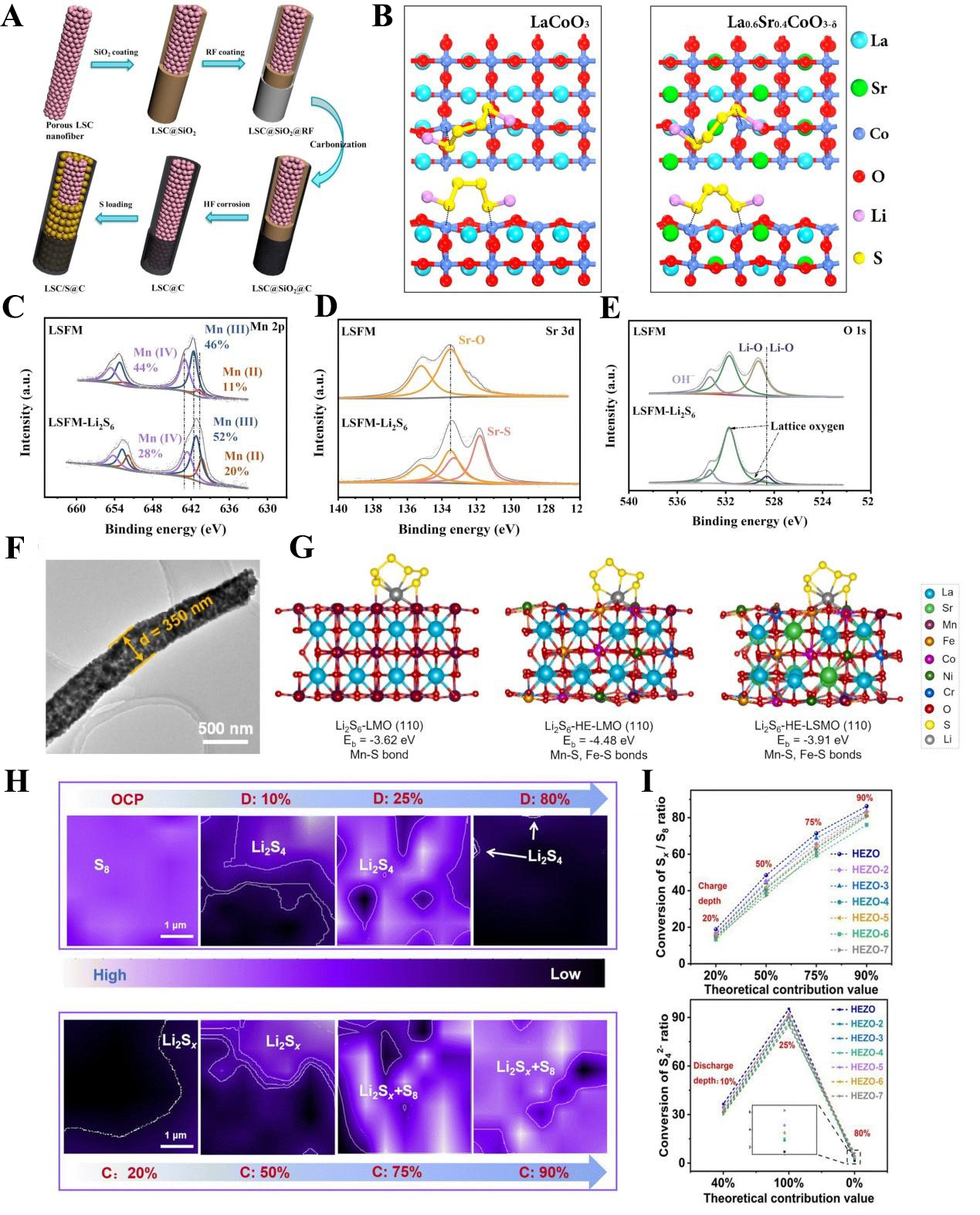
fig5
Figure 5. (A) Preparation process of LSC perovskite-based host for composite sulfur electrode; (B) Optimized Li2S4 adsorption structure on the surface of LaCoO3 and LSC. The figures are quoted with permission from Hao et al.[99] XPS tests before and after Li2S6 adsorption on LSFM (C) Mn 2p; (D) Sr 3d; (E) O 1s. The figures are quoted with permission from Jin et al.[100]; (F) HETEM image of HE-LSMO. (G) Binding energy comparison of Li2S6 adsorption on the surface of LMO, HE-LMO, and HE-LSMO. The figures are quoted with permission from Tian et al.[105]; (H) In-situ electrochemical Raman 2D spectra of HEZO-based sulfur electrode at different charge/discharge depths; (I) Actual utilization of Li2S4 in discharge process and charge process. The figures are quoted with permission from Zhou et al.[106]. LSC: La0.6Sr0.4CoO3-δ; XPS: X-ray photoelectron spectroscopy; LSFM: La0.3Sr0.7Fe0.2Mn0.8O3; OCP: open circuit potential; HRTEM: high-resolution transmission electron microscopy; HE-LSMO: La0.8Sr0.2(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3); HE-LMO: La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3); HEZO: (La0.15Nd0.15Sm0.40Eu0.15Gd0.15)2Zr2O7.