Subtype-specific roles of nigrostriatal dopaminergic neurons in motor and associative learning
Abstract
Aim: Define the subtype-specific contributions of nigrostriatal dopaminergic neurons (DANs) to motor and non-motor behaviors by comparing Calbindin 1-positive (Calb1+) and Aldehyde dehydrogenase 1a1-positive (Aldh1a1+) DANs.
Methods: Intersectional genetic strategy and chemogenetic inhibition were applied to selectively silence Calb1+ or Aldh1a1+ DANs in mice. An adeno-associated viral vector (AAV-CreOn-FlpOn-hM4Di-P2A-mCherry) was stereotactically delivered into the substantia nigra pars compacta of double knock-in lines ThFlp; Calb1IRESCre or ThFlp; Aldh1a1CreERT2. Following expression, subtype-specific neuronal inhibition was induced with a designer receptor exclusively activated by designer drugs (DREADD) ligand, and the mice were assessed in assays of voluntary movement, motor skill learning, and early associative learning behavior.
Results: Chemogenetic inhibition of either Calb1+ or Aldh1a1+ DANs produced a marked reduction in voluntary movement and impaired acquisition of motor skills, indicating that both subtypes are necessary for normal motor function and learning. In contrast, only inhibition of Calb1+ DANs altered early associative-learning performance, revealing a dissociable, subtype-specific role for Calb1+ neurons in reinforcement-related behavior that was not observed with Aldh1a1+ neuron inhibition.
Conclusion: Both Calb1+ and Aldh1a1+ nigrostriatal DANs are key regulators of movement and motor learning, with Calb1+ neurons additionally modulating reward-based associative learning. These findings highlight the functional heterogeneity of nigrostriatal DAN subtypes and identify potential therapeutic targets for addressing motor and non-motor deficits in Parkinson’s disease.
Keywords
INTRODUCTION
Parkinson’s disease (PD) is pathologically characterized by the widespread loss of midbrain dopaminergic neurons (DANs), especially those in the substantia nigra pars compacta (SNc)[1-4]. We and others have demonstrated that Aldehyde dehydrogenase 1A1-positive (ALDH1A1+) DANs in the ventral tier of SNc are particularly vulnerable in individuals with PD, as well as in PD-related rodent and primate models[5-9]. Recent single-cell and single-nuclei RNA sequencing studies have identified multiple molecularly distinct midbrain DAN subtypes[10-12]. While midbrain DANs regulate various behavioral processes, including both motor and non-motor functions[10,13-15], the roles of specific DAN subtypes in regulating behaviors remain to be fully explored.
ALDH1A1+ DANs comprise two-thirds of the DANs in the human and rodent SNc[5,16] and one-third in the rodent ventral tegmental area (VTA)[16]. In the SNc, these neurons contribute to locomotion control and are crucial for motor skill learning in rodents[16]. While ALDH1A1- DANs, located in the dorsal tier of the SNc, are also affected by PD, they are less vulnerable than ALDH1A1+ DANs[5]. However, the role of ALDH1A1- SNc DANs in motor control and learning remains unclear[11,16,17].
Since Calbindin 1 (CALB1) is predominantly expressed by ALDH1A1- DANs[9,17], we employed an intersectional genetic approach using ThFlp[18]; Calb1IRESTCre[19] double knock-in (KI) mice, combined with a Cre and Flp-dependent designer receptors exclusively activated by designer drugs (DREADD) system[20], to selectively inhibit Calb1+ DAN subtypes during behavioral tests. In parallel, we generated ThFlp;
METHODS
Mouse work
All mouse studies were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committees (IACUC) of the Intramural Research Program of the National Institute on Aging (NIA), NIH. ThFlp KI mice (strain: C57BL/6N-Thtm1Awar/Mmmh; Stock# 050618-MU; MMRRC item: 050618-MU-HET) were obtained from the Mutant Mouse Regional Resource Center (MMRRC). Calb1IRESCre mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA; JAX# 028532). Aldh1a1CreERT2 mice were generated in our lab previously[16] and are available from the MMRRC (Stock# 065802-UCD). Th+/Flp heterozygous KI mice were crossed with Calb1+/IESCre heterozygous or Aldh1a1+/CreERT2 heterozygous KI mice to generate Th+/Flp; Calb1+/IRESCre and Th+/Flp; Aldh1a1+/CreERT2 double heterozygous KI lines, respectively. Mice used for viral injections were between 2 and 4 months of age. They were housed in groups of 2-5 animals under a 12-hour light/12-hour dark cycle with ad libitum access to water and a standard diet. Both male and female mice were included in the experiments, and all behavioral tasks were conducted during the light cycle. Littermates were randomly assigned to different experimental groups before the start of the study.
Adeno-associated viral vectors
The adeno-associated viral (AAV)-EF1a-Con/Fon-mCherry vector[21] was obtained from Addgene (#137132, Watertown, MA, USA). The AAV-EF1a-Con/Fon-GiDREADD-P2A-mCherry vector was constructed based on the pAAV-nEF-Con/Fon-ChR2-mCherry plasmid[21]. The DNA cassette was designed by splitting the reading frame of hM4Di-P2A-mCherry into three fragments, which were synthesized de novo and incorporated into the pAAV-EF-Con/Fon-GiDREADD-P2A-mCherry vector.
Animal surgery and stereotaxic injections
We selected adult male and female mice, aged 20-24 weeks and weighing > 20 g, for stereotactic injection as described previously[22]. In brief, mice were deeply anesthetized with isoflurane, and the head fur was shaved and cleaned with iodine solution. The anesthetized mice were then placed on a stereotactic frame (Stoelting) equipped with stabilizing ear bars and a mouthpiece delivering a steady flow of 1.5% isoflurane and 2% oxygen. The levels of oxygen and isoflurane were continuously monitored throughout the procedure. A midline scalp incision was made to expose the skull, and Bregma and Lambda coordinates were used for precise brain positioning. A small hole was drilled over the target region using a 0.8 mm stereotaxic drill. Injection coordinates for the midbrain were determined based on the standard mouse brain atlas. Following the injection, the needle remained in place for 5 min to allow viral diffusion and prevent backflow along the needle tract upon withdrawal. The scalp was then closed using non-absorbable sutures and Vetbond tissue adhesive (3M), and an antibiotic ointment was applied. 350 nL of AAV vectors [original titer:
Brain sectioning and immunohistochemistry
Mice were anesthetized with pentobarbital, and transcardial perfusion was performed with a controlled pressure device with 100-150 mL ice-cold 1× PBS, followed by 100 mL 4% paraformaldehyde (PFA) in
For immunohistochemistry, 35-40-µm-thick free-floating coronal sections were briefly washed once for
Confocal microscopy and image analysis
Fluorescent staining images were acquired using a Zeiss LSM 780 and LSM 980 confocal microscopes (Zeiss). Whole coronal sections were initially captured using a 10× objective lens. Higher-magnification images of regions of interest were then obtained using a 20× objective lens with z-stack intervals. The 10× images were 512 × 512 pixels, while all 20× images were at least 1,024 × 1,024 pixels. Counts were taken from comparable sections across the rostro-caudal axis of the midbrain. Imaging was performed using three to four fluorescence channels with the following filters: Alexa Fluor 405, 488, 555, and 647. Each channel was imaged separately to eliminate potential signal crosstalk between channels.
Cell counting
Two coronal brain sections containing the SNc were selected from each mouse (n = 5 Aldh1a1CreERT2; THFlp and 3 Calb1Cre; THFlp). RFP+, ALDH1A1+, and TH+ cells were quantified per section using NeuroInfo analysis software (MBF Bioscience). Cell detection was performed using the “Detect Cells” pipeline, and regions of interest (ROIs) were manually delineated around the SNc using TH immunostaining and the Allen Brain Atlas as anatomical references. ROIs were drawn by placing connected contour points with the mouse and closed by selecting “Close Contour”. The previously drawn ROI was then selected to proceed to the preview and detection step. Channel-specific detection parameters were defined as follows: large diameter =
DREADD ligand JHU37160 drug preparation
JHU37160-dihydrochloride (Cat# HB6261, Hello Bio, Inc., Princeton, NJ, USA), a high-affinity and highly potent water-soluble DREADD agonist[23], is stored as a powder at -20 °C. A new 5 mg vial was dissolved in 16.666 mL of sterile 0.9% sodium chloride solution to achieve a final concentration of 0.3 mg/mL. The solution was aliquoted into 5 mL Eppendorf tubes, labeled, and stored at -20 °C for up to one month. On the day of injection, one tube was thawed and diluted 10-fold with sterile saline before use. Mice received intraperitoneal injections of JHU37160 (0.3 mg/kg bodyweight) 30 min before each motor or feeding test.
Behavioral assessment
Habituation of animals
For all behavioral studies, mice were housed in groups, with males and females housed in separate cages. Mouse cages were transported on a cart covered with lab coat from the animal home room to the rodent behavior core. Mice were then habituated in a small holding room for 20-30 min before starting the behavior. The paradigm was cleaned with 70% alcohol and dried before placing the animal in the designated space.
Motor coordination and activity tests
Open-field test for spontaneous locomotion
To measure the motor activity, we used the Photobeam Activity System-Open field (PAS-OF, San Diego Instruments) test. The chambers were cleaned with 70% alcohol, turned on red lights, and mice were placed in their designated chambers according to their IDs. The mice explored the chambers, and their movements were recorded by two sets of 4 × 8 photobeams for 30 min. Infrared photo beams at two different heights tracked mouse movements: the lower beams recorded x- and y-axis positions, termed fine and ambulation movements, while the upper beams counted rearing or vertical movements. The PAS-OF system accurately recorded all beam interruptions caused by mouse movements including ambulation, fine, and rearing behavior. It utilizes a database to store all results in a single Microsoft Access database file in table format, which could be easily exported as CSV files. We then run the analysis program R scripts to extract the different parameters of locomotion. The system recorded any activity as ambulatory movement when three or more different light beams were interrupted consecutively, whereas it was recorded as fine movement when only adjacent light beams were disrupted alternately.
Rotarod test for motor skill learning
One week after the open field activity test, we assessed the motor skill learning of mice using accelerating rotarod tests, following a procedure similar to our previous study[16]. We cleaned the 5-lane rotarod instrument (Panlab, Harvard Apparatus, Holliston, MA) with 70% alcohol and kept the rod dry. We then gently placed the mice on the rotating rod and gradually increased the speed linearly from 4 to 40 revolutions per minute over a 5-minute trial. The duration the mice stayed on the rod was recorded as latency to fall. Ten trials were conducted per day for six consecutive days, with a 2-3-minute interval between each trial.
Operant conditioning using Feeding Experimentation Device 3
Feeding Experimentation Device 3
The Feeding Experimentation Device 3 (FED3) is a compact battery-powered operant device designed for feeding and training rodents within their home cage environment[24], purchased fully assembled from Open Ephys (Open Ephys Production Site, Lisbon, Portugal). It features a dual nose-poke system with left and right sensors, a beeper, a multi-color LED strip, a pellet dispenser that dispenses pellets, an output jack for equipment connection, a pre-loaded ClassicFED3 program, and a user communication screen. All behavioral activity interacting with the device is logged onto an onboard microSD card for future analysis.
Food restriction
Rodents were food-restricted overnight one to two days before starting the feeding device experiment. During the restriction period, body weight, eating time, and reward intake were recorded daily and maintained in a log accessible to the facility veterinarian in the animal holding room. If an animal’s body weight dropped below 85% of its initial weight, it was given unrestricted access to food and monitored until full recovery. Mice were individually housed in a new cage containing a FED3 device for a 2-hour training session period. The pellets dispensed from the FED3 device were the only food source available during the training session. After training, they were returned to their original group home cage and given unlimited access to regular chow for three hours, starting 30 min after the session ended.
Free feeding
A FED3 device, half-filled with grain-based dustless pellet reward (Bio-Serv, 20 mg, Product # F0163, Flemington, NJ, USA), was placed in a rodent’s cage. The device was powered on and set to “free feeding” mode, allowing mice to retrieve pellets in the food magazine without requiring a nose poke. Half an hour following either JHU37160 or saline injections, mice were placed in the new cage and given access to the FED3 device for a 2-hour session. In this free-feeding paradigm, pellet availability was independent of nose-poking behavior. The pellet sensor detected when a pellet was removed from the magazine and automatically dispensed another pellet. After one or two sessions, mice became habituated to the FED3 pellet-dispensing system and were transitioned to fixed-ratio training.
Fixed ratio 1 schedules of reinforcement
Mice transitioned from a free-feeding mode to a fixed-ratio 1 (FR1) reinforcement training schedule, where they received pellets through active nose-poking in a similar experimental setting with a FED3 device and single housing. Each experiment started half an hour following either JHU37160 or saline injections. The device was powered on and set to “FR1” mode. During FR1 sessions, a nose poke in the “active” port triggered the immediate delivery of a single 20mg pellet reward in the magazine, while a nose poke in the “inactive” port did not result in pellet delivery. In all experiments, the left nose poke was designated as the active port, while the right nose poke was designated as the inactive port. Correct nose-poking behavior was reinforced with immediate audio and visual cues. There was no punishment after poking an inactive port. To assess the reinforcement learning behavior, mice were trained on consecutive days with 2-hour FR1 sessions. Performance data from the first to the third sessions were used for the analysis. Accuracy was calculated as the count of pokes on the active port divided by the total poke count in a session. Mice were trained with the FR1 task for 3-7 sessions.
Statistics
Data were analyzed using Prism 10 (GraphPad) and custom R code. Statistical details for each experiment are provided in the figure legends. Results are presented as means ± SEM. Statistical significance was assessed using unpaired parametric t-tests and two-way ANOVA with multiple comparisons. Significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
RESULTS
Selectively targeting of mouse Calb1+ SNc DANs using intersectional genetics and chemogenetic approaches
A recent study reported that the activity of Calb1+ SNc DANs is negatively correlated with movement acceleration[17]. However, the direct impact of Calb1+ DANs on locomotion remains unclear. To investigate the function of this DAN subtype, we generated ThFlp; Calb1IRESTCre (TC) double KI mice, enabling selective heterologous gene expression in Calb1+ DANs.
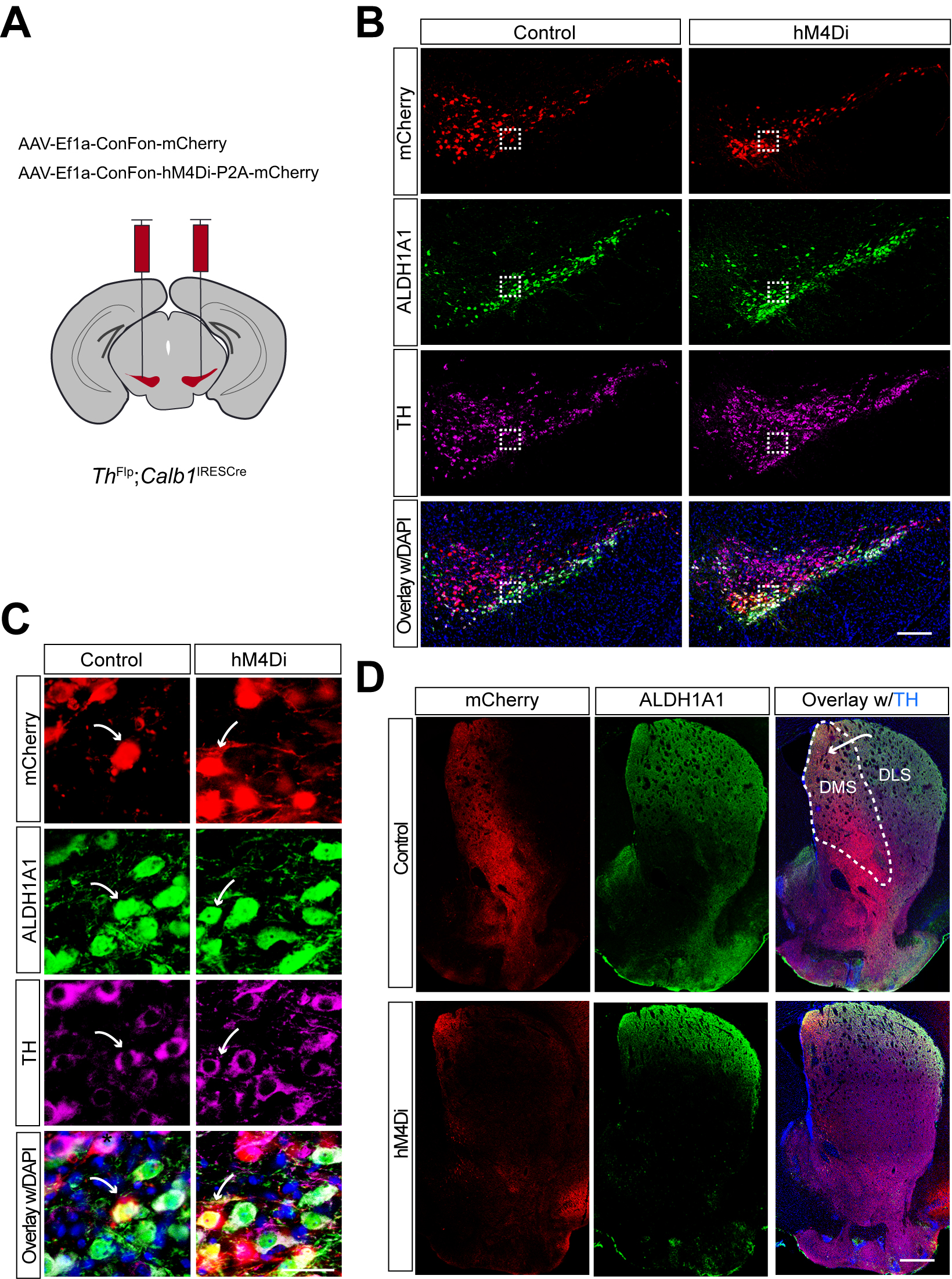
To specifically inhibit Calb1+ SNc DAN activity, we performed bilateral stereotactic injections of AAV vectors expressing either a Cre- and Flp-dependent inhibitory DREADD, hM4Di (AAV9-CreOn/FlpOn-hM4Di-P2A-mCherry), or a control red fluorescent protein mCherry (AAV9-CreOn/FlpOn-mCherry), into the SNc of TC double KI mice [Figure 1A]. Immunostaining confirmed selective expression of hM4Di and mCherry in Calb1+ SNc DANs [Figure 1B], which are primarily located in the dorsal tier of SNc and account for approximately 30% of SNc DANs[16]. Notably, when counterstaining sections with ALDH1A1 antibodies, we observed that while most mCherry-positive DANs were ALDH1A1-negative, a small fraction (5.8%) of mCherry-positive neurons in the dorsomedial SNc were also ALDH1A1-positive (arrows, Figure 1B and C). Further examination of the projection pattern of Calb1+ SNc DANs in the dorsal striatum revealed that their axon terminals are primarily distributed in the dorsomedial striatum (DMS) [Figure 1D].
Figure 1. Targeted expression of the chemogenetic inhibitor hM4Di in Calb1+ nigrostriatal DANs. (A) Schematic depicting the stereotactic delivery of control mCherry or hM4Di AAVs in the SNc of ThFlp; Calb1IRESCre (TC) double KI mice; (B) Representative images showing mCherry (red), ALDH1A1 (green), and TH (magenta) expression in the SNc of control mCherry (left panel) and hM4Di (right panel)-expressing mice. Scale bar: 1 mm; (C) Magnified images of the boxed areas in (B), with arrows indicating neurons co-expressing mCherry and ALDH1A1. Scale bar: 100 mm; (D) Representative images illustrating mCherry (red), ALDH1A1 (green), and TH (magenta) staining in the striatum of control mCherry-expressing mice. Scale bar: 1 mm. Calb1+: Calbindin 1-positive; DANs: dopaminergic neurons; AAVs: adeno-associated viruses; SNc: substantia nigra pars compacta; KI: knock-in; TH: Tyrosine Hydroxylase.
Together, the use of TC double KI mice combined with local injection provides a precise approach to selectively targeting Calb1+ SNc DANs for genetic and functional manipulations.
Chemogenetic inhibition of Calb1+ SNc DANs suppresses spontaneous movements
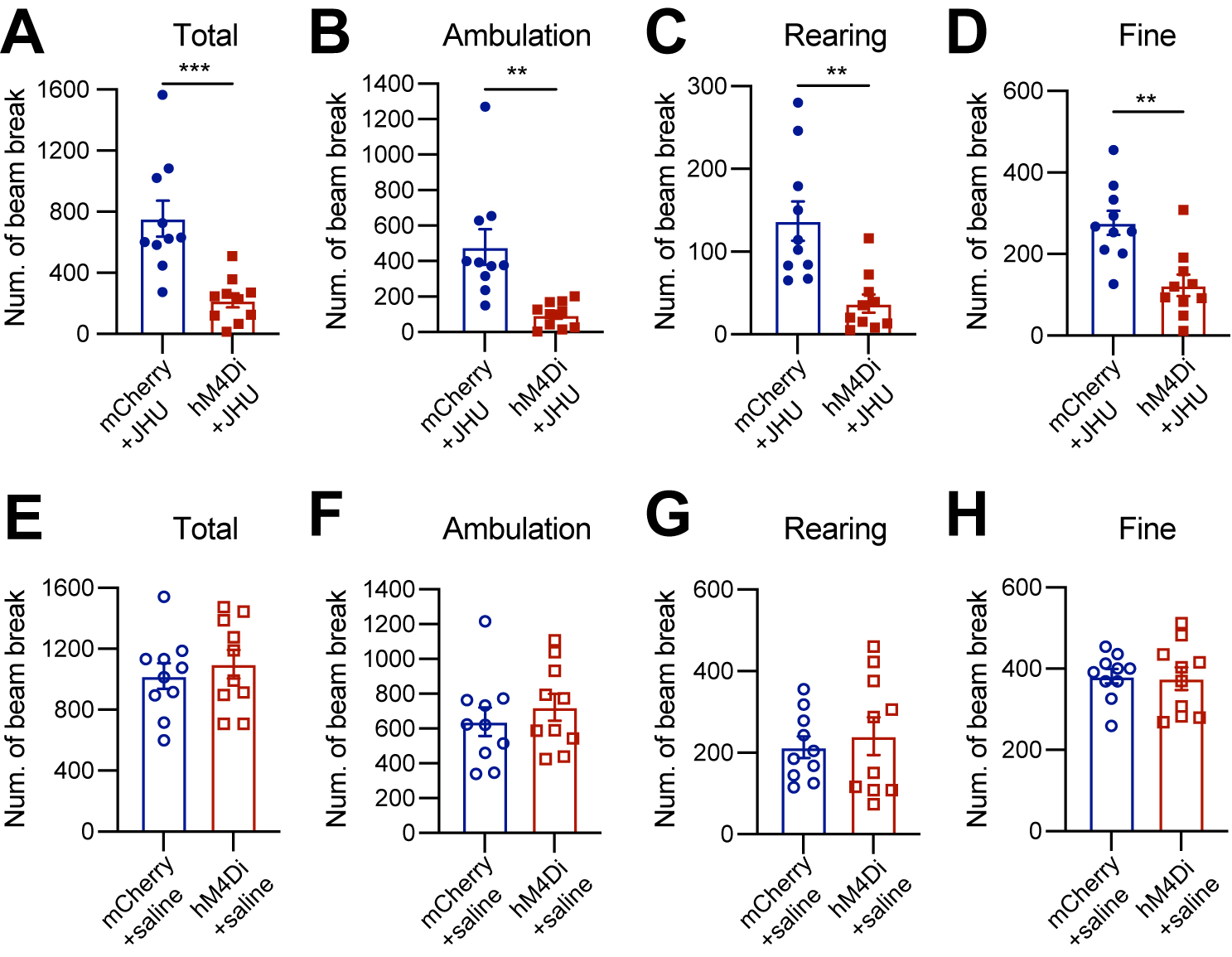
Six to eight weeks after stereotactic injection of control and DREADD adeno-associated viruses (AAVs), we used the beam break-based open-field test to assess spontaneous movements following intraperitoneal injection of the DREADD activator JHU37160[23]. We observed a marked reduction in total movement, ambulatory movement, rearing, and fine movement [Figure 2A-D]. By contrast, intraperitoneal saline injections had no effect on locomotion or rearing [Figure 2E-H]. These findings underscore the critical role of Calb1+ SNc DANs in regulating spontaneous movements.
Figure 2. Chemogenetic inhibition of Calb1+ nigrostriatal DANs impairs spontaneous locomotion. (A-D) Quantification of total (A)
Chemogenetic inhibition of Calb1+ SNc DANs severely impairs motor skill learning
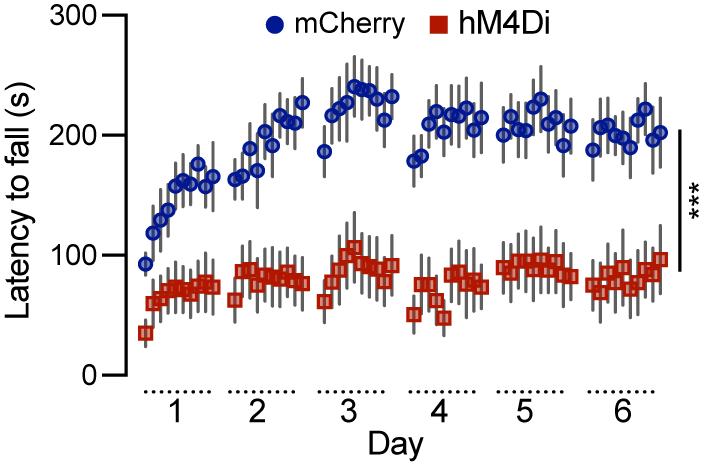
To investigate the role of Calb1+ SNc DANs in motor skill learning, we conducted rotarod motor skill learning tests[16,25] on TC mice that received bilateral injections of control and DREADD AAVs into the SNc. Chemogenetic inhibition with JHU37160 severely impaired motor skill acquisition in hM4Di-expressing mice compared to mCherry-expressing control mice [Figure 3]. These data demonstrate that Calb1+ SNc DANs play a crucial role in the acquisition of skilled movements.
Figure 3. Chemogenetic inhibition of Calb1+ nigrostriatal DANs severely impairs rotarod motor skill learning. Rotarod motor skill learning in TC double KI mice injected with either control mCherry [n = 10, 3 Male (M)/7 Female (F)] or hM4Di AAVs (n = 10, 4 M/6 F) in the SNc. Mice underwent one training session per day for six consecutive days, with JHU37160 administered prior to each session. Data are presented as mean ± SEM. Two-way ANOVA, mCherry vs. hM4Di effect: F (1, 18) = 22.2, ***P = 0.0002. Calb1+: Calbindin 1-positive; DANs: dopaminergic neurons; TC: ThFlp; Calb1IRESTCre; KI: knock-in; AAVs: adeno-associated viruses; SNc: substantia nigra pars compacta; SEM: standard error of the mean; ANOVA: analysis of variance.
Chemogenetic inhibition of Calb1+ SNc DANs affects the reward learning
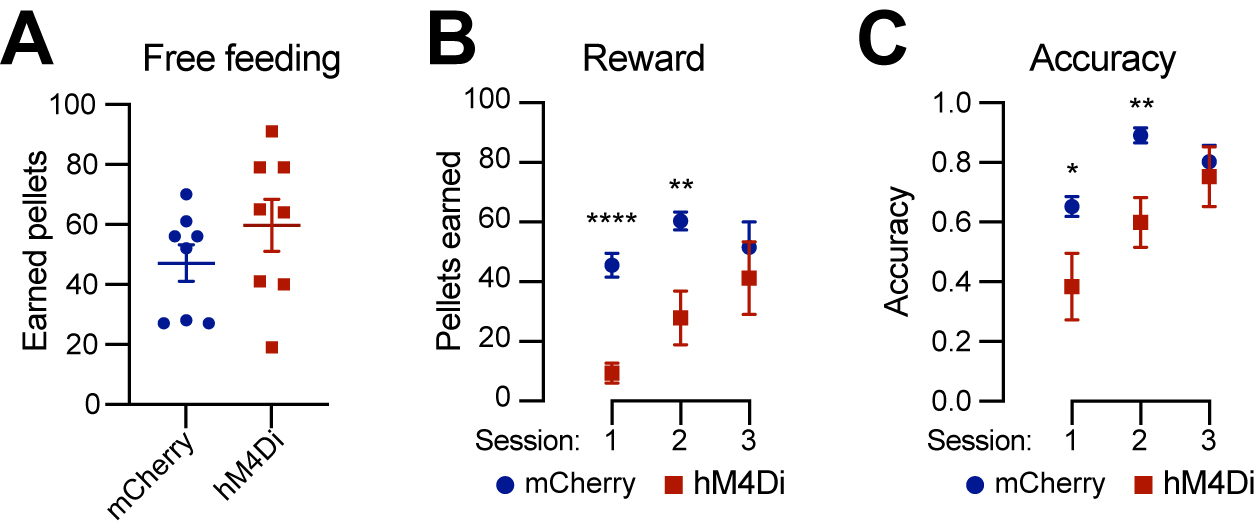
DANs are involved in various non-motor functions, including reward-related behavior such as seeking rewards, avoidance, and motivation[10,26,27]. However, the specific role of the Calb1+ SNc DAN subtype in reward-related behavior remains unclear. To address this, we investigated their involvement in simple reward-related functions using a home cage-based feeding experimentation device (FED3) under food-restricted conditions[24]. We assessed performance in both free-feeding, where a food pellet was immediately dispensed following the previous pellet retrieval, and fixed ratio 1 (FR1) reinforcement, where a correct nose-poke to the designated port was required to earn a pellet, schedules. During a 2-hour free-feeding session, DREADD experimental mice and control mice collected a similar number of pellets [Figure 4A], indicating no difference between the two groups in pellet consumption when pellets were easily accessible. However, in the FR1 schedule, where mice needed to learn an action-outcome association to obtain a pellet, over first three days, DREADD mice initially exhibited a lower pellet acquisition following chemogenetic inhibition of Calb1+ SNc DANs but gradually caught up with pellet acquisition rate of control mice [Figure 4B]. Additionally, Calb1+ DAN-inhibited mice showed reduced accuracy in poking the assigned ports during the first two training sessions but gradually improved and achieved similar accuracy in the subsequent session [Figure 4C] and maintained the same to controls beyond day 3 (data not shown). These findings suggest that Calb1+ SNc DANs contribute to early stages of associative learning and reward-seeking behavior, though their influence diminishes over time, leading to comparable performance between experimental and control groups in later stages.
Figure 4. Chemogenetic inhibition of Calb1+ nigrostriatal DANs affects food-seeking behavior. (A) Quantification of rewards earned by control mCherry [n = 8, 3 Male (M)/5 Female (F)] and hM4Di (n = 8, 4 M/4 F)-expressing TC double KI mice during a 2-hour free-feeding session on the FED3 device. Data are presented as mean ± SEM; unpaired t-test, P = 0.251; (B) Quantification of pellets earned in FR1 training on FED3 across three sessions. Two-way ANOVA with Tukey’s multiple comparison test: ****P < 0.0001 (session 1), **P = 0.0086 (session 2), and 0.5036 (session 3); (C) Quantification of nose-poking accuracy in FR1 training on FED3 across three sessions. Two-way ANOVA with Tukey’s multiple comparison test: *P = 0.0495 (session 1), **P = 0.0096 (session 2), and 0.6679 (session 3). Calb1+: Calbindin 1-positive; DANs: dopaminergic neurons; TC: ThFlp; Calb1IRESTCre; KI: knock-in; FED3: Feeding Experimentation Device 3; SEM: standard error of the mean; FR1: fixed-ratio 1; ANOVA: analysis of variance.
Selectively targeting of Aldh1a1+ SNc DANs using intersectional mouse genetics and chemogenetic approaches
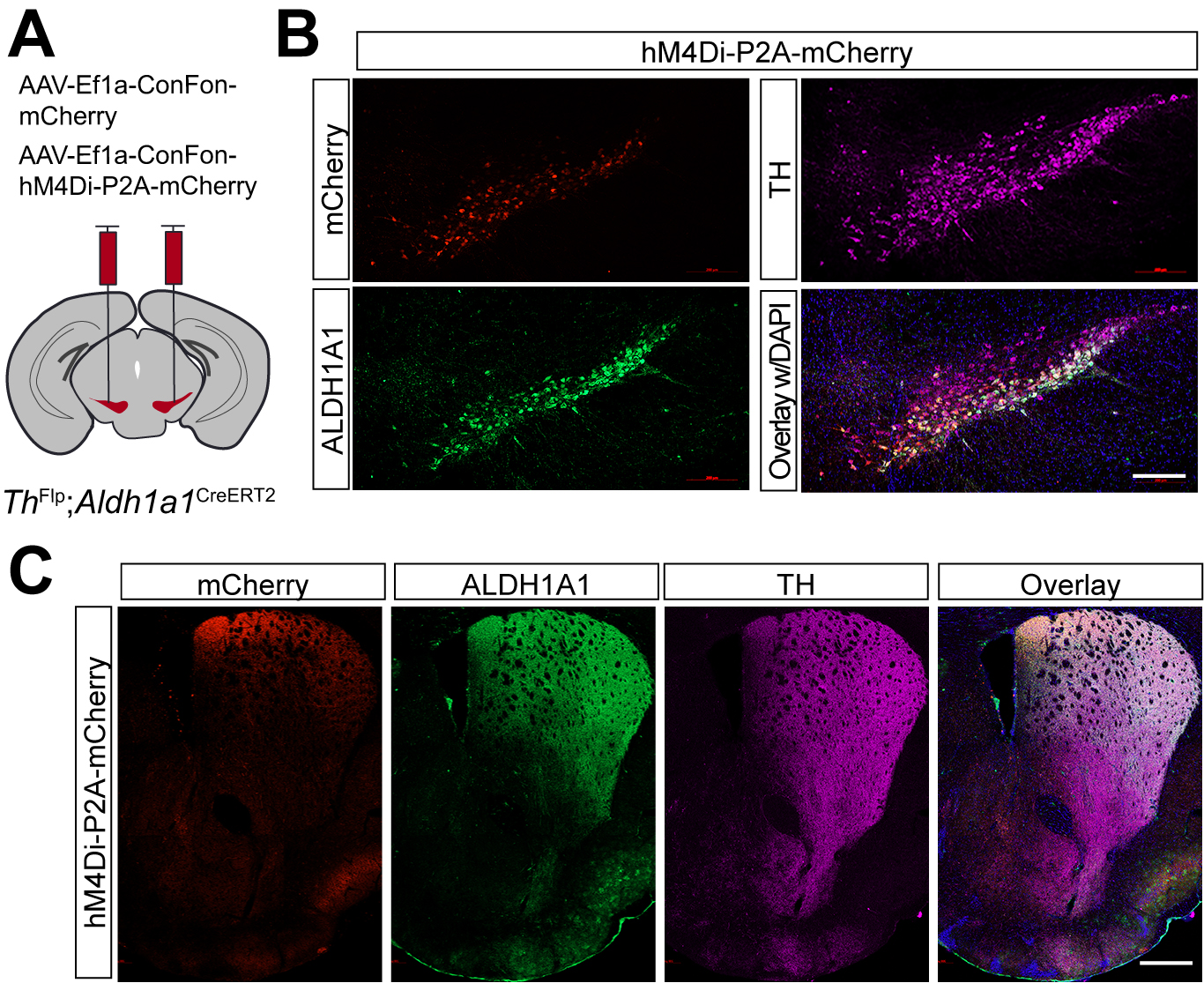
Compared to the chemogenetic inhibition of Calb1+ SNc DANs, genetic ablation of Aldh1a1+ SNc DANs in a previous study resulted in only a modest impairment of locomotion[16]. To directly compare the roles of Aldh1a1+ and Calb1+ SNc DANs in locomotion within the same experimental paradigm, we generated ThFlp; Aldh1a1CreERT2 (TA) double KI mice and selectively inhibited Aldh1a1+ SNc DAN activity using the same chemogenetic approach applied to Calb1+ SNc DANs [Figure 5A].
Figure 5. Targeted expression of the chemogenetic inhibitor hM4Di in Aldh1a1+ nigrostriatal DANs. (A) Schematic depicting the stereotactic delivery of control mCherry or hM4Di AAVs in the SNc of ThFlp; Aldh1a1CreERT2 (TA) double KI mice; (B) Representative images showing mCherry (red), ALDH1A1 (green), and TH (magenta) expression in the SNc of control mCherry (left panel) and hM4Di (right panel)-expressing mice. Scale bar: 1 mm; (C) Representative images illustrating mCherry (red), ALDH1A1 (green) and TH (magenta) staining in the striatum of control mCherry-expressing mice. Scale bar: 1 mm. Aldh1a1+: Aldehyde dehydrogenase 1A1-positive; DANs: dopaminergic neurons; AAVs: adeno-associated viruses; SNc: substantia nigra pars compacta; KI: knock-in; TH: Tyrosine Hydroxylase.
Immunostaining confirmed selective expression of hM4Di and mCherry in approximately 44% of Aldh1a1+ SNc DANs, which are primarily located in the ventral tier of the SNc [Figure 5B]. Examination of their projection patterns in the dorsal striatum revealed axon terminals distributed in both the DMS and dorsolateral striatum (DLS) [Figure 5C]. Together, the use of TA double KI mice combined with stereotaxic injection enables selective targeting of Aldh1a1+ SNc DANs for genetic and functional manipulations.
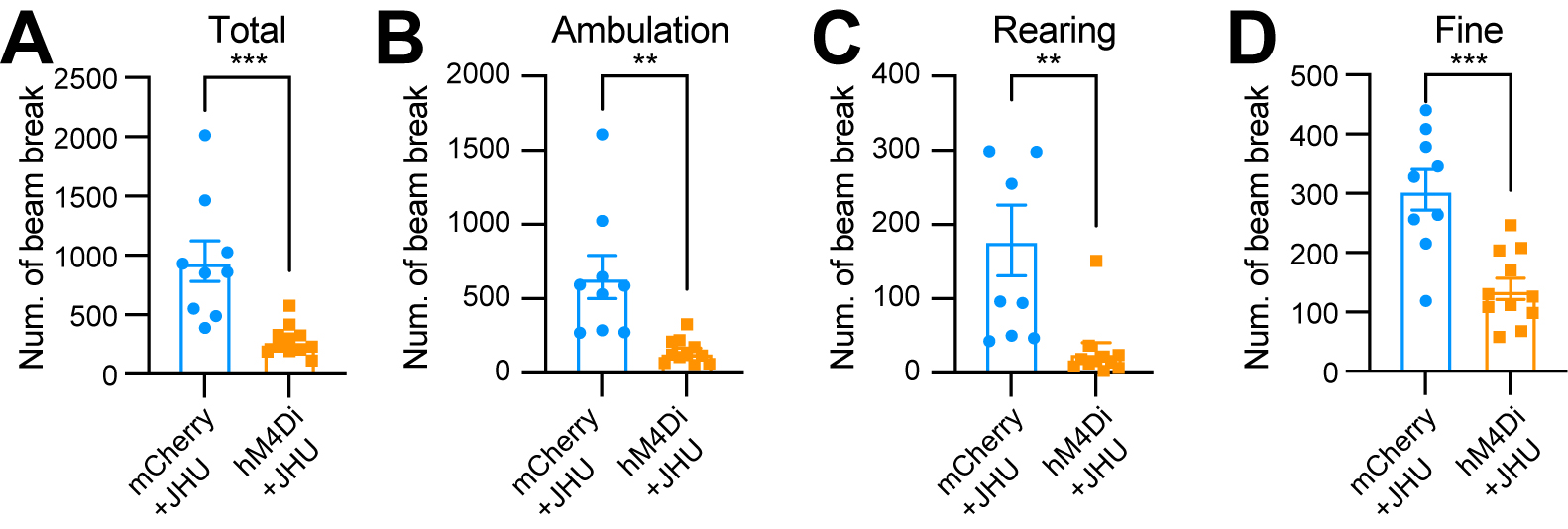
Chemogenetic inhibition of Aldh1a1+ SNc DANs suppresses spontaneous movements
Using an open-field test to assess spontaneous movements following intraperitoneal injection of the DREADD activator JHU37160, we observed a marked reduction in total movement, ambulatory movement, rearing, and fine movement [Figure 6A-D]. These findings reveal a critical acute role of Aldh1a1+ SNc DANs in regulating spontaneous movements.
Figure 6. Chemogenetic inhibition of Aldh1a1+ nigrostriatal DANs impairs spontaneous locomotion. (A-D) Quantification of total (A)
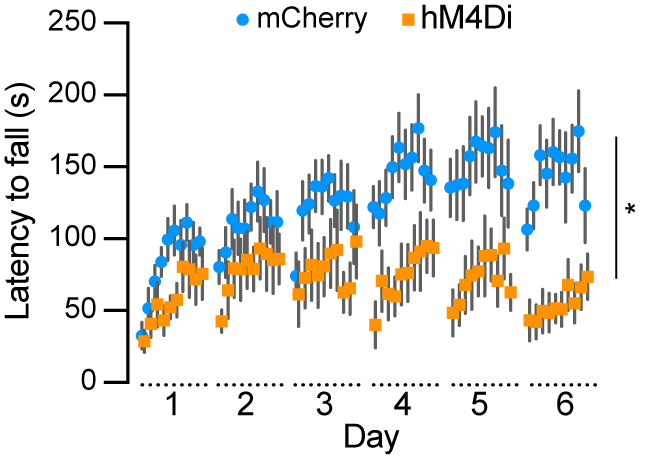
Chemogenetic inhibition of Aldh1a1+ SNc DANs severely impairs motor skill learning
To examine the role of Aldh1a1+ SNc DANs in motor skill learning, we conducted rotarod tests on TA double KI mice that received bilateral injections of control and hM4Di AAVs into the SNc. Chemogenetic inhibition with JHU37160 severely impaired motor skill acquisition in hM4Di-expressing mice compared to mCherry-expressing control mice [Figure 7]. These results confirm previous findings in Aldh1a1+ SNc DAN-ablated mice[16] and further demonstrate that Aldh1a1+ SNc DANs are essential for acquiring skilled movements.
Figure 7. Chemogenetic inhibition of Aldh1a1+ nigrostriatal DANs severely impairs rotarod motor skill learning. Rotarod motor skill learning in TA double KI mice injected with either control mCherry [n = 9, 8 Male (M)/1 Female (F)] or hM4Di AAVs (n = 11, 5 M/6 F) in the SNc. Mice underwent one training session per day for six consecutive days, with JHU37160 administered prior to each session. Data are presented as mean ± SEM. Two-way ANOVA, mCherry vs. hM4Di effect: F (1, 18) = 8.227, *P = 0.012. Aldh1a1+: Aldehyde dehydrogenase 1A1-positive; DANs: dopaminergic neurons; TA: ThFlp; Aldh1a1CreERT2; KI: knock-in; AAVs: adeno-associated viruses; SNc: substantia nigra pars compacta; SEM: standard error of the mean; ANOVA: analysis of variance.
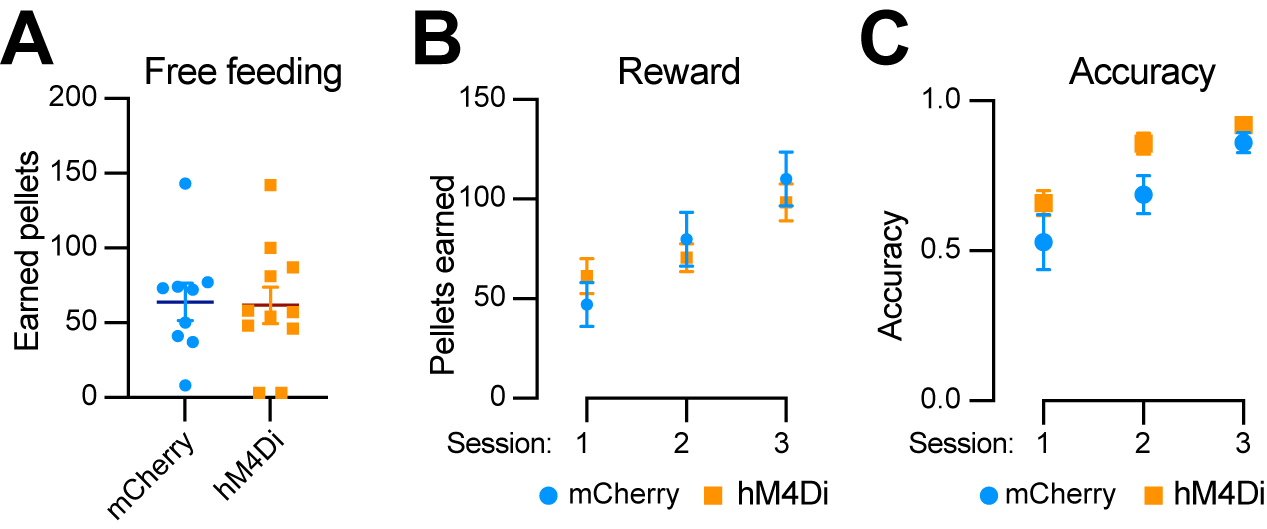
Chemogenetic inhibition of Aldh1a1+ SNc DANs does not affect the simple reward learning
To determine whether Aldh1a1+ SNc DANs are involved in reward and associative learning related behaviors, we examined the performance of Aldh1a1+ DAN-inhibited mice in both free-feeding and FR1 reinforcement schedules. Chemogenetic inhibition of Aldh1a1+ SNc DANs had no defect on free feeding, reward acquisition, or nose-poking accuracy [Figure 8A-C]. These findings suggest that Aldh1a1+ SNc DANs do not play a significant role in the simple associative learning and reward-seeking behaviors, in contrast to that of Calb1+ SNc DANs.
Figure 8. Chemogenetic inhibition of Aldh1a1+ nigrostriatal DANs does not affect food-seeking behavior. (A) Quantification of rewards earned by control mCherry [n = 9, 8 Male (M)/1 Female (F)] and hM4Di (n = 11, 5 M/6 F) AAV-injected TA double KI mice during a 2-hour free-feeding session on the FED3 device. Data are presented as mean ± SEM; unpaired t-test, P = 0.251; (B) Quantification of pellets earned in FR1 training on FED3 across three sessions. Two-way ANOVA with Tukey’s multiple comparison test: P = 0.3325 (session 1), 0.5549 (session 2), and 0.4928 (session 3); (C) Quantification of nose-poking accuracy in FR1 training on FED3 across three sessions. Two-way ANOVA with Tukey’s multiple comparison test: P = 0.5333 (session 1), 0.1080 (session 2), and 0.4305 (session 3). Aldh1a1+: Aldehyde dehydrogenase 1A1-positive; DANs: dopaminergic neurons; AAV: adeno-associated virus; TA: ThFlp; Aldh1a1CreERT2; KI: knock-in; FED3: Feeding Experimentation Device 3; SEM: standard error of the mean; FR1: fixed-ratio 1; ANOVA: analysis of variance.
DISCUSSION
In this study, we employed intersectional genetics and a chemogenetic approach to selectively manipulate the activity of Calb1+ and Aldh1a1+ nigrostriatal DAN subtypes in TC or TA double KI mice during Open field, rotarod motor skill learning, and FED3 reward learning tests. We found that inhibition of either DAN subtype suppressed locomotion and impaired motor skill learning, while inhibition of the Calb1+ subtype also slowed down reward-based reinforcement learning. These findings demonstrate that both DAN subtypes in SNc are essential for promoting locomotion and motor skill learning, with Calb1+ neurons additionally contributing to reward associative learning.
We previously demonstrated that genetic ablation of Aldh1a1+ nigrostriatal DANs resulted in a modest reduction in locomotor activity but completely abolished rotarod motor skill learning[16]. Interestingly, while chemogenetic inhibition of Aldh1a1+ DANs similarly caused severe motor skill learning deficits, it led to a more pronounced reduction in locomotor activity compared to genetic ablation. The underlying reason for this discrepancy remains unclear; however, we speculate that the rapid and transient inhibition of Aldh1a1+ DAN activity via chemogenetics may not trigger a robust compensatory response from other neural circuits, unlike genetic ablation, which gradually leads to neuronal loss over weeks[16].
While the locomotion-promoting function of Aldh1a1+ nigrostriatal DANs is well established[16,17], the role of the Calb1+ subtype remains less defined. A recent study reported that Calb1+ DAN activity correlates with deceleration, as measured by calcium transients in axons or somas of specific DAN subtypes while head-fixed mice ran on a treadmill[17]. This correlation with deceleration suggests a potential role for Calb1+ DANs in locomotion suppression. However, using chemogenetic manipulation in freely moving mice, we demonstrated that inhibition of Calb1+ DAN activity led to a severe reduction in locomotor activity, consistent with the general role of basal ganglia dopamine transmission in promoting movement.
Furthermore, to explore the involvement of Calb1+ DANs in reward-related behaviors, we employed the FED3 system. Our findings revealed that Calb1+ DANs contribute to early associative learning and reward-seeking behaviors, as chemogenetic inhibition disrupted food pellet acquisition and performance accuracy, particularly during the initial phase of the FR1 sessions. These results align with previous studies highlighting the role of Calb1+ nigrostriatal DANs in reward processing[17].
It is worth noting that a small fraction of DANs located in the ventromedial region of the SNc co-express both Aldh1a1 and Calb1. These neurons project their axonal fibers primarily to the DMS. However, their exact roles in motor control, learning, and reward responses remain unknown. Given that both Aldh1a1+ and Calb1+ DAN subtypes promote locomotion and motor learning, it is likely that this dual-positive subtype also facilitates these functions. However, future studies employing additional intersectional genetic manipulations will be necessary to specifically investigate the functional contributions of this distinct DAN population. DMS is known for goal-directed behavior and flexible action control[28-30]. We therefore hypothesize that the Calb1+/Aldh1a1+ DANs deliver dopamine signals to DMS that encode value and prediction errors in flexible, value-based, and goal-directed control of behavior.
Although our experiments establish the necessity of Calb1+ and Aldh1a1+ SNc DANs for movement and motor learning, we did not perform parallel activation tests. Given the preferential projections of Calb1+ DANs to DMS, we predict that selective activation would increase spontaneous locomotor vigor and accelerate motor learning, with possible enhancement of early associative learning. In contrast, activating Aldh1a1+ DANs is likely to increase locomotor vigor and motor-skill acquisition with a stronger impact on sensorimotor/habit-like performance than on early associative learning. Future work employing intersectional hM3Dq or temporally precise optogenetic activation will directly test these sufficiency predictions.
Advances in single-cell and single-nucleus RNA sequencing have provided valuable insights into the complexity and heterogeneity of DAN subtypes in the SNc[10,11,31-33]. By leveraging the distinct molecular markers identified in these neuronal subtypes, our study highlights the importance of systematically defining their functions. This approach will enhance our understanding of the diverse motor and non-motor phenotypes observed in PD.
DECLARATIONS
Acknowledgments
The authors thank members of the Cai Lab and the Rodent Behavioral Core of the Intramural Research Program, National Institute of Mental Health, for helpful suggestions and technical assistance.
Authors’ contributions
Conceived and wrote the manuscript and prepared the figures with inputs from all authors: Cai H
Designed and performed histology and behavioral experiments, conducted data analyses, prepared figures, wrote the methods and figure legends, and wrote the manuscript for the TC double KI mice: Habib A
Designed and performed histology and behavioral experiments and conducted data analyses for the TA double KI mice: Riccobono G
Performed histology: Tian L
Generated the cohort of TA mice: Basu D
Built the dual control hM4Di plasmid: Sun L
Performed stereotactic surgery: Chang L
Contributed to the histological analysis: Martinez Smith VM
Designed FED3 experiments, contributed to data analysis, and edited the manuscript: Wang L
Edited and provided critical feedback: Le W
All authors read and approved the final manuscript.
Availability of data and materials
The datasets and materials generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This work is supported in part by the Intramural Research Programs of the National Institute on Aging, NIH (HC, ZIA AG000944, AG000928), the Rodent Behavioral Core of the Intramural Research Program of the National Institute of Mental Health (MH002952), and the National Natural Science Foundation of China (WDL, 32220103006). The contributions of the NIH author(s) were made as part of their official duties as NIH federal employees, are in compliance with agency policy requirements, and are considered Works of the United States Government. However, the findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
Conflicts of interest
Le W is the Editor-in-Chief of the journal Ageing and Neurodegenerative Diseases; Cai H is an Editorial Board member of the journal Ageing and Neurodegenerative Diseases. They were not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
All mouse studies were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committees (IACUC) of the Intramural Research Program of the National Institute on Aging (NIA) (Proposal #464-LNG-2027).
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Alberico SL, Cassell MD, Narayanan NS. The vulnerable ventral tegmental area in Parkinson’s disease. Basal Ganglia. 2015;5:51-5.
2. Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;18:251-9.
3. Fu Y, Paxinos G, Watson C, Halliday GM. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J Chem Neuroanat. 2016;76:98-107.
4. Weisenhorn DM, Giesert F, Wurst W. Diversity matters - heterogeneity of dopaminergic neurons in the ventral mesencephalon and its relation to Parkinson’s disease. J Neurochem. 2016;139 Suppl 1:8-26.
5. Liu G, Yu J, Ding J, et al. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J Clin Invest. 2014;124:3032-46.
6. Cai H, Liu G, Sun L, Ding J. Aldehyde dehydrogenase 1 making molecular inroads into the differential vulnerability of nigrostriatal dopaminergic neuron subtypes in Parkinson’s disease. Transl Neurodegener. 2014;3:27.
7. Carmichael K, Evans RC, Lopez E, et al. Function and regulation of ALDH1A1-positive nigrostriatal dopaminergic neurons in motor control and Parkinson’s disease. Front Neural Circuits. 2021;15:644776.
8. Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS. Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem Res Toxicol. 2014;27:1359-61.
9. Poulin JF, Zou J, Drouin-Ouellet J, Kim KY, Cicchetti F, Awatramani RB. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014;9:930-43.
10. Carmichael K, Sullivan B, Lopez E, Sun L, Cai H. Diverse midbrain dopaminergic neuron subtypes and implications for complex clinical symptoms of Parkinson’s disease. Ageing Neur Dis. 2021;1:4.
11. Gaertner Z, Azcorra M, Dombeck DA, Awatramani R. Molecular heterogeneity in the substantia nigra: a roadmap for understanding PD motor pathophysiology. Neurobiol Dis. 2022;175:105925.
12. Poulin JF, Gaertner Z, Moreno-Ramos OA, Awatramani R. Classification of midbrain dopamine neurons using single-cell gene expression profiling approaches. Trends Neurosci. 2020;43:155-69.
13. Goldstein DS. Biomarkers, mechanisms, and potential prevention of catecholamine neuron loss in Parkinson disease. Adv Pharmacol. 2013;68:235-72.
14. Hegarty SV, Sullivan AM, O’Keeffe GW. Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol. 2013;379:123-38.
15. Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14:223-36; discussion 222.
16. Wu J, Kung J, Dong J, et al. Distinct connectivity and functionality of aldehyde dehydrogenase 1A1-positive nigrostriatal dopaminergic neurons in motor learning. Cell Rep. 2019;28:1167-1181.e7.
17. Azcorra M, Gaertner Z, Davidson C, et al. Unique functional responses differentially map onto genetic subtypes of dopamine neurons. Nat Neurosci. 2023;26:1762-74.
18. Poulin JF, Caronia G, Hofer C, et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 2018;21:1260-71.
19. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133-40.
20. Pei Y, Rogan SC, Yan F, Roth BL. Engineered GPCRs as tools to modulate signal transduction. Physiology. 2008;23:313-21.
21. Fenno LE, Ramakrishnan C, Kim YS, et al. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron. 2020;107:836-853.e11.
22. Liu Z, Yang N, Dong J, et al. Deficiency in endocannabinoid synthase DAGLB contributes to early onset Parkinsonism and murine nigral dopaminergic neuron dysfunction. Nat Commun. 2022;13:3490.
23. Bonaventura J, Eldridge MAG, Hu F, et al. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat Commun. 2019;10:4627.
24. Matikainen-Ankney BA, Earnest T, Ali M, et al. An open-source device for measuring food intake and operant behavior in rodent home-cages. Elife. 2021;10:e66173.
25. Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124-34.
27. Coddington LT, Dudman JT. Learning from action: reconsidering movement signaling in midbrain dopamine neuron activity. Neuron. 2019;104:63-77.
28. Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513-23.
29. Thorn CA, Atallah H, Howe M, Graybiel AM. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66:781-95.
30. Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464-76.
31. Yaghmaeian Salmani B, Lahti L, Gillberg L, et al. Transcriptomic atlas of midbrain dopamine neurons uncovers differential vulnerability in a Parkinsonism lesion model. Elife. 2024;12:RP89482.
32. Martirosyan A, Ansari R, Pestana F, et al. Unravelling cell type-specific responses to Parkinson’s disease at single cell resolution. Mol Neurodegener. 2024;19:7.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].