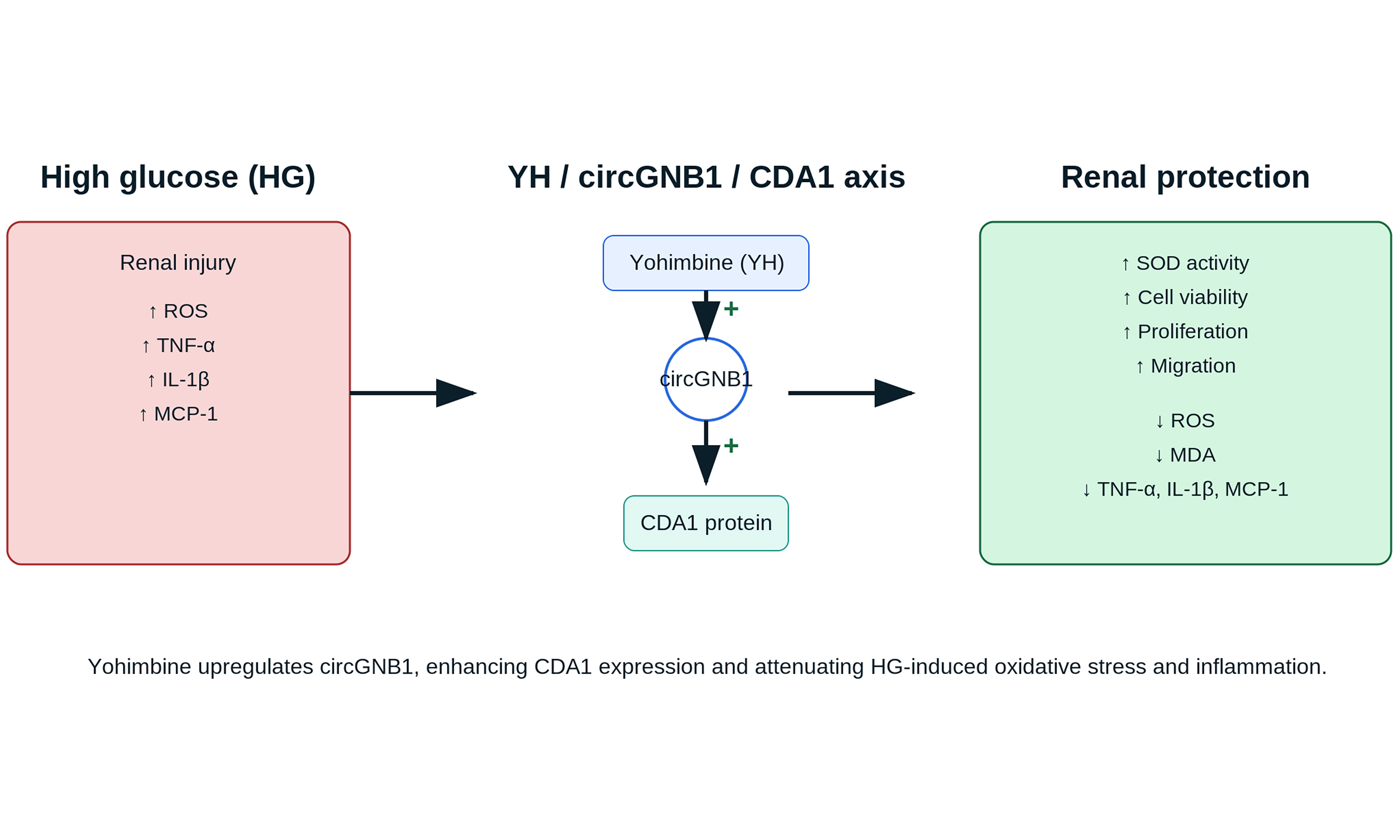
Yohimbine attenuates oxidative stress through circGNB1/CDA1 axis in diabetic nephropathy
Abstract
Aim: To investigate the role of yohimbine (YH) in diabetic nephropathy (DN) progression and identify its underlying molecular mechanisms.
Methods: Human renal proximal tubular epithelial cells (HK-2) were cultured with high glucose (HG) and treated with varying doses of YH. Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. Inflammatory markers and oxidative stress were measured. Male diabetic C57BLKS/J-LepR (db/db) mice received YH (2.5, 5, or 10 mg/kg) daily for eight weeks. Circular RNA (circRNA) microarray analysis was performed on HG-induced HK-2 cells. RNA pull-down assays combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS) were used to identify circGNB1-interacting proteins. Cellular localization and protein expression were determined through fractionation, RNA fluorescence in situ hybridization (RNA-FISH), and western blotting.
Results: YH significantly enhanced viability of HG-induced HK-2 cells while reducing inflammatory factors and oxidative stress markers. These protective effects were confirmed in db/db mice, where YH reduced blood glucose, urinary albumin-to-creatinine ratio, and blood urea nitrogen levels. CircRNA microarray identified circGNB1 as the most significantly upregulated gene in YH-treated HG-induced cells. RNA pull-down assays identified cell division autoantigen 1 (CDA1) as a circGNB1-interacting protein. CircGNB1 positively regulated CDA1 expression at the protein level in HK-2 cells. CDA1 knockdown significantly decreased cell viability and counteracted the protective effects of YH treatment.
Conclusion: Our study identifies a novel YH/circGNB1/CDA1 axis in DN progression. YH attenuates oxidative stress through upregulation of circGNB1, which interacts with and regulates CDA1 protein expression, thereby mitigating HG-induced renal cell damage. These findings provide new insights for developing therapeutic strategies for DN.
Keywords
INTRODUCTION
Diabetic nephropathy (DN), a significant microvascular complication of diabetes mellitus, affects approximately half of all patients with type I or II diabetes[1,2]. DN is characterized by extracellular matrix accumulation, basement membrane thickening, and glomerular hypertrophy linked to mesangial cell proliferation[3]. Its pathogenesis involves multiple interconnected pathways, with oxidative stress, chronic inflammation, and progressive fibrosis serving as key drivers of renal dysfunction. Recent studies have identified circular RNAs (circRNAs) as important regulators in DN progression, with circHIPK2 and circRNA_010383 demonstrating roles in renal fibrosis and inflammatory responses, respectively. These alterations progress to renal insufficiency, fibrosis, podocyte damage, and ultimately end-stage renal disease[4]. Addressing this global health challenge requires deeper understanding of underlying mechanisms and new therapeutic targets.
Yohimbine (YH), an indole alkaloid common in weight loss supplements, possesses anti-inflammatory and antioxidant properties with potential in treating organ fibrosis. Research suggests that α-2 adrenergic receptor targeting, a mechanism of YH action, may effectively alleviate fibrotic conditions[5]. YH demonstrates benefits in treating erectile dysfunction, cardiovascular diseases, inflammation, and cancer with relatively few side effects[6-9]. However, despite the demonstrated therapeutic potential of yohimbine in various conditions, its effects in DN have not been previously investigated. To our knowledge, this study represents the first investigation of yohimbine’s therapeutic potential specifically in DN.
circRNAs represent a novel class of endogenous non-coding RNAs formed through reverse splicing of exons or introns into covalently closed loops[10,11]. This structure provides resistance to exonuclease degradation, enhancing stability compared to linear RNA counterparts[12]. Emerging evidence indicates critical roles for circRNAs in DN pathogenesis[13-15].
Given the emerging importance of circRNAs in DN pathogenesis and the potential therapeutic benefits of targeting oxidative stress pathways, we hypothesized that yohimbine’s protective effects might involve novel circRNA-mediated mechanisms. Our study investigated YH’s role in DN and its molecular mechanisms. Human renal proximal tubular epithelial cells (HK-2) exposed to high glucose (HG) and treated with YH showed significantly enhanced viability and reduced inflammatory and oxidative stress markers. These protective effects were validated in db/db mice. CircRNA microarray analysis identified circGNB1 as the most upregulated gene in YH-treated HG-induced cells. Through RNA pull-down assays and liquid chromatography-tandem mass spectrometry (LC-MS/MS), we identified cell division autoantigen 1 (CDA1) as a circGNB1-interacting protein. Further experiments confirmed that circGNB1 positively regulates CDA1 protein expression and that CDA1 mediates YH’s protective effects in DN. These findings establish a novel YH/circGNB1/CDA1 axis in DN pathophysiology that could inform therapeutic development.
MATERIALS AND METHODS
Cell culture and treatment
HK-2 were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and maintained in low glucose Dulbecco’s modified Eagle’s Medium (DMEM, 5.5 mmol/L) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin in a humidified atmosphere (5% CO2, 37 °C, 95% humidity). To establish an in vitro model of DN, HK-2 cells were divided into normal glucose (NG,
Animal studies
Eight-week-old male C57BL/KS/J-LepR (db/db) mice (n = 32) and non-diabetic db/m controls (n = 32) were acquired from Shanghai Animal Center (Shanghai, China). Animals were maintained under standard conditions (22 ± 2 °C, 12-h light/dark cycle) with free access to food and water. After one week of acclimatization, mice were randomly divided into eight groups (n = 8 per group): (1) db/m + vehicle; (2)
Cell viability assessment
Cells were seeded (4 × 104/well) in 96-well plates and incubated for 12 h before treatment with varying YH concentrations for 48 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) solution (5 mg/mL) was then added for 4 h, followed by Dimethyl sulfoxide (CH2)2SO (DMSO) solubilization of formazan crystals. Absorbance was measured at 570 nm using a BioTek microplate reader.
Biochemical analysis
Fasting blood glucose was monitored biweekly using an Omron HEA-230 glucometer. Urinary albumin and creatinine levels were measured every four weeks using commercial kits (Biovision, USA). Blood urea nitrogen (BUN) was determined at study conclusion using kits from Arbor Assays. Post-euthanasia, kidneys were harvested for RNA, protein, and histological examinations. BUN was measured as an indicator of renal function, recognizing that this parameter can be influenced by non-renal factors and may not exclusively reflect glomerular filtration capacity.
Oxidative stress assessment
Tissue and cell samples were homogenized in appropriate buffers. Malondialdehyde (MDA) and nitrite levels were quantified using the Griess Reagent Nitrite Measurement Kit (Invitrogen, #13547), and Superoxide Dismutase (SOD) Activity Assay Kit (Cayman Chemical, #706002) was determined according to manufacturer protocols (Cayman Chemical #706002). Measurements were performed on a BioTek multimode plate reader.
Intracellular reactive oxygen species measurement
HK-2 cells were seeded in 100 mm dishes (5 × 105 cells/dish) and cultured overnight. Following experimental treatments for 24 h, cells were harvested, and proteins extracted using Cell lysis buffer (Biovision, #1067-100). Cytochrome Oxidase Activity was measured using Cytochrome Oxidase Activity Kit (Biovision, #K287-100) per manufacturer’s instructions.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from tissues and cells was isolated using Mini-BEST Universal RNA Extraction kit (TaKaRa, Japan). For circRNA and mRNA analysis, reverse transcription was performed with SYBR Green Master Mix (TaKaRa) on a PCR LightCycler480 (Roche). Expression was normalized to endogenous β-actin, with fold changes calculated using the 2^(-ΔΔCt) method. Primers sequences included: circGNB1 F: 5’-AGAATCCAAATGCGCACGAG-3’, R: 5’-GAAGGGCTTCTGGTCTGGTC-3’; GNB1 mRNA F: 5’-AGCTTGACCAGTTACGGCAG-3’, R: 5’-TCCTCGTGCGCATTTGGATT-3’; GAPDH F: 5’-CTGGGCTACACTGAGCACC-3’, R: 5’-AAGTGGTCGTTGAGGGCAATG-3’; β-actin F: 5’-AGAGCTACGAGCTGCCTGAC-3’, R: 5’-AGCACTGTGTTGGCGTACAG-3’.
Western blot analysis
Proteins were extracted using Total Cell Protein Extraction Kit (KeyGen Biotechnology, China) and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to poly (vinylidene fluoride) (PVDF) membranes and blocking with 2% bovine serum albumin (BSA, Beyotime Biotechnology), membranes were incubated overnight at 4 °C with Primary antibodies: CDA1 (1:1,000, Abcam, #ab123456), GAPDH (1:1,000, Cell Signaling Technology, #5174). Following washing, membranes were treated with HRP-conjugated anti-rabbit IgG (1:1,000, Proteintech, #SA00001-2) for 1 h at room temperature. Protein bands were visualized using chemiluminescence detection and quantified with ImageJ software, with GAPDH serving as internal control.
circRNAs microarray analysis
RNA was extracted from HG and YH-treated HK-2 cells using Trizol reagent (Invitrogen) and quantified with NanoDrop ND-1000 (Thermo Scientific). CircRNA microarray analysis was conducted by Kangchen Corporation following Arraystar protocols, with hybridization performed according to Arraystar Mouse circRNA Array procedures. The microarray was scanned using an Agilent G2505C Scanner. Differentially expressed circRNAs were identified using fold-change > 2.0 and P < 0.05 as selection criteria. The most significantly upregulated circRNA was prioritized for functional validation studies.
RNase R treatment
Total RNA samples (2 μg) were treated with 3 U/μg RNase R at 37 °C for 15 min to degrade linear RNA. The resulting RNA was purified using an RNA clean kit according to manufacturer’s protocol.
Subcellular fractionation
Cells were suspended in hypotonic buffer, incubated on ice for 10 min, and centrifuged at 5,000 g for 5 min to collect cytoplasmic fractions. Nuclear pellets were washed, resuspended in RIPA buffer, and lysed for
RNA-fluorescence in situ hybridization analysis
The RiboTM Fluorescent in situ Hybridization Kit (RiboBio) was employed following manufacturer’s protocol. Cy3-labeled probes targeting circGNB1 were hybridized with paraformaldehyde-fixed HK-2 cells at 37 °C overnight in hybridization buffer. After washing and 4’,6-diamidino-2-phenylindole (DAPI) counterstaining, fluorescent signals were visualized using a confocal microscope (OLYMPUS FV1000).
EdU proliferation assay
Cells (1 × 105) were seeded in 24-well plates and cultured for 20 h before incubation with EdU reagent for
Migration assay
Cells were resuspended in serum-free DMEM at 2 × 105 cells/mL. A 100 μL cell suspension was added to the upper chamber of a transwell insert, while 600 μL DMEM containing 10% FBS was placed in the lower chamber. After 24-h incubation, non-migrated cells were removed, and migrated cells were fixed, stained with crystal violet, and quantified by microscopy.
RNA pull-down and mass spectrometry
Cell lysates were homogenized in pull-down lysis buffer and centrifuged at 12,000 rpm for 15 min. Supernatants were incubated with biotinylated probes and streptavidin magnetic beads (Thermo, #65601) at 4 °C. Captured proteins were analyzed by mass spectrometry or western blotting.
For mass spectrometry, immunoprecipitated proteins were resolved by SDS-PAGE, excised, and treated with 10 mmol/L dithiothreitol and alkylated with 55 mmol/L iodoacetamide. After trypsin digestion, samples were desalted and analyzed by LC-MS/MS.
RNA immunoprecipitation
RNA immunoprecipitation (RIP) was performed using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore) according to manufacturer’s instructions. Cells were lysed, and lysates were stored at -80 °C. After antibody incubation and magnetic bead binding, RNA was purified for PCR analysis.
ELISA for cytokine analysis
Levels of interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein 1 (MCP-1) in human kidney-2 (HK-2) cells were measured using commercial Enzyme-Linked Immunosorbent Assay (ELISA) kits: IL-1β (Puregene, India, #PG-ELI-001), TNF-α (Puregene, India, #PG-ELI-002), MCP-1 (Puregene, India, #PG-ELI-003) following manufacturer’s protocols. Absorbance was determined using a BioTek multimode reader.
Statistical analysis
All data were analyzed using GraphPad Prism v9.0 and expressed as mean ± SD. Group comparisons were performed using one- or two-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. Statistical significance was denoted as *P < 0.05, **P < 0.01, and ***P < 0.001. For cell culture experiments, n represents independent biological replicates (separate cell culture preparations), with each experiment performed in triplicate technical replicates. For animal studies, n represents individual animals (n = 8 per group as specified). Data from technical replicates were averaged to generate single values for each biological replicate before statistical analysis.
RESULTS
Yohimbine inhibits HK-2 cell injury induced by HG
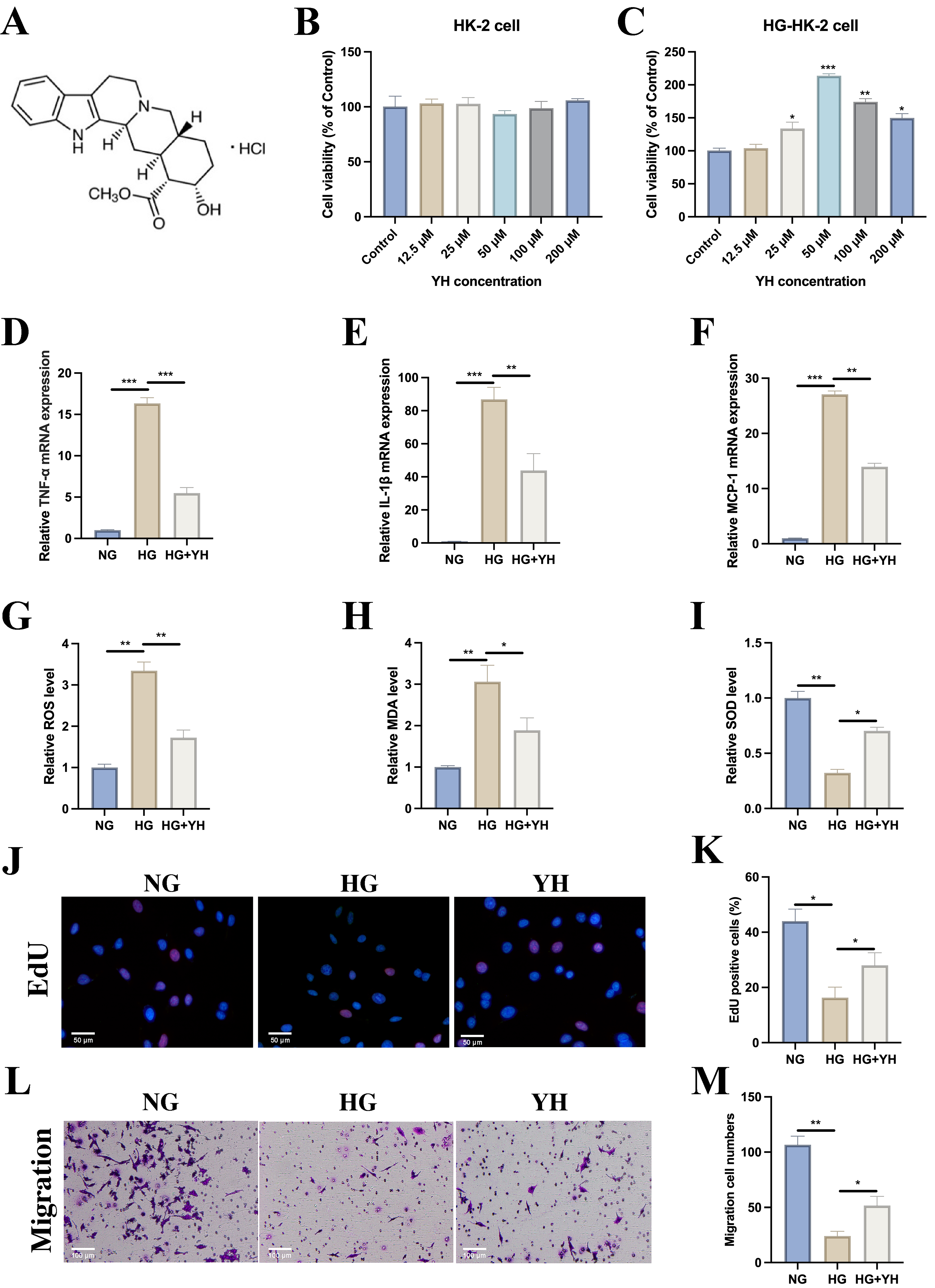
Yohimbine [Figure 1A], an indole alkaloid with established anti-inflammatory and antioxidant properties, was investigated for its protective effects against HG-induced cellular damage. To investigate yohimbine’s (YH) therapeutic potential in DN, we first examined its effect on HK-2 cell viability under HG conditions. Treatment with HG (30 mM) for 48 h significantly reduced HK-2 cell viability to approximately 65% of NG controls and markedly increased inflammatory markers (TNF-α, IL-1β, MCP-1) and oxidative stress indicators [reactive oxygen species (ROS), MDA], while decreasing antioxidant enzyme (SOD) activity, confirming the establishment of a DN-like cellular damage model. These inflammatory mediators (TNF-α, IL-1β, and MCP-1) are key contributors to DN progression, where chronic inflammation drives fibrosis, endothelial dysfunction, and progressive renal damage. The significant reduction of these markers by yohimbine treatment suggests its potential to interrupt the inflammatory cascade central to DN pathogenesis. MTT assays revealed that while YH alone did not significantly affect cell viability at any tested concentration [Figure 1B], it markedly enhanced survival of HG-exposed cells, with optimal effects at 50 μM [Figure 1C]. Further analyses demonstrated that YH treatment substantially reduced inflammatory mediators including TNF-α, IL-1β, and MCP-1 in HG-challenged cells [Figure 1D-F]. Given the established connection between oxidative damage and DN progression, we measured oxidative stress markers and found that YH significantly attenuated HG-induced ROS, MDA, and improved SOD activity [Figure 1G-I]. Additionally, YH counteracted the suppressive effects of HG on cell proliferation and migration as determined by EdU and transwell assays, respectively [Figure 1J-M].
Figure 1. YH inhibits HK-2 cell injury induced by HG. (A) Chemical structure of YH; (B and C) MTT assay results showing cell viability under various treatments; (D-F) Relative expression of inflammatory markers (TNF-α, IL-1β, MCP-1) across different experimental groups; (G-I) Oxidative stress parameters including MDA, ROS, and SOD activities; (J-M) Cellular proliferation assessed by EdU incorporation (Scale bar = 20 μm) and migration capacity (Scale bar = 100 μm) determined by transwell assay. *P < 0.05; **P < 0.01; ***P < 0.001. HK-2: Human kidney-2; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; TNF-α: tumor necrosis factor alpha; IL-1β: interleukin-1 beta; MCP-1: monocyte chemoattractant protein-1; MDA: malondialdehyde; ROS: reactive oxygen species; SOD: superoxide dismutase; EdU: 5-ethynyl-2’-deoxyuridine; NG: normal glucose; HG: high glucose; YH: yohimbine.
Yohimbine mitigates DN in db/db mice
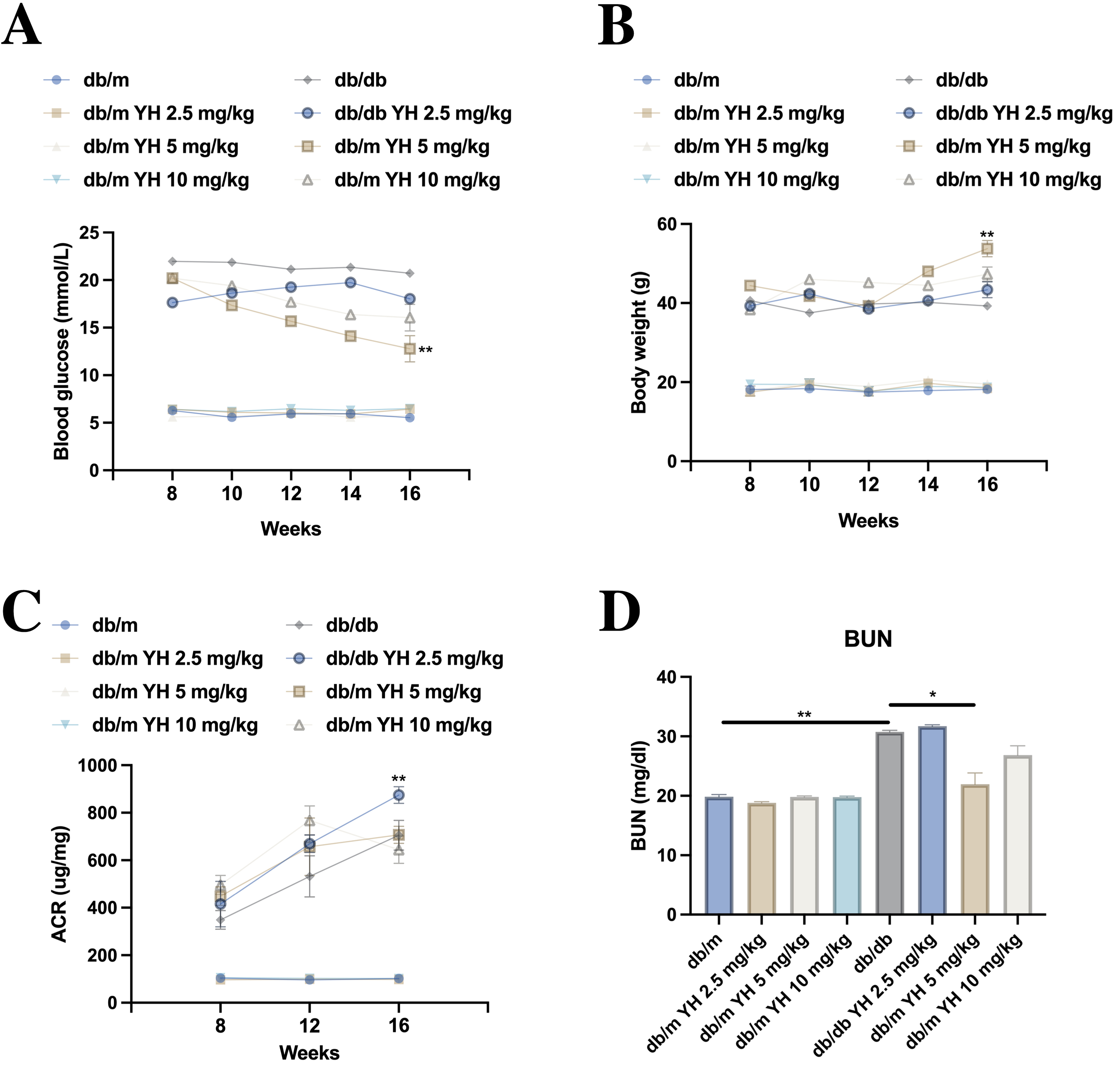
To evaluate the effectiveness of yohimbine (YH) in slowing the progression of DN in a live model, we established eight experimental groups (n = 8 per group) of male mice: four groups of non-diabetic db/m mice (vehicle and three YH doses) and four groups of diabetic db/db mice (vehicle and three YH doses). The animals received daily oral gavage of either vehicle or YH at 2.5, 5, or 10 mg/kg for eight weeks. Throughout the study, diabetic
Figure 2. YH mitigates diabetic nephropathy in db/db mice. (A) Blood glucose measurements in eight experimental groups (n = 8 per group): db/m control, db/m + YH (2.5, 5, and 10 mg/kg), and db/db mice with or without YH treatment (2.5, 5, and 10 mg/kg) administered daily by gavage for eight weeks; (B) Body weight comparisons between control and diabetic mice with or without YH intervention; (C) ACR evaluated from urine samples collected every four weeks; (D) Terminal BUN levels measured from orbital sinus samples at study conclusion. *P < 0.05; **P < 0.01 compared to untreated db/db group. YH: Yohimbine; ACR: albumin-to-creatinine ratio; BUN: blood urea nitrogen.
CircGNB1 is upregulated by Yohimbine in vitro and in vivo
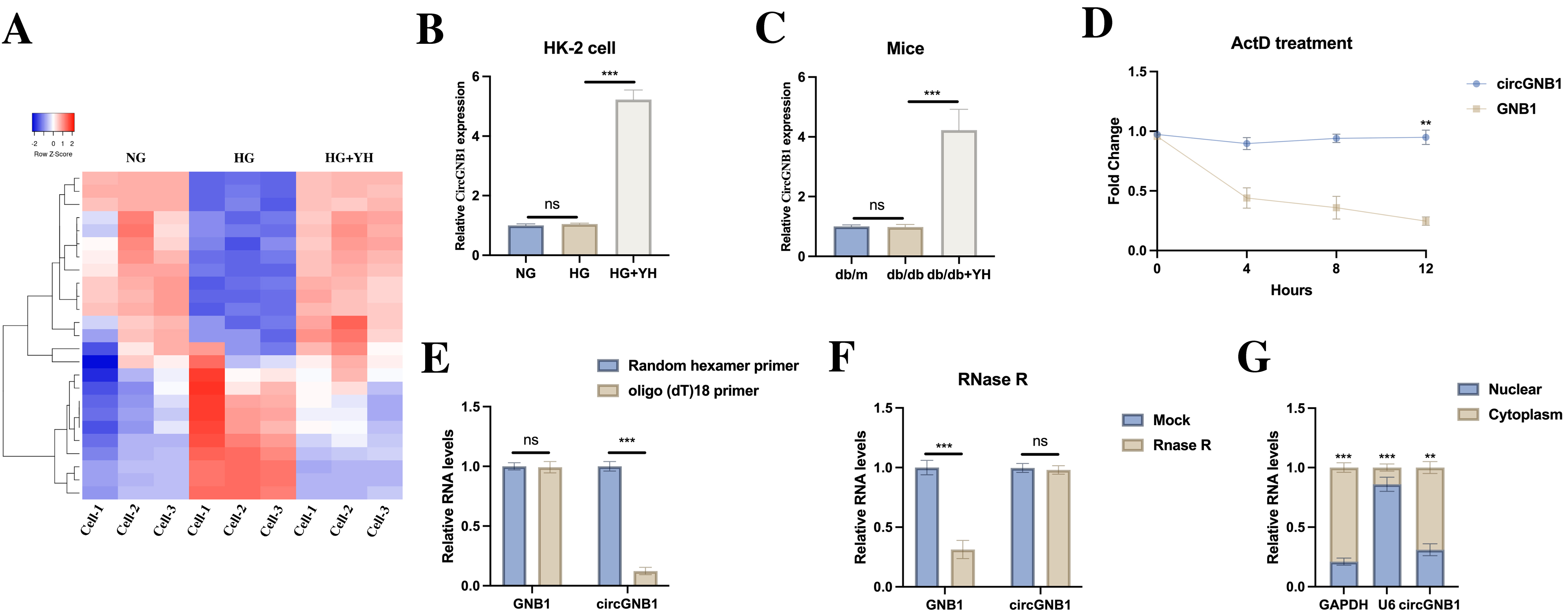
To explore the molecular mechanisms underlying YH’s protective effects, we performed circRNA microarray analysis on HK-2 cell models [Figure 3A]. The analysis identified circGNB1 (hsa_circ_0008702) as the most significantly upregulated circRNA in YH-treated HG-exposed cells compared to HG-only controls. Quantitative PCR confirmed this upregulation both in vitro [Figure 3B] and in renal tissues from YH-treated db/db mice [Figure 3C]. To verify circGNB1’s characteristics, we conducted actinomycin D transcription inhibition assays, which revealed greater stability of circGNB1 compared to linear GNB1 mRNA [Figure 3D]. Further characterization using random hexamer versus oligo(dT)18 primers demonstrated that circGNB1 lacks a poly-A tail [Figure 3E]. RNase R digestion experiments confirmed resistance to exonuclease degradation, a hallmark of CircRNA structure [Figure 3F]. Subcellular fractionation and RNA fluorescence in situ hybridization (RNA-FISH) analyses showed predominant cytoplasmic localization of circGNB1 in HK-2 cells [Figure 3G].
Figure 3. CircGNB1 upregulation by YH in vitro and in vivo. (A) CircRNA microarray analysis of differentially expressed circRNAs; (B) CircGNB1 expression in NG, HG, and YH-treated HG cells; (C) CircGNB1 levels in renal tissues from different treatment groups; (D) RNA stability assessment after actinomycin D treatment; (E) Reverse transcription efficiency using random hexamer versus oligo(dT)18 primers; (F) RNase R resistance test comparing circular and linear RNA forms; (G) Subcellular localization of circGNB1 in HK-2 cells determined by fractionation and FISH analysis (Scale bar = 20 μm). **P < 0.01; ***P < 0.001. HG: High glucose; YH: yohimbine; NG: normal glucose; circRNA: circular RNA; RNA-seq: RNA sequencing; RT: reverse transcription; FISH: fluorescence in situ hybridization; GNB1: guanine nucleotide-binding protein subunit beta-1; circGNB1: circular RNA derived from the GNB1 gene; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; U6: U6 small nuclear RNA.
CircGNB1 is crucial for the role of Yohimbine in DN
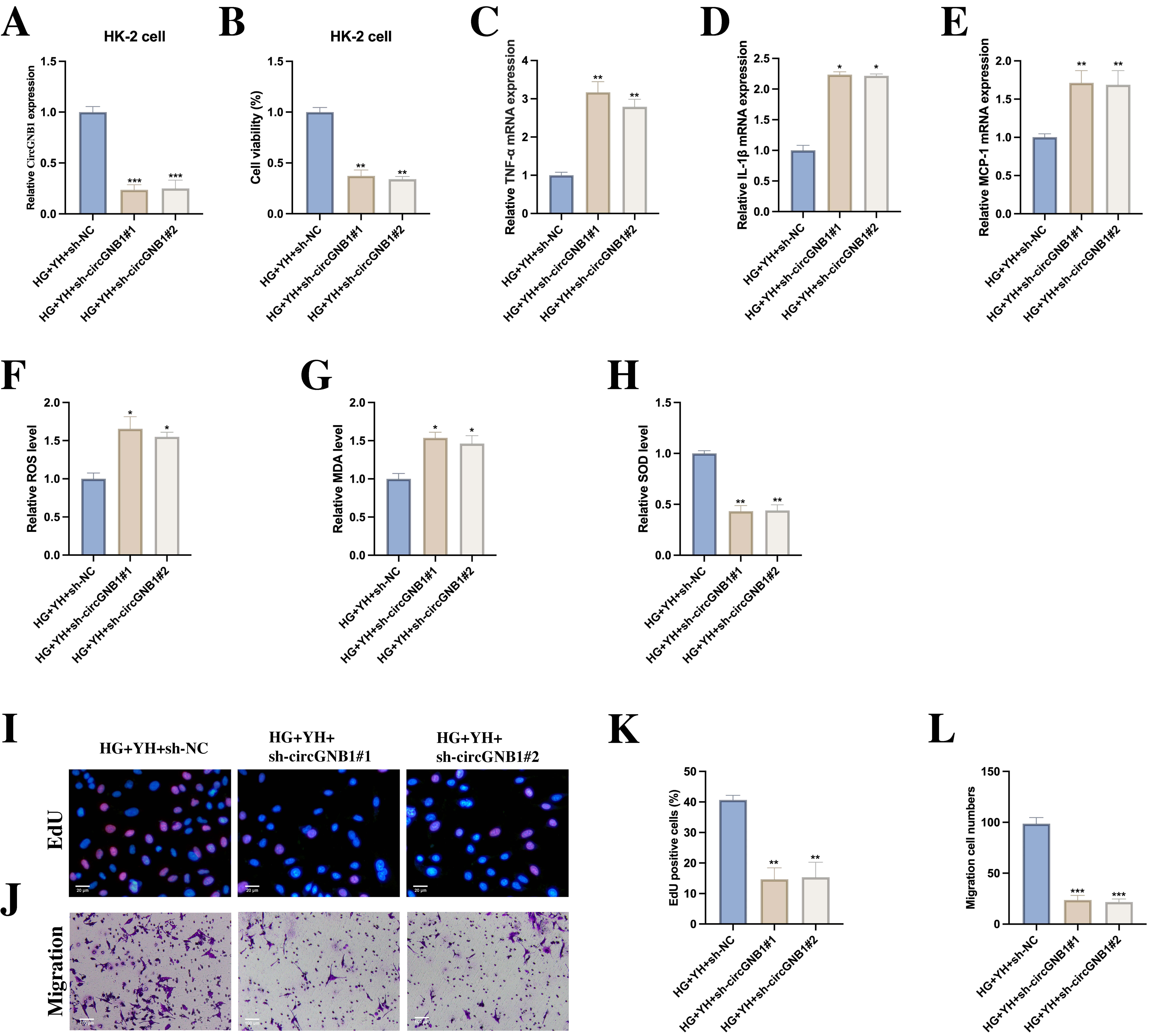
Having established circGNB1’s upregulation by YH, we investigated its functional significance using siRNA-mediated knockdown in cell models. Transfection with circGNB1-specific siRNAs significantly reduced circGNB1 expression [Figure 4A] and abolished YH’s beneficial effects on cell viability in HG-exposed HK-2 cells [Figure 4B]. Notably, circGNB1 silencing increased inflammatory marker expression (TNF-α, IL-1β, MCP-1) in YH-treated, HG-induced cells [Figure 4C-E]. When assessing oxidative stress parameters, we found that circGNB1 knockdown completely reversed YH’s antioxidant effects, as evidenced by increased oxidative stress markers in HG-treated cells [Figure 4F-H]. Similarly, circGNB1 depletion negated YH’s beneficial impact on cell proliferation and migration capacities [Figure 4I-L]. CircRNA microarray analysis identified multiple differentially expressed circRNAs in YH-treated HG-exposed cells. CircGNB1 (hsa_circ_0008702) was selected for further investigation based on the following criteria: (1) highest fold-change upregulation (> 3.5-fold) among all significantly altered circRNAs; (2) statistical significance (P < 0.001); and (3) potential relevance to cellular stress responses based on its parental gene GNB1’s known functions in signal transduction. These comprehensive findings establish circGNB1 as a critical mediator of YH’s protective actions in DN models.
Figure 4. CircGNB1 mediates YH’s protective effects in diabetic nephropathy. (A) Verification of circGNB1 knockdown efficiency; (B) Cell viability analysis following siRNA transfection; (C-E) Inflammatory marker expression after circGNB1 depletion in YH-treated, HG-induced cells; (F-H) Oxidative stress indicators in circGNB1-silenced conditions; (I-L) Cell proliferation (Scale bar = 20 μm) and migration assessments (Scale bar = 100 μm) following circGNB1 knockdown. *P < 0.05; **P < 0.01; ***P < 0.001. YH: Yohimbine; HG: high glucose; circGNB1: circular RNA GNB1; siRNA: small interfering RNA; sh-NC: short hairpin RNA negative control.
CircGNB1 interacts with CDA1
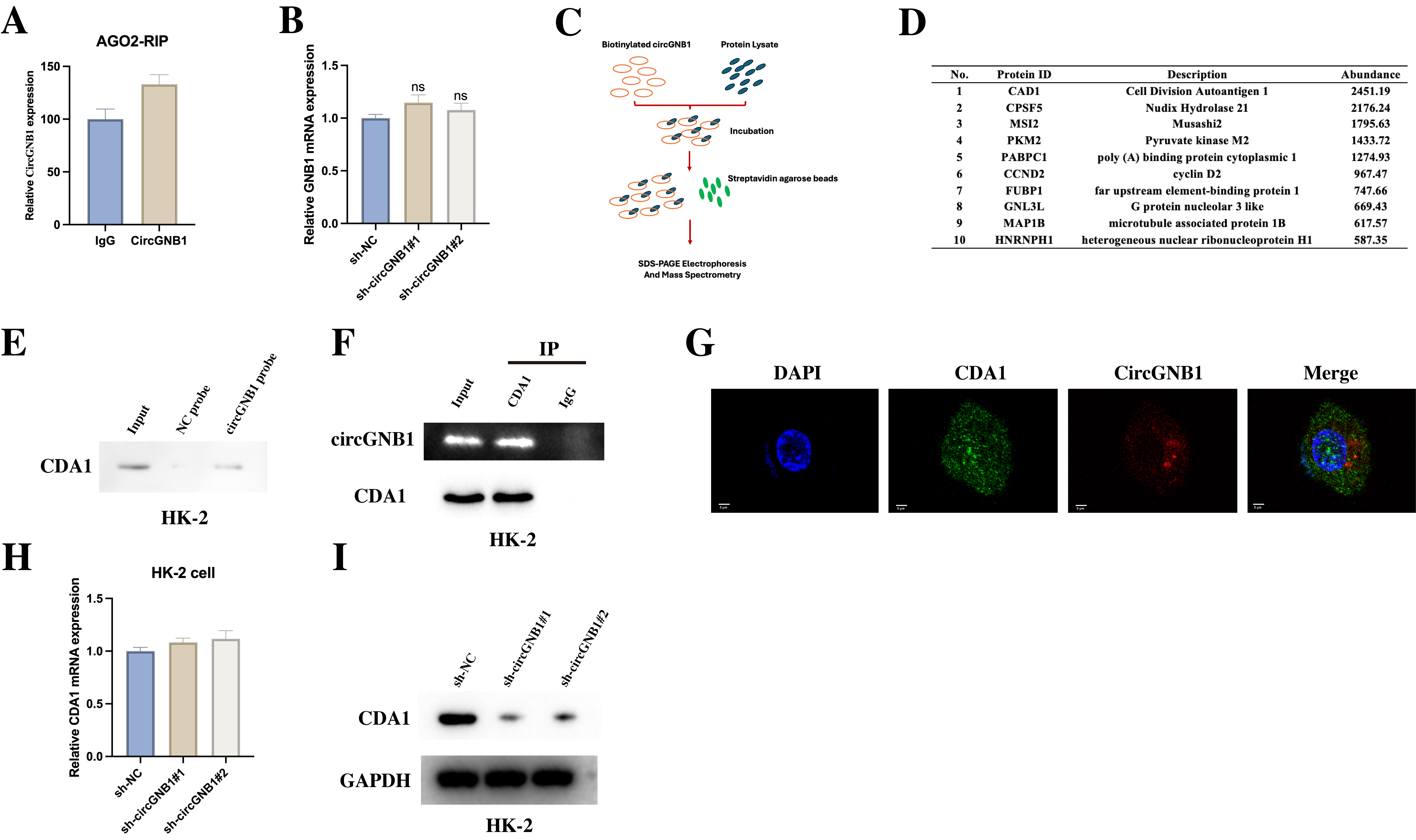
To investigate potential microRNA-mediated mechanisms, we performed Argonaute 2 (AGO2) RIP assays, which showed minimal enrichment of circGNB1 with AGO2 protein [Figure 5A], suggesting that circGNB1 does not primarily function through microRNA sponging mechanisms. Additionally, knockdown of circGNB1 did not affect the expression of its linear counterpart GNB1 mRNA [Figure 5B], indicating that circGNB1’s effects are independent of its parental gene regulation and supporting its distinct functional role as a circRNA. We performed RNA pull-down assays followed by LC-MS/MS analysis, which identified CDA1 as a potential interacting partner [Figure 5C and D]. This interaction was validated using RIP and RNA pull-down assays [Figure 5E-H]. Notably, CDA1 is primarily localized in the cytoplasm, consistent with circGNB1’s distribution pattern. Colocalization studies using FISH and immunofluorescence confirmed that circGNB1 and CDA1 co-localize within the cytoplasmic compartment of HK-2 cells [Figure 5G]. Intriguingly, while circGNB1 knockdown did not affect CDA1 mRNA levels, it significantly reduced CDA1 protein expression [Figure 5H and I], suggesting that circGNB1 modulates CDA1 at the post-transcriptional level, possibly by enhancing protein stability or translation efficiency.
Figure 5. CircGNB1 interaction with CDA1. (A) AGO2 RIP assay results; (B) Expression analysis of linear GNB1 in cells with circGNB1 knockdown; (C) RNA pull-down and LC-MS/MS analysis workflow; (D) Identified proteins enriched with biotinylated probes; (E and F) Validation of interaction between CDA1 and circGNB1 by RNA pull-down and RIP assays; (G) Colocalization visualized by FISH and immunofluorescence (Scale bar = 5 μm); (H and I) qRT-PCR and western blot analyses of CDA1 expression in circGNB1-depleted cells.
CDA1 is responsible for the role of YH in DN
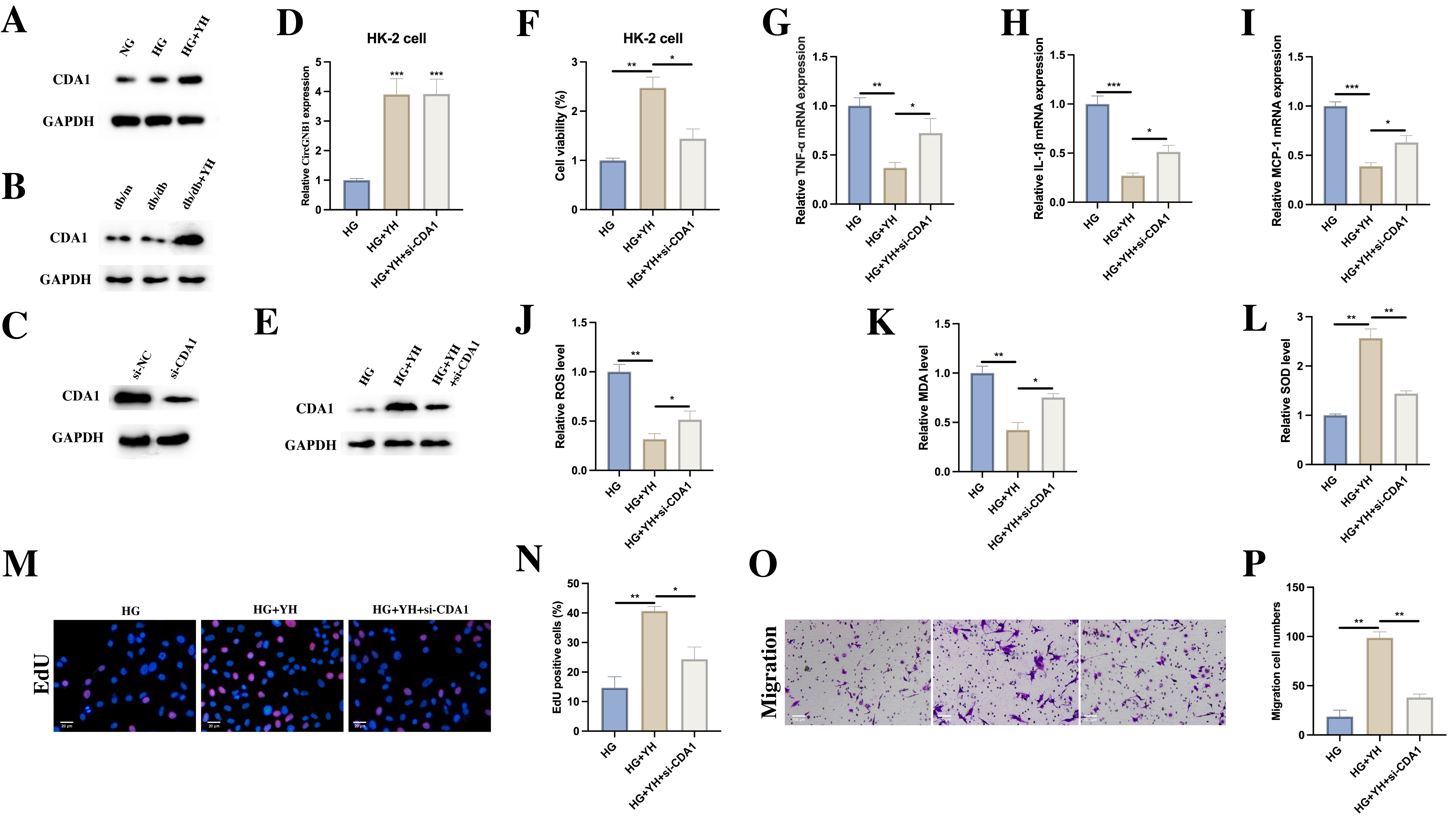
We next examined CDA1’s involvement in YH’s renoprotective actions. Western blot analysis demonstrated that YH treatment upregulated CDA1 expression in both cultured HK-2 cells and renal tissues from db/db mice [Figure 6A and B]. Additionally, quantitative RT-PCR analysis confirmed increased CDA1 mRNA levels in YH-treated conditions [Figure 6C]. The dose-dependent effects of YH on CDA1 expression were further validated across multiple concentrations [Figure 6D], and time-course analysis revealed progressive upregulation of CDA1 following YH treatment [Figure 6E]. To evaluate CDA1’s functional role, we performed siRNA-mediated silencing in HG-exposed, YH-treated cells. CDA1 knockdown significantly diminished the cell viability improvements conferred by YH [Figure 6F] and reversed YH-mediated suppression of inflammatory mediators [Figure 6G-I]. Similarly, CDA1 depletion counteracted YH’s beneficial effects on oxidative stress markers [Figure 6J-L] and cellular functions, including proliferation and migration capacities [Figure 6M-P]. These results establish that CDA1 is indispensable for mediating YH’s protective effects against HG-induced cellular damage and dysfunction, functioning as a downstream effector in the YH-circGNB1 signaling axis.
Figure 6. CDA1’s role in YH’s therapeutic effects on diabetic nephropathy. (A-C) Western blot analysis of CDA1 expression in cells and tissues; (D and E) CircGNB1 and CDA1 expression measured by qRT-PCR and western blot in various experimental conditions; (F) MTT assay results after CDA1 knockdown; (G-I) Inflammatory marker levels following CDA1 silencing in YH-treated HG-induced cells; (J-L) Oxidative stress parameter changes with CDA1 depletion; (M-P) Cell proliferation (Scale bar = 20 μm) and migration capabilities assessment (Scale bar = 100 μm) after CDA1 knockdown. *P < 0.05; **P < 0.01; ***P < 0.001. CDA1: Cell division autoantigen 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; qRT-PCR: quantitative real-time polymerase chain reaction; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; YH: yohimbine; HG: high glucose; si-NC: small interfering negative control; si-CDA1: small interfering CDA1; TNF-α: tumor necrosis factor alpha; IL-1β: interleukin-1 beta; MCP-1: monocyte chemoattractant protein 1; ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase; EdU: 5-ethynyl-2’-deoxyuridine.
DISCUSSION
Our study has identified a novel YH/circGNB1/CDA1 axis that plays a critical role in attenuating oxidative stress in DN. We demonstrated that yohimbine significantly enhances the viability of HG-induced HK-2 cells while reducing inflammatory factors and oxidative stress markers. These protective effects were confirmed in db/db mice, where YH treatment effectively reduced blood glucose, urinary ACR, and BUN levels. Through circRNA microarray analysis, we identified circGNB1 as the most significantly upregulated gene in YH-treated HG-induced cells, establishing it as a key mediator of YH’s protective actions.
The development and progression of DN involve multiple pathways and mediators, with oxidative stress being particularly prominent among these factors[17,18]. Oxidative stress occurs when there is an excessive buildup of ROS due to an imbalance between oxidants and antioxidants, leading to oxidative damage in the body[19]. While moderate ROS-induced damage can often be repaired, excessive ROS production that exceeds the body’s self-regulatory mechanisms can cause irreversible cellular damage or death[20]. Conditions such as hyperglycemia and hypoxia are known to induce oxidative stress and inflammatory responses. Furthermore, the release of ROS not only causes tissue oxidative stress damage but also promotes the aggregation of inflammatory cells and the production of inflammatory cytokines, growth factors, and transcription factors, all of which are involved in the pathological processes of DN[21,22]. Previous studies have reported that YH is an important regulator of oxidative stress in various cellular activities[23-26]. Our results also confirmed that YH is capable of aborting the oxidative stress in HK-2 cells evoked by HG. It is noteworthy that yohimbine’s well-established α2-adrenergic receptor antagonist activity may also contribute to its renoprotective effects, as α2-adrenergic signaling has been implicated in fibrotic processes. However, our identification of the circGNB1/CDA1 axis suggests that yohimbine’s protective mechanisms in DN extend beyond its adrenergic properties and involve novel post-transcriptional regulatory pathways.
Our experiments revealed that YH upregulates CDA1 expression via circGNB1. Studies have reported that circGNB1 is involved in the progression of Triple-Negative Breast Cancer, glioma, and osteoarthritis[27-29]. However, in HK-2 cells, we found that circGNB1 is not capable of binding microRNAs but has protein binding potential[27]. Following experiments confirmed that CDA1 is responsible for the role of YH in DN progression. The identification of CDA1 as a downstream effector of circGNB1 is particularly intriguing given CDA1’s established roles in fibrotic pathways. Given that TGF-β signaling and extracellular matrix regulation are central to DN progression, CDA1’s upregulation by the YH/circGNB1 axis may exert renoprotective effects beyond oxidative stress attenuation. Future studies should examine CDA1’s potential connections to TGF-β signaling pathways to fully elucidate its therapeutic relevance in DN.
Our study has partially unveiled the function of YH in DN progression, while there are still more works to do to fully understand the way YH exerts its function. Firstly, although the clinical significance of YH in DN is important to validate, there is still a long way to go. Secondly, we should also generate different DN models, both in vitro and in vivo, to comprehensively elucidate YH function. In addition, the downstream pathway of CDA1 in DN is also another research direction for the following study. Thirdly, the downstream signaling pathways remain to be fully elucidated. Given CDA1’s role in oxidative stress attenuation, potential involvement of antioxidant pathways such as Nrf2/Keap1 signaling or anti-inflammatory pathways including NF-κB modulation warrants future investigation.
Limitations
Our study has several limitations. Most importantly, we did not perform histological evaluation of renal tissue. While we demonstrated improvements in functional parameters (ACR, BUN), histopathological analysis including glomerular morphology, tubular damage, and interstitial fibrosis would provide more direct evidence of yohimbine’s renoprotective effects. Additionally, inclusion of more specific renal injury markers such as KIM-1, NGAL, eGFR, and cystatin C would strengthen our findings beyond the current biochemical parameters. Other limitations include the use of HK-2 cells and db/db mice which may not fully replicate human DN, incomplete exploration of CDA1’s downstream signaling mechanisms, and the need to characterize the structural basis of circGNB1-CDA1 interaction. Future studies addressing these limitations, particularly histological assessment and comprehensive biomarker evaluation, would enhance the translational potential of our findings.
In summary
Our study is the first to demonstrate yohimbine’s protective effects in DN, establishing a foundation for future clinical investigations of this compound in renal complications of diabetes. Our study unveiled the function of YH in DN progression and partially explored the underlying mechanisms; a novel YH/circGNB1/CDA1 axis has been identified. Our results provide a partial explanation for the role of YH in DN progression and may offer novel insights for the development of therapeutic targets for DN.
DECLARATIONS
Acknowledgments
We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance for this study.
Authors’ contributions
Conceptualization, methodology, cell culture experiments, molecular biology assays, data analysis, writing-original draft: Ge X
Animal experiments, biochemical analyses, data collection, formal analysis, writing-review and editing: Du J
Methodology, RNA pull-down and mass spectrometry, protein-RNA interaction studies, data analysis, writing-review and editing: Li W
Animal experiments, biochemical analyses, oxidative stress measurements: Peng Z
Cell culture experiments, functional assays, ELISA analyses: Gao J
Statistical analysis, data visualization, writing-review and editing: Peng W
Conceptualization, supervision, project administration, funding acquisition, writing-review and editing: Huang S
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
Not applicable.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
All animal experiments strictly followed the National Institutes of Health guidelines for the care and use of laboratory animals and received ethical approval from the Ethics Committee of Shanghai Tongren Hospital (ethics approval number: 2021-044).
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Kato M, Natarajan R. Diabetic nephropathy--emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10:517-30.
2. AKh. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361-81.
3. Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13:311-8.
4. Sharma D, Bhattacharya P, Kalia K, Tiwari V. Diabetic nephropathy: new insights into established therapeutic paradigms and novel molecular targets. Diabetes Res Clin Pract. 2017;128:91-108.
5. Schwinghammer UA, Melkonyan MM, Hunanyan L, et al. α2-adrenergic receptor in liver fibrosis: implications for the adrenoblocker mesedin. Cells. 2020;9:456.
6. Wibowo DNSA, Soebadi DM, Soebadi MA. Yohimbine as a treatment for erectile dysfunction: a systematic review and meta-analysis. Turk J Urol. 2021;47:482-8.
7. Lin Y, Zhu X, Yao WZ, Yang YL, A LT, Chen L. Yohimbine protects against endotoxin-induced acute lung injury by blockade of alpha 2A adrenergic receptor in rats. Chin Med J. 2011;124:1069-74.
8. Jabir NR, Firoz CK, Zughaibi TA, et al. A literature perspective on the pharmacological applications of yohimbine. Ann Med. 2022;54:2861-75.
9. Miksa M, Das P, Zhou M, et al. Pivotal role of the alpha2A-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLoS One. 2009;4:e5504.
10. Huang R, Zhang Y, Han B, et al. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy. 2017;13:1722-41.
11. Qu S, Yang X, Li X, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141-8.
12. Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31-44.
13. Peng F, Gong W, Li S, et al. circRNA_010383 acts as a sponge for miR-135a, and its downregulated expression contributes to renal fibrosis in diabetic nephropathy. Diabetes. 2021;70:603-15.
14. Hu W, Han Q, Zhao L, Wang L. Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-β1. J Cell Physiol. 2019;234:1469-76.
15. Zhang SJ, Chen X, Li CP, et al. Identification and characterization of circular RNAs as a new class of putative biomarkers in diabetes retinopathy. Invest Ophthalmol Vis Sci. 2017;58:6500-9.
16. Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159:433-6.
17. Bahreini E, Rezaei-Chianeh Y, Nabi-Afjadi M. Molecular mechanisms involved in intrarenal renin-angiotensin and alternative pathways in diabetic nephropathy - a review. Rev Diabet Stud. 2021;17:1-10.
18. Dawood AF, Maarouf A, Alzamil NM, et al. Metformin is associated with the inhibition of renal artery AT1R/ET-1/iNOS axis in a rat model of diabetic nephropathy with suppression of inflammation and oxidative stress and kidney injury. Biomedicines. 2022;10:1644.
19. Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763.
20. Rotariu D, Babes EE, Tit DM, et al. Oxidative stress - complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed Pharmacother. 2022;152:113238.
21. Mehta MM, Weinberg SE, Chandel NS. Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol. 2017;17:608-20.
22. Wang Y, Cui J, Liu M, Shao Y, Dong X. Schisandrin C attenuates renal damage in diabetic nephropathy by regulating macrophage polarization. Am J Transl Res. 2021;13:210-22.
23. Sharma N, Sistla R, Andugulapati SB. Yohimbine ameliorates liver inflammation and fibrosis by regulating oxidative stress and Wnt/β-catenin pathway. Phytomedicine. 2024;123:155182.
24. Iciek M, Górny M, Kotańska M, Bilska-Wilkosz A, Kaczor-Kamińska M, Zagajewski J. Yohimbine alleviates oxidative stress and suppresses aerobic cysteine metabolism elevated in the rat liver of high-fat diet-fed rats. Molecules. 2023;28:2025.
25. Ansari MM, Khan HA. Yohimbine hydrochloride ameliorates collagen type-II-induced arthritis targeting oxidative stress and inflammatory cytokines in Wistar rats. Environ Toxicol. 2017;32:619-29.
26. Veeram A, Shaikh TB, Kaur R, Chowdary EA, Andugulapati SB, Sistla R. Yohimbine treatment alleviates cardiac inflammation/injury and improves cardiac hemodynamics by modulating pro-inflammatory and oxidative stress indicators. Inflammation. 2024;47:1423-43.
27. Liang Y, Shen L, Ni W, et al. CircGNB1 drives osteoarthritis pathogenesis by inducing oxidative stress in chondrocytes. Clin Transl Med. 2023;13:e1358.
28. Hu J, Zhang G, Wang Y, et al. CircGNB1 facilitates the malignant phenotype of GSCs by regulating miR-515-5p/miR-582-3p-XPR1 axis. Cancer Cell Int. 2023;23:132.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].