Exploring the interplay of Ti-Sn co-doping in photoelectrochemical water splitting of hematite nanowires
Abstract
Photoelectrochemical water splitting is a promising alternative for sustainable energy production, addressing the growing need for clean energy sources. Hematite is a potential semiconductor for this process due to its abundance, low cost, non-toxicity, and stability. However, bare-hematite-based photoelectrochemical cells face challenges such as low photocurrent density, requiring innovative strategies to improve efficiency. This study explores the combined effects of three key approaches: enhancing crystallinity through high-temperature annealing, increasing specific surface area via nanostructuring, and improving photoanode conductivity through heteroatom doping. Hematite nanowires were synthesized using a hydrothermal method, with Ti-doping introduced during hydrothermal synthesis and subsequent Sn co-doping during an 800 °C annealing process, which also improved crystallinity. The introduction of Ti dopant significantly increased the photocurrent density under simulated solar illumination from 0.03 mA·cm-2 to 0.63 mA·cm-2. Co-doping with Ti and Sn further enhanced performance to 1.27 mA·cm-2. The research explores how heteroatom doping influences the properties of hematite and examines its interaction with high-temperature annealing. These findings are significant for advancing the design of efficient nanostructures for energy conversion applications.
Keywords
INTRODUCTION
In recent years, rapid industrial growth and societal development have led to a significant increase in fossil fuel consumption[1]. Consequently, the world faces two major challenges: environmental pollution and energy resource depletion[2,3]. Direct production of hydrogen from renewable resources such as sunlight and water using a photoelectrochemical (PEC) cell holds promise as a cost-effective approach for harvesting and storing solar energy[4]. The use of hematite (α-Fe2O3) photoanodes in PEC water splitting has attracted significant interest because of its abundance, very good long-term stability[5], and appropriate bandgap
To improve the hematite photocurrent density, two parameters can be manipulated: recombination reaction time (τ1) and carrier (hole or electron) drift time (τ2). Increasing τ1 (reducing recombination rate) can be achieved by passivating surface states to diminish the positive charge accumulation on the surface[10]. Additionally, improving crystallinity by reducing the number of grain boundaries, which act as
Nanostructuring significantly enhances PEC performance by expediting photogenerated carrier extraction and improving charge separation[14,15]. Nanostructuring results in benefits such as larger surface area, better light absorption, reduced electron-hole recombination, and enhanced electronic properties due to quantum confinement effects[16-18]. Notably, the one-dimensional structure of nanowires (NWs), influenced by quantum confinement, shifts the absorption spectrum towards the visible range, thereby enhancing photoactivity.
The inclusion of impurity atoms, whether intentionally or unintentionally, within the hematite lattice has been demonstrated to significantly enhance photocurrent[12,19]. Incorporating high concentrations (ranging from 0.5% to 20% atomic) of impurities such as Si[20], Sn[11], Se[21], Cr[22], Pt[23], Mn[24], Cr[25], Ge[26], W[27] or Rh[28] has been achieved mostly through physical and chemical deposition, spray pyrolysis, or hydrothermal techniques[6,8,20,29-32]. Also, it has been shown that surface modification of hematite photoanodes can contribute to improving their PEC properties[33-36]. While most dopants yield modest improvements in PEC performance of hematite photoanodes, notable enhancements have been observed with Si, Ti and Sn doping[37]. In many studies, dopants are introduced into nanostructured hematite during the material growth process, allowing control over doping concentration. Particularly, Ti doping via hydrothermal techniques has become a primary method for improving hematite photocurrent by increasing donor density and carrier density, thus leading to higher solar-to-hydrogen conversion efficiency[38,39].
While some studies have addressed Ti-doping[8,40-42], most do not delve deeply into understanding the influence of Ti on the nanostructure. Furthermore, the high-temperature annealing strategy can unintentionally dope the hematite photoanodes with Sn[11,12,43]. Although the effect of Sn doping has been explored, the interaction between Sn and Ti through intentional crossover doping has not been studied.
In this study, three strategies were combined to enhance hematite photoanode performance: (i) optimization of thermal annealing to enhance separation efficiency by increasing τ1, (ii) nanostructuring to decrease τ2 by reducing carrier migration distance and improving specific surface area, and (iii) dual-doping to enhance carrier mobility, thereby increasing τ1 and reducing τ2. For this purpose, hematite NWs obtained through the hydrothermal method were doped with Ti in a controlled manner during photoanode fabrication, while Sn-doping was achieved through Sn4+ incorporation into the hematite structure from the fluorine-doped tin oxide (FTO) substrate during thermal annealing[12].
This work aims to understand how the different strategies described above contribute to improving the efficiency of hematite photoanodes. For this purpose, this study successfully characterizes the impact of annealing conditions on dual doping with Ti and Sn and their interactions within the in-depth concentration profile using Rutherford Backscattering Spectroscopy (RBS). Moreover, the PEC performance evaluation of the photoanodes was conducted by determining the photocurrent
EXPERIMENTAL
Photoanodes synthesis
α-Fe2O3 NWs, with and without Ti doping, were prepared by hydrothermal method[44]. FTO coated glass substrates (Solaronix, Aubonne), with dimensions 1.2 × 2.5 cm2, were previously cleaned as reported by Francisco et al.[45]. 7 Ω·square-1 and 10 Ω·square-1 substrates were used for the samples that followed the
Two annealing treatment approaches were studied: the one-step annealing approach, which involves annealing at 600 ºC for 3 h, and the two-step annealing approach, which includes a first step at 550 ºC for
Photoanodes characterization
Morphological characterization
The morphological characterization was performed using FE-SEM (FEI Quanta 400 ESEM FEG, FEI, Oregon) at CEMUP, University of Porto. The diameter (D) and thickness of NWs were estimated using ImageJ software[47].
Structural characterization
The structural analysis was performed using XRD (Rigaku SmartLab, Rigaku, Tokyo) with Cu Kα radiation (1.540593 Å) in parallel-beam mode with grazing incidence omega angles. Measurements were made in a 2θ range from 20° to 80°, with 0.02° increments and 0.5 s accumulation time. Raman spectroscopy was also used to confirm the crystalline phase and ordering of the photoanodes. The Raman scattering was measured using a Renishaw inVia Qontor Spectrometer, using an excitation laser with a 633 nm polarized line of
Surface compositional characterization.
To study the surface composition of the samples, XPS characterization was carried out. A Phoibos 150 EP MCD analyzer was used (SPECS Group, Berlin). The undoped 800 ºC sample was irradiated with Al Kα
Bulk compositional characterization
On the other hand, to obtain additional information about the bulk composition, thickness and roughness, Rutherford ion backscattering experiments were performed with a 5 MV terminal voltage Tandetron accelerator located at “Centro de Microanálisis de Materiales”[49]. Experiments were conducted using a
Photoelectrochemical impedance spectroscopy
The electrical properties of the photoanodes were examined by PEIS. In this technique, a small sinusoidal potential perturbation is applied to the system and the amplitude and phase shift of the resulting current response are recorded. A BioLogic SP-150 potentiostat (Bio-Logic, Seyssinet-Pariset, France) was employed. A frequency range between 0.1 Hz and 100 kHz was covered with a modulation signal of 10 mV[55]. The EIS spectra were then fitted to an appropriate electrical equivalent circuit using ZView software (Scribner Associates Inc., Southern Pines).
Photoelectrochemical performance
The PEC performance of the hematite photoanodes was assessed based on the J-V curves, stability tests, intrinsic solar-to-chemical (ISTC) conversion efficiency, and IPCE measurements.
The photocurrent J-V curves were performed using a class B solar simulator with a 150 W Xe lamp (Oriel, Newport) under dark and 1-sun simulated sunlight (100 mW·cm-2, AM 1.5 G). The photoanodes were immersed in 1.0 M NaOH electrolyte solution using a “cappuccino” PEC cell[5,51], with an illuminated surface area of 0.528 cm2 and a three-electrode configuration: Ag/AgCl/saturated KCl electrode as the reference electrode, a platinum wire as the counter-electrode and the hematite NW photoanodes as the working electrode. The photocurrent was assessed under front-side illumination, which resulted in higher photocurrent than backside illumination, as the transport of generated electrons from the illuminated hematite through the FTO layer is easier than transport of holes (with a short diffusion length of 2-4 nm) through the hematite layer[12,52,53].
The stability tests were performed at a constant voltage of 1.23 VRHE (voltage vs. reversible hydrogen electrode) for two hours, using the same conditions described above. The ISTC conversion efficiency was determined from the photocurrent density vs. potential curves measured under dark and illumination, in a three-electrode configuration[5,54]. According to Rothschild’s model[54], the ISTC is defined by
with ηel the electrolysis efficiency, Jphoto the generated photocurrent, Vphoto the correspondent photopotential and Udark the potential applied to the photoanode to reach the respective dark current[5,54]. Furthermore, the intrinsic photovoltaic power Plight for the photoanodes is given by
Also, the fill factor (FF) at the maximum power point (MPP) is determined by
where VMPP and JMPP indicate the values of photopotential and photocurrent at the MPP, respectively; VOC and JSC represent the open circuit voltage and short circuit current, respectively.
IPCE measurements were carried out using a 150 W continuous-wave Xe lamp, with a computer-controlled monochromator. A scan was performed for wavelengths between 330 nm and 650 nm. The scanning speed was 2 nm/s. Measurements were performed at VRHE = 1.45 V. The IPCE can be calculated from the photocurrent measured at each wavelength, based on the expressions proposed in previous reports[4,7].
RESULTS AND DISCUSSION
Morphological characterization
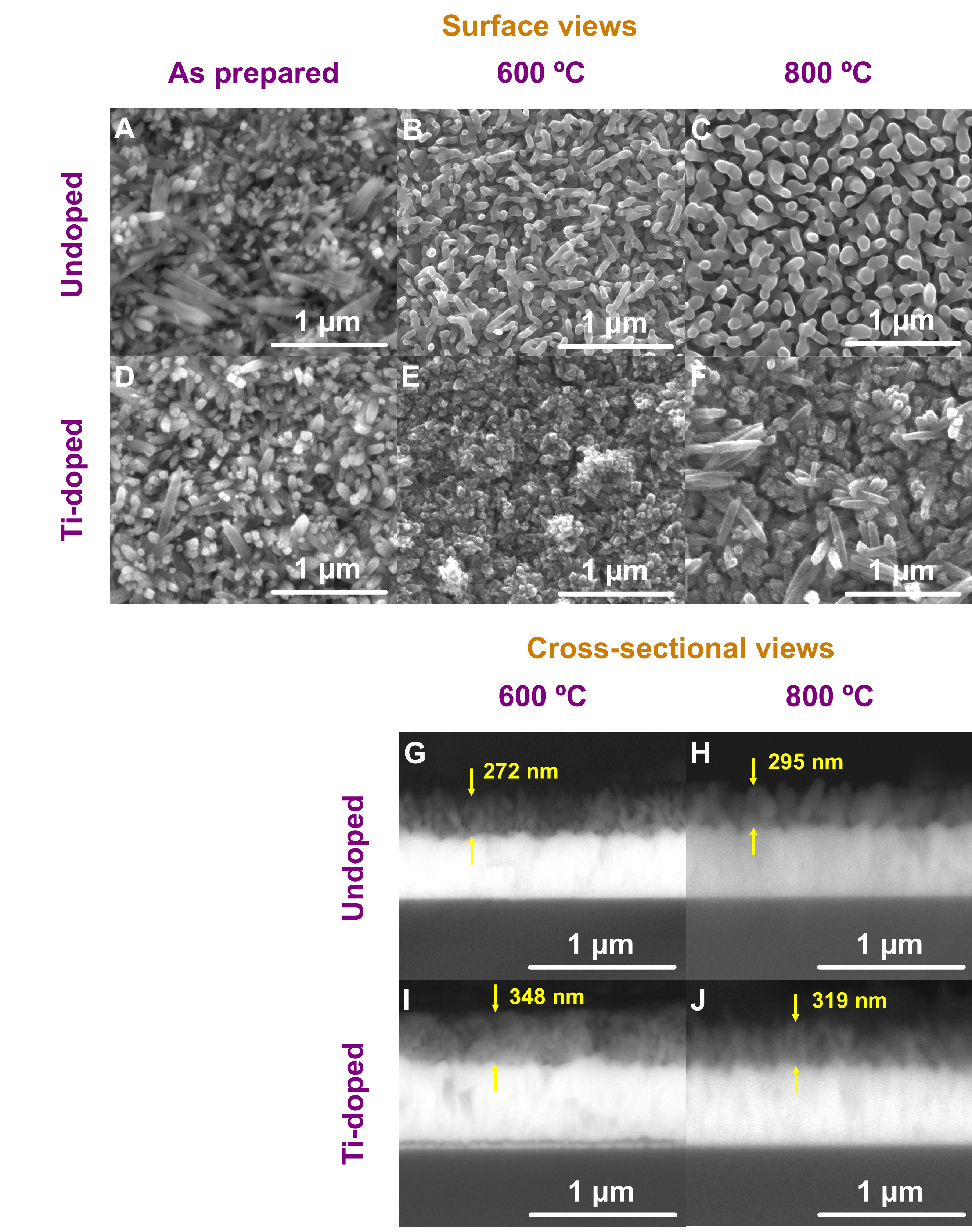
The morphology of the prepared photoanodes was assessed through FE-SEM, as shown in Figure 1A-F. The as-prepared NWs were observed to be fully formed during the initial stage of the autoclaving process. This formation is attributed to the thermodynamically stable nucleation of Fe3+ ions in solution, under controlled temperature and pressure conditions[44].
Figure 1. FE-SEM surface images of (A) undoped NWs after hydrothermal synthesis; and after (B) one-step and (C) two-step annealing processes; (D) Ti-doped NWs after hydrothermal synthesis, and after (E) one-step and (F) two-step annealing processes;
For the undoped samples, following a one-step annealing process at 600 ºC, the NWs exhibited a rounder morphology. Furthermore, the D of the NWs notably increased when subjected to a second annealing step at 800 ºC for 20 min. Specifically, D measurements from top-view FE-SEM images revealed an increase from 63 ± 9 nm to 97 ± 19 nm when considering the one-step and the two-step annealing processes, respectively. The observed increase is attributed to the fusion between neighboring NWs during the
In the case of Ti-doped photoanodes, a less ordered structure was obtained after one-step annealing at
Figure 1G-J shows FE-SEM cross-sectional views of the samples. The thickness of the hematite photoanodes was estimated, revealing that for the undoped samples, the hematite layer measures approximately 272 nm after annealing at 600 ºC. The thickness slightly increases to 295 nm following the final annealing step. In contrast, Ti-doped samples exhibit a higher thickness of about 348 nm when annealed at 600 ºC. However, the high-temperature annealing step at
Structural analysis
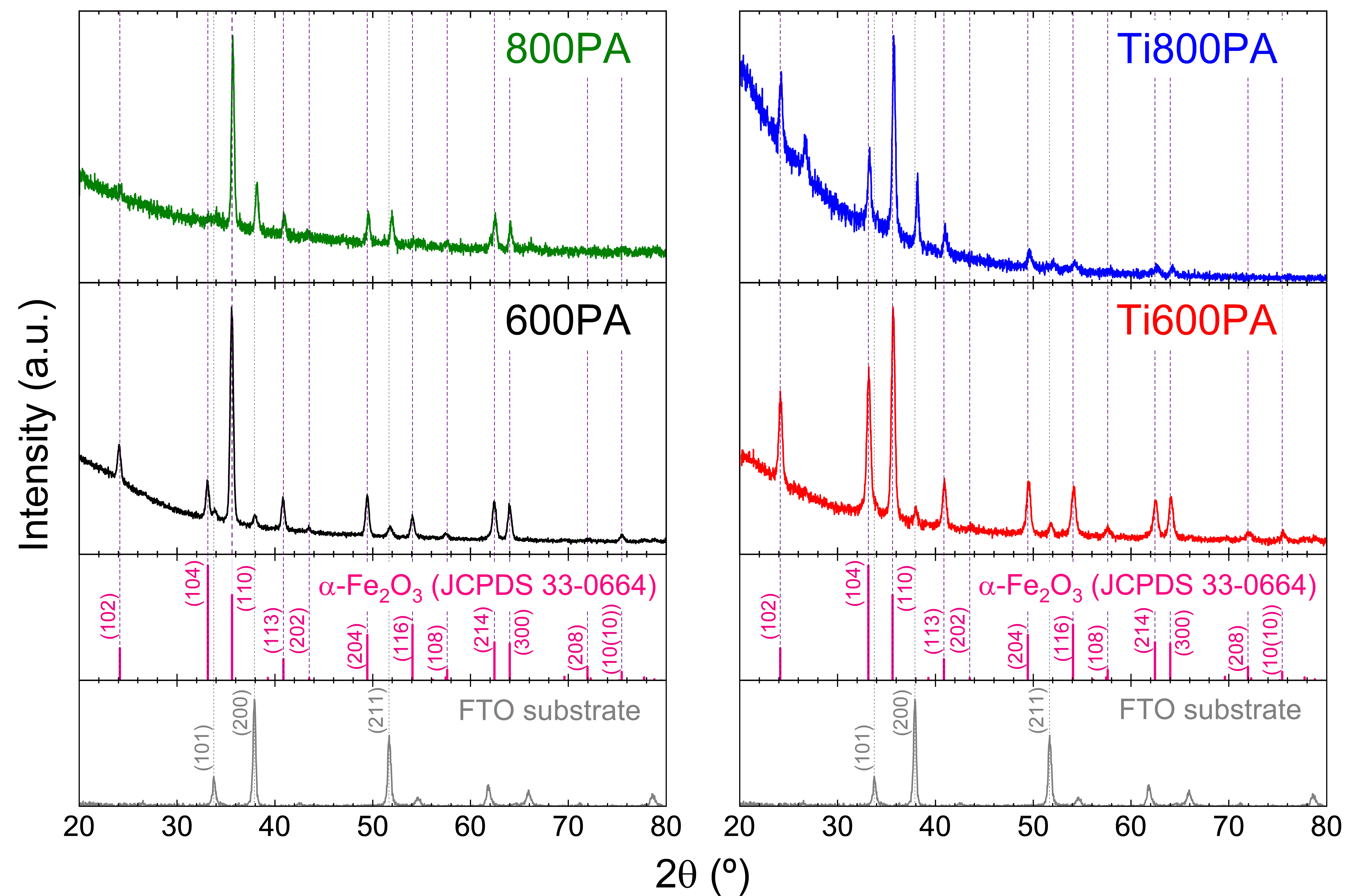
Figure 2 shows the XRD patterns for both bare and Ti-doped hematite NWs, subjected to the
Figure 2. XRD patterns of the undoped and Ti-doped hematite NWs, subjected to one-step (600 °C) and two-step (800 °C) annealing processes. The α-Fe2O3 reference pattern (JCPDS 33-0664), and the diffractogram corresponding to the FTO substrate are also shown. XRD: X-ray diffraction; NWs: nanowires; FTO: fluorine-doped tin oxide.
The α-Fe2O3 lattice structure consists of an alternation of iron bilayers and oxygen layers, aligned parallel to the (001) basal plane. Within each bilayer, Fe3+ atoms have parallel spins, while the spins in adjacent bilayers are antiparallel[61,62]. Electrons can move by hopping within the iron bilayers, but electron exchange between neighboring bilayers is spin forbidden by Hund’s rule. Consequently, the conductivity of hematite is found to be four orders of magnitude higher within the (001) basal plane (e.g., in [110] direction) than in the orthogonal directions[61,62]. Therefore, having a preferential orientation of the crystallites along the [110] direction is expected to enhance the efficiency of the PEC process[63].
In addition to the peak associated with plane (110), the peaks associated with planes (102), (104), (113), (204), (116), (108), (214) and (300) can be also appreciated.
The crystallite size D and inhomogeneous strain ϵ have been estimated for each sample using the Williamson-Hall model[64,65], as explained in the supplementary information. Williamson-Hall plots are shown in Supplementary Figure 1. With the two-step annealing scheme, an increase from D = 27 ± 4 nm to D = 37 ± 12 nm for the undoped and from D = 23 ± 3 nm to D = 30 ± 5 nm for the Ti-doped photoanodes is observed. This is ascribed to the hematite crystallinity improvement promoted by temperature[11,13,29]. For
The cell parameters obtained for the hematite structure by the Rietveld Refinement, and the calculated unit cell volume V are shown in Table 1. It is observed that the undoped sample subjected to a two-step annealing process presents a slightly larger unit cell than the sample subjected to a one-step annealing process. However, this difference is minimal and could be attributed to the incorporation of Sn4+, which has an ionic radius of 0.69 Å, slightly larger than that of Fe3+, at 0.65 Å, within the structure[70]. In the case of the Ti-doped samples, while the unit cell volume for the sample subjected to a single-step annealing process is comparable to that of the undoped sample, a significantly greater reduction in unit cell volume is observed when the annealing occurs at 800 ºC. This is likely due to the incorporation of Ti4+, which has an ionic radius of 0.61 Å, smaller than that of Fe3+, and suggests that the higher temperature of 800 ºC facilitates a more effective integration of Ti into the α-Fe2O3 structure compared to the 600 ºC annealing process[70].
Cell parameters obtained from the Rietveld Refinement for each of the samples. The unit cell volume is also shown
| Sample | 600PA | Ti600PA | 800PA | Ti800PA |
| a (Å) | 5.0299 | 5.0295 | 5.0322 | 5.0174 |
| c (Å) | 13.7335 | 13.7467 | 13.7339 | 13.7085 |
| Volume (Å3) | 300.9 | 301.1 | 301.2 | 298.9 |
Supplementary Figure 2 shows the Raman spectra of different synthesized photoanodes. The spectra are consistent with the α-Fe2O3 structure, with the peaks at 244 cm-1, 293 cm-1, 410 cm-1 and 612 cm-1 corresponding to doubly degenerate Eg modes and those at 225 cm-1 and 497 cm-1 to single degenerate A1g modes[71-73]. The full width at half maximum (FWHM) of each peak is shown in Supplementary Table 2. A greater broadening of the peaks is observed for the Ti-doped samples. This is consistent with the smaller crystallite size observed for the Ti-doped samples in relation to the undoped ones.
Surface composition
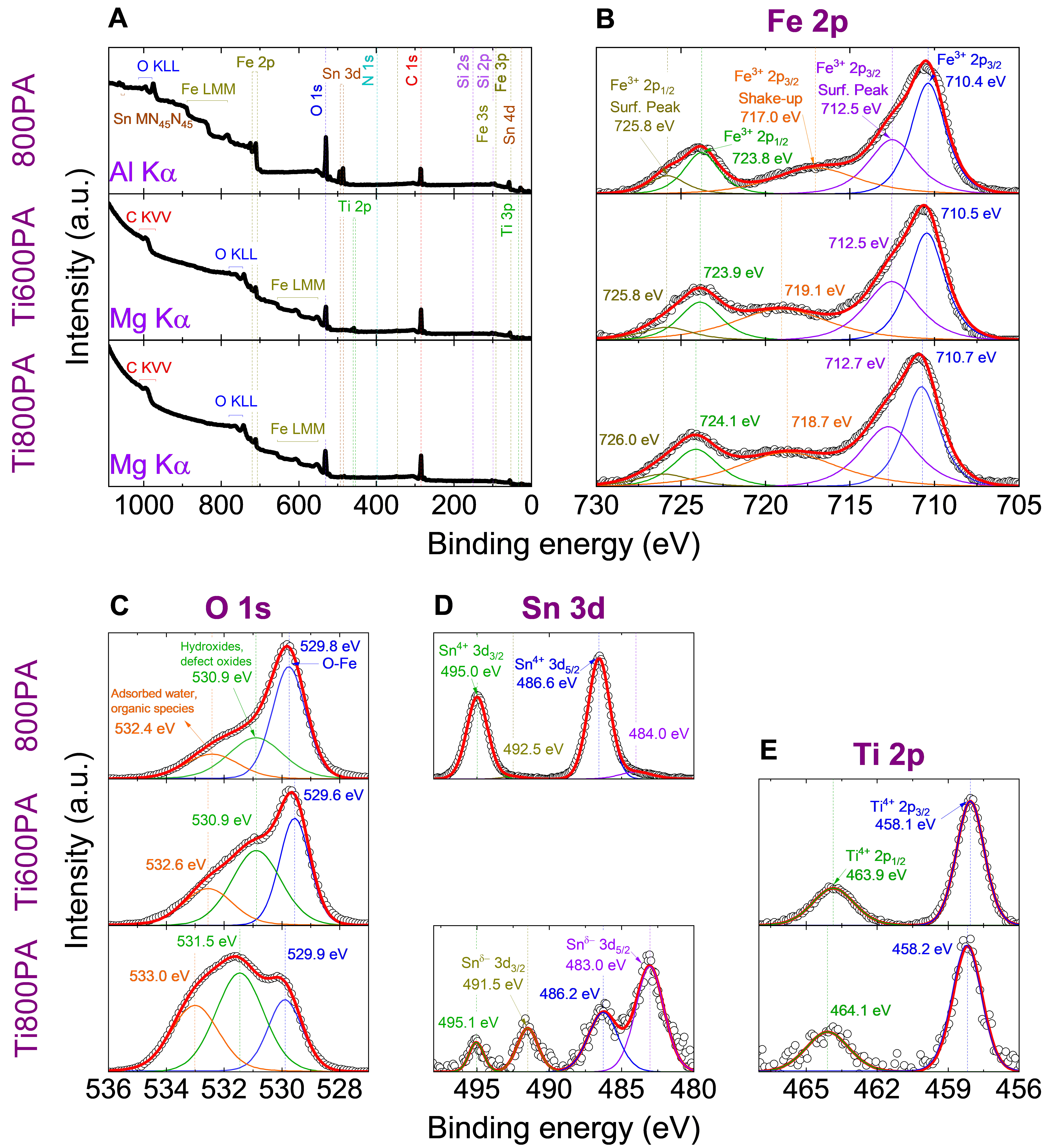
To assess the surface composition, an XPS study was performed on the 800PA, Ti600PA and Ti800PA photoanodes. The full survey for each sample and the high-resolution spectra corresponding to Fe 2p, O 1s, Sn 3d and Ti 2p are shown in Figure 3. For high-resolution spectra, the background was considered by applying a Shirley baseline model[74].
Figure 3. XPS spectra collected from the 800PA, Ti600PA and Ti800PA photoanodes: (A) full survey; (B) Fe 2p; (C) O 1s; (D) Sn 3d; (E) Ti 2p. For high-resolution spectra, the background was deduced by applying a Shirley baseline model. XPS: X-ray photoelectron spectroscopy.
In the full survey spectra, shown in Figure 3A, it is observed that the most intense signals correspond to the characteristic Fe and O peaks of hematite. Also, presence of Sn coming from the diffusion from the FTO substrate can be observed, along with Ti, for the samples doped with this element. In addition, a significant amount of C is observed, mainly due to adventitious carbon contamination during air exposure. In addition, traces of Si are observed at a very low concentration, probably due to small impurities introduced by the glass substrate itself. Also, a very small signal associated with N is appreciated, probably due to traces of the precursors.
The spectral region associated with the Fe 2p photoemission peak is shown in Figure 3B. Two split
The O 1s spectral region is shown in Figure 3C. This region has been fitted with three Gaussian - Lorentzian peaks corresponding to lattice oxygen at the lowest BE (mainly Fe-O), to defective surface oxides or surface hydroxides at the medium BE, and to absorbed water or organic O at the highest BE[82,83]. The Fe-O peak is located at 529.6-529.9 eV, which is consistent with the reported BE in α-Fe2O3[39,84]. It is also worth mentioning that the percentage of the oxygen that forms the Fe-O bond, and the total oxygen is 51 %, 37 % and 23 % for the 800PA, Ti600PA and Ti800PA samples, respectively. Since the XPS measurements were performed after the PEC study, these differences may simply be due to small variations in the amount of
In Figure 3D, the spectral region corresponding to Sn 3d is shown. For the 800PA, two peaks at ~486.6 eV and ~495.0 eV are observed, corresponding to the photoemission peaks associated with the 3d5/2 and 3d3/2 levels of Sn, respectively, which are consistent with the Sn4+ oxidation state[85,86]. Additionally, two weak peaks are observed at lower BE than the previous ones. The BE of these peaks is significantly lower than that expected for metallic tin (485.0 eV for the 3d5/2 peak)[87], so they must be negatively charged Sn species. Similar effects have been reported by other authors[88,89]. For the 800PA sample, more than 93% of the Sn is in the Sn4+ state; in the case of the Ti800PA sample, this percentage is reduced to 36%, with the remaining 64% corresponding to negatively charged Sn species. In both cases, no metallic Sn is observed. The origin of these negatively charged species may be due to the incorporation of some Sn ions into the hematite structure, replacing O, or to the formation of some SnNax species on the surface during PEC measurements. In this case, Na may not be observed in the survey spectrum because its sensitivity factor is four times lower than that of Sn, whose concentration is already low. For the Ti600PA sample, the amount of Sn on the surface is lower than the detection limit of the technique, suggesting a barrier effect of the Ti doping, which prevents Sn diffusion towards the photoanode surface.
Finally, Figure 3E shows the spectral region corresponding to Ti 2p, for the Ti-doped samples. In both cases, two peaks at ~458.1 eV and ~464.0 eV are observed, corresponding to the photoemission peaks associated with Ti levels 2p3/2 and 2p1/2, respectively. For titanium (IV) oxide, the position of the 2p3/2 peak would be expected to be located at ~458.7 eV. This shift to lower BE has also been reported by other authors for
A quantitative estimation of the surface composition has been performed based on the intensity of the signals associated with each element. For this, the sensitivity factors provided by the manufacturer of the analyzer have been considered. When conducting this calculation, the carbon and oxygen that do not form Fe-O bonds have not been considered, since they are essentially surface features present on the outfacing side of the sample. Thus, Table 2 shows the relative concentration of Olattice, Fe, Sn and Ti for each of the samples.
Surface composition of the different samples estimated by XPS
| Sample | 800C | Ti600PA | Ti800PA |
| Olattice (at.%) | 62.4 | 65.3 | 62.7 |
| Fe (at.%) | 28.3 | 25.5 | 35.5 |
| Sn (at.%) | 8.3 | | 1.2 | |
| Ti (at.%) | -- | 9.2 | 1.4 |
A higher concentration of Ti on the surface is observed for an annealing temperature of 600 ºC (9.2 at.%) compared to 800 ºC (1.4 at.%). Furthermore, it should be noted that for the two samples annealed at 800 ºC, the Sn concentration in the undoped sample is about seven times higher than in the Ti-doped sample. This variation may be attributed to the more defined morphology and ordering of the NWs of the undoped sample. As a result, part of the analyzed area may correspond to an area with a very low hematite thickness, facilitating better diffusion of Sn towards the surface for this sample (no Ti barrier).
In-depth compositional characterization.
While XPS is a very useful technique to determine with great precision the composition and chemical state of the different elements present on the outmost (5-10 nm) of the sample, using Rutherford Backscattering Spectrometry (RBS) makes it possible to determine the in-depth distribution of the different elements present in the photoanodes[94,95].
Supplementary Figure 3 shows the RBS spectra obtained for the 800PA, Ti600PA and Ti800PA samples. Only the relevant part of the spectra, corresponding to the hematite photoanode and the FTO layer, is shown. To simulate the diffusion of Sn from the FTO substrate and the incorporation of Ti, the experimental data have been fitted, simulating up to twenty layers corresponding to hematite doped with different atomic percentages of Ti and Sn, on top of up to thirteen more layers containing Sn and O to simulate the FTO substrate. C was also considered when performing the simulations to consider possible organic contamination present in the samples.
According to the highest energy edge of the Fe signal, located at ~3,205 keV for all the samples, the presence of this element on the surface is identified. Furthermore, for the Ti-doped samples, the presence of Ti on the surface is also observed in both samples, as per its high energy edge, located at ~3,056 keV. The lower energy edges of the Fe and Ti signal, along with the high energy edge of the Sn signal, are quite smooth. This effect is particularly prominent in the undoped sample annealed at 800 ºC. This phenomenon results from a combination of Sn diffusion from the FTO substrate towards the surface, and the more defined morphology and ordering of the NWs for the undoped samples. Consequently, not all the incident ions pass through the same amount of hematite, leading to non-uniform energy loss among the ions that reach the FTO layer.
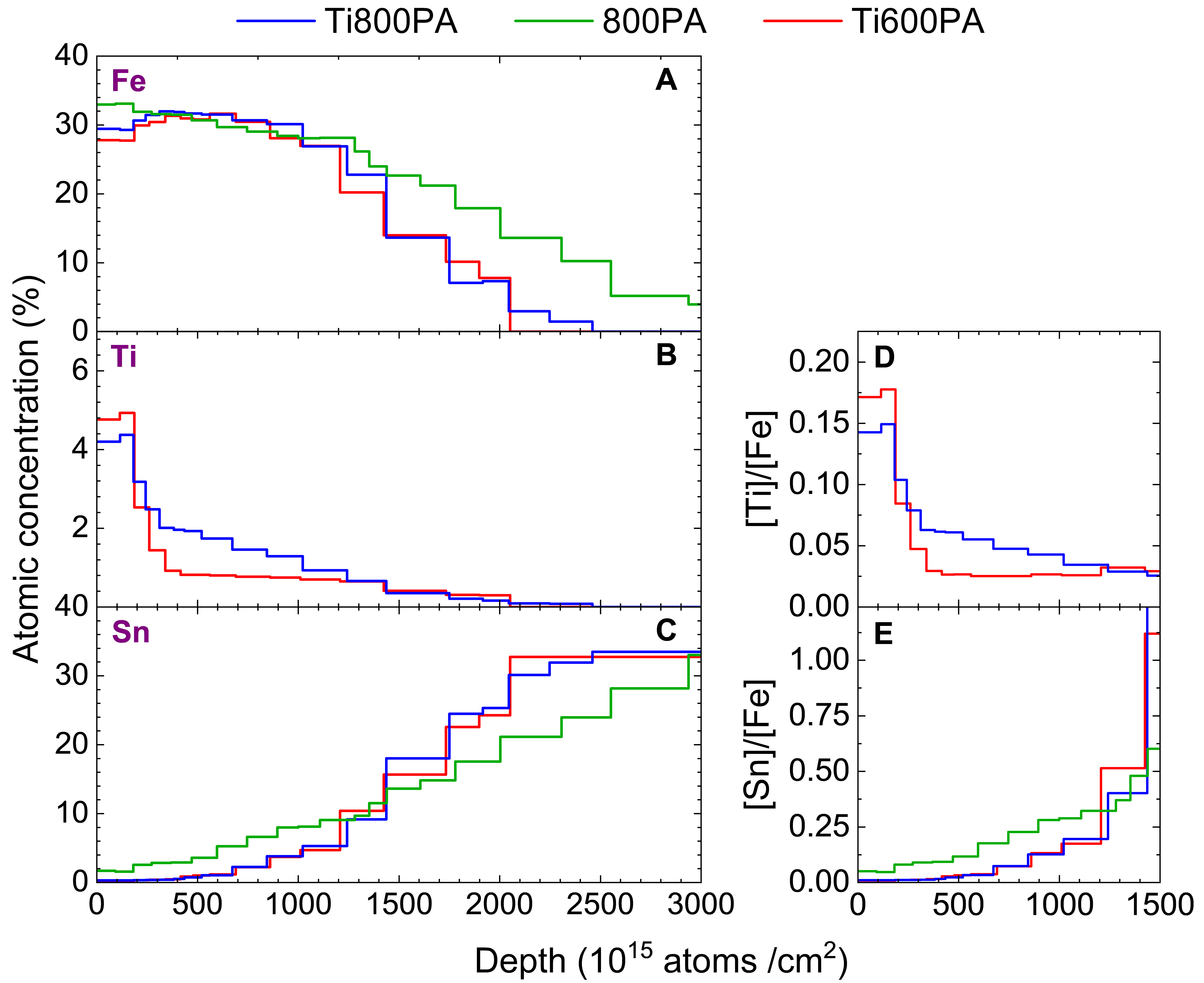
Figure 4 shows the depth concentration profile of Fe, Ti, and Sn and the variation of the [Sn]/[Fe] and
Figure 4. In-depth concentration profiles of (A) Fe; (B) Ti and (C) Sn obtained from the analysis of the RBS spectra, for the 800PA, Ti600PA and Ti800PA samples; (D) [Ti]/[Fe] ratio, and (E) [Sn]/[Fe] ratio depth profiles. RBS: Rutherford backscattering spectrometry.
In relation to the Ti concentration, it is observed that, although in both cases the [Ti]/[Fe] ratio is greater in the most superficial part of the sample than in bulk, the difference is greater for the 600 ºC annealed sample. This is consistent with the results observed by XPS. However, the difference in Ti concentration between the two samples is small compared to the results obtained by XPS. This may be due to the surface sensitivity of XPS, which analyzes only the first 5-10 nm of the sample. In contrast, while RBS can estimate a depth profile, it is not the appropriate technique to resolve the exact concentration of the most superficial part. Additionally, Ti analysis by RBS is challenging due to its low concentration, low Rutherford
Regarding the bulk Ti concentration, it is higher for the 800 ºC annealed sample. This suggests that the nucleation and growth of FeO(OH) NWs occurs in the first stage of the synthesis process, with Ti being incorporated later, mostly on the surface. Through thermal annealing processes, the diffusion of Ti throughout the Fe2O3 matrix is favored. The higher the temperature, the greater this effect is confirmed to be.
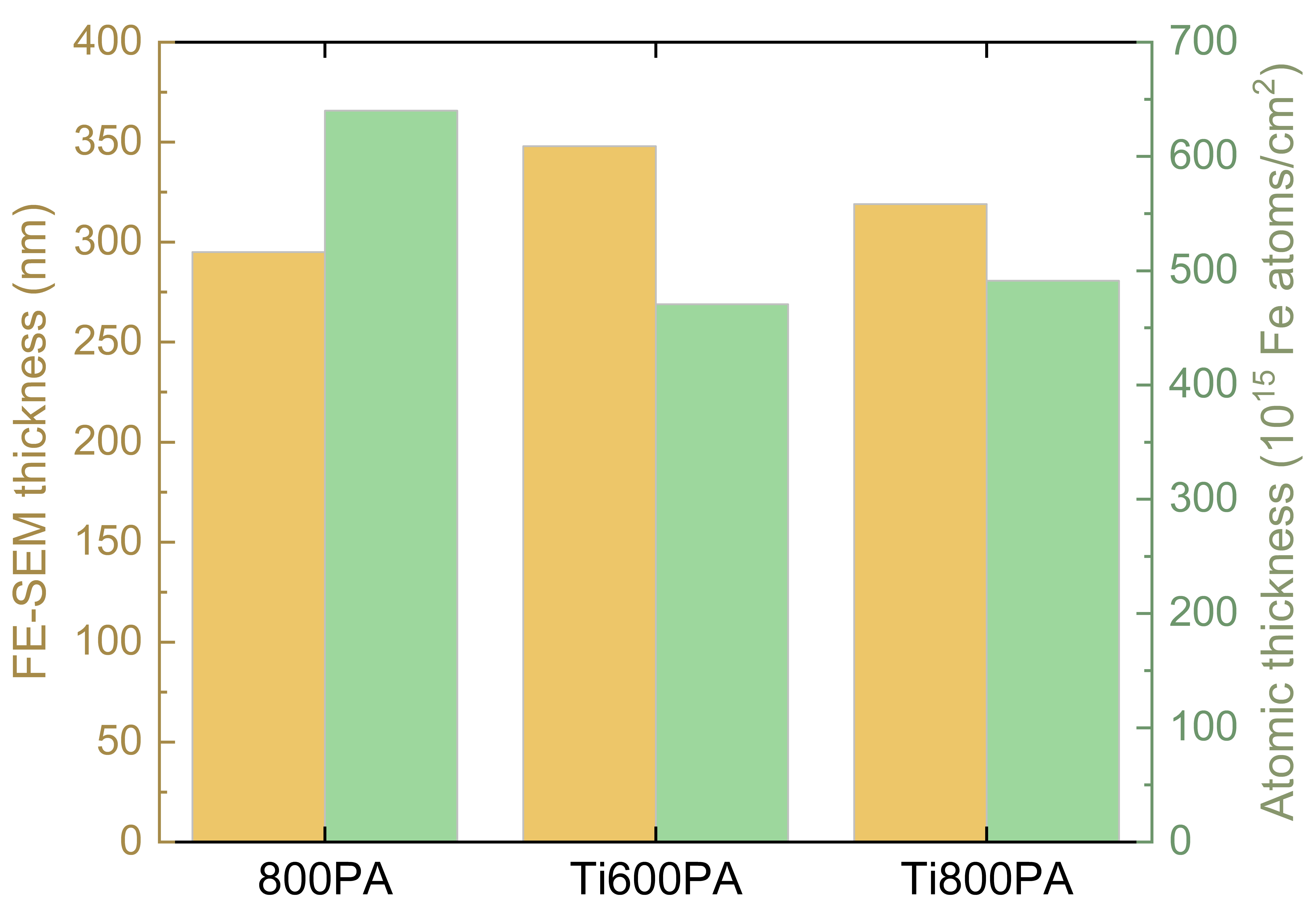
By considering the atomic thickness of each layer used in the model and the percentage of Fe in each simulated layer, it is possible to estimate the total atomic thickness of the hematite layer, measured in Fe atoms/cm2, for each sample. This is shown in Figure 5, along with the thickness measured by SEM. For the undoped sample annealed at 800 ºC, the atomic thickness is approximately 6.4 × 1017 Fe atoms/cm2. For the doped samples, the atomic thickness is 4.7 × 1017 Fe atoms/cm2 and 4.9 × 1017 Fe atoms/cm2 for the one-step and two-step annealing samples, respectively. This indicates that Ti doping reduces the total amount of Fe atoms in the sample by 20%-30%. However, the thickness measured by SEM for the Ti-doped samples is slightly higher than for the undoped samples. This suggests that the volume occupied by voids inside the doped samples is higher than in the case of the undoped samples, probably leading to a larger porosity for the Ti-doped photoanodes. Additionally, the fact that the two doped samples present a similar amount of Fe atoms is expected since, although the distribution of these in the sample could change during the annealing processes, their incorporation is necessarily done during the hydrothermal synthesis process.
PEIS characterization
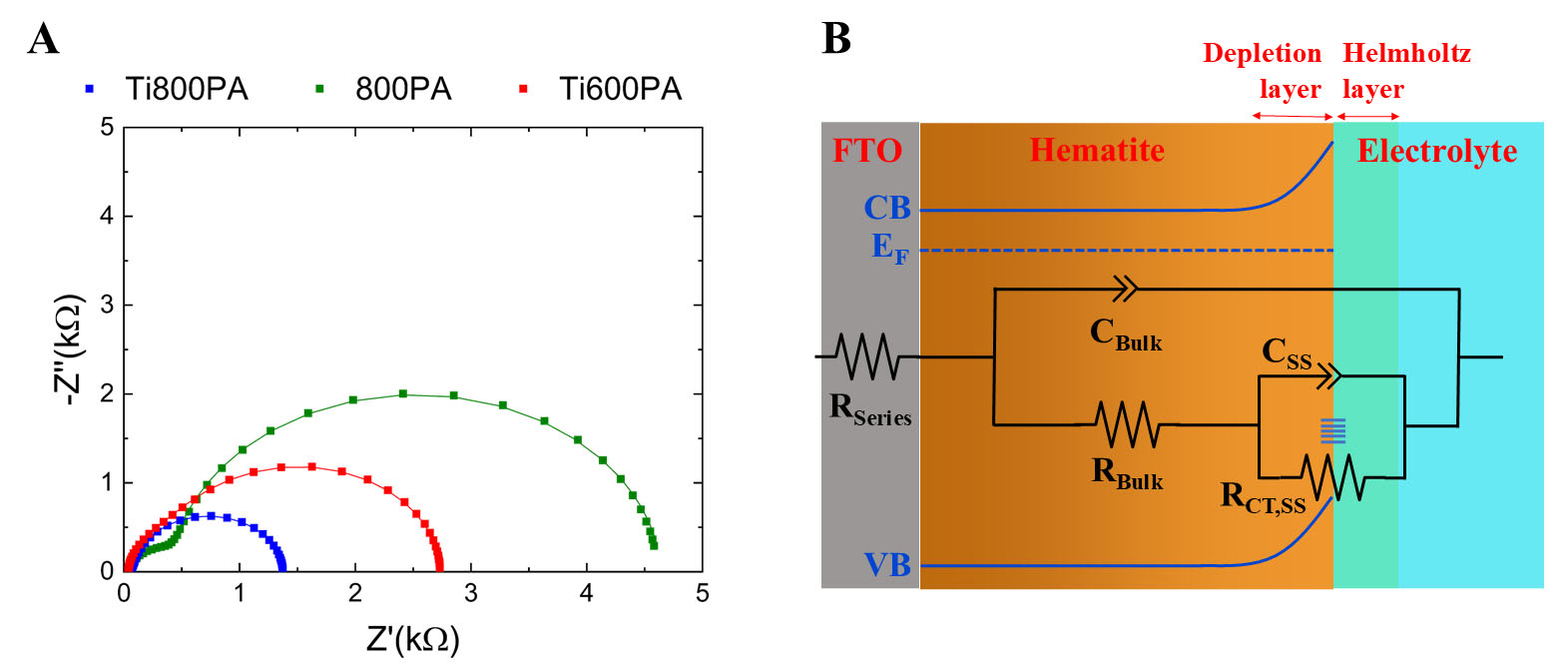
Figure 6A shows the Nyquist plot of the Ti600PA, 800PA and Ti800PA photoanodes. Likewise, Figure 6B shows a schematic representation of the equivalent circuit used to fit the experimental data. The circuit consists of the following elements: (1) A series resistance RSeries, corresponding mainly to the small resistance of the FTO substrate; (2) A resistance RBulk, associated with the recombination of electron-hole pairs in bulk, in parallel with a capacitance CBulk associated with charge accumulation in bulk; (3) A resistance RCT;SS, corresponding to the charge transfer resistance from the surface states to the electrolyte, in parallel with its corresponding capacitance CSS[9,96]. Capacitors were modeled as constant phase elements (CPEs), to consider the possibility of non-uniform current distribution[51].
Figure 6. (A) Nyquist impedance plots of the Ti600PA, 800PA and Ti800PA photoanodes obtained under illumination and VRHE = 1 V; (B) Equivalent electrical circuit used to fit the impedance data under illumination. FTO: Fluorine-doped tin oxide.
Table 3 shows the values of the different elements of the equivalent circuit, for the three samples analyzed. It is observed how the RSeries is similar and small in all cases, of about RSeries ~30-60 Ω. Concerning the resistance associated with the recombination of electron-hole pairs in bulk, it is minimal for the Ti800PA sample, obtaining a value of 134 Ω, higher for the 800PA sample (516 Ω), and maximum for the Ti600PA sample (1,111 Ω). This indicates that both Ti doping and high-temperature annealing contribute to the reduction of the recombination of electron-hole pairs in bulk. The reduction of Rbulk with Ti doping is consistent with the incorporation of Ti4+ cations into the α-Fe2O3 structure[38,59]. Also, the reduction of Rbulk with increasing annealing temperature is consistent with the increase in crystallinity observed for the 800 ºC annealed samples, which contributes to the reduction of non-radiative recombination at grain boundaries[97-100], as well as to the incorporation of Sn4+ cations at the interface with the FTO substrate when annealing at
Value of each element of the considered equivalent circuit, for the Ti600PA, 800PA and Ti800PA photoanodes
| Sample | Ti600PA | 800PA | Ti800PA |
| RSeries (Ω) | 37 | 52 | 49 |
| RBulk (Ω) | 1111 | 516 | 134 |
| CBulk (μF) | 4.1 | 4.3 | 5.0 |
| RCT,SS (Ω) | 1590 | 4080 | 1192 |
| CSS (μF) | 7.8 | 24.4 | 5.8 |
On the other hand, the resistance associated with the charge transfer between the surface states and the electrolyte RCT,SS is maximum for the 800PA sample (4,080 Ω), and lower for the Ti-doped samples (1,590 Ω and 1,192 Ω for the Ti600PA and Ti800PA samples, respectively). This suggests that the presence of Ti4+ ions at the surface prevents positive charge accumulation at the hematite surface and decreases the surface recombination of the trapped electrons with holes from the valence band, favoring an increment of the quantum efficiency of the system.
Photoelectrochemical performance
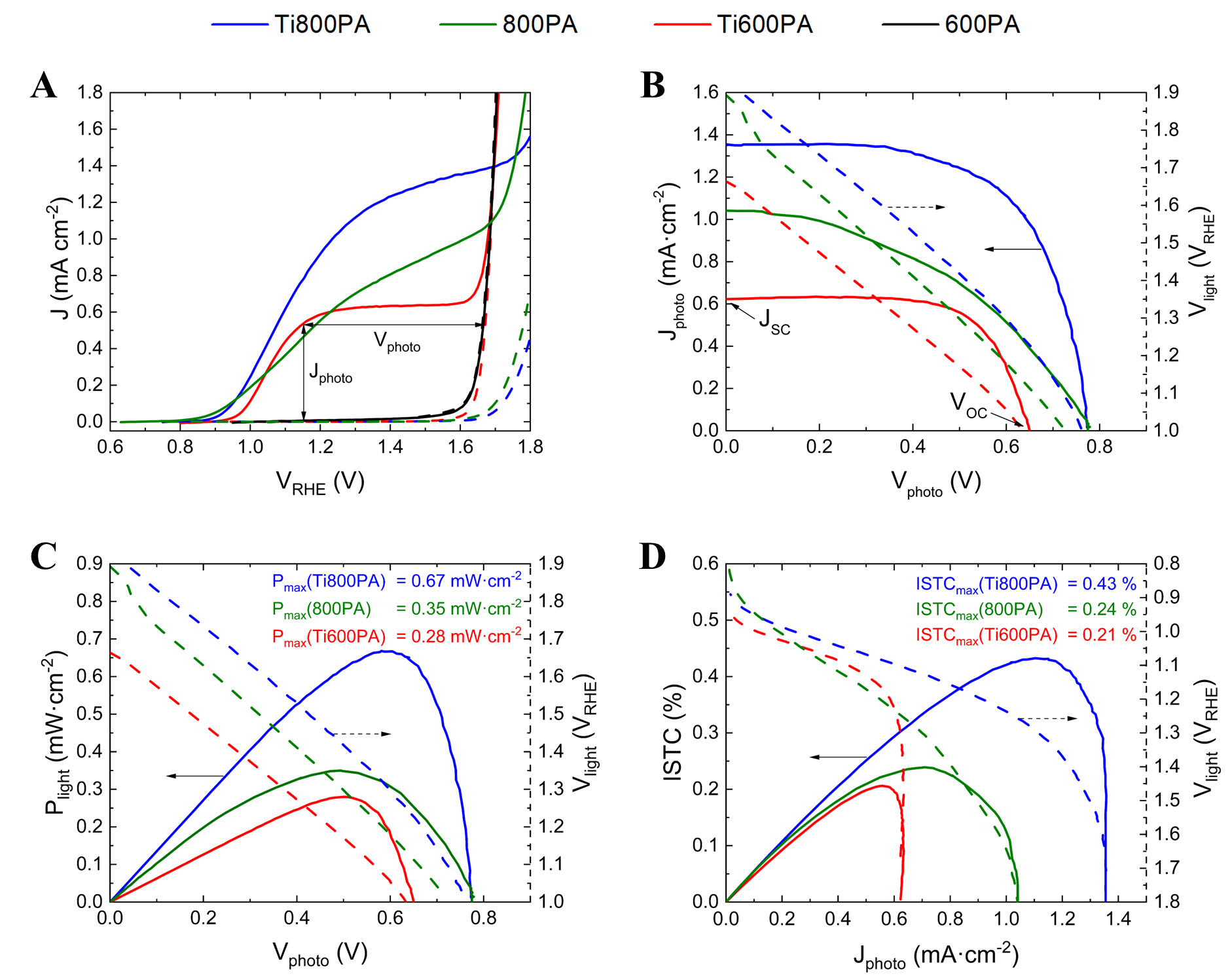
Figure 7A shows the current J-V characteristics for the synthesized α-Fe2O3 photoanodes in the dark and under simulated sunlight. For the undoped one-step annealed sample, J is very low, ~0.02 mA·cm-2 (at
Figure 7. (A) J-V curves for the 600PA, Ti600PA, 800PA, Ti800PA photoanodes, obtained under simulated solar illumination (solid lines), and in the dark (dashed lines); (B) Jphoto as a function of Vphoto; (C) Intrinsic photovoltaic power Plight as a function of Vphoto; (D) ISTC efficiency as a function of Jphoto. For (b-d), the potential Vlight applied to the photoanode under simulated solar illumination to obtain each Vphoto/Jphoto value is shown with dashed lines. J-V: Photocurrent density-voltage; ISTC: intrinsic solar-to-chemical.
The improvement of J under sunlight illumination for the Ti-doped samples, compared to the undoped ones, is mainly due to the presence of Ti4+ ions on the surface, which prevent the accumulation of positive charges. Therefore, there is a decrease in the surface recombination of trapped electrons with holes in the valence band, as shown by PEIS measurements. This favors the quantum efficiency of the photoanodes. Furthermore, the reduction in bulk resistance observed, due to the presence of Ti4+ ions, also contributes to improving the quantum efficiency of the photoanodes.
On the other hand, the improvement of J for the samples subjected to a two-step annealing scheme, compared to the one-step annealed samples, is essentially due to a decrease in bulk recombination, as observed by PEIS measurements. This is associated, on the one hand, with an increase in crystallite size, observed by XRD, as well as with the possible incorporation of Sn4+ cations at the interface with the FTO substrate when annealing at 800 ºC. Furthermore, the greater preferential orientation in the (110) direction of these samples also favors a greater photocurrent, since the conductivity in the [110] direction is much higher than in directions orthogonal to it[61,62]. However, despite the improvement in the photocurrent with Ti and Sn co-doping, the photocurrent value obtained is not yet comparable to reported values of up to
However, while for the undoped 800 ºC annealed sample, the onset potential is ~0.90 VRHE, an anodic shift in the onset potential is observed for the Ti-doped samples (~0.93 VRHE and ~0.97 VRHE for the 800 ºC and 600 ºC annealed samples, respectively). This is possibly due to the additional donor levels introduced by surface doping with Ti, which affects band bending at the semiconductor-electrolyte interface[107]. Theoretical research by Toroker also indicated that a much larger overpotential was needed for water oxidation when hematite was doped with Ti on the surface[108]. Additionally, the Ti-doped samples present a step photocurrent rise. This has been reported to be associated with a reduction in recombination in surface states[109], consistent with what was observed by PEIS.
No significant variation of Jdark is observed when the photoanodes are doped with Ti. However, a notable decrease in Jdark is observed when the samples are subjected to the two-step annealing treatment, compared to when they are subjected to the one-step annealing treatment, consistent with previous studies[9,12].
The photocurrent Jphoto and photopotential Vphoto were extracted from the J-V curves, as shown in Figure 7A.
The intrinsic photovoltaic power Plight of the photoanodes as a function of Vphoto is shown in Figure 7C. This way, the conditions for working at the MPP can be determined. For the Ti600PA and the 800PA photoanodes, maximum powers of 0.28 mW·cm-2 (at Vlight = 1.17 VRHE) and 0.35 mW·cm-2 (at
The FF at the MPP was calculated for each photoanode applying eq. (3). For the Ti-doped samples, a FF at MPP of 64 % and 68 % was obtained for the photoanodes subjected to two-step and one-step annealing processes, respectively. This value is much higher than the one obtained for the undoped 800 ºC annealed sample (FF at MPP of 44 %). Previous works have reported that the greater the FF, the greater the charge extraction compared to the recombination of electron-hole pairs[110,111]. Therefore, the fact that the Ti-doped samples present a higher FF indicates that charge extraction is favored by Ti-doping, consistent with the reduction of RCT;SS observed by PEIS, which is associated with a reduction of the positive charge accumulation at the hematite surface.
To evaluate the efficiency of the Ti-doped photoanodes in the conversion of solar into chemical energy necessary for water splitting, the ISTC conversion efficiency was determined by applying eq. (1). Figure 7D shows the plot of ISTC efficiency as a function of Jphoto. ISTC maximum efficiencies of 0.21 % (at
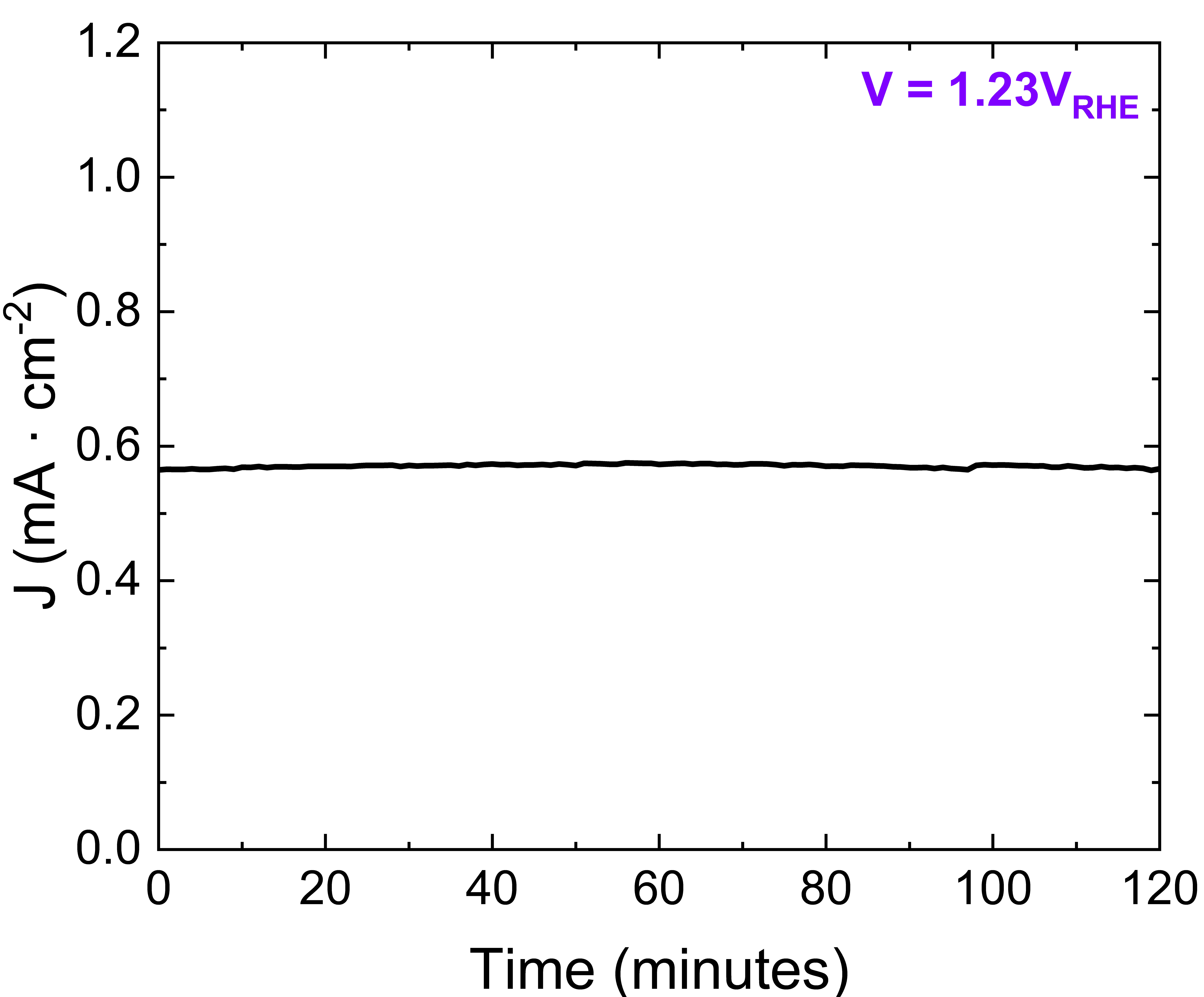
A stability test was performed using the one-step annealed Ti-doped α-Fe2O3 sample [Figure 8]. J remained stable during a continuous measurement over 2 h applying a bias potential of 1.23 VRHE under simulated
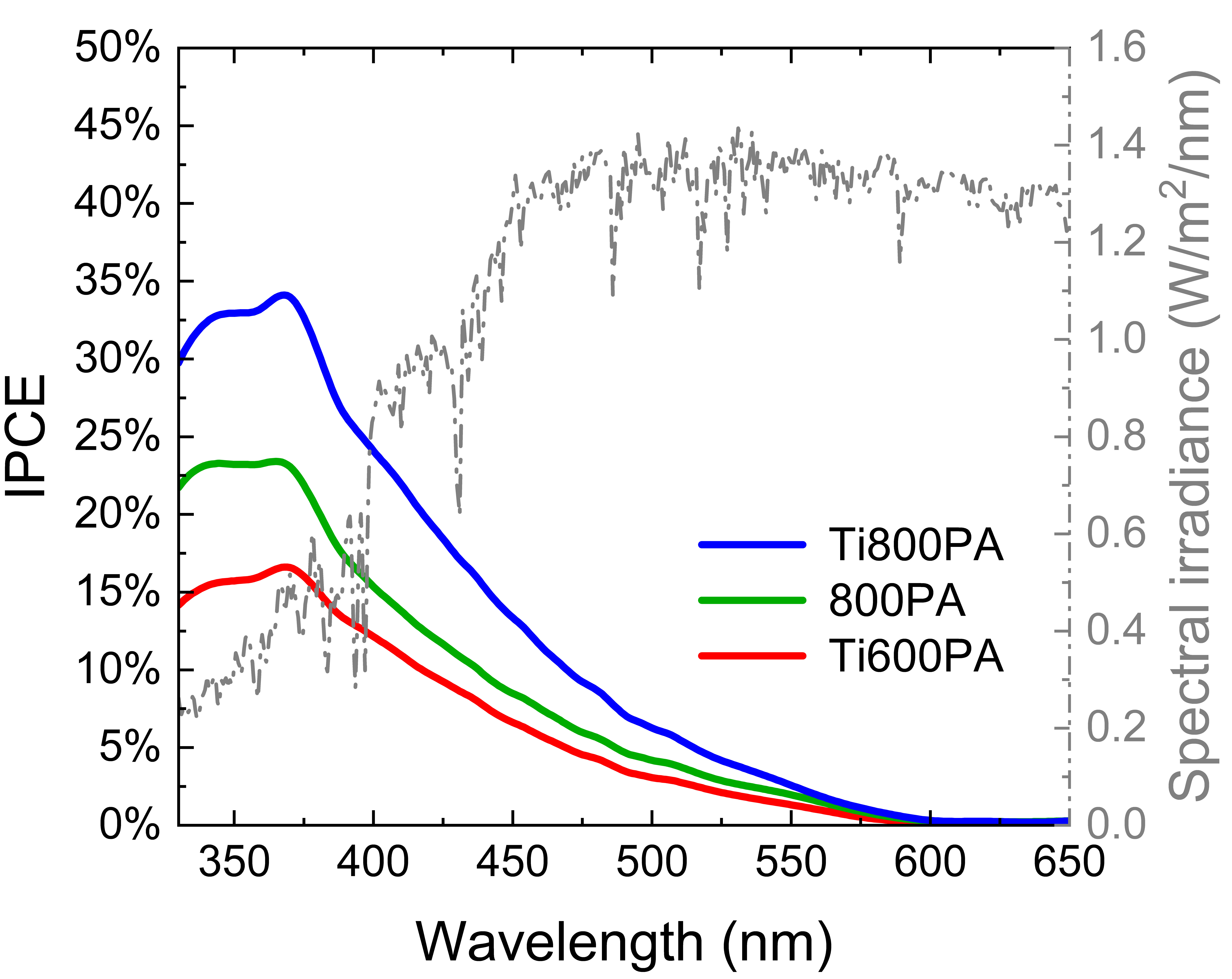
To better understand the higher photocurrent obtained for Ti-doped photoanodes, IPCE measurements at VRHE = 1.45 V were performed as a function of the wavelength of the incident light, as shown in Figure 9. As can be seen, the Ti800PA sample presents the highest IPCE at all wavelengths, with a maximum of 34%, while the 800PA and Ti600PA samples present maximum values of 23% and 17%, respectively. These results indicate a positive effect of Sn and Ti co-doping on water oxidation photoactivity. Interestingly, the
CONCLUSIONS
Ti-doped hematite NWs were synthesized via hydrothermal synthesis, and the impact of applying a
By deepening our comprehension of the impact of heteroatom doping on the properties of hematite semiconductors, and the possibility of applying this together with high-temperature annealing processes, this study lays the ground for the design and enhancement of efficient materials with high specific surface areas for sustainable chemical energy conversion applications.
DECLARATIONS
Acknowledgments
The authors thank the “Centro de Microanalisis de Materiales” for the beam time with reference
Authors’ contributions
Conceptualization: Araújo, J. P.; Apolinario, A.; de Sousa, C. T.
Data curation: Fernández-Alonso, F. J.; Quiterio, P.; Vilarinho, R.
Formal analysis, investigation: Fernández-Alonso, F. J.; Quiterio, P.; Vilarinho, R.; Manso-Silván, M.; Torres-Costa, V.; Apolinario, A.; de Sousa, C. T.
Funding adquisition: Araújo, J. P.; de Sousa, C. T.
Methodology: Fernández-Alonso, F. J.; Quiterio, P.; Vilarinho, R.; Torres-Costa, V.; Apolinario, A.; de Sousa, C. T.
Project administrarion: Araújo, J. P.; de Sousa, C. T.
Resources: Mendes, A.; Manso-Silván, M.; Torres-Costa, V.; Apolinario, A.; de Sousa, C. T.
Supervision: Araújo, J. P.; Manso-Silván, M.; Torres-Costa, V.; Apolinario, A.; de Sousa, C. T.
Visualization: Fernández-Alonso, F. J.; Quiterio, P.; Vilarinho, R.
Writing - original draft: Fernández-Alonso, F. J.; Quiterio, P.; de Sousa, C. T.
Writing - review and editing: Fernández-Alonso, Manso-Silván, M.; Torres-Costa, V.; Apolinario, A.; de Sousa, C. T.
Availability of data and materials
The data are available upon request from the authors.
Financial support and sponsorship
This research was financially supported by the grants PID2022-141080OB-C22 and CNS2024-154729, funded by MCIN/AEI. Fernández-Alonso, F.J. acknowledges the Formación de Profesorado Universitario programme, ref. FPU22/04365. de Sousa, C. T. acknowledges the programme Atracción de Talento (CAM), ref. 2020-T1/IND-19889. Apolinário, A. acknowledges FCT- Fundação para a Ciência e a Tecnologia DL57/2016 (Ref. DL 57/2016/CP1454/CT0017; https://doi.org/10.54499/DL57/2016/CP1454/CT0017) projects H2FlexiPEC’s (ref. 2022.07332.PTDC; (http://doi.org/10.54499/2022.07332.PTDC), 2024.00223.CERN; H2INNOVATE (NORTE-01-0145-FEDER-000076); Laboratório de Física para Materiais e Tecnologias Emergentes (LaPMET), Instituto de Física de Materiais Avançados, Nanotecnologia e Fotónica Universidade do Porto (IFIMUP)-UIDB/04968/2020 (https://doi.org/10.54499/UIDB/04968/2020); UIDP/04968/2020 (https://doi.org/10.54499/UIDP/04968/2020). This article is based upon work from COST Action NETPORE, CA20126, supported by COST (European Cooperation in Science and Technology).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Jackson, R. B.; Saunois, M.; Bousquet, P.; et al. Increasing anthropogenic methane emissions arise equally from agricultural and fossil fuel sources. Environ. Res. Lett. 2020, 15, 071002.
2. Sudhaik, A.; Parwaz, K. A. A.; Raizada, P.; et al. Strategies based review on near-infrared light-driven bismuth nanocomposites for environmental pollutants degradation. Chemosphere 2022, 291, 132781.
3. Ullah, S.; Chishti, M. Z.; Majeed, M. T. The asymmetric effects of oil price changes on environmental pollution: evidence from the top ten carbon emitters. Environ. Sci. Pollut. Res. 2020, 27, 29623-35.
5. Dias, P.; Vilanova, A.; Lopes, T.; Andrade, L.; Mendes, A. Extremely stable bare hematite photoanode for solar water splitting. Nano. Energy. 2016, 23, 70-9.
6. Sivula, K.; Le, F. F.; Grätzel, M. Solar water splitting: progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432-49.
7. Murphy, A.; Barnes, P.; Randeniya, L.; et al. Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen. Energy. 2006, 31, 1999-2017.
8. Tamirat, A. G.; Rick, J.; Dubale, A. A.; Su, W. N.; Hwang, B. J. Using hematite for photoelectrochemical water splitting: a review of current progress and challenges. Nanoscale. Horiz. 2016, 1, 243-67.
9. Dias, P.; Andrade, L.; Mendes, A. Hematite-based photoelectrode for solar water splitting with very high photovoltage. Nano. Energy. 2017, 38, 218-31.
10. Formal F, Tétreault N, Cornuz M, Moehl T, Grätzel M, Sivula K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737-43.
11. Ling, Y.; Wang, G.; Wheeler, D. A.; Zhang, J. Z.; Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano. Lett. 2011, 11, 2119-25.
12. Quitério, P.; Apolinário, A.; Navas, D.; et al. Photoelectrochemical water splitting: thermal annealing challenges on hematite nanowires. J. Phys. Chem. C. 2020, 124, 12897-911.
13. Zandi, O.; Hamann, T. W. Enhanced water splitting efficiency through selective surface state removal. J. Phys. Chem. Lett. 2014, 5, 1522-6.
14. Bassi, P. S.; Antony, R. P.; Boix, P. P.; Fang, Y.; Barber, J.; Wong, L. H. Crystalline Fe2O3/Fe2TiO5 heterojunction nanorods with efficient charge separation and hole injection as photoanode for solar water oxidation. Nano. Energy. 2016, 22, 310-8.
15. Zhou, D.; Fan, K. Recent strategies to enhance the efficiency of hematite photoanodes in photoelectrochemical water splitting. Chin. J. Catal. 2021, 42, 904-19.
16. Ni, S.; Wang, D.; Guo, F.; et al. Efficiency improvement of TiO2 nanowire arrays based dye-sensitized solar cells through further enhancing the specific surface area. J. Cryst. Growth. 2019, 505, 62-8.
17. Dhara, A.; Show, B.; Baral, A.; et al. Core-shell CuO-ZnO p-n heterojunction with high specific surface area for enhanced photoelectrochemical (PEC) energy conversion. Solar. Energy. 2016, 136, 327-32.
18. Haider, Z.; Yim, H. W.; Lee, H. W.; Kim, H. Surface and bulk modification for advanced electrode design in photoelectrochemical water splitting. Int. J. Hydrogen. Energy. 2020, 45, 5793-815.
19. Park, J.; Kang, J.; Chaule, S.; Jang, J. Recent progress and perspectives on heteroatom doping of hematite photoanodes for photoelectrochemical water splitting. J. Mater. Chem. A. 2023, 11, 24551-65.
20. Dias, P.; Lopes, T.; Andrade, L.; Mendes, A. Temperature effect on water splitting using a Si-doped hematite photoanode. J. Power. Sources. 2014, 272, 567-80.
21. Niu, S.; Jiang, W. J.; Wei, Z.; et al. Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation. J. Am. Chem. Soc. 2019, 141, 7005-13.
22. Wang, J.; Xiang, Y.; Zhang, W.; et al. Integrating Cr doped FeOOH into FeSe2 nanoparticles for efficient water oxidation at large current densities. Fuel 2023, 351, 128827.
23. Lin, J.; Zhang, X.; Zhou, L.; Li, S.; Qin, G. Pt-doped α-Fe2O3 photoanodes prepared by a magnetron sputtering method for photoelectrochemical water splitting. Mater. Res. Bull. 2017, 91, 214-9.
24. Gurudayal; Chiam, S. Y.; Kumar, M. H.; et al. Improving the efficiency of hematite nanorods for photoelectrochemical water splitting by doping with manganese. ACS. Appl. Mater. Interfaces. 2014, 6, 5852-9.
25. Shen, S.; Jiang, J.; Guo, P.; Kronawitter, C. X.; Mao, S. S.; Guo, L. Effect of Cr doping on the photoelectrochemical performance of hematite nanorod photoanodes. Nano. Energy. 2012, 1, 732-41.
26. Liu, J.; Cai, Y.; Tian, Z.; et al. Highly oriented Ge-doped hematite nanosheet arrays for photoelectrochemical water oxidation. Nano. Energy. 2014, 9, 282-90.
27. Shen, S.; Kronawitter, C. X.; Jiang, J.; Mao, S. S.; Guo, L. Surface tuning for promoted charge transfer in hematite nanorod arrays as water-splitting photoanodes. Nano. Res. 2012, 5, 327-36.
28. Zhang, M.; Luo, W.; Li, Z.; Yu, T.; Zou, Z. Surface modification of hematite photoanode films with rhodium. Rare. Met. 2011, 30, 38-41.
29. Sivula, K.; Zboril, R.; Le, F. F.; et al. Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J. Am. Chem. Soc. 2010, 132, 7436-44.
30. Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Grätzel, M. Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C. 2009, 113, 772-82.
31. Malviya, K. D.; Dotan, H.; Shlenkevich, D.; Tsyganok, A.; Mor, H.; Rothschild, A. Systematic comparison of different dopants in thin film hematite (α-Fe2O3) photoanodes for solar water splitting. J. Mater. Chem. A. 2016, 4, 3091-9.
32. Zhang, Y.; Ji, H.; Ma, W.; Chen, C.; Song, W.; Zhao, J. Doping-promoted solar water oxidation on hematite photoanodes. Molecules 2016, 21, 868.
33. Quang, N. D.; Van, P. C.; Le, D. D.; et al. Fluorine-surface-modified tin-doped hematite nanorod array photoelectrodes with enhanced water oxidation activity. Appl. Surf. Sci. 2021, 558, 149898.
34. Quang N, Cao Van P, Majumder S, Jeong JR, Kim D, Kim C. Rational construction of S-doped FeOOH onto Fe2O3 nanorods for enhanced water oxidation. J. Colloid. Interface. Sci. 2022, 616, 749-58.
35. Barroso, M.; Mesa, C. A.; Pendlebury, S. R.; et al. Dynamics of photogenerated holes in surface modified α-Fe2O3 photoanodes for solar water splitting. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 15640-5.
36. Kim, J. H.; Kim, J. M.; Pan, Z.; Sohn, W. Y. Revealing the roles of surface treatments on hematite (α-Fe2O3) photoanode in the shift of the onset potential. J. Photochem. Photobiol. A. Chem. 2023, 445, 115037.
37. Franking, R.; Li, L.; Lukowski, M. A.; et al. Facile post-growth doping of nanostructured hematite photoanodes for enhanced photoelectrochemical water oxidation. Energy. Environ. Sci. 2013, 6, 500-12.
38. Pu, A.; Deng, J.; Li, M.; et al. Coupling Ti-doping and oxygen vacancies in hematite nanostructures for solar water oxidation with high efficiency. J. Mater. Chem. A. 2014, 2, 2491.
39. Su, J.; Zhou, J.; Zong, S.; Zhou, Z.; Liu, C.; Feng, B. The effect of thermal annealing on the interfacial properties and photoelectrochemical performance of Ti doped Fe2O3 nanowire arrays. RSC. Adv. 2016, 6, 99851-8.
40. Peng, Y.; Ruan, Q.; Lam, C. H.; et al. Plasma-implanted Ti-doped hematite photoanodes with enhanced photoelectrochemical water oxidation performance. J. Alloys. Compd. 2021, 870, 159376.
41. Shen, S.; Kronawitter, C. X.; Wheeler, D. A.; et al. Physical and photoelectrochemical characterization of Ti-doped hematite photoanodes prepared by solution growth. J. Mater. Chem. A. 2013, 1, 14498.
42. Zhang, P.; Kleiman-shwarsctein, A.; Hu, Y.; et al. Oriented Ti doped hematite thin film as active photoanodes synthesized by facile APCVD. Energy. Environ. Sci. 2011, 4, 1020.
43. Ling, Y.; Li, Y. Review of Sn-doped hematite nanostructures for photoelectrochemical water splitting. Part. Part. Syst. Charact. 2014, 31, 1113-21.
44. Vayssieres, L.; Beermann, N.; Lindquist, S.; Hagfeldt, A. Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: application to iron(III) oxides. Chem. Mater. 2001, 13, 233-5.
45. Francisco, F.; Dias, P.; Ivanou, D.; Santos, F.; Azevedo, J.; Mendes, A. Synthesis of Host-guest hematite photoelectrodes for solar water splitting. ChemNanoMat 2019, 5, 911-20.
46. Yang, T.; Kang, H.; Jin, K.; et al. An iron oxide photoanode with hierarchical nanostructure for efficient water oxidation. J. Mater. Chem. A. 2014, 2, 2297-305.
47. Schneider, C. A.; Rasband, W. S.; Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671-5.
48. Seah, M. P.; Dench, W. A. Quantitative electron spectroscopy of surfaces: a standard data base for electron inelastic mean free paths in solids. Surf. Interface. Anal. 1979, 1, 2-11.
49. Redondo-cubero, A.; Borge, M. J. G.; Gordillo, N.; et al. Current status and future developments of the ion beam facility at the centre of micro-analysis of materials in Madrid. Eur. Phys. J. Plus. 2021, 136, 1085.
50. Mayer, M. SIMNRA, a simulation program for the analysis of NRA, RBS and ERDA. In: The fifteenth international conference on the application of accelerators in research and industry; Denton, Texas (USA): AIP; 1999. pp. 541-4. Available from: https://pubs.aip.org/aip/acp/article/475/1/541-544/953322. [Last accessed on 21 Mar 2025].
51. Lopes, T.; Andrade, L.; Le, F. F.; Gratzel, M.; Sivula, K.; Mendes, A. Hematite photoelectrodes for water splitting: evaluation of the role of film thickness by impedance spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 16515-23.
52. Dare-edwards, M. P.; Goodenough, J. B.; Hamnett, A.; Trevellick, P. R. Electrochemistry and photoelectrochemistry of iron(III) oxide. J. Chem. Soc,. Faraday. Trans. 1. 1983, 79, 2027.
53. Ahn, H.; Kwak, M.; Lee, J.; Yoon, K.; Jang, J. Nanoporous hematite structures to overcome short diffusion lengths in water splitting. J. Mater. Chem. A. 2014, 2, 19999-20003.
54. Dotan, H.; Mathews, N.; Hisatomi, T.; Grätzel, M.; Rothschild, A. On the solar to hydrogen conversion efficiency of photoelectrodes for water splitting. J. Phys. Chem. Lett. 2014, 5, 3330-4.
55. Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S. C. Probing the photoelectrochemical properties of hematite (α-Fe2O3 ) electrodes using hydrogen peroxide as a hole scavenger. Energy. Environ. Sci. 2011, 4, 958-64.
56. Morrish, R.; Rahman, M.; MacElroy, J. M.; Wolden, C. A. Activation of hematite nanorod arrays for photoelectrochemical water splitting. ChemSusChem 2011, 4, 474-9.
57. Zhao, X.; Feng, J.; Wang, N.; et al. The Influence of Ti doping on morphology and photoelectrochemical properties of hematite grown from aqueous solution for water splitting. Energy. Tech. 2018, 6, 2188-99.
58. Mazzaro, R.; Boscolo, B. S.; Natali, M.; et al. Hematite nanostructures: an old material for a new story. Simultaneous photoelectrochemical oxidation of benzylamine and hydrogen production through Ti doping. Nano. Energy. 2019, 61, 36-46.
59. Deng, J.; Zhong, J.; Pu, A.; et al. Ti-doped hematite nanostructures for solar water splitting with high efficiency. J. Appl. Phys. 2012, 112, 084312.
60. Li, L.; Zhang, H.; Liu, C.; Liang, P.; Mitsuzaki, N.; Chen, Z. The effect of annealing regime and electrodeposition time on morphology and photoelecrochemical performance of hematite converted from nanosheet γ-FeOOH. J. Photochem. Photobiol. A. Chem. 2019, 369, 8-15.
61. Kay, A.; Cesar, I.; Grätzel, M. New benchmark for water photooxidation by nanostructured alpha-Fe2O3 films. J. Am. Chem. Soc. 2006, 128, 15714-21.
62. Iordanova, N.; Dupuis, M.; Rosso, K. M. Charge transport in metal oxides: a theoretical study of hematite alpha-Fe2O3. J. Chem. Phys. 2005, 122, 144305.
63. Zhang, H.; He, Y.; Bao, X.; et al. Fabrication of hematite photoanode consisting of (110)-oriented single crystals. ChemSusChem 2023, 16, e202300666.
64. Suryanarayana, C.; Norton, M. G. X-ray diffraction. 1th ed. Boston, MA: Springer US; 1998. Available from: http://link.springer.com/10.1007/978-1-4899-0148-4. [Last accessed on 21 Mar 2025].
65. Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta. Metall. 1953, 1, 22-31.
66. Maabong, K.; Machatine, A. G.; Mwankemwa, B. S.; et al. Nanostructured hematite thin films for photoelectrochemical water splitting. Physica. B. 2018, 535, 67-71.
67. Apolinário, A.; Lopes, T.; Costa, C.; Araújo, J. P.; Mendes, A. M. Multilayered WO3 nanoplatelets for efficient photoelectrochemical water splitting: the role of the annealing ramp. ACS. Appl. Energy. Mater. 2019, 2, 1040-50.
68. Oliveira, G.; Machado, P.; Pires, A.; Pereira, A.; Araújo, J.; Lopes, A. Magnetocaloric effect and refrigerant capacity in polycrystalline YCrO3. J. Phys. Chem. Solids. 2016, 91, 182-8.
69. Proenca, M. P.; Sousa, C. T.; Pereira, A. M.; et al. Size and surface effects on the magnetic properties of NiO nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 9561-7.
70. Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. A. 1976, 32, 751-67.
71. Bhandary, N.; Singh, A. P.; Ingole, P. P.; Basu, S. Enhanced photoelectrochemical performance of electrodeposited hematite films decorated with nanostructured NiMnOx. RSC. Adv. 2016, 6, 35239-47.
72. Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 2013, 15, 8166.
73. Sarma, S. K.; Mohan, R.; Shukla, A. Structural, opto-electronic and photoelectrochemical properties of tin doped hematite nanoparticles for water splitting. Mater. Sci. Semicond. Process. 2020, 108, 104873.
74. Shirley, D. A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B. 1972, 5, 4709-14.
75. Zhang, J.; Liu, X.; Wang, L.; et al. Synthesis and gas sensing properties of α-Fe2O3@ZnO core-shell nanospindles. Nanotechnology 2011, 22, 185501.
76. Fu, Y.; Wang, R.; Xu, J.; et al. Synthesis of large arrays of aligned α-Fe2O3 nanowires. Chem. Phys. Lett. 2003, 379, 373-9.
77. Kang, M. J.; Yu, H.; Lee, W.; Cha, H. G. Efficient Fe2O3/C-g-C3N4 Z-scheme heterojunction photocatalyst prepared by facile one-step carbonizing process. J. Phys. Chem. Solids. 2019, 130, 93-9.
78. Biesinger, M. C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681.
79. Grosvenor, A. P.; Kobe, B. A.; Biesinger, M. C.; Mcintyre, N. S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface. Anal. 2004, 36, 1564-74.
80. Hao, S.; Wang, H.; Yang, R.; et al. Corn-like mesoporous SnO2/α-Fe2O3 heterostructure for superior TEA sensing performance. Appl. Phys. A. 2021, 127, 4350.
81. Sun, L.; Wu, W.; Yang, S.; et al. Template and silica interlayer tailorable synthesis of spindle-like multilayer α-Fe2O3/Ag/SnO2 ternary hybrid architectures and their enhanced photocatalytic activity. ACS. Appl. Mater. Interfaces. 2014, 6, 1113-24.
82. Biesinger, M. C.; Payne, B. P.; Grosvenor, A. P.; Lau, L. W.; Gerson, A. R.; Smart, R. S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717-30.
83. Payne, B.; Biesinger, M.; Mcintyre, N. X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces. J. Electron. Spectrosc. Relat. Phenom. 2011, 184, 29-37.
84. Wang, L.; Nguyen, N. T.; Zhang, Y.; Bi, Y.; Schmuki, P. Enhanced solar water splitting by swift charge separation in Au/FeOOH sandwiched single-crystalline Fe2O3 nanoflake photoelectrodes. ChemSusChem 2017, 10, 2720-7.
85. Tian, C. M.; Li, W.; Lin, Y. M.; et al. Electronic structure, optical properties, and photoelectrochemical activity of Sn-doped Fe2O3 thin films. J. Phys. Chem. C. 2020, 124, 12548-58.
86. Wang, J.; Perry, N. H.; Guo, L.; Vayssieres, L.; Tuller, H. L. On the theoretical and experimental control of defect chemistry and electrical and photoelectrochemical properties of hematite nanostructures. ACS. Appl. Mater. Interfaces. 2019, 11, 2031-41.
87. Ye, F.; Zhao, B.; Ran, R.; Shao, Z. Facile mechanochemical synthesis of nano SnO2/graphene composite from coarse metallic Sn and graphite oxide: an outstanding anode material for lithium-ion batteries. Chem. Eur. J. 2014, 20, 4055-63.
88. Mancipe, S.; Martínez, J. J.; Pinzón, C.; Rojas, H.; Solis, D.; Gómez, R. Effective photocatalytic degradation of Rhodamine B using tin semiconductors over hydrotalcite-type materials under sunlight driven. Catal. Today. 2021, 372, 191-7.
89. Baggetto, L.; Ganesh, P.; Meisner, R. P.; et al. Characterization of sodium ion electrochemical reaction with tin anodes: experiment and theory. J. Power. Sources. 2013, 234, 48-59.
90. Lee, M. H.; Park, J. H.; Han, H. S.; et al. Nanostructured Ti-doped hematite (α-Fe2O3) photoanodes for efficient photoelectrochemical water oxidation. Int. J. Hydrogen. Energy. 2014, 39, 17501-7.
91. Chae, S. Y.; Rahman, G.; Joo, O. Elucidation of the structural and charge separation properties of titanium-doped hematite films deposited by electrospray method for photoelectrochemical water oxidation. Electrochim. Acta. 2019, 297, 784-93.
92. Lian, X.; Yang, X.; Liu, S.; et al. Enhanced photoelectrochemical performance of Ti-doped hematite thin films prepared by the sol-gel method. App. Surf. Sci. 2012, 258, 2307-11.
93. Niu, Y.; Zhou, Y.; Niu, P.; Shen, H.; Ma, Y. Effects of Ti doping on hematite photoanodes: more surface states. J. Nanosci. Nanotechnol. 2019, 19, 3437-46.
94. Barradas, N. P. Rutherford backscattering analysis of thin films and superlattices with roughness. J. Phys. D. Appl. Phys. 2001, 34, 2109-16.
95. Barradas, N. P.; García, N. C.; Redondo-cubero, A.; Shen, G.; Kung, P.; Pau, J. Analytical simulation of RBS spectra of nanowire samples. Nucl. Instrum. Methods. Phys. Res. B. 2016, 371, 116-20.
96. Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water oxidation at hematite photoelectrodes: the role of surface states. J. Am. Chem. Soc. 2012, 134, 4294-302.
97. Jrad, F.; Naceur, J. B.; Ouertani, R.; Chtourou, R. Photo-electrochemical impedance spectroscopy analysis of hydrothermally synthesized β-In2S3 thin film photo-anodes. Physica. E. 2019, 114, 113585.
98. Katsuki, T.; Zahran, Z. N.; Tanaka, K.; et al. Facile fabrication of a highly crystalline and well-interconnected hematite nanoparticle photoanode for efficient visible-light-driven water oxidation. ACS. Appl. Mater. Interfaces. 2021, 13, 39282-90.
99. Peerakiatkhajohn, P.; Yun, J. H.; Chen, H.; Lyu, M.; Butburee, T.; Wang, L. Stable hematite nanosheet photoanodes for enhanced photoelectrochemical water splitting. Adv. Mater. 2016, 28, 6405-10.
100. Liu, G.; Karuturi, S. K.; Chen, H.; et al. Tuning the morphology and structure of disordered hematite photoanodes for improved water oxidation: a physical and chemical synergistic approach. Nano. Energy. 2018, 53, 745-52.
101. Qin, D.; Li, Y.; Wang, T.; et al. Sn-doped hematite films as photoanodes for efficient photoelectrochemical water oxidation. J. Mater. Chem. A. 2015, 3, 6751-5.
102. Bak, A.; Choi, W.; Park, H. Enhancing the photoelectrochemical performance of hematite (α-Fe2O3) electrodes by cadmium incorporation. Appl. Catal. B. Environ. 2011, 110, 207-15.
103. Jha, B. K.; Chaule, S.; Jang, J. Enhancing photocatalytic efficiency with hematite photoanodes: principles, properties, and strategies for surface, bulk, and interface charge transfer improvement. Mater. Chem. Front. 2024, 8, 2197-226.
104. Yoon, K.; Park, J.; Lee, H.; et al. Unveiling the role of the ti dopant and viable Si doping of hematite for practically efficient solar water splitting. ACS. Catal. 2022, 12, 5112-22.
105. Liu, H.; Fan, X.; Li, Y.; Guo, H.; Jiang, W.; Liu, G. Hematite-based photoanodes for photoelectrochemical water splitting: performance, understanding, and possibilities. J. Environ. Chem. Eng. 2023, 11, 109224.
106. Jeon, T. H.; Moon, G.; Park, H.; Choi, W. Ultra-efficient and durable photoelectrochemical water oxidation using elaborately designed hematite nanorod arrays. Nano. Energy. 2017, 39, 211-8.
107. Iandolo, B.; Zhang, H.; Wickman, B.; Zorić, I.; Conibeer, G.; Hellman, A. Correlating flat band and onset potentials for solar water splitting on model hematite photoanodes. RSC. Adv. 2015, 5, 61021-30.
108. Toroker, M. C. Theoretical insights into the mechanism of water oxidation on nonstoichiometric and titanium-doped Fe2O3(0001). J. Phys. Chem. C. 2014, 118, 23162-7.
109. Kamimura, J.; Bogdanoff, P.; Abdi, F. F.; et al. Photoelectrochemical properties of GaN photoanodes with cobalt phosphate catalyst for solar water splitting in neutral electrolyte. J. Phys. Chem. C. 2017, 121, 12540-5.
110. Bartesaghi, D.; Pérez, I. C.; Kniepert, J.; et al. Competition between recombination and extraction of free charges determines the fill factor of organic solar cells. Nat. Commun. 2015, 6, 7083.
111. Dias, P.; Lopes, T.; Meda, L.; Andrade, L.; Mendes, A. Photoelectrochemical water splitting using WO3 photoanodes: the substrate and temperature roles. Phys. Chem. Chem. Phys. 2016, 18, 5232-43.
112. Krol, R.; Grätzel, M. Photoelectrochemical hydrogen production. 1th ed. New York: Springer; 2012. Available from: https://link.springer.com/book/10.1007/978-1-4614-1380-6. [Last accessed on 21 Mar 2025].
113. Yu, Q.; Meng, X.; Wang, T.; Li, P.; Ye, J. Hematite films decorated with nanostructured ferric oxyhydroxide as photoanodes for efficient and stable photoelectrochemical water splitting. Adv. Funct. Materials. 2015, 25, 2686-92.
114. Li, C.; Li, A.; Luo, Z.; et al. Surviving high-temperature calcination: ZrO2-induced hematite nanotubes for photoelectrochemical water oxidation. Angew. Chem. Int. Ed. 2017, 56, 4150-5.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].