fig6
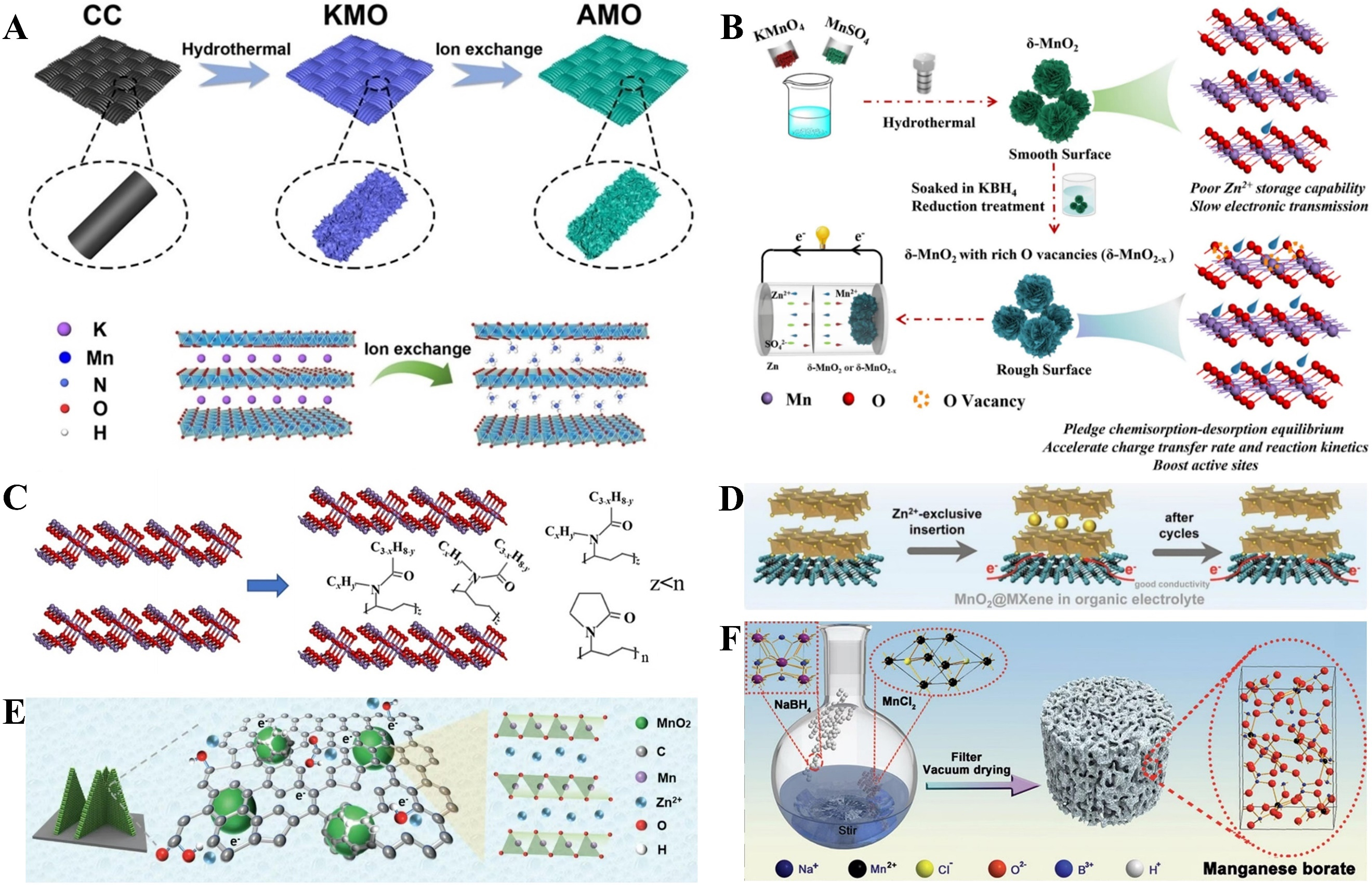
Figure 6. (A) Illustration of the synthesis of AMO. Reprinted with permission[106]. Copyright 2023, John Wiley and Sons; (B) The preparation procedure for δ-MnO2 with rich oxygen vacancies (δ-MnO2-x). Reprinted with permission[109]. Copyright 2023, Elsevier; (C) PVP intercalation in δ-MnO2. Reprinted with permission[108]. Copyright 2023, John Wiley and Sons; (D) Ti3C2 MXene nanosheets with good conductivity enable fast electron transfer kinetics. Reprinted with permission[109]. Copyright 2023, Elsevier; (E) The advantages of










