs, loading into nanomaterials for fusion with donor cells, or co-incubation with mother cells[119-121]. Drugs can also be loaded by electroporation, ultrasound, hypo-osmotic dialysis, and extrusion into EVs[122-125]. Jia et al. employed electroporation to encapsulate curcumin within EVs, successfully enabling its traversal of the blood-brain barrier and facilitating targeted drug delivery to the brain[126]. Lee et al. used lipid loading of various functional groups to achieve a wide variety of functionalities in EVs[127]. Saari et al. adeptly synthesized paclitaxel-loaded EVs via the incubation of EVs derived from Lymph Node Carcinoma of the Prostate (LNCaP) and PC-3 prostate cancer (PCa) cells with paclitaxel at a temperature of 22 °C for a period of 1 h[119].
AI-enabled construction of EVs
AI is an important component of computing. With the relentless progress of technology, it is enhancing numerous conventional industries and substantially boosting operational efficiency. Remarkably, there has been a proposal to leverage AI to empower the field of biomedicine[128-130]. Bai et al. introduced an innovative concept of applying AI to organoid research[131]. Incorporating AI holds the potential to significantly improve the design and construction of organoids, facilitating dynamic monitoring of culture conditions. We believe that similar approaches can accelerate research on EV engineering. Configuring distinct functional components to confer specific functionalities on EVs is a labor-intensive process. However, by applying predefined screening criteria, AI can rapidly identify the required components, thereby substantially enhancing efficiency. Furthermore, AI can offer more efficient modification techniques tailored to diverse cell sources and their respective characteristics[132]. Deep learning-based computational platforms also enable the precise prediction of bone-homing peptides and receptor-specific ligands, thereby enhancing the precision of targeted EV delivery[133]. In addition, the integration of multi-omics data through advanced AI algorithms allows for the systematic identification and selection of optimal therapeutic cargo, specifically tailored to address the complexities of bone-aging pathologies. Machine learning-driven optimization of EV production parameters improves scalability, yield, and batch-to-batch reproducibility, effectively addressing critical challenges in the manufacturing process. Furthermore, AI-powered real-time quality control systems that leverage high-throughput imaging and spectral analysis ensure stringent batch consistency and adherence to clinical standards. Collectively, these synergistic AI-driven strategies are poised to accelerate the development of safe, efficient, and clinically translatable engineered EVs, offering promising solutions for combating bone aging.
BONE AGING MICROENVIRONMENT AND DISEASE
Bone is a dynamic organ whose structure and function are precisely regulated by various mechanisms, such as hormonal changes, oxidative stress, genetic factors, and chronic inflammation[3,134]. These processes become progressively altered with age, contributing to bone aging. The decline in estrogen levels disrupts the balance between osteoblasts and osteoclasts, resulting in increased bone resorption[72]. Oxidative stress, induced by the accumulation of reactive oxygen species (ROS), impairs osteoblast function and disrupts bone remodeling[135]. Genetic factors contribute to reduced bone mineral density and increased bone fragility, while chronic inflammatory microenvironments further promote bone resorption and suppress bone formation. The interplay of these factors contributes to a progressive deterioration in bone quality and heightened susceptibility to conditions such as OP and OA[134]. OP is characterized by reduced bone mineral density and microstructural deterioration, whereas OA is marked by articular cartilage degeneration and the formation of osteophytes. Therefore, a comprehensive understanding of bone aging and its underlying mechanisms is crucial for the development of innovative therapeutic strategies targeting OP and OA.
APPLICATIONS OF EVs FOR OP
OP is a systemic bone metabolic disorder distinguished by reduced bone mass and disruption of skeletal microarchitecture. Scholars have identified that EVs play a pivotal role in the modulation of bone homeostasis and are currently recognized as promising targets for addressing OP[136]. Next, we summarize the roles of MEVs, BEVs, and PEVs in the OP [Table 1].
EVs from various cell origins and their physiological significance in OP
| Type | Cell source of EVs | Function | Reference |
| MEVs | DCs | Modulate local immune reactions | [137] |
| ECs | Target bone tissue | [138] | |
| Osteoblasts | Promote differentiation of BMSCs into osteoblasts | [139] | |
| AFSCs | Attenuate steroid-induced oxidative stress | [140] | |
| DPSCs | Regulate telomerase activity and reduce osteoclast population | [141,142] | |
| USCs | Deliver osteoprotegerin to promote osteogenesis | [143] | |
| hiPSC-MSCs | Promote angiogenesis and bone regeneration | [144] | |
| BMSCs | Upregulate osteogenic effectors and decrease osteoclast numbers | [145] | |
| AMSCs | Inhibit activation of the NLRP3 inflammasome in osteoclasts | [146] | |
| UCMSCs | Enhance bone formation, diminish bone marrow fat accumulation, and mitigate bone resorption | [147] | |
| BEVs | AKK | Promote bone formation | [67] |
| PM | Inhibit osteoclast formation | [148] | |
| PEVs | Yam | Promote osteoblast formation | [69] |
| Pueraria lobata | Enhance BMSC differentiation via increased autophagy | [68] |
MEVs
Numerous studies have proved that MSCs of various origins can achieve therapeutic effects on OP by modulating bone homeostasis, stimulating vascular regeneration, and inhibiting the activation of nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammatory vesicles[149]. Notably,
Figure 6. The role of MEVs in OP. (A) EVs expressing RANK from BMSCs were designed to remodel bone homeostasis for the treatment of OP. Copyright 2024 American Chemical Society; (B) EVs derived from M2 macrophages can promote the differentiation of osteoclast precursor cells into M2 macrophages and, through the upregulation of glutamate, reduce bone resorption and enhance bone repair. Copyright 2025 Elsevier Inc; (C) The condition of skeletal muscle directly affects the quantity and functionality of the EVs secreted by it. These EVs can be endocytosed by BMSCs, promoting the glycolysis process through the delivery of LDHA and subsequently regulating osteogenic differentiation. Copyright 2023 Elsevier Inc; (D) By leveraging biominerals and EVs with bone-targeting capabilities, a multifunctional biomimetic EV system can be engineered. This system not only enhances the osteogenic differentiation of BMSCs but also demonstrates the capacity to induce angiogenesis and promote collagen mineralization. Copyright 2025 American Chemical Society. MEVs: Muscle-derived extracellular vesicles; OP: osteoporosis; EVs: extracellular vesicles; RANK: receptor activator of nuclear factor kappa-B; BMSCs: bone marrow mesenchymal stem cells; LDHA: lactate dehydrogenase A.
The expression of gene transcripts meticulously regulates the differentiation process of MSCs, directing them toward either adipocyte or osteoblast lineages[151]. However, MEVs from osteoblasts of OP patients showed that the reduced osteogenic rather than proliferative capacity of BMSCs, reflecting the influence of the bone microenvironment on cell metabolism in OP[152]. MEVs secreted by osteoblasts and osteoclasts first attracted the interest of researchers. Wei et al. reported that MEVs from osteoblasts can significantly enhance the osteogenic differentiation of BMSCs via positive feedback[139]. Li et al. also disclosed that osteoclast-secreted MEVs inhibited bone formation in osteoblasts by delivering miR-214-3p[153]. Therefore, strategies that inhibit MEV secretion by osteoclasts may offer a fresh perspective for the therapeutic intervention of OP. Similarly, EVs from different sources of stem cells have attracted the interest of researchers. For example, MEVs secreted by amniotic fluid stem cells (AFSCs) can treat OP by attenuating steroid-induced oxidative stress[140]. MEVs released by dental pulp stem cells (DPSCs) demonstrate the ability to regulate telomerase activity, consequently leading to a reduction in the count of tartrate-resistant acidic phosphatase (TRAP)-positive osteoclasts[141,142]. MEVs secreted by urine-derived stem cells (USCs) can deliver osteoprotegerin (OPG) and restore bone density for the OP treatment[143]. Other MEVs secreted by stem cells, including those from human induced pluripotent stem cells (hiPSC-MSCs)[144], BMSCs[145], adipose-derived mesenchymal stem cells (AMSCs)[146], and umbilical cord mesenchymal stem cells (UCMSCs)[147], have also shown efficacy in treating OP. Collectively, MEVs from diverse stem cell sources provide a completely new direction for OP therapy.
BEVs
Fecal microbiota transfer is a developing therapeutic modality. Liu et al. demonstrated that transplanting gut microbes from children effectively treated ovariectomy (OVX)-induced OP in mice[67]. It was reported that AKK played an important role. Following this, the direct application of AKK to OVX-induced mice demonstrated therapeutic efficacy in addressing OP. Additionally, the therapeutic effect produced by AKK was significantly reduced after inhibiting the production of BEVs with GW4869. In contrast, direct administration of BEVs produced by AKK secretion to OVX-induced mice produced a therapeutic effect comparable to that of the parent strain. Therefore, it can be concluded that BEV produced by gut microorganisms can modulate bone homeostasis and thereby achieve a therapeutic effect on OP [Figure 7]. This finding reveals a novel intestinal-bone axis model of bone metabolism regulation.
Figure 7. Overview of the role of BEVs in OP. (A) Anchor bone-targeting peptides onto the membrane of BEVs and achieve targeted treatment of osteoporosis by delivering endogenous miRNAs. Copyright 2023, Elsevier Ltd; (B) BMP-2 and CXCR4 components were synthesized to construct the recombinant plasmid pClyA-BMP-2-CXCR4, which facilitated the generation of the recombinant probiotic ECN-pClyA-BMP-2-CXCR4. This recombinant probiotic was then cultured to produce BEVs with bone-targeting and osteogenic capabilities. Copyright 2025, Wiley Periodicals LLC. BEVs: Bacterial extracellular vesicles; OP: osteoporosis; miRNAs: microRNAs; BMP-2: bone morphogenetic protein-2; CXCR4: C-X-C chemokine receptor type 4; pClyA: plasmid carrying cytolysin A; ECN: Escherichia coli Nissle 1917.
Recently, Wang et al. also found that BEVs derived from the gut microorganism Proteus mirabilis (PM) could inhibit osteoclastogenesis and bone resorption[148]. Their study showed that PM-secreted BEVs induced mitochondrial dysfunction through collapse of mitochondrial membrane potential, elevated intracellular ROS levels, and modulation of Bax, Bcl-2, caspase-3, and cytochrome c expression, along with strong inhibition of miR-96-5p expression. Overall, PM-secreted BEVs possess osteoprotective functions, further enriching the emerging regulatory paradigm of BEVs-mediated gut-bone axis.
Each of the previously mentioned studies has illuminated a nascent paradigm in the coordination of bone metabolism, presenting a fresh therapeutic avenue for addressing OP. In particular, a solid foundation has been established for using probiotics, along with functional BEVs, in the OP treatment. We also expect that more BEVs will be discovered to further enrich the gut-bone axis regulation model.
PEVs
Previous studies have demonstrated the pivotal role of Chinese herbs in the treatment of OP[154]. Following the confirmation of the substantial role played by EVs in regulating the bone microenvironment between osteoblasts and osteoclasts, researchers shifted their focus to PEVs. Exosome-like vesicles derived from yam and Pueraria lobata have been reported to alleviate OP by modulating osteoblasts or by degrading trimethylamine-N-oxide (TMAO) and enhancing autophagy, as shown in Figure 8[68,69].
Figure 8. Overview of the role of PEVs in OP (A) EVs of yam origin, when administered orally, stimulate osteoblast formation, thereby effectively alleviating OP. Copyright 2023, Elsevier B.V.; (B) Pueraria lobata-derived EVs promote osteogenesis through enhanced autophagy. Copyright 2023, Elsevier B.V. PEVs: Plant-derived extracellular vesicles; OP: osteoporosis; EVs: extracellular vesicles; TMAO: trimethylamine-N-oxide; OVX: ovariectomy; PELNs: pueraria lobata-derived exosome-like nanovesicles; YNVs: yam-derived exosome-like nanovesicles; S-YNVs: small size of YNVs.
In a recent breakthrough, Huang et al. achieved the successful isolation and characterization of PEVs from yam. The incorporation of these PEVs into the osteoblast culture medium yielded noteworthy results, notably stimulating the proliferation, differentiation, and mineralization of osteoblasts[69]. Subsequently, a comparative assessment of the osteogenic activity between PEVs and the saponin elements present in yam (specifically, components such as dioscin and diosgenin employed in OP treatment) unveiled the heightened osteogenic activity of PEVs. Notably, only a small amount of saponins could be extracted from a large amount of yam, whereas a large amount of PEVs could be extracted from a small amount of yam. Bioinformatics analysis further indicated that the osteogenic activity of PEVs is induced by p-p38/BMP-2-dependent activation of the Runx2 pathway. Finally, the OVX-induced OP symptoms were successfully alleviated by oral administration in OVX-induced OP mice. Surprisingly, the PEVs were able to maintain alkaline phosphatase (ALP) activity after storage at -80 °C for one year and remained non-biological toxicity at higher concentrations.
In 2023, Zhan et al. also found the ability of Pueraria lobata-derived EVs to alleviate OP. Furthermore, they identified TMAO, a metabolite associated with the gut microbiota, as a prospective target for OP therapy[68]. Similarly, the researchers successfully isolated and characterized the PEVs, which were added to the BMSC medium and successfully stimulated the differentiation and mineralization of BMSCs. Subsequently, researchers proceeded to analyze the differences in the fecal microbiota between normal individuals and those diagnosed with OP. The study revealed a significant increase in Clostridium in the feces of OP patients, accompanied by an anomalous elevation of TMAO in the serum of individuals afflicted with OP. Interestingly, Clostridium can produce TMAO, which inhibits osteoblast mineralization and the expression of associated osteogenic proteins. More interestingly, these effects were reversed after treatment with PEVs. Bioinformatics analysis suggested that the osteogenic activity of PEVs is mediated by regulating TMAO-regulated autophagy in BMSCs. Finally, the researchers again verified that PEVs could reverse TMAO-induced OP in a rat model.
APPLICATIONS OF EVs FOR OA
EVs, as mediators of intercellular communication, have been extensively studied in the pathogenesis and diagnosis of OA[155]. Similarly, owing to their exceptional biological properties, EVs are regarded as promising biopharmaceuticals and drug delivery vehicles[156]. While MEVs from various origins exhibit considerable potential for the OA diagnosis and treatment, BEVs and PEVs have not yet garnered significant attention from researchers. This section focuses on the research progress of using MEVs in the treatment of OA. We also propose potential therapeutic strategies informed by previous studies, integrating the advantages of BEVs and PEVs [Figure 9].
Figure 9. EVs from different sources for OA therapy. The existing and potential future donors of EVs are comprehensively introduced. EVs of organoid, bacterial, and plant may be potential therapeutic agents. Created in BioRender. Bigbone B. (2025) https://BioRender.com/wpbir74. EVs: Extracellular vesicles; OA: osteoarthritis; BMSCs: bone marrow mesenchymal stem cells; ADSCs: adipose-derived stem cells; SMSCs: synovium-derived mesenchymal stem cells; EMSCs: endometrium-derived mesenchymal stem cells; UMSCs: umbilical cord mesenchymal stem cells; AMSCs: amniotic mesenchymal stem cells; AFSCs: amniotic fluid stem cells; SHED: stem cells from human exfoliated deciduous teeth; CPCs: chondroprogenitor cells; PRP: platelet-rich plasma; hypACT: hyperacute serum.
MEVs
Different sources of MEVs have shown varied roles in OA progression. MEVs have been extensively studied as biomarkers for OA diagnosis[157]. Here, we provide only a brief overview. EVs derived from blood, synovial fluid within the joint cavity, and tissue sources in patients with OA exhibit distinct differences compared to those from healthy individuals[158-160]. These differences offer significant potential for early OA diagnosis, especially in cases where symptoms are not yet apparent. Notably, EVs derived from synovial fluid are rich in substances secreted by joint structures, reflecting the status of OA. They also have the potential to identify different OA subtypes based on their distinct contents[161]. It is important to note that EV-based diagnostic methods primarily target specific joints, and their reliability diminishes when multiple joints are involved. This section focuses on summarizing the roles of different MEVs in OA treatment [Table 2].
The role of MEVs in the treatment of OA
| Cell source of EVs | Functions | Reference |
| BMSCs | Regulate macrophage polarization, inhibit inflammation in OA, and suppress IL-1β-induced chondrocyte senescence and apoptosis | [162-164] |
| ADSCs | Inhibit M1 macrophage infiltration in the synovium and suppress the inflammatory response by targeting the NF-κB signaling pathway | [165,166] |
| SMSCs | Inhibit PTEN expression to suppress inflammation and apoptosis; target HMGB1 to alleviate the progression of OA | [167,168] |
| SHED | Inhibit chondrocyte inflammation | [169] |
| AMSCS | Promote macrophage polarization and inhibit inflammatory T cells | [170] |
| UMSCs | Inhibit chondrocyte ROS production and regulate macrophage polarization | [171,172] |
| EMSCs | Increase type II collagen synthesis and inhibit ADAMTS5 expression to protect chondrocytes and suppress IL-1β-induced MMP13 production | [173,174] |
| AFSCs | Promote cartilage repair through TGF-β | [175] |
| hypACT | Promote COL2A1 and ACAN expression and enhance chondrocyte anabolic activity | [176] |
| PRP | Inhibit TNF-α and activate the Wnt/β-catenin signaling pathway | [177] |
| Monocytes | Enhance chondrocyte differentiation and function | [178] |
| Chondrocytes | Inhibit inflammation and regulate mitochondrial function | [179] |
| Tendon cells | Promote MSC differentiation into tendon cells | [180] |
| Sea cucumber | Inhibit synovial inflammation | [181] |
| Antler velvet | Promote MSC proliferation and inhibit inflammation and MSC senescence | [182] |
| Milk | Regulate immunity and inhibit inflammation | [183] |
Stem cell-based treatments for OA have proven to be highly promising approaches[184]. However, tumorigenicity and immune rejection continue to be significant obstacles in stem cell therapy[185,186]. The advent of EVs presents an optimal solution to these issues. He et al. demonstrated that EVs derived from BMSCs can upregulate COL2A1 expression and downregulate matrix metalloproteinase-13 (MMP13) expression, thereby promoting chondrocyte repair and maintaining extracellular matrix stability[187]. Meanwhile, Zhang et al. found that BMSC-derived EVs could attenuate the progression of OA by modulating macrophage polarization[164]. Woo et al. demonstrated that EVs derived from adipose mesenchymal stem cells (ADSCs) effectively inhibit M1 macrophage infiltration in the synovium, thereby reducing chondrocyte catabolism[165]. It is worth mentioning that ADSC-EVs have demonstrated an extremely strong therapeutic role in early OA. Researchers have also found that EVs derived from synovial mesenchymal stem cells (SMSCs) can deliver miR-129-5p and alleviate OA progression by targeting high-mobility group box 1[167]. Luo et al. found that EVs of human exfoliated deciduous teeth stem cells (SHED) also delivered MiR-100-5p to inhibit inflammation in OA[169]. EVs derived from amniotic membrane mesenchymal stem cells (AM-MSCs), UMSCs, embryonic mesenchymal stem cells (EMSCs), and AFSCs have also shown therapeutic potential for OA[170,171,173,175].
Additionally, EVs from body fluids, adult cells, marine organisms, and antler sources have attracted the attention of researchers. These donor cells tend to have simpler functions than stem cells, which also means their quality is more controllable. Blood-derived EVs have been shown to reduce pain and inflammation by intra-articular injection[188]. Macrophages are closely associated with the progression of OA. Bai et al. found that EVs derived from M2-type macrophages, which deliver SOX9 to host cells, enhance chondrocyte function[178]. Cartilage is a crucial component of the joint, responsible for maintaining extracellular matrix homeostasis. The state of chondrocytes significantly influences the progression of OA. Zheng et al. demonstrated that EVs originating from cartilage can alleviate OA by modulating mitochondrial and immune responses[179]. Similarly, the tendon is a structure that maintains joint stability, and its disruption often leads to OA[189]. Xu et al. demonstrated that EVs derived from tendon cells can promote the differentiation of mesenchymal stem cells into tendon cells via transforming growth factor β (TGF-β) signaling[180]. Marine organisms are rich sources of biomaterials, with sea cucumbers particularly attracting researchers’ interest due to their strong regenerative abilities. Jo et al. employed collagenase treatment on lyophilized sea cucumber extracellular matrix to obtain EVs and demonstrated their ability to decrease COX-2 expression and reduce synovial inflammation[181]. This study demonstrates the potential of marine-derived EVs in treating OA. Antler velvet, which is a unique organ capable of periodic complete regeneration, has also been studied. Lei et al. discovered that EVs derived from deer antlers can alleviate OA by reducing P16 and P21 protein levels, β-galactosidase activity, and senescence-associated secretory phenotype gene expression[182].
With the rapid advancement of organoid technology, EVs derived from organoids have garnered increasing attention. These EVs, obtained through 3D culture, are regarded as more representative of in vivo metabolism[190]. Recently, EVs derived from retinal organoids have been shown to protect retinal epithelial cells from damage by regulating fatty acid metabolism[191]. A comparison between organoid-derived EVs (OEVs) and human embryonic stem cell-derived EVs revealed that OEVs exhibit greater selectivity in protein loading. It is worth noting that OEVs are enriched with proteins involved in immune regulation and retinal development. This study demonstrated that OEV has better efficacy than 2D culture-derived EVs. Researchers have successfully constructed cartilage-like organs and achieved cartilage repair by utilizing induced pluripotent stem cells (iPSCs) and BMSCs[192,193]. However, cartilage-like organ-derived EVs have not been thoroughly investigated. These OEVs may offer enhanced therapeutic effects and provide additional insights into the pathogenesis of OA. Further exploration by researchers is warranted.
While natural MEVs have shown promising therapeutic potential, modified EVs offer superior targeting, delivery efficiency, and therapeutic efficacy. The modification methods have been outlined previously. Here, we focus on the application of engineered EVs in OA. Current strategies for treating OA using engineered MEVs are broadly categorized into targeting enhancement and therapeutic capacity improvement [Figure 10]. Xu et al. engineered E7-MEVs by fusing the MSC-binding peptide E7 to the membrane protein LAMP2B of MEVs, thereby conferring targeting ability[194]. E7-MEVs successfully delivered kartogenin to synovial MSCs, promoting chondrocyte formation. Additionally, chondrocyte-affinity peptide (CAP) was fused with membrane proteins to create cartilage-targeted CAP-MEVs[104]. CAP-MEVs specifically deliver miR-140 to chondrocytes, thereby effectively inhibiting cartilage-degrading proteases. Additionally, enhancing the anti-inflammatory capacity of MEVs is also a powerful therapeutic strategy. A large number of herbs contain anti-inflammatory activity, such as curcumin, icariin, and quercetin. However, stability and bioavailability issues often limit the application of these compounds. Numerous studies have demonstrated that EVs obtained from BMSCs and ADSCs pretreated with curcumin exhibit enhanced cartilage protection[195,196]. Similarly, EVs derived from MSCs pretreated with hypoxia exhibit high expression of specific miRNAs, thereby enhancing cartilage repair[197,198]. It is evident that the delivery of specific miRNAs or therapeutic agents can significantly enhance therapeutic efficacy. Researchers have utilized gene transfection and electroporation to deliver miR-126-3p, miR-127-3p, miR-361-5p, and kartogenin to mitigate the progression of OA[194,199-201].
Figure 10. MEVs for OA therapy. (A) MEVs loaded with curcumin are obtained through co-culture with ADSCs, and these MEVs exhibit excellent cartilage-protective effects. Copyright 2022 Springer Nature; (B) Exogenous miR-223 is loaded into MEVs via electroporation to enhance their therapeutic efficacy, followed by surface modification with a type II collagen-targeting peptide to confer targeting capability. These bifunctional MEVs exhibit strong therapeutic effects against OA. Copyright 2023 Elsevier; (C) An injectable multifunctional therapeutic system was developed by encapsulating T cell-derived EVs into lubricative hydrogel microspheres. The inflammatory microenvironment was effectively modulated, cartilage homeostasis was promoted, and cartilage surface friction was reduced by this system, thereby advancing cartilage remodeling and OA treatment. Copyright 2024 American Chemical Society; (D) The hybrid nanoparticles, fabricated through the hybridization of nicotinamide-encapsulated and Col2A1 antibody-modified liposomes with TGF-β1-overexpressing EVs, were demonstrated to exhibit targeted delivery, anti-inflammatory, and cartilage-protective capabilities, effectively mitigating the progression of OA. Copyright 2024 American Chemical Society. MEVs: Macrophage-derived extracellular vesicles; OA: osteoarthritis; ADSCs: adipose-derived stem cells; miR: microRNA; EVs: extracellular vesicles; Col2A1: type II collagen alpha 1 chain; TGF-β1: transforming growth factor beta 1.
BEVs
In recent years, BEVs have seen increasing application in bone diseases[202]. Using synthetic biology techniques, Liu et al. successfully displayed BMP-2 and CXCR4 on the membrane surface of BEVs, endowing them with both targeting and therapeutic capabilities[203]. This study indicates that BEVs hold great promise for bone disease treatment due to their highly modifiable properties. Additionally, the ease of culturing bacteria at an industrial scale enhances their potential for future clinical applications. Notably, both natural and engineered BEVs have shown therapeutic potential for OP[72]. However, there is a notable lack of studies on the use of BEVs for OA treatment. In this section, we propose potential strategies for utilizing BEVs in OA treatment to advance the field. Rapid advancements in synthetic biology have enabled effective customization of BEVs[86]. For example, we engineered BEVs by fusing CAP to their membrane proteins, thereby enhancing targeting ability. This approach facilitates the precise delivery of a large number of carriers to chondrocytes. Electroporation is employed to load drugs or miRNAs into BEVs for therapeutic effects. This method represents a promising strategy for OA treatment using BEVs. Additionally, strategies targeting macrophage polarization and immune response modulation have been shown to effectively alleviate OA[171]. However, the use of EVs derived from human umbilical cord mesenchymal stem cells (hUCMSCs) still faces the common issue associated with MEVs, such as low yield. It is clear that the emergence of BEVs perfectly addresses this issue. We can easily polarize macrophages by displaying IL-4 on the membrane surface of BEVs[204]. Simultaneously, by displaying CAP on the membrane surface of BEVs, we can engineer them with the ability to target cartilage for immune modulation. Furthermore, different regulators can be presented on BEV membranes to modulate mechanisms involved in OA pathogenesis, enhancing therapeutic effects. Additionally, Liu et al. demonstrated that BEVs from the gut of children exhibit a natural therapeutic role in OP treatment[67]. This study proposes a novel mechanism of gut-bone communication, laying the foundation for the development of oral BEVs. The role of gut microbiota in influencing OA progression through the gut-bone axis is well established[205,206]. Similarly, it is valuable for researchers to investigate whether BEVs derived from gut microbes have therapeutic potential for OA [Figure 11].
Figure 11. Both natural and engineered BEVs are promising therapeutic agents for OA therapy. BEVs play a crucial role in the gut-bone axis communication. Therefore, besides using these bacteria directly to alleviate OA, utilizing EVs derived from these bacteria may offer a superior treatment option. Additionally, both engineered strains and BEVs can be used to produce functional BEVs. These engineered BEVs exhibit enhanced properties, including reduced toxicity, high yield, targeted delivery to chondrocytes, and improved cartilage repair capabilities, thus providing greater potential for OA treatment. Created in BioRender. Bigbone B. (2025) https://BioRender.com/cw92itq. BEVs: Bacterial extracellular vesicles; OA: osteoarthritis; EVs: extracellular vesicles.
PEVs
Currently, strategies for treating OA based on PEVs are virtually non-existent. However, it has been confirmed that MEVs with plant pretreatment possess enhanced cartilage repair capabilities[195,196]. Anti-inflammatory treatment is a crucial component of OA management. Recent research indicates that PEVs exhibit strong anti-inflammatory capabilities [Table 3].
Mechanisms of action of anti-inflammatory PEVs
| Source of EVs | Mechanism | Reference |
| Ginger | Promote IL-10 and inhibit TNF-α, IL-6, and NLRP3 | [207] |
| Grapefruit | Regulate macrophage HO-1 expression and inhibit IL-1β and TNF-α | [208] |
| Cabbage | Inhibit macrophage COX-2, IL-1β, and IL-6 expression | [209] |
| Blueberry | Reverse the effects of TNF-α and IL-6 | [210] |
| Grape Nut Garlic Ginseng | Inhibit TNF-α and NF-κB Inhibit the TNF-α pathway and reduce TNFPQ protein expression Inhibit NLRP3 inflammasome activation Promote M1 macrophage polarization to M2 macrophages | [211] [212] [213] [138] |
Notably, EVs derived from ginger have demonstrated therapeutic effects in conditions such as inflammatory bowel disease, autoimmune liver disease, and rheumatoid arthritis[61,207,214]. It is anticipated that ginger-derived EVs may help mitigate the progression of OA. Given the abundance of plant sources, many underexplored plants, such as quercetin, warrant further investigation. The fusion of PEVs and BEVs presents a highly promising direction. PEVs possess strong anti-inflammatory capabilities, while BEVs can be easily endowed with targeting abilities. The hybridized EVs formed through this fusion combine both anti-inflammatory and targeting properties, effectively avoiding the inefficiency associated with cargo loading via electroporation mentioned in the previous section. However, research on PEVs-based treatments for OA has been relatively slow and requires more extensive exploration by researchers.
ADVANTAGES AND CHALLENGES OF EVs
EVs exhibit distinct characteristics depending on their species of origin, yet they undoubtedly hold great potential in biomedicine. MEVs have been studied for a considerable time, while recent attention has shifted toward BEVs and PEVs. Here, we briefly outline the benefits and challenges of EVs to support the field’s rapid growth [Figure 12].
Figure 12. The main strengths of EVs as they exist today and the challenges they will face. Created in BioRender. Bigbone B. (2025) https://BioRender.com/gyl8jqc. EVs: Extracellular vesicles.
Advantages
Due to their structural characteristics, the three different classes of EVs share several common advantages. Their nanoscale size and good biocompatibility make them a new generation of drug delivery carriers and hold great promise for vaccine delivery[215,216]. Additionally, EVs are easily modifiable, with many studies demonstrating enhanced therapeutic effects through various modification strategies[93]. Meanwhile, EVs exhibit diverse functional roles and have been shown to participate in multiple disease contexts[217].
This makes BEVs an exceedingly appealing choice for prospective industrial production, holding considerable promise for commercialization. Compared with MEVs, BEVs can directly modify parental strains without worrying about ethical issues. There is no doubt that BEVs can be used as biomarkers for bacterial infections. This provides a new direction for rapid clinical diagnosis.
The most significant features of PEVs are that they are environmentally friendly, non-polluting, and sustainably available with few ethical concerns. Meanwhile, large-scale production of PEVs is relatively easy due to their wide range of sources and easy scale production of plants[218]. Investigations have consistently demonstrated that the effectiveness of PEVs remains unaltered post oral administration. Additionally, these PEVs showcase exceptional stability when stored for extended periods at -80 °C, establishing an exceedingly favorable scenario for potential clinical applications and for the commercial transport[69]. It is worth mentioning that for Chinese herbal medicines, there are long-term medication experiences and corresponding efficacy references, and the combination of EVs can effectively guide researchers to discover new disease targets[68].
Challenges
A common limitation of EVs is the lack of efficient standard isolation and purification methods, which may further affect the reproducibility of experiments. Additionally, EVs exhibit limited targeting ability and low efficacy; however, engineering modifications can be a more effective solution to these problems[219]. Meanwhile, the complexity of the contents encapsulated in EVs and the variability of EV contents from different sources lead to safety uncertainties, which must be overcome for successful clinical translation. It is worth mentioning that the mechanism of EV content sorting remains unclear and requires further investigation and validation[149]. Although EVs are known to carry miRNAs and other bioactive molecules, recent studies have raised concerns about the actual copy number per vesicle. It has been estimated that the average number of specific miRNAs per EV is often less than one, which brings into question their physiological relevance under normal dosing[220]. However, evidence also suggests that sustained exposure and paracrine amplification may overcome this limitation[221]. Moreover, EV uptake efficiency varies by cell type and tissue, with phagocytic cells showing higher endocytosis rates, while non-phagocytic cells exhibit more limited internalization. These factors highlight the need for dose optimization and enhanced delivery systems in EV-based therapies.
For BEVs, the lack of specific proteins presents a considerable challenge in characterization[41]. The cultivation of bacteria, as outlined in the protocol for engineering modifications of parental strains and the subsequent overexpression of heterologous proteins, demands low-temperature induction. Nevertheless, this requirement proves counterproductive for attaining industrial-scale production and impeding the commercialization efforts of BEVs[85]. In addition, the pathogenicity factors contained in BEVs are important issues that need to be addressed.
The most significant disadvantage of PEVs is the long growth cycle of the plant, which is also not conducive to future industrialization and commercialization. The lack of appropriate biomarkers for PEVs also poses a challenge to their characterization, hindering their development to some extent[58]. Meanwhile, there are still few studies on the storage conditions of PEVs, and their efficacy may be affected by various aspects such as temperature and pH.
CONCLUSIONS AND PROSPECTS
This review provides a comprehensive summary of the latest advancements in MEVs, BEVs, and PEVs within the context of bone aging research. Although MEVs significantly influence the progression of bone metabolism, their yield and purity remain relatively low, limiting their further development[149,155,222]. Consequently, more readily accessible BEVs and PEVs have attracted our attention. Recent studies have substantiated that BEVs and PEVs can effectively modulate bone metabolism[67,68]. Furthermore, due to their higher yield and more closely aligned in vivo metabolism compared to traditional MEVs, OEVs may develop into a crucial component of MEVs[223]. Accordingly, we propose the innovative concept of utilizing OEVs to treat bone aging-related diseases, while also summarizing the therapeutic advantages of each EV type.
Although various types of EVs possess distinct characteristics and advantages, their selection for therapeutic purposes should primarily be guided by biological functions, disease context, and the immunological profile of the recipient tissue. Optimal administration strategies should be tailored to disease-specific requirements. Local injection ensures high drug concentrations at the target site with minimal systemic clearance, offering clear benefits for joint-focused disorders such as OA. Conversely, systemic infusion provides extensive biodistribution, which may be preferable for mitigating generalized bone loss in OP. Beyond serving as anti-bone-aging nanocarriers, EVs can be combined with functional biomaterials - including mesoporous inorganics, metallic scaffolds, and hydrogels - to enable controlled release and synergistic therapeutic effects, thereby expediting tissue regeneration.
Moreover, MEVs can carry a range of bioactive molecules from parent cells, effectively reflecting their cellular state[224]. By comparing and analyzing the content differences in MEVs under healthy and aging conditions, and monitoring their changes, the stage of bone aging diseases can be accurately assessed[225,226]. The concentration of specific biomarkers in MEVs is significantly correlated with disease stage, aiding in the determination of the patient’s pathological state and the timely adjustment of treatment strategies for precision medicine[226]. Thus, MEVs show great potential as biomarkers for diagnosing bone aging diseases.
Bacteria benefit from established high-density culture methods and advanced gene editing technologies, facilitating the large-scale isolation and purification of BEVs[136,222]. Furthermore, the rapid advancement of synthetic biology offers vast potential for the customization of engineered BEVs in bone aging[227]. Furthermore, we present the concept of AI-enabled engineering modifications of EVs, which may represent a promising direction for EV engineering. Indeed, BEVs possess the capacity to cross the intestinal epithelial barrier and interact with immune cells to initiate immune regulation[228-230]. Consequently, employing BEV-based oral bacterial strategies to modulate immune cells may represent a promising avenue for OP treatment. One of the best-characterized EV-mediated mechanisms in bone remodeling involves receptor activator of nuclear factor kappa B-ligand (RANKL)- and receptor activator of nuclear factor kappa B (RANK)-containing EVs. Recent studies have demonstrated that EVs can transfer RANKL to osteoclast precursors, promoting osteoclastogenesis via reverse signaling[231]. This EV-mediated pathway plays a central role in bone resorption, particularly in OP, and may serve as a target for therapeutic intervention using decoy receptors or neutralizing strategies.
PEVs, readily sourced and sustainably obtained, facilitate the efficient large-scale isolation and purification process[58,218]. They offer exceptionally high safety profiles and have been utilized as biotherapeutics or drug delivery vehicles[61,232]. Furthermore, compared to natural products, PEVs demonstrate enhanced stability, wider applicability, and reduced side effects. Relative to BEVs, PEVs offer advantages in isolation and purification, immunogenicity, biocompatibility, and source availability[72]. Their potent anti-inflammatory effects have been substantiated in the context of autoimmune diseases. Therefore, whether utilized as drug delivery vehicles or biotherapeutics, PEVs present significant potential for bone aging.
As organoid research advances rapidly, OEVs are poised to receive increased attention. Trentesaux et al. have employed synthetic biology to engineer organoids and augment their regenerative capabilities[233]. Our team has likewise introduced the concept of AI-enabled organoids[131]. By leveraging AI to guide the development of organoids and integrating it with synthetic biology techniques, we can customize OEVs for bone aging.
Recent studies have revealed that EVs can regulate the homeostasis of key organelles in recipient cells, thereby offering novel therapeutic pathways beyond traditional miRNA/protein signaling. Mitochondrial-derived EVs can transfer mitochondrial components - or even intact mitochondria - to recipient osteoblasts and BMSCs, restoring their bioenergetic function and alleviating oxidative stress[234]. Additionally, cells experiencing lysosomal dysfunction secrete large EVs containing mitochondrial fragments, which are taken up by macrophages, altering their metabolic and immune profiles[235]. EVs also modulate autophagy and mitophagy through PI3K/Akt/mTOR-regulated miRNAs, maintaining organelle quality control. In osteogenic models, MSC-derived EVs enhance autophagy, promoting the survival and differentiation of osteoblasts under oxidative stress. Furthermore, EVs can influence lysosomal and endoplasmic reticulum stress responses, suppressing the activation of the NLRP3 inflammasome in inflammatory microenvironments[236]. Finally, EVs derived from muscle or stem cells can deliver metabolic enzymes to reprogram the energy metabolism of BMSCs, supporting bone regeneration processes[150].
Despite the promising therapeutic potential of EVs in bone aging-related disorders, several critical challenges remain unresolved. Currently, standardized protocols for the isolation and purification of diverse EV subtypes are lacking, and multiple variables within the preparation process can significantly influence their bioactivity and stability. These limitations impede both fundamental research and clinical translation. Consequently, it is imperative to establish robust and standardized methods for EV separation and purification, systematically elucidate their underlying biological mechanisms, and integrate well-designed clinical trials to facilitate the advancement of EV-based therapies for bone aging diseases.
DECLARATIONS
Authors’ contributions
Designed the review framework, collected literature, and drafted the manuscript: Tang L.
Collected and analyzed literature, prepared figures: Wang P
Organized and analyzed literature: Sheng S
Integrated content, analyzed literature: Yang H
Assisted in literature collection and figure preparation, and revised the manuscript: Jiang Y
Assisted in literature organization, content proofreading: Jing Y
Provided manuscript revision, logical integration: Liu H
Supervised the study, guided key content, and finalized the manuscript: Su J
Availability of data and materials
Not applicable.
AI and AI-assisted tools statement
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (82202344), the Jiangsu Province Natural Science Foundation (BK20241808), the Fujian Province Natural Science Foundation (2024J01221), and the Jiangsu Province Youth Science and Technology Talent Support Project (JSTJ-2024-097).
Conflicts of interest
Liu H and Su J are Guest Editors of the Special Issue “Extracellular Vesicles in Disease Diagnosis and Treatment” in the journal Extracellular Vesicles and Circulating Nucleic Acids, hold editorial roles in the journal - Liu H as a Junior Editorial Board member and Su J as an Associate Editor. They were not involved in any steps of the editorial process for this manuscript, including reviewer selection, manuscript handling, or decision making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Fang H, Deng Z, Liu J, Chen S, Deng Z, Li W. The mechanism of bone remodeling after bone aging. Clin Interv Aging. 2022;17:405-15.
2. Chandra A, Rajawat J. Skeletal aging and osteoporosis: mechanisms and therapeutics. Int J Mol Sci. 2021;22:3553.
3. Cui J, Shibata Y, Zhu T, Zhou J, Zhang J. Osteocytes in bone aging: advances, challenges, and future perspectives. Ageing Res Rev. 2022;77:101608.
4. Viganò M, Pennestrì F, Listorti E, Banfi G. Proximal hip fractures in 71,920 elderly patients: incidence, epidemiology, mortality and costs from a retrospective observational study. BMC Public Health. 2023;23:1963.
5. Song S, Guo Y, Yang Y, Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. 2022;237:108168.
6. Hu Y, Li X, Zhi X, et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 2021;22:e52481.
7. Witwer KW, Goberdhan DC, O'Driscoll L, et al. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182.
8. O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585-606.
9. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748-59.
11. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208.
12. Zhang T, Ma S, Lv J, et al. The emerging role of exosomes in Alzheimer’s disease. Ageing Res Rev. 2021;68:101321.
13. Chronopoulos A, Kalluri R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene. 2020;39:6951-60.
14. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
16. Fonseka P, Marzan AL, Mathivanan S. Introduction to the community of extracellular vesicles. Subcell Biochem. 2021;97:3-18.
17. Sharma S, Masud MK, Kaneti YV, et al. Extracellular vesicle nanoarchitectonics for novel drug delivery applications. Small. 2021;17:e2102220.
18. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17.
19. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89.
20. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968-77.
21. Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-25.
22. Liu DA, Tao K, Wu B, et al. A phosphoinositide switch mediates exocyst recruitment to multivesicular endosomes for exosome secretion. Nat Commun. 2023;14:6883.
23. Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605-19.
24. Lee EY, Choi DY, Kim DK, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425-36.
25. Schrempf H, Koebsch I, Walter S, Engelhardt H, Meschke H. Extracellular Streptomyces vesicles: amphorae for survival and defence. Microb Biotechnol. 2011;4:286-99.
26. Brown L, Kessler A, Cabezas-Sanchez P, Luque-Garcia JL, Casadevall A. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol Microbiol. 2014;93:183-98.
27. Albuquerque PC, Nakayasu ES, Rodrigues ML, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695-710.
28. Vallejo MC, Nakayasu ES, Longo LV, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 2012;7:e39463.
29. Rodrigues ML, Nakayasu ES, Oliveira DL, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58-67.
30. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13-24.
31. Burdett ID, Murray RG. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974;119:1039-56.
32. Kadurugamuwa JL, Clarke AJ, Beveridge TJ. Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol. 1993;175:5798-805.
33. Roier S, Zingl FG, Cakar F, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515.
34. Turnbull L, Toyofuku M, Hynen AL, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220.
35. Bauwens A, Kunsmann L, Karch H, Mellmann A, Bielaszewska M. Antibiotic-mediated modulations of outer membrane vesicles in Enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob Agents Chemother. 2017:61.
37. Wang J, Ding Y, Wang J, et al. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell. 2010;22:4009-30.
38. Hatsugai N, Iwasaki S, Tamura K, et al. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev. 2009;23:2496-506.
39. Halperin W, Jensen WA. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18:428-43.
40. An Q, van Bel AJ, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2:4-7.
41. Liu H, Geng Z, Su J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Extracell Vesicles Circ Nucl Acids. 2022;3:63-86.
42. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, rna cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301-12.
43. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-7.
44. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83.
45. Shockman GD, Barrett JF. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501-27.
46. Jiang Y, Kong Q, Roland KL, Curtiss R 3rd. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int J Med Microbiol. 2014;304:431-43.
47. Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A. 2010;107:19002-7.
48. Olaya-Abril A, Prados-Rosales R, McConnell MJ, et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014;106:46-60.
49. Lee JH, Choi CW, Lee T, Kim SI, Lee JC, Shin JH. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS One. 2013;8:e73196.
50. Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620-30.
51. Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414.
52. Kojer K, Riemer J. Balancing oxidative protein folding: the influences of reducing pathways on disulfide bond formation. Biochim Biophys Acta. 2014;1844:1383-90.
53. Sjöström AE, Sandblad L, Uhlin BE, Wai SN. Membrane vesicle-mediated release of bacterial RNA. Sci Rep. 2015;5:15329.
54. Bitto NJ, Chapman R, Pidot S, et al. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep. 2017;7:7072.
55. Pérez-Cruz C, Delgado L, López-Iglesias C, Mercade E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS One. 2015;10:e0116896.
56. Li J, Azam F, Zhang S. Outer membrane vesicles containing signalling molecules and active hydrolytic enzymes released by a coral pathogen Vibrio shilonii AK1. Environ Microbiol. 2016;18:3850-66.
57. Sarvarian P, Samadi P, Gholipour E, et al. Application of emerging plant-derived nanoparticles as a novel approach for nano-drug delivery systems. Immunol Invest. 2022;51:1039-59.
58. Han R, Wu Y, Han Y, Liu X, Liu H, Su J. Engineered plant extracellular vesicles for autoimmune diseases therapy. Nano Res. 2024;17:2857-73.
59. Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6:1312-21.
60. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11:87.
61. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321-40.
62. Li D, Yao X, Yue J, et al. Advances in bioactivity of microRNAs of plant-derived exosome-like nanoparticles and milk-derived extracellular vesicles. J Agric Food Chem. 2022;70:6285-99.
63. Gardiner C, Di Vizio D, Sahoo S, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945.
64. Wu Q, Wang W, Zhang C, et al. Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics. Nat Commun. 2023;14:6541.
65. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789-804.
66. Li J, Lin S, Vanhoutte PM, Woo CW, Xu A.
67. Liu JH, Chen CY, Liu ZZ, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. 2021;8:2004831.
68. Zhan W, Deng M, Huang X, et al. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhacning autophagy. J Control Release. 2023;364:644-53.
69. Hwang JH, Park YS, Kim HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184-98.
70. Yang M, Liu X, Luo Q, Xu L, Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnology. 2020;18:100.
71. Chen L, Qin H, Zhao R, et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci Transl Med. 2021;13:eabc2816.
72. Liu H, Li M, Zhang T, et al. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J. 2022;450:138309.
73. Xie J, Li Q, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022;40:1173-94.
74. Zhou H, Peng K, Wang J, et al. Aloe-derived vesicles enable macrophage reprogramming to regulate the inflammatory immune environment. Front Bioeng Biotechnol. 2023;11:1339941.
75. Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161.
76. Gonda A, Kabagwira J, Senthil GN, et al. Exosomal survivin facilitates vesicle internalization. Oncotarget. 2018;9:34919-34.
77. Yao Z, Qiao Y, Li X, et al. Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon-induced antiviral activity. J Virol. 2018;92:e01578-18.
78. Heusermann W, Hean J, Trojer D, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213:173-84.
79. Sung BH, von Lersner A, Guerrero J, et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun. 2020;11:2092.
80. Arya SB, Collie SP, Parent CA. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2024;34:90-108.
81. Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447-58.
82. Cerezo-Magaña M, Christianson HC, van Kuppevelt TH, Forsberg-Nilsson K, Belting M. Hypoxic induction of exosome uptake through proteoglycan-dependent endocytosis fuels the lipid droplet phenotype in glioma. Mol Cancer Res. 2021;19:528-40.
83. Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380-5.
84. Tang T, An B, Huang Y, et al. Materials design by synthetic biology. Nat Rev Mater. 2021;6:332-50.
85. Li Y, Zhao R, Cheng K, et al. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 2020;14:16698-711.
86. Cheng K, Zhao R, Li Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12:2041.
87. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5.
88. Whitley JA, Cai H. Engineering extracellular vesicles to deliver CRISPR ribonucleoprotein for gene editing. J Extracell Vesicles. 2023;12:e12343.
89. Kempen DH, Lu L, Hefferan TE, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245-52.
90. Hay JJ, Rodrigo-Navarro A, Petaroudi M, et al. Bacteria-based materials for stem cell engineering. Adv Mater. 2018;30:e1804310.
91. Liu Y, Berendsen AD, Jia S, et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101-13.
92. Lu Y, Li H, Wang J, et al. Engineering bacteria‐activated multifunctionalized hydrogel for promoting diabetic wound healing. Adv Funct Mater. 2021;31:2105749.
93. Hu Y, Li X, Zhang Q, et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905-13.
94. Thomas SC, Madaan T, Kamble NS, Siddiqui NA, Pauletti GM, Kotagiri N. Engineered bacteria enhance immunotherapy and targeted therapy through stromal remodeling of tumors. Adv Healthc Mater. 2022;11:e2101487.
95. Zhang Y, Malzahn AA, Sretenovic S, Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat Plants. 2019;5:778-94.
96. Demirer GS, Silva TN, Jackson CT, et al. Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat Nanotechnol. 2021;16:243-50.
97. Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483-94.
98. Lv Q, Cheng L, Lu Y, et al. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7:2000515.
99. Li Z, Zhou X, Wei M, et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19-28.
100. Kim H, Yun N, Mun D, et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Biophys Res Commun. 2018;499:803-8.
101. Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem. 2015;290:8166-72.
102. Zhang C, Guo F, Chang M, et al. Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/p190RhoGAP/RhoA/ROCK/NF-κB signaling axis and glycocalyx enhancement. Exp Cell Res. 2019;384:111596.
103. Liang G, Zhu Y, Ali DJ, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18:10.
104. Liang Y, Xu X, Li X, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938-47.
105. Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomedicine. 2018;13:585-99.
106. Liu HY, Kumar R, Zhong C, et al. Rapid capture of cancer extracellular vesicles by lipid patch microarrays. Adv Mater. 2021;33:e2008493.
107. Yang L, Han D, Zhan Q, et al. Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics. 2019;9:7680-96.
108. Tamura R, Uemoto S, Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274-84.
109. Nakase I, Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep. 2015;5:10112.
110. Cheng H, Fan JH, Zhao LP, et al. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials. 2019;211:14-24.
111. Gao X, Ran N, Dong X, et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10:eaat0195.
112. Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467.
113. Koo S, Sohn HS, Kim TH, et al. Ceria-vesicle nanohybrid therapeutic for modulation of innate and adaptive immunity in a collagen-induced arthritis model. Nat Nanotechnol. 2023;18:1502-14.
114. Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30:e1706759.
115. Chen Q, Huang G, Wu W, et al. A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020;32:e1908185.
116. Salunkhe S, Dheeraj
117. Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12:6830-42.
118. Lin Y, Wu J, Gu W, et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5:1700611.
119. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727-37.
120. Pascucci L, Coccè V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262-70.
121. Yong T, Zhang X, Bie N, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838.
122. Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139-48.
123. Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519-30.
124. Wei H, Chen J, Wang S, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomedicine. 2019;14:8603-10.
125. Sun L, Fan M, Huang D, et al. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271:120761.
126. Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302-16.
127. Lee J, Lee H, Goh U, et al. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl Mater Interfaces. 2016;8:6790-5.
128. Zhou HY, Yu Y, Wang C, et al. A transformer-based representation-learning model with unified processing of multimodal input for clinical diagnostics. Nat Biomed Eng. 2023;7:743-55.
129. Zhang K, Liu X, Shen J, et al. Clinically applicable AI system for accurate diagnosis, quantitative measurements, and prognosis of COVID-19 pneumonia using computed tomography. Cell. 2020;181:1423-33.e11.
130. Monzel AS, Hemmer K, Kaoma T, et al. Machine learning-assisted neurotoxicity prediction in human midbrain organoids. Parkinsonism Relat Disord. 2020;75:105-9.
131. Bai L, Wu Y, Li G, Zhang W, Zhang H, Su J. AI-enabled organoids: construction, analysis, and application. Bioact Mater. 2024;31:525-48.
132. Guo H, Huang X. Engineered exosomes for future gene-editing therapy. Biomater Transl. 2022;3:240-2.
133. García-Barberán V, Gómez Del Pulgar ME, Guamán HM, Benito-Martin A. The times they are AI-changing: AI-powered advances in the application of extracellular vesicles to liquid biopsy in breast cancer. Extracell Vesicles Circ Nucl Acids. 2025;6:128-40.
134. Wang J, Zhang Y, Wang S, Wang X, Jing Y, Su J. Bone aging and extracellular vesicles. Sci Bull. 2024;69:3978-99.
135. Yang J, Luo J, Tian X, Zhao Y, Li Y, Wu X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants. 2024;13:394.
136. Fang F, Yang J, Wang J, et al. The role and applications of extracellular vesicles in osteoporosis. Bone Res. 2024;12:4.
137. Elashiry M, Elashiry MM, Elsayed R, et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J Extracell Vesicles. 2020;9:1795362.
138. Song H, Li X, Zhao Z, et al. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019;19:3040-8.
139. Wei Y, Tang C, Zhang J, et al. Extracellular vesicles derived from the mid-to-late stage of osteoblast differentiation markedly enhance osteogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2019;514:252-8.
140. Gatti M, Beretti F, Zavatti M, et al. Amniotic fluid stem cell-derived extracellular vesicles counteract steroid-induced osteoporosis in vitro. Int J Mol Sci. 2020;22:38.
141. Sonoda S, Murata S, Nishida K, et al. Extracellular vesicles from deciduous pulp stem cells recover bone loss by regulating telomerase activity in an osteoporosis mouse model. Stem Cell Res Ther. 2020;11:296.
142. Nakao Y, Fukuda T, Zhang Q, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306-24.
143. Chen CY, Rao SS, Tan YJ, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019;7:18.
144. Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836-49.
145. Xiao F, Zuo B, Tao B, et al. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann Transl Med. 2021;9:798.
146. Zhang L, Wang Q, Su H, Cheng J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J Biosci Bioeng. 2021;131:671-8.
147. Hu Y, Zhang Y, Ni CY, et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics. 2020;10:2293-308.
148. Wang T, Mo L, Ou J, et al.
149. Jiang Y, Li J, Xue X, Yin Z, Xu K, Su J. Engineered extracellular vesicles for bone therapy. Nano Today. 2022;44:101487.
150. Ma S, Xing X, Huang H, et al. Skeletal muscle-derived extracellular vesicles transport glycolytic enzymes to mediate muscle-to-bone crosstalk. Cell Metab. 2023;35:2028-43.e7.
151. Scheideler M, Elabd C, Zaragosi LE, et al. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics. 2008;9:340.
152. Niedermair T, Lukas C, Li S, et al. Influence of extracellular vesicles isolated from osteoblasts of patients with Cox-arthrosis and/or osteoporosis on metabolism and osteogenic differentiation of BMSCs. Front Bioeng Biotechnol. 2020;8:615520.
153. Li D, Liu J, Guo B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872.
154. He J, Li X, Wang Z, et al. Therapeutic anabolic and anticatabolic benefits of natural Chinese medicines for the treatment of osteoporosis. Front Pharmacol. 2019;10:1344.
155. Liu Z, Zhuang Y, Fang L, Yuan C, Wang X, Lin K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact Mater. 2023;22:423-52.
156. Peng Q, Mu H. The potential of protein-nanomaterial interaction for advanced drug delivery. J Control Release. 2016;225:121-32.
157. Yin B, Ni J, Witherel CE, et al. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. 2022;12:207-31.
158. Zhang X, Huebner JL, Kraus VB. Extracellular vesicles as biological indicators and potential sources of autologous therapeutics in osteoarthritis. Int J Mol Sci. 2021;22:8351.
159. Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42:2865-72.
160. Rosenthal AK, Gohr CM, Ninomiya J, Wakim BT. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011;63:401-11.
161. Kolhe R, Hunter M, Liu S, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7:2029.
162. Jin Y, Xu M, Zhu H, et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25:9281-94.
163. Wang X, Li Z, Cui Y, Cui X, Chen C, Wang Z. Exosomes isolated from bone marrow mesenchymal stem cells exert a protective effect on osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front Cell Dev Biol. 2021;9:644380.
164. Zhang J, Rong Y, Luo C, Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging. 2020;12:25138-52.
165. Woo CH, Kim HK, Jung GY, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9:1735249.
166. Cavallo C, Merli G, Borzì RM, et al. Small extracellular vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-κB dependent inflammatory/catabolic environment of osteoarthritis. Sci Rep. 2021;11:1053.
167. Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987.
168. Lu L, Wang J, Fan A, et al. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J Gene Med. 2021;23:e3379.
169. Luo P, Jiang C, Ji P, Wang M, Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 2019;10:216.
170. Ragni E, Papait A, Perucca Orfei C, et al. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: Anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10:1044-62.
171. Li K, Yan G, Huang H, et al. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnology. 2022;20:38.
172. Li X, Wang Y, Cai Z, Zhou Q, Li L, Fu P. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol Int. 2021;45:2096-106.
173. Wang Y, Yu D, Liu Z, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189.
174. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47.
175. Zavatti M, Beretti F, Casciaro F, Bertucci E, Maraldi T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors. 2020;46:106-17.
176. Otahal A, Kramer K, Kuten-Pella O, et al. Effects of extracellular vesicles from blood-derived products on osteoarthritic chondrocytes within an inflammation model. Int J Mol Sci. 2021;22:7224.
177. Liu X, Wang L, Ma C, Wang G, Zhang Y, Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res. 2019;14:470.
178. Bai J, Zhang Y, Zheng X, et al. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11:763.
179. Zheng L, Wang Y, Qiu P, et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine. 2019;14:3193-212.
180. Xu T, Xu M, Bai J, et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71:57-65.
181. Jo SH, Kim C, Park SH. Novel marine organism-derived extracellular vesicles for control of anti-inflammation. Tissue Eng Regen Med. 2021;18:71-9.
182. Lei J, Jiang X, Li W, et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13:220-6.
183. Arntz OJ, Pieters BC, Oliveira MC, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. 2015;59:1701-12.
184. Song Y, Zhang J, Xu H, et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. 2020;24:121-30.
185. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-60.
186. Damjanov I, Andrews PW. Teratomas produced from human pluripotent stem cells xenografted into immunodeficient mice - a histopathology atlas. Int J Dev Biol. 2016;60:337-419.
187. He L, He T, Xing J, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:276.
188. Tassara M, De Ponti A, Barzizza L, et al. Autologous conditioned serum (ACS) for intra-articular treatment in osteoarthritis: retrospective report of 28 cases. Transfus Apher Sci. 2018;57:573-7.
189. Cai Z, Xin Z, Wang H, Wang C, Liu X. Extracellular vesicle-contained thrombospondin 1 retards age-related degenerative tendinopathy by rejuvenating tendon stem/progenitor cell senescence. Small. 2024;20:e2400598.
190. Liu H, Sun J, Wang M, Wang S, Su J, Xu C. Intestinal organoids and organoids extracellular vesicles for inflammatory bowel disease treatment. Chem Eng J. 2023;465:142842.
191. Gao H, Zeng Y, Huang X, et al. Extracellular vesicles from organoid-derived human retinal progenitor cells prevent lipid overload-induced retinal pigment epithelium injury by regulating fatty acid metabolism. J Extracell Vesicles. 2024;13:e12401.
192. Abe K, Yamashita A, Morioka M, et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat Commun. 2023;14:804.
193. Shen C, Wang J, Li G, et al. Boosting cartilage repair with silk fibroin-DNA hydrogel-based cartilage organoid precursor. Bioact Mater. 2024;35:429-44.
194. Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539.
195. Li S, Stöckl S, Lukas C, et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res Ther. 2021;12:252.
196. Xu C, Zhai Z, Ying H, Lu L, Zhang J, Zeng Y. Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J Nanobiotechnology. 2022;20:123.
197. Rong Y, Zhang J, Jiang D, et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325-42.
198. Wan S, Bao D, Li J, et al. Extracellular vesicles from hypoxic pretreated urine-derived stem cells enhance the proliferation and migration of chondrocytes by delivering miR-26a-5p. Cartilage. 2022;13:19476035221077401.
199. Zhou Y, Ming J, Li Y, et al. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021;7:37.
200. Dong J, Li L, Fang X, Zang M. Exosome-encapsulated microRNA-127-3p released from bone marrow-derived mesenchymal stem cells alleviates osteoarthritis through regulating CDH11-mediated Wnt/β-catenin pathway. J Pain Res. 2021;14:297-310.
201. Tao Y, Zhou J, Wang Z, et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg Chem. 2021;113:104978.
202. Li M, Fang F, Sun M, Zhang Y, Hu M, Zhang J. Extracellular vesicles as bioactive nanotherapeutics: an emerging paradigm for regenerative medicine. Theranostics. 2022;12:4879-903.
203. Liu H, Song P, Zhang H, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. 2024;13:e12429.
204. Divakaruni AS, Hsieh WY, Minarrieta L, et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab. 2018;28:490-503.e7.
205. Rahman SO, Bariguian F, Mobasheri A. The potential role of probiotics in the management of osteoarthritis pain: current status and future prospects. Curr Rheumatol Rep. 2023;25:307-26.
206. Wen M, Wang J, Ou Z, et al. Bacterial extracellular vesicles: a position paper by the microbial vesicles task force of the Chinese society for extracellular vesicles. Interdiscip Med. 2023;1:e20230017.
207. Zhuang X, Deng ZB, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713.
208. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522-34.
209. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6:4321-32.
210. De Robertis M, Sarra A, D’Oria V, et al. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 cells. Biomolecules. 2020;10:742.
211. Zhang H, Wang L, Li C, et al. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464.
212. Aquilano K, Ceci V, Gismondi A, et al. Adipocyte metabolism is improved by TNF receptor-targeting small RNAs identified from dried nuts. Commun Biol. 2019;2:317.
213. Song H, Canup BSB, Ngo VL, Denning TL, Garg P, Laroui H. Internalization of garlic-derived nanovesicles on liver cells is triggered by interaction with CD98. ACS Omega. 2020;5:23118-28.
214. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm. 2019;16:2690-9.
215. Matías J, Brotons A, Cenoz S, et al. Oral immunogenicity in mice and sows of enterotoxigenic Escherichia Coli outer-membrane vesicles incorporated into zein-based nanoparticles. Vaccines. 2019;8:11.
216. Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater. 2022;14:169-81.
217. Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958-66.
218. Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29:2424-40.
219. Ji N, Wang F, Wang M, Zhang W, Liu H, Su J. Engineered bacterial extracellular vesicles for central nervous system diseases. J Control Release. 2023;364:46-60.
220. Andreu Z, Rivas E, Sanguino-Pascual A, et al. Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J Extracell Vesicles. 2016;5:31655.
221. Yang L, Jiang S. Adipose tissue-derived extracellular vesicle microRNAs: diagnostic biomarkers for the pathophysiology associated with obesity. Precis Chem. 2025;3:480-91.
222. Ho MY, Liu S, Xing B. Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential. Nano Converg. 2024;11:28.
223. Liu H, Su J. Organoid extracellular vesicle-based therapeutic strategies for bone therapy. Biomater Transl. 2023;4:199-212.
224. Kumar MA, Baba SK, Sadida HQ, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9:27.
225. Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5:18.
226. Shi H, Jiang X, Xu C, Cheng Q. MicroRNAs in serum exosomes as circulating biomarkers for postmenopausal osteoporosis. Front Endocrinol. 2022;13:819056.
227. McCarty NS, Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019;37:181-97.
228. Yue Y, Xu J, Li Y, et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat Biomed Eng. 2022;6:898-909.
229. Ou Z, Situ B, Huang X, et al. Single-particle analysis of circulating bacterial extracellular vesicles reveals their biogenesis, changes in blood and links to intestinal barrier. J Extracell Vesicles. 2023;12:e12395.
230. Chen X, Li Q, Xie J, Nie S. Immunomodulatory effects of probiotic-derived extracellular vesicles: opportunities and challenges. J Agric Food Chem. 2024;72:19259-73.
231. Yang Z, Yang Z, Ding L, et al. Nanoengineering multifunctional extracellular vesicles availably mitigate bone loss in osteoporosis through binding to RANKL and rebalancing the Treg/Th17 cells. Chem Eng J. 2023;467:143391.
232. Kim SQ, Kim KH. Emergence of edible plant-derived nanovesicles as functional food components and nanocarriers for therapeutics delivery: potentials in human health and disease. Cells. 2022;11:2232.
233. Trentesaux C, Yamada T, Klein OD, Lim WA. Harnessing synthetic biology to engineer organoids and tissues. Cell Stem Cell. 2023;30:10-9.
234. Liang W, Sagar S, Ravindran R, et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat Commun. 2023;14:5031.
235. Ma J, Shen M, Yue D, Wang W, Gao F, et al. Extracellular vesicles from BMSCs prevent glucocorticoid-induced BMECs injury by regulating autophagy via the PI3K/Akt/mTOR pathway. Cells. 2022;11:2104.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data








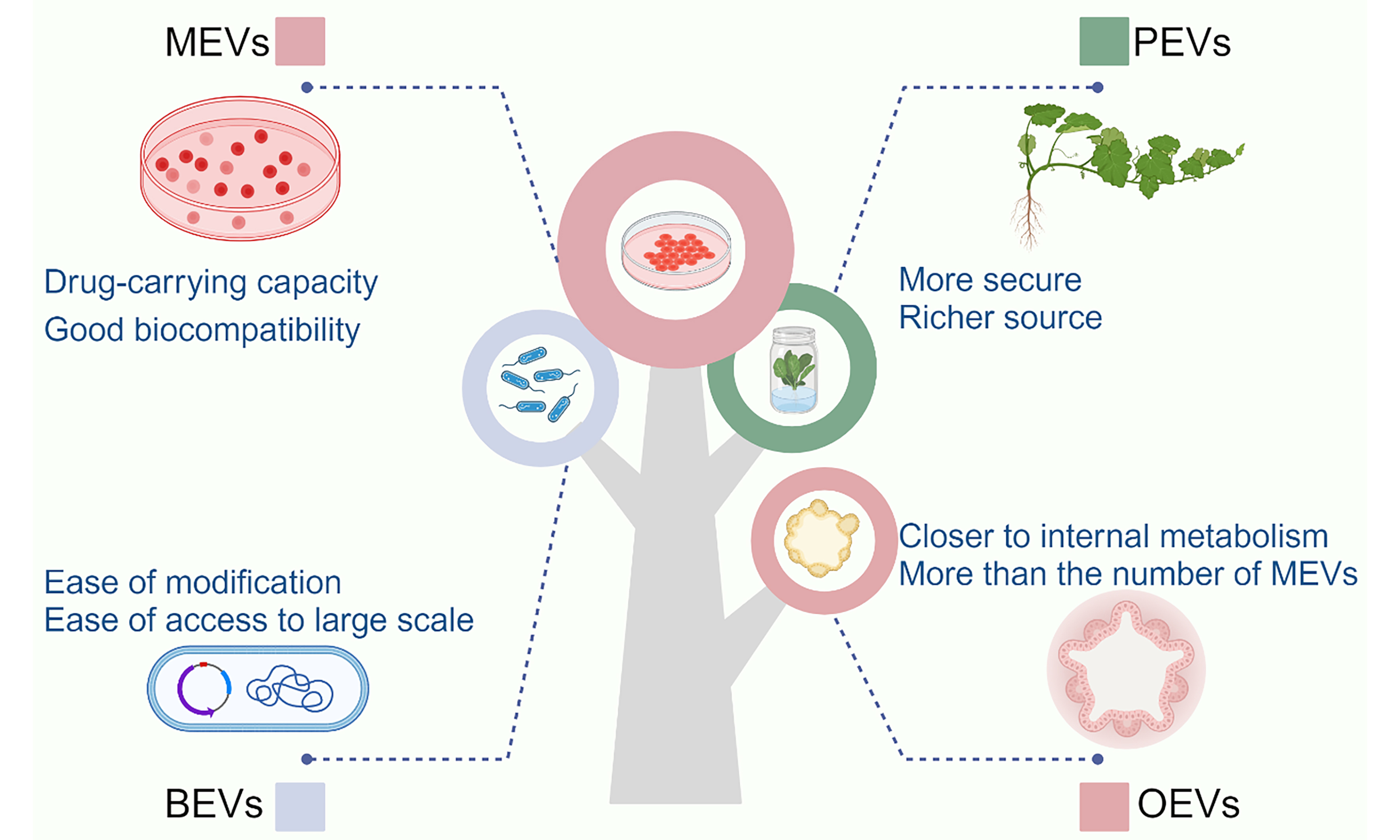
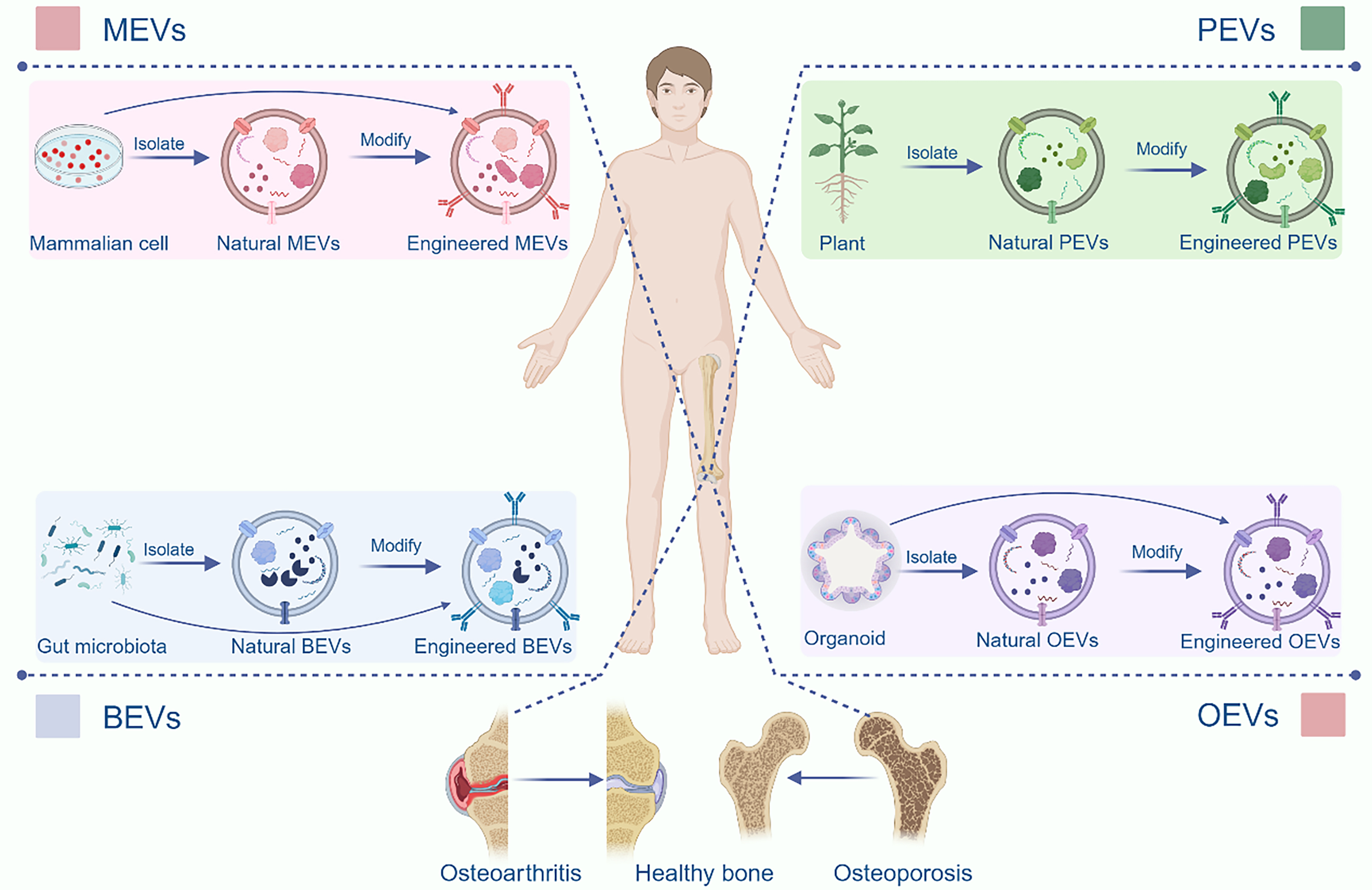
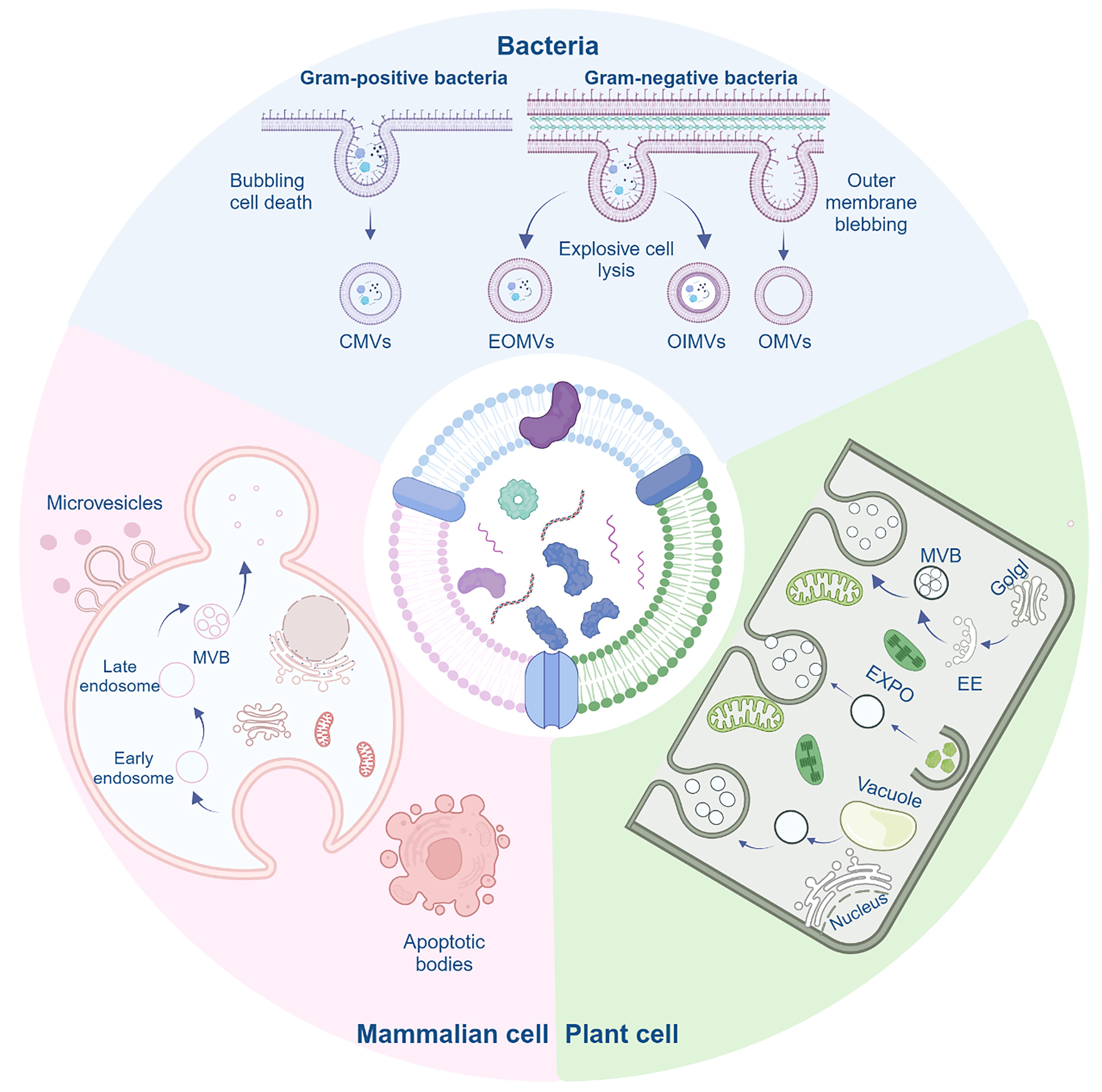
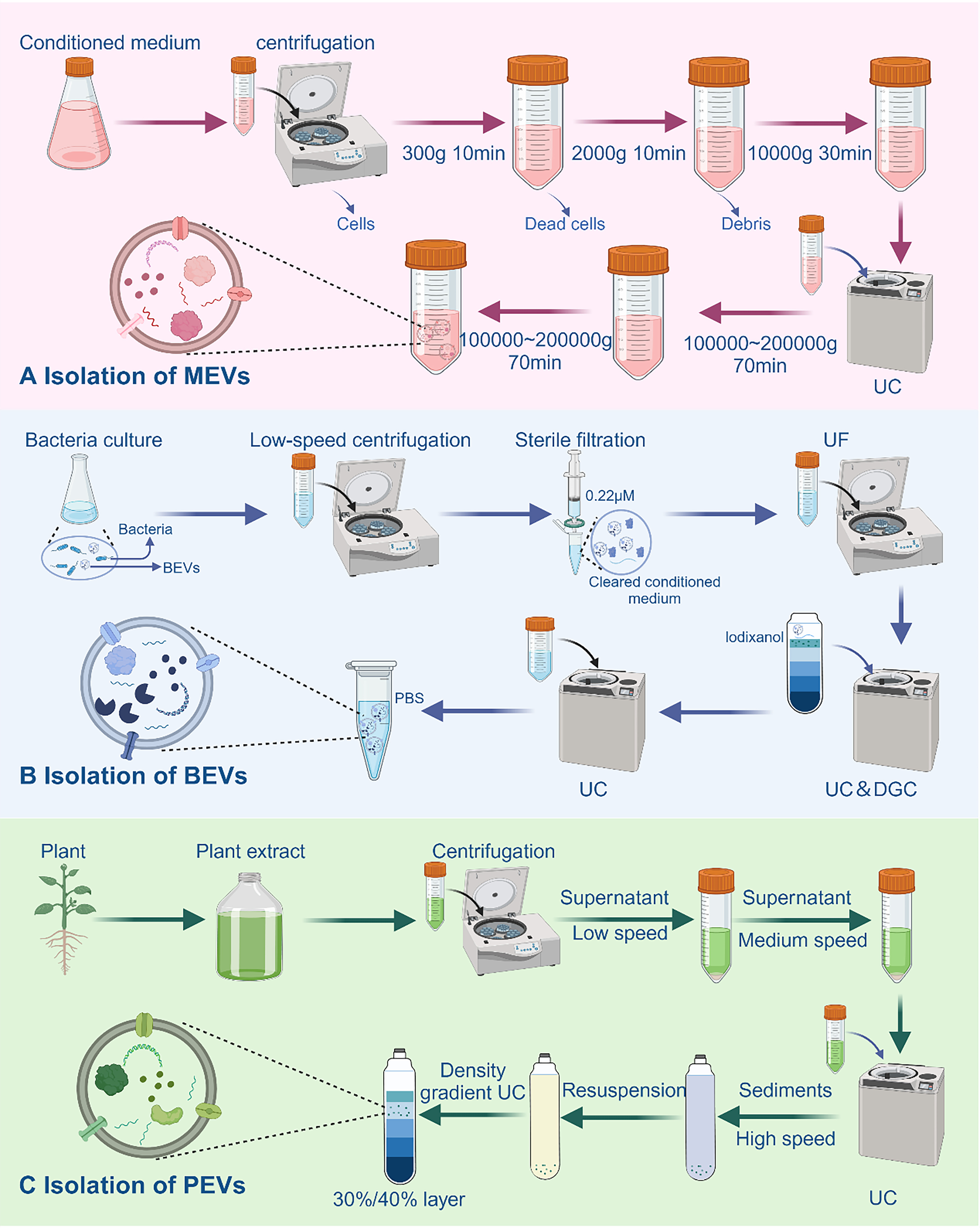
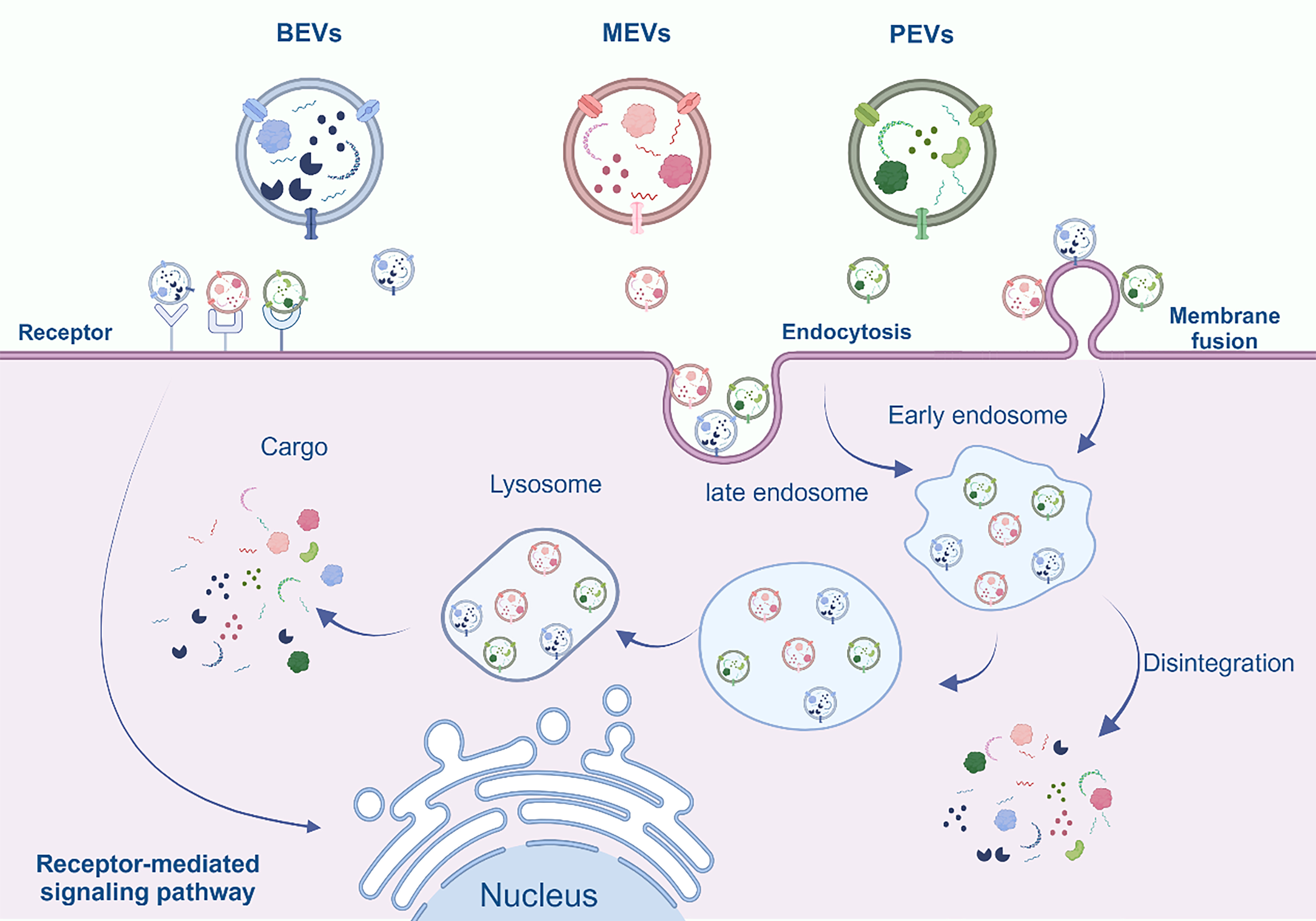
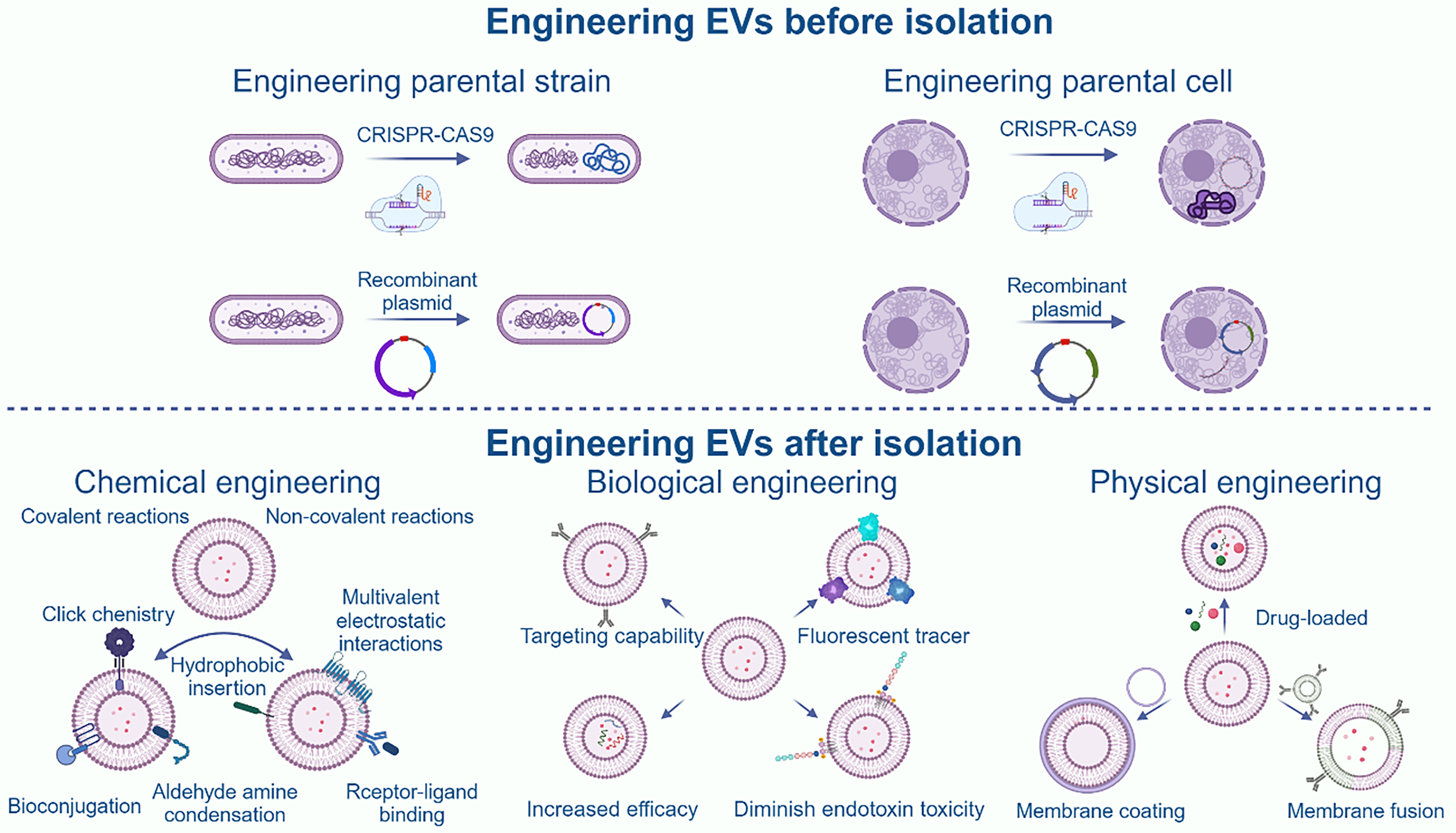
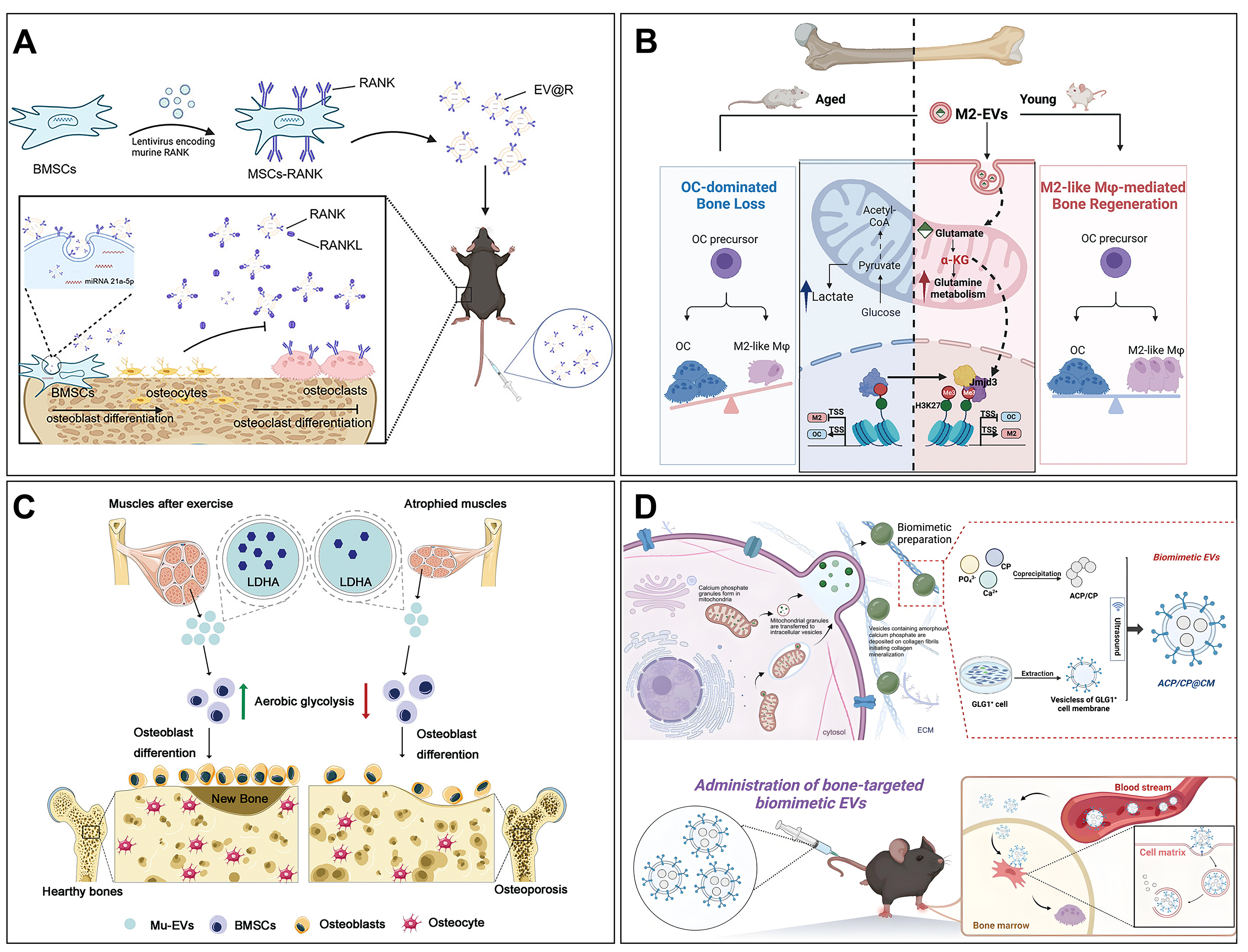
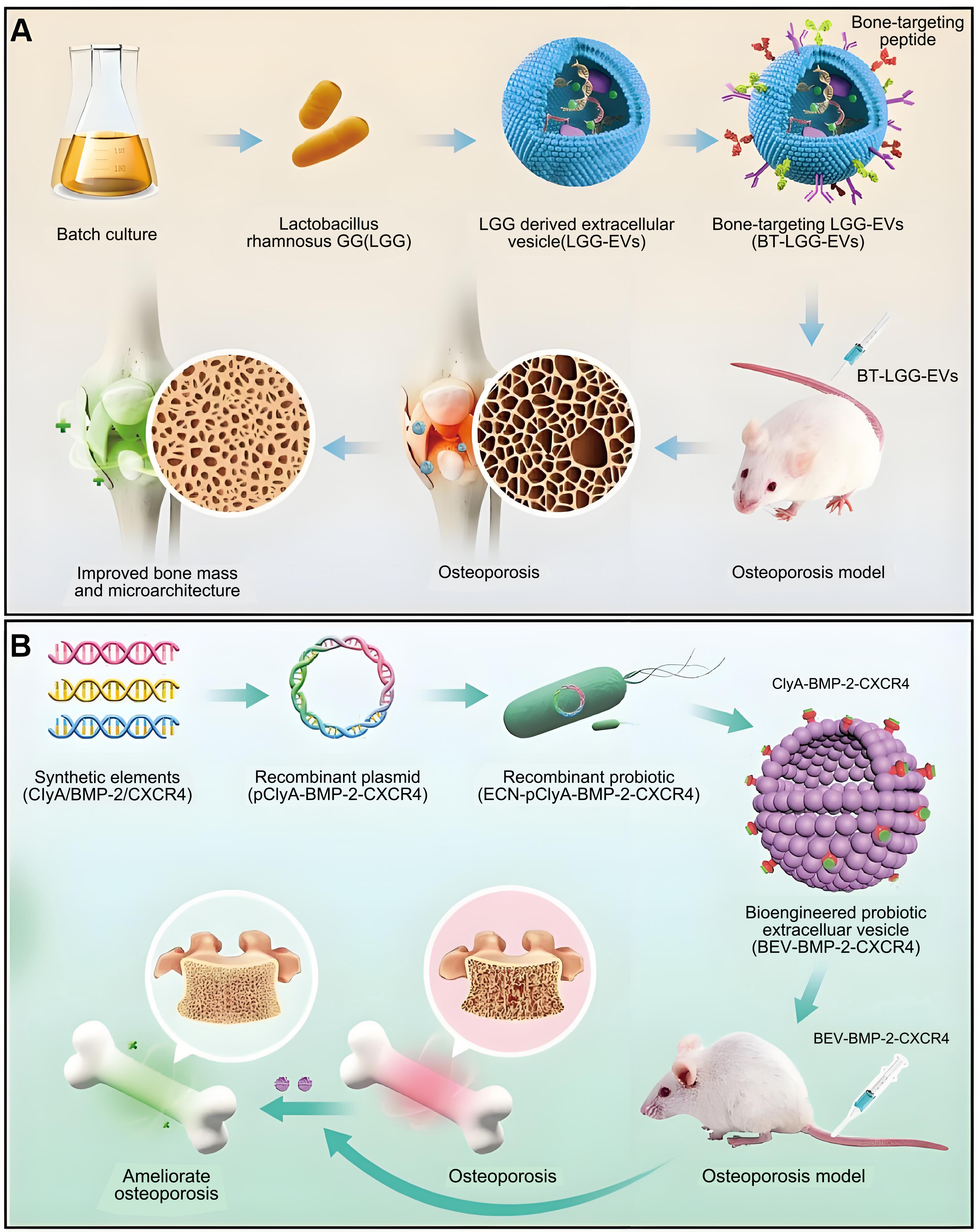
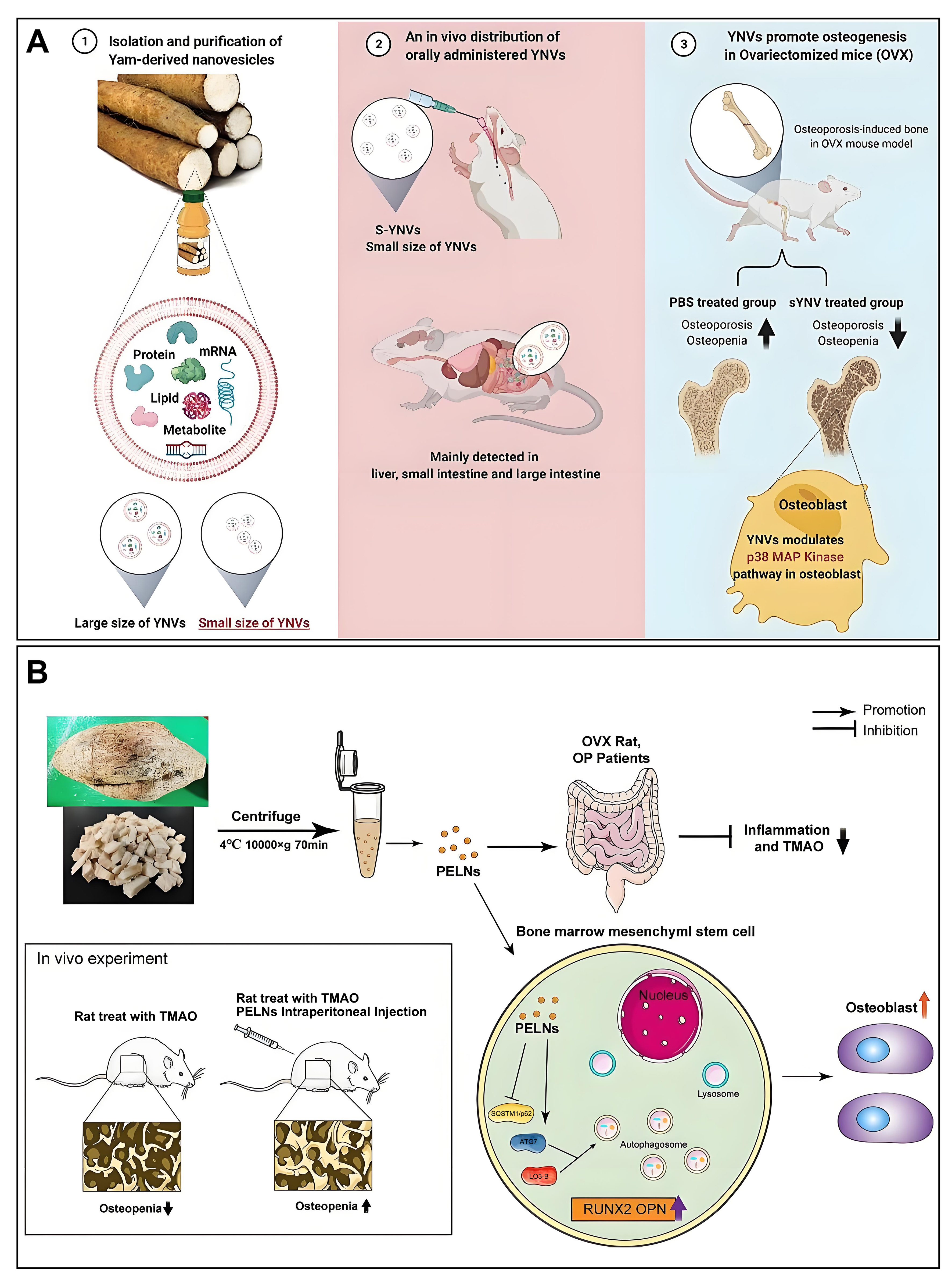
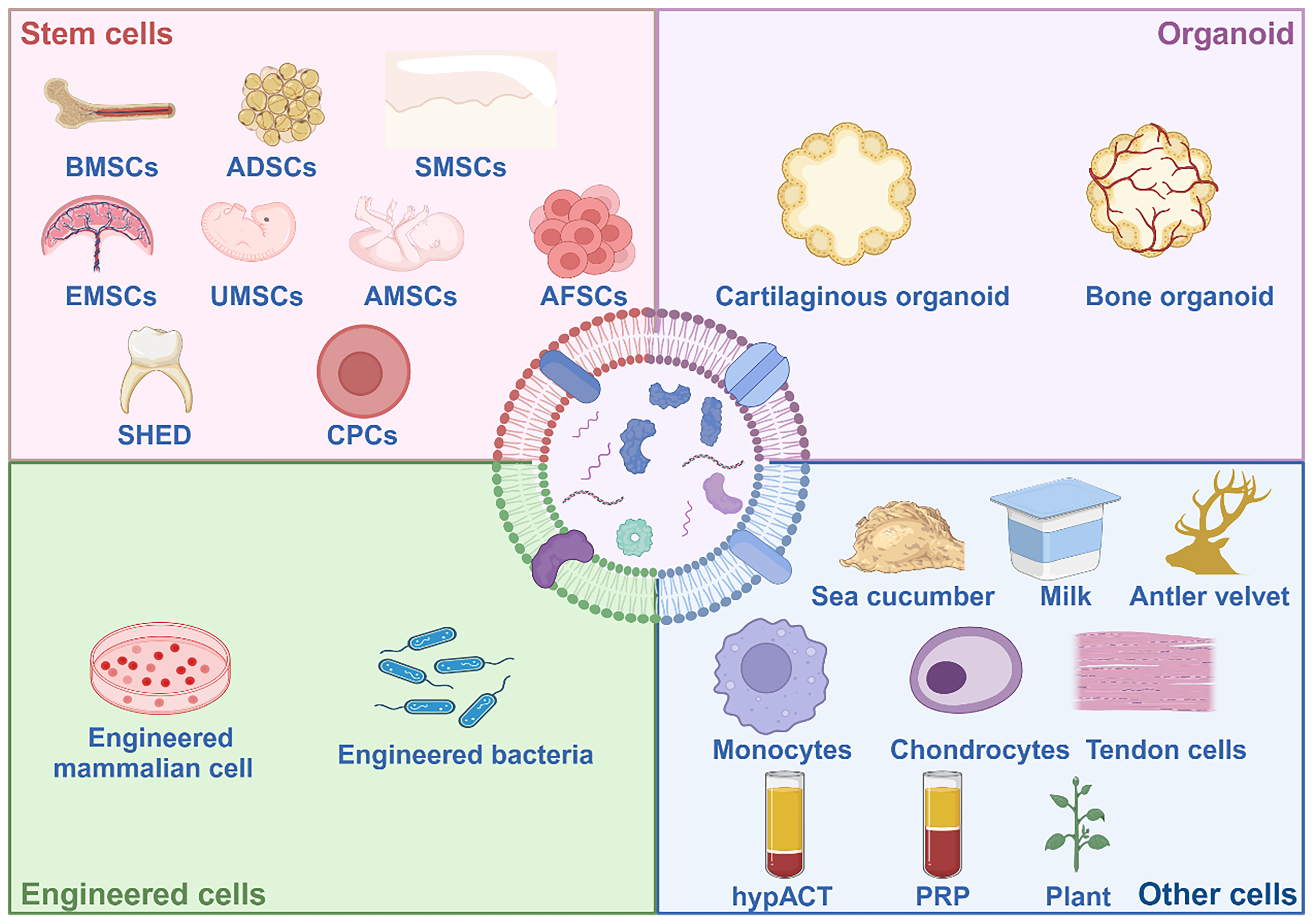
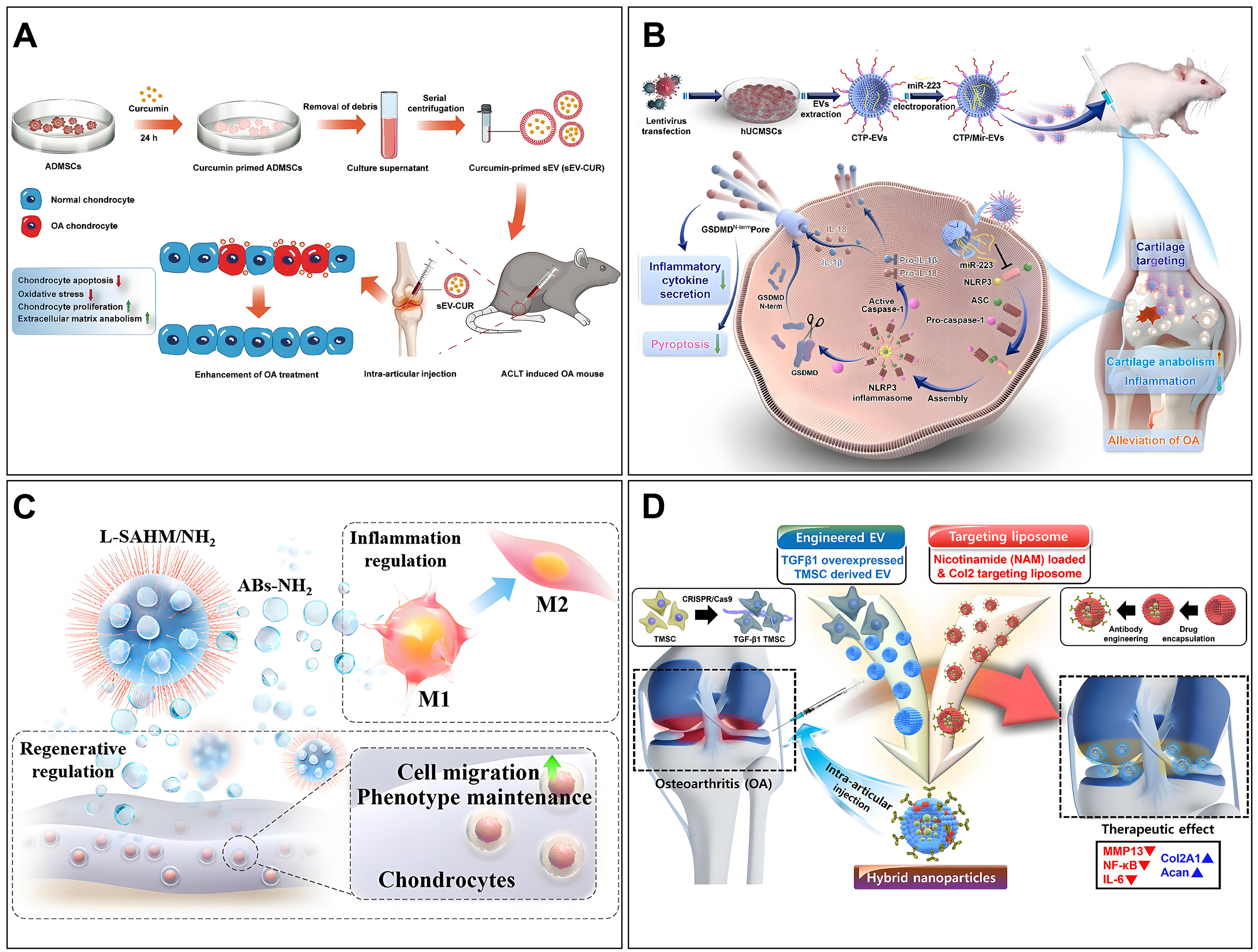
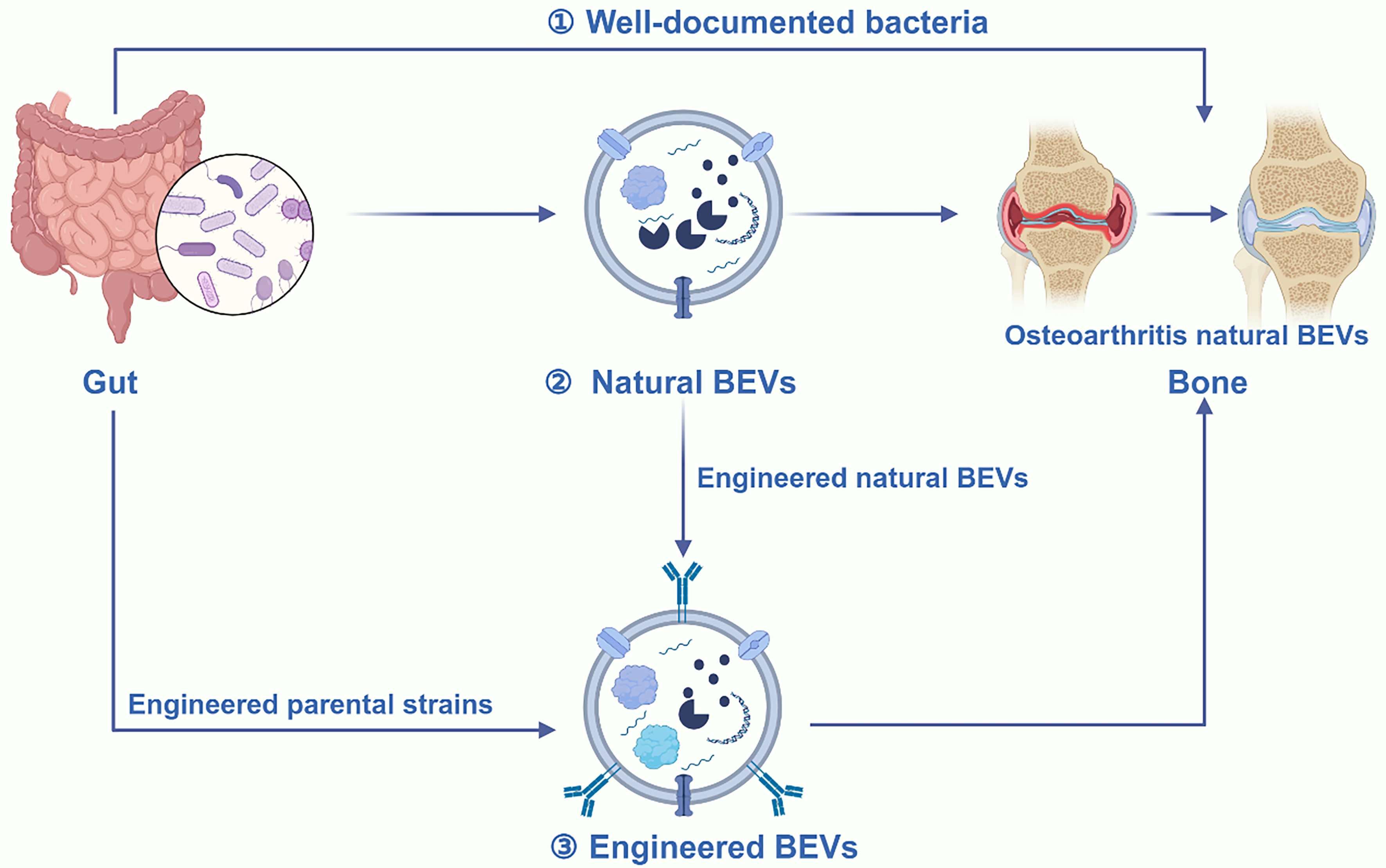
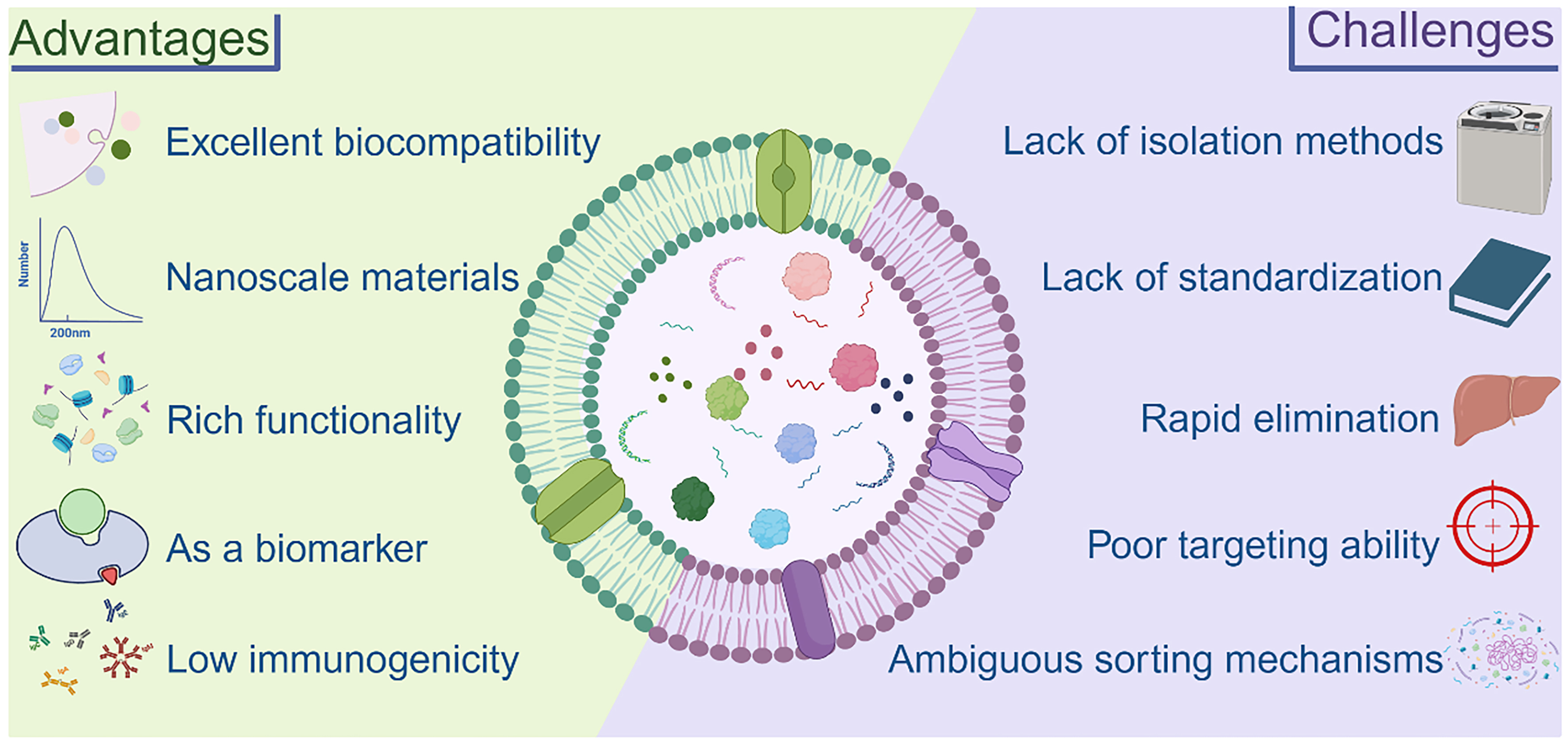











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].