Cumulative metabolic, behavioral, and early-life factors for MASLD in Chinese children with validation in U.S. adolescents
Abstract
Aim: This study aimed to explore the cumulative effects of metabolic, behavioral, and early-life risk factors on metabolic dysfunction-associated steatotic liver disease (MASLD).
Methods: Data were obtained from a school-based longitudinal survey conducted in Beijing in 2023. Logistic regression models were used to examine independent associations, construct a risk score, and assess the cumulative effects of risk factors on pediatric MASLD. The risk score was further validated using data from the National Health and Nutrition Examination Survey (NHANES) 2017-2020 cycles.
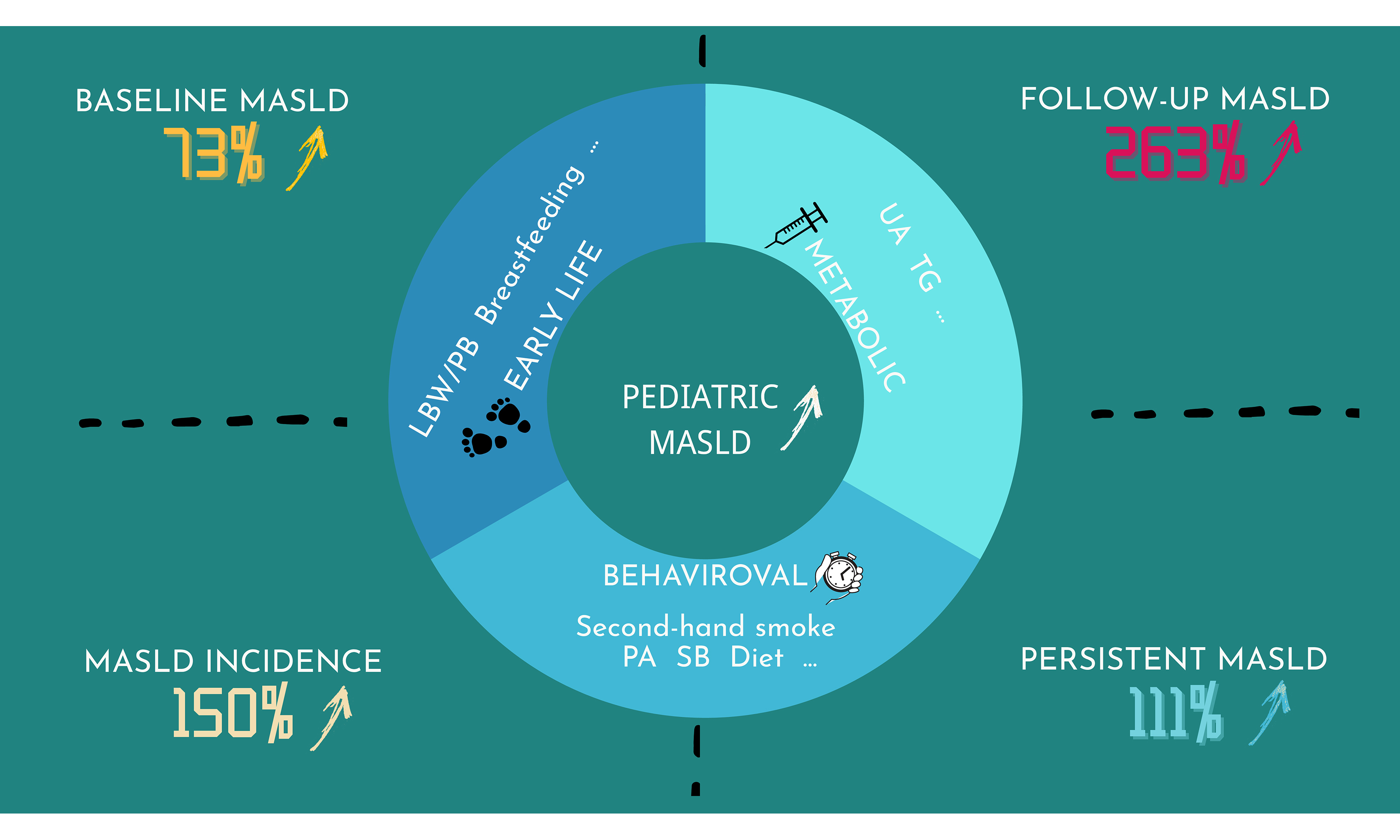
Results: The prevalence of MASLD among Chinese children was 4.4% at baseline and 7.6% at follow-up. Baseline MASLD was significantly associated with exposure to second-hand smoke (OR = 2.36) and sedentary behavior (SB, OR = 3.21). Each one-unit increase in the risk score was associated with a 73% higher risk of MASLD at baseline, with similar cumulative effects observed in the NHANES cohort. At follow-up, each unit increase in the score corresponded to a 263% higher risk of MASLD. Furthermore, the risk of incident MASLD and persistent MASLD increased by 150% and 111%, respectively, for each unit increase in the score.
Conclusion: Within a conceptual framework addressing multiple levels of risk, we found that metabolic, behavioral, and early-life factors exert cumulative effects on pediatric MASLD. These effects were evident despite the substantial differences between the Chinese and U.S. populations. Targeted intervention strategies informed by this framework - such as improving the early-life environment, promoting healthier lifestyle behaviors, and maintaining favorable metabolic profiles - are essential for the management of pediatric MASLD.
Keywords
INTRODUCTION
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a newly adopted term that replaces non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated fatty liver disease (MAFLD)[1]. MASLD is now recognized as the leading cause of chronic liver disease, affecting nearly 24% of the global population[2]. With rapid economic and social changes, accompanied by more sedentary lifestyles, less physical activity (PA), and excessive calorie intake from unhealthy diets[3], the prevalence of MASLD is expected to continue increasing alongside the growing rates of obesity. China, which has one of the largest obese populations worldwide, is projected to experience an increase in MASLD cases from 246.33 million in 2016 to 314.58 million by 2030[4]. Although the prevalence in children is lower than in adults, the burden of MASLD in children is also concerning, as they may develop more severe steatosis due to unawareness[5]. These trends underscore the need to investigate the risk factors of MASLD to inform policy-making and guide preventive interventions.
Previous studies have examined a range of risk factors for MASLD in adults, including genetic[6], metabolic, behavioral[7], and early-life factors. Compared with genetic factors, metabolic, behavioral, and early-life factors are modifiable and have a significant effect on the burden of non-communicable diseases. Among metabolic factors, obesity is considered the strongest risk factor for MASLD[8], while waist-to-height ratio (WHtR)[9], triglyceride (TG), and uric acid (UA) have also been shown to be correlated with MASLD[10]. Behavioral factors such as exposure to second-hand smoke[11], PA[12], sedentary behavior (SB)[13], and dietary patterns[12] are likewise linked to MASLD. In terms of early-life factors, low birth weight (LBW)[14], preterm birth (PB), and lack of breastfeeding[15] may increase susceptibility to MASLD. However, relatively few studies have investigated these risk factors in children, and very few have explored their cumulative effects. Considering that the histological features of MASLD in children differ from those in adults, and that their pathogenesis, prognosis, and treatment responses may also vary[8], it is necessary to assess the individual and combined effects of metabolic, behavioral, and early-life factors in pediatric populations.
Building on findings from adult studies, we hypothesized that metabolic, behavioral, and early-life factors are independently associated with MASLD in children, and that cumulative effects of these risk factors exist. The objectives of this study were to: (1) explore the independent and cumulative associations of metabolic, behavioral, and early-life factors with MASLD in children and (2) develop a nomogram for predicting MASLD risk in pediatric populations. We examined these associations using cross-sectional data, validated them in the National Health and Nutrition Examination Survey (NHANES), and further confirmed them with longitudinal data. The results of this study may help identify key modifiable risk factors requiring greater attention, provide evidence to guide future policy and interventions, and ultimately contribute to reducing the burden of MASLD and promoting overall health and well-being.
METHODS
Study population
Participants were selected from a cluster-randomized clinic trial conducted between April and December 2023 in Changping District, Beijing. The detailed study design has been published elsewhere[16,17]. Briefly, at baseline, a multistage stratified cluster sampling method was applied. In the first stage, 12 schools were selected from regions with different levels of economic development; six were randomly assigned to the intervention group (receiving myopia and obesity interventions), and the remaining six to the control group. In the second stage, classes were randomly selected from grades 1 to 4 and grade 7 (age range: 6-14 years). All students who provided informed consent, along with their parents, were included. In total, 597 students underwent abdominal ultrasonography and fasting blood tests for cross-sectional analysis. For longitudinal analysis, only 289 students from the control group were included. Comparisons between the populations included in the cross-sectional and longitudinal analyses are presented in Supplementary Table 1. The study was approved and supervised by the Ethics Committee of Review Board of Peking University Health Science Center (Approval No. 00001052-22018).
Diagnosis of MASLD
Hepatic steatosis was diagnosed using ultrasound scanners (GE Vivid i, Probo Medical, USA; M9, Mindray Medical, China) based on the presence of hepatorenal echo contrast, increased liver parenchymal brightness, deep attenuation, and vascular blurring[18]. A diagnosis of MASLD was made when hepatic steatosis was present in combination with one or more of the following five criteria: (1) Body mass index (BMI) ≥ the 85th percentile for age/sex or waist circumference (WC) > the 95th percentile; (2) fasting plasma glucose (FPG) ≥ 5.6 mmol/L; (3) blood pressure ≥ the 95th percentile[19], or ≥ 130/80 mmHg for children < 13 years and ≥ 130/85 mmHg for children ≥ 13 years; (4) TG concentration ≥ 1.15 mmol/L for children < 10 years and ≥ 1.70 mmol/L for children ≥ 10 years; and (5) high-density lipoprotein cholesterol (HDL-C) concentration ≤ 1.0 mmol/L[1].
Anthropometric measurements, questionnaire surveys, and clinical examination
Height, weight, and WC were measured via standardized procedures by well-trained examiners. Blood pressure was measured three times while participants were at rest. BMI and WHtR were calculated using the following formulas: BMI (kg/m²) = weight (kg)/height squared (m²); WHtR = WC (cm)/height (cm). To ensure accuracy, 5% of students were randomly selected for repeat measurements. Blood samples were analyzed in a certified laboratory, with 5% randomly reanalyzed for quality control. Two structured questionnaires - a student version and a parent version - were administered. These were developed based on preliminary and pilot studies and were found to be feasible and reliable for this research[16], with high internal consistency (Cronbach’s α = 0.921). The questionnaires collected information on exposure to second-hand smoke, PA, SB, diet, birth date, birth weight, PB, and breastfeeding history.
Definitions
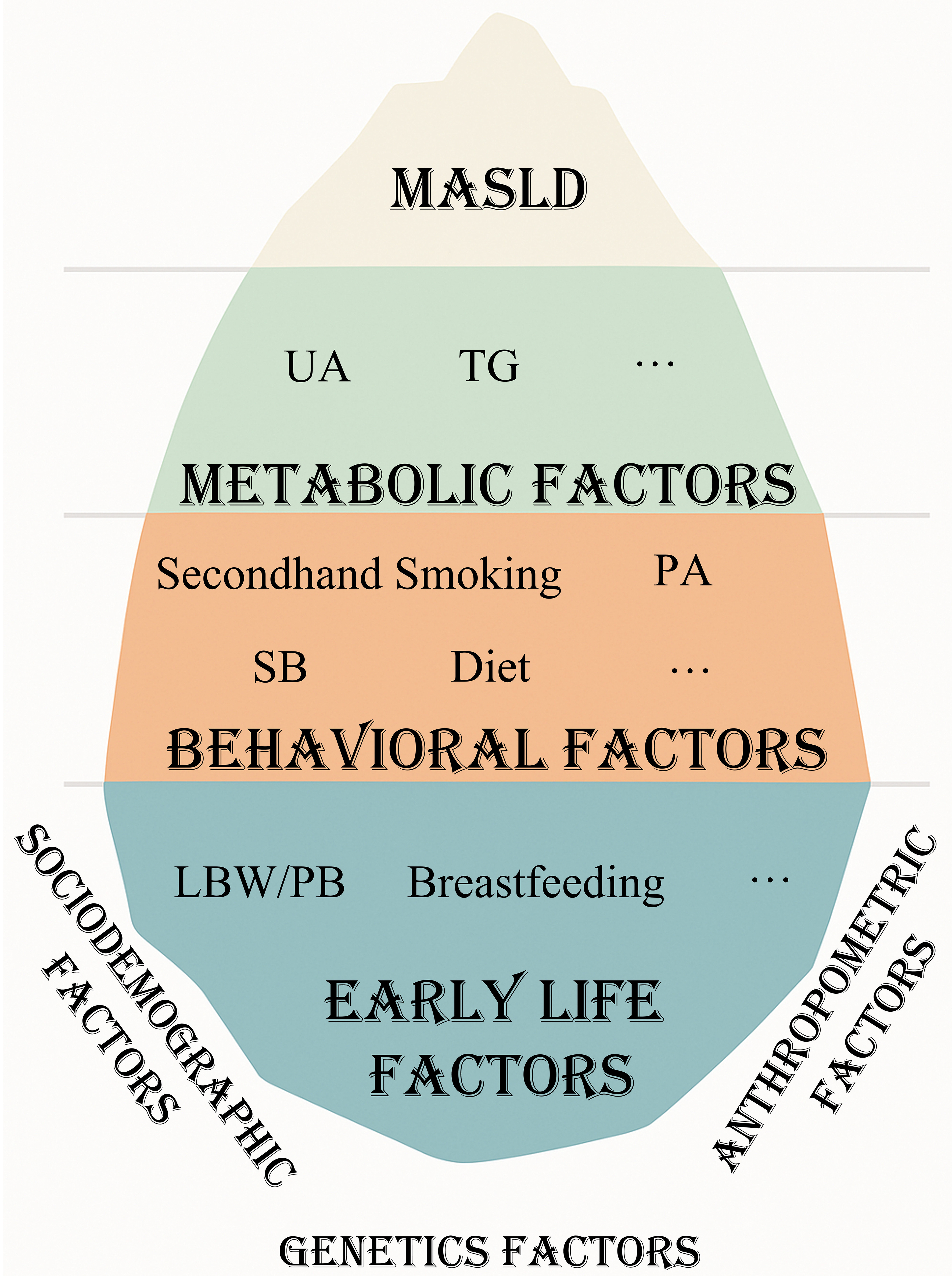
Based on previous adult studies, we constructed a conceptual framework of three levels of risk factors for MASLD: metabolic, behavioral, and early-life factors [Figure 1].
Figure 1. The iceberg model of MASLD: conceptual framework of risk factors. Figure generated using Microsoft PowerPoint. UA: Uric acid; TG: triglyceride; LBW/PB: low birth weight/ preterm birth; PA: physical activity; SB: sedentary behavior; MASLD: metabolic dysfunction-associated steatotic liver disease.
Metabolic factors
UA and TG were included in the analysis. Children were categorized into two groups for each indicator, according to the Guideline for the Diagnosis and Management of Hyperuricemia and Gout in China (2019)[20] and the Chinese Guidelines for Lipid Management (2023)[21] [Table 1].
Definitions of metabolic, early-life, and modifiable behavioral factors associated with MASLD
| Risk factors | Definition | Reference category |
| Metabolic factors | ||
| UA | Classified into two categories: (1) > 0.42 mmol/L; (2) ≤ 0.42 mmol/L | ≤ 0.42 mmol/L |
| TG | Classified into two categories: (1) ≥ 1.1 mmol/L (children < 10 years) or ≥ 1.5 mmol/L (children ≥ 10 years); (2) < 1.1 mmol/L (children < 10 years) or < 1.5 mmol/L (children ≥ 10 years) | < 1.1 mmol/L (children < 10 years) and < 1.5 mmol/L (children ≥ 10 years) |
| Behavioral factors | ||
| Exposure to second-hand smoke | Number of days exposed to second-hand smoke in the past 7 days. Classified into: (1) 0 days; (2) 1-7 days | 0 days |
| PA | Average daily MVPA during the past week.Classified into: (1) ≥ 70 min/day; (2) < 70 min/day | ≥ 70 min/day |
| SB | Average daily sedentary time. Classified into: (1) ≤ 6 h/day; (2) > 6 h/day | ≤ 6 h/day |
| Diet | Number of days with adequate intake of fruits, vegetables, and protein in the past 7 days. Classified into: (1) 0 days; (2) 1-7 days | 1-7 days |
| Early-life factors | ||
| LBW/PB | Children with birth weight < 2,500 g were classified as LBW; those with gestational age < 37 weeks were classified as PB. Classified into: (1) No LBW and no PB; (2) LBW or PB | No LBW and no PB |
| Breastfeeding history | Classified into two categories: (1) ≥ 6 months; (2) < 6 months | ≥ 6 months |
Behavioral factors
Behavioral risk factors included second-hand smoke, PA, SB, and diet habits. Each was dichotomized as shown in Table 1.
Early-life factors
Breastfeeding history was classified as ≥ 6 months or < 6 months. Children with a birth weight < 2,500 g were categorized as having LBW, and those with a gestational age < 37 weeks as PB[22]. LBW/PB referred to children with either LBW or PB. Detailed definitions are provided in Table 1.
Data imputation
We used multivariate imputation by chained equations (R package “mice”) to address missing values for early-life and behavioral risk factors, with anthropometric characteristics, age, sex, school, and grade as predictors. A comparison of datasets before and after imputation is presented in Supplementary Table 2.
Statistical analysis
Basic characteristics of the children
Continuous variables with normal distributions are presented as means with standard errors (SEs), whereas those with non-normal distributions are reported as medians with interquartile ranges (IQRs). Categorical variables are shown as frequencies and percentages. To assess group differences, we used one-way ANOVA, the Kruskal-Wallis test, and either the Chi-square test or Fisher’s exact test, as appropriate. We also estimated prevalence rates and examined sex-specific differences in risk factor levels.
Model construction for independent effects in cross-sectional data
We applied multivariate binary logistic regression to construct the crude model (Model 1), with reference categories displayed in Table 1. Model 2 additionally adjusted for WHtR group (≥ 0.48 vs. < 0.48), Model 3 further adjusted for sex and age group, and Model 4 additionally accounted for the cluster effect of school. We also developed a nomogram to predict MASLD, enabling comparison of factor importance and providing a user-friendly tool to assess childhood MASLD risk[23].
Risk score construction and cumulative effects in cross-sectional data
Based on the results of Models 1 to 4, we constructed a risk score to capture the combined influence of risk factors on MASLD. For factors with odds ratio (OR) > 1, the reference category was assigned a score of 0 and the alternative category a score of 1. For factors with OR < 1, the scoring was reversed. Total scores were then summed across all risk factors. We used a generalized additive model (GAM) to examine the relationship between MASLD and the risk score and to test for nonlinear associations. Logistic regression was also applied, with the same adjustment sequence as above (Models 1-4).
Model validation for independent and cumulative effects in longitudinal data
The same models were applied to longitudinal data to validate both independent and cumulative effects of metabolic, behavioral, and early-life factors on MASLD. Children without MASLD at baseline who developed MASLD during follow-up were classified as the incident MASLD group, whereas those with MASLD at both baseline and follow-up were classified as the persistent MASLD group. We evaluated the cumulative effects of risk factors on both MASLD incidence and persistence.
Sensitivity analyses
We performed sensitivity analyses by reclassifying breastfeeding duration, PA, and SB using cutoffs of 12 months, 60 min, and 300 min, respectively, to assess model robustness. Additionally, the effect size (β) from the independent associations was applied as a weight when constructing risk scores in both cross-sectional and longitudinal analyses.
10-fold cross-validation
We used 10-fold cross-validation to further validate the model. Participants were randomly divided into 10 equal subsets. For each subset, the model was trained on the remaining nine and tested on the current subset. Model performance was evaluated by averaging log-likelihood error measures.
External validation
Due to data accessibility, we validated our risk score in a cross-sectional dataset of 1,584 adolescents aged 12-19 years from the 2017-2020 NHANES cycles, who had complete data on body measurements, TG, second-hand smoke exposure, PA, and birth weight[24]. Hepatic steatosis was defined as controlled attenuation parameter > 248 dB/m.
Figures were created using R version 4.2.1 and Microsoft PowerPoint. All statistical analyses were performed in R version 4.2.1, and a two-tailed P < 0.05 was considered statistically significant.
RESULTS
General characteristics of the participants
In the baseline survey, 597 children aged 6 to 14 years were enrolled, including 297 boys (49.7%) and 300 girls (50.3%). Boys had a higher prevalence of overweight (15.8% vs. 14.0%) and obesity (24.2% vs. 16.7%) compared with girls. Boys also showed a higher WHtR, a higher prevalence of UA, and a higher rate of not being breastfed, whereas girls were more likely to have non-optimal PA and SB [Supplementary Table 3]. The overall prevalence of MASLD was 4.4%, higher in boys (7.4%) than in girls (1.3%). Children with MASLD had higher WHtR and were more likely to have elevated UA levels (34.6% vs. 14.0%) and a non-optimal diet (20.8% vs. 6.8%). Among the 289 children included in the follow-up survey, 137 (47.4%) were boys and 152 (52.6%) were girls. Boys again had a higher WHtR than girls [Supplementary Table 3]. The prevalence of MASLD was 7.6%. Children with MASLD had higher WHtR and were more likely to present with elevated UA (31.8% vs. 13.5%) and TG (40.9% vs. 9.7%), no breastfeeding history (35.3% vs. 13.8%), and exposure to second-hand smoke (63.6% vs. 32.6%) [Table 2].
Basic features of participants by MASLD status
| Cross-sectional | Longitudinal | |||||
| No MASLD | MASLD | P | No MASLD | MASLD | P | |
| N | 571 | 26 | 267 | 22 | ||
| Sociodemographic and anthropometric factors | ||||||
| Age at baseline, year* | 9.00 (8.00, 10.00) | 9.00 (8.00, 10.00) | 0.396 | 9.00 (8.00, 11.00) | 10.00 (9.00, 10.75) | 0.083 |
| Girls** | 296 (51.8) | 4 (15.4) | 0.001 | 144 (53.9) | 8 (36.4) | 0.173 |
| WHtR* | 0.42 (0.39, 0.46) | 0.55 (0.52, 0.58) | < 0.001 | 0.42 (0.40, 0.45) | 0.54 [0.50, 0.59] | < 0.001 |
| Nutrition status** | < 0.001 | < 0.001 | ||||
| Non-overweight/obese | 386 (67.6) | 0 (0.0) | 185 (69.3) | 1 (4.5) | ||
| Overweight | 86 (15.1) | 3 (11.5) | 45 (16.9) | 3 (13.6) | ||
| Obese | 99 (17.3) | 23 (88.5) | 37 (13.9) | 18 (81.8) | ||
| Metabolic factors | ||||||
| High UA** | 80 (14.0) | 9 (34.6) | 0.009 | 36 (13.5) | 7 (31.8) | 0.044 |
| High TG** | 74 (13.0) | 6 (23.1) | 0.235 | 26 (9.7) | 9 (40.9) | < 0.001 |
| Early-life factors | ||||||
| LBW/PB | 30 (15.2) | 4 (30.8) | 0.278 | 13 (14.9) | 3 (37.5) | 0.255 |
| No breastfeeding** | 90 (19.0) | 6 (35.3) | 0.176 | 28 (13.8) | 6 (35.3) | 0.045 |
| Behavioral factors | ||||||
| Exposure to second-hand smoke** | 181 (31.7) | 12 (46.2) | 0.185 | 87 (32.6) | 14 (63.6) | 0.007 |
| Non-optimal PA** | 392 (68.7) | 21 (80.8) | 0.275 | 181 (67.8) | 16 (72.7) | 0.811 |
| Non-optimal SB** | 111 (36.2) | 6 (42.9) | 0.822 | 51 (32.1) | 5 (41.7) | 0.716 |
| Non-optimal diet** | 36 (6.8) | 5 (20.8) | 0.030 | 21 (8.5) | 3 (15.0) | 0.568 |
Independent effects of metabolic, behavioral, and early-life factors associated with MASLD
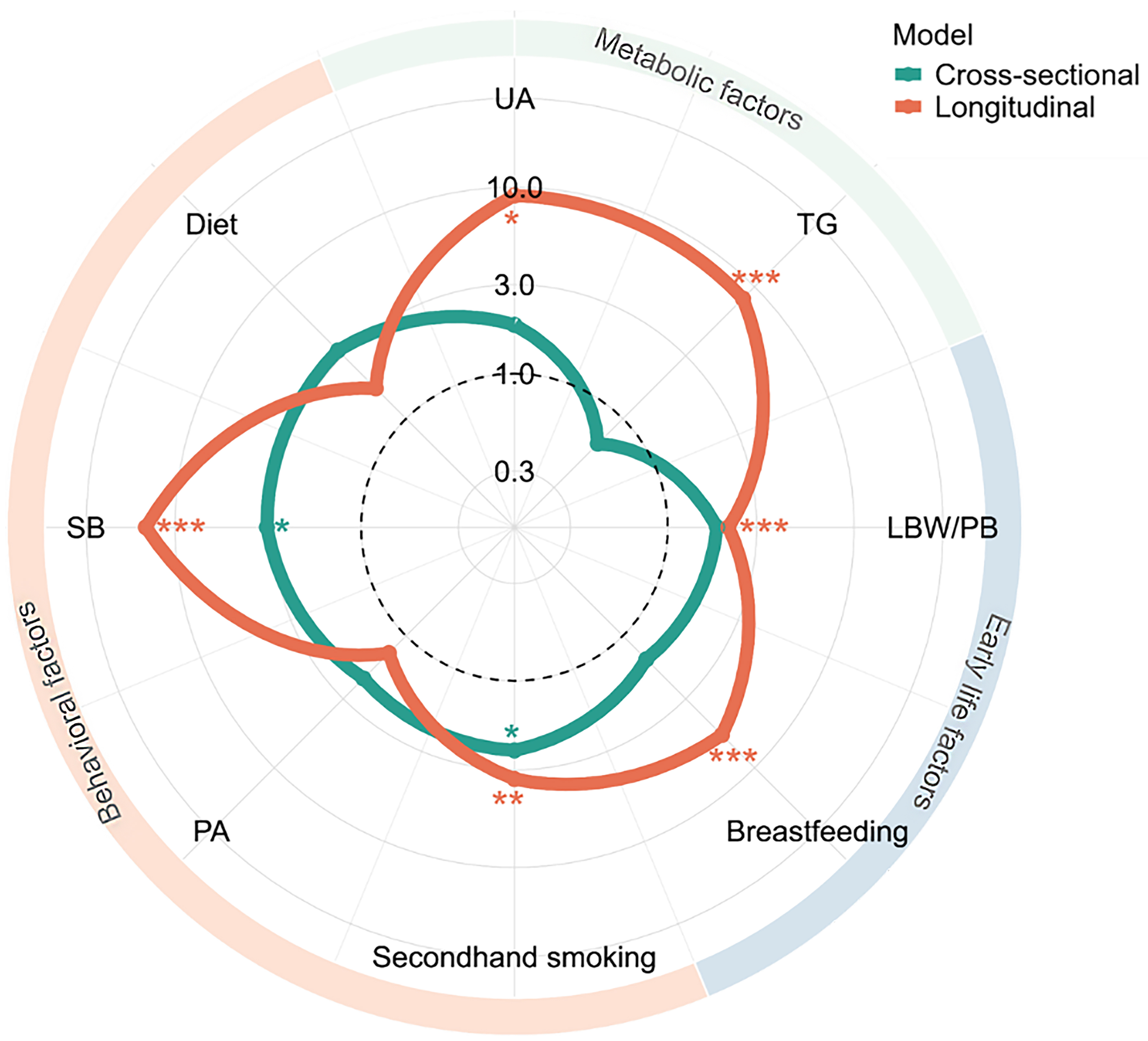
In the final multivariate model, two factors were independently associated with MASLD: exposure to second-hand smoke [OR = 2.36, 95% confidence interval (CI): 1.07-5.17] and non-optimal SB (OR = 3.21, 1.02-10.11), after adjustment for WHtR, sex, age group, and school clustering [Figure 2 and Supplementary Figure 1A]. Sensitivity analyses produced results consistent with the main analysis [Supplementary Figure 2A]. The log-likelihood error for 10-fold cross-validation was 0.039 [Supplementary Table 4].
Figure 2. Independent effects of metabolic, behavioral, and early-life factors on baseline and follow-up MASLD. Numbers represent ORs for each factor. Models were adjusted for age, sex, WHtR group, and school clustering. Figure generated using R 4.2.1. UA: Uric acid; TG: triglyceride; LBW/PB: low birth weight/ preterm birth; PA: physical activity; SB: sedentary behavior; MASLD: metabolic dysfunction-associated steatotic liver disease; WHtR: waist-to-height ratio; OR: odds ratio. *P < 0.05; **P < 0.01; ***P < 0.001.
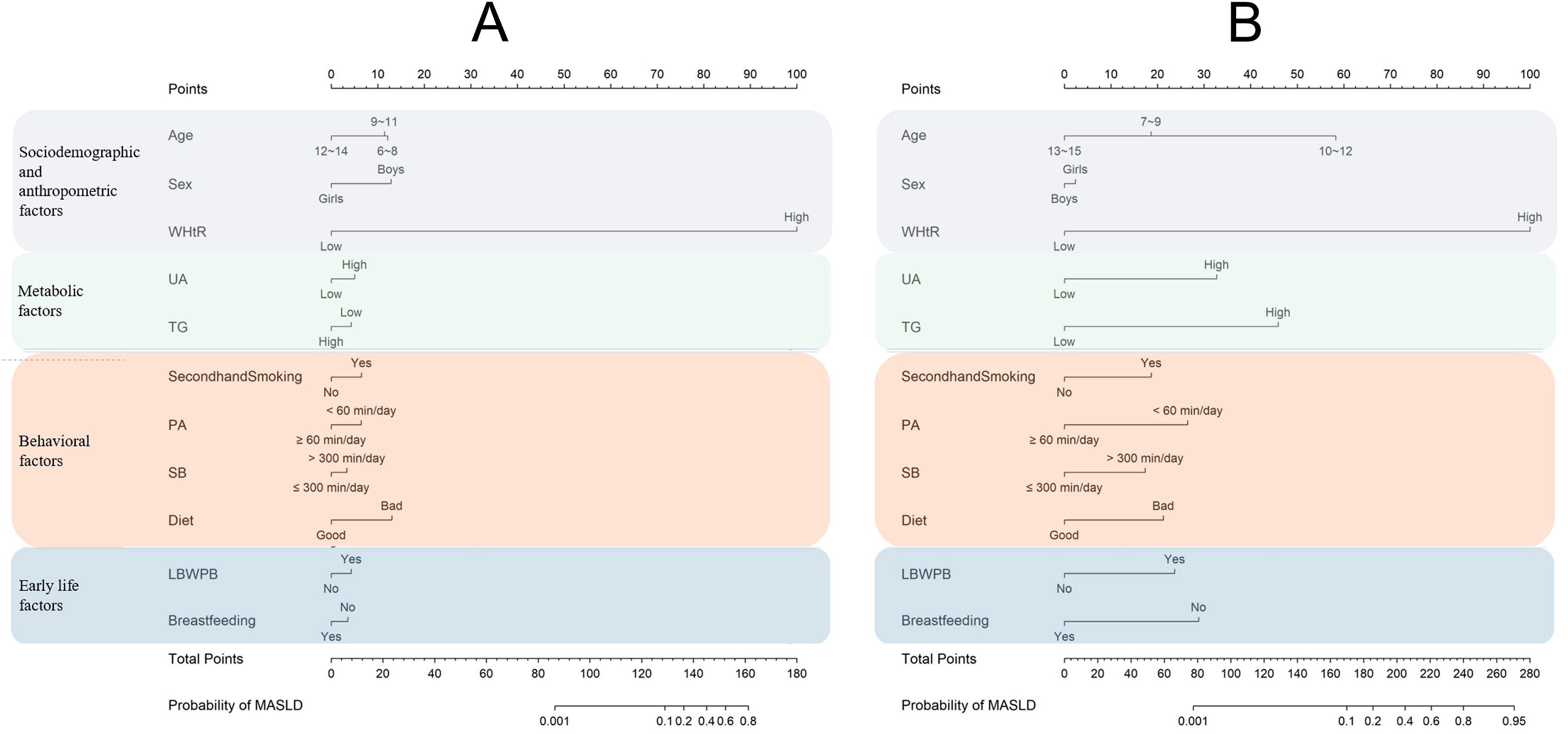
We used eight metabolic, behavioral, and early-life factors, together with age, sex, and WHtR, to construct a nomogram for predicting MASLD [Figure 3A]. WHtR had the strongest effect on MASLD. After controlling for WHtR, sex, and age group, non-optimal SB, non-optimal diet, and second-hand smoke exposure contributed the greatest point values among the eight factors. The total score and corresponding probability of MASLD are shown at the bottom of the nomogram. Sensitivity analyses yielded results consistent with the main analysis [Supplementary Figure 3A].
Figure 3. Nomograms of sociodemographic and anthropometric, metabolic, behavioral, and early-life factors for (A) baseline MASLD and (B) follow-up MASLD. Figure generated using R 4.2.1. WHtR: Waist-to-height ratio; UA: uric acid; TG: triglyceride; LBW/PB: low birth weight/ preterm birth; PA: physical activity; SB: sedentary behavior; MASLD: metabolic dysfunction-associated steatotic liver disease.
Cumulative effects of metabolic, behavioral, and early-life factors associated with MASLD
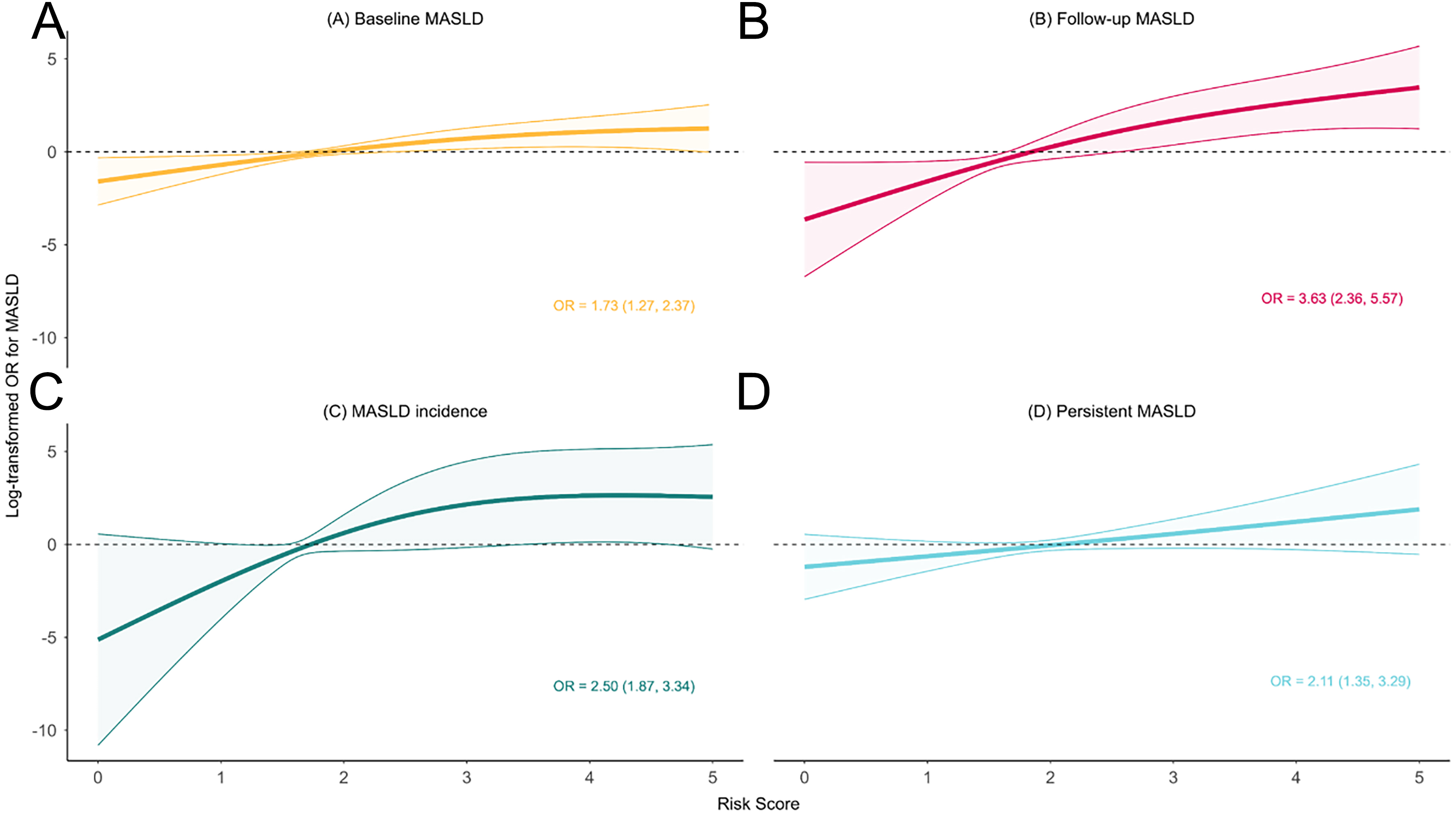
We observed a linear relationship between the cumulative risk score and the log-transformed OR for MASLD. After adjusting for WHtR, sex, age group, and school clustering, each unit increase in the risk score was associated with a 73% higher risk of MASLD (95%CI: 27%-137%) [Figure 4 and Supplementary Figure 4]. Sensitivity analyses confirmed these findings [Supplementary Figures 5 and 6]. The log-likelihood error for 10-fold cross-validation was 0.033 [Supplementary Table 4]. In U.S adolescents, where only four factors were available, each unit increase in the risk score was associated with a 20% higher risk of MASLD (95%CI: 0.4%-43%) [Supplementary Figure 7].
Figure 4. Cumulative effects of metabolic, behavioral, and early-life factors on (A) baseline MASLD; (B) follow-up MASLD; (C) MASLD incidence; and (D) persistent MASLD. Models were adjusted for age, sex, WHtR group, and school clustering. Figure generated using R 4.2.1. OR: Odds ratio; MASLD: metabolic dysfunction-associated steatotic liver disease; WHtR: waist-to-height ratio.
Independent and cumulative effects in longitudinal analyses
In longitudinal analyses, six baseline factors were independently associated with follow-up MASLD after adjustment for baseline WHtR, sex, age group, and school clustering: high UA, high TG, second-hand smoke exposure, non-optimal SB, LBW/PB, and no breastfeeding history [Figure 2 and Supplementary Figure 1B]. Sensitivity analyses yielded consistent results [Supplementary Figure 2B]. The log-likelihood error for 10-fold cross-validation was 0.050 [Supplementary Table 4]. After adjustment for baseline WHtR, sex, and age group, non-optimal SB, high UA, and high TG contributed the highest point values among the eight factors in the nomogram [Figure 3B]. Sensitivity analyses again confirmed the findings
Each unit increase in the cumulative risk score was associated with a 263% higher risk of follow-up MASLD (95%CI: 136%-457%) [Figure 4]. Sensitivity analyses were consistent [Supplementary Figures 5 and 6]. The log-likelihood error for 10-fold cross-validation was 0.045 [Supplementary Table 4]. Furthermore, the risk of incident MASLD and persistent MASLD increased by 150% (95%CI: 87%-234%) and 111% (95%CI: 35%-229%), respectively, for each unit increase in the risk score [Figure 4 and Supplementary Figure 4]. The log-likelihood errors for 10-fold cross-validation were 0.038 and 0.037, respectively [Supplementary Table 5].
DISCUSSION
Using the updated definition of MASLD, we investigated metabolic, behavioral, and early-life factors associated with MASLD in children aged 6 to 14 years in Beijing, China, and further validated these associations in U.S. adolescents. In our study, 4.4% (26/597) of children were diagnosed with MASLD at baseline, whereas the prevalence increased to 7.6% (22/289) at follow-up, with higher rates observed in boys than girls. Several modifiable metabolic, behavioral, and early-life factors were identified as being associated with MASLD risk. Additionally, we observed a dose-response relationship between the cumulative risk score and baseline MASLD in both Chinese and U.S. adolescents. In longitudinal analyses, the risk score was also associated with follow-up MASLD, MASLD incidence, and persistent MASLD.
At baseline and follow-up, 4.4% and 7.6% of children had MASLD, respectively, which is higher than the rates reported in a 2007 study[25] but consistent with findings from several recent studies[26-28]. This trend may reflect the gradual development of metabolic changes in children with long-term obesity[29,30]. Boys were at higher risk than girls, consistent with previous reports[31], likely reflecting sex differences in childhood obesity. These findings highlight the importance of sex-specific interventions.
Previous studies have indicated that the WHtR is a strong predictor of pediatric MASLD[17]. Accordingly, we adjusted for WHtR while exploring associations between metabolic, behavioral, and early-life factors and MASLD. After controlling for age, sex, WHtR, and school cluster effects, cross-sectional analyses revealed that exposure to second-hand smoke and non-optimal SB (> 6 h per day) were significantly associated with pediatric MASLD. In longitudinal analyses, high UA, high TG, LBW/PB, and lack of breastfeeding were associated with follow-up MASLD. These findings align with previous studies[10-13]. Mechanistically, second-hand smoke may cause liver damage by activating fibroblasts and pro-fibrotic pathways or through nicotinic acetylcholine receptors[32]. Breast milk oligosaccharides might promote beneficial gut microbiota, offering protection against MASLD[33], and mouse experiments reveal that breast milk-derived extracellular vesicles can effectively alleviate liver steatosis and insulin resistance in MASLD mice induced by a high-fat diet by inhibiting lipogenesis and enhancing lipolysis[34]. Notably, non-optimal SB emerged as the strongest factor affecting pediatric MASLD in both cross-sectional and longitudinal analyses, even after adjusting for confounders. This supports previous evidence that SB is an independent risk factor for pediatric MASLD[35], underscoring the importance of interventions to reduce sedentary time in children. For metabolic factors, UA and TG were not significant in cross-sectional analyses, unlike in adults[10]; however, longitudinally, both were significantly associated with MASLD and ranked as the second and third most influential factors. Elevated UA may contribute to MASLD via insulin resistance and mitochondrial oxidative stress[36]. The lack of significance for TG in cross-sectional analyses could reflect a transient protective effect against metabolic disturbances, masking short-term impacts[37]. Nevertheless, follow-up data indicate that TG levels significantly influence future MASLD development, likely through inflammatory mechanisms[37]. These results emphasize the importance of maintaining healthy metabolic profiles to prevent MASLD in children.
Constructing cumulative risk scores is a common approach[38] to simplify assessment and highlight the impact of multiple factors. In this study, we developed a cumulative risk score for metabolic, behavioral, and early-life factors. We observed a clear dose-response relationship, with MASLD risk increasing as the number of risk factors rose. Similar trends were observed in the NHANES adolescent population, even though not all risk factors were included. Specifically, each unit increase in the risk score was associated with a 73% increase in baseline MASLD risk, a 263% increase in follow-up MASLD, a 150% increase in MASLD incidence, and a 111% increase in persistent MASLD. These findings highlight the importance of addressing multiple modifiable risk factors and implementing targeted interventions for high-risk children and their families.
This study comprehensively examined metabolic, behavioral, and early-life factors and their cumulative effects on pediatric MASLD under the updated definition. The NHANES adolescent population was used for external validation. Hepatic steatosis was assessed using semi-quantitative ultrasonography, distinguishing this study from older investigations[39]. Our findings emphasize the importance of multidimensional interventions targeting metabolic, behavioral, and early-life factors. Such interventions require coordinated efforts across individual, family, and school levels to promote healthier behaviors and environments that can reduce pediatric MASLD prevalence.
Several limitations should be noted. First, the sample size was relatively small, and focusing on specific regions and age groups limited subgroup analyses by sex or pubertal status. Previous studies suggest these differences are important[40-45], indicating the need for future research focusing on boys and pre-pubertal children, as they are at greater risk of MASLD, and the specific mechanisms and interventions still warrant further exploration and validation in large populations[46]. Second, due to data limitations, surrogate biomarkers of liver fibrosis (e.g., ALT: AST ratio, fibrosis-4 index) were unavailable[47,48], and other metabolic factors, such as insulin resistance and glycated hemoglobin A1C, were not included. Third, the short follow-up interval may have limited detection of true changes in MASLD prevalence; the observed increase over eight months raises the possibility of selection bias. Thus, the findings of longitudinal analysis should be interpreted with caution. Fourth, while external validation was performed, not all risk factors were captured in NHANES, longitudinal data were not available, age ranges differed, and ethnic differences existed. Despite attenuated effects, significant associations suggest some generalizability. Future work should establish a large, cross-ethnic, longitudinal pediatric MASLD data-sharing platform. Fifth, recall bias may have affected early-life and behavior factor measurements; however, our questionnaire was developed from pilot studies and considered feasible[16]. Finally, unmeasured confounders could have influenced findings.
In conclusion, metabolic, behavioral, and early-life factors significantly influence pediatric MASLD risk, and their cumulative effects can rapidly increase risk in both Chinese and U.S. children and adolescents. Interventions targeting children and their families to improve early-life conditions, promote healthier lifestyles, and maintain optimal metabolic health are essential to reduce these risks and the overall burden of pediatric MASLD.
DECLARATIONS
Acknowledgments
The authors thank the team and participants of the National Health and Nutrition Examination Survey.
Authors’ contributions
Conceived this study: Liu Y, Song Y
Accessed the data, did the primary analysis, formulated the figure and table, and drafted the first version of the manuscript: Liu Y
Collected the data: Liu Y, Shi D, Chen Z, Dang J, Cai S, Wang Y, Liu J, Guo L, Li Y, Cui Y
Contributed to the interpretation of the results and the review of the manuscript: Shi D, Chen Z, Dang J, Cai S
Critically reviewed and substantially edited the manuscript: Song J, Li J, Dong Y
Responsible for general supervision and had final responsibility for the decision to submit for publication: Song Y
All authors reviewed the article, read the final version of the manuscript, and approved the submission.
Availability of data and materials
The datasets used and/or analyzed during the current study are available within this Article and its Supplementary Materials. Further data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82273654) and Capital’s Funds for Health Improvement and Research (Grant No. 2022-1G-4251).
Conflicts of interest
Song Y is a Guest Editor of the Special Issue Metabolic Heterogeneity in Childhood Obesity and Preventive Strategy. Song Y was not involved in any steps of the editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was approved and supervised by the Ethics Committee of Review Board of Peking University Health Science Center (Approval No. 00001052-22018). All participants and their parents provided informed consent prior to the study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Rinella ME, Lazarus JV, Ratziu V, et al; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-56.
2. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20.
3. Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7:276-88.
4. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904.
5. Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. 2016;150:1798-810.
6. Chen Y, Du X, Kuppa A, et al. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat Genet. 2023;55:1640-50.
7. Yuan Q, Wang H, Gao P, et al. Prevalence and risk factors of metabolic-associated fatty liver disease among 73,566 individuals in Beijing, China. Int J Environ Res Public Health. 2022;19:2096.
8. Goldner D, Lavine JE. Nonalcoholic fatty liver disease in children: unique considerations and challenges. Gastroenterology. 2020;158:1967-83.e1.
9. Zong X, Kelishadi R, Hong YM, et al. Establishing international optimal cut-offs of waist-to-height ratio for predicting cardiometabolic risk in children and adolescents aged 6-18 years. BMC Med. 2023;21:442.
10. Chen YL, Li H, Li S, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21:212.
11. Liu E, Li Q, Pan T, Chen Y. Association between secondhand smoke exposure and nonalcoholic fatty liver disease in the general U.S. adult nonsmoker population. Nicotine Tob Res. 2024;26:663-8.
12. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-46.
13. Tsunoda K, Kitano N, Kai Y, Jindo T, Uchida K, Arao T. Dose-response relationships of accelerometer-measured sedentary behaviour and physical activity with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1330-9.
14. Ebrahimi F, Yao J, Hagström H, et al. Birth weight, gestational age, and risk of pediatric-onset MASLD. JAMA Netw Open. 2024;7:e2432420.
15. Querter I, Pauwels NS, De Bruyne R, et al. Maternal and perinatal risk factors for pediatric nonalcoholic fatty liver disease: a systematic review. Clin Gastroenterol Hepatol. 2022;20:740-55.
16. Wang X, Dang J, Liu J, et al. A cluster randomized trial of a comprehensive intervention nesting family and clinic into school centered implementation to reduce myopia and obesity among children and adolescents in Beijing, China: study protocol. BMC Public Health. 2023;23:1435.
17. Liu Y, Wang Y, Xing Y, et al. Establish a noninvasive model to screen metabolic dysfunction-associated steatotic liver disease in children aged 6-14 years in China and its applications in high-obesity-risk countries and regions. Lancet Reg Health West Pac. 2024;49:101150.
18. Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954-61.
19. Flynn JT, Kaelber DC, Baker-Smith CM, et al; SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017; 140(3):e20171904. Pediatrics. 2017;140:e20173035.
20. Chinese Society of Endocrinology CMA. Guideline for the diagnosis and management of hyperuricemia and gout in China (2019). Chin J Endocrinol Metab. 2020;36:1-13. (in Chinese).
21. Committee on the Chinese Guidelines for Lipid Management. [Chinese guidelines for lipid management (2023)]. Zhonghua Xin Xue Guan Bing Za Zhi. 2023;51:221-55.
22. Care of Preterm or Low Birthweight Infants Group. Electronic address: [email protected]; Care of Preterm or Low Birthweight Infants Group. New WHO recommendations for the care of preterm or low birthweight infants have the potential to transform maternal and newborn health-care delivery. Lancet. 2022;400:1828-31.
23. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-70.
24. prevention UScfdca. National Health and Nutrition Examination Survey. Available from: https://www.cdc.gov/nchs/nhanes/index.html. [Last accessed on 3 Sep 2025].
25. Zhou YJ, Li YY, Nie YQ, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419-24.
26. Yang S, Zhong J, Ye M, et al. Association between the non-HDL-cholesterol to HDL-cholesterol ratio and non-alcoholic fatty liver disease in Chinese children and adolescents: a large single-center cross-sectional study. Lipids Health Dis. 2020;19:242.
27. Li M, Shu W, Zunong J, et al. Predictors of non-alcoholic fatty liver disease in children. Pediatr Res. 2022;92:322-30.
28. Zhang X, Wan Y, Zhang S, et al. Nonalcoholic fatty liver disease prevalence in urban school-aged children and adolescents from the Yangtze River delta region: a cross-sectional study. Asia Pac J Clin Nutr. 2015;24:281-8.
29. Luo D, Ma N, Liu Y, et al. Long-term trends and urban-rural disparities in the physical growth of children and adolescents in China: an analysis of five national school surveys over three decades. Lancet Child Adolesc Health. 2023;7:762-72.
30. Dong Y, Lau PWC, Dong B, et al. Trends in physical fitness, growth, and nutritional status of Chinese children and adolescents: a retrospective analysis of 1·5 million students from six successive national surveys between 1985 and 2014. Lancet Child Adolesc Health. 2019;3:871-80.
31. Obita G, Alkhatib A. Disparities in the prevalence of childhood obesity-related comorbidities: a systematic review. Front Public Health. 2022;10:923744.
32. She D, Jiang S, Yuan S. Association between serum cotinine and hepatic steatosis and liver fibrosis in adolescent: a population-based study in the United States. Sci Rep. 2024;14:11424.
33. Quek SXZ, Tan EX, Ren YP, et al. Factors early in life associated with hepatic steatosis. World J Hepatol. 2022;14:1235-47.
34. Jiang X, Wu Y, Zhong H, et al. Human milk-derived extracellular vesicles alleviate high fat diet-induced non-alcoholic fatty liver disease in mice. Mol Biol Rep. 2023;50:2257-68.
35. Kim D, Konyn P, Cholankeril G, Ahmed A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by fibroscan. Clin Gastroenterol Hepatol. 2022;20:e1438-55.
36. Sun Q, Zhang T, Manji L, et al. Association between serum uric acid and non-alcoholic fatty liver disease: an updated systematic review and meta-analysis. Clin Epidemiol. 2023;15:683-93.
37. Lee E, Korf H, Vidal-Puig A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J Hepatol. 2023;78:1048-62.
38. Li Z, Kong Y, Chen S, et al. Independent and cumulative effects of risk factors associated with stillbirths in 50 low- and middle-income countries: a multi-country cross-sectional study. EClinicalMedicine. 2022;54:101706.
39. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-90.
40. Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61-71.e15.
41. Burra P, Zanetto A, Schnabl B, et al. Hepatic immune regulation and sex disparities. Nat Rev Gastroenterol Hepatol. 2024;21:869-84.
42. Burra P, Bizzaro D, Gonta A, et al; Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021;41:1713-33.
43. Lonardo A, Suzuki A. Sexual dimorphism of NAFLD in adults. Focus on clinical aspects and implications for practice and translational research. J Clin Med. 2020;9:1278.
44. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457-69.
45. Suzuki A, Abdelmalek MF, Schwimmer JB, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:786-94.
46. Arnold AP, Klein SL, McCarthy MM, Mogil JS. Male-female comparisons are powerful in biomedical research - don’t abandon them. Nature. 2024;629:37-40.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].