Manganese dioxide as cathode for aqueous zinc-ion batteries: reaction mechanisms, optimization strategies and further prospects
Abstract
Aqueous zinc-ion batteries (AZIBs), as one of the most promising energy storage devices, have attracted widespread attention owing to their abundant resources, environmental friendliness, and high safety. As a crucial component of AZIBs, the electrochemical performance of cathode materials plays a decisive role in battery performance, thus necessitating in-depth investigations into the structure and properties of cathode materials. Manganese dioxide (MnO2), as a cathode material for AZIBs, has garnered significant interest owing to advantages such as the low cost of manganese, stable structure, simple synthesis process, and abundant raw materials. Additionally, it exhibits high specific capacity and tunable cycling performance. However, MnO2 as a cathode in AZIBs is plagued by structural deformation, side reactions, and the Jahn-Teller effect during cycling. Therefore, it is essential to comprehensively review the research progress, reaction mechanisms, and optimization strategies of MnO2 in AZIBs.
Herein, MnO2 is taken as the research focus. Firstly, we comprehensively summarize the development status and research progress of MnO2 materials as cathodes for AZIBs. Subsequently, we conduct an in-depth analysis of the structural evolution and Zn2+ storage mechanisms of MnO2 during cycling, including the conversion reaction mechanism, Zn2+ intercalation mechanism, dissolution-deposition mechanism, and H+/Zn2+ co-intercalation mechanism. Building on this, various optimization strategies such as structural control, morphological regulation, defect engineering, and electrolyte development are systematically reviewed. Finally, we outline future research directions for high-performance MnO2 cathodes, put forward a rational research roadmap to maximize the electrochemical properties of MnO2, and facilitate the construction of stable AZIBs.
Keywords
INTRODUCTION
The energy crisis and environmental pollution have become increasingly severe, making the development of novel sustainable energy storage and conversion technologies an urgent priority[1-4]. As a new-generation clean and green energy technology, electrochemical energy storage has emerged as a research hotspot[5]. Among various energy storage technologies, lithium-ion batteries (LIBs) hold a prominent position in the energy storage market. Their high energy density and relatively stable performance render them the preferred choice for applications such as portable electronic devices and electric vehicles[6]. However, the scarcity and uneven distribution of lithium resources have resulted in a continuous rise in its price, severely impeding the development and large-scale application of LIBs[7-9]. Additionally, the organic electrolytes used in LIBs are flammable and explosive, posing significant safety risks, particularly in large-scale energy storage scenarios[10-13]. In contrast, aqueous zinc-ion batteries (AZIBs), with their unique advantages, offer new prospects for the energy storage market[13-16]. AZIBs utilize zinc metal-derived from abundant and widely distributed zinc resources-as electrode materials, leading to relatively low costs and ensuring the sustainability of battery production[17-20] Furthermore, the aqueous electrolytes employed in AZIBs effectively eliminate flammable and toxic hazards throughout the entire lifecycle, including production, usage, and recycling[14,15,21]. Moreover, AZIBs exhibit high energy density and excellent cycle stability, enabling them to meet the demands of diverse application scenarios[22-24]. Nevertheless, due to the relatively late initiation of research on AZIBs, the development of electrode materials and the investigation of energy storage mechanisms remain immature. In particular, the power density and cycling performance of cathode materials have become key factors restricting their commercialization[25,26]. Therefore, exploring the synthesis of stable cathode materials with high energy density and long cycling life is crucial for advancing the development of AZIBs[27,28].
Vanadium-based oxides, manganese-based (Mn-based) oxides, layered materials, and Prussian blue analogs are the most commonly studied and well-investigated cathode materials for AZIBs[29,30]. Thus, the exploration of novel cathode materials has emerged as a research focus[31]. Among various cathode materials, Mn-based oxides, such as manganese dioxide (MnO2), MnO, Mn2O3, Na2Mn3O7, ZnMn2O4, etc., have been widely reported as cathode materials for AZIBs, attributed to manganese’s abundant reserves, eco-friendliness, low cost, ready availability, and multiple redox states[32-36]. The performance of cathode materials is influenced by their type, properties, structure, and composition[37-40]. MnO2 stands out as a promising cathode material for AZIBs due to its high capacity and favorable discharge plateau[41-43]. The fundamental building block of manganese dioxide is the MnO6 octahedron, where the Mn4+ ion is situated at the center of the distorted octahedron, with the six oxygen atoms occupying the vertices. The basic unit of manganese dioxide is the MnO6 octahedron. The crystal structure of MnO2 is diverse, and the MnO6 octahedrons are arranged by common angle or common edge to form different crystal structures such as tunnel (α-MnO2, β-MnO2, ε-MnO2, γ-MnO2, etc.), layer (δ-MnO2), or spinel (λ-MnO2)[44]. α-MnO2 exhibits relatively high stability and well-suited tunnel structures, enabling the rapid reversible insertion and facile deintercalation of Zn2+ ions[45-48]. β-MnO2 is recognized as the most stable phase; however, its structure is difficult to accommodate Zn2+ intercalation/deintercalation, thus delivering a relatively low capacity.
Layered manganese dioxide materials exhibit advantages such as low cost, high theoretical capacity, and high operating voltage. However, MnO2 with different crystal structures undergoes significantly distinct and multiple structural transitions during Zn2+ insertion and extraction processes[61-63]. Additionally, issues such as poor electronic conductivity, slow Zn2+ reaction kinetics, volume expansion, and the dissolution of divalent manganese during cycling significantly limit the specific capacity and cycle stability, thereby undermining its application potential in large-scale energy storage systems[64-66]. Furthermore, the diverse crystal structures of MnO2 and the complexity of its electrochemical reactions complicate investigations into the specific internal storage mechanisms, thereby hindering the further development of optimal modification strategies[67-70]. Hence, studying the ion storage mechanism and modifying the properties of manganese dioxide is of significant importance[71-74].
Numerous studies on MnO2 cathodes with different crystal structures for AZIBs have been reported, revealing significant differences in ion storage mechanisms and electrochemical properties[75-77]. Additionally, various reports have focused on modifying the tunnel structure, regulating morphology, engineering defects, and other factors of MnO2 to enhance its zinc storage capability[78-81]. To comprehensively investigate MnO2 cathodes for AZIBs, it is essential to summarize their storage mechanisms and modification strategies. Herein, as shown in Figure 1, this review systematically elaborates on the Zn2+ storage mechanisms and various modification strategies of MnO2 cathode materials in AZIBs, emphasizing their potential for improving performance and sustainability. We discuss the cycling performance of MnO2 and its corresponding zinc storage mechanisms, including the conversion reaction mechanism, Zn2+ insertion/extraction mechanism, dissolution-deposition mechanism, and H+/Zn2+
REACTION MECHANISMS
The storage mechanism of MnO2 as a cathode in AZIBs involves complex electrochemical reactions. The continuous advancement and integration of in-situ characterization techniques are critical for driving progress in battery research and energy storage technologies. Dynamic data obtained via in-situ characterization hold greater value than static data from ex-situ methods, particularly in investigating battery ion storage mechanisms. In recent years, significant advancements in various in-situ characterization techniques-such as in-situ X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and in-situ impedance spectroscopy-have enabled real-time monitoring of the structural evolution of electrode materials during charge-discharge processes, which has played a pivotal role in analyzing the ion storage mechanisms of electrode materials. Characterization techniques including orthogonal microscopy, atomic force microscopy (AFM), and in-situ Raman spectroscopy can effectively detect changes in the surface morphology and composition of electrodes, providing insights for optimizing electrode design. These in-situ approaches are crucial for optimizing electrode materials, analyzing reaction kinetics, and exploring redox mechanisms in high-performance energy storage systems. In-situ characterization techniques will aid in guiding the structural and functional design of electrode materials, elucidating their working mechanisms, and thereby facilitating the sustainable development of MnO2 cathodes[70].
The electrochemical reactions of MnO2 involve the insertion/desorption of Zn2+ and H+. In AZIBs, MnO2 cathodes face key challenges in regulating the solvation structure of Zn2+. Zn2+ exists in the electrolyte as hexacoordinate hydrated ions [Zn(H2O)6]2+, and the stability of its solvation sheath significantly influences ion migration behavior. When Zn2+ intercalates into the MnO2 lattice, the energy barrier of the hydration layer must be overcome to achieve desolvation. The thermodynamic and kinetic characteristics of this process directly determine the efficiency of electrode reactions. Upon migrating to the electrode interface, hydrated Zn2+ forms temporary coordination with oxygen atoms on the MnO2 surface, gradually displacing water molecules to complete desolvation, and ultimately intercalates into the lattice as dehydrated ions. Currently, molecular dynamics simulations and in-situ characterization techniques have revealed that the reversibility and kinetic matching of the desolvation process are key factors limiting the cycle life of
Zn2+ insertion/extraction mechanism
During the discharge process of AZIBs, zinc foil at the anode loses electrons, releasing zinc ions (Zn2+), which then migrate toward the cathode material. The cathode material accepts electrons and undergoes one or more reversible reduction reactions. Manganese (Mn) undergoes a valence reduction from +4 to +3, while Zn2+ intercalates into the cathode material. During the charge process, the cathode undergoes an oxidation reaction, releasing the previously intercalated Zn2+, which migrates back to the anode and undergoes a reduction reaction to form metallic zinc or intercalate into the zinc foil lattice. This is referred to as the Zn2+ insertion/extraction mechanism, one of the earliest discovered and most prevalent energy storage mechanisms in AZIBs. Early studies revealed that during discharge, zinc at the cathode is oxidized to Zn2+, which migrates through the electrolyte and intercalates into the tunnel structure of α-MnO2, reacting with MnO2 to form ZnMn2O4. Concurrently, Mn is reduced from the +4 to the +3 oxidation state. The electrochemical reaction can be expressed as follows:
During charging, the process is reversed: Zn2+ is deintercalated from the cathode, returns to the electrolyte, and is ultimately reduced to metallic zinc, which deposits at the anode. Subsequent studies have revealed that distinct mechanisms govern Zn2+ intercalation into α-MnO2. As shown in Figure 2A, in investigations of zinc-ion batteries using α-MnO2/graphene scrolls (MGS) as cathode materials, Zn2+ was found to intercalate into the α-MnO2 tunnels during the first cycle, converting it into layered Zn-buserite until Zn2+ accommodation is no longer feasible. This process is irreversible. In subsequent cycles, Zn2+ undergoes reversible intercalation/extraction within the generated layered structure[83]. MnO2 encompasses α-MnO2
Figure 2. (A) The two-step intercalation mechanism of MGS cathode. Reprinted with permission[83]. Copyright 2018, John Wiley and Sons; (B) Different crystal structures of MnO2 and the zinc storage mechanisms. Reprinted with permission[84]. Copyright 2020, John Wiley and Sons; (C) The reaction pathway of Zn-insertion in γ-MnO2 cathode. Reprinted with permission[85]. Copyright 2015, American Chemical Society; (D) Cell structure and working mechanism of AZIBs with δ-MnO2 cathode. Reprinted with permission[87]. Copyright 2024, John Wiley and Sons. MGS: α-MnO2/graphene scrolls; AZIBs: aqueous zinc-ion batteries.
Among MnO2 with layered crystal structures, δ-MnO2 features a two-dimensional planar configuration with large interlayer spacing, facilitating faster diffusion and intercalation of Zn2+. As shown in Figure 2D, in zinc-ion batteries (ZIBs), the layered δ-MnO2 nanosheet cathode undergoes structural transformation during Zn2+ intercalation, converting into spinel-type ZnMn2O4 and the intermediate Mn(II)
Manganese is reduced from the +4 to the +3 and +2 oxidation states, respectively. Subsequent studies have revealed that zinc cations are progressively intercalated into the δ-MnO2 layers in the aqueous electrolyte through a two-stage process, while Zn2+ deintercalation from the MnO2 matrix occurs in two steps[88]. It is noteworthy that research on the Zn2+ intercalation/deintercalation mechanism of tunnel-structured MnO2 is relatively mature. In recent years, emerging studies have primarily focused on co-intercalation mechanisms and other related mechanisms. However, the Zn2+ intercalation/deintercalation mechanism of layered MnO2 still warrants further investigation.
H+/Zn2+ Co-insertion mechanism
The co-insertion mechanism of hydrogen ions (H+) and Zn2+ is crucial for enhancing the electrochemical performance of batteries. As shown in Figure 3A, the co-insertion mechanism in AZIBs involves the simultaneous intercalation of H+ and Zn2+ into cathode materials such as MnO2[89]. Distinct from traditional Zn2+-based energy storage mechanisms, this co-insertion strategy enables higher capacity and faster reaction kinetics. Owing to their smallest ionic radius and lowest mass, H+ typically contributes to high capacity and rapid dynamics in batteries. In Figure 3B, a reversible H+/NH4+ co-insertion/extraction mechanism was established in the zinc-manganese dioxide aqueous battery system, and it was found that such a mechanism significantly improves the rate capability and cycling stability of AZIBs[32]. For Figure 3C, the co-insertion mechanism enhances ion diffusion within MnO2 through a synergistic effect. Moreover, benefiting from reversible structural evolution, the battery maintains long-term stable operation[90]. Several approaches have been reported to reduce the detrimental incorporation of H+ and promote Zn2+ uptake in aqueous systems. Notable progress has been achieved in this field, particularly through the modulation of water activity, regulation of Zn2+ hydration behavior, and development of subzero-temperature batteries[91]. In Figure 3D, a strategy to tailor the electrochemical behavior of layered δ-MnO2 by introducing Cu2+ ions was developed[92]. This approach enables a reversible process involving Zn2+ insertion and H+ conversion within the modified δ-MnO2 electrode, thus forming an efficient H+/Zn2+ co-insertion/extraction mechanism. The findings indicate that Cu2+ incorporation into δ-MnO2 facilitates favorable ion intercalation/deintercalation, which is attributed to strong ionic interactions with oxygen atoms.
Figure 3. (A) Zn2+ intercalation and H+ conversion reaction mechanism in δ-MnO2. Reprinted with permission[89]. Copyright 2019, John Wiley and Sons; (B) The insertion energies of H+ or NH4+ ions in MnO2. Reprinted with permission[32]. Copyright 2021, John Wiley and Sons; (C) The discharge of tunnel-like ε-MnO2 stockpiled H+ and Zn2+. Reprinted with permission[90]. Copyright 2022, Elsevier; (D) The synthetic process of CuMO electrode. Reprinted with permission[92]. Copyright 2022, Elsevier; (E) Schematic illustration of the preparation of TMA-MnO2. Reprinted with permission[1]. Copyright 2024, Elsevier.
Given the significant difference in ionic radii between Zn2+ and H+, the interlayer distance of MnO2 is expected to play a critical role in regulating the intercalation competition between Zn2+ and H+ at the MnO2 surface. Based on this premise, it has been demonstrated that a MnO2/tetramethylammonium (TMA) composite with expanded interlayer spacing (denoted as TMA-MnO2) serves as a robust cathode material for AZIBs [Figure 3E][1]. These studies suggest that expanding the interlayer spacing is a viable strategy to promote Zn2+ uptake while suppressing the competing intercalation of H+. This approach effectively inhibits the concurrent formation of zinc hydroxide sulfate (ZHS) and enhances the structural stability of MnO2. Sluggish Zn2+ insertion kinetics in existing MnO2-based materials have been identified as a key issue, leading to the formation of irreversible inert phases on the electrode surface and thus substantial capacity loss. To address this, researchers have emphasized that preventing the formation of such irreversible inert phases is essential for improving the cycling performance of zinc-manganese batteries[93]. They proposed that engineering MnO2 with a layered structure can accelerate Zn2+ intercalation/deintercalation and preserve structural integrity, thereby inhibiting the formation of the inert ZnMn2O4 phase.
Zn2+ insertion mechanism
The pH value of Zn/MnO2 batteries varies during charge-discharge cycles, which confirms the occurrence of H+ transformation. The formation of MnOOH also provides strong evidence for the H+ intercalation-induced transformation reaction mechanism. As shown in Figure 4A, compared with the traditional Zn2+ intercalation/deintercalation mechanism, the transformation reaction mechanism enables direct charge transfer involving two or more charge carriers. Under acidic conditions, MnO2 reacts with H+ to form MnOOH[94]. However, excess OH- will combine with Zn2+ to form precipitates on the cathode surface {such as Zn4SO4(OH)6·nH2O shown in Figure 4B and ZnSO4[Zn(OH)2]3·xH2O shown in Figure 4C}[93,95]. Additionally, under specific conditions, MnOOH can further react with H+ to generate MnO2 and Mn2+. The equation for this conversion reaction is as follows:
Figure 4. (A) Conversion reaction mechanism. Reprinted with permission[94]. Copyright 2022, Elsevier; (B) A side reaction of the AZIBs conversion reaction to form Zn4SO4(OH)6·nH2O. Reprinted with permission[93]. Copyright 2022, Elsevier; (C) A side reaction of the AZIBs conversion reaction to form ZnSO4[Zn(OH)2]3·xH2O. Reprinted with permission[95]. Copyright 2022, Elsevier; (D) Dynamic compensation mechanism for the AZIBs with the Zn-Ce electrolyte. Reprinted with permission[97]. Copyright 2023, Royal Society of Chemistry; (E) Diagram illustrating phase transformations with MnO2 cathode in zinc sulfate. Reprinted with permission[98]. Copyright 2022, Elsevier. AZIBs: Aqueous zinc-ion batteries.
Additionally, the detection of MnOOH diffraction peaks further confirms the existence of a conversion reaction mechanism.
By investigating the ion transport and reaction behaviors during cycling, researchers identified diffraction peaks corresponding to Zn4SO4(OH)6·nH2O and MnOOH in the δ-MnO2-4 h/CC system during charge-discharge processes[96]. These results confirm that H+ is inserted into MnO2, and the increase in electrolyte pH induced by H+ insertion promotes the formation of Zn4SO4(OH)6·nH2O on the electrode surface. Similarly, to elucidate the electrochemical energy storage mechanism of the A-MnO2 cathode, researchers observed that the phase composition of the A-MnO2 cathode during the reaction process includes thin sheets with reversible formation/dissolution behaviors, specifically basic zinc sulfate {Zn4SO4(OH)6·4H2O, BZS}. The formation of BZS is associated with increased pH and Zn2+ concentration in the vicinity of the cathode[92]. Additionally, the detection of MnOOH diffraction peaks further confirms the existence of a conversion reaction mechanism.
Studies have shown that the capacity of MnO2 cathodes often degrades due to manganese dissolution. To address this, researchers introduced Ce(SO4)2 as an additive into the electrolyte of Zn/MnO2 batteries. They found that MnOOH, formed via the reaction between Ce4+ and Mn2+, is attracted by H+ and deposited on the cathode [Figure 4D]. This MnOOH is subsequently converted into MnO2, achieving dynamic compensation and effectively ensuring capacity stability[97]. Furthermore, researchers investigated the irreversibility of different MnO2 polymorph cathodes during charge-discharge cycles in weakly acidic ZnSO4 aqueous electrolytes, and analyzed its close correlation with conversion reactions. A schematic diagram illustrating the phase transition of the MnO2 cathode in aqueous zinc sulfate solution is presented in Figure 4E. It is worth noting that current studies on the conversion reaction mechanism remain limited, with few reports available, and research in this field is still dominated by the traditional intercalation-deintercalation mechanism[98].
Deposition-dissolution mechanism
The deposition-dissolution mechanism of MnO2 as a cathode in AZIBs essentially involves a redox reaction coupled with Mn valence state changes and Zn2+ migration during cycling, accompanied by the dissolution and re-deposition of MnO2[51]. The reversibility of MnO2 in the deposition-dissolution mechanism is jointly regulated by the intrinsic properties of the material (e.g., structure and morphology), electrolyte characteristics (e.g., pH and ionic composition), and operating conditions (e.g., temperature and voltage). Among these factors, Mn dissolution, Mn loss, and structural phase transitions are core challenges[49]. Strategies such as crystal structure optimization, electrolyte regulation, and interface engineering can effectively suppress side reactions, enhance the reversibility of the mechanism, and facilitate the practical application of aqueous zinc-ion batteries[35]. As in-depth research into the role of MnO2 in AZIBs advances, growing evidence indicates that the dissolution-deposition phenomenon of MnO2 is widespread.
Researchers have noted that ZHS, often regarded as a by-product of proton consumption at the electrolyte interface, can potentially be utilized as a cathode material, exhibiting cycling characteristics similar to those of MnO2 cathodes[61]. As shown in Figure 5A, researchers analyzed the reversible formation and dissolution of ZHS during charge-discharge cycles. The ZHS cathode exhibits markedly reduced capacity in electrolytes lacking Mn2+[99]. During the interaction between ZHS and Mn2+, the presence of abundant OH- lowers the electrooxidation potential of Mn2+. Thus, with the assistance of ZHS, Mn2+ may be re-deposited as manganese oxides [Figure 5B][100]. This mechanism emphasizes that the electrochemical activity of ZHS stems from the liberation and reintegration of Mn2+, providing a new perspective for understanding the energy storage mechanism of MnO2.
Figure 5. (A) The proposed charge storage mechanism of the β-MnO2 cathode. Reprinted with permission[99]. Copyright 2018, Royal Society of Chemistry; (B) The reaction mechanism for the α-MnO2. Reprinted with permission[100]. Copyright 2022, Elsevier; (C) The dissolution of MnO2 in acidic electrolytes. Reprinted with permission[101]. Copyright 2022, Royal Society of Chemistry; (D) Cycling profiles of the ZHS and the corresponding SEM images. Reprinted with permission[102]. Copyright 2023, Royal Society of Chemistry; (E) The reaction mechanism of the MnO2/CC electrode. Reprinted with permission[103]. Copyright 2022, Royal Society of Chemistry; (F) Mn-based competitive capacity evolution protocol. Reprinted with permission[104]. Copyright 2020, Elsevier. SEM: Scanning electron microscopy; ZHS: zinc hydroxide sulfate.
Additionally, traditional views suggest that the incorporation of Mn2+ can suppress manganese dissolution, thereby enhancing the battery’s ability to maintain cycling performance over multiple charge-discharge cycles. However, this explanation does not fully account for the observed fluctuations in battery capacity following the addition of Mn2+ to ZnSO4 electrolytes. To address this phenomenon, an in-depth study monitored changes in Mn2+ concentration and electrolyte pH at different stages of cycling processes. As shown in Figure 5C, the cycle curves are divided into four regions: the activation region, the H+/Zn2+ dominated region, the Mn2+ dominated region, and the final attenuation region[101]. Proton-coupled reactions (PCRs) are closely associated with energy conversion and storage in AZIBs. Specifically, pH changes regulate the formation and dissolution of Mn2+ and MnO2, which in turn affect the cycling capacity of AZIBs. Consequently, the behavior of Mn2+ is tightly linked to the pH level in the vicinity of the electrode/electrolyte interface. Notably, during battery charging, proton concentration significantly impacts the deposition potential of Mn2+. Due to the high electrolyte pH, manganese oxides are more readily formed [Figure 5D][102]. During discharge, hydrogen evolution reactions and irreversible H+ deintercalation reactions consume H+, which cannot be regenerated during subsequent charging. This leads to an increase in electrolyte pH, thereby promoting the deposition of manganese oxides onto the cathode [Figure 5E][103].
Therefore, elucidating the intrinsic mechanism of the MnO2/Mn2+ dissolution-deposition phenomenon, clarifying the reaction mechanism of aqueous zinc-manganese batteries [Figure 5F], explaining capacity fluctuations in Zn-MnO2 batteries, and developing high-performance aqueous zinc-manganese secondary batteries are critical issues requiring in-depth investigation at present[104].
OPTIMIZATION STRATEGIES
Structural control
When MnO2 serves as the cathode material for AZIBs, variations in its crystal structure (e.g., α, β, γ, δ types) exert a substantial influence on the electrochemical performance, and crystal transformation may take place during the cycling process[60]. α-MnO2 features a layered structure characterized by a substantial interlayer spacing, which facilitates the intercalation of Zn2+ and endows it with a relatively high initial capacity. Nevertheless, during the cycling process, the structure is liable to transform into β-MnO2 as a result of the dissolution of Mn2+, thereby leading to capacity attenuation[53]. The compact tunnel structure of β-MnO2 restrains the dissolution of Mn and exhibits excellent cycling stability. However, the narrow tunnels restrict the diffusion of Zn2+ and result in a relatively low capacity. γ-MnO2 is a mixed structure of α and β, characterized by both high capacity and a certain degree of cycling stability[58]. However, δ-MnO2 (layered hydrated structure) exhibits poor capacity and rate performance because the interlayer water molecules impede ion transport. Crystal form transformation is typically driven by the dissolution-deposition mechanism (e.g., the re-deposition of Mn2+ into different crystal forms subsequent to dissolution), or induced by lattice stress resulting from the intercalation of Zn2+[55]. Among these, β-MnO2 exhibits superior reversibility owing to its robust structural rigidity and the arduousness of crystal form transformation. In contrast, the layered structure of α-MnO2 endows it with a high capacity. Nevertheless, the cycle life is restricted because of the irreversibility of crystal form transformation. The equilibrium between capacity and cycling stability can be optimized by regulating the synthesis conditions (e.g., temperature and pH value) or introducing heterogeneous ions (such as Zn2+ and Co2+) to stabilize the target crystal form[59].
The structural collapse of MnO2 can be effectively alleviated, and its structural stability enhanced, through structural engineering strategies including interlayer intercalation, in-situ coordination, and introduction of oxygen vacancies[105]. Researchers designed ammonium ion pre-embedded layered δ-MnO2 nanosheets (denoted as AMO) as the cathode for AZIBs[106]. During ion-exchange reactions, ammonium cations can replace the initially intercalated potassium ions in pristine δ-MnO2 nanosheets. As shown in Figure 6A, the embedded ammonium ions act as interlayer pillars, expanding the lattice spacing of AMO and forming a hydrogen-bonding network. Consequently, AMO mitigates the Jahn-Teller effect and enables fast Zn2+ diffusion kinetics. The robust AMO cathode exhibits excellent electrochemical performance, including high specific capacity, superior rate capability, and extended cycle life. As illustrated in Figure 6B, typical
Figure 6. (A) Illustration of the synthesis of AMO. Reprinted with permission[106]. Copyright 2023, John Wiley and Sons; (B) The preparation procedure for δ-MnO2 with rich oxygen vacancies (δ-MnO2-x). Reprinted with permission[109]. Copyright 2023, Elsevier; (C) PVP intercalation in δ-MnO2. Reprinted with permission[108]. Copyright 2023, John Wiley and Sons; (D) Ti3C2 MXene nanosheets with good conductivity enable fast electron transfer kinetics. Reprinted with permission[109]. Copyright 2023, Elsevier; (E) The advantages of
As depicted in Figure 6D, closely linked MnO2@MXene hetero-nanosheets were synthesized via in-situ polymerization of dopamine on Ti3C2@MXene nanosheets, followed by a redox reaction between KMnO4 and polydopamine[109]. During this process, thin layer-like δ-MnO2 nanosheets were vertically deposited on MXene nanosheets, substantially promoting rapid electron/ion transfer and enhancing structural stability. Notably, the MnO2@MXene hetero-nanosheets exhibit a unique Zn2+ storage mechanism in Zn(OTf)2/TEP organic electrolytes, overcoming the limitations and side reactions associated with unprotonated storage in traditional aqueous electrolytes. Consequently, the MnO2@MXene hetero-nanosheet-based thin-film cathode delivers excellent Zn storage performance. MnO2 nanocrystal-carbon hybrid frameworks were constructed via in-situ reactions between a rationally designed dimethylimidazole-based MOF array and KMnO4. The self-supported arrays, consisting of a carbon skeleton and MnO2 nanocrystals, endow the
As illustrated in Figure 6F, an amorphous manganese borate (denoted as a-MnBOx) cathode material for AZIBs was designed, featuring irregular coordination between divalent Mn2+ ions and BO33- planar triangular or BO45- tetrahedral structures[111]. The boric acid ligand effectively stabilizes Mn2+ in its divalent state and enables a-MnBOx to form a 3D stable porous framework with a large specific surface area
Morphological control
The morphological regulation of MnO2, such as nanowires, nanosheets, hollow structures, and hierarchical structures, exerts a substantial influence on their electrochemical performance. Distinct morphologies optimize the storage behavior of Zn2+ by altering the specific surface area, ion diffusion path, structural flexibility, and electron transport capacity of MnO2. Firstly, there exists one-dimensional morphology, encompassing nanowires, nanorods, and so forth. Their structural characteristics involve directional ion transport channels, robust structural flexibility, and outstanding electron conduction ability, which together contribute to stable cycling performance. Furthermore, two-dimensional morphology, such as nanosheets and nanoplates, exhibits large specific surface areas and uniform dispersion properties. These attributes facilitate efficient ion diffusion and enhanced electrochemical reactions, contributing to improved energy density and cycling stability. Additionally, three-dimensional morphologies, such as hierarchical nanostructures and porous frameworks, offer abundant active sites and effective pathways for ion and electron transport. These unique architectures further enhance the overall performance of electrochemical devices by promoting rapid kinetic processes and facilitating mass transport. Regarding material regulation, template methods, including the SiO2 template and metal-organic framework template, are predominantly employed to precisely control the three-dimensional morphology. Additionally, self-assembly methods, such as the sol-gel method and self-assembly process, can be utilized to prepare hierarchical nanostructures and regulate the pore size distribution[113].
Currently, the morphology regulation of aqueous zinc-manganese batteries encompasses three main categories. The first category includes template method, self-assembly method, and solvothermal method[114]. These approaches can effectively modulate the morphology and structure of materials, thereby enhancing electrochemical performance. Wang et al. prepared novel manganese-deficient MnO2
Figure 7. (A) Structural and morphological analyses. Reprinted with permission[58] Copyright 2024, John Wiley and Sons; (B) The illustration of the formation of δ-MnO2 nanosheets. Reprinted with permission[114]. Copyright 2019, Elsevier; (C) Schematic sketch of the synthesis and possible cycling process of β-MnO2 and δ-MnO2 submicrospheres. Reprinted with permission[116]. Copyright 2019, Elsevier; (D) Schematic illustration of the MnO2/MXene superlattice synthesis. Reprinted with permission[117]. Copyright 2023, American Chemical Society.
The third category involves combining manganese oxides with conductive materials (e.g., carbon nanotubes and graphene) to effectively improve the electrical conductivity and mechanical strength of electrodes. Guo et al. constructed a high-performance Zn/MnO2 battery based on salt anions in the aqueous electrolyte. As depicted in Figure 7C, MnO2 herein exhibits a vertically aligned nanosheet structure[116], which is crucial for the cycling stability of Zn/MnO2 aqueous batteries. Wang et al. fabricated 2D MnO2/MXene superlattices through liquid-phase preparation of single-layer MnO2 and Ti3C2Tx MXene nanosheets [Figure 7D]. In this process, single-layer MnO2 nanosheets and single-layer MXene nanosheets are arranged at intervals[117]. The MXene nanosheets can separate the MnO2 nanosheets and prevent them from aggregating. MXene nanosheets can separate MnO2 nanosheets to prevent aggregation; moreover, they can enhance electrical conductivity, maintain the stability of overall structural performance, and further enable rapid electron transport. Additionally, the regularly arranged and planarly stacked MnO2 nanosheets and MXene nanosheets can provide exposed active sites and enhance ion diffusion capability.
Overall, the morphology regulation of aqueous zinc-manganese batteries constitutes a multidimensional and comprehensive research domain. Diverse morphology regulation strategies interact synergistically, enhancing key metrics such as electrochemical performance, cycling stability, and safety of the batteries from multiple perspectives[118]. Morphology regulation offers a significant approach for enhancing the performance of AZIBs cathode materials by optimizing the structural dimension, pore network, and interface characteristics of MnO2. In the future, it is essential to integrate computational simulation to design morphological structures that possess both high capacity and long service life, and to advance the practical application of AZIBs. This lays a solid foundation for the practical application of aqueous
Defect engineering
The introduction of various defects into MnO2 cathode materials via atomic vacancies, element doping, or defect engineering can significantly modulate the electrochemical properties of AZIBs[119]. These defects create additional ion transport channels, which reduce Zn2+ diffusion resistance, accelerate charge transfer, and thus enhance rate capability[120]. The increased number of active sites facilitates electrochemical reactions, thereby improving the material’s adsorption and storage capacity for Zn2+ and further increasing the specific capacity of the battery[121]. Furthermore, defects can mitigate volume changes in the electrode during cycling, leading to enhanced structural stability. Oxygen vacancies facilitate the intercalation of Zn2+ by modulating the intercalation process of MnO2, offering a more accommodating pathway. Simultaneously, the oxygen vacancy, functioning as a positively charged defect, can attract Zn2+ via Coulombic interaction, thereby reducing the energy barrier associated with intercalation. Appropriate lattice defects can regulate the D-band center of MnO2, which strengthens the binding energy with Zn2+ and enhances the reversibility of the reaction. Nevertheless, excessive distortion will disrupt the periodicity of the layered structure, leading to capacity attenuation. Manganese vacancies can effectively optimize the electronic structure, create ion storage sites, inhibit dissolution, and stabilize the structure. Optimizing the electronic structure and ionic transport path of manganese dioxide through defect engineering is a crucial strategy for enhancing the performance of AZIBs[67].
As shown in Figure 8A, the transformation of α-MnO2 nanowires into Mo-Vo nanoparticles (as cathodes with rich surface oxygen defects) is achieved by adjusting annealing conditions in argon[122]. The small size of Mo-Vo nanoparticles can effectively promote the spatially uniform distribution of volume stress during ion intercalation, thereby improving the structural stability of the Mo-Vo cathode. By regulating the surface oxygen defects of MnO2, the Zn2+ intercalation pseudocapacitive behavior in the Mo-Vo cathode is significantly enhanced, which in turn improves cycling stability and redox kinetics. Amorphous MnO2 nanosheets with abundant oxygen vacancies were prepared using sodium carboxymethyl cellulose (CMC) as a capping agent, as illustrated in Figure 8B[123]. During growth, CMC preferentially attaches to the (003) faces of MnO2, guiding its crystal growth and regulating its morphology. The small nanosheets expose abundant edge sites and surface oxygen vacancies, which facilitate the insertion/extraction of H+ and Zn2+ in ZIBs. Additionally, CMC stabilizes Mn3+ during preparation and inhibits the Jahn-Teller effect. A novel
Figure 8. (A) The transition of nanowire (α-MnO2) to nanoparticle (MO-Vo). Reprinted with permission[122]. Copyright 2022, American Chemical Society; (B) Schematic drawing for fabricating the different MnO2 samples. Reprinted with permission[123]. Copyright 2022, John Wiley and Sons; (C) Pristine MnO2 and Al-intercalated MnO2. Reprinted with permission[124]. Copyright 2021, Elsevier; (D) Fabrication process of the MnO2@CC and MnO2-x@CC. Reprinted with permission[125]. Copyright 2021, American Chemical Society; (E) Schematic mechanism for the formation of hierarchical spheres with interlaced nanosheets via the F-doping of β-MnO2. Reprinted with permission[126]. Royal Society of Chemistry.
Development of electrolytes
The electrolyte functions as a “connector” between the positive and negative electrodes, offering them a practical operating environment. With the advancement of AZIBs, diverse zinc salts have been employed as electrolytes, primarily encompassing ZnSO4, Zn(ClO4)2, ZnCl2, Zn(CF3SO3)2, Zn(CH3COO)2, and
In general, conventional inorganic salt electrolytes for AZIBs have certain limitations, such as low Coulombic efficiency (below 75%) and a narrow electrochemical stability window (ESW)[127]. Researchers reported a method of applying Zn(CF3SO3)2 organic electrolyte in AZIBs, which exhibits high Coulombic efficiency (100%) and a wide electrochemical window. As shown in Figure 9A, oxygen evolution side reactions are significantly inhibited, which is much weaker than in ZnSO4 electrolytes[128]. Further experimental data demonstrate excellent reversibility and fast Zn deposition/stripping kinetics in the Zn(CF3SO3)2 electrolyte system. In aqueous rechargeable zinc-ion batteries (ARZIBs), prior to Zn2+ deposition, the zinc plate surface has inherent roughness. During deposition, protruding tips inevitably form, which increase the electric field strength and lead to the fastest ion deposition and dendrite formation. Figure 9B shows that a small amount of diethyl ether (Et2O) as an electrolyte additive can significantly improve the performance of Zn-MnO2 cells. An appropriate amount of Et2O molecules effectively inhibits zinc dendrite formation on the zinc anode[129], thereby enhancing the cycling stability of rechargeable aqueous zinc-ion batteries. The four-in-one functional additive triethyl phosphate (TEP) was incorporated into the conventional ZnSO4 electrolyte. Experimental results showed that TEP participates in forming a dense inorganic interface. The dendrite-free zinc anode exhibits higher stability and improved corrosion resistance. This enhances the battery’s stability and lifespan, as shown in Figure 9C[130]. Hao et al. reported an example of improving Zn/LiFePO4 battery performance by adding sodium benzenesulfonate (SDB) additives. In Figure 9D, the SDB-containing electrolyte enables smooth anodic Zn2+ deposition (instead of vertical growth of lamellar Zn dendrites) while enhancing Li+ migration in LiFePO4[131]. Researchers also reported a method to modify battery performance by adding Na+ to the electrolyte, with the main mechanism depicted in Figure 9E[132]. Na+ can form a positive electrostatic protective layer on the surface of zinc protrusions, fundamentally suppressing zinc dendrite formation. This results in highly reversible zinc stripping/plating processes with high Coulombic efficiency.
Figure 9. (A) Cyclic voltammograms of Zn electrode in 1M Zn(CF3SO3)2. Reprinted with permission[128]. Copyright 2016, American Chemical Society; (B) Schematics of morphology evolution for Zn anodes with and without Et2O additive. Reprinted with permission[129]. Copyright 2019, Elsevier; (C) Zn deposition process in blank ZnSO4 and TEP-containing electrolytes. Reprinted with permission[130]. Copyright 2024, John Wiley and Sons; (D) Schematic illustration of the hybrid aqueous Zn/LFP battery. Reprinted with permission[131]. Copyright 2019, John Wiley and Sons; (E) Schematic diagram about the effect of Na2SO4 additive. Reprinted with permission[132]. Copyright 2018, Springer Nature; (F) Schematic illustration of the Zn-MnO2 cell assembled in a UV-vis cuvette. Reprinted with permission[105]. Copyright 2022, Elsevier; (G) Schematic diagram of additives on the capacity and voltage. Reprinted with permission[133]. Copyright 2021, Elsevier; (H) Feasibility study for the designed Zn-Mn hybrid aqueous battery. Reprinted with permission[134]. Copyright 2020, John Wiley and Sons; (I) Schematic diagram of formation of stable coordinated hydrated structures. Reprinted with permission[135]. Copyright 2022, John Wiley and Sons; (J) Theory-Driven Design of a Cationic Accelerator for
In aqueous rechargeable Zn-MnO2 cells, the electrolyte pH gradually shifts alkaline during reactions, hindering reversible electrochemical processes. As shown in Figure 9F, a straightforward electrolyte modification approach was utilized. By comparing the pH of the electrolyte containing a pH indicator with its initial value, the proton release associated with Mn2+ additive oxidation and the stable voltage at the end of the charging cycle were determined[105]. The effect of floating voltage was also evaluated to maintain electrolyte pH stability. To date, electrolyte regulation via additives remains the simplest and most convenient method to modulate cathode voltage and capacity. Various additives (including Na+, Mg2+,
Other strategies
The interface regulation strategy can notably enhance the electrochemical performance. This is achieved by optimizing the electrode - electrolyte interface through the use of surface-coated carbon-based materials or metal oxide materials. By doing so, ion-selective channels are constructed to inhibit the dissolution of Mn and reduce the internal interface resistance when MnO2 serves as the cathode of AZIBs. The fundamental advantages of these strategies reside in suppressing side reactions, accelerating ion transport, and stabilizing the interface structure, offering a crucial approach to overcome the bottlenecks of cycle life and rate performance in AZIBs. As depicted in Figure 10A, manganese-based Prussian blue analog (Mn-PBA) is chosen as a seed layer to offer a stable surface for MnO2 electrodeposition[136]. Due to the substantial specific surface area of Mn-PBA, the deposition/dissolution kinetics between Mn2+ and MnO2 are notably enhanced. In Figure 10B, the inherent zwitterionic groups on sulfobetaine chains can offer distinct ion migration channels for positive and negative ions, which considerably promotes electrolyte ionic transport[137]. As illustrated in Figure 10C, this insight can only be gained after isolating the electrochemical reactions on the surface of MnO2 and eliminating any MnO2 deposits on the metal collector within the electrochemical cell[138]. As shown in Figure 10D, acesulfame potassium, serving as a co-solute with strong polarity, is incorporated into a conventional low-concentration aqueous electrolyte. This incorporation enhances the ionic conductivity of the electrolyte and accelerates the desolvation process of zinc ions at the electrode/electrolyte interface[139]. Figure 10E presents a comprehensive MnO2/Mn2+ redox chemistry, which is put forward to account for the abnormal electrochemical behavior of the β-MnO2 cathode. This suggests that a reversible dissolution/deposition process of layered manganese oxides takes place subsequent to the initial MnO2 dissolution reaction[100]. It reveals a new perspective for studying the working mechanism of AZIBs.
Figure 10. (A) Schematic illustration of ZnMn-PBA as a seed layer to assist MnO2 deposition-dissolution for cathode-free AZIBs. Reprinted with permission[136]. Copyright 2024, John Wiley and Sons; (B) Schematic of the fabrication of the ZSC-gel matrix. Reprinted with permission[137]. Copyright 2020, John Wiley and Sons; (C) Transformation of the electrochemical interface at a dynamic δ-MnO2 cathode. Reprinted with permission[138]. Copyright 2021, John Wiley and Sons; (D) Schematic diagram of the zinc anode status. Reprinted with permission[139]. Copyright 2025, John Wiley and Sons; (E) The schematic diagram of the proposed charge storage mechanism of the β-MnO2 cathode. Reprinted with permission[100]. Copyright 2022, Elsevier. AZIBs: Aqueous zinc-ion batteries.
CONCLUSIONS AND FURTHER PROSPECTS
MnO2, as a cathode material for AZIBs, has garnered significant attention owing to abundant manganese reserves, low cost, and high reversible capacity. As illustrated in Figure 11, with the advancement of MnO2 research, the ion storage mechanisms of MnO2 as a cathode in AZIBs during cycling primarily include the conversion reaction mechanism, Zn2+ intercalation mechanism, dissolution-deposition mechanism, and
Figure 11. Summary of the ion storage mechanisms, optimization strategies, and prospects of MnO2 for future high-performance AZIBs. AZIBs: Aqueous zinc-ion batteries.
The aforementioned modification strategies are mutually complementary, offering a multidimensional solution for the efficient application of MnO2 cathode materials in AZIBs. These strategies significantly enhance the cycle stability and rate capability of MnO2, thereby enabling its more efficient utilization. Regarding the current limitations of MnO2 as a cathode for AZIBs, future research directions will primarily focus on the following aspects:
Development of multifunctional materials: MnO2 is constrained by inherent drawbacks, such as poor electronic conductivity, inferior rate capability, and inadequate cycling stability, thus highlighting the critical need for developing multifunctional materials. Integrating MnO2 with conductive materials (e.g., graphene, carbon nanotubes) to fabricate multifunctional composites enhances the electrical conductivity of MnO2, increases the specific surface area of the material, and offers more active sites, thereby boosting its electrochemical reaction efficiency during Zn2+ insertion/extraction. Furthermore, surface modification techniques can enhance the conductivity and structural stability of MnO2, leading to improved overall battery performance. Controlling the particle size, morphology, and structure of MnO2 via nanotechnology increases its specific surface area, provides more active sites, and improves Zn2+ intercalation/deintercalation efficiency. Notably, engineering MnO2 into a 3D network structure helps mitigate volume expansion during Zn2+ insertion and reduces the risk of electrode structural rupture. Through these modification strategies, the electrochemical performance of MnO2 can be significantly enhanced, particularly its stability under long-term cycling and performance at high current densities.
Electrode/electrolyte interface optimization: The electrolyte/electrode interface in zinc-ion batteries is critical to the long-term stability and safety of AZIBs. The issues of zinc dendrite formation and interfacial instability can be addressed by designing appropriate electrolytes and interface layers. Electrolyte additives, such as organic acids and zinc salts, can modulate the deposition behavior of metallic zinc, suppress the uneven deposition of Zn2+, and inhibit dendrite growth. Furthermore, introducing a thin, conductive interlayer (e.g., conductive polymers, inorganic/organic composites) between the MnO2 cathode and zinc anode can effectively facilitate ion and electron transport, reduce side reactions at the electrode/electrolyte interface, and prevent corrosion between MnO2 and metallic zinc. Additionally, constructing a protective layer on the electrode surface using highly conductive polymers (e.g., polyaniline) or conductive carbon materials not only enhances interfacial stability but also promotes the smooth intercalation of Zn2+ during cycling, inhibits dendrite growth, and contributes to the development of stable AZIBs.
In-depth investigation of the zinc storage mechanism: Advanced characterization techniques, such as in-situ synchrotron radiation X-ray diffraction, are employed to explore the dynamic changes in microstructure, morphology, and composition of MnO2 materials during Zn2+ insertion and adsorption. Density functional theory (DFT) calculations are utilized to determine the optimal configuration of Zn2+ intercalated into MnO2. Moreover, the structural transformation, electrode/electrolyte interface stability, ion transport mechanisms, and characteristics of the cathode electrolyte interphase (CEI) film of MnO2 during cycling processes are systematically studied. The principles governing ion transport, interface stability rules, and criteria for structural stability are elaborated. Additionally, the correlation between microstructure and cycling performance remains to be clarified, and the storage behavior of sodium ions is analyzed. The integration of theoretical and experimental approaches provides crucial insights for optimizing MnO2 materials, offers theoretical support for enhancing their efficiency and durability in energy storage devices, and guides structural optimization in the development of MnO2-based composite materials.
Environmental friendliness and sustainable development: With the growing demand for AZIBs, the recycling and reuse of spent batteries have become increasingly critical. Recovering and reusing manganese from waste zinc-based batteries can not only reduce the cost of raw material procurement but also mitigate resource scarcity and environmental pollution. Although zinc-ion batteries pose lower environmental risks compared to lithium-ion batteries, substantial research efforts are still required for their waste treatment and recycling during large-scale application. The development of efficient recycling technologies enables the effective recovery of elements such as manganese and zinc from batteries, thereby reducing reliance on natural resources and enhancing the sustainability of the energy storage industry. Furthermore, the recovered manganese can be utilized to synthesize new MnO2 materials for battery cathodes, forming a closed-loop recycling system to improve resource recycling efficiency. This process not only minimizes the environmental impact of waste batteries but also reduces the demand for natural ores, thus further promoting the green development of aqueous zinc-ion batteries.
High-performance AZIBs hold great promise for large-scale grid energy storage applications. It is crucial to continuously optimize the performance of AZIBs, further enhance their energy density, long-cycle stability, cost-effectiveness, and recyclability, and advance their commercialization. However, to date, in the context of Zn//MnO2 batteries, critical challenges remain, including the ambiguity surrounding the internal mechanisms of intercalation-deintercalation and dissolution-deposition, as well as the failure to achieve high energy density. Addressing how to effectively integrate these two mechanisms, exploring the development of high-performance manganese-based electrodes, and fabricating practical aqueous zinc-ion batteries with high energy density and excellent cycling stability thus demands more in-depth investigation.
DECLARATIONS
Authors’ contributions
Developed the concept: Liu, Z.; Zhang, H.
Conducted the frame of the paper: Zhang, H.; Gong, Y.; Wang, L.
Gathered the initial characterizations and data analysis: Wang, J.; Gong, Z.
Collected the various works of literature: Huang, Z.; Zhou, L.
Written the manuscript, helped to polish this paper: Zhang, H.; Zha, D.
Helped to polish this paper: Du, Y.; Zhang, Q.; Liu, Z.
All authors discussed the results and commented on the manuscript. All authors have read and approved the final version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
The work was financially supported by the National Natural Science Foundation of China (No. 52204308) and the Natural Science Foundation of Liaoning Province (2023-MSBA-101).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Zhang, A.; Zhang, X.; Zhao, H.; et al. MnO2 superstructure cathode with boosted zinc ion intercalation for aqueous zinc ion batteries. J. Colloid. Interface. Sci. 2024, 669, 723-30.
2. Liang, Z.; Tian, F.; Yang, G.; Wang, C. Enabling long-cycling aqueous sodium-ion batteries via Mn dissolution inhibition using sodium ferrocyanide electrolyte additive. Nat. Commun. 2023, 14, 3591.
3. Wu, L.; Fu, H.; Li, S.; et al. Phase-engineered cathode for super-stable potassium storage. Nat. Commun. 2023, 14, 644.
4. Xu, J.; Zhang, J.; Pollard, T. P.; et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 2023, 614, 694-700.
5. Caracciolo, L.; Madec, L.; Petit, E.; et al. Electrochemical redox processes involved in carbon-coated KVPO4F for high voltage K-ion batteries revealed by XPS analysis. J. Electrochem. Soc. 2020, 167, 130527.
6. Zheng, J.; Sun, C.; Wang, Z.; et al. Double ionic-electronic transfer interface layers for all-solid-state lithium batteries. Angew. Chem. Int. Ed. 2021, 60, 18448-53.
7. Xu, S.; Xu, R.; Yu, T.; et al. Decoupling of ion pairing and ion conduction in ultrahigh-concentration electrolytes enables wide-temperature solid-state batteries. Energy. Environ. Sci. 2022, 15, 3379-87.
8. Zhang, Q.; Cheng, X.; Wang, C.; Rao, A. M.; Lu, B. Sulfur-assisted large-scale synthesis of graphene microspheres for superior potassium-ion batteries. Energy. Environ. Sci. 2021, 14, 965-74.
9. Wang, Y.; Yang, H.; Xu, J.; et al. Competitive coordination of sodium ions for high-voltage sodium metal batteries with fast reaction speed. J. Am. Chem. Soc. 2024, 146, 7332-40.
10. Zhang, Q.; Wang, L.; Wang, J.; et al. Low-temperature synthesis of edge-rich graphene paper for high-performance aluminum batteries. Energy. Storage. Mater. 2018, 15, 361-7.
11. Cao, W.; Zhang, E.; Wang, J.; et al. Potato derived biomass porous carbon as anode for potassium ion batteries. Electrochim. Acta. 2019, 293, 364-70.
12. Cheng, N.; Fan, L.; Liu, Z.; et al. Fluorine atom-inducing graphene oxide in situ coating SnPO composites as anode for sodium ion batteries. Mater. Today. Energy. 2019, 11, 174-81.
13. Wang, D.; Liu, Z.; Gao, X.; Gu, Q.; Zhao, L.; Luo, W. Massive anionic fluorine substitution two-dimensional δ-MnO2 nanosheets for high-performance aqueous zinc-ion battery. J. Energy. Storage. 2023, 72, 108740.
14. Ma, K.; Li, Q.; Hong, C.; Yang, G.; Wang, C. Bi doping-enhanced reversible-phase transition of α-MnO2 raising the cycle capability of aqueous Zn-Mn batteries. ACS. Appl. Mater. Interfaces. 2021, 13, 55208-17.
15. Shen, X.; Wang, X.; Zhou, Y.; et al. Highly reversible aqueous Zn-MnO2 battery by supplementing Mn2+-mediated MnO2 deposition and dissolution. Adv. Funct. Mater. 2021, 31, 2101579.
16. Li, C.; Chi, X.; Huang, J.; Wu, J.; Liu, Y. Reversible transformation of a zinc salt-boosted high areal capacity manganese dioxide cathode for energy-dense and stable aqueous zinc batteries. ACS. Appl. Energy. Mater. 2022, 5, 1478-86.
17. Ding, S.; Zhang, M.; Qin, R.; et al. Oxygen-deficient β-MnO2@graphene oxide cathode for high-rate and long-life aqueous zinc ion batteries. Nano-Micro. Lett. 2021, 13, 173.
18. Le, T.; Sadique, N.; Housel, L. M.; et al. Discharging behavior of hollandite α-MnO2 in a hydrated zinc-ion battery. ACS. Appl. Mater. Interfaces. 2021, 13, 59937-49.
19. Chen, X.; Ruan, P.; Wu, X.; Liang, S.; Zhou, J. Crystal structures, reaction mechanisms, and optimization strategies of MnO2 cathode for aqueous rechargeable zinc batteries. Acta. Phys. Chim. Sin. 2022, 38, 2111003-0.
20. Xie, J.; Yu, F.; Zhao, J.; et al. An irreversible electrolyte anion-doping strategy toward a superior aqueous Zn-organic battery. Energy. Storage. Mater. 2020, 33, 283-9.
21. Zeng, X.; Liu, J.; Mao, J.; et al. Toward a Reversible Mn4+ /Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy. Mater. 2020, 10, 1904163.
22. Wang, K.; Zhang, X.; Han, J.; et al. High-performance cable-type flexible rechargeable Zn battery based on MnO2@CNT fiber microelectrode. ACS. Appl. Mater. Interfaces. 2018, 10, 24573-82.
23. Zheng, J.; Shi, P.; Chen, C.; et al. Reinforced bonding of Mo-doped MnO2 with ammonium-ion as cathodes for durable aqueous MnO2-Zn batteries. Sci. China. Mater. 2023, 66, 3113-22.
24. Wang, K.; Luo, D.; Ma, Q.; Lai, X.; He, L.; Chen, Z. Advanced in situ and operando characterization techniques for zinc-ion batteries. Energy. Technol. 2024, 12, 2400199.
25. Zhou, W.; Fan, H. J.; Zhao, D.; Chao, D. Cathodic electrolyte engineering toward durable Zn-Mn aqueous batteries. Natl. Sci. Rev. 2023, 10, nwad265.
26. Chen, N.; Wang, W.; Ma, Y.; et al. Aqueous zinc-chlorine battery modulated by a MnO2 redox adsorbent. Small. Methods. 2024, 8, 2201553.
27. Huang, J.; Wang, Z.; Hou, M.; et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906.
28. Li, Y.; Wang, S.; Salvador, J. R.; et al. Reaction mechanisms for long-life rechargeable Zn/MnO2 batteries. Chem. Mater. 2019, 31, 2036-47.
29. Nguyen, T. N.; Iranpour, B.; Cheng, E.; Madden, J. D. W. Washable and stretchable Zn-MnO2 rechargeable cell. Adv. Energy. Mate. 2022, 12, 2103148.
30. Wang, Z.; Fang, Y.; Shi, J.; Ma, Z.; Qu, X.; Li, P. Conversion reaction of the zinc sulfate hydroxide activated by voltage modulation for high-performance aqueous Zn/MnO2 batteries. Adv. Energy. Mater. 2024, 14, 2303739.
31. Gong, Z.; Liu, Z.; Gao, X. W.; et al. Constructing cyclic hydrogen bonding to suppress side reactions and dendrite formation on zinc anodes. Chem. Eur. J. 2024, 30, e202402558.
32. Wang, S.; Yuan, Z.; Zhang, X.; et al. Non-metal ion co-insertion chemistry in aqueous Zn/MnO2 batteries. Angew. Chem. Int. Ed. 2021, 60, 7056-60.
33. Chen, H.; Zhao, L. K.; Li, S. D.; et al. Suppression of structural degradation in molybdenum-modified layered oxides for high-performance potassium-ion batteries. J. Colloid. Interface. Sci. 2025, 695, 137733.
34. Liao, X.; Pan, C.; Yan, H.; Zhu, Y.; Pan, Y.; Yin, C. Polyaniline-functionalized graphene composite cathode with enhanced Zn2+ storage performance for aqueous zinc-ion battery. Chem. Eng. J. 2022, 440, 135930.
35. Zhang, T.; Wu, X. W.; Jiang, J. B. Energy storage mechanism, issue and modification strategies of vanadiumbased cathode materials for aqueous zinc ion batteries. Chinese. J. Rare. Metals. 2023, 47, 399-424.
36. Yin, C.; Pan, C.; Pan, Y.; Hu, J. Hierarchical spheroidal MOF-derived MnO@C as cathode components for high-performance aqueous zinc ion batteries. J. Colloid. Interface. Sci. 2023, 642, 513-22.
37. Zhang, Q.; Gao, X.; Liu, X.; et al. Flexible wearable energy storage devices: materials, structures, and applications. Battery. Energy. 2024, 3, 20230061.
38. Sambandam, B.; Mathew, V.; Ahmad, N. F.; Kim, S.; Song, M.; Kim, J. Aqueous rechargeable zinc-metal batteries: a critical analysis. ACS. Energy. Lett. 2024, 9, 3058-65.
39. Zhang, A.; Yin, X.; Saadoune, I.; Wei, Y.; Wang, Y. Zwitterion intercalated manganese dioxide nanosheets as high-performance cathode materials for aqueous zinc ion batteries. Small 2024, 20, 2402811.
40. Zhang, Q.; Zhao, J.; Chen, X.; et al. Unveiling the energy storage mechanism of MnO2 polymorphs for zinc-manganese dioxide batteries. Adv. Funct. Mater. 2024, 34, 2306652.
41. Ding, H.; Zhang, Q.; Liu, Z.; et al. TiO2 quantum dots decorated multi-walled carbon nanotubes as the multifunctional separator for highly stable lithium sulfur batteries. Electrochim. Acta. 2018, 284, 314-20.
42. Liu, Y.; Xiang, K.; Zhou, W.; Deng, W.; Zhu, H.; Chen, H. Investigations on tunnel-structure MnO2 for utilization as a high-voltage and long-life cathode material in aqueous ammonium-ion and hybrid-ion batteries. Small 2024, 20, 2308741.
43. Paik, S.; Choi, I.; Lee, S.; Nam, K. W. Chelating effects of polyphenolic biomolecules to improve β-MnO2 cathode performance for aqueous rechargeable zinc-ion batteries. ACS. Appl. Mater. Interfaces. 2024, 16, 50775-84.
44. Xia, J.; Zhou, Y.; Zhang, J.; et al. Triggering high capacity and superior reversibility of manganese oxides cathode via magnesium modulation for Zn//MnO2 batteries. Small 2023, 19, 2301906.
45. Yin, C.; Pan, C.; Pan, Y.; Hu, J.; Fang, G. Proton self-doped polyaniline with high electrochemical activity for aqueous zinc-ion batteries. Small. Methods. 2023, 7, 2300574.
46. Li, G.; Sun, L.; Zhang, S.; et al. Developing cathode materials for aqueous zinc ion batteries: challenges and practical prospects. Adv. Funct. Mater. 2024, 34, 2301291.
47. Li, H.; Liu, Z.; Tang, Y.; Liang, S.; Fang, G. Copper-based materials in anode electrode of aqueous zinc metal batteries. cMat 2024, 1, e25.
48. Li, L.; Yin, C.; Han, R.; Zhong, F.; Hu, J. CNT composite β-MnO2 with fiber cable shape as cathode materials for aqueous zinc-ion batteries. Inorg. Chem. 2024, 63, 13100-9.
49. Yin, C.; Pan, C.; Pan, Y.; Hu, J. Hollow Mn-Co-O@C yolk-shell microspheres with carbon shells as cathodes derived from a double-metal MOF for aqueous zinc-ion batteries. ACS. Sustain. Chem. Eng. 2023, 11, 12397-405.
50. Yin, C.; Wang, H.; Pan, C.; Li, Z.; Hu, J. Constructing MOF-derived V2O5 as advanced cathodes for aqueous zinc ion batteries. J. Energy. Storage. 2023, 73, 109045.
51. Han, R.; Pan, Y.; Du, C.; et al. Eu doping β-MnO2 as cathode materials for high specific capacity aqueous zinc ion batteries. J. Energy. Storage. 2024, 80, 110250.
52. Lin, C.; He, L.; Xiong, P.; et al. Adaptive ionization-induced tunable electric double layer for practical Zn metal batteries over wide pH and temperature ranges. ACS. Nano. 2023, 17, 23181-93.
53. Xiong, P.; Lin, C.; Wei, Y.; et al. Charge-transfer complex-based artificial layers for stable and efficient Zn metal anodes. ACS. Energy. Lett. 2023, 8, 2718-27.
54. Li, Y.; Li, Y.; Liu, Q.; et al. Revealing the dominance of the dissolution-deposition mechanism in aqueous Zn-MnO2 batteries. Angew. Chem. Int. Ed. 2024, 63, e202318444.
55. Kitchaev, D. A.; Dacek, S. T.; Sun, W.; Ceder, G. Thermodynamics of phase selection in MnO2 framework structures through alkali intercalation and hydration. J. Am. Chem. Soc. 2017, 139, 2672-81.
56. Lian, S.; Sun, C.; Xu, W.; et al. Built-in oriented electric field facilitating durable Zn MnO2 battery. Nano. Energy. 2019, 62, 79-84.
57. Xiong, P.; Zhang, Y.; Zhang, J.; et al. Recent progress of artificial interfacial layers in aqueous Zn metal batteries. EnergyChem 2022, 4, 100076.
58. Wang, T.; Jin, J.; Zhao, X.; Qu, X.; Jiao, L.; Liu, Y. Unraveling the anionic redox chemistry in aqueous zinc-MnO2 batteries. Angew. Chem. Int. Ed. 2024, 63, e202412057.
59. Aguilar, I.; Brown, J.; Godeffroy, L.; et al. A key advance toward practical aqueous Zn/MnO2 batteries via better electrolyte design. Joule 2025, 9, 101784.
60. Fu, H.; Huang, S.; Wang, C.; et al. Exploring hybrid electrolytes for Zn metal batteries. Adv. Energy. Mater. , 2025, 2501152.
61. Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus nonoxide cathode materials for aqueous Zn batteries: an insight into the charge storage mechanism and consequences thereof. ACS. Appl. Mater. Interfaces. 2019, 11, 674-82.
62. Pan, H.; Ellis, J. F.; Li, X.; Nie, Z.; Chang, H. J.; Reed, D. Electrolyte effect on the electrochemical performance of mild aqueous zinc-electrolytic manganese dioxide batteries. ACS. Appl. Mater. Interfaces. 2019, 11, 37524-30.
63. Yadav, G. G.; Turney, D.; Huang, J.; Wei, X.; Banerjee, S. Breaking the 2 V barrier in aqueous zinc chemistry: creating 2.45 and 2.8 V MnO2-Zn aqueous batteries. ACS. Energy. Lett. 2019, 4, 2144-6.
64. Zhang, X.; Wu, S.; Deng, S.; et al. 3D CNTs networks enable MnO2 cathodes with high capacity and superior rate capability for flexible rechargeable Zn-MnO2 batteries. Small. Methods. 2019, 3, 1900525.
65. Zhang, Y.; Deng, S.; Luo, M.; et al. Defect promoted capacity and durability of N-MnO2-x branch arrays via low-temperature NH3 treatment for advanced aqueous zinc ion batteries. Small 2019, 15, 1905452.
66. Gao, X.; Wu, H.; Li, W.; et al. H+-insertion boosted α-MnO2 for an aqueous Zn-ion battery. Small 2020, 16, 1905842.
67. Zhang, M.; Tong, Y.; Sun, Z.; et al. Two-dimen sional covalent organic framework with synergistic active centers for efficient electrochemical sodium storage. Chem. Mater. 2023, 35, 4873-81.
68. Ding, C.; Wang, Y.; Li, C.; Wang, J.; Zhang, Q.; Huang, W. Constructing ultra-stable, high-energy, and flexible aqueous zinc-ion batteries using environment-friendly organic cathodes. Chem. Sci. 2024, 15, 4952-9.
69. Su, J.; Zhang, M.; Tian, H.; et al. Synergistic π-conjugation organic cathode for ultra-stable aqueous aluminum batteries. Small 2024, 20, 2312086.
70. Tong, Y.; Sun, Z.; Wang, J.; Huang, W.; Zhang, Q. Covalent organic framework containing dual redox centers as an efficient anode in Li-ion batteries. SmartMat 2022, 3, 685-94.
71. Zhao, L.; Gao, X.; Gu, Q.; et al. Realizing a dendrite-free metallic-potassium anode using reactive prewetting chemistry. eScience 2024, 4, 100201.
72. Qin, Z.; Song, Y.; Yang, D.; et al. Enabling reversible MnO2/Mn2+ transformation by Al3+ addition for aqueous Zn-MnO2 hybrid batteries. ACS. Appl. Mater. Interfaces. 2022, 14, 10526-34.
73. Zuo, Y.; Liu, P.; Ling, L.; et al. Boosted H+ intercalation enables ultrahigh rate performance of the δ-MnO2 cathode for aqueous zinc batteries. ACS. Appl. Mater. Interfaces. 2022, 14, 26653-61.
74. Huang, X.; Liu, X.; Li, H.; Zhao, Q.; Ma, T. Revealing the real charge carrier in aqueous zinc batteries based on polythiophene/manganese dioxide cathode. Small. Struct. 2023, 4, 2200221.
75. Li, J.; Mu, J.; Liu, Z.; et al. Boosting potassium-based dual ion battery with high energy density and long lifespan by red phosphorous. J. Power. Sources. 2023, 571, 233054.
76. Chen, Y.; Zhao, L.; Zhou, J.; et al. Advances in the use of carbonaceous scaffolds for constructing stable composite Li metal anodes. New. Carbon. Mater. 2023, 38, 698-718.
77. Yu, W.; Ge, J.; Hu, Y.; et al. Hybrid high-performance aqueous batteries with potassium-based cathode||zinc metal anode. Sci. China. Mater. 2023, 66, 923-31.
78. Kim, S. J.; Wu, D.; Sadique, N.; et al. Unraveling the dissolution-mediated reaction mechanism of α-MnO2 cathodes for aqueous Zn-ion batteries. Small 2020, 16, 2005406.
79. Li, G.; Chen, W.; Zhang, H.; et al. Membrane-free Zn/MnO2 flow battery for large-scale energy storage. Adv. Energy. Mater. 2020, 10, 1902085.
80. Wang, C.; Wang, M.; He, Z.; Liu, L.; Huang, Y. Rechargeable aqueous zinc-manganese dioxide/graphene batteries with high rate capability and large capacity. ACS. Appl. Energy. Mater. 2020, 3, 1742-8.
81. Wang, S. B.; Ran, Q.; Yao, R. Q.; et al. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634.
82. Liu, Y.; Shi, Q.; Wu, Y.; Wang, Q.; Huang, J.; Chen, P. Highly efficient dendrite suppressor and corrosion inhibitor based on gelatin/Mn2+ Co-additives for aqueous rechargeable zinc-manganese dioxide battery. Chem. Eng. J. 2021, 407, 127189.
83. Wu, B.; Zhang, G.; Yan, M.; et al. Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 2018, 14, 1703850.
84. Jiang, Y.; Ba, D.; Li, Y.; Liu, J. Noninterference revealing of “layered to layered” zinc storage mechanism of δ-MnO2 toward neutral Zn-Mn batteries with superior performance. Adv. Sci. 2020, 7, 1902795.
85. Alfaruqi, M. H.; Mathew, V.; Gim, J.; et al. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609-20.
86. Alfaruqi, M. H.; Gim, J.; Kim, S.; et al. A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications. Electrochem. Commun. 2015, 60, 121-5.
87. Meng, L.; Zhu, Y.; Lu, Y.; et al. Rechargeable Zn-MnO2 batteries: progress, challenges, rational design, and perspectives. ChemElectroChem 2024, 11, e202300495.
88. Liu, Y.; Zhi, J.; Sedighi, M.; et al. Mn 2+ ions confined by electrode microskin for aqueous battery beyond intercalation capacity. Adv. Energy. Mater. 2020, 10, 2002578.
89. Jin, Y.; Zou, L.; Liu, L.; et al. Joint charge storage for high-rate aqueous zinc-manganese dioxide batteries. Adv. Mater. 2019, 31, 1900567.
90. Zhang, R.; Liang, P.; Yang, H.; et al. Manipulating intercalation-extraction mechanisms in structurally modulated δ-MnO2 nanowires for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2022, 433, 133687.
91. Zhao, W.; Kong, Q.; Wu, X.; et al. ε-MnO2@C cathode with high stability for aqueous zinc-ion batteries. Appl. Surf. Sci. 2022, 605, 154685.
92. Li, Y.; Li, X.; Duan, H.; et al. Aerogel-structured MnO2 cathode assembled by defect-rich ultrathin nanosheets for zinc-ion batteries. Chem. Eng. J. 2022, 441, 136008.
93. Liao, Y.; Chen, H.; Yang, C.; et al. Unveiling performance evolution mechanisms of MnO2 polymorphs for durable aqueous zinc-ion batteries. Energy. Storage. Mater. 2022, 44, 508-16.
94. Yadav, P.; Kumari, N.; Rai, A. K. A review on solutions to overcome the structural transformation of manganese dioxide-based cathodes for aqueous rechargeable zinc ion batteries. J. Power. Sources. 2023, 555, 232385.
95. An, N.; Xin, J.; Li, W.; et al. 3D Binder-free conjugated microporous polymer carbon Aerogels@MnO2 cathode for high-performance aqueous zinc ion batteries. Appl. Surf. Sci. 2022, 599, 153881.
96. Wang, H.; Liang, M.; Gao, J.; et al. Robust structural stability of flower-like δ-MnO2 as cathode for aqueous zinc ion battery. Colloids. Surf. A. Phys. Eng. Aspects. 2022, 643, 128804.
97. Lai, G.; Ruan, P.; Hu, X.; et al. Dynamic compensation of MnOOH to mitigate the irregular dissolution of MnO2 in rechargeable aqueous Zn/MnO2 batteries. J. Mater. Chem. A. 2023, 11, 15211-8.
98. Siamionau, U.; Aniskevich, Y.; Mazanik, A.; et al. Rechargeable zinc-ion batteries with manganese dioxide cathode: how critical is choice of manganese dioxide polymorphs in aqueous solutions? J. Power. Sources. 2022, 523, 231023.
99. Zhao, S.; Han, B.; Zhang, D.; et al. Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries. J. Mater. Chem. A. 2018, 6, 5733-9.
100. Li, H.; Yao, H.; Sun, X.; et al. Interface regulated MnO2/Mn2+ redox chemistry in aqueous Zn ion batteries. Chem. Eng. J. 2022, 446, 137205.
101. Yang, H.; Zhou, W.; Chen, D.; et al. The origin of capacity fluctuation and rescue of dead Mn-based Zn-ion batteries: a Mn-based competitive capacity evolution protocol. Energy. Environ. Sci. 2022, 15, 1106-18.
102. Ye, X.; Han, D.; Jiang, G.; et al. Unraveling the deposition/dissolution chemistry of MnO2 for high-energy aqueous batteries. Energy. Environ. Sci. 2023, 16, 1016-23.
103. Cui, S.; Zhang, D.; Zhang, G.; Gan, Y. Reaction mechanism for the α-MnO2 cathode in aqueous Zn ion batteries revisited: elucidating the irreversible transformation of α-MnO2 into Zn-vernadite. J. Mater. Chem. A. 2022, 10, 25620-32.
104. Qiu, C.; Zhu, X.; Xue, L.; et al. The function of Mn2+ additive in aqueous electrolyte for Zn/δ-MnO2 battery. Electrochim. Acta. 2020, 351, 136445.
105. Perez-antolin, D.; Sáez-bernal, I.; Colina, A.; Ventosa, E. Float-charging protocol in rechargeable Zn-MnO2 batteries: unraveling the key role of Mn2+ additives in preventing spontaneous pH changes. Electrochem. Commun. 2022, 138, 107271.
106. Yao, H.; Yu, H.; Zheng, Y.; et al. Pre-intercalation of ammonium ions in layered δ-MnO2 nanosheets for high-performance aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202315257.
107. Wang, Y.; Zhang, Y.; Gao, G.; et al. Effectively modulating oxygen vacancies in flower-like δ-MnO2 nanostructures for large capacity and high-rate zinc-ion storage. Nano-Micro. Lett. 2023, 15, 219.
108. Zhang, A.; Zhao, R.; Wang, Y.; et al. Hybrid superlattice-triggered selective proton grotthuss intercalation in δ-MnO2 for high-performance zinc-ion battery. Angew. Chem. Int. Ed. 2023, 62, e202313163.
109. Wu, L.; Mei, Y.; Liu, Y.; et al. Interfacial synthesis of strongly-coupled δ-MnO2/MXene heteronanosheets for stable zinc ion batteries with Zn2+-exclusive storage mechanism. Chem. Eng. J. 2023, 459, 141662.
110. Xu, X.; Chen, Y.; Li, W.; et al. Achieving ultralong-cycle zinc-ion battery via synergistically electronic and structural regulation of a MnO2 nanocrystal-carbon hybrid framework. Small 2023, 19, 2207517.
111. Li, X.; Ji, C.; Shen, J.; et al. Amorphous heterostructure derived from divalent manganese borate for ultrastable and ultrafast aqueous zinc ion storage. Adv. Sci. 2023, 10, 2205794.
112. Li, W.; Wang, D. Conversion-type cathode materials for aqueous Zn metal batteries in nonalkaline aqueous electrolytes: progress, challenges, and solutions. Adv. Mater. 2023, 2304983.
113. Li, X.; Xu, Z.; Qian, Y.; Hou, Z. In-situ regulated competitive proton intercalation and deposition/dissolution reaction of MnO2 for high-performance flexible zinc-manganese batteries. Energy. Storage. Mater. 2022, 53, 72-8.
114. Guo, C.; Liu, H.; Li, J.; et al. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim. Acta. 2019, 304, 370-7.
115. Yu, B.; Lu, L.; He, Y.; et al. Hierarchical porous CS@Ce-MnO2 as cathode for energy-dense and long-cycling flexible aqueous zinc-ion batteries. J. Colloid. Interface. Sci. 2024, 654, 56-65.
116. Guo, C.; Zhou, Q.; Liu, H.; et al. A case study of β- and δ-MnO2 with different crystallographic forms on ion-storage in rechargeable aqueous zinc ion battery. Electrochim. Acta. 2019, 324, 134867.
117. Wang, Y.; Liu, L.; Wang, Y.; Qu, J.; Chen, Y.; Song, J. Atomically coupled 2D MnO2/MXene superlattices for ultrastable and fast aqueous zinc-ion batteries. ACS. Nano. 2023, 17, 21761-70.
118. Chen, H.; Cai, S.; Wu, Y.; Wang, W.; Xu, M.; Bao, S. Successive electrochemical conversion reaction to understand the performance of aqueous Zn/MnO2 batteries with Mn2+ additive. Mater. Today. Energy. 2021, 20, 100646.
119. Silapasom, W.; Kao-ian, W.; Wannapaiboon, S.; Opchoei, M.; Kheawhom, S. Enhancing zinc-ion batteries: PEDOT-MnO2 cathodes for superior stability and capacity. Radiat. Phys. Chem. 2024, 223, 111935.
120. Zhang, N.; Cheng, F.; Liu, J.; et al. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405.
121. Guo, X.; Zhou, J.; Bai, C.; Li, X.; Fang, G.; Liang, S. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater. Today. Energy. 2020, 16, 100396.
122. Han, K.; Wang, Z.; An, F.; et al. Boosting aqueous Zn/MnO2 batteries via a synergy of edge/defect-rich cathode and dendrite-free anode. ACS. Appl. Mater. Interfaces. 2022, 14, 4316-25.
123. Zhang, X.; Ma, X.; Bi, H.; et al. Carboxymethylcellulose induced the formation of amorphous MnO2 nanosheets with abundant oxygen vacancies for fast ion diffusion in aqueous zinc-ion batteries. Adv. Funct. Mater. 2025, 35, 2411990.
124. Chen, C.; Shi, M.; Zhao, Y.; Yang, C.; Zhao, L.; Yan, C. Al-intercalated MnO2 cathode with reversible phase transition for aqueous Zn-ion batteries. Chem. Eng. J. 2021, 422, 130375.
125. Zhao, J.; Xu, Z.; Zhou, Z.; et al. A safe flexible self-powered wristband system by integrating defective MnO2-x nanosheet-based zinc-ion batteries with perovskite solar cells. ACS. Nano. 2021, 15, 10597-608.
126. Kim, S.; Koo, B.; Jo, Y.; et al. Defect engineering via the F-doping of β-MnO2 cathode to design hierarchical spheres of interlaced nanosheets for superior high-rate aqueous zinc ion batteries. J. Mater. Chem. A. 2021, 9, 17211-22.
127. Lee, B.; Seo, H. R.; Lee, H. R.; et al. Critical role of pH evolution of electrolyte in the reaction mechanism for rechargeable zinc batteries. ChemSusChem 2016, 9, 2948-56.
128. Zhang, N.; Cheng, F.; Liu, Y.; et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 2016, 138, 12894-901.
129. Xu, W.; Zhao, K.; Huo, W.; et al. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano. Energy. 2019, 62, 275-81.
130. Wang, P.; Zhou, H.; Zhong, Y.; Sui, X.; Sun, G.; Wang, Z. Dendrite-free Zn metal anodes with boosted stability achieved by four-in-one functional additive in aqueous rechargeable zinc batteries. Adv. Energy. Mater. 2024, 14, 2401540.
131. Hao, J.; Long, J.; Li, B.; et al. Toward high-performance hybrid Zn-Based batteries via deeply understanding their mechanism and using electrolyte additive. Adv. Funct. Mater. 2019, 29, 1903605.
132. Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656.
133. Guo, S.; Qin, L.; Zhang, T.; et al. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries. Energy. Storage. Mater. 2021, 34, 545-62.
134. Chao, D.; Ye, C.; Xie, F.; et al. Atomic engineering catalyzed MnO2 electrolysis kinetics for a hybrid aqueous battery with high power and energy density. Adv. Mater. 2020, 32, 2001894.
135. Chuai, M.; Yang, J.; Tan, R.; et al. Theory-driven design of a cationic accelerator for high-performance electrolytic MnO2-Zn batteries. Adv. Mater. 2022, 34, 2203249.
136. Qi, Y.; Li, F.; Sheng, H.; et al. Seed-assisted reversible dissolution/deposition of MnO2 for long-cyclic and green aqueous zinc-ion batteries. Small 2024, 20, 2404312.
137. Mo, F.; Chen, Z.; Liang, G.; et al. Zwitterionic sulfobetaine hydrogel electrolyte building separated positive/negative ion migration channels for aqueous Zn-MnO2 batteries with superior rate capabilities. Adv. Energy. Mater. 2020, 10, 2000035.
138. Becknell, N.; Lopes, P. P.; Hatsukade, T.; et al. Employing the dynamics of the electrochemical interface in aqueous zinc-ion battery cathodes. Adv. Funct. Mater. 2021, 31, 2102135.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data







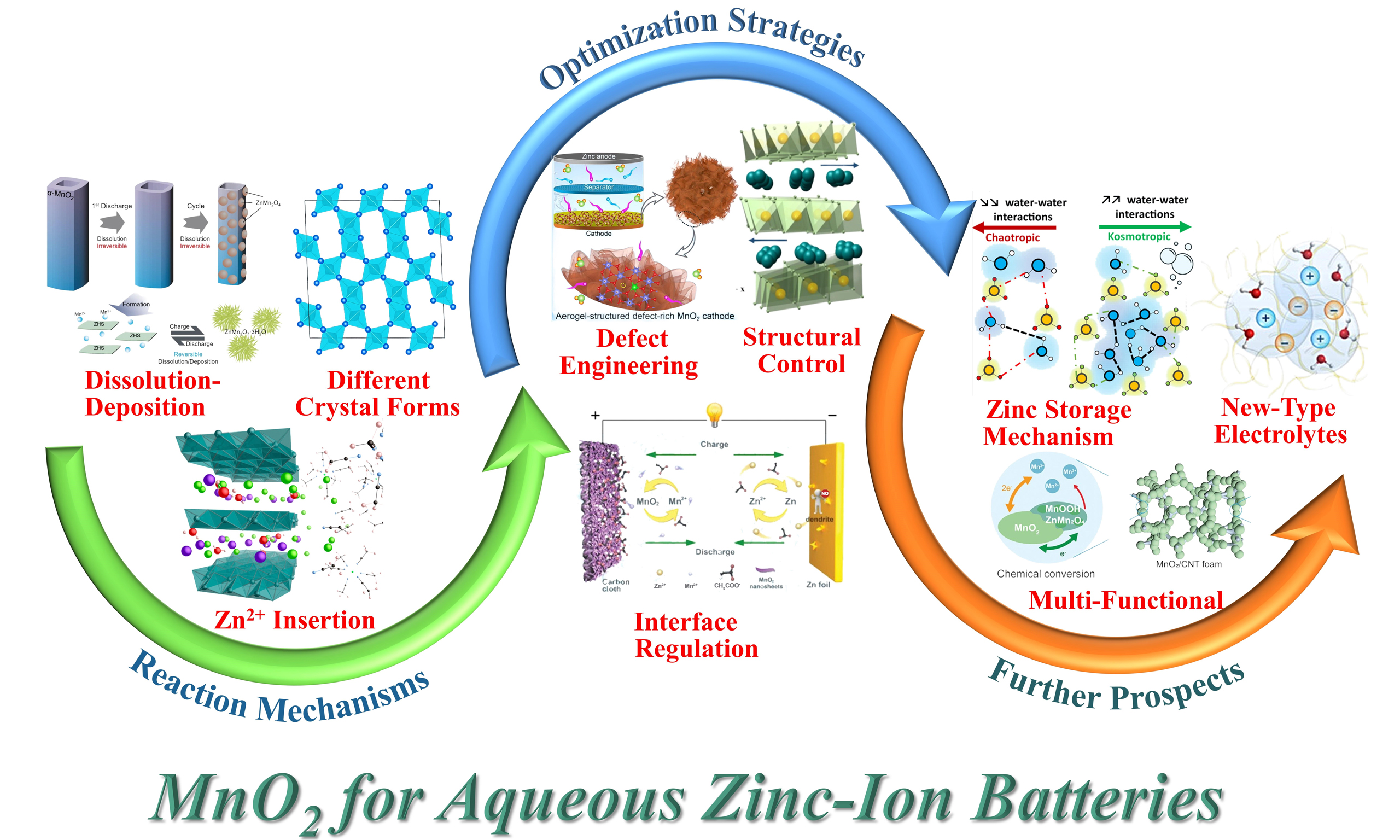
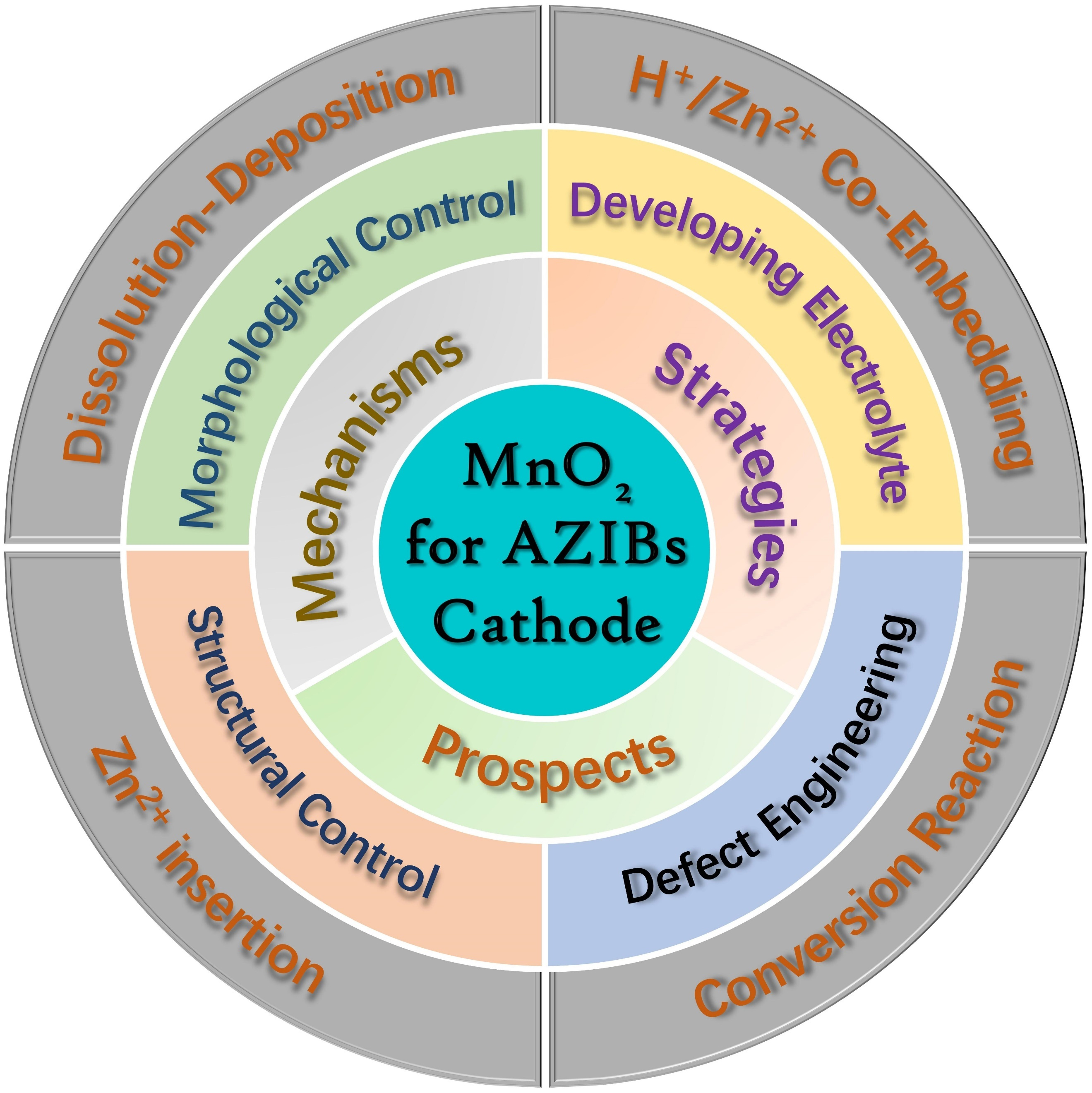
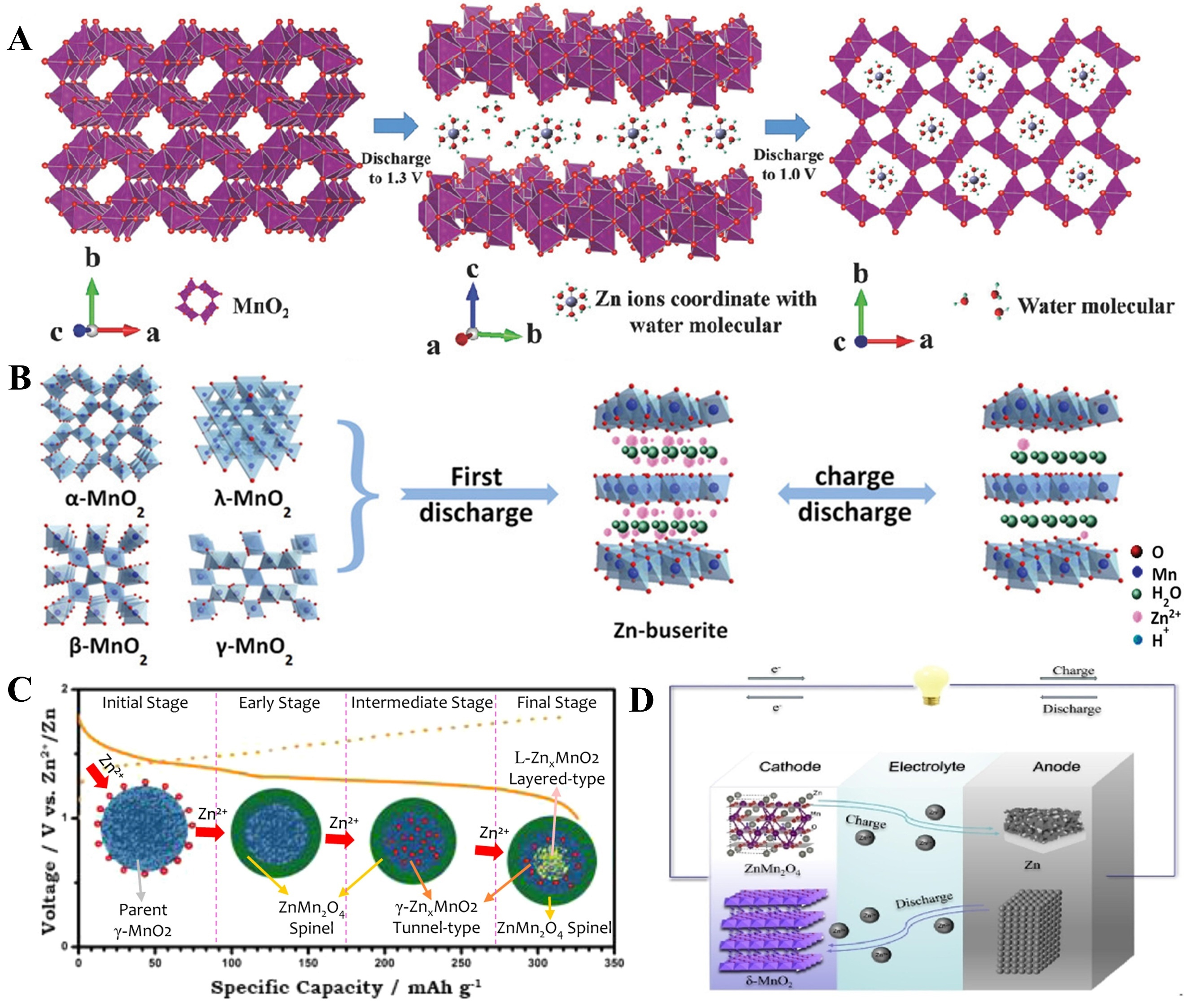
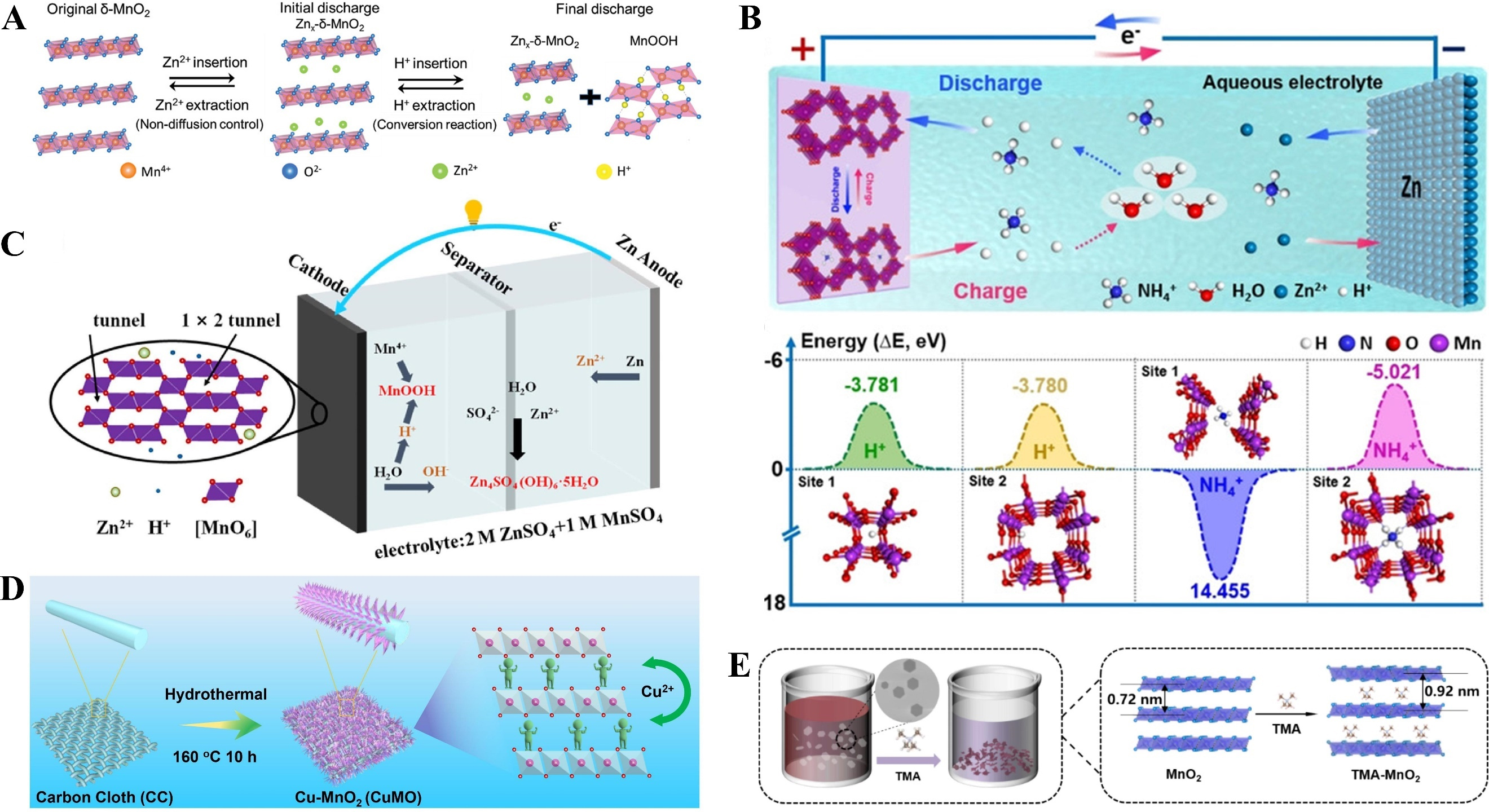
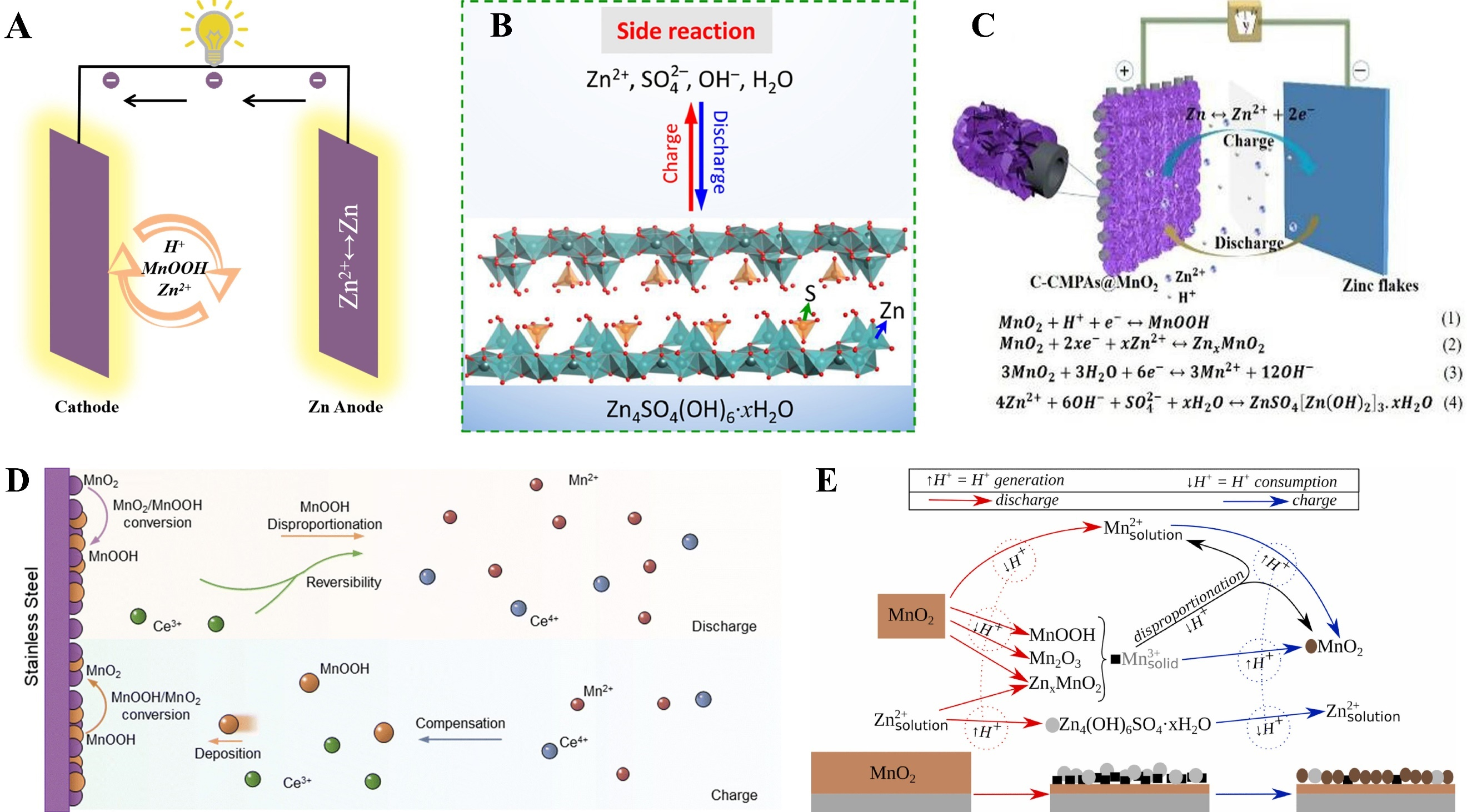
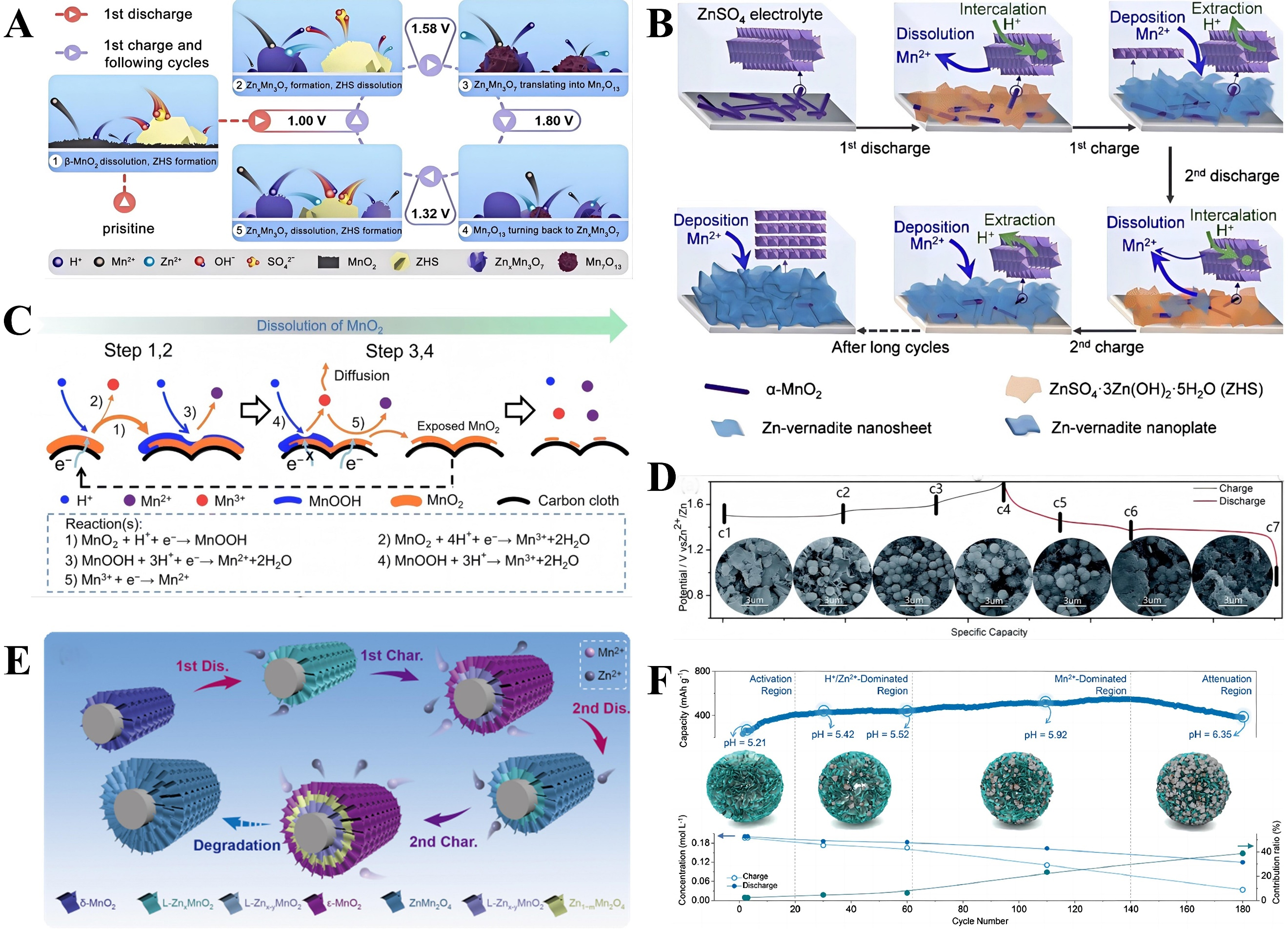
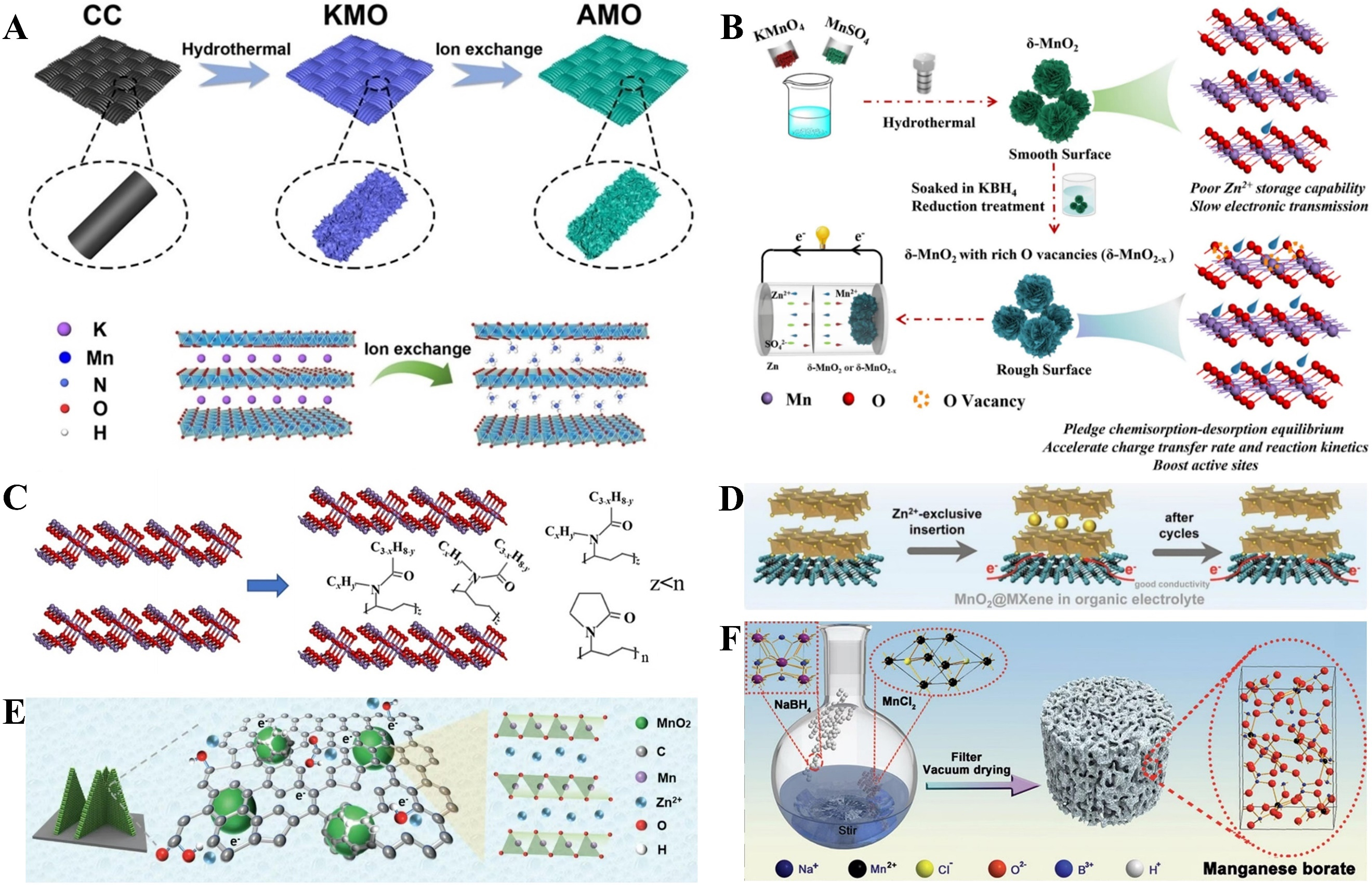
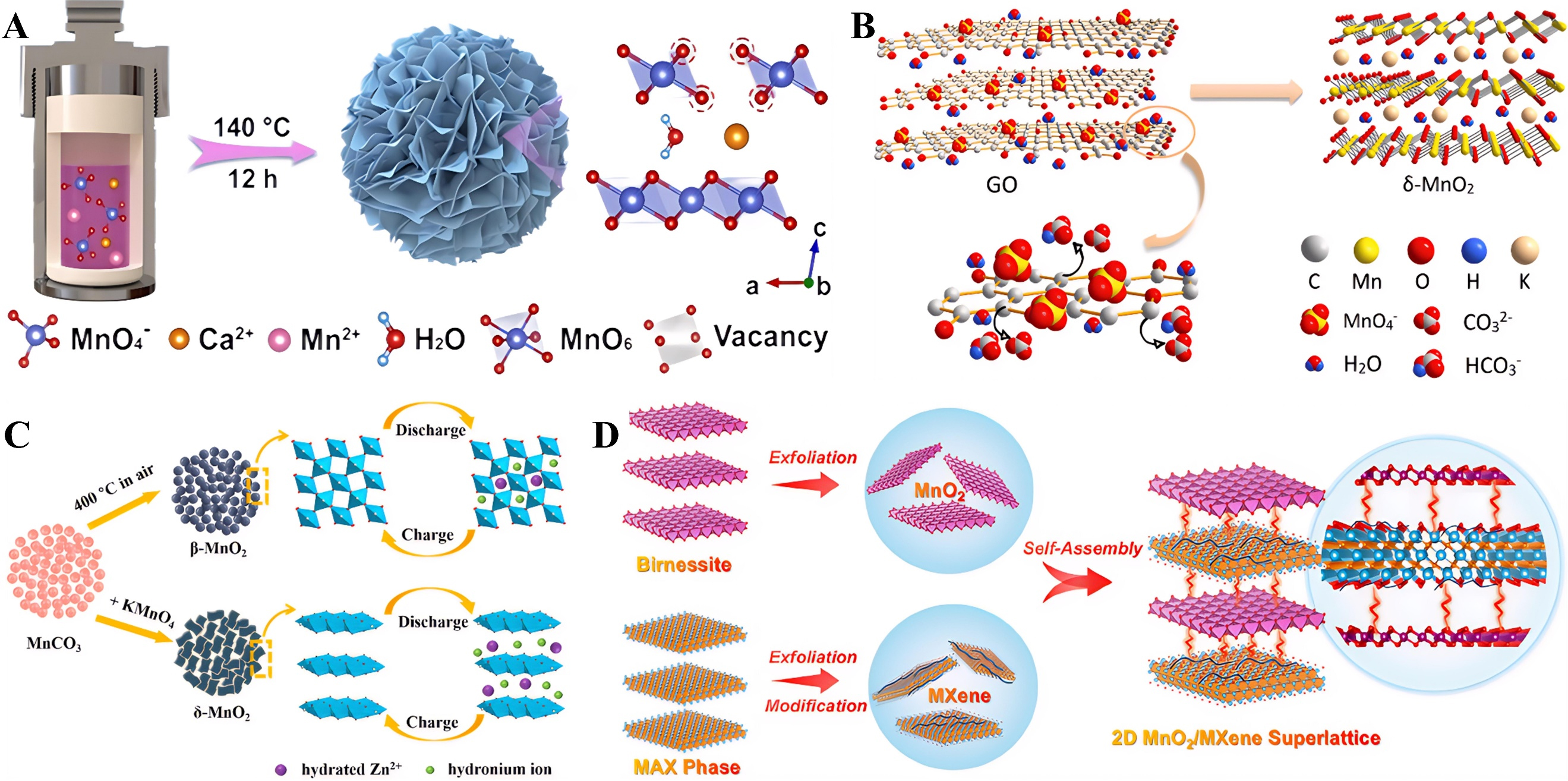
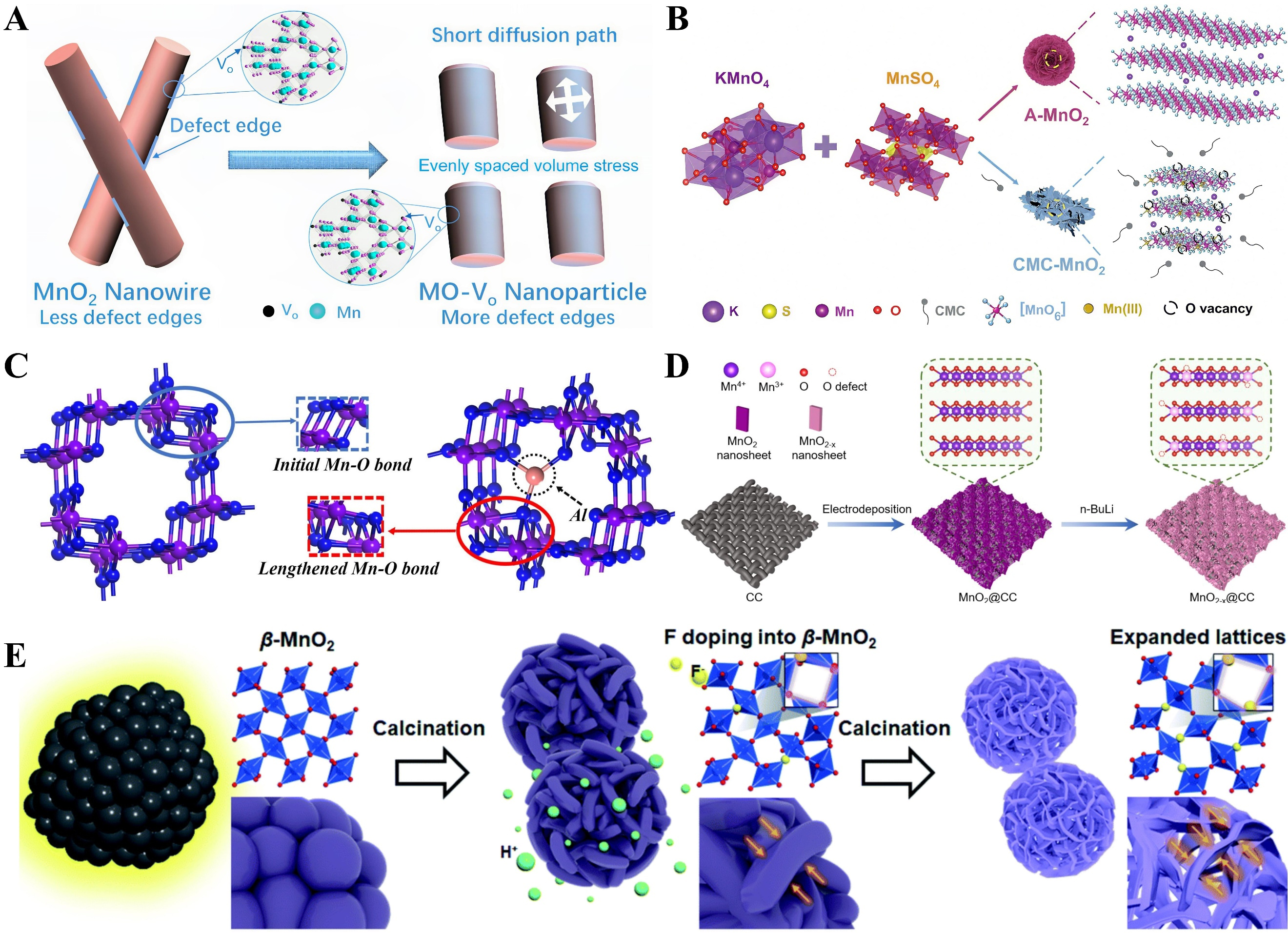
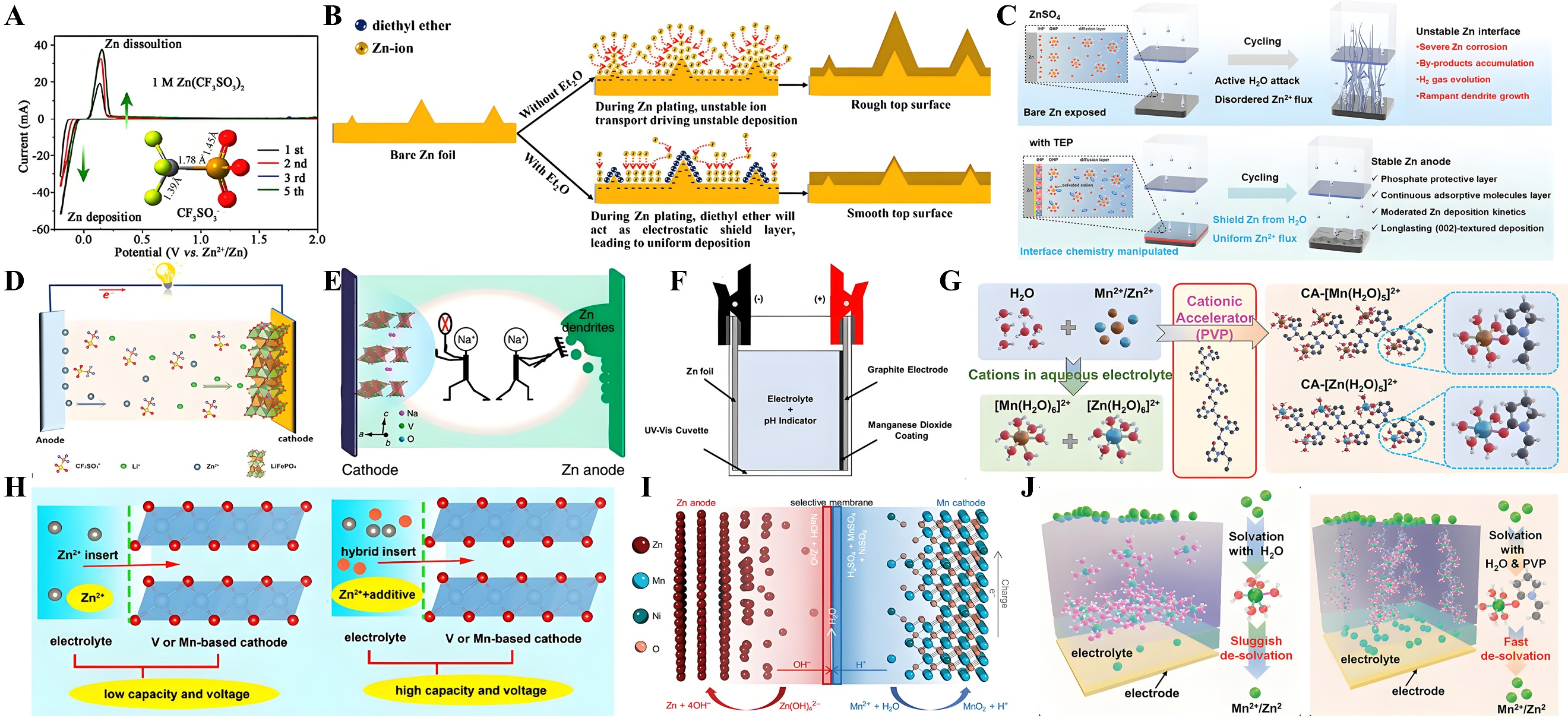
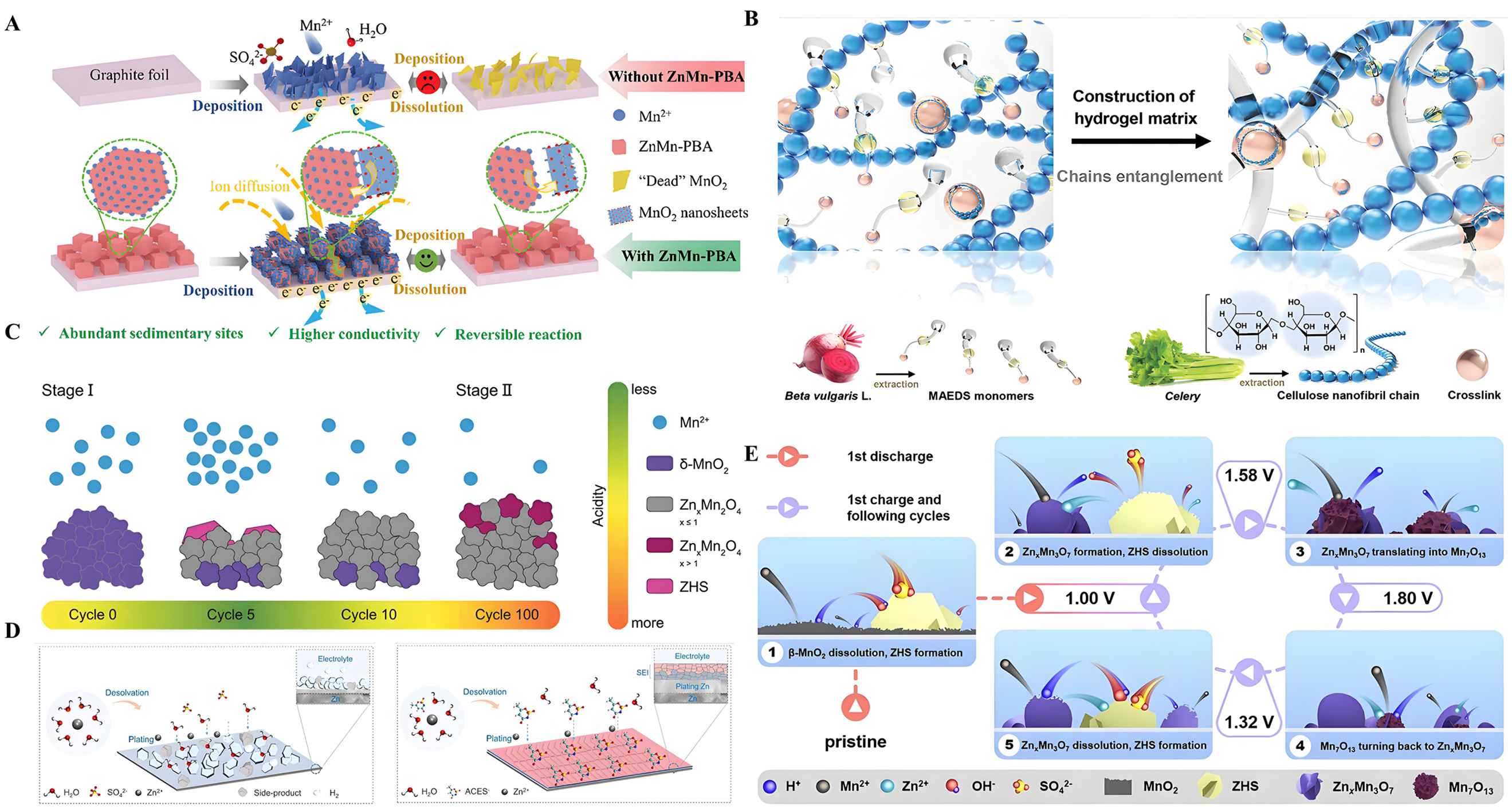
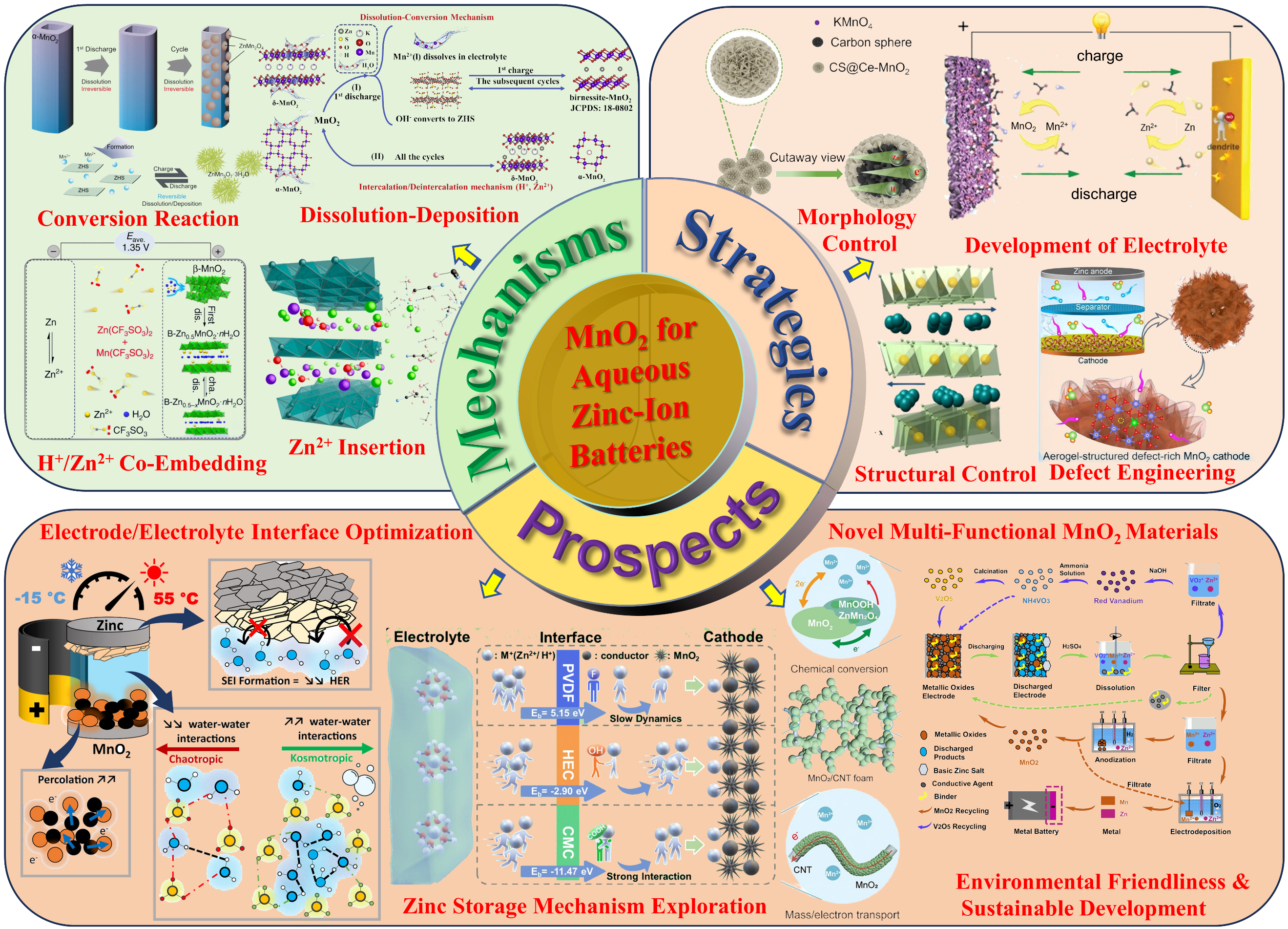













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].