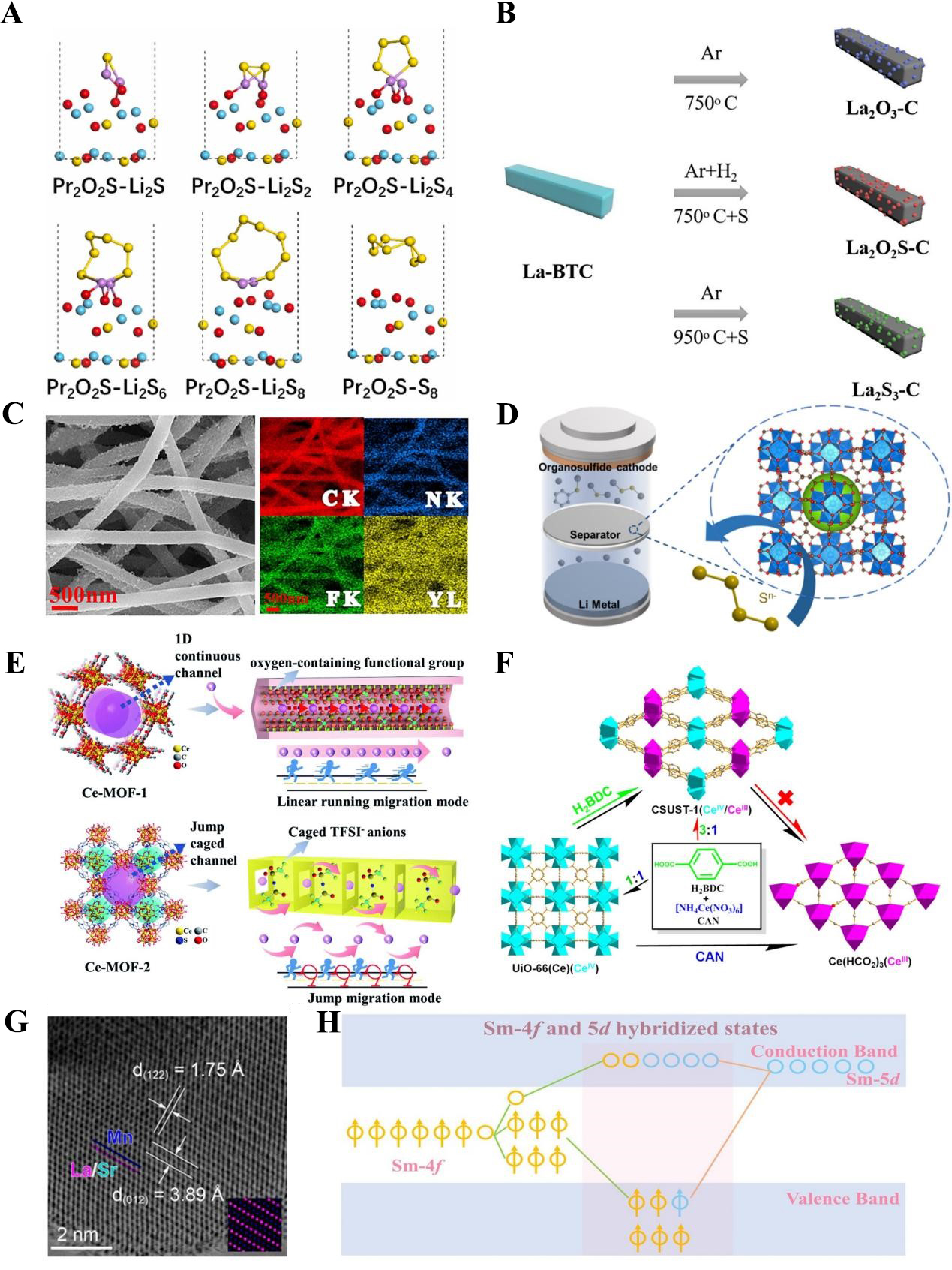
fig8
Figure 8. (A) Optimized structure of polysulfide species adsorption on Pr2O2S. This figure is quoted with permission from Wang et al.[131]; (B) Schematic illustration of La-BTC as a precursor for preparing La2O3-C, La2O2S-C, and La2S3-C. This figure is quoted with permission from Sun et al.[132]; (C) SEM image of YF3 nanofiber and corresponding EDS mapping. This figure is quoted with permission from Deng et al.[133]; (D) Schematic illustration of MOF-801 modified separator. This figure is quoted with permission from Zhao et al.[137]; (E) Li+ transportation mechanism in Ce-MOF with 1D channels. This Figure is quoted with permission from Song et al.[138]; (F) Schematic illustration of CSUST-1 with mixed-valent preparation process. This figure is quoted with permission from Jin et al.[140]; (G) STEM image of Sr2+ doped perovskite (LSMO-0.3). This figure is quoted with permission from Hao et al.[141]; (H) Scenario of Sm 4f-5d hybridization. This figure is quoted with permission from Zhou et al.[142]. BTC: 1,3,5-benzenetricarboxylate; La-BTC: lanthanum-benzenetricarboxylate metal-organic framework; SEM: scanning electron microscopy; EDS: energy-dispersive X-ray spectroscopy; MOF: Metal-organic framework; MOF-801: Zr-Fumarate MOF; STEM: scanning transmission electron microscopy; CSUST: Changsha University of Science and Technology; LSMO-0.3: La0.7Sr0.3MnO3.