Unraveling resistance to immune checkpoint inhibitors in HNSCC: from mechanisms to combination therapies
Abstract
Head and neck squamous cell carcinoma (HNSCC), which arises from the mucosal linings of the oral cavity, pharynx, and larynx, represents the most prevalent head and neck malignancy. This cancer is notable for its elevated incidence and substantial mortality. The intricate anatomy of the region contributes to marked tumor heterogeneity, rendering the pursuit of effective therapeutic regimens a crucial aspect of enhancing clinical outcomes. Recently, the advent of immune checkpoint blockade, particularly agents targeting programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4, has introduced significant advancements within the oncological landscape, including for HNSCC. The introduction of immune checkpoint inhibitors, specifically the PD-1 blockers pembrolizumab and nivolumab, has established a new therapeutic standard for recurrent/metastatic HNSCC (R/M HNSCC). However, the clinical benefit is not universal, as a primary challenge remains the high incidence of treatment resistance. Consequently, a majority of patients (approximately 60%-70%) with R/M HNSCC derive minimal or no benefit from this form of immunotherapy, highlighting the critical need to understand the underlying resistance mechanisms. This review comprehensively discusses the types of immunotherapy resistance in HNSCC and the underlying mechanisms contributing to resistance. Furthermore, it reviews current strategies to overcome immunotherapy resistance, providing new perspectives for improving therapeutic efficacy in HNSCC.
Keywords
INTRODUCTION
Globally, cancers of the head and neck impose a significant burden, being ranked as the seventh most common malignancy. Epidemiological studies from 2020 recorded around 890,000 new diagnoses and 450,000 deaths attributable to this disease group[1]. A concerning upward trend in incidence has been observed, and forecasts indicate a potential 30% increase by the end of this decade, culminating in roughly 1.08 million new cases per year by 2030[2]. Tobacco smoking is a major risk factor for head and neck squamous cell carcinoma (HNSCC), significantly increasing disease susceptibility. Additionally, alcohol consumption, environmental pollutant exposure, and viral infections - specifically human papillomavirus (HPV) and Epstein-Barr virus (EBV) - are recognized as high-risk factors for HNSCC development. HPV and EBV infections are known etiological contributors to oropharyngeal cancer and nasopharyngeal cancer, respectively[3].
The programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis has emerged as a cornerstone for immunotherapy in HNSCC. Agents targeting this pathway, such as pembrolizumab and nivolumab, have established clinical efficacy, leading to their regulatory approval. The current first-line standard of care for recurrent/metastatic HNSCC (R/M HNSCC) disease often involves a combination of immune checkpoint inhibitors (ICIs) with platinum. Beyond this established setting, the therapeutic landscape is expanding, with ongoing clinical investigations exploring ICIs concurrently with chemoradiotherapy for locally advanced HNSCC and as a neoadjuvant approach prior to surgical resection[4]. Despite significant advancements in immunotherapy for R/M HNSCC, overall survival (OS) rates remain limited. The data show that immunotherapy has improved the 5-year survival rate from 5.0% to 15.4%-23.9%; however, approximately 60% of patients eventually develop resistance, and only one-third of patients achieve long-term disease control[5].
This review provides a comprehensive focus on resistance mechanisms, offers an integrated framework for classifying and addressing different types of resistance, and provides updated, specific and actionable strategies for overcoming resistance in clinical practice in HNSCC.
CURRENT STATUS OF IMMUNOTHERAPY FOR HNSCC
ICIs, such as anti-PD-1 monoclonal antibodies (mAbs), have become the standard first-line treatment for R/M HNSCC and are recommended by multiple guidelines[6-8]. Among the clinically approved anti-PD-1 immunotherapies are nivolumab, pembrolizumab, and toripalimab, all of which are humanized immunoglobulin G4 (IgG4) mAbs.
Their primary mechanism involves blocking the engagement of PD-1 with its ligands (PD-L1/PD-L2), thereby preventing the negative regulation of T-cell activity. This intervention reinvigorates tumor-specific T cells, promoting their activation, proliferation, and cytotoxic capabilities against tumor cells. The restored anti-tumor immunity is largely mediated through the reactivation of cluster of differentiation 8 positive (CD8+) cytotoxic T lymphocytes (CTLs) and the enhanced phagocytic function of antigen-presenting cells (APCs)[9]. In addition to the approved anti-PD-1 mAbs, PD-L1 checkpoint inhibitors primarily bind to the programmed cell death ligand on the surface of tumor cells. Here, we present some key clinical trials of immunotherapy in R/M HNSCC[5,10-17] [Table 1].
Key clinical trials of immunotherapy in R/M HNSCC
| Trial name | Phase | Population | Treatment Arms | ORR (%) | Median OS (months) | Grade 3/4 TRAEs (%) | Key findings |
| KEYNOTE-012[10] | Ib | R/M HNSCC | Pembrolizumab | 18 | 8.0 | 9 | First trial showing efficacy of anti-PD-1 in HNSCC |
| CheckMate-141[18] | III | Platinum-refractory R/M HNSCC | Nivolumab vs. SOC | 13.3 vs. 5.8 | 7.7 vs. 5.1 | 15.3 vs. 36.9 | FDA approval for nivolumab in second-line treatment of HNSCC |
| KEYNOTE-055[12] | II | Platinum and cetuximab refractory R/M HNSCC | Pembrolizumab | 16 | 8 | 15 | Pembrolizumab exhibited clinically meaningful anti-tumor activity and an acceptable safety profile |
| KEYNOTE-040[19] | III | Platinum-refractory R/M HNSCC | Pembrolizumab vs. SOC | 22.6 vs. 16.7 | 8.4 vs. 6.9 | 13 vs. 35 | Significant OS improvement with pembrolizumab |
| KEYNOTE-048[13] | III | R/M HNSCC | Pembrolizumab vs. Pembrolizumab + chemotherapy vs. EXTREME | Undisclosed | 11.5 vs. 13.0 vs. 10.7 | 55 vs. 85 vs. 83 | Led to 1st line approval in CPS ≥ 1 patients |
| CheckMate-651[14] | III | R/M HNSCC | Nivolumab plus ipilimumab vs. EXTREME | 34.1 vs. 36.0 | 13.9 vs. 13.5 | 70.7 vs. 28.2 | No significant OS improvement |
| KEYNOTE-412[15] | III | Unresected locally advanced HNSCC | Pembrolizumab plus chemoradiotherapy vs. placebo plus chemoradiotherapy | - | Not reached vs. 47.7 | 92 vs. 88 | Pembrolizumab plus chemoradiotherapy did not significantly improve EFS |
| CheckMate-714[16] | II | Platinum-refractory R/M HNSCC | Nivolumab plus ipilimumab vs. Nivolumab | 13.2 vs. 118.3 | Undisclosed | 5.7 vs. 3.7 | No significant PFS and OS improvement |
| KESTREL[17] | III | R/M HNSCC | Durvalumab + tremelimumab vs. durvalumab vs. EXTREME | Undisclosed | 10.9 vs. 11.2 vs. 10.9 | 8.9 vs. 19.1 vs. 53.1 | No significant OS improvement |
Despite the transformative impact of PD-1/PD-L1 checkpoint inhibitors on the management of R/M HNSCC, a significant clinical challenge persists. The efficacy of this immunotherapeutic strategy is markedly limited by primary resistance, which prevents durable responses in a substantial majority (over 60%) of patients, underscoring the need to overcome this barrier to improve outcomes. Future trials should prioritize biomarker-driven patient stratification and explore rational combinations with radiotherapy or targeted agents to expand durable response rates.
FACTORS PREDICTING IMMUNOTHERAPY EFFICACY
As previously mentioned, immunotherapy is currently effective in the treatment of R/M HNSCC and is undergoing many clinical trials in both first-line and later-line settings. Currently, the expression levels of PD-L1 and tumor mutation burden (TMB) have been recommended by guidelines for evaluating the efficacy of immunotherapy in HNSCC. However, immunotherapy, guided by predictive biomarkers, is not effective for all patients. Besides, HPV status and the characteristics of tumor immune infiltration (TIL) in immunotherapy for HNSCC, based on existing clinical trial results, lack sufficient evidence and need further study [Figure 1]. Therefore, finding biomarkers that predict the efficacy of immune response treatment has become an urgent task.
Figure 1. Biomarkers to evaluate the efficacy of immunotherapy. PD-L1 IHC staining and TMB validated by clinical guidelines, HPV status and characteristics of TIL are under study. PD-L1: Programmed death-ligand 1; IHC: immunohistochemistry; TMB: tumor mutation burden; HPV: human papillomavirus; TIL: tumor immune infiltration; R/M HNSCC: recurrent/metastatic head and neck squamous cell carcinoma; CPS: combined positive score; 5-FU: 5-fluorouracil.
PD-L1 expression
Within the predictive biomarker landscape for immune checkpoint inhibition, the expression level of PD-L1 holds significant prominence. Its evaluation in tumor samples typically relies on two principal methodologies: tumor proportion score (TPS), which quantifies expression on tumor cells alone, and combined positive score (CPS), which provides a more comprehensive assessment by incorporating staining on both tumor and associated immune cells[20-22] [Table 2]. Clinical data from various studies, including the phase II KEYNOTE-055 trial (NCT02255097), have established a general association between higher PD-L1 expression (using a CPS ≥ 1% cutoff) and improved OS (18% vs. 12% in CPS < 1% patients). However, the documented efficacy in some PD-L1-negative cohorts confirms that this biomarker, while informative, lacks absolute predictive power, highlighting the contribution of other resistance and response mechanisms[12]. Clinical guidelines now strongly endorse pembrolizumab, either as monotherapy or in combination with platinum and 5-fluorouracil (5-FU), as a first-line treatment for R/M HNSCC in patients with a CPS ≥ 1%. This recommendation is substantiated by the KEYNOTE-048 (NCT02358031) and KEYNOTE-040 (NCT02252042) trials, which demonstrated a concordant association between higher CPS scores and enhanced efficacy of immunotherapy[13,20]. Supporting evidence also comes from the CheckMate-141 trial (NCT02105636), which utilized the TPS method. In this trial, patients treated with nivolumab whose tumors had a TPS > 1% showed improved progression-free survival (PFS), although a significant OS benefit was not observed across all groups[23]. For individuals with a CPS < 1%, the recommended regimen is pembrolizumab combined with platinum and 5-FU. Meanwhile, both pembrolizumab and nivolumab remain therapeutic options for patients with platinum-refractory R/M HNSCC, irrespective of their CPS status[24].
Comparison of PD-L1 assessment methods in HNSCC
| Feature | TPS | CPS |
| Definition | Percentage of tumor cells with PD-L1 membrane staining | Sum of PD-L1 staining tumor cells and immune cells per 100 tumor cells |
| Cells evaluated | Tumor cells only | Tumor cells + immune cells |
| Advantages | Simple, standardized | More comprehensive immune assessment |
| Limitations | Ignores immune cell PD-L1 expression | More complex scoring system |
| Clinical utility | Predictive in some cancers | Better sensitivity in HNSCC |
| Preferred cutoff | ≥ 50% | ≥ 1% |
TMB and microsatellite instability
The concept of TMB provides a metric for the genomic alteration frequency in cancers, defined as the number of protein-altering (nonsynonymous) mutations accumulated per megabase of the sequenced coding region. An elevated mutational load is hypothesized to increase the repertoire of tumor neoantigens presented on the cell surface. This heightened antigenic diversity promotes immunogenic recognition, thereby potentially sensitizing tumors to immunotherapies that unleash T-cell cytotoxicity, such as anti-PD-1/PD-L1 agents. The KEYNOTE-158 (NCT02628067) study evaluated the correlation between TMB and the efficacy of pembrolizumab in solid tumors, showing that pembrolizumab treatment in patients with TMB ≥ 10 mut/mb resulted in a higher overall response rate (ORR) (29% vs. 6%)[25]. Additionally, the KEYNOTE-012 (NCT01848834) study analyzed the data on TMB and pembrolizumab treatment in R/M HNSCC, finding a positive correlation between TMB and ORR. In the subgroup analysis, TMB ≥ 175 mut/exome performed better than TMB < 175 mut/exome; however, the median OS showed no significant improvement, and there was no correlation between TMB and tumor tissue CPS scores[26]. Therefore, American Society of Clinical Oncology (ASCO) guidelines recommend a weak recommendation for pembrolizumab in patients with high TMB and rare
HPV status
Infection with high-risk HPV is an established etiological factor for a distinct subset of HNSCC. HPV-positive (HPV+) tumors constitute a distinct clinical and biological entity compared to HPV-negative (HPV-) HNSCC, characterized by divergent genetic profiles, mutational patterns, and immune phenotypes[27]. Nevertheless, the value of HPV status as a predictive marker for immunotherapy efficacy remains poorly defined and continues to be a focus of intensive research[28,29]. The immunogenic nature of HPV, driven by the persistent expression of viral oncoproteins (E6/E7), typically induces a robust anti-tumor immune response. This immunological feature is well reflected in the tumor microenvironment (TME) of HPV+ HNSCC: such TMEs are frequently characterized by a more pronounced inflammatory phenotype, including increased infiltration of CD8+ CTLs, cluster of differentiation 4 positive (CD4+) T helper 1 (Th1) cells, and APCs, as well as higher concentrations of pro-inflammatory cytokines and chemokines. Several clinical trials have investigated the correlation between HPV status and ICI responses. While early subgroup analyses from CheckMate 141 and KEYNOTE-040 suggested limited survival benefit of PD-1 inhibitors in HPV+ HNSCC[11,19], subsequent evidence reveals this reflects quantitative limitations in predictive power rather than true inefficacy. HPV+ tumors exhibit marked heterogeneity: A subset with intact antigen presentation machinery and high CD8+ TIL density shows robust responses[30,31]. Thus, HPV status alone is insufficient for patient selection; it must be integrated with spatial immune mapping and genomic classifiers.
Characteristics of TIL
In addition to PD-L1 expression, TMB, and MSI status, there have been new explorations into biomarkers for predicting the efficacy of immunotherapy in HNSCC. Similar to lung cancer and melanoma, HNSCC exhibits significant TIL. Some studies suggest that tumor lymphocytic infiltration is associated with prognosis. For instance, the density of immune cell infiltration in HNSCC has been established as a positive prognostic indicator for patients receiving ICIs. Studies indicate that immunologically “hot” tumors, which are markedly enriched with CD8+ T cells and TILs, orchestrate potent anti-tumor responses via the localized production of cytokines including interferon-γ (IFN-γ), perforin, and granzymes[32]. Complementing this, research has also highlighted the role of tumor-infiltrating natural killer (NK) cells in predicting favorable responses to immunotherapy. A key mechanism involves IFN-γ secretion by NK cells, which activates Th1 lymphocytes and subsequently contributes to the reversal of an immunosuppressive milieu, enhancing the overall efficacy of the treatment[33].
In the future, validation of composite biomarkers - incorporating genomic, immunophenotypic, and microenvironmental features - is critical to better stratify patients. Future studies should also explore dynamic biomarkers and liquid biopsies to capture temporal evolution of resistance. Ultimately, advancing predictive accuracy will require multimodal algorithms that reconcile tumor intrinsic features with immune contexture, enabling more personalized and effective immunotherapy strategies in HNSCC.
MECHANISMS OF IMMUNE THERAPY RESISTANCE IN HNSCC
Definition of immune therapy resistance
Resistance to ICIs is typically categorized into three main types based on the underlying mechanism and therapeutic timeline. (1) The first, resistance driven by target loss, arises when the TME lacks the molecular targets (e.g., PD-1/PD-L1) necessary for the antibody to engage, rendering anti-PD-1/PD-L1 monotherapy ineffective from the outset; (2) The second, primary resistance, describes a scenario where tumors, despite showing histologic evidence of immune cell infiltration and PD-L1 expression, derive no clinical benefit from initial anti-PD-1 treatment; (3) The third, acquired resistance, occurs when patients exhibit a significant initial response to ICIs, but subsequently experience disease progression during the course of therapy[34]. Target loss resistance refers specifically to loss of the drug target (e.g., PD-L1 downregulation), which can occur as one mechanism of acquired resistance; in contrast, acquired resistance encompasses broader mechanisms that develop after initial response to therapy, which may include target loss as one component. According to clinical manifestations, immune therapy resistance can also be categorized into four types: ① immune response not activated; ② immune response activated but suppressed by certain mechanisms; ③ pre-existing heterogeneous and resistant tumor cells before treatment; and ④ acquired resistance mutations during immune therapy[35].
Common resistance mechanisms in immune therapy for head and neck cancer
While ICIs, particularly mAbs targeting the PD-1/PD-L1 axis, have revolutionized oncology by providing durable responses in a subset of patients, their clinical benefit remains limited by a low ORR of 15%-20%[35]; a primary challenge is the development of resistance, which significantly curtails long-term survival benefits. The underlying mechanisms are multifaceted, involving a complex interplay between tumor-intrinsic factors, host immunity, and a suppressive TME [Figure 2].
Figure 2. Mechanisms for immune therapy resistance in HNSCC. Created in BioRender. Yu, Y. (2025) https://BioRender.com/2oorj0t. HNSCC: Head and neck squamous cell carcinoma; MHC-1: major histocompatibility complex-1; PD-L1: programmed death-ligand 1; PD-1: programmed death-1; VEGF: vascular endothelial growth factor.
Cancer immune surveillance and immune editing
The theory of cancer immune surveillance posits that the immune system maintains body homeostasis by recognizing and clearing tumor cells, but tumors can escape this process through immune editing[36]. Immune editing consists of three stages: elimination, equilibrium, and escape. In HNSCC, immune editing primarily promotes resistance in the following mechanisms: (1) Antigen presentation defects: Tumor cells downregulate the expression of major histocompatibility complex I (MHC-I) or antigen-processing related transporter (TAP) to reduce the presentation of tumor-associated antigens (TAAs), thereby evading immune recognition[37]. For example, the loss of MHC-I in HNSCC is significantly associated with resistance to PD-1 inhibitors[38]; (2) Loss of immunogenic antigens: Under immune pressure, tumor cells selectively lose highly immunogenic antigens (such as HPV-related E6/E7 proteins) through epigenetic silencing or gene mutations, preventing recognition by T-cells[39]; (3) Induction of immune tolerance: Tumor cells release immunosuppressive factors [such as interleukin (IL)-10 and transforming growth factor-beta (TGF-β)] that induce the expansion of regulatory T cells (Tregs), which suppress the function of effector T cells[40].
Immune escape and immune suppression
The limited efficacy of immunotherapies in HNSCC can be attributed to a multifaceted network of immunosuppressive mechanisms operating within the TME. A well-characterized strategy employed by tumor cells to bypass the cytotoxic activity of T cells involves the high expression of PD-L1, which is often more pronounced in HPV-associated HNSCC. Ligation of PD-1 by PD-L1 effectively induces a state of functional exhaustion in antigen-specific T cells[13]. The observation that therapeutic outcomes are not consistently predicted by PD-L1 expression levels alone suggests that resistance may be driven by the engagement of other inhibitory immune checkpoints, including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3)[41]. Beyond direct checkpoint interaction, the immunosuppressive niche is critically shaped by infiltrating myeloid cells. Tumor-associated macrophages (TAMs) suppress T-cell function through the release of soluble factors such as IL-10 and arginase-1. In parallel, myeloid-derived suppressor cells (MDSCs) induce T-cell apoptosis via the production of reactive oxygen species (ROS) and nitric oxide, creating a formidable barrier to an effective anti-tumor immune response[42].
A fundamental distinction in the mechanisms of resistance to PD-1 vs. PD-L1 inhibitors stems from their differential binding specificities and consequent biological impacts. PD-1 inhibitors, including nivolumab and pembrolizumab, function by directly binding to the PD-1 receptor on T cells. This action provides broad-spectrum inhibition by preventing the receptor’s engagement with both of its known ligands, PD-L1 and PD-L2. The comprehensive blockade of PD-1 signaling can, however, trigger a compensatory feedback mechanism, leading to the pronounced upregulation of alternative co-inhibitory receptors such as LAG-3 and TIM-3 on exhausted T-cell populations[41,43]. Additionally, resistance to PD-1 inhibitors frequently correlates with T-cell-intrinsic alterations, such as JAK1/2 mutations that disrupt IFN-γ signaling and reduce antigen presentation[35].
Conversely, PD-L1 inhibitors (e.g., atezolizumab, durvalumab) bind specifically to PD-L1 on tumor or stromal cells, leaving the PD-1/PD-L2 axis intact. This allows persistent PD-L2-mediated immunosuppression through alternative receptors such as repulsive guidance molecule B (RGMb), promoting adaptive resistance[44]. Tumor cells may also exploit PD-L1-independent pathways, including CD80 transendocytosis, which diminishes costimulatory signals for T-cell activation[45]. TME further differentiates resistance: PD-L1 inhibitors are more susceptible to resistance driven by MDSCs expressing PD-L2 or V-domain Ig suppressor of T cell activation (VISTA), whereas PD-1 resistance is more associated with regulatory T-cell infiltration[46].
Spatial heterogeneity amplifies these differences. PD-L1 expression is highly variable within tumors, and PD-L1 inhibitors require direct target engagement on cancer cells, leading to resistance from “immune deserts” lacking T-cell infiltration or PD-L1-negative clones that evade therapy[47]. In contrast, PD-1 inhibitors act on circulating and tumor-infiltrating lymphocytes, making efficacy dependent on systemic T-cell functionality but vulnerable to peripheral tolerance mechanisms[48]. Overcoming these resistance mechanisms may thus require tailored strategies - LAG-3/TIM-3 co-inhibition for PD-1 resistance, and targeting PD-L2 or myeloid compartments for PD-L1 resistance[49].
Besides, the immunosuppressive role of vascular endothelial growth factor (VEGF) extends beyond angiogenesis to include direct and indirect modulation of the immune landscape. VEGF signaling coordinates a complex immunosuppressive program by inhibiting dendritic cell (DC) maturation, reducing T-cell infiltration, and inducing inhibitory cells such as Tregs and MDSCs[50]. At the vascular level, VEGF-A-induced abnormal angiogenesis generates chaotic, leaky vessels with poor perfusion, leading to persistent hypoxia. This stabilizes hypoxia-inducible factor alpha (HIF-1α), which upregulates PD-L1 expression on tumor-associated endothelial cells and malignant cells, fostering an environment that excludes effector immune cells and facilitates immune evasion[51]. Concurrently, VEGF inhibits the activation of nuclear factor kappa-B (NF-κB) in hematopoietic progenitor cells, preventing DC differentiation. This leads to a reduced number of functional DCs, thus weakening the antigen - presenting ability and subsequent activation of T cells against tumor cells[52]. These mechanisms collectively establish VEGF as a non-redundant orchestrator of ICI resistance beyond its angiogenic role.
Gene mutations and heterogeneity in tumor cells
The high mutational burden in HNSCC should theoretically enhance immunogenicity, but gene heterogeneity and clonal evolution lead to resistance in several ways: (1) Driver gene mutations: Mutations in tumor protein 53 (TP53), notch receptor 1 (NOTCH1), and related genes inhibit T-cell infiltration by activating the wingless-int (WNT)/β-catenin pathway[53]. Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations promote PD-L1 expression, leading to an immunosuppressive phenotype[54]; (2) Clonal heterogeneity: Multiple subclones exist within the tumor, and some subclones carrying immune escape-related mutations [e.g., Janus kinase 1/2 (JAK1/2) deletions] are selectively expanded under treatment pressure[55]; (3) Genomic instability: Chromosomal instability (CIN) weakens the innate immune response by inducing defects in interferon signaling pathways [e.g., inactivation of the stimulator of interferon response CGAMP interactor 1 (STING) pathway][56].
Impact of TME
TME is a complex ecosystem composed of tumor cells, diverse stromal cells (including immune cells, fibroblasts, endothelial cells, etc.), and the extracellular matrix (ECM), which collectively play a critical role in the pathogenesis, progression, metastasis, and treatment response of HNSCC. The cellular components of the TME exhibit significant heterogeneity; for instance, CD8+ CTLs serve as the principal anti-tumor effector cells; however, their functional capacity is frequently impaired due to exhaustion. In contrast, Tregs primarily exert immunosuppressive activities. Moreover, TAMs and MDSCs facilitate tumor immune escape through the secretion of immunosuppressive mediators. The spatial imbalance in the distribution of these cellular components and their functional phenotypes - termed the spatial heterogeneity of the TME - represents a key determinant of therapeutic resistance, particularly to interventions such as ICIs, in HNSCC.
Cancer-associated fibroblasts (CAFs) are pivotal components of TME that actively contribute to immune evasion by secreting TGF-β, a process that directly suppresses T cell infiltration into tumor sites. TGF-β signaling in CAFs promotes the creation of physical and molecular barriers, which exclude CTLs from penetrating tumor nests and accessing cancer cells, thereby limiting effective anti-tumor immune responses[57,58]. Specifically, CAF-derived TGF-β enhances the secretion of immunosuppressive factors and chemokines, such as C-X-C motif chemokine ligand 12 (CXCL12), that coat tumor cells and establish a milieu permissive for immune quiescence[59]. This secretion further recruits Tregs, amplifying the immunosuppressive microenvironment through increased TGF-β levels and fostering Treg infiltration, which collectively exhausts and excludes effector T cells[60]. Consequently, the TGF-β-dependent signaling in CAFs represents a key axis for modulating immune dynamics, highlighting its potential as a therapeutic target to overcome T cell exclusion and enhance immunotherapy efficacy in solid tumors.
Metabolic reprogramming, a hallmark of cancer and immune disorders, involves a shift from oxidative phosphorylation to glycolysis, leading to significant lactate accumulation in the TME[61]. This excessive lactate buildup creates an acidic immunosuppressive milieu that directly impairs CTL function by interfering with metabolic pathways in immune cells, promoting CTL exhaustion and dysfunction[62]. Specifically, lactate accumulation alters nutrient uptake and intracellular metabolic signaling in CTLs, disrupting their differentiation and activation[63]. Mechanistically, lactate accumulation facilitates lactylation, a post-translational modification that modifies both histone and non-histone proteins, regulating gene expression through epigenetic changes such as histone H3 lysine 18 lactylation (H3K18la). This reshapes transcriptional programs in immune cells and suppresses CTL-mediated immunity[64]. Lactate-derived lactylation acts as a key regulator in the immunosuppressive TME, reinforcing CTL exhaustion and limiting their persistence[65]. Such metabolic-epigenetic crosstalk highlights how lactate not only functions as a metabolic byproduct but also orchestrates immunosuppressive signaling that inhibits CTL efficiency, presenting a therapeutic target for overcoming immunosuppression.
Tumor heterogeneity in the TME is characterized by distinct immune phenotypes, including immune-inflamed tumors with abundant T cell infiltration and immune-desert tumors with a paucity of CD8+ T cells, leading to differential therapeutic outcomes. Immune-inflamed tumors are more responsive to immune checkpoint blockade (ICB) but can develop resistance through immunosuppressive niches formed by CAFs and macrophages, which spatially exclude CD8+ T cells and contribute to local immune evasion[66]. In contrast, immune-desert tumors exhibit innate resistance to ICB due to an immune-deficient microenvironment incapable of initiating effective anti-tumor responses[67-69]. Spatial heterogeneity, influenced by metabolic pressures and stromal interactions, promotes the emergence of drug-resistant clones and immune escape. For instance, the dynamic interplay between tumor cells and the ECM spatially restricts immune cell access, while desert regions correlate with non-expanded T cell receptor (TCR) clones and reduced MHC-I expression, exacerbating therapeutic resistance[70-72]. Comprehensive spatial analysis underscores the need for personalized strategies, such as enhancing immune cell infiltration in desert microenvironments or disrupting immunosuppressive niches in inflamed tumors, to overcome resistance[73,74]. Addressing this heterogeneity is critical for developing innovative immunomodulatory strategies.
Resistance to ICIs in HNSCC stems from multifactorial mechanisms, including tumor-intrinsic genomic heterogeneity, immunosuppressive TME dynamics, and compensatory immune checkpoint upregulation. Spatiotemporal mapping of resistance mechanisms via single-cell and spatial transcriptomics will be critical to identify context-specific therapeutic vulnerabilities for personalized interventions.
STRATEGIES TO OVERCOME IMMUNE THERAPY RESISTANCE
For patients with intrinsic and adaptive resistance to PD-1 inhibitors, more effective treatment methods need to be developed to reverse this phenomenon. Several mechanisms have been proposed to overcome immune resistance and improve patient prognosis: (I) Enhancing the tumor’s accessibility to the immune system; (II) Increasing T-cell infiltration within the tumor; (III) Removing barriers to T-cell trafficking within the tumor; (IV) Enhancing T/NK cell function; (V) Addressing genomic and epigenetic dysfunctions[75]. The key to overcoming immune therapy resistance lies in combining other drug targets to promote anti-tumor responses [Figure 3].
Figure 3. Therapeutic strategies and related mechanisms to overcome immune therapy resistance in HNSCC. Created in BioRender. Yu, Y. (2025) https://BioRender.com/2sr7zb0. HNSCC: Head and neck squamous cell carcinoma; DAMPs: damage associated molecular patterns; CAR-T cell: chimeric antigen receptor T cells; TCR: T cell receptor; TIGIT: T cell immune receptor with Ig and ITIM domains (Ig: immunoglobulin domain; ITIM: immunoreceptor tyrosine-based inhibitory motif); LAG3: lymphocyte activation gene 3; VISTA: V-domain Ig suppressor of T cell activation; TIM3: T cell immunoglobulin and mucin domain-containing protein 3; TLR9: Toll-like receptor 9; TGFB: transforming growth factor beta; CSF1R: colony-stimulating factor 1 receptor; CCR5: C-C motif chemokine receptor 5; CXR2: C-X-C motif chemokine receptor 2; CXCR: C-X-C motif chemokine receptor; NK cell: natural killer cell; M2-TAM: M2 tumor-associated macrophages; ICI: immune checkpoint inhibitor.
Chemotherapy
Increasing tumor visibility to the immune system is critical for improving the efficacy of immune therapies. A major therapeutic strategy is inducing tumor cell death through cytotoxic chemotherapy[76]. After cell death, a large number of tumor antigens, pro-inflammatory cytokines, damage-associated molecules, calreticulin, adenosine triphosphate (ATP), etc., are released into the TME, recruiting APCs and triggering subsequent T-cell activation[77,78].
Chemotherapeutic agents exhibit cytotoxic effects on Tregs and MDSCs within the TME - two cell populations commonly recognized for their immunosuppressive properties[76]. In the treatment of HNSCC, cisplatin exerts its anti-tumor effects not only through direct cytotoxicity but also by modulating the host immune system. It enhances type I interferon and IFN-γ signaling, leading to the upregulation of PD-L1, and increases the permeability of granzyme B released by CTLs, thereby improving immune-mediated cell killing[79,80]. At the molecular level, chemotherapy-induced immunogenic cell death (ICD) exposes calreticulin on the cell surface and releases ATP and high mobility group box 1 (HMGB1), which enhance DC maturation and antigen presentation[81].
Advanced delivery platforms (e.g., ROS-responsive nanosystems) further optimize synergy by spatial-temporal coordination of drug release and immune activation[82]. This multimodal approach addresses the complexity of multidrug resistance (MRD) by concurrently targeting tumor cell-intrinsic resistance mechanisms (e.g., ferroptosis evasion[83]) and extrinsic immunosuppressive factors. The integration of chemotherapy’s direct cytotoxicity with immunotherapy’s immune memory effects creates a positive feedback loop that sustains tumor suppression.
Radiotherapy
Radiotherapy kills cancer cells mainly through two mechanisms: high-energy radiation directly affects intracellular DNA or induces ROS. Radiation causes DNA single- or double-strand breaks, leading to apoptosis if the tumor cells cannot properly repair the damaged genetic material. Radiotherapy can upregulate immune responses locally or systemically, indirectly inducing anti-tumor immune responses, which can affect immune therapy. Current research suggests that the immune response induced by radiotherapy involves several key mechanisms: (I) Ionizing radiation triggers ICD, stimulating the recruitment and differentiation of tumor-specific T lymphocytes[84]; (II) Radiotherapy activates the cyclic GMP-AMP synthase (cGAS; GMP: guanosine monophosphate, AMP: adenosine monophosphate)-STING signaling pathway, initiating type I interferon responses[85,86]; (III) Radiotherapy enhances the expression of antigen-presenting molecules on tumor cells[87,88]; (IV) Radiotherapy promotes the release of inflammatory factors, remodeling the tumor immune microenvironment[89,90]; (V) Radiotherapy upregulates the expression of immune checkpoints and death receptors on tumor cells[84]. There are some clinical trials involving the combination of radiotherapy and immunotherapy for HNSCC, mostly prospective phase I/II studies and retrospective analyses [Table 3]. The integration of ICIs with radiotherapy in HNSCC has yielded mixed outcomes across clinical trials. In the NCT02609503 trial, which enrolled 29 platinum-ineligible stage III/IV patients, concurrent pembrolizumab and radiotherapy led to a 2-year PFS of 71% and OS of 75%. However, grade 3-4 lymphopenia occurred in 59% of participants, alongside typical radiation-related toxicities[91]. Conversely, the phase III KEYNOTE-412 (NCT03040999) study demonstrated that adding pembrolizumab to chemoradiotherapy failed to significantly improve event-free survival (EFS) compared to placebo in an unselected locally advanced HNSCC population. High-grade adverse events (AEs) were frequent in both groups (92% with pembrolizumab vs. 88% with placebo), with no new safety signals identified[15]. Similarly, the NCT02952586 trial reported no significant PFS or OS benefit from adding avelumab to cisplatin-based radiotherapy[92]. The timing, radiotherapy dosage/fractionation regimen, and potential beneficiary populations of radiotherapy combined with immunotherapy need further validation through more clinical trials.
Clinical trials of combination therapy with chemoradiotherapy in HNSCC
Combination with other immune therapies
CTLA-4 inhibitors
Simultaneously blocking inhibitory checkpoints or activating stimulatory pathways can maximally enhance effector T-cell function [Table 4]. Although the combination of anti-PD-1/PD-L1 and anti-CTLA-4 agents has demonstrated superior efficacy over monotherapy in multiple tumor types, its clinical benefits in specific malignancies such as HNSCC require further precise delineation. The mechanistic basis for this synergy stems from the competitive binding of CTLA-4 to cluster of differentiation 80/cluster of differentiation 86 (CD80/CD86) ligands on APCs, which attenuates the costimulatory signals delivered through cluster of differentiation 28 (CD28) and consequently suppresses the initial activation of T cells. In contrast, anti-CTLA-4 antibodies not only block ligand engagement by binding CTLA-4 with high affinity but may also deplete or functionally inhibit Tregs within the TME, thereby alleviating immunosuppression and potentiating anti-tumor immunity. Nonetheless, clinical outcomes have been variable; for instance, the phase III EAGLE trial (NCT02369874) in R/M HNSCC revealed no statistically significant improvement in OS with durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA-4) compared to durvalumab monotherapy[93]. Beyond the PD-1/PD-L1 and CTLA-4 pathways, other co-inhibitory receptors - including TIM-3, LAG-3, and T cell immune receptor with Ig and ITIM domains (TIGIT; Ig: immunoglobulin domain, ITIM: immunoreceptor tyrosine-based inhibitory motif), which are expressed on tumor-infiltrating lymphocytes - have emerged as promising next-generation targets for reversing T-cell exhaustion and reinvigorating anti-tumor immune responses.
Clinical studies of other immunotherapeutic approaches in HNSCC
| Agent | Description | Phase | Therapeutic comparison | Target drug-resistant types | Sponsor |
| Tremelimumab[93] | mAb to human CTLA-4 | III | Durvalumab | Synergistically activate immune initiation; overcome chemotherapy resistance; transform the “cold tumor” microenvironment | AstraZeneca |
| Relatlimab[94] | mAb to human LAG-3 | II | Nivolumab | Compensatory LAG-3 upregulation; T cell exhaustion | Bristol-Myers Squibb |
| TSR-033[95] | mAb to human LAG-3 | I | Dostarlimab (anti-PD-1) and Cobolimab (anti-TIM-3) plus Bevacizumab (anti-VEGFA) and FOLFIRI (folinic acid/leucovorin, 5-FU and irinotecan) | Compensatory LAG-3 upregulation; T cell exhaustion | Tesaro |
| Fianlimab[96] | mAb to human LAG-3 | I | Cemipilimab (anti-PD-1) | Compensatory LAG-3 upregulation; T cell exhaustion | Regeneron/Sanofi |
| BI754111[97] | mAb to human LAG-3 | II | Ezabenlimab (anti-PD-1) | Compensatory LAG-3 upregulation; T cell exhaustion | Boehringer Ingelheim |
| FS118[98] | Bispecific anti-LAG-3-anti-PD-L1 | I/II | N/A | CD8+ TIL cells exhausted | F-Star |
| XmAb22841[99] | Bispecific LAG-3/CTLA-4 antibody | I | N/A | T cell exhaustion; Treg-mediated TME inhibition | Xencor |
| Tebotelimab[100] | DART anti-LAG-3-anti-PD-L1 | II | Enoblituzumab (anti-B7-H3) | CD8+ TIL cells exhausted | MacroGenics |
| Eftilagimod alpha[101] | LAG-3-Ig agonist | II | Pembrolizumab (anti-PD-1); avelumab (anti-PD-L1) | Primary drug resistance in PD-L1 low expression/negative tumors; acquired resistance following PD-1/PD-L1 inhibitor therapy | Immutep |
| Sabatolimab[102] | mAb to human TIM-3 | I/Ib | Spartalizumab (anti-PD-1) | Inhibition of T cells and NK cells | Novartis |
| RO-7121661[103] | Bispecific anti-TIM-3-anti-PD-L1 | I | N/A | T cell exhaustion; TIM-3 is compensatorily upregulated, establishing bypass immunosuppression | Roche |
| AZD-7789[104] | Bispecific anti-TIM-3-anti-PD-L1 | I/IIa | N/A | T cell exhaustion; TIM-3 is compensatorily upregulated, establishing bypass immunosuppression | AstraZeneca |
| Tiragolumab[105] | mAb to human TIGIT | II | Atezolizumab (anti-PD-L1) | Compensatory upregulation of TIGIT; remodel the tumor immune microenvironment | Roche |
| OMP-313M32[106] | mAb to human TIGIT | I | Nivolumab (anti-PD-1) | Compensatory upregulation of TIGIT; remodel the tumor immune microenvironment | Mereo BioPharma 5 |
| Enoblituzumab[100] | B7-H3 inhibitor | I/II | Pembrolizumab (anti-PD-1) | Immunosuppressive function of B7-H3 | MacroGenics |
| SD-101[107] | Synthetic CpG-ODN agonist of TLR9 | Ib/II | Pembrolizumab (anti-PD-1) | T-cell exhaustion and compensatory immunosuppression | Dynavax Technologies |
| GSK3359609[108] | ICOS agonist antibody | III | Pembrolizumab (anti-PD-1) | PD-L1 low expression/negative tumor; T cell exhaustion or immunosuppressive microenvironment | GlaxoSmithKline |
LAG-3 inhibitors
LAG-3, an immunoinhibitory receptor structurally homologous to CD4, is upregulated on T cells following activation and maintains persistent expression under conditions of chronic antigen exposure, such as cancer and persistent viral infections[109-111]. Its expression extends beyond T cells to encompass various immune cells, including NK cells, B cells, and DCs. On conventional T cells, LAG-3 functions as a critical negative regulator, suppressing T-cell activation and cytokine production, thereby contributing to a state of T-cell exhaustion[112]. Additionally, LAG-3 is expressed on forkhead box P3 positive (FOXP3+) Treg cells, leading to an immunosuppressive state[113]. Although LAG-3 blockade alone did not show benefits in preclinical studies, when combined with PD-1 inhibitors, it significantly enhanced tumor control in tumor models. The pivotal RELATIVITY-047 trial (NCT03470922), a global, randomized, double-blind phase II/III study, evaluated the combination therapy Opdualag (anti-LAG-3 plus anti-PD-1) vs. nivolumab monotherapy in patients with unresectable or metastatic melanoma. The results demonstrated a superior median PFS of 10.1 months for the Opdualag group compared to 4.6 months for the nivolumab group. The safety profile was manageable, with common immune-related AEs (irAEs) including thyroiditis and rash[114]. Based on these findings, the U.S. FDA granted approval for this combination in 2022. Consequently, multiple phase I and II clinical trials are ongoing to further explore the efficacy of LAG-3 inhibitors, both as monotherapy and in combination regimens, in other malignancies, including HNSCC[43].
TIM-3 inhibitors
TIM-3, a member of the T cell immunoglobulin mucin (TIM) family of immunoregulatory proteins, functions as a multifaceted inhibitory receptor within the TME. Initially characterized as a marker for IFN-γ-producing CD4+ (Th1) and CD8+ T cells, subsequent research has revealed that its expression extends to a diverse array of immune cells[115]. These include Tregs, MDSCs, NK cells, and mast cells, indicating that TIM-3-targeted therapeutic strategies may exert immunomodulatory effects through multiple cellular pathways[116-118]. Preclinical investigations have demonstrated that concurrent blockade of the TIM-3 and PD-1 pathways can induce significant tumor regression, a finding that has accelerated the clinical translation of anti-TIM-3 agents[119,120]. Among the most advanced candidates are sabatolimab (MBG453) and cobolimab (TSR-022). Sabatolimab has received Fast Track designation from the FDA for use in combination with hypomethylating agents (HMAs) for the treatment of myelodysplastic syndromes (MDS)[121]. Meanwhile, cobolimab is under evaluation in phase II/III clinical trials for various solid tumors, including lung cancer, hepatocellular carcinoma, and melanoma, exploring its efficacy in combination with PD-1 inhibitors and/or chemotherapeutic drugs. Encouragingly, a phase I trial investigating cobolimab in combination with a PD-1 inhibitor for lung cancer reported an ORR of 42.9%, alongside a favorable tolerability profile without serious treatment-emergent AEs[122].
TIGIT inhibitors
TIGIT, a newly identified co-inhibitory receptor, was discovered in 2009[123]. Its expression pattern varies across immune cell populations: it is transiently induced on T cells following TCR stimulation, while being constitutively expressed on NK cells and multiple T-cell subsets, such as Tregs, type 1 regulatory T (Tr1) cells, follicular helper T (Tfh) cells, and functionally impaired CD8+ T cells[124]. Emerging evidence has further demonstrated TIGIT expression on regulatory B cells and innate immune cell populations[125], suggesting that this receptor may exert regulatory effects in immune responses that extend beyond its well-documented roles. Upon engagement with its cognate ligands, TIGIT exerts suppressive effects on T-cell activation and impairs NK cell functionality[126]. Moreover, within the TME, TIGIT mediates tumor immune escape through dual mechanisms: direct inhibition of effector CD8+ T cells and augmentation of the immunosuppressive properties of Tregs[127]. A current phase II trial (CITYSCAPE-02, NCT03563716) is comparing the efficacy of PD-L1 inhibitors alone vs. a combination of TIGIT inhibitors with PD-L1 inhibitors in NSCLC patients. Results showed that the combination therapy group had a significantly higher ORR compared to the monotherapy group, with both PFS and OS improving[128]. Besides NSCLC, other cancers are also being tested for the potential of TIGIT blockade as a therapeutic target. In the SKYCRAPER-09 trial (NCT03563716), the efficacy of tiragolumab combined with atezolizumab in R/M HNSCC was compared with a placebo[129]. Additionally, multiple phase II/III clinical trials are ongoing in lung cancer, liver cancer, kidney cancer, gastrointestinal tumors, and HNSCC with anti-TIGIT/anti-PD-1/anti-PD-L1 combined with chemotherapy, and we look forward to the results of these trials in the future[49].
B7 homolog 3 protein inhibitors
B7 homolog 3 protein (B7-H3) is a dual-function immune checkpoint molecule with both costimulatory and co-inhibitory properties, whose expression spans cancer cells and bone marrow-derived immune cells - including T lymphocytes, B lymphocytes, monocytes/macrophages, and DCs[130]. Notably, elevated B7-H3 expression levels in tumor cells and immune-infiltrating cells within the TME have been linked to the development of tumor immune evasion[131]. Emerging evidence suggests that B7-H3 promotes tumor proliferation, metastasis, and therapeutic resistance. In oral squamous cell carcinoma, B7-H3 overexpression is linked to increased tumor aggressiveness and higher mortality rates, with blockade suppressing tumor formation[132]. These findings highlight B7-H3 as a promising therapeutic target. A phase I trial evaluated the B7-H3 inhibitor enoblituzumab combined with the anti-PD-1 inhibitor retifanlimab across multiple tumor types, showing an ORR of 33.3% in 18 HNSCC patients, including five partial responses (PRs) and one complete response (CR)[133]. These promising phase I results have led to subsequent phase II/III trials to assess the safety and anti-tumor activity of combination therapy.
Inducible T cell costimulatory agonists
The ICOS/inducible T cell costimulator (ICOS, also called CD278) pathway, an activation-induced T-cell costimulatory receptor, plays a critical role in modulating T-cell proliferation, survival, and differentiation[134]. ICOS ligand (ICOSL) is expressed by APCs and various tumor cells within the microenvironment. ICOS ligation triggers downstream signaling that exerts pleiotropic effects across T-cell subsets, potentially enhancing CD8+ T-cell cytotoxicity while simultaneously amplifying the immunosuppressive function of Tregs[135]. Although preclinical studies supported a synergistic effect between ICOS agonism and PD-1 blockade, clinical development faced challenges, as evidenced by the termination of the phase II/III INDUCE-3 (NCT04128696) and INDUCE-4 (NCT04428333) trials of the ICOS agonist feladilimab in R/M HNSCC due to insufficient efficacy[108]. Subsequent research has shifted toward novel bispecific antibodies, such as izuralimab (XmAb23104), which targets both PD-1 and ICOS to concurrently block inhibitory signaling and provide agonist costimulation.
TLR agonists
Toll-like receptor 9 (TLR9) agonists have been widely studied in both monotherapy and combination therapy for cancer treatment. Combination therapy with TLR9 agonists and immunotherapy has shown a synergistic effect, enhancing anti-tumor immune responses. TLR9 TLR9 agonists are capable of activating B lymphocytes and plasmacytoid DCs (pDCs), which in turn enhances the secretion of chemokines and cytokines. This immunomodulatory effect optimizes the tumor immune microenvironment and facilitates T cell-driven anti-tumor immune responses[136]. A phase Ib clinical trial (ClinicalTrials.gov: NCT02680184) investigated the therapeutic efficacy and safety profile of intratumoral administration of the Toll-like receptor (TLR) agonist Vidutolimod in combination with a PD-1 inhibitor. In this study involving 44 patients, 11 individuals achieved either a PR or CR, with a median PFS of 2.8 months. These results highlight the potential of TLR9 agonists to reverse PD-1 inhibitor resistance in patients with advanced melanoma[137]. Another clinical study (NCT02521870) evaluated the safety and efficacy of SD-101 - a synthetic CpG oligodeoxynucleotide (CpG-ODN) acting as a TLR9 agonist - in combination with pembrolizumab for the treatment of R/M HNSCC. Among the 28 enrolled patients, the ORR was recorded at 22%[138].
Research and development of emerging drugs or approaches
Epidermal growth factor receptor inhibitors
Epidermal growth factor receptor (EGFR) is an established therapeutic target for HNSCC. There is a theoretical rationale for combining EGFR inhibitors with ICIs. Preclinical studies have demonstrated that EGFR signaling can influence the TME by modulating cytokines such as IL-6, TGF-β, and progranulin, thereby suppressing immune-mediated anti-cancer effects. EGFR activation induces the expression of PD-L1 and other immunosuppressive factors[139]. Cetuximab is a mAb used for HNSCC treatment that inhibits EGFR signaling. The NCT01218048 study revealed that after cetuximab treatment, PD-1 expression on CD8+ TILs increased significantly and was closely associated with treatment response, providing a theoretical basis for combination therapy. A phase II trial evaluated pembrolizumab plus cetuximab in platinum-refractory recurrent HNSCC (33 patients). The ORR was 45%, with a median duration of response (DOR) of 14.9 months and no severe treatment-related toxicity[140]. Multiple phase I-II trials of PD-1/PD-L1 plus EGFR inhibitors in R/M HNSCC are complete [Table 5]; an ongoing phase III trial assesses cetuximab/radiotherapy with avelumab in locally advanced HNSCC[146].
Clinical studies combining immunotherapy with other targeted drugs in HNSCC
| Trial name | Phase | Comparison | Overcome drug-resistant types | Enrolled patients | Outcome measures |
| NCT03082534[140] | II | Cetuximab (EGFR inhibitor) + pembrolizumab (anti-PD-1) | Cold TME; immune escape triggered by EGFR-targeted therapy | 33 | OS |
| NCT02938273[133] | I | Cetuximab (EGFR inhibitor) + radiotherapy + avelumab (anti-PD-L1) | Primary microenvironment-mediated resistance; compensatory immunosuppression | 10 | Efficacy and safety |
| NCT03691714[141] | II | Cetuximab (EGFR inhibitor) + durvalumab (anti-PD-L1) | EGFR-driven adaptive immune escape; antigen presentation-deficient resistance | Ongoing | OS |
| NCT03370276[142] | II | Cetuximab (EGFR inhibitor) + nivolumab (anti-PD-1) | Cold TME; immune escape | 95 | OS |
| NCT02501096[143] | Ib/II | Lenvatinib (VEGFR inhibitor) + pembrolizumab (anti-PD-1) | Vascular barrier-mediated primary resistance; clonal evolution-mediated acquired resistance | 22 | ORR PFS DOR |
| NCT04199104[144] | III | Pembrolizumab (anti-PD-1) ± lenvatinib (VEGFR inhibitor) | Vascular barrier-mediated primary resistance; clonal evolution-mediated acquired resistance | 511 | ORR PFS OS |
| NCT02178722[145] | I/II | Pembrolizumab (anti-PD-1) + epacadostat (IDO1 inhibitor) | Tryptophan metabolism-driven adaptive resistance; DC dysfunction resistance | 38 | ORR DCR |
A meta-analysis of seven trials showed cetuximab + anti-PD-1 significantly improved ORR and 1-year OS in HPV-negative R/M HNSCC vs. monotherapy, but no benefit in HPV-positive disease. However, subgroup analysis indicated no significant effect in HPV-positive diseases[147].
VEGFR inhibitors
Lenvatinib acts as a broad-spectrum receptor tyrosine kinase (RTK) inhibitor, which exerts robust inhibitory effects on major mediators of tumor angiogenesis and progression. These key regulatory targets include vascular endothelial growth factor receptors 1-3 (VEGFR1-3), fibroblast growth factor receptors 1-4 (FGFR1-4), platelet-derived growth factor receptor α (PDGFRα), as well as the KIT proto-oncogene, receptor tyrosine kinase (KIT) and ret proto-oncogene (RET) kinases. Clinically, this agent has secured approval from FDA for the treatment of several malignancies, specifically radioactive iodine-refractory differentiated thyroid carcinoma, advanced renal cell carcinoma, and unresectable hepatocellular carcinoma. The KEYNOTE-146 phase Ib/II trial (NCT02501096) assessed the efficacy and safety of pembrolizumab combined with lenvatinib in HNSCC, showing improved ORR and a median PFS of 7.6 months [95% confidence interval (CI) 4.2-12.6]. Treatment-related AEs primarily included fatigue, appetite loss, hypertension, diarrhea, and nausea, without severe toxic effects[148]. Based on these findings, a phase III trial (LEAP-010, NCT04199104) was initiated to evaluate pembrolizumab with or without lenvatinib in R/M HNSCC[149]. Results showed that in PD-L1 CPS ≥ 1 R/M HNSCC patients, the combination therapy significantly improved PFS and ORR, but OS was not prolonged. The safety profile was consistent with previous reports, although 156 (61.4%) patients in the combination therapy group experienced treatment-related AEs, compared to 45 (17.8%) in the placebo group.
In addition, multiple studies have investigated the efficacy of combining VEGFR inhibitors with ICIs in the treatment of metastatic or recurrent HNSCC. For instance, a phase 1/2 trial evaluating ramucirumab (a mAb targeting VEGFR2) in combination with pembrolizumab for recurrent or metastatic HNSCC demonstrated encouraging results. In this trial, ORR reached 55%, highlighting the superiority of the combination therapy over single-agent immunotherapy in improving response rates[150]. Additionally, a phase II trial of pembrolizumab combined with cabozantinib (a multi-targeted RTK inhibitor including VEGFR) achieved promising outcomes, meeting the primary endpoint of improved ORR[151]. Cabozantinib modulates immune cell function within the TME by targeting VEGFR and other pathways, thereby synergizing with pembrolizumab to enhance tumor control.
Indoleamine-2,3-dioxygenase 1 inhibitors
Indoleamine-2,3-dioxygenase 1 (IDO1), a key immunomodulatory enzyme, catalyzes the initial and rate-limiting step of tryptophan catabolism along the kynurenine pathway, thereby suppressing CTL activity and contributing to an immunosuppressive TME[152]. In HNSCC, elevated expression of IDO1 is frequently observed within malignant cells, as well as in TAMs and DCs, and this overexpression is correlated with unfavorable patient prognosis[153]. IDO1 plays a role in T cell exhaustion and inhibition while promoting Treg cell activation and MDSC infiltration[154,155]. The selective IDO1 inhibitor epacadostat was developed to counteract this mechanism. Studies have also suggested that IDO1 activity is closely related to ICI resistance. A phase I/II clinical trial evaluating epacadostat combined with pembrolizumab in HNSCC showed an ORR of 34%[156]. However, the CheckMate 9NA/ECHO-310 phase III study (NCT03342352), which assessed the same treatment combination in melanoma, yielded poor results, leading to early study termination[157].
Antibody–drug conjugates
Antibody–drug conjugates (ADCs) are hybrid therapeutic agents composed of mAbs and small-molecule cytotoxic payloads, where the two components are covalently linked via a chemical linker. The mAb moiety of ADCs is specifically designed to bind to tumor-specific antigens (TSAs) or TAAs, enabling targeted delivery of the cytotoxic agent to cancerous cells. This unique targeted mechanism endows ADCs with two key advantages: enhanced safety profiles and an expanded therapeutic window compared to conventional chemotherapeutics. Furthermore, the antibody moiety of ADCs interacts with immune effector cells in the TME, triggering antibody-dependent cellular cytotoxicity (ADCC) to enhance anti-tumor effects[158]. A NCT04223856 trial evaluated Enfortumab Vedotin (ADC) plus Pembrolizumab as first-line therapy for advanced urothelial carcinoma. Results showed that the combination group had significantly longer PFS (12.5 vs. 6.3 months) and OS (31.5 vs. 16.1 months) than the standard chemotherapy group[159]. The combination of ADCs and ICIs may offer a new potential treatment approach for R/M HNSCC. Based on data from other cancer types, further research into this combination therapy is warranted.
Oncolytic viruses
Oncolytic viruses represent a distinctive category of viruses characterized by their ability to selectively proliferate within cancer cells, trigger apoptotic cell death in these malignant targets, while leaving normal, non-tumorigenic tissues intact[160]. RP1, a herpes simplex virus type 1 (HSV-1)-based oncolytic immunotherapeutic agent, is engineered to express two key bioactive molecules: human granulocyte-macrophage colony-stimulating factor (GM-CSF) and the chimeric fusion protein Gibbon Ape Leukemia Virus envelope glycoprotein (GALV-GP-R). This dual-expression design strengthens the eradication of tumor cells by potentiating the activation of systemic anti-tumor immune responses. The phase I/II IGNYTE study (NCT03767348) assessed the therapeutic efficacy of RP1 in combination with nivolumab among melanoma patients who had previously experienced treatment failure with PD-1 inhibitors. Study outcomes indicated that at the 12-month follow-up, the ORR reached 33.6%. Further stratification showed an objective response rate of 31.4% and a CR rate of 12.2%. Notably, the mean DOR surpassed 35 months, and the combined regimen exhibited favorable tolerability in the study cohort[161]. Beyond RP1, other oncolytic viruses - including vaccinia virus, vesicular stomatitis virus, and measles virus - are currently under clinical investigation for the treatment of head and neck cancer[162,163].
Nanomedicine approaches for cancer treatment
The insufficient accumulation of ICIs within HNSCC tumors constitutes a critical barrier to their therapeutic effectiveness. This limitation is mainly attributed to aberrant tumor vascularization, elevated interstitial fluid pressure, and a dense ECM[164]. Nano-drug delivery systems (NDDSs) have emerged as a promising strategy for the targeted delivery of diverse ICIs, with the majority of current research focusing on PD-1/PD-L1 inhibitors. These nanosystems enable the site-specific delivery of various ICI types - including antibodies, nucleic acids, and small-molecule inhibitors - to tumor tissues, thereby boosting anti-tumor potency. Furthermore, NDDSs can be engineered for dual ICI co-delivery, such as the simultaneous transport of PD-1/PD-L1 and CTLA-4 inhibitors[165]. Collectively, NDDSs have not only enhanced the therapeutic efficacy of ICIs but also mitigated the incidence of irAEs. Nanomedicine approaches address the pharmacokinetic limitations that have hampered traditional combination therapies. For example, glutathione (GSH)-sensitive polyethylene glycol-poly-L-lysine (PEG-PLL) micelles are surface-modified with angiopep-2 peptide [to penetrate the blood-brain barrier (BBB)] and co-load anti-PD-L1 and paclitaxel (PTX) to achieve chemo-immunotherapy for glioma[166]; Hollow mesoporous organosilicon nanoparticle-coated Fe-MOF loaded with doxorubicin (DOX), which can locally release DOX after surgery to induce ICD, combined with anti-PD-1 to inhibit postoperative recurrence and brain metastasis of 4T1 breast cancer[167]. Man@pSiNPs-erastin (mannose-modified porous silicon nanoparticles loaded with erastin), targeting TAM to induce ferroptosis, combined with anti-PD-1 to inhibit liver cancer[168]; Cu2WS4-PEG nanozyme induces ferroptosis through the Kelch-like ECH associated protein 1 (KEAP1)/nuclear factor erythroid 2–related factor 2 (NRF2)/heme oxygenase 1 (HMOX1)/glutathione peroxidase 4 (GPX4) pathway, and enhances immunoradiotherapy in combination with radiotherapy and anti-PD-L1[169].
In conclusion, NDDSs have unique advantages and potential in the treatment of R/M HNSCC, and various development strategies can help them maximize therapeutic effects and reduce side effects in treatment.
Emerging therapeutic paradigms: beyond conventional combinatorial approaches
Neoadjuvant ICB
Neoadjuvant ICIs leverage intact tumor antigenicity to prime systemic anti-tumor immunity prior to surgery. This approach induces in situ formation of tertiary lymphoid structures (TLS) and clonal T-cell expansion, enhancing micrometastasis eradication. TLS are lymph node-like structures formed in chronic inflammatory or TMEs. While not endogenously formed at birth, these structures exhibit functional similarities to lymph nodes: they recruit immune cell populations - including T lymphocytes, B lymphocytes, and DCs - and facilitate localized immune responses. Within the TLS microenvironment, DCs capture TAAs and present them to naive T cells. Subsequently, the activated T cells undergo clonal proliferation under the stimulation of cytokines (e.g., IL-2), yielding a large pool of effector T cells that share identical antigenic specificity. This process enhances the recognition and cytotoxic capacity against tumor cells. In tumors, the presence of TLS is often correlated with improved immune infiltration and better prognosis, as they provide an organized immunological niche where immune cells can be activated and proliferate. For resectable HNSCC patients undergoing neoadjuvant nivolumab therapy, the surgical procedure was successfully accomplished in roughly 88% of participants across all study arms. The median duration of follow-up stood at 38.3 months. With respect to 36-month EFS outcomes, distinct differences were observed between the pembrolizumab and control groups: in the population with a CPS of 10 or higher, the EFS rates were 59.8% and 45.9%, respectively; whereas in the CPS ≥ 1 subgroup, the rates were 58.2% vs. 44.9%; and in the total population, 57.6% vs. 46.4%[170]. Mechanistically, ICIs expanded tumor-reactive T-cell clones post-surgery, reduced MDSCs, and induced systemic CD8+ memory differentiation driving abscopal effects[171]. Phase III trials (e.g., KEYNOTE-689, NCT03765918) now validated pathologic response as a surrogate endpoint for OS[172,173].
Microbiome modulation: the gut-immune axis as a therapeutic target
Based on provided literature, the gut microbiome significantly modulates ICI efficacy in cancers, including HNSCC, through intricate mechanisms involving immune crosstalk: (1) Molecular Mimicry: Bacterial peptides can structurally resemble tumor antigens. For instance, peptides derived from Bacteroides fragilis exhibit cross-reactivity with TP53 antigens[174]. This mimicry potentially triggers or modifies T-cell responses targeting tumors; (2) Metabolite Production: Microbial metabolites profoundly influence immune cell differentiation and function. Short-chain fatty acids (SCFAs), produced by commensal bacteria during fiber fermentation, promote the differentiation and function of Tregs[175]. Conversely, the microbial metabolite inosine enhances anti-tumor Th1 responses, potentiating ICI activity[176]; (3) DC Priming: Specific commensal bacteria directly prime DCs, essential for initiating adaptive immunity. Akkermansia muciniphila, a species associated with positive ICI responses, enhances DC function leading to the upregulation of IL-12. This, in turn, promotes the recruitment and activation of cytotoxic CD8+ T cells within the TME[177].
Clinically, modulation strategies such as Fecal Microbiota Transplantation (FMT) exploit these mechanisms to overcome resistance. Transplanting microbiota from ICI-responsive donors to refractory patients enriches beneficial taxa (e.g., Ruminococcaceae) and has demonstrated the capacity to double objective response rates compared to control groups (32% vs. 15%) in refractory cancers[178]. Importantly, the microbiome’s impact extends beyond the gut. Within the HNSCC context, a high abundance of Fusobacterium in the salivary microbiome has been negatively correlated with resistance to PD-1 inhibitors, suggesting that modulating both gut and local oral/respiratory microbiota represents a viable therapeutic avenue to enhance ICI efficacy and circumvent resistance[179]. Collectively, targeting the microbiome-immune axis is emerging as a critical strategy to optimize immunotherapy outcomes.
Multimodal therapeutic innovations
CAR-NK cell therapy: precision off-the-shelf immunity
Chimeric antigen receptor-modified NK (CAR-NK) cells are a pivotal breakthrough for overcoming solid tumor resistance. Via natural cytotoxicity receptors, they exert MHC-unrestricted cytotoxicity, and their PD-1- and TIM-3- phenotype avoids cytokine release syndrome (CRS) and neurotoxicity. The University of Texas MD Anderson Cancer Center spearheaded a phase I/IIa clinical study (NCT03056339) to assess the safety and efficacy of cord blood-derived cluster of differentiation 19 (CD19)-targeted CAR-NK cells (designated CAR19/IL-15 NK cells) in patients with relapsed or refractory CD19-positive B-cell hematological malignancies, such as non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Results from this trial demonstrated a cumulative ORR of 48.6% across the cohort, with substantial variability by disease subtype: 100% ORR in low-grade NHL, 67% in CLL, and 41% in diffuse large B-cell lymphoma (DLBCL). The 1-year OS was 68%, with no graft-versus-host disease (GVHD) or neurotoxicity observed[180]. Critically, CAR-NKs persisted > 90 days post-infusion, confirming durable engraftment[181]. Targeting diverse antigens [e.g., EGFR deletion variant III (EGFRvIII), MHC class I polypeptide-related sequence A (MICA) and MHC class I polypeptide-related sequence B (MICB), natural killer group 2 member D (NKG2D) ligands] enhances tumor specificity while leveraging “off-the-shelf” availability[182].
STING agonists: activating innate immune surveillance
Stimulator of interferon genes (STING) agonists reprogram immunosuppressive TMEs by binding cyclic guanosine monophosphate-adenosine monophosphate (cGAMP), triggering TANK binding kinase 1 (TBK1)/interferon regulatory factor 3 (IRF3) phosphorylation and type I interferon production. This signaling cascade facilitates the maturation of DCs and enhances the infiltration of CD8+ T cells into the TME. In the context of HNSCC, a phase II clinical trial (NCT03937141) investigated the combination of ADU-S100 and pembrolizumab in adult patients with PD-L1-positive R/M HNSCC. The findings indicated that the ADU-S100 plus pembrolizumab regimen exhibited favorable tolerability and successfully induced abscopal effects[183]. Novel delivery systems - including pH-sensitive nanoparticles targeting TAMs and sustained-release intratumoral implants (e.g., MK-2118) - optimize bioavailability and reduce systemic toxicity[1,184].
Epigenetic modulators: resetting immune evasion mechanisms
Histone deacetylase inhibitors (HDACi; e.g., entinostat) and enhancer of zeste homolog 2 (EZH2) inhibitors (EZH2i; e.g., tazemetostat) reverse epigenetic silencing of immunogenic pathways. HDACi downregulate Treg-suppressive genes [FOXP3, cytotoxic T-lymphocyte-associated protein 4 (CTLA4)], while EZH2i reactivate endogenous retroviruses, inducing “viral mimicry” via dsRNA sensing pathways. The ongoing phase II ENCORE601 trial (NCT02437136) evaluates entinostat plus pembrolizumab in multiple tumors. In 76 treated NSCLC patients, the ORR was 9.2% (below the pre-specified threshold), median PFS was 2.8 months (95%CI 1.5-4.1), and median OS was 11.7 months (95%CI 7.6-13.4), with no new toxicities (including irAEs) observed. However, the combination missed its primary efficacy endpoint[185]. Demethylation of the interferon gamma (IFNG) promoter in circulating T cells served as a predictive biomarker for therapeutic response[186]. These agents also enhance chimeric antigen receptor T-cell (CAR-T)/NK cell function by reducing exhaustion and promoting memory phenotypes[187].
In conclusion, a growing body of clinical and preclinical evidence indicates that synergistic treatment modalities can induce potent and durable anti-tumor responses in R/M HNSCC. This approach provides a potential pathway to achieving long-term disease control in historically intractable HNSCC subsets. Future research must focus on optimizing these combinations to maximize efficacy while diligently managing the associated toxicities.
CONCLUSION
Resistance to immunotherapy in HNSCC is the result of complex, intertwined mechanisms involving both intrinsic tumor characteristics and extrinsic regulation by the TME. Future research should focus on the following directions: (1) developing multi-omics technologies to analyze the dynamic evolution of resistance; (2) exploring combination therapeutic strategies targeting both tumor cells and the microenvironment; (3) achieving precise classification based on biomarkers such as TMB and the spatial distribution of PD-L1; (4) developing efficient and low-toxicity immunotherapy combination models. Additionally, novel immunotherapies, such as CAR-T therapy and bispecific antibodies, hold promise for overcoming current therapeutic limitations. Through interdisciplinary collaboration and translational research, the ultimate goal is to comprehensively overcome immunotherapy resistance in HNSCC.
DECLARATIONS
Acknowledgements
In this manuscript, Figure 1 was drafted using Adobe Illustrator (AI), Figures 2, 3, and the Graphical Abstract were created using BioRender elements, and the final compositions were completed in AI. We thank Yu Yu for assistance with Figures 2, 3, and graphical abstract creation using BioRender.com. [Graphical Abstract was created in BioRender. Yu, Y. (2025) https://BioRender.com/ym2sj7z].
Authors’ contributions
Involved in manuscript preparation: Zhao W, Duan H, Jiang S
Revised the manuscript: Luo Q, Yuan B
All authors have read and approved the review.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Natural Science Foundation of Hunan Province, China (Grant Nos. 2025JJ80866, 2025JJ60776, and 2024JJ9162). The project was also supported by the Health Commission of Hunan Province (Grant Nos. B202302068083 and C202303109144).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: a 2023 update. Drugs. 2023;83:217-48.
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63.
3. Elmusrati A, Wang J, Wang C. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int J Oral Sci. 2021;13:131.
4. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92.
5. Harrington K, Cohen E, Soulières D, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab in recurrent or metastatic head and neck squamous cell carcinoma (KEYNOTE-040): subgroup analysis by pattern of disease recurrence. Oral Oncol. 2023;147:106587.
6. Caudell JJ, Gillison ML, Maghami E, et al. NCCN Guidelines® Insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20:224-34.
7. Keam B, Machiels JP, Kim HR, et al. Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck. ESMO Open. 2021;6:100309.
8. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for head and neck cancer 2018 (English version). Chin J Cancer Res. 2019;31:84-98.
9. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
10. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956-65.
11. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51.
12. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542-9.
13. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-28.
14. Haddad RI, Harrington K, Tahara M, et al. Nivolumab plus ipilimumab versus EXTREME regimen as first-line treatment for recurrent/metastatic squamous cell carcinoma of the head and neck: the final results of CheckMate 651. J Clin Oncol. 2023;41:2166-80.
15. Machiels J, Tao Y, Licitra L, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2024;25:572-87.
16. Harrington KJ, Ferris RL, Gillison M, et al. Efficacy and safety of nivolumab plus ipilimumab vs nivolumab alone for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck: the phase 2 CheckMate 714 randomized clinical trial. JAMA Oncol. 2023;9:779-89.
17. Psyrri A, Fayette J, Harrington K, et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol. 2023;34:262-74.
18. Harrington KJ, Ferris RL, Blumenschein G Jr, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18:1104-15.
19. Cohen EEW, Soulières D, Le Tourneau C, et al.; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156-67.
20. Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7:184.
21. Huang Z, Zheng S, Ding S, et al. Prognostic role of programmed cell death ligand-1 expression in head and neck cancer treated with programmed cell death protein-1/programmed cell death ligand-1 inhibitors: a meta-analysis based on clinical trials. J Cancer Res Ther. 2021;17:676-87.
22. Emancipator K, Huang L, Aurora-Garg D, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol. 2021;34:532-41.
23. Ferris RL, Licitra L, Fayette J, et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by prior cetuximab use. Clin Cancer Res. 2019;25:5221-30.
24. Yilmaz E, Ismaila N, Bauman JE, et al. Immunotherapy and biomarker testing in recurrent and metastatic head and neck cancers: ASCO Guideline. J Clin Oncol. 2023;41:1132-46.
25. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353-65.
26. Haddad RI, Seiwert TY, Chow LQM, et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer. 2022;10:e003026.
27. Stein AP, Saha S, Kraninger JL, et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21:138-46.
28. Argiris A, Li S, Ghebremichael M, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol. 2014;25:1410-6.
29. Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365-73.
30. Sahovaler A, Kim MH, Mendez A, et al. Survival outcomes in human papillomavirus–associated nonoropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146:1158-66.
31. Wang H, Wei J, Wang B, et al. Role of human papillomavirus in laryngeal squamous cell carcinoma: a meta-analysis of cohort study. Cancer Med. 2020;9:204-14.
32. Nguyen N, Bellile E, Thomas D, et al.; Head and Neck SPORE Program Investigators. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074-84.
33. Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829.
34. Kim TK, Herbst RS, Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 2018;39:624-31.
35. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707-23.
36. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329-60.
37. Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57:1719-26.
38. Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥ 25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142-52.
39. Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733-41.
40. Wilson EB, Brooks DG. The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol. 2011;350:39-65.
41. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
42. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-68.
43. Andrews LP, Cillo AR, Karapetyan L, Kirkwood JM, Workman CJ, Vignali DA. Molecular pathways and mechanisms of LAG3 in cancer therapy. Clin Cancer Res. 2022;28:5030-9.
44. Park JS, Gazzaniga FS, Wu M, et al. Targeting PD-L2–RGMb overcomes microbiome-related immunotherapy resistance. Nature. 2023;617:377-85.
45. Zhao Y, Lee CK, Lin CH, et al. PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51:1059-73.e9.
46. Ren D, Hua Y, Yu B, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19:19.
47. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-7.
49. Joller N, Anderson AC, Kuchroo VK. LAG-3, TIM-3, and TIGIT: distinct functions in immune regulation. Immunity. 2024;57:206-22.
50. Navasardyan I, Bonavida B. Regulation of T cells in cancer by nitric oxide. Cells. 2021;10:2655.
51. Basheeruddin M, Qausain S. Hypoxia-inducible factor 1-alpha (HIF-1α) and cancer: mechanisms of tumor hypoxia and therapeutic targeting. Cureus. 2024;16:e70700.
52. Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096-103.
53. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-67.
54. Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761-9.
55. Mcgranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463-9.
56. Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467-72.
57. van Dorp J, van der Heijden MS. The bladder cancer immune micro-environment in the context of response to immune checkpoint inhibition. Front Immunol. 2023;14:1235884.
58. Cao Y, Wen E, Chen Q, Li X, Wang Z. Multifunctional ICG-SB@Lip-ZA nanosystem focuses on remodeling the inflammatory-immunosuppressive microenvironment after photothermal therapy to potentiate cancer photothermal immunotherapy. Adv Healthc Mater. 2025;14:e2402211.
59. Yan R, Moresco P, Gegenhuber B, Fearon DT. T cell-mediated development of stromal fibroblasts with an immune-enhancing chemokine profile. Cancer Immunol Res. 2023;11:1044-54.
60. Liu Z, Zhang Z, Zhang Y, et al. Spatial transcriptomics reveals that metabolic characteristics define the tumor immunosuppression microenvironment via iCAF transformation in oral squamous cell carcinoma. Int J Oral Sci. 2024;16:267.
61. Dai E, Wang W, Li Y, Ye D, Li Y. Lactate and lactylation: behind the development of tumors. Cancer Lett. 2024;591:216896.
62. Shi H, Chen S, Chi H. Immunometabolism of CD8+ T cell differentiation in cancer. Trends Cancer. 2024;10:610-26.
63. Imani S, Farghadani R, Roozitalab G, et al. Reprogramming the breast tumor immune microenvironment: cold-to-hot transition for enhanced immunotherapy. J Exp Clin Cancer Res. 2025;44:131.
64. Wu W, Zhang J, Sun H, et al. Glycolysis induces abnormal transcription through histone lactylation in T-cell acute lymphoblastic leukemia. Genomics Proteomics Bioinformatics. 2025;23:qzaf029.
65. Wang S, Huang T, Wu Q, et al. Lactate reprograms glioblastoma immunity through CBX3-regulated histone lactylation. J Clin Invest. 2024;134:e176851.
66. Pich-Bavastro C, Yerly L, Di Domizio J, Tissot-Renaud S, Gilliet M, Kuonen F. Activin A-mediated polarization of cancer-associated fibroblasts and macrophages confers resistance to checkpoint immunotherapy in skin cancer. Clin Cancer Res. 2023;29:3498-513.
67. Yan J, Chen Y, Patel AJ, et al. Tumor-intrinsic PRC2 inactivation drives a context-dependent immune-desert microenvironment and is sensitized by immunogenic viruses. J Clin Invest. 2022;132:e153437.
68. Zhu Z, Zhao Q, Song W, et al. A novel cuproptosis-related molecular pattern and its tumor microenvironment characterization in colorectal cancer. Front Immunol. 2022;13:940774.
69. Zheng G, Lu Y, Yang Z, et al. Immune desert in MMR-deficient tumors predicts poor responsiveness of immune checkpoint inhibition. Front Immunol. 2023;14:1142862.
70. Vredevoogd DW, Peeper DS. Heterogeneity in functional genetic screens: friend or foe? Front Immunol. 2023;14:1162706.
71. Lauss M, Phung B, Borch TH, et al. Molecular patterns of resistance to immune checkpoint blockade in melanoma. Nat Commun. 2024;15:3075.
72. Mancini C, Lori G, Pranzini E, Taddei ML. Metabolic challengers selecting tumor-persistent cells. Trends Endocrinol Metab. 2024;35:263-76.
73. Abdulrahman Z, Slieker RC, Mcguire D, Welters MJP, van Poelgeest MIE, van der Burg SH. Single-cell spatial transcriptomics unravels cell states and ecosystems associated with clinical response to immunotherapy. J Immunother Cancer. 2025;13:e011308.
74. van Weverwijk A, de Visser KE. Mechanisms driving the immunoregulatory function of cancer cells. Nat Rev Cancer. 2023;23:193-215.
75. Aldea M, Andre F, Marabelle A, Dogan S, Barlesi F, Soria JC. Overcoming resistance to tumor-targeted and immune-targeted therapies. Cancer Discov. 2021;11:874-99.
76. Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507-19.
77. Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16:123-35.
78. Galluzzi L, Vitale I, Warren S, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:e000337.
79. Leduc C, Adam J, Louvet E, et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open. 2018;3:e000257.
80. Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2021;21:37-50.
81. Garg AD, More S, Rufo N, et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. OncoImmunology. 2017;6:e1386829.
82. Wang J, Zhang H, Lv J, et al. A tumor-specific ROS self-supply enhanced cascade-responsive prodrug activation nanosystem for amplified chemotherapy against multidrug-resistant tumors. Acta Biomaterialia. 2023;164:522-37.
83. Fu J, Li T, Yang Y, et al. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials. 2021;268:120537.
84. Fucikova J, Kepp O, Kasikova L, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11:1013.
85. Long Y, Guo J, Chen J, et al. GPR162 activates STING dependent DNA damage pathway as a novel tumor suppressor and radiation sensitizer. Sig Transduct Target Ther. 2023;8:1224.
86. Du S, Chen G, Yang P, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys. 2022;112:1243-55.
87. Jin WJ, Zangl LM, Hyun M, et al. ATM inhibition augments type I interferon response and antitumor T-cell immunity when combined with radiation therapy in murine tumor models. J Immunother Cancer. 2023;11:e007474.
88. Seyedin SN, Hasibuzzaman MM, Pham V, et al. Combination therapy with radiation and PARP inhibition enhances responsiveness to anti-PD-1 therapy in colorectal tumor models. Int J Radiat Oncol Biol Phys. 2020;108:81-92.
89. Liu S, Wang W, Hu S, et al. Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity. Cell Death Dis. 2023;14:679.
90. Wang Y, Liu ZG, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25:1709-17.
91. Weiss J, Sheth S, Deal AM, et al. Concurrent definitive immunoradiotherapy for patients with stage III–IV head and neck cancer and cisplatin contraindication. Clin Cancer Res. 2020;26:4260-7.
92. Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450-62.
93. Ferris R, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942-50.
94. Ferris RL, Gooding WE, Chiosea SI, et al. Neoadjuvant nivolumab alone or in combination with relatlimab or ipilimumab in resectable head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2023;41:6018.
95. Perez-Santos M, Anaya-Ruiz M, Villafaña-Diaz L, Sánchez Esgua G. Approaches for development of LAG-3 inhibitors and the promise they hold as anticancer agents. Expert Opin Drug Discov. 2022;17:1341-55.
96. Cho BC, Hamid O, Zhu X, et al. A phase 1 study of fianlimab (anti–LAG-3) in combination with cemiplimab (anti–PD-1) in patients with advanced HNSCC. J Clin Oncol. 2024;42:6038.
97. Patel MR, Johnson M, Winer I, et al. Ezabenlimab (BI 754091), an anti-PD-1 antibody, in patients with advanced solid tumours. Cancer Immunol Immunother. 2024;73:89.
98. Yap TA, Lorusso PM, Wong DJ, et al. A phase 1 first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1 in patients with advanced cancer and PD-L1 resistance. Clin Cancer Res. 2023;29:888-98.
99. Giri VK, McDermott DF, Zaemes J. The emerging role of lymphocyte-activation gene 3 targeting in the treatment of solid malignancies. Cancer. 2025;131:e35892.
100. Obara G, Sun J, Loo D, Bohac GC. Phase 2 trial of enoblituzumab plus retifanlimab or tebotelimab in first-line treatment of patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). J Clin Oncol. 2022;40:TPS6102.
101. Forster M, Brana I, Pousa AL, et al. Eftilagimod alpha (soluble LAG3 protein) combined with pembrolizumab as second-line therapy for patients with metastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2024;30:3726-34.
102. Curigliano G, Gelderblom H, Mach N, et al. Phase I/Ib clinical trial of sabatolimab, an anti–TIM-3 antibody, alone and in combination with spartalizumab, an anti–PD-1 antibody, in advanced solid tumors. Clin Cancer Res. 2021;27:3620-9.
103. Sun M, Yang P, Wang W, et al. Advancements in the research of immune checkpoint inhibitors for the treatment of advanced esophageal squamous cell carcinoma. Am J Cancer Res. 2024;14:1981-98.
104. Malhotra J, Huang A, Amini A, Lee P. Novel immunotherapeutics for the treatment of non-small cell lung cancer (NSCLC) resistant to PD-1/PD-L1 inhibitors. Cancers. 2024;16:3603.
105. Cohen E, Fayette J, Harrington K, et al. 927TiP SKYSCRAPER-09: a phase II, randomised, double-blinded study of atezolizumab (Atezo) + tiragolumab (Tira) and atezo + placebo as first-line (1L) therapy for recurrent/metastatic (R/M) PD-L1+ squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol. 2021;32:S814-5.
106. Mettu NB, Ulahannan SV, Bendell JC, et al. A phase 1a/b open-label, dose-escalation study of etigilimab alone or in combination with nivolumab in patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2022;28:882-92.
107. Cohen EEW, Nabell L, Wong DJ, et al. Intralesional SD-101 in combination with pembrolizumab in anti-PD-1 treatment-naïve head and neck squamous cell carcinoma: results from a multicenter, phase II trial. Clin Cancer Res. 2022;28:1157-66.
108. Hansen AR, Stanton TS, Hong MH, et al. INDUCE-3: a randomized, double-blind study of GSK3359609 (GSK609), an inducible T-cell co-stimulatory (ICOS) agonist antibody, plus pembrolizumab (PE) versus placebo (PL) plus PE for first-line treatment of PD-L1-positive recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2020;38:TPS6591.
109. Grosso JF, Goldberg MV, Getnet D, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659-69.
110. Woo S, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917-27.
111. Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393-405.
112. Grebinoski S, Zhang Q, Cillo AR, et al. Autoreactive CD8+ T cells are restrained by an exhaustion-like program that is maintained by LAG3. Nat Immunol. 2022;23:868-77.
113. Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503-13.
114. Tawbi HA, Schadendorf D, Lipson EJ, et al.; RELATIVITY-047 Investigators. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
115. Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536-41.
116. Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141-3.
117. Phong BL, Avery L, Sumpter TL, et al. Tim-3 enhances FcεRI-proximal signaling to modulate mast cell activation. J Exp Med. 2015;212:2289-304.
118. Ndhlovu LC, Lopez-Vergès S, Barbour JD, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734-43.
119. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-94.
120. Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733-8.
121. Brunner AM, Esteve J, Porkka K, et al. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients (Pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): final analysis from a phase Ib study. Blood. 2021;138:244.
122. Falchook GS, Ribas A, Davar D, et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). J Clin Oncol. 2022;40:2504.
123. Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48-57.
124. Anderson A, Joller N, Kuchroo V. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989-1004.
125. Yamada T, Tatematsu M, Takasuga S, et al. TIGIT mediates activation-induced cell death of ILC2s during chronic airway allergy. J Exp Med. 2023:220.
126. Brauneck F, Fischer B, Witt M, et al. TIGIT blockade repolarizes AML-associated TIGIT+ M2 macrophages to an M1 phenotype and increases CD47-mediated phagocytosis. J Immunother Cancer. 2022;10:e004794.
127. Kurtulus S, Sakuishi K, Ngiow SF, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2024;134:e183278.
128. Cho BC, Abreu DR, Hussein M, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022;23:781-92.
129. Chu X, Tian W, Wang Z, Zhang J, Zhou R. Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: mechanisms and clinical trials. Mol Cancer. 2023;22:1800.
130. Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269-74.
131. Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899-906.
132. Chen J, Chen C, Ku K, et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc Natl Acad Sci U S A. 2015;112:13057-62.
133. Elbers JBW, Al-Mamgani A, Tesseslaar MET, et al. Immuno-radiotherapy with cetuximab and avelumab for advanced stage head and neck squamous cell carcinoma: results from a phase-I trial. Radiother Oncol. 2020;142:79-84.
134. Amatore F, Gorvel L, Olive D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin Ther Targets. 2018;22:343-51.
135. Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 2020;5:e000544.
136. Dongye Z, Li J, Wu Y. Toll-like receptor 9 agonists and combination therapies: strategies to modulate the tumour immune microenvironment for systemic anti-tumour immunity. Br J Cancer. 2022;127:1584-94.
137. Ribas A, Medina T, Kirkwood JM, et al. Overcoming PD-1 blockade resistance with CpG-A Toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 2021;11:2998-3007.
138. Cohen EEW, Nabell L, Wong DJL, et al. Phase 1b/2, open label, multicenter study of intratumoral SD-101 in combination with pembrolizumab in anti-PD-1 treatment naïve patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2019;37:6039.
139. Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910-23.
140. Sacco AG, Chen R, Worden FP, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883-92.
141. Gulati S, Crist M, Riaz MK, et al. Durvalumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, nonrandomized, phase II clinical trial. Clin Cancer Res. 2023;29:1906-15.
142. Wang X, Li T, Slebos RJC, et al. Clinical significance of peripheral T-cell repertoire in head and neck squamous cell carcinoma treated with cetuximab and nivolumab. Cancer Immunol Immunother. 2025;74:142.
143. Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946-58.
144. Siu LL, Burtness B, Cohen EEW, et al. Phase III LEAP-010 study: first-line pembrolizumab with or without lenvatinib in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2020;38:TPS6589.
145. Gangadhar TC, Hamid O, Smith DC, et al. Preliminary results from a Phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J Immunother Cancer. 2015;3:O7.
146. dos Santos LV, Abrahão CM, William WN. Overcoming resistance to immune checkpoint inhibitors in head and neck squamous cell carcinomas. Front Oncol. 2021;11:596290.
147. Zhang S, Zheng M, Nie D, et al. Efficacy of cetuximab plus PD-1 inhibitor differs by HPV status in head and neck squamous cell carcinoma: a systematic review and meta-analysis. J Immunother Cancer. 2022;10:e005158.
148. Taylor MH, Rasco DW, Brose MS, et al. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2018;36:6016.
149. Licitra L, Tahara M, Harrington K, et al. Pembrolizumab with or without lenvatinib as first-line therapy for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): phase 3 LEAP-010 study. Int J Radiat Oncol Biol Phys. 2024;118:e2-3.
150. Adkins D, Ley JC, Liu J, Oppelt P. Ramucirumab in combination with pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma: a single-centre, phase 1/2 trial. Lancet Oncol. 2024;25:888-900.
151. Saba NF, Steuer CE, Ekpenyong A, et al. Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial. Nat Med. 2023;29:880-7.
152. Economopoulou P, Kladi-Skandali A, Strati A, et al. Prognostic impact of indoleamine 2,3-dioxygenase 1 (IDO1) mRNA expression on circulating tumour cells of patients with head and neck squamous cell carcinoma. ESMO Open. 2020;5:e000646.
153. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193-207.
154. Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147-54.
155. Holmgaard R, Zamarin D, Li Y, et al. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep. 2015;13:412-24.
156. Hamid O, Bauer TM, Spira AI, et al. Epacadostat plus pembrolizumab in patients with SCCHN: Preliminary phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:6010.
157. Long GV, Dummer R, Hamid O, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083-97.
158. Wang X, Zhang H, Wang Y, et al. DNA sensing via the cGAS/STING pathway activates the immunoproteasome and adaptive T-cell immunity. EMBO J. 2023;42:e110597.
159. Powles T, Valderrama BP, Gupta S, et al.; EV-302 Trial Investigators. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390:875-88.
160. Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6:140.
161. Wong MKK, Sacco JJ, Robert C, et al. Efficacy and safety of RP1 combined with nivolumab in patients with anti–PD-1–failed melanoma from the IGNYTE clinical trial. J Clin Oncol. 2024;42:9517.
162. Dong H, Li M, Yang C, et al. Combination therapy with oncolytic viruses and immune checkpoint inhibitors in head and neck squamous cell carcinomas: an approach of complementary advantages. Cancer Cell Int. 2023;23:2846.
163. Wang Z, Sun P, Li Z, Xiao S. Clinical advances and future directions of oncolytic virotherapy for head and neck cancer. Cancers. 2023;15:5291.
164. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62.
165. Guo C, Lin L, Wang Y, Jing J, Gong Q, Luo K. Nano drug delivery systems for advanced immune checkpoint blockade therapy. Theranostics. 2025;15:5440-80.
166. Zhang Z, Xu X, Du J, et al. Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy. Nat Commun. 2024;15:44963.
167. Qian M, Jiang G, Guo W, Huang R. A biodegradable nanosuspension locally used for inhibiting postoperative recurrence and brain metastasis of breast cancer. Nano Lett. 2024;24:3165-75.
168. Tang B, Zhu J, Wang Y, et al. Targeted xCT-mediated ferroptosis and protumoral polarization of macrophages is effective against HCC and enhances the efficacy of the anti-PD-1/L1 response. Adv Sci. 2023;10:e2203973.
169. Fu S, Li Y, Shen L, et al. Cu2WS4-PEG nanozyme as multifunctional sensitizers for enhancing immuno-radiotherapy by inducing ferroptosis. Small. 2024;20:e2309537.
170. Uppaluri R, Haddad RI, Tao Y, et al.; KEYNOTE-689 Investigators. Neoadjuvant and adjuvant pembrolizumab in locally advanced head and neck cancer. N Engl J Med. 2025;393:37-50.
171. Orland MD, Ullah F, Yilmaz E, Geiger JL. Immunotherapy for head and neck squamous cell carcinoma: present and future approaches and challenges. JCO Oncol Pract. 2024;20:1588-95.
172. De Felice F, Cattaneo CG, Franco P. Radiotherapy and systemic therapies: focus on head and neck cancer. Cancers. 2023;15:4232.
173. Liu C, Li M, Liu X, et al. Evaluating the efficacy and safety of different neoadjuvant immunotherapy combinations in locally advanced HNSCC: a systematic review and meta-analysis. Front Immunol. 2024;15:1467306.
174. Yu X, Li W, Li Z, Wu Q, Sun S. Influence of microbiota on tumor immunotherapy. Int J Biol Sci. 2024;20:2264-94.
175. Elkrief A, Pidgeon R, Maleki Vareki S, Messaoudene M, Castagner B, Routy B. The gut microbiome as a target in cancer immunotherapy: opportunities and challenges for drug development. Nat Rev Drug Discov. 2025;24:685-704.
176. Hadjigeorgiou AG, Harkos C, Mishra AK, et al. Mathematical modeling and association analysis decipher the impact of the gut microbiome on cancer immunotherapy. Cancer Res. 2025;85:3139-55.
177. Xia L, Zhu X, Wang Y, Lu S. The gut microbiota improves the efficacy of immune-checkpoint inhibitor immunotherapy against tumors: from association to cause and effect. Cancer Lett. 2024;598:217123.
178. Zheng Q, Zhong Y, Lian H, et al. Gut microbial signatures associated with clinical remission in inflammatory bowel disease treated with biologics: a comprehensive multi-cohort analysis. United European Gastroenterol J. 2025;13:1263-77.
179. He Y, Mohapatra G, Asokan S, Nobs SP, Elinav E. Microbiome modulation of antigen presentation in tolerance and inflammation. Curr Opin Immunol. 2024;91:102471.
180. Marin D, Li Y, Basar R, et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat Med. 2024;30:772-84.
181. Hosseinalizadeh H, Wang LS, Mirzaei H, Amoozgar Z, Tian L, Yu J. Emerging combined CAR-NK cell therapies in cancer treatment: finding a dancing partner. Mol Ther. 2025;33:2406-25.
182. Lamers-Kok N, Panella D, Georgoudaki AM, et al. Natural killer cells in clinical development as non-engineered, engineered, and combination therapies. J Hematol Oncol. 2022;15:164.
183. Zandberg D, Ferris R, Laux D, et al. 71P A phase II study of ADU-S100 in combination with pembrolizumab in adult patients with PD-L1+ recurrent or metastatic HNSCC: preliminary safety, efficacy and PK/PD results. Ann Oncol. 2020;31:S1446-7.
184. Masarwy R, Breier D, Stotsky-Oterin L, et al. Targeted CRISPR/Cas9 lipid nanoparticles elicits therapeutic genome editing in head and neck cancer. Adv Sci. 2025;12:e2411032.
185. Hellmann MD, Jänne PA, Opyrchal M, et al. Entinostat plus pembrolizumab in patients with metastatic NSCLC previously treated with anti–PD-(L)1 therapy. Clin Cancer Res. 2021;27:1019-28.
186. Li Y, Wang J, Zhou L, et al. DNMT1 inhibition reprograms T cells to NK-like cells with potent antitumor activity. Sci Immunol. 2025;10:eadm8251.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data







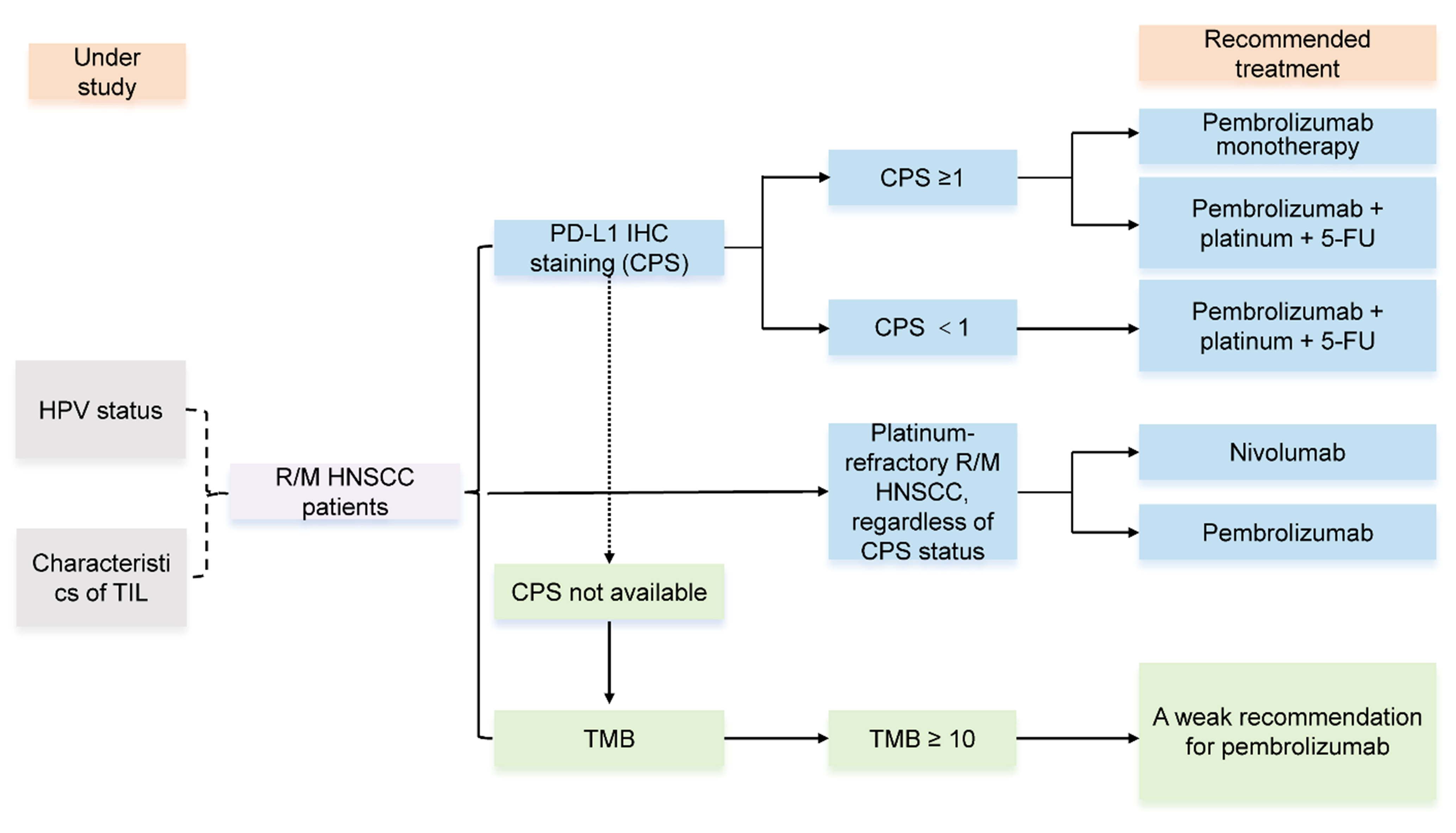
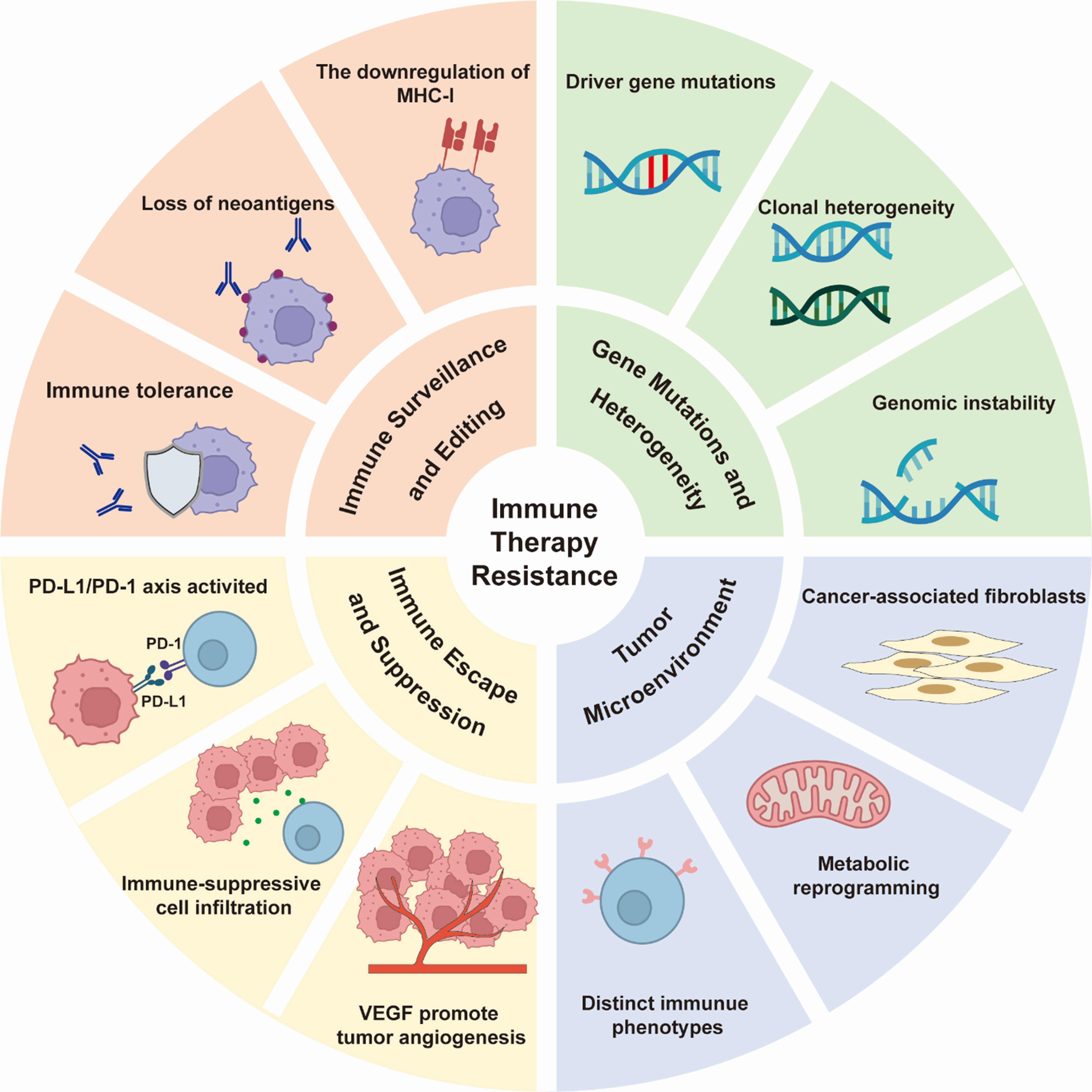
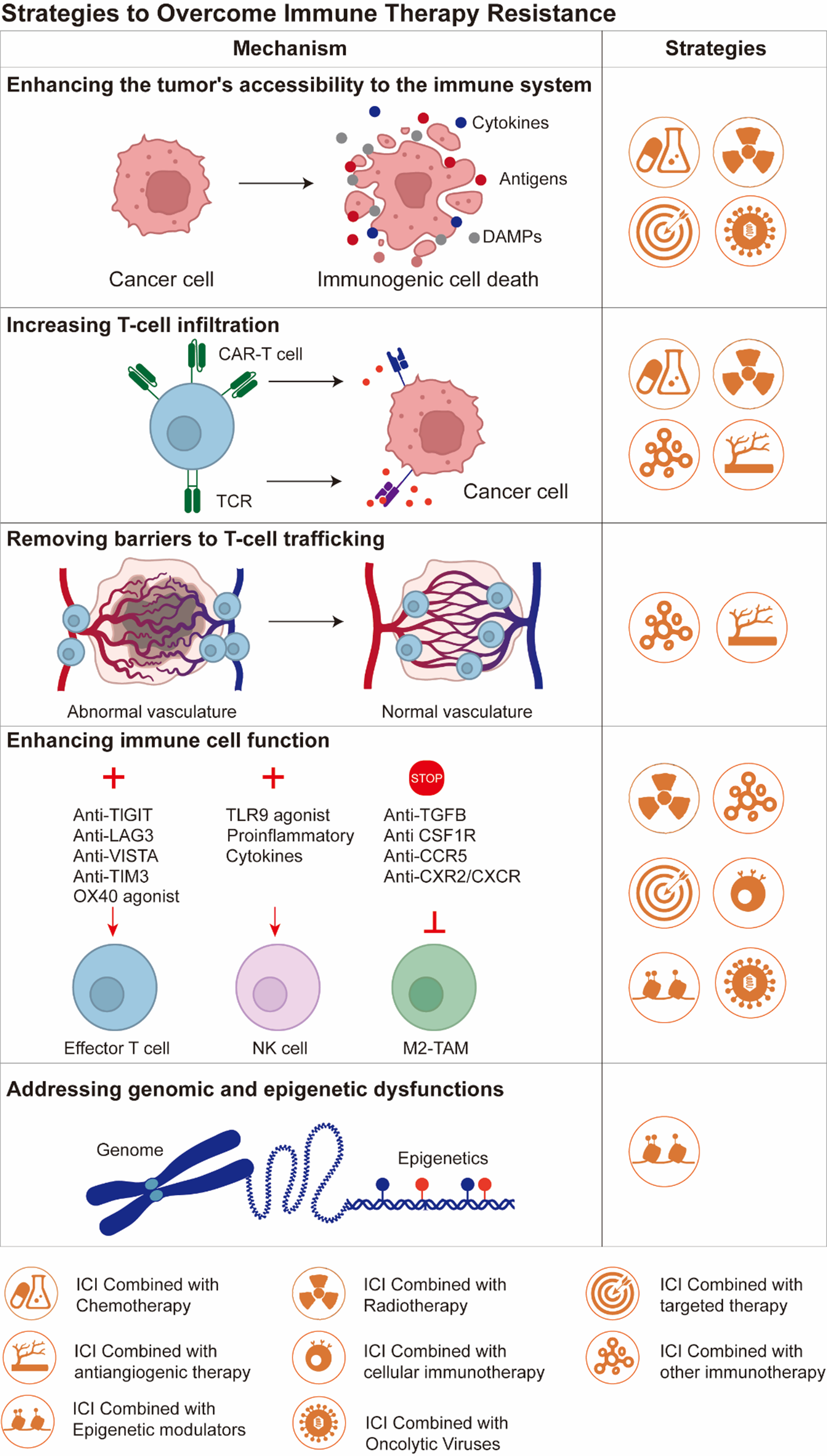












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].