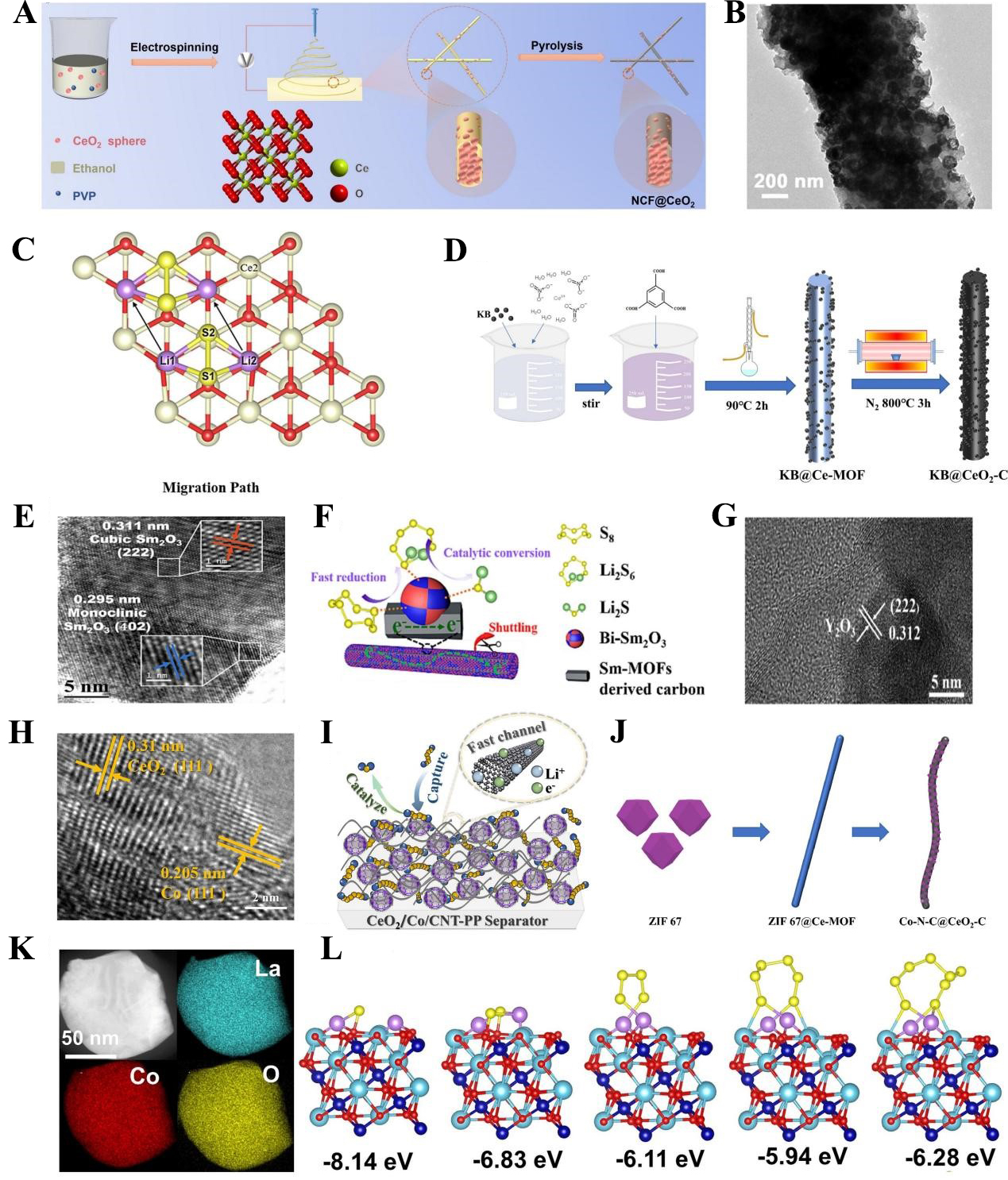
fig7
Figure 7. (A) Schematic diagram of NCF@CeO2 preparation by electrospinning; (B) HRTEM NCF@CeO2 morphology. The figures are quoted with permission from Wen et al.[122]; (C) Migration path of Li2S2 on the surface of CeO2 (111). This figure is quoted with permission from Yu et al.[124]; (D) Schematic illustration of KB@CeO2-C preparation process. This figure is quoted with permission from Zhang et al.[125]; (E) HRTEM image of bi-Sm2O3; (F) Schematic diagram of polysulfide species conversion to Li2S enhanced by Bi- Sm2O3. The figures are quoted with permission from Lai et al.[126]; (G)TEM image of Y2O3. The figures are quoted with permission from Qiao et al.[127]; (H) HRTEM image of CeO2/Co/carbon nanotube; (I) Schematic illustration of enhanced mechanism by CeO2/Co/CNT-PP separator. The Figures are quoted with permission from Zhang et al.[128]; (J) Schematic illustration of Co-N-C@CeO2-C preparation process. This figure is quoted with permission from Jin et al.[129]; (K) EDS mapping of LaCoO3; (L) Optimized structures of different polysulfide species adsorption on LaCoO3 and calculated adsorption energy. The figures are quoted with permission from Wang et al.[130]. MOF: Metal-organic framework; NCF@CeO2: nitrogen-doped carbon fibers based on cerium dioxide spheres; HRTEM: high-resolution transmission electron microscopy; KB: Ketjen Black; CNT: carbon nanotube; PP: polypropylene; EDS: energy-dispersive X-ray spectroscopy.