6-PPD quinone-inhibited retinoic acid synthesis mediates toxicity through feedback loop between ALH-3/DHS-19-SEX-1 axis and intestinal signals in Caenorhabditis elegans
Abstract
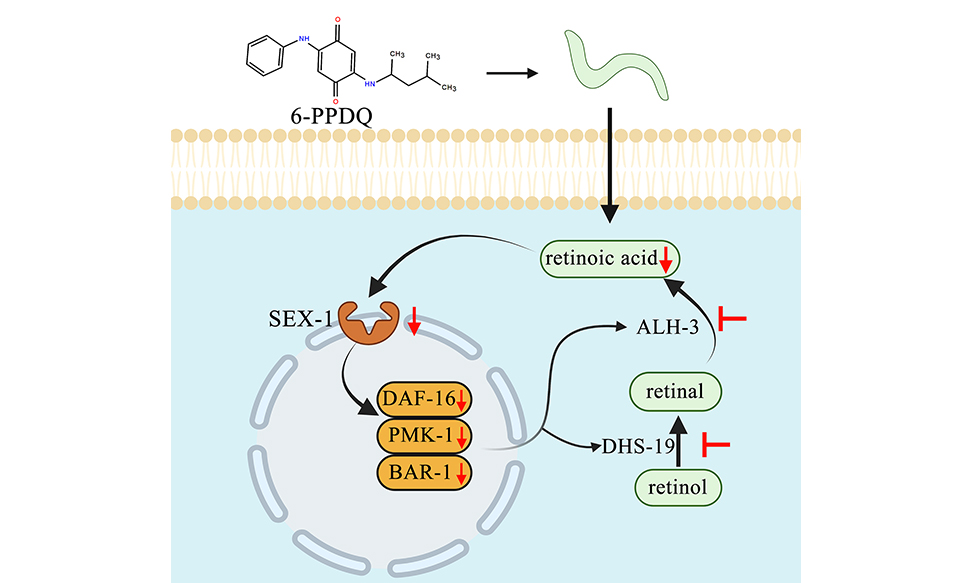
Due to its widespread occurrence in environments, the exposure risk to 6-PPD quinone (6-PPDQ) is receiving increasing attention. Considering the crucial role of retinoic acid in regulating physiological state, we investigated the effects of 6-PPDQ on retinoic acid synthesis and the underlying mechanisms in nematodes. Retinoic acid content was reduced by 6-PPDQ (0.1-10 μg/L). Expression of enzyme genes alh-3 governing retinoic acid synthesis and dhs-19 governing retinal synthesis was decreased by 6-PPDQ. Retinoic acid content was reduced by alh-3 and dhs-19 RNA interference (RNAi). Additionally, alh-3 and dhs-19 RNAi conferred susceptibility to 6-PPDQ toxicity, and these two genes functioned in intestine to modulate 6-PPDQ toxicity. In the intestine, alh-3 and dhs-19 expressions were decreased by intestinal pmk-1, bar-1, and daf-16 RNAi, and pmk-1, bar-1, and daf-16 RNAi reduced retinoic acid content and induced susceptibility to 6-PPDQ toxicity. Additionally, 6-PPDQ decreased expression of sex-1 encoding retinoic acid receptor. After 6-PPDQ exposure, sex-1 expression was decreased by alh-3 and dhs-19 RNAi, and sex-1 RNAi further inhibited pmk-1, bar-1, and daf-16 expressions. sex-1 RNAi caused susceptibility to 6-PPDQ toxicity. Moreover, 6-PPDQ-induced toxicity and decrease in sex-1 expression were suppressed by retinoic acid treatment. Therefore, 6-PPDQ disrupted retinoic acid synthesis, which was linked to toxicity induction through the formation of a feedback loop between the alh-3/dhs-19-sex-1 axis and intestinal signals.
Keywords
INTRODUCTION
Initially, 6-PPD quinone (6-PPDQ) was considered as a cause for coho salmon lethality[1]. It can be transformed from N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine (6-PPD) added in tire to delay oxidation, after reaction with ozone through different pathways[2-4]. After that, besides the aquatic environment, 6-PPDQ occurrence was found in other environments[5-9]. Additionally, 6-PPDQ was bioavailable to organisms[10-13] and accumulated in multiple organs, causing corresponding toxicity in mice[14-19]. Detection of 6-PPDQ in human-related samples, such as urine, suggested its potential health relevance[20-21].
With detection in environmental samples, potential 6-PPDQ toxicity at environmentally relevant concentrations (ERCs) has received attention. Tens of μg/L or ng/L were observed to belong to ERCs of 6-PPDQ[22-25]. Caenorhabditis elegans (C. elegans) shows high sensitivity to pollutant exposure[26-27], making it suitable to detect their toxicity at ERCs[28-32]. In nematodes, not only intestinal damage but also toxicity on locomotion behavior and reproductive capacity were caused by 6-PPDQ[33-35]. In cells, mitochondrial function was disrupted by 6-PPDQ[36-38], which results from suppression in mitochondrial unfolded protein response (UPR) and mitophagy[39-41]. C. elegans is also important to elucidate regulatory mechanisms of metabolic processes[42-43]. Metabolisms of amino acids[44-45], vitamin D3[46], and citric acid cycle[47] were significantly disrupted by 6-PPDQ. Moreover, 6-PPDQ-induced disruption in several metabolisms can be further found in offspring[48-49].
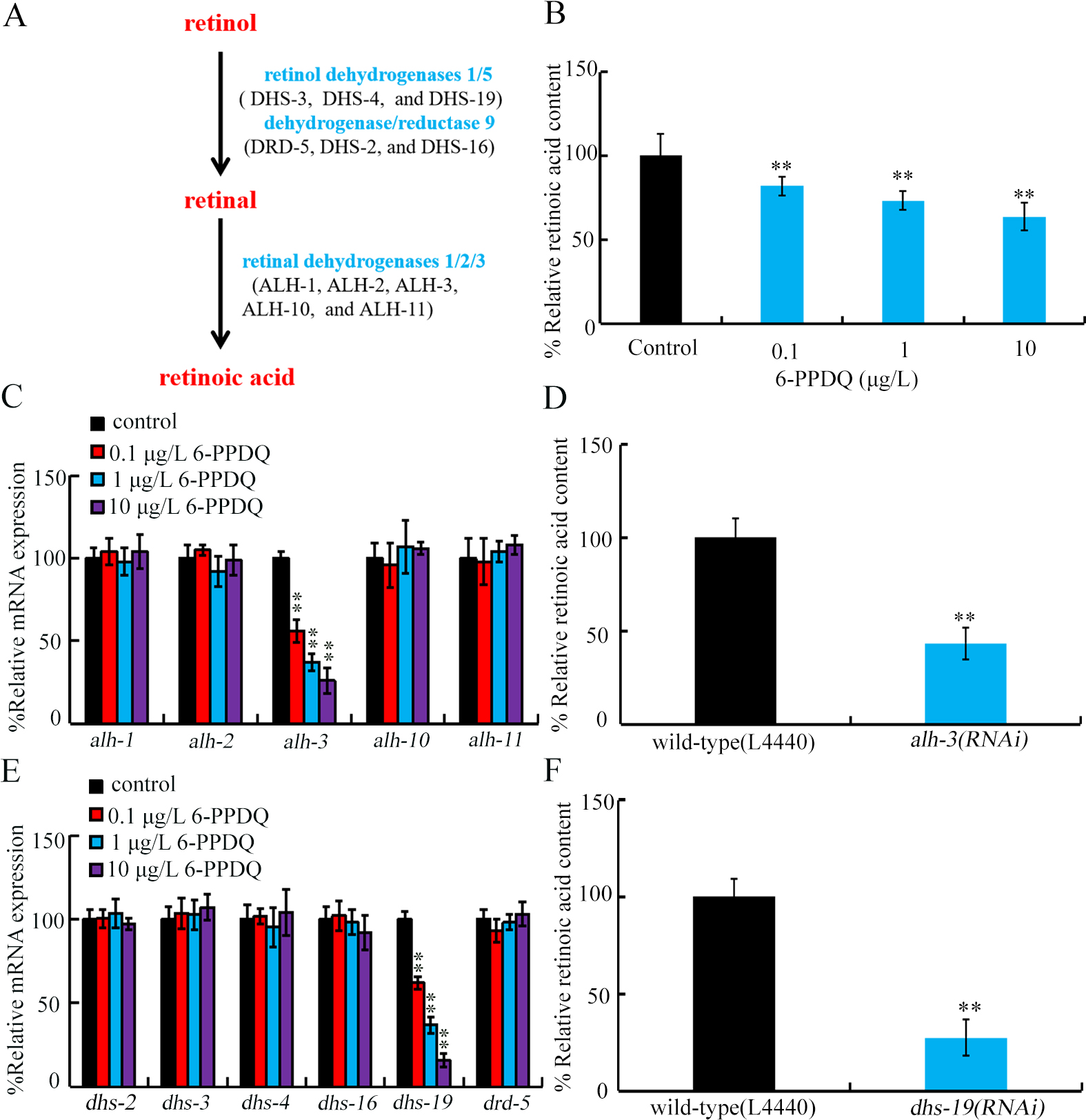
Retinoic acid, a bioactive metabolite of retinol, plays a crucial role in regulating physiological state of organisms[50-51]. Additionally, retinoic acid can help organisms resist damage from environmental pollutants, such as benzo[a]pyrene[52]. Synthesis of C. elegans retinoic acid involves a two-step enzymatic process [Figure 1A]. Initially, retinol is oxidized to retinal by retinol dehydrogenases/reductases encoded by dhs-3, dhs-4, and dhs-19, drd-5, dhs-2, and dhs-16[53]. Subsequently, retinal is further oxidized to retinoic acid by retinaldehyde dehydrogenases encoded by alh-1, alh-2, alh-3, alh-10, and alh-11[31]. In nematodes, predicted retinoic acid receptors include SEX-1, NHR-1, NHR-10, NHR-35, NHR-64, and NHR-61[53], which are supposed to have binding potential to retinoic acid and further regulate expression of downstream targets. We aimed to determine the association between inhibition in retinoic acid synthesis and 6-PPDQ-induced toxicity. Thus, effects of 6-PPDQ on synthesis of retinoic acid and expression of related enzyme genes were examined. Moreover, we determined the underlying mechanism for association between inhibition in retinoic acid synthesis and 6-PPDQ toxicity. The obtained data highlighted a 6-PPDQ risk in suppressing retinoic acid synthesis, which was linked to toxicity formation via a feedback regulatory mechanism.
Figure 1. Effect of 6-PPDQ exposure on retinoic acid content and expression of enzyme genes governing synthesis of retinoic acid from retinal and synthesis of retinal from retinol. (A) Metabolic control of retinoic acid synthesis; (B) Retinoic acid content in 0.1-10 μg/L 6-PPDQ-exposed nematodes. **P < 0.01 vs. control; (C) Effect of 6-PPDQ exposure on expressions of alh-1, alh-2, alh-3, alh-10, and alh-11. **P < 0.01 vs. control; (D) Effect of RNAi of alh-3 on retinoic acid content in 6-PPDQ-exposed nematodes. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01 vs. wild-type (L44440); (E) Effect of 6-PPDQ exposure on expressions of dhs-2, dhs-3, dhs-4, dhs-16, dhs-19, and drd-5. **P < 0.01 vs. control. (F) Effect of RNAi of dhs-19 on retinoic acid content in 6-PPDQ-exposed nematodes. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01 vs. wild-type (L44440). 6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone; RNAi: RNA interference.
EXPERIMENTAL
C. elegans maintenance
Nematode growth medium (NGM) supplemented with Escherichia coli (E. coli) OP50 was used for animal maintenance[54]. Adults were treated with a bleaching solution (2% HOCl, 0.45 M NaOH) to allow eggs to be released and develop into L1 larvae[55].
6-PPDQ exposure
Based on reported ERCs, the working concentrations of 6-PPDQ (Toronto Research Chemicals Co.) were set to 0.1-10 μg/L[22]. C. elegans were exposed to 6-PPDQ from L1 larvae for 6.5 days[56]. The 6-PPDQ solutions were changed daily, and OP50 (4 × 106 CFUs) was added to meet the nutritional requirements of the larvae.
Retinoic acid content
Retinoic acid content was determined according to the manufacturer’s protocol (Shanghai Tongwei Bio-industrial Co). Nematodes were weighed, homogenized, and centrifuged. The supernatant was used to measure absorbance at 450 nm. Retinoic acid content was calculated using the kit-provided standard curve and equation. Experiments were repeated three times.
Gene expression
mRNA was prepared using TRIzol, and reverse-transcribed for complementary DNA (cDNA) synthesis. Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted using SYBR Green RT-qPCR master mix. tba-1 was used to normalize gene expressions using the 2^- ΔΔCt method[57]. Three biological replicates were performed. Primer sequences are listed in Supplementary Table 1.
RNA interference (RNAi)
To silence candidate genes through RNA interference (RNAi), animals were fed with double-stranded RNA (dsRNA)-producing bacterial strains[58]. RNAi constructs targeting specific genes were generated in the L4440 vector[59]. VP303 is a transgenic tool for RNAi of intestinal genes. RNAi efficiency was shown in Supplementary Figures 1 and 2.
Endpoints
To detect reactive oxygen species (ROS) generation, animals were incubated with 1 μM CM-H2DCFDA (5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate acetyl ester) for 2 h in darkness[34]. After washing with K buffer to remove unbound ROS probe, semi-quantitative analysis of fluorescence images was performed using ImageJ. For the locomotion assay, head thrashing (number of head swings in one minute) and body bending (number of times the nematode body bends) were measured as described[60]. Brood size reflecting reproductive capacity refers to the total number of offspring[39]. Brood size was counted until animals stopped egg-laying. Fifty animals were examined.
Pharmacological treatment
Following 6-PPDQ exposure (10 μg/L), nematodes were transferred into and treated with 15 μM retinoic acid for 24 h[61]. Experiments were conducted in triplicate.
Data analysis
One-way or two-way analysis of variance (ANOVA) (for multi-factor comparison) followed by post-hoc test was applied to determine significant differences among groups. P-value of < 0.01 (**) was deemed statistically significant.
RESULTS AND DISCUSSION
6-PPDQ reduced retinoic acid content
Retinoic acid content was inhibited by 0.1-10 μg/L 6-PPDQ [Figure 1B]. Retinoic acid reduction was concentration-dependent in 6-PPDQ-exposed nematodes [Figure 1B].
6-PPDQ inhibited retinoic acid synthesis from retinal
Among retinal dehydrogenase genes governing synthesis of retinoic acid from retinal, expressions of alh-1, alh-2, alh-10, and alh-11 were not changed by 6-PPDQ [Figure 1C]. In contrast, alh-3 expression was decreased by 6-PPDQ [Figure 1C]. Moreover, retinoic acid content was inhibited by alh-3 RNAi [Figure 1D].
6-PPDQ affected expression of enzyme gene governing retinal synthesis from retinol
Considering that retinal is synthesized from retinol, we further examine effect of 6-PPDQ on expression of genes encoding retinol dehydrogenases/reductases. Among these genes, expressions of dhs-2, dhs-3, dhs-4, dhs-16, and drd-5 were not altered by 6-PPDQ [Figure 1E]. In contrast, dhs-19 expression was decreased by 6-PPDQ [Figure 1E]. Moreover, retinoic acid content was also inhibited by dhs-19 RNAi [Figure 1F].
RNAi of alh-3 and dhs-19 caused susceptibility to 6-PPDQ toxicity
The 6-PPDQ caused intestinal toxicity, such as induction of intestinal ROS[35]. As shown in Figure 2A, 6- intestinal ROS was increased by alh-3 and dhs-19 RNAi. Additionally, 6-PPDQ inhibited locomotion[62] and reduced reproductive capacity[33], effects further enhanced by alh-3 and dhs-19 RNAi [Figure 2B and C].
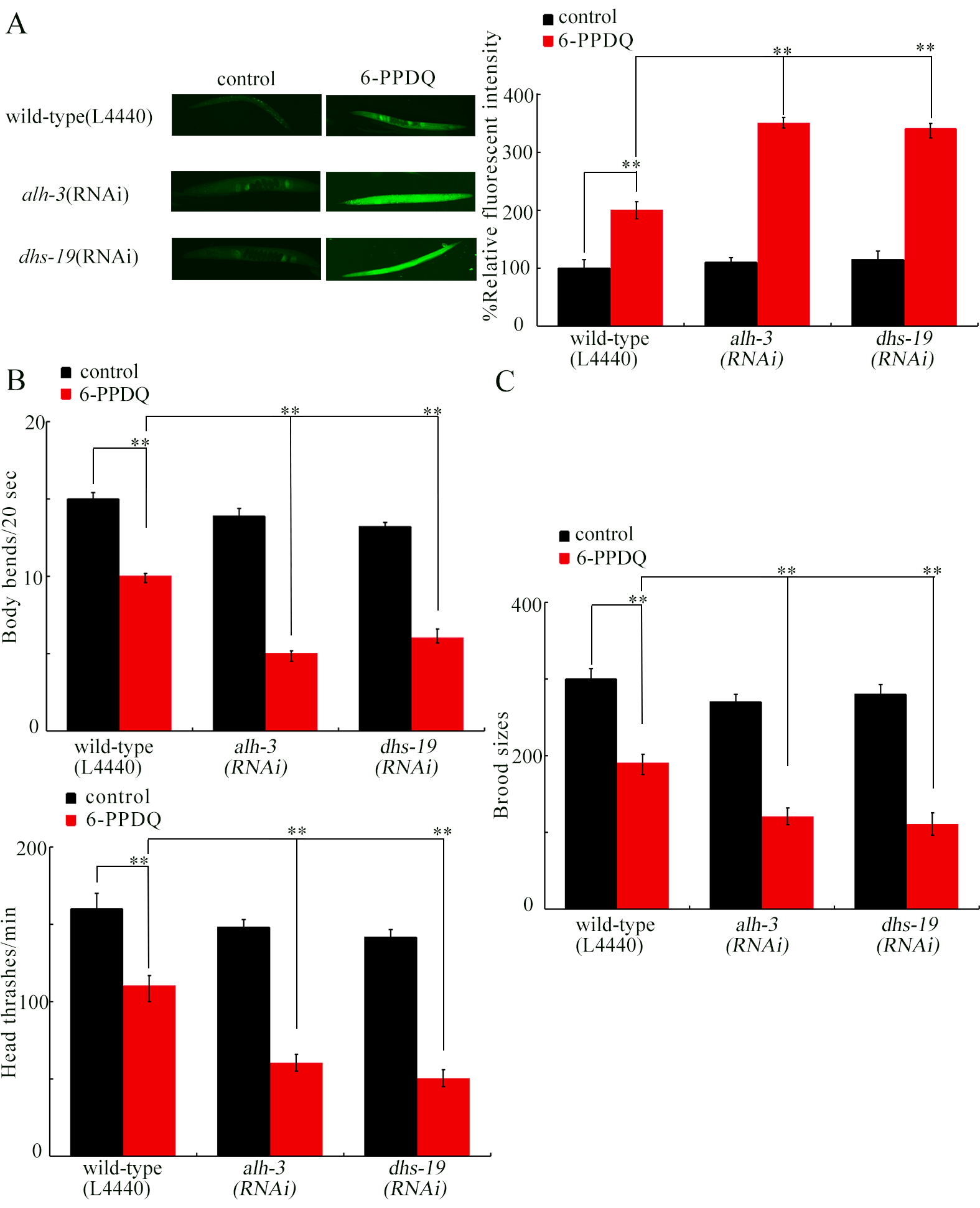
Figure 2. Effect of RNAi of alh-3 and dhs-19 on 6-PPDQ toxicity in inducing ROS generation (A); decreasing locomotion (B); and reducing brood size (C). Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01. RNAi: RNA interference; 6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone.
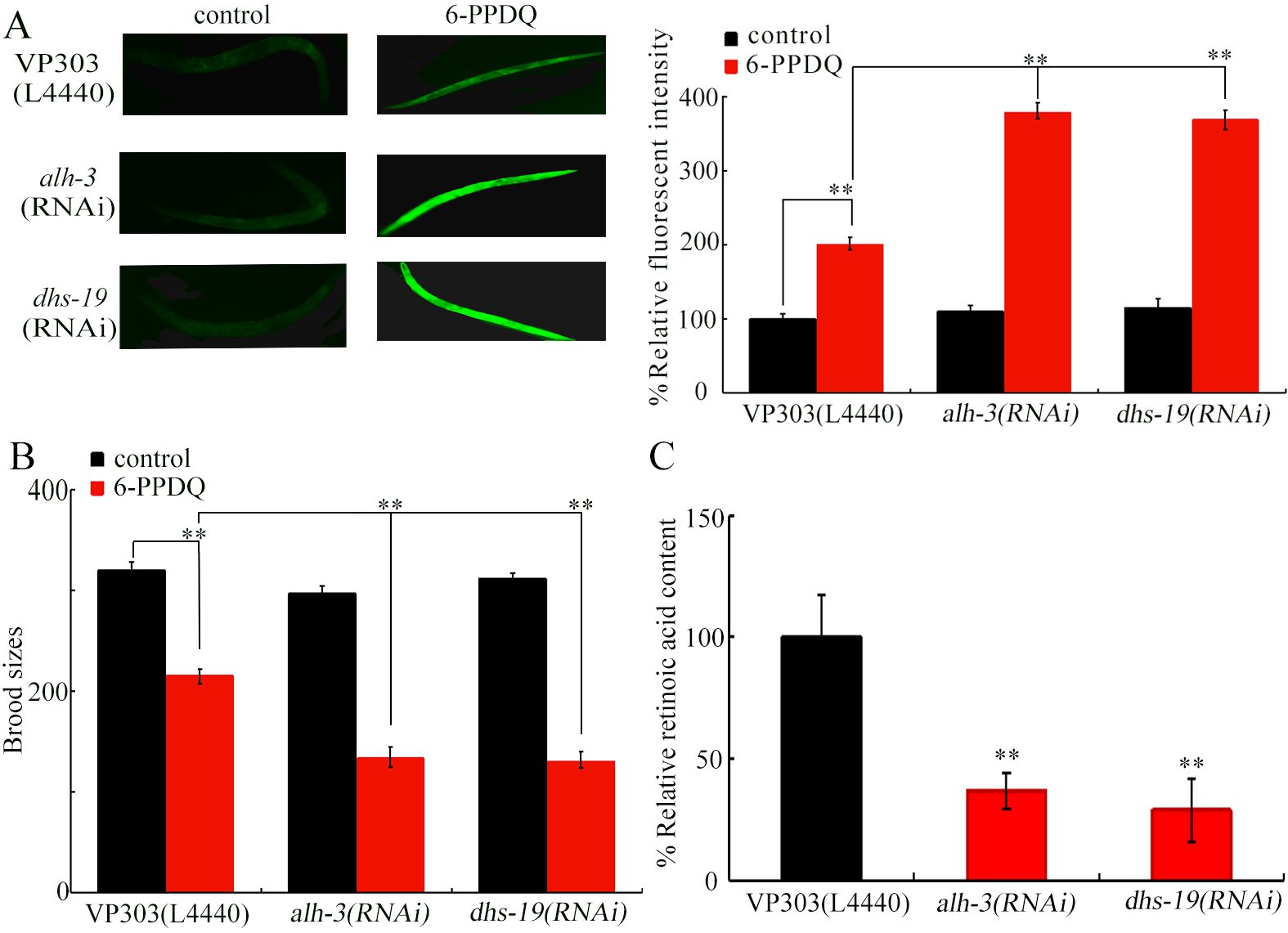
Figure 3. Effect of intestinal RNAi of alh-3 and dhs-19 on 6-PPDQ toxicity. (A) Effect of intestinal RNAi of alh-3 and dhs-19 on 6-PPDQ toxicity in inducing ROS generation. **P < 0.01; (B) Effect of intestinal RNAi of alh-3 and dhs-19 on 6-PPDQ toxicity in reducing brood size. **P < 0.01; (C) Effect of intestinal RNAi of alh-3 and dhs-19 on retinoic acid content in 6-PPDQ-exposed nematodes. **P < 0.01 vs. VP303 (L4440). Exposure concentration of 6-PPDQ was 10 μg/L. RNA interference; 6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone; ROS: reactive oxygen species.
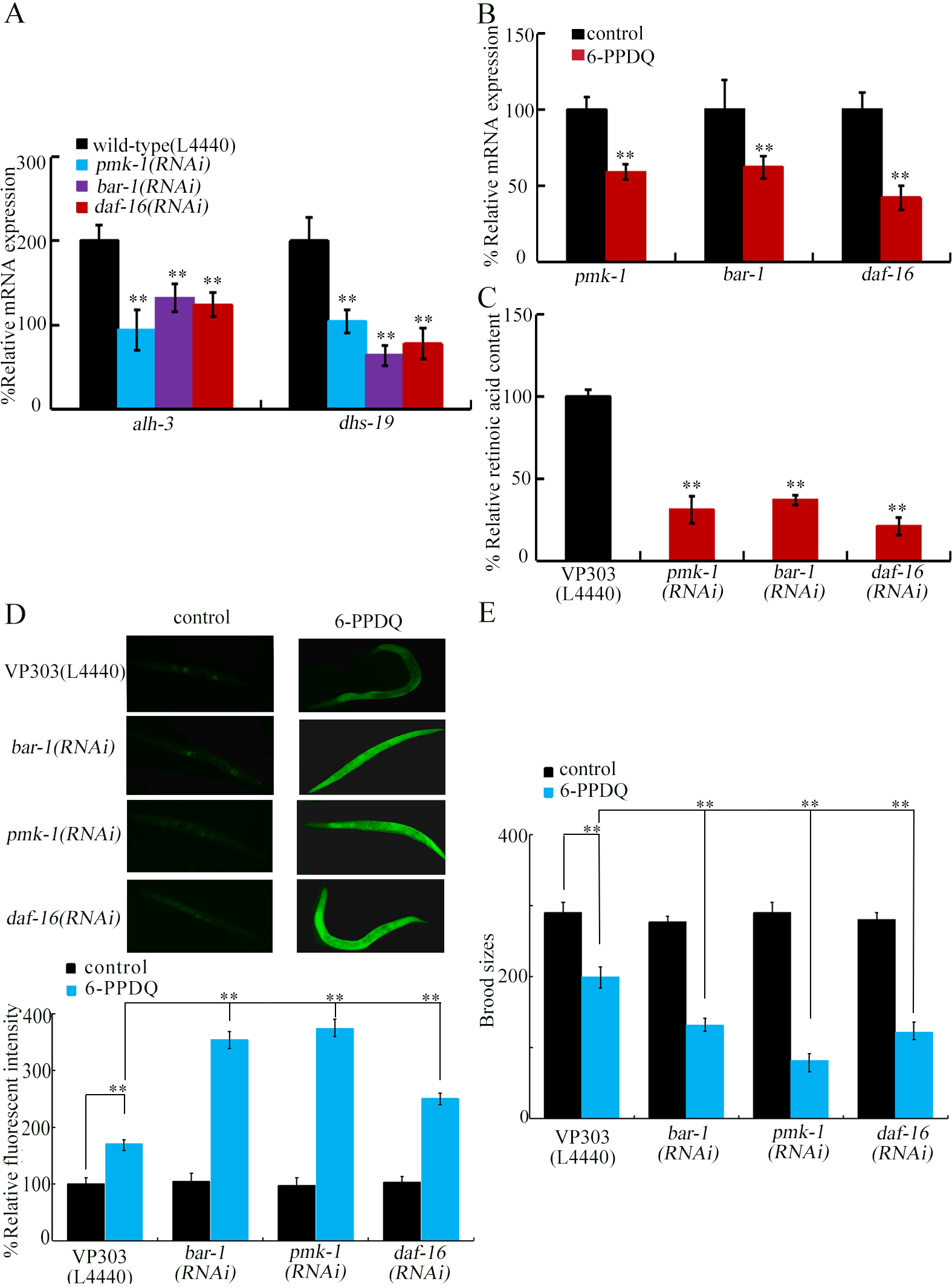
Figure 4. Effect of intestinal RNAi of pmk-1, bar-1, and daf-16 on expressions of alh-3 and dhs-19 in 6-PPDQ-exposed nematodes. (A) Expressions of intestinal alh-3 and dhs-19 in 6-PPDQ-exposed nematodes with intestinal RNAi of pmk-1, bar-1, and daf-16. Thirty intact intestines were isolated for qRT-PCR analysis. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01 vs. VP303 (L4440); (B) Effect of 6-PPDQ exposure on intestinal expressions of pmk-1, bar-1, and daf-16. Thirty intact intestines were isolated for qRT-PCR analysis. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01 vs. control; (C) Effect of intestinal RNAi of pmk-1, bar-1, and daf-16 on retinoic acid content in 6-PPDQ-exposed nematodes. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01 vs. VP303(L4440); (D) Effect of intestinal RNAi of pmk-1, bar-1, and daf-16 on 6-PPDQ toxicity in causing ROS generation. Exposure concentration of 6-PPDQ was 10 μg/L.**P < 0.01; (E) Effect of intestinal RNAi of pmk-1, bar-1, and daf-16 on 6-PPDQ toxicity in decreasing brood sizes. Exposure concentration of 6-PPDQ was 10 μg/L.**P < 0.01. RNA interference; 6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone; qRT-PCR: quantitative real-time polymerase chain reaction.
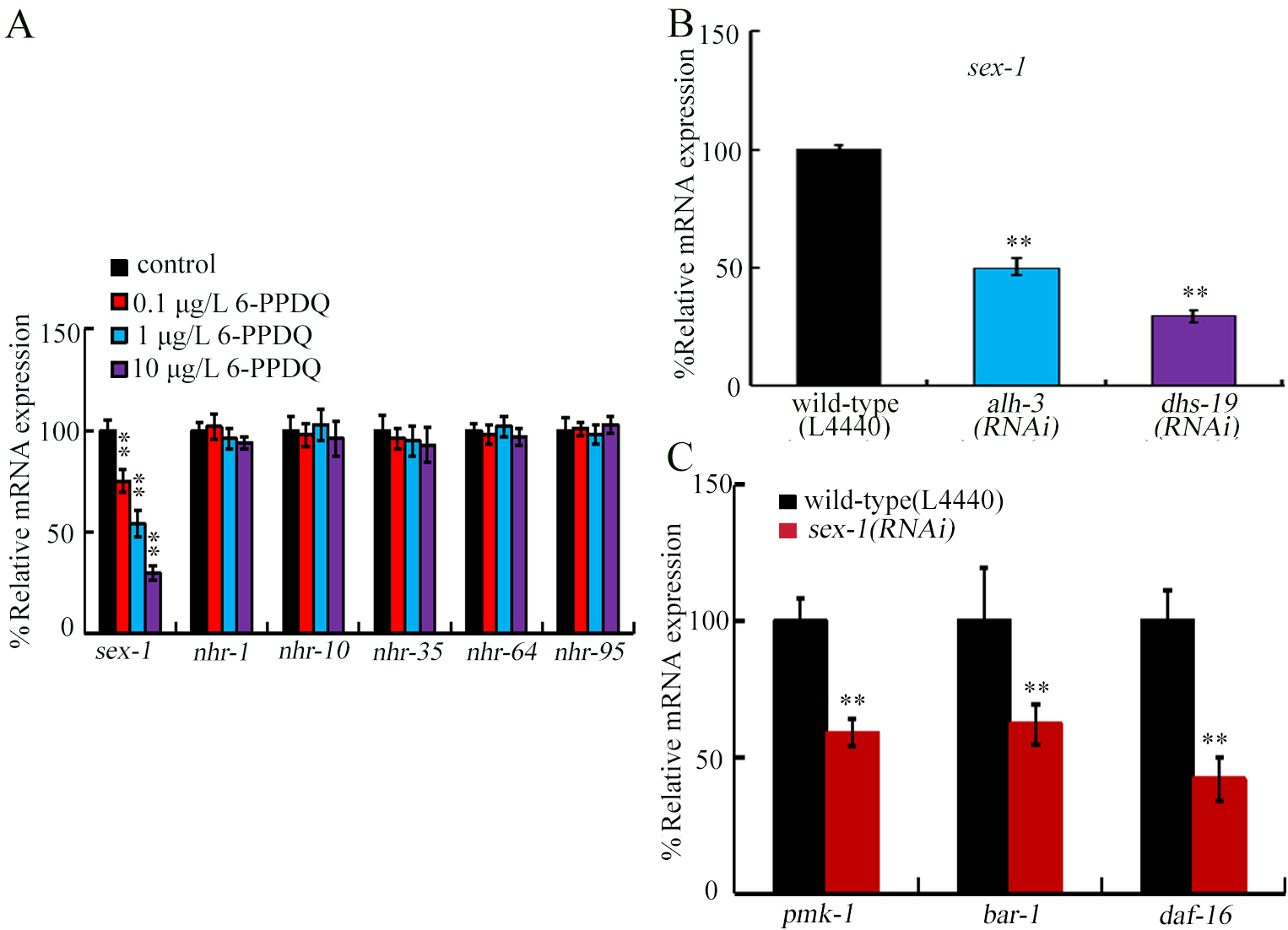
Figure 5. Effect of 6-PPDQ exposure on the expression of genes encoding potential retinoic acid receptors. (A) Effect of 6-PPDQ exposure on expressions of sex-1, nhr-1, nhr-10, nhr-35, nhr-64, and nhr-91. **P < 0.01 vs. control; (B) Effect of intestinal RNAi of alh-3 and dhs-19 on expression of sex-1 in 6-PPDQ-exposed nematodes. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01 vs. wild-type (L4440); (C) Effect of RNAi of sex-1 on intestinal expressions of pmk-1, bar-1, and daf-16 in 6-PPDQ-exposed nematodes. Thirty intact intestines were isolated for qRT-PCR analysis. Exposure concentration of 6-PPDQ was 10 μg/L.**P < 0.01 vs. wild-type (L4440).6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone; RNAi: RNA interference; qRT-PCR: quantitative real-time polymerase chain reaction.
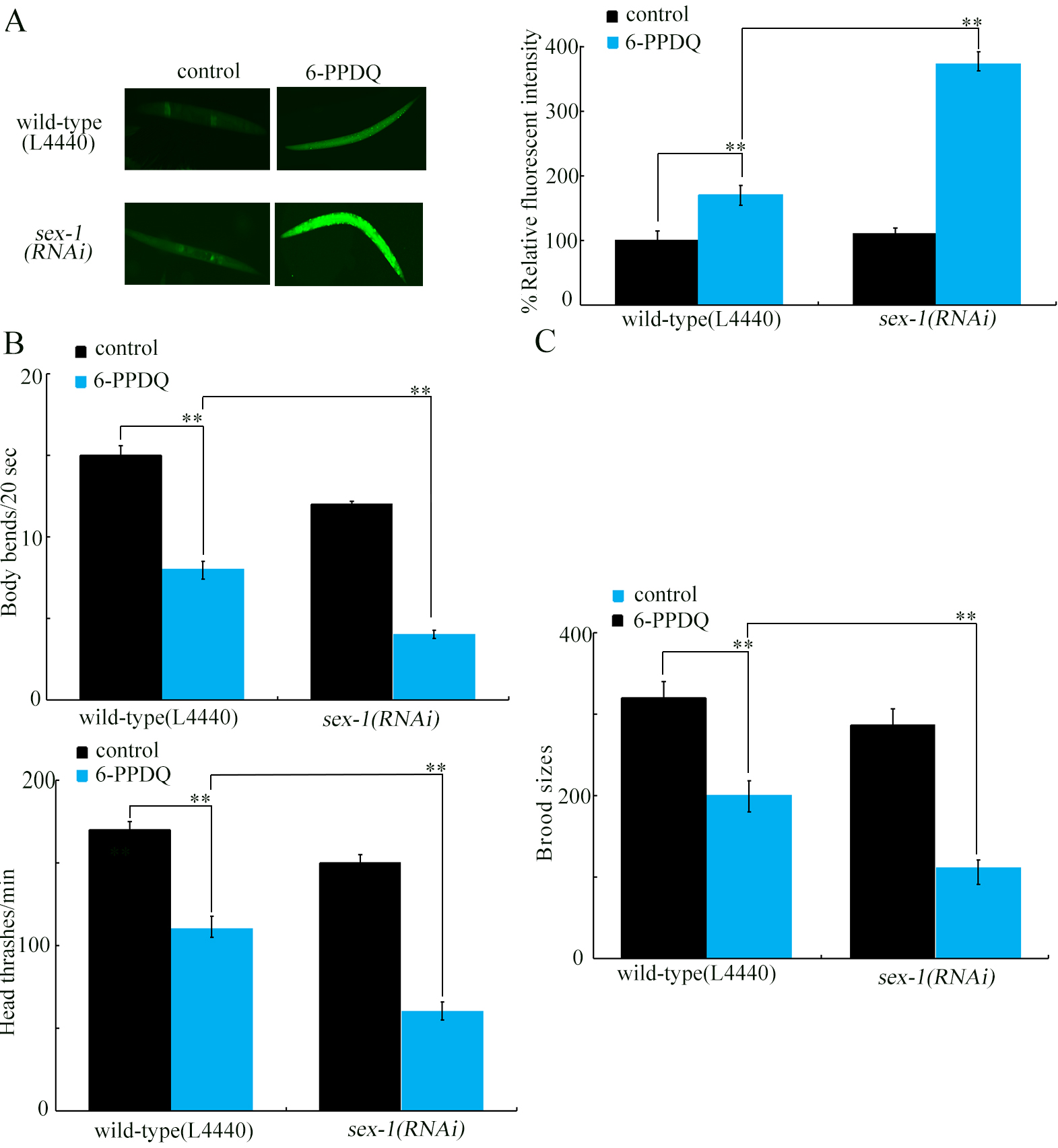
Figure 6. Effect of RNAi of sex-1 on 6-PPDQ toxicity. (A) Effect of RNAi of sex-1 on 6-PPDQ toxicity in inducing ROS generation; (B) Effect of RNAi of sex-1 on 6-PPDQ toxicity in decreasing locomotion behavior; (C) Effect of RNAi of sex-1 on 6-PPDQ toxicity in reducing brood size. Exposure concentration of 6-PPDQ was 10 μg/L. **P < 0.01. RNA interference; 6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone; ROS: reactive oxygen species.
Intestinal activity of ALH-3 and DHS-19 in controlling 6-PPDQ toxicity
Considering requirement of retinol absorption from intestinal lumen to body of nematodes[63-64], we next focused on examination of intestinal function of ALH-3 and DHS-19 expressed in intestine (https://wormbase.org/). The 6-PPDQ-induced ROS generation was strengthened by intestinal alh-3 and dhs-19 RNAi [Figure 3A], and 6-PPDQ-caused reduction in brood size was enhanced by intestinal RNAi of alh-3 and dhs-19 [Figure 3B]. Therefore, ALH-3 and DHS-19 functioned in the intestine to modulate 6-PPDQ toxicity. Due to a defect in locomotion in the VP303 strain, we did not further evaluate the locomotion phenotype. Additionally, retinoic acid content was inhibited by intestinal alh-3 and dhs-19 RNAi [Figure 3C].
Identification of PMK-1, BAR-1, and DAF-16 as upstream regulators for ALH-3 and DHS-19 to regulate 6-PPDQ toxicity
Some intestinal molecular signals participate in the control of pollutant toxicity[65]. Among them, PMK-1 is p38 mitogen-activated protein kinase (MAPK)[66], BAR-1 is β-catenin[67], and DAF-16 is forkhead box O (FOXO) transcriptional factor[68]. After 6-PPDQ exposure, intestinal alh-3 and dhs-19 expressions were decreased by intestinal pmk-1, bar-1, and daf-16 RNAi [Figure 4A]. Intestinal pmk-1, bar-1, and daf-16 expressions could meanwhile be inhibited by 6-PPDQ [Figure 4B]. After 6-PPDQ exposure, retinoic acid content was inhibited by intestinal pmk-1, bar-1, and daf-16 RNAi [Figure 4C]. Moreover, 6-PPDQ toxicity was enhanced by intestinal pmk-1, bar-1, and daf-16 RNAi [Figure 4D and E], suggesting susceptibility of pmk-1(RNAi), bar-1(RNAi), and daf-16(RNAi) to 6-PPDQ toxicity.
Screen of genes encoding potential retinoic acid receptors in response to 6-PPDQ
Among genes encoding potential retinoic acid receptors, nhr-1, nhr-10, nhr-35, nhr-64, and nhr-91 expressions were not changed by 6-PPDQ [Figure 5A]. In contrast, sex-1 expression was decreased by 6-PPDQ [Figure 5A]. Moreover, after 6-PPDQ exposure, sex-1 expression was inhibited by intestinal alh-3 and dhs-19 RNAi [Figure 5B], suggesting response of sex-1 expression to 6-PPDQ.
SEX-1 was involved in controlling 6-PPDQ toxicity
After 6-PPDQ exposure, intestinal pmk-1, bar-1, and daf-16 expressions were suppressed by sex-1 RNAi [Figure 5C], suggesting involvement of SEX-1 in regulating stress response. The 6-PPDQ-induced ROS generation, locomotion inhibition, and reduced brood size were further enhanced by sex-1 RNAi [Figure 6A-C]. These results demonstrated the function of SEX-1 in controlling 6-PPDQ toxicity.
Pharmacological effect of retinoic acid against 6-PPDQ toxicity in wild-type
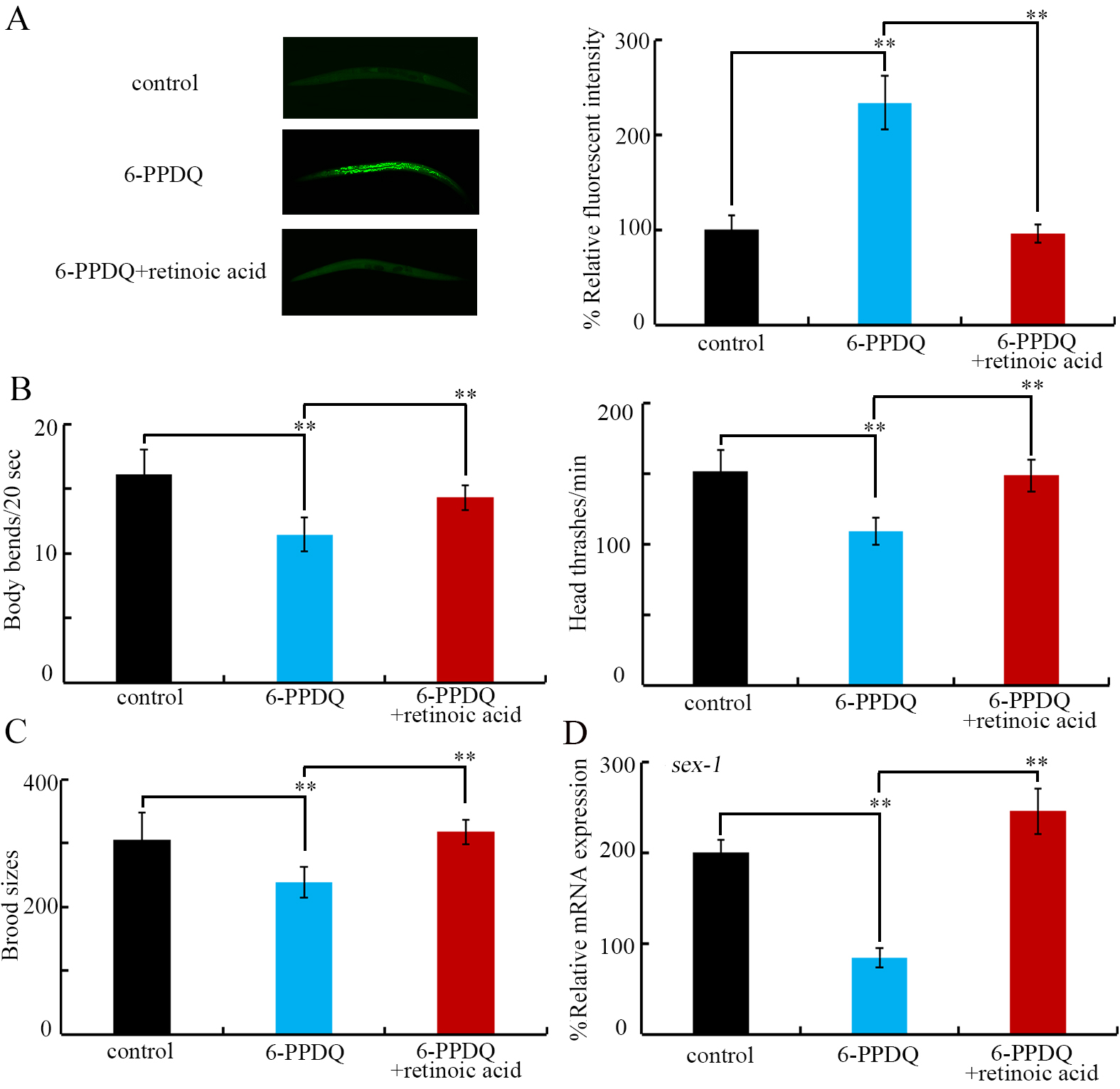
After 6-PPDQ exposure, ROS generation was suppressed by retinoic acid treatment [Figure 7A]; locomotion was accelerated by retinoic acid [Figure 7B], and brood size was increased by retinoic acid [Figure 7C]. More importantly, 6-PPDQ-caused decrease in sex-1 expression was inhibited by following retinoic acid treatment [Figure 7D].
Figure 7. Effect of treatment with retinoic acid (15 μM) on toxicity of 6-PPDQ (10 μg/L) in wild-type nematodes. (A) Effect of treatment with retinoic acid on 6-PPDQ toxicity in inducing ROS generation in wild-type nematodes; (B) Effect of treatment with retinoic acid on 6-PPDQ toxicity in decreasing locomotion in wild-type nematodes; (C) Effect of treatment with retinoic acid on 6-PPDQ toxicity in reducing brood size wild-type nematodes; (D) Effect of treatment with retinoic acid on sex-1 expression in 6-PPDQ-exposed nematodes. **P < 0.01. 6-PPDQ: N’-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone; ROS: reactive oxygen species.
In mammals, 6-PPDQ disrupts several aspects of metabolism[69-70]. In nematodes, 6-PPDQ also caused disruption in some metabolisms[44-47]. We further found a reduction in retinoic acid content by 6-PPDQ [Figure 1B]. This suggested that multiple aspects of metabolism would be disrupted by 6-PPDQ.
For biochemical basis, observed decrease in retinoic acid content by 6-PPDQ was due to inhibition in retinoic acid synthesis. Firstly, this reduction was associated with inhibition of its synthesis from retinal. Expression of retinal dehydrogenase gene alh-3 was decreased by 6-PPDQ [Figure 1C], and retinoic acid content was inhibited by alh-3 RNAi [Figure 1D]. Secondly, it was also related to retinal synthesis inhibition from retinol. Expression of dehydrogenase gene dhs-19 was decreased by 6-PPDQ [Figure 1E], and retinoic acid content was also reduced by dhs-19 RNAi [Figure 1F]. That is, reduced synthesis of retinal by 6-PPDQ possibly further contributed to reduction in retinoic acid content.
Moreover, evidence was provided to show an association between inhibition in retinoic acid synthesis and 6-PPDQ toxicity. RNAi of alh-3 and dhs-19 governing retinoic acid synthesis caused susceptibility to 6-PPDQ-induced toxicities [Figure 2]. These 6-PPDQ toxicities were suppressed by retinoic acid treatment
ALH-3 and DHS-19 acted in intestine to modulate 6-PPDQ toxicity (Figure 3A and 3B). Additionally, ALH-3 and DHS-19 functioned in intestine to control retinoic acid content [Figure 3C]. In C. elegans, intestine is the pivotal organ in response to pollutant exposure[72]. Enzyme genes governing retinoic acid synthesis also acted in this organ to regulate pollutant toxicity. Besides in the intestine, alh-3 and dhs-19 are also expressed in neurons, epidermis, muscle, and/or germline (https://wormbase.org). Functions of alh-3 and dhs-19 in these tissues need to be further determined.
pmk-1, bar-1, and daf-16 were identified as upregulators of intestinal alh-3 and dhs-19. Intestinal pmk-1, bar-1, and daf-16 participated in control of toxicity of multiple pollutants[65], suggesting their crucial and conserved function in modulating stress response. Intestinal pmk-1, bar-1, and daf-16 controlled 6-PPDQ toxicity, as reflected in their susceptibility to 6-PPDQ-induced intestinal and reproductive toxicities [Figure 4D and E]. Additionally, after 6-PPDQ exposure, intestinal pmk-1, bar-1, and daf-16 regulated retinoic acid content, as indicated by changes in intestinal alh-3 and dhs-19 expression and retinoic acid content [Figure 4A and C]. Moreover, pmk-1, bar-1, and daf-16 expressions were decreased by 6-PPDQ [Figure 4B]. Thus, 6-PPDQ caused inhibition in retinoic acid synthesis, which was under the control of some intestinal signals. This also implied that observed inhibition in retinoic acid synthesis and decrease in alh-3 and dhs-19 expressions reflect a kind of stress response after pollutant exposure.
Among predicted retinoic acid receptor genes, sex-1 responded to 6-PPDQ. sex-1 expression was inhibited by 6-PPDQ [Figure 5A]. Some evidence was provided to support the role of SEX-1 as a retinoic acid receptor. Firstly, sex-1 expression was inhibited by alh-3 and dhs-19 RNAi [Figure 5B], which have been proven to govern retinoic acid synthesis [Figure 1D and F]. This further indicated formation of alh-3/dhs-19-sex-1 signaling axis. Secondly, 6-PPDQ-induced inhibition in sex-1 expression was suppressed by following retinoic acid treatment [Figure 7D]. Among predicted retinoic acid receptor genes, only sex-1 expression was changed by 6-PPDQ [Figure 5A], suggesting sensitivity of sex-1 expression to 6-PPDQ. For other predicted retinoic acid receptor genes, the sensitivity of their expression may be limited to a response to pollutant exposure. Or, they may respond specially to other pollutant exposures.
Using different endpoints, we confirmed susceptibility of sex-1(RNAi) to 6-PPDQ toxicity [Figure 6]. This was consistent with observed decrease in retinoic acid content and expressions of alh-3 and dhs-19
Moreover, we raised a feedback loop formed between alh-3/dhs-19-sex-1 signaling axis and intestinal signals (pmk-1, bar-1, and daf-16) to control retinoic acid content and toxicity of 6-PPDQ-exposed nematodes. As described above, after 6-PPDQ exposure, alh-3 and dhs-19 expressions were inhibited by intestinal pmk-1, bar-1, and daf-16 RNAi [Figure 4A], and intestinal pmk-1, bar-1, and daf-16 expressions were further decreased by sex-1 RNAi [Figure 5C]. This suggested that a feedback regulatory mechanism existed to drive and amplify toxicity induction and inhibition in retinoic acid synthesis in 6-PPDQ-exposed nematodes.
the current work was performed in C. elegans, and some of the metabolic regulations from retinol to retinoic acid in C. elegans may be different from those in mammals. The underlying mechanisms for effect of 6-PPDQ exposure on metabolism from retinol to retinoic acid need to be further examined in mammals. Additionally, 6-PPDQ exposure in nematodes reflects a form of feeding exposure. In contrast, exposure of mammals to 6-PPDQ can be performed through multiple exposure routes. Moreover, effect of 6-PPDQ on retinoic synthesis in multiple organs in mammals also needs to be investigated.
CONCLUSIONS
Together, we detected reduction in retinoic acid content by 6-PPDQ. This observed reduction was due to inhibition in expression of enzyme genes of alh-3 and dhs-19. Intestinal alh-3 and dhs-19 RNAi caused a reduction in retinoic acid content and susceptibility to 6-PPDQ toxicity. This suggested a link between reduced retinoic acid content and 6-PPDQ-induced toxicity, which was confirmed by pharmacological treatment of 6-PPDQ-exposed nematodes with retinoic acid. Additionally, 6-PPDQ inhibited receptor gene sex-1 expression. Moreover, a feedback loop was formed between alh-3/dhs-19-sex-1 axis and some intestinal signals to control 6-PPDQ toxicity. Our data demonstrated that 6-PPDQ exposure poses a risk of inhibiting retinoic acid synthesis, which is linked to toxicity through the formation of a feedback regulatory mechanism.
DECLARATIONS
Acknowledgments
The authors declare that Excel and Photoshop were used to generate the figures in this manuscript.
Authors’ contributions
Methodology: Hu, D.; Wang, Y.; Hu, G.
Data analysis: Hu, D.; Wang, Y.; Hu, G.
Design, review & editing: Liu, R.; Wang, D.
All authors approved the final manuscript.
Availability of data and materials
The data during the current study are available in Supplementary Materials.
Financial support and sponsorship
This study was supported by a grant from the Open Fund for Key Laboratory of Environmental Pollution Health Risk Assessment in South China Institute of Environmental Sciences, Ministry of Ecology and Environment (KLEPHRA-2024-2).
Conflicts of interest
Dayong Wang is the Guest Editor of the Journal of Environmental Exposure Assessment. He had no involvement in the review or editorial process of this manuscript, including but not limited to reviewer selection, evaluation, or the final decision, while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Tian, Z.; Zhao, H.; Peter, K. T.; et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185-9.
2. Hua, X.; Wang, D. Tire-rubber related pollutant 6-PPD quinone: a review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard. Mater. 2023, 459, 132265.
3. Zhao, H. N.; Hu, X.; Tian, Z.; et al. Transformation products of tire rubber antioxidant 6PPD in heterogeneous gas-phase ozonation: identification and environmental occurrence. Environ. Sci. Technol. 2023, 57, 5621-32.
4. Li, K.; Li, W.; Chen, Z.; Ye, Z. Accelerated transformation of 6PPD to 6PPD-Q in tire wear particles driven by roadway manganese oxides and dry-wet cycles: interfacial catalysis coupled with climatic stressors. Water. Res. 2025, 288, 124741.
5. Kazmi, S. S. U. H.; Xu, Q.; Tayyab, M.; et al. Navigating the environmental dynamics, toxicity to aquatic organisms and human associated risks of an emerging tire wear contaminant 6PPD-quinone. Environ. Pollut. 2024, 356, 124313.
6. Liang, Y.; Zhu, F.; Li, J.; et al. P-phenylenediamine antioxidants and their quinone derivatives: a review of their environmental occurrence, accessibility, potential toxicity, and human exposure. Sci. Total. Environ. 2024, 948, 174449.
7. Chen, X.; He, T.; Yang, X.; et al. Analysis, environmental occurrence, fate and potential toxicity of tire wear compounds 6PPD and 6PPD-quinone. J. Hazard. Mater. 2023, 452, 131245.
8. Zhang, Y.; Yan, L.; Wang, L.; Zhang, H.; Chen, J.; Geng, N. A nation-wide study for the occurrence of PPD antioxidants and 6PPD-quinone in road dusts of China. Sci. Total. Environ. 2024, 922, 171393.
9. Ren, S.; Xia, Y.; Wang, X.; et al. Development and application of diffusive gradients in thin-films for in-situ monitoring of 6PPD-Quinone in urban waters. Water. Res. 2024, 266, 122408.
10. Ihenetu, S. C.; Xu, Q.; Khan, Z. H.; et al. Environmental fate of tire-rubber related pollutants 6PPD and 6PPD-Q: a review. Environ. Res. 2024, 258, 119492.
11. Jin, R.; Venier, M.; Chen, Q.; Yang, J.; Liu, M.; Wu, Y. Amino antioxidants: a review of their environmental behavior, human exposure, and aquatic toxicity. Chemosphere 2023, 317, 137913.
12. Bohara, K.; Timilsina, A.; Adhikari, K.; et al. A mini review on 6PPD quinone: a new threat to aquaculture and fisheries. Environ. Pollut. 2024, 340, 122828.
13. Wang, W.; Huang, G.; Miao, F.; Zhao, Z.; Cai, Z. Biotransformation of tire-derived 6PPD and 6PPD-Q in soil nematode caenorhabditis elegans: unraveling novel Phosphorylation Products and Distinct Kinetic Profiles. Environ. Sci. Technol. 2025, 59, 14625-36.
14. He, W.; Gu, A.; Wang, D. Four-week repeated exposure to tire-derived 6-PPD quinone causes multiple organ injury in male BALB/c mice. Sci. Total. Environ. 2023, 894, 164842.
15. Yu, H.; Zhang, W.; Wang, D.; et al. Exposure to 6PPD-Q induces dysfunctions of ovarian granulosa cells: its potential role in PCOS. J. Hazard. Mater. 2025, 486, 137037.
16. Yao, K.; Kang, Q.; Liu, W.; Chen, D.; Wang, L.; Li, S. Chronic exposure to tire rubber-derived contaminant 6PPD-quinone impairs sperm quality and induces the damage of reproductive capacity in male mice. J. Hazard. Mater. 2024, 470, 134165.
17. Zhao, H. N.; Thomas, S. P.; Zylka, M. J.; Dorrestein, P. C.; Hu, W. Urine excretion, organ distribution, and placental transfer of 6PPD and 6PPD-quinone in mice and potential developmental toxicity through nuclear receptor pathways. Environ. Sci. Technol. 2023, 57, 13429-38.
18. Fang, L.; Fang, C.; Di, S.; et al. Oral exposure to tire rubber-derived contaminant 6PPD and 6PPD-quinone induce hepatotoxicity in mice. Sci. Total. Environ. 2023, 869, 161836.
19. Yang, Y.; Sun, N.; Lv, J.; et al. Environmentally realistic dose of tire-derived metabolite 6PPD-Q exposure causes intestinal jejunum and ileum damage in mice via cannabinoid receptor-activated inflammation. Sci. Total. Environ. 2024, 918, 170679.
20. Shi, R.; Zhang, Z.; Zeb, A.; et al. Environmental occurrence, fate, human exposure, and human health risks of p-phenylenediamines and their quinones. Sci. Total. Environ. 2024, 957, 177742.
21. Deng, M.; Ji, X.; Peng, B.; Fang, M.
22. Wan, X.; Liang, G.; Wang, D. Potential human health risk of the emerging environmental contaminant 6-PPD quinone. Sci. Total. Environ. 2024, 949, 175057.
23. Black, G. P.; De, Parsia. M.; Uychutin, M.; Lane, R.; Orlando, J. L.; Hladik, M. L. 6PPD-quinone in water from the San Francisco-San Joaquin Delta, California, 2018-2024. Environ. Monit. Assess. 2025, 197, 369.
24. Xu, S.; Wang, Q.; Lao, J. Y.; et al. Typical tire additives in river water: leaching, transformation, and environmental risk assessment. Environ. Sci. Technol. 2024, 58, 18940-9.
25. Rauert, C.; Charlton, N.; Okoffo, E. D.; et al. Concentrations of tire additive chemicals and tire road wear particles in an Australian urban tributary. Environ. Sci. Technol. 2022, 56, 2421-31.
26. McDonough, C. M.; Xu, H. S.; Guo, T. L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283-300.
27. Wu, J.; Shao, Y.; Hua, X.; Li, Y.; Wang, D. Photo-aged polylactic acid microplastics causes severe transgenerational decline in reproductive capacity in C. elegans: insight into activation of DNA damage checkpoints affected by multiple germline histone methyltransferases. Environ. Pollut. 2025, 382, 126697.
28. Yang, Y. F.; Chen, P. J.; Liao, V. H. Nanoscale zerovalent iron (nZVI) at environmentally relevant concentrations induced multigenerational reproductive toxicity in Caenorhabditis elegans. Chemosphere 2016, 150, 615-23.
29. Song, M.; Ruan, Q.; Wang, D. Comparison of transgenerational neurotoxicity between pristine and amino-modified nanoplastics in C. elegans. Toxics 2024, 12, 555.
30. Shang, Y.; Chen, K.; Ni, H.; et al. Environmentally relevant concentrations of perfluorobutane sulfonate impair locomotion behaviors and healthspan by downregulating mitophagy in C. elegans. J. Hazard. Mater. 2024, 480, 135938.
31. Liu, H.; Wu, Y.; Wang, Z. Long-term exposure to polystyrene nanoparticles at environmentally relevant concentration causes suppression in heme homeostasis signal associated with transgenerational toxicity induction in Caenorhabditis elegans. J. Hazard. Mater. 2023, 459, 132124.
32. Wang, X.; Zhang, J.; Cao, Z.; Dai, L.; Zhao, Q.; Du, H. Multigenerational toxicity and transcriptomic changes induced by environmentally relevant concentrations of microcystin-LR and -RR in Caenorhabditis elegans. Toxicol. Lett. 2025, 410, 58-66. Erratum in: 2025;411:122.
33. Liu, Z.; Bian, Q.; Wang, D. 6-PPD quinone induces response of nuclear hormone receptors in the germline associated with formation of reproductive toxicity in Caenorhabditis elegans. J. Hazard. Mater. 2025, 495, 138815.
34. Wang, Y.; Wang, D. Transgenerational intestinal toxicity of 6-PPD quinone in causing ROS production, enhancement in intestinal permeability and suppression in innate immunity in C. elegans. Environ. Pollut. 2024, 363, 125208.
35. Song, M.; Ruan, Q.; Wang, D. Paeoniflorin alleviates toxicity and accumulation of 6-PPD quinone by activating ACS-22 in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2024, 286, 117226.
36. Hua, X.; Wang, D. 6-PPD quinone causes alteration in ubiquinone-mediated complex III associated with toxicity on mitochondrial function and longevity in Caenorhabditis elegans. J. Environ. Chem. Eng. 2025, 13, 116571.
37. Hua, X.; Liang, G.; Chao, J.; Wang, D. Exposure to 6-PPD quinone causes damage on mitochondrial complex I/II associated with lifespan reduction in Caenorhabditis elegans. J. Hazard. Mater. 2024, 472, 134598.
38. Hua, X.; Wang, D. 6-PPD quinone at environmentally relevant concentrations activates feedback response of electron transport chain to mediate damage on mitochondrial function and longevity in Caenorhabditis elegans. Environ. Chem. Ecotox. 2025, 7, 2356-65.
39. Hua, X.; Wang, D. Transgenerational response of germline histone acetyltransferases and deacetylases to nanoplastics at predicted environmental doses in Caenorhabditis elegans. Sci. Total. Environ. 2024, 952, 175903.
40. Hua, X.; Wang, D. An environmentally relevant concentration of 6-PPD quinone inhibits two types of mitophagy to cause mitochondrial dysfunction and lifespan reduction in Caenorhabditis elegans. Environ. Sci. Process. Impacts. 2025, 27, 1928-40.
41. Hua, X.; Wang, D. 6-PPD quinone at environmentally relevant concentrations induced damage on longevity in C. elegans: mechanistic insight from inhibition in mitochondrial UPR response. Sci. Total. Environ. 2024, 954, 176275.
42. Salzer, L.; Witting, M.
43. Rubio-Tomás, T.; Tavernarakis, N. Lipid metabolism and ageing in Caenorhabditis elegans: a complex interplay. Biogerontology 2022, 23, 541-57.
44. Wang, W.; Li, Y.; Wang, D. Exposure to 6-PPD quinone disrupts adsorption and catabolism of leucine and causes mitochondrial dysfunction in Caenorhabditis elegans. Toxics 2025, 13, 544.
45. Wang, Y.; Hu, G.; Wang, D. Increased S-adenosyl methionine strengthens the suppression in mitochondrial unfolded protein response induced by 6-PPD quinone at environmentally relevant concentrations in Caenorhabditis elegans. Environ. Pollut. 2025, 386, 127231.
46. Wu, J.; Li, L.; Hu, D.; Liu, R.; Bian, Q.; Wang, D. Environmentally relevant concentrations of 6-PPDQ disrupt vitamin D3 adsorption and receptor function in Caenorhabditis elegans. Environ. Sci. Process. Impacts. 2025, 27, 2798-808.
47. Wan, X.; Liang, G.; Wang, D. 6-PPD quinone at environmentally relevant concentrations disrupts citric acid cycle in Caenorhabditis elegans: role of reduction in acetyl CoA and pyruvate contents. Environ. Chem. Ecotox. 2025, 7, 1119-29.
48. Liu, Z.; Li, Y.; Wang, D. Transgenerational response of glucose metabolism in Caenorhabditis elegans exposed to 6-PPD quinone. Chemosphere 2024, 367, 143653.
49. Wang, Y.; Wu, J.; Wang, D. 6-PPD quinone causes lipid accumulation across multiple generations differentially affected by metabolic sensors and components of COMPASS complex in Caenorhabditis elegans. Environ. Pollut. 2025, 366, 125539.
51. Ghyselinck, N. B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502.
52. Mohanty, R.; Das, S. K.; Patri, M. Modulation of benzo[a]pyrene induced anxiolytic-like behavior by retinoic acid in zebrafish: involvement of oxidative stress and antioxidant defense system. Neurotox. Res. 2017, 31, 493-504.
53. Joseph, P. A.; Everts, H. B.; Gumienny, T. L. Establishing C. elegans as a model to study the function of vitamin A metabolism. TWUSJ 2021;1:16-30. Available from: https://twusj-ojs-twu.tdl.org/twusj/article/view/11 (accessed 2025-11-17).
55. Wang, Y.; Wang, D. Effect of disruption in the intestinal barrier function during the transgenerational process on nanoplastic toxicity induction in Caenorhabditis elegans. Environ. Sci:. Nano. 2025, 12, 2741-9.
56. Wang, W.; Li, Y.; Wang, D. Long-Term Exposure to 6-PPD quinone inhibits glutamate synthesis and glutamate receptor function associated with its toxicity induction in Caenorhabditis elegans. Toxics 2025, 13, 434.
57. Wu, T.; He, J.; Ye, Z.; et al. Aged biodegradable nanoplastics enhance body accumulation associated with worse neuronal damage in Caenorhabditis elegans. Environ. Sci. Technol. 2025, 59, 4352-63.
58. Wu, J.; Shao, Y.; Hua, X.; Wang, D. Activated hedgehog and insulin ligands by decreased transcriptional factor DAF-16 mediate transgenerational nanoplastic toxicity in Caenorhabditis elegans. J. Hazard. Mater. 2024, 480, 135909.
59. Wu, J.; Shao, Y.; Hua, X.; Wang, Y.; Wang, D. Nanoplastic at environmentally relevant concentrations induces toxicity across multiple generations associated with inhibition in germline G protein-coupled receptor CED-1 in Caenorhabditis elegans. Chemosphere 2024, 364, 143011.
60. Wu, J.; Shen, S.; Wang, D. 6-PPD quinone at environmentally relevant concentrations induces immunosenescenece by causing immunosuppression during the aging process. Chemosphere 2024, 368, 143719.
61. Joshi, P.; Chia, S.; Yang, X.; et al. Combinations of vitamin A and vitamin E metabolites confer resilience against amyloid-β aggregation. ACS. Chem. Neurosci. 2023, 14, 657-66.
62. Hua, X.; Feng, X.; Liang, G.; Chao, J.; Wang, D. Exposure to 6-PPD quinone at environmentally relevant concentrations causes abnormal locomotion behaviors and neurodegeneration in Caenorhabditis elegans. Environ. Sci. Technol. 2023, 57, 4940-50.
63. Bang, Y. J. Vitamin A: a key coordinator of host-microbe interactions in the intestine. BMB. Rep. 2023, 56, 133-9.
64. Li, Y.; Wei, C. H.; Hodges, J. K.; Green, M. H.; Ross, A. C. Priming with retinoic acid, an active metabolite of vitamin A, increases vitamin A uptake in the small intestine of neonatal rats. Nutrients 2021, 13, 4275.
65. Wang, D. Y. Target organ toxicology in Caenorhabditis elegans. Springer Nature Singapore Pte Ltd, 2019.
66. Qu, M.; Liu, Y.; Xu, K.; Wang, D. Activation of p38 MAPK signaling-mediated endoplasmic reticulum unfolded protein response by nanopolystyrene particles. Adv. Biosyst. 2019, 3, e1800325.
67. Shao, H.; Kong, Y.; Wang, D. Response of intestinal signaling communication between the nucleus and peroxisome to nanopolystyrene at a predicted environmental concentration. Environ. Sci:. Nano. 2020, 7, 250-61.
68. Shao, H.; Han, Z.; Krasteva, N.; Wang, D. Identification of signaling cascade in the insulin signaling pathway in response to nanopolystyrene particles. Nanotoxicology 2019, 13, 174-88.
69. Jia, K.; Sun, J.; Du, Q.; et al. Mass spectrometry imaging unveils the metabolic effect of 6ppd-quinone in exposed mice. Environ. Sci. Technol. 2025, 59, 4282-91.
70. Wang, L.; Tang, W.; Sun, N.; et al. Low-dose tire wear chemical 6PPD-Q exposure elicit fatty liver via promoting fatty acid biosynthesis in ICR mice. J. Hazard. Mater. 2025, 489, 137574.
71. Liu, Y.; Xu, S.; Zhang, C.; et al. Hydroxysteroid dehydrogenase family proteins on lipid droplets through bacteria, C. elegans, and mammals. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids. 2018, 1863, 881-94.
72. Wang, D. Y. Toxicology at environmentally relevant concentrations in Caenorhabditis elegans. Springer Nature Singapore Pte Ltd, 2022.
73. Carmi, I.; Kopczynski, J. B.; Meyer, B. J. The nuclear hormone receptor SEX-1 is an X-chromosome signal that determines nematode sex. Nature 1998, 396, 168-73.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].