Engineering hydrogel-based conformal epidermal electrodes for human-machine interaction
Abstract
Hydrogels, with their skin-like physicochemical properties, offer great potential as flexible electrodes for electrophysiological interfaces and high-quality biosignal acquisition in human-machine interaction systems. However, traditional hydrogel electrodes often face issues such as mechanical mismatch with skin, low electrical conductivity, and poor adhesion, which hinder stable biosignal acquisition with high signal-to-noise ratio. To address these challenges, optimizing conductivity, compliance, and adhesion is crucial. This perspective reviews recent advancements in hydrogel electrode optimization, materials design, interface engineering, human-machine interaction applications, and future directions for hydrogel-based biointerfaces.
Keywords
INTRODUCTION
With the rapid advance of flexible electronics, wearable sensors that form high-performance interfaces with human skin are gradually replacing conventional rigid devices and have become indispensable in health monitoring[1-3], active rehabilitation[4,5], and intelligent human-machine interaction (HMI)[6-8]. Among the various soft electronic materials under development, hydrogels stand out because their physicochemical characteristics closely resemble those of human skin[9-14]. When used as epidermal electrodes, hydrogel devices can record diverse biosignals - such as electroencephalograms (EEG) and surface electromyograms (sEMG) - and supply them as high-value inputs to HMI systems[15,16].
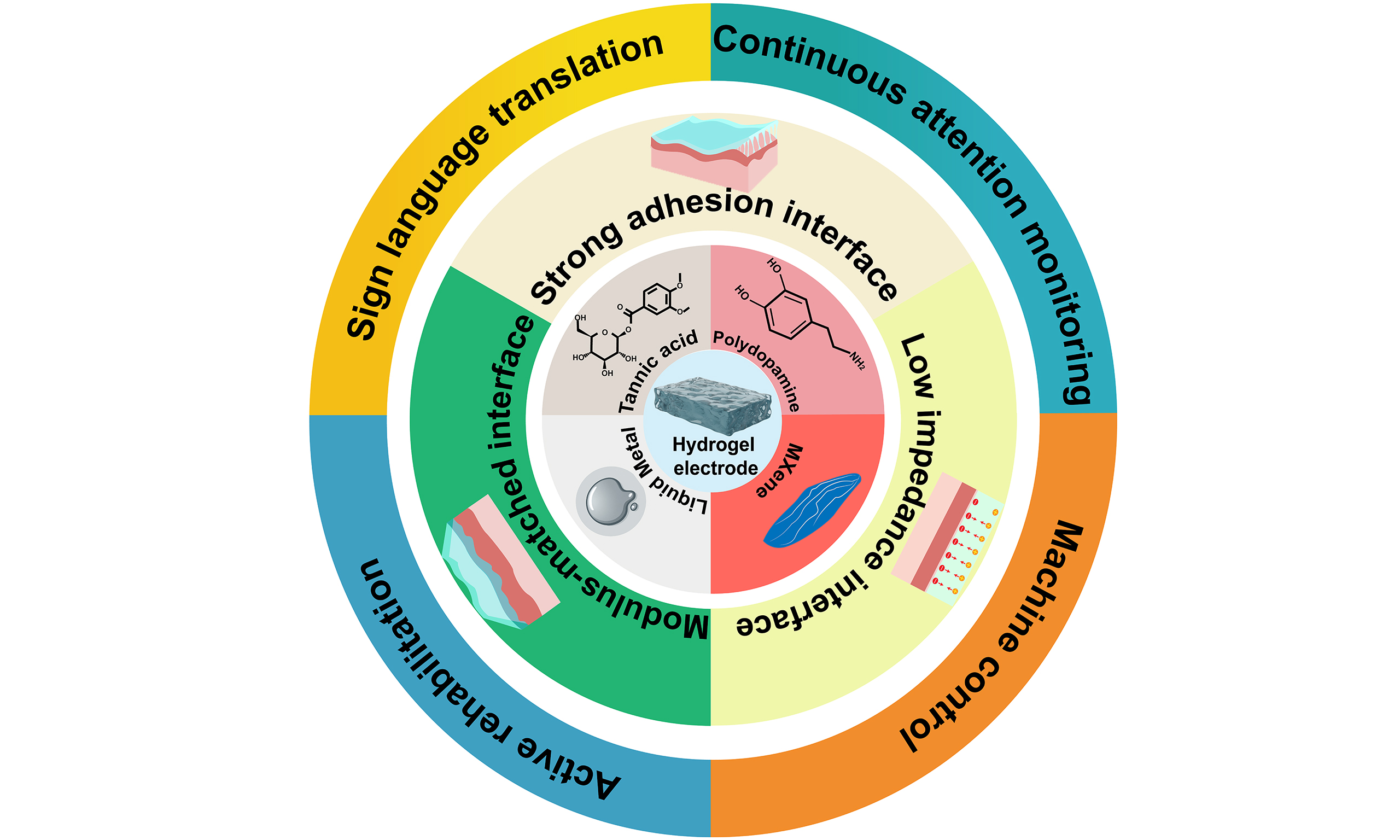
High-performance HMI platforms rely on electrophysiological signals with a high signal-to-noise ratio (SNR)[17]. Achieving such quality depends on a low-impedance hydrogel-skin interface that minimizes signal attenuation and external interference[18]. Excellent electrical conductivity of hydrogel electrodes is demanded to support efficient signal transmission. Simultaneously, sufficient mechanical flexibility is required to conform intimately to the microtopography of the skin[19]. Moreover, strong adhesive properties are necessary to prevent electrode displacement during movement and enable stable and continuous biosignal monitoring [Figure 1A][20]. However, conventional hydrogels often suffer from poor conductivity, weak adhesion, and mechanical mismatch with skin, compromising signal quality[19]. Therefore, next-generation hydrogel electrodes must be engineered with conductive additives, ions, adhesion enhancers, and multidimensional structural optimizations to ensure long-term, stable collection of high-quality physiological data for advanced HMI applications.
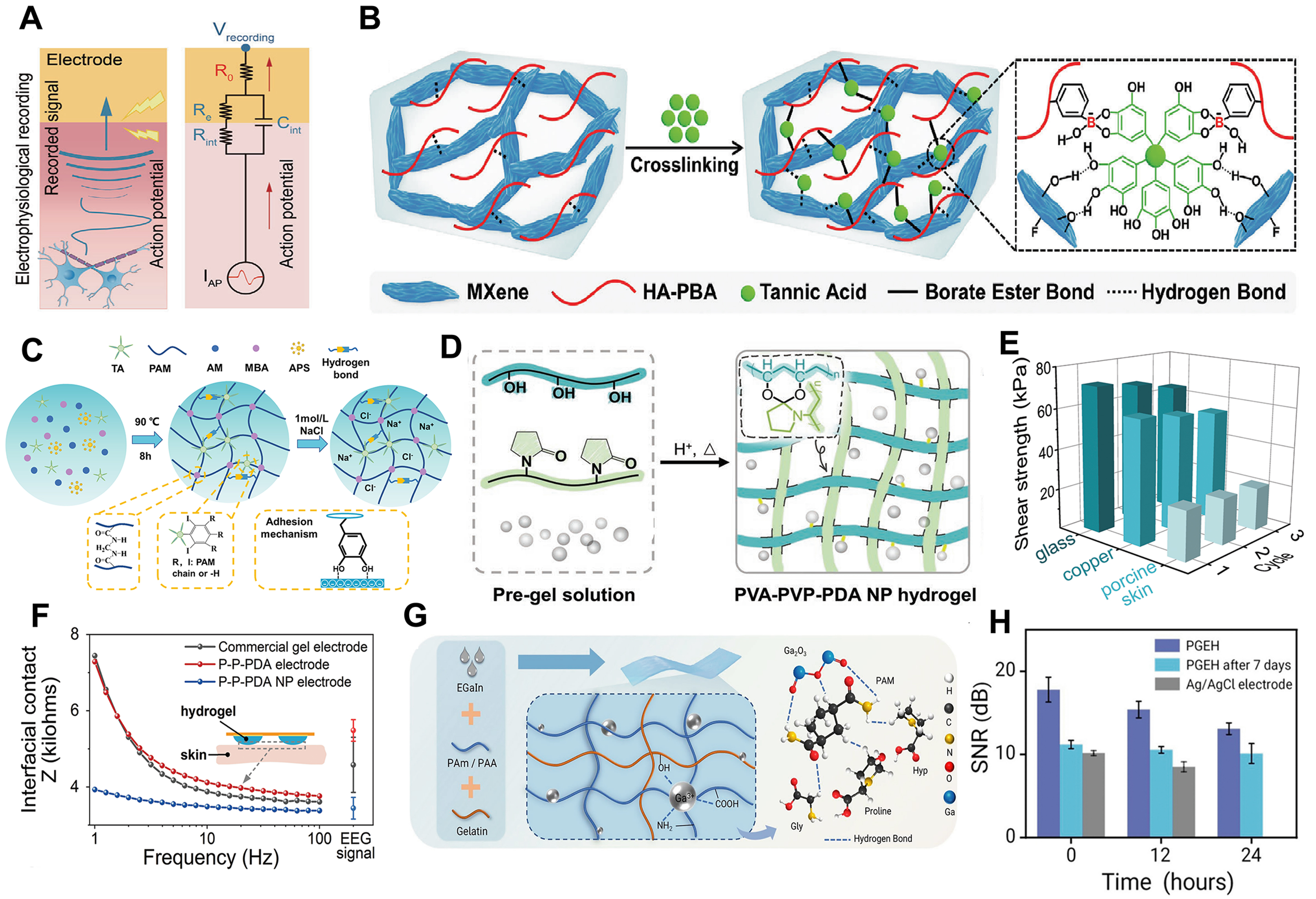
Figure 1. (A) Schematic illustration of a high-performance hydrogel-skin interface[20]. Reprinted with permission. Copyright 2025, John Wiley & Sons; (B) Fabrication schematic of MXene-HA-PBA-TA-PAM hydrogel. Reprinted with permission[24]. Copyright 2024, John Wiley & Sons; (C) Preparation process of TA-NaCl-PAM hydrogel. Reprinted with permission[17]. Copyright 2023, John Wiley & Sons; (D) Schematic illustration of PVA-PVP-PDA NP hydrogel preparation. Reprinted with permission[19]. Copyright 2023, John Wiley & Sons; (E) Repeatable adhesion behavior of the PVA-PVP-PDA NP hydrogel on porcine skin, copper, and glass surfaces[19]. Reprinted with permission. Copyright 2023, John Wiley & Sons; (F) Interfacial impedance comparison among commercial gel electrodes, PVA-PVP-PDA hydrogel electrodes, and PDA NP-loaded PVA-PVP hydrogels over 1-100 Hz[19]. Reprinted with permission. Copyright 2023, John Wiley & Sons; (G) Schematic of the preparation process for the PGEH[25]. Reprinted with permission. Copyright 2025, Springer Nature; (H) Comparison of SNR among the three electrodes during long-term EMG monitoring[25]. Reprinted with permission. Copyright 2025, Springer Nature. HA-PBA: Phenylboronic acid-grafted hyaluronic acid; TA: tannic acid; PAM: polyacrylamide; PVA: polyvinyl alcohol; PVP: polyvinylpyrrolidone; PDA: polydopamine; NP: nanoparticle; PGEH: polyacrylamide/gelatin/EGaIn hydrogel; SNR: signal-to-noise ratio; EMG: electromyograms; EEG: electroencephalograms; AM: acrylamide; MBA: N,N’-methylenebisacrylamide; APS: ammonium persulfate.
PERFORMANCE OPTIMIZATION STRATEGIES AND INTERFACE ENGINEERING OF HYDROGEL ELECTRODES
The three-dimensional network of hydrogels offers intrinsic porosity for incorporating functional fillers, enabling optimization of conductivity, adhesion, and mechanical compliance to enhance skin-electrode interfaces[21,22]. Common strategies include adding fillers such as tannic acid (TA) and MXene, which improve both conductivity and adhesion, ensuring a low-impedance and stable skin-electrode interface[23]. For example, Wan et al. integrated MXene nanosheets into a self-healing hydrogel made of TA-phenylboronic acid-grafted hyaluronic acid (HA-PBA) [Figure 1B][24], resulting in a stable skin interface that supports multimodal biosignal acquisition and additional functions such as ultraviolet (UV) protection and photothermal therapy via MXene’s photothermal conversion ability. Furthermore, Wu et al. introduced TA into a polyacrylamide (PAM) hydrogel and immersed it in sodium chloride (NaCl) solution, enhancing ionic conductivity and enabling high-performance signal acquisition from hairy skin surfaces [Figure
Table 1 summarizes key performance metrics and applications of these optimized hydrogel electrodes, highlighting their potential for future interface engineering and multifunctional integration, with higher SNR than commercial electrodes.
Summary of performance and applications of hydrogel electrodes in recent studies
| Materials | Conductivity (S·m-1) | Adhesiveness (kPa) | Stretchability (strain) | Modulus (kPa) | SNR (dB) | Application |
| CMC-PDA-PEDOT:PSS-PA hydrogel[26] | 0.08 | 6 | 400% | 100 | 30 vs. 30a) | sEMG |
| PVA-PEI-CaCl2 hydrogel[27] | 0.6 | 10 | 1,400% | 15 | 8.5 vs. 6.3 | sEMG, EEG |
| AgNPs/MXene/GG/Alg-PBA[23] | 0.005 | NAb) | 250% | NAb) | 16 vs. 10a) | sEMG, ECG |
| fCNT/TA/PVA/PAA[28] | 40 | 49 | 1,000% | 100 | NAb) | sEMG |
| AgNW hydrogel[29] | 1 | NAb) | 200% | NAb) | NAb) | sEMG |
| SOH hydrogel[30] | 0.8 | NAb) | 250% | NAb) | 30 vs. 30a) | sEMG |
| PVA-PVP-PEDOT:PSS hydrogel[31] | 0.6 | NAb) | 150% | 8 | 15 vs. 9a) | sEMG, ECG |
| AgNPs/MXene/GG/Alg-PBA hydrogel[32] | 0.02 | 19 | 200% | 2,000 | 17.5 | ECG |
| DAT[33] | 0.8 | 19 | 1,200% | 20 | 21 vs. 15a) | sEMG |
DEVELOPMENT AND APPLICATION OF INTELLIGENT HMI SYSTEMS BASED ON HYDROGEL ELECTRODES
Optimized hydrogel electrodes enable low-impedance skin-electrode interfaces, facilitating the acquisition of high SNR physiological signals, making them ideal for various HMI applications. These electrodes are integrated into signal acquisition systems and combined with neural networks for real-time signal classification, supporting applications such as sign language recognition, active rehabilitation, attention monitoring, and remote health management.
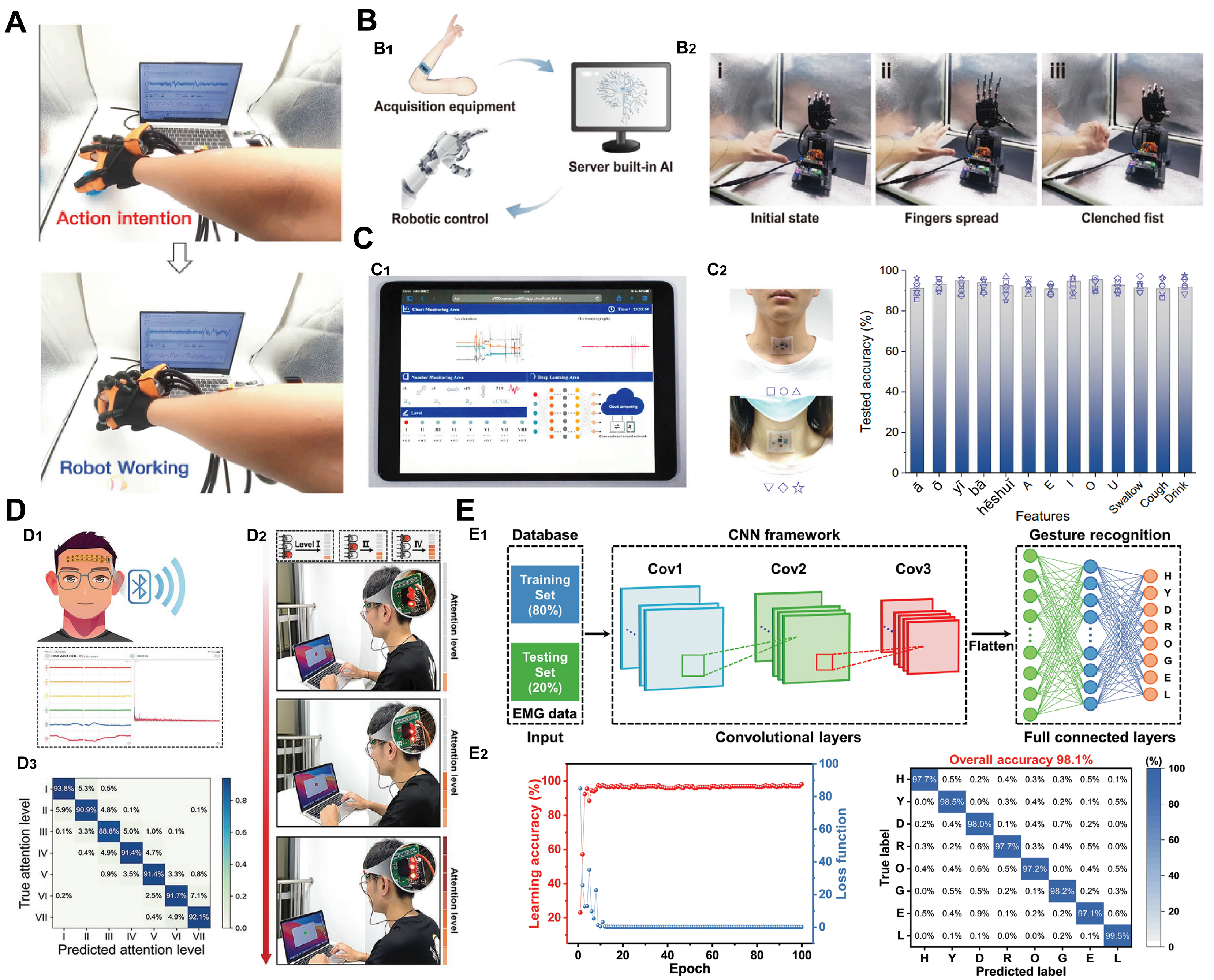
For example, Wu et al. developed a dual-modality sensing platform using sEMG hydrogel electrodes and foam-based pressure sensors, enabling real-time hand gesture classification and driving a rehabilitation glove for stroke patients [Figure 2A][17]. Xie et al. designed hydrogel electrodes with antibacterial properties, which, when integrated into a dual-channel sEMG system, enabled real-time prosthetic hand control through AI-based recognition [Figure 2B][34]. Gao et al. used Ag nanowire-modified hydrogel electrodes in a stretchable throat patch, combined with an accelerometer, to classify vocalization and swallowing, supporting remote monitoring and rehabilitation [Figure 2C][29]. Han et al. incorporated high-adhesion hydrogel electrodes into EEG acquisition circuits for real-time attention state classification, demonstrating potential for brain-machine interfaces [Figure 2D][19]. Wan et al. used MXene-modified hydrogel electrodes to collect sEMG signals for sign language translation, enabling communication between hearing-impaired individuals and the public [Figure 2E][24].
Figure 2. (A) Real-time active rehabilitation system based on dual-modality sensing of muscle force and sEMG[17]. Reprinted with permission. Copyright 2023, John Wiley & Sons; (B) Antibacterial hydrogel electrodes for prosthetic control in HMI applications: B1 System operation schematic; B2 Real-time prosthetic hand control via dual-channel sEMG signals[34]. Reprinted with permission. Copyright 2025, American Chemical Society; (C) Throat motion monitoring and rehabilitation using sEMG and accelerometer-based dual-modality sensing: C1 Cloud-based interface for real-time throat motion tracking; C2 Classification accuracy of 13 throat movements from two subjects[29]. Reprinted with permission. Copyright 2023, Springer Nature; (D) Continuous attention level assessment system: D1 Schematic of wireless EEG acquisition system; D2 Confusion matrix of attention classification results; D3 Real-time system interface for sustained attention evaluation[19]. Reprinted with permission. Copyright 2023, John Wiley & Sons; (E) Machine learning-assisted sign language recognition based on sEMG: E1 CNN-based algorithm framework for sign language translation; E2 Post-training accuracy/loss curves and confusion matrix for eight sign gestures[24]. Reprinted with permission. Copyright 2024, John Wiley & Sons. HMI: Human-machine interaction; sEMG: surface electromyograms; EEG: electroencephalograms; CNN: convolutional neural network.
CHALLENGE AND PERSPECTIVES
Despite significant advances in hydrogel electrode materials and device integration for HMI applications, several challenges hinder their large-scale and long-term deployment:
First, Hydrogel electrodes are prone to dehydration due to temperature and humidity fluctuations, which reduces conductivity and increases impedance, compromising signal stability. While strategies such as hydrogen bonding networks and glycerol incorporation help with water retention, achieving week-long stability without losing flexibility or conductivity remains a challenge[35]. Optimizing the crosslinking method to enhance the hydrogel’s three-dimensional network could improve water absorption and dehydration resistance. Additionally, mechanical deformation and contamination can damage the hydrogel, further degrading signal quality and device lifespan.
Second, Most hydrogel electrodes target a single electrophysiological signal (e.g., sEMG), requiring additional sensors for multimodal HMI, thus increasing size, cost, and complexity[36]. Advances in multimodal epidermal sensors, circuit design, and data fusion algorithms could allow hydrogel electrodes to measure multiple signals (e.g., sEMG, strain, pressure, or temperature) simultaneously on the same platform. Innovations in structural and circuit design can enable integrated physiological signal sensing.
Next, electromagnetic interference (EMI) can disrupt signal acquisition, especially at high frequencies, affecting signal stability and the SNR[37-40]. Hydrogel electrodes must have sufficient EMI resistance. Using liquid metal for EMI shielding is common, but its efficiency needs improvement in specific frequency ranges[41]. Designing gradient or porous structures could enhance shielding in targeted directions or achieve absorption-based shielding.
Finally, due to their excellent biocompatibility, hydrogel electrodes hold great potential for human rehabilitation applications. In future research, they could serve as interface materials for brain-computer interfaces, sensing brain electrical signals to enable human control of external machinery, thus helping patients who are unable to move independently regain mobility.
DECLARATIONS
Authors’ contributions
Xiao, M., Zhang, X., and Luo, Y. contributed equally to this work.
Wrote the original draft: Xiao, M.; Zhang, X.; Luo, Y.
Supervised, reviewed, and revised the manuscript: Xie, R.; Tao, K.; Wu, J.
Availability of data and materials
Not applicable.
Financial support and sponsorship
The authors acknowledge financial support from the National Natural Science Foundation of China (22475243), the Foundation of the state key Laboratory of Transducer Technology (No. SKT2301), the Open Project Program of Fujian Key Laboratory of Special Intelligent Equipment Measurement and Control, Fujian Special Equipment Inspection and Research Institute, China (No. FJIES2024KF02).
Conflicts of interest
Wu, J. is a Guest Editor of the journal Soft Science. Wu, J. was not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling and decision making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Mahato, K.; Saha, T.; Ding, S.; Sandhu, S. S.; Chang, A.; Wang, J. Hybrid multimodal wearable sensors for comprehensive health monitoring. Nat. Electron. 2024, 7, 735-50.
2. Walter, J. R.; Xu, S.; Rogers, J. A. From lab to life: how wearable devices can improve health equity. Nat. Commun. 2024, 15, 44634.
3. Someya, T.; Bao, Z.; Malliaras, G. G. The rise of plastic bioelectronics. Nature 2016, 540, 379-85.
4. Cai, C.; Liu, T.; Meng, X.; et al. Lightweight and mechanically robust cellulosic triboelectric materials for wearable self-powered rehabilitation training. ACS. Nano. 2025, 19, 396-405.
5. Lee, H.; Lee, S.; Kim, J.; et al. Stretchable array electromyography sensor with graph neural network for static and dynamic gestures recognition system. npj. Flex. Electron. 2023, 7, 246.
6. Xia, H.; Zhang, Y.; Rajabi, N.; et al. Shaping high-performance wearable robots for human motor and sensory reconstruction and enhancement. Nat. Commun. 2024, 15, 1760.
7. Xu, R.; She, M.; Liu, J.; et al. Skin-friendly and wearable iontronic touch panel for virtual-real handwriting interaction. ACS. Nano. 2023, 17, 8293-302.
8. Sun, D.; Feng, Y.; Sun, S.; et al. Transparent, self-adhesive, conductive organohydrogels with fast gelation from lignin-based self-catalytic system for extreme environment-resistant triboelectric nanogenerators. Adv. Funct. Mater. 2022, 32, 2201335.
9. Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Progress of research on conductive hydrogels in flexible wearable sensors. Gels 2024, 10, 144.
10. Kim, J.; Kim, Y.; Lee, J.; Shin, M.; Son, D. Wearable liquid metal composite with skin-adhesive chitosan-alginate-chitosan hydrogel for stable electromyogram signal monitoring. Polymers 2023, 15, 3692.
11. Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable sensors-enabled human-machine interaction systems: from design to application. Adv. Funct. Mater. 2021, 31, 2008936.
12. Dang, C.; Zhang, F.; Li, Y.; et al. Lithium bonds enable small biomass molecule-based ionoelastomers with multiple functions for soft intelligent electronics. Small 2022, 18, e2200421.
13. Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-strength, tough, and self-healing nanocomposite physical hydrogels based on the synergistic effects of dynamic hydrogen bond and dual coordination bonds. ACS. Appl. Mater. Interfaces. 2017, 9, 28305-18.
14. Shao, C.; Meng, L.; Wang, M.; et al. Mimicking dynamic adhesiveness and strain-stiffening behavior of biological tissues in tough and self-healable cellulose nanocomposite hydrogels. ACS. Appl. Mater. Interfaces. 2019, 11, 5885-95.
15. Yang, S.; Cheng, J.; Shang, J.; et al. Stretchable surface electromyography electrode array patch for tendon location and muscle injury prevention. Nat. Commun. 2023, 14, 6494.
16. Liang, Q.; Xia, X.; Sun, X.; et al. Highly stretchable hydrogels as wearable and implantable sensors for recording physiological and brain neural signals. Adv. Sci. 2022, 9, e2201059.
17. Wang, H.; Ding, Q.; Luo, Y.; et al. High-performance hydrogel sensors enabled multimodal and accurate human-machine interaction system for active rehabilitation. Adv. Mater. 2024, 36, e2309868.
18. Chang, Y.; Wang, L.; Li, R.; et al. First decade of interfacial iontronic sensing: from droplet sensors to artificial skins. Adv. Mater. 2021, 33, e2003464.
19. Han, Q.; Zhang, C.; Guo, T.; et al. Hydrogel nanoarchitectonics of a flexible and self-adhesive electrode for long-term wireless electroencephalogram recording and high-accuracy sustained attention evaluation. Adv. Mater. 2023, 35, e2209606.
20. Lu, J.; Li, Q.; Huang, Q.; et al. A highly sensitive surface electrode for electrophysiological monitoring. Adv. Funct. Mater. 2025, 35, 2421132.
21. Liu, S.; Rao, Y.; Jang, H.; Tan, P.; Lu, N. Strategies for body-conformable electronics. Matter 2022, 5, 1104-36.
22. Lu, Y.; Yang, G.; Wang, S.; et al. Stretchable graphene-hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2024, 7, 51-65.
23. Li, M.; Zhang, Y.; Lian, L.; et al. Flexible accelerated-wound-healing antibacterial mxene-based epidermic sensor for intelligent wearable human-machine interaction. Adv. Funct. Mater. 2022, 32, 2208141.
24. Wang, W.; Zhou, H.; Xu, Z.; Li, Z.; Zhang, L.; Wan, P. Flexible conformally bioadhesive MXene hydrogel electronics for machine learning-facilitated human-interactive sensing. Adv. Mater. 2024, 36, e2401035.
25. Zheng, K.; Zheng, C.; Zhu, L.; et al. Machine learning enabled reusable adhesion, entangled network-based hydrogel for long-term, High-Fidelity EEG Recording and Attention Assessment. Nanomicro. Lett. 2025, 17, 281.
26. Huang, X.; Chen, C.; Ma, X.; et al.
27. Liu, Y.; Wang, C.; Xue, J.; et al. Body temperature enhanced adhesive, antibacterial, and recyclable ionic hydrogel for epidermal electrophysiological monitoring. Adv. Healthc. Mater. 2022, 11, e2200653.
28. Park, J.; Kim, J. Y.; Heo, J. H.; et al. Intrinsically nonswellable multifunctional hydrogel with dynamic nanoconfinement networks for robust tissue-adaptable bioelectronics. Adv. Sci. 2023, 10, e2207237.
29. Xu, H.; Zheng, W.; Zhang, Y.; et al. A fully integrated, standalone stretchable device platform with in-sensor adaptive machine learning for rehabilitation. Nat. Commun. 2023, 14, 7769.
30. Wu, J.; Xian, J.; He, C.; Lin, H.; Li, J.; Li, F. Asymmetric wettability hydrogel surfaces for enduring electromyographic monitoring. Adv. Mater. 2024, 36, e2405372.
31. Chen, J. X. M.; Chen, T.; Zhang, Y.; et al. Conductive bio-based hydrogel for wearable electrodes via direct ink writing on skin. Adv. Funct. Mater. 2024, 34, 2403721.
32. Bai, Z.; Wang, X.; Huang, M.; et al. Smart battery-free and wireless bioelectronic platform based on a nature-skin-derived organohydrogel for chronic wound diagnosis, assessment, and accelerated healing. Nano. Energy. 2023, 118, 108989.
33. Ye, Y.; Guo, J.; Wang, A.; et al. Starfish tube feet inspired hydrogel electrode for durable underwater sEMG acquisition. Chem. Eng. J. 2024, 496, 153882.
34. Sun, Y.; Xiao, M.; Tang, Z.; et al. Preparation of active on-demand antibacterial hydrogel epidermis electrodes based on flora balance strategy for intelligent prostheses. ACS. Appl. Mater. Interfaces. 2025, 17, 37231-42.
35. Wang, W.; Chen, F.; Fang, L.; Li, Z.; Xie, Z. Reversibly stretchable organohydrogel-based soft electronics with robust and redox-active interfaces enabled by polyphenol-incorporated double networks. ACS. Appl. Mater. Interfaces. 2022, 14, 12583-95.
36. Cai, P.; Wan, C.; Pan, L.; et al. Locally coupled electromechanical interfaces based on cytoadhesion-inspired hybrids to identify muscular excitation-contraction signatures. Nat. Commun. 2020, 11, 2183.
37. Park, S. Y.; Choi, S. J.; Kim, J. C.; Joe, D. J.; Lee, H. E. Self-healable and conductive hydrogel nanocomposite with high environmental stability for electromagnetic-interference-free electrocardiography patches. Energy. Environ. Mater. , e70039.
38. Wang, D.; Xue, H.; Xia, L.; et al. A tough semi-dry hydrogel electrode with anti-bacterial properties for long-term repeatable non-invasive EEG acquisition. Microsyst. Nanoeng. 2025, 11, 105.
39. Guo, J.; Zhang, T.; Hao, X.; et al. Aramid nanofiber/MXene-reinforced polyelectrolyte hydrogels for absorption-dominated electromagnetic interference shielding and wearable sensing. Nanomicro. Lett. 2025, 17, 271.
40. Li, X.; Jiang, M.; Du, Y.; et al. Self-healing liquid metal hydrogel for human-computer interaction and infrared camouflage. Mater. Horiz. 2023, 10, 2945-57.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].