Advances of soft robotics for gastrointestinal tract applications
Abstract
Gastrointestinal (GI) robots overcome the limitations of conventional endoscopy, offering a noninvasive and precise approach for diagnosing and treating major GI diseases including colorectal cancer, gastric cancer, inflammatory bowel disease, and peptic ulcers. However, due to the dynamic, tortuous, mucus-rich, and pH-variable environment of GI tract, GI robots face serious challenges in achieving precise localization, stable operation, and reliable sensing. Leveraging the properties of tissue-matched modulus, adaptive deformation, and bioinspired design, soft robots can conform to the intestinal wall, minimizing mechanical damage and thereby offering new strategies for efficient sensing and treatment of GI diseases. Hereinto, this review presents a comprehensive GI soft robot system by integrating materials, structural designs, actuation modes, and physiological adaptability to achieve multifunctional performance and clinical reliability. Specifically, materials are classified into biocompatible elastomers, smart responsive polymers, and functionalized conductive materials to guide material selection and structural optimization. Subsequently, structural engineering methods including continuous encapsulation designs, modular-reconfigurable architectures, and biomimetic frameworks are introduced, alongside diverse actuation strategies of field-driven, fluid-driven, and chemically driven autonomous mechanisms. Following that, preclinical applications are highlighted as navigation and localization, drug delivery and controlled release, in vivo sensing, as well as minimally invasive manipulation and therapy. In the future, this integrated GI soft robotics technology will fundamentally reshape precision medicine, enabling a more intelligent and personalized therapeutic platform, and driving the evaluation of next-generation GI soft robotics.
Keywords
INTRODUCTION
Soft robots, with their high degrees of freedom, large deformability, flexibility, and strong environmental adaptability, have become a research focus in robotics[1,2]. Their advantages in flexible deformation, actuation, and gripping offer great potential for applications in deep-sea exploration, medical rehabilitation, and pipeline inspection[2,3]. For instance, Li et al. developed a deep-sea soft robot based on dielectric elastomers capable of operating at a depth of 10,900 m[4]; Xie et al. designed a bioinspired octopus arm integrated with flexible electronics for sensing, locomotion, and interaction in complex environments[5].
The human digestive tract transports nutrients and expels waste, and its dysfunction directly affects health. Soft robots designed for the gastrointestinal (GI) tract operate within its constrained environment by integrating sensing, actuation, power supply, and communication systems, progressively enabling precise diagnosis and treatment of GI diseases. In specific clinical scenarios, GI soft robots are capable of performing GI tract biopsies and sampling intestinal microbiota for early screening and detection of colorectal cancer[21-23]. Furthermore, Mimee et al. integrated a probiotic sensor into the robot to enable effective detection of GI bleeding[24]. Additionally, capsule endoscopy enables direct visualization of the GI mucosal surface and, when integrated with deep learning, facilitates the detection of pathological changes such as bleeding, ulcers, polyps, and inflammation[25,26]. Besides, soft robots integrated into endoscopes assist in esophageal and colonic stricture dilation and stent placement[27,28], thereby improving surgical precision and safety. A system designed by Del Bono et al. can autonomously inflate and expand colonic tissue[29], reducing potential tissue damage while maintaining the colonic lumen patency. Meanwhile, site-specific drug delivery represents another critical application area[30]. For example, Zhao et al. reported a protein-based soft robot capable of navigating closed spaces within the GI tract and releasing drugs on demand for precise therapy[31]. For gastric ulcer treatment, Zhou et al. developed an acid-triggered charge-switching antibacterial hydrogel soft robot that effectively accelerates gastric mucosal wound healing[32]. In parallel, for chronic GI disorders, Abramson et al. designed an orally ingestible soft robot that adheres to the GI wall and achieves precise control of gastric motility disorders through electrical stimulation[33]. Overall, these representative preclinical devices demonstrate the feasibility of integrating multifunctional soft robots into the complex GI environment and lay a solid foundation for intelligent, multimodal diagnostics and therapeutics for GI diseases in the future.
However, as one of the most complex organ systems, the GI tract poses a highly challenging physiological environment for soft robot design and application. Firstly, the GI tract exhibits multiscale, multilayered structural features and dynamic behaviors. Its folded surface, viscous mucus layer, and distinctive peristaltic motility hinder the precise localization and stable retention of soft robots[34,35], while GI peristalsis, luminal folding, and the viscous mucosal layer further impede mechanical propulsion. These challenges necessitate soft robots with a high degree of freedom, compact and reconfigurable structures, and reliable responses to external field-driven or autonomous actuation. Moreover, the GI tract exhibits pronounced pH gradients[36], with a strongly acidic stomach environment (pH 1-4), near-neutral pH in the small intestine (pH 6.5-7), and mildly alkaline conditions in the colon (pH 7-8). These pH variations impose specific requirements on the selection of materials for soft robotic systems. Variations in pH necessitate that materials for soft robotics either possess robust chemical stability and corrosion resistance, or are pH-sensitive to enable an intelligent response to pH gradients[37,38]. In addition, digestive enzymes and diverse microbiota communities further affect the stability and functionality of soft robots[39,40]. Natural polymers (such as gelatin, collagen, and cellulose) and certain synthetic polyesters, including polylactic acid (PLA) and polycaprolactone (PCL), are susceptible to enzymatic degradation by digestive enzymes[41,42]. Their rapid decomposition and dissolution can lead to structural failure of soft robots. Furthermore, metallic functional components (including actuators, sensors, and embedded circuits) are vulnerable to corrosion by bodily fluids, which may impair the core operational capabilities of the robot and result in complete functional failure[43,44]. Therefore, soft robots must be either encapsulated or designed to strategically exploit GI physiological features for targeted activation. The complex physiological environment of the GI tract necessitates systematic innovations in material selection, structural design, actuation control, and functional implementation to enable safe and effective in vivo diagnostics and therapeutics.
This review aims to systematically delineate the latest advances in GI soft robotics field by integrating insights across four key dimensions: materials science, structural engineering, actuation strategies, and functional implementations [Figure 1]. A function-oriented material classification system is proposed, encompassing three major categories: biocompatible soft elastomers, smart responsive materials, and functionalized conductive and magnetic materials. Subsequently, three structural design strategies (namely continuous-encapsulated, modular-reconfigurable, and biomimetic morphologies) are summarized. Furthermore, the diverse actuation mechanisms, including field-driven, fluid-driven, and chemical reaction-driven methods, are systematically analyzed with respect to their applicability and technical characteristics. Finally, the multifunctional applications are thoroughly elaborated, ranging from fundamental drug delivery to complex integrated diagnosis and therapy. Through this review, we aspire to guide theoretical and technological advances in soft robotics, and aim to accelerate the clinical translation of GI soft robotic technologies from laboratory to clinics.
Figure 1. Overview of GI soft robots. The inner circle illustrates the gastric rugae and intestinal villi structure of the GI tract, as well as the functions of the GI robot for drug release and colon cancer sampling. The outer circle presents the aspects of GI soft robot, including materials, structure, actuation, and applications. Created with BioRender.com. GI: Gastrointestinal.
MATERIALS
Considering the complex physiological characteristics of GI tract, materials should possess mechanical properties matched to biological tissues to minimize tissue damage. Meanwhile, to ensure safe operation
Biocompatible soft elastomeric materials
Biocompatible elastomeric materials, including elastomers and hydrogels, constitute the fundamental material platform for GI soft robotics, with their selection directly influencing the safety and functionality of the robots. Elastomers (such as silicone rubber and polyurethane) primarily serve as the main structural materials of the robot. Their core function is mechanical matching, requiring elastic modulus (typically ranging from tens of kPa to tens of MPa[50]) aligned with GI soft tissues (~10 kPa) to minimize tissue damage and accommodate the deformation of the luminal environment. Additionally, their high fracture strain and superior fatigue resistance enable them to withstand cyclic strains, facilitating repetitive motions such as peristalsis and grasping. Silicone-based materials, as the most widely applied matrix materials [such as polysiloxanes, particularly polydimethylsiloxane (PDMS), and Ecoflex], exhibit low modulus, high strain capacity, and low hysteresis[51]; PDMS-based soft robots have been extensively utilized as carriers and compliant actuators within simulated gastric and intestinal fluids. When combined with bioadhesive agents[52], the robot demonstrated reliable operation exceeding 25 h under cyclic mechanical loading, effectively mimicking peristaltic environments. Similarly, the soft robot fabricated from Ecoflex exhibited remarkable structural stability after prolonged immersion in acidic solutions (pH = 1)[53], maintaining motion performance with negligible change in velocity (0.21 cm·s-1 pre-immersion vs. 0.20 cm·s-1 post-immersion), indicative of superior corrosion resistance. Further validation in ex vivo porcine stomach models confirmed the structural integrity and operational feasibility of the robot, underscoring its potential for applications such as targeted bacterial infection therapy within the GI tract. Hydrogels, as three-dimensional (3D) polymer networks with high water content[54], have attracted considerable attention for their unique hydrophilicity and bioactivity, serving key roles in cargo loading, controlled release, and mucoadhesion. Natural polymer hydrogels, including alginate, gelatin, and chitosan, both possess excellent biocompatibility and tunable mechanical properties via crosslinked networks[55,56]. Moreover, hydrogels exhibit drug-loading capabilities. As illustrated in Figure 2A, sequential ingestion of a crosslinker solution containing calcium and dithiol crosslinkers, followed by a drug-loaded polymer solution of alginate and four-arm poly(ethylene glycol)-maleimide, forms a tough hydrogel in the stomach capable of withstanding pressures ≥ 13 kPa[57], making it an ideal material for GI soft-robotic drug delivery. Hydrogels can also enhance adhesion performance; for instance, Figure 2B depicts robust hydrogel adhesives designed for gastric perforation repair that are acid-resistant[58]. Thermoplastic elastomers, such as polyurethanes[59], demonstrate distinct advantages in applications requiring frequent deformation due to their excellent elastic recoverability and ease of processing.
Figure 2. Material selection strategies for GI soft robots. (A) Liquid in situ-forming tough hydrogels form in vivo through sequential oral administration of crosslinker and polymer solutions, utilizing alginate and four-arm PEG-maleimide networks to create tough double-network systems capable of controlled drug release. Reprinted with permission from[57]. Copyright 2024, Springer Nature; (B) Poly(N-acryloyl aspartic acid) hydrogels demonstrate strong tissue adhesion through hydrogen bond crosslinked networks with Janus patch design, enabling gastric perforation repair while preventing. Reprinted with permission from[58]. Copyright 2022, Elsevier Ltd; (C) Ca-alginate-chitosan/protamine/silica capsules provide gastric protection for encapsulated indomethacin via acid-resistant composite shells, then enable rapid and controlled drug release in small intestine through pH-triggered opening of microchannels. Reprinted with permission from[63]. Copyright 2023, Elsevier Ltd; (D) Different response shapes of a novel SMP/hydrogel hybrid actuator. Reprinted with permission from[68]. Copyright 2023, MDPI; (E) Soft robot design mimics natural skin’s hierarchical structure, integrating electronic sensing layers with artificial muscle components for adaptive robotic functionality. Reprinted with permission from[80]. Copyright 2024, Springer Nature. GI: Gastrointestinal; SMP: shape memory polymer.
Smart responsive materials
Smart responsive materials endow GI soft robots with the capability to perceive environmental changes and autonomously react, representing a critical technological foundation for precision medicine. pH-sensitive materials exploit the pronounced pH gradient along the GI tract to achieve site-specific actuation[60]. Polymers such as poly(acrylic acid) (PAA) and carboxymethyl chitosan (CMCS) exhibit significant swelling behavior changes in response to varying pH levels, making them ideal platforms for stomach-protective and intestine-targeted release systems[61,62]. For example, Wen et al. developed Ca-alginate-based Janus capsules wherein hydroxypropyl methylcellulose phthalate (HPMCP) enteric microspheres dissolve under intestinal pH conditions to form microchannels[63], enabling PAA to swell under alkaline environments and generate propulsion forces for targeted release in the intestine [Figure 2C]. Thermoresponsive polymers, such as poly(N-isopropylacrylamide) (PNIPAM)[64], possess a lower critical solution temperature (LCST) near body temperature, facilitating phase transitions triggered by physiological heat or external stimuli. Ma et al. engineered a nanocellulose-mediated PNIPAM layer exhibiting hydrophobic contraction starting at
Functionalized conductive and magnetic materials
Functionalized conductive and magnetic materials constitute a vital material foundation for enabling remote actuation and multifunctional integration in GI soft robotics. Magnetic fillers are essential for wireless control with remote actuation forces. Specifically, hard magnetic materials, such as neodymium-iron-boron (NdFeB), possess high remanence and coercivity[73], making them ideal candidates for magnetic actuation in GI soft robots. The application of an external alternating magnetic field (AMF) enables precise navigation, localization, and actuation of these robots. In contrast, ferromagnetic or soft magnetic materials (such as iron and iron oxides) exhibit magnetostrictive behavior[74], undergoing deformation when subjected to external magnetic fields. Iron oxides, when used at appropriate dosages, demonstrate biocompatibility and can generate Joule heating under alternating high-frequency magnetic fields, making them suitable for magnetothermal therapy[75,76]. In addition, they can serve as triggers for thermo-responsive material deformation and controlled drug release. Conductive elastomers incorporate conductive fillers to endow electrical functionalities. Typical conductive inclusions include
Material constraints and imaging considerations
In summary, biocompatible soft elastomers, stimuli-responsive materials, and functionalized conductive/magnetic materials all present distinct advantages in GI soft robots. However, to ensure reliable operation in the complex GI environment, these materials must satisfy a series of quantitative physiological and engineering constraints. Table 1 summarizes the critical material-related requirements for GI soft robotic systems with quantitative indicators, including elastic modulus ranges relevant to mucosal compliance, adhesion strength, pH/temperature responsiveness, allowable temperature during actuation, imaging compatibility, and in vivo residence/biodegradation timelines. By mapping specific material classes (elastomers, hydrogels, SMPs/LCEs, conductive/magnetic fillers, etc.) to these metrics, Table 1 provides a concise reference for evaluating suitability and guiding material selection in the GI environment.
Key material classes for GI soft robotics and their performance parameters compared with GI tract
| Category | Primary function | Elastic modulus (for mucosal compliance) | Mucoadhesive strength | pH/Temperature transition point | Allowable temperature | Imaging compatibility | In vivo residence time/biodegradation period | References |
| GI tract | N/A | 1-10 kPa Intestine: 20-40 kPa | N/A | Esophagus pH: 5-7 Stomach pH: 1-4 Intestine pH: 6.5-7 | < 60 ℃ (At 60 ℃ protein coagulates) | MRI (assessment of inflammation and tissue characteristics) X-ray/CT Ultrasound imaging | Gastric emptying (2-5 h), small bowel transit (2-6 h), colonic transit (10-59 h) and whole gut transit (10-73 h) | [84-88] |
| Elastomer | Structural framework, mechanical matching, cyclic strain | 10 kPa-20 Mpa | Matrix-dependent (typically requires modification) | N/A | > 60 ℃ (excellent thermal stability) | Generally compatible (ultrasound: excellent; CT/MRI: filler-dependent) | Mostly non-degradable; some degradable elastomers available | [89-92] |
| Hydrogels | Mucoadhesion, drug loading | 1-100 kPa PAA hydrogel: 6.7-9.3 kPa Ca-alginate hydrogels: 10-100 kPa | 4.31-170 kPa | pH-responsive swelling/degradation (e.g., chitosan at pH < 6.5; PAA at pH 6-8) | < 60 ℃ (prone to dehydration) | Pure hydrogels show poor imaging contrast; compatibility improves when combined with various contrast agents | Hours to days (biodegradable) | [54,92-97] |
| SMP/LCE | Shape fixation; stimuli-responsive | MPa (rubbery state) → GPa (glassy state) | Tunable | Tg/Tni: ~35-60 °C (actuation trigger) | N/A (intrinsically thermally actuated) | Generally compatible (US: excellent; CT/MRI: filler-dependent) | Tunable from days to biostable (dependent on chemical structure) | [98-101] |
| Liquid metal/Conductive composite | Circuits and sensing | Matrix-dependent (typically kPa to MPa) | Matrix-dependent | N/A | > 60 ℃ (excellent thermal conductivity) | CT-incompatible (high density); MRI/US (compatibility tunable). Radiogallium compounds as tumor imaging agents | Permanent (non-biodegradable) | [77,79,102-104] |
| Magnetic filler | Remote actuation and heating | Matrix-dependent | Matrix-dependent | N/A | N/A (serves as the heat source) | MRI: incompatible (artifact), CT/US: excellent | Permanent (non-biodegradable) | [74,76,104,105] |
In clinical imaging applications, it is essential to fully consider their compatibility to avoid imaging artifacts, localized overheating, and potential biosafety risks. Magnetic resonance imaging (MRI), a core modality for abdominal clinical imaging[84], can be significantly affected by NdFeB permanent magnets, which cause significant magnetic field distortions in MRI, resulting in severe geometric distortion artifacts. In contrast, Fe3O4 superparamagnetic nanoparticles primarily affect signal intensity by altering local magnetic susceptibility, producing contrast enhancement or signal artifacts[106]. Highly conductive gallium-based LMs may induce eddy currents under alternating radiofrequency fields[107], leading to localized heating and risk of tissue burns[108]. Mitigation strategies include the use of superparamagnetic fillers, reduction of filler concentration, polymer encapsulation layers, and optimization of MRI sequences [e.g., short echo time (TE) and spin-echo sequences][109,110].
In CT, high atomic number metals such as LMs absorb substantial X-rays, producing starburst-like beam hardening artifacts that can be alleviated by dilution or substitution with low-concentration contrast agents such as bismuth salts[111,112]. Conventional X-ray radiography also relies on high contrast between metal and tissue. However, large metal components cause shadow artifacts, necessitating improved encapsulation designs or artifact suppression algorithms[113].
By contrast, ultrasound imaging is highly compatible with most soft materials, such as hydrogels and elastomers, due to matched acoustic impedance with soft tissue, and active contrast can be achieved through microbubbles or using echogenic contrast agents[114]. Among common conductive polymers and nanomaterials, PEDOT:PSS and CNTs are nearly “invisible” in MRI/CT with weak ultrasound echoes, making them suitable for stealth tracking. AgNWs and MXene, owing to higher atomic numbers or conductive paramagnetic properties, provide contrast in CT, X-ray, and MRI but may generate speckle artifacts in ultrasound[115]. Graphene requires loading with high-Z metals or microbubble contrast agents to enhance imaging visibility[116]. Overall, material selection should balance functional performance and imaging requirements depending on application scenarios, with ultrasound or low-dose CT preferred for real-time tracking and safety assessment [Table 2].
Imaging compatibility of materials in GI soft robotics
| Imaging modality | Physical principle | Compatible material examples | Incompatible/risky material examples | Major effects and risks | Mitigation strategies | References |
| MRI | Magnetic field and RF pulses | Pure hydrogels, most elastomers, low-concentration Fe3O4 | NdFeB magnets, high-concentration magnetic fillers, LMs | Severe artifacts or complete imaging failure, local heating risk, displacement forces due to magnetic gradients | Use superparamagnetic particles, dilute fillers, polymer encapsulation | [106,108,109,117,118,111,112,119] |
| Ultrasound | Acoustic wave reflection | Hydrogels, elastomers, microbubbles | Large dense metal structures (e.g., NdFeB, LMs) | lower spatial resolutions, speckle artifacts | Add microbubbles, use of echogenic contrast agents | [114,120-122] |
STRUCTURAL DESIGN
Structural design constitutes a critical aspect for realizing the intended functions of GI soft robots, necessitating the optimization of their locomotion performance, environmental adaptability, and operational capabilities while ensuring compliance with biocompatibility and safety requirements.
Continuous and encapsulated structure
Continuous and encapsulated structure represents a pivotal technological strategy for achieving both drug protection and site-specific release in GI soft robotics. Capsule-shaped architectures, a classical encapsulation design, fully protect internal functional components with continuous outer shells. As illustrated in Figure 3A, a core-shell configuration comprising a hydrogel-encapsulated magnetic LM exhibits remarkable mechanical resilience[123], enduring compressive strains up to 85% and impact forces equivalent to free falls from heights exceeding 14 meters, while demonstrating passive deformability, active rolling, and toughness. The RoboCap system employs grooved and protruding spiral surface designs to enable simultaneous mucus clearance and drug dispersion[124], resulting in a 20- to 40-fold enhancement in bioavailability compared to conventional oral administration. Meanwhile, tubular structures capitalize on the inherent luminal geometry of the GI tract[125], achieving propulsion via coordinated peristaltic and expansion motions. For instance, an untethered soft robot integrates capsule and tubular components[126], with the tube facilitating propulsion through peristalsis and expansion [Figure 3B].
Figure 3. Design strategies of continuous and encapsulated structures, as well as modular and reconfigurable structures. (A) The hydrogel-coated containment capsule features a hierarchical core-shell architecture with remotely controlled thermal ablation capabilities via magnetic robotic arm manipulation under X-ray imaging guidance. Reprinted with permission from[123]. Copyright 2025, Oxford University Press; (B) Schematic illustration (i) and images (ii) of tubular robot expansion and peristaltic motion enabled by liquid-gas phase transition. Reprinted with permission from[126]. Copyright 2024, Springer Nature; (C) pH-responsive jack-in-the-box device deploys self-extending microneedle arrays to penetrate mucus and sustain localized drug release. Reprinted with permission from[131]. Copyright 2022, American Association for the Advancement of Science; (D) Self-folding chain exhibits modular self-folding architecture that transforms from linear configuration into large functional assemblies with multiple domains at the catheter tip. Reprinted with permission from[133]. Copyright 2023, Springer Nature; (E) The low-friction soft robot incorporates densely arranged cone structures with hydrophobic surface decoration to achieve ultra-low friction locomotion in viscous GI environments. Reprinted with permission from[53]. Copyright 2024, Beijing Institute of Technology Press. GI: Gastrointestinal.
Modular and reconfigurable structure
Modular and reconfigurable structure enables the execution of complex functions through the assembly and rearrangement of discrete functional units, representing a significant development direction in soft robotic architecture. A magnetically actuated origami millirobot[132], based on the Kresling pattern, dynamically interacts with its environment to perform rolling, flipping, and swimming motions, thus enabling amphibious locomotion. As illustrated in Figure 3D, a self-folding soft robot chain combines elastic energy storage with magnetic assembly to autonomously transform from a slender[133], catheter-constrained configuration into a larger functional structure. The multi-module MagCaps integrates two soft valve components containing different concentrations of NdFeB particles in parallel[134], thereby achieving sequential or simultaneous release of distinct drugs by modulating only the amplitude of a single external magnetic field. A magnetic multilayer soft robot employs serially connected modules to provide a multi-degree-of-freedom motion and enable on-demand separation and reconfiguration for targeted adhesion[52]. Moreover, a low-friction soft robot partitions its body and foot into modular functional zones
Biomimetic structure
Biomimetic structure design, inspired by the locomotion mechanisms and morphological features of natural organisms, offers soft robots efficient movement strategies and enhanced environmental adaptability. The specific mechanisms involved encompass six aspects: geometric structure design, morphological features, material functional integration, mechanical responsiveness, tunable stiffness, and multimodal locomotion. To demonstrate this concept, researchers have developed various prototypes inspired by nature. The self-orienting millimeter-scale applicator adopts a mono-monostatic body design modeled after the leopard tortoise shell[136], characterized by a shifted center of mass and a high-curvature upper shell. This bioinspired configuration enables rapid self-orientation within the gastric environment and imparts robustness against perturbations from peristaltic motion and fluid turbulence, thereby ensuring adaptability across fluids of varying viscosity and enabling precise and targeted drug delivery. As shown in Figure 4A, the starfish-inspired structure utilizes the contraction and expansion of star-shaped arms to achieve temperature-controlled drug release[67]. Meanwhile, an octopus-inspired soft robot mimics suction cups through a constricted orifice, disk-like infundibulum, and dome-shaped acetabulum [Figure 4B][137]. By manipulating magnetic gradient fields, it generates dynamically controllable adhesion and detachment within the GI tract. Furthermore, Figure 4C illustrates an untethered magnetic robot modeled after the overlapping scales of a pangolin. This robot combines a flexible polymer matrix with metallic scales[138]. Under radiofrequency fields, it attains localized heating above 70 ℃, demonstrating potential for noninvasive hyperthermia within the GI tract. A microneedle robot inspired by the pufferfish’s spiny organs expands to a diameter of 14 millimeters by absorbing intestinal fluids, forming a spiked vesicle[139]. This soft robot structure can withstand and harness radial peristaltic pressure to penetrate the mucosal tissue with barbed drug-loaded microneedles. A stiffness-tunable velvet worm-inspired soft robot employs magnetorheological materials to replicate the worm’s gland-based self-thickening secretion[140], achieving controllable adhesion. Finally, a climbing plant-inspired actuator leverages the coiling and twisting mechanisms of plant tendrils to realize multimodal deformation[141]. This actuator has been employed to fabricate a biomimetic quadruped robot capable of executing inchworm-like crawling locomotion [Figure 4D]. To sum up, bionic structural design significantly improves the locomotion efficiency and adhesion capability of GI soft robots while providing versatile strategies for actuation control and physiological task execution within the complex GI environment.
Figure 4. Structural designs to optimize the locomotion, environmental adaptability, and operational capabilities of GI soft robots. (A) Bio-inspired soft carrier with a starfish-mimetic structure, featuring a central disc and radiating arms. Reprinted with permission from[67]. Copyright 2023, Wiley‐VCH; (B) OISR’s biomimetic suction-cup architecture stores drug payloads and employs magnetic-gradient adhesion (i) and multimodal rolling motions for controlled gastric release (ii). Reprinted with permission from[137]. Copyright 2024, MDPI; (C) Pangolin-inspired robot features overlapping metallic scale architecture that enables magnetic field-controlled locomotion while providing Joule heating capabilities exceeding 70 ℃ for therapeutic applications. Reprinted with permission from[138]. Copyright 2023, Springer Nature; (D) Climbing plant-inspired smart tendrils demonstrating flower wrapping (i), alongside a schematic of an inchworm-inspired robot designed for locomotion on flat surfaces (ii). Reprinted with permission from[141]. Copyright 2024, Wiley‐VCH. GI: Gastrointestinal.
ACTUATION METHODS
Actuation methods are the core technology to GI soft robots, governing locomotion performance and directly affecting control precision, response speed, energy efficiency, and operational safety. The unique GI environment precludes direct use of conventional motor-driven approaches, necessitating the development of innovative actuation technologies tailored to these biological conditions.
Field-driven actuation
Field-driven actuation enables wireless control of soft robots via external physical fields, and is considered as the most promising actuation approach for GI applications. Remote magnetic actuation offers strong tissue penetration and precise control capabilities. For example, a tri-reconfigurable magnetic robot achieves accurate navigation under a 7.42 mT magnetic field by embedding Fe3O4 magnetic nanoparticles[142]. It reaches a maximum rolling speed of 7.23 mm·s-1 with a positioning error as low as
Figure 5. Field-driven and fluid-driven technologies for GI soft robots. (A) Near-infrared laser irradiation induces photothermal transformation of SMP PatchBots, with peak surface temperatures captured by NIR imaging. Reprinted with permission from[146]. Copyright 2025, IOP Publishing Ltd; (B) Multimode control via ultrasound and external magnetic fields enables on-demand switching between distinct locomotion behaviors. Reprinted with permission from[135]. Copyright 2024, Wiley‐VCH; (C) Hybrid electrothermal-magnetic actuation with in situ reprogrammable magnetic driving layer and conductive heating achieves versatile gastroscopic robot motions and pose sensing. Reprinted with permission from[148]. Copyright 2023, Science and Technology Review Publishing House; (D) Pneumatic FOD design integrates FA, CA, and rigid actuator modules (RA-IM, RA-EM) for complex bending and extension. Reprinted with permission from[155]. Copyright 2023, IEEE; (E) Liquid-gas phase-change STPAM exhibits reversible inflation and deflation under thermal cycling, as shown in thermal and optical images. Reprinted with permission from[161]. Copyright 2022, Elsevier B.V. GI: Gastrointestinal; FA: fluidic actuator; CA: compliant actuator.
Fluid-driven actuation
Fluid-driven actuation generates driving force for soft robots by compressing fluids, offering high actuation power and smooth response characteristics. Pneumatic actuation is the most classical form of fluid driving and achieves deformation of soft structures through controlled air inflation and deflation. For instance, a flexible over-the-tube device incorporates three pneumatically driven actuators [Figure 5D][155]. By precisely regulating air pressure, it enables gripping, advancing, and anchoring of the soft tether within a colonoscope. Melancon et al. integrated bistable origami modules into pneumatic actuators to develop modular origami structures with varying stability[156], capable of locomotion through multimodal deformation. Hydraulic actuation leverages the incompressibility of liquids to transmit pressure and achieve precise control. Zhu et al. developed a hydraulically driven, motorless soft robot with a three-degree-of-freedom omnidirectional manipulator that supports lesion lifting, precise cutting, and en bloc foreign body removal[157]. A handheld hydraulic soft robot uses a closed hydraulic system combined with an inner hydraulic filament artificial muscle to achieve bidirectional bending angles up to 180°[158], with an average response time of approximately 0.0482 s. Fluidic actuation achieves robust and flexible motion by regulating internal fluid pressure to mimic biological peristalsis or bending movements[159]. It is well-suited for applications requiring high environmental adaptability, excellent flexibility, and safe contact with the GI wall. Its advantages include a strong driving force, simple structure, and high compatibility with soft tissues. However, it often relies on external air or fluid supplies, which limits the miniaturization of the robot and its ability to operate untethered[160]. To address the bulkiness of traditional pneumatic systems, researchers have developed a pumpless actuation technology based on liquid-gas phase change [Figure 5E][161]. A bidirectional thermoelectric-driven system controls heating and cooling of phase-change materials to inflate and deflate pneumatic chambers. Moreover, Beatty et al. introduced the FibroSensing Dynamic Soft Reservoir (FSDSR)[162], where pressure applied to an external reservoir compresses an inner therapeutic reservoir, enabling active drug release and delivery. Therefore, pumpless fluid-driven soft robots offer a novel actuation paradigm for GI soft robotics, combining rapid response and precise control. This integration provides robust power support for complex medical interventions. In conclusion, the development trend of fluid-driven systems lies in overcoming dependence on external supplies and advancing toward miniaturization and wireless operation, thereby enabling greater portability and autonomy.
Chemical autonomous actuation
Chemical autonomous actuation converts chemical energy directly into self-propulsion, eliminating the need for external power sources, and making it particularly ideal for in vivo applications. Bubble propulsion, driven by gases from chemical reactions, is a common strategy. For example, a rocket-inspired effervescent micromotor rapidly produces CO2 bubbles through the acid-base neutralization reaction between sodium bicarbonate and citric acid [Figure 6A][163]. The resultant thrust propels the micromotor at an average speed reaching 750 µm·s-1, offering a novel strategy for oral delivery of macromolecular drugs such as insulin. Micromotors designed for GI drug delivery typically feature a core-shell architecture consisting of a reactive metallic core and an inert shell[164]. In the dual-responsive micromotor pill[165], magnesium particles react with intestinal fluid to generate hydrogen bubbles, achieving a chemical propulsion speed of 41.6 µm·s-1. Magnesium-based propulsion provides sufficient thrust while producing biocompatible reaction byproducts. Self-diffusiophoretic propulsion harnesses concentration and electrochemical gradients to achieve directed migration of molecules or ions. A dual-pH-responsive hydrogel actuator utilizes differential swelling of polyacrylamide (PAAm) and PAA to produce bidirectional bending with response times between 5 and 20 min[166], offering an effective trigger mechanism for lipophilic drug delivery in GI pH environments. A Janus double-chamber capsule generates internal pressure through pH-responsive swelling of PAA polymers, enabling reactive pumping-controlled release via microchannels[63]. Catalytic reaction-based actuation employs enzyme catalysis to convert biochemical energy. For instance, NO delivery microcapsules leverage reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS) at inflammatory sites to catalyze L-arginine into NO[167], achieving combined antibacterial and anti-inflammatory effects [Figure 6B]. The selectivity of catalytic reactions enables activation of the robot in specific physiological or pathological environments, improving therapeutic precision. Chemical autonomous actuation relies on chemical reactants stored within soft robots to generate propulsion through chemical reactions, enabling self-powered motion[168]. This actuation method is well-suited for fully autonomous microrobots that operate without external fields or equipment, with applications including localized in vivo cruising and positioning. Its advantages include high autonomy and miniaturization, although it is limited by low thrust and challenges in controlling the direction and speed of movement[169]. Future advancements in chemical actuation aim to enhance reaction efficiency, regulate reaction kinetics, and optimize biocompatibility, thereby providing a technological foundation for fully autonomous biomedical robots.
Figure 6. Chemical propulsion and other actuation technologies for GI soft robots. (A) Effervescent chemical reaction in rocket-like micromotors generates gas bubbles for self-propelled oral drug delivery in vivo. Reprinted with permission from[163]. Copyright 2023, Wiley‐VCH; (B) Catalytic degradation of P@G@NG releases NO in a multistage sequence along the GI tract to target colitis and modulate intestinal microbiota. Reprinted with permission from[167]. Copyright 2024, Wiley‐VCH; (C) Water-absorption-driven barbed microneedles swell and retain in tissue under peristaltic forces, with layered barb geometry optimizing retention strength. Reprinted with permission from[139]. Copyright 2024, American Association for the Advancement of Science; (D) Biohybrid algae-macrophage nanoparticle robots combine self-propulsion and immune cell targeting to treat inflammatory colitis, modified from (Modified from[174]). Created with BioRender.com. GI: Gastrointestinal.
Other actuation methods
Beyond the primary actuation techniques, the field of GI soft robotics also explores moisture-triggered and emerging biohybrid drive systems. Moisture-responsive soft robots absorb fluids within the GI tract and exhibit significant physical property changes. As illustrated in Figure 6C, a superabsorbent hydrogel-based robot swells to a diameter of 14 mm upon exposure to intestinal fluid[139]. This expansion, combined with intestinal peristaltic pressure, enables barbed microneedles to penetrate the mucosa, facilitating painless oral delivery of insulin. Wei et al. designed a composite film actuator[170]. The strong humidity sensitivity of cellulose nanofibers (CNF) and graphene oxide (GO), coupled with the rapid desorption properties of hydrophobic CNTs and the porous 3D nanochannels, results in fast and reliable moisture responsiveness. Under a relative humidity of 91%, another composite actuator produces bending angles up to 190 degrees[171]. Relying solely on moisture-driven actuation within the GI tract is limited by insufficient driving force and poor environmental adaptability. Therefore, integration with other responsive mechanisms, such as magnetic and chemical stimuli, is necessary to enhance locomotion performance. Biohybrid actuation combines the autonomous motility of living cells with the controllability of artificial materials[172], improving biocompatibility and biodegradability of soft medical robots. Magnetic particle-immobilized soft microrobots (MSCSMs) exemplify such biohybrid systems[173]. Mesenchymal stem cells provide exceptional tissue-like softness and biocompatibility, enabling adaptive in vivo morphology changes through reconfigurable transformations. By integrating the robust motility of green algae with the binding affinity of macrophage membrane-coated nanoparticles, Li et al. developed a biohybrid microrobot capable of capturing and removing multiple proinflammatory cytokines in vitro [Figure 6D][174]. Although these novel actuation technologies remain in exploratory stages, they open new avenues for diversifying the capabilities of soft robotic systems.
FUNCTIONS AND APPLICATIONS
The functionalities and applications of GI soft robots represent the ultimate manifestation of their technological value, encompassing multiple levels ranging from fundamental navigation and localization to complex minimally invasive procedures. Based on clinical requirements and technological implementations, their primary functions can be categorized into four major areas: navigation and localization, drug delivery and controlled release, in vivo information acquisition, and minimally invasive manipulation and therapy. These functional modules can operate independently or synergistically through system integration, thereby providing comprehensive technical support for the diagnosis, treatment, and monitoring of GI diseases.
Navigation and localization
Significant advancements have been made in the precise intraluminal locomotion and stable positioning of GI soft robots. Magnetic actuation navigation systems enable free movement within 3D space through precise control of external magnetic fields. As shown in Figure 7A, a magnetic multilayer soft robot employs interlayer magnetic interactions to achieve on-demand detachment and simultaneous multi-target therapy[52]. When the external magnetic field strength exceeds 15 mT, precise motion control is possible, allowing flexible navigation on the gastric tissue surface alongside prolonged adhesion. The shape-morphing magnetic patch robot (PatchBot) achieves complex morphological transformations via multilayer architecture and near-infrared laser actuation[146], exhibiting high maneuverability and selective adhesion capabilities. Adaptation to the mucus-covered environment remains a critical challenge for GI navigation. A low-friction soft robot driven by AMF demonstrates efficient crawling on mucus-coated soft tissue surfaces[53]. Its hydrophobic coating increases the surface contact angle beyond 120°, effectively minimizing mucus adhesion resistance. The development of real-time localization technologies provides robust support for precise navigation. Figure 7B illustrates a radio frequency identification (RFID)-based positioning system that utilizes received signal strength indication (RSSI) to achieve centimeter-level spatial resolution with a maximum detection depth of 15 cm[82]. This system offers effective control of robots in visually occluded environments. A magnetically-actuated ultrasound capsule integrates magnetic actuation with ultrasound imaging[175], allowing real-time in vivo tracking and delivering visual feedback for complex navigation tasks. A hydrogel-coated containment capsule of magnetic LM not only enables remote wireless magnetic hyperthermia therapy but also exhibits excellent radiopacity under X-ray imaging due to the high density of LM[123], facilitating accurate in vivo localization and tracking. Localization techniques frequently combine medical imaging modalities, such as ultrasound and X-ray, with visual feedback to enhance navigation accuracy and safety[176]. GI soft robots predominantly rely on external magnetic fields as wireless actuation sources. Combined with multimodal locomotion strategies, this approach effectively overcomes challenges imposed by GI tract peristalsis, mucus layers, and complex anatomical geometries[177-179]. Nevertheless, the effective range of magnetic actuation remains limited, and reliance on large magnetic field generation equipment is significant. Further efforts are required to improve system portability and clinical applicability.
Figure 7. GI soft robots for precise navigation, localization, and targeted drug delivery within the digestive tract. (A) Magnetic multilayer soft robot achieves on-demand targeted drug delivery and adhesion to gastric ulcers through programmable flipping and translational motions under ultrasound guidance. Reprinted with permission from[52]. Copyright 2024, Springer Nature; (B) RFID-equipped millipede-inspired soft robot enables precise magnetic localization and controlled intragastric navigation for site-specific drug delivery throughout the digestive tract. Reprinted with permission from[82]. Copyright 2023, Elsevier Inc.; (C) A magnetic living hydrogel designed for targeted GI localization and retention via magnetic navigation. Reprinted with permission from[181]. Copyright 2021, Wiley‐VCH; (D) Programmable soft robot dispenses four distinct drugs with reprogrammable sequences and dosages at multiple targeted regions using magnetic two-anchor crawling locomotion. Reprinted with permission from[182]. Copyright 2024, Wiley‐VCH. GI: Gastrointestinal; RFID: radio frequency identification.
Drug delivery and controlled release
Drug delivery and therapeutic functions represent some of the most clinically valuable applications of GI soft robots. These systems significantly enhance the precision and efficacy of pharmacological treatments through targeted delivery, controlled release technologies, and protection of macromolecular drugs. The pH gradient within the GI environment provides a natural trigger mechanism for intelligent drug delivery systems. For instance, a double-layered polysaccharide hydrogel (DPH) leverages pH differences to achieve gut-specific release of probiotics[128]. The probiotics are protected by the closed cage structure of the DPH in the acidic gastric environment and are subsequently released at a rate of 90% within 6 h upon exposure to intestinal fluids. The probiotic count is increased by 239.5-fold compared to controls. A rocket-inspired effervescent micromotor uses chemically generated bubbles to propel and efficiently deliver macromolecular drugs such as insulin to the intestine[163]. Localized treatment is enabled by precise robot positioning and prolonged retention, facilitating high-concentration targeted drug administration. Nevertheless, the GI mucus layer and epithelial barrier impose limitations on drug penetration depth, local bioavailability, and retention time at target sites, thereby reducing therapeutic efficacy. To overcome these obstacles, several strategies have been proposed, including permeation enhancers to improve mucosal penetration, microneedles or mechanical anchors to enhance local retention, magnetic targeting for prolonged localized release, and modulation of release kinetics to align with the in vivo residence time of the robotic device, thereby optimizing therapeutic outcomes. Wu et al. developed insulin-loaded, milk-derived exosomes (EXO@INS), enriched with negatively charged phospholipids and hydrophilic proteins[180]. Their exosomal membranes serve as natural permeation enhancers, markedly improving traversal of the mucus barrier and demonstrating substantial potential for insulin delivery. A swellable microneedle robot enhances mucosal adhesion through peristaltic relaxation and barbed[139], hook-like features, thereby overcoming gastric acid and enzymatic barriers to facilitate trans-mucosal penetration and sustained drug release within the intestine. As shown in Figure 7C, magnetically responsive hydrogels demonstrate site-specific retention in the murine intestine for up to seven days through attraction by external magnets[181]. The integrated genetically engineered bacteria can detect biomarkers such as heme, thus enabling combined diagnostic and therapeutic functions. Tough in situ-forming gastric double-network hydrogels finely modulate the release rate by adjusting parameters such as network density and geometric dimensions[57]. Using this hydrogel as a carrier, lumefantrine exhibits an area under the curve (AUC) comparable to the free drug, delivering equivalent total drug dosage at lower plasma concentrations and potentially mitigating drug toxicity. As depicted in Figure 7D, a multimodal reconfigurable capsule robot utilizes modular design to fulfill multi-site drug delivery requirements[182], providing selective release of multiple therapeutics and sustained treatment effects. To conclude, soft robotic drug delivery systems have shown promising therapeutic potential in clinical models, including diabetes[124], inflammatory bowel disease, intestinal microbiota modulation[167], and colorectal cancer[183]. However, systematic optimization is still required to address limitations related to drug-loading capacity, controlled dosage release, and long-term stability.
In vivo information acquisition
GI soft robots integrate diverse in vivo information collection capabilities, including sample acquisition, biochemical sensing, and physiological monitoring. For example, Sun et al. developed a multimodal responsive magnetically actuated capsule featuring a unique dual-layer magnetic soft valve structure
Figure 8. GI soft robots for in vivo information acquisition and minimally invasive therapies. (A) Magnetically actuated capsule robot performs targeted tissue sampling through four-stage operation: swinging, sliding, rolling, and high-frequency magnetic field-triggered drug release. Reprinted with permission from[134]. Copyright 2024, Springer Nature; (B) Triboelectric E-armor system integrates multimodal tactile sensing for real-time detection and acquisition of colonoscopic examination data. Reprinted with permission from[188]. Copyright 2025, Wiley‐VCH; (C) Hydraulic-driven soft endoscopic system features 3-DOF cutting arm and teleoperated grasper for minimally invasive colon lesion manipulation and surgical intervention. Reprinted with permission from[157]. Copyright 2025, Beijing Institute of Technology Press; (D) Ingestible robotic interface delivers programmable electrical stimulation via near-field inductive coupling, adhering to gastric mucosa for chronic neuromodulation therapy. Reprinted with permission from[196]. Copyright 2024, Springer Nature. GI: Gastrointestinal.
Minimally invasive manipulation and therapy
Minimally invasive procedures represent a crucial direction in the development of GI soft robot technology. These encompass biopsy, suturing, cutting, mucosal repair, foreign body removal, and functional actuation. A soft inflatable cable-driven parallel robot provides five degrees of freedom in spatial motion and variable stiffness control through pneumatic inflation and cable actuation[194]. This system enables suturing tasks during GI endoscopic surgery. As shown in Figure 8C, Zhu et al. developed a biomimetic grasper-electrocautery hybrid system actuated by magnetic and hydraulic dual soft drives[157]. Remote operation is achieved by pressure transmission, allowing submillimeter precision grasping and cauterizing within the colon. Nguyen et al. designed a motor-free soft robot that achieves positional errors below 300 µm[195]. By combining hydraulic actuation with microelectrodes and optical fibers, the robot can perform electrical impedance-based cancer lesion detection and radio-frequency ablation. It also supports in situ 3D printing for internal wound healing. These systems emphasize compliance and precise control in both structural design and actuation methods, significantly reducing the risk of tissue damage while improving surgical safety and accuracy. Beyond assisting surgical maneuvers, soft robots are also applied in the treatment of GI disorders. Nan et al. developed an ingestible, battery-free robotic interface driven by near-field inductive coupling technology [Figure 8D][196]. This device delivers noninvasive GI electrical stimulation, effectively increasing ghrelin levels. Wu et al. reported a biodegradable, self-powered electronic bandage fabricated from soft materials that accelerates intestinal wound healing[197]. Addressing postoperative ileus,
CONCLUSION AND OUTLOOK
This review primarily elucidates the current technical characteristics and application scenarios of GI soft robots from materials, structures, actuations, and functional applications. Firstly, we summarize materials applied in GI soft robots, where biocompatible soft elastomers serve as the foundation, functional materials impart intelligent responsiveness, and the combination of various materials is increasingly adopted. Secondly, we focus on structural engineering, outlining design strategies from continuous encapsulation to modular-reconfigurable structures, and exploring the integration of existing robotic functional units with biomimetic structures. Furthermore, we systematically categorize major actuation manners of field-driven, fluid-driven, and self-driven chemical reactions, highlighting the diverse evolution of actuation mechanisms for the complex GI environment. Finally, we extensively explore the applications of GI soft robots across multiple levels, spanning navigation, localization, and minimally invasive interventions.
However, GI soft robots still face a range of challenges. Firstly, precise navigation and targeting remain limitations due to the complex intestinal environment, where robots struggle to reach specific sites and oral delivery faces premature degradation in gastric acid and poor targeting in the intestine. Meanwhile, limited battery life remains a major challenge for GI soft robots, as current devices typically have low-capacity power sources that restrict operating time and overall performance. Moreover, environmental adaptability is also critical, as GI soft robots must navigate the uneven GI surface. Current technologies, however, lack precise positional control, fail to conform to lesion sites, and leave certain regions inaccessible, creating diagnostic and therapeutic blind spots. Besides, current soft robots face issues in precision, flexibility, and accessibility within confined GI spaces, while these robots must operate safely in vivo and be excreted or degraded, imposing strict demands on material selection and design.
GI soft robotics technology is entering a new era characterized by noninvasiveness, targeted functionality, and intelligent capabilities. In response to the associated challenges, feasible solutions and a clear development roadmap are proposed. In the short term, over the next 12 to 18 months, four major technological breakthroughs can address key current bottlenecks. Firstly, in navigation, localization, and drug delivery, integrated magnetic-RFID-ultrasound tracking technologies can achieve sub-centimeter spatial accuracy, while enteric coatings or pH-responsive polymer encapsulation can overcome premature degradation in the gastric environment and improve intestinal targeting in oral delivery. Secondly, regarding power and endurance, incorporating wireless power transfer and energy harvesting modules or employing battery-free triggering mechanisms can significantly extend operational time and enhance overall performance. Thirdly, to improve environmental adaptability, low-friction coatings, variable-stiffness materials, and shape-memory structures can be applied to robot surfaces to achieve effective attachment and detachment control on the uneven GI mucosa. Lastly, for operational safety, controllable biodegradable or excretable materials should be selected, guided by comprehensive toxicological evaluation systems to minimize in vivo residual risks.
In the long term, GI soft robots must meet critical requirements for clinical translation. First, they must demonstrate in vivo biocompatibility and safety by utilizing biocompatible materials to prevent toxic reactions and immune rejection, while ensuring safety for long-term implantation or in vivo operation. Second, comprehensive large animal safety and efficacy evaluations conforming to Good Laboratory Practice (GLP) standards are required to reliably validate performance and comply with regulatory requirements. Third, the robots must ensure compatibility with clinical imaging modalities such as X-ray, CT, and MRI to avoid significant interference and imaging artifacts. Furthermore, safety thresholds for localized tissue heating and electromagnetic stimulation should be established to protect surrounding tissues from damage.
By fulfilling these preclinical prerequisites, GI soft robots are expected to evolve from simple drug carriers into highly integrated diagnostic and therapeutic platforms, driving precision medicine toward minimally invasive, intelligent, and personalized approaches. Ultimately, they will become a new generation of noninvasive, targeted, and smart systems for the diagnosis and treatment of GI diseases. In terms of diagnosis, they are suitable for home-based assessment of acute symptoms, integrating big data and hospital networks to enable coordinated management. For therapy, these robots can achieve precise drug release and long-term sustained delivery, significantly enhancing drug utilization and enabling home treatment of chronic diseases. In summary, GI soft robots are reshaping digestive healthcare by surpassing the spatial and functional limits of conventional medical tools. Their development paves the way for next-generation systems towards minimally invasive, site-specific, and intelligent diagnosis and therapy of GI diseases.
DECLARATIONS
Authors’ contributions
Designed and organized the project: Du, M.; Xie, Z.; Wang, X.
Manuscript writing: Du, M.
Manuscript supervision: Xie, Z.; Wang, X.
All authors have given approval to the final version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (No. 52406062), and the Fundamental Research Funds for the Central Universities (JKF-20240778).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Aubin, C. A.; Gorissen, B.; Milana, E.; et al. Towards enduring autonomous robots via embodied energy. Nature 2022, 602, 393-402.
2. Wehner, M.; Truby, R. L.; Fitzgerald, D. J.; et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 2016, 536, 451-5.
3. Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143-53.
4. Li, G.; Chen, X.; Zhou, F.; et al. Self-powered soft robot in the Mariana Trench. Nature 2021, 591, 66-71.
5. Xie, Z.; Yuan, F.; Liu, J.; et al. Octopus-inspired sensorized soft arm for environmental interaction. Sci. Robot. 2023, 8, eadh7852.
6. Hu, W.; Lum, G. Z.; Mastrangeli, M.; Sitti, M. Small-scale soft-bodied robot with multimodal locomotion. Nature 2018, 554, 81-5.
8. Yoon, J. E.; Chung, J.; Park, S.; et al. Evaluation of gait-assistive soft wearable robot designs for wear comfort, focusing on electroencephalogram and satisfaction. IEEE. Robot. Autom. Lett. 2024, 9, 8834-41.
9. Kim, Y.; Parada, G. A.; Liu, S.; Zhao, X. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329.
10. Piskarev, Y.; Sun, Y.; Righi, M.; et al. Fast-response variable-stiffness magnetic catheters for minimally invasive surgery. Adv. Sci. 2024, 11, e2305537.
11. Davy, J.; Dean, T. P.; Greenidge, N. J.; et al. Magnetic fluid-driven vine robots for minimally invasive tissue biopsy sampling. Adv. Intell. Syst. 2025, 7, 2400827.
12. Proietti, T.; O’Neill, C.; Gerez, L.; et al. Restoring arm function with a soft robotic wearable for individuals with amyotrophic lateral sclerosis. Sci. Transl. Med. 2023, 15, eadd1504.
13. Gu, G.; Zhang, N.; Xu, H.; et al. A soft neuroprosthetic hand providing simultaneous myoelectric control and tactile feedback. Nat. Biomed. Eng. 2023, 7, 589-98.
15. Song, X.; Sun, R.; Wang, R.; et al. Puffball-inspired microrobotic systems with robust payload, strong protection, and targeted locomotion for on-demand drug delivery. Adv. Mater. 2022, 34, e2204791.
16. Li, Y.; Dong, D.; Qu, Y.; et al. A multidrug delivery microrobot for the synergistic treatment of cancer. Small 2023, 19, e2301889.
17. Pang, Y.; Xu, X.; Chen, S.; et al. Skin-inspired textile-based tactile sensors enable multifunctional sensing of wearables and soft robots. Nano. Energy. 2022, 96, 107137.
18. Ashok, A.; Nguyen, T. K.; Barton, M.; et al. Flexible nanoarchitectonics for biosensing and physiological monitoring applications. Small 2023, 19, e2204946.
19. Lee, S. H.; Kim, Y. S.; Yeo, M. K.; et al. Fully portable continuous real-time auscultation with a soft wearable stethoscope designed for automated disease diagnosis. Sci. Adv. 2022, 8, eabo5867.
20. Zhou, W.; Xiong, P.; Ge, Y.; et al. Amoeba-inspired soft robot for integrated tumor/infection therapy and painless postoperative drainage. Adv. Sci. 2024, 11, e2407148.
21. Soto, F.; Purcell, E.; Ozen, M. O.; et al. Robotic pill for biomarker and fluid sampling in the gastrointestinal tract. Adv. Intell. Syst. 2022, 4, 2200030.
22. Nejati, S.; Wang, J.; Sedaghat, S.; et al. Smart capsule for targeted proximal colon microbiome sampling. Acta. Biomater. 2022, 154, 83-96.
23. Del-rio-ruiz, R.; Romualdo, da. Silva. D. R.; Suresh, H.; et al. Soft autonomous ingestible device for sampling the small-intestinal microbiome. Device 2024, 2, 100406.
24. Mimee, M.; Nadeau, P.; Hayward, A.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018, 360, 915-8.
25. Steiger, C.; Abramson, A.; Nadeau, P.; Chandrakasan, A. P.; Langer, R.; Traverso, G. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 2019, 4, 83-98.
26. Yogapriya, J.; Chandran, V.; Sumithra, M. G.; Anitha, P.; Jenopaul, P.; Suresh, Gnana. Dhas. C. Gastrointestinal tract disease classification from wireless endoscopy images using pretrained deep learning model. Comput. Math. Methods. Med. 2021, 2021, 5940433.
27. Manfredi, L.; Capoccia, E.; Ciuti, G.; Cuschieri, A. A soft pneumatic inchworm double balloon (SPID) for colonoscopy. Sci. Rep. 2019, 9, 11109.
28. Zhang, L.; Ren, L.; Li, S.; et al. A water strider-inspired intestinal stent actuator for controllable adhesion and unidirectional biofluid picking. Mater. Today. Bio. 2024, 28, 101216.
29. Del, Bono. V.; McCandless, M.; Gerald, A.; et al. A soft robotic “Add-on” for colonoscopy: increasing safety and comfort through force monitoring. Npj. Robot. 2025, 3, 15.
30. Weitschies, W.; Müller, L.; Grimm, M.; Koziolek, M. Ingestible devices for studying the gastrointestinal physiology and their application in oral biopharmaceutics. Adv. Drug. Deliv. Rev. 2021, 176, 113853.
31. Zhao, H.; Yu, B.; Yu, D.; et al. Electrochemical-genetic programming of protein-based magnetic soft robots for active drug delivery. Adv. Sci. 2025, 12, e2503404.
32. Zhou, Z.; Wang, L.; Yang, D.; et al. Acid-triggered charge-switchable antibacterial hydrogel for accelerated healing of gastric mucosal wounds. ACS. Nano. 2025, 19, 17533-53.
33. Abramson, A.; Dellal, D.; Kong, Y. L.; et al. Ingestible transiently anchoring electronics for microstimulation and conductive signaling. Sci. Adv. 2020, 6, eaaz0127.
34. Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver. Physiol. 2001, 280, G922-9.
35. Huizinga, J. D.; Lammers, W. J. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am. J. Physiol. Gastrointest. Liver. Physiol. 2009, 296, G1-8.
36. Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183-96.
37. Zhao, L.; Ren, Z.; Liu, X.; Ling, Q.; Li, Z.; Gu, H. A multifunctional, self-healing, self-adhesive, and conductive sodium alginate/poly(vinyl alcohol) composite hydrogel as a flexible strain sensor. ACS. Appl. Mater. Interfaces. 2021, 13, 11344-55.
38. Rodríguez-rodríguez, R.; Carreón-álvarez, C.; Cruz-medina, C. A.; et al. A review of pH-responsive chitosan-based hydrogels for drug delivery applications. Eur. Polym. J. 2025, 237, 114173.
41. Li, C.; Guo, C.; Fitzpatrick, V.; et al. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61-81.
42. Morsada, Z.; Hossain, M. M.; Islam, M. T.; Mobin, M. A.; Saha, S. Recent progress in biodegradable and bioresorbable materials: from passive implants to active electronics. Appl. Mater. Today. 2021, 25, 101257.
43. Li, R.; Wang, L.; Kong, D.; Yin, L. Recent progress on biodegradable materials and transient electronics. Bioact. Mater. 2018, 3, 322-33.
44. Singh, R.; Bathaei, M. J.; Istif, E.; Beker, L. A review of bioresorbable implantable medical devices: materials, fabrication, and implementation. Adv. Healthc. Mater. 2020, 9, e2000790.
45. Zhalmuratova, D.; Chung, H. Reinforced gels and elastomers for biomedical and soft robotics applications. ACS. Appl. Polym. Mater. 2020, 2, 1073-91.
46. Du, N.; Fan, Y.; Huang, H.; Guan, Y.; Nan, K. Stimuli-responsive hydrogel actuators for skin therapeutics and beyond. Soft. Sci. 2024, 4, 35.
47. Wang, K.; Jia, Y.; Zhao, C.; Zhu, X. Multiple and two-way reversible shape memory polymers: design strategies and applications. Prog. Mater. Sci. 2019, 105, 100572.
48. Chen, S.; Wang, H.; Zhao, R.; Rao, W.; Liu, J. Liquid metal composites. Matter 2020, 2, 1446-80.
49. Rus, D.; Tolley, M. T. Design, fabrication and control of soft robots. Nature 2015, 521, 467-75.
50. Lötters, J. C.; Olthuis, W.; Veltink, P. H.; Bergveld, P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J. Micromech. Microeng. 1997, 7, 145-7.
51. Li, J.; Yao, Z.; Meng, X.; et al. High-fidelity, low-hysteresis bionic flexible strain sensors for soft machines. ACS. Nano. 2024, 18, 2520-30.
52. Chen, Z.; Wang, Y.; Chen, H.; et al. A magnetic multi-layer soft robot for on-demand targeted adhesion. Nat. Commun. 2024, 15, 644.
53. Wang, B.; Chen, Y.; Ye, Z.; et al. Low-friction soft robots for targeted bacterial infection treatment in gastrointestinal tract. Cyborg. Bionic. Syst. 2024, 5, 0138.
54. Park, C. S.; Kang, Y.; Na, H.; Sun, J. Hydrogels for bioinspired soft robots. Prog. Polym. Sci. 2024, 150, 101791.
55. Kuang, X.; Arıcan, M. O.; Zhou, T.; Zhao, X.; Zhang, Y. S. Functional tough hydrogels: design, processing, and biomedical applications. Acc. Mater. Res. 2023, 4, 101-14.
56. Lu, Z.; Sheng, R.; Zhang, W.; Chen, J. Strong and tough hydrogels fabricated through molecular and structural engineering and their biomedical applications. Chem. Eng. J. 2025, 508, 160728.
57. Liu, G. W.; Pickett, M. J.; Kuosmanen, J. L. P.; et al. Drinkable in situ-forming tough hydrogels for gastrointestinal therapeutics. Nat. Mater. 2024, 23, 1292-9.
58. Yu, J.; Qin, Y.; Yang, Y.; et al. Robust hydrogel adhesives for emergency rescue and gastric perforation repair. Bioact. Mater. 2023, 19, 703-16.
59. Wang, H.; Li, T.; Li, J.; Zhao, R.; Ding, A.; Xu, F. Structural engineering of polyurethanes for biomedical applications. Prog. Polym. Sci. 2024, 151, 101803.
60. Ding, H.; Tan, P.; Fu, S.; et al. Preparation and application of pH-responsive drug delivery systems. J. Control. Release. 2022, 348, 206-38.
61. Gao, W.; Yu, X.; Zhang, C.; et al. Facile fabrications of poly (acrylic acid)-mesoporous zinc phosphate/polydopamine Janus nanoparticles as a biosafe photothermal therapy agent and a pH/NIR-responsive drug carrier. Acta. Biomater. 2024, 187, 328-39.
62. Huang, W.; Ying, R.; Wang, W.; et al. A macroporous hydrogel dressing with enhanced antibacterial and anti-inflammatory capabilities for accelerated wound healing. Adv. Funct. Mater. 2020, 30, 2000644.
63. Wen, S.; Ju, X.; Liu, W.; et al. Ca-alginate-based janus capsules with a pumping effect for intestinal-targeted controlled release. Engineering 2023, 24, 114-25.
64. Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702.
65. Ma, Y.; Lu, Y.; Yue, Y.; et al. Nanocellulose-mediated bilayer hydrogel actuators with thermo-responsive, shape memory and self-sensing performances. Carbohydr. Polym. 2024, 335, 122067.
66. Delaey, J.; Dubruel, P.; Van, Vlierberghe. S. Shape-memory polymers for biomedical applications. Adv. Funct. Mater. 2020, 30, 1909047.
67. Heunis, C. M.; Wang, Z.; de, Vente. G.; Misra, S.; Venkiteswaran, V. K. A magnetic bio-inspired soft carrier as a temperature-controlled gastrointestinal drug delivery system. Macromol. Biosci. 2023, 23, e2200559.
68. Peng, S.; Cao, X.; Sun, Y.; et al. Polyurethane shape memory polymer/ph-responsive hydrogel hybrid for bi-function synergistic actuations. Gels 2023, 9, 428.
69. Wang, S.; Wang, X.; Luo, Y.; Liang, Y. A comprehensive review of conventional and stimuli-responsive delivery systems for bioactive peptides: from food to biomedical applications. Adv. Compos. Hybrid. Mater. 2025, 8, 1053.
70. Choi, S. H.; Kim, J. H.; Ahn, J.; et al. Phase patterning of liquid crystal elastomers by laser-induced dynamic crosslinking. Nat. Mater. 2024, 23, 834-43.
71. Yao, Y.; He, E.; Xu, H.; et al. Enabling liquid crystal elastomers with tunable actuation temperature. Nat. Commun. 2023, 14, 3518.
72. Song, C.; Zhang, Y.; Bao, J.; et al. Light-responsive programmable shape-memory soft actuator based on liquid crystalline polymer/polyurethane network. Adv. Funct. Mater. 2023, 33, 2213771.
73. Zhao, R.; Kim, Y.; Chester, S. A.; Sharma, P.; Zhao, X. Mechanics of hard-magnetic soft materials. J. Mech. Phys. Solids. 2019, 124, 244-63.
75. Llacer-Wintle, J.; Rivas-Dapena, A.; Chen, X. Z.; et al. Biodegradable small-scale swimmers for biomedical applications. Adv. Mater. 2021, 33, e2102049.
76. Bai, S.; Hou, S.; Chen, T.; Ma, X.; Gao, C.; Wu, A. Magnetic nanoparticle-mediated hyperthermia: from heating mechanisms to cancer theranostics. TIMS 2024, 2, 100051.
77. Wang, Y.; Qin, W.; Yang, M.; et al. High linearity, low hysteresis Ti3C2Tx MXene/AgNW/liquid metal self-healing strain sensor modulated by dynamic disulfide and hydrogen bonds. Adv. Funct. Mater. 2023, 33, 2301587.
78. Yang, J.; Fan, Y.; Xiong, X.; et al. Highly conductive and adhesive wearable sensors based on PVA/PAM/SF/PEDOT:PSS double network hydrogels. Appl. Phys. A. 2024, 130, 7329.
79. Tian, Z.; Xue, J.; Xiao, X.; Du, C.; Han, Z.; Liu, Y. Untethered multifunctional biomimetic soft actuator with programmable shape deformation capabilities and localized maneuverability. Sens. Actuators. B. Chem. 2024, 410, 135678.
80. Zhang, L.; Xing, S.; Yin, H.; et al. Skin-inspired, sensory robots for electronic implants. Nat. Commun. 2024, 15, 4777.
81. Shen, Y.; Jin, D.; Fu, M.; et al. Reactive wetting enabled anchoring of non-wettable iron oxide in liquid metal for miniature soft robot. Nat. Commun. 2023, 14, 6276.
82. Ye, Z.; Zheng, L.; He, J.; et al. Liquid-metal soft electronics coupled with multi-legged robots for targeted delivery in the gastrointestinal tract. Device 2024, 2, 100181.
83. Zhang, L.; Chen, L.; Xu, L.; Zhao, H.; Wen, R.; Xia, F. Gastrointestinal-peristalsis-inspired hydrogel actuators for NIR-controlled transport of viscous liquids. Adv. Mater. 2023, 35, e2212149.
84. Sun, B.; Liu, J.; Li, S.; Lovell, J. F.; Zhang, Y. Imaging of gastrointestinal tract ailments. J. Imaging. 2023, 9, 115.
85. Song, E.; Huang, Y.; Huang, N.; Mei, Y.; Yu, X.; Rogers, J. A. Recent advances in microsystem approaches for mechanical characterization of soft biological tissues. Microsyst. Nanoeng. 2022, 8, 77.
86. Wang, Y.; Shen, J.; Handschuh-Wang, S.; Qiu, M.; Du, S.; Wang, B. Microrobots for targeted delivery and therapy in digestive system. ACS. Nano. 2023, 17, 27-50.
87. Kumar, P.; Fleischer, D. E. Thermal therapy for gastrointestinal bleeding. Gastrointest. Endosc. Clin. N. Am. 1997, 7, 593-609.
88. Lee, Y. Y.; Erdogan, A.; Rao, S. S. How to assess regional and whole gut transit time with wireless motility capsule. J. Neurogastroenterol. Motil. 2014, 20, 265-70.
89. Hines, L.; Petersen, K.; Lum, G. Z.; Sitti, M. Soft actuators for small-scale robotics. Adv. Mater. 2017, 29, 1603483.
90. Liao, X.; Zhu, Z.; Zhang, Y.; Xiang, Z.; Lin, S.; Shang, J. Synthesis and evaluation of biodegradable copolyimide elastomer with tunable mechanical and thermal properties. Mater. Today. Commun. 2022, 31, 103573.
91. Wrede, P.; Remlova, E.; Chen, Y.; Deán-ben, X. L.; Sitti, M.; Razansky, D. Synergistic integration of materials in medical microrobots for advanced imaging and actuation. Nat. Rev. Mater. 2025.
92. Liu, Q.; Lou, P.; Sun, Z.; et al. Bio-based elastomers: design, properties, and biomedical applications. Adv. Mater. 2025, 37, e2417193.
94. An, H.; Zhang, M.; Huang, Z.; et al. Hydrophobic cross-linked chains regulate high wet tissue adhesion hydrogel with toughness, anti-hydration for dynamic tissue repair. Adv. Mater. 2024, 36, e2310164.
95. Khan, M. T.; Rehman, T. U.; Shah, L. A.; Yoo, H. Development of acrylic acid-agar-based adhesive hydrogel: influence of tannic acid concentration on adhesion performance. Int. J. Adhes. Adhesives. 2025, 142, 104088.
96. Peng, W.; Lai, Y.; Jiang, Y.; et al. Charge balance transition enabled Janus hydrogel for robust wet-tissue adhesion and anti-postoperative adhesion. Bioact. Mater. 2025, 52, 123-38.
97. Mo, C.; Zhang, W.; Zhu, K.; et al. Advances in injectable hydrogels based on diverse gelation methods for biomedical imaging. Small. Methods. 2024, 8, e2400076.
98. Kong, Q.; Tan, Y.; Zhang, H.; et al. Mimosa-inspired body temperature-responsive shape memory polymer networks: high energy densities and multi-recyclability. Adv. Sci. 2024, 11, e2407596.
99. Li, D.; Sun, Y.; Li, X.; et al. 3D printing of near-ambient responsive liquid crystal elastomers with enhanced nematic order and pluralized transformation. ACS. Nano. 2025, 19, 7075-87.
100. Ye, Z.; Hou, P.; Zhang, L. Preparation and characterization of PLA/TPU/HA enhanced shape memory blends. Mater. Today. Commun. 2025, 47, 113023.
101. Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J. M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211-68.
102. Chen, S.; Xing, W.; Wang, H.; et al. A bottom-up approach to generate isotropic liquid metal network in polymer-enabled 3D thermal management. Chem. Eng. J. 2022, 439, 135674.
103. Chitambar, C. R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public. Health. 2010, 7, 2337-61.
104. Hargreaves, B. A.; Worters, P. W.; Pauly, K. B.; Pauly, J. M.; Koch, K. M.; Gold, G. E. Metal-induced artifacts in MRI. AJR. Am. J. Roentgenol. 2011, 197, 547-55.
105. Ali, A.; Zafar, H.; Zia, M.; et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49-67.
106. Ma, D.; Chen, J.; Luo, Y.; Wang, H.; Shi, X. Zwitterion-coated ultrasmall iron oxide nanoparticles for enhanced T1-weighted magnetic resonance imaging applications. J. Mater. Chem. B. 2017, 5, 7267-73.
107. Wang, X.; Fan, L.; Zhang, J.; et al. Printed conformable liquid metal e-skin-enabled spatiotemporally controlled bioelectromagnetics for wireless multisite tumor therapy. Adv. Funct. Mater. 2019, 29, 1907063.
108. Shellock, F. G. Radiofrequency energy-induced heating during mr procedures: a review. J. Magn. Reson. Imaging. 2000, 12, 30-6.
109. Shen, Z.; Wu, A.; Chen, X. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol. Pharm. 2017, 14, 1352-64.
110. Mhlanga, N.; Mphuthi, N.; Van, der. Walt. H.; Nyembe, S.; Mokhena, T.; Sikhwivhilu, L. Nanostructures and nanoparticles as medical diagnostic imaging contrast agents: a review. Mater. Today. Chem. 2024, 40, 102233.
111. D. Pitfalls. in. Musculoskeletal. Radiology. , Peh, W.C.G., Eds.; Cham, Springer International Publishing; 2017; pp 45-59.
112. Naha, P. C.; Hsu, J. C.; Kim, J.; et al. Dextran-coated cerium oxide nanoparticles: a computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. ACS. Nano. 2020, 14, 10187-97.
113. Akagi, M.; Nakamura, Y.; Higaki, T.; et al. Deep learning reconstruction improves image quality of abdominal ultra-high-resolution CT. Eur. Radiol. 2019, 29, 6163-71.
114. Zlitni, A.; Gambhir, S. S. Molecular imaging agents for ultrasound. Curr. Opin. Chem. Biol. 2018, 45, 113-20.
115. Mohammadlou B, Ippolito S, FitzPatrick J, Upadhyay P, Burnett TL, Gogotsi Y. Characterization of MXene-based materials by X-Ray computed tomography. Small. Methods. 2025, 9, e2500262.
116. Lin, J.; Chen, X.; Huang, P. Graphene-based nanomaterials for bioimaging. Adv. Drug. Deliv. Rev. 2016, 105, 242-54.
117. Shellock, F. G.; Crues, J. V. MR procedures: biologic effects, safety, and patient care. Radiology 2004, 232, 635-52.
118. Lee, B.; Lee, Y.; Lee, N.; Kim, D.; Hyeon, T. Design of oxide nanoparticles for biomedical applications. Nat. Rev. Mater. 2025, 10, 252-67.
119. Xiong, Z.; Feng, C.; Tang, J.; et al. Rationally constructing the theranostics hydrogels for targeted CT imaging and healing of inflammatory bowel disease. Chem. Eng. J. 2025, 513, 162986.
120. Sridharan, B.; Lim, H. G. Exosomes and ultrasound: the future of theranostic applications. Mater. Today. Bio. 2023, 19, 100556.
122. Beyer, T.; Bailey, D. L.; Birk, U. J.; et al. Medical physics and imaging-a timely perspective. Front. Phys. 2021, 9, 634693.
123. Shen, Y.; Cao, J.; Zhou, E.; et al. Tough hydrogel-coated containment capsule of magnetic liquid metal for remote gastrointestinal operation. Natl. Sci. Rev. 2025, 12, nwaf042.
124. Srinivasan, S. S.; Alshareef, A.; Hwang, A. V.; et al. RoboCap: robotic mucus-clearing capsule for enhanced drug delivery in the gastrointestinal tract. Sci. Robot. 2022, 7, eabp9066.
125. Consumi, V.; Lindenroth, L.; Merlin, J.; Stoyanov, D.; Stilli, A. Design and evaluation of the SoftSCREEN capsule for colonoscopy. IEEE. Robot. Autom. Lett. 2023, 8, 1659-66.
126. Hao, B.; Wang, X.; Dong, Y.; et al. Focused ultrasound enables selective actuation and Newton-level force output of untethered soft robots. Nat. Commun. 2024, 15, 5197.
127. Nan, M.; Go, G.; Song, H.; et al. Multistimulus-responsive miniature soft actuator with programmable shape-morphing design for biomimetic and biomedical applications. Adv. Funct. Mater. 2024, 34, 2401776.
128. Huang, W.; Wang, W.; Wang, W.; Hao, Y.; Xue, C.; Mao, X. A double-layer polysaccharide hydrogel (DPH) for the enhanced intestine-targeted oral delivery of probiotics. Engineering 2024, 34, 187-94.
129. Zhang, X.; Chen, G.; Fu, X.; Wang, Y.; Zhao, Y. Magneto-responsive microneedle robots for intestinal macromolecule delivery. Adv. Mater. 2021, 33, e2104932.
130. Levy, J. A.; Straker, M. A.; Stine, J. M.; Beardslee, L. A.; Ghodssi, R. Magnetically triggered ingestible capsule for localized microneedle drug delivery. Device 2024, 2, 100438.
131. Chen, W.; Wainer, J.; Ryoo, S. W.; et al. Dynamic omnidirectional adhesive microneedle system for oral macromolecular drug delivery. Sci. Adv. 2022, 8, eabk1792.
132. Ze, Q.; Wu, S.; Dai, J.; et al. Spinning-enabled wireless amphibious origami millirobot. Nat. Commun. 2022, 13, 3118.
133. Gu, H.; Möckli, M.; Ehmke, C.; et al. Self-folding soft-robotic chains with reconfigurable shapes and functionalities. Nat. Commun. 2023, 14, 1263.
134. Sun, Y.; Zhang, W.; Gu, J.; et al. Magnetically driven capsules with multimodal response and multifunctionality for biomedical applications. Nat. Commun. 2024, 15, 1839.
135. Li, Q.; Niu, F.; Yang, H.; et al. Magnetically actuated soft microrobot with environmental adaptative multimodal locomotion towards targeted delivery. Adv. Sci. 2024, 11, e2406600.
136. Abramson, A.; Caffarel-Salvador, E.; Khang, M.; et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363, 611-5.
137. Tong, D.; Zhao, Y.; Wu, Z.; et al. Octopus-inspired soft robot for slow drug release. Biomimetics 2024, 9, 340.
138. Soon, R. H.; Yin, Z.; Dogan, M. A.; et al. Pangolin-inspired untethered magnetic robot for on-demand biomedical heating applications. Nat. Commun. 2023, 14, 3320.
139. Gao, X.; Li, J.; Li, J.; Zhang, M.; Xu, J. Pain-free oral delivery of biologic drugs using intestinal peristalsis-actuated microneedle robots. Sci. Adv. 2024, 10, eadj7067.
140. Min, H.; Bae, D.; Jang, S.; et al. Stiffness-tunable velvet worm-inspired soft adhesive robot. Sci. Adv. 2024, 10, eadp8260.
141. Li, W.; Lou, C.; Liu, S.; et al. Climbing plant-inspired multi-responsive biomimetic actuator with transitioning complex surfaces. Adv. Funct. Mater. 2025, 35, 2414733.
142. Chen, W.; Chen, X.; Yang, M.; et al. Triple-configurational magnetic robot for targeted drug delivery and sustained release. ACS. Appl. Mater. Interfaces. 2021, 13, 45315-24.
143. Lum, G. Z.; Ye, Z.; Dong, X.; et al. Shape-programmable magnetic soft matter. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E6007-15.
144. Liu, D.; Liu, X.; Chen, Z.; et al. Magnetically driven soft continuum microrobot for intravascular operations in microscale. Cyborg. Bionic. Syst. 2022, 2022, 9850832.
145. Liu, H.; Chu, H.; Yuan, H.; et al. Bioinspired multifunctional self-sensing actuated gradient hydrogel for soft-hard robot remote interaction. Nanomicro. Lett. 2024, 16, 69.
146. Wei, T.; Hu, Y.; Yang, M.; Shi, C.; Hu, C. A magnetic patch robot with photothermal-activated multi-modality for targeted anti-postoperative adhesion. Int. J. Extrem. Manuf. 2025, 7, 055502.
147. Ying, B.; Nan, K.; Zhu, Q.; et al. An electroadhesive hydrogel interface prolongs porcine gastrointestinal mucosal theranostics. Sci. Transl. Med. 2025, 17, eadq1975.
148. Zhang, L.; Zhao, S.; Zhou, X.; et al. A magnetic-driven multi-motion robot with position/orientation sensing capability. Research 2023, 6, 0177.
149. Chen, Z.; Chen, H.; Fang, K.; Liu, N.; Yu, J. Magneto-thermal hydrogel swarms for targeted lesion sealing. Adv. Healthc. Mater. 2025, 14, e2403076.
150. Li, W.; Sang, M.; Lou, C.; et al. Triple-responsive soft actuator with plastically retentive deformation and magnetically programmable recovery. ACS. Nano. 2023, 17, 24042-54.
151. Ebrahimi, N.; Bi, C.; Cappelleri, D. J.; et al. Magnetic actuation methods in bio/soft robotics. Adv. Funct. Mater. 2021, 31, 2005137.
152. Li, H.; Jiang, S.; Deng, Q.; et al. Programmable magnetic hydrogel robots with drug delivery and physiological sensing capabilities. Mater. Today. 2025, 87, 66-76.
153. Kaynak, M.; Dirix, P.; Sakar, M. S. Addressable acoustic actuation of 3D printed soft robotic microsystems. Adv. Sci. 2020, 7, 2001120.
154. Wu, S.; Lee, S. J.; Zhu, Y. Thermally actuated soft robotics. Adv. Mater. 2025, Epub ahead of print.
155. Huan, Y.; Ren, X.; Firrincieli, A.; et al. Flexible over-the-tube device for soft-tethered colonoscopy. IEEE/ASME. Trans. Mechatron. 2024, 29, 1611-21.
156. Melancon, D.; Forte, A. E.; Kamp, L. M.; Gorissen, B.; Bertoldi, K. Inflatable origami: multimodal deformation via multistability. Adv. Funct. Mater. 2022, 32, 2201891.
157. Zhu, K.; Nguyen, C. C.; Sharma, B.; et al. Development of a bioinspired soft robotic system for teleoperated endoscopic surgery. Cyborg. Bionic. Syst. 2025, 6, 0289.
158. Nguyen, C. C.; Teh, T.; Thai, M. T.; et al. A handheld hydraulic soft robotic device with bidirectional bending end-effector for minimally invasive surgery. IEEE. Trans. Med. Robot. Bionics. 2023, 5, 590-601.
159. Zhang, C.; Duan, Y.; Jiao, Z.; et al. Functional fluid-based soft robotic actuation. Adv. Mater. 2025, 37, e2502669.
160. Siéfert, E.; Reyssat, E.; Bico, J.; Roman, B. Bio-inspired pneumatic shape-morphing elastomers. Nat. Mater. 2019, 18, 24-8.
161. Yoon, Y.; Park, H.; Lee, J.; et al. Bioinspired untethered soft robot with pumpless phase change soft actuators by bidirectional thermoelectrics. Chem. Eng. J. 2023, 451, 138794.
162. Beatty, R.; Mendez, K. L.; Schreiber, L. H. J.; et al. Soft robot-mediated autonomous adaptation to fibrotic capsule formation for improved drug delivery. Sci. Robot. 2023, 8, eabq4821.
163. Cai, L.; Chen, G.; Sun, L.; et al. Rocket-inspired effervescent motors for oral macromolecule delivery. Adv. Mater. 2023, 35, e2210679.
164. Karshalev, E.; Zhang, Y.; Esteban-Fernández, de. Ávila. B.; et al. Micromotors for active delivery of minerals toward the treatment of iron deficiency anemia. Nano. Lett. 2019, 19, 7816-26.
165. An, Z.; Lin, E.; Wu, Z.; Kang, Y. Dual-responsive micromotor pill for targeted retention in the intestines in vivo. J. Mater. Chem. B. 2025, 13, 1296-301.
166. Han, Z.; Wang, P.; Mao, G.; et al. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS. Appl. Mater. Interfaces. 2020, 12, 12010-7.
167. Fu, Y. J.; Zhao, X.; Wang, L. Y.; et al. A gas therapy strategy for intestinal flora regulation and colitis treatment by nanogel-based multistage NO delivery microcapsules. Adv. Mater. 2024, 36, e2309972.
168. Wang, J.; Wu, S.; Zhang, W.; et al. Selective decorating Ag and MnOx nanoparticles on halloysite and used as micromotor for bacterial killing. Appl. Clay. Sci. 2022, 216, 106352.
169. Feng, Y.; An, M.; Liu, Y.; Sarwar, M. T.; Yang, H. Advances in chemically powered micro/nanorobots for biological applications: a review. Adv. Funct. Mater. 2023, 33, 2209883.
170. Wei, J.; Jia, S.; Guan, J.; Ma, C.; Shao, Z. Robust and highly sensitive cellulose nanofiber-based humidity actuators. ACS. Appl. Mater. Interfaces. 2021, 13, 54417-27.
171. Sun, L.; Che, L.; Li, M.; et al. Reinforced nacre-like MXene/sodium alginate composite films for bioinspired actuators driven by moisture and sunlight. Small 2024, 20, e2406832.
172. Chen, Z.; Chen, J.; Jung, S.; et al. Bioinspired and biohybrid soft robots: principles and emerging technologies. Matter 2025, 8, 102045.
173. Wang, B.; Chan, K. F.; Yuan, K.; et al. Endoscopy-assisted magnetic navigation of biohybrid soft microrobots with rapid endoluminal delivery and imaging. Sci. Robot. 2021, 6, eabd2813.
174. Li, Z.; Duan, Y.; Zhang, F.; et al. Biohybrid microrobots regulate colonic cytokines and the epithelium barrier in inflammatory bowel disease. Sci. Robot. 2024, 9, eadl2007.
175. Yang, Z.; Liu, L.; Li, Z.; Jiao, Y.; Zhang, L.; Cui, Y. A magnetically-actuated ultrasound capsule endoscope (MUSCE) for endoluminal imaging in tubular environments. IEEE. Robot. Autom. Lett. 2025, 10, 2590-7.
176. Xu, Z.; Wu, Z.; Yuan, M.; Chen, Y.; Ge, W.; Xu, Q. Versatile magnetic hydrogel soft capsule microrobots for targeted delivery. iScience 2023, 26, 106727.
177. Wang, C.; Mzyk, A.; Schirhagl, R.; Misra, S.; Venkiteswaran, V. K. Biocompatible film-coating of magnetic soft robots for mucoadhesive locomotion. Adv. Mater. Technol. 2023, 8, 2201813.
178. Liu, R.; Chen, Y.; Zhen, Y.; Zhang, J. A magnetic capsule robot with an exoskeleton to withstand esophageal pressure and delivery drug in stomach. IEEE. Robot. Autom. Lett. 2024, 9, 11802-9.
179. Hu, X.; Zhou, Y.; Li, M.; Wu, J.; He, G.; Jiao, N. Catheter-assisted bioinspired adhesive magnetic soft millirobot for drug delivery. Small 2024, 20, e2306510.
180. Wu, L.; Wang, L.; Liu, X.; et al. Milk-derived exosomes exhibit versatile effects for improved oral drug delivery. Acta. Pharm. Sin. B. 2022, 12, 2029-42.
181. Liu, X.; Yang, Y.; Inda, M. E.; et al. Magnetic living hydrogels for intestinal localization, retention, and diagnosis. Adv. Funct. Mater. 2021, 31, 2010918.
182. Yang, Z.; Xu, C.; Lee, J. X.; Lum, G. Z. Magnetic miniature soft robot with reprogrammable drug-dispensing functionalities: toward advanced targeted combination therapy. Adv. Mater. 2024, 36, e2408750.
183. Kumawat, A.; Karmakar, M.; Ghoroi, C. pH-responsive, reactive oxygen species scavenging and highly swellable nanogel for colon-targeted oral drug delivery. ACS. Appl. Nano. Mater. 2024, 7, 18964-78.
184. Xiao, B.; Xu, Y.; Edwards, S.; Balakumar, L.; Dong, X. Sensing mucus physiological property in situ by wireless millimeter-scale soft robots. Adv. Funct. Mater. 2024, 34, 2307751.
185. You, S. S.; Gierlach, A.; Schmidt, P.; et al. An ingestible device for gastric electrophysiology. Nat. Electron. 2024, 7, 497-508.
186. Wang, C.; Wu, Y.; Dong, X.; Armacki, M.; Sitti, M. In situ sensing physiological properties of biological tissues using wireless miniature soft robots. Sci. Adv. 2023, 9, eadg3988.
187. Inda-Webb, M. E.; Jimenez, M.; Liu, Q.; et al. Sub-1.4 cm3 capsule for detecting labile inflammatory biomarkers in situ. Nature 2023, 620, 386-92.
188. Sun, Y.; Chen, T.; Li, D.; et al. Stretchable, multiplexed, and bimodal sensing electronic armor for colonoscopic continuum robot enhanced by triboelectric artificial synapse. Adv. Mater. 2025, 37, e2502203.
189. Sahafi, A.; Wang, Y.; Rasmussen, C. L. M.; et al. Edge artificial intelligence wireless video capsule endoscopy. Sci. Rep. 2022, 12, 13723.
190. De, la. Paz. E.; Maganti, N. H.; Trifonov, A.; et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 2022, 13, 7405.
191. Li, Y.; Halwah, A.; Bhuiyan, S. R. A.; Yao, S. Bio-inspired untethered robot-sensor platform for minimally invasive biomedical sensing. ACS. Appl. Mater. Interfaces. 2023, 15, 58839-49.
192. Sharma, S.; Ramadi, K. B.; Poole, N. H.; et al. Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics. Nat. Electron. 2023, 6, 242-56.
193. Madhvapathy, S. R.; Bury, M. I.; Wang, L. W.; et al. Miniaturized implantable temperature sensors for the long-term monitoring of chronic intestinal inflammation. Nat. Biomed. Eng. 2024, 8, 1040-52.
194. Yang, J.; Zhou, Z.; Runciman, M.; Avery, J.; Sun, Z.; Mylonas, G. A soft inflatable cable-driven parallel robot with a variable stiffness end-effector for advanced interventional endoscopy. IEEE. Trans. Biomed. Eng. 2025, 72, 2794-803.
195. Nguyen, C. C.; Davies, J.; Ashok, A.; et al. Motor-free soft robots for cancer detection, surgery, and in situ bioprinting. Adv. Healthc. Mater. 2025, 14, e2404623.
196. Nan, K.; Wong, K.; Li, D.; et al. An ingestible, battery-free, tissue-adhering robotic interface for non-invasive and chronic electrostimulation of the gut. Nat. Commun. 2024, 15, 6749.
197. Wu, H.; Wang, Y.; Li, H.; et al. Accelerated intestinal wound healing via dual electrostimulation from a soft and biodegradable electronic bandage. Nat. Electron. 2024, 7, 299-312.
198. Srinivasan, S. S.; Dosso, J.; Huang, H. W.; et al. An ingestible self-propelling device for intestinal reanimation. Sci. Robot. 2024, 9, eadh8170.
199. Wang, Y.; Hu, X.; Cui, L.; et al. Bioinspired handheld time-share driven robot with expandable DoFs. Nat. Commun. 2024, 15, 768.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data






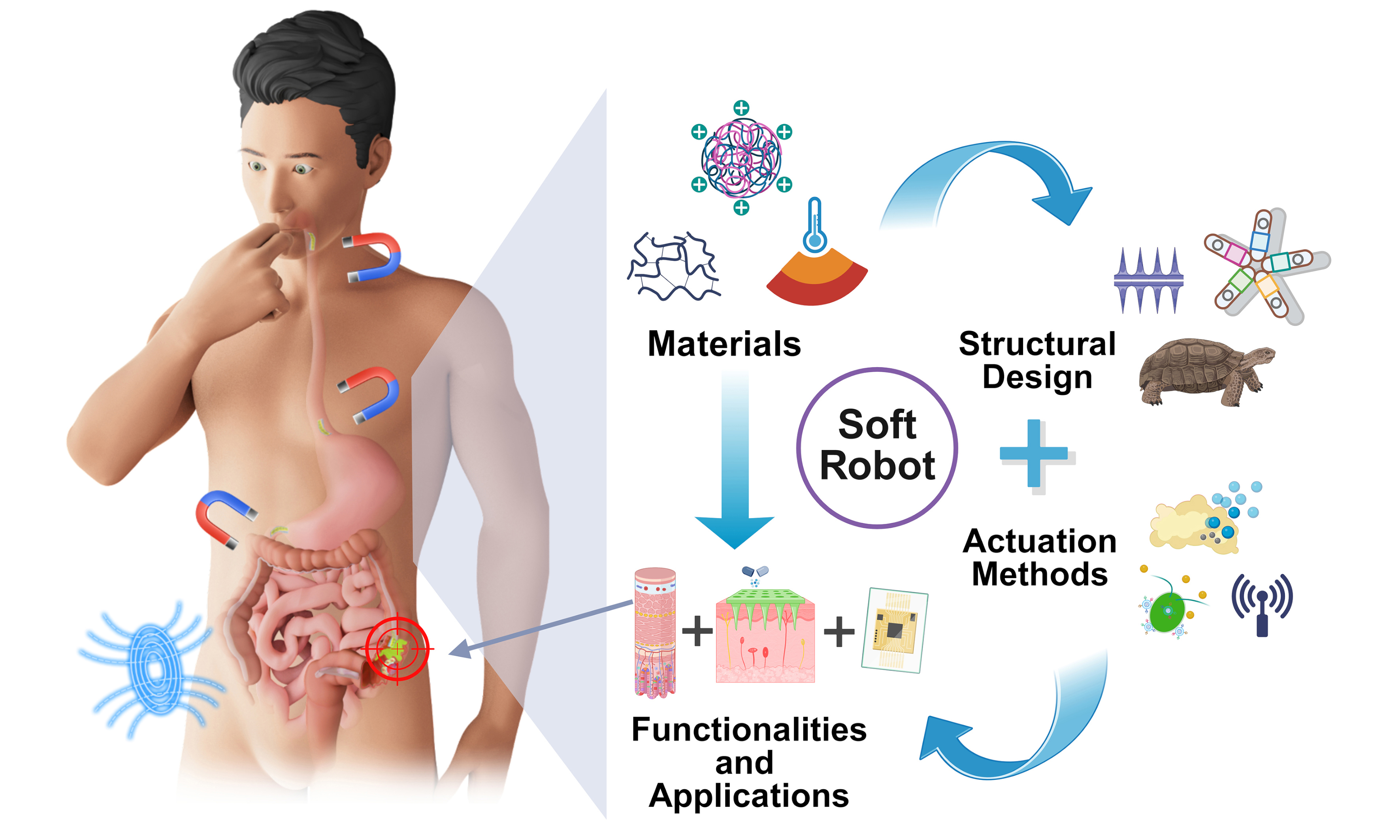
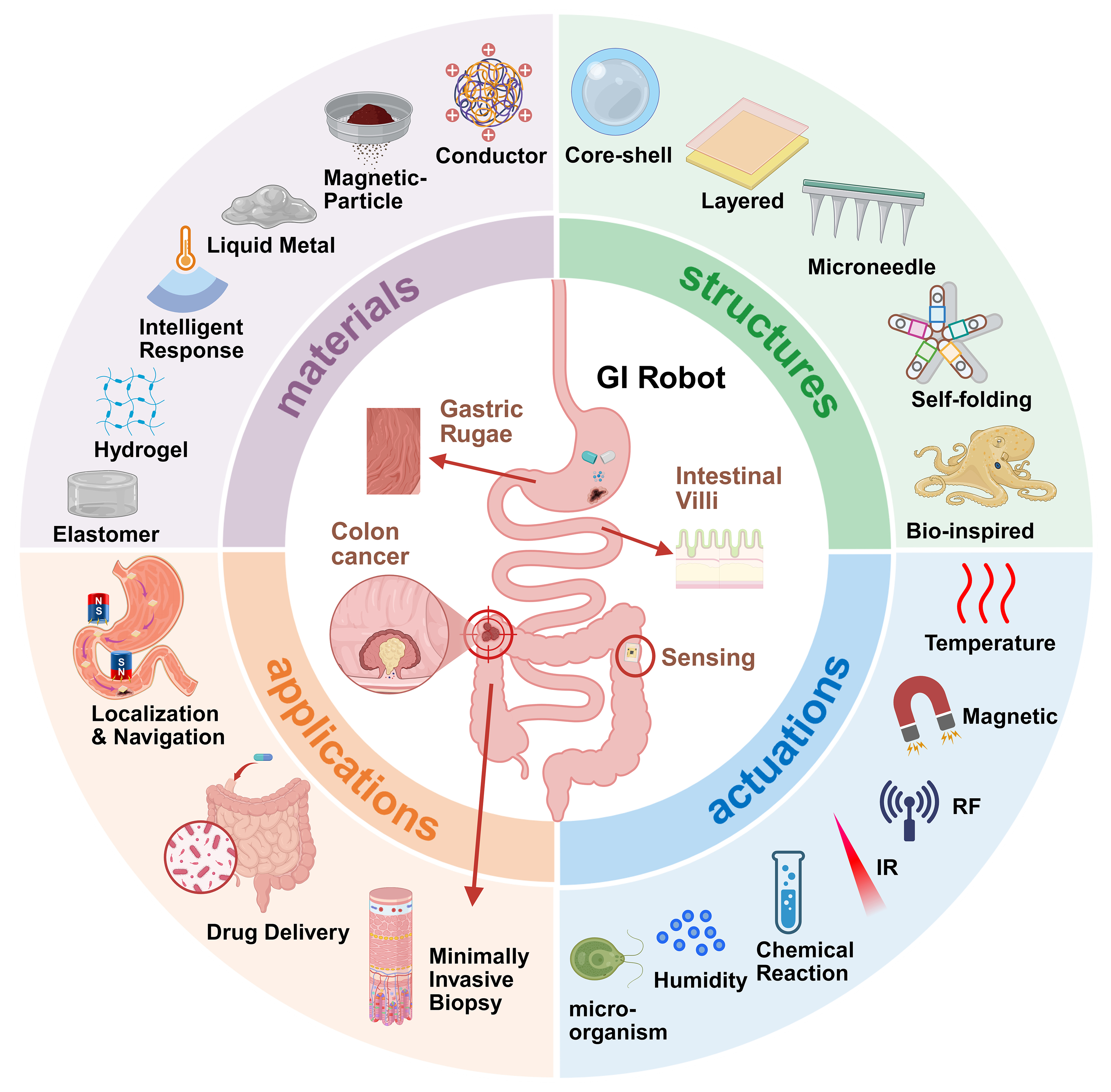
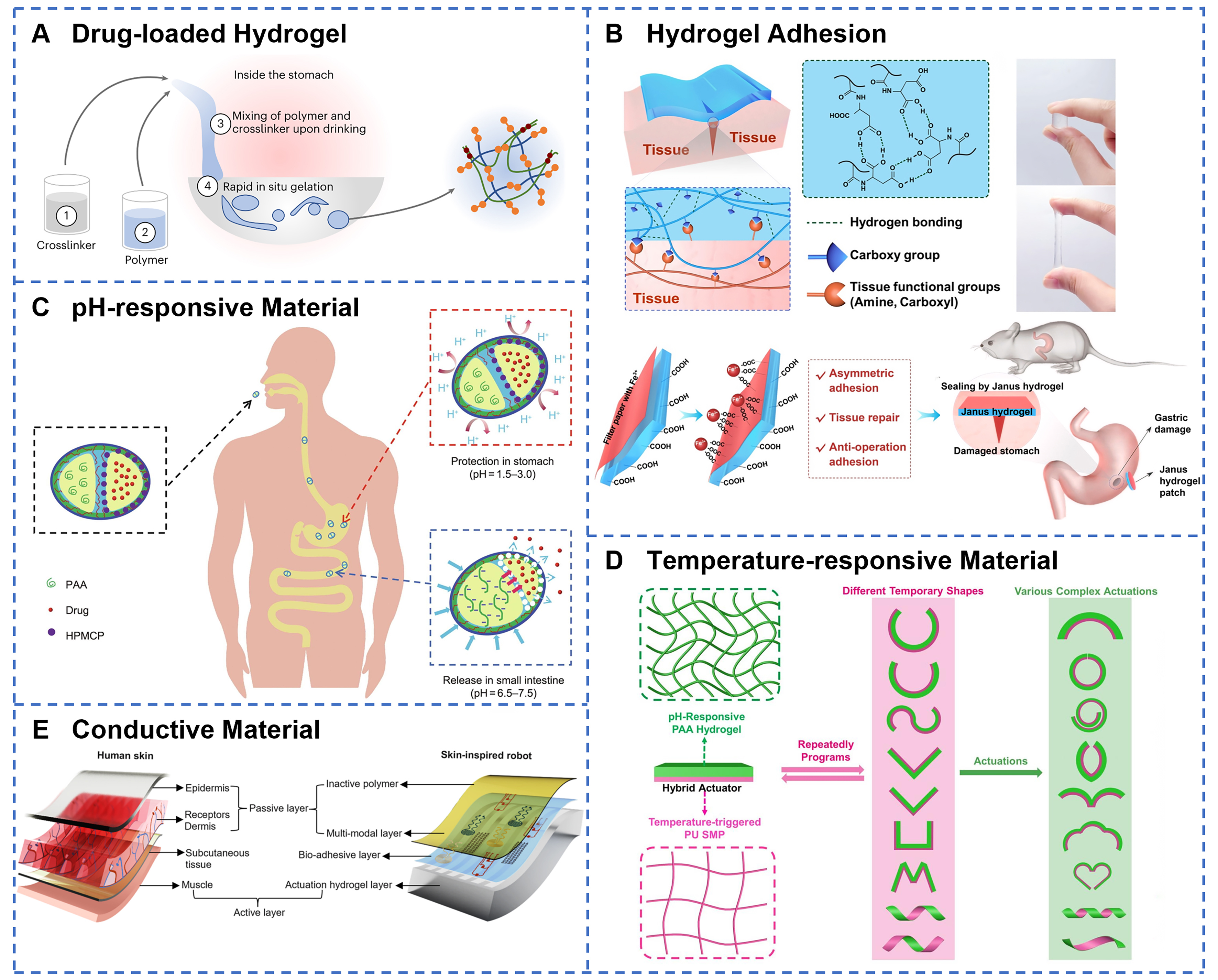
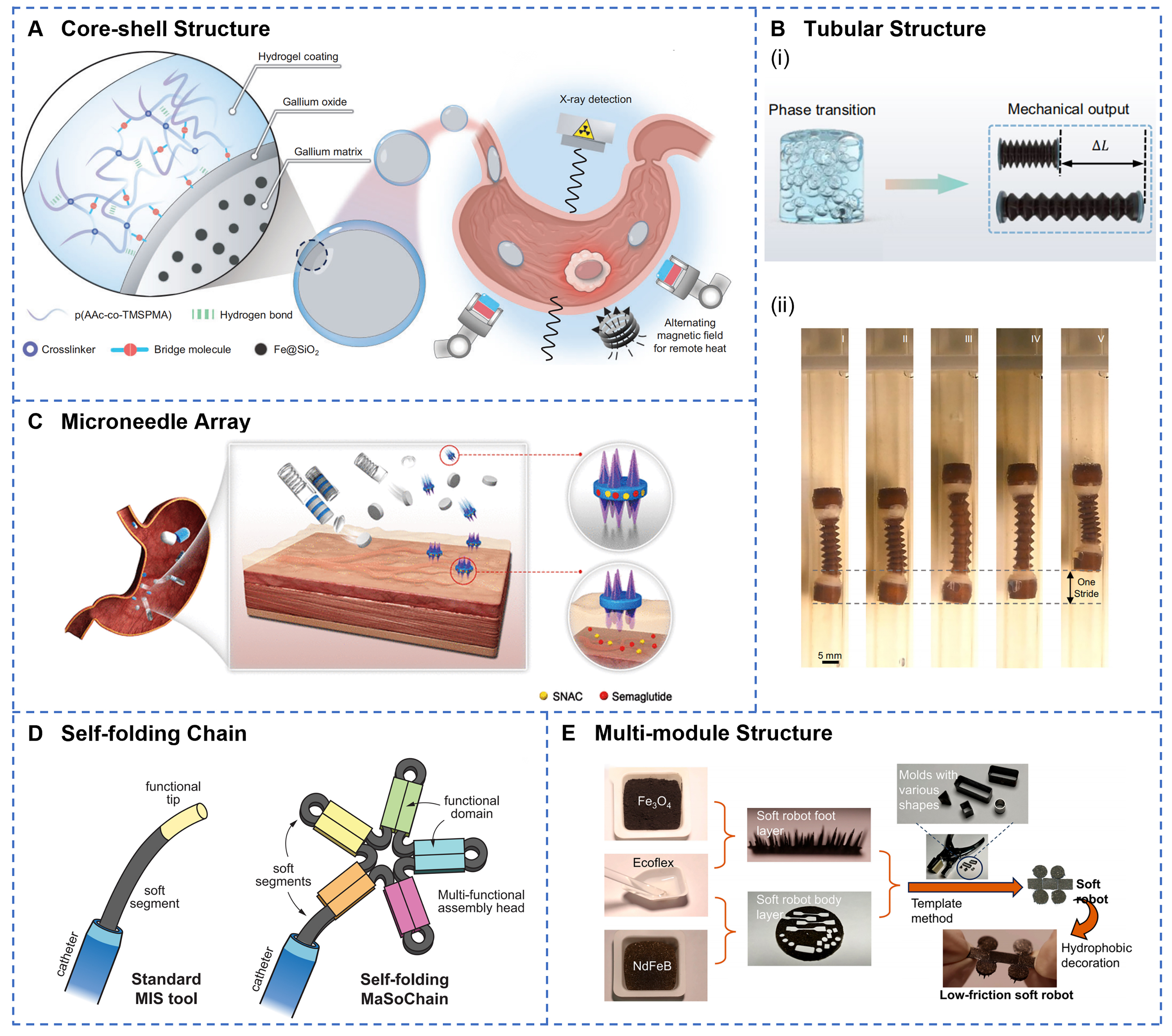
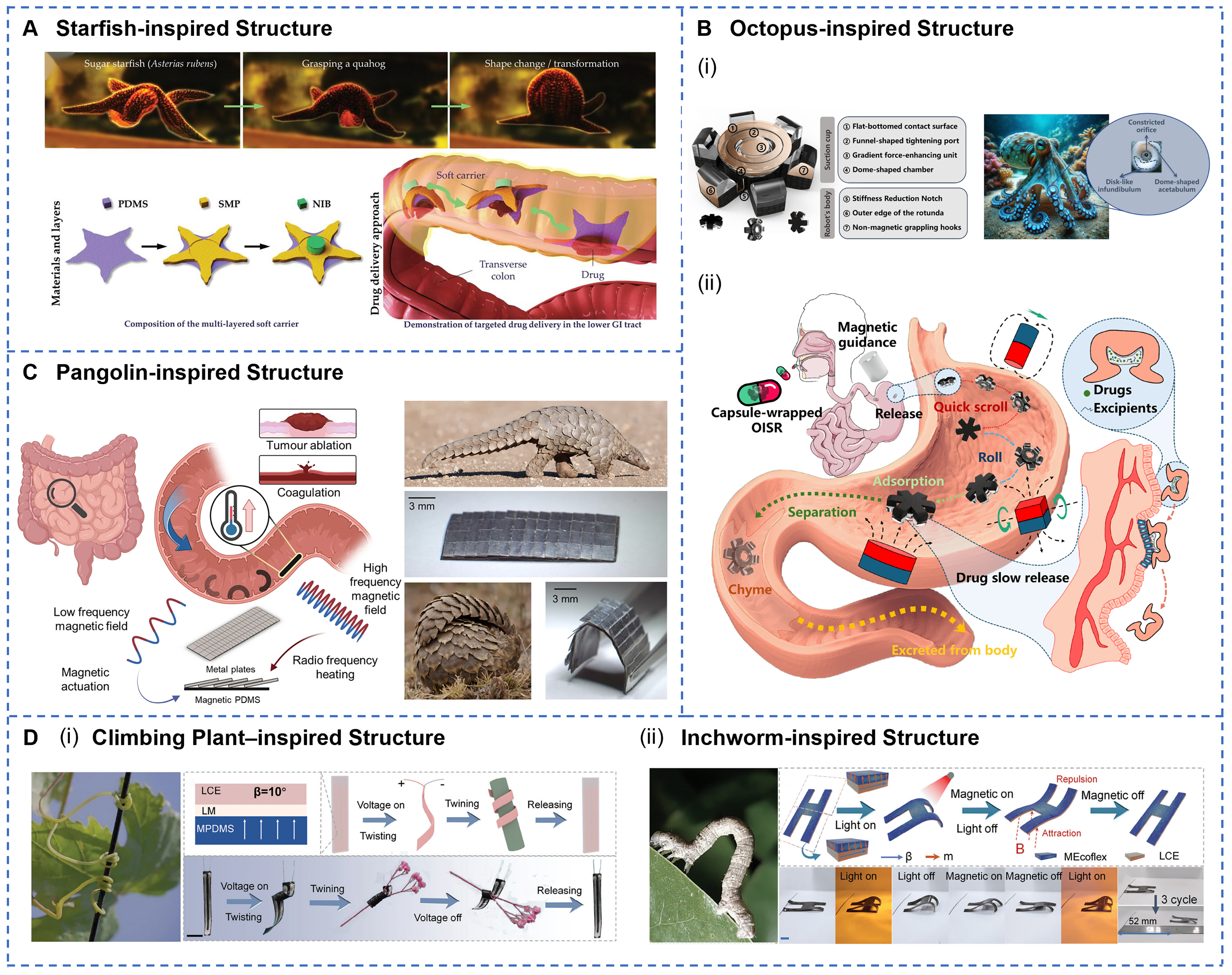
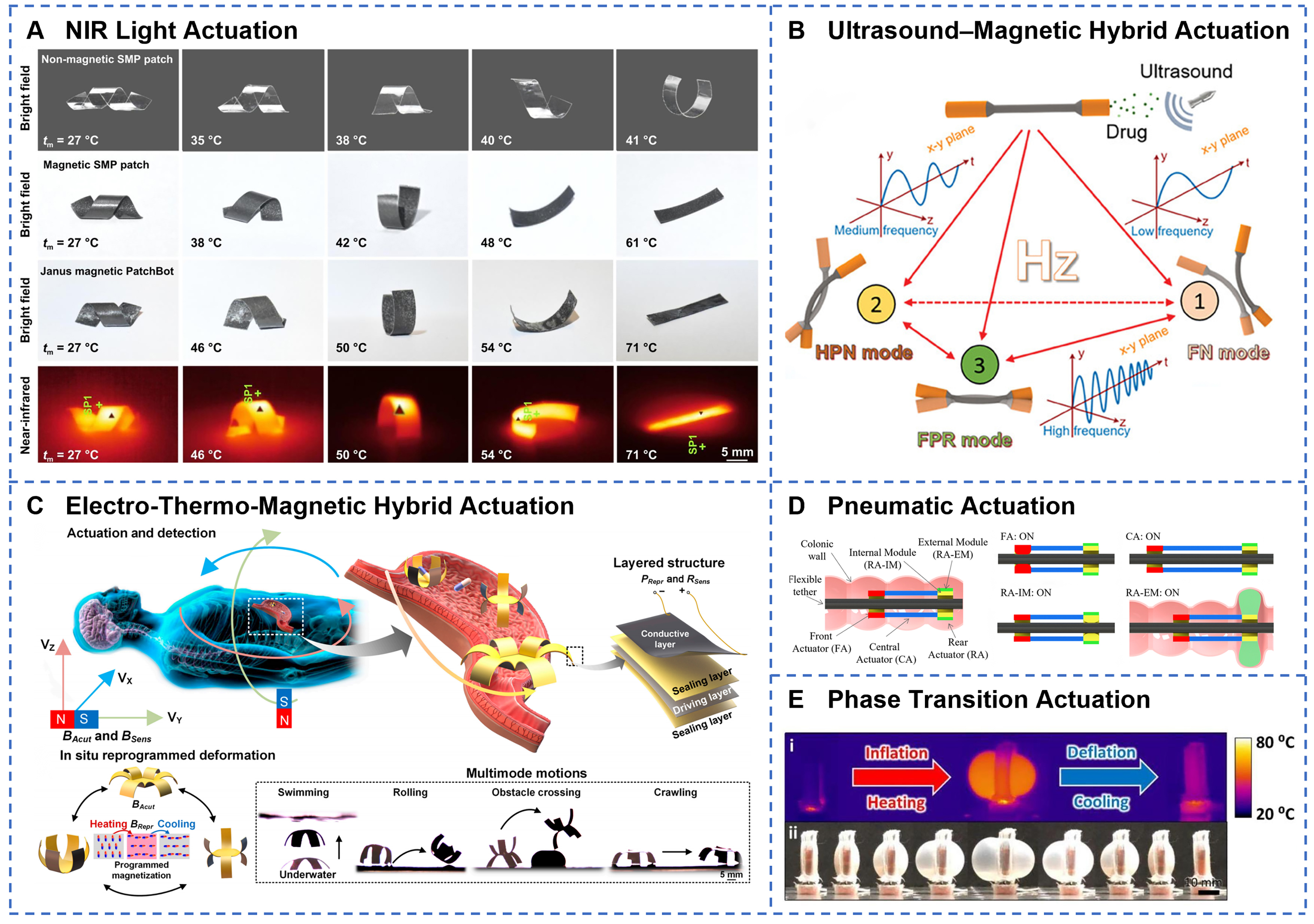
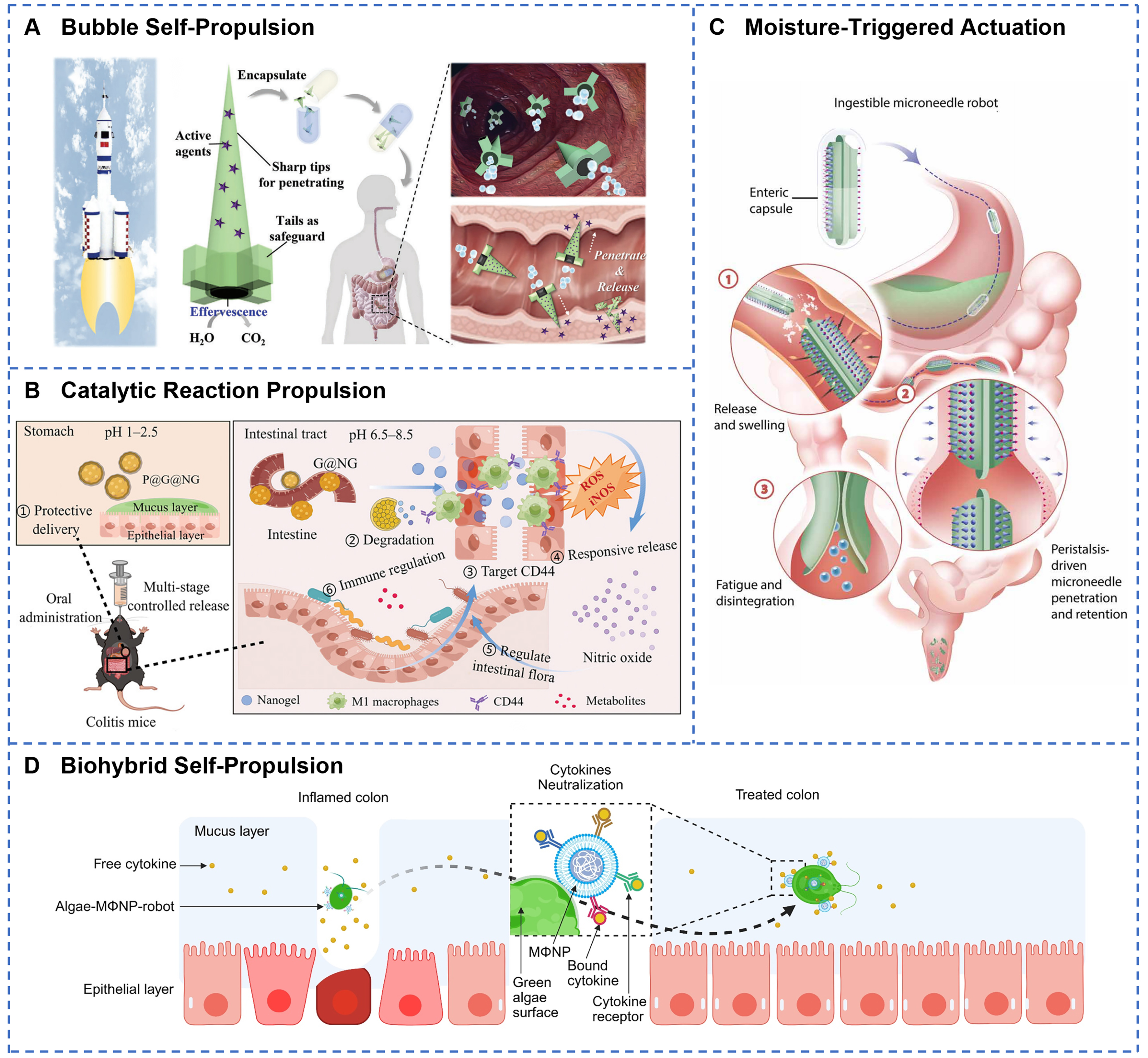
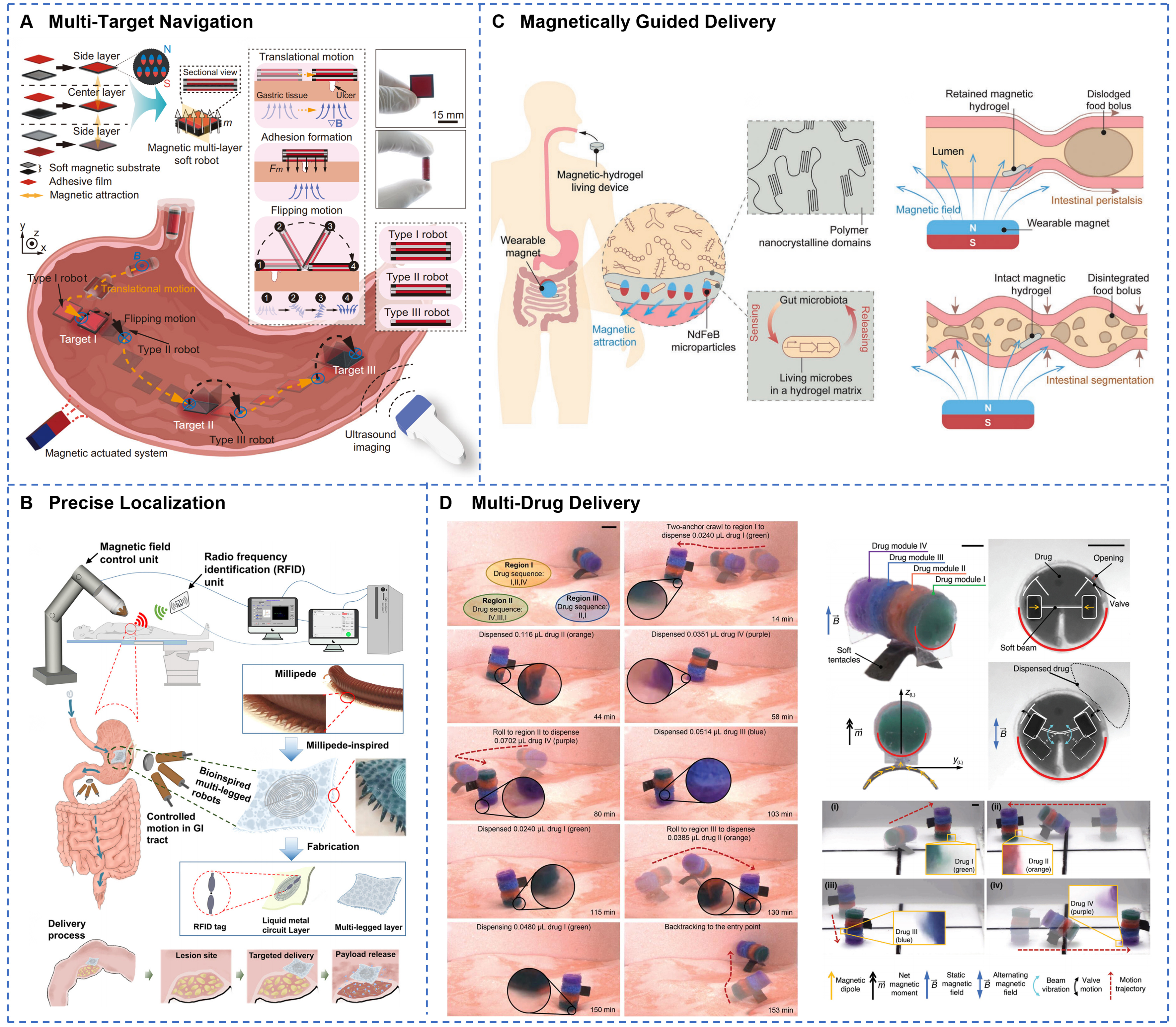
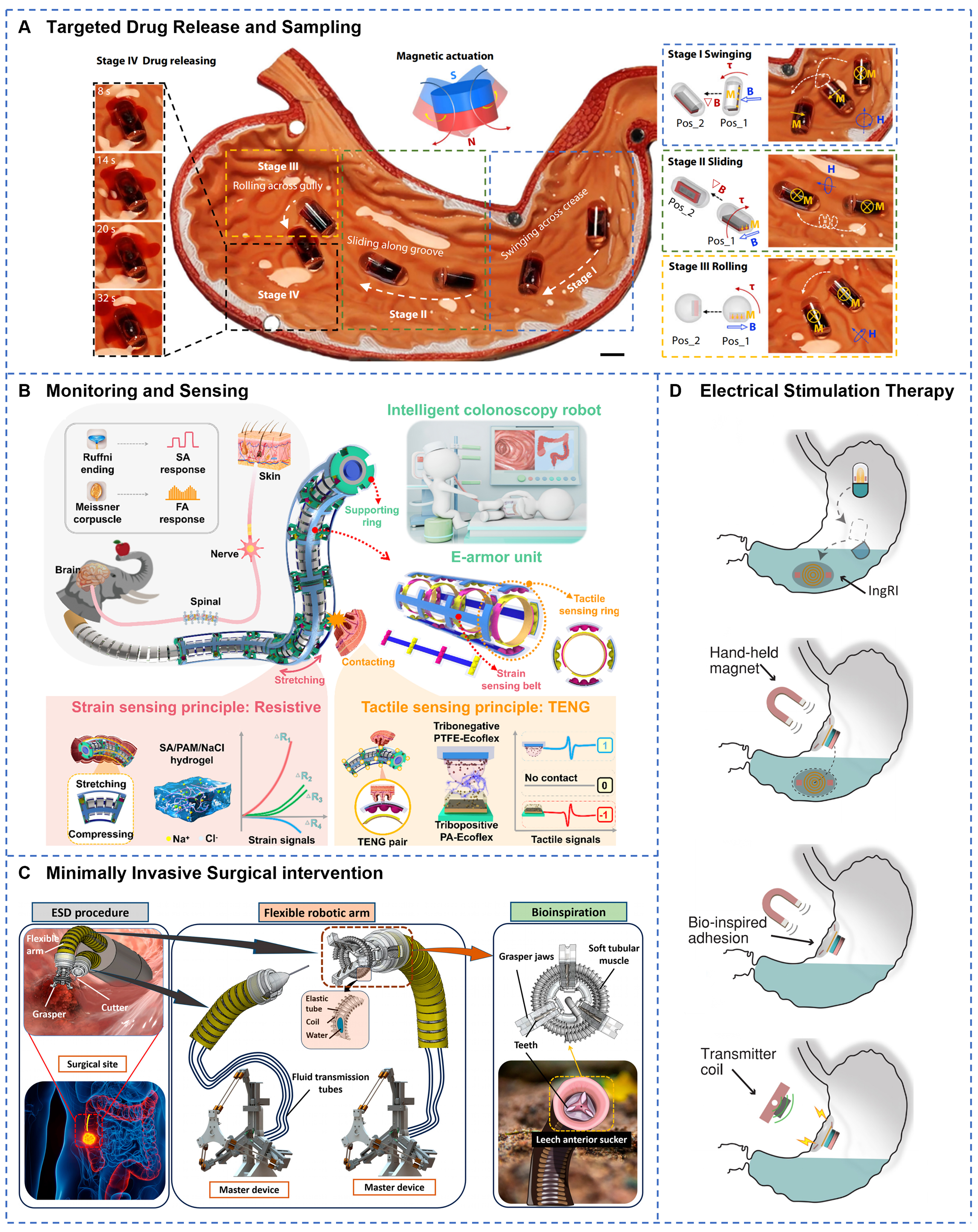












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].