fig3
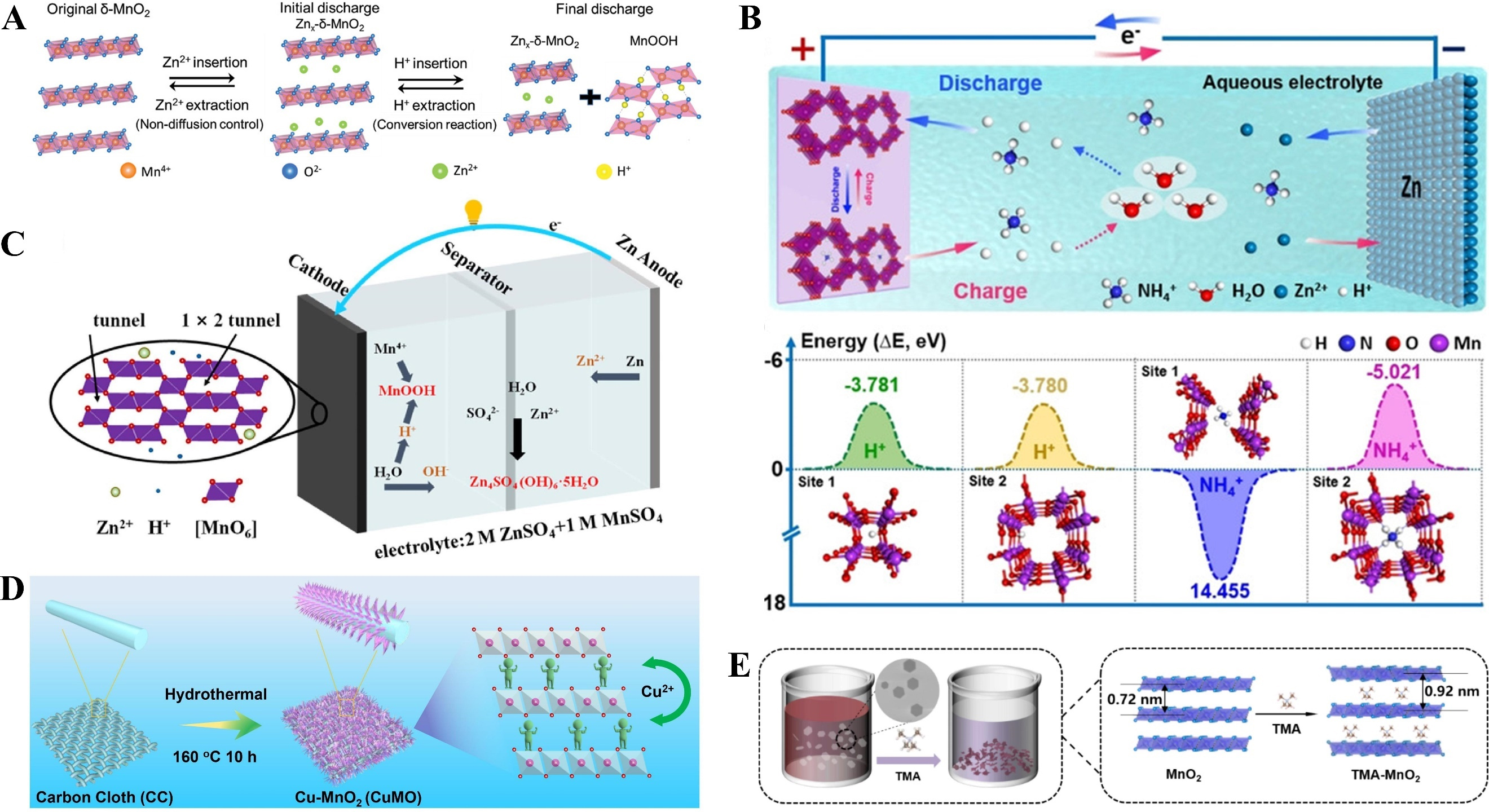
Figure 3. (A) Zn2+ intercalation and H+ conversion reaction mechanism in δ-MnO2. Reprinted with permission[89]. Copyright 2019, John Wiley and Sons; (B) The insertion energies of H+ or NH4+ ions in MnO2. Reprinted with permission[32]. Copyright 2021, John Wiley and Sons; (C) The discharge of tunnel-like ε-MnO2 stockpiled H+ and Zn2+. Reprinted with permission[90]. Copyright 2022, Elsevier; (D) The synthetic process of CuMO electrode. Reprinted with permission[92]. Copyright 2022, Elsevier; (E) Schematic illustration of the preparation of TMA-MnO2. Reprinted with permission[1]. Copyright 2024, Elsevier.










