Rare-earth-based strategies for lithium-sulfur batteries: enhancing multi-electron conversion reaction kinetics
Abstract
Lithium-sulfur batteries (LSBs) are considered promising alternatives to conventional lithium-ion batteries (LIBs) because of their high energy density, natural abundance of sulfur, and environmental benignity. Nevertheless, their practical application is hindered by issues including the shuttle effect, severe volume fluctuations, poor conductivity, and lithium dendrite growth at the anode. In recent years, rare-earth (RE) elements, benefiting from their unique unsaturated 4f orbital configurations, have shown great potential in tackling these issues. In particular, RE compounds not only anchor soluble polysulfides via RE-sulfur (S) bonding and catalyze their conversion to S/Li2S in the cathode, but also improve electrolyte function by facilitating lithium ion (Li+) transport, mitigating parasitic reactions, and reinforcing interfacial stability. This review systematically highlights the electronic structures, variable valence states, and strong chemical affinities of RE elements, all of which contribute to their multifunctional roles in LSBs. Furthermore, we summarize the progress of RE-based approaches across different components of LSBs, including cathode hosts, separators/interlayers, electrolyte additives, and solid-state electrolytes. Looking forward, we outline critical challenges and propose emerging directions for leveraging RE-based materials to realize practical, high-performance LSB technologies.
Keywords
INTRODUCTION
Since the birth of the steam engine during the first industrial revolution, various power technologies have made indelible contributions to the development of social productivity. However, the widespread use of traditional fossil fuels has led to severe and often irreversible environmental pollution, intensified the greenhouse effect, and exacerbated the imbalance between the supply and demand of natural resources[1-3]. Clean renewable energy is conducive to alleviating carbon footprints, which is a strategy vigorously developed by governments and societies around the world to achieve “carbon neutrality”. Rechargeable batteries as a key energy storage device of renewable energy play an irreplaceable role in large-scale smart grids, electrified transportation, and portable equipment owing to their favorable characteristics, including high efficiency, simple operation, and electrochemical stability[4,5].
As the most representative battery system, the energy density of lithium-ion batteries (LIBs) has increased from 100 to 280 Wh/kg in the past 30 years, which has triggered the revolution of energy storage devices[6,7]. Due to the limited crystallographic sites available for charge-carrier storage in intercalation-type electrodes, LIBs can hardly satisfy today’s rapidly increasing energy demands. For instance, power batteries related to electric vehicles urgently need higher energy density (≥ 500 Wh/kg) to achieve greater mileage (≥ 300 miles) between charges[8,9]. In addition, the energy storage system based on LIBs accounts for 50% of the cost of electric vehicles, which further hinders the electrification of transportation[10]. There is an urgent need to explore cheaper electrode materials and new electrochemical mechanisms, such as conversion and alloying, beyond intercalation[11,12].
Lithium-sulfur batteries (LSBs) based on conversion-based cathode material, orthorhombic cyclic octasulfur (α-S8), are the most promising to break through the current bottleneck of energy density. The electrochemical reaction between metallic lithium (Li) anode and sulfur (S) cathode can be expressed as 16Li + S8 ↔ 8Li2S, which is reflected as 2 voltage plateaus at about 2.3 and 2.1 V in the discharge process [Figure 1A][13-15]. In LSBs, the high-voltage plateau corresponds to the conversion of elemental sulfur into long-chain lithium polysulfides (LiPSs), followed by further reduction to Li2S2/Li2S at the low-voltage plateau. This distinctive multi-step reaction involves 16 electrons and affords a theoretical capacity of 1,672 mAh/g and an energy density of 2,600 Wh/kg. Coupled with abundance, low toxicity, and global accessibility of sulfur, these advantages position LSBs as cost-effective and environmentally friendly candidates for future large-scale energy storage[16-19].
Figure 1. (A) Charge-discharge voltage profiles of LSBs under galvanostatic conditions; (B) Schematic diagram of bottlenecks in LSBs; (C) The publication numbers of LSBs from 2009 to 2024. LSB: Lithium-sulfur battery.
Nevertheless, although the unique intrinsic material characteristics and multi-electron conversion process of sulfur phase change (S8 ↔ S82- ↔ S62- ↔ S42- ↔ Li2S2 ↔ Li2S) give LSBs more advantages than LIBs on energy density, they also hinder the large-scale practical application of current LSBs. First and foremost, S8 and Li2S2/Li2S are both electrically and ionically insulating (the electronic conductivity of sulfur and Li2S is about 10-30 and 10-14 S/cm, respectively, at room temperature), which greatly decreases the utilization of the active sulfur materials and has posed major issues for practical capacity in the past few decades[20,21]. In addition, due to density difference between the initial reactant sulfur (2.03 g/cm3) and the product Li2S (1.67 g/cm3), the sulfur phase transition causes volume expansion and contraction of the electrode, resulting in electrode cracking and deactivation during cycling in LSBs[22,23]. For the reaction intermediates polysulfides, their solubility in organic electrolytes is the main cause of the notorious “shuttle effect”. Nevertheless, continuous dissolution of LiPSs is also necessary to enable electron transfer and expose unreacted sulfur, thus promoting deeper sulfur participation in the conversion process[24-26]. Another well-known problem is the defects encountered on the lithium metal anode side, including the interface side reaction caused by the thermodynamic instability of Li, the production of lithium dendrite caused by uneven space charge layer on the anode surface, and the increase of internal resistance caused by “dead Li” produced by detached lithium dendrite[27,28]. All these deficiencies eventually lead to the low coulomb efficiency, short service life and unsatisfactory actual energy density of LSBs [Figure 1B].
Limited by the technical bottleneck of sulfur cathode and electrolyte materials, the problems of low discharge capacity and fast capacity attenuation in cycling have not been appropriately settled in the decades after the birth of LSBs in 1962. Until 2009, Ji et al. at University of Waterloo pioneered the idea of using conductive mesoporous carbon framework as the host material of sulfur cathodes, achieving a major breakthrough in improving conductivity, alleviating repeated volume transformation of sulfur cathodes and dissolution of LiPSs[29]. Since then, research on LSBs has undergone rapid growth, leading to an explosive increase in the number of related publications [Figure 1C].
Researchers worldwide are committed to improving the electrochemical performance of LSBs from the aspects of the electrolyte, separator, current collector, anode and cathode[30-39]. No matter which part of LSBs is modified or improved, endowing novel materials with unique functionality is always the core strategy and has received the greatest attention in the field. For example, porous cathode hosts with physical space limitation for sulfur, catalysts with chemical adsorption and catalytic effect for LiPSs, alternative electrolyte systems to replace traditional organic ethers, electrolyte additives to improve coulomb efficiency, multifunctional polymer binder, artificial solid electrolyte interphase (SEI) layers on the lithium metal anode host and its surface, and solid electrolytes[40-44]. Most of these functional materials come from nanostructured inorganic compounds, polymers, metals, porous carbon-based materials, and their composites, all of which are composed of various basic elements. We highlight the cells in the periodic table to represent the elements that have been applied to LSBs [Figure 2A]. Among the explored elements for advanced energy storage, rare-earth (RE) elements have recently gained growing recognition for their potential in LSBs.
Figure 2. (A) The elements used in LSBs including non-metallic, transition metal, and RE metal; (B) Publication numbers on the role of RE elements in LSBs in various forms; (C) Schematic diagram of electronic orbits of RE elements. LSB: Lithium-sulfur battery; RE: rare-earth.
Herein, we present a comprehensive review of recent advances in RE-based functional materials for LSBs. We systematically summarize the structural features, chemical states, and synthesis strategies of RE compounds and elucidate the mechanisms by which they enhance electrochemical performance. The discussion is organized by battery components: cathode hosts, separators/interlayers, electrolyte additives and interphase regulators, and solid-state electrolytes. We place special emphasis on the multifaceted roles of RE elements in immobilizing LiPSs, catalyzing their conversion, facilitating Li+ migration at the anode, and suppressing lithium dendrite growth. We also highlight the contributions of RE-based strategies to LSB development and assess their prospects for future commercialization. We hope this review will inspire new research directions and guide the rational design of RE-based materials to broaden the practical application of LSBs and achieve optimized electrochemical performance.
BASIC PROPERTIES OF RARE-EARTH ELEMENTS IN LITHIUM-SULFUR BATTERIES
Fundamental physicochemical properties of rare-earth elements
RE elements are characterized by similar ground-state electronic configurations and include the 15 lanthanides - lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu) - together with two group IIIB elements, scandium (Sc) and yttrium (Y)[45]. Owing to their versatile electronic structures and unique physicochemical properties, these elements have been widely utilized in diverse industries such as petroleum processing, metallurgy, ceramics, glass manufacturing, textiles, and permanent magnetic materials, earning the title of “industrial vitamins”. RE elements have an empty or unfilled 4f electronic layer structure and the resulting diversified electronic energy levels, leading to rich and unique energy storage and catalytic properties of RE elements and their compounds[46-49]. Therefore, they also play an indispensable key role in the research and development of LSBs. Figure 2B shows the number of publications in which RE elements have been applied to LSBs in various forms in recent years. It is obvious that the introduction of RE elements and their derivatives into the LSB system to activate their energy storage characteristics has been a research hotspot worldwide in recent years. Nevertheless, there is little literature and reports summarizing LSBs based on RE-based materials.
Among the 17 RE elements, Sc and Y are different from lanthanides in that their ground state electronic configurations have no 4f electrons, i.e., [Ar]3d14s2 and [Kr]4d15s2, respectively. In the case of ground state electronic configurations of 15 lanthanides, La, Ce, Gd, and Lu are the [Xe]4fn-15d16s2 and other lanthanides are [Xe]4fn6s2, all of which have unfilled 4f orbitals [Figure 2C][47]. Nevertheless, they all have the same atomic structure-oriented properties; that is, the two electrons in the outermost layer and one electron in the sub-outer layer are easy to lose, so as to form a stable trivalent ion state (RE3+). This feature makes RE ions have the potential to combine with different single-phase materials, which is very important for the design of crystalline multicomponent materials suitable for different use scenarios, including catalysis, luminescence, and magnetic modulation[50,51]. For instance, Ce elements can be doped into nanomaterials as Lewis acid active sites to catalyze CO conversion, selective oxidation of organic molecules and soot combustion[52]. The repeated gain and loss of electrons of Ce between trivalent (Ce3+) and tetravalent (Ce4+) states endows Ce-based materials with the function of local oxygen storage and release, thus affecting the catalytic effect. In addition, some aliovalent dopants based on RE elements (e.g., Pr) doped into the cerium oxide host can introduce oxygen vacancies, which is also conducive to improving the oxygen storage capacity and catalytic performance[53]. The transition metal-Sm alloy has served as a permanent magnet at room temperature for high-density magnetic recording and energy storage due to the exchange interaction between the conduction electrons of transition metals and the local spin-polarized electrons of Sm[54]. Nd-based nanoparticles are usually used as NIR (near-infrared) nanophosphors in biological imaging field because their excitation and emission are contained in the optical window (700-1,100 nm)[55]. Interestingly, the up-conversion emission of Nd3+ can be realized at around 382 nm and 413 nm by reasonably designing the core-shell structure of composite material[56]. These cases demonstrated the tunability and versatility of RE elements, as well as their nonnegligible contribution in related fields by combining with the current advanced nanotechnology. These unique properties have also been proven by researchers to be suitable for solving the notorious problems in the LSB system, such as aggravated shuttle effect, uncontrollable lithium dendrite growth, hindered multi-electron conversion reaction kinetics, and so on.
Theoretical perspectives on the role of rare-earth elements in LSBs
Density functional theory (DFT), a powerful tool in materials science, enables atomic- and electronic-scale insights into interactions within RE-Li-S systems. It provides theoretical support for understanding experimental phenomena and guiding electrode/electrolyte design. The performance of LSBs depends on multi-electron reactions, including sulfur reduction, polysulfide dissolution/shuttle, and interfacial side reactions. Benefiting from their unique 4f configuration and high physicochemical activity, RE species regulate these processes by tuning reaction pathways and suppressing side reactions. DFT further clarifies RE functions via electron density, bonding characteristics, and energy barrier evolution.
For instance, Zhang et al. utilized a La2O3/carbon composite system as a research model. By employing first-principles calculations, they systematically uncovered the synergistic mechanism through which this composite enhances the cycling stability and rate performance of LSBs[57]. Their findings revealed that the migration of polysulfides and the cleavage of dimers (e.g., Li2S2) predominantly occur on the surface of La2O3. In contrast, the carbon-based component provides rapid migration channels for the two-step lithium ion (Li+) insertion/extraction process, the bulk diffusion of lithium, and the transport of dimers. This result further confirms that the synergistic effect between La2O3 and the carbon substrate during the catalytic conversion of polysulfides arises from their collaborative participation in regulating reaction pathways and improving mass transfer efficiency. It is not a mere combination of the catalytic activity of La2O3 and the electrical conductivity of the carbon material.
In addition to the design of composite electrodes, another key strategy to enhance the regulatory role of REs involves modifying their coordination environment via non-metallic element doping. This modification adjusts the splitting energy of the valence orbitals at the metal center, thereby strengthening the interactions of metal-sulfur bonds and optimizing the adsorption and conversion efficiency of polysulfides[58]. Within electrolyte systems, RE-based additives also demonstrate significant regulatory potential, primarily by facilitating the formation of stable SEI films enriched with REs. Ren et al. conducted research in this area, focusing on a systematic investigation of the ionic transport behavior of La-doped LiF when used as a component of the SEI film on lithium metal anodes[59]. Their results indicated that La doping effectively alleviates the excessive periodic grain boundary spacing, which is caused by the lattice mismatch between LiF and lithium metal during lithium deposition. Specifically, the grain boundary spacing was reduced from 0.478 nm to 0.306 nm. Concurrently, La doping led to the formation of a more stable vacancy defect structure in the grain boundary region and decreased the Li+ transport energy barrier to 0.789 eV. This modification not only facilitates the rapid migration and uniform deposition of Li+ but also makes a significant contribution to suppressing the growth of lithium dendrites and extending the cycle life of batteries.
To directly link RE-related active sites to kinetics, we adopt three cross-comparable metrics: (1) LiPS adsorption energies for S8, Li2S8/6/4, Li2S2, and Li2S computed on explicit RE sites; (2) Li+ diffusion coefficients; and (3) ionic conductivity. Supplementary Table 1 benchmarks adsorption energies of sulfur species on RE-containing surfaces. RE oxides exhibit stronger adsorption than carbon, and defect/non-stoichiometric or anion-engineered phases further enhance binding: ScO0.95 > Sc2O3, LaNiO3-x > LaNiO3, and CeO2-xSx > CeO2. RE sulfides/oxysulfides (e.g., La2S3, La2O2S) display the strongest interaction with S8 and Li2Sx, making them effective for capturing and activating long-chain species, although they may overbind the end products (Li2S2/Li2S) and risk surface passivation. In contrast, halides/oxychlorides (CeF3, LaOCl) and LaCO3OH show moderate adsorption, which is favorable for product desorption and sustained conversion. The single-atom Ce/N4 site gives intermediate binding for Li2S6, indicating that isolated RE sites can activate LiPSs without excessive adhesion. Supplementary Table 2 compiles Li+-transport metrics for RE-enabled electrolyte systems: Li+ diffusion coefficients during the Li2S4 → Li2S regime for RE electrolyte additives and ionic conductivity for RE-containing solid electrolytes. A clear trend emerges: sulfide/halide electrolytes reach ionic conductivity ~ 10-3 S cm-1, exceeding garnet Li7La3Zr2O12 (LLZO, 4.0 × 10-4 S cm-1) and perovskite LLTO (2.0 × 10-5 S cm-1), while organic Nd(OTf)3 raises Li+ diffusion coefficients versus inorganic nitrates.
CATHODE MATERIALS BASED ON RARE-EARTH COMPOUNDS
The sulfur cathode plays a decisive role in determining the electrochemical performance of LSBs. A conventional sulfur cathode is generally fabricated by coating a mixture of sulfur active material, conductive additives, and polymer binders onto a carbon or aluminum current collector[60]. As we all know, conductive agents and binders mainly play a role in increasing the conductivity and electrode integrity, but they have limited impact on the electrochemical performance of LSBs. In recent years, researchers have focused on designing sulfur host materials to mitigate volume change, inhibit polysulfide dissolution and accelerate conversion reaction kinetics. Most excellent sulfur hosts have three-dimensional (3D) connected electron channels and smooth ion diffusion paths, which are conducive to overcoming the low conductivity of sulfur and accommodating the volume transition caused by the conversion reaction. In addition, the outstanding ones also have appropriate chemisorption for polysulfide intermediates and catalytic effect for polysulfide conversion. Most of the previous reports are devoted to the heteroatom doping, metal compound modification or surface functionalization of carbon-based materials.
Rare-earth oxides as sulfur hosts
As the pioneering work of applying RE elements to the sulfur cathode of LSB, Zheng et al. introduced lanthanum oxide (La2O3) as an additive to replace part of the conductive agent into the sulfur cathode in 2006[61]. They found that La2O3 has the potential to adsorb polysulfides and catalyze their redox reactions. Zhang et al. clarified the mechanism of La2O3 and conductive nitrogen-rich mesoporous carbons (NMC) synergistically improving the electrochemical performance of LSBs from the perspective of theoretical calculation with DFT[57]. They systematically investigated the adsorption configurations, migration pathways, and diffusion energy barriers of LiPSs species on La2O3 and NMC surfaces [Figure 3A and B]. Unlike the conventional understanding, La2O3 not only exhibits strong polysulfide adsorption but also provides a polar surface that facilitates smooth LiPSs migration and accelerates the cleavage of LiPSs dimers. The cooperative functions of La2O3 and NMC extend beyond simple adsorption and conductivity, acting as a synergistic system that jointly catalyzes polysulfide conversion. Building on this early insight, subsequent studies shifted from simple mixing to architecture-guided incorporation of La2O3. A representative advance involved embedding La2O3 quantum dots (QDs) a few nanometers deep into carbon nanorods that self-assemble into microspheres, so that thin carbon layers protect the QDs from direct electrolyte contact while still allowing Li+/solvent penetration [Figure 3C][62]. This design prevents oversaturation and deactivation of catalytic sites, maintains high surface area, and leverages strong La-O-Li interactions to immobilize LiPSs and accelerate S8 → Li2S conversion. Electrochemically, such La2O3-carbon microspheres delivered 1,392 mAh/g at 0.25C, and sustained 0.044% capacity fade per cycle over 1,000 cycles at 2C. They achieved an areal capacity of 6.31 mAh/cm2 at 0.1C with strong capacity retention at a sulfur loading of ~ 5.5 mg/cm2. Subsequently, when integrated with various carbon-based materials, lithium lanthanum titanate (LLTO) has been shown to exhibit distinct properties. Notably, it can also function as a cathode host material. Yeh et al. demonstrated that LLTO can serve as an effective cathode additive for LSBs under high sulfur loading and lean electrolyte[63]. A hot-pressed sulfur-LLTO electrode (6 mg/cm2) exhibited uniform LLTO dispersion, which enhanced ionic transport, facilitated polysulfide adsorption, and catalyzed their conversion. These results established La-based oxides as durable, catalytically active hosts for LSB cathodes.
Figure 3. (A) Migration path, Li-O and S-La bond lengths profile of Li2S2 diffusion on the surface of La2O3; (B) The energy profile for Li2S2 → Li2S conversion process catalyzed by La2O3. The figures are quoted with permission from Zhang et al.[57]; (C) Schematic of the solvothermal route to carbon microspheres (CMs) with and without embedded La2O3 quantum dots (QDs). Note that adding La precursors produces pronounced differences in sphere morphology. This figure is quoted with permission from Dai et al.[62]; (D) Preparation process of sulfur electrode with CNT and CeO2. The figures are quoted with permission from Xiao et al.[78]; (E) Electronic density difference of different models (i.e., CeO2, Cu-CeO2, and Cu-CeO2-x). The figures are quoted with permission from Hou et al.[82]. DMF: BTC: 1,3,5-benzenetricarboxylate; CNT: carbon nanotube; NMC: nitrogen-rich mesoporous carbons.
Beyond La2O3, various other RE oxides (e.g., Y2O3, CeO2, Ga2O3, Sc2O3, Nd2O3, Sm2O3) have been utilized as adsorption-catalytic regulators in the host materials of LSBs. The differences in their performance are summarized in Supplementary Table 3[64-77]. Among these RE oxides, Ce, the most abundant lanthanide, combines metallic reactivity with chemical stability. This unique combination makes CeO2-based composite host materials the most extensively studied in this field. Compositing CeO2 with carbon-based materials is a common modification strategy, as it enables the synergistic optimization of both polysulfide anchoring capability and charge transport properties. For instance, Xiao et al. synthesized CeO2-doped carbon nanotube (CeO2@CNT) host materials via a hydrothermal method [Figure 3D][78]. In this composite, uniformly dispersed nanoscale CeO2 particles suppress the shuttling of polysulfides through chemical bonding interactions. Meanwhile, one-dimensional (1D) carbon nanotubes (CNTs) with a high aspect ratio provide rapid transport pathways for ions and electrons. The CeO2@CNT/S cathode exhibited excellent specific capacities of 1,359 mAh/g and 715 mAh/g at current densities of 0.1C and 1C, respectively. To further enhance the polysulfide anchoring effect, researchers have also modulated interfacial interactions by doping the carbon substrate with non-metallic elements. Wang et al. loaded CeO2 onto nitrogen-doped carbon nanofibers (CeO2@CNF)[79]. The resulting CeO2@CNF/S cathode maintained favorable cycling stability even at a high current density of 2C. The exposed crystal planes of CeO2 have a critical impact on its adsorption and catalytic performance. Wei et al. designed and synthesized CeO2 nanomaterials with controllable morphologies, featuring exposed (110)/(100)/(111), (100), and (111) crystal planes[80]. They revealed that CeO2 nanorods with exposed (110)/(100) planes and defective (111) planes effectively inhibit the shuttling of long-chain polysulfides through the formation of strong Ce-S and Li-O bonds. Additionally, the defect-rich structure (consisting of oxygen vacancies and Ce3+ ions) on the surface of CeO2 nanorods acts as additional active sites, which further enhances the adsorption of polysulfides.
In recent years, modifying CeO2 nanorods through defect engineering and doping has emerged as a key strategy to enhance their synergistic adsorption-catalytic performance. Azam et al. developed composite host materials by loading bimetallic nickel/cobalt oxides (NiCo2Ox) onto CeO2 nanorods[81]. CeO2 surface defects provide strong binding sites for LiPSs, while NiCo2Ox ensures fast catalytic conversion; together, they form a synergistic host that enhances kinetics and suppresses shuttle. Hou et al. further prepared Cu-CeO2-x/carbon nanofiber (CNF) composites with a heterointerface via electrospinning followed by stepwise carbonization [Figure 3E][82]. In this process, Cu2+ ions dispersed within the CNFs gradually diffuse and are trapped by defects in CeO2. This not only stabilizes the Cu clusters and prevents their agglomeration but also induces the formation of abundant oxygen vacancies in CeO2. By generating oxygen vacancies, CeO2 achieves bandgap narrowing, improved electronic conductivity, and lower reaction barriers. Such interfacial engineering promotes rapid polysulfide-to-Li2S conversion, leading to long-term stability with 626 mAh/g retained after 800 cycles at 2C (0.046% fading per cycle). Moreover, Fe2+-doped and carbon-supported CeO2 composites exhibit comparable enhancements in both cycling life and rate capability[83]. Doping CeO2 with non-metallic elements is another effective strategy for regulating its adsorption and catalytic performance. Fan et al. proposed a chlorine modification strategy for CeO2 (CeO2-Cl)[84]. Cl doping significantly enhances the adsorption energy of CeO2 for polysulfides and alters the adsorption mechanism.
Rare-earth hydroxides and fluorides for polysulfide regulation
Metal hydroxides have also been shown to effectively enhance the performance of LSBs, owing to their combined advantages of structural stability and polysulfide anchoring capability[85-90]. Tian et al. utilized a spray-drying method to fabricate a 3D layered micro-spherical host material, which consists of oxygen-deficient lanthanum hydroxide [La(OH)3] and reduced graphene oxide (rGO)[91]. The densely packed micro-spherical structure of host material establishes a continuous ion transport network, which shortens the free path of ion migration [Figure 4A]. Meanwhile, the layered and stacked rGO provides sufficient buffer space to accommodate the volume expansion of sulfur during charge-discharge cycles, thereby ensuring the structural integrity of the electrode. La(OH)3 with oxygen defects [oxygen-deficient La(OH)3] can enhance polysulfide anchoring by forming La-S bonds. The synergistic effect of lithophilic oxygen and sulfiphilic lanthanum significantly improves the binding strength of La(OH)3 toward Li2S6, with the binding energy increasing from -1.88 eV [pristine La(OH)3] to -5.03 eV [oxygen-deficient La(OH)3], thereby suppressing Li2S6 dissolution and shuttle [Figure 4B].
Figure 4. (A) Morphology of composite sulfur electrode and corresponding EDS mapping; (B) Optimized polysulfide adsorption structures and calculated binding energy. The figures are quoted with permission from Tian et al.[91]; (C) HRTEM images of YF3 nanofiber and corresponding EDS mapping; (D) Optimized YF3 structure. The figures are quoted with permission from Hao et al.[92]; (E) Preparation process of CoF3co-doping into YF3 carbon nanofiber and corresponding morphologies. This figure is quoted with permission from Wang et al.[93]; (F) HRTEM image of Hollow- CeF3 and corresponding EDS mapping; (G) Optimized polysulfide adsorption structures on the surface of CeF3 and CeO2; (H) Adsorption energy comparison between CeF3 and CeO2. The figures are quoted with permission from Liu et al.[94]. PVP: Polyvinyl pyrrolidone; PTFE: polytetrafluoroethylene; EDS: energy-dispersive X-ray spectroscopy; HRTEM: high-resolution transmission electron microscopy.
Compared to RE oxides, RE fluorides theoretically exhibit superior polysulfide adsorption performance owing to the high electronegativity of fluorine, which strengthens the polar interactions between RE fluorides and polysulfides. To date, significant progress has been achieved in relevant research. Hao et al. synthesized 1D CNFs doped with semi-enclosed yttrium fluoride (YF3) via an electrospinning method [Figure 4C and D][92]. The semi-closed carbon architecture associated with YF3 suppresses polysulfide leakage more effectively than open-structured carbons. Dispersed YF3 nanoparticles also enable the generation of fluorine-rich electrode interfaces. Benefiting from these effects, YF3 cathodes sustained 709.8 mAh/g after 600 cycles at 1C, with only 0.043% capacity decay per cycle. Wang et al. further improved anchoring capacity by introducing CoF3 into YF3-modified porous CNFs [Figure 4E][93]. Through the synergistic anchoring effect of YF3 and CoF3, the cycling performance was further enhanced.
Departing from conventional strategies that load RE fluorides onto pre-formed hosts, Liu et al. developed a novel approach that first synthesized hollow cerium fluoride (CeF3) nanocages (h-CeF3), and then encapsulated active material sulfur within the nanocage cavities via a melt-dispersion process [Figure 4F][94]. The hollow structure of h-CeF3 directly accommodates the volume expansion of sulfur during charge-discharge cycles, mitigating electrode structural degradation. Additionally, DFT calculations revealed that h-CeF3 exhibits a significantly higher adsorption energy for long-chain polysulfides compared to cerium oxide [Figure 4G and H].
Beyond single RE compounds, perovskite materials incorporating REs have attracted considerable attention as host materials for LSBs, stemming from their inherent advantages, including stable crystal structures, abundant surface defects/oxygen vacancies, and strong polar adsorption capabilities for polysulfides[95-98]. Perovskites have the general composition ABX3, where the A-site is typically occupied by large-radius cations (e.g., REs, methylammonium, Cs+), the B-site by small-radius metal cations (e.g., Ti4+, Pb2+, Sn2+), and the X-site by halide or oxygen anions (e.g., O2⁻, Cl⁻, Br-). A key advantage of these materials lies in their tunable properties, wherein their adsorption and catalytic performances for polysulfides can be flexibly modulated through doping at either the A-site or B-site.
Rare-earth perovskites and high-entropy compounds for catalytic enhancement
Hao et al. pioneered the application of La0.6Sr0.4CoO3-δ (LSC) perovskite as a host material for composite cathodes in LSBs[99]. They employed SiO2 templates combined with acid etching to fabricate dual-core nanowires that effectively improve mass transport pathways within the electrode [Figure 5A]. DFT calculations further revealed the mechanism underlying the enhanced performance of LSC. Sr doping significantly strengthens the ability of LSC to anchor polysulfides via Co-S bonds, reducing the binding energy from -1.95 eV [for pure LaCoO3 (LCO)] to -2.95 eV [Figure 5B]. Bader charge analysis provided additional insights into this mechanism. For the (110) plane of the materials, the average valence of Co atoms was +0.93 in LCO and +0.92 in LSC, while the average valence of S atoms bonded to Co was -0.71 in LCO and -0.79 in LSC. This indicates that Sr doping increases the charge difference between Co and S atoms, thereby enhancing the surface bonding energy between LSC and polysulfides. Benefiting from these improvements, the LSC/S@C composite cathode delivered a discharge specific capacity of 996 mAh/g at 0.5C when the sulfur loading was 2.1 mg/cm2.
Figure 5. (A) Preparation process of LSC perovskite-based host for composite sulfur electrode; (B) Optimized Li2S4 adsorption structure on the surface of LaCoO3 and LSC. The figures are quoted with permission from Hao et al.[99] XPS tests before and after Li2S6 adsorption on LSFM (C) Mn 2p; (D) Sr 3d; (E) O 1s. The figures are quoted with permission from Jin et al.[100]; (F) HETEM image of HE-LSMO. (G) Binding energy comparison of Li2S6 adsorption on the surface of LMO, HE-LMO, and HE-LSMO. The figures are quoted with permission from Tian et al.[105]; (H) In-situ electrochemical Raman 2D spectra of HEZO-based sulfur electrode at different charge/discharge depths; (I) Actual utilization of Li2S4 in discharge process and charge process. The figures are quoted with permission from Zhou et al.[106]. LSC: La0.6Sr0.4CoO3-δ; XPS: X-ray photoelectron spectroscopy; LSFM: La0.3Sr0.7Fe0.2Mn0.8O3; OCP: open circuit potential; HRTEM: high-resolution transmission electron microscopy; HE-LSMO: La0.8Sr0.2(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3); HE-LMO: La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3); HEZO: (La0.15Nd0.15Sm0.40Eu0.15Gd0.15)2Zr2O7.
In another study, Zhou et al. synthesized La0.3Sr0.7Fe0.2Mn0.8O3 (LSFM) perovskite via dual Sr/Fe metal doping and further constructed an LSFM/Ti3C2Tx composite as a cathode host[100]. X-ray photoelectron spectroscopy (XPS) analysis confirmed the polysulfide anchoring mechanism. The shifts in the peak positions and changes in the content of Mn species with different valence states, along with the appearance of characteristic Sr-S peaks and Li-O peaks, collectively demonstrated that Mn, Sr, and lattice oxygen defects serve as the primary active sites for polysulfide anchoring [Figure 5C-E].
High-entropy alloys (HEAs) composed of five or more elements readily induce lattice distortion due to differences in electron density and atomic radius among their constituent elements. This lattice distortion generates numerous surface defects, which act as active sites for chemical reactions. In recent years, such high-entropy materials have also been explored as host materials for LSB, leveraging their unique structural and chemical properties[101-104]. Tian et al. prepared La0.8Sr0.2(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3) (HE-LSMO) nanofibers via electrospinning–calcination [Figure 5F][105]. They found that while LaMnO3 anchors polysulfides mainly through Li-O and Mn-S bonds, HE-LSMO and La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3) (HE-LMO) also involve Fe-S interactions [Figure 5G].
Zhou et al. elucidated how active-site structure governs catalytic performance in high-entropy systems and mapped the electrocatalytic conversion of Li2S4[106]. First, adsorption experiments demonstrated that Li2S4 preferentially adsorbs onto Zr sites, while RE metals (La, Nd, Sm, Eu, Gd) exhibited lower catalytic activity for Li2S4 conversion compared to Zr. Based on this observation, the researchers synthesized high-entropy ceramics (La0.15Nd0.15Sm0.40Eu0.15Gd0.15)2Zr2O7 (HEZO) using Sm2Zr2O7 as the matrix, where Sm was substituted with a high-entropy mixture of RE elements (La, Nd, Sm, Eu, Gd). They confirmed that RE elements can modulate the electronic structure of Zr, thereby altering its catalytic properties for polysulfide conversion. Figure 5H is the in-situ electrochemical Raman two-dimensional (2D) projection mapping at different charge/discharge depths, vigorously demonstrating that the kinetic conversion of polysulfides is greatly enhanced under charge/discharge state. As the discharge depth increases, the proportion of S8 continues to decrease and is progressively replaced by polysulfides, which behaves oppositely during the charge process. Additionally, the theoretical contribution of Li2S4 at different charge/discharge depths closely matches experimental results, confirming the reliability of using S8 peak projection area as a reference for monitoring the phase evolution [Figure 5I].
BINDER SYSTEMS INCORPORATING RARE-EARTH COMPONENTS
In a composite sulfur electrode, binder accounts for only 2-10 wt.% yet exerts a critical regulatory role in determining battery performance. Beyond maintaining the structural integrity of electrodes during charge-discharge process, it also ensures the continuity of internal electron/ion transport pathways, which is indispensable for stable battery operation[107-109]. Currently, polyvinylidene fluoride (PVDF) dominates as the commercial cathode binder, however, it suffers from notable limitations. First, PVDF requires dissolution in the toxic solvent N-methylpyrrolidone (NMP), which conflicts with the growing demand for green manufacturing processes. Second, PVDF is prone to swelling in organic electrolytes and exhibits weak affinity toward long-chain LiPSs. These drawbacks collectively impede the commercialization of LSBs[110,111].
Eco-friendly biomaterials as binder for LSBs
To address these challenges, researchers have identified that binders rich in polar functional groups (e.g., hydroxyl, amino, carboxyl) can chemically adsorb long-chain polysulfides and offer a viable strategy to suppress the shuttle effect and further improve battery performance[112-114]. Nevertheless, developing high-performance binders via environmentally benign approaches remains a core research bottleneck. In recent years, the use of eco-friendly biomaterials, either directly or after simple modification, as LSB binders has emerged as a highly promising technical route. For example, bio-based binders such as gum arabic (GA), carboxymethyl cellulose (CMC), and chitosan possess abundant polar moieties (e.g., sulfonic acid, hydroxyl, and amino groups) in their molecular backbones[115-117]. These groups can trap and immobilize polysulfides through physical adsorption or chemical interactions, thereby mitigating the loss of active sulfur species.
Despite this potential, bio-based binders still face significant hurdles that limit their practical application. Their poor rate performance renders them unsuitable for high-power scenarios, low sulfur loading capabilities restrict improvements in battery energy density, and low coulombic efficiency results in substantial energy wastage. In addition, when paired with lithium metal anodes, they tend to induce excessive lithium metal consumption and rapid electrolyte depletion. Subsequent studies have attempted to address these issues by optimizing the polysulfide adsorption capacity of polar functional groups and regulating ion transport kinetics within composite sulfur cathodes. However, new challenges have arisen: graft modification via chemical crosslinking often involves complex separation and purification procedures, and the synthesis reactions are highly sensitive to reaction conditions - making large-scale production economically unfeasible.
To circumvent the limitations of chemical crosslinking, hydrogen-bond crosslinking strategies have gained increasing attention in recent years. Studies have shown that multifunctional binders prepared via hydrogen-bond crosslinking can introduce additional active sites, which not only enhance polysulfide anchoring but also optimize Li+ migration pathways[60]. However, current research on such binders primarily focuses on boosting polysulfide adsorption, while overlooking a fundamental issue: the shuttle effect caused by electrolyte-soluble long-chain polysulfides originates essentially from the sluggish kinetics of their conversion to short-chain polysulfides. Building on bio-based binders, introducing RE ions (e.g., Ce3+/La3+/Y3+) to coordinate in aqueous media with biomass ligands (CMC/alginate, tannic acid, etc.) forms tunable metal - organic networks. Moreover, compared with PVDF binders, which rely on costly NMP-based processing, RE-modified binders generally add some material/synthesis cost yet can retain aqueous processability while offering markedly stronger polysulfide anchoring and improved cycling/rate performance.
Rare-earth metal-based supramolecular complexes
Targeting this critical bottleneck that the slow kinetic conversion of long-chain polysulfides (e.g., Li2S8, Li2S6, and Li2S4) to short-chain species (e.g., Li2S2 and Li2S), Zhou et al. proposed a novel strategy for preparing multifunctional binders based on supramolecular chemistry principles[118]. Specifically, they synthesized a GB-Y (gelatin and boric acid cross-linked with rare earth yttrium-pyridine complex) binder by leveraging hydrogen-bonding interactions between a water-soluble matrix and an yttrium-pyridine complex [Figure 6A][118]. This binder exhibits three key advantages: (1) Its molecular structure contains abundant polar functional groups (e.g., carbonyl, amino), which enable direct chemical anchoring of polysulfides. DFT calculations revealed that numerous oxygen atoms in GB-Y act as Li-affinity sites, adsorbing polysulfides via O-Li bonds with an adsorption energy as high as 4.17 eV; (2) The synergy between hydrogen-bonding interactions and strong chemical bonding endows the binder with excellent mechanical properties, allowing it to effectively preserve electrode structural integrity even after 100 cycles at 1C; (3) The incorporated RE metal ion Y3+ exhibits exceptional electrocatalytic activity, which significantly accelerates the kinetic conversion of polysulfides, particularly promoting the transformation of short-chain polysulfides into the final discharge product Li2S.
Figure 6. (A) Schematic illustration of GB-Y binder preparation and enhancing polysulfides kinetic conversion mechanism. The figure is quoted with permission from Zhou et al.[118]; (B) Optimized hydrogen-bonded CMC-Ce binder configuration; (C) Optimized structures of different polysulfide species adsorption on CMC-Ce binder and corresponding binding energy profiles; (D) Li+ migration paths on CMC-Ce binder. The figures are quoted with permission from Zhou et al.[119]. GB-Y: gelatin and boric acid cross-linked with rare earth yttrium-pyridine complex; Pdc-Y: rare earth yttrium-pyridine complex; CMC: carboxymethyl cellulose.
Lewis acidity of rare-earth metals in hydrogen-bonding networks
By virtue of these advantages, LSBs assembled with the GB-Y binder achieve superior cycling stability and rate performance. Similarly, Chen et al. prepared a multifunctional CMC-Ce binder via in situ hydrogen-bond crosslinking, using CMC as the matrix and a RE metal Ce complex as the modifier [Figure 6B][119]. They systematically investigated the mechanism by which Ce enhances polysulfide adsorption and facilitates ion migration[119]. DFT calculations demonstrated that the binding energies between the CMC-Ce binder and various polysulfides were significantly higher than those of PVDF and pure CMC, confirming CMC-Ce’s superior polysulfide anchoring capability [Figure 6C]. Analysis of the optimized adsorption configurations revealed that polysulfides are primarily adsorbed via polar functional groups (e.g., -OH, -O-) in CMC-Ce. This enhanced adsorption arises from the introduced rare-earth metal complex (Pdc-Ce), which modulates the electron density of CMC, optimizes the S-P orbital interactions between Li and O, and thereby substantially increases the binding energy. Furthermore, Pdc-Ce in CMC-Ce acts as a Lewis acid site, which regulates the electron density and spatial distribution of lithophilic groups (e.g., -OH, -COO-), effectively promoting lithium-ion migration. This is further supported by DFT-calculated Li+ migration energy barriers. CMC-Ce exhibits a minimum energy barrier of only 0.57 eV, much lower than that of conventional binders [Figure 6D]. Benefiting from their excellent polysulfide adsorption capacity and rapid ion transport kinetics, LSBs based on the CMC-Ce binder deliver exceptional performance: a discharge specific capacity of 1,542.5 mAh g-1 at 0.5C (far exceeding that of other binders), a rate capability of up to 20C, and stable cycling for 600 cycles at 5C.
SEPARATOR AND INTERLAYER STRATEGIES WITH RARE-EARTH MATERIALS
Unlike cathode hosts, which mainly provide conductive frameworks and catalytic sites for sulfur redox reactions, separators and interlayers function primarily as physical and chemical barriers in LSBs. Incorporating RE compounds into these components not only suppresses polysulfide diffusion through strong chemical affinity and coordination, but also accelerates redox conversion at the separator/electrolyte interface. In addition, RE species can facilitate ionic transport, stabilize interfacial structures, and mitigate shuttle-induced side reactions, thereby ensuring high capacity retention and extended cycling life.
However, during repeated charge-discharge cycles, challenges such as the shuttle effect and active material loss arising from sluggish polysulfide conversion cannot be fully addressed by optimizing cathode matrices alone. To further suppress the shuttle effect, Manthiram and co-workers first introduced the concept of an “intermediate layer” in 2012[120], which is strategically positioned outside the cathode and can be regarded as a “secondary electrode”. Compared with the simple physical adsorption of carbon materials and the weak chemical interactions of organic compounds, polar metal compounds, particularly RE-based species, offer stronger binding with polysulfides and catalytic activity to accelerate their conversion, thus providing a more effective strategy to mitigate shuttle-related issues.
Structural design of rare-metal oxides for polysulfide blocking
RE oxides have attracted significant attention in separator/interlayer research due to their superior adsorption of polysulfides. Current studies primarily focus on La-, Y-, Ce-, and Nd-based systems. The core design principle for these RE-based separator/intermediate typically involves compositing RE compounds with carbon materials. This approach leverages the strong adsorption capacity of REs to immobilize active materials and the excellent electronic conductivity of carbon materials to establish efficient charge transport channels, and promotes the kinetic conversion of polysulfides through a synergistic “adsorption-catalysis” mechanism. As a representative example, Zheng et al. fabricated a composite of Nd2O3 and rGO as a coating for commercial polypropylene (PP) separators (Nd2O3/RGO/PP)[121]. This modified separator not only effectively mitigated polysulfide shuttling but also significantly enhanced battery cycling stability. The assembled full cell achieved stable cycling for 1,000 cycles at a high current density of 2C, with a single-cycle capacity loss rate of only 0.053%.
Among various RE metal oxides, CeO2 has been the most extensively studied for separator modification. Wen et al. prepared N-doped nanofiber separators embedded with CeO2 spheres (NCF@CeO2) via electrospinning combined with carbonization [Figure 7A][122]. The N-doped nanofiber skeleton forms a continuous conductive network, which accelerates charge transfer at the cathode/separator interface and ensures rapid kinetic conversion of polysulfides [Figure 7B]. Meanwhile, the hollow CeO2 structure effectively suppresses polysulfide migration through its strong adsorption capacity. Benefiting from this synergy, the Li-S full cell based on NCF@CeO2 achieved stable cycling for 1,000 cycles at 1C, with a single-cycle capacity loss of only 0.063%. Furthermore, CeO2 with a nanorod morphology has also been investigated for modification of the separator/intermediate layer[123]. Yu et al. theoretically elucidated the mechanism by which CeO2/KB (Ketjen Black) enhances LSB performance as a separator modification material [Figure 7C][124]. Their results showed that during catalytic conversion, polysulfides preferentially adsorb onto the CeO2 surface. In the subsequent transformation of Li2S2 to Li2S, Li2S2 first migrates to the KB surface, undergoes lithiation and diffusion to form Li2S2, and finally migrates to the CeO2 surface where it decomposes into the final discharge product Li2S. The migration of Li2S2 is the rate-limiting step of this process, with a Gibbs free energy of 0.85 eV. The synergy between CeO2 and KB is critical for polysulfide conversion, where CeO2 promotes polysulfide migration and decomposition, and KB provides charge transfer pathways for the lithiation process.
Figure 7. (A) Schematic diagram of NCF@CeO2 preparation by electrospinning; (B) HRTEM NCF@CeO2 morphology. The figures are quoted with permission from Wen et al.[122]; (C) Migration path of Li2S2 on the surface of CeO2 (111). This figure is quoted with permission from Yu et al.[124]; (D) Schematic illustration of KB@CeO2-C preparation process. This figure is quoted with permission from Zhang et al.[125]; (E) HRTEM image of bi-Sm2O3; (F) Schematic diagram of polysulfide species conversion to Li2S enhanced by Bi- Sm2O3. The figures are quoted with permission from Lai et al.[126]; (G)TEM image of Y2O3. The figures are quoted with permission from Qiao et al.[127]; (H) HRTEM image of CeO2/Co/carbon nanotube; (I) Schematic illustration of enhanced mechanism by CeO2/Co/CNT-PP separator. The Figures are quoted with permission from Zhang et al.[128]; (J) Schematic illustration of Co-N-C@CeO2-C preparation process. This figure is quoted with permission from Jin et al.[129]; (K) EDS mapping of LaCoO3; (L) Optimized structures of different polysulfide species adsorption on LaCoO3 and calculated adsorption energy. The figures are quoted with permission from Wang et al.[130]. MOF: Metal-organic framework; NCF@CeO2: nitrogen-doped carbon fibers based on cerium dioxide spheres; HRTEM: high-resolution transmission electron microscopy; KB: Ketjen Black; CNT: carbon nanotube; PP: polypropylene; EDS: energy-dispersive X-ray spectroscopy.
In terms of preparation methods, most RE oxides for separator/interlayer modification are obtained by high-temperature calcination of corresponding RE salts, with a small portion synthesized using metal-organic frameworks (MOFs) as precursors. The advantage of MOF-derived RE oxides is that they retain the porous structure of the MOF while generating more active defects, both of which enhance their adsorption and catalytic performance toward polysulfides. Among these, research on Ce-containing MOFs as precursors for CeO2 preparation has been the most intensive. For instance, Zhang et al. mixed KB with a Ce-MOF and calcined the mixture at 800 °C in a tube furnace to prepare a KB@ CeO2-C composite, which was used as an intermediate layer [Figure 7D][125]. At a sulfur loading of 2.8 mg/cm2, the battery based on this composite exhibited stable cycling for over 800 cycles at 0.5C.
Beyond Ce-based MOFs, studies on other RE-based MOF precursors have also made progress. Lai et al. prepared biphasic Sm2O containing both cubic and monoclinic phases via high-temperature carbonization of a Sm-based MOF [Figure 7E][126]. Bi-Sm2O3 derived from MOF precursors preserves the intrinsic porosity and high surface area of the parent structure, which strengthens its ability to confine polysulfides. DFT results indicated that the exposed (402) facet exhibits strong affinity toward LiPSs, while the (222) facet in C-Sm2O3 further promotes redox kinetics [Figure 7F]. As a result, the assembled LSBs delivered 860.1 mAh/g at 2C. Moreover, Y-containing MOFs employed as separator precursors provided effective LiPS immobilization [Figure 7G], enabling cells to reach 900.0 mAh/g at 0.5C with a sulfur loading of 3 mg/cm2[127].
To further improve the redox efficiency of polysulfides, elemental doping has become a key strategy for optimizing RE oxide performance. Zhang et al. prepared a CeO2/Co/CNT bimetallic electrocatalyst composite via high-temperature carbonization and used it to modify commercial PP separators [Figure 7H][128]. Based on the ultraviolet visible (UV-vis) visible adsorption test, the shuttle effect is greatly alleviated by CeO2-Co/CNT. In this composite, CeO2 traps polysulfides on its surface, Co accelerates their conversion to Li2S via high catalytic activity, and CNTs provide channels for rapid ion/electron transport [Figure 7I]. The modified separator enabled the cell to deliver a remarkably high discharge capacity of 1377.05 mAh/g at 1C. Jin et al. employed ZIF-67 (a Co-based MOF) and a Ce-MOF as dual precursors to synthesize a Co-N-C@CeO2-C coated separator through high-temperature carbonization [Figure 7J][129]. In this design, Co-N-C nanoparticles create charge imbalance and generate abundant oxygen vacancies and catalytic sites, while the CeO2-C matrix provides strong polysulfide immobilization. Acting as metal catalytic centers, the Co-N-C domains further accelerate polysulfide conversion. With a sulfur loading of 2.8 mg/cm2, the assembled battery delivered an initial capacity of 915.9 mAh/g at 0.5C.
For La-based RE oxides, doping with catalytically active metals (e.g., Fe, Co, Ni) further enhances their affinity for polysulfides. Wang et al. synthesized LCO with a unique honeycomb structure via the sol-gel method[130]. This honeycomb structure not only facilitates electrolyte permeation but also provides additional active sites [Figure 7K]. DFT calculations confirmed that the introduction of Co enhances La-S and Li-O interactions, enabling effective anchoring of polysulfides [Figure 7L]. The Li-S full cell based on this material retained a discharge specific capacity of 549.0 mAh/g after 1,000 cycles at 1C.
Rare-earth sulfides and fluorides as functional coatings
Beyond RE oxides, research on sulfide-based RE materials for separator/intermediate layer modification is also advancing. Wang et al. used Pr-BTC (a Pr-based MOF) as a precursor to synthesize porous two-dimensional carbon nanosheets doped with Pr2O2S, which was then used as a modification coating for commercial PP separators [Figure 8A][131]. DFT results showed that the Pr-O and Pr-S bonds in Pr2O2S not only improve material stability but also confer excellent polysulfide adsorption and catalytic capabilities. Meanwhile, Pr2O2S-C retains the high porosity of the parent MOF, facilitating electrolyte permeation. Sun et al. compared the battery performance of different La-based compounds (La2O3-C, La2O2S-C, La2S3-C) as separator modification materials[132]. Using La-BTC as the precursor, these composites were prepared by adjusting the calcination temperature and atmosphere [Figure 8B]. DFT simulations revealed that compared with La2O3 and La2S3, La2O2S exhibits moderate polysulfide adsorption, making it more advantageous for separator/intermediate layer modification and exhibiting the best discharge performance.
Figure 8. (A) Optimized structure of polysulfide species adsorption on Pr2O2S. This figure is quoted with permission from Wang et al.[131]; (B) Schematic illustration of La-BTC as a precursor for preparing La2O3-C, La2O2S-C, and La2S3-C. This figure is quoted with permission from Sun et al.[132]; (C) SEM image of YF3 nanofiber and corresponding EDS mapping. This figure is quoted with permission from Deng et al.[133]; (D) Schematic illustration of MOF-801 modified separator. This figure is quoted with permission from Zhao et al.[137]; (E) Li+ transportation mechanism in Ce-MOF with 1D channels. This Figure is quoted with permission from Song et al.[138]; (F) Schematic illustration of CSUST-1 with mixed-valent preparation process. This figure is quoted with permission from Jin et al.[140]; (G) STEM image of Sr2+ doped perovskite (LSMO-0.3). This figure is quoted with permission from Hao et al.[141]; (H) Scenario of Sm 4f-5d hybridization. This figure is quoted with permission from Zhou et al.[142]. BTC: 1,3,5-benzenetricarboxylate; La-BTC: lanthanum-benzenetricarboxylate metal-organic framework; SEM: scanning electron microscopy; EDS: energy-dispersive X-ray spectroscopy; MOF: Metal-organic framework; MOF-801: Zr-Fumarate MOF; STEM: scanning transmission electron microscopy; CSUST: Changsha University of Science and Technology; LSMO-0.3: La0.7Sr0.3MnO3.
Additionally, RE fluorides have been explored for separator/intermediate layer modification. Deng et al. synthesized YF3-doped polyacrylonitrile (PAN) CNFs (YF3-PAN-CNF) via electrospinning and carbonization [Figure 8C][133]. The high-aspect-ratio CNFs establish rapid charge transfer channels, while the strong chemical bonding between lithophilic YF3 and polysulfides effectively suppresses the shuttle effect, effectively improving the cycling stability and discharge performance.
Rare-earth-containing MOFs as coating
Notably, MOFs themselves can be directly used as separator coatings, and their tunable functional groups and exposed metal active sites enable efficient polysulfide adsorption[134-136]. For example, Zhao et al. coated commercial PP separators with MOF-801 [Figure 8D][137]. With a pore size of 6 Å (smaller than that of polysulfide molecules), MOF-801 acts as a physical barrier and effectively mitigates the shuttle effect via a “pore sieving” mechanism. Song et al. used Ce-MOF-1 with 1D pore characteristics, where pores are formed by oxygen-containing ligands as a separator coating [Figure 8E][138]. Compared with cage-like frameworks, their research showed that Li+ have a twofold lower migration energy barrier in the 1D channels of Ce-MOF-1, enabling stable lithium stripping/deposition for over 3,000 h at an ultrahigh current density of 15 mA/cm2. Su et al. modified the ligands of a Ce-MOF (UIO-66-NH2) by introducing -NH2 groups[139]. The newly introduced active sites further enhanced polysulfide adsorption, thereby mitigating the shuttle effect. In Ce-MOF systems, Ce typically exists in the +3 or +4 oxidation state. Jin et al. synthesized CSUST-1, which features isolated mixed-valent Ce (+3, +4) sites [Figure 8F][140]. The isolated arrays of Ce (+3, +4) in CSUST-1 act as redox couples, and when combined with the abundant oxygen vacancies, they significantly enhance both the redox kinetics of polysulfides and the lithium-ion transport rate.
Emerging rare-earth-based materials for separator and interlayer
Perovskite materials have attracted growing attention in LSB research in recent years due to their tunable adsorption and catalytic properties. Hao et al. combined XAFS and X-ray computed tomography (CT) to establish a continuous multiscale analysis model, which precisely revealed how dopant elements regulate the relationship between perovskite structure and LSB performance [Figure 8G][141]. Their study showed that Sr2+ doping induces partial conversion of Mn3+ to Mn4+ while altering the coordination environment of Mn. This not only enhances the material’s electronic conductivity but also strengthens the interaction between Mn cations and polysulfides, ultimately leading to superior electrochemical performance.
As a new class of catalytic materials, single-atom catalysts differ from traditional nanoparticles or cluster-based catalysts. Their isolated single atoms maximize atomic utilization and interact more easily with reactant molecules, significantly boosting catalytic activity. Zhou et al. were the first to synthesize a Sm-based RE single-atom catalyst[142]. Their research revealed that the unique Mott transition between the 4f65d0 and 4f55d1 valence electron configurations of Sm-N3C3 enables f-d-p orbital hybridization with polysulfides, accelerating polysulfide conversion and Li2S decomposition [Figure 8H]. The LSB based on this catalyst retained 84.3% of its capacity after 2,000 stable cycles at 4C.
ELECTROLYTE ADDITIVES BASED ON RARE-EARTH SPECIES
In liquid LSBs, the electrolyte is the Li+-transport medium that governs cycling stability, rate capability, and safety. It comprises solvent, lithium salt, and additives; the solvent–salt matrix forms the Li+-conducting medium enabling transport between the cathode and anode[143-146]. Additives, though minor in content, are crucial for tuning electrode–electrolyte reactions and optimizing the SEI. In current mainstream LSB electrolyte systems, the solvent is predominantly a binary mixture of 1,3-dioxolane (DOL) and dimethyl ether (DME), while the lithium salt is mainly lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). Lithium nitrate (LiNO3) is commonly added to form a robust SEI enriched in LiF and Li3N, which suppresses dendrites and limits electrolyte decomposition[147]. As performance requirements for LSB continue to escalate, traditional additives struggle to meet the growing demands for higher cycling stability and safety. Against this backdrop, RE metal compounds have emerged as promising candidates for electrolyte additives, owing to their unique electronic structures and chemical reactivity.
Inorganic salts containing rare-earth elements
To date, the most intensively studied category of RE-based electrolyte additives for LSB batteries is RE nitrate. Liu et al. pioneered the integration of lanthanum nitrate [La(NO3)3] into LSB electrolytes, laying the foundation for research on RE nitrates as additives[148]. Systematic studies demonstrate that La(NO3)3 participates in interfacial reactions to form a passivation layer on the lithium metal anode surface during battery cycling, which is enriched with a La/Li sulfide composite [Figure 9A and B]. The passivation layer serves two critical functions: on the one hand, it effectively blocks direct contact between electrolyte components and lithium metal, reducing unnecessary Li consumption and electrolyte degradation; on the other hand, it guides the uniform deposition of lithium metal, avoiding electrode structural damage and capacity decay caused by random dendrite growth. Together, these effects significantly extend the battery’s cycle life and enhance its safety. Beyond La(NO3)3, research on yttrium nitrate [Y(NO3)3] as an electrolyte additive has also yielded positive outcomes, with a mechanism of action analogous to that of La(NO3)3. Studies confirm that Y(NO3)3 similarly induces the formation of a stable passivation layer on the lithium metal anode, which is rich in yttrium sulfide (Y2S3) and provides interfacial protection comparable to that of the La/Li sulfide composite. Through inhibiting dendritic lithium deposition and reducing electrolyte-lithium side reactions, the cycling performance of LSB is significantly prolonged [Figure 9C-E][149].
Figure 9. (A) EDS maps of Li anode after 100 cycles; (B) Schematic illustration of mechanism of La(NO3)3 as electrolyte additive. The Figures are quoted with permission from Liu et al.[148]; (C) HUMO and LUMO profiles of Li+ NO3, Y 3+NO3, DOL, DME, and TFSI-; (D) In-situ electrochemical deposition optical images of Li/Li symmetrical battery with 1% Y(NO3)3 as electrolyte additive; (E) Schematic illustration of uniform deposition enhanced by Y(NO3)3 as electrolyte additive. The figures are quoted with permission from Hao et al.[149]; (F) FTIR spectra of Li2S6 in DME/DOL and DME/DOL with Nd(OTf)3; (G) Comparison CV tests of Nd(OTf)3 as symmetrical battery electrolyte additive; (H) XPS S 2p plot of cycled Li anode with Nd(OTf)3 as electrolyte additive. The figures are quoted with permission from Yen et al.[150]. EDS: Energy-dispersive X-ray spectroscopy; HUMO: highest occupied molecular orbital; LUMO: lowest unoccupied molecular orbital; DOL: 1,3-dioxolane; Y(NO3)3: yttrium nitrate; DME: dimethyl ether; TFSI-: bis(trifluoromethanesulfonyl)imide anion; FTIR: Fourier transform infrared spectroscopy; Nd(OTf)3: neodymium trifluoromethanesulfonate; CV: cyclic voltammetry; XPS: X-ray photoelectron spectroscopy.
Organic salts containing rare-earth elements
In addition to RE nitrates, researchers have explored other classes of RE compounds as electrolyte additives, with RE trifluoromethanesulfonates emerging as a key focus. Addressing the unique requirements of anode-less LSB, the Manthiram group systematically investigated the role of neodymium trifluoromethanesulfonate [Nd(OTf)3] as an electrolyte additive[150]. Their study revealed that Nd(OTf)3 optimizes battery performance through multiple synergistic mechanisms: (1) Leveraging its unique electronic structure, Nd3+ modulates the solvation shell configuration of Li+, altering the transport pathways and kinetic behavior of Li+ in the electrolyte; (2) Nd3+ exhibits strong adsorption affinity for polysulfides, enabling chemical immobilization of free polysulfides in the electrolyte to minimize shuttle-related capacity loss. Additionally, its intrinsic catalytic activity may partially accelerate the kinetic conversion of polysulfides [Figure 9F and G]; (3) XPS analysis of the SEI layer on the cycled lithium metal surface provided further insights. After adding Nd(OTf)3, the intensity of the polysulfide characteristic peak in the S 2p spectrum of the SEI layer was significantly lower than that of the additive-free control sample. Moreover, characteristic signals of Nd-S bonds were detected, confirming that Nd(OTf)3 promotes the formation of an SEI layer enriched with Nd-S species [Figure 9H]. This RE-doped SEI layer exhibits superior stability and ionic conductivity, effectively protecting the lithium metal anode while ensuring efficient Li+ transport. Concurrently, the presence of Nd(OTf)3 facilitates the uniform deposition of lithium metal, further suppressing dendrite growth. These combined effects provide critical support for enhancing the performance of anode-less LSBs.
SOLID-STATE ELECTROLYTES MODIFIED BY RARE-EARTH COMPOUNDS
Solid-state LSBs fundamentally eliminate the core issue of polysulfide shuttling in liquid electrolytes through the intrinsic properties of solid-state electrolytes. They also significantly enhance the energy density and safety of batteries, making them a critical development direction in the LSB field[151-153]. However, solid-state systems still face three major challenges: (1) sulfur exhibits extremely poor electrical conductivity, leading to low active material utilization; (2) significant impedance exists at the electrode-solid electrolyte interface, hindering ion transport; and (3) lithium metal anodes are prone to dendrite growth, which poses safety risks and causes capacity decay[154-156].
RE doping can stabilize solid electrolytes through coupled bulk-grain-boundary-interphase effects[153]. Aliovalent RE3+/RE4+ substitution creates beneficial Li-vacancies and widens diffusion bottlenecks, increasing ionic conductivity and homogenizing Li+ flux at the Li|SE contact. RE-assisted sintering promotes densification and grain growth, thereby reducing porosity and grain-boundary resistance. At the interface, RE cations tend to segregate and react to form ultrathin, electronically insulating yet Li+-permeable layers (e.g., RE-oxides/fluorides/oxyfluorides), which suppress parasitic reactions, lower interfacial impedance, and raise the dendrite initiation threshold by blocking electron pathways while increasing local shear modulus.
LLZO and garnet-type solid-state electrolytes with RE doping
RE-based materials have demonstrated substantial application value in ceramic solid electrolytes due to their unique structural and chemical properties, providing critical support for optimizing the performance of solid-state LSBs[157-159]. To simultaneously address the issues of ion conduction efficiency and lithium dendrite suppression, researchers have proposed a “dual-layer hybrid electrolyte” design strategy to achieve targeted optimization through functional zoning. The electrolyte layer facing the sulfur cathode prioritizes high ionic conductivity to ensure rapid reaction of active materials, while the layer facing the lithium anode balances ionic conduction with mechanical strength to withstand the stress generated by lithium dendrite growth. Based on this strategy, Liu et al. designed a dual-layer hybrid solid electrolyte (DLHSE) with a ceramic framework [Figure 10A][160]. The cathode side employs NASICON-type Li1+xAlxTi2-x(PO4)3 (LATP), leveraging its high ionic conductivity to accelerate cathode reactions; the anode side uses garnet-type LLZO, which relies on its excellent mechanical properties to suppress lithium dendrites. To further improve electrode-electrolyte interface contact and reduce interfacial impedance, the researchers immersed the DLHSE in a liquid electrolyte. The permeated ether-based electrolyte not only enhanced the wettability of the solid-solid interface but also established rapid ion transport channels. LSB employing this electrolyte demonstrated remarkable long-term stability, retaining 802 mAh/g after 500 cycles at 0.2C, and even achieving a discharge capacity of 7 Ah at 0.1C in pouch-cell configurations.
Figure 10. (A) Schematic diagram of DLHSE preparation process. The figures are quoted with permission from Liu et al.[160]; (B) Schematic diagram of composite sulfur electrode and PEO/LiTFSI-LLTO preparation. This figure is quoted with permission from Zhu et al.[161]; (C) HRTEM images of (Li4(BH4)3I)@75LLZTO after ball milling; (D) Rate performance of Li/SSE/SPAN at 100 ℃. The figures are quoted with permission from Lv et al.[166]; (E) Schematic diagram of synergetic interface optimization of all solid-state LSBs; (F) Nyquist plots of Li7P2.9Y0.1S10.8I0.2 at different temperatures; (G) Cycling performance of the SC-Li7P3S11, SbSn-Li7P3S11, and SbSn-Li7P2.9Y0.1S10.8I0.2 batteries at 0.05C. The figures are quoted with permission from Zhao et al.[171]; (H) SEM image of Li3HoBr6 and corresponding EDS mapping; (I) Out-of-plane migration pathway of Li+ from original [Oct] → [Tetra] → nearest [Oct]; (J) Migration energy barrier of Li+ from original [Oct] → [Tetra] → nearest [Oct]. The figures are quoted with permission from Shi et al.[173]. DLHSE: Double-layer hybrid solid-state electrolyte; PEO: poly(ethylene oxide); LiTFSI: lithium bis(trifluoromethanesulfonyl)imide; TFSI⁻: bis(trifluoromethanesulfonyl)imide anion; LLTO: Li0.33La0.56TiO3; HRTEM: high-resolution transmission electron microscopy; LLZTO: Li6.4La3Zr1.4Ta0.6O12; SSE: solid-state electrolyte; SPAN: sulfurized polyacrylonitrile; LSB: lithium-sulfur battery.
Compared with inorganic solid electrolytes, polymer solid electrolytes (e.g., PEO, PVDF) offer superior flexibility and processability, which substantially improve their interfacial compatibility with electrodes. Leveraging this advantage, researchers have further optimized interfacial performance through “polymer-inorganic synergy” approaches. For example, Zhu et al. incorporated PEO during the preparation of sulfur cathodes; the flexibility of PEO filled the gaps between the electrode and electrolyte, significantly enhancing interfacial compatibility [Figure 10B][161]. Wang et al. added LLTO/C nanofibers to the cathode, where LLTO provided efficient ion transport channels, while the carbon component constructed an electronic transport network[162]. This synergistic design enabled dual-channel (ion/electron) transport within the cathode, effectively overcoming the insulating nature of sulfur[162].
As a representative RE-based inorganic solid electrolyte, LLZO has great potential for high ionic conductivity. However, pure-phase LLZO suffers from low conductivity, poor structural stability, and inadequate interfacial compatibility, limiting its practical application. To address these shortcomings, researchers have adopted two key optimization strategies: elemental doping and surface modification. For elemental doping, introducing elements such as Ta and Nb induces LLZO to form a stable cubic phase and increases the concentration of lithium vacancies in the lattice. The higher density of lithium vacancies creates additional “channels” for Li+ migration, effectively reducing the ion transport energy barrier and significantly improving conductivity[163,164]. For surface modification, the core goal is to eliminate the lithophilic LiOH/Li2CO3 layer that forms on the surface of LLZTO when exposed to air, which severely hinders Li+ transport. Wang et al. used in-situ modification to coat the surface of LLZTO with an amphoteric covalent organic framework denoted as LLZTO@HUT4[165]. This modification not only improved the compatibility of the organic-inorganic interface but also enhanced the mechanical properties of the electrolyte membrane through intermolecular interactions, substantially boosting lithium-ion transport efficiency. Lv et al. coated the LLZTO surface with a complex hydride Li4(BH4)3I [Figure 10C][166]. After modification, the electrolyte conductivity increased dramatically from 8.29 × 10-6 S/cm to 1.10 × 10-2 S/cm[166]. SPAN (sulfurized polyacrylonitrile) batteries based on this electrolyte achieved a discharge specific capacity of 1149 mAh/g at 0.2C, with a capacity retention rate of up to 91% after 100 cycles [Figure 10D]. Additionally, Ruan et al. proposed an innovative “in-situ reaction modification” method using phosphoric acid to react with the inert LiOH/Li2CO3 components on the LLZTO surface, generating a uniform Li3PO4 modification layer in-situ[167]. This layer not only reduces the surface ion migration energy barrier but also forms a robust SEI layer during cycling and this dual effect effectively minimizes interfacial impedance.
Sulfide and halide solid-state electrolytes incorporating RE elements
Beyond oxide solid-state electrolytes, sulfide solid-state electrolytes have become another key research focus, as their room-temperature ionic conductivity (10-3-10-2 S/cm) far exceeds that of oxides and polymers[168-170]. However, in solid-state systems, the absence of liquid electrolytes significantly restricts the redox kinetics of sulfur - especially at high sulfur loadings, where active materials struggle to fully participate in reactions. Therefore, when designing sulfur cathodes, researchers focus on constructing dual ion/electron channels, and RE metal doping has emerged as a key strategy to optimize electrolyte performance. Zhao et al. adopted a dual-doping strategy using Y2S3 and LiI to synthesize the sulfide solid-state electrolyte Li7P2.9Y0.1S10.8I0.2, which was then combined with an SbSn/S-C alloyed cathode [Figure 10E][171]. On one hand, the doped Y element optimized the interfacial contact between the cathode and electrolyte, accelerating ion transport and achieving a high room temperature conductivity [Figure 10F]. On the other hand, SbSn alloying enhanced the internal electronic conductivity of the cathode. The synergy of these two factors enabled efficient utilization of active materials under high loading conditions. Based on this system, the all-solid-state LSBs maintained a discharge specific capacity of 1,163.5 mAh/g after 50 cycles at room temperature and 0.05C [Figure 10G].
Furthermore, the air sensitivity of sulfide solid-state electrolytes is a major obstacle to their industrialization, and the introduction of Ce2S3 provides a solution[172]. Ce doping not only improves the chemical stability of the sulfide electrolyte but also maintains a high conductivity of 7.7 × 10-4 S/cm at room temperature while expanding the electrochemical window to 5 V. DFT analysis showed that Ce inhibits the decomposition of highly conductive P2S72- into PS43- and P2S64-, thereby preserving the structural and performance stability of the electrolyte. RE halides have also emerged as a new research direction for solid-state electrolytes, thanks to their wide electrochemical windows and high-voltage stability. Shi et al. synthesized a novel RE-based halide solid-state electrolyte, Li3HoBr6, which exhibits an impressive room-temperature ionic conductivity of 1.1 × 10-3 S/cm [Figure 10H][173]. DFT simulations revealed the source of this high conductivity. LHB crystals contain four Li+ migration pathways, and the pathway perpendicular to the crystal plane has the lowest migration energy barrier and highest efficiency, providing a structural basis for rapid ion transport [Figure 10I and J].
CONCLUSION AND OUTLOOK
Summary
This review has summarized recent advances in RE-based materials across various LSB components, including cathodes, separators/interlayers, electrolyte additives, and solid-state electrolytes, with emphasis on the underlying mechanisms of performance improvement. Supplementary Table 4 confirms that the benefits of RE strategies generally persist under high loading, lean electrolyte, and pouch-cell formats, with representative areal capacities of ~ 2-9 mAh/cm2 and low per-cycle fade where reported. Such advantages highlight the vital role of RE compounds in overcoming the intrinsic limitations of polysulfide chemistry and advancing the practical deployment of LSBs.
Challenges
Despite remarkable progress, several challenges remain for RE-based strategies in LSBs [Figure 11]:
Figure 11. Schematic illustration of the major challenges and perspectives for RE-based strategies in lithium-sulfur batteries (LSBs). SEI: Solid electrolyte interphase; RE: rare-earth; LSB: lithium-sulfur battery.
(1) Imbalanced research focus.
Current research has predominantly concentrated on cathode hosts and separator/interlayer systems, where RE-based oxides such as La2O3 and CeO2 have served as model compounds. In contrast, the exploration of RE-based electrolyte additives, lithium anode protection layers, and solid-state electrolyte modifications remains relatively limited. Since these components play equally crucial roles in ensuring overall battery stability and performance, future studies need to broaden their scope beyond cathode-centered designs.
(2) Limited material diversity.
Among the wide variety of RE compounds, most studies are confined to oxides and hydroxides, whereas other classes such as sulfides, fluorides, nitrides, carbides, and organometallic complexes are rarely investigated. These less-explored materials may possess stronger catalytic activity, higher electronic conductivity, or better stability under electrochemical conditions. For example, RE sulfides and nitrides could provide more favorable electronic structures for facilitating multi-electron polysulfide redox, while RE carbides may offer enhanced conductivity and structural robustness. Expanding the library of RE materials is therefore essential for discovering superior candidates.
(3) Restricted element utilization.
Of the 17 RE elements, practical applications in LSBs have so far been limited mainly to La, Ce, Y, Nd, Gd, and Sm. This represents only about half of the available RE series, leaving significant untapped potential. Less common RE elements such as Tb, Dy, Er, and Yb, with their distinct ionic radii and electronic configurations, may offer unique catalytic pathways or adsorption behaviors. A more systematic exploration of the full RE series is necessary to establish clear element-property-performance correlations.
(4) Lack of rational design principles.
In many studies, the design strategies for cathode hosts and separators/interlayers are overly similar, often relying on heavy oxide or hydroxide coatings. While such approaches may work well for cathode hosts, they are less suitable for separators and interlayers, where minimizing inactive mass is essential for achieving high energy density. Failure to account for the lightweighting principle compromises the energy-to-weight ratio of the entire battery. Thus, it is imperative to establish distinct design principles tailored to each component, for example, high-density active site construction in cathode hosts versus ultrathin, lightweight yet catalytically active coatings in separators/interlayers.
(5) Sustainability and techno-economic constraints.
Beyond electrochemical metrics, the practical deployment of RE-enabled LSBs is limited by sustainability and cost. RE resources are unevenly distributed, exposing supply chains to concentration, price volatility, and geopolitical risk. Mining and refining carry high CAPEX/OPEX and non-trivial environmental footprints (energy/reagent intensity, tailings, wastewater management)[47]. These burdens must be weighed against performance gains, especially under practical operating conditions (high sulfur loading, lean electrolyte, limited Li excess, pouch cells). A further challenge is the lack of standardized sustainability metrics (e.g., RE mass per Wh, recyclability/readiness), which hampers transparent benchmarking across studies.
Perspectives
Looking ahead, several research directions are recommended to unlock the full potential of RE-based materials in LSBs [Figure 11]:
(1) Diversifying RE chemistries.
Future studies should extend beyond conventional RE oxides to systematically investigate sulfides, nitrides, carbides, fluorides, and organometallic complexes. These materials offer unique electronic structures and bonding characteristics that may provide superior polysulfide anchoring, enhanced conductivity, or improved electrochemical stability. The rational combination of different RE compounds into composite or hybrid structures may further unlock synergistic effects.
(2) Post-Li (e.g., Na, K) systems.
Transferring RE strategies to sodium sulfur (NSB) and potassium sulfur (KSB) systems requires addressing kinetics that differ from those in LSBs. Larger Na+/K+ radii and weaker desolvation shift polysulfide speciation and solubility, alter nucleation pathways, and favor more fragile, resistive product layers (Na2S, K2S). These factors slow solid-solid conversion, amplify volume change, and raise interfacial and charge-transfer resistance[174]. RE-based cathode hosts and catalysts can lower barriers for S ↔ Na2S/K2S by providing polar RE-S binding sites and redox-active centers that facilitate chain-length scission and product detachment. Tailoring adsorption strength is critical: strong capture for long-chain Na/K polysulfides, but moderate binding for Na2S/K2S to avoid passivation.
(3) Advanced characterization and integrated approaches.
The catalytic mechanisms of RE species, especially the role of 4f orbitals during polysulfide conversion, remain poorly understood. The application of advanced in-situ/operando techniques is critical to reveal structure-activity relationships and guide rational material design. Beyond mechanism elucidation, advanced characterization should also quantify the stability of active components under cycling by tracking RE dissolution/crossover and active-site evolution using H-cell tests coupled with UV-vis spectroscopy and inductively coupled plasma-optical emission spectroscopy (ICP-OES), as well as operando XPS, X-ray absorption fine structure (XAFS), and time-of-flight secondary ion mass spectrometry (ToF-SIMS). These diagnostics connect leaching- or interphase-driven changes to kinetic decay, guiding the design of more durable RE architectures. DFT has proven to be an indispensable tool for elucidating atomic- and electronic-scale interactions of RE species with polysulfides. Moving forward, machine learning and high-throughput computational screening should be integrated with DFT to accelerate the discovery of new RE-based materials with optimal catalytic and structural properties. Experimentally, advanced synthesis methods such as atomic layer deposition, solvothermal growth, and single-atom dispersion strategies should be further explored to precisely control the distribution and activity of RE species.
(4) SEI engineering.
Preliminary studies suggest that RE-based salts or nitrides can promote the formation of RE-enriched SEIs, which may improve interfacial stability and suppress dendrite growth. However, the mechanisms governing the nucleation, composition, and long-term stability of RE-modified SEIs remain poorly understood. Moreover, extending these insights to NSB and KSB systems requires accounting for key mechanistic and engineering differences. The larger ionic radii and weaker desolvation energies of Na+/K+ alter polysulfide speciation/solubility and tend to form more fragile, resistive SEIs; fluoride-rich layers (NaF/KF) often exhibit lower ion transport than LiF, and volume changes at the alkali metal exacerbate interfacial cracking. Combining systematic experimental characterization with DFT simulations and molecular dynamics modeling will be critical to uncover the underlying processes.
(5) Pathways to practical and responsible deployment.
Looking ahead, advancing RE-enabled LSBs demands an integrated strategy that simultaneously addresses performance, manufacturability, and responsibility. Priority should be given to reducing dependence on critical RE elements through low-dosage designs, element diversification toward less-critical species, and green, scalable synthesis routes that employ benign solvents, lower temperatures, and process intensification[51]. Equally important is closing the materials loop by developing clean, closed-loop recycling for end-of-life LIBs/LSBs, implemented under design-for-recycling principles. To guide choices objectively, sustainability and cost must be quantified alongside electrochemical metrics by embedding life-cycle assessment (LCA), techno-economic analysis (TEA), and social-LCA/traceability. Finally, studies should report application-relevant benchmarks - such as RE mass per Wh, recyclability considerations, and practical performance - to accelerate translation from laboratory demonstrations to manufacturable, durable, and responsible LSB technologies.
Overall, RE-based materials hold great promise for tackling the intrinsic challenges of LSBs and accelerating their commercialization. Through continued interdisciplinary efforts, particularly the precise modulation of f-orbital properties combined with advanced experimental and theoretical methodologies, the practical realization of RE-based strategies in next-generation high-energy-density batteries can be anticipated.
DECLARATIONS
Acknowledgments
Dr. Q. Wu acknowledges the support of the Alexander von Humboldt Research Fellowship.
Authors’ contributions
Writing - original draft: Zhou, F.
Supervision: Wu, Q.; Xu, J.; Cao, F.
Review & Editing: Wu, Q.; Meng, J.; Xu, J.; Cao, F.
Availability of data and materials
All data supporting this review are contained within the article and its Supplementary Materials. Source studies are cited throughout the text.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval and consent to participate
None.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
2. Xiao, J.; Shi, F.; Glossmann, T.; Burnett, C.; Liu, Z. From laboratory innovations to materials manufacturing for lithium-based batteries. Nat. Energy. 2023, 8, 329-39.
3. Li, J.; Gao, L.; Pan, F.; et al. Engineering strategies for suppressing the shuttle effect in lithium-sulfur batteries. Nanomicro. Lett. 2023, 16, 12.
4. Vera, M. L.; Torres, W. R.; Galli, C. I.; Chagnes, A.; Flexer, V. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth. Environ. 2023, 4, 149-65.
5. Zeng, X.; Li, M.; Abd El‐hady, D.; et al. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy. Mater. 2019, 9, 1900161.
6. Yang, Z.; Lu, Y.; Liu, X.; Li, F.; Chen, J. Application of high energy X-ray diffraction and Rietveld refinement in layered lithium transition metal oxide cathode materials. Nano. Res. 2023, 16, 9954-67.
8. Duan, J.; Wang, K.; Teng, L.; et al. Nanofibrous covalent organic frameworks as the cathode, separator, and anode for batteries with high energy density and ultrafast-charging performance. ACS. Nano. 2024, 18, 29189-29202.
9. Jiang, F.; Du, Y. F.; Guo, J. X.; et al. Thermoresponsive solid electrolyte interphase enables safe lithium-sulfur batteries with high energy density. Energy. Environ. Sci. 2025, 18, 4925-4933.
10. Zhou, S.; Shi, J.; Liu, S.; et al. Visualizing interfacial collective reaction behaviour of Li-S batteries. Nature. 2023, 621, 75-81.
11. Yao, Y.; Rui, X.; Bai, R.; et al. Roadmap for next-generation electrochemical energy storage technologies: secondary batteries and supercapacitors. ACS. Nano. 2025, 19, 30568-30687.
12. Cao, R.; Yu, Y.; Li, C. Metal fluorides emerging as fast-charging and high-capacity cathodes from the viewpoint of open framework strategies. EnLab. 2025, 2, 240017.
13. Yari, S.; Conde Reis, A.; Pang, Q.; Safari, M. Performance benchmarking and analysis of lithium-sulfur batteries for next-generation cell design. Nat. Commun. 2025, 16, 5473.
14. Wu, Y.; Huang, J.; Zhang, Z.; et al. Recent advances in functionalized separators for shuttle-free and dendrite-free lithium/sodium-sulfur batteries. Energy. Mater. 2025, 5, 500027.
15. Wu, C. C.; Chan, T. C.; Chung, S. H. Metal-based composite sulfur cathodes for lithium-sulfur electrochemical cells. Commun. Mater. 2025, 6, 111.
16. Ye, C.; Li, H.; Chen, Y.; et al. The role of electrocatalytic materials for developing post-lithium metal||sulfur batteries. Nat. Commun. 2024, 15, 4797.
17. Yao, Z.; Zou, Y.; Liu, S.; et al. Reactivity descriptors for sulfur redox kinetics in lithium-sulfur batteries: from mechanistic insights to machine learning driven catalyst design. Chem. Soc. Rev. 2025, 54, 9161-91.
18. Huang, X.; Li, X.; Zhao, L.; et al. Manipulating sulfur redox kinetics in rechargeable metal-sulfur batteries: fundamental principles and universal methodologies. Adv. Mater. 2025, 37, 2419089.
19. Zhang, X.; Zhang, X.; Wang, X.; Cui, G.; Pan, H.; Sun, W. Engineering spin states of metal sites toward advanced lithium-sulfur batteries. Energy. Environ. Sci. 2025, 18, 3553-3567.
20. Zeng, L.; Zhu, J.; Chu, P. K.; et al. Catalytic effects of electrodes and electrolytes in metal-sulfur batteries: progress and prospective. Adv. Mater. 2022, 34, 2204636.
21. Chung, S.; Chang, C.; Manthiram, A. Progress on the critical parameters for lithium-sulfur batteries to be practically viable. Adv. Funct. Mater. 2018, 28, 1801188.
22. Liu, X.; Li, Z.; Liao, X.; et al. A three-dimensional nitrogen-doped graphene framework decorated with an atomic layer deposited ultrathin V2O5 layer for lithium sulfur batteries with high sulfur loading. J. Mater. Chem. A. 2020, 8, 12106-13.
23. Huang, L.; Li, J.; Liu, B.; et al. Electrode design for lithium-sulfur batteries: problems and solutions. Adv. Funct. Mater. 2020, 30, 1910375.
24. Fang, R.; Zhao, S.; Sun, Z.; Wang, D.; Cheng, H.; Li, F. More reliable lithium-sulfur batteries: status, solutions and prospects. Adv. Mater. 2017, 29, 1606823.
25. Lim, W.; Kim, S.; Jo, C.; Lee, J. A comprehensive review of materials with catalytic effects in Li-S batteries: enhanced redox kinetics. Angew. Chem. Int. Ed. Engl. 2019, 58, 18746-57.
26. Pang, Q.; Liang, X.; Kwok, C. Y.; Nazar, L. F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy. 2016, 1, 16132.
27. Hong, H.; Che Mohamad, N. A. R.; Chae, K.; Marques Mota, F.; Kim, D. H. The lithium metal anode in Li-S batteries: challenges and recent progress. J. Mater. Chem. A. 2021, 9, 10012-38.
28. Han, Z.; Li, S.; Wu, Y.; Yu, C.; Cheng, S.; Xie, J. Challenges and key parameters in exploring the cyclability limitation of practical lithium-sulfur batteries. J. Mater. Chem. A. 2021, 9, 24215-40.
29. Ji, X.; Lee, K. T.; Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500-6.
30. Huang, X. Y.; Zhao, C. Z.; Kong, W. J.; et al. Tailoring polymer electrolyte solvation for 600 Wh kg-1 lithium batteries. Nature. 2025, 646, 343-50.
31. Wang, T.; He, J.; Zhu, Z.; et al. Heterostructures regulating lithium polysulfides for advanced lithium‐sulfur batteries. Adv. Mater. 2023, 35, 2303520.
32. Xia, S.; Xu, X.; Wu, W.; et al. Advancements in functionalized high-performance separators for lithium-sulfur batteries. Mater. Sci. Eng. R. Rep. 2025, 163, 100924.
33. Jung, S. Y.; Park, J. Y.; Yu, S. H. Recent advances in electrolyte design for optimized lithium polysulfides solvation in lithium-sulfur batteries. Energy. Mater. 2025, 5, 500125.
34. Zhou, Q.; Xiong, X.; Peng, J.; et al. Tailored engineering on the interface between lithium metal anode and solid-state electrolytes. Energy. Environ. Mater. 2024, 8, e12831.
35. Li, Z.; Zhou, C.; Hua, J.; et al. Engineering oxygen vacancies in a polysulfide-blocking layer with enhanced catalytic ability. Adv. Mater. 2020, 32, 1907444.
36. Wu, Q.; Chen, K.; Shadike, Z.; Li, C. Relay-type catalysis by a dual-metal single-atom system in a waste biomass derivative host for high-rate and durable Li-S batteries. ACS. Nano. 2024, 18, 13468-83.
37. Wu, Q.; Zhou, X.; Xu, J.; Cao, F.; Li, C. Adenine derivative host with interlaced 2D structure and dual lithiophilic-sulfiphilic sites to enable high-loading Li-S batteries. ACS. Nano. 2019, 13, 9520-32.
38. Wu, Q.; Yao, Z.; Zhou, X.; Xu, J.; Cao, F.; Li, C. Built-in catalysis in confined nanoreactors for high-loading Li-S batteries. ACS. Nano. 2020, 14, 3365-77.
39. Wu, Q.; Shadike, Z.; Xu, J.; Cao, F.; Li, C. Integrated reactor architecture of conductive network and catalytic nodes to accelerate polysulfide conversion for durable and high-loading Li-S batteries. Energy. Storage. Mater. 2023, 55, 73-83.
40. Liu, R.; Wei, Z.; Peng, L.; et al. Establishing reaction networks in the 16-electron sulfur reduction reaction. Nature. 2024, 626, 98-104.
41. Li, H.; Meng, R.; Ye, C.; et al. Developing high-power Li||S batteries via transition metal/carbon nanocomposite electrocatalyst engineering. Nat. Nanotechnol. 2024, 19, 792-9.
42. Wu, Q.; Zheng, Y.; Guan, X.; Xu, J.; Cao, F.; Li, C. Dynamical SEI reinforced by open-architecture MOF film with stereoscopic lithiophilic sites for high-performance lithium-metal batteries. Adv. Funct. Mater. 2021, 31, 2101034.
43. Zhou, J.; Holekevi Chandrappa, M. L.; Tan, S.; et al. Healable and conductive sulfur iodide for solid-state Li-S batteries. Nature. 2024, 627, 301-5.
44. Kim, J. T.; Su, H.; Zhong, Y.; et al. All-solid-state lithium-sulfur batteries through a reaction engineering lens. Nat. Chem. Eng. 2024, 1, 400-10.
45. Zheng, B.; Fan, J.; Chen, B.; et al. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 2022, 122, 5519-603.
46. Liu, J.; Kong, X.; Zheng, L.; Guo, X.; Liu, X.; Shui, J. Rare earth single-atom catalysts for nitrogen and carbon dioxide reduction. ACS. Nano. 2020, 14, 1093-101.
47. Shuai, Z.; Zhu, Y.; Gao, P.; Han, Y. Rare earth elements resources and beneficiation: a review. Miner. Eng. 2024, 218, 109011.
48. Jiang, Y.; Fu, H.; Liang, Z.; Zhang, Q.; Du, Y. Rare earth oxide based electrocatalysts: synthesis, properties and applications. Chem. Soc. Rev. 2024, 53, 714-63.
49. Su, P.; Song, F.; Cao, J.; Yan, C. H.; Tang, Y. Rare earth complex-based functional materials: from molecular design and performance regulation to unique applications. Acc. Chem. Res. 2025, 58, 218-30.
50. Zhou, B.; Shi, B.; Jin, D.; Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015, 10, 924-36.
51. Ghosh, B.; Vapnik, H.; Kim, H. E.; et al. Electrochemical separation and clean energy applications of rare earth elements. Chem. Rev. 2025, 125, 7965-8023.
52. Othman, A.; Gowda, A.; Andreescu, D.; et al. Two decades of ceria nanoparticle research: structure, properties and emerging applications. Mater. Horiz. 2024, 11, 3213-66.
53. Takada, R.; Yao, H. Praseodymium-doped cerium oxide (PrxCexO2-δ) nanoparticles with high water dispersibility: the nature of Pr-related optical transitions studied by MCD spectroscopy. J. Phys. Chem. C. 2025, 129, 5461-71.
54. Ochirkhuyag, T.; Hong, S. C.; Odkhuu, D. Intrinsic hard magnetism and thermal stability of a ThMn12-type permanent magnet. NPJ. Comput. Mater. 2022, 8, 193.
55. Carbonati, T.; Cionti, C.; Cosaert, E.; Nimmegeers, B.; Meroni, D.; Poelman, D. NIR emitting GdVO4:Nd nanoparticles for bioimaging: the role of the synthetic pathway. J. Alloys. Compd. 2021, 862, 158413.
56. Zhu, Q.; Sun, T.; Chung, M. N.; et al. Yb3+-sensitized upconversion and downshifting luminescence in Nd3+ ions through energy migration. Dalton. Trans. 2018, 47, 8581-4.
57. Zhang, X.; Chen, M.; Zhang, Q.; Liu, J.; Wu, Y. New insights into synergistic effects of La2O3 and nitrogen doped carbon for improved redox kinetics in lithium-sulfur batteries: a computational study. Appl. Surf. Sci. 2021, 563, 150172.
58. Han, X.; Zhang, Z.; Xu, X. Single atom catalysts supported on N-doped graphene toward fast kinetics in Li-S batteries: a theoretical study. J. Mater. Chem. A. 2021, 9, 12225-35.
59. Ren, Y.; Qi, Z.; Ma, X.; et al. First-principles study of the effect of mechanical strength on ion transport in La-doped LiF-SEI on the Li (001) surface. Mater. Today. Chem. 2021, 20, 100451.
60. Sun, R.; Hu, J.; Shi, X.; et al. Water-soluble cross-linking functional binder for lowcost and high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2021, 31, 2104858.
61. Zheng, W.; Hu, X. G.; Zhang, C. F. Electrochemical properties of rechargeable lithium batteries with sulfur-containing composite cathode materials. Electrochem. Solid-State. Lett. 2006, 9, A364.
62. Dai, S.; Sun, C.; Zhang, Y.; et al. Carbon microspheres built of La2O3 quantum dots-implanted nanorods: superb hosts with ultra-long Li2Sn-catalysis durability. J. Colloid. Interface. Sci. 2023, 640, 320-8.
63. Yeh, P. H.; Chung, S. H. Incorporation of electronically and ionically conductive additives in high-loading sulfur cathodes in lean-electrolyte lithium-sulfur cells. Electrochim. Acta. 2024, 502, 144794.
64. Huang, Z.; Zhu, Y.; Kong, Y.; et al. Efficient Synergism of chemisorption and wackenroder reaction via heterostructured La2O3-Ti3C2Tx -embedded carbon nanofiber for high-energy lithium-sulfur pouch cells. Adv. Funct. Mater. 2023, 33, 2303422.
65. Xing, Y.; Zhang, M.; Guo, J.; et al. Effect of CeO2-x-CNT/S cathode on the electrochemical performance of lithium-sulfur batteries. J. Solid. State. Chem. 2022, 316, 123642.
66. Wang, Y.; Yu, H.; Bi, M.; et al. Gadolinium oxide nanorods decorated Ketjen black@sulfur composites as functional catalyzing polysulfides conversion in lithium/sulfur batteries. Int. J. Energy. Res. 2022, 46, 16050-60.
67. Mai, H.; Wang, Q.; Sun, L.; et al. Nonstoichiometric scandium oxide hybridized in N-doped porous graphitic carbon promotes the rate capability of lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2023, 15, 41426-37.
68. Wang, Y.; Yu, H.; Majeed, A.; et al. Yttrium oxide nanorods as electrocatalytic polysulfides traps for curbing shuttle effect in lithium-sulfur batteries. J. Alloys. Compd. 2022, 891, 162074.
69. Li, X.; Zhang, L.; Ding, Z.; He, Y. Ultrafine Nd2O3 nanoparticles doped carbon aerogel to immobilize sulfur for high performance lithium-sulfur batteries. J. Electroanal. Chem. 2017, 799, 617-24.
70. Li, X.; Ding, Z.; Zhang, L.; Tang, R.; He, Y. Enhanced performance of lithium sulfur batteries with sulfur embedded in Sm2O3 -doped carbon aerogel as cathode material. Electrochim. Acta. 2017, 241, 197-207.
71. Shu, M.; Dong, Y.; Ni, M.; et al. Strategically engineered metal cluster-rare earth oxide heterojunction catalyst for high-performance lean electrolyte lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2025, 17, 4961-71.
72. He, B.; Wang, Z.; Li, G.; Liu, S.; Gao, X. Perovskite transition metal oxide of nanofibers as catalytic hosts for lithium-sulfur battery. J. Alloys. Compd. 2022, 918, 165660.
73. Zou, K.; Zhou, T.; Chen, Y.; et al. Defect Engineering in a multiple confined geometry for robust lithium-sulfur batteries. Adv. Energy. Mater. 2022, 12, 2103981.
74. Feng, W.; Yang, H.; Pu, Z.; Zhang, L. Study of CNTs-MoS2/CeO2 composites for lithium-sulfur battery performance. Ionics. 2022, 28, 2781-91.
75. Arshad, H. M. U.; Liu, S.; Li, G. R.; Gao, X. P. La2MoO6 as an effective catalyst for the cathode reactions of lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2022, 14, 5247-5256.
76. Yang, T.; Xiao, J.; Sun, X.; Song, Y.; He, C. Facilitating the polysulfides conversion kinetics by porous LaOCl nanofibers towards long-cycling lithium-sulfur batteries. Chin. Chem. Lett. 2025, 36, 109691.
77. Lin, Y.; Tang, W.; Wu, S.; et al. Alleviating the self-discharge and enhancing the polysulphides conversion kinetics with LaCO3OH nanocrystals decorated hierarchical porous carbon. Chem. Eng. J. 2023, 452, 139091.
78. Xiao, D.; Lu, C.; Chen, C.; Yuan, S. CeO2-webbed carbon nanotubes as a highly efficient sulfur host for lithium-sulfur batteries. Energy. Storage. Mater. 2018, 10, 216-22.
79. Wang, H.; Zhang, B.; Zeng, X.; et al. 3D porous carbon nanofibers with CeO2-decorated as cathode matrix for high performance lithium-sulfur batteries. J. Power. Sources. 2020, 473, 228588.
80. Wei, Z.; Li, J.; Wang, R. Surface engineered polar CeO2-based cathode host materials for immobilizing lithium polysulfides in high-performance Li-S batteries. Appl. Surf. Sci. 2022, 580, 152237.
81. Azam, S.; Wei, Z.; Wang, R. Adsorption-catalysis design with cerium oxide nanorods supported nickel-cobalt-oxide with multifunctional reaction interfaces for anchoring polysulfides and accelerating redox reactions in lithium sulfur battery. J. Colloid. Interface. Sci. 2023, 635, 466-80.
82. Hou, Q.; Wang, K.; Zheng, W.; et al. Eliminating bandgap between Cu-CeO2-x heterointerface enabling fast electron transfer and redox reaction in Li-S batteries. Energy. Storage. Mater. 2023, 63, 102983.
83. Qi, W.; Jiang, W.; Ling, R.; Yang, C.; Wang, Y.; Cao, B. Highly active CeO2-x/Fe interfaces enable fast redox conversion of polysulfides for high-performance lithium-sulfur batteries. Electrochim. Acta. 2022, 419, 140402.
84. Fan, X.; Feng, Z.; Zeng, M.; et al. Tuning the surface structure of CeO2 nanoparticles by chlorine-doped strategy to improve the polysulfide reaction kinetic for lithium sulfur battery. Colloids. Surf. A:. Physicochem. Eng. Asp. 2023, 659, 130571.
85. Cui, J.; Li, Z.; Wang, G.; Guo, J.; Shao, M. Layered double hydroxides and their derivatives for lithium-sulfur batteries. J. Mater. Chem. A. 2020, 8, 23738-55.
86. Zhang, J.; Hu, H.; Li, Z.; Lou, X. W. D. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2016, 55, 3982-6.
87. Dai, C.; Hu, L.; Wang, M. Q.; et al. Uniform α-Ni(OH)2 hollow spheres constructed from ultrathin nanosheets as efficient polysulfide mediator for long-term lithium-sulfur batteries. Energy. Storage. Mater. 2017, 8, 202-8.
88. Dong, W.; Wu, Z.; Guo, Y.; et al. Construction transition metal hydroxides on pyridinic/pyrrolic nitrogen co-doped carbon as high performance cathodes for lithium-sulfur batteries. J. Power. Sources. 2025, 645, 237186.
89. Chen, L.; Cao, G.; Li, Y.; et al. A review on engineering transition metal compound catalysts to accelerate the redox kinetics of sulfur cathodes for lithium-sulfur batteries. Nanomicro. Lett. 2024, 16, 97.
90. Wang, X.; Han, J.; Luo, C.; et al. Coordinated adsorption and catalytic conversion of polysulfides enabled by perovskite bimetallic hydroxide nanocages for lithium-sulfur batteries. Small. 2021, 17, 2101538.
91. Tian, Y.; Zhao, Y.; Zhang, Y.; Ricardez-Sandoval, L.; Wang, X.; Li, J. Construction of oxygen-deficient La(OH)3 nanorods wrapped by reduced graphene oxide for polysulfide trapping toward high-performance lithium/sulfur batteries. ACS. Appl. Mater. Interfaces. 2019, 11, 23271-23279.
92. Hao, Y.; Wang, L.; Liang, Y.; et al. Bifunctional semi-closed YF3-doped 1D carbon nanofibers with 3D porous network structure including fluorinating interphases and polysulfide confinement for lithium-sulfur batteries. Nanoscale. 2019, 11, 21324-39.
93. Wang, X.; Hao, Y.; Wang, G.; et al. YF3/CoF3 co-doped 1D carbon nanofibers with dual functions of lithium polysulfudes adsorption and efficient catalytic activity as a cathode for high-performance Li-S batteries. J. Colloid. Interface. Sci. 2022, 607, 922-32.
94. Liu, L.; Guo, Y.; Qi, Z.; et al. Confinement and electrocatalysis of cerium fluoride nanocages to boost the lithium-sulfur batteries performance. Small. Structures. 2022, 3, 2200050.
95. Yang, D.; Han, Y.; Li, M.; et al. Highly conductive quasi‐1D hexagonal chalcogenide perovskite Sr8Ti7S21 with efficient polysulfide regulation in lithium-sulfur batteries. Adv. Funct. Mater. 2024, 34, 2401577.
96. Hou, W.; Feng, P.; Guo, X.; et al. Catalytic mechanism of oxygen vacancies in perovskite oxides for lithium-sulfur batteries. Adv. Mater. 2022, 34, 2202222.
97. Chen, M.; Huang, C.; Li, Y.; et al. Perovskite-type La0.56Li0.33TiO3 as an effective polysulfide promoter for stable lithium-sulfur batteries in lean electrolyte conditions. J. Mater. Chem. A. 2019, 7, 10293-302.
98. Kong, L.; Chen, X.; Li, B.; et al. A bifunctional perovskite promoter for polysulfide regulation toward stable lithium-sulfur batteries. Adv. Mater. 2017, 30, 1705219.
99. Hao, Z.; Zeng, R.; Yuan, L.; et al. Perovskite La0.6Sr0.4CoO3-δ as a new polysulfide immobilizer for high-energy lithium-sulfur batteries. Nano. Energy. 2017, 40, 360-8.
100. Jin, Q.; Niu, Y.; Lin, S.; et al. La0.3Sr0.7Fe0.2Mn0.8O3/Ti3C2Tx sulfur host with high tap density enabling the high volumetric energy density of lithium-sulfur batteries. J. Alloys. Compd. 2023, 943, 169200.
101. Gonçalves, J. M. Santos, É. A.; Martins, P. R.; Silva, C. G.; Zanin, H. Emerging medium- and high-entropy materials as catalysts for lithium-sulfur batteries. Energy. Storage. Mater. 2023, 63, 102999.
102. Han, F.; Wang, Z.; Jin, Q.; et al. High-entropy alloy electrocatalysts bidirectionally promote lithium polysulfide conversions for long-cycle-life lithium-sulfur batteries. ACS. Nano. 2024, 18, 15167-76.
103. Du, M.; Geng, P.; Pei, C.; et al. High-entropy Prussian blue analogues and their oxide family as sulfur hosts for lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209350.
104. Wang, Z.; Ge, H.; Liu, S.; Li, G.; Gao, X. High-entropy alloys to activate the sulfur cathode for lithium-sulfur batteries. Energy. Environ. Mater. 2022, 6, e12358.
105. Tian, L.; Zhang, Z.; Liu, S.; Li, G.; Gao, X. High-entropy perovskite oxide nanofibers as efficient bidirectional electrocatalyst of liquid-solid conversion processes in lithium-sulfur batteries. Nano. Energy. 2023, 106, 108037.
106. Zhou, L.; Liu, H.; Liu, J. X.; et al. Unconventional catalytic kinetics of dual field regulated pyrochlore-type high-entropy ceramics towards the Li2S4 intermediate. Energy. Environ. Sci. 2025, 18, 6809-22.
107. Mu, P.; Sun, C.; Gao, C.; et al. Dual network electrode binder toward practical lithium-sulfur battery applications. ACS. Energy. Lett. 2023, 8, 3733-41.
108. Bhattacharya, P.; Nandasiri, M. I.; Lv, D.; et al. Polyamidoamine dendrimer-based binders for high-loading lithium-sulfur battery cathodes. Nano. Energy. 2016, 19, 176-86.
109. Zhang, L.; Yan, H.; Zhou, J.; et al. High-performance organohydrogel artificial muscle with compartmentalized anisotropic actuation under microdomain confinement. Adv. Mater. 2023, 35, 2202193.
110. Wang, W.; Hua, L.; Zhang, Y.; Wang, G.; Li, C. A conductive binder based on mesoscopic interpenetration with polysulfides capturing skeleton and redox intermediates network for lithium sulfur batteries. Angew. Chem. Int. Ed. Engl. 2024, 63, e202405920.
111. Guo, R.; Yang, Y.; Huang, X. L.; et al. Recent advances in multifunctional binders for high sulfur loading lithium-sulfur batteries. Adv. Funct. Mater. 2023, 34, 2307108.
112. Han, P.; Chung, S. H.; Chang, C. H.; Manthiram, A. Bifunctional binder with nucleophilic lithium polysulfide immobilization ability for high-loading, high-thickness cathodes in lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2019, 11, 17393-9.
113. Zhao, B.; Zhang, Z.; Wang, Y.; Jin, Y.; Gao, H. A novel type of multifunctional binder for improved cycle stability of lithium-sulfur battery. J. Solid. State. Electrochem. 2019, 23, 1269-78.
114. Han, P.; Chung, S. H.; Manthiram, A. Designing a high-loading sulfur cathode with a mixed ionic-electronic conducting polymer for electrochemically stable lithium-sulfur batteries. Energy. Storage. Mater. 2019, 17, 317-24.
115. Wang, H.; Zheng, P.; Yi, H.; et al. Low-cost and environmentally friendly biopolymer binders for Li-S batteries. Macromolecules. 2020, 53, 8539-47.
116. Huang, Y.; Shaibani, M.; Gamot, T. D.; et al. A saccharide-based binder for efficient polysulfide regulations in Li-S batteries. Nat. Commun. 2021, 12, 5375.
117. Kim, S.; Cho, M.; Lee, Y. Multifunctional Chitosan-rGO network binder for enhancing the cycle stability of Li-S batteries. Adv. Funct. Mater. 2020, 30, 1907680.
118. Zhou, F.; Mei, Y.; Wu, Q.; Li, H.; Xu, J.; Chen, H. Sulfur electrode tolerance and polysulfide conversion promoted by the supramolecular binder with rare-earth catalysis in lithium-sulfur batteries. Energy. Storage. Mater. 2024, 67, 103315.
119. Zhou, F.; Mei, Y.; Wu, Q.; et al. Lewis acid-optimized hydrolysable catalytic binder with dynamic hydrogen-bonded framework for durable lithium-sulfur batteries. Chem. Eng. J. 2025, 507, 160009.
120. Su, Y. S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium-sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. (Camb). 2012, 48, 8817.
121. Zheng, L.; Zhu, Z.; Kuai, Y.; et al. Modification of separators with neodymium oxide/graphene composite to enhance lithium-sulfur battery performance. J. Rare. Earths. 2024, 42, 1730-9.
122. Wen, G.; Zhang, X.; Shi, Z.; et al. Sphere-in-fiber hybrid of N-doped carbon/cerium dioxide as an interlayer material with superior electrocatalytic performance for lithium sulfide precipitation and conversion. J. Colloid. Interface. Sci. 2022, 619, 106-15.
123. Jin, L.; Chen, J.; Qian, X.; Cheng, J.; Hao, Q.; Zhang, K. CeO2-C nanorods obtained by high-temperature carbonization of Ce-MOF as separator coating for Li-S battery. Colloids. Surf. A:. Physicochem. Eng. Asp. 2023, 657, 130443.
124. Yu, Z.; Peng, L.; Kang, X.; Li, Y.; Li, A.; Chang, Y. First-principles investigation of the catalytic mechanism of CeO2/KB composite separator in Li-S batteries. J. Electroanal. Chem. 2024, 953, 118004.
125. Zhang, K.; Jin, L.; Chen, J.; et al. Ketjen Black@Ce-MOF derived KB@CeO2-C as separator coating for lithium sulfur batteries. J. Energy. Storage. 2024, 78, 110006.
126. Lai, X.; Chen, Q.; Wang, H.; et al. Metal-organic framework-derived heterostructural Bi-phased Sm2O3 as a novel sulfur immobilizer for high-performance lithium-sulfur batteries. J. Energy. Storage. 2023, 74, 109520.
127. Qian, X.; Hao, Q.; Zhao, S.; Jin, L.; Li, B.; Xu, H. Application of Y-MOF-CNT-derived Y2O3-C@CNT composites in lithium-sulfur battery separators. Langmuir. 2024, 40, 23529-37.
128. Zhang, Z.; Wang, F.; Li, Z.; et al. Construction of CeO2/Co/CNT electrocatalyst with adsorption-catalytic synergistic effect for the suppression of the shuttle effect in lithium sulfur batteries. J. Energy. Storage. 2024, 94, 112259.
129. Jin, L.; Zhang, K.; Chen, J.; et al. ZIF67@Ce-MOF derived Co-N-C@CeO2-C for separator modification of lithium sulfur batteries. Mater. Res. Bull. 2024, 175, 112785.
130. Wang, W.; Zhang, Q.; Wan, J.; Liu, J.; Wang, L.; He, M. Constructing honeycomb-structured LaCoO3 as an efficient polysulfide converter on the separator to achieve practical lithium-sulfur batteries. Appl. Surf. Sci. 2024, 661, 160031.
131. Wang, D.; Zhao, X.; Yin, D.; Du, M.; Qu, C.; Feng, M. Metal-organic frameworks derived Pr2O2S-doped porous carbon nanosheets as efficient adsorption-catalytic separator coating materials for lithium-sulfur batteries. J. Power. Sources. 2025, 635, 236540.
132. Sun, L.; Zhang, W.; Fu, J.; et al. Highly active rare earth sulfur oxides used for membrane modification of lithium sulfur batteries. Chem. Eng. J. 2023, 457, 141240.
133. Deng, N.; Peng, Z.; Tian, X.; et al. Yttrium trifluoride doped polyacrylonitrile based carbon nanofibers as separator coating layer for high performance lithium-metal batteries. J. Colloid. Interface. Sci. 2023, 634, 949-962.
134. Zhao, T.; Xiao, P.; Nie, S.; Luo, M.; Zou, M.; Chen, Y. Recent progress of metal-organic frameworks based high performance batteries separators: a review. Coord. Chem. Rev. 2024, 502, 215592.
135. Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational design of MOF-based materials for next-generation rechargeable batteries. Nanomicro. Lett. 2021, 13, 203.
136. Xie, Z.; Cao, B.; Yue, X.; et al. Metal organic frameworks-based cathode materials for advanced Li-S batteries: a comprehensive review. Nano. Res. 2024, 17, 2592-618.
137. Zhao, R.; Ren, H.; Si, Y.; Li, G.; Fu, Y. MOF801(Ce)-modified polypropylene separator as efficient barrier for lithium-organosulfide batteries. Electrochim. Acta. 2023, 447, 142116.
138. Song, C. L.; Luo, J. R.; Ma, L. Y.; et al. Dendrite-free lithium metal batteries achieved with Ce-MOF membrane coating with one-dimensional continuous oxygen-containing channels for rapid migration of Li ions. J. Mater. Chem. A. 2022, 10, 18248-55.
139. Su, Y.; Wang, W.; Wang, W.; Wang, A.; Huang, Y.; Guan, Y. Cerium-based MOF as a separator coating for high-performance lithium-sulfur batteries. J. Electrochem. Soc. 2022, 169, 030528.
140. Jin, H. G.; Wang, M.; Wen, J. X.; et al. Oxygen vacancy-rich mixed-valence cerium MOF: an efficient separator coating to high-performance lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2021, 13, 3899-910.
141. Hao, Z.; Chen, J.; Lu, X.; et al. Precisely visit the performance modulation of functionalized separator in Li-S batteries via consecutive multiscale analysis. Energy. Storage. Mater. 2022, 49, 85-92.
142. Zhou, R.; Ren, Y.; Li, W.; et al. Rare earth single-atom catalysis for high-performance Li-S full battery with ultrahigh capacity. Angew. Chem. Int. Ed. Engl. 2024, 63, e202405417.
143. Xiao, P.; Yun, X.; Chen, Y.; et al. Insights into the solvation chemistry in liquid electrolytes for lithium-based rechargeable batteries. Chem. Soc. Rev. 2023, 52, 5255-316.
144. Li, X.; Zhao, R.; Fu, Y.; Manthiram, A. Nitrate additives for lithium batteries: mechanisms, applications, and prospects. eScience. 2021, 1, 108-23.
145. Chen, W.; Li, B.; Zhao, C.; et al. Electrolyte regulation towards stable lithium-metal anodes in lithium-sulfur batteries with sulfurized polyacrylonitrile cathodes. Angew. Chem. Int. Ed. Engl. 2020, 59, 10732-45.
146. Deng, T.; Wang, J.; Zhao, H.; et al. Dynamically regulating polysulfide degradation via organic sulfur electrolyte additives in lithium-sulfur batteries. Adv. Energy. Mater. 2024, 14, 2402319.
147. Ye, Y.; Song, M. K.; Xu, Y.; et al. Lithium nitrate: a double-edged sword in the rechargeable lithium-sulfur cell. Energy. Storage. Mater. 2019, 16, 498-504.
148. Liu, S.; Li, G. R.; Gao, X. P. Lanthanum nitrate as electrolyte additive to stabilize the surface morphology of lithium anode for lithium-sulfur battery. ACS. Appl. Mater. Interfaces. 2016, 8, 7783-9.
149. Hao, X.; Mao, Y.; Zhu, T.; et al. Yttrium-containing solid electrolyte interphase safeguards lithium anodes in lithium-sulfur batteries. ACS. Sustainable. Chem. Eng. 2024, 12, 3691-701.
150. Yen, Y. J.; Manthiram, A. Anode-free lithium-sulfur batteries with a rare-earth triflate as a dual-function electrolyte additive. ACS. Appl. Mater. Interfaces. 2024, 16, 34997-5005.
151. Xing, C.; Chen, H.; Qian, S.; et al. Regulating liquid and solid-state electrolytes for solid-phase conversion in Li-S batteries. Chem. 2022, 8, 1201-30.
152. Kim, J. T.; Rao, A.; Nie, H. Y.; et al. Manipulating Li2S2/Li2S mixed discharge products of all-solid-state lithium sulfur batteries for improved cycle life. Nat. Commun. 2023, 14, 6404.
153. Song, S.; He, F.; Xia, Q.; et al. Research advances in rare-earth-based solid electrolytes for all-solid-state batteries. Small. 2025, 21, 2502008.
154. Gicha, B. B.; Tufa, L. T.; Nwaji, N.; Hu, X.; Lee, J. Advances in all-solid-state lithium-sulfur batteries for commercialization. Nanomicro. Lett. 2024, 16, 172.
155. Su, Y.; Ren, S.; Lin, Q.; et al. In situ solid electrolyte ionic pathway formation in high sulfur loading cathodes for high-performance all-solid-state lithium-sulfur batteries. Adv. Energy. Mater. 2025, 15, 2500363.
156. Liang, F.; Wang, S.; Liang, Q.; et al. Insight into all-solid-state Li-S batteries: challenges, advances, and engineering design. Adv. Energy. Mater. 2024, 14, 2401959.
157. Shi, C.; Takeuchi, S.; Alexander, G. V.; et al. High sulfur loading and capacity retention in bilayer garnet sulfurized-polyacrylonitrile/lithium-metal batteries with gel polymer electrolytes. Adv. Energy. Mater. 2023, 13, 2301656.
158. Shi, C.; Hamann, T.; Takeuchi, S.; et al. 3D asymmetric bilayer garnet-hybridized high-energy-density lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2022, 15, 751-60.
159. Shi, C.; Alexander, G. V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E. D. All-solid-state garnet type sulfurized polyacrylonitrile/lithium-metal battery enabled by an inorganic lithium conductive salt and a bilayer electrolyte architecture. ACS. Energy. Lett. 2023, 8, 1803-10.
160. Liu, Y.; Han, J.; Baek, D. H.; Woo Kim, H.; Ahn, J. H.; Kim, J. K. Unlocking high-energy solid-state lithium-sulfur batteries with an innovative double-layer hybrid solid electrolyte. Chem. Eng. J. 2024, 496, 153647.
161. Zhu, P.; Yan, C.; Zhu, J.; et al. Flexible electrolyte-cathode bilayer framework with stabilized interface for room-temperature all-solid-state lithium-sulfur batteries. Energy. Storage. Mater. 2019, 17, 220-5.
162. Wang, L.; Yin, X.; Li, B.; Zheng, G. W. Mixed ionically/electronically conductive double-phase interface enhanced solid-state charge transfer for a high-performance all-solid-state Li-S battery. Nano. Lett. 2021, 22, 433-40.
163. Luo, Y.; Dong, D.; Zhou, J.; Wang, Y.; Xue, Z.; Jiang, X. Solid-state lithium-sulfur battery chemistries achieving excellent room-temperature cycle performance by high-quality Li7La3Zr2O12-based electrolyte. J. Alloys. Compd. 2023, 935, 168112.
164. Ma, K.; Chen, B.; Li, C. X.; Thangadurai, V. Improvement of the Li-ion conductivity and air stability of the Ta-doped Li7La3Zr2O12 electrolyte via Ga co-doping and its application in Li-S batteries. J. Mater. Chem. A. 2024, 12, 3601-15.
165. Wang, S.; Li, M.; Yan, G.; et al. Squaraine-linked zwitterionic COF modified LLZTO nanoparticles for high performance polymer composite electrolytes in Li-S batteries. Nanoscale. 2023, 15, 12961-71.
166. Lv, Y.; Zhang, T.; Hu, Z.; et al. High critical current density in Li6.4La3Zr1.4Ta0.6O12 electrolyte via interfacial engineering with complex hydride. Rare. Metals. 2023, 43, 692-701.
167. Ruan, Y.; Lu, Y.; Huang, X.; et al. Acid induced conversion towards a robust and lithiophilic interface for Li-Li7La3Zr2O12 solid-state batteries. J. Mater. Chem. A. 2019, 7, 14565-74.
168. Nomura, Y.; Yamamoto, K. Advanced characterization techniques for sulfide-based solid-state lithium batteries. Adv. Energy. Mater. 2023, 13, 2203883.
169. Ren, D.; Lu, L.; Hua, R.; et al. Challenges and opportunities of practical sulfide-based all-solid-state batteries. eTransportation. 2023, 18, 100272.
170. Liu, S.; Zhou, L.; Zhong, T.; Wu, X.; Neyts, K. Sulfide/polymer composite solid-state electrolytes for all-solid-state lithium batteries. Adv. Energy. Mater. 2024, 14, 2403602.
171. Zhao, B.; Zhou, C.; Chen, P.; Gao, X. Synergistic interfacial optimization for high-sulfur-content all-solid-state lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2024, 16, 4679-88.
172. Mirtaleb, A.; Wang, R. A highly stable and conductive cerium‐doped Li7P3S11 glass‐ceramic electrolyte for solid‐state lithium-sulfur batteries. J. Am. Ceram. Soc. 2024, 107, 3800-12.
173. Shi, X.; Zeng, Z.; Sun, M.; et al. Fast Li-ion conductor of Li3HoBr6 for stable all-solid-state lithium-sulfur battery. Nano. Lett. 2021, 21, 9325-31.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data




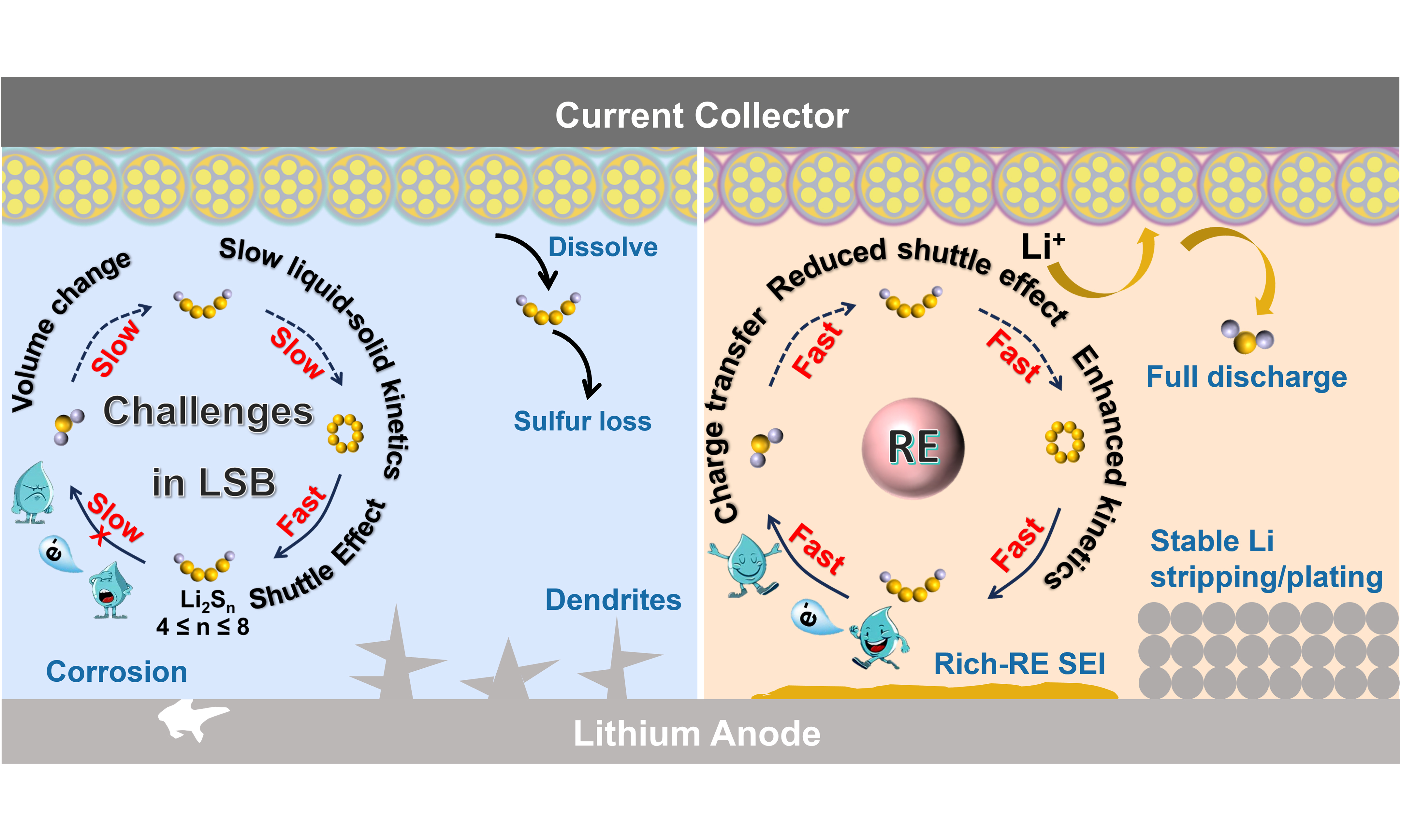
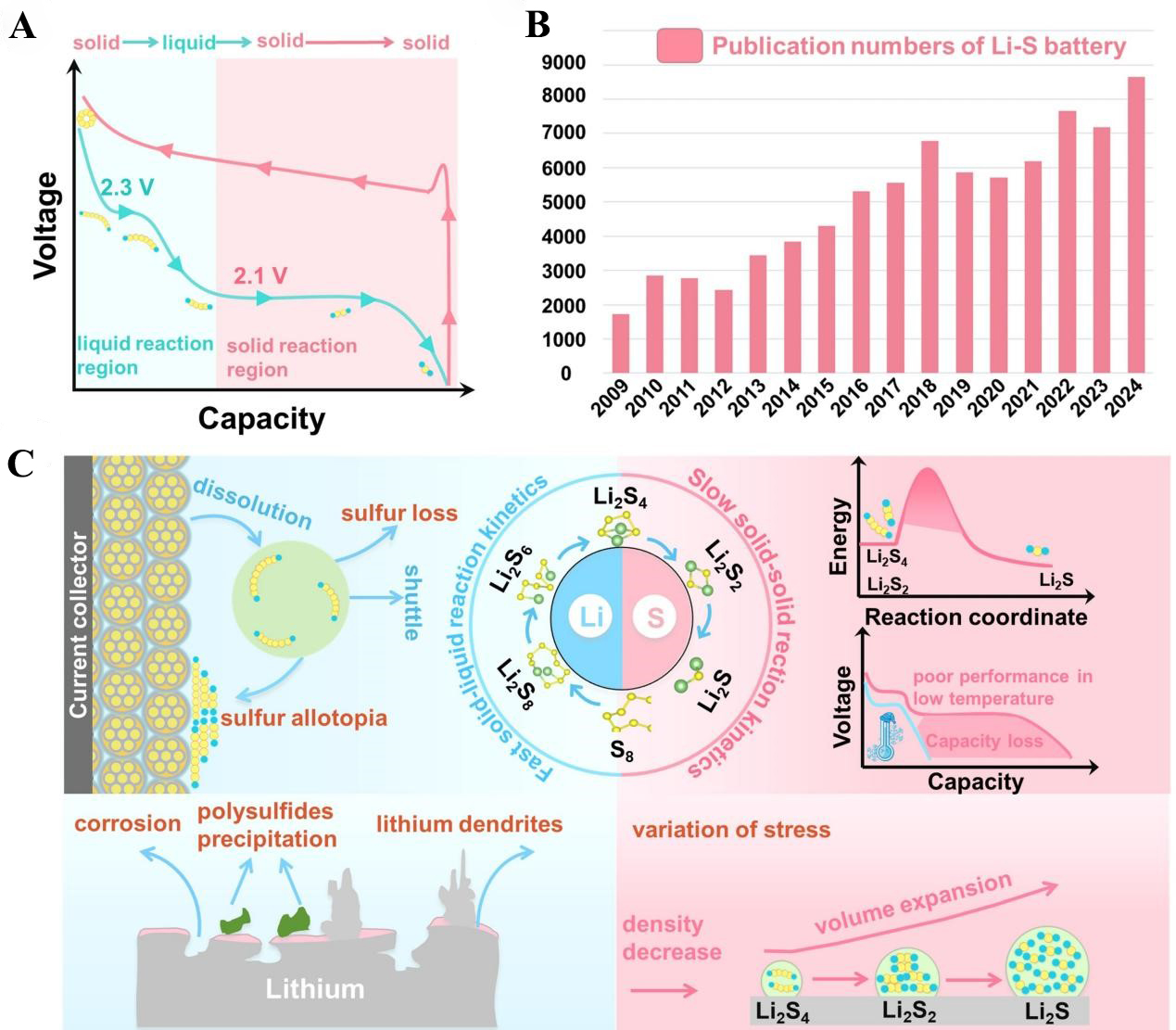

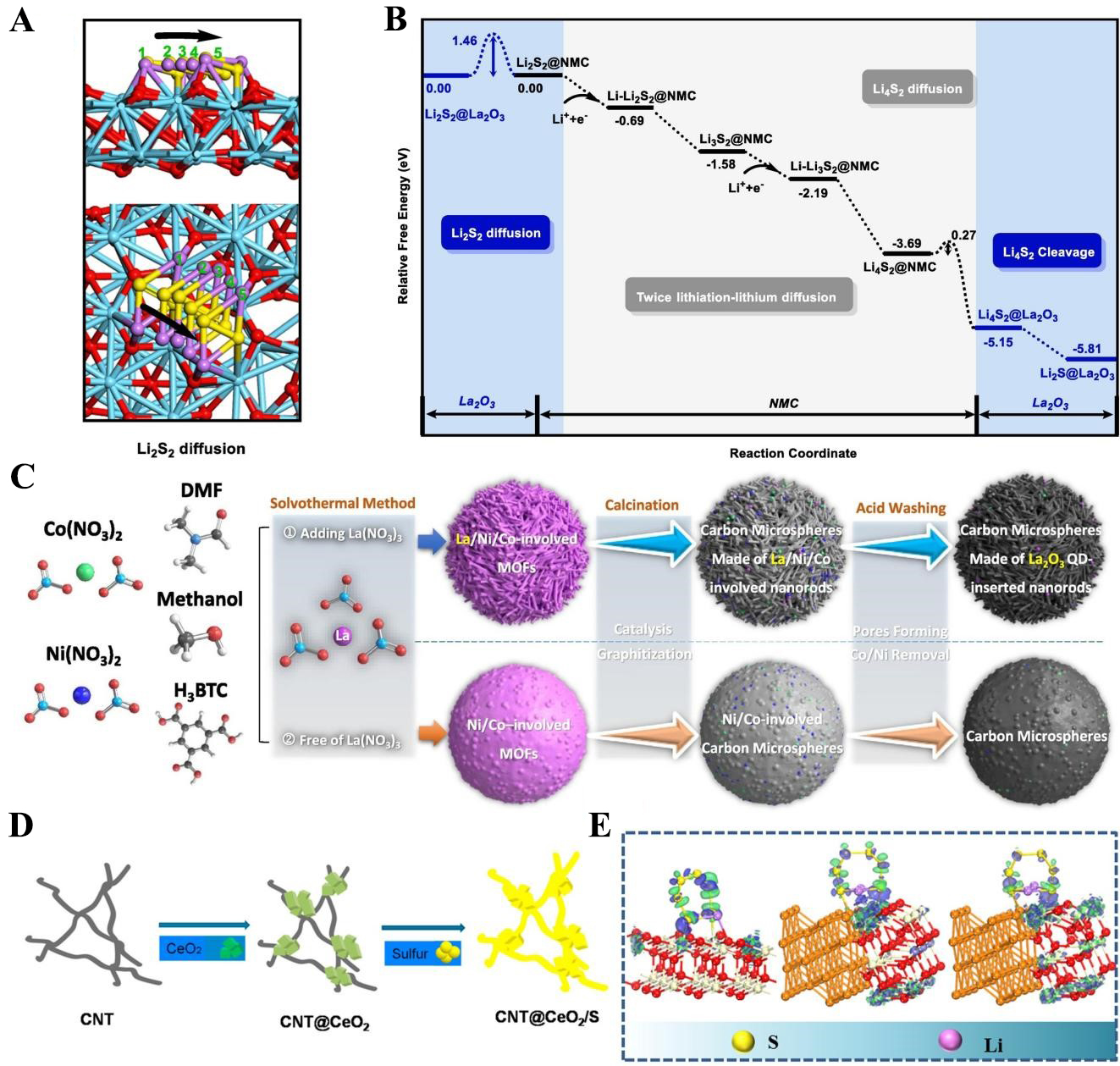
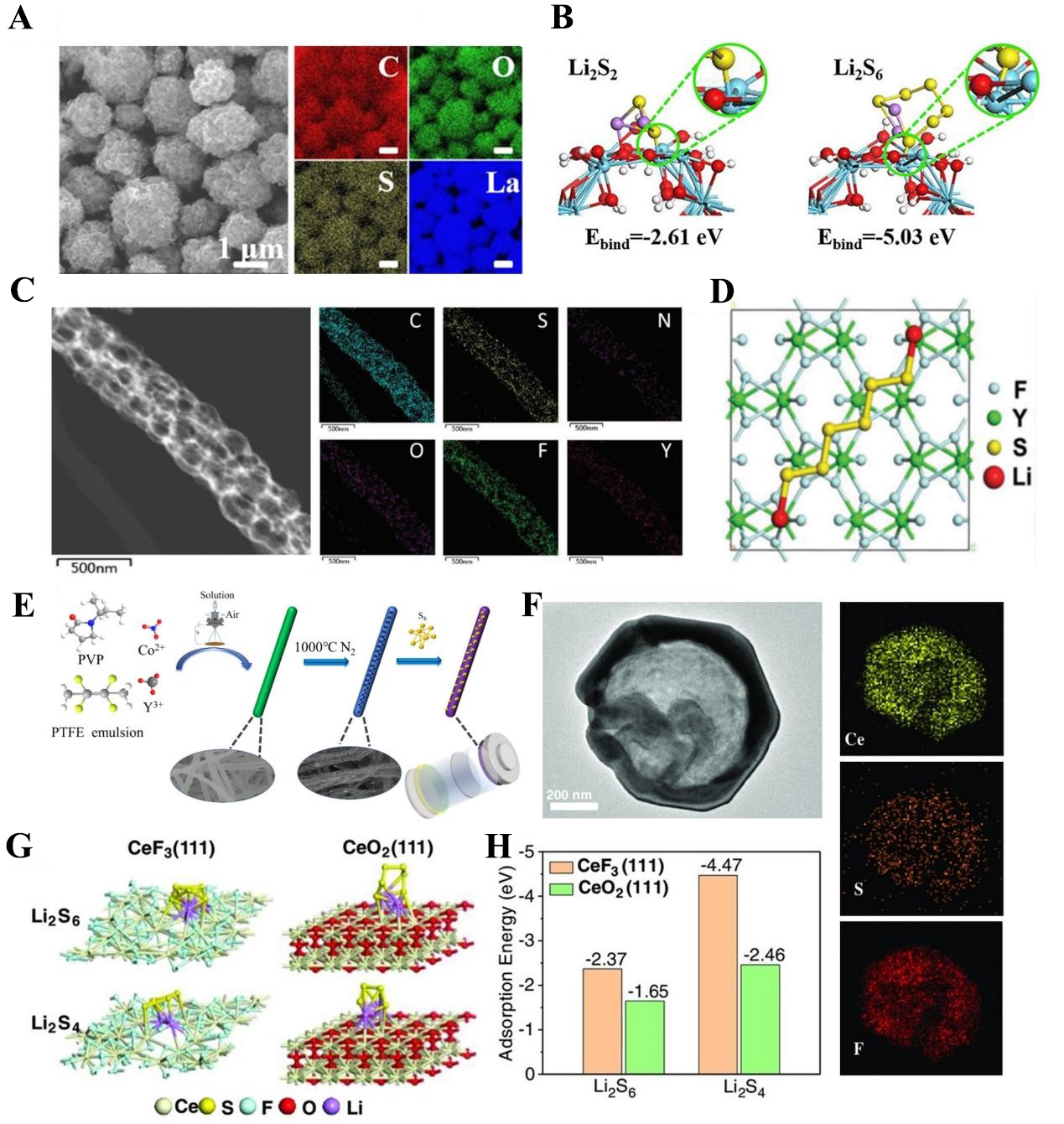
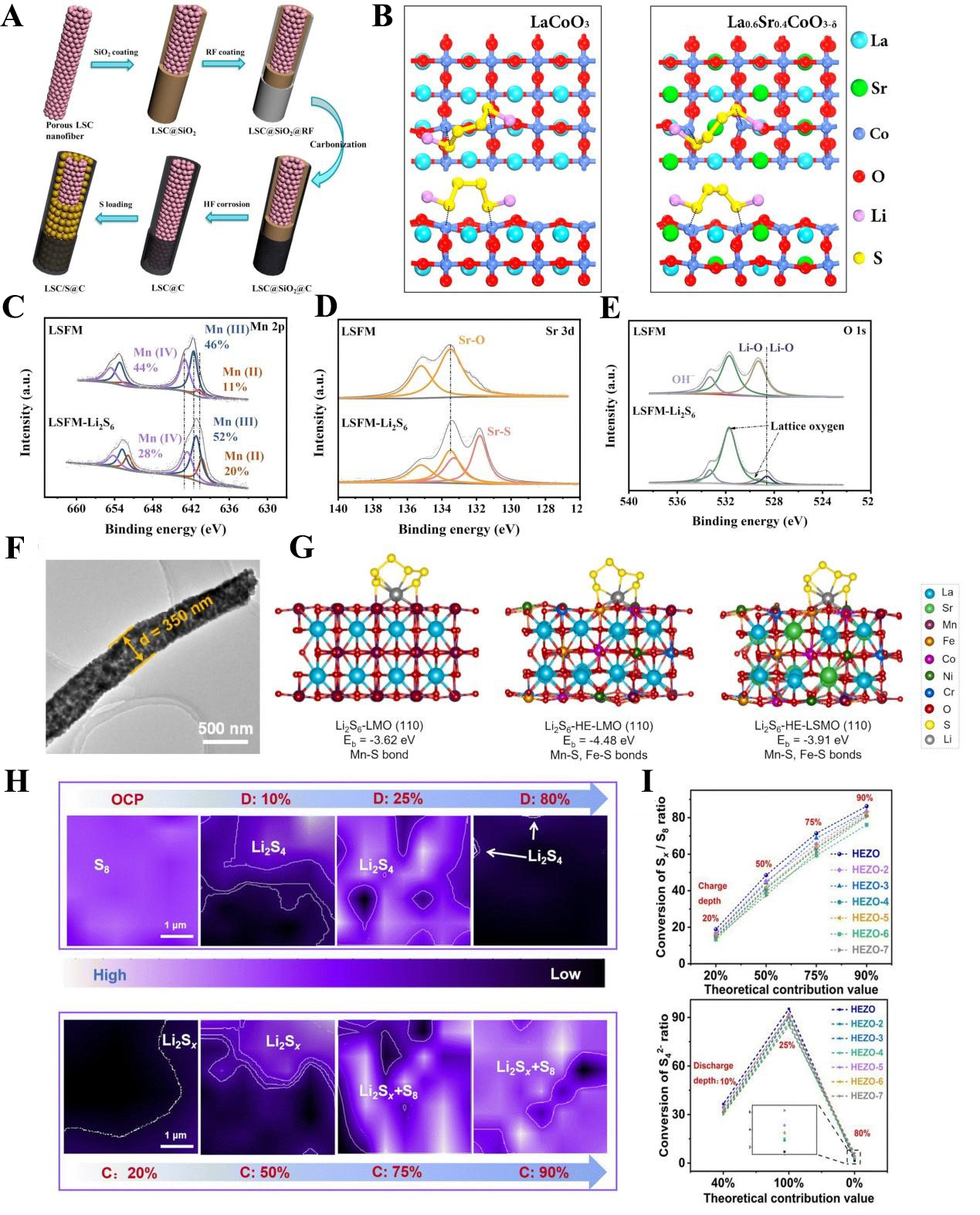
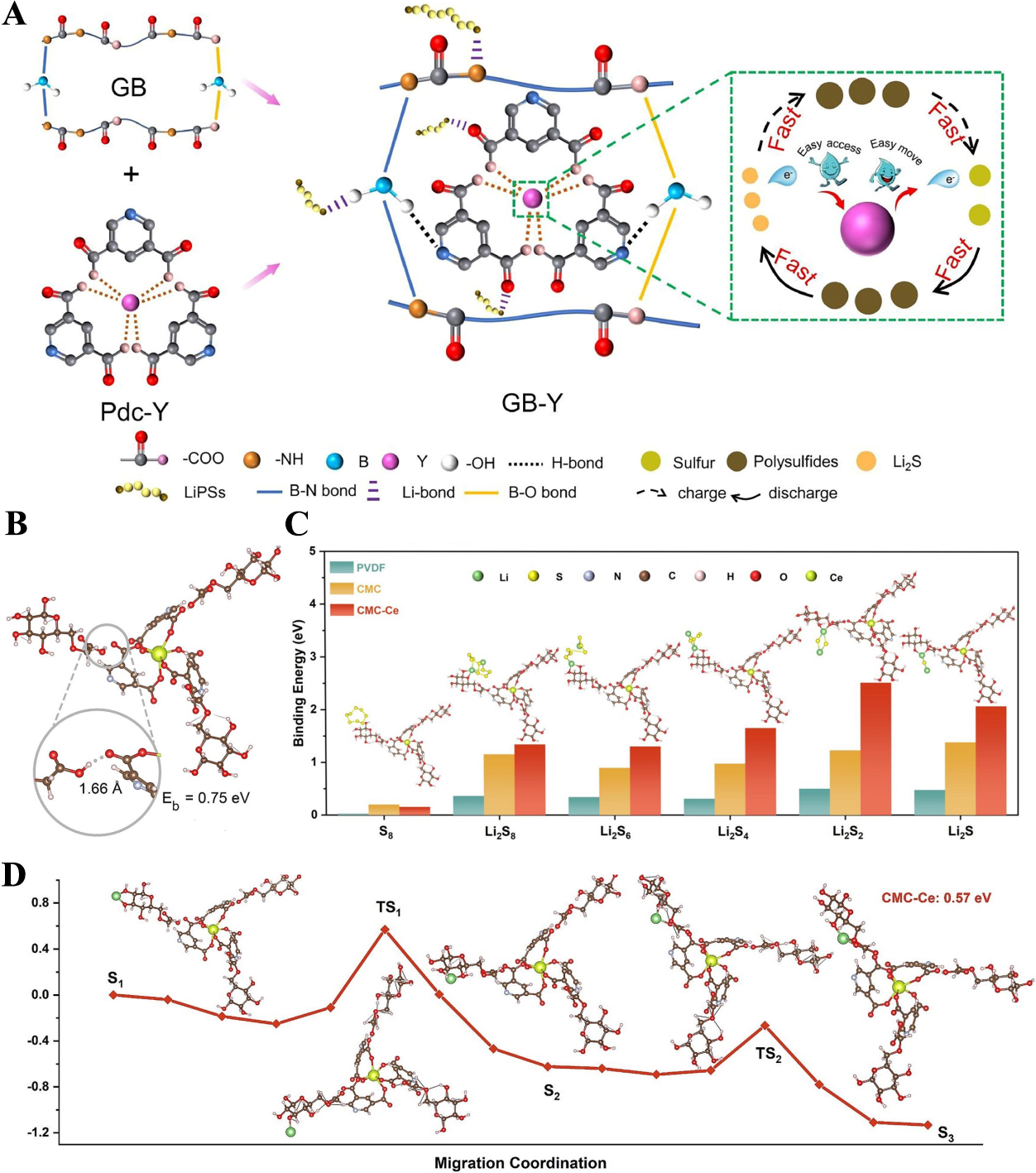
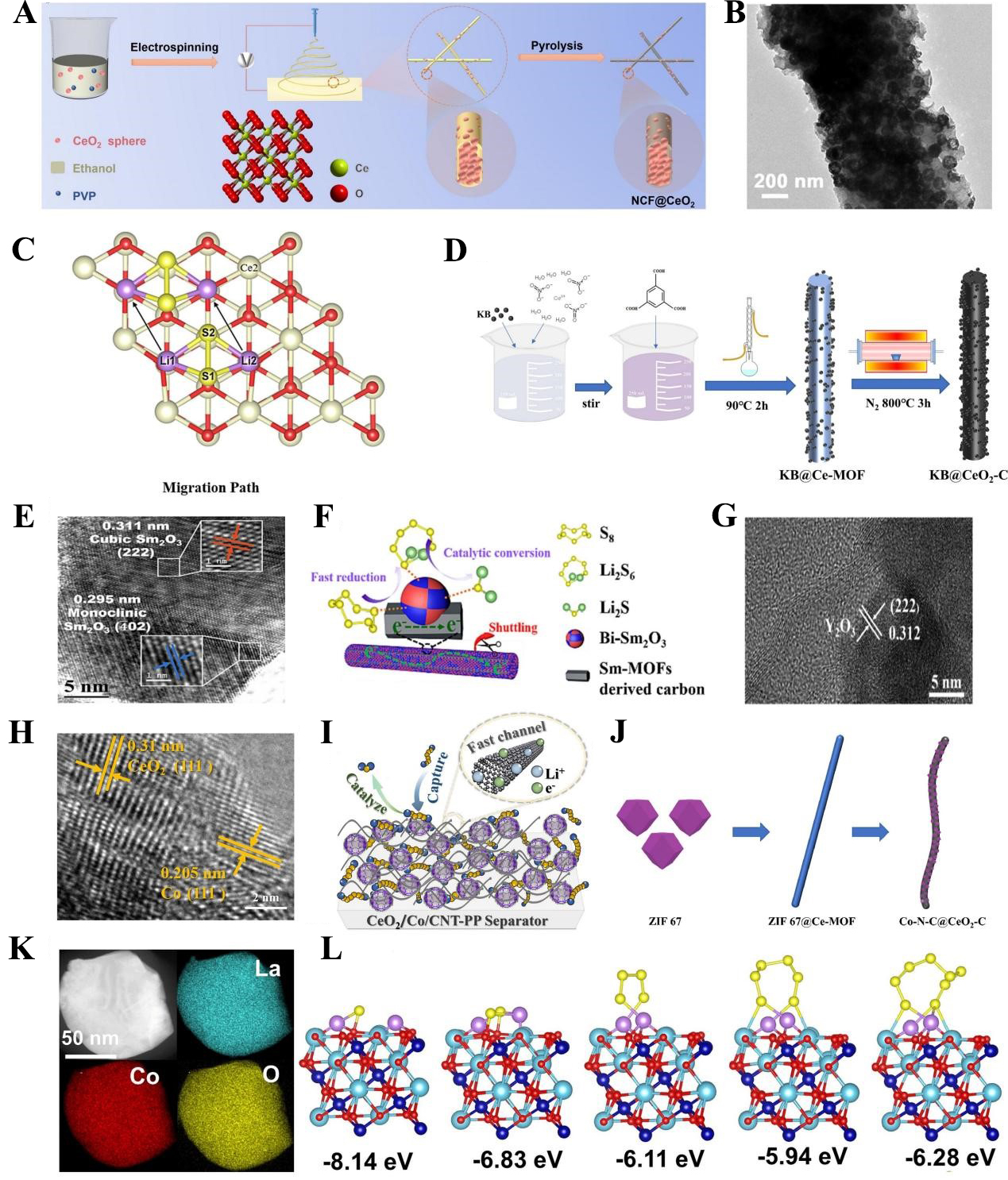
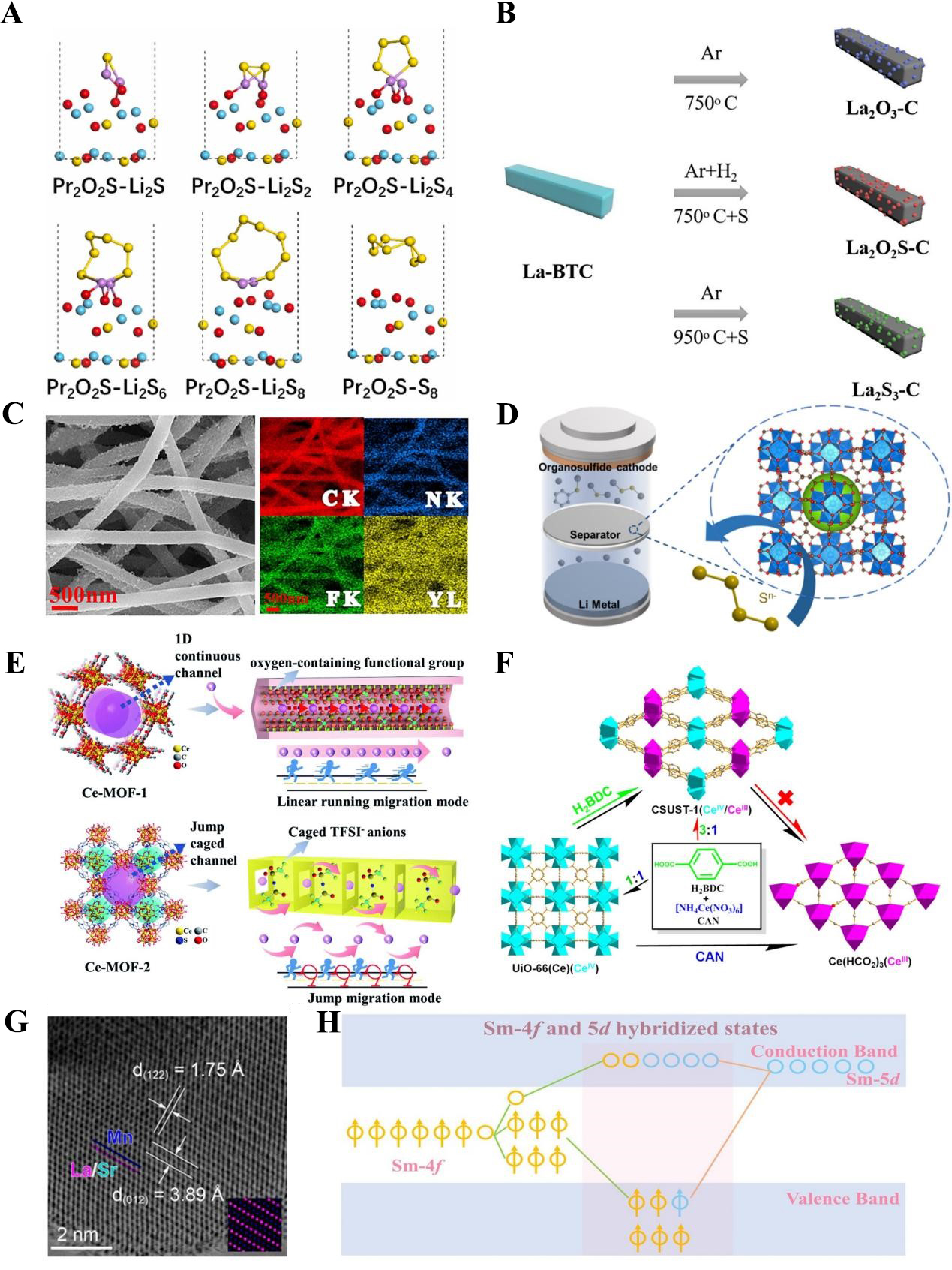










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].