fig9
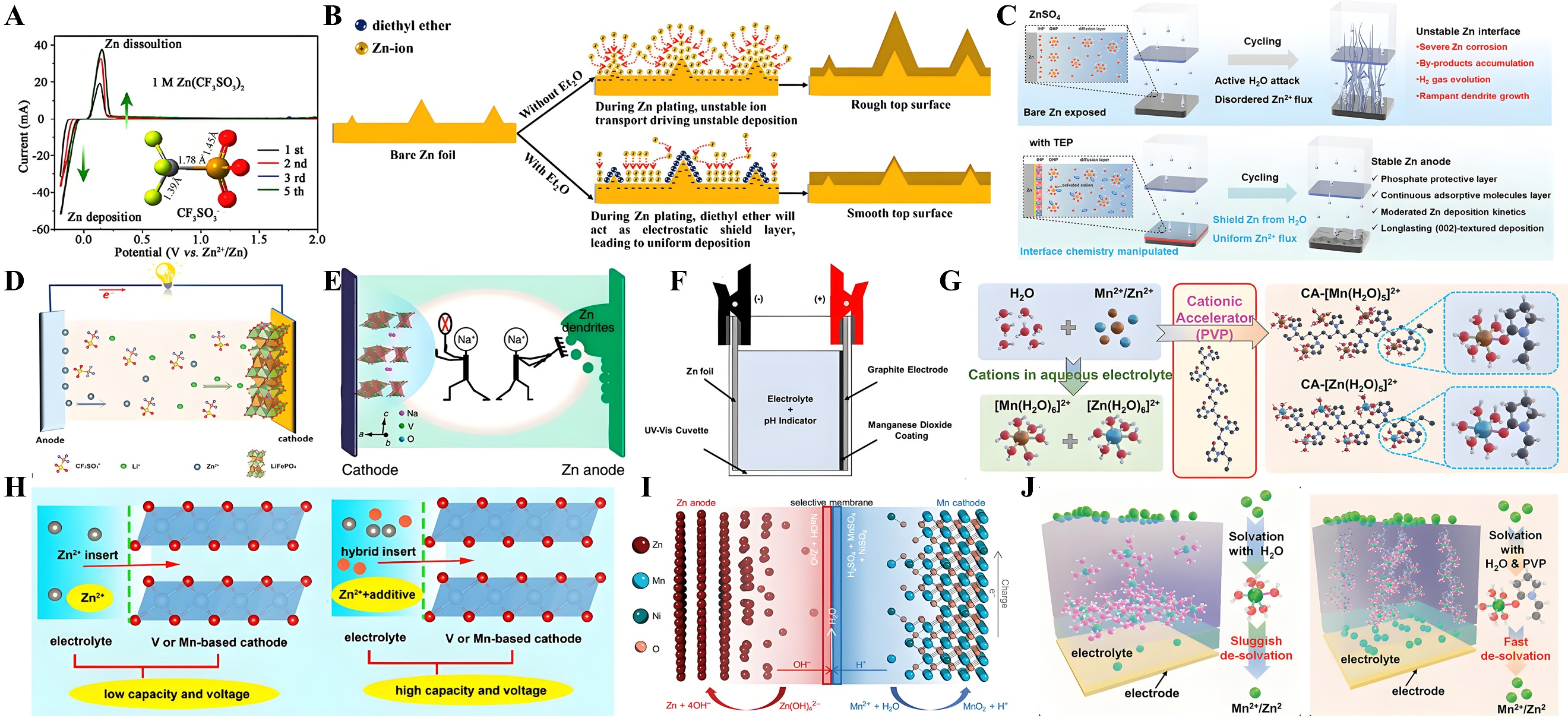
Figure 9. (A) Cyclic voltammograms of Zn electrode in 1M Zn(CF3SO3)2. Reprinted with permission[128]. Copyright 2016, American Chemical Society; (B) Schematics of morphology evolution for Zn anodes with and without Et2O additive. Reprinted with permission[129]. Copyright 2019, Elsevier; (C) Zn deposition process in blank ZnSO4 and TEP-containing electrolytes. Reprinted with permission[130]. Copyright 2024, John Wiley and Sons; (D) Schematic illustration of the hybrid aqueous Zn/LFP battery. Reprinted with permission[131]. Copyright 2019, John Wiley and Sons; (E) Schematic diagram about the effect of Na2SO4 additive. Reprinted with permission[132]. Copyright 2018, Springer Nature; (F) Schematic illustration of the Zn-MnO2 cell assembled in a UV-vis cuvette. Reprinted with permission[105]. Copyright 2022, Elsevier; (G) Schematic diagram of additives on the capacity and voltage. Reprinted with permission[133]. Copyright 2021, Elsevier; (H) Feasibility study for the designed Zn-Mn hybrid aqueous battery. Reprinted with permission[134]. Copyright 2020, John Wiley and Sons; (I) Schematic diagram of formation of stable coordinated hydrated structures. Reprinted with permission[135]. Copyright 2022, John Wiley and Sons; (J) Theory-Driven Design of a Cationic Accelerator for










