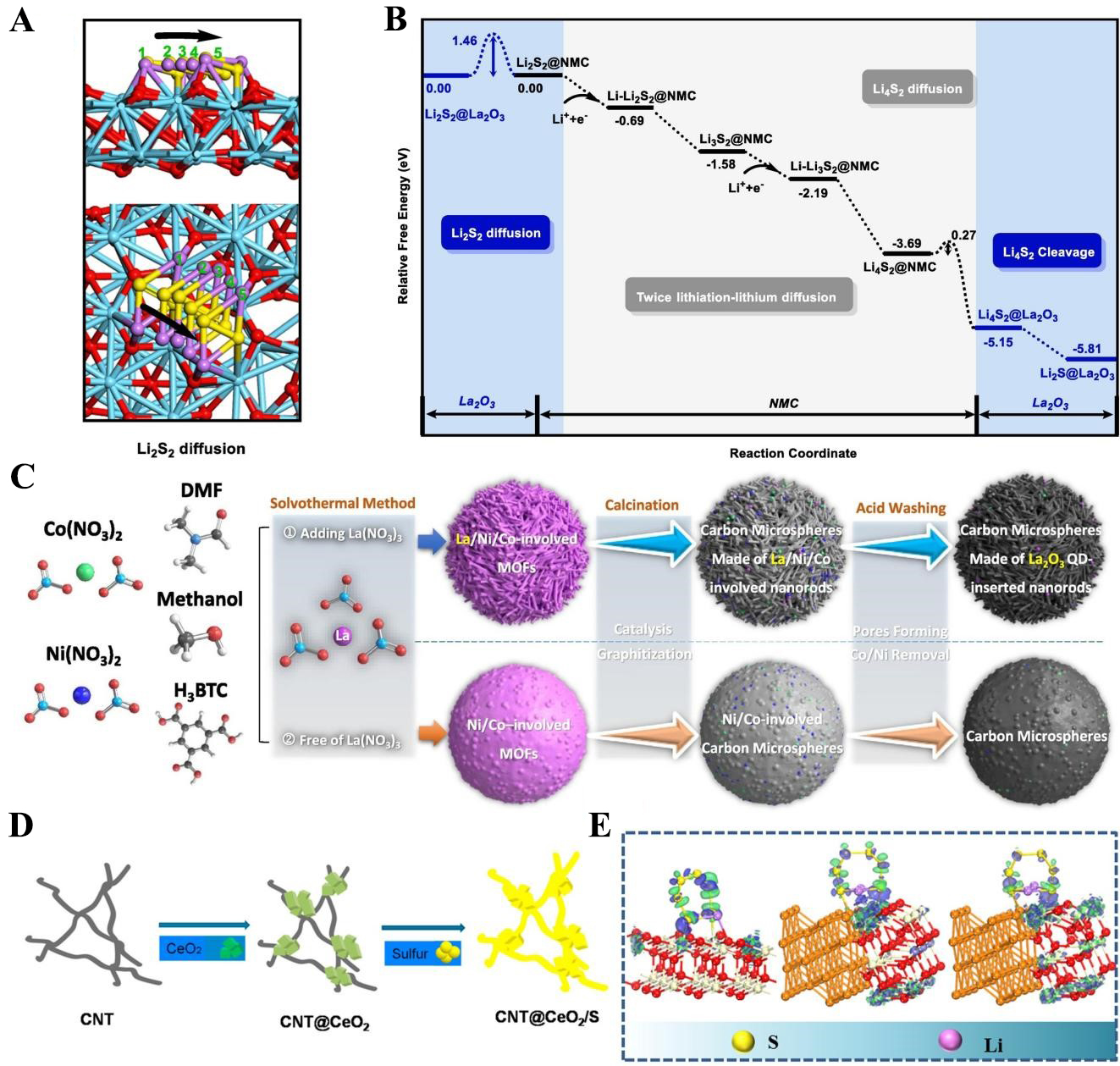
fig3
Figure 3. (A) Migration path, Li-O and S-La bond lengths profile of Li2S2 diffusion on the surface of La2O3; (B) The energy profile for Li2S2 → Li2S conversion process catalyzed by La2O3. The figures are quoted with permission from Zhang et al.[57]; (C) Schematic of the solvothermal route to carbon microspheres (CMs) with and without embedded La2O3 quantum dots (QDs). Note that adding La precursors produces pronounced differences in sphere morphology. This figure is quoted with permission from Dai et al.[62]; (D) Preparation process of sulfur electrode with CNT and CeO2. The figures are quoted with permission from Xiao et al.[78]; (E) Electronic density difference of different models (i.e., CeO2, Cu-CeO2, and Cu-CeO2-x). The figures are quoted with permission from Hou et al.[82]. DMF: BTC: 1,3,5-benzenetricarboxylate; CNT: carbon nanotube; NMC: nitrogen-rich mesoporous carbons.