fig5
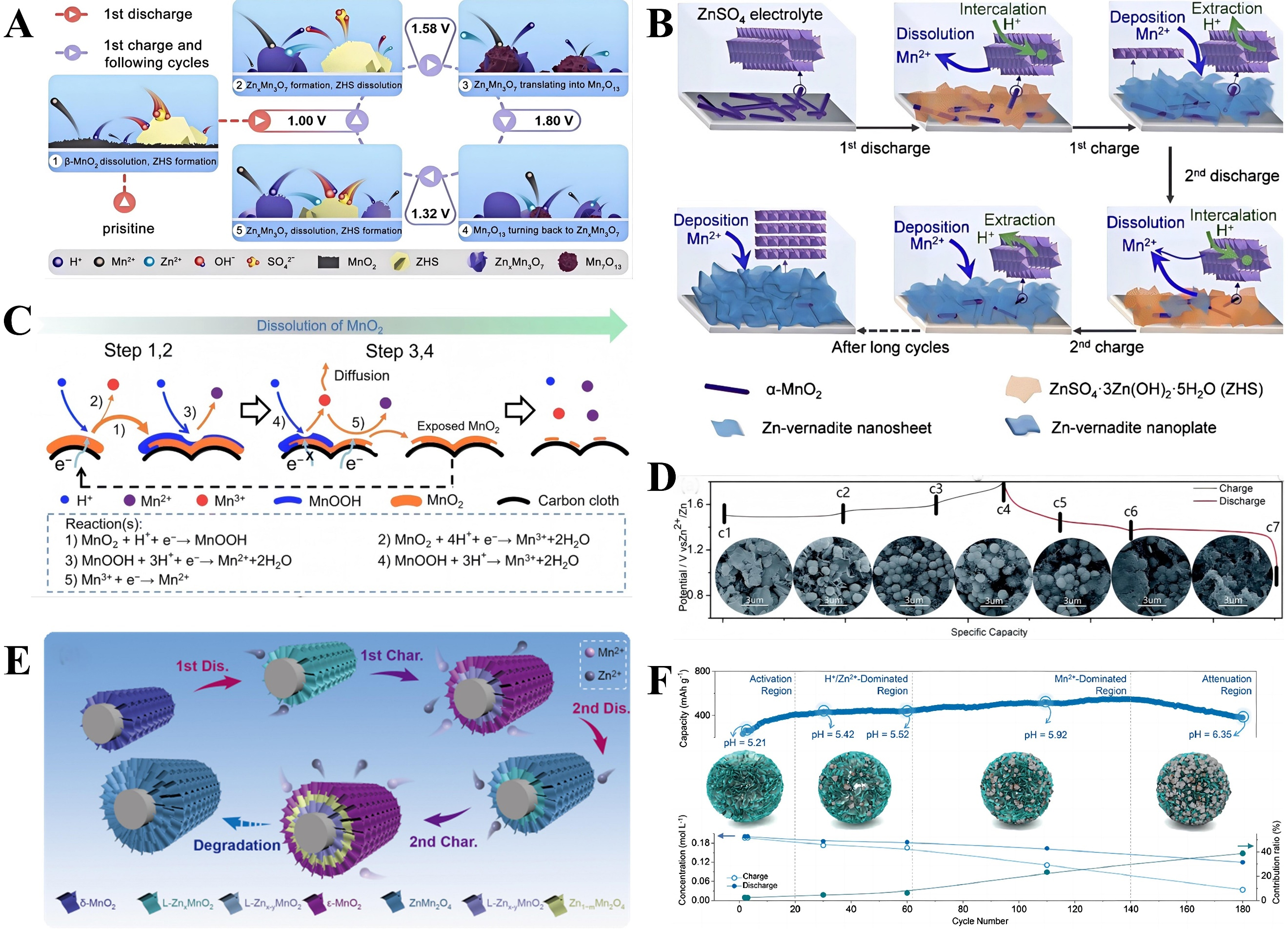
Figure 5. (A) The proposed charge storage mechanism of the β-MnO2 cathode. Reprinted with permission[99]. Copyright 2018, Royal Society of Chemistry; (B) The reaction mechanism for the α-MnO2. Reprinted with permission[100]. Copyright 2022, Elsevier; (C) The dissolution of MnO2 in acidic electrolytes. Reprinted with permission[101]. Copyright 2022, Royal Society of Chemistry; (D) Cycling profiles of the ZHS and the corresponding SEM images. Reprinted with permission[102]. Copyright 2023, Royal Society of Chemistry; (E) The reaction mechanism of the MnO2/CC electrode. Reprinted with permission[103]. Copyright 2022, Royal Society of Chemistry; (F) Mn-based competitive capacity evolution protocol. Reprinted with permission[104]. Copyright 2020, Elsevier. SEM: Scanning electron microscopy; ZHS: zinc hydroxide sulfate.










