Association of exposure to phenols, pesticides, and phthalates with hepatic steatosis and MASLD in adolescents: the potential role of inflammation and lifestyle factors
Abstract
Aim: Limited evidence exists on the effects of endocrine-disrupting chemical exposure on metabolic dysfunction-associated steatotic liver disease (MASLD) in adolescents. We aimed to assess the effects of multiple chemicals on the hepatic steatosis index (HSI) and MASLD in adolescents, and to further explore the potential roles of inflammation and lifestyle factors.
Methods: Associations between chemical exposures and HSI/MASLD were examined using generalized linear models, restricted cubic spline analysis, weighted quantile sum regression, and Bayesian kernel machine regression. Mediation analysis was conducted to evaluate whether inflammation mediated these relationships.
Results: Among 2,163 adolescents (median age 15 years), 490 (22.7%) were diagnosed with MASLD. Bisphenol A, mono-ethyl phthalate, mono-(carboxyoctyl) phthalate, and mono-benzyl phthalate (MBzP) were significantly associated with HSI or MASLD. Both weighted quantile sum and Bayesian kernel machine regression consistently indicated a positive correlation between chemical mixtures and MASLD, with MBzP and bisphenol A identified as key contributors. Mediation analysis showed that white blood cells partially mediated the associations of MBzP with HSI and MASLD, and of mono-(carboxynonyl) phthalate with MASLD. Sedentary behavior and physical activity further modulated the combined effects of chemical mixtures on MASLD.
Conclusion: Exposure to phenols, pesticides, and phthalates was significantly associated with HSI or MASLD, with white blood cells acting as a mediator. Reducing sedentary behavior and increasing physical activity may mitigate the adverse impacts of chemical mixtures on MASLD.
Keywords
INTRODUCTION
Metabolic dysfunction-associated steatotic liver disease (MASLD), originally known as non-alcoholic fatty liver disease, was terminologically updated to highlight the metabolic dysfunction underlying the condition[1]. As rates of childhood obesity continue to rise, MASLD has emerged as the predominant etiology of pediatric chronic liver disease[2]. Recent research has reported that MASLD affects approximately 13% of the pediatric population and 47% of children with obesity globally[3]. MASLD can progress to metabolic dysfunction-associated steatohepatitis (MASH), liver cirrhosis, and hepatocellular carcinoma, while also increasing the risk of type 2 diabetes, cardiovascular disease, and chronic kidney disease[4-6], thereby placing a significant burden on healthcare systems and societies worldwide. Excessive energy intake, insufficient physical activity, and a predisposed genetic background are well-established risk factors for MASLD[7]. However, with the rapid advances in the use of environmental chemicals, endocrine disrupting chemicals (EDCs) have been recognized as major environmental contributors to the progression of MASLD, including persistent EDCs (e.g., polychlorinated biphenyls and perfluorinated chemicals)[8], phthalates, bisphenol A (BPA), and others[9].
EDCs are a class of exogenous substances that interfere with hormone synthesis, metabolism, and/or regulation, and may have adverse effects on human health by contributing to developmental, reproductive, metabolic, and immune-related diseases[10,11]. This immunotoxicity is mechanistically exemplified by BPA, for which accumulating evidence from animal studies indicates that exposure induces elevated oxidative stress in the spleen, subsequently triggering mitochondrial dysfunction in splenocytes and ultimately contributing to cellular and tissue damage in mammalian systems[12,13]. Non-persistent EDCs, such as chlorophenols, phenols and phthalates, have become increasingly widespread and persistent due to their prolonged use in daily products over the years (e.g., food packaging, construction materials, pesticides, cleaning and personal care products)[14,15]. Their unique physicochemical properties allow them to be easily released into the environment, consequently resulting in pervasive human exposure via inhalation, ingestion, and dermal contact[14]. Notably, due to their immaturity in physiology, anatomy, behavior, and toxicokinetics, children may be particularly susceptible to the effects of EDCs[10,16]. This underscores the epidemiological importance of assessing the link between EDC exposure in the pediatric population and long-term health outcomes.
Mounting evidence increasingly points to a potential association between higher exposure to EDCs and the development of MASLD in adults[17-20]. However, few epidemiological studies have assessed the relationships between exposure to these EDCs and MASLD in children and adolescents. A single study based on the National Health and Nutrition Examination Survey (NHANES) data found that elevated levels of BPA exposure raised the likelihood of MASLD among Hispanic adolescents[21]. Additionally, some evidence suggests a link between prenatal or childhood exposure to EDCs and an increased risk of liver injury in children and adolescents[22,23]. Over the past few years, an expanding body of research has emphasized the importance of considering the complex exposure patterns, significant correlations, and synergistic interactions among environmental chemicals[20,22,24]. Given that the body is unavoidably exposed to numerous chemicals simultaneously, this can result in additive, synergistic, or antagonistic interactions among concurrently administered chemicals[25]. Consequently, it is essential to implement novel analytical strategies to elucidate the correlations between various EDC exposures and MASLD in adolescents, while accounting for these intricate relationships.
Some evidence from experiments has also highlighted the harmful effects of EDCs on the development of MASLD, with inflammation possibly playing a key role. A recent in vitro study has demonstrated BPA’s active involvement in hepatic inflammation, driven by the release of pro-inflammatory cytokines[26].
Unhealthy lifestyles, including smoking, drinking, physical inactivity, insufficient sleep, and poor diet, have been reported to increase the risk of MASLD[30,31]. Several studies indicate that adopting a healthy lifestyle can help mitigate the harmful effects of environmental pollutants[32-34]. For instance, Aimuzi et al. found that individuals with a healthier diet may experience fewer adverse effects from organophosphate esters on MASLD[32]. Another study suggested that a healthy lifestyle can reduce the harmful impact of polychlorinated biphenyls and organochlorine pesticides on MASLD[33]. However, it remains unclear whether lifestyle factors can modify the association between EDC exposure and MASLD in adolescents.
The hepatic steatosis index (HSI), a non-intrusive method for assessing hepatic steatosis, has been confirmed to have good predictive performance[35]. Thus, in this study, we adopted HSI as a non-invasive tool to assess MASLD in adolescents. Additionally, the application of HSI as another primary outcome could more effectively elucidate the relationship between EDCs and the extent of hepatic steatosis[36]. Given this background, we extracted data on two phenols, two chlorophenol pesticides, and ten phthalate metabolites from the NHANES dataset. We then investigated the single and combined effects of these chemicals on HSI and MASLD in adolescents. We also assessed the potential mediating effects of the inflammation index and the potential modifying effects of lifestyle factors.
METHODS
Study population
The data for this study were obtained from the NHANES conducted between 2005 and 2016. NHANES is a nationally representative cross-sectional survey that collects data on diet, nutritional status, health, and health behaviors. It assesses the physical health and nutrition levels of noninstitutionalized children and adults in the United States (https://www.cdc.gov/nchs/nhanes/). Initially, 60,936 participants were included. After excluding those with incomplete liver function data (n = 22,480), individuals aged 20 or older
Measurement of chemicals in urine
The urine samples were stored at -20 °C and subsequently shipped to the National Center for Environmental Health for testing. Phenols and chlorophenol pesticides were quantified using online solid-phase extraction combined with high-performance liquid chromatography and tandem mass spectrometry. Phthalate metabolites were measured using high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Concentrations below the LOD were assigned a value of LOD/√2, following NHANES recommendations. To adjust for urine dilution in spot samples, metabolite levels were corrected for creatinine concentrations. Urinary creatinine levels (g/L) were determined by the enzymatic Roche Cobas 6000 system. Detailed experimental methods are available on the NHANES website (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2005/DataFiles/EPH_D.htm).
In this study, we included chemicals with metabolite detection frequencies of 85% or higher[37]. Thus, the final analysis included 14 chemicals: 2 phenols [BPA and bisphenol-3 (BP3)], 2 chlorophenol pesticides [2,4-dichlorophenol (2,4-DCP) and 2,5-dichlorophenol (2,5-DCP)], and 10 phthalate metabolites [MEP, mono-n-butyl phthalate (MBP), mono (carboxyoctyl) phthalate (MCOP), mono (2-ethyl-5-carboxypentyl) phthalate (MECPP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(3-carbox ypropyl) phthalate (MCPP), mono-benzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono-(carboxynonyl) phthalate (MCNP), and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP)].
Definition of outcomes
The extent of hepatic steatosis was assessed using the HSI, calculated as follows: HSI = 8 × [alanine aminotransferase (ALT, IU/L)/aspartate aminotransferase (AST, IU/L)] + body mass index (BMI, kg/m²) + 2 for females + 2 for individuals with diabetes[35]. A previously published cutoff value of 36 was applied to define the existence of hepatic steatosis[35]. MASLD is diagnosed by hepatic steatosis (HSI ≥ 36) and one or more of the following cardiometabolic risk factors: (1) BMI ≥ the 85th percentile based on age- and sex-specific criteria; (2) diabetes or prediabetes (including self-reported diabetes/prediabetes, glycosylated hemoglobin (HbA1c) > 5.7%, fasting blood glucose ≥ 5.6 mmol/L, or 2-h postprandial glucose levels
Measurement of inflammatory markers
Lymphocytes, Neu, white blood cells (WBC), and platelet counts (expressed as × 103 /μL) were measured using automated hematology analyzers. The neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII) were calculated using NLR = Neu count/lymphocyte count[39]; SII = (platelet count × Neu count) / lymphocyte count[40].
Covariates and lifestyle factors
We extracted the following covariates from the NHANES database: age, gender, race/ethnicity, family income-to-poverty ratio (PIR), BMI (categorized as normal/underweight, overweight, or obesity according to World Health Organization (WHO) age- and sex-specific reference standards[41]), total calorie intake, diabetes status, serum cotinine, ALT, AST, alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) levels, along with session of sample collection, survey time period, and NHANES cycles.
Data on lifestyle factors, including sedentary behavior, physical activity, sleep patterns, smoking status, and dietary patterns, were collected using structured questionnaires and 24-h dietary recalls. Sedentary behavior was classified as high (≥ 7 h/day) or low (< 7 h/day) based on self-reported sitting time[42-44]. Physical activity was defined as the sum of weekly minutes spent on moderate and vigorous physical activities. Following the physical activity recommendations, physical activity was grouped into low (< 420 min/week) or moderate to high (≥ 420 min/week)[45]. Healthy sleep patterns were defined as 8-10 h/day for adolescents aged
Statistical analysis
For basic characteristics, continuous variables were expressed as median interquartile range (IQR) due to right-skewed distributions, and categorical variables were expressed as frequencies (n) and percentages (%). Group comparisons were conducted utilizing the Wilcoxon rank-sum test or the χ2 test. Due to right-skewed distribution, serum cotinine, ALT, ALP, GGT, Neu, WBC, and creatinine-corrected chemicals were ln-transformed to better approximate the normal distributions. Spearman correlation analysis assessed correlations among individual chemicals. We employed weighted generalized linear regression models (GLMs) to evaluate the relationship of individual chemicals with HSI and MASLD. The chemical concentrations were ln-transformed as continuous variables or grouped into quartiles as categorical variables for further analysis. Trend test was performed by assigning the median value of each quartile in the regression model. Covariates included age, gender, race/ethnicity, BMI, PIR, total calorie intake, diabetes status, and ln-transformed serum cotinine, ALT, ALP, and GGT levels. Subgroup analyses by sex (male and female) were conducted to identify potential sex-specific variations. In addition, restricted cubic spline (RCS) analysis was performed to examine the possible nonlinear dose-response relationships between individual chemicals with HSI and MASLD, with the 10th percentile serving as the reference[48].
The weighted quantile sum (WQS) regression and Bayesian kernel machine regression (BKMR) models were utilized to investigate the association of chemical mixtures with HSI and MASLD. WQS regression is a multivariate approach that assesses the joint impacts of multiple chemicals on health outcomes and quantifies the relative importance of each chemical[49]. The dataset was split into training (40%) and validation (60%) sets, using 1,000 bootstrap samples. BKMR employs a kernel function to flexibly model both individual and combined impacts of exposure mixtures on HSI and MASLD, utilizing 10,000 iterations of the Markov Chain Monte Carlo algorithm[50]. Within this analytical framework, to facilitate the selection of one component within a prespecified group of correlated components, chemical exposures were stratified by their common exposure sources into three groups: Group 1, phenols; Group 2, pesticides; Group 3, phthalates[24,50]. We further explored: (1) the joint impacts of chemical mixtures on health outcomes compared to the medians; (2) the single effects and potential interactions of chemical mixtures using both univariate and bivariate exposure-response functions; (3) the key chemicals contributing most to HSI and MASLD by calculating the posterior inclusion probability (PIP).
Subsequently, mediation analyses were conducted to examine whether inflammatory markers (such as WBC, Neu, NLR, and SII) mediated the associations of chemicals with HSI and MASLD. Specifically, we investigated the relationships between these chemicals and inflammatory markers, as well as the effects of these markers on HSI and MASLD[51]. To evaluate effect modification by lifestyle factors, we conducted subgroup analyses based on sedentary behavior, physical activity, sleep patterns, smoking status, and dietary quality. Interactions between these variables and chemical mixture exposure, operationalized using cross-product terms, were formally tested in WQS models. Then, we estimated subgroup results by plugging in chemical weights as obtained from the above overall models[52].
Several sensitivity analyses were performed to verify the robustness of our main results. Firstly, we repeated the GLMs and WQS models with additional adjustments of WBC, Neu, sedentary behavior, and physical activity. Secondly, in light of prior evidence linking prescription medication use to inflammation[53], the mediation analysis was repeated after excluding users of these medications. All statistical analyses were conducted using R software (version 4.4.2, with packages “survey”, “rcs”, “gWQS”, “bkmr”, “mediation”); a two-tailed P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Table 1 provides the socio-demographic characteristics of the study population. Of the 2,163 adolescents included, the median age was 15 years, with 47.0% being male. Among them, 490 (22.7%) were diagnosed with MASLD, while the remaining 1,673 (77.3%) served as controls. Adolescents with MASLD exhibited a higher age and a lower PIR compared with controls (P < 0.05). Additionally, compared to the non-MASLD group, the MASLD group had a higher likelihood of being Mexican American, a greater proportion of obesity, elevated levels of ALT, GGT, and serum cotinine, and reduced levels of ALP. No significant differences were observed between the cases and controls in terms of gender, AST levels, blood sample collection session, survey period, and NHANES cycles.
Characteristics of participants included in the study
| Items | Overall (n = 2,163) | Non-MASLD (n = 1,673) | MASLD (n = 490) | P |
| Age (years) | 15 (14, 17) | 15 (13, 17) | 16 (14, 18) | < 0.001 |
| Gender | ||||
| Male | 1,016 (47.0) | 762 (45.5) | 254 (51.8) | 0.353 |
| Female | 1,147 (53.0) | 911 (54.5) | 236 (48.2) | |
| Race/ethnicity | ||||
| Mexican American | 546 (25.2) | 395 (23.6) | 151 (30.8) | 0.003 |
| Other Hispanic | 193 (8.9) | 155 (9.3) | 38 (7.8) | |
| Non-Hispanic White | 638 (29.5) | 638 (29.5) | 120 (24.5) | |
| Non-Hispanic Black | 575 (26.6) | 426 (25.5) | 149 (30.4) | |
| Other race | 211 (9.8) | 179 (10.7) | 32 (6.5) | |
| PIR | ||||
| ≤ 1.0 | 678 (31.3) | 516 (30.8) | 162 (33.1) | 0.019 |
| 1.0-3.0 | 921 (42.6) | 703 (42.0) | 218 (44.5) | |
| ≥ 3.0 | 564 (26.1) | 454 (27.1) | 110 (22.4) | |
| BMI (kg/m2) | ||||
| Normal or underweight | 1,207 (55.8) | 1,205 (72.0) | 2 (0.4) | < 0.001 |
| Overweight | 487 (22.5) | 399 (23.8) | 88 (18.0) | |
| Obesity | 469 (21.7) | 69 (4.1) | 400 (81.6) | |
| Serum cotinine (ug/L) | 0.05 (0.01, 0.60) | 0.04 (0.01, 0.54) | 0.08 (0.02, 0.82) | < 0.001 |
| ALT (U/L) | 17 (14, 21) | 16 (13, 19) | 23 (17, 33) | < 0.001 |
| AST (U/L) | 22 (19, 26) | 22 (20, 26) | 23 (19, 28) | 0.386 |
| GGT (U/L) | 13 (11, 17) | 13 (10, 16) | 18 (13, 24) | < 0.001 |
| ALP (U/L) | 98 (71, 172) | 104 (72, 194) | 87 (69, 126) | < 0.001 |
| Total calories intake (kcal/day) | 1,938 (1,486, 2,518) | 1,996 (1,517, 2,559) | 1,840 (1,348, 2,365) | 0.043 |
| Diabetes | ||||
| No | 1,857 (85.9) | 1,468 (87.7) | 389 (79.4) | 0.001 |
| Boardline | 289 (13.4) | 200 (12.0) | 89 (18.2) | |
| Yes | 17 (0.8) | 5 (0.3) | 12 (2.4) | |
| Session of sample collection | ||||
| Morning | 1,035 (47.9) | 830 (49.6) | 205 (41.8) | 0.265 |
| Afternoon | 703 (32.5) | 531 (31.7) | 172 (35.1) | |
| Evening | 425 (19.6) | 312 (18.6) | 113 (23.1) | |
| Six-month time period when surveyed | ||||
| November 1st-April 30th | 1,083 (50.1) | 820 (49.0) | 263 (53.7) | 0.303 |
| May 1st-October 31st | 1,080 (49.9) | 853 (51.0) | 227 (46.3) | |
| NHANES cycles | ||||
| 2005-2006 | 580 (26.8) | 451 (27.0) | 129 (26.3) | 0.346 |
| 2007-2008 | 311 (14.4) | 248 (14.8) | 63 (12.9) | |
| 2009-2010 | 336 (15.5) | 260 (15.5) | 76 (15.5) | |
| 2011-2012 | 299 (13.8) | 228 (13.6) | 71 (14.5) | |
| 2013-2014 | 337 (15.6) | 261 (15.6) | 76 (15.5) | |
| 2015-2016 | 300 (13.9) | 225 (13.4) | 75 (15.3) | |
Supplementary Table 1 summarizes the detection frequency, geometric mean, arithmetic mean, and specific percentiles of the concentrations of 14 chemicals. MEP exhibited the highest geometric mean concentration (193.59 ng/mL), followed by BP3 (137.72 ng/mL) and MECPP (26.41 ng/mL). Spearman correlation analysis observed moderate to strong positive correlations among multiple chemicals, with the highest correlation observed between MEHHP and MEOHP (r = 0.98; Supplementary Figure 2).
Individual effect of multiple chemicals on HSI and MASLD risk
In the linear regression model, we observed that the MEP (quartile 2 vs. quartile 1: β = 0.451;
Associations between single urinary chemicals and HSI and MASLD risk
| Chemicals | Continuous | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Ptrend |
| HSI, Linear regression model, β (95%CI) | ||||||
| Phenols | ||||||
| BPA | -0.088 (-0.274, 0.097) | Ref. | 0.401 (-0.116, 0.917) | 0.326 (-0.136, 0.789) | 0.022 (-0.524, 0.569) | 0.468 |
| BP3 | -0.064 (-0.170, 0.043) | Ref. | 0.221 (-0.310, 0.751) | 0.235 (-0.287, 0.757) | -0.094 (-0.653, 0.466) | 0.246 |
| Pesticides | ||||||
| 2,4-DCP | -0.013 (-0.140, 0.114) | Ref. | 0.071 (-0.481, 0.623) | -0.372 (-0.807, 0.062) | 0.125 (-0.364, 0.614) | 0.515 |
| 2,5-DCP | -0.008 (-0.099, 0.083) | Ref. | -0.042 (-0.539, 0.456) | -0.343 (-0.833, 0.147) | 0.001 (-0.520, 0.522) | 0.716 |
| Phthalates | ||||||
| MEP | 0.016 (-0.117, 0.149) | Ref. | 0.451 (0.021, 0.880)* | 0.205 (-0.183, 0.594) | 0.092 (-0.405, 0.588) | 0.607 |
| MBP | -0.106 (-0.331, 0.120) | Ref. | -0.061 (-0.568, 0.445) | 0.317 (-0.238, 0.873) | -0.520 (-1.015, -0.025) | 0.007 |
| MCOP | 0.133 (-0.045, 0.311) | Ref. | 0.404 (-0.129, 0.938) | 0.431 (-0.009, 0.872) | 0.517 (0.075, 0.960)* | 0.167 |
| MECPP | -0.134 (-0.328, 0.060) | Ref. | -0.176 (-0.653, 0.301) | -0.21 (-0.595, 0.174) | -0.205 (-0.723, 0.312) | 0.675 |
| MEHHP | -0.087 (-0.246, 0.073) | Ref. | 0.261 (-0.327, 0.849) | -0.206 (-0.592, 0.180) | -0.03 (-0.492, 0.431) | 0.662 |
| MCPP | 0.069 (-0.128, 0.266) | Ref. | 0.179 (-0.293, 0.652) | -0.015 (-0.547, 0.518) | 0.231 (-0.291, 0.753) | 0.422 |
| MBzP | 0.192 (0.004, 0.380)* | Ref. | -0.218 (-0.694, 0.258) | 0.005 (-0.463, 0.473) | 0.260 (-0.322, 0.841) | 0.164 |
| MiBP | 0.053 (-0.167, 0.273) | Ref. | 0.409 (-0.037, 0.855) | 0.132 (-0.294, 0.557) | 0.195 (-0.271, 0.660) | 0.947 |
| MCNP | 0.098 (-0.138, 0.334) | Ref. | 0.144 (-0.319, 0.607) | 0.036 (-0.453, 0.524) | 0.012 (-0.567, 0.591) | 0.835 |
| MEOHP | -0.076 (-0.241, 0.089) | Ref. | 0.199 (-0.193, 0.591) | -0.180 (-0.631, 0.272) | -0.118 (-0.576, 0.340) | 0.463 |
| MASLD, Logistic regression model, OR (95%CI) | ||||||
| Phenols | ||||||
| BPA | 1.324 (0.986, 1.778) | Ref. | 2.002 (0.865, 4.635) | 1.910 (0.755, 4.830) | 2.375 (1.168, 4.832) | 0.056 |
| BP3 | 0.878 (0.729, 1.058) | Ref. | 0.916 (0.463, 1.811) | 0.715 (0.291, 1.757) | 0.567 (0.250, 1.288) | 0.237 |
| Pesticides | ||||||
| 2,4-DCP | 1.076 (0.886, 1.305) | Ref. | 1.108 (0.450, 2.729) | 1.458 (0.650, 3.270) | 1.118 (0.554, 2.260) | 0.947 |
| 2,5-DCP | 1.046 (0.925, 1.183) | Ref. | 0.661 (0.330, 1.324) | 0.897 (0.408, 1.970) | 0.951 (0.532, 1.699) | 0.612 |
| Phthalates | ||||||
| MEP | 1.138 (0.912, 1.421) | Ref. | 2.257 (0.873, 5.839) | 1.133 (0.434, 2.954) | 2.361 (0.947, 5.885) | 0.122 |
| MBP | 1.164 (0.819, 1.655) | Ref. | 1.079 (0.473, 2.458) | 1.851 (0.845, 4.057) | 1.351 (0.586, 3.112) | 0.635 |
| MCOP | 0.993 (0.783, 1.259) | Ref. | 1.813 (0.912, 3.606) | 1.241 (0.560, 2.752) | 1.365 (0.659, 2.828) | 0.887 |
| MECPP | 1.000 (0.785, 1.275) | Ref. | 0.665 (0.293, 1.511) | 1.504 (0.650, 3.480) | 0.739 (0.332, 1.648) | 0.467 |
| MEHHP | 1.050 (0.833, 1.324) | Ref. | 2.204 (0.822, 5.905) | 1.441 (0.732, 2.837) | 1.792 (0.853, 3.767) | 0.549 |
| MCPP | 1.035 (0.778, 1.378) | Ref. | 0.722 (0.302, 1.723) | 0.936 (0.417, 2.101) | 0.706 (0.287, 1.735) | 0.607 |
| MBzP | 1.471 (1.156, 1.872)* | Ref. | 1.024 (0.438, 2.394) | 1.431 (0.645, 3.176) | 2.524 (1.237, 5.148)* | 0.016 |
| MiBP | 1.152 (0.824, 1.611) | Ref. | 0.860 (0.385, 1.921) | 0.832 (0.377, 1.833) | 1.115 (0.504, 2.470) | 0.515 |
| MCNP | 1.152 (0.824, 1.611) | Ref. | 2.141 (0.928, 4.938) | 0.903 (0.339, 2.403) | 2.163 (0.899, 5.205) | 0.183 |
| MEOHP | 1.081 (0.851, 1.374) | Ref. | 1.399 (0.644, 3.039) | 1.511 (0.673, 3.395) | 1.417 (0.675, 2.976) | 0.638 |
The subgroup analysis stratified by sex suggested a positive relationship between BPA, MCPP, and HSI in males, while MiBP showed a significant correlation with HSI in females (all P < 0.05;
Combined effect of chemical mixtures on HSI and MASLD risk
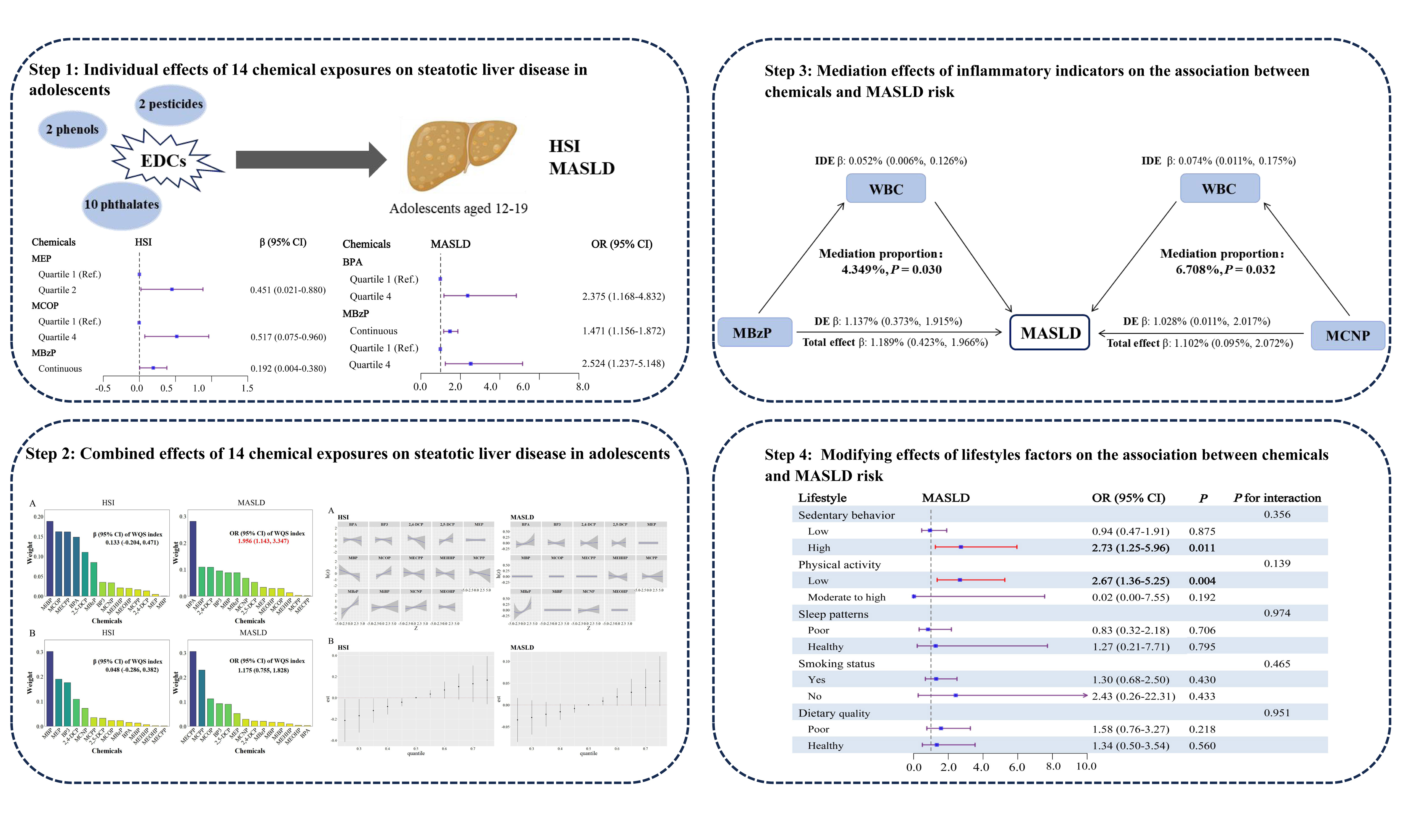
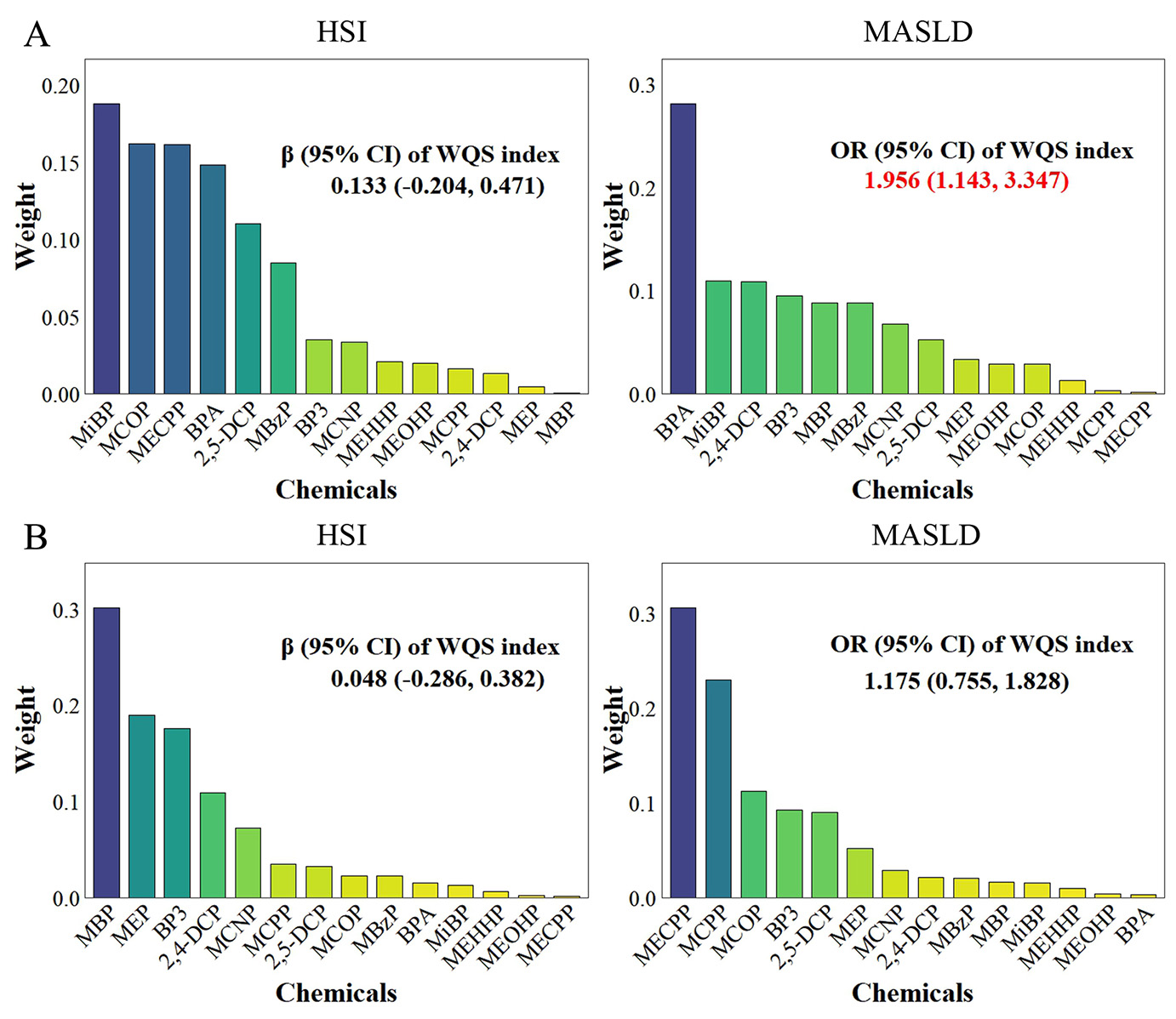
The WQS models indicated a statistically significant correlation between chemical mixtures and MASLD (OR = 1.956; 95%CI = 1.143-3.347), with BPA being the top-weighted chemical positively correlated with MASLD, accounting for 28% of the weight. Nevertheless, no statistically significant correlation was identified between the chemical mixtures and the HSI [Figure 1A]. Additionally, no statistically significant negative correlation was observed between the chemical mixtures and the outcomes [Figure 1B].
Figure 1. Associations and component weights of WQS index with HSI and MASLD based on the positive (A) and negative (B) WQS models. Models adjusted for age, gender, race/ethnicity, BMI, PIR, total calories intake, diabetes and ln-transformed serum cotinine, ALT, ALP, and GGT levels.
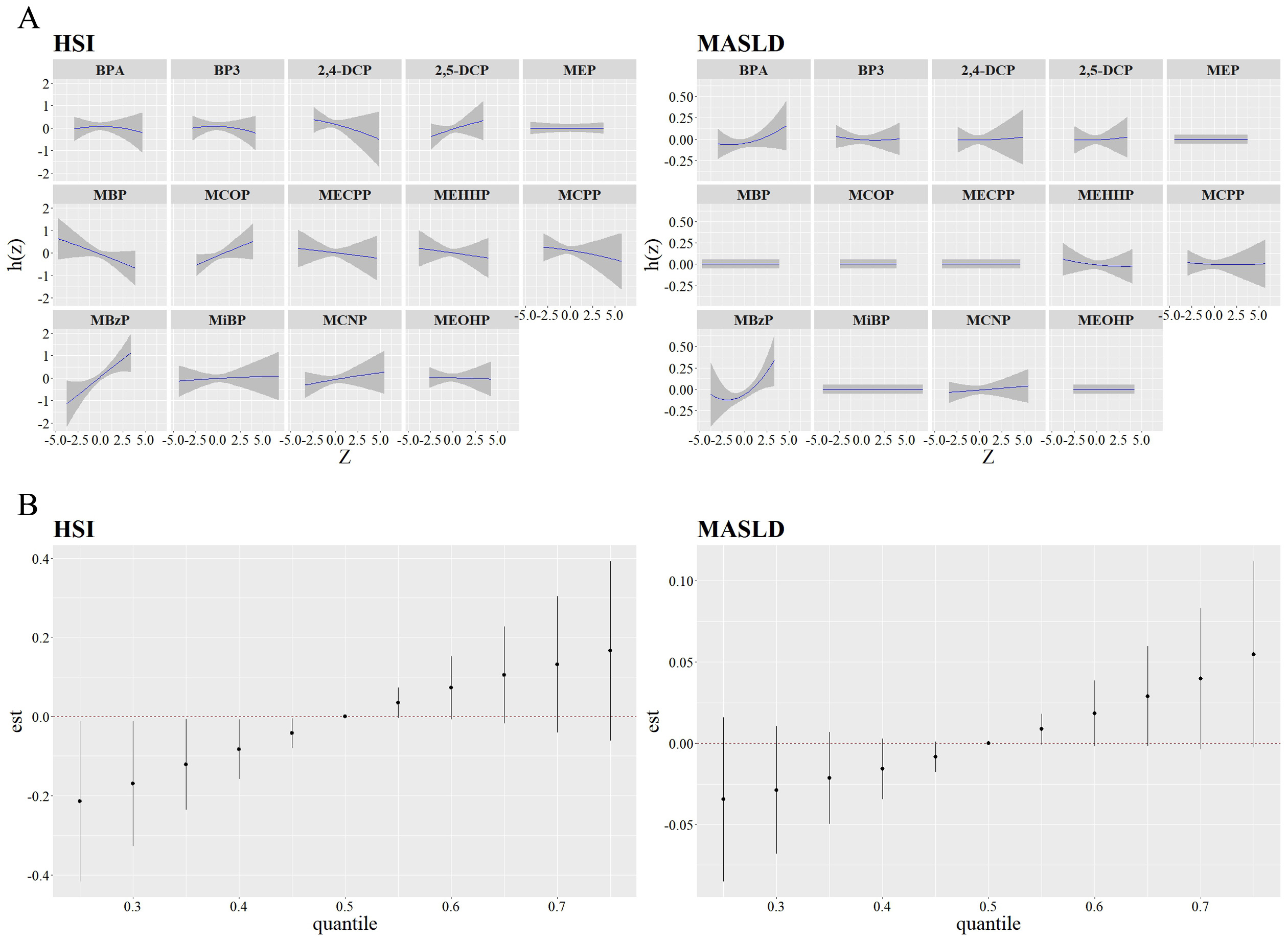
BKMR analyses revealed that MBzP had a positive linear relationship with HSI, as well as a nonlinear relationship with MASLD [Figure 2A]. Besides, HSI and MASLD risk exhibited increasing trends when all the chemicals were at or above their 60th percentile, compared to the 50th percentile [Figure 2B]. In addition, phthalates had the highest group PIP for HSI (0.303), with MBzP being the most important contributor (cond PIP = 0.564). In contrast, phenols had the highest group PIP for MASLD (0.186), with BPA as the most contributor (cond PIP = 0.989) [Supplementary Table 4]. No significant interaction was found in the bivariate exposure-response analysis [Supplementary Figure 5].
Figure 2. Univariate exposure-response function between the chemicals with HSI and MASLD (A), and Overall effect of the mixture on HSI and MASLD estimated using BKMR (B). Models adjusted for age, gender, race/ethnicity, BMI, PIR, total calories intake, diabetes and ln-transformed serum cotinine, ALT, ALP, and GGT levels.
Mediation effects of inflammatory indicators on the relationship of chemicals with HSI and MASLD risk
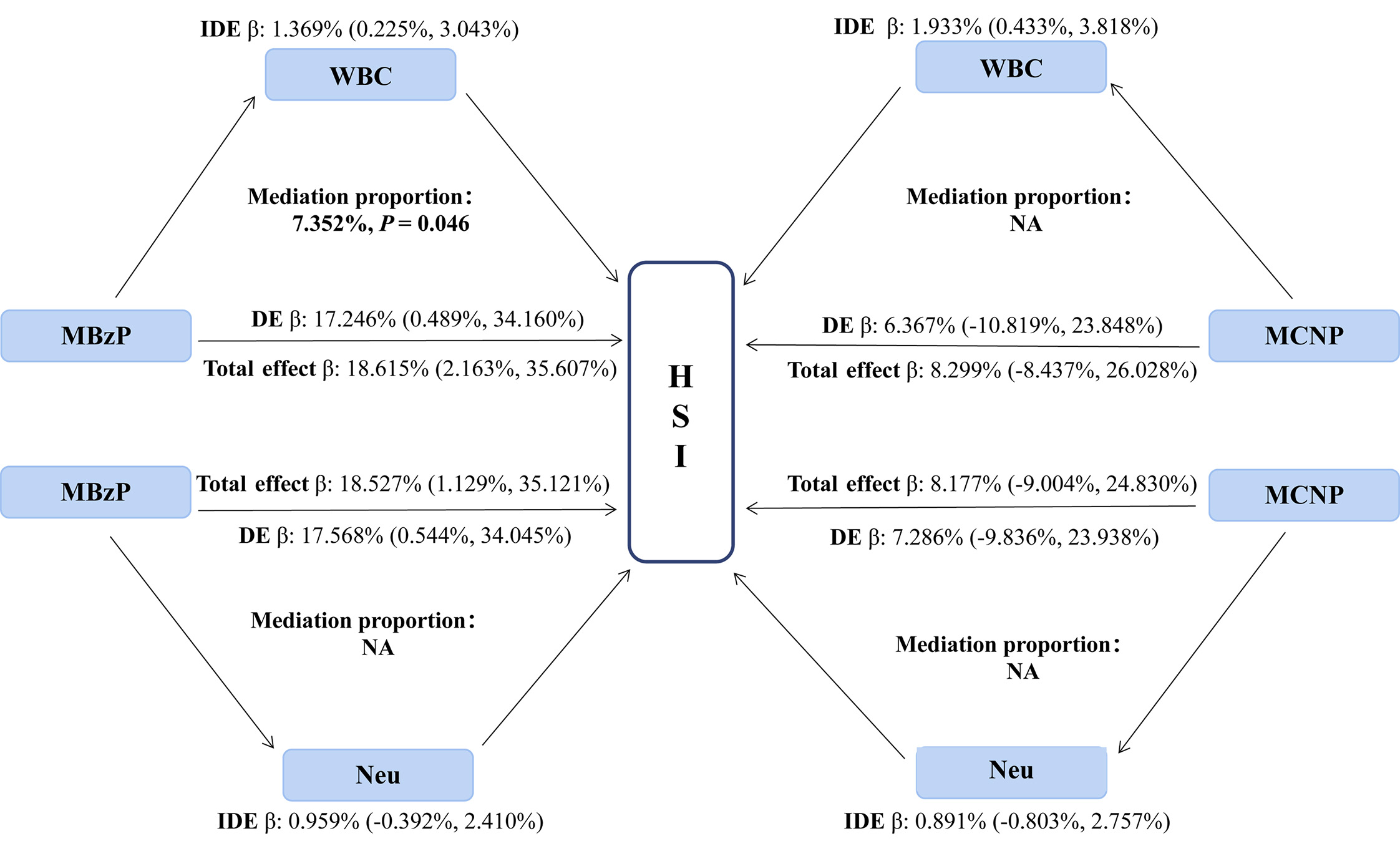
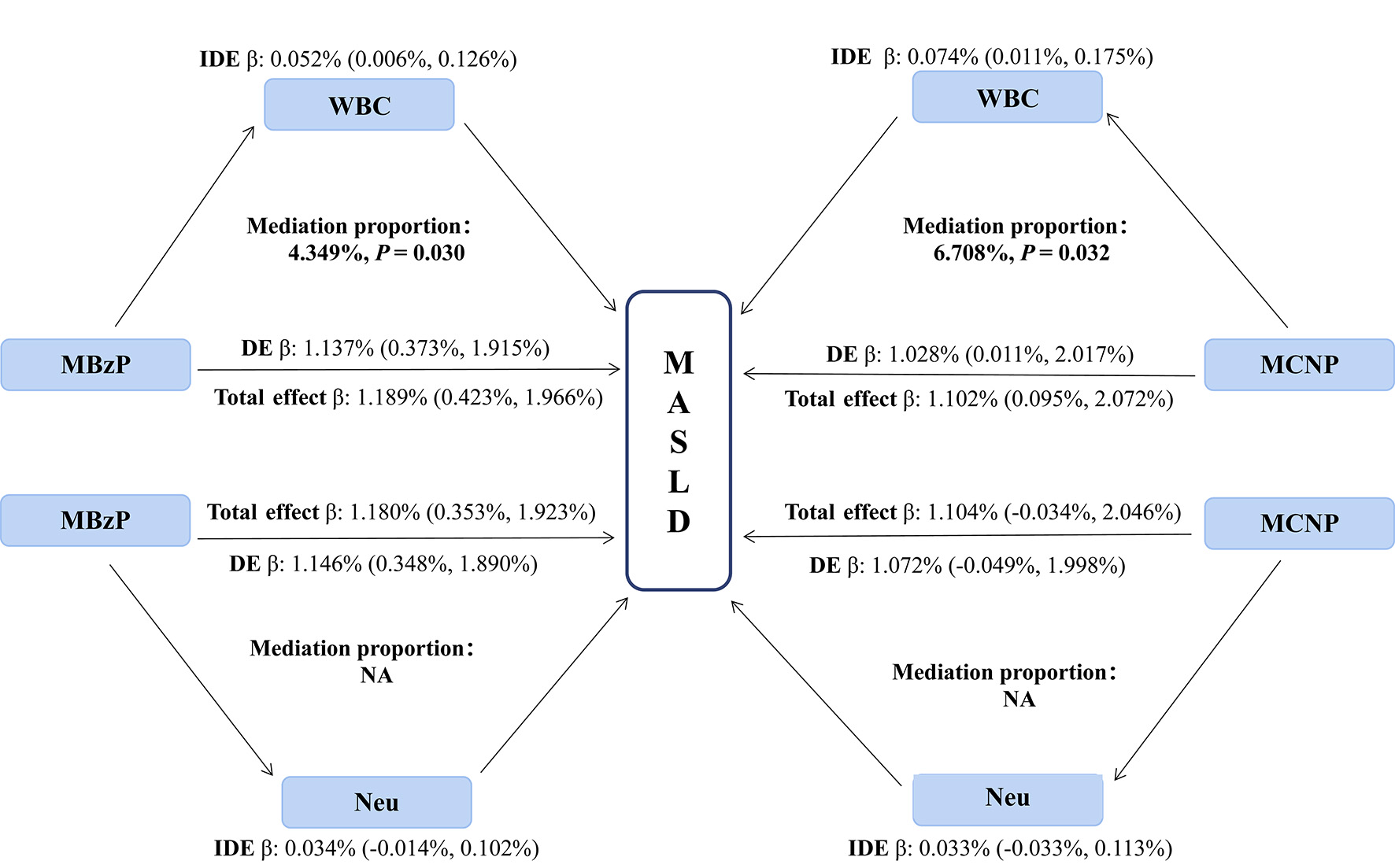
We performed a linear regression analysis between 14 chemicals and inflammatory indicators (WBC, Neu, NLR, and SII), revealing that MEP, MBP, MECPP, MEHHP, MCPP, MBzP, MCNP, and MEOHP were positively correlated with WBC or Neu (all P < 0.05; Supplementary Table 5). Subsequently, we identified positive correlations between WBC and Neu with both HSI and MASLD (all P < 0.05;
Figure 3. Mediating effects and proportions of immune cells on association between urine phthalate and HSI. WBC: White blood cell counts; Neu: neutrophil; MBzP: mono benzyl phthalate; MCNP: mono-(carboxynonyl) phthalate; IDE: indirect effect; DE: direct effect; HSI: hepatic steatosis index.
Figure 4. Mediating effects and proportions of immune cells on association between urine phthalate and MASLD. WBC: White blood cell counts; Neu: neutrophil; MBzP: mono benzyl phthalate; MCNP: mono-(carboxynonyl) phthalate; IDE: indirect effect; DE: direct effect; MASLD: metabolic dysfunction-associated steatotic liver disease.
Modifying effects of lifestyle factors on the association between chemicals and MASLD risk
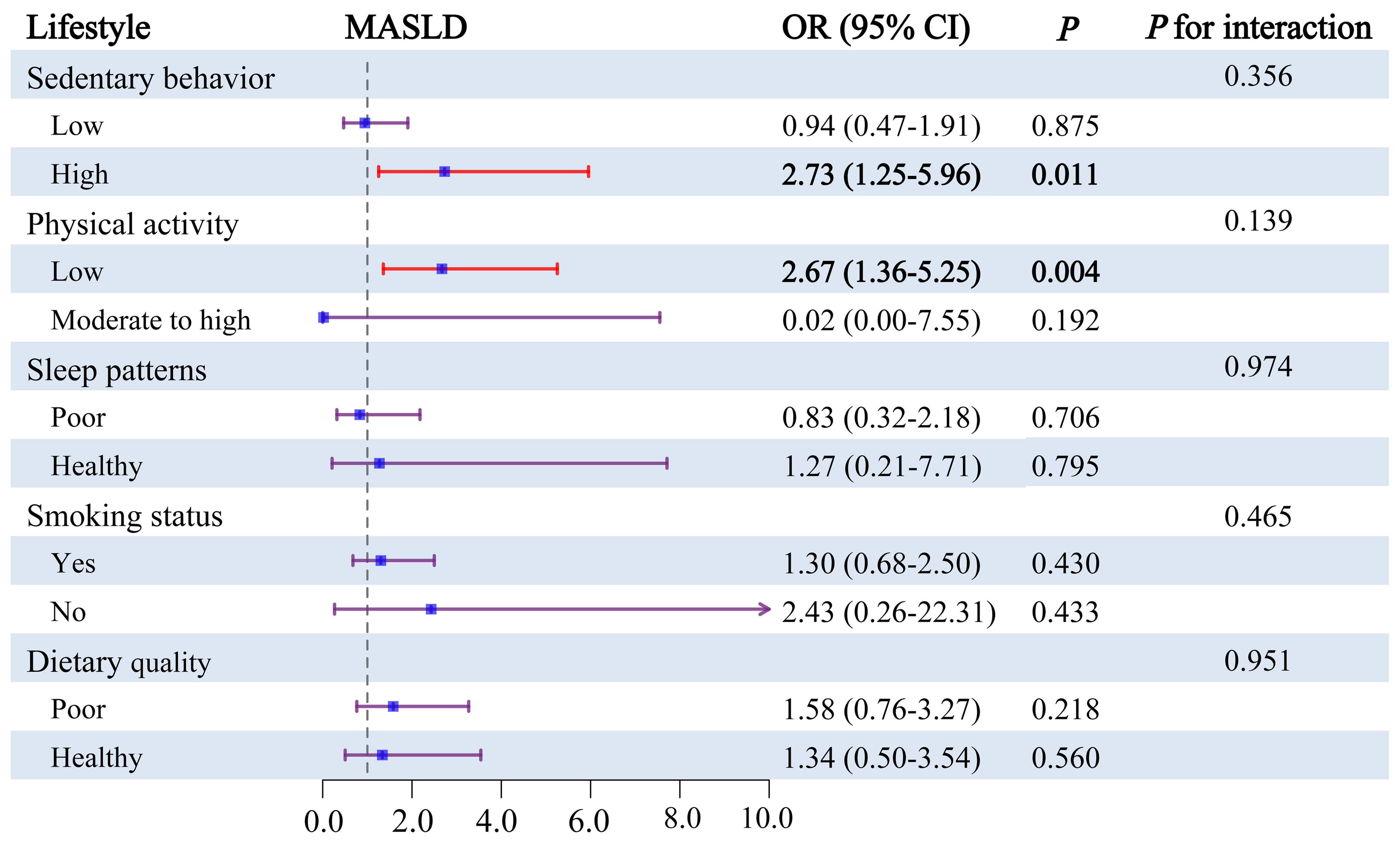
We further explored the modulatory role of lifestyle factors in the joint effects of chemical exposure on MALSD by WQS regression models, encompassing sedentary behavior, physical activity, sleep patterns, smoking status, and dietary quality [Figure 5]. Among adolescents with high sedentary behavior, the joint effect of chemicals exhibited a positive correlation with MASLD (OR = 2.73, 95%CI = 1.25-5.96). Similarly, among adolescents with low physical activity, the combined effect of chemicals was linked to a higher risk of MASLD (OR = 2.67, 95%CI = 1.36-5.25). However, among adolescents with other lifestyle factors, the joint effect of chemicals showed no significant correlation with MASLD. We also tried to evaluate the interaction effects of lifestyle factors on the relationship between chemical co-exposure and MASLD, but no significant interaction was observed.
Figure 5. Subgroup analysis for the joint effects of lifestyle and urinary chemicals mixture on the risks of MAFLD. Weights obtained from WQS models using interaction terms for the respective subgroup variables and pollutants were plugged into subgroup models to achieve comparability of subgroup results with main analysis. Models adjusted for age, gender, race/ethnicity, BMI, PIR, total calories intake, diabetes and ln-transformed serum cotinine, ALT, ALP, and GGT levels. MASLD: metabolic dysfunction-associated steatotic liver disease.
Sensitivity analysis
First, the GLMs and WQS regression models showed similar findings to those above, after further adjustment for WBC, Neu, sedentary behavior, and physical activity as covariates [Supplementary Table 9 and Supplementary Figure 6]. Furthermore, we repeated the mediation analysis after excluding users of prescription medications, which showed that WBC significantly mediated the associations between MBzP and HSI, as well as between MCNP and MASLD [Supplementary Tables 10 and 11].
DISCUSSION
In this research, we explored the correlations between exposure to phenols, parabens, and phthalates and both HSI and MASLD, while investigating the potential mediating effects of inflammatory markers and the modifying effects of lifestyle factors. First, the GLMs revealed significant associations of MEP, MCOP, and MBzP with HSI, as well as of BPA and MBzP with MASLD. Second, the WQS and BKMR models indicated a significant correlation between chemical mixtures and MASLD, with MBzP and BPA identified as key contributors. Moreover, mediation analysis showed that WBC mediates the associations between MBzP and both MASLD and HSI, as well as between MCNP and MASLD. Additionally, our study also revealed the modulatory role of sedentary behavior and physical activity in the combined effects of chemical exposure on MALSD.
MASLD is a multifactorial disorder that is closely associated with obesity, impaired glucose tolerance, lipid metabolism, inflammation, type 2 diabetes, and cardiovascular disease[4,7,54]. A survey study performed by Verstraete et al. indicated a positive association between BPA and MASLD, aligning with our findings[21]. BPA, a widely recognized environmental endocrine disruptor, has been increasingly linked to obesity, insulin resistance, and hepatic metabolic alterations in epidemiological studies[16,55]. A study by Huc et al. suggests that BPA promotes steatosis by affecting disturbances in mitochondrial function, lipid accumulation and inflammation responses[26]. Concurrently, murine studies reveal that BPA not only induces splenic mitochondrial dysfunction through localized oxidative stress but also causes morphological changes, including dilation of the splenic sinuses, alterations to the white and red pulp, and disrupted cellular structures[12,13]. This pathological profile notably parallels the hepatic mitochondrial disturbances reported by Huc et al.[26]. Beyond hepatic impacts, animal evidence indicates that the spleen is a sensitive extrahepatic target of BPA, whose estrogen-mimetic activity may disrupt immune responses, underscoring the need for further research to fully understand the implications for human health[56]. Our study also identified that MBzP was significantly correlated with MASLD, indirectly supporting previous findings[57]. Existing evidence demonstrates a significant correlation between MBzP exposure and a higher risk of obesity and insulin resistance[57,58], which are recognized risk factors for MASLD. The potential mechanism may involve MBzP modulating fatty acid catabolism by impacting the expression of genes related to lipid metabolism[59]. In addition, our study observed that some individual chemicals were not significantly associated with the outcomes (such as BP3 and 2,4-DCP). A possible explanation involves population heterogeneity in toxicokinetics, such as differences in metabolic capacity, detoxification efficiency, and excretion rates. This variability may dilute the observed effect estimates and obscure a true association at the population level. Another plausible explanation for the non-significant findings is differences in exposure characteristics. For example, the half-life of 2,4-DCP is relatively short (4 to 30 min)[60], and a single spot urine measurement may not accurately reflect long-term internal exposure, leading to exposure dose levels being below the biological threshold necessary to elicit a measurable effect on HSI or MASLD.
The WQS and BKMR models revealed a significant correlation between chemical mixtures and MASLD in adolescents, with phenols and phthalates as the primary contributors, consistent with prior research. For instance, an epidemiologic study in the Korean adult population showed that exposure to a combination of EDCs (including phenols, pesticides, phthalates, parabens, and pyrethroids) is significantly associated with non-alcoholic fatty liver disease (NAFLD)[20]. Similar results were observed in a survey study evaluating the correlation between exposure to phthalates and NAFLD in US adults[17]. These associations may be explained by several possible mechanisms. First, in vitro studies showed that exposure to phenols and phthalates could over-activate certain receptors, such as peroxisome proliferator-activated receptor γ (PPAR-γ) and estrogen receptor ER-α, the activation of which could up-regulate adipose gene expression related to insulin resistance, contributing to an elevated risk of insulin resistance and diabetes[61,62]. These mechanisms are further supported by animal studies. For instance, a study by Ding et al. indicated that DEHP exposure could further inhibit glucose uptake, glycogen synthesis, and lipid/protein metabolism, and impair cardiovascular and hepatic functions in female P-T2DM mice, which is consistent with impaired glucose homeostasis and insulin resistance[63]. Second, a study of Yu et al. indicated the possible harmful impact of phthalate exposures on the human liver, which partially supports our findings[64]. Third, previous research has demonstrated that phenols and phthalates are significantly associated with obesity, impaired glucose tolerance, and cardiovascular disease, which are risk factors for MASLD[14,65].
Furthermore, we tested the potential mediating role of inflammatory markers in the associations between chemicals and MASLD. The results indicated that WBC partially mediated these associations. A study by Chen et al. suggested that phthalates may trigger inflammatory responses by activating the PI3K/AKT and NF-κB inflammasome signaling pathways[66]. The animal experiment research suggested that phthalates (DEHP and MEHP) could enhance lipid synthesis while inhibiting lipid breakdown in hepatocytes through inflammatory pathways, thereby causing lipid accumulation[67]. As key components of the immune system, WBC plays a crucial role in mediating inflammatory responses, which is underlying pathophysiological mechanisms in the development of MASLD[68]. In this study, we observed that certain chemicals (MEP, MBP, MECPP, MEHHP, and MEOHP) had a significant correlation with WBC and Neu, largely consistent with findings from a study based on the Korean population[69]. Moreover, we found that WBC was positively correlated with MASLD. Therefore, we propose that certain chemicals may affect WBC levels, trigger inflammatory responses, and thereby contribute to an elevated risk of MASLD, emphasizing the potential role of inflammation in this process. In addition, oxidative stress represents another pivotal mechanism through which EDCs may contribute to MASLD pathogenesis. Although the present study did not directly assess oxidative stress mediators due to the limitations in biomarker availability within the NHANES database, prior mechanistic research has indicated that EDCs (such as phthalates and BPA) can induce excessive production of reactive oxygen species (ROS)[70-72]. This oxidative burden promotes hepatic lipid peroxidation, impairs mitochondrial function, and triggers apoptotic pathways, thereby accelerating steatosis and liver injury. Therefore, we recommend that future epidemiological studies incorporate specific biomarkers of oxidative damage (e.g., malondialdehyde (MDA) or 8-hydroxy-2'-deoxyguanosine (8-OHdG)) and antioxidant capacity to explore the contribution of this pathway.
Finally, our study explored the modulatory role of lifestyle factors in the combined impacts of chemical mixture exposure on MASLD. Specifically, the correlation between chemical exposures and MASLD was significant in subgroups with high sedentary behavior and low physical activity, whereas a significant effect was not observed in groups with low sedentary behavior and moderate-to-high physical activity. The results are partially aligned with prior research demonstrating the impacts of environmental pollutants and lifestyle factors on multiple health outcomes. For instance, a study by Lei et al. demonstrated that physical inactivity and an inflammatory diet could worsen the adverse influences of diisobutyl phthalate on MASLD[19]. A cohort study among Chinese adults observed that physical activity could mitigate the adverse influences of air pollution on metabolic syndrome[73], which indirectly supports our findings to some extent, as MASLD is viewed as the liver manifestation of metabolic syndrome[2]. However, the potential mechanisms through which sedentary behavior and physical activity modify this association remain unclear, and more research is needed to clarify these mechanisms.
Our research has several advantages. First, this investigation comprehensively explores the impacts of single and combined chemical exposures on MASLD in adolescents. Second, we found the mediating role of WBC in chemical exposure and MASLD. Third, our findings suggest that reducing sedentary behavior and increasing physical activity could be effective strategies to mitigate the adverse effects of chemical exposure on MASLD. Nonetheless, the study has some limitations. First, owing to the cross-sectional design of NHANES, it could not confirm the causal associations of chemical exposure with HSI or MASLD; thus, further prospective studies are needed to confirm these results. Second, the evaluation of chemicals at a single point in time cannot accurately capture individuals' actual exposure levels due to data limitations. Finally, assessing mediating role of WBC and the modifying effects of sedentary behavior and physical activity still needs to be further confirmed in cell models and animal experiments.
In conclusion, our study indicated that single or combined exposure to multiple chemicals (phenols, pesticides, and phthalates) was positively correlated with HSI or MASLD. MBzP and BPA were the major contributors to these associations. WBC levels were further identified as partially mediating the significant correlation between phthalate exposure and outcomes. Additionally, reducing sedentary behavior and increasing physical activity could mitigate the adverse influences of chemical mixtures on MASLD.
DECLARATIONS
Acknowledgments
All authors thank NHANES for providing publicly available data.
Authors’ contributions
Conceptualization, methodology, data curation, writing - original draft, supervision: Yao X
Methodology, formal analysis, data curation, writing - original draft, visualization:Tang c
Data curation, writing - original draft: Li Y
Methodology, validation, data curation: You C
Methodology, writing - review & editing: Zhang H
Resources, writing - review & editing, supervision: Gu X
Resources, supervision, writing - review & editing, funding acquisition: Liu K
Conceptualization, project administration, resources, supervision, writing - review & editing, funding acquisition: Zhang L
Availability of data and materials
The data used in this study are publicly available at the NHANES database.
Financial support and sponsorship
This work was funded by National Natural Science Foundation of China (No. 82401002); Top medical expert team of Wuxi Taihu Talent Plan (Nos. YXTD202101, GDTD202105, DJTD202106); Medical Key Discipline Program of Wuxi Health Commission (Nos. CXTD2021005, ZDXK2021007); Medical Key Discipline Program of Wuxi Health Commission (Nos. ZDXK2021007, CXTD2021005); Scientific Research Program of Wuxi health Commission (Nos. Z202109, M202208, Q202162, Z202214); Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (No. BJ2023090); and Wuxi Science and Technology Development Fund (Nos. N20202003, Y20222001, K20241001).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study utilized publicly available data from the National Health and Nutrition Examination Survey (NHANES), conducted by the U.S. Centers for Disease Control and Prevention (CDC). All NHANES protocols have been approved by the NCHS Ethics Review Board. Continuation of Protocol #2005-06 (NHANES 2005-2010); Continuation of Protocol #2011-17 (NHANES 2011-2017). The analysis strictly used 2005-2016 data under these protocols. Written informed consent was obtained from each participant before participation in the study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-86.
2. Zhang L, El-Shabrawi M, Baur LA, et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med. 2024;5:797-815.e2.
3. Lee EJ, Choi M, Ahn SB, et al. Prevalence of nonalcoholic fatty liver disease in pediatrics and adolescents: a systematic review and meta-analysis. World J Pediatr. 2024;20:569-80.
4. Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707.
5. Sun DQ, Targher G, Byrne CD, et al. An international Delphi consensus statement on metabolic dysfunction-associated fatty liver disease and risk of chronic kidney disease. Hepatobiliary Surg Nutr. 2023;12:386-403.
6. Zhou XD, Targher G, Byrne CD, et al. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int. 2023;17:773-91.
7. Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic fatty liver disease in children. Semin Liver Dis. 2018;38:1-13.
8. Deierlein AL, Rock S, Park S. Persistent endocrine-disrupting chemicals and fatty liver disease. Curr Environ Health Rep. 2017;4:439-49.
9. Pan K, Xu J, Xu Y, Wang C, Yu J. The association between endocrine disrupting chemicals and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Pharmacol Res. 2024;205:107251.
10. Ghassabian A, Vandenberg L, Kannan K, Trasande L. Endocrine-disrupting chemicals and child health. Annu Rev Pharmacol Toxicol. 2022;62:573-94.
12. Dong Y, Zhai L, Zhang L, Jia L, Wang X. Bisphenol A impairs mitochondrial function in spleens of mice via oxidative stress. Mol Cell Toxicol. 2013;9:401-6.
13. Shaibi T, Balug HN, Ben-Othman ME, et al. Exposure to low-dose bisphenol A induces spleen damage in a murine model: potentially through oxidative stress? Open Vet J. 2022;12:23-32.
14. Li D, Suh S. Health risks of chemicals in consumer products: a review. Environ Int. 2019;123:580-7.
15. Rivera-Núñez Z, Kinkade CW, Zhang Y, et al. Phenols, parabens, phthalates and puberty: a systematic review of synthetic chemicals commonly found in personal care products and girls' pubertal development. Curr Environ Health Rep. 2022;9:517-34.
16. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161-73.
17. Cai S, Fan J, Ye J, Rao X, Li Y. Phthalates exposure is associated with non-alcoholic fatty liver disease among US adults. Ecotoxicol Environ Saf. 2021;224:112665.
18. Kim D, Yoo ER, Li AA, et al. Elevated urinary bisphenol A levels are associated with non-alcoholic fatty liver disease among adults in the United States. Liver Int. 2019;39:1335-42.
19. Lei R, Xue B, Tian X, et al. The association between endocrine disrupting chemicals and MAFLD: evidence from NHANES survey. Ecotoxicol Environ Saf. 2023;256:114836.
20. Park B, Kim B, Kim CH, Oh HJ, Park B. Association between endocrine-disrupting chemical mixtures and non-alcoholic fatty liver disease with metabolic syndrome as a mediator among adults: A population-based study in Korea. Ecotoxicol Environ Saf. 2024;276:116310.
21. Verstraete SG, Wojcicki JM, Perito ER, Rosenthal P. Bisphenol a increases risk for presumed non-alcoholic fatty liver disease in Hispanic adolescents in NHANES 2003-2010. Environ Health. 2018;17:12.
22. Midya V, Colicino E, Conti DV, et al. Association of prenatal exposure to endocrine-disrupting chemicals with liver injury in children. JAMA Netw Open. 2022;5:e2220176.
23. Xiang S, Dong J, Li X, Li C. Urine phthalate levels and liver function in US adolescents: analyses of NHANES 2007-2016. Front Public Health. 2022;10:843971.
24. Zhang Y, Dong T, Hu W, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int. 2019;123:325-36.
25. Billionnet C, Sherrill D, Annesi-Maesano I. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol. 2012;22:126-41.
26. Huc L, Lemarié A, Guéraud F, Héliès-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol In Vitro. 2012;26:709-17.
27. Su HY, Lai CS, Lee KH, Chiang YW, Chen CC, Hsu PC. Prenatal exposure to low-dose di-(2-ethylhexyl) phthalate (DEHP) induces potentially hepatic lipid accumulation and fibrotic changes in rat offspring. Ecotoxicol Environ Saf. 2024;269:115776.
28. Nowak K, Jabłońska E, Ratajczak-Wrona W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int. 2019;125:350-64.
29. Liu K, Tang S, Liu C, et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front Immunol. 2024;15:1337241.
30. He P, Zhang Y, Ye Z, et al. A healthy lifestyle, life's essential 8 scores and new-onset severe NAFLD: a prospective analysis in UK biobank. Metabolism. 2023;146:155643.
31. Wang L, Yi J, Guo X, Ren X. Associations between life's essential 8 and non-alcoholic fatty liver disease among US adults. J Transl Med. 2022;20:616.
32. Aimuzi R, Xie Z, Qu Y, Jiang Y, Luo K. Associations of urinary organophosphate esters metabolites and diet quality with nonalcoholic/metabolic dysfunction-associated fatty liver diseases in adults. Ecotoxicol Environ Saf. 2023;254:114720.
33. Liu Q, Fan G, Bi J, et al. Associations of polychlorinated biphenyls and organochlorine pesticides with metabolic dysfunction-associated fatty liver disease among Chinese adults: effect modification by lifestyle. Environ Res. 2024;240:117507.
34. Zhang Y, Fu Y, Guan X, et al. Associations of ambient air pollution exposure and lifestyle factors with incident dementia in the elderly: a prospective study in the UK Biobank. Environ Int. 2024;190:108870.
35. Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-8.
36. Yang M, Su W, Li H, et al. Association of per- and polyfluoroalkyl substances with hepatic steatosis and metabolic dysfunction-associated fatty liver disease among patients with acute coronary syndrome. Ecotoxicol Environ Saf. 2023;264:115473.
37. Hu P, Pan C, Su W, et al. Associations between exposure to a mixture of phenols, parabens, and phthalates and sex steroid hormones in children 6-19 years from NHANES, 2013-2016. Sci Total Environ. 2022;822:153548.
38. Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-56.
39. Tudurachi BS, Anghel L, Tudurachi A, Sascău RA, Stătescu C. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and monocyte/HDL-cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci. 2023;24:14378.
40. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212-22.
41. World Health Organization (WHO). Child growth standards; 2006. Available from: https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age [Last accessed on 12 Sep 2025].
42. Ku PW, Steptoe A, Liao Y, Hsueh MC, Chen LJ. A cut-off of daily sedentary time and all-cause mortality in adults: a meta-regression analysis involving more than 1 million participants. BMC Med. 2018;16:74.
43. Lau JH, Nair A, Abdin E, et al. Prevalence and patterns of physical activity, sedentary behaviour, and their association with health-related quality of life within a multi-ethnic Asian population. BMC Public Health. 2021;21:1939.
44. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8:e80000.
45. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-8.
46. Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40-3.
47. Shams-White MM, Pannucci TE, Lerman JL, et al. Healthy eating index-2020: review and update process to reflect the dietary guidelines for Americans,2020-2025. J Acad Nutr Diet. 2023;123:1280-8.
48. Huang S, Zhong D, Lv Z, et al. Associations of multiple plasma metals with the risk of metabolic syndrome: A cross-sectional study in the mid-aged and older population of China. Ecotoxicol Environ Saf. 2022;231:113183.
49. Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015;20:100-20.
50. Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493-508.
51. Wu L, Zhang J, Xin Y, et al. Associations between phenols, parabens, and phthalates and depressive symptoms: The role of inflammatory markers and bioinformatic insights. Ecotoxicol Environ Saf. 2024;286:117191.
52. Feng C, Yang B, Wang Z, et al. Relationship of long-term exposure to air pollutant mixture with metabolic-associated fatty liver disease and subtypes: a retrospective cohort study of the employed population of Southwest China. Environ Int. 2024;188:108734.
53. Takefuji Y. Drug-induced inflammation: a review of literature. Chem Biol Interact. 2024;404:111282.
54. Gofton C, Upendran Y, Zheng MH, George J. MAFLD: how is it different from NAFLD? Clin Mol Hepatol. 2023;29:S17-31.
55. Kasongo AA, Leroux M, Amrouche-Mekkioui I, Belhadji-Domecq M, Aguer C. BPA exposure in L6 myotubes increased basal glucose metabolism in an estrogen receptor-dependent manner but induced insulin resistance. Food Chem Toxicol. 2022;170:113505.
56. Gear RB, Belcher SM. Impacts of bisphenol A and ethinyl estradiol on male and female CD-1 mouse spleen. Sci Rep. 2017;7:856.
57. Shi X, Wang W, Feng J, Ma X, Xu M, Wang C. Gender-specific abdominal fat distribution and insulin resistance associated with organophosphate esters and phthalate metabolites exposure. Environ Pollut. 2024;349:123959.
58. Shoshtari-Yeganeh B, Zarean M, Mansourian M, et al. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environ Sci Pollut Res Int. 2019;26:9435-42.
59. Zhou J, Shu R, Yu C, et al. Exposure to low concentration of trifluoromethanesulfonic acid induces the disorders of liver lipid metabolism and gut microbiota in mice. Chemosphere. 2020;258:127255.
60. Somani SM, Khalique A. Distribution and metabolism of 2,4-dichlorophenol in rats. J Toxicol Environ Health. 1982;9:889-97.
61. Zhang W, Shen XY, Zhang WW, Chen H, Xu WP, Wei W. Di-(2-ethylhexyl) phthalate could disrupt the insulin signaling pathway in liver of SD rats and L02 cells via PPARγ. Toxicol Appl Pharmacol. 2017;316:17-26.
62. Kratochvil I, Hofmann T, Rother S, et al. Mono(2-ethylhexyl) phthalate (MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) but not di(2-ethylhexyl) phthalate (DEHP) bind productively to the peroxisome proliferator-activated receptor γ. Rapid Commun Mass Spectrom. 2019;33 Suppl 1:75-85.
63. Ding Y, Gao K, Liu Y, et al. Transcriptome analysis revealed the mechanism of the metabolic toxicity and susceptibility of di-(2-ethylhexyl)phthalate on adolescent male ICR mice with type 2 diabetes mellitus. Arch Toxicol. 2019;93:3183-206.
64. Yu L, Yang M, Cheng M, et al. Associations between urinary phthalate metabolite concentrations and markers of liver injury in the US adult population. Environ Int. 2021;155:106608.
65. Lucas A, Herrmann S, Lucas M. The role of endocrine-disrupting phthalates and bisphenols in cardiometabolic disease: the evidence is mounting. Curr Opin Endocrinol Diabetes Obes. 2022;29:87-94.
66. Chen L, Chen J, Xie CM, Zhao Y, Wang X, Zhang YH. Maternal disononyl phthalate exposure activates allergic airway inflammation via stimulating the phosphoinositide 3-kinase/akt pathway in rat pups. Biomed Environ Sci. 2015;28:190-8.
67. Zhang Y, Qian H, Wang J, et al. Di-(2-ethylhexyl) phthalate (DEHP) promoted hepatic lipid accumulation by activating Notch signaling pathway. Environ Toxicol. 2023;38:1628-40.
68. Michalopoulou E, Thymis J, Lampsas S, et al. The triad of risk: linking MASLD, cardiovascular disease and type 2 diabetes; from pathophysiology to treatment. J Clin Med. 2025;14:428.
69. Kim H, Kil M, Han C. Urinary phthalate metabolites and anemia: findings from the Korean national environmental health survey (2015-2017). Environ Res. 2022;215:114255.
70. Li CL, Yao ZY, Zhang YF, et al. Bisphenols exposure and non-alcoholic fatty liver disease: from environmental trigger to molecular pathogenesis. Front Endocrinol. 2025;16:1606654.
71. Pirozzi C, Lama A, Annunziata C, et al. Oral bisphenol A worsens liver immune-metabolic and mitochondrial dysfunction induced by high-fat diet in adult mice: cross-talk between oxidative stress and inflammasome pathway. Antioxidants. 2020;9:1201.
72. Zhang Y, Wang S, Zhao T, et al. Mono-2-ethylhexyl phthalate (MEHP) promoted lipid accumulation via JAK2/STAT5 and aggravated oxidative stress in BRL-3A cells. Ecotoxicol Environ Saf. 2019;184:109611.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].