Biodegradable, self-powered E-skin for contactless respiratory and epidermal humidity monitoring
Abstract
Humidity monitoring is vital for respiratory health assessment, yet conventional sensors lack flexibility, back-end power supply, and environmental friendliness, demanding flexible, self-powered, and biodegradable devices. Here, we developed a flexible, fully biodegradable self-powered electronic skin (E-skin) that seamlessly integrates micro-supercapacitors (MSCs) and a humidity sensor. Highly hygroscopic agarose (AG) was selected as both the substrate and gel electrolyte, with Ti3C2Tx MXene nanosheet-based interdigital electrode arrays patterned on its surface via in-situ fabrication. No metal interconnects or polymer binders were introduced during the whole fabrication process. The MSCs based on AG/sweat gel electrolyte exhibited high area capacitance (15.6 mF·cm-2), long-term cycling stability (up to 10,000 cycles), and desired biodegradable properties. Due to the strong interaction between AG and MXene with water molecules by abundant hydrophilic groups such as hydroxyl, the humidity sensor has high sensitivity and a good linear relationship within the range of 11%-97% relative humidity. The integrated E-skin system enables real-time monitoring of human breathing patterns (including the mouth and nose), as well as the humidity levels of non-contact finger touch and skin. This work paves the way for sustainable, self-sufficient wearable electronics in personalized respiratory monitoring.
Keywords
INTRODUCTION
As key indicators of human health (e.g., thermoregulation, emotional states), non-contact humidity monitoring is essential for tracking respiratory patterns and skin moisture[1-5] while avoiding skin irritation and infection risks. Although traditional humidity sensors show promise in healthcare and human-machine interfaces, they still face critical limitations, including (i) Rigidity and poor wearability causing discomfort during prolonged use; (ii) Insufficient energy autonomy and reliance on external power sources; (iii) Inadequate performance (such as slow response, limited sensitivity) hindering real-time physiological monitoring. In addition, toxic compounds and/or non-biodegradable components have exacerbated e-waste pollution. It is estimated that global e-waste will reach 74 million tons by 2030[6]. Consequently, flexible self-powered humidity sensors with high sensitivity, rapid response, and full biodegradability are urgently needed to enable comfortable, eco-friendly health monitoring.
Fundamentally, the operating principle of most humidity sensors can be explained by the Grotthuss chain reaction[7,8], commonly simplified as a proton hopping process: H2O + H3O+ = H3O+ + H2O. Based on this mechanism and measurement strategies, the humidity sensors are categorized into resistive, capacitive, impedance, and voltage types. It is key to develop high-performance humidity sensing materials with high accuracy and good physicochemical stability. To date, nano-carbon materials such as carbon nanotubes and graphene[9-11], transition metal oxides[12], and molybdenum disulfide[13] are widely used in the manufacture of various humidity sensors due to their advantages of high specific surface area, abundant hydrophilic functional groups, and ideal electronic response characteristics. However, due to inherent material limitations, single-component active sensing materials generally cannot simultaneously achieve high humidity sensitivity and fast response/recovery kinetics. To address this trade-off, chemical doping and structural engineering strategies are typically employed to optimize humidity sensing performance. For instance, designing porous architectures in active materials or interdigital electrodes significantly enhances water vapor absorption/desorption kinetics[14,15]. Element doping provides precise control over the electronic properties of active sensing materials[16], such as modulating band structures, carrier mobility, and surface reactivity to tailor humidity-dependent electrical responses. Despite significant progress made, there are limitations in balancing humidity sensitivity and response/recovery speed. Recently, two-dimensional
In this work, we engineered a fully biodegradable, self-powered electronic skin (E-skin) through seamless integration of MXene-based micro-supercapacitors (MSCs) and a humidity sensor. Highly hygroscopic agarose (AG) serves dual roles as a flexible substrate and gel electrolyte. Self-assembled Ti3C2Tx MXene films are directly patterned into interdigital arrays on an AG substrate and treated by acid etching to expand the interlayer spacing. No metal interconnects and the elimination of polymer binders are required between the components. Abundant hydroxyl groups in AG and etched MXene enable high-sensitivity humidity sensing [range: 11%-97% relative humidity (RH)]. Sweat-activated MSCs achieve a capacitance of 15.6 mF·cm-2 with cycling stability up to 10,000 cycles. After charging, it can continuously supply energy to the sensor. Because the materials used in E-skin are all biocompatible and biodegradable, they can be discarded at will after use and be oxidized and decomposed by moisture in the environment or absorbed by microorganisms in the soil. This integrated system monitors breathing rates (mouth/nose) and non-contact finger humidity in real time, demonstrating a sustainable paradigm for wearable diagnostics while resolving flexibility, power supply, and e-waste challenges simultaneously.
EXPERIMENTAL
Synthesis of delaminated Ti3C2Tx MXene nanoflakes
Ti3C2Tx MXene was prepared by selective etching of Al from Ti3AlC2 using in-situ production of hydrofluoric acid as previously reported in the literature. First, 2 g of Ti3AlC2 MAX phase (Mn+1AXn, where
Preparation of AG/sweat electrolyte
According to physiologically relevant standards for simulating human sweat (e.g., European Standard EN 1811:2011), artificial sweat electrolyte was prepared via the following procedure: 0.5 wt.% of NaCl, 0.1 wt.% of KCl, 0.1 wt.% of urea, and 0.1 wt.% of lactic acid were added to DI water, and the pH was adjusted to 6.5 ± 0.05 by the addition of 0.1 M NaOH. The AG/sweat gel was produced by an AG gelation process. Subsequently, 0.1 g AG powder was dissolved in 10 mL artificial sweat and stirred vigorously at 90 °C for
Fabrication of MXene-based MSC and humidity sensor
The glass plate (1.5 × 2 cm2) was ultrasonically cleaned in acetone, hydrochloric acid, and DI water sequentially for 5 min each to remove the dirt on its surface. Following that, 1 mL AG/sweat gel was evenly dropped onto the glass and dried in the air for three days to obtain a flexible substrate. Then, the Ti3C2Tx solution was directly spray-coated on the as-fabricated AG/sweat substrate at a constant temperature of
Materials characterization
The surface morphology of MXene was characterized using field emission scanning electron microscopy (FE-SEM, TESCAN MIRA3 XMU). Transmission electron microscopy (TEM), high-resolution TEM (HRTEM) images, and selected area electron diffractive (SAED) patterns were obtained on an FEI Tecnai F30 at 300 kV. The chemical structures were imaged by X-ray diffraction (XRD, Philips, X’pert pro, Cu Kα, 0.154056 nm), Raman spectroscopy (JY-HR800 micro-Raman, 532 nm wavelength YAG laser), and X-ray photoelectron spectrometry (XPS, PHI-5702, Mg KR X-ray, 1,253.6 eV).
Electrochemical measurements
Electrochemical tests of MXene-based MSC were conducted using an electrochemical workstation (CHI 660E) at room temperature. Cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) measurements were performed at various scan rates and current densities in the same potential window, respectively. Electrochemical impedance spectroscopy (EIS) was performed at the open circuit potential (OCP) with a frequency range from 0.1 Hz to 1 MHz.
Humidity-sensing measurement
The humidity-sensing properties of the MXene humidity sensor were investigated in various humidity conditions obtained by saturated solutions of different salts. The RH values were in saturated salt solution: LiCl (11%), MgCl2 (33%), Mg(NO3)2 (54%), NaCl (75%), KCl (85%), and K2SO4 (97%). The humidity performance tests were performed in a semiconductor characterization system (Keithley 2400).
RESULTS AND DISCUSSION
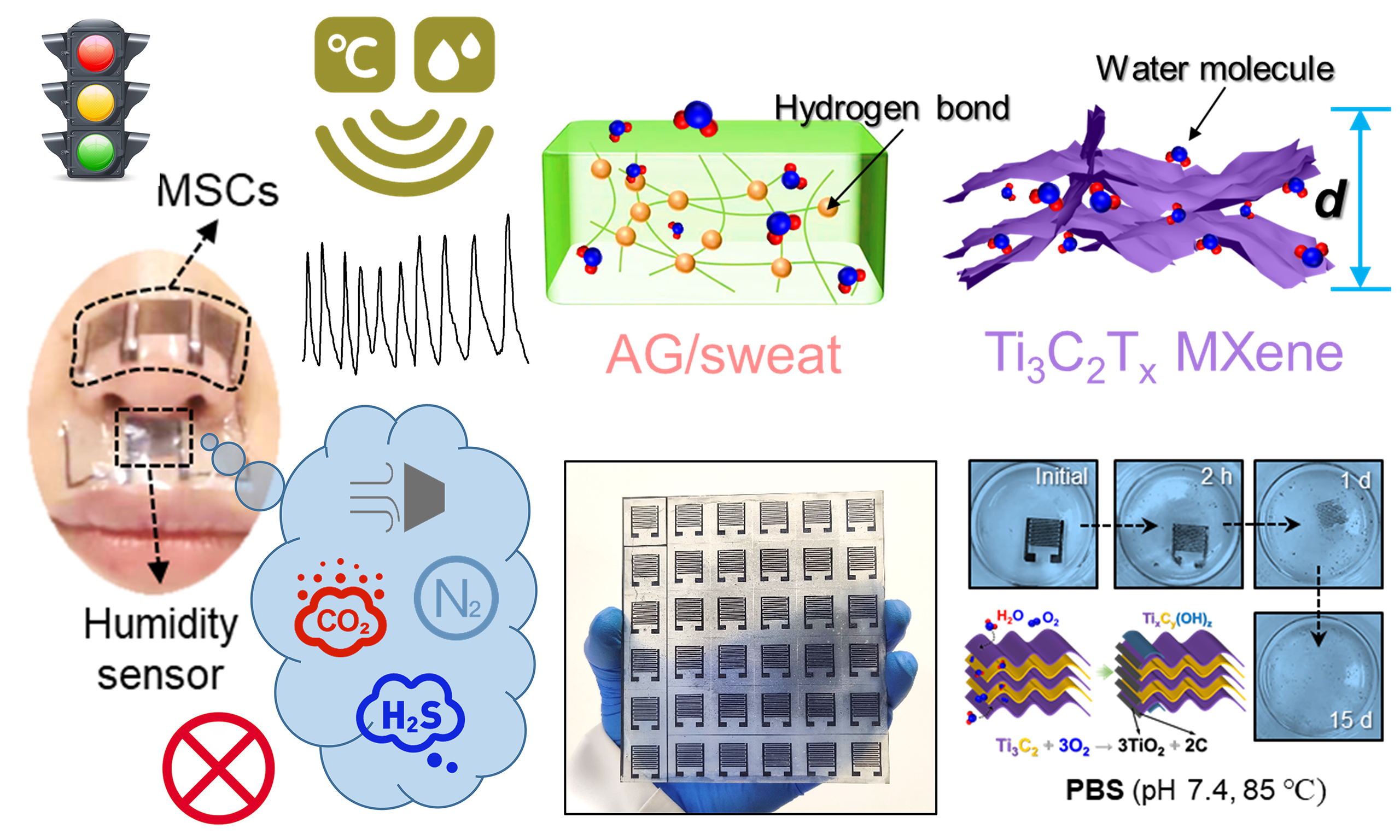
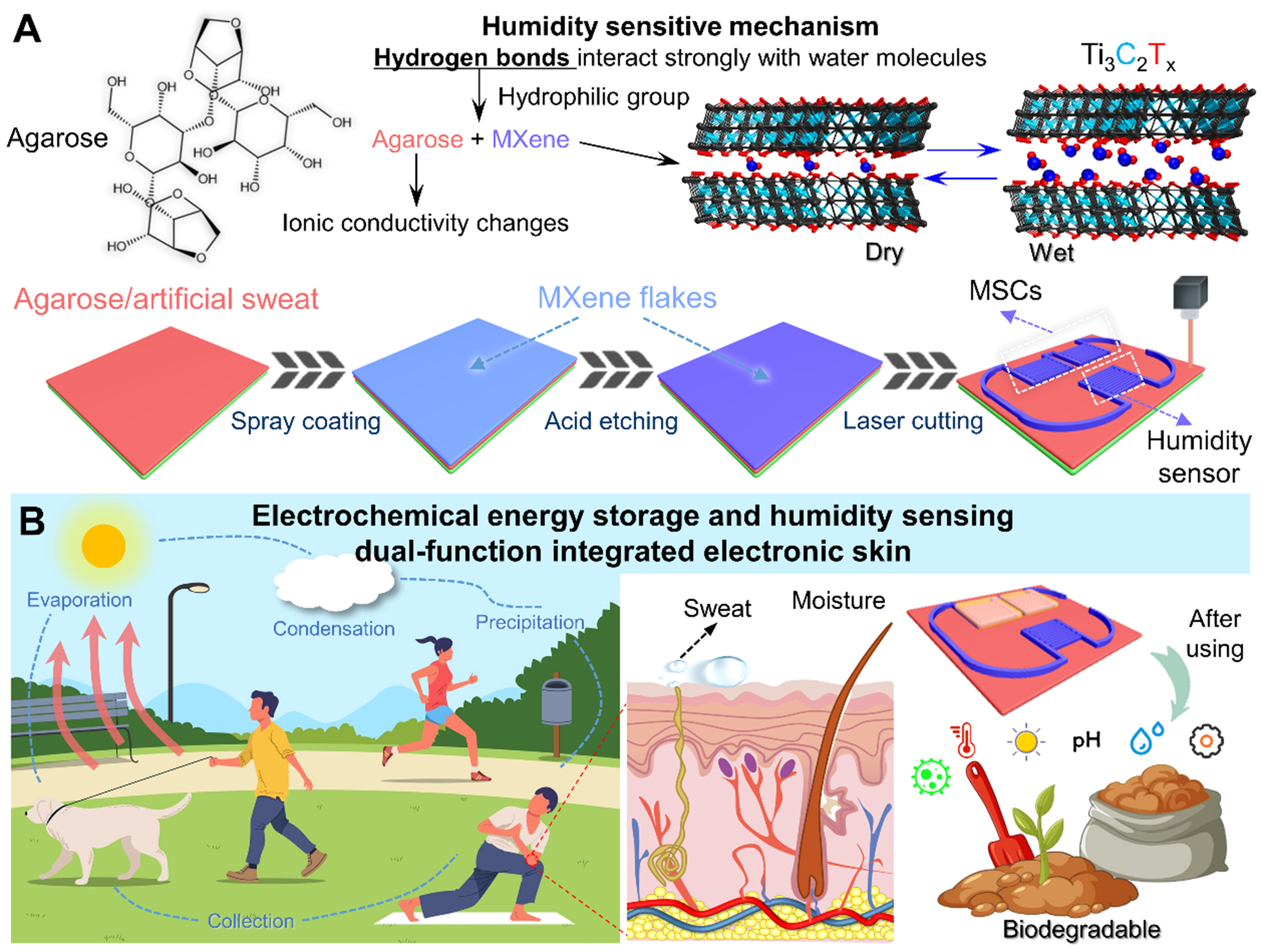
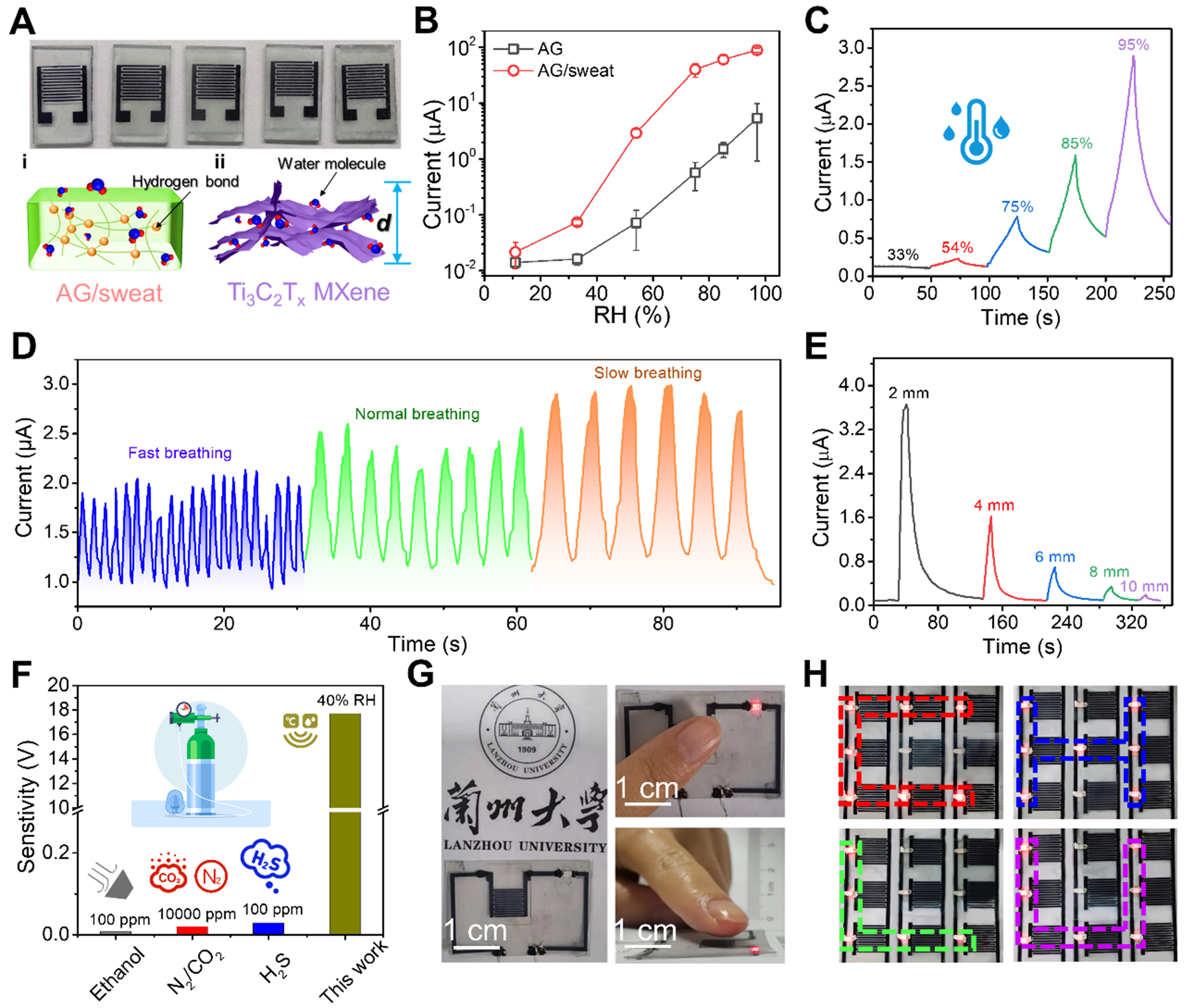
Figure 1A schematically illustrates the fabrication procedure of the MXene-based E-skin. First, the Al atomic layers were selectively etched from the Ti3AlC2 MAX phase and exfoliated to obtain Ti3C2Tx MXene nanoflakes. Uniform MXene films were spray-coated on flexible AG/sweat substrates, where abundant hydroxyl groups (-OH) in AG synergize with MXene’s -OH/-F terminals to create a hydrophilic network for enhanced humidity adsorption [Supplementary Figure 1]. To mitigate oxidation and expand Ti3C2Tx interlayer spacing for improved ion accessibility, MXene films were pretreated with concentrated H2SO4, simultaneously optimizing electrode conductivity and hydration kinetics. Interdigital electrodes were then patterned via direct laser scribing [Supplementary Figure 2], which provides a scalable, mask-free alternative to traditional micromachining that reduces costs. These identical MXene electrodes serve dual purposes: as MSC collectors leveraging acid-enhanced interlayer channels, and as humidity sensors where MXene/AG hydrophilic groups enable real-time vapor detection. The AG/sweat film functions as both an electrolyte and a humidity-sensitive matrix. Figure 1B depicts the E-skin’s operational scenarios in environmental and epidermal humidity monitoring. Relevant studies have shown that people feel most comfortable living in an environment with a RH of 40%-60%[30]. Ambient humidity extremes (< 40% or > 60% RH) correlate with exacerbated respiratory diseases and impaired skin barrier function, highlighting the need for precision monitoring. The environmental water cycles (comprising ambient moisture collection, evaporation, condensation, and precipitation[31]) and epidermal monitoring (such as pre-sweat moisture, perspiration) are illustrated. All materials used are biocompatible and naturally degrade under environmental conditions after use. They can be discarded without a recycling agreement and will not leave any ecologically toxic residues.
Figure 1. (A) Schematic diagram of the preparation of flexible self-powered E-skin based on MXene and its humidity sensing mechanism; (B) Application scenarios of the dual-functional E-skin integrating electrochemical energy storage and humidity sensing: non-contact ambient humidity monitoring and epidermal moisture perception. Post-use disposal requires no recycling, leaving no environmental pollution. Some image elements designed by Freepik (https://www.freepik.com/). E-skin: Electronic skin; MSCs: micro-supercapacitors.
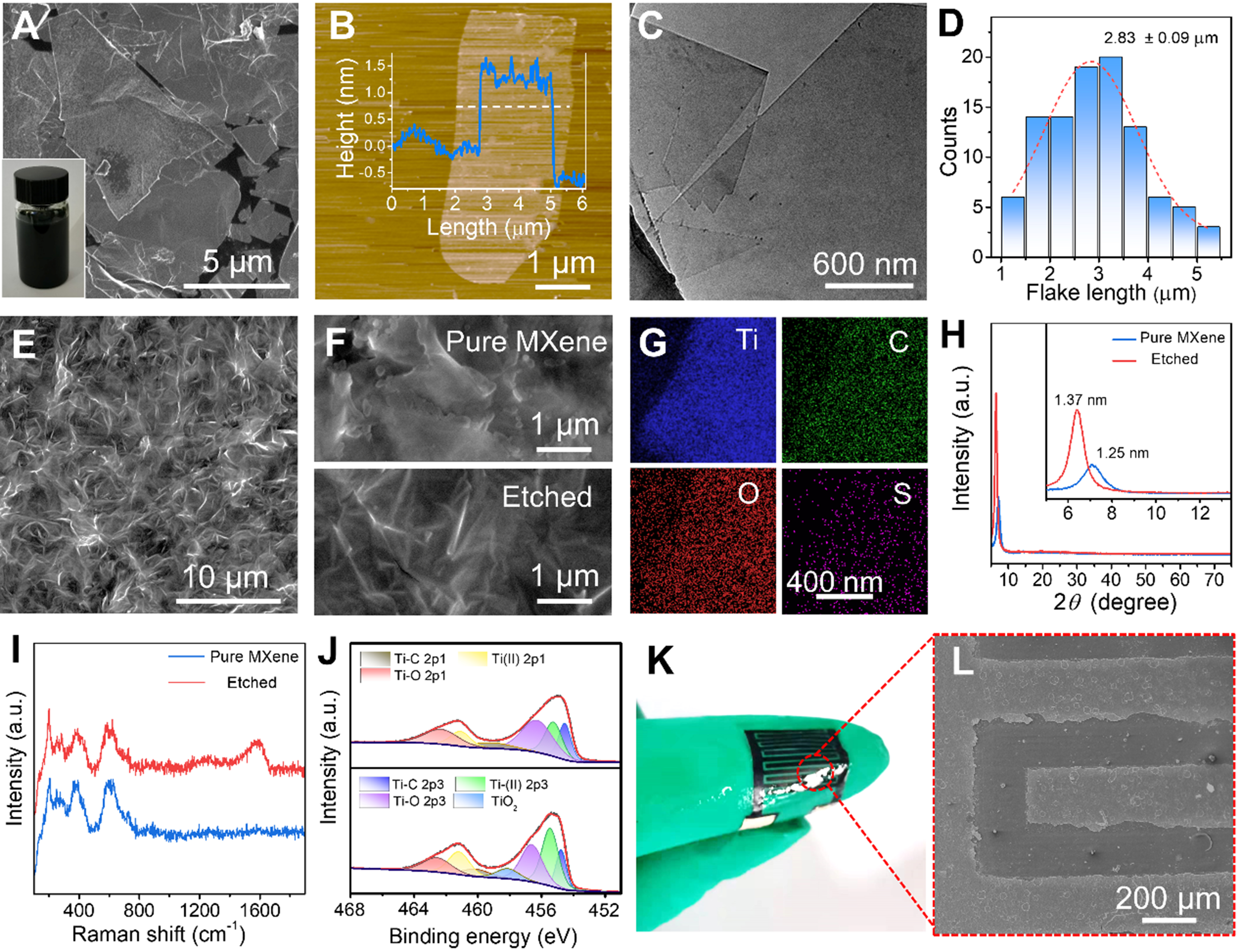
Structural characterization of Ti3C2Tx MXene nanoflakes for integrated MSCs and sensors reveals key features. Scanning electron microscopy (SEM) images [Figure 2A and Supplementary Figure 3A] show that the ultrathin flake morphology of MXene is similar to that of graphene. The inset of Figure 2A shows an aqueous solution of MXene with a concentration of 5 mg·mL-1, which is well dispersed and has no precipitation. As shown in Figure 2B, atomic force microscopy (AFM) confirmed monolayer thickness (about 1.5 nm), while SAED [Supplementary Figure 3B] and TEM results [Figure 2C, Supplementary Figure 3C and D] verified the typical hexagonal crystalline structure. The mean lateral size of MXene nanoflakes statistically measured was 2.83 ± 0.09 μm [Figure 2D]. Thanks to the good hydrophilicity of the AG/sweat substrate, MXene nanoflakes can be uniformly spray-coated on its surface and self-assembled to form a continuous conductive network [Figure 2E]. Crucially, concentrated H2SO4 etching serves dual purposes: improving electrochemical performance and optimizing humidity sensing. In addition, SEM images reveal significant surface restructuring post-etching [Figure 2E]. Although pure MXene films show smooth basal planes [Figure 2F], H2SO4-treated counterparts exhibit increased surface roughness and microstructure, which enhances hydrophilicity and active sites. Despite this roughened morphology [Supplementary Figure 3E and F], the fabrication process ensures excellent device uniformity. Statistical analysis [Supplementary Figure 4A] of the sheet resistance across a large-area spray-coated film (10 × 10 cm2, n = 10 points) showed an average value of 24.2 Ω/□ with a relative standard deviation (RSD) of 18.5%. Furthermore, the performance of a batch of laser-scribed devices (6 × 6 array) exhibited high consistency, confirming the reproducibility of our manufacturing approach [Supplementary Figure 4B]. SEM-energy-dispersive X-ray spectrometry (EDS) elemental mapping [Figure 2G] shows the uniform distribution of Ti, C, and O elements, and the S element is derived from sulfate ions (SO42-). Compared with pure MXene, due to the column-supported effect, the (002) peak of Ti3C2Tx MXene has a small angular shift after protons (H+) and SO42- intercalation [Figure 2H], indicating that its interlayer spacing has increased. The D and G peaks of carbon atom vibrations related to sp2 hybridization in the Raman spectrum of etched Ti3C2Tx MXene become distinct [Figure 2I], which might be due to structural changes, such as alterations in interlayer spacing and surface functional groups. It can be seen from the XRD data [Supplementary Figure 5A] that the core crystal lattice of the AG substrate remains intact after etching. Acid etching treatment also helps to remove TiOx nanoparticles from Ti3C2Tx (verified by XPS results in Figure 2J and Supplementary Figure 5B), thereby eliminating charge transfer obstacles and enhancing conductivity. Surface roughening and microporosity further amplify hydrophilic interactions with -OH/-F terminals. Laser-patterned interdigitated electrodes demonstrate skin-conformable flexibility [Figure 2K] and maintain structural integrity with 400 μm width/gaps [Figure 2L].
Figure 2. (A) Typical SEM image of MXene flakes. Inset is a photograph of MXene ink dispersed in a mixed solution of water; (B) AFM image of Ti3C2Tx flakes. Inset is the corresponding height profile along the crossed lines; (C) TEM image of MXene flakes; (D) Histogram of lateral dimensions of Ti3C2Tx flakes; (E) SEM images of spray-coated Ti3C2Tx MXene film; (F) SEM images of Ti3C2Tx MXene films before and after acid etching; (G) EDS mapping of MXene flakes; (H) XRD patterns, (I) Raman spectra, and (J) XPS Ti 2p spectra of
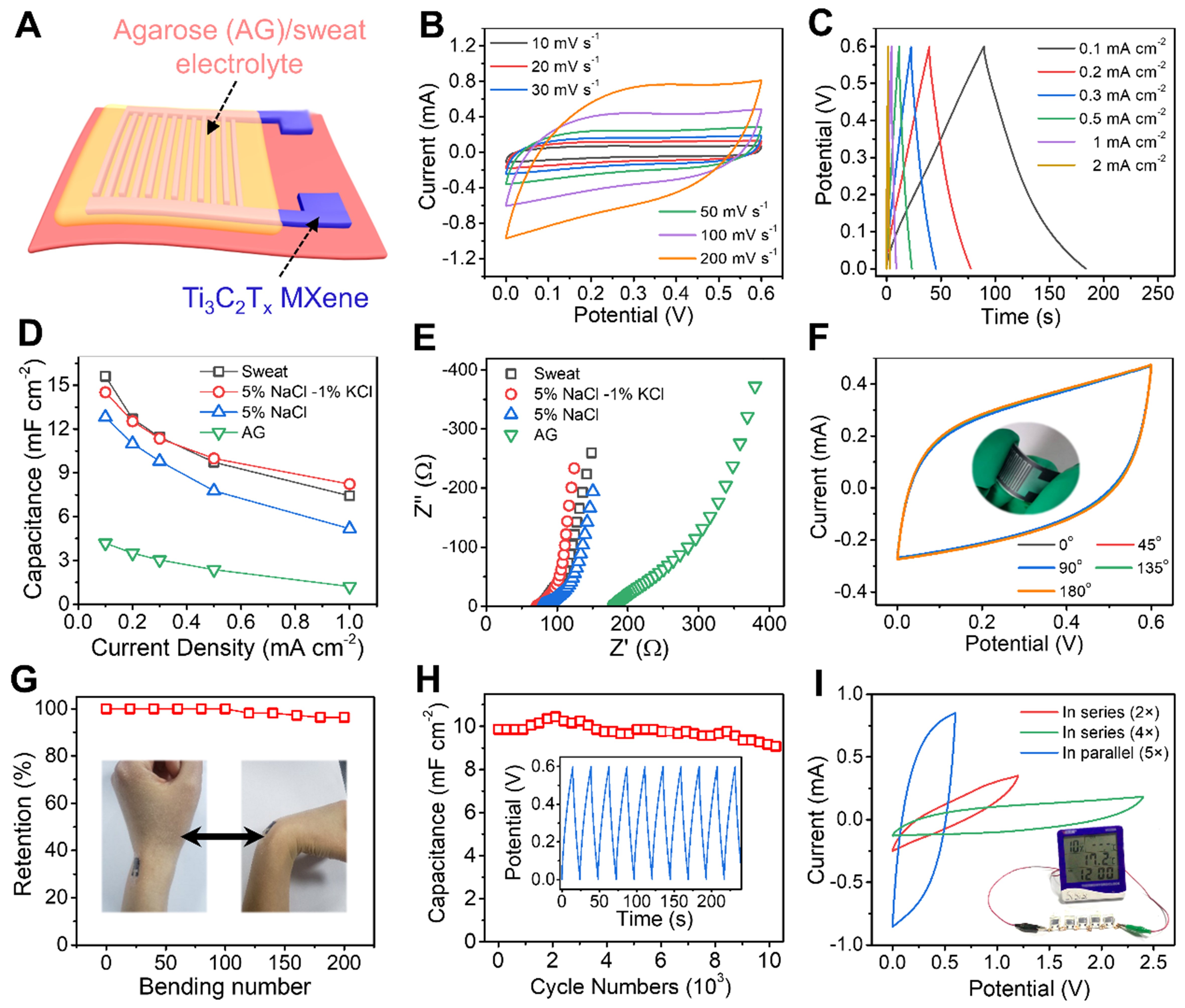
To establish a robust and stable structure for promoting electron conduction and ion diffusion, a planar MXene-based interdigital electrode pattern is designed, and then AG/sweat gel electrolyte is coated on its surface as an MSC device [Figure 3A]. Compared with widely used corrosive acid-base electrolytes, sweat is a natural biological electrolyte produced by the sweat glands of the skin, featuring non-toxicity and environmental friendliness. Figure 3B reveals near-rectangular CV profiles, confirming ideal capacitive behavior. Symmetrical GCD curves [Figure 3C] demonstrate efficient ion transport. The acid-etched MXene-based MSC achieves 15.6 mF·cm-2 at 0.1 mA·cm-2 (vs. 10.3 mF·cm-2 for pure MXene, Figure 3D and Supplementary Figure 6), surpassing most carbon-based counterparts. Remarkably, it retains 7.4 mF·cm-2 (47.4%) at 1 mA·cm-2, highlighting exceptional rate capability. This enhancement directly stems from the acid-etched structural modification. The expansion of interlayer spacing accelerates ion diffusion, while surface micropores maximize the utilization of active sites. Electrochemical analysis with sweat components [Supplementary Figure 7A and B] shows NaCl/KCl-dominated capacitance: AG/NaCl (12.8 mF·cm-2), AG/NaCl-KCl (14.5 mF·cm-2), confirming Na+/K+/Cl- as primary charge carriers. The concentrations of these primary ions (0.5 wt.% NaCl, 0.1 wt.% KCl) were selected based on well-established, physiologically relevant standards for simulating human sweat, ensuring the electrolyte accurately replicates the ionic environment encountered in real-world epidermal applications. Consequently, the device’s performance is expected to be robust against the natural fluctuation of minor organic components in sweat, as its functionality is primarily governed by these stable salt concentrations. At high current densities, NaCl-KCl as electrolyte salts outperforms sweat [Figure 3D], indicating urea/lactate inhibition of ion mobility. This slight inhibitory effect represents a deliberate and acceptable trade-off, as the inclusion of these organic components
Figure 3. (A) Schematic diagram of MSC device based on Ti3C2Tx MXene interdigital electrode and AG/sweat electrolyte; (B) Cyclic voltammograms at different scan rates; (C) GCD curves and (D) the obtained capacitance at different current densities; (E) Comparison of EIS spectra of MSC devices with and without electrolytes and different types of electrolyte salts; (F) CV curves of the device bent at different angles; (G) Capacitance retention during the bending process of the device attached to the wrist area; (H) The cycling stability of the device at a certain current density (the inset shows the typical GCD curves); (I) CV curves of multiple MSCs connected in parallel and series. MSC: Micro-supercapacitor; AG: agarose; GCD: galvanostatic charge-discharge; EIS: electrochemical impedance spectroscopy; CV: cyclic voltammetry.
In addition to electrochemical energy storage, the MSC device can also be used as a humidity sensor. Leveraging synergistic humidity sensitivity from AG’s hydrophilic hydroxyl groups[32,33] and MXene’s tunable interlayer spacing[34,35], the integrated sensor achieves high-performance non-contact humidity monitoring. Several fabricated sensors demonstrate structural uniformity [Figure 4A]. The sensing mechanism operates via Grotthuss proton hopping (H3O+ migration) and ionic conduction (Na+/K+/Cl- migration), including: (i) hydrogen bonding with H2O in AG/sweat electrolyte modulates ionic conductivity; (ii) humidity-dependent reversible expansion/contraction between layers facilitates water adsorption/desorption. Typically, under low RH conditions, the proton-hopping conduction mainly depends on the absorption of small amounts of water molecules. As RH increases, proton conduction becomes easier and H3O+ and OH- ions are readily produced, creating ionic conduction. Meanwhile, the ions in sweat (Na+, K+, Cl-, etc.) are ionized, which contributes to the conductivity process. Under higher RH conditions, the adsorbed water molecules gradually form a continuous water layer, accelerating the flow of water molecules. To confirm the existence of the sensing mechanism mentioned above, we tested the current response with and without sweat electrolyte salts in the AG of the sensor as a function of humidity. The specific ionic concentration is critical, as it directly governs the baseline conductivity and the magnitude of the current change. The chosen physiologically-relevant concentrations provide an optimal ionic strength, creating a strong, measurable humidity-dependent signal without causing short-circuiting or saturation effects that might occur with excessively high salt concentrations. The AG/sweat electrolyte
Figure 4. (A) Photos of humidity sensors based on interdigital electrodes and the humidity sensing mechanisms of their various parts; (B) The influence of sweat electrolytes on the sensor response current when RH changes; (C) Response and recovery characteristics of the sensor at different RH levels, with RH switching intervals of 25 s; (D) Nose-breathing response curve with different breathing frequencies; (E) Contactless sensing: effect of finger distance from sensor height on response current; (F) Selectivity evaluation of the humidity sensor against common breath volatiles. Some image elements designed by Freepik (https://www.freepik.com/); (G) Photographs of the contactless switch sensing system; (H) Programmable 3 × 3 humidity sensors and LED arrays to be used to display the shapes of the letters “C”, “H”, “L”, and “U”. RH: Relative humidity; LED: light-emitting diode; AG: agarose.
To visualize non-contact operation, the humidity sensor was integrated into a 5 V direct current (DC) circuit controlling a red light-emitting diode (LED). Approaching fingers increased circuit current, illuminating the LED; withdrawal decreased current, extinguishing it [Figure 4G]. Additionally, a 3 × 3 sensor array [Supplementary Figure 15A] was fabricated to assess spatial resolution. Using 3 mm-thick polyvinyl chloride (PVC) letter masks (“C”, “H”, “L”, and “U”), moistened palms created heterogeneous humidity fields. Leveraging the sensors’ slow-recovery kinetics, LED illumination persisted for 20 s post-hand removal, enabling clear letter identification through differential LED activation states [Figure 4H and Supplementary Figure 15B]. Supplementary Table 1 compared the comprehensive performance of our multifunctional E-skin with that of several recently reported humidity sensors, showing that the modest individual performance is a trade-off for achieving full integration, biodegradability, and self-powering capability in one platform. While the response and recovery times are on the order of seconds, which is longer than that of some nanostructured sensors on solid substrates, this performance is governed by the water molecule kinetics within the biodegradable AG hydrogel matrix. This temporal resolution is fully compatible with the intended real-time applications, such as monitoring human respiration (typically 0.1-0.3 Hz) and non-contact switching. The pursuit of complete biodegradability and self-powering capability necessitates a balanced compromise in ultra-fast response.
It is worth noting that the performance of humidity-sensitive, self-powered devices can be influenced by ambient temperature, primarily due to its effect on ionic conductivity in the gel electrolyte and charge carrier mobility. While the present study characterized the sensing performance at a stable room temperature (~25 °C) to establish a baseline, future implementations for real-world applications would require strategies to decouple this temperature-humidity cross-sensitivity. Our controlled experiments at 25, 35, and 45 °C [Supplementary Figure 16] confirmed that elevated temperature enhances both capacitance and humidity response due to improved ion mobility, underscoring the necessity of the proposed compensation strategy. A feasible approach, without altering the current device architecture, would be to integrate a separate, dedicated temperature sensor on the same E-skin platform. In addition, MXene-based humidity sensors can be specifically designed or encapsulated to be sensitive only to thermal changes while isolated from humidity variations (e.g., with a hydrophobic layer). The real-time temperature data acquired could then be used to calibrate and compensate the humidity sensor’s output signal via a predefined algorithm, enabling accurate measurements in fluctuating thermal environments.
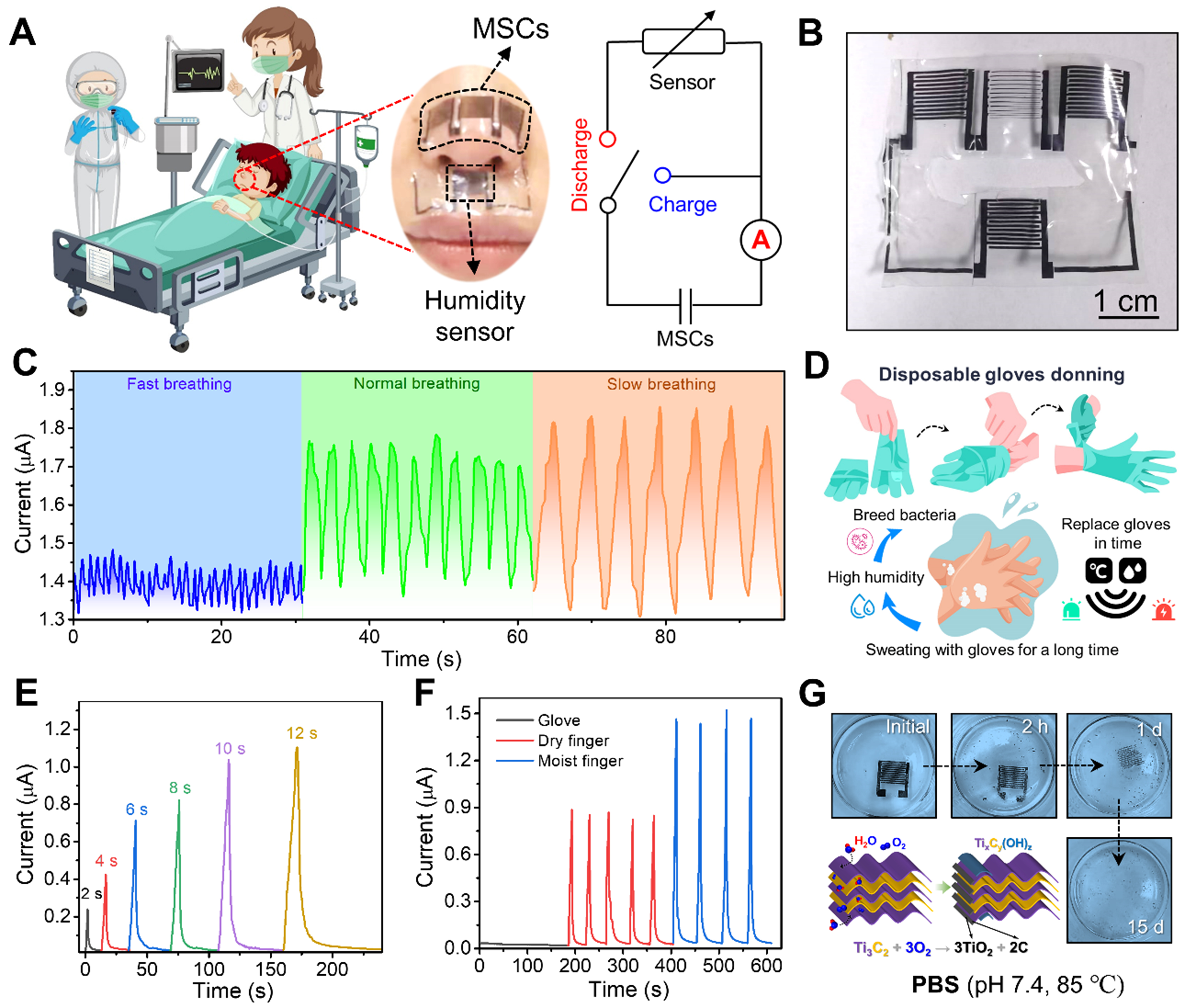
The self-powered E-skin integrates MXene-based MSCs and humidity sensors into a unified sensing platform. Clinically, this integrated E-skin [Figure 5A] can be deployed near the nostrils for breathing analysis during medical procedures. Hollow-structured device [Figure 5B] ensures a secure nasal fit while maintaining skin breathability. Sub-nasally mounted sensors record pathological breathing patterns (slow:
Figure 5. (A) Self-powered application demonstration, deployment location, and operating principle of E-skin for health monitoring. Some image elements designed by Freepik (https://www.freepik.com/); (B) A photo of seamlessly integrated E-skin. The hollow design in the middle facilitates conformal fit near the nose; (C) Nose-breathing humidity response curve at different breathing frequencies; (D) Humidity sensing applications related to the use of disposable gloves; (E) Relationship between the time that an ungloved finger stays near the sensor and the current; (F) Changes in the relative currents of the sensors near the dry and wet fingers with or without gloves; (G) Photographs of the time-sequential dissolution of MXene-based interdigital electrodes immersed in PBS (0.1 M, pH 7.4) at 85 °C. Schematic of the corresponding degradation mechanism. E-skin: Electronic skin; PBS: phosphate-buffered saline; MSCs: micro-supercapacitors.
CONCLUSIONS
This work establishes a paradigm for sustainable contactless respiratory and epidermal humidity monitoring through the seamless integration of energy autonomy and sensing in a fully biodegradable E-skin platform. The dual-functional MXene interdigital electrode architecture simultaneously addresses core challenges of incompatibility between energy supply and humidity sensing. Energy-storage enhancement (boost to 15.6 mF·cm-2) stems from expanded interlayer spacing and TiOx removal, facilitating rapid ion diffusion in MSCs. Humidity sensitivity optimization leverages the supramolecular interaction between hydrophilic functional groups and water molecules to achieve medical-grade linear response (11%-97% RH), with a sensitivity as high as 3,000. Critically, the sweat electrolyte system transcends conventional toxic electrolytes by exploiting physiological NaCl/KCl ion transport while mitigating organic metabolite interference. This bio-integration enables precision respiration analysis and real-time glove humidity tracking. The E-skin is disposable and can be programmed to degrade after use, reducing environmental impact. It is important to note that the acid pretreatment and the device’s biodegradable, short-lifecycle nature are strategic approaches to mitigate the impact of MXene oxidation, a fundamental challenge for long-term use. For future applications requiring extended longevity, strategies such as deposition of ultra-thin protective layers or integration with more stable polymers could be explored[37,38]. Future work will explore epidermal enzyme-triggered degradation at physiological temperatures for implantable diagnostics. Scaling production via roll-to-roll laser scribing could enable low-cost population-level deployment in respiratory disease management.
DECLARATIONS
Acknowledgments
Some of the icons used in the manuscript are sourced from Freepik (https://www.freepik.com/).
Authors’ contributions
Prepared MXene and agarose films, performed most measurements, simulations, and characterization, processed the raw data, and wrote the draft: Zhang, X.; Yue, Q.; Sheng, H.; Lan, W.
Participated in the partial experimental measurements: Yue, Q.; Yuan, J.; Ma, L.; Zhang, H.
Participated in the planning of the experimental analysis: Shao, M.; Su, Q.
Participated in the design of the experimental scheme and the revision of the manuscript: Bi, H.; Hu, J.
Designed the main experimental scheme, guided the experimental implementation, and revised the manuscript: Sheng, H.; Lan, W.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (62374077, 62404088), the Key Research and Development Projects in Gansu Province (25YFFA030), the Joint Research Fund of Gansu Province (24JRRA818), the Key Project of Natural Science Foundation of Gansu Province (24JRRA395), Natural Science Foundation of Gansu Province (25JRRA659), Natural Science Foundation of Shandong Province (ZR2024QB198), Doctoral Fund of University of Heze (XY23BS37), the Open Research Fund Program of Tsinghua University Flexible Electronic Technology Laboratory, and the Supporting Fund for Young Researchers from Lanzhou University.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study involved only non-invasive data collection through the non-invasive placement of the device on the skin and did not involve any invasive procedures or pose risks to human health. In accordance with Article 32 of the “Measures for the Ethical Review of Life Science and Medical Research Involving Human Subjects (Trial)”, this study qualifies for exemption from ethical review. All participants provided informed consent before their involvement in the experiment.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Zhang, Q.; Ren, Z.; Jia, P.; et al. An ultra-miniaturized fiber humidity sensor based on near-parallel ion pathways induced efficient water-electricity conversion. Adv. Mater. 2025, 37, e2411558.
2. Zhang, Z.; Li, J.; Chen, H.; et al. Scalable fabrication of uniform fast-response humidity field sensing array for respiration recognition and contactless human-machine interaction. Adv. Funct. Mater. 2025, 35, 2502583.
3. Zhang, M.; Duan, Z.; Yuan, Z.; Jiang, Y.; Tai, H. Observing mixed chemical reactions at the positive electrode in the high-performance self-powered electrochemical humidity sensor. ACS. Nano. 2024, 18, 34158-70.
4. Ma, J.; Sun, H.; Chu, Z.; et al. Self-powered and self-calibrated sensing system for real-time environmental monitoring. Sci. Adv. 2025, 11, eadw3745.
5. Zhang, H.; Su, K.; Yin, S.; Yang, Z. Recent progress of flexible wearable respiratory sensing and monitoring system. J. Funct. Mater. Devices. 2021, 27, 281-91. https://www.jfmd.net.cn/en/article/id/5dfec71e-4922-4b42-b648-105dd2bfd4f3. (accessed 29 Oct 2025).
6. Vishwakarma, A.; Kanaujia, K.; Hait, S. Chapter 2 - Global scenario of E-waste generation: trends and future predictions. In Global E-waste management strategies and future implications, Arya, S.; Kumar, S., Eds.; Elsevier: 2023; pp. 13-30.
7. Lu, Y.; Yang, G.; Shen, Y.; Yang, H.; Xu, K. Multifunctional flexible humidity sensor systems towards noncontact wearable electronics. Nanomicro. Lett. 2022, 14, 150.
8. Trung, V. D.; Zhao, W.; Le, P.; et al. Iontronic dual pressure-humidity sensor based on poly(vinyl alcohol)/phosphoric acid/Ni-Al layered double hydroxide hydrogel@melamine sponge for advanced wearable electronics. Mater. Res. Bull. 2025, 186, 113357.
9. Li, S.; Zhang, Y.; Liang, X.; et al. Humidity-sensitive chemoelectric flexible sensors based on metal-air redox reaction for health management. Nat. Commun. 2022, 13, 5416.
10. Liang, A.; Chen, X. A non-contact porous composite fiber paper-based humidity sensor for wearable breathing and skin humidity monitoring. J. Mater. Chem. A. 2024, 12, 29081-91.
11. Lu, S.; Fang, Z.; Lei, M.; et al. Self-healable graphene-cellulose nanofibril composite with strain/humidity responsivity for wearable respiratory monitoring. Carbon 2025, 242, 120473.
12. Paolucci, V.; De Santis, J.; Ricci, V.; Lozzi, L.; Giorgi, G.; Cantalini, C. Bidimensional engineered amorphous a-SnO2 interfaces: synthesis and gas sensing response to H2S and humidity. ACS. Sens. 2022, 7, 2058-68.
13. Mei, L.; Gao, Z.; Yang, R.; et al. Phase-switchable preparation of solution-processable WS2 mono- or bilayers. Nat. Synth. 2025, 4, 303-13.
14. Li, T.; Zhao, T.; Zhang, H.; et al. A skin-conformal and breathable humidity sensor for emotional mode recognition and non-contact human-machine interface. npj. Flex. Electron. 2024, 8, 290.
15. Wu, J.; Zhang, S.; Gu, Q.; Zhang, Q. Recent progress in covalent organic frameworks for flexible electronic devices. FlexMat 2024, 1, 160-72.
16. Kan, Z.; Hu, W.; Hu, C.; et al. Time-frequency domain NO2-humidity sensor with full-range tolerance based on Pt single-atom sensitized Nb2CTx nanosheets. Adv. Mater. 2025, 37, e2506463.
17. Zhou, Z.; Song, Q.; Huang, B.; Feng, S.; Lu, C. Facile fabrication of densely packed Ti3C2 MXene/nanocellulose composite films for enhancing electromagnetic interference shielding and electro-/photothermal performance. ACS. Nano. 2021, 15, 12405-17.
18. Chen, B.; Lu, Z.; Feng, S.; Zhou, Z.; Lu, C. Redox-active nitroxide radicals grafted onto MXene: boosting energy storage via improved charge transfer and surface capacitance. ACS. Energy. Lett. 2023, 8, 1096-106.
19. Huang, M.; Lu, J.; Ji, J.; et al. Non-contact humidity monitoring: boosting the performance of all-printed humidity sensor using PDDA-modified Ti3C2Tx nanoribbons. Chem. Eng. J. 2024, 485, 149633.
20. Zhang, H.; Xu, X.; Huang, M.; et al. Interlayer cross-linked MXene enables ultra-stable printed paper-based flexible sensor for real-time humidity monitoring. Chem. Eng. J. 2024, 495, 153343.
21. Liu, T.; Qu, D.; Guo, L.; et al. MXene/TPU composite film for humidity sensing and human respiration monitoring. Adv. Sens. Res. 2024, 3, 2300014.
22. Lu, Y.; Wang, M.; Wang, D.; et al. Flexible impedance sensor based on Ti3C2Tx MXene and graphitic carbon nitride nanohybrid for humidity-sensing application with ultrahigh response. Rare. Met. 2023, 42, 2204-13.
23. Huang, B.; Wu, S.; Liu, J.; Liu, J.; Peng, B.; Zhou, Z. Aerosol jet printing of polyelectrolyte-modified MXene ink for a multifunctional humidity and temperature flexible sensor. Chem. Eng. J. 2025, 519, 165403.
24. Ni, Y.; Zang, X.; Yang, Y.; et al. Environmental stability stretchable organic hydrogel humidity sensor for respiratory monitoring with ultrahigh sensitivity. Adv. Funct. Mater. 2024, 34, 2402853.
25. Zhang, C. J.; Pinilla, S.; Mcevoy, N.; et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes). Chem. Mater. 2017, 29, 4848-56.
26. Soomro, R. A.; Zhang, P.; Fan, B.; Wei, Y.; Xu, B. Progression in the oxidation stability of MXenes. Nanomicro. Lett. 2023, 15, 108.
27. Sheng, H.; Ma, Y.; Zhang, H.; et al. Integration of supercapacitors with sensors and energy-harvesting devices: a review. Adv. Mater. Technol. 2024, 9, 2301796.
28. Wang, Y.; Zhao, Y.; Yu, L.; et al. Deformation-tolerant, wireless-charging microbatteries for seamlessly integrated omnidirectional stretchable electronics. Sci. Adv. 2025, 11, eads6892.
29. Sheng, H.; Jiang, L.; Wang, Q.; et al. A soft implantable energy supply system that integrates wireless charging and biodegradable Zn-ion hybrid supercapacitors. Sci. Adv. 2023, 9, eadh8083.
30. Arundel, A. V.; Sterling, E. M.; Biggin, J. H.; Sterling, T. D. Indirect health effects of relative humidity in indoor environments. Environ. Health. Perspect. 1986, 65, 351-61.
31. Worden, J.; Noone, D.; Bowman, K.; Tropospheric Emission Spectrometer Science Team and Data contributors. Importance of rain evaporation and continental convection in the tropical water cycle. Nature 2007, 445, 528-32.
32. Lin, S.; Ma, S.; Chen, K.; et al. A humidity-driven film with fast response and continuous rolling locomotion. Chem. Eng. J. 2024, 495, 153294.
33. Quan, X.; Zhu, K.; Liu, Y.; Yan, B. Bionic luminescent sensors based on covalent organic frameworks: auditory, gustatory, and olfactory information monitoring for multimode perception. ACS. Nano. 2025, 19, 3852-64.
34. Zhang, C.; Ma, Y.; Zhang, X.; et al. Two-dimensional transition metal carbides and nitrides (MXenes): synthesis, properties, and electrochemical energy storage applications. Energy. Environ. Mater. 2020, 3, 29-55.
35. Chen, M.; Fan, Q.; Chen, K.; Majkova, E.; Huang, Q.; Liang, K. MXene materials: pioneering sustainable energy storage solutions. Carbon. Neutral. 2024, 3, 493-500.
36. Tian, Y.; Hou, P.; Zhang, H.; et al. Theoretical insights on potential-dependent oxidation behaviors and antioxidant strategies of MXenes. Nat. Commun. 2024, 15, 10099.
37. Chen, B.; Lu, Z.; Chen, X.; Zhou, Z.; Lu, C. Two-electron conversion nitroxide radicals-based electrode synergistically enhancing charge storage in water-in-salt electrolyte. Chem. Eng. J. 2024, 490, 151768.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].