Large-scale hydrogen storage-transportation equipment safety and accident chain interruption keys for petrochemical industry
Abstract
As global energy demands escalate, hydrogen has gained increasing recognition as a viable alternative fuel and critical energy carrier for future industrial systems. This review examines unique safety challenges associated with industrial-scale hydrogen storage and transportation infrastructure within petroleum and chemical processing sectors. Systematic analysis of hydrogen storage and transportation equipment failure mechanisms reveals three primary risk dimensions: material degradation from hydrogen embrittlement, gas leakage in high-pressure configurations, and combustion/detonation hazards in complex industrial settings. A targeted accident prevention framework emerges through three synergistic strategies: (1) High-performance material engineering for pressure vessels and pipeline networks, (2) Smart monitoring architectures for leak detection in large-scale installations, and (3) Customized deflagration suppression systems for chemical plant applications. By synthesizing cutting-edge hydrogen safety technologies with industrial case analyses, the study proposes an integrated safety management paradigm combining material innovation, predictive monitoring, and explosion mitigation. These technical countermeasures address operational requirements of petrochemical enterprises in hydrogen infrastructure development while establishing comprehensive safety protocols for industrial hydrogen utilization. The insights offer implementable strategies for enabling secure, large-scale hydrogen deployment in energy-intensive petroleum refining and chemical production environments.
Keywords
INTRODUCTION
Hydrogen is widely regarded as a clean secondary energy source, prized for its high calorific value, abundant availability, and potential to serve as a transformative energy carrier in the 21st century. Rapid advancements in hydrogen production, storage, and fuel cell technologies have propelled its adoption as a critical component of global decarbonization strategies[1]. China’s Medium and Long-term Development Plan for the Hydrogen Energy Industry (2021-2035) underscores hydrogen’s pivotal role in future energy systems, framing it as both a catalyst for green energy transitions and a cornerstone of strategic industrial development. However, the very properties that make hydrogen attractive - its odorless, colorless nature and high energy density - also introduce significant safety risks. Its wide flammability range (4-75 vol% in air), exceptionally low ignition energy (as little as 0.017 millijoules), and rapid flame propagation speeds (exceeding 970 meters/second under certain concentrations) create inherent hazards[2]. Moreover, hydrogen’s reactivity with materials during storage and transport can induce structural failures, such as hydrogen embrittlement, compromising containment systems and increasing leakage risks - a precursor to catastrophic fires or explosions.
Historical incidents starkly illustrate these dangers [Figure 1][3-7]. In 2007, a hydrogen transfer accident in Muskingum, Ohio, involving a ruptured disk, corroded ventilation, and flawed infrastructure design led to fatal gas accumulation and ignition. Similarly, the 2019 KJøRBO Uno-X station explosion in Norway was traced to undertorqued bolts on high-pressure tanks, where gradual leaks overwhelmed venting capacity, halting regional fuel-cell vehicle deployments. At Tsinghua University (2015), improper chemical storage near hydrogen systems triggered a fire and cylinder rupture, exposing deficiencies in hazard zoning. The 2019 AB Specialty Silicones disaster in Illinois, caused by uncontrolled hydrogen release during chemical production, highlighted failures in gas detection and ventilation, claiming four lives. South Korea’s 2019 tank explosion, driven by oxygen-hydrogen mixing during electrolysis and defective safety systems, unleashed energy equivalent to 50 kg of TNT. These cases collectively reveal systemic vulnerabilities - material degradation, procedural noncompliance, and inadequate risk mitigation - that span design, operation, and emergency response.
Figure 1. Typical hydrogen incidents. (A) A fatal hydrogen explosion at a power plant in Muskingum, Ohio. (B) A hydrogen explosion occurred at KJøRBO Uno-X hydrogenation station in Sandvika, Norway. (C) Laboratory hydrogen explosion in Tsinghua University. (D) An explosion at AB Specialty Silicones in Waukegan, Illinois. (E) Damage to nearby buildings after the explosion of a hydrogen storage tank in South Korea.
Current hydrogen storage equipment in petrochemical installations inherits and amplifies the systemic flaws demonstrated in historical disasters. The operational triad of high-pressure containments, extended pipeline networks, and multimodal transfer nodes creates uniquely magnified risks that demand industry-specific safety frameworks. These systems concentrate hydrogen's intrinsic hazards through three synergistic pathways: penetrative molecular behavior accelerating material degradation beyond conventional gas service, hyper-diffusive leak dynamics modulated by industrial infrastructure configurations, and cascading combustion risks inherent to large-scale hydrogen deployment. This complexity challenges conventional safety paradigms, as demonstrated by persistent gaps between laboratory prototypes and operational realities - where theoretical models inadequately predict degradation kinetics under industrial service conditions, while detection systems struggle with hydrogen's unique dispersion characteristics. Our analysis confronts this challenge through a threefold lens: first, deconstructing hydrogen's failure mechanisms across material, leak, and combustion systems; second, evaluating emerging mitigation technologies against industrial scalability criteria; third, proposing a risk-cascade management framework that integrates advanced metallurgy with predictive monitoring and active suppression systems. By bridging materials science, fluid dynamics, and safety engineering, this review establishes a paradigm for transforming petroleum industry's hydrogen infrastructure from vulnerability concentration points into resilient energy arteries.
SAFETY ISSUES OF HSTE
The petroleum industry grapples with hydrogen’s tripartite menace: material degradation (embrittlement, blistering) under high-pressure cyclic loads, unpredictable leak dynamics (under-expanded jets, stratified flammability in confined spaces), and cascading combustion risks (spontaneous ignition, detonation transitions). Hydrogen’s ultralow ignition thresholds, synergized with residual hydrocarbons and turbulent dispersion in refinery networks, create hypergolic hotspots. Legacy infrastructure’s incompatibility with hydrogen permeation and shockwave-driven pressure spikes further exacerbates latent risks inherent to petrochemical ecosystems.
Hydrogen-induced material failure
Currently, hydrogen storage and transportation primarily utilize four methods: high-pressure gas storage, cryogenic liquid storage, liquid organic hydrogen carriers, and solid-state storage[8-11]. High-pressure gaseous hydrogen storage technology is relatively mature but offers lower energy density, while liquid hydrogen storage provides higher energy density but requires an energy-intensive liquefaction process. Liquid organic and solid-state storage and transportation are safer; however, due to limitations in technological maturity, they have not yet been widely applied in large-scale production. From a cost-benefit perspective, pipeline transportation and high-pressure storage are currently the preferred options. Therefore, austenitic stainless steels (such as AISI 316, 304, 316L, 304L, and other 300 series)[12,13], carbon steels (including Cr-Mo, Cr-Mo-Ni, Cr-Mo-V, etc.)[14-16], and aluminum alloys (such as 5000 series Al-Mg, 3000 series Al-Mn, 7000 series Al-Zn-Mg, etc.)[17-19] are widely used materials in the manufacturing of hydrogen storage and transportation equipment (HSTE) and infrastructure.
Hydrogen storage and transportation pose significant risks due to the potential for material failure, which can severely damage pipelines and storage tanks. When exposed to high pressure, hydrogen atoms have the ability to penetrate metal structures, compromising their internal integrity and increasing the risk of catastrophic failures. The detrimental effects of hydrogen on materials manifest in several forms, including hydrogen embrittlement, permeation, blistering, and high-temperature hydrogen attack. Among these, hydrogen embrittlement is notably the most prevalent type of damage. This phenomenon typically arises when hydrogen atoms dissolve in metals and subsequently recombine into hydrogen molecules. This recombination produces localized stress concentrations that can surpass the mechanical strength of the metal, thereby initiating microcracks within the structure [Figure 2A][20]. In pipelines and storage tanks, the embrittlement process is intricate, involving the formation of solid solutions, hydrides, and gaseous byproducts with the metals or their additives. This interaction significantly weakens the bonds at the grain boundaries of the metal, diminishes ductility, and enhances brittleness, which ultimately leads to microcracking and pitting[21]. The process of hydrogen-induced cracking comprises six critical steps: the production of hydrogen atoms, their adsorption on the material's surface, absorption into the bulk material, diffusion through the metal lattice, local accumulation of hydrogen atoms, and the eventual initiation of cracks induced by hydrogen[22]. This sequence of events underscores the complex challenges faced in preventing hydrogen-related damage in infrastructures intended for hydrogen handling.
Figure 2. Hydrogen embrittlement phenomena and mechanisms. (A) Schematic illustration of hydrogen trapping sites within materials at the atomic level. (B) Diagram outlining the HEDE mechanism, highlighting the tensile separation of atoms due to weakened interatomic bonds influenced by hydrogen in the lattice, adsorbed on surfaces, and present at interfaces between particles and the matrix. (C) Diagram depicting the HELP mechanism, characterized by localized plastic deformation and microvoid coalescence in areas of high hydrogen concentration. (D) Diagram illustrating the AIDE mechanism, which facilitates crack propagation via alternate-slip movements from crack tips, enhancing crack and void merging in the plastic zone. Reproduced with permission[20], Copyright 2022 Hydrogen Energy Publications LLC. Published by Elsevier Ltd.
Hydrogen diffusion can be expressed through thermodynamic factors, quantified by Sievert's law which states that the dissolved hydrogen concentration (CH)dis is proportional to the square root of the hydrogen gas's partial pressure (PH2)gas,
Three primary physical models elucidate the processes through which hydrogen diffusion precipitates material degradation, as depicted in Figure 2B-D. These models include the hydrogen-enhanced decohesion (HEDE), hydrogen-enhanced local plasticity (HELP), and adsorption-induced dislocation emission (AIDE) mechanisms. Initially posited by Pfeil in 1926, the HEDE model suggests that hydrogen diffusion weakens the cohesive strength among metal atoms[32,33]. Conversely, the HELP model argues that hydrogen presence increases the mobility of dislocations, which in turn causes plastic deformation within the metal lattice[34,35]. Integrating elements of both HEDE and HELP, the AIDE model proposes that hydrogen atoms, once adsorbed at stress concentration sites such as crack tips, promote either intergranular or transgranular crack propagation, thereby diminishing the material's strength[36]. Given the diverse interactions between hydrogen and metals, the mechanisms leading to hydrogen embrittlement and their combined effects are complex and variable, necessitating precise theoretical approaches for their analysis. In pioneering work, Nagumo[37] identified hydrogen-vacancy synergies via thermal desorption analysis, later formalizing the hydrogen-enhanced strain-induced vacancy (HESIV) theory to explain hydrogen-aggravated fracture[38]. The mechanism attributes failure to hydrogen-enhanced vacancy formation and stabilization, evidenced by positron annihilation spectroscopy[39] and molecular dynamics simulations[40]. These vacancy clusters nucleate nanovoids that coalesce into micropores, reducing ductile crack resistance - a phenomenon corroborated by elevated pore density in hydrogen-exposed steels and analogous results in cast iron/alloys. Subsequent studies, such as the hydrogen adatom model by Hou et al.[41] and the energy calculations by Hickel et al.,[42] further validated thermodynamic feasibility of HESIV. However, HESIV’s reliance on preexisting crack-tip plasticity prompted its integration with complementary mechanisms. For instance, the nanovoid coalescence (NVC) model[43] unifies HESIV with HEDE and HELP, reflecting observations of mixed brittle/ductile fracture modes. While HESIV clarifies vacancy-mediated failure, its inability to fully explain plasticity origins underscores the necessity of multi-mechanistic frameworks for comprehensive hydrogen degradation models[44].
Hydrogen permeation and blistering are the main causes of aging in composite material-lined pressure vessels[45]. As the smallest diatomic molecules, with a covalent radius of only 37 × 10-12 m, hydrogen molecules can easily penetrate materials such as polymers, with their permeation rate increasing with temperature and storage pressure. Under high pressure, hydrogen gas is readily adsorbed onto plastic liners; if the depressurization rate exceeds the gas diffusion escape rate, blistering of the plastic liner can occur, leading to cracks and failure events in the load-bearing composite material. High-temperature hydrogen attack primarily occurs when system temperatures exceed 200 °C, at which point hydrogen molecules or atoms can rapidly penetrate metals. The interaction of penetrating hydrogen molecules or atoms with impurity elements in the metal (such as carbon) often forms gaseous products (such as methane), causing significant internal pressure[46]. Due to the small size of hydrogen atoms and their high mobility when dissolved in metals, rapid formation of bulges, cracks, or elongated pores within the metal can occur.
Hydrogen gas leak and diffusion
Hydrogen safety incidents often stem from leaks followed by rapid diffusion. When hydrogen gas under high pressure is released into the air, it forms a jet which, based on the pressure differential between the source and the ambient environment, can manifest in one of three distinct states as illustrated in
Figure 3. Hydrogen leakage and diffusion process. (A) Schematic diagram depicting hydrogen jets influenced by various mechanisms. (B) Illustration of successful ignition in an environment with a hydrogen concentration exceeding 10%, using a flow rate of
The exit densiometric Froude number (Fr), a crucial metric in the study of subsonic free jets, quantifies the balance between momentum and buoyancy forces. It is defined as the ratio of the exit velocity of the gas to the square root of the product of gravitational acceleration, nozzle diameter, and the relative density difference between the ambient air and the exiting hydrogen:
In practical applications, Swain et al. carried out experiments on a horizontal hydrogen jet flowing at 556 standard liters per minute (SLPM), using concentration sensors to create a concentration contour map depicted in Figure 3B[52]. Conversely, Houf et al. studied a vertical axisymmetric hydrogen leak through a 1.905-mm orifice at a constant mass flow rate, using Rayleigh scattering to map the concentration distribution shown in Figure 3C[53]. Accurately calibrating these concentration contours remains challenging, with fixed-point sampling methods predominantly used[54]. Nonetheless, further research is necessary to improve sampling accuracy and to understand how the measurement apparatus influences the morphology of the jet.
When the storage-to-ambient pressure ratio surpasses the critical value (approximately 1.9 for hydrogen), an under-expanded jet forms, exhibiting a complex impact structure, uneven velocity distribution, and a Mach disk at its terminus, which separates the supersonic and subsonic regions [Figure 3D]. The characteristics of a Mach disk, such as its formation, position, and diameter, are shaped by several factors including the pressure ratio, the nozzle diameter, and the nozzle's geometric design[55,56]. Ruggles et al. employed a schlieren system to visually document the shock wave structure in an under-expanded jet formed under a 10:1 pressure ratio with a nozzle outlet diameter of 0.75 mm. Their images distinctly show a barrel-shaped core, the boundary layer of the jet, a prominent Mach disk, and a reflected wave downstream of the Mach disk, as depicted in Figure 3E[57]. Crist et al. discovered a correlation between the pressure ratio and the spatial placement of the Mach disk. This relationship indicates that the disk's position is proportionate to the square root of the ratio of the critical or stagnation pressure to the ambient pressure, modified by a function of the specific heat ratio, which ranges from 0.65 to 0.67[58]. They also found that the dimensionless diameter of the Mach disk and its position from the nozzle share a direct, linear relationship, suggesting that as the pressure conditions within the jet change, so does the scale and location of the Mach disk in a predictable manner[59]. This relationship underscores the complexity of the interactions within the jet, driven by both thermodynamic and geometric factors.
Hydrogen leakage into confined spaces significantly affects the formation, evolution, and kinetics of hydrogen-air mixtures[60]. Two leakage modes can be identified based on the leak flow rate
The Kumamoto group conducted research to compare the ease of ignition and explosion risks between these models, discovering that the minimum ignition energy (MIE) is lowest in environments where the hydrogen concentration is between 20% and 30%, as shown in Figure 3H[64]. This phenomenon arises from the interplay of chemical reactivity near stoichiometry, flame thickness stability, and preferential diffusion effects. Specifically, hydrogen-air mixtures in this range balance optimal fuel-oxygen ratios for rapid chain-branching reactions while minimizing heat losses through reduced flame thickness and enhanced laminar burning velocity. Additionally, hydrogen’s high diffusivity amplifies flame stretch effects, locally enriching fuel concentration at flame fronts and further lowering ignition energy thresholds. Consequently, the "filling box" model, with its enriched hydrogen concentrations, ignites more readily due to these synergistic factors. In contrast, Nikolaev noted that the "fading up box" model, despite its premixed nature, exhibits an approximately 8% higher combustible volume compared to the "filling box" model for identical leak volumes[65]. This disparity highlights how differences in dispersion dynamics - such as localized fuel enrichment versus premixed homogeneity - directly influence combustion risks, underscoring the need for model-specific safety strategies.
Hydrogen gas combustion and explosion
Hydrogen combustion and explosion can lead to greatly elevated temperatures and pressures, thereby heightening the risks associated. Hydrogen's MIE in air is merely 4% of that for methane, indicating its greater propensity for ignition. When ignited with a low energy source, hydrogen ignites in a laminar phase, achieving a calorific value as high as 143 kJ/g, double that of natural gas, which intensifies the combustion chain reaction[66]. As displayed in Figure 4A, in oxygen-enriched settings, hydrogen's MIE can plummet to as low as 0.0056 mJ[67,68], enhancing its susceptibility to ignition. Following this phase, the flame's inherent instability, coupled with the interaction of diverse pressure waves, leads to turbulent combustion. This shift not only accelerates the flame speed but may also escalate to detonation. Particularly critical is hydrogen's extremely low MIE, which makes high-pressure hydrogen leaks susceptible to auto-ignition, resulting in jet fires. These jet fires are marked by high-speed turbulent flames that exert considerable momentum in a specific direction, as shown in Figure 4B[69]. In scenarios where gaseous hydrogen is stored in pressurized vessels, the significant pressure differential between the interior of the vessel and the external environment propels the fuel out with high momentum.
Figure 4. Hydrogen ignition and explosion. (A) MIE of H2, CH4 and C3H8 in air. Reproduced with permission[67], Copyright 2023, Springer Fachmedien Wiesbaden GmbH. (B) Schematic of hydrogen self-ignition within a tube, depicted from top to bottom: the setup prior to rupture; the initial formation of a shockwave; the creation of vortex rings and reactions in both the core and boundary layer regions, triggered by interactions between shockwaves and the boundary layer; reactions occurring primarily in the core region; and the eventual merging of the two reactive zones. Reproduced with permission[69], Copyright 2010, The Combustion Institute. Published by Elsevier Inc. (C) Time histories of overpressure from ignition experiments with 29.3% hydrogen concentrations. Reproduced with permission[95], Copyright 2012, Elsevier Ltd. (D) High-speed images from hydrogen-air cloud detonation test. Reproduced with permission[98], Copyright 2007, Elsevier Ltd. (E) Temperature contours, Schlieren images, and pressure contours capture the final stage of DDT in an uneven H2/Air mixture with a 20% concentration. Reproduced with permission[100], Copyright 2022, Elsevier Ltd.
The rapid expulsion of high-pressure hydrogen facilitates turbulent mixing with ambient air, creating conditions where ignition risks emerge through interconnected mechanisms. While the reverse Joule-Thomson effect generates a modest temperature rise during expansion (e.g., 9~18 K at 50 MPa), this alone is insufficient to reach hydrogen’s autoignition threshold (858 K). However, such heating reduces the MIE, amplifying risks when combined with electrostatic sparks - a hazard driven not by pure hydrogen but by impurities (e.g., particulates or droplets) accelerated in supersonic jets, where friction or impact can generate sparks exceeding hydrogen’s ultralow MIE. Dominating spontaneous combustion scenarios, diffusion ignition occurs when shock waves from rapid release compress and heat air to critical temperatures, as demonstrated in confined tubes by Wolanski and Wojcicki[70], where shock reflections at structural discontinuities enhance turbulent mixing and localized heating. These mechanisms synergize dynamically: turbulent mixing (e.g., in complex pipe geometries) accelerates hydrogen-air diffusion, while Joule-Thomson heating lowers MIE, creating conditions where even weak electrostatic discharges or residual shock wave energy can trigger ignition. Experimental studies, such as the work of Grune et al.[71] with impurity-free systems and the visualization of flame formation in mixing layers by Kim et al.,[72] underscore the need for holistic safety protocols that address these coupled risks in both confined and open environments.
The Joule-Thomson effect, which is pivotal in adiabatic gas flow dynamics through conduits, manifests when gas traverses a throttling device such as a pressure-reducing valve, porous plug, or orifice, inducing both a drop in pressure and a change in temperature. Specifically for hydrogen, the Joule-Thomson cooling effect can lower the temperature to -80 °C. However, when hydrogen gas at high pressure is released into ambient conditions, it experiences the inverse Joule-Thomson effect - a thermodynamic phenomenon governed by hydrogen’s negative Joule-Thomson coefficient above its inversion temperature. This unique property causes temperature to rise during free expansion, contrary to most gases. While this alone does not reach the self-ignition temperature of hydrogen, it does contribute to conditions that favor self-ignition[73]. In high-pressure hydrogen leaks, electrostatic ignition may occur due to hydrogen's low MIE and wide ignition range[74]. Charged particles, especially when not properly grounded, can accumulate significant electrical potential; for instance, one investigation observed static voltages reaching up to 9 kV at a ventilation outlet, attributed to iron oxide particles mobilized after a leak[75]. Additionally, diffusion ignition occurs primarily through the interaction of shock waves with the hydrogen-air mix, where the intensity of the shock wave raises the gas temperature and increases the probability of spontaneous combustion. Research has investigated these mechanisms in tubes or shock tubes following high-pressure hydrogen releases[76-78]. Variations in pipeline structure significantly impact self-ignition risks[79,80], as changes in cross-sectional area induce complex flow patterns that enhance ignition probabilities. In pipes with variable interiors, vertical walls generate stronger shock waves and turbulence compared to expansion tubes, which can substantially lower critical relief pressures[81]. Other ignition mechanisms include hot surface ignition, where localized high-temperature surfaces ignite hydrogen-oxygen mixtures[82], and mechanical ignition caused by friction and impact[83].
The classification of hydrogen explosions into four categories - expansion deflagration, mixture deflagration, detonation, and deflagration-to-detonation (DDT) - is intrinsically linked to the chain-branching reactions governing hydrogen/oxygen combustion dynamics. These phenomena arise from the competition between chain-branching and chain-termination mechanisms in hydrogen oxidation. For instance, when a hydrogen leak forms a combustible cloud, ignition triggers deflagration driven by radical proliferation (e.g., H, O, OH). This process aligns with the first and second explosion limits of the hydrogen/oxygen mixture, where chain-branching reactions (H + O2 → OH + O) dominate at low-to-intermediate pressures, enabling rapid heat release and flame acceleration[84]. However, if the mixture transitions to high-pressure conditions near the third explosion limit, three-body termination reactions
Pressure vessel burst (PVB) and boiling liquid expanding vapor explosion (BLEVE) are two major catastrophic outcomes. PVB occurs when a high-pressure gas tank bursts due to factors such as malfunctioning safety devices, increased internal pressure from external heat sources such as fires, or tank failure from hydrogen embrittlement and material damage[89,90]. A small crack might only cause a jet fire, whereas a larger one could lead to a fireball and deflagration. BLEVE happens when a liquid hydrogen container fails due to collision or when hydrogen leaks into the vacuum layer from an internally fatigued wall[91,92]. Damage to the insulation layer causes a rapid temperature increase, overheating the liquid hydrogen. Upon reaching the overheating threshold, rapid boiling and evaporation escalate internal pressure until the container bursts. A significant crack or fracture can cause spontaneous combustion of escaping hydrogen, heating the remaining liquid hydrogen and further increasing pressure, resulting in a physical explosion and a large fireball with intense thermal radiation. At ambient conditions, the density of coexisting gas-liquid hydrogen (20.37 K) exceeds that of air, causing leaked hydrogen to hover near the ground, where it absorbs heat and gradually mixes with air to form a flammable hydrogen-air cloud[93].
Hydrogen-air cloud deflagrations are common in hydrogen explosions. When hydrogen fully mixes with air to form a large cloud, a vapor cloud explosion (VCE) occurs if it reaches the flammable limit. Compared to other gases such as methane, unconfined hydrogen-air clouds can generate significant overpressure, and confined clouds are even more dangerous, producing higher blast overpressure [Figure 4C][94-96]. Hydrogen-air clouds can also detonate, resulting in much greater blast loading[97]. Detonation, unlike deflagration, involves the adiabatic compression of the leading shock wave, inducing faster ignition and heat release, with much lower reaction times and higher overpressures. Groethe et al. conducted field tests, detonating a 30% hydrogen-air cloud with C-4 explosive, reaching flame speeds of about 1,980 m/s and peak overpressures five times higher than deflagration at the same concentration [Figure 4D][98]. Under certain conditions, commonly in confined space or blocked environment, a laminar flame accelerates, generating compression waves that merge into shock waves, leading to deflagration-to-detonation transition (DDT). DDT occurs as the flame accelerates continuously before suddenly transitioning to detonation[99,100], typically within microseconds to tens of microseconds [Figure 4E]. Boundary layer effects increase the flame surface area, enhancing the reaction rate, heat release, and flame stretching. As flame surface speeds increase, pressure waves coalesce into a leading shock wave. Once sufficiently accelerated, the flame surface and shock wave couple, creating a feedback loop that increases temperature and pressure, culminating in detonation[101,102].
Predicting DDT remains challenging due to its microsecond-scale dynamics, which often restrict experimental analysis to qualitative observations. Optical methods such as schlieren imaging and shadowgraphy exploit density gradients to resolve detonation structures (e.g., Mach stems, incident shocks) in confined geometries, though such confinement introduces artifacts such as velocity deficits and amplified transverse waves[103-105]. Advanced laser diagnostics, including hydroxyl radical planar laser-induced fluorescence (OH-PLIF) and nitric oxide PLIF (NO-PLIF), provide deeper insights: OH-PLIF maps reaction zones via intermediate OH radicals, while NO-PLIF tracks shock-heated regions using seeded NO
HYDROGEN INCIDENT CHAIN INTERRUPTION STRATEGY
The disaster incidents during the hydrogen storage and transportation process are diverse and occur abruptly, making emergency warnings challenging. Interrupting the chain of accidents is crucial in preventing such incidents, focusing on three key aspects: first, the safety design of hydrogen storage containers and transportation pipeline materials; second, high spatiotemporal resolution and rapid response hydrogen leak detection; and third, the construction of explosion and detonation isolation mechanisms.
Material safety design for HSTE
In designing safe hydrogen storage and transportation facilities, a critical step involves selecting and engineering materials with tailored resistance to hydrogen permeation and embrittlement. As illustrated in Figure 5A, this selection process must balance physical and chemical properties, including chemical composition, microstructure, yield/tensile strength, weldability, thermal stability, and manufacturability. Material suitability depends on hydrogen state (liquid, gas, or solid), operational conditions (temperature, pressure, cyclic loading), and design criteria such as hydrogen permeability, HE sensitivity, and mechanical robustness. For instance, austenitic steels with > 7% Ni are widely used for hydrogen containers due to their low hydrogen diffusivity (Deff) and high solubility, attributed to their fcc structure. However, their stability under cryogenic conditions must be carefully assessed, as stress-induced martensitic transformation at subzero temperatures can exacerbate HE susceptibility[109,110]. To mitigate this, advanced alloying strategies incorporate nitrogen (N) and manganese (Mn) to stabilize austenite while introducing nano-sized precipitates (e.g., TiC, NbC) as hydrogen traps. These carbides, with binding energies of 40-70 kJ/mol, drastically reduce Deff by immobilizing hydrogen at interfaces or within precipitates[111,112]. Grain boundary (GB) engineering further optimizes performance: Σ5 and Σ9 boundaries act as preferential trapping sites, while high-angle GBs in nickel alloys are tailored to balance short-circuit diffusion and trapping[113,114]. Aluminum alloys, favored for their lightweight properties, require microstructural modifications to offset their inherent strength limitations. For example, 7xxx series alloys leverage coherent T-phase precipitates (superior to η-phase) to redistribute hydrogen from GBs to grain interiors, reducing interfacial decohesion[115,116]. Additions of zirconium (Zr) form Al3Zr dispersoids, which act as high-capacity hydrogen traps, enhancing HE resistance without compromising ductility[117]. Innovative material designs exploit solute heterogeneity to achieve synergistic properties. As shown in Figure 5B and C, Mn-Al-Si steels engineered with Mn-rich buffer zones suppress strain-induced martensite formation, maintaining austenite stability under deformation while resisting hydrogen-assisted cracking[118]. Similarly, high-entropy alloys such as CoCrFeMnNi utilize atomic-scale disorder and nanotwinning to limit hydrogen enrichment and dislocation impingement at GBs, achieving exceptional HE resistance[119,120]. Advanced coatings and surface treatments (e.g., laser melting, ultrasonic nanocrystallizing) on metallic components further reduce hydrogen uptake by altering surface energy barriers or introducing compressive residual stresses[121]. Ultimately, hydrogen-resistant materials demand a holistic approach integrating alloy chemistry, phase stability, defect engineering, and processing techniques. These strategies collectively ensure compliance with operational demands while addressing the trilemma of strength, permeability, and embrittlement resistance.
Figure 5. Hydrogen embrittlement prevention strategies. (A) Schematic representation of configurations designed to prevent hydrogen embrittlement through the selection of appropriate materials. (B) Conceptual diagram depicting the propagation of a hydrogen-induced crack traversing a specifically designed, solute-rich buffer region; the solute concentration profile and associated crack resistance are illustrated schematically on the right. (C) High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) reveals Mn-rich zones in austenite clusters/grains. Reproduced with permission[118], Copyright 2021, The Author(s). (D) Application of a surface treatment. (E) Hydrogen permeation through disordered nanostructured tungsten films. Reproduced with permission[129], Copyright 2012 Elsevier B.V. (F) Cross-sectional bright-field TEM image of Al45Ti38W17 thin film revealing non-columnar growth from 100C6 steel substrate with robust interfacial adhesion. Reproduced with permission[130], Copyright 2021 Elsevier B.V. (G) Selection of an appropriate design by avoiding sharp variations. (H) The distribution of residual stress after multi-impact SP treatment. (I) TEM image of SP (0.50 MPa) specimen surface layer showing dense dislocation networks at martensite boundaries, with lath structure elimination, confirming SP-induced dislocation density enhancement. Reproduced with permission[149], Copyright 2016 Elsevier Ltd.
Polymers such as polyethylene (PE) and polyvinyl chloride (PVC) are widely employed in structural components of hydrogen storage and transportation systems (e.g., piping, tanks) due to their low cost and chemical inertness[122]. However, for sealing applications in high-pressure hydrogen environments - particularly in dynamic systems such as compressors, valves, and fuel cell connectors - advanced elastomers such as fluorinated elastomers (FKM), acrylonitrile-butadiene rubber (NBR), and brominated butyl rubber (BIIR) are prioritized[123-125]. These materials uniquely address the dual challenges of hydrogen permeation resistance and mechanical reliability. FKM, for instance, leverages its highly polar C-F bonds to minimize hydrogen diffusion by stiffening the polymer matrix and reducing free volume, while its inherent thermal stability ensures performance under cyclic pressure fluctuations. NBR, enriched with nitrile groups, provides a polar backbone that impedes hydrogen solubility; when compounded with high-surface-area fillers (e.g., carbon black), it further reduces permeability by adsorbing hydrogen at filler-matrix interfaces and elongating diffusion paths. BIIR, modified with bromine, enhances intermolecular cohesion through halogen-induced polarity, effectively lowering both hydrogen solubility and diffusion rates. In contrast, while PE and PVC may suffice for static, low-pressure components, their susceptibility to hydrogen embrittlement, higher permeability, and limited elasticity under repetitive stress render them unsuitable for sealing roles. For example, PVC’s rigidity and PE’s semi-crystalline structure can lead to microcrack formation under high-pressure hydrogen exposure, accelerating failure. Recent advancements in nanocomposites (e.g., graphene oxide-reinforced NBR)[126] and multilayer barrier coatings[127] further optimize these elastomers by introducing nanoscale tortuosity and interfacial blocking effects. Notably, even these advanced elastomers cannot fully reconcile the trade-offs between hydrogen impermeability, mechanical durability, and processability - a trilemma that fundamentally constrains monolithic material solutions for hydrogen storage and transportation systems.
As an alternative solution, developing innovative surface modification techniques to create composites that integrate the advantageous characteristics of various materials is the most viable option [Figure 5D]. Metal plating composites are a widely utilized surface treatment technique, with their effectiveness in resisting hydrogen largely depending on the metallurgical interactions between the plating material and the base metal. In cases where there is no interaction, such as with silver or copper platings on stainless steel, the plating may offer little to no reduction in hydrogen permeability. Conversely, significant interactions can result in the formation of a continuous intermetallic layer, such as when aluminum is plated onto a cobalt-nickel alloy, which markedly decreases hydrogen permeability. Research has also explored several ternary metal alloys as potential hydrogen permeation barriers. For instance, Zn-Ni-Cd alloy coatings have demonstrated superior permeation blocking capabilities compared to pure Cd and Zn-Ni coatings[128]. This enhanced performance is attributed to the inclusion of Cd, which not only reduces hydrogen evolution but also increases the composite reaction constant, thereby effectively slowing hydrogen diffusion kinetics. Lakdhar and colleagues have applied Al-Ti-W coatings to 100C6 steel substrates utilizing magnetron sputtering technology, as shown in Figure 5E and F[129]. With an increase in the tungsten content within the coating, both mechanical properties and resistance to hydrogen absorption improve. Notably, the
Compared to metals, polymers used as protective coatings have very limited mechanical strength, but their low hydrogen permeability is attractive. Table 1 lists the hydrogen permeation characteristics of some typical commercial thermoplastic plastics and elastomers in high-pressure hydrogen environments, where FKM and high-density PE (HDPE) exhibit relatively lower hydrogen permeation rates[131-134]. Due to the presence of semi-crystalline, ordered, and amorphous regions in the microstructure of thermoplastic plastics, their response to exposure to high-pressure hydrogen is not as pronounced. Copolymer-based coatings are another potential approach to control hydrogen diffusion through molecular-scale architectural design. By strategically arranging polar/nonpolar segments, these systems suppress hydrogen solubility while ensuring mechanical robustness. Ethylene vinyl alcohol (EVOH) copolymers exemplify this strategy, where hydroxyl groups form interlocking hydrogen-bonding networks to immobilize hydrogen molecules, with barrier efficiency governed by crystallinity modulation through ethylene/vinyl alcohol ratio optimization[135]. Complementary fluorinated systems such as polyvinylidene fluoride (PVDF) exploit fluorine’s low polarizability to inhibit hydrogen adsorption at polymer interfaces[136]. Hybrid architectures further integrate lamellar nanofillers (e.g., h-boron nitride) to synergize chemical resistance with physical barrier effects - the nanofillers extend gas diffusion pathways through tortuous geometric blocking while preserving the copolymer’s structural integrity[137]. This dual mechanism couples polarity-driven solubility reduction with steric hindrance, addressing both hydrogen permeation and environmental durability. Such molecular-to-microstructural engineering enables multifunctional coatings compatible with pipeline service conditions, including humidity and mechanical stress, without requiring explicit layer separation for corrosion protection.
Hydrogen permeability comparison between typical metals and polymers
| Metals [132] | Permeability | Polymer [133-136] | Permeability |
| Ferritic steels | 0.3 | Polytetrafluoroethylene (PTFE) | 0.032 |
| Austenitic steels | 0.09-0.12 | Fluoroelastomer (FKM) | 0.035 |
| Vanadium | 290 | High-density polyethylene (HDPE) | 0.0082 |
| Titanium | 75 | Ethylene propylene diene monomer (EPDM) | 0.079 |
| Tungsten | 4.3 × 10-5 | Nitrile butadiene (NBR) | 0.05 |
| Molybdenum | 0.12 | Polyvinylidene fluoride (PVDF) | 0.015 |
| Nickel | 1.2 | Ethylene vinyl alcohol (EVOH) | 1.2 × 10-5-2.3 × 1 0-4 |
| Iron | 1.8 | Brominated butyl rubber (BIIR) | 0.0023-0.0087 |
Recent advancements in deposition technologies have led to the development of ceramic materials that exhibit high strength, thermal resistance, and low thermal expansion, making them ideal candidates for hydrogen permeation barriers. Among these, oxide ceramics are among the most recognized coatings for this purpose. According to recent research by Levchuk et al., a 1-micron-thick α-alumina coating, applied via filtered arc discharge technology, can reduce the hydrogen permeability of a stainless-steel substrate by a factor of 1,000[138]. Other similar materials include alumina (Al2O3)[139], zirconia (ZrO2)[140], erbium oxide
Shot peening (SP) is another effective and commonly employed technique for enhancing the surface properties of industrial components, complementing traditional coating processes [Figure 5G]. This method leverages the cold working hardening effect, wherein spherical shots strike the target surface, resulting in surface deformation and the induction of residual compressive stress. These changes enhance the mechanical strength and ductility of the surface while simultaneously inhibiting the diffusion of hydrogen atoms. SP is frequently used to improve hardness, fatigue strength, and crack resistance, particularly against wear and corrosion-induced damage[147,148]. Numerous studies have confirmed that SP treatment provides substantial protection against hydrogen degradation for materials such as X80 pipeline steel, low carbon steel, and pearlitic steel. Samples subjected to SP exhibited superior mechanical properties after hydrogen exposure compared to untreated specimens. Additionally, earlier research underscored the importance of mechanisms such as grain refinement, increased GB and dislocation density, and the establishment of compressive residual stress in limiting hydrogen ingress into the metal, as illustrated in Figure 5H and I[149].
In practical engineering applications, the storage and transportation of natural gas blended with hydrogen have emerged as the preferred industrial solution for ensuring operability and safety, attracting significant global research interest. Investigative teams across various countries have examined the compatibility of conventional pipeline steels in high-pressure hydrogen environments. Meng's analysis of the mechanical properties of X70 and X80 pipeline steels mixed with natural gas revealed that while both steels retained their yield and tensile strengths, their notched tensile strength diminished, and the rate of fatigue crack growth markedly increased. His findings indicated that increasing the hydrogen content to 50% in natural gas at a pressure of 12 MPa significantly reduced the plasticity and fracture toughness of X80 steel, accelerating fatigue crack growth[150]. The European Union's NATURALHY project determined that hydrogen concentrations of up to 50% in natural gas do not critically compromise the safety or durability of pipeline steel[151]. Additionally, Li et al.[152] and Lei et al.[153] investigated the use of polymer pipes, such as PE and PVC, for hydrogen transport. They acknowledged these materials' high compatibility while expressing concerns regarding their aging and greater hydrogen permeability compared to metal pipes. Further research by Hardie et al.,[154] Slifka et al.,[155] and Drexler et al.[156] suggested that lower-grade steels could be suitable for hydrogen pipelines. Before hydrogen can be blended into natural gas pipelines, thorough validation of the pipelines' adaptability is essential. This process includes assessing and testing steel grade, pressure, chemical composition, and toughness at various hydrogen ratios through compatibility tests and practical blending trials.
Current studies have yet to fully clarify the detailed mechanisms of hydrogen damage or the synergistic effects involved. Advanced techniques such as Transmission Electron Microscopy (TEM) and Atom Probe Tomography (APT) have provided insights into hydrogen embrittlement. Recent advancements in molecular simulations have also filled gaps in experimental research and helped validate findings. However, most investigations have concentrated on pure hydrogen or mixtures with nitrogen, often neglecting the potential interactions with contaminants such as sulfides and carbon oxides. In situ tensile tests under hydrogen conditions are vital for assessing the integrity of high-pressure hydrogen pipelines and storage. Unfortunately, research aimed at quantifying hydrogen damage, predicting its occurrence, and developing prevention strategies remains insufficient.
Leakage awareness for HSTE
The awareness of leakage situations in hydrogen storage and transportation is a complex task involving multiple management activities, aimed at preventing emergencies, controlling social damage, and reducing public impact. With the rapid advancement of technologies such as sensors, the Internet of Things (IoT), machine learning, 3D simulation, digital twins, drones, navigation, and satellite communication, adopting proactive awareness strategies through smart technology has become a trend. This approach plays a crucial role in enhancing the timeliness, effectiveness, and comprehensiveness of chemical disaster management and emergency response. The strategy primarily consists of four key components: intelligent sensing technology and devices, dynamic IoT systems, precise disaster situation deduction, and an emergency command platform.
In the realm of hydrogen gas sensing terminal equipment, current hydrogen sensors, based on their working principles and testing methods, can be classified into optical, acoustic, thermal conductivity, catalytic, work function, resistive, and electrochemical types. These sensors mainly rely on changes in refractive index, spectral absorption, temperature, mass structure, and electrical properties caused by the interaction between sensitive material elements and hydrogen gas. They generate optical or electrical sensing signals through digital-analog conversion[157]. Each type has its advantages and disadvantages. For example, optical sensors have strong specificity and fast signal transmission but are limited by equipment size and investment cost, affecting their deployment density and the refinement of monitoring spatiotemporal scales. Acoustic, thermal conductivity, and work function sensors are small in size and low in power consumption but are troubled by long-term stability, target selectivity, and hysteresis issues, respectively. Traditional catalytic, electrochemical, and resistive hydrogen sensors generally offer relatively high sensitivity and sampling frequency, simpler component structures, and convenient signal processing. However, they consume more power, are often restricted by power supply line installation standards, and lack flexibility in expanding monitoring points and inspection routes. Moreover, their performance in detection range, response rate, anti-interference capability, and environmental adaptability often leaves much to be desired in complex environments or when dealing with multi-component mixed target atmospheres. In fact, no specific type of hydrogen sensor currently meets all application needs, providing ample room for the development of novel hydrogen sensor technologies.
Compared to conventional sensors, MEMS (micro-electromechanical systems) gas sensors offer advantages such as low power consumption, high selectivity, easy scalability, high integration, and a wide dynamic measuring range. They effectively overcome the monopolization, high consumption, and inefficiency of traditional detection methods, representing an optimal balance of "cost-market-comprehensive characteristics"[158]. The introduction of novel nanosensitive materials and advanced microstructured chips, along with cross-scale integration, can significantly enhance the adaptability of monitoring elements, expand the detection spatiotemporal coverage, and improve the accuracy of monitoring results and risk event identification in complex operational environments. At the present stage, the development bottlenecks for MEMS gas sensors applied in hydrogen storage and transportation primarily focus on the rational screening of sensitive materials [Figure 6A][159]; the design and optimization of new principle chip structures [Figure 6B][160]; on-chip array integration and precise loading technology for sensitive film layers[161]; the unification of comprehensive characteristics of sensing devices, process compatibility, and mass production capability; multi-component gas edge algorithm with low resource consumption
Figure 6. Intelligent emergency command strategy for hydrogen leakage disaster management. (A) On-demand artificial hydrogen olfaction achieved through the use of combinatorial high-throughput screening approaches. Reproduced with permission: Copyright 2020, The Authors[159]. (B) Design and schematic of the fabrication process for a low-power micro-sensing chip. Reproduced with permission: Copyright 2016, IOP Publishing Ltd.[160]. (C) Smart monitoring terminals integration for real-time gas identification by deep learning algorithm. Reproduced with permission: Copyright 2019, The Authors[162], and permission: Copyright 2019, The Authors[170]. (D) A dynamic wireless sensing network, created by integrating both fixed and mobile safety sensing devices, where optimizing the network's matrix topology significantly enhances system robustness. Reproduced with permission: Copyright 2020, Elsevier B.V.[167]. (E) Integrating and merging information from multi-source heterogeneous data, including collection, analysis, processing, and dissemination. (F) The dynamic IoT system, equipped with an early warning safety strategy, consolidates monitoring from hydrogen disaster sources and potential risk areas. It offers an intuitive interface for seamless human-computer interaction, facilitating emergency management and supporting decision-making processes. Reproduced with permission: Copyright 2017, Springer International Publishing Switzerland[177].
Dynamic sensing in IoT systems, based on wireless sensor network (WSN) architecture, typically features characteristics such as large-scale deployment, self-organization, random placement, and frequent changes in network topology in the context of hazardous gas leak monitoring applications. The widespread distribution of sensor nodes, limited energy supply, and communication bandwidth pose challenges to the design and management of WSNs [Figure 6D]. Particularly in harsh outdoor environments or emergency situations, the network's dynamism, reliability, and resilience are problematic, and accurate environmental monitoring is compromised as the network scales. Therefore, designing network protocols requires considering energy-saving and damage-resistant topology control strategies to enhance network robustness and adaptability, ensuring network connectivity and coverage, thereby extending the network's lifespan. Early topology control primarily focused on energy saving and balancing energy consumption through power control, hierarchical structuring, and sleep strategies[164,165]. However, these methods, based on static models, overlook the uncertainties in practical applications and lack effective fault tolerance mechanisms. To address network damage and node failures, researchers have proposed strategies such as increasing node redundancy in recent years, but this may reduce the network's energy efficiency and lifespan. Recently, to adapt to dynamic changes in network environments, researchers have explored dynamic model methods including node regeneration technology and data link reconstruction, improving the network's self-healing capabilities and cost-effectiveness[166,167]. The introduction of dynamic models, especially combined with low-power MEMS microsensors and modern technologies such as drones and tracked robots, significantly enhances the feasibility of implementing dynamic topology control. With the unique demands of high-risk industries such as oil and petrochemicals on industrial IoT, current research focuses on constructing logical links, node configuration, reducing path lengths, and improving network clustering coefficients, with relatively less study on network robustness and vulnerability. Therefore, there is an urgent need to integrate WSNs with complex network dynamics, considering the impact of node mobility, wake/sleep states, and changes in communication range on topology changes[168]. By applying new network theories to study topology structures under unstable and uncertain environments, such as power optimization, clustering, network deployment, and scale-free topology evolution, WSN topology control can be achieved from a perspective of overall performance optimization.
The analysis of hydrogen leak disasters necessitates the integration and fusion of diverse safety perception data from multiple sources. Given the notable differences in spatiotemporal distribution, accuracy, and data consistency across fixed and mobile sensor networks, employing efficient fusion techniques to filter out erroneous or abnormal data is essential [Figure 6E]. This step enhances data usability and reduces the volume of data transmission[169]. Moreover, it is crucial to account for the spatiotemporal variability of these data sources, structured disaster scenario representations, and factors such as regional characteristics, system operations, maintenance, and historical accident data. This comprehensive approach allows for an in-depth analysis of environments prone to disasters, the factors causing these disasters, and the entities affected[170]. Utilizing tools such as event trees and interpretive structural models facilitates the analysis of transmissibility between nodes in disaster scenarios. By focusing on the causal, derivative, and coupling relationships among disaster risk factors, it is possible to construct network models for typical disaster scenarios. These models cover individual disasters, coupled disasters, and secondary disasters[171,172]. Techniques such as principal component analysis and cluster analysis aid in identifying the characteristics of factors causing hazardous chemical disasters. A thorough deduction of disaster scenario evolution enables the dynamic analysis of disaster propagation paths under various emergency rescue scenarios. This analysis can predict the initial outbreak points, secondary derivative points, and the spatiotemporal evolution of disasters.
Additionally, integrating probabilistic quantitative models of disaster scenarios with system dynamics and dynamic Bayesian networks (DBNs) - and incorporating sensor-driven real-time data - enables precise simulation, prediction, and mitigation of hydrogen storage and transportation risks[173,174]. For instance, in a high-pressure storage facility, sensor networks continuously monitor parameters such as pressure, temperature, and hydrogen concentration. When a pressure anomaly is detected (e.g., a valve malfunction), probabilistic models simulate potential failure pathways (e.g., leakage → dispersion → ignition), while system dynamics models predict hydrogen plume behavior under ambient conditions. Concurrently, DBNs update risk probabilities in real time by assimilating sensor data, enabling adaptive decision-making. This framework was validated in a case study at a hydrogen refueling station, where it reduced false alarms by 30% and prevented a critical incident by triggering automated valve closure and ventilation within 5 s of detecting a 10% pressure deviation. Such integration not only enhances predictive accuracy but also enables proactive risk mitigation, as demonstrated by Patel et al., where AI-CFD hybrid models cut computational costs by 50% while maintaining 95% precision in leak localization[175,176].
The development of an emergency command platform unites monitoring systems for diverse disaster sources and hazard points, creating a Dynamic Real-Time Digital Map (DRDM, Figure 6F). Utilizing 3D geographic information systems, oblique photography, and other 3D visualization technologies, this platform vividly displays the sources and scenarios of hazardous chemical disasters on a map[177]. Linked to IoT real-time data, the DRDM showcases the precise locations and safety conditions of various sensing devices (including both fixed and mobile detection equipment), mobile disaster sources (such as transport vehicles), and rescue equipment[178]. By leveraging data from multi-sensor terminals across varying environmental conditions, the platform establishes specific thresholds for anomaly information, ensuring coverage of disaster sources and real-time alerts under diverse operational scenarios. Recognizing the nuanced impacts of different disaster risk factors and the intensity of disaster-causing elements on affected carriers is crucial. This necessitates the integration of damage mechanisms, crucial decision-making nodes, and the evolution of states, followed by the implementation of tiered warning systems[179]. To facilitate a tiered early warning system for hazardous chemical incidents, based on dynamic, multisource heterogeneous data, the fuzzy comprehensive evaluation method is employed to develop situation assessment and early warning models[180]. Furthermore, utilizing pair analysis, probabilistic neural networks, and disaster safety assessment techniques, the platform constructs a disaster early warning database and an emergency management response paradigm, integrating warning extents and secondary/derivative characteristics[181]. This ensures that the early warning information disseminated to governments, enterprises, and individuals, among other disaster-prepared entities, is timely, accessible, and distinctly categorized.
Deflagration and detonation mitigation for HSTE
Mitigating deflagration and detonation serves as the final line of defense for the safety of hydrogen storage and transportation equipment. As in conventional gas or dust explosion scenarios, the strategies for mitigation can be broadly classified into passive and active methods. Passive methods function autonomously without requiring additional control units, activating solely based on the physical effects of an explosion, DDT, or detonation itself. In contrast, active methods rely on detectors and control units to implement targeted preventive measures prior to the occurrence of an explosion.
Passive explosion suppression technology was initially implemented in the coal mining industry, where passive barriers are widely utilized. This method leverages the kinetic energy of the pressure wave generated by an explosion. Upon detonation, a pre-installed barrier is displaced by the pressure wave, releasing inert materials that create a flame-retardant mist. Passive barriers can be categorized into different types, such as dust barriers and water barriers [Figure 7A], depending on the inert material employed[182,183]. From an installation perspective, they are divided into centralized and distributed systems. Stone dust barriers have been widely used to prevent coal dust explosions since the 1920s, but they have limitations: high wind speeds can reduce their effectiveness, and they are prone to clogging due to moisture over long exposures. In contrast, water barriers are easy to maintain, cost less, and can effectively extinguish flames by dispersing water mist into the path of the explosion flames, as well as moistening nearby dust and airflow, thereby effectively suppressing flame propagation. Beyond passive barriers, ventilation systems are also a common means of preventing explosions, functioning by releasing explosive pressure that may accumulate in enclosed spaces[184]. Flame arresters in flameless venting devices allow gas to be vented while preventing flame spread[185]. Since Humphry Davy first used fine metal mesh as a flame arrester in 1815, porous materials have been widely studied for their excellent flame-suppressing performance. Studies show that the pore size, material, and thickness of porous materials significantly impact the speed of flame propagation and the suppression of combustion pressure waves [Figure 7B and C]. In microchannels (planar gaps, annular gaps, capillaries, etc.), flame propagation and DDT can accelerate due to wall constraints and boundary layer effects; however, in subsequent detonation propagation, there is insufficient detonation, inversely proportional to the narrow gap height and initial pressure[186,187]. Overall, when the pore size is small, porous materials act as damping permeation media; when the pore size is larger, porous materials transform into turbulent elements, leading to increased combustion area and intensified combustion.
Figure 7. Passive and active methods for hydrogen explosion and detonation suppression. (A) Distributed bagged dust barrier (top) and multi-layered water trough barrier (bottom). Reproduced with permission[182], Copyright 2022, Elsevier B.V. (B) Test of explosion venting without (left) and with a flameless venting device (right). Reproduced with permission[185], Copyright 2008, The Authors. (C) Porous materials hinder explosion spread through several physical mechanisms, including energy dissipation, acoustic damping, heat absorption, flame quenching, and thermal expansion and deformation. Reproduced with permission[186], Copyright 2023, Elsevier Ltd. (D) Morphology and structure of various typical porous materials: expanded aluminum mesh, spherical non-metallic substances, metallic foams, ceramic foams and aerogels. Reproduced with permission[187], Copyright 2020 Hydrogen Energy Publications LLC., permission[193], Copyright 2021, Taylor & Francis and permission[195], Copyright 2017, Elsevier Ltd. (E) Active explosion suppression system built on the high-pressure fire extinguisher. Reproduced with permission[196], Copyright 2021, The Author(s). (F) The principle of the active explosion suppression system. Reproduced with permission[198], Copyright 2021, The Author(s). (G) Graphical overview of the components in an active barrier system. Reproduced with permission[197], Copyright 2015, The Author(s).
Currently, mesh aluminum alloys (MAAs) and polyurethane foam are commonly utilized as explosion-proof materials[188,189]. In the early 1980s, the U.S. established a military standard (MIL-B-87162) for employing expanded aluminum mesh to mitigate aircraft fuel tank explosions. This standard was later revised to MIL-B-87162A in 1994 to enhance the evaluation methods for mesh MAAs. However, the U.S. military discontinued MIL-B-87162A in 2004 due to challenges related to the loading and unloading processes, high maintenance costs, and metal fragments obstructing fuel inlets, leading to a shift away from metal-based explosion suppression materials[190]. Today, such materials are predominantly found in civilian applications. In contrast to MAAs, foam copper, with its three-dimensional porous structure, offers exceptional sound absorption and thermal conductivity. Additionally, its superior ductility minimizes cracking under shock wave impacts, allowing for a degree of deformation that effectively cushions shock waves and enhances reusability. Some pioneering research indicates that just 3 cm of foam copper can significantly suppress explosions during the DDT[191-193]. Recently, new non-metallic materials, particularly silica/nitride ceramic aerogels, have gained substantial research interest [Figure 7D]. The unique network structure of aerogels provides low thermal conductivity, lightweight density, high flexibility, and excellent compressive performance, positioning them as optimal candidates for shock absorption and explosion suppression[194,195]. Nonetheless, limited production capacity restricts their commercial availability, keeping them in the developmental phase.
Passive explosion suppression technologies typically cannot guarantee optimal deployment timing, and active suppression methods equipped with triggering devices can effectively compensate for this deficiency
In contrast, the fluorine found in hydrofluorocarbons is effective at scavenging active free radicals such as H, O, and OH, thereby interrupting chain reactions and demonstrating superior suppression capabilities for hydrogen explosions[203,204]. However, the high-pressure storage and release requirements for hydrofluorocarbons limit their broader application. Recently, researchers have introduced modified perfluorohexanone dry water, a technique that integrates water mist with perfluorohexanone to mitigate the storage challenges associated with fluorocarbon compounds such as CHF3, C2HF5, CF3CHFCF3[205]. When modified perfluorohexanone dry water is used at a mass fraction of 16.70 wt% and a concentration of
CONCLUSION AND OUTLOOK
This review delves into the crucial aspects of operational safety and security for storage and transportation equipment, showcasing a variety of effective technical innovations and safety management strategies. Advancements in material safety design, leakage detection, and mitigation technologies for deflagration and detonation can markedly diminish the safety risks in hydrogen storage and transport. However, the impending surge in green hydrogen demand - projected by IEA to reach 150 million tons by 2030 - necessitates balancing technical innovation with evolving policy frameworks and cost-benefit dynamics. Four priority research directions emerge:
(i) Coupled damage mechanisms and safety design for large-volume hydrogen storage containers, including studies on hydrogen-induced material degradation under complex loads, extreme environments, and with heterogeneous materials, strategies to prevent brittle fracture, and overpressure protection in extreme conditions;
(ii) Damage failure mechanisms and prevention of hydrogen embrittlement in long-distance hydrogen pipelines, uncovering deterioration mechanisms in pipeline materials and welds within hydrogen environments, developing a compatibility assessment system, and establishing safety thresholds and damage control methods;
(iii) Quick detection of hydrogen gas leaks and diffusion in large-scale green hydrogen facilities, understanding leak and diffusion patterns in large containers and across regional pipelines, and developing rapid response leak detection methods with high spatiotemporal resolution;
(iv) Evolution of hydrogen gas combustion and explosion in complex settings and deflagration and detonation mitigation mechanisms, creating models to predict behaviors and outcomes of hydrogen gas deflagration and detonation, investigating porous materials' suppression effects and flame quenching mechanisms, and elucidating mitigation mechanisms in typical scenarios.
The global policy landscape presents both drivers and challenges for implementing these solutions. Major economies have escalated hydrogen ambitions, with the US targeting 50 million tons of green hydrogen by 2050 and China's 2021-2035 plan allocating $20B+ for hydrogen infrastructure. Paradoxically, 78% of surveyed nations lack unified hydrogen safety regulations, creating fragmented compliance landscapes. Urgent standardization efforts should integrate three dimensions: Aligning material testing protocols with ISO/TC 197 guidelines; Establishing cross-border certification for hydrogen-compatible welding technologies; Developing risk-based zoning frameworks for large-scale storage facilities.
Cost-benefit analyses reveal critical leverage points: While PEM electrolyzer costs have fallen 60% since 2015 to $800/kW, green hydrogen production remains 2-3× more expensive than gray hydrogen. Techno-economic modeling shows that combining advanced leakage detection (reducing losses by 15%-20%) with optimized composite pipelines (cutting transport costs to $0.3-0.5/kg/100 km) could achieve grid parity in heavy transport by 2035. Notably, China's "West-East Hydrogen Transmission" megaproject demonstrates that scaling 50 MPa pipelines to 3,000 km lengths lowers LCOH by 38% compared to trucking.
To accelerate commercialization, three synergistic strategies prove essential: First, integrating hydrogen safety R&D with national carbon markets through tradable risk mitigation credits. Second, establishing "Hydrogen Valleys" that co-locate offshore wind farms, underground pipelines, and industrial users to validate circular economy models. Third, implementing tiered subsidy schemes that prioritize technologies with dual safety-cost benefits, such as self-sealing composite tanks showing 25% lower lifecycle costs than conventional designs.
DECLARATIONS
Acknowledgments
The authors acknowledge funding from the Key Research and Development Plan of Shaanxi Province.
Authors’ contributions
Investigation, validation, formal analysis, writing, and visualization: Wang, J.
Conceptualization, methodology, investigation, writing, visualization, supervision, project administration, and funding acquisition: Zhao, Y.
Availability of data and materials
All discussed data originate from previously published studies properly cited throughout the text. For access to original datasets, readers should refer to the corresponding references provided in the bibliography.
Financial support and sponsorship
This work was financially supported by the Key Research and Development Plan of Shaanxi Province (No. 2024GX-ZDCYL-01-06).
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Espegren, K.; Damman, S.; Pisciella, P.; Graabak, I.; Tomasgard, A. The role of hydrogen in the transition from a petroleum economy to a low-carbon society. Int. J. Hydrogen. Energy. 2021, 46, 23125-38.
2. Yao, Z.; Deng, H.; Dong, J.; et al. On explosion characteristics of premixed syngas/air mixtures with different hydrogen volume fractions and ignition positions. Fuel 2021, 288, 119619.
3. Case study: power plant hydrogen explosion. 2022. Available from: https://wha-international.com/case-study-power-plant-hydrogen-explosion/ [Last accessed on 30 May 2025].
4. Hydrogen refuelling plant explodes in Norway. Available from: https://ctif.org/news/hydrogen-refuelling-plant-explodes-norway [Last accessed on 30 May 2025].
5. Student killed in chemistry lab blast. 2025. Available from: https://www.chinadaily.com.cn/china/2015-12/19/content_22750853.htm [Last accessed on 30 May 2025].
6. Hydrogen blast led to deaths at US silicones plant. 2019. Available from: https://cen.acs.org/safety/industrial-safety/Hydrogen-blast-led-deaths-US/97/web/2019/12 [Last accessed on 30 May 2025].
7. Hydrogen tank explosion kills 2 in Gangneung. 2019. Available from: https://www.koreatimes.co.kr/www/nation/2024/02/113_269400.html [Last accessed on 30 May 2025].
8. Moradi, R.; Groth, K. M. Hydrogen storage and delivery: review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen. Energy. 2019, 44, 12254-69.
9. Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen. Energy. 2013, 38, 14595-617.
10. Wang, Z.; Wang, Y.; Afshan, S.; Hjalmarsson, J. A review of metallic tanks for H2 storage with a view to application in future green shipping. Int. J. Hydrogen. Energy. 2021, 46, 6151-79.
11. Hassan, I.; Ramadan, H. S.; Saleh, M. A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: review, analysis and perspectives. Renew. Sustain. Energy. Rev. 2021, 149, 111311.
12. Park, W. S.; Yoo, S. W.; Kim, M. H.; Lee, J. M. Strain-rate effects on the mechanical behavior of the AISI 300 series of austenitic stainless steel under cryogenic environments. Mater. Des. 2010, 31, 3630-40.
13. Desisto, T. S.; Carr, L. C. Low temperature mechanical properties of 300 series stainless steel and titanium. In: Timmerhaus KD, editor. Advances in cryogenic engineering. Boston: Springer US; 1961. pp. 577-86.
14. Matsunaga, H.; Yoshikawa, M.; Kondo, R.; Yamabe, J.; Matsuoka, S. Slow strain rate tensile and fatigue properties of Cr-Mo and carbon steels in a 115 MPa hydrogen gas atmosphere. Int. J. Hydrogen. Energy. 2015, 40, 5739-48.
15. Yoon, S. J.; Lee, H. J.; Yoon, K. B.; Ma, Y. W.; Baek, U. B. Hydrogen damage in 34CrMo4 pressure vessel steel with high tensile strength. J. Mech. Sci. Technol. 2018, 32, 637-46.
16. Ritchie, R. O.; Parker, E. R.; Spencer, P. N.; Todd, J. A. A new series of advanced 3Cr-Mo-Ni steels for thick section pressure vessels in high temperature and pressure hydrogen service. J. Mater. Energy. Syst. 1984, 6, 151-62.
17. Zhu, Z.; Hu, Z.; Seet, H. L.; et al. Recent progress on the additive manufacturing of aluminum alloys and aluminum matrix composites: Microstructure, properties, and applications. Int. J. Mach. Tool. Manu. 2023, 190, 104047.
18. Verstraete, D.; Hendrick, P.; Pilidis, P.; Ramsden, K. Hydrogen fuel tanks for subsonic transport aircraft. Int. J. Hydrogen. Energy. 2010, 35, 11085-98.
19. Rometsch, P. A.; Zhu, Y.; Wu, X.; Huang, A. Review of high-strength aluminium alloys for additive manufacturing by laser powder bed fusion. Mater. Des. 2022, 219, 110779.
20. Laadel, N.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation barriers for hydrogen embrittlement prevention in metals - A review on mechanisms, materials suitability and efficiency. Int. J. Hydrogen. Energy. 2022, 47, 32707-31.
21. Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen related degradation in pipeline steel: a review. Int. J. Hydrogen. Energy. 2018, 43, 14584-617.
22. Lynch, S. P. 2 - Hydrogen embrittlement (HE) phenomena and mechanisms. Stress Corrosion Cracking. Elsevier; 2011. pp. 90-130.
23. Robertson, I. M.; Sofronis, P.; Nagao, A.; et al. Hydrogen embrittlement understood. Metall. Mater. Trans. B. 2015, 46, 1085-103.
24. Nagumo, M. Diffusion and transport of hydrogen. Fundamentals of hydrogen embrittlement. Singapore: Springer Nature; 2023. pp. 77-93.
25. Forcey, K.; Ross, D.; Simpson, J.; Evans, D. Hydrogen transport and solubility in 316L and 1.4914 steels for fusion reactor applications. J. Nucl. Mater. 1988, 160, 117-24.
26. Ishikawa, T.; Mclellan, R. The diffusivity of hydrogen in aluminum. Acta. Metall. 1986, 34, 1091-5.
27. Bhadeshia, H. K. D. H. Prevention of hydrogen embrittlement in steels. ISIJ. Int. 2016, 56, 24-36.
28. Taxak, M.; Kumar, S.; Kalekar, B. B.; Krishnamurthy, N. Effect of nickel addition on the solubility of hydrogen in tantalum. Int. J. Hydrogen. Energy. 2013, 38, 7561-8.
29. Yan, E.; Sun, L.; Xu, F.; et al. Changes in microstructure, solidification path and hydrogen permeability of Nb-Hf-Co alloy by adjusting Hf/Co ratio. Int. J. Hydrogen. Energy. 2016, 41, 1391-400.
30. Saeki, Y.; Yamada, Y.; Ishikawa, K. Relationship between hydrogen permeation and microstructure in Nb-TiCo two-phase alloys. J. Alloys. Compd. 2015, 645, S32-5.
31. Kim, K. H.; Park, H. C.; Lee, J.; Cho, E.; Lee, S. M. Vanadium alloy membranes for high hydrogen permeability and suppressed hydrogen embrittlement. Scr. Mater. 2013, 68, 905-8.
32. Pfeil, L. B.; Carpenter, H. C. H. The effect of occluded hydrogen on the tensile strength of iron. Proc. R. Soc. Lond. A. 1926, 112, 182-95.
33. Beachem, C. D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Trans. 1972, 3, 441-55.
34. Depover, T.; Verbeken, K. The detrimental effect of hydrogen at dislocations on the hydrogen embrittlement susceptibility of Fe-C-X alloys: an experimental proof of the HELP mechanism. Int. J. Hydrogen. Energy. 2018, 43, 3050-61.
35. Martin, M. L.; Dadfarnia, M.; Nagao, A.; Wang, S.; Sofronis, P. Enumeration of the hydrogen-enhanced localized plasticity mechanism for hydrogen embrittlement in structural materials. Acta. Mater. 2019, 165, 734-50.
36. Dwivedi, S. K.; Vishwakarma, M. Hydrogen embrittlement in different materials: a review. Int. J. Hydrogen. Energy. 2018, 43, 21603-16.
37. Nagumo, M. Function of hydrogen in embrittlement of high-strength steels. ISIJ. Int. 2001, 41, 590-8.
38. Nagumo, M. Hydrogen related failure of steels - a new aspect. Mater. Sci. Technol. 2004, 20, 940-50.
39. Sakaki, K.; Kawase, T.; Hirato, M.; et al. The effect of hydrogen on vacancy generation in iron by plastic deformation. Scr. Mater. 2006, 55, 1031-4.
40. Wen, M.; Zhang, L.; An, B.; Fukuyama, S.; Yokogawa, K. Hydrogen-enhanced dislocation activity and vacancy formation during nanoindentation of nickel. Phys. Rev. B. 2009, 80, 094113.
41. Hou, J.; Kong, X. S.; Wu, X.; Song, J.; Liu, C. S. Predictive model of hydrogen trapping and bubbling in nanovoids in bcc metals. Nat. Mater. 2019, 18, 833-9.
42. Hickel, T.; Nazarov, R.; Mceniry, E. J.; Leyson, G.; Grabowski, B.; Neugebauer, J. Ab Initio based understanding of the segregation and diffusion mechanisms of hydrogen in steels. JOM 2014, 66, 1399-405.
43. Neeraj, T.; Srinivasan, R.; Li, J. Hydrogen embrittlement of ferritic steels: observations on deformation microstructure, nanoscale dimples and failure by nanovoiding. Acta. Mater. 2012, 60, 5160-71.
44. Barrera, O.; Bombac, D.; Chen, Y.; et al. Understanding and mitigating hydrogen embrittlement of steels: a review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251-90.
45. Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of hydrogen safety during storage, transmission, and applications processes. J. Loss. Prev. Process. Ind. 2021, 72, 104569.
46. Li, H.; Cao, X.; Liu, Y.; et al. Safety of hydrogen storage and transportation: an overview on mechanisms, techniques, and challenges. Energy. Rep. 2022, 8, 6258-69.
47. Wang, C.; Zhao, L.; Qu, J.; Xiao, Y.; Deng, J.; Shu, C. Minireview on the leakage ignition and flame propagation characteristics of hydrogen: advances and perspectives. Energy. Fuels. 2023, 37, 5653-66.
48. Genovese, M.; Cigolotti, V.; Jannelli, E.; Fragiacomo, P. Current standards and configurations for the permitting and operation of hydrogen refueling stations. Int. J. Hydrogen. Energy. 2023, 48, 19357-71.
49. Schefer, R.; Houf, W.; Williams, T. Investigation of small-scale unintended releases of hydrogen: momentum-dominated regime. Int. J. Hydrogen. Energy. 2008, 33, 6373-84.
50. Veser, A.; Kuznetsov, M.; Fast, G.; et al. The structure and flame propagation regimes in turbulent hydrogen jets. Int. J. Hydrogen. Energy. 2011, 36, 2351-9.
51. Kotchourko, N.; Kuznetsov, M.; Kotchourko, A.; Grune, J.; Lelyakin, A.; Jordan, T. Concentration measurements in a round hydrogen jet using background oriented schlieren (BOS) technique. Int. J. Hydrogen. Energy. 2014, 39, 6201-9.
52. Swain, M.; Filoso, P.; Swain, M. An experimental investigation into the ignition of leaking hydrogen. Int. J. Hydrogen. Energy. 2007, 32, 287-95.
53. Houf, W.; Schefer, R. Analytical and experimental investigation of small-scale unintended releases of hydrogen. Int. J. Hydrogen. Energy. 2008, 33, 1435-44.
54. Han, S. H.; Chang, D.; Kim, J. S. Experimental investigation of highly pressurized hydrogen release through a small hole. Int. J. Hydrogen. Energy. 2014, 39, 9552-61.
55. Birch, A. D.; Hughes, D. J.; Swaffield, F. Velocity decay of high pressure jets. Combust. Sci. Technol. 1987, 52, 161-71.
56. Caramia, G.; Amirante, R.; De Palma, P. Unsteady RANS simulations of under-expanded hydrogen jets for internal combustion engines. Int. J. Hydrogen. Energy. 2024, 96, 849-59.
57. Ruggles, A.; Ekoto, I. Ignitability and mixing of underexpanded hydrogen jets. Int. J. Hydrogen. Energy. 2012, 37, 17549-60.
58. Crist, S.; Glass, D. R.; Sherman, P. M. Study of the highly underexpanded sonic jet. AIAA. J. 1966, 4, 68-71.
59. Velikorodny, A.; Kudriakov, S. Numerical study of the near-field of highly underexpanded turbulent gas jets. Int. J. Hydrogen. Energy. 2012, 37, 17390-9.
60. Mo, F.; Liu, B.; Wang, H.; She, X.; Teng, L.; Kang, X. Study on hydrogen dispersion in confined space with complex air supply and exhaust system. Int. J. Hydrogen. Energy. 2022, 47, 29131-47.
61. Han, H.; Chang, X.; Duan, P.; Li, Y.; Zhu, J.; Kong, Y. Study on the leakage and diffusion behavior of hydrogen-blended natural gas in utility tunnels. J. Loss. Prev. Proc. Ind. 2023, 85, 105151.
62. Baines, W. D.; Turner, J. S. Turbulent buoyant convection from a source in a confined region. J. Fluid. Mech. 1969, 37, 51-80.
64. Kumamoto, A.; Iseki, H.; Ono, R.; Oda, T. Measurement of minimum ignition energy in hydrogen-oxygen-nitrogen premixed gas by spark discharge. J. Phys. Conf. Ser. 2011, 301, 012039.
65. Wang, Z.; Li, S.; Jin, Z.; Li, Z.; Liu, Q.; Zhang, K. Oil and gas pathway to net-zero: review and outlook. Energy. Strategy. Rev. 2023, 45, 101048.
66. Kim, H. J.; Chung, S. H.; Sohn, C. H. Numerical calculation of minimum ignition energy for hydrogen and methane fuels. KSME. Int. J. 2004, 18, 838-46.
67. Klell, M.; Eichlseder, H.; Trattner, A. Internal combustion engines. Hydrogen in automotive engineering. Wiesbaden: Springer Fachmedien; 2023. pp. 193-249.
68. Ono, R.; Nifuku, M.; Fujiwara, S.; Horiguchi, S.; Oda, T. Minimum ignition energy of hydrogen-air mixture: effects of humidity and spark duration. J. Electrostat. 2007, 65, 87-93.
69. Lee, H. J.; Kim, Y. R.; Kim, S.; Jeung, I. Experimental investigation on the self-ignition of pressurized hydrogen released by the failure of a rupture disk through tubes. Proc. Combust. Inst. 2011, 33, 2351-8.
70. Wolański, P.; Wójcicki, S. Stabilization of coal dust-air mixture by bluff bodies. Symp. (Int). Combust. 1975, 15, 1295-302.
71. Grune, J.; Sempert, K.; Kuznetsov, M.; Jordan, T. Experimental study of ignited unsteady hydrogen releases from a high pressure reservoir. Int. J. Hydrogen. Energy. 2014, 39, 6176-83.
72. Kim, Y. R.; Lee, H. J.; Kim, S.; Jeung, I. A flow visualization study on self-ignition of high pressure hydrogen gas released into a tube. Proc. Combust. Inst. 2013, 34, 2057-64.
73. Don, W. G.; Robert, H. P. Perry's chemical engineers' handbook. McGraw-Hill Education: New York; 2008. Available from: https://www.accessengineeringlibrary.com/content/book/9780071422949 [Last accessed on 30 May 2025].
74. Merilo, E.; Groethe, M.; Adamo, R.; Schefer, R.; Houf, W.; Dedrick, D. Self-ignition of hydrogen releases through electrostatic discharge induced by entrained particulates. Int. J. Hydrogen. Energy. 2012, 37, 17561-70.
75. Imamura, T.; Mogi, T.; Wada, Y. Control of the ignition possibility of hydrogen by electrostatic discharge at a ventilation duct outlet. Int. J. Hydrogen. Energy. 2009, 34, 2815-23.
76. Yamada, E.; Kitabayashi, N.; Hayashi, A. K.; Tsuboi, N. Mechanism of high-pressure hydrogen auto-ignition when spouting into air. Int. J. Hydrogen. Energy. 2011, 36, 2560-6.
77. Wang, Z.; Pan, X.; Wang, Q.; et al. Experimental study on spontaneous ignition and flame propagation of high-pressure hydrogen release through tubes. Int. J. Hydrogen. Energy. 2019, 44, 22584-97.
78. Mogi, T.; Wada, Y.; Ogata, Y.; Koichi Hayashi, A. Self-ignition and flame propagation of high-pressure hydrogen jet during sudden discharge from a pipe. Int. J. Hydrogen. Energy. 2009, 34, 5810-6.
79. Wasim, M.; Djukic, M. B.; Ngo, T. D. Influence of hydrogen-enhanced plasticity and decohesion mechanisms of hydrogen embrittlement on the fracture resistance of steel. Eng. Fail. Anal. 2021, 123, 105312.
80. Li, P.; Duan, Q.; Zeng, Q.; Jin, K.; Chen, J.; Sun, J. Experimental study of spontaneous ignition induced by sudden hydrogen release through tubes with different shaped cross-sections. Int. J. Hydrogen. Energy. 2019, 44, 23821-31.
81. Duan, Q.; Xiao, H.; Gao, W.; Gong, L.; Sun, J. Experimental investigation of spontaneous ignition and flame propagation at pressurized hydrogen release through tubes with varying cross-section. J. Hazard. Mater. 2016, 320, 18-26.
82. Rubtsov, N. M.; Chernysh, V. I.; Tsvetkov, G. I.; Troshin, K. Y.; Shamshin, I. O. Ignition of hydrogen-methane-air mixtures over Pd foil at atmospheric pressure. Mendeleev. Commun. 2019, 29, 469-71.
83. Song, C.; Jiang, H.; Gao, W. High temperature generated by sliding metal friction and its effectiveness as an ignition source for hydrogen. J. Loss. Prev. Proc. Ind. 2022, 79, 104833.
84. Mueller, M. A.; Kim, T. J.; Yetter, R. A.; Dryer, F. L. Flow reactor studies and kinetic modeling of the H2/O2 reaction. Int. J. Chem. Kinet. 1999, 31, 113-25.
85. Sánchez, A. L.; Fernández-Tarrazo, E.; Boivin, P.; Liñán, A.; Williams, F. A. Ignition time of hydrogen-air diffusion flames. Comptes. Rendus. Mécanique. 2012, 340, 882-93.
86. Sánchez, A. L.; Fernández-Tarrazo, E.; Williams, F. A. The chemistry involved in the third explosion limit of H2-O2 mixtures. Combust. Flame. 2014, 161, 111-7.
87. Wang, X.; Law, C. K. An analysis of the explosion limits of hydrogen-oxygen mixtures. J. Chem. Phys. 2013, 138, 134305.
88. Liang, W.; Law, C. K. An analysis of the explosion limits of hydrogen/oxygen mixtures with nonlinear chain reactions. Phys. Chem. Chem. Phys. 2018, 20, 742-51.
89. Molkov, V.; Dadashzadeh, M.; Kashkarov, S.; Makarov, D. Performance of hydrogen storage tank with TPRD in an engulfing fire. Int. J. Hydrogen. Energy. 2021, 46, 36581-97.
90. Molkov, V.; Cirrone, D.; Shentsov, V.; Dery, W.; Kim, W.; Makarov, D. Dynamics of blast wave and fireball after hydrogen tank rupture in a fire in the open atmosphere. Int. J. Hydrogen. Energy. 2021, 46, 4644-65.
91. Cirrone, D.; Makarov, D.; Molkov, V. Rethinking “BLEVE explosion” after liquid hydrogen storage tank rupture in a fire. Int. J. Hydrogen. Energy. 2023, 48, 8716-30.
92. Baraza, X. The boiling liquid expanding vapour explosion (BLEVE): a bibliometric review and futur trends. J. Loss. Prev. Process. Ind. 2023, 83, 105104.
93. Holborn, P.; Benson, C.; Ingram, J. Modelling hazardous distances for large-scale liquid hydrogen pool releases. Int. J. Hydrogen. Energy. 2020, 45, 23851-71.
94. Mukhim, E. D.; Abbasi, T.; Tauseef, S.; Abbasi, S. A method for the estimation of overpressure generated by open air hydrogen explosions. J. Loss. Prev. Process. Ind. 2018, 52, 99-107.
95. Pitts, W. M.; Yang, J. C.; Blais, M.; Joyce, A. Dispersion and burning behavior of hydrogen released in a full-scale residential garage in the presence and absence of conventional automobiles. Int. J. Hydrogen. Energy. 2012, 37, 17457-69.
96. Thomas, J. K.; Eastwood, C.; Goodrich, M. Are unconfined hydrogen vapor cloud explosions credible? Process. Saf. Prog. 2015, 34, 36-43.
97. Sun, X.; Li, Q.; Xu, M.; Wang, L.; Guo, J.; Lu, S. Experimental study on the detonation propagation behaviors through a small-bore orifice plate in hydrogen-air mixtures. Int. J. Hydrogen. Energy. 2019, 44, 15523-35.
98. Groethe, M.; Merilo, E.; Colton, J.; Chiba, S.; Sato, Y.; Iwabuchi, H. Large-scale hydrogen deflagrations and detonations. Int. J. Hydrogen. Energy. 2007, 32, 2125-33.
99. Ciccarelli, G.; Dorofeev, S. Flame acceleration and transition to detonation in ducts. Prog. Energy. Combust. Sci. 2008, 34, 499-550.
100. Shamsadin Saeid, M. H.; Khadem, J.; Emami, S.; Ghodrat, M. Effect of diffusion time on the mechanism of deflagration to detonation transition in an inhomogeneous mixture of hydrogen-air. Int. J. Hydrogen. Energy. 2022, 47, 23411-26.
101. Zhou, S.; Luo, Z.; Wang, T.; He, M.; Li, R.; Su, B. Research progress on the self-ignition of high-pressure hydrogen discharge: a review. Int. J. Hydrogen. Energy. 2022, 47, 9460-76.
102. Dziemińska, E.; Hayashi, A. K. Auto-ignition and DDT driven by shock wave - Boundary layer interaction in oxyhydrogen mixture. Int. J. Hydrogen. Energy. 2013, 38, 4185-93.
103. Melguizo-Gavilanes, J.; Ballossier, Y.; Faria, L. Experimental and theoretical observations on DDT in smooth narrow channels. Proc. Combust. Inst. 2021, 38, 3497-503.
104. Urtiew, P. A.; Oppenheim, A. K.; Saunders, S. O. Experimental observations of the transition to detonation in an explosive gas. Proc. R. Soc. Lond. A. 1966, 295, 13-28.
105. Zeldovich, Y. Regime classification of an exothermic reaction with nonuniform initial conditions. Combust. Flame. 1980, 39, 211-4.
106. Gamezo, V. N.; Ogawa, T.; Oran, E. S. Flame acceleration and DDT in channels with obstacles: effect of obstacle spacing. Combust. Flame. 2008, 155, 302-15.
107. Han, W.; Gao, Y.; Law, C. K. Flame acceleration and deflagration-to-detonation transition in micro- and macro-channels: an integrated mechanistic study. Combust. Flame. 2017, 176, 285-98.
108. Goodwin, G. B.; Oran, E. S. Premixed flame stability and transition to detonation in a supersonic combustor. Combust. Flame. 2018, 197, 145-60.
109. Zhang, L.; Li, Z.; Zheng, J.; et al. Effect of strain-induced martensite on hydrogen embrittlement of austenitic stainless steels investigated by combined tension and hydrogen release methods. Int. J. Hydrogen. Energy. 2013, 38, 8208-14.
110. Zhang, L.; Wen, M.; Imade, M.; Fukuyama, S.; Yokogawa, K. Effect of nickel equivalent on hydrogen gas embrittlement of austenitic stainless steels based on type 316 at low temperatures. Acta. Mater. 2008, 56, 3414-21.
111. Zhang, S.; Liu, S.; Wan, J.; Liu, W. Effect of Nb-Ti multi-microalloying on the hydrogen trapping efficiency and hydrogen embrittlement susceptibility of hot-stamped boron steel. Mater. Sci. Eng. A. 2020, 772, 138788.
112. Lin, Y.; Mccarroll, I. E.; Lin, Y.; Chung, W.; Cairney, J. M.; Yen, H. Hydrogen trapping and desorption of dual precipitates in tempered low-carbon martensitic steel. Acta. Mater. 2020, 196, 516-27.
113. Di Stefano, D.; Mrovec, M.; Elsässer, C. First-principles investigation of quantum mechanical effects on the diffusion of hydrogen in iron and nickel. Phys. Rev. B. 2015, 92, 224301.
114. Ramunni, V. P.; Pascuet, M. I.; Castin, N.; Rivas, A. M. The influence of grain size on the hydrogen diffusion in bcc Fe. Comput. Mater. Sci. 2021, 188, 110146.
115. López Freixes, M.; Zhou, X.; Zhao, H.; et al. Revisiting stress-corrosion cracking and hydrogen embrittlement in 7xxx-Al alloys at the near-atomic-scale. Nat. Commun. 2022, 13, 4290.
116. Zhao, H.; Chakraborty, P.; Ponge, D.; et al. Hydrogen trapping and embrittlement in high-strength Al alloys. Nature 2022, 602, 437-41.
117. Safyari, M.; Moshtaghi, M.; Hojo, T.; Akiyama, E. Mechanisms of hydrogen embrittlement in high-strength aluminum alloys containing coherent or incoherent dispersoids. Corros. Sci. 2022, 194, 109895.
118. Sun, B.; Lu, W.; Gault, B.; et al. Chemical heterogeneity enhances hydrogen resistance in high-strength steels. Nat. Mater. 2021, 20, 1629-34.
119. Zhao, Y.; Lee, D.; Seok, M.; et al. Resistance of CoCrFeMnNi high-entropy alloy to gaseous hydrogen embrittlement. Scr. Mater. 2017, 135, 54-8.
120. Fu, Z.; Yang, B.; Gan, K.; et al. Improving the hydrogen embrittlement resistance of a selective laser melted high-entropy alloy via modifying the cellular structures. Corros. Sci. 2021, 190, 109695.
121. Baek, S.; He, S.; Jang, M.; Back, D.; Jeong, D.; Park, S. Ultrasonic nanocrystal surface modification effect on reduction of hydrogen embrittlement in Inconel-625 parts fabricated via additive manufacturing process. J. Manuf. Processes. 2023, 108, 685-95.
122. Barth, R. R.; Simmons, K. L.; Marchi, C. S. Polymers for hydrogen infrastructure and vehicle fuel systems: applications, properties, and gap analysis. 2013. Available from: https://www.osti.gov/servlets/purl/1104755 [Last accessed on 30 May 2025].
123. Zaghdoudi, M.; Kömmling, A.; Böhning, M.; Jaunich, M. Ageing of elastomers in air and in hydrogen environment: a comparative study. Int. J. Hydrogen. Energy. 2024, 63, 207-16.
124. Fujiwara, H.; Ono, H.; Nishimura, S. Degradation behavior of acrylonitrile butadiene rubber after cyclic high-pressure hydrogen exposure. Int. J. Hydrogen. Energy. 2015, 40, 2025-34.
125. Luo, G.; Pang, B.; Luo, X.; Wang, Y.; Zhou, H.; Zhao, L. Brominated butyl rubber anticorrosive coating and its self-healing behaviors. Chin. J. Polym. Sci. 2023, 41, 297-305.
126. Zhang, X.; Xue, X.; Yin, Q.; et al. Enhanced compatibility and mechanical properties of carboxylated acrylonitrile butadiene rubber/styrene butadiene rubber by using graphene oxide as reinforcing filler. Compos. Part. B. Eng. 2017, 111, 243-50.
127. Kapuscinsky, N.; Ignatusha, P.; Islam, A.; Ezzine, M.; Du, N.; Meek, K. M. Polymeric coatings for preventing hydrogen embrittlement in industrial storage and transmission systems. ACS. Appl. Eng. Mater. 2024, 2, 2488-503.
128. Kim, H.; Popov, B. N.; Chen, K. S. Comparison of corrosion-resistance and hydrogen permeation properties of Zn-Ni, Zn-Ni-Cd and Cd coatings on low-carbon steel. Corros. Sci. 2003, 45, 1505-21.
129. Nemanič, V.; Zajec, B.; Dellasega, D.; Passoni, M. Hydrogen permeation through disordered nanostructured tungsten films. J. Nucl. Mater. 2012, 429, 92-8.
130. Lakdhar, I.; Alhussein, A.; Capelle, J.; Creus, J. Al-Ti-W alloys deposited by magnetron sputtering: effective barrier to prevent steel hydrogen embrittlement. Appl. Surf. Sci. 2021, 567, 150786.
131. Li, Y.; Barzagli, F.; Liu, P.; et al. Mechanism and evaluation of hydrogen permeation barriers: a critical review. Ind. Eng. Chem. Res. 2023, 62, 15752-73.
132. Menon, N. C.; Kruizenga, A. M.; Alvine, K. J.; San Marchi, C.; Nissen, A.; Brooks, K. Behaviour of polymers in high pressure environments as applicable to the hydrogen infrastructure. ASME 2016 Pressure Vessels and Piping Conference; 2016.
133. Jung, J.; Kim, I.; Kim, K. Evaluation of hydrogen permeation characteristics in rubbery polymers. Curr. Appl. Phys. 2021, 21, 43-9.
134. Zhang, S.; Yuan, S.; Pei, L.; et al. Innovating a EVOH composite coating towards outstanding H2 barrier and anti-corrosion properties. Chem. Eng. J. 2024, 499, 156327.
135. Jana, S.; Parthiban, A.; Rusli, W. Polymer material innovations for a green hydrogen economy. Chem. Commun. 2025, 61, 3233-49.
136. Yang, X.; Qin, L.; Wang, L.; Ding, R.; Shi, L.; Lv, B. Scalable synthesis of quasi-monodispersed BN colloidal nanocrystals by “solvent cutting” and their anti-electrochemical corrosion coating. Chem. Eng. J. 2018, 333, 191-9.
137. Nemanič, V. Hydrogen permeation barriers: basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy. 2019, 19, 451-7.
138. Levchuk, D.; Koch, F.; Maier, H.; Bolt, H. Deuterium permeation through Eurofer and α-alumina coated Eurofer. J. Nucl. Mater. 2004, 328, 103-6.
139. Zhang, G.; Dou, S.; Lu, Y.; Shi, Y.; Lai, X.; Wang, X. Mechanisms for adsorption, dissociation and diffusion of hydrogen in hydrogen permeation barrier of α-Al2O3: the role of crystal orientation. Int. J. Hydrogen. Energy. 2014, 39, 610-9.
140. Hatano, Y.; Zhang, K.; Hashizume, K. Fabrication of ZrO2 coatings on ferritic steel by wet-chemical methods as a tritium permeation barrier. Phys. Scr. 2011, 2011, 014044.
141. Chikada, T.; Suzuki, A.; Koch, F.; Maier, H.; Terai, T.; Muroga, T. Fabrication and deuterium permeation properties of erbia-metal multilayer coatings. J. Nucl. Mater. 2013, 442, S592-6.
142. Engels, J.; Houben, A.; Rasinski, M.; Linsmeier, C. Hydrogen saturation and permeation barrier performance of yttrium oxide coatings. Fusion. Eng. Des. 2017, 124, 1140-3.
143. He, D.; Li, S.; Liu, X.; et al. Preparation of Cr2O3 film by MOCVD as hydrogen permeation barrier. Fusion. Eng. Des. 2014, 89, 35-9.
144. Nemanič, V.; Mcguiness, P. J.; Daneu, N.; Zajec, B.; Siketić, Z.; Waldhauser, W. Hydrogen permeation through silicon nitride films. J. Alloys. Compd. 2012, 539, 184-9.
145. Matějíček, J.; Veverka, J.; Nemanič, V.; et al. Characterization of less common nitrides as potential permeation barriers. Fusion. Eng. Des. 2019, 139, 74-80.
146. Liu, Y.; Huang, S.; Ding, J.; Yang, Y.; Zhao, J. Vanadium carbide coating as hydrogen permeation barrier: a DFT study. Int. J. Hydrogen. Energy. 2019, 44, 6093-102.
147. Bagheri, S.; Guagliano, M. Review of shot peening processes to obtain nanocrystalline surfaces in metal alloys. Surf. Eng. 2009, 25, 3-14.
148. Bagherifard, S. Enhancing the structural performance of lightweight metals by shot peening. Adv. Eng. Mater. 2019, 21, 1801140.
149. Li, X.; Zhang, J.; Wang, Y.; Ma, M.; Shen, S.; Song, X. The dual role of shot peening in hydrogen-assisted cracking of PSB1080 high strength steel. Mater. Des. 2016, 110, 602-15.
150. Meng, B.; Gu, C.; Zhang, L.; et al. Hydrogen effects on X80 pipeline steel in high-pressure natural gas/hydrogen mixtures. Int. J. Hydrogen. Energy. 2017, 42, 7404-12.
151. Shirvill, L.; Roberts, T.; Royle, M.; Willoughby, D.; Sathiah, P. Experimental study of hydrogen explosion in repeated pipe congestion - Part 2: effects of increase in hydrogen concentration in hydrogen-methane-air mixture. Int. J. Hydrogen. Energy. 2019, 44, 3264-76.
152. Li, X.; Shao, P.; Wang, J.; Huang, L.; Dong, Z.; Zhong, F. Study on the permeability behaviour of hydrogen doped natural gas in polyethylene pipeline. J. Phys. Conf. Ser. 2024, 2713, 012001.
153. Lei, Y.; Hosseini, E.; Liu, L.; Scholes, C.; Kentish, S. Internal polymeric coating materials for preventing pipeline hydrogen embrittlement and a theoretical model of hydrogen diffusion through coated steel. Int. J. Hydrogen. Energy. 2022, 47, 31409-19.
154. Hardie, D.; Charles, E.; Lopez, A. Hydrogen embrittlement of high strength pipeline steels. Corros. Sci. 2006, 48, 4378-85.
155. Slifka, A. J.; Drexler, E. S.; Nanninga, N. E.; et al. Fatigue crack growth of two pipeline steels in a pressurized hydrogen environment. Corros. Sci. 2014, 78, 313-21.
156. Drexler, E. S.; Slifka, A. J.; Amaro, R. L.; et al. Fatigue crack growth rates of API X70 pipeline steel in a pressurized hydrogen gas environment. Fatigue. Fract. Eng. Mat. Struct. 2014, 37, 517-25.
157. Koo, W. T.; Cho, H. J.; Kim, D. H.; et al. Chemiresistive hydrogen sensors: fundamentals, recent advances, and challenges. ACS. Nano. 2020, 14, 14284-322.
158. Guo, M.; Brewster Ii, J. T.; Zhang, H.; Zhao, Y.; Zhao, Y. Challenges and opportunities of chemiresistors based on microelectromechanical systems for chemical olfaction. ACS. Nano. 2022, 16, 17778-801.
159. Jeong, S. Y.; Kim, J. S.; Lee, J. H. Rational design of semiconductor-based chemiresistors and their libraries for next-generation artificial olfaction. Adv. Mater. 2020, 32, e2002075.
160. Marasso, S. L.; Tommasi, A.; Perrone, D.; et al. A new method to integrate ZnO nano-tetrapods on MEMS micro-hotplates for large scale gas sensor production. Nanotechnology 2016, 27, 385503.
161. Zhao, Y.; Zhang, H.; Zhang, S.; Zhao, Y. Toward highly trustable miniaturized semiconductor gas sensors. Matter 2022, 5, 1985-9.
162. Park, S. Y.; Kim, Y.; Kim, T.; Eom, T. H.; Kim, S. Y.; Jang, H. W. Chemoresistive materials for electronic nose: progress, perspectives, and challenges. InfoMat 2019, 1, 289-316.
163. Al-Karaki, J. N.; Kamal, A. E. Routing techniques in wireless sensor networks: a survey. IEEE. Wirel. Commun. 2004, 11, 6-28.
164. Jain, D.; Shukla, P. K.; Varma, S. Energy efficient architecture for mitigating the hot-spot problem in wireless sensor networks. J. Ambient. Intell. Human. Comput. 2023, 14, 10587-604.
165. Liu, J.; Zhao, Z.; Ji, J.; Hu, M. Research and application of wireless sensor network technology in power transmission and distribution system. Intell. Converged. Netw. 2020, 1, 199-220.
166. Sanjeevi, P.; Prasanna, S.; Siva Kumar, B.; Gunasekaran, G.; Alagiri, I.; Vijay Anand, R. Precision agriculture and farming using Internet of Things based on wireless sensor network. Trans. Emerging. Telecommun. Technol. 2020, 31, e3978.
167. Fu, X.; Pace, P.; Aloi, G.; Yang, L.; Fortino, G. Topology optimization against cascading failures on wireless sensor networks using a memetic algorithm. Comput. Netw. 2020, 177, 107327.
168. Gama, K.; Touseau, L.; Donsez, D. Combining heterogeneous service technologies for building an internet of things middleware. Comput. Commun. 2012, 35, 405-17.
169. Xie, X.; Tian, Y.; Wei, G. Deduction of sudden rainstorm scenarios: integrating decision makers' emotions, dynamic Bayesian network and DS evidence theory. Nat. Hazards. 2023, 116, 2935-55.
170. Fang, C.; Li, H.; Li, L.; et al. Smart electronic nose enabled by an all-feature olfactory algorithm. Adv. Intell. Syst. 2022, 4, 2200074.
171. Men, J.; Chen, G.; Yang, Y.; Reniers, G. An event-driven probabilistic methodology for modeling the spatial-temporal evolution of natural hazard-induced domino chain in chemical industrial parks. Reliab. Eng. Syst. Saf. 2022, 226, 108723.
172. Xu, Z.; Liu, X.; Xu, W.; Sun, B.; Liu, X.; Xu, D. Analysis on the disaster chain evolution from gas leak to explosion in urban utility tunnels. Eng. Fail. Anal. 2022, 140, 106609.
173. Men, J.; Chen, G.; Zeng, T. Multi-hazard coupling effects in chemical process industry - Part II: research advances and future perspectives on methodologies. IEEE. Syst. J. . 2023, 17, 1637-47.
174. Ramzali, N.; Lavasani, M. R. M.; Ghodousi, J. Safety barriers analysis of offshore drilling system by employing fuzzy event tree analysis. Saf. Sci. 2015, 78, 49-59.
175. Park, B.; Kim, Y.; Paik, S.; Kang, C. Numerical and experimental analysis of jet release and jet flame length for qualitative risk analysis at hydrogen refueling station. Process. Saf. Environ. Prot. 2021, 155, 145-54.
176. Patel, P.; Baalisampang, T.; Arzaghi, E.; Garaniya, V.; Abbassi, R.; Salehi, F. Computational analysis of the hydrogen dispersion in semi-confined spaces. Proc. Saf. Environ. Prot. 2023, 176, 475-88.
177. Atzmueller, M.; Becker, M.; Molino, A.; Mueller, J.; Peters, J.; Sîrbu, A. Applications for environmental sensing in EveryAware. In: Loreto V, Haklay M, Hotho A, et al., editors. Participatory sensing, opinions and collective awareness. Cham: Springer International Publishing; 2017. pp. 135-55.
178. Fan, C.; Zhang, C.; Yahja, A.; Mostafavi, A. Disaster city digital twin: a vision for integrating artificial and human intelligence for disaster management. Int. J. Inf. Manag. 2021, 56, 102049.
179. Basu, S.; Roy, S.; DasBit, S. A post-disaster demand forecasting system using principal component regression analysis and case-based reasoning over smartphone-based DTN. IEEE. Trans. Eng. Manag. 2019, 66, 224-39.
180. Gong, Z.; Wang, Y.; Wei, G.; Li, L.; Guo, W. Cascading disasters risk modeling based on linear uncertainty distributions. Int. J. Dis. Risk. Reduct. 2020, 43, 101385.
181. Dong, M.; Meng, Y.; Qin, C.; Li, T.; Zhang, T.; Zhao, D. Coupling evolution effect between security system vulnerability and security incident in petrochemical plants. J. Loss. Prev. Process. Ind. 2022, 75, 104682.
182. Ray, S. K.; Khan, A. M.; Mohalik, N. K.; Mishra, D.; Mandal, S.; Pandey, J. K. Review of preventive and constructive measures for coal mine explosions: an Indian perspective. Int. J. Min. Sci. Technol. 2022, 32, 471-85.
183. Cheng, J. A historical review of identifying and mitigating mine gas explosions. explosions in underground coal mines. Cham: Springer International Publishing; 2018. pp. 15-50.
184. Snoeys, J.; Going, J. E.; Taveau, J. R. Advances in dust explosion protection techniques: flameless venting. Proc. Eng. 2012, 45, 403-13.
185. Snoeys, J.; Going, J. E.; Taveau, J. R. Dust explosion protection using flameless venting. Bulk. Solids. Handl. 2008, 31, 733-8.
186. Cao, J.; Wu, J.; Zhao, Y.; Cai, J.; Bai, Y.; Pang, L. Suppression effects of energy-absorbing materials on natural gas explosion in utility tunnels. Energy 2023, 281, 128262.
187. Song, X.; Zuo, X.; Yang, Z.; Chen, J.; Xie, L.; Li, B. The explosion-suppression performance of mesh aluminum alloys and spherical nonmetallic materials on hydrogen-air mixtures. Int. J. Hydrogen. Energy. 2020, 45, 32686-701.
188. Pang, L.; Wang, C.; Han, M.; Xu, Z. A study on the characteristics of the deflagration of hydrogen-air mixture under the effect of a mesh aluminum alloy. J. Hazard. Mater. 2015, 299, 174-80.
189. Yang, Z.; Zhao, K.; Song, X.; Li, B.; Zhang, D.; Xie, L. Effects of mesh aluminium alloys and propane addition on the explosion-suppression characteristics of hydrogen-air mixture. Int. J. Hydrogen. Energy. 2021, 46, 34998-5013.
190. Zhou, S.; Gao, J.; Luo, Z.; et al. Effects of mesh aluminium alloy and aluminium velvet on the explosion of H2/air, CH4/air and C2H2/air mixtures. Int. J. Hydrogen. Energy. 2021, 46, 14871-80.
191. Li, Y.; Zhao, Q.; Liu, L.; Chen, X.; Huang, C.; Yuan, B. Investigation on the flame and explosion suppression of hydrogen/air mixtures by porous copper foams in the pipe with large aspect ratio. J. Loss. Prev. Process. Ind. 2022, 76, 104744.
192. Li, Y.; Zhao, Q.; Chen, X.; et al. Effect of copper foam on the explosion suppression in hydrogen/air with different equivalence ratios. Fuel 2023, 333, 126324.
193. Ustolin, F.; Paltrinieri, N.; Berto, F. Loss of integrity of hydrogen technologies: a critical review. Int. J. Hydrogen. Energy. 2020, 45, 23809-40.
194. Zhuang, C.; Wang, Z.; Zhang, Y.; et al. Effect of porous materials on explosion venting overpressure and flame of CH4/air premixed gas. Combust. Sci. Technol. 2023, 195, 508-29.
195. Xie, Q.; Wen, H.; Ren, Z.; Liu, H.; Wang, B.; Wolanski, P. Effects of silicone rubber and aerogel blanket-walled tubes on H2/air gaseous detonation. J. Loss. Prev. Process. Ind. 2017, 49, 753-61.
196. Szkudlarek, Z.; Janas, S. Active protection of work area against explosion of dust-gas mixture. Int. J. Coal. Sci. Technol. 2021, 8, 674-84.
197. Plessis JJL. Active explosion barrier performance against methane and coal dust explosions. Int. J. Coal. Sci. Technol. 2015, 2, 261-8.
198. Lesiak, P.; Bąk, D.; Maloziec, D.; Grabarczyk, M.; Kołaczkowski, A. Evaluation of the effectiveness of active HRD systems for dust explosion suppression in a technology demonstrator system. Saf. Fire. Technol. 2019, 53, 46-67.
199. van Wingerden, M.; Skjold, T.; Roosendans, D.; Dutertre, A.; Pekalski, A. Chemical inhibition of hydrogen-air explosions: literature review, simulations and experiments. Proc. Saf. Environ. Prot. 2023, 176, 1120-9.
200. Yan, C.; Bi, M.; Li, Y.; Gao, W. Effects of nitrogen and carbon dioxide on hydrogen explosion behaviors near suppression limit. J. Loss. Prev. Process. Ind. 2020, 67, 104228.
201. Holborn, P.; Battersby, P.; Ingram, J.; Averill, A.; Nolan, P. Estimating the effect of water fog and nitrogen dilution upon the burning velocity of hydrogen deflagrations from experimental test data. Int. J. Hydrogen. Energy. 2013, 38, 6882-95.
202. Li, Y.; Bi, M.; Zhou, Y.; Gao, W. Hydrogen cloud explosion suppression by micron-size water mist. Int. J. Hydrogen. Energy. 2022, 47, 23462-70.
203. Linteris, G. T.; Burgess, D. R.; Takahashi, F.; Katta, V. R.; Chelliah, H. K.; Meier, O. Stirred reactor calculations to understand unwanted combustion enhancement by potential halon replacements. Combust. Flame. 2012, 159, 1016-25.
204. Gao, M.; Bi, M.; Ye, L.; et al. Suppression of hydrogen-air explosions by hydrofluorocarbons. Proc. Saf. Environ. Prot. 2021, 145, 378-87.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data








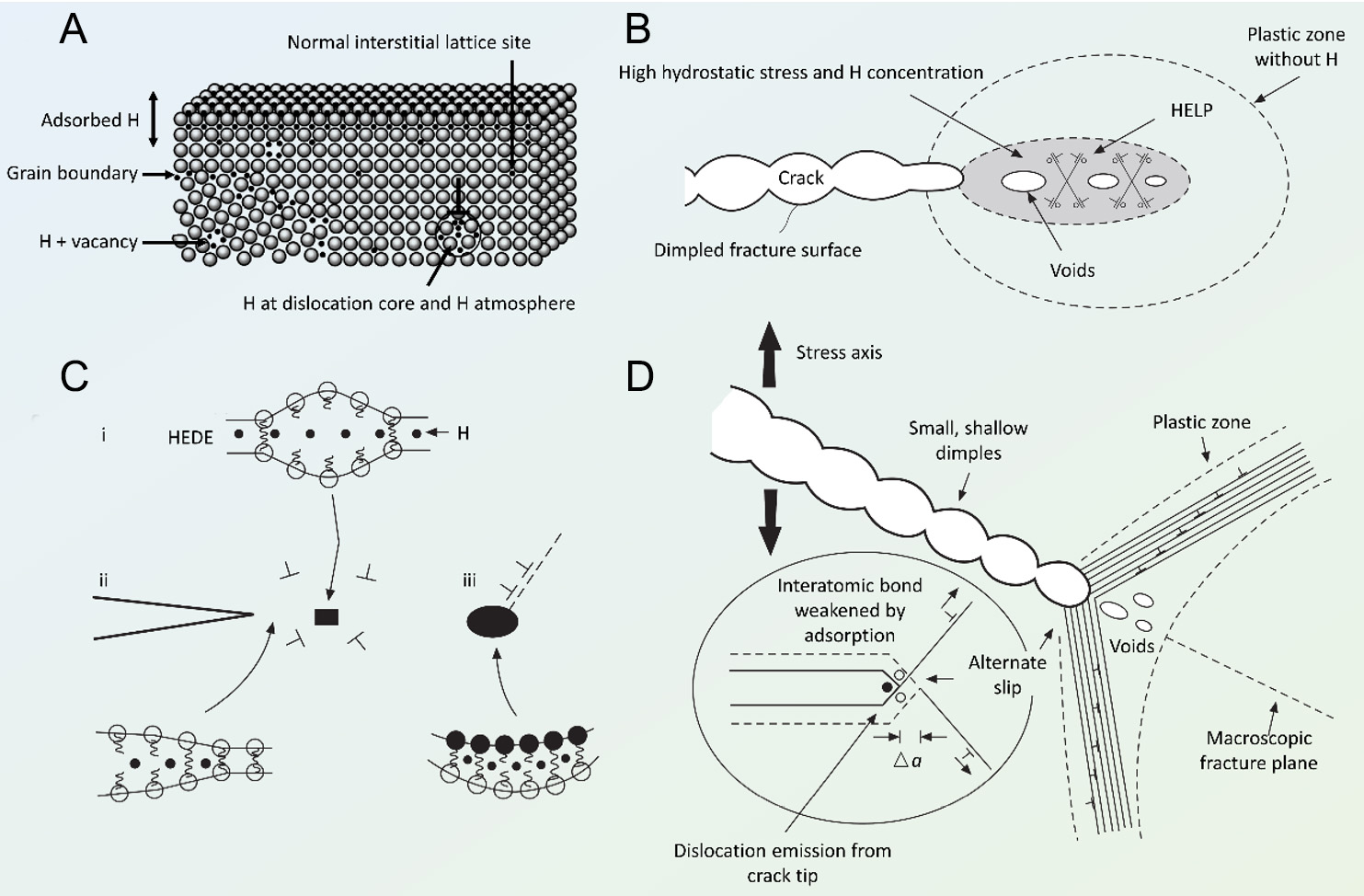
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].