Flexible optical waveguides for health: a review of the current status and technological perspectives
Abstract
In response to the increasing demand for the prevention and control of chronic noncommunicable diseases, people are paying growing attention to the application of flexible optical waveguides in health assistance, and the specific functions of flexible optical waveguides are gradually enriched in the process. This review systematically explains the research progress of flexible optical waveguides in human health assistance. An analysis of the sensing principles used in flexible optical waveguides for signal sensing is provided. The specific applications of flexible optical waveguides in human health assistance are categorized into three main areas: invasive biomedical diagnosis and therapy, contact physiological information monitoring, and interactive soft robots. From the perspective of materials science, a comprehensive analysis is conducted on commonly used materials and their properties for flexible optical waveguides in human health assistance. Furthermore, the sensing principles and specific applications of flexible optical waveguides are provided, aiming to provide theoretical support and technological innovation direction for the construction of a new generation of intelligent health monitoring systems. The unique advantages of flexible optical waveguides in sensing, especially in human physiological signal sensing, are demonstrated through detailed theoretical analyses. Their specific applications in human health assistance are summarized under each category. Finally, this review proposes evolution paths for flexible optical waveguides by addressing current bottlenecks through material innovation (e.g., hybrids, metasurfaces), functional enhancement (e.g., self-powered sensing), and system integration (e.g., miniaturization, Internet of Things platforms).
Keywords
INTRODUCTION
In recent years, the global attention paid to human health has significantly increased, and health needs have become multidimensional and refined. The Fourteenth General Programme of Work (GPW14) 2025-2028, published by the World Health Organization (WHO), states that chronic noncommunicable diseases have become the leading threat to global health, causing approximately 41 million deaths annually, accounting for 74% of all global deaths[1]. Among these diseases, the incidence of cardiovascular diseases, diabetes, and cancer continues to rise, with cardiovascular diseases alone causing 17.9 million deaths annually[2]. The management of these diseases is highly dependent on long-term, accurate monitoring of physiological parameters, such as real-time tracking of blood glucose fluctuations in diabetic patients and dynamic monitoring of blood pressure and hemodynamic changes in patients with cardiovascular disease[3]. In terms of clinical treatment, taking cancer as an example, common pharmacological treatments and electrical stimulation therapies have had little effect, and cancer remains the second leading cause of death worldwide. Clinical treatments would be much more efficient if they could be directed at a more fundamental cellular level by causing or inhibiting neuronal activity in brain regions or even directly targeting diseased cells without damaging healthy cells[4]. In the face of this situation, there is a need for health assistance equipment with high sensitivity, low cost, portability, real-time monitoring ability, and efficient diagnosis and treatment characteristics to build a more resilient health insurance system.
As an important part of health assistance, health sensing, which involves monitoring physiological signals from the human body for health condition monitoring and disease prevention, has gained considerable momentum. Augustus Desiré Waller first captured human electrocardiographic signals (ECGs) via a capillary galvanometer that occupied an entire room in 1887, and by 1903, Willem Einthoven, who won the 1924 Nobel Prize, had improved the string galvanometer, enabling the clinical use of the ECGs, which led to the beginning of biosignal monitoring[5,6]. The devices of that time were groundbreaking, but they were bulky and had low sensitivity, making them less practical in reality. Since then, advancements in sensor technology have witnessed persistent refinements and evolutions: transitioning from bulky, inherently rigid sensors posing physical risks to the human body to highly integrated and miniaturized variants, and ultimately progressing into the contemporary epoch of flexible sensors[7]. To date, different kinds of flexible sensors have been used for health monitoring; for example, flexible electrochemical sensors based on detecting target analytes in tears[8-10], saliva[11,12], sweat[13,14], and so on for physiological signal monitoring. These sensors are often characterized by high performance, miniaturization, and low cost; however, they are susceptible to electromagnetic interference and highly dependent on the electrolyte and the environmental conditions of the electrolyte, such as the pH and temperature; thus, their long-term stability and biocompatibility must be improved[15]. Another commonly used flexible sensor is the strain sensor, which can be divided into four types, namely, resistive[16-18], capacitive[19,20], piezoelectric[21], and friction electric[22], which realize sensing through the detection of deformation or pressure-induced electrical signals and are commonly used to monitor physiological signals, such as joint and muscle movements, pulse rates, heart rates (HRs), and respiratory rates[23]. Similar to electrochemical sensors, these sensors are also susceptible to electromagnetic interference and have problems such as low sensitivity, high power usage, and susceptibility to environmental interference[24,25]. For the mainstream electrical sensors, including the two types mentioned above, invasive electrical sensors inevitably induce inflammatory reactions due to the implantation of foreign bodies such as electrodes, whereas noninvasive electrical sensors also have safety concerns due to electrical hazards, the inability to resist electromagnetic interference, and poor biocompatibility[26,27]. To overcome these challenges, sensors composed of flexible optical waveguide systems, which operate on the principle of optical modulation rather than electrical modulation, have emerged as safe and reliable alternatives.
Flexible optical waveguides are well established in the sensing field for measuring various physical, chemical, and biological quantities such as displacement[28], velocity[29], temperature[30], weight[31], pressure[32], strain[33], pH[34], humidity[35], and so on. In terms of performance, flexible optical waveguides can achieve ≥ 30 V/m immunity to electromagnetic interference, exceeding the immunity of electrochemical sensors; and even after prolonged physiological monitoring, flexible optical waveguides are generally offset within < 0.5%, and their optical modulation principle eliminates electrochemical drift, a key limitation of enzyme-based electrochemical sensors that require frequent recalibration[26]. In addition, the waveguides enable multiparameter sensing with a single device, reducing system complexity compared to hybrid electrical arrays[36]. Furthermore, due to their high biocompatibility, high sensitivity, and deep penetration range, flexible optical waveguides possess unique advantages in human health monitoring and sensing[36-39], and are commonly used for long-term monitoring of human physiological signals, disease prevention, and clinical diagnosis[40-42]. In addition to sensing, owing to the biocompatibility of their materials and the further development of photobiology, flexible optical waveguides can also be used for minimally invasive or even noninvasive medical clinical treatment, and after surgery, they can also be used for rehabilitation, assisted living support, etc., with a broad range of applications[43,44].
In this work, based on a review of flexible optical waveguides for human health assistance, we systematically classify and summarize the current development status and specific applications of flexible optical waveguides in the field of human health assistance, as well as their possible future development trends, as presented in Figure 1[45-56]. We classify the materials commonly used for preparing flexible optical waveguide systems, summarizing the basic properties and uniqueness of the three primary material types: hydrogels, elastomers, and biodegradable materials. Building on this foundation, we will then examine the fundamental principles that enable flexible optical waveguide systems to function in sensing applications, offering readers deeper insight into how these systems are leveraged for practical use. To illustrate their versatility, we further review and analyze concrete application examples across three key domains: invasive biomedical diagnosis and therapy, contact-based physiological information monitoring, and interactive soft robotics, highlighting their transformative potential in these fields.
Figure 1. System diagram of flexible optical waveguides for human health assistance[45-56]. Copyright 2020, Elsevier; Copyright 2023, Wiley; Copyright 2020, Wiley; Copyright 2024, American Chemical Society; Copyright 2020, American Chemical Society; Copyright 2022, American Chemical Society; Copyright 2023, Opto-Electronic Journals Group; Copyright 2024, American Association for the Advancement of Science.
MATERIALS FOR FLEXIBLE OPTICAL WAVEGUIDES
The advent of rapid material science advancements has facilitated the utilization of an array of innovative materials in the fabrication and packaging of optical waveguides that impart enhanced flexibility. Traditional rigid materials (such as silicon and carbon-based materials) have many advantages, such as anti-electromagnetic interference, light weight, low optical loss, sensitive response, etc., and are widely used in optical fiber communication, remote sensing, imaging, etc.[57-59]. However, these materials are not suitable for preparing flexible optical waveguides. Specifically, first, such conventional materials are hard, brittle, and sharp, making them unsuitable for use as wearable flexible optical waveguide materials; second, these materials are not biocompatible, which can easily lead to inflammation and immune reactions if they are used as implantable flexible optical waveguide materials.
While traditional rigid materials are unable to adapt to human tissue deformation due to their hard and brittle properties, flexible materials can achieve a Young’s modulus that matches that of soft tissue due to their molecular connectivity properties. This mechanical adaptability effectively solves the stress shielding problem of implantable devices and avoids the occurrence of inflammatory reactions. Among the flexible materials, those that can be used for photoconductive and biological applications still need to possess several necessary properties[60]. First, it should exhibit a high transparency to minimize energy loss and improve the propagation efficiency. Second, the refractive index (RI) should satisfy the requirements of the total internal reflection (TIR) mechanism. Third, the mechanical properties of the materials, such as high tensile strength, high breaking stress, and high Young’s modulus, are crucial for achieving flexible fiber sensor preparation. In addition, implantable flexible optical waveguide materials must also be nontoxic and highly biocompatible to protect the host tissues. Some representative flexible optical waveguide preparation materials and related properties are summarized in Table 1. In this section, some of the properties of these materials, including hydrogels, elastomers, and biodegradable polymers, are described and discussed in detail.
Summary of typical materials used in the fabrication of flexible optical waveguides and their relevant properties
| Material | RI | Optical loss | Flexibility metrics | Structural metrics | Other properties | Ref. | |
| Hydrogel | PEG | 1.35-1.47 | 0.17-25 dB/cm | Max elongation: 300%-2,000% | Young’s modulus: 1-44 kPa | Nontoxic | [61-64] |
| PEGDA | 1.33-1.48 | / | Elongation at break: 20%-80% | Elastic modulus: 30 kPa - 85 MPa | Tensile strength: 1.5-4.0 MPa (highly cross-linked); 36 kPa -20 MPa (composite) Nontoxic | [65-67] | |
| PAM | 1.46-1.50 | 1-11 dB/cm | Max elongation: 13%-74% | Young’s modulus: 20-27 MPa | / | [68] | |
| Elastomer | PDMS | 1.41-1.47 | 0.5 dB/cm | Max elongation: 95%-140% | Young’s modulus: 0.57-3.7 MPa | / | [69-74] |
| Eco-flex | 1.40 | 68.6%-78% | Elongation at break: 900% | Shear modulus: 1.3-35 kPa | Tensile strength: 200 psi Shore hardness: 00-30 | [75-77] | |
| Solaris | / | 80% | Max elongation: 290% | Young’s modulus: 1.08 MPa Shear modulus: 0.6-175 kPa | Shore A hardness: 15 Tensile strength: 180 psi | [75,78] | |
| Biodegradable polymers | PLA | 1.46-1.47 | 1.5 dB/cm | Max elongation: 3%-100% | Young’s modulus: 2.7-7 GPa | Biodegradation speed: 1 week to 4 months Nonelastic | [79-81] |
| PU | 1.46 | 2 dB/cm | Max elongation: 10% | Young’s modulus: 0.3 MPa | / | [82] | |
Hydrogels
Hydrogels are promising materials for biomedical applications because of their biocompatibility and ability to incorporate functional groups for sensing. As a cross-linked network of hydrophilic polymers, a hydrogel contains a large amount of water and exhibits excellent biocompatibility[83]. Its 3-dimensional (3D) polymer network [Figure 2A] is connected by physical entanglement or chemical bonding [Figure 2B and C[56]], and each of the chains is not fixed but can be relatively freely rotated and bent, which makes a hydrogel highly flexible as an optical waveguide and able to undergo elastic deformation within a certain range[84]. The optical and mechanical properties of hydrogels are affected by their polymer content, molecular weight, and cross-linking density, which can be adjusted to closely match the optical and mechanical properties of soft tissues for a wide range of biomedical applications[85]. In addition, as shown in Figure 2D[86], the pore size of hydrogels can be controlled by changing the relevant properties of the polymer precursor. This feature makes hydrogels attractive materials for preparing functional biophotonic waveguides that provide drug delivery and controlled drug release[87,88]. In this section, we will focus on three hydrogel materials, polyethylene glycol (PEG), poly (ethylene glycol) diacrylate (PEGDA), and polyacrylamide (PAM), which are commonly used to prepare flexible optical waveguides for human health.
Figure 2. Characterization of hydrogel and features of flexible optical waveguides prepared from it. (A) Schematic of the three-dimensional structure of hydrogels; (B) Physical cross-linking of hydrogels including (a) hydrogen bonding interactions, (b) electrostatic interactions, and (c) hydrophobic interactions; (C) Chemical cross-linking of hydrogels including (a) chemically reactive polymerization, (b) free-radical polymerization, and (c) photopolymerization[56]. Copyright 2024, American Association for the Advancement of Science; (D) SEM images of hydrogels with PEGDA and PEGDA/ALG as examples. (a) SEM images of PEGDA hydrogels. (b-d) SEM images of PEGDA/ALG hydrogels with gradually increasing alginate concentration. It can be seen that the hydrogel pore size decreases gradually with the increase of alginate concentration[86]. Copyright 2023, Elsevier; (E) Case of flexible optical waveguide prepared with PEG. (a) Preparation process of PEG hydrogel. The UV photo cross-linking was first performed through a mold, followed by the passage of dichloromethane to remove the core, and finally, the alginate shell was generated by dip-coating on the surface of the core. (b) Physical drawing of the prepared 1 m-long optical fiber. (c) Light conduction of an optical waveguide sandwiched between two pieces of thin pig tissue[94]. Copyright 2015, Wiley; (F) Chemical formula of PEGDA; (G) Uniform luminescence after fiber-coupled blue light based on PAM[105]. Copyright 2018, Wiley. SEM: Scanning electron microscope; PEGDA: poly (ethylene glycol) diacrylate; ALG: alginates; PEG: polyethylene glycol; UV: ultraviolet; PAM: polyacrylamide.
PEG
PEG hydrogels are usually prepared by cross-linking PEGDA in an aqueous solution and have antifouling properties or anti-protein-adsorption properties similar to those of PEGDA, as well as excellent biocompatibility, optical transparency, no immunoreactivity, etc.[85]. The mechanical and optical properties of PEG hydrogels can be adjusted by adjusting their molecular weight and water content[89]. In addition, owing to its excellent biocompatibility, optical transparency, and lack of immunoreactivity, PEG has promising potential for applications in the fields of biosensing[90], tissue engineering[91], surface coating of nanoparticles[92], and so on.
One of the major limitations of photoconductive structures in biomedical applications is that the effective light transmission distance is smaller than the organ scale. The organ-scale distance in the human body is more than 10 cm, whereas the 1/e attenuation range of waveguides is no more than a few centimeters[93]. Choi et al. addressed this limitation by preparing a core-cladding hydrogel fiber with an overall step RI by the process depicted in Figure 2E(a) using 80%-90% w/v PEG material as the core and a 1%-2% w/v alginate hydrogel as the cladding [see Figure 2E(b) for the actual picture][94]. As shown in Figure 2E(c), the optical loss of this fiber in pork tissue is about 0.42 dB/cm (λ = 402 nm) with a 1/e attenuation distance of over 10 cm. At the same time, they exploited the properties of hydrogels by infiltrating functional molecules into the hydrogels to produce fluorescence, amplified spontaneous emission, whispering gallery mode (WGM) lasers, and photothermal devices, giving PEG hydrogel optical fibers rich functionality.
PEGDA
PEGDA is a PEG derivative containing acrylate groups, with two acrylate groups in the main chain of PEG, as shown in Figure 2F. These acrylate groups can be rapidly cured into a hydrogel by a photoinitiated free radical polymerization reaction to form a cross-linked network when irradiated with ultraviolet (UV) or visible light[95]. This structure can enhance the optical transparency, toughness, and stress-cracking resistance of the material[96]. In addition, factors such as the amount of photoinitiator, the amount of monomer, and the light intensity significantly affect the polymerization rate and gelation behavior of hydrogels[97-99]. Specifically, the polymerization rate of the hydrogel polymerization precursors linearly increases with the square root of the photoinitiator dosage, monomer dosage, and light intensity, whereas the gel transition time exponentially decreases with these factors. The tunable mechanical and optical properties and flexible chemical modification possibilities make PEGDA ideal for use as an optical waveguide material[100].
In addition, the light-curing characteristics of PEGDA allow precise control of the curing process of the material, which is particularly important for the fabrication of optical waveguide materials, as it ensures that the precise geometries and optical properties of the optical waveguide are suitable for precise transmission of optical signals in vivo[101]. This precise control is achieved through a photolithography process in which a photosensitive material is used to form the desired optical waveguide pattern after exposure to UV light, thereby reducing the optical loss and improving the transmission efficiency of the optical waveguide.
PAM
PAM, a hydrogel material commonly used in the preparation of optical waveguides, is a linear, water-soluble polymer with many amide groups in its molecular chain[102]. This structure endows PAM with excellent hydrophilicity and biocompatibility, enabling it to exhibit nontoxicity and good histocompatibility in biomedical applications[85,103]. Compared with PEG and PEGDA, PAM has greater mechanical strength and a longer service life in biomedical applications. This is mainly attributed to its linear polymer structure, which endows PAM with good film-forming properties and mechanical strength, enabling it to exhibit excellent light transmission properties among optical waveguide materials. This also makes PAM promising for tissue engineering applications. For example, nanohydroxyapatite (nHAP) particles can be introduced into a PAM hydrogel system to prepare nHAP/PAM nanocomposite hydrogels, which can be used to make scaffolds for bone tissue repair[104].
More possibilities for the application of PAM in optical waveguide technology are enabled through chemical modification, polymerization with other materials, and the introduction of a cladding-core structure[68]. For example, through one-step polymerization and cross-linking of alginate and PAM precursors, alginate–PAM hydrogel optical fibers with a low modulus [the Young’s modulus of the fiber slowly increases from 48 to 90 kPa with increasing acrylamide (AAm) concentration] and high stretchability (120%-140% of the original length under the condition of little change in the electrical conductivity) can be prepared[105]. The feasibility of the use of these fibers for chronic optogenetic brain conditioning was demonstrated in free-moving animals, as shown in Figure 2G[105].
Elastomers
Elastomers are characterized by high flexibility and an elastic recovery ability, making them reliable materials for the fabrication and modification of flexible optical waveguides. The source of these outstanding capabilities is some of their structural features at the micromolecular level, such as polymer chains and cross-linking points. The soft segments in the polymer chain produce large deformations, giving the material a high degree of flexibility. A high proportion of soft segments in the molecular chain leads to a lower Young’s modulus of elastomers. In contrast, cross-linking points act as interchain junctions, restricting molecular movement and providing elastic recovery of the network structure. According to different types of cross-linking, elastomers can be divided into thermoset elastomers (TSEs) and thermoplastic elastomers (TPEs), which differ in the fabrication and application areas of different flexible optical waveguides[106]. Together, these materials significantly expand the design and application potential of flexible optical waveguides for human health assistance.
TSEs
The intermolecular structure of a TSE is formed by chemical cross-linking, forming a 3D network structure with irreversible cross-linking bonds. Therefore, a TSE has a high degree of mechanical stability due to its resistance to temperature and fatigue. Moreover, considering the different optical properties and biocompatibilities, the TSEs most commonly used in flexible optical waveguides are polydimethylsiloxane (PDMS) and the Eco-flex series.
Characterized by low cost, high elasticity, thermal stability, and high transmittance in the near-ultraviolet to near-infrared regions, PDMS is an important material for preparing flexible optical waveguides[107]. PDMS, with an RI of approximately 1.41 and a transmittance of nearly 95% in the visible spectrum, is widely used in waveguides requiring high optical clarity. The Sylgard® 184 silicone is one of the most commonly utilized commercial formulations of PDMS. The properties of PDMS, including its mechanical strength[108], optical clarity[109], and gas permeability[110], can be significantly influenced by varying the ratio of monomers to curing agent. Compared to Sylgard® 184, Solaris has lower Shore A Hardness (15), indicating that it has better elasticity and can provide sufficiently sensitive bending feedback to pressure changes. Additionally, Solaris has a clear color, which will contribute to obtaining sufficiently strong signals; the fabrication process of Solaris is presented in Figure 3A[56]. Another commonly used type of TSE is Eco-flex, which shares many of the excellent properties of PDMS. Nevertheless, Eco-flex is softer and more malleable because of the specially designed silicone groups in its chemistry. Owing to its excellent skin safety, Eco-flex has become a popular choice for manufacturing prosthetics and artificial skin. Notably, after curing, Eco-flex rubbers are translucent rather than optically transparent, which partially limits their use as waveguide materials. Figure 3B shows that a liquid bladder sensor encapsulated with the Eco-flex material for human wrist pulse wave monitoring demonstrates excellent flexibility[111]. Figure 3C indicates that PDMS can be used as an overpack for flexible skin-friendly sensors[112].
Figure 3. Typical role of TSE materials in flexible optical waveguides. (A) Solaris elastomer as part of a flexible optical waveguide sensing channel[49]. Copyright 2024, American Chemical Society; (B) Eco-flex as an encapsulation material for flexible optical waveguides[111]. Copyright 2018, Wiley; (C) PDMS as an overpack for flexible skin-friendly sensors[112]. Copyright 2018, Wiley. TSE: Thermoset elastomer; PDMS: polydimethylsiloxane.
TSEs typically need to consist of blended and cross-linked two-component compositions. They are prone to bubble formation during optical waveguide preparation and molding, which affects their optical properties. In addition, optical waveguides prepared from these materials with commonly used preparation methods are limited in length and are thicker[56].
TPEs
The intermolecular structure of TPEs is formed by physical cross-linking through phase separation, resulting in a two-phase morphology that is reversible under thermal processing. This characteristic enables TPEs to exhibit recyclability and reprocessability, making them suitable for sustainable manufacturing. Owing to their tunable mechanical and optical properties, TPEs are widely employed in applications ranging from wearable sensors and smart textiles to biomedical devices and optical waveguides.
TPEs demonstrate versatile optical and mechanical properties that make them highly suitable for flexible optical waveguide applications. For example, Geniomer 200 exhibits a high optical transmittance of over 90% across the visible spectrum (400-700 nm), making it ideal for waveguide designs requiring minimal signal attenuation[113]. Similarly, tetrafluoroethylene hexafluoropropylene vinylidene fluoride (THV), with an RI range of 1.34-1.36, provides excellent compatibility for step-index optical fibers. In terms of the mechanical performance, Geniomer 200 achieves a tensile strength of approximately 15 MPa and an elongation at break exceeding 600%, ensuring robustness under significant mechanical stress. THV further offers exceptional chemical resistance and retains its mechanical integrity even after prolonged exposure to solvents, acids, and bases. Similarly, StarClear 1044 and Daikin T-530 have been utilized to create stretchable step-index optical fibers, leveraging their excellent optical clarity and adaptability to coextrusion and thermal stretching[114]. These properties collectively position TPEs as a compelling choice for high-performance, durable, and adaptable optical waveguide systems. Polystyrene-ethylene-butene-styrene (SEBS) is a linear triblock copolymer that can be tailored by adjusting the ratio of its hard phase (polystyrene, PS) to its soft phase (ethylene-butene, EB), as well as its molecular weight, to control the softening temperature and viscoelastic properties. SEBS has been shown to thermally stretch at high viscosities (above 103 Pa·s), enabling encapsulation of soft and hard materials with various microstructures[115].
Compared to TSEs, TPEs minimize risks such as bubble formation during processing and enable the fabrication of longer and thinner optical fibers. However, their performance under extreme thermal or mechanical conditions may be limited, which can be addressed through the use of material blends or nanocomposites.
Biodegradable polymers
Biodegradable polymers are a promising class of materials for flexible optical waveguides used in health-related applications, offering the dual benefits of sustainability and biocompatibility. These polymers are designed to naturally degrade over time, reducing the long-term environmental impact and minimizing the need for removal or disposal. In the context of human health assistance, biodegradable polymers are beneficial for short-term biomedical applications, such as implantable medical devices, tissue scaffolds, and biodegradable sensors, for which gradual degradation after use is desirable.
Among the most commonly employed biodegradable polymers are polylactic acid (PLLA), polycaprolactone (PCL), and polyhydroxyalkanoate (PHA)[60]. PLLA, derived from renewable resources such as cornstarch or sugarcane, has been widely studied for use in flexible optical waveguides for biomedical applications because of its biocompatibility, optical clarity, and ability to degrade into nontoxic products, as shown in Figure 4A[79,116]. PLA is particularly valuable in applications in which the degradation of the material over time aligns with the temporary nature of the device, such as optical biosensors and wearable health monitoring systems. The PLLA degradation rate can be controlled by adjusting the molecular weight, which influences the performance of the material in vivo. Owing to its low melting point and slower degradation rate, PCL is used in controlled-release drug delivery systems and implantable optical devices that require gradual degradation to support tissue regeneration[117]. This property makes PCL ideal for optical waveguides or implantable sensors in drug delivery. Additionally, PHA, a biopolymer produced by microorganisms, has shown potential for use in biodegradable medical devices and sustainable biomedical packaging, providing a viable alternative for applications requiring environmental and biological compatibility. The flexibility, tensile stress-strain curves, transparency, and transmission spectra of biodegradable PHA films are shown in Figure 4B[118]. To demonstrate the biocompatibility of poly(L-lactic acid) and poly(L-lactic acid-co-glycolic acid) optical fibers, researchers cocultured bone marrow-derived mesenchymal stem cells (BMSCs) with these fibers for one week[47]. Figure 4C[47] shows the cell morphology on the fiber surface, and the cells uniformly adhered to the fiber surface and remained healthy, indicating that the fibers and their degradation products are ideally biocompatible.
Figure 4. Characterization and testing of biodegradable polymer materials. (A) Characterization of surface morphology for PLLA films and fibers degraded in vitro[116]. Copyright 2018, Wiley; (B) Flexibility, tensile stress-strain curves, transparency, and transmission spectra of biodegradable PHA films[118]. Copyright 2023, Wiley; (C) Biocompatibility test of PLLA & PLGA fiber with SEM[47]. Copyright 2020, Wiley. PLLA: Polylactic acid; PHA: polyhydroxyalkanoate; PLGA: poly lactic-co-glycolic acid; SEM: scanning electron microscope.
The use of biodegradable polymers in flexible optical waveguides for health applications reduces plastic waste and enhances medical device safety and functionality. These materials can be tailored to degrade at specific rates to match the biological needs of the application, whether for biosensors, implants, or health monitoring systems. Despite their advantages, challenges such as controlling the degradation rate and improving the mechanical properties remain. Ongoing research is focused on optimizing these materials for longer-lasting performance and more efficient manufacturing, thus ensuring their broader adoption in the healthcare industry.
SENSING PRINCIPLES OF FLEXIBLE OPTICAL WAVEGUIDES
Flexible optical waveguides have become a transformative technology for human health monitoring and biomedical applications because of their unique ability to be integrated with soft biological tissues to provide real-time, high-precision sensing[60]. The input signal of flexible optical waveguides is usually the light signal generated by light-emitting diodes (LEDs), fluorescence, or other light sources. Due to physiological changes such as pulse pressure, biomarker concentration, temperature, and so on, the flexible optical waveguide undergoes microbending loss[119], RI change[120], or fluorescence change[121], so that the light inside the waveguide is modulated accordingly, and it becomes the optical signal at the output. Changes in the input and output light signals, such as intensity[122] and phase[123], can reflect the characteristics of physiological changes. Flexible optical waveguides can also convert the output optical signal into an electrical signal with the support of photodetectors or spectrometers. This not only allows for a clearer presentation of the changes in the input and output optical signals, but also enables sophisticated signal analysis, feature extraction, such as peak detection and spectral analysis, as well as refinement of these signals through algorithms or machine learning, etc., to derive the corresponding health parameters. By utilizing these interactions, flexible optical waveguides can be used to detect and quantify various physiological parameters, making them indispensable tools in wearable devices, implantable sensors, and diagnostic systems[37].
The sensing mechanisms of flexible optical waveguides can be broadly categorized into three main groups: optical loss-based sensing, fluorescence-based sensing, and spectral-based sensing. Put simply, optical loss-based sensing utilizes the attenuation of light due to bending or absorption, whereas fluorescence-based sensing utilizes the emission properties of embedded phosphors. In spectral-based sensing, in contrast, changes in the spectral properties of light, such as wavelength shifts or interference patterns, are analyzed to extract information about the external environment. These different sensing principles enable a wide range of biomedical challenges, from monitoring vital signs to detecting biochemical information changes in real time, to be addressed with flexible optical waveguides.
Optical loss-based sensing
The principle of optical loss-based sensing is based on the TIR mechanism, in which sensing is realized by measuring the loss incurred during light transmission. Sensors applying this principle are often used to sense stress or strain conditions. Depending on the cause of the optical loss, this sensing principle can be categorized into two types: one in which the loss occurs due to deformation and another in which the loss occurs due to absorption of light by the material.
For the sensing principle of light loss due to deformation, according to Maxwell’s system of equations, when a rapidly decaying evanescent wave (EW) exists in the low RI layer, its penetration thickness dp can be expressed as
where θl is the angle of incidence, and the other quantities are constant for the same beam of light entering a fixed sensor[109]. When dp is greater than the cladding thickness of the optical fiber, part of the EW passes through the cladding, generating optical loss, and when bending deformation occurs, θl decreases while dp increases; thus, the optical loss increases. The change in optical loss corresponds to the change in strain or stress in the sensor, and this relationship can be established through reconstruction algorithms[124], simulation fitting[125], and so on. Therefore, by monitoring the output optical loss, the stress or strain in the optical fiber can be fed back to realize sensing.
On this basis, according to the degree of bending deformation, bending deformation can be divided into microbending and macrobending, as shown in Figure 5A[126]. Microbending refers to when the optical fiber bending radius is relatively comparable to the fiber radius and the fiber bends in a periodic pattern, such as sawtooth or corrugated. In the case of sawtooth microbending, for example, an optical fiber passes between two toothed plates with a mechanical cycle, as shown in Figure 5B[119]. Owing to the presence of sawteeth, when the sawtooth layer is shifted by an external force, the optical fiber bends accordingly, resulting in a change in the output power. Hu et al. reported that when the microbending period is set, the greater the number of microbending cycles (that is, the greater the number of teeth of the sawtooth pattern) is, the greater the sensitivity of the sensor[119]. They used this principle to design and prepare a microbending optical fiber sensor that can be used for respiratory monitoring. Macrobending refers to when the optical fiber bending radius is much larger than the fiber radius, approximately on the order of magnitude of a few centimeters, such as ring-shaped, U-shaped, spiral[127], equal-amplitude sinusoidal, and variable-amplitude sinusoidal bending[128]. This macrobending design places the sensor under no strain or stress and results in a large optical loss. When strain or stress is generated, the deformation is reduced, and the transmitted light intensity is significantly increased, achieving high-sensitivity sensing[129]. The sensitivity of the sensor is related to the complexity of the macrobending structure. Al-Lami et al. designed and compared the feasibility and sensitivity of four macrobending sensor structures, namely, U-shaped, droplet-shaped, knot-shaped, and figure-eight shaped structures, as shown in Figure 5C[130], for monitoring the curvature displacements of human joints[130]. The figure-eight shape had the highest sensor sensitivity of -0.477 dB/°, whereas the sensitivity of the U-shaped and knot-shaped structures was only approximately half that of the figure-eight structure.
Figure 5. Overview of sensing principles based on optical loss. (A) Schematic diagram of two bending deformations of the optical fiber[126]. Copyright 2023, Elsevier. (a) Micro-bending. (b) U-shaped macro-bending; (B) Stress changes lead to optical loss changes through changes in sawtooth bending of the optical fiber[119]. Copyright 2016, Elsevier; (C) Three different macro-bending[130]. Copyright 2024, Elsevier. (a) Droplet-shaped. (b) Knot shape. (c) Figure-of-eight shape; (D) Schematic of the sensing principle of a stretchable optical fiber sensor doped with dye molecules[131]. Copyright 2022, Elsevier.
Another type of optical loss is generated by absorption of transmitted light by a dopant material, the principle of which is shown in Figure 5D[131]. This type of optical loss is common in stretchable optical sensors. The sensing mechanism of these strain sensors is mostly based on dye absorption spectroscopy, following the Bouguer–Lambert–Beer law[132]:
where A(λ, l) is the absorbance, k(λ) denotes the molar absorption coefficient, c is the concentration of the dye molecules, l is the length of the optical fiber, l0 is the original length of the optical fiber, and ε is the strain; that is, the absorbance of the dye molecule changes proportionally with the length of the optical path l through which the light passes. At the same time, the spectrum T(λ, l) of the transmitted light undergoes a corresponding attenuation:
where D(λ, ε) denotes the variation in attenuation under strain, and α(ε) denotes the optical coupling loss at the junction. With Equations (3) and (4), the relationship between the spectrum of transmitted light at the output and the strain of stretchable optical sensors is established, thus realizing sensing.
However, the Bouguer–Lambert–Beer law can be rigorously applied only if certain prerequisites are met, such as the incident light being monochromatic and parallel or perpendicular, the absorbing substance being a homogeneous system, and no scattering, fluorescence, or photochemistry of the radiation interacting with the substance occurring[133]. The reality of complex practical situations often fails to fulfill these assumptions, leading to inaccurate predictions. Instead, the non-ideal situation is often characterized by a non-linear relationship between material deformation and light attenuation[134]. This discrepancy stems from competing physical mechanisms: microscopic scattering dominates at low strains. This problem can be solved by systematically integrating the scattering parameters into the classical framework, thereby improving the prediction accuracy and extending the applicability of the law to complex real systems[135,136].
Optical sensors based on the optical loss principle have the advantages of being simple, easy to operate, and inexpensive[137]. However, since these sensors rely completely on optical loss, any fluctuations in optical loss due to fluctuations in the light source or other non-investigative factors will affect the stability and accuracy of the sensor[138].
Fluorescence-based sensing
Fluorescence sensing technology uses fluorescence signals to detect various substances. It is widely used in chemical analysis, biomedicine, and environmental monitoring. The basic principle is that certain substances (usually fluorescent probes or molecules) emit fluorescence at a specific wavelength when irradiated by an external excitation light source (e.g., UV or visible light)[139]. The intensity, wavelength, and lifetime of the fluorescence signals can reflect the concentration, characteristics, and existence of the substance under test.
Fluorescence optical fiber sensing is an innovative application that combines fluorescence sensing and optical fiber technology and is widely used in many fields, such as biomedicine, environmental monitoring, and industrial process control. By combining fluorescent probes with optical fiber technology, high-sensitivity, long-distance, real-time monitoring can be achieved while avoiding the limitations of traditional sensors (e.g., the need for direct contact with the sample)[140]. In the biomedical field, fluorescent optical fiber sensing technology has many unique advantages and can be efficiently used in a variety of areas, such as disease diagnosis, molecular labeling, cellular imaging, and real-time monitoring. Fluorescent optical fiber sensors can be used to extract rich information from different features of fluorescence signals (e.g., the fluorescence intensity, wavelength, and lifetime), and these distinct methods of fluorescence data analysis may provide diverse diagnostic tools for biomedical applications.
Currently, most reported flexible fluorescent optical fiber sensors mainly utilize fluorescence intensity demodulation. Analysis of the fluorescence intensity is the most common way of analyzing fluorescence data, quantifying the concentration of a target substance, or detecting the presence of a biomarker by measuring the intensity of light emitted by a probe molecule upon excitation. The fluorescence intensity is proportional to the concentration of fluorescent molecules in a sample and is therefore widely used for quantitative analysis. For example, in tumor marker detection, fluorescent optical fiber sensors combined with specific fluorescent probes can be used to detect trace amounts of tumor markers (such as carcinoembryonic antigen) in blood or tissues and to assess the presence and concentration of tumors through changes in the fluorescence intensity. In cellular analysis, when monitoring changes in the internal environment of cells (e.g., calcium ion concentration and pH changes), changes in the fluorescence intensity can reflect the cellular status in real time, providing information for early diagnosis of diseases.
Measuring the absolute fluorescence intensity is a straightforward analysis method, but this method can be influenced by variations in the laser excitation power and other environmental factors. Consequently, the fluorescence intensity ratio (FIR) technique has emerged as a more reliable optical sensing method. The FIR technique involves measuring the ratio of the intensities of fluorescence signals at different wavelengths:
where I1 and I2 are the fluorescence intensities of two probes at different wavelengths. Typically, this method employs two different fluorescent probes, each of which emits a fluorescence signal at a distinct wavelength after being excited[141]. These probes are specifically chosen so that each fluorescence signal responds differently to the target substance being measured.
The FIR method reduces the effect of external interference, reduces the dependence on the probe concentration, increases the sensitivity and resolution, etc. Li et al. hydrophobically modified a pH-sensitive ratiometric fluorescent probe with a sol-gel material and physically encapsulated it on the surface of an optical fiber with an antibleaching agent[142]. The 8-hydroxy-1,3,6-styrene trisulfonic acid (HPTS) used in this study has two excitation bands near 375 and 450 nm that exhibit different pH dependences, making it suitable for use in the FIR method to monitor the pH to confirm the boundaries of cancer cells. In addition, by modifying multiple indicators on the fiber surface, multiple biomarkers can also be simultaneously monitored, reducing cross-sensitivity[143].
Spectrum-based sensing
Spectrum-based optical sensing relies on analyzing the spectral properties of light after its interactions with a target medium, such as reflection, scattering, and interference, to extract spectral information related to specific external parameters or medium properties. This principle is widely used in a variety of flexible optical waveguide sensors, including those based on fiber Bragg gratings (FBGs) and interference sensors.
The FBG sensor features high sensitivity, miniaturization, flexibility, and resistance to electromagnetic interference[144]. It operates by measuring the wavelength shift of the Bragg peak, as shown in Figure 6A[55]. When broadband light passes through the FBG, only a specific wavelength of light (λB) that satisfies the Bragg condition is reflected.
Figure 6. Overview of spectral-based sensing principles. (A) Schematic diagram of the FBG sensing principle[55]. Copyright 2019, MDPI; (B) Schematic diagram of the interferometric systems sensing principle[37]. Copyright 2024, Elsevier; (C) Sensing for respiratory rate monitoring using reflectance spectroscopy[146]. Copyright 2022, MDPI. FBG: Fiber Bragg grating.
When external conditions such as strain or temperature change, these parameters change accordingly, resulting in a shift in the center wavelength of the FBG. The relationship between the wavelength shift and grating period can be simply established by[40]
where neff is the effective RI. FBG sensing is based on an external parameter X, which causes a change in the neff, which in turn changes the λB to thus achieve sensing. Compared with optical sensors, which are based on optical loss, FBG sensors are minimally affected by fluctuations in the power of the light source and have a better signal-to-noise ratio (SNR) for high-precision applications. However, a demodulation system is necessary for FBG sensors, which increases the complexity and cost of the device and operation[145].
Interferometric sensors function by sensing the phase change caused by light as shown in Figure 6B[37]. Interferometers commonly used for human health monitoring include Michelson interferometers, Mach–Zehnder interferometers[147], Fabry–Perot interferometers[148], etc., and they have similar working principles. According to the principle of double-beam interference, the output light intensity can be expressed as[149]:
where I1 and I2 are the intensities of the two beams of light and ΔΦ is the phase difference between the two beams of light, λ is the wavelength of the light, L is the distance over which the light passes, and Δneff is the difference in the RIs of the media through which the two beams of light pass. It indicates that when the optical waveguide is subjected to external forces or changes in the RI due to temperature changes, the optical range difference between the two beams of light changes, and the corresponding phase difference and interference light intensity also change, thus realizing sensing. Zhao et al. implemented human breath sensing using a Fabry–Perot interferometer to measure the reflectance spectrum, as shown in Figure 6C[146], experimentally demonstrating the stability, high sensitivity, and practicality of interferometry for human health sensing applications.
APPLICATIONS OF FLEXIBLE OPTICAL WAVEGUIDES IN HUMAN HEALTH AIDS
Flexible optical waveguides have demonstrated wide-ranging potential in biomedical and engineering contexts due to their unique combination of optical performance, mechanical adaptability, and biocompatibility. In recent years, an increasing number of researchers have developed devices using different types of flexible optical waveguides for human health diagnosis, monitoring, or assistance. In general, human health information includes in vivo information such as blood (blood glucose, blood oxygen, and other biomarkers), physiological cell and gene information; surface information such as body temperature, cardiac output (heartbeat, HR, etc.), blood pressure, respiration, and electrolyte balance (sweat, humidity, and pH); and extracorporeal information such as plantar pressures, joint flexion, tooth grinding, and facial muscle twitching. Invasive or noninvasive devices fabricated using flexible optical waveguides can enable diagnosis or monitoring of the above human health signals. To provide readers with a clear overview before delving into detailed case studies, we summarize in Table 2 the representative categories of flexible optical waveguides, their performance indicators, and specific biomedical applications. This comparative summary highlights not only the material and performance characteristics but also examples of how each category is being applied in invasive biomedical diagnosis and therapy, contact physiological information monitoring, and interactive soft robots.
Representative categories of flexible optical waveguides, their performance indicators, and specific biomedical applications
| Waveguide category | Optical performance | Mechanical properties | Biocompatibility and degradability | Biomedical applications | Ref. |
| Hydrogel-based | Transmission loss: ~0.2-25 dB/cm; visible–NIR | Flexible, but limited robustness | Biocompatible; non-biodegradable | • Invasive diagnosis and therapy: in situ analytical detection, biomedical therapy • Contact monitoring: cardiorespiratory function assessment | [68,150,151] |
| Elastomer-based | Transmission loss: ~0.1-0.5 dB/cm; visible–IR | Highly stretchable (> 100%); durable | Biocompatible; non-biodegradable | • Contact monitoring: motion pattern recognition, cardiorespiratory function assessment • Interactive robots | [49,52,152-155] |
| Biodegradable polymer | Transmission loss: ~0.5-2 dB/cm; visible–NIR | Moderate flexibility; limited lifetime | Biocompatible; biodegradable | • Invasive diagnosis and therapy: biomedical therapy | [47] |
| Hybrid/nanocomposite (e.g., polymer + nanoparticles, nanofibers) | Transmission loss: < 0.5 dB/cm; broadband | Tunable flexibility; enhanced robustness | Partially biocompatible; non-biodegradable | • Invasive diagnosis and therapy: drug delivery, in situ analytical detection, biomedical therapy • Contact monitoring: motion pattern recognition, cardiorespiratory function assessment • Interactive robots | [48,54,156-159] |
This comparative framework not only clarifies the trade-offs among different waveguide platforms in terms of optical, mechanical, and biological performance but also highlights their translational relevance across diverse biomedical scenarios. Guided by this framework, the following subsections provide a detailed analysis of the state-of-the-art applications of flexible optical waveguides in invasive biomedical diagnosis and therapy, contact physiological information monitoring, and interactive soft robots.
Invasive biomedical diagnosis and therapy
The human immune system is highly exclusive, making biocompatibility or biodegradability crucial for modern medical devices. Within tissues, photons interact with biological matter through various processes, producing various effects on tissues and cells[160]. In this context, flexible optical waveguides fabricated from different materials for use in invasive biomedical devices have been extensively explored, opening new possibilities for biomedical applications. Researchers have made notable progress in the use of flexible optical waveguides for drug delivery, in situ analytical detection, and biomedical therapy.
Drug delivery
Conventional drug delivery systems for tumors and many chronic diseases rely primarily on the passive transport of small-molecule drugs through the body. The effectiveness of this transport is closely related to the physicochemical properties of the drug molecule, such as the drug solubility and drug dissociation constant[161]. However, these physicochemical properties of drugs may lead to limitations in the therapeutic efficacy for the disease[162] and to excessive accumulation in nontarget organs[163]. Thus, flexible optical waveguide-based intelligent drug delivery systems that enable controlled release of drugs in the body have been developed in recent years.
When light is used as a stimulus, optical fibers can be used to remotely manipulate drug release. In addition, spatial and temporal control of drug release can be achieved by varying the light intensity and exposure time to precisely regulate the corresponding dose[164,165]. As shown in Figure 7A[45], Kurochkin et al. presented a method for photothermally activating and releasing encapsulated substances from the surface of a photosensitive polymer 3D microstructured film (PTMF) in picogram quantities in different environments using laser radiation delivered by multimode optical fibers[45]. Sui et al. presented a novel structured polymer optical fiber (POFs) with an ultrahigh numerical aperture (NA) for drug delivery and neuromodulation field[156]. The optical waveguide developed was made of three polymer materials-polycarbonate (PC), polysulfone (PSU), and fluorinated ethylene propylene (FEP)-and had improved flexibility and fiber lighting angle compared with traditional silicon optical fibers, making it suitable for use as an implantable material.
Figure 7. Typical application examples of drug delivery and in vivo analyte detection. (A) Demonstration of the feasibility of laser-triggered photothermal unsealing of PTMF chambers in three different environments: air (a), deionized water (b), and 1% agarose gel (b), with laser beams transmitted through optical fibers[45]. Copyright 2020, Elsevier; (B) Functionalization processes in silicon dioxide and hydrogel fibers (a and b) and glucose-boron complexation in the hydrogel matrix inducing positive volumetric shift (c)[168]. Copyright 2019, Elsevier; (C) Schematic diagram of the ARC/Ag/MOF sensor structure[175]. Copyright 2023, Elsevier; (D) Polymerization of the sensing gel at the tip of the optical fiber during the manufacturing process of the OF sensor (a) and integration of AuNPs into PBA-based hydrogel OF sensor (b)[176]. Copyright 2024, Wiley; (E) Experimental setup for detection of hemoglobin not drawn to scale (a-e)[177]. Copyright 2023, Elsevier; (F) Schematic illustration of npRDW TFs for neurotransmitters’ SERS signal detection (a) and optical images (b) and SEM images of the npRDW TF (c)[46]. Copyright 2023, Wiley. PTMF: Polymer 3D microstructured film; ARC: ab initio REPEAT charge; MOF: metal-organic framework; OF: optical fiber; AuNPs: Au nanoparticles; PBA: phenylboronic acid; npRDW: nonplanar repeated dewetting; TFs: tapered fibers; SERS: surface-enhanced Raman spectroscopy; SEM: scanning electron microscope.
In situ analytical detection
In-situ analytical detection plays a crucial role in biomedical diagnostics by enabling real-time monitoring of analytes such as blood glucose, blood oxygen, and various biomarkers. These analytes provide valuable insights into the state of health and disease, facilitating early detection and management of conditions such as diabetes, respiratory distress, and cardiovascular diseases. By integrating waveguides into sensors and probes, researchers can develop minimally invasive diagnostic tools capable of continuous monitoring within biological tissues[166]. This section delves into recent advancements in the use of flexible optical waveguides for in-situ analytical detection, elucidating their transformative potential in advancing healthcare through early disease detection and tailored therapeutic interventions.
Blood glucose
Monitoring of blood glucose levels is paramount in the management of diabetes, a chronic condition affecting millions of people worldwide. Flexible optical waveguides offer promising avenues for continuous and minimally invasive monitoring of blood glucose. Currently, sensors based on diffraction gratings[167], optical fibers[168,169], holographic sensors[170], tapered optical fibers[171], phenylboronic acid (PBA)-based hydrogel gratings[172], and nanostructured optical fibers[173] have been successfully applied to monitoring of glucose concentrations. However, their performances in terms of concentration measurement ranges, detection times, and detection limits widely vary. Selected flexible optical waveguide glucose sensors with high sensing performance are described in the following section. Fluorescent hydrogel fibers that enable long-term in vivo glucose monitoring were reported long ago, but this technique does not apply to individuals with skin pigmentation or tissue light scattering and is affected by the epidermal thickness[174]. Yetisen et al. obtained quantitative glucose readings via changes in the intensity of light transmitted through hydrogel optical fibers functionalized with PBA[68]. Elsherif et al. built on previous research to develop an optical fiber probe for continuous glucose monitoring under physiological conditions [Figure 7B[168]] on the basis of cutting-edge functionalization of silica and biocompatible hydrogel fibers[168]. In recent years, optical fiber surface plasmon resonance (SPR) sensing technology has been used to successfully measure various biochemical indicators in the human body because of its high sensitivity and stability. Zheng et al. developed a reflective optical fiber SPR dual-parameter biosensor for simultaneous detection of glucose and cholesterol concentrations. The sensor was coated with Au nanoparticles (AuNPs) to modulate the resonance wavelength and enhance the sensor sensitivity, whereas P-mercaptophenylboronic acid (PMBA) and β-cyclodextrin (β-CD) were chosen as sensitive materials to avoid cross-sensitivity[157]. In addition, the 3D hybrid array Ag/metal-organic framework (MOF) multi-plasma resonator cavity system [Figure 7C[175]] for SPR sensing proposed by Li et al. filled the gap of D-shaped plastic optical fiber (D-POF) SPR sensors in the field of high-sensitivity detection of low glucose concentrations[175]. As shown in Figure 7D[176], Ahmed
Blood oxygen and other biomarkers
Blood oxygen levels and various biomarkers serve as critical indicators of physiological health and disease status. By leveraging flexible optical waveguides, researchers have developed innovative techniques for real-time, noninvasive monitoring, paving the way for personalized healthcare interventions. Deng et al. described a highly sensitive hemoglobin detection method based on polarization-differential spectrophotometry with excellent detection accuracy and sensitivity [Figure 7E[177]], which can be applied to the early diagnosis of diseases[177]. Similarly, Rahad et al. performed hemoglobin concentration measurements using a novel RI nanosensor based on a metal-insulator-metal (MIM) waveguide[178]. Luo
Biomedical therapy
Biomedical therapeutic applications of flexible optical waveguides leverage the unique properties of photons through photochemical or photophysical mechanisms. These mechanisms encompass a spectrum of techniques, including photothermal therapy (PTT), photodynamic therapy (PDT), photobiomodulation (PBM), and optogenetic therapy. The integration of flexible optical waveguides enhances the efficacy and safety of these therapeutic modalities by facilitating targeted light delivery to specific anatomical sites with minimal invasiveness[181]. In this section, the diverse applications of flexible optical waveguides in biomedical therapy are explored, highlighting their role in advancing therapeutic interventions for various diseases and medical conditions.
Photomedicine
Currently, phototherapy for cancer consists mainly of PTT and PDT. In PTT, the conversion of light energy into heat is used to selectively target and destroy cancer cells[182]. Flexible optical waveguides can precisely deliver the necessary light to tumor sites, minimizing damage to surrounding healthy tissues and enhancing treatment specificity. PDT involves the use of light-activated photosensitizers to produce reactive oxygen species that can kill cancer cells or pathogens[183,184]. The use of flexible optical waveguides in PDT allows accurate delivery of light to deep tissues, improving the effectiveness and precision of the therapy. An upconversion nanoparticle (UCNP) is the ideal wireless transducer for PDT, converting near-infrared light that penetrates deep tissues into visible light for phototherapy. As shown in Figure 8A, Teh et al. developed a biocompatible UCNP implant delivered in flexible hydrogel optical waveguides[48]. The system was successful in achieving chronic PDT in an unfettered and noninvasive manner in a mouse model of glioblastoma. The application of photomedicine treatments in deep tissues is often challenging because of the possible risk of hyperthermia (damage to normal tissues)[160]. Recently, Chen et al. prepared a temperature-adaptive hydrogel fiber-based optical waveguide (THFOW) [Figure 8B], which can eliminate deep tumor cells through thermally modulated interventional photomedicine[151]. According to the study results, the THFOW showed good light propagation properties and thermal sensitivity along with soft tissue affinity and was effective in eliminating tumor cells and reducing the risk of overheating in a mouse model.
Figure 8. (A) The UCNPs implant successfully guided NIR light at the maximum bending angle (a), tissue penetration evaluation of UCNPs implant in synthetic tissue model (b), and NIR transmission of UCNPs implants (c)[48]. Copyright 2020, Wiley; (B) Light transmission within the THFOW (a), laser light (λ = 515 nm) propagation within porcine tissue through implanted THFOW2500 (b), and schematic illustration of the controllable PTT in vivo (c)[151]. Copyright 2022, Springer Nature; (C) Optical images of tissues with and without biodegradable fibers under green laser irradiation (a), and diagram of the SD rat bone defect model established procedure (b-g)[47]. Copyright 2020, Wiley. UCNPs: Upconversion nanoparticles; NIR: near-infrared; THFOW: temperature-adaptive hydrogel fiber-based optical waveguide; PTT: photothermal therapy.
PBM
PBM uses low-level laser or light therapy to stimulate cellular function and promote tissue repair[185]. Several mechanisms have been widely hypothesized to elucidate these effects[186,187]. Although these mechanisms are still poorly understood, significant progress has been made for PBM in the development of wearable devices for wound healing[188], traumatic brain injury[189], and cognitive improvement[190,191]. Flexible optical waveguides enable efficient delivery of light to targeted areas, which further enhances the therapeutic effect. A smart textile made of POFs with V-grooves and cotton yarn was designed for low-intensity phototherapy and exhibited excellent optical and thermal properties[192]. PBM therapy has shown good results in bone regeneration treatment. As shown in Figure 8C, Jiang et al. fabricated implantable and biodegradable poly (L-lactic acid) and poly (L-lactic-co-glycolic acid) photoconductors for bone regeneration[47]. In the study, accelerated bone regeneration and repair processes were achieved by introducing green light into defective bone structures using a light waveguide in a rodent model. Conventional PBM treatments are limited in that only a very small amount of effective light reaches the neural tissues of the spinal cord. Zuo et al. and Liang et al. developed a method of delivering near-infrared light to deeper structures of the spinal column using flexible optical fibers and verified that the fiber-diffused light had no side effects on normal tissues in a piglet model[193,194].
Optogenetics
Optogenetic therapy involves the use of light to control cells within living tissue that have been genetically modified to express light-sensitive ion channels[195]. The advantages of optogenetic therapy over other techniques include small wounds and high temporal and spatial resolution[196]. Flexible optical waveguides provide a minimally invasive means to deliver precise light pulses, facilitating control of neuronal activity for research and potential treatment of neurological disorders. Systems that utilize optical waveguides for optogenetic applications can be broadly classified into two categories: wired optogenetic systems and wireless optogenetic systems[197]. Gutierrez et al. completed an optogenetic study on a mouse model using an optical fiber-based wired system[198]. However, owing to the fixed light source and the limited length of the optical fiber, this wired optogenetic system could limit the subject’s range of motion during the experiment[199]. Recent studies have demonstrated the feasibility of wireless optogenetic systems that combine the three procedures of light-activated retinoid delivery, photo delivery, and subsequent electrical recording in a miniature, flexible, all-polymer waveguide device[195,200,201]. The multifunctional neural probe introduced by Park et al. consists of an optical waveguide, six electrodes, and two microfluidic channels fabricated by fiber-optic drawing[200]. The flexible probe is made entirely of polymers and polymer composites that minimize the tissue response, enabling long-term, multimodal, high-fidelity detection of brain circuits.
Contact physiological information monitoring
Long-term monitoring of physiological information is a key application area of flexible optical waveguides beyond invasive biomedical diagnostics. Researchers are developing a wide range of photonic textiles, wearable devices, and Internet of Things (IoT) furniture for long-term monitoring using various flexible optical waveguides. In this section, we report research advances in contact physiological monitoring devices based on four different usage scenarios: motion pattern recognition, cardiorespiratory function assessment, sleep state recording, and pronunciation detection.
Motion pattern recognition
Several physiological changes can occur when the human body moves, the most intuitive of which can involve joint movement, muscle contraction, and plantar pressure. Monitoring these physiological signals for motion pattern recognition can help the public better understand their exercise health status and support specific scenarios, such as athlete training and physician diagnosis.
Gait
Gait is an important indicator for assessing human health, and people with conditions such as Parkinson’s disease, diabetes, and stroke often exhibit unique gait characteristics, which can be used to analyze details such as the plantar pressure, step count, and walking speed. Therefore, developing devices for gait monitoring is promising for preventing falls in elderly individuals, assisting athletes in training, and improving shoe design. Domingues et al. designed an insole with an FBG network for remote gait analysis, and the study showed that this IoT monitoring device successfully enabled monitoring and analysis of the plantar pressure in the standing and walking phases of gait[202]. However, the FBG optical fiber used in this study may present potential safety hazards because of its insufficient flexibility. Recently, the POF-based smart insole [Figure 9A[49]] developed by Xiang et al. demonstrated good performance in gait monitoring[49]. Avellar et al. demonstrated smart pants for biomechanical and activity recognition by developing a POF to achieve controlled modulation measurement coupled with a light source with low cost, high reliability, and flexibility[203]. Zhang et al. proposed a noncontact bendable sensitive sensor that uses a semiring optical fiber (SROF) waveguide made of polymethyl methacrylate (PMMA) integrated with a wearable knee brace[152]. The sensor responded synchronously with the subject’s muscle deformation during movement, and four abnormal gaits and one normal gait were identified through data processing. With respect to health assistive device design, Leal-Junior et al. proposed a 3D printing-supported gait assistive and rehabilitation device with embedded POFs, enabling pressure and microclimate change assessment[204].
Figure 9. (A) The S-POF sensing part embedded in the key position of the insole is combined with the smartphone (a), plot of signal data for three gait cycles (b), and the direction of gait causes differences in plantar pressure distribution (c)[49]. Copyright 2024, American Chemical Society; (B) PDMS fiber under the relaxing and stretching states (a), schematic design and board design (b and c), and wearable sensor testing on the wrist and elbow (d and e)[158]. Copyright 2024, Optica Publishing Group; (C) Schematic diagram of the WPOMF sensor (a), potential applications of the WPOMF sensor (b), and one-dimensional convolutional neural network-assisted sensor pronunciation recognition experiment (c and d)[154]. Copyright 2024, American Chemical Society. S-POF: Solaris polymer optical fiber; PDMS: polydimethylsiloxane; WPOMF: wavy polymer optical microfiber.
Joint movement
In addition to the gait information reflected by the plantar pressure and changes in the leg muscles when standing, the movement of the joints (knees, elbows, etc.) of the human body when walking also contains much health information. Wang et al. proposed a wearable D-shaped POF sensing system based on machine learning for human motion recognition[153]. The designed wearable sports cuffs were worn on the elbow and knee joints of the human body, and good results were achieved in the recognition of six types of movement, such as walking, running, and climbing stairs. As shown in Figure 9B, Jiang et al. recently proposed self-powered mechanoluminescent elastic optical waveguides combined with flexible circuits, which were successfully applied in the monitoring of the bending motions of fingers, wrists, and elbows, showing excellent sensing performance[158]. The stretchable TPE optical fibers proposed by Leber et al. can reliably assess extreme mechanical stimuli, and their utility was demonstrated in a scaffold used for tracking motion tracking[114]. Zhou et al. reported a self-powered stretchable fiber-optic strain sensor with a distributed sensing capability based on mechanoluminescent optical fibers, in which mechanoluminescent phosphors that emitted light of different colors were discretely integrated into the housing of an elastomeric fiber[205]. The sensor acquired bending information from different parts of the finger joints and used it for complex gesture judgment.
Micromotion
Micromotion of the body, such as throat vibrations, muscle twitches, and teeth clenching, plays an equally important role in physiological health monitoring. As shown in Figure 9C[154], Wang et al. proposed a novel wearable optical microfiber smart sensor based on wavy polymer optical microfibers (WPOMF) and successfully conducted behavioral detection experiments with this sensor[154]. With the assistance of artificial intelligence (AI), the WPOMF sensor placed on the larynx achieved articulatory recognition of key medical monitoring words. Qian et al. presented a flexible and sensitive mechanoluminescent device with an elastic modulus modified by a nano-dopant, which enabled light emission driven by muscle movement, i.e., the photonic skin phenomenon[112]. An interactive mouthguard based on a mechanoluminescence-driven optical fiber sensor can be used for the operation of an occlusal control device, as proposed by Hou et al.[206]. The sensor can be used to operate computers, smartphones, and wheelchairs through occlusion, showing great promise for applications.
Cardiorespiratory function assessment
Cardiorespiratory function is one of the most important indicators of human health. Many diseases, although difficult to detect in the early stages, can be detected based on subtle changes in cardiorespiratory function. Thus, flexible optical waveguide devices to monitor physiological information over long periods have been developed, offering significant potential for health monitoring. This section reports advanced cases of flexible optical waveguide applications in pulse wave, heartbeat, and respiration monitoring.
Pulse waves
Monitoring pulse waves is essential for assessing cardiovascular health. Koyama et al. reported the use of a plastic FBG optical sensor to measure pulsating strain at the fingertip[207]. They successfully applied this sensor to pulse rate estimation, overcoming the safety hazards of quartz fiber grating sensors. Li et al. developed a microfiber optical sensor with a PDMS hybrid plasmonic microfiber knot resonator PDMS sandwich structure for monitoring clinical physiological signals[208]. The sensor was successfully used for wrist pulse wave and finger pulse wave monitoring, showing excellent sensitivity. Liang et al. presented a wearable flexible sensor combining ultrafine optical fibers as optical waveguides that can be used to accurately detect pulse waves, HRs, and blood pressure[209]. Additionally, using PDMS-encapsulated microfibers, Wang et al. reported a flexible strain sensor with an extremely high sensitivity and very low detection limits[210]. This Sagnac interferometer may provide new ideas for the design of wearable devices for underwater human physiological signal detection. Pan et al. reported a flexible liquid-filled fiber adapter (FLFFA) based on the principle of intensity sensing and successfully implemented sensing in areas such as real-time monitoring of the wrist pulse[211]. The sensor consists of a flexible glycerin-filled tube and two silica fibers, and as light propagates through it, part of the light is radiated from the FLFFA into the environment, causing a loss of light intensity. Recently, Li et al. presented an optical fiber sensor-assisted smartwatch for accurate continuous blood pressure monitoring, which also uses liquid filling [Figure 10A][155]. The liquid capsule in the smartwatch allows spatial insensitivity and free alignment according to Pascal’s principle while also improving the coupling between the sensor and the body.
Figure 10. (A) Schematic of the blood pressure monitoring system (a), Grid sensing area on the wrist and arterial pulse signals collected by sensors from different sites (b), typical pulse wave signals with features defined (c), and estimation process of the blood pressure(d)[155]. Copyright 2023, Springer Nature; (B) Strain tests of LGPs with the strain values ranging from 0% to 50% (a), IR image of LGP embedded with a AgNW heater (b), design schematic of stretchable pulsed oximetry (c), and PPG signal was acquired using μ-LEDs with 630 and 850 nm (d)[50]. Copyright 2020, American Chemical Society; (C) Schematic representation of the proposed flexible photonic interferometer (a and b), analysis of bi-directional stretching of sensors (c and d), and the system’s real-time reaction for monitoring breathing (e)[51]. Copyright 2023, Wiley. LGPs: Light guide panels; IR: infrared; AgNW: silver nanowires; PPG: photoplethysmogram; LEDs: light-emitting diodes.
Heartbeat
Accurate heartbeat monitoring is critical for early diagnosis and management of cardiac conditions. Bonefacino et al. presented a PMMA-based doped polymer FBG and successfully applied it to heartbeat monitoring of the brachial artery and chest[212]. A new dopant material, diphenyl disulfide, was reportedly used in this FBG, which exhibited at least 15 times higher sensitivity than silica glass fibers. Rein et al. proposed diode fibers for fabric-based optical communications, which were processed by a scalable hot-drawing process for electrically connected diode fibers[57]. When light-emitting and light-detecting fibers are placed 5 mm apart and a finger is placed on them, the reflected light, which is sensitive to the blood circulation in blood vessels close to the skin, can be recorded. HR measurements revealed that these devices have potential for use in a whole-fabric physiological state monitoring system. Furthermore, Bae et al. presented a new method for fabricating stretchable optical waveguides [Figure 10B] that can be combined with integrated functional devices for multifunctional healthcare monitoring[50]. Based on this platform, the communication of photoplethysmogram (PPG) information, including HR, oxygen saturation, respiratory rate, coughing, and sighing, was successfully demonstrated. Lo Presti et al. proposed a fiber-optic-based skin interface biosensor (i.e., a smart patch) capable of simultaneously monitoring the HR and respiration rate (RR)[213]. The smart patch was shown to estimate the RR and HR with high fidelity under different breathing conditions and common daily body postures.
Respiratory
Common human respiration monitoring can be realized by monitoring chest and abdominal expansion, expiratory humidity, or respiratory airflow. Recently, various innovative approaches have been developed. For example, Yi et al. presented a novel fiber-optic humidity sensor based on tapered microfibers coated with deposited gelatin, which demonstrated an excellent respiratory mode sensing capability in nasal breathing humidity monitoring[214]. Bao et al. reported a wearable all-fiber-optic flexible humidity transducer for respiratory monitoring that recognizes different respiratory patterns and allows extraction of the respiratory frequency from the sensor response[215]. Further expanding the scope, Zhang et al. presented cantilevered optical micro- and nanofibers (MNFs) for a multifunctional fiber-optic airflow sensor, in which the cantilever was made of a PDMS substrate encapsulated by MNFs[216]. These optimized sensors enable real-time detection and recognition of various respiratory signals, including normal breathing, deep breathing, and coughing. Zheng et al. developed a multifunctional active plasma platform based on a moisture-driven metal–hydrogel–metal hypersurface and demonstrated an example of its use as a high-performance optical respiration sensor[150]. This innovation highlights the potential of combining different materials for enhanced sensor performance. As shown in Figure 10C, Mishra et al. presented a wearable system with a bidirectional stretchable and skin-mountable balloon shape[51]. This spherical waveguide encapsulated in a PDMS film is based on the interference between the core and higher-order cladding modes due to single-mode fiber (SMF) bending, which enables human respiration and pulse wave monitoring. Additionally, Zhang et al. proposed a PMMA–PDMS fiber with good stretchability and achieved functional validation in various aspects, such as respiratory monitoring[217]. Finally, Shen et al. proposed a wearable WaveFlex sensor that incorporates a flexible wavy POF, which enables monitoring of physiological signals such as respiration of the human body in different postures[218].
Interactive soft robots
Interactive soft robotics represents a rapidly evolving field with significant implications for healthcare and assistive technologies. These systems, often composed of flexible materials integrated with optical waveguides, are designed to interact with human users safely and adaptively. In this context, researchers have investigated various configurations and materials to enhance the functionality and integration of soft robotics in medical and assistive devices. Notable progress has been made in developing interactive soft robotic systems, such as robotic prosthetics and assistive robotic hands. These advancements enable more intuitive and responsive interfaces between robots and users, improving the effectiveness of rehabilitation and daily assistance. This section reviews recent advancements in interactive soft robotics, focusing on their development and application in enhancing human–machine interactions (HMIs).
Unlike devices used for physiological information monitoring, some wearable devices have already realized human–computer interactions. Various devices based on flexible optical waveguides for recognizing gesture information and reconstructing 3D models have been developed[52,114,219-221], providing new ideas for bionic mechanical manufacturing. Khan et al. developed a novel strain sensor based on an optomechanical concept, in which the device senses the magnitude of the strain it experiences through a change in the transmittance of a flexible optical waveguide prepared from an Eco-flex/MoS2 material[221]. Additionally, the sensor was applied to develop a glove to achieve effective control of different parts of the bionic robot NAO. In another bionic stretchable optical fiber sensor proposed by Li et al. [Figure 11A], optical fibers were embedded into Eco-flex films to form Lindernia nummularifolia (LN) structures[52]. The sensor has an excellent response to strain and the bending angle and can translate real-time human motion signals into control commands for HMI applications. Researchers have further developed immersive rehabilitation training systems and remote robot-assisted motion stacking games for disabled and sick people, which have rich application prospects. In addition to HMIs, devices that realize human–machine–environment interactions exist. For example, to realize real-time dynamic monitoring of exposure to viruses or bacteria in the environment, a wearable freeze-dried, cell-free (wFDCF) platform was developed by Nguyen et al.[159]. The reported wFDCF POF system apparel involves attaching a fabric-based module to a wearable POF spectrometer with wireless connectivity, which is then integrated with a wireless mobile app. The app enables continuous cloud-based data logging, signal processing, geolocation tracking, and real-time control of various detector components from a smartphone or other connected digital device.
Figure 11. (A) Design and fabrication of the BSOS sensor (a), definition of robotic arm control rules based on the BSOS sensor output signal (b), and HMI demonstration of the finger-driven robotic arm for precise handling of paper cups (c)[52]. Copyright 2022, American Chemical Society; (B) Schematic illustration of the tactile sensor (a), real-time response recorded from different finger sensors for grasping and releasing a tennis ball under various gestures (b), intelligent robotics integrated with tactile sensors to perceive material hardness, roughness, and shape of objects (c-e)[54]. Copyright 2023, Wiley; (C) Schematic diagram of the OFN sensing system (a), finite element simulation of strain distribution in OFN sensors under stress (b-d), exploded diagram of the robotic tactile finger (e), and the tri-axial force sensing signals and snapshots during the cutting experiment and the unlocking experiment, respectively (f and g)[53]. Copyright 2023, Opto-Electronic Journals Group. BSOS: Bioinspired stretchable optical fiber-based sensor; HMI: human–machine interaction; OFN: optical fiber knot.
Another application of interactive soft robots is bionic mechanical grippers, the development of which has exploded. Owing to the advantages of the materials and sensing ability of flexible optical waveguides, many well-performing soft grippers and even bionic robots have been reported in recent years[222,223]. As shown in Figure 11B, Guo et al. used AuNPs and elastomer composites to make flexible plasma fibers and proposed a flexible optical tactile sensor[54]. The hardness, roughness, and shape of an object can be sensed via this sensor integrated into a robotic hand. The tactile perception of the human hand when grasping includes the perception of static friction or slippage in addition to the perception of a positive pressure[224]. Therefore, artificial tactile sensing, especially friction measurement and slip detection, plays a crucial role in robot operation[225,226]. The optical microfiber-based flexible tactile sensors inspired by finger skin designed by Jiang et al. have force sensing and slip detection capabilities[227]. When connected to a robotic gripper, the soft sensor successfully distinguishes between soft and hard objects, measures the grasping force, and detects object slip, making it suitable for robotic grasping and manipulation. In some recent studies, inspired by topological mechanics, Pan et al. developed optical fiber knot (OFN) sensors that can be used for slip detection and friction measurements [Figure 11C][53]. The twisted structure of the knot allows the sensor to sense not only loads along the fiber direction but also slippage and triaxial forces through customized detection algorithms. By utilizing the advantages of both optical and electrical dual modes, Shang et al. developed a dual-mode haptic sensor that enables the detection of slippage as well as instantaneous adjustment of the clamping force[228]. In this study, a microstructured piezoresistive layer was constructed for static pressure sensing, and a triboelectrification-induced electroluminescence (TIEL) layer was constructed for dynamic sensing of the sliding force.
FUTURE PERSPECTIVES ON THE TECHNOLOGICAL DEVELOPMENT OF FLEXIBLE OPTICAL WAVEGUIDES
Flexible optical waveguides have emerged as transformative technologies in biomedical applications, offering distinct advantages over conventional optical fibers, such as enhanced flexibility, biocompatibility, and integrability with wearable and implantable systems. Despite significant advancements, several issues must be addressed to facilitate their widespread clinical and industrial adoption. This section outlines key areas for future research and technological development.
Advancing material design and fabrication techniques
The development of novel materials and scalable fabrication processes is crucial for the next generation of flexible optical waveguides. Current materials, including hydrogels, elastomers, and biodegradable polymers, show promise but must be optimized in terms of mechanical robustness, optical performance, and long-term stability[229-231]. Future research should explore high-performance hybrid materials that combine nanocomposites, functionalized polymers, and bioinspired materials to create waveguides with tunable mechanical and optical properties, enhancing the durability and signal integrity. Additionally, improving fabrication methods is essential for scalable production. Existing techniques such as photopolymerization, thermal drawing, and microfluidic molding often fall short in precision and consistency for large-scale manufacturing. Advancements in roll-to-roll processing, 3D printing, and electrospinning could increase the scalability and reproducibility needed for flexible optical waveguides. Moreover, integrating optical waveguides with microfluidics, soft electronics, and bioelectronic interfaces may enable multifunctional capabilities, expanding their use in applications such as real-time health monitoring[158].
Enhancing functional performance and sensing capabilities
Flexible optical waveguides operate primarily on the basis of optical loss, fluorescence, and spectral modulation principles, but their sensing performance can be significantly enhanced by integrating them with emerging photonic and nanophotonic technologies. One promising approach is to incorporate metastructures and plasmonic nanoparticles into optical waveguides to create metasurface-assisted devices. This strategy could enhance light–matter interactions, enabling ultrahigh sensitivity for biochemical and physiological sensing applications[232,233]. Another avenue for improvement is the development of stimuli-responsive optical materials, such as thermochromic, mechanochromic, or bioresponsive materials, which would allow waveguides to dynamically adjust their optical properties in response to environmental changes, thereby improving the real-time adaptability[234]. Furthermore, integrating self-powered sensing capabilities into optical waveguide systems by incorporating triboelectric nanogenerators, piezoelectric elements, or biofuel cells could eliminate the need for external power sources. This integration would enable autonomous and long-term sensing applications, enhancing the practicality and versatility of flexible optical waveguides[205,220].
Overcoming challenges in device miniaturization and system integration
The practical deployment of flexible optical waveguides often faces challenges because of their reliance on bulky optical components such as power meters and signal demodulators. To address this, one approach is to integrate miniaturized photonic circuits. At the electronic level, co-integration of photodetectors (e.g., silicon or organic photodiodes) with low-noise transimpedance amplifiers, programmable-gain readout, and on-chip analog-to-digital conversion can reduce parasitics and improve SNR, while compact microcontrollers enable on-node preprocessing (filtering, demodulation, feature extraction) to lower data rates and power. By incorporating on-chip waveguide structures, silicon photonics, and quantum dot-based light sources, compact, portable, and high-performance optical sensing systems can be developed. This would not only reduce the footprint of the device but also enhance its overall functionality. Another key direction is the development of wireless and IoT-enabled platforms. Optical sensors equipped with Bluetooth or 5G capabilities can facilitate continuous, remote health monitoring by seamlessly transmitting real-time physiological data to cloud-based analytics platforms[217,235]. System-level design for such platforms should consider tight power and thermal budgets (e.g., duty-cycling of sources and readout, adaptive sampling), electromagnetic compatibility/shielding, time-synchronized driving and detection, and robust demodulation to maintain performance under ambient light and motion. Data integrity, on-device compression, secure transmission, and firmware/OTA update pathways further influence clinical readiness and maintainability. Additionally, improving optical coupling mechanisms is essential for enhancing device reliability and reducing signal losses. Advanced alignment techniques and micro-optical components can significantly increase the waveguide-to-fiber and waveguide-to-detector coupling efficiencies, ensuring seamless integration with existing optical infrastructure and optimizing the performance of flexible optical waveguides. Finally, biocompatible encapsulation, hermetic sealing around electronic interfaces, and reliable flexible interconnects with appropriate strain-relief are critical to preserve calibration, bandwidth, and longevity during repeated deformation and sterilization cycles.
CONCLUSION AND OUTLOOK
We have comprehensively reviewed the design and diverse biomedical applications of flexible optical waveguides. By exploring the unique optical and mechanical properties of advanced materials such as hydrogels, elastomers, and biodegradable polymers, our analysis underscores how these flexible platforms overcome the limitations of traditional rigid systems. These waveguides enable high-sensitivity sensing, minimally invasive diagnosis, and targeted therapeutic intervention, thus laying a solid foundation for real-time health monitoring and enhanced clinical performance.
Looking ahead, flexible optical waveguides represent a rapidly evolving technology with significant potential not only in biomedical applications but also in neuroengineering and interactive soft robotics. However, to achieve their full potential, key challenges in material innovation, functional enhancement, miniaturization, and system integration must be addressed. Future research should focus on developing scalable fabrication techniques, intelligent self-powered sensing systems, and multimodal photonic integration strategies. Overcoming these hurdles will be pivotal in advancing next-generation healthcare technologies and establishing flexible optical waveguides as core components in emerging medical and engineering applications. Regulatory considerations, clinical translation barriers, and commercialization pathways are critical factors for the widespread adoption of flexible optical waveguide-based devices. Regulatory approval processes must be navigated, ensuring that these devices meet safety and efficacy standards. Additionally, overcoming clinical translation challenges, such as biocompatibility and long-term stability, will be key to successful deployment in healthcare settings. Commercialization efforts will require the development of cost-effective manufacturing processes, along with market acceptance, to realize the full potential of these technologies in real-world applications.
DECLARATIONS
Authors’ contributions
Writing - original draft: Yang, C.; Chen, S.; Zou, Y.; Ren, Y.
Writing - review and editing: Wang, Z.; Xiao, K.; Leal-Junior, A.; Kumar, S.; Min, R.
Funding acquisition, supervision: Min, R.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This research was funded by the National Key R&D Program of China (2022YFE0140400); the National Natural Science Foundation of China (62405027, 62111530238, 62003046); The work of R. Min was supported by the Tang Scholar of Beijing Normal University.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. World Health Organization. Regional Office for South-East Asia. Draft Fourteenth General Programme of Work (GPW14) 2025-2028. https://iris.who.int/handle/10665/373010. (accessed 8 Sep 2025).
2. World Health Organization. Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). (accessed 8 Sep 2025).
3. Du, Y.; Kim, J. H.; Kong, H.; et al. Biocompatible electronic skins for cardiovascular health monitoring. Adv. Healthc. Mater. 2024, 13, e2303461.
5. Caffè, A.; Animati, F. M.; Iannaccone, G.; Rinaldi, R.; Montone, R. A. Precision medicine in acute coronary syndromes. J. Clin. Med. 2024, 13, 4569.
6. Ahmed, M. R.; Newby, S.; Potluri, P.; Mirihanage, W.; Fernando, A. Emerging paradigms in fetal heart rate monitoring: evaluating the efficacy and application of innovative textile-based wearables. Sensors 2024, 24, 6066.
7. Yadav, A.; Yadav, N.; Wu, Y.; Ramakrishna, S.; Hongyu, Z. Wearable strain sensors: state-of-the-art and future applications. Mater. Adv. 2023, 4, 1444-59.
8. Liao, Y. T.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B. P. A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE. J. Solid. State. Circuits. 2012, 47, 335-44.
9. Yao, H.; Liao, Y.; Lingley, A. R.; et al. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 2012, 22, 075007.
10. Thomas, N.; Lähdesmäki, I.; Parviz, B. A contact lens with an integrated lactate sensor. Sens. Actuators. B. Chem. 2012, 162, 128-34.
11. Mannoor, M. S.; Tao, H.; Clayton, J. D.; et al. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763.
12. Kim, J.; Valdés-Ramírez, G.; Bandodkar, A. J.; et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632-6.
13. Guinovart, T.; Parrilla, M.; Crespo, G. A.; Rius, F. X.; Andrade, F. J. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst 2013, 138, 5208-15.
14. Bandodkar, A. J.; Molinnus, D.; Mirza, O.; et al. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603-9.
15. Bandodkar, A. J.; Wang, J. Non-invasive wearable electrochemical sensors: a review. Trends. Biotechnol. 2014, 32, 363-71.
16. Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS. Nano. 2014, 8, 5154-63.
17. Park, B.; Kim, J.; Kang, D.; et al. Dramatically enhanced mechanosensitivity and signal-to-noise ratio of nanoscale crack-based sensors: effect of crack depth. Adv. Mater. 2016, 28, 8130-7.
18. Liu, Z.; Qi, D.; Guo, P.; et al. Thickness-gradient films for high gauge factor stretchable strain sensors. Adv. Mater. 2015, 27, 6230-7.
19. Kim, S. R.; Kim, J. H.; Park, J. W. Wearable and transparent capacitive strain sensor with high sensitivity based on patterned Ag nanowire networks. ACS. Appl. Mater. Interfaces. 2017, 9, 26407-16.
20. Yao, S.; Zhu, Y. Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale 2014, 6, 2345-52.
21. Chen, Z.; Qu, C.; Yao, J.; Zhang, Y.; Xu, Y. Two-stage micropyramids enhanced flexible piezoresistive sensor for health monitoring and human-computer interaction. ACS. Appl. Mater. Interfaces. 2024, 16, 7640-9.
22. Zhou, M.; Xu, Y.; Wang, C.; et al. Amorphous TiO2 inverse opal anode for high-rate sodium ion batteries. Nano. Energy. 2017, 31, 514-24.
23. Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced carbon for flexible and wearable electronics. Adv. Mater. 2019, 31, e1801072.
24. Kim, K. H.; Hwang, A.; Song, Y.; et al. 3D hierarchical nanotopography for on-site rapid capture and sensitive detection of infectious microbial pathogens. ACS. Nano. 2021, 15, 4777-88.
25. Qu, X.; Xue, J.; Liu, Y.; Rao, W.; Liu, Z.; Li, Z. Fingerprint-shaped triboelectric tactile sensor. Nano. Energy. 2022, 98, 107324.
26. Yu, Q.; Zhang, Y. N.; Jiang, L.; Li, L.; Li, X.; Zhao, J. Flexible optical fiber sensor for non-invasive continuous monitoring of human physiological signals. Small. Methods. 2025, 9, e2401368.
27. Guo, J.; Zhou, B.; Yang, C.; Dai, Q.; Kong, L. Stretchable and temperature-sensitive polymer optical fibers for wearable health monitoring. Adv. Funct. Mater. 2019, 29, 1902898.
28. Li, R.; Zhang, T.; Yu, Y.; Jiang, Y.; Zhang, X.; Wang, L. Flexible multilayer substrate based optical waveguides: applications to optical sensing. Sens. Actuators. A. Phys. 2014, 209, 57-61.
29. Sekine, M.; Furuya, M. Development of measurement method for temperature and velocity field with optical fiber sensor. Sensors 2023, 23, 1627.
30. Zhang, M.; Liu, Z.; Zhang, Y.; et al. Spider silk as a flexible light waveguide for temperature sensing. J. Lightwave. Technol. 2023, 41, 1884-9.
31. Funnell, A. C.; Thomas, P. J. Design of a flexible weight sensor using optical fibre macrobending. Sensors 2023, 23, 912.
32. Zhang, H.; Wu, J.; Gao, C. Research on the fabrication and parameters of a flexible fiber optic pressure sensor with high sensitivity. Photonics 2024, 11, 919.
33. To, C.; Hellebrekers, T.; Jung, J.; Yoon, S. J.; Park, Y. A soft optical waveguide coupled with fiber optics for dynamic pressure and strain sensing. IEEE. Robot. Autom. Lett. 2018, 3, 3821-7.
34. Pathak, A. K.; Singh, V. K. A wide range and highly sensitive optical fiber pH sensor using polyacrylamide hydrogel. Opt. Fiber. Technol. 2017, 39, 43-8.
35. Ren, Y.; Mormile, P.; Petti, L.; Cross, G. H. Optical waveguide humidity sensor with symmetric multilayer configuration. Sens. Actuators. B. Chem. 2001, 75, 76-82.
36. Liang, L.; Xie, F.; Jin, L.; Yang, B.; Sun, L.; Guan, B. Optical microfiber biomedical sensors: classification, applications, and future perspectives. Adv. Sens. Res. 2025, 4, 2400185.
37. Jha, R.; Mishra, P.; Kumar, S. Advancements in optical fiber-based wearable sensors for smart health monitoring. Biosens. Bioelectron. 2024, 254, 116232.
38. Zha, B.; Wang, Z.; Ma, L.; et al. Intelligent wearable photonic sensing system for remote healthcare monitoring using stretchable elastomer optical fiber. IEEE. Internet. Things. J. 2024, 11, 17317-29.
39. Luo, Z.; Li, M.; Kong, X.; et al. Advance on fiber optic-based biosensors for precision medicine: from diagnosis to therapy. Interdiscip. Med. 2023, 1, e20230022.
40. Quandt, B. M.; Scherer, L. J.; Boesel, L. F.; Wolf, M.; Bona, G. L.; Rossi, R. M. Body-monitoring and health supervision by means of optical fiber-based sensing systems in medical textiles. Adv. Healthc. Mater. 2015, 4, 330-55.
41. Li, W.; Long, Y.; Yan, Y.; et al. Wearable photonic smart wristband for cardiorespiratory function assessment and biometric identification. Opto. Electron. Adv. 2025, 8, 240254.
42. Wang, Y.; Zhou, Y.; Qi, L.; Zhang, Y. Soft optical fibers for biomedical and wearable technologies: current trends and future prospects. Adv. Funct. Mater. 2025, 2507712.
43. De Chiara, F.; Wang, S.; Liu, H. Creating a soft tactile skin employing fluorescence based optical sensing. IEEE. Robot. Autom. Lett. 2020, 5, 3375-81.
44. Otsuka, R.; Zhang, S.; Hayashi, K.; Sassa, F. Active optical sensor microrobot equipped with multi-DoF gripper arm based on kinetic electronics. IEEE. Sens. Lett. 2023, 7, 1-4.
45. Kurochkin, M. A.; Sindeeva, O. A.; Brodovskaya, E. P.; et al. Laser-triggered drug release from polymeric 3-D micro-structured films via optical fibers. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 110, 110664.
46. Zheng, D.; Pisano, F.; Collard, L.; et al. Toward plasmonic neural probes: SERS detection of neurotransmitters through gold-nanoislands-decorated tapered optical fibers with sub-10 nm gaps. Adv. Mater. 2023, 35, e2200902.
47. Jiang, Y.; Qi, W.; Zhang, Q.; et al. Green light-based photobiomodulation with an implantable and biodegradable fiber for bone regeneration. Small. Methods. 2020, 4, 1900879.
48. Teh, D. B. L.; Bansal, A.; Chai, C.; et al. A flexi-PEGDA upconversion implant for wireless brain photodynamic therapy. Adv. Mater. 2020, 32, e2001459.
49. Xiang, K.; Liu, M.; Chen, J.; et al. AI-assisted insole sensing system for multifunctional plantar-healthcare applications. ACS. Appl. Mater. Interfaces. 2024, 16, 32662-78.
50. Bae, S. H.; Kim, D.; Chang, S. Y.; et al. Hybrid integrated photomedical devices for wearable vital sign tracking. ACS. Sens. 2020, 5, 1582-8.
51. Mishra, P.; Sahu, P. K.; Kumar, H.; Jha, R. Human pulse and respiration monitoring: reconfigurable and scalable balloon-shaped fiber wearables. Adv. Mater. Technol. 2023, 8, 2300429.
52. Li, T.; Su, Y.; Chen, F.; et al. Bioinspired stretchable fiber-based sensor toward intelligent human-machine interactions. ACS. Appl. Mater. Interfaces. 2022, 14, 22666-77.
53. Pan, J.; Wang, Q.; Gao, S.; et al. Knot-inspired optical sensors for slip detection and friction measurement in dexterous robotic manipulation. Opto. Electronic. Adv. 2023, 6, 230076.
54. Guo, J.; Shang, C.; Gao, S.; Zhang, Y.; Fu, B.; Xu, L. Flexible plasmonic optical tactile sensor for health monitoring and artificial haptic perception. Adv. Mater. Technol. 2023, 8, 2201506.
55. Gong, Z.; Xiang, Z.; OuYang, X.; et al. Wearable fiber optic technology based on smart textile: a review. Materials 2019, 12, 3311.
56. Gan, J.; Yang, A.; Guo, Q.; Yang, Z. Flexible optical fiber sensing: materials, methodologies, and applications. Adv. Devices. Instrum. 2024, 5, 0046.
57. Rein, M.; Favrod, V. D.; Hou, C.; et al. Diode fibres for fabric-based optical communications. Nature 2018, 560, 214-8.
58. Koeppel, M.; Sharma, A.; Podschus, J.; et al. Doppler optical frequency domain reflectometry for remote fiber sensing: erratum. Opt. Express. 2021, 29, 24193.
59. Stellinga, D.; Phillips, D. B.; Mekhail, S. P.; et al. Time-of-flight 3D imaging through multimode optical fibers. Science 2021, 374, 1395-9.
60. Guo, J.; Yang, C.; Dai, Q.; Kong, L. Soft and stretchable polymeric optical waveguide-based sensors for wearable and biomedical applications. Sensors 2019, 19, 3771.
61. Choi, M.; Choi, J. W.; Kim, S.; Nizamoglu, S.; Hahn, S. K.; Yun, S. H. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics. 2013, 7, 987-94.
62. Okumura, Y.; Ito, K. The polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv. Mater. 2001, 13, 485-7.
63. Browning, M. B.; Wilems, T.; Hahn, M.; Cosgriff-Hernandez, E. Compositional control of poly(ethylene glycol) hydrogel modulus independent of mesh size. J. Biomed. Mater. Res. A. 2011, 98, 268-73.
64. Gaharwar, A. K.; Rivera, C. P.; Wu, C. J.; Schmidt, G. Transparent, elastomeric and tough hydrogels from poly(ethylene glycol) and silicate nanoparticles. Acta. Biomater. 2011, 7, 4139-48.
65. Musumeci, G.; Loreto, C.; Castorina, S.; Imbesi, R.; Leonardi, R.; Castrogiovanni, P. New perspectives in the treatment of cartilage damage. Poly(ethylene glycol) diacrylate (PEGDA) scaffold. A review. Ital. J. Anat. Embryol. 2013, 118, 204-10.
66. Zhang, Z. F.; Ma, X.; Wang, H.; Ye, F. Influence of polymerization conditions on the refractive index of poly(ethylene glycol) diacrylate (PEGDA) hydrogels. Appl. Phys. A. 2018, 124, 1713.
67. Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S. A.; Aria, A. I. Additive manufacturing and physicomechanical characteristics of PEGDA hydrogels: recent advances and perspective for tissue engineering. Polymers 2023, 15, 2341.
68. Yetisen, A. K.; Jiang, N.; Fallahi, A.; et al. Glucose-sensitive hydrogel optical fibers functionalized with phenylboronic acid. Adv. Mater. 2017, 29, 1606380.
69. Kwok, S. J. J.; Kim, M.; Lin, H. H.; et al. Flexible optical waveguides for uniform periscleral cross-linking. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2596-602.
70. Martincek, I.; Pudis, D.; Chalupova, M. Technology for the preparation of PDMS optical fibers and some fiber structures. IEEE. Photon. Technol. Lett. 2014, 26, 1446-9.
71. Lu, C.; Park, S.; Richner, T. J.; et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Sci. Adv. 2017, 3, e1600955.
72. Martincek, I.; Pudis, D.; Gaso, P. Fabrication and optical characterization of strain variable PDMS biconical optical fiber taper. IEEE. Photon. Technol. Lett. 2013, 25, 2066-9.
73. Wang, Z.; Volinsky, A. A.; Gallant, N. D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 2014, 131, app.41050.
74. Johnston, I. D.; Mccluskey, D. K.; Tan, C. K. L.; Tracey, M. C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017.
75. Darby, D. R.; Cai, Z.; Mason, C. R.; Pham, J. T. Modulus and adhesion of Sylgard 184, Solaris, and Ecoflex 00-30 silicone elastomers with varied mixing ratios. J. Appl. Polym. Sci. 2022, 139, e52412.
76. Li, Y.; Hu, J.; Cao, D.; Wang, S.; Dasgupta, P.; Liu, H. Optical-waveguide based tactile sensing for surgical instruments of minimally invasive surgery. Front. Robot. AI. 2021, 8, 773166.
77. Vaicekauskaite, J.; Mazurek, P.; Vudayagiri, S.; Skov, A. L. Mapping the mechanical and electrical properties of commercial silicone elastomer formulations for stretchable transducers. J. Mater. Chem. C. 2020, 8, 1273-9.
78. Cheng, X.; Miao, L.; Su, Z.; et al. Controlled fabrication of nanoscale wrinkle structure by fluorocarbon plasma for highly transparent triboelectric nanogenerator. Microsyst. Nanoeng. 2017, 3, 16074.
79. Nizamoglu, S.; Gather, M. C.; Humar, M.; et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun. 2016, 7, 10374.
80. Kim, M.; An, J.; Kim, K. S.; et al. Optical lens-microneedle array for percutaneous light delivery. Biomed. Opt. Express. 2016, 7, 4220-7.
81. Bergström, J. S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330-40.
82. Zhao, H.; O’Brien, K.; Li, S.; Shepherd, R. F. Optoelectronically innervated soft prosthetic hand via stretchable optical waveguides. Sci. Robot. 2016, 1, eaai7529.
83. Hu, L.; Chee, P. L.; Sugiarto, S.; et al. Hydrogel-based flexible electronics. Adv. Mater. 2023, 35, e2205326.
84. Sadeque, M. S. B.; Chowdhury, H. K.; Rafique, M.; et al. Hydrogel-integrated optical fiber sensors and their applications: a comprehensive review. J. Mater. Chem. C. 2023, 11, 9383-424.
85. Shabahang, S.; Kim, S.; Yun, S. H. Light-guiding biomaterials for biomedical applications. Adv. Funct. Mater. 2018, 28, 1706635.
86. Nurlidar, F.; Rahayu, D. P.; Lasmawati, D.; Yunus, A. L.; Heryani, R.; Suryani, N. A simple method for the simultaneous encapsulation of ciprofloxacin into PEGDA/alginate hydrogels using gamma irradiation. Arab. J. Chem. 2023, 16, 104793.
87. Tiwari, G.; Tiwari, R.; Sriwastawa, B.; et al. Drug delivery systems: an updated review. Int. J. Pharm. Investig. 2012, 2, 2-11.
88. Tibbitt, M. W.; Dahlman, J. E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704-17.
89. Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639-56.
90. Sun, S.; Cui, Y.; Yuan, B.; et al. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647.
91. Paneer Selvam, S.; Ayyappan, S.; I Jamir, S.; Sellappan, L. K.; Manoharan, S. Recent advancements of hydroxyapatite and polyethylene glycol (PEG) composites for tissue engineering applications - a comprehensive review. Eur. Polym. J. 2024, 215, 113226.
92. Zhang, Y.; Zhang, J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J. Colloid. Interface. Sci. 2005, 283, 352-7.
93. Xin, H.; Li, Y.; Liu, X.; Li, B.
94. Choi, M.; Humar, M.; Kim, S.; Yun, S. H. Step-index optical fiber made of biocompatible hydrogels. Adv. Mater. 2015, 27, 4081-6.
95. Li, W.; Lin, M.; Wang, C.; et al.
96. Jin, H.; Yoon, S. S.; Kim, S. C. Synthesis and characterization of interpenetrating polymer networks from polyurethane and poly(ethylene glycol) diacrylate. J. Appl. Polym. Sci. 2008, 109, 805-12.
97. Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of furan derivative as LED light photoinitiator: one-pot, low usage, photobleaching for light color 3D printing. Dyes. Pigments. 2019, 165, 467-73.
98. Kaastrup, K.; Aguirre-Soto, A.; Wang, C.; Bowman, C. N.; Stansbury, J.; Sikes, H. D. UV-Vis/FT-NIR in situ monitoring of visible-light induced polymerization of PEGDA hydrogels initiated by eosin/triethanolamine/O2. Polym. Chem. 2016, 7, 592-602.
99. Fairbanks, B. D.; Schwartz, M. P.; Bowman, C. N.; Anseth, K. S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702-7.
100. Sun, G.; Pan, X.; Zhong, Y.; Chen, E.; Huang, Y.; Shao, J. Blue light induced polymerization kinetics of polyethylene glycol diacrylate hydrogel. Polym. Mater. Sci. Eng. 2022.
101. LeValley, P. J.; Noren, B.; Kharkar, P. M.; Kloxin, A. M.; Gatlin, J. C.; Oakey, J. S. Fabrication of functional biomaterial microstructures by in situ photopolymerization and photodegradation. ACS. Biomater. Sci. Eng. 2018, 4, 3078-87.
102. Bae, J.; Park, J.; Kim, S.; et al. Tailored hydrogels for biosensor applications. J. Ind. Eng. Chem. 2020, 89, 1-12.
103. Liu, R.; Fan, X.; Fu, X.; et al. Synthesis and properties of thermo-sensitive PEG hydrogel. Fine. Chem. 2018, 35, 429-36. http://en.cnki.com.cn/Article_en/CJFDTotal-JXHG201803011.htm. (accessed 8 Sep 2025).
104. Li, Z.; Mi, W.; Wang, H.; Su, Y.; He, C. Nano-hydroxyapatite/polyacrylamide composite hydrogels with high mechanical strengths and cell adhesion properties. Colloids. Surf. B. Biointerfaces. 2014, 123, 959-64.
105. Wang, L.; Zhong, C.; Ke, D.; et al. Ultrasoft and highly stretchable hydrogel optical fibers for in vivo optogenetic modulations. Adv. Opt. Mater. 2018, 6, 1800427.
106. Shanks, R. A.; Kong, I. General purpose elastomers: structure, chemistry, physics and performance. In Advances in Elastomers I: Blends and Interpenetrating Networks; Visakh, P. M., Thomas, S., Chandra, A. K., Mathew, Aji. P., Eds.; vol 11; Springer: Berlin, Heidelberg, 2013; pp. 11-45.
107. Miranda, I.; Souza, A.; Sousa, P.; et al. Properties and applications of PDMS for biomedical engineering: a review. J. Funct. Biomater. 2021, 13, 2.
108. Khanafer, K.; Duprey, A.; Schlicht, M.; Berguer, R. Effects of strain rate, mixing ratio, and stress-strain definition on the mechanical behavior of the polydimethylsiloxane (PDMS) material as related to its biological applications. Biomed. Microdevices. 2009, 11, 503-8.
109. Prajzler, V.; Nekvindova, P.; Spirkova, J.; Novotny, M. The evaluation of the refractive indices of bulk and thick polydimethylsiloxane and polydimethyl-diphenylsiloxane elastomers by the prism coupling technique. J. Mater. Sci. Mater. Electron. 2017, 28, 7951-61.
110. Lamberti, A.; Marasso, S. L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. RSC. Adv. 2014, 4, 61415-9.
111. Fan, X.; Huang, Y.; Ding, X.; et al. Alignment-free liquid-capsule pressure sensor for cardiovascular monitoring. Adv. Funct. Mater. 2018, 28, 1805045.
112. Qian, X.; Cai, Z.; Su, M.; et al. Printable skin-driven mechanoluminescence devices via nanodoped matrix modification. Adv. Mater. 2018, 30, e1800291.
113. Rothmaier, M.; Luong, M. P.; Clemens, F. Textile pressure sensor made of flexible plastic optical fibers. Sensors 2008, 8, 4318-29.
114. Leber, A.; Cholst, B.; Sandt, J.; Vogel, N.; Kolle, M. Stretchable thermoplastic elastomer optical fibers for sensing of extreme deformations. Adv. Funct. Mater. 2019, 29, 1802629.
115. Qu, Y.; Nguyen-Dang, T.; Page, A. G.; et al. Superelastic multimaterial electronic and photonic fibers and devices via thermal drawing. Adv. Mater. 2018, 30, e1707251.
116. Fu, R.; Luo, W.; Nazempour, R.; et al. Implantable and biodegradable poly(l-lactic acid) fibers for optical neural interfaces. Adv. Opt. Mater. 2018, 6, 1700941.
117. Qin, H.; Owyeung, R. E.; Sonkusale, S. R.; Panzer, M. J. Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing. J. Mater. Chem. C. 2019, 7, 601-8.
118. Wu, S. D.; Hsu, S. H.; Ketelsen, B.; et al. Fabrication of eco-friendly wearable strain sensor arrays via facile contact printing for healthcare applications. Small. Methods. 2023, 7, e2300170.
119. Hu, H.; Sun, S.; Lv, R.; Zhao, Y. Design and experiment of an optical fiber micro bend sensor for respiration monitoring. Sens. Actuators. A. Phys. 2016, 251, 126-33.
120. Praveena, S.; Melwin, G.; Babu, P. R.; Senthilnathan, K. MoS2 sensitized tapered fiber optic evanescent wave sensor for refractive index based glucose sensing application. Curr. Appl. Phys. 2025, 77, 46-56.
121. Li, Y.; Luo, S.; Gui, Y.; Wang, X.; Tian, Z.; Yu, H. Difunctional hydrogel optical fiber fluorescence sensor for continuous and simultaneous monitoring of glucose and pH. Biosensors 2023, 13, 287.
122. Li, J.; Li, H.; Long, Z.; Meng, L.; Guo, H.; Lv, M. Wearable multifunctional optical sensor based on Er3+/Yb3+ co-doped Gd2O3 nanoparticles and tapered U-shaped fiber. Opt. Lett. 2025, 50, 281-4.
123. Li, H.; Ma, S.; Ding, M.; et al. New class of optical blood glucose sensors based on a PMMA Mach–Zehnder interferometer. ACS. Photonics. 2024, 11, 1684-92.
124. Levi, A.; Piovanelli, M.; Furlan, S.; Mazzolai, B.; Beccai, L. Soft, transparent, electronic skin for distributed and multiple pressure sensing. Sensors 2013, 13, 6578-604.
125. Peng, W.; Liao, Q.; Song, H. A nanograting-based flexible and stretchable waveguide for tactile sensing. Nanoscale. Res. Lett. 2021, 16, 23.
126. He, R.; Shen, L.; Wang, Z.; et al. Optical fiber sensors for heart rate monitoring: a review of mechanisms and applications. Results. Opt. 2023, 11, 100386.
127. Youn, J.; Mun, H.; Jang, S.; Kyung, K. Highly stretchable-compressible coiled polymer sensor for soft continuum manipulator. Smart. Mater. Struct. 2022, 31, 015043.
128. Huang, X.; Yang, M.; Liu, T.; Su, H.; Cui, X. An approach on a new variable amplitude waveform sensor. Optik 2017, 132, 52-66.
129. Yun, S.; Jeong, J.; Mun, S.; Kyung, K. A highly stretchable optical strain sensor monitoring dynamically large strain for deformation-controllable soft actuator. Smart. Mater. Struct. 2021, 30, 105020.
130. Al-Lami, S. S.; Atea, H.; Salman, A. M.; Al-Janabi, A. Adjustable optical fiber displacement-curvature sensor based on macro-bending losses with a coding of optical signal intensity. Sens. Actuators. A. Phys. 2024, 373, 115403.
131. Min, R.; Hu, X.; Pereira, L.; et al. Polymer optical fiber for monitoring human physiological and body function: a comprehensive review on mechanisms, materials, and applications. Opt. Laser. Technol. 2022, 147, 107626.
132. Guo, J.; Niu, M.; Yang, C. Highly flexible and stretchable optical strain sensing for human motion detection. Optica 2017, 4, 1285.
133. Mäntele, W.; Deniz, E. UV-VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2017, 173, 965-8.
134. Wang, W.; Li, Z.; Zhao, R.; He, Y.; Tao, G.; Hou, C. Stretchable polymer optical fiber with an unusual relationship between optical loss and elongation. J. Lightwave. Technol. 2024, 42, 3370-5.
135. Huong, A.; Ngu, X. Quantitative analysis of spectroscopy data for skin oximetry. In Proceedings of the 3rd International Conference on Biomedical and Bioinformatics Engineering. Association for Computing Machinery; 2016. pp. 46-9.
136. Fuente, D.; Lizama, C.; Urchueguía, J. F.; Conejero, J. A. Estimation of the light field inside photosynthetic microorganism cultures through Mittag-Leffler functions at depleted light conditions. J. Quant. Spectrosc. Radiat. Transf. 2018, 204, 23-6.
137. Nagar, M. A.; Janner, D. Polymer-based optical guided-wave biomedical sensing: from principles to applications. Photonics 2024, 11, 972.
138. Pendão, C.; Silva, I. Optical fiber sensors and sensing networks: overview of the main principles and applications. Sensors 2022, 22, 7554.
139. Jakubowski, K.; Huang, C.; Boesel, L. F.; Hufenus, R.; Heuberger, M. Recent advances in photoluminescent polymer optical fibers. Curr. Opin. Solid. State. Mater. Sci. 2021, 25, 100912.
140. Shin, Y. H.; Teresa, Gutierrez-Wing. M. T.; Choi, J. W. Review - recent progress in portable fluorescence sensors. J. Electrochem. Soc. 2021, 168, 017502.
141. Wang, Q.; Liao, M.; Lin, Q.; Xiong, M.; Mu, Z.; Wu, F. A review on fluorescence intensity ratio thermometer based on rare-earth and transition metal ions doped inorganic luminescent materials. J. Alloys. Compd. 2021, 850, 156744.
142. Li, Z.; Lan, N.; Cheng, Z.; et al.
143. Stich, M. I.; Fischer, L. H.; Wolfbeis, O. S. Multiple fluorescent chemical sensing and imaging. Chem. Soc. Rev. 2010, 39, 3102-14.
145. Bilro, L.; Alberto, N.; Pinto, J. L.; Nogueira, R. Optical sensors based on plastic fibers. Sensors 2012, 12, 12184-207.
146. Zhao, C.; Liu, D.; Cai, Z.; et al. A wearable breath sensor based on fiber-tip microcantilever. Biosensors 2022, 12, 168.
147. Sirkis, T.; Beiderman, Y.; Agdarov, S.; Beiderman, Y.; Zalevsky, Z. Blood pulse wave velocity and pressure sensing via fiber based and free space based optical sensors. Nanoscale. Imag. Sens. Actuat. Biomed. Appl. 2017, 10077, 100770A.
148. Measures, R. M. Fiber-optic-based smart structures. In Encyclopedia of Physical Science and Technolog. 2003. pp. 769-802.
149. Shao, M.; Yuan, Y.; Liu, Y.; Fu, H.; Qiao, X. All-fiber michelson interferometer for heart rate and breath monitoring. IEEE. Sensors. J. 2024, 24, 23909-17.
150. Zheng, M.; Shen, Y.; Zou, Q.; et al. Moisture-driven switching of plasmonic bound states in the continuum in the visible region. Adv. Funct. Mater. 2023, 33, 2209368.
151. Chen, G.; Hou, K.; Yu, N.; et al. Temperature-adaptive hydrogel optical waveguide with soft tissue-affinity for thermal regulated interventional photomedicine. Nat. Commun. 2022, 13, 7789.
152. Zhang, W.; Ju, L.; Jia, H.; Ding, X.; Feng, Y. Semiring-optic-fiber (SROF) sensor-based abnormal gait recognition via monitoring muscle activation. IEEE. Sensors. J. 2023, 23, 19307-17.
153. Wang, S.; Liu, B.; Wang, Y.; et al. Machine-learning-based human motion recognition via wearable plastic-fiber sensing system. IEEE. Internet. Things. J. 2023, 10, 17893-904.
154. Wang, Z.; Chen, Z.; Ma, L.; et al. Optical microfiber intelligent sensor: wearable cardiorespiratory and behavior monitoring with a flexible wave-shaped polymer optical microfiber. ACS. Appl. Mater. Interfaces. 2024, 16, 8333-45.
155. Li, L.; Sheng, S.; Liu, Y.; et al. Automatic and continuous blood pressure monitoring via an optical-fiber-sensor-assisted smartwatch. PhotoniX 2023, 4, 99.
156. Sui, K.; Meneghetti, M.; Kaur, J.; Sørensen, R. J. F.; Berg, R. W.; Markos, C. Drug delivery and optical neuromodulation using a structured polymer optical fiber with ultra-high NA. In Proceedings of the Optogenetics and Optical Manipulation. 2023.
157. Zheng, W. L.; Zhang, Y. N.; Li, L. K.; Li, X. G.; Zhao, Y. A plug-and-play optical fiber SPR sensor for simultaneous measurement of glucose and cholesterol concentrations. Biosens. Bioelectron. 2022, 198, 113798.
158. Jiang, Q.; Liang, X.; Chen, Z.; et al. Wearable strain sensor integrating mechanoluminescent fiber with a flexible printed circuit. Opt. Lett. 2024, 49, 1221-4.
159. Nguyen, P. Q.; Soenksen, L. R.; Donghia, N. M.; et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 2021, 39, 1366-74.
160. Yun, S. H.; Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008.
161. Serajuddin, A. T. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 1999, 88, 1058-66.
162. Savjani, K. T.; Gajjar, A. K.; Savjani, J. K. Drug solubility: importance and enhancement techniques. ISRN. Pharm. 2012, 2012, 195727.
163. Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151-67.
164. Dreiss, C. A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid. Interface. Sci. 2020, 48, 1-17.
165. Sandoval-Yañez, C.; Escobar, L.; Amador, C. A. The advantages of polymeric hydrogels in calcineurin inhibitor delivery. Processes 2020, 8, 1331.
166. Hu, Y.; Minzioni, P.; Hui, J.; Yun, S.; Yetisen, A. K. Fiber optic devices for diagnostics and therapy in photomedicine. Adv. Opt. Mater. 2024, 12, 2400478.
167. Bajgrowicz-Cieslak, M.; Alqurashi, Y.; Elshereif, M. I.; Yetisen, A. K.; Hassan, M. U.; Butt, H. Optical glucose sensors based on hexagonally-packed 2.5-dimensional photonic concavities imprinted in phenylboronic acid functionalized hydrogel films. RSC. Adv. 2017, 7, 53916-24.
168. Elsherif, M.; Hassan, M. U.; Yetisen, A. K.; Butt, H. Hydrogel optical fibers for continuous glucose monitoring. Biosens. Bioelectron. 2019, 137, 25-32.
169. Guo, J.; Zhou, B.; Du, Z.; Yang, C.; Kong, L.; Xu, L. Soft and plasmonic hydrogel optical probe for glucose monitoring. Nanophotonics 2021, 10, 3549-58.
170. Davies, S.; Hu, Y.; Blyth, J.; Jiang, N.; Yetisen, A. K. Reusable dual-photopolymerized holographic glucose sensors. Adv. Funct. Mater. 2023, 33, 2214197.
171. Ujah, E.; Lai, M.; Slaughter, G. Ultrasensitive tapered optical fiber refractive index glucose sensor. Sci. Rep. 2023, 13, 4495.
172. Wen, X.; Liu, Y.; Liu, Q.; et al. Glucose sensing based on hydrogel grating incorporating phenylboronic acid groups. Opt. Express. 2022, 30, 47541-52.
173. Ahmed, I.; Elsherif, M.; Ali, M.; Al Ghaferi, A.; Mohammad, B.; Butt, H. Photonic hydrogel for continuous glucose monitoring using smartphone readout. Mater. Design. 2023, 231, 112065.
174. Heo, Y. J.; Shibata, H.; Okitsu, T.; Kawanishi, T.; Takeuchi, S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 13399-403.
175. Li, Y.; Liu, W.; Liu, R.; et al. 3D hybrid arrayed Ag/MOF multi-plasmon resonant cavity system for high-performance SPR sensing. Opt. Laser. Technol. 2023, 167, 109825.
176. Ahmed, I.; El Turk, S.; Al Ghaferi, A.; Samad, Y. A.; Butt, H. Nanocomposite hydrogel-based optical fiber probe for continuous glucose sensing. Small. Sci. 2024, 4, 2300189.
177. Deng, C.; Zhao, Q.; Gan, Y.; et al. High-sensitivity hemoglobin detection based on polarization-differential spectrophotometry. Biosens. Bioelectron. 2023, 241, 115667.
178. Rahad, R.; Rakib, A.; Haque, M. A.; Sharar, S. S.; Sagor, R. H. Plasmonic refractive index sensing in the early diagnosis of diabetes, anemia, and cancer: an exploration of biological biomarkers. Results. Phys. 2023, 49, 106478.
179. Luo, M.; Wang, Q. A reflective optical fiber SPR sensor with surface modified hemoglobin for dissolved oxygen detection. Alex. Eng. J. 2021, 60, 4115-20.
180. Safaee, M. M.; Gravely, M.; Roxbury, D. A wearable optical microfibrous biomaterial with encapsulated nanosensors enables wireless monitoring of oxidative stress. Adv. Funct. Mater. 2021, 31, 2006254.
181. Guo, Y.; Zheng, J.; Wang, Z.; Chen, G.; Hou, K.; Zhu, M. Biocompatible optical fiber for photomedical application. Giant 2023, 16, 100195.
182. Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 2013, 42, 530-47.
183. Brown, S. B.; Brown, E. A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet. Oncol. 2004, 5, 497-508.
184. Lai, B.; Loshchenov, M.; Douplik, A.; et al. Three-dimensional fluence rate measurement and data acquisition system for minimally invasive light therapies. Rev. Sci. Instrum. 2009, 80, 043104.
185. Zein, R.; Selting, W.; Hamblin, M. R. Review of light parameters and photobiomodulation efficacy: dive into complexity. J. Biomed. Opt. 2018, 23, 1-17.
186. Huang, Y. Y.; Sharma, S. K.; Carroll, J.; Hamblin, M. R. Biphasic dose response in low level light therapy - an update. Dose. Response. 2011, 9, 602-18.
187. de Freitas, L. F.; Hamblin, M. R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE. J. Sel. Top. Quantum. Electron. 2016, 22, 7000417.
188. Courtois, E.; Guy, J. B.; Axisa, F.; et al. Photobiomodulation by a new optical fiber device: analysis of the in vitro impact on proliferation/migration of keratinocytes and squamous cell carcinomas cells stressed by X-rays. Lasers. Med. Sci. 2021, 36, 1445-54.
189. Naeser, M. A.; Zafonte, R.; Krengel, M. H.; et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J. Neurotrauma. 2014, 31, 1008-17.
190. Pan, W. T.; Liu, P. M.; Ma, D.; Yang, J. J. Advances in photobiomodulation for cognitive improvement by near-infrared derived multiple strategies. J. Transl. Med. 2023, 21, 135.
191. Lee, T. L.; Ding, Z.; Chan, A. S. Can transcranial photobiomodulation improve cognitive function? A systematic review of human studies. Ageing. Res. Rev. 2023, 83, 101786.
192. Shen, J.; Chui, C.; Tao, X. Luminous fabric devices for wearable low-level light therapy. Biomed. Opt. Express. 2013, 4, 2925-37.
193. Zuo, X.; Liang, Z.; Zhang, J.; et al. Photobiomodulation and diffusing optical fiber on spinal cord’s impact on nerve cells from normal spinal cord tissue in piglets. Lasers. Med. Sci. 2022, 37, 259-67.
194. Liang, Z.; Lei, T.; Wang, S.; et al. Photobiomodulation by diffusing optical fiber on spinal cord: a feasibility study in piglet model. J. Biophotonics. 2020, 13, e201960022.
195. Canales, A.; Jia, X.; Froriep, U. P.; et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015, 33, 277-84.
196. Tan, P.; He, L.; Huang, Y.; Zhou, Y. Optophysiology: illuminating cell physiology with optogenetics. Physiol. Rev. 2022, 102, 1263-325.
197. Tsakas, A.; Tselios, C.; Ampeliotis, D.; Politi, C.; Alexandropoulos, D. (INVITED)Review of optical fiber technologies for optogenetics. Results. Opt. 2021, 5, 100168.
198. Gutierrez, D. V.; Mark, M. D.; Masseck, O.; et al. Optogenetic control of motor coordination by Gi/o protein-coupled vertebrate rhodopsin in cerebellar Purkinje cells. J. Biol. Chem. 2011, 286, 25848-58.
199. Chen, S.; Wang, Z.; Xiao, K.; et al. A comprehensive review of optical fiber technologies in optogenetics and their prospective developments in future clinical therapies. Opt. Laser. Technol. 2024, 179, 111332.
200. Park, S.; Guo, Y.; Jia, X.; et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 2017, 20, 612-9.
201. Sharma, K.; Jäckel, Z.; Schneider, A.; Paul, O.; Diester, I.; Ruther, P. Multifunctional optrode for opsin delivery, optical stimulation, and electrophysiological recordings in freely moving rats. J. Neural. Eng. 2021, 18, 066013.
202. Domingues, M. F.; Alberto, N.; Leitao, C. S. J.; et al. Insole optical fiber sensor architecture for remote gait analysis - an e-health solution. IEEE. Internet. Things. J. 2019, 6, 207-14.
203. Avellar, L.; Frizera, A.; Leal-Junior, A. POF Smart Pants: a fully portable optical fiber-integrated smart textile for remote monitoring of lower limb biomechanics. Biomed. Opt. Express. 2023, 14, 3689-704.
204. Leal-Junior, A. G.; Díaz, C. R.; Pontes, M. J.; Marques, C.; Frizera, A. Polymer optical fiber-embedded, 3D-printed instrumented support for microclimate and human-robot interaction forces assessment. Opt. Laser. Technol. 2019, 112, 323-31.
205. Zhou, H.; Wang, X.; He, Y.; et al. Distributed strain sensor based on self-powered, stretchable mechanoluminescent optical fiber. Adv. Intell. Syst. 2023, 5, 2300113.
206. Hou, B.; Yi, L.; Li, C.; et al. An interactive mouthguard based on mechanoluminescence-powered optical fibre sensors for bite-controlled device operation. Nat. Electron. 2022, 5, 682-93.
207. Koyama, S.; Haseda, Y.; Ishizawa, H.; Okazaki, F.; Bonefacino, J.; Tam, H. Measurement of pulsation strain at the fingertip using a plastic FBG sensor. IEEE. Sensors. J. 2021, 21, 21537-45.
208. Li, J.; Chen, J.; Xu, F. Sensitive and wearable optical microfiber sensor for human health monitoring. Adv. Mater. Technol. 2018, 3, 1800296.
209. Liang, H.; Wang, Y.; Kan, L.; et al. Wearable and multifunctional self-mixing microfiber sensor for human health monitoring. IEEE. Sensors. J. 2023, 23, 2122-7.
210. Wang, X.; Zhou, H.; Chen, M.; et al. Wearable ultrasensitive and rapid human physiological monitoring based on microfiber Sagnac interferometer. Sci. China. Inf. Sci. 2024, 67, 3870.
211. Pan, J.; Jiang, C.; Zhang, Z.; Zhang, L.; Wang, X.; Tong, L. Flexible liquid-filled fiber adapter enabled wearable optical sensors. Adv. Mater. Technol. 2020, 5, 2000079.
212. Bonefacino, J.; Tam, H. Y.; Glen, T. S.; et al. Ultra-fast polymer optical fibre Bragg grating inscription for medical devices. Light. Sci. Appl. 2018, 7, 17161.
213. Lo Presti, D.; Bianchi, D.; Massaroni, C.; Gizzi, A.; Schena, E. A soft and skin-interfaced smart patch based on fiber optics for cardiorespiratory monitoring. Biosensors 2022, 12, 363.
214. Yi, Y.; Jiang, Y.; Zhao, H.; Brambilla, G.; Fan, Y.; Wang, P. High-performance ultrafast humidity sensor based on microknot resonator-assisted mach-zehnder for monitoring human breath. ACS. Sens. 2020, 5, 3404-10.
215. Bao, W.; Chen, F.; Lai, H.; Liu, S.; Wang, Y. Wearable breath monitoring based on a flexible fiber-optic humidity sensor. Sens. Actuators. B. Chem. 2021, 349, 130794.
216. Zhang, Z.; Kang, Y.; Yao, N.; et al. A multifunctional airflow sensor enabled by optical micro/nanofiber. Adv. Fiber. Mater. 2021, 3, 359-67.
217. Zhang, H.; Wang, Z.; Teng, C.; Kumar, S.; Li, X.; Min, R. Wearable cardiorespiratory sensor for real-time monitoring with smartphone integration. IEEE. Trans. Instrum. Meas. 2024, 73, 1-10.
218. Shen, L.; Wang, Z.; Xiao, K.; et al. WaveFlex sensor: advancing wearable cardiorespiratory monitoring with flexible wave-shaped polymer optical fiber. IEEE. J. Select. Topics. Quantum. Electron. 2024, 30, 1-9.
219. Bai, H.; Li, S.; Barreiros, J.; Tu, Y.; Pollock, C. R.; Shepherd, R. F. Stretchable distributed fiber-optic sensors. Science 2020, 370, 848-52.
220. Yang, W.; Gong, W.; Gu, W.; et al. Self-powered interactive fiber electronics with visual-digital synergies. Adv. Mater. 2021, 33, e2104681.
221. Khan, H.; Soomro, A. M.; Samad, A.; et al. Highly sensitive mechano-optical strain sensors based on 2D materials for human wearable monitoring and high-end robotic applications. J. Mater. Chem. C. 2022, 10, 932-40.
222. Qu, J.; Mao, B.; Li, Z.; et al. Recent progress in advanced tactile sensing technologies for soft grippers. Adv. Funct. Mater. 2023, 33, 2306249.
223. Xin, Y.; Zhou, X.; Bark, H.; Lee, P. S. The role of 3D printing technologies in soft grippers. Adv. Mater. 2024, 36, e2307963.
224. Mason, M. T. Toward robotic manipulation. Annu. Rev. Control. Robot. Auton. Syst. 2018, 1, 1-28.
225. Billard, A.; Kragic, D. Trends and challenges in robot manipulation. Science 2019, 364, eaat8414.
227. Jiang, C.; Zhang, Z.; Pan, J.; Wang, Y.; Zhang, L.; Tong, L. Finger-skin-inspired flexible optical sensor for force sensing and slip detection in robotic grasping. Adv. Mater. Technol. 2021, 6, 2100285.
228. Shang, K.; He, C.; Zhou, J.; et al. Optical and electrical dual-mode tactile sensor with interlinked interfaces recording normal force and slip for closed-loop robotics. Chem. Eng. J. 2023, 475, 146279.
229. Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal. Transduct. Target. Ther. 2021, 6, 426.
230. Cywar, R. M.; Rorrer, N. A.; Hoyt, C. B.; Beckham, G. T.; Chen, E. Y. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83-103.
231. Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent progress in natural biopolymers conductive hydrogels for flexible wearable sensors and energy devices: materials, structures, and performance. ACS. Appl. Bio. Mater. 2021, 4, 85-121.
232. Yu, H.; Peng, Y.; Yang, Y.; Li, Z. Plasmon-enhanced light–matter interactions and applications. npj. Comput. Mater. 2019, 5, 184.
233. Liu, C.; Wu, T.; Lalanne, P.; Maier, S. A. Enhanced light-matter interaction in metallic nanoparticles: a generic strategy of smart void filling. Nano. Lett. 2024, 24, 4641-8.
234. Guo, Q.; Zhang, X. A review of mechanochromic polymers and composites: from material design strategy to advanced electronics application. Compos. Part. B. Eng. 2021, 227, 109434.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data






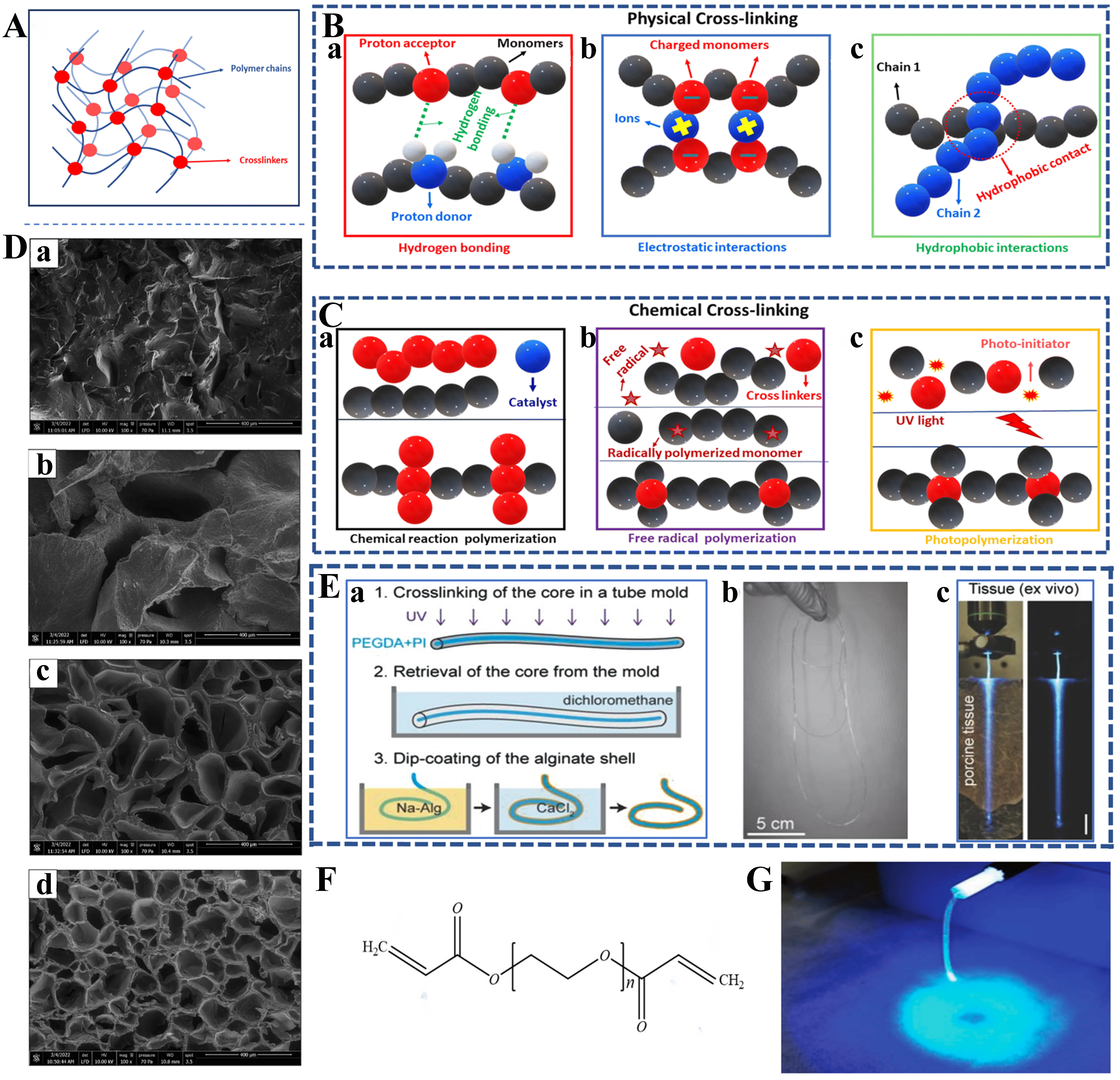
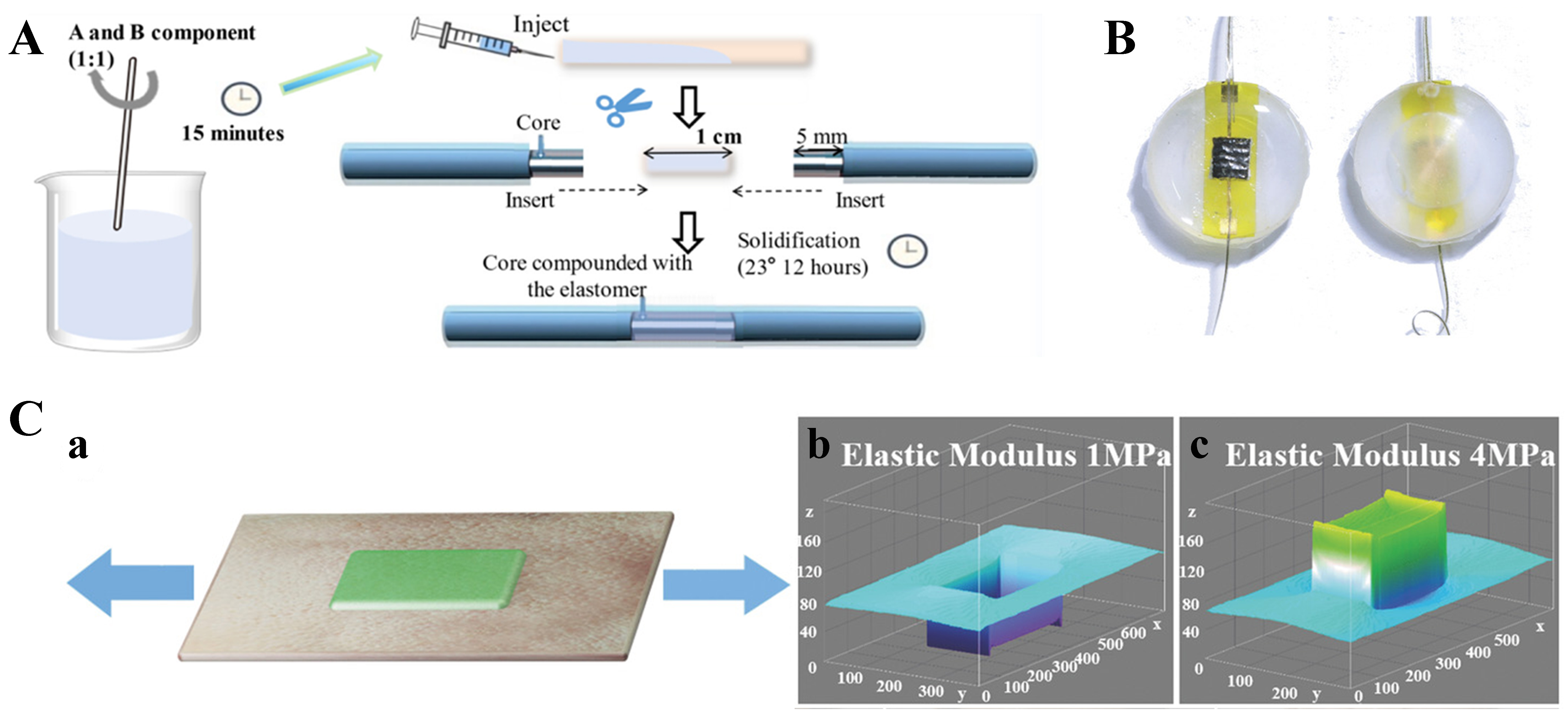
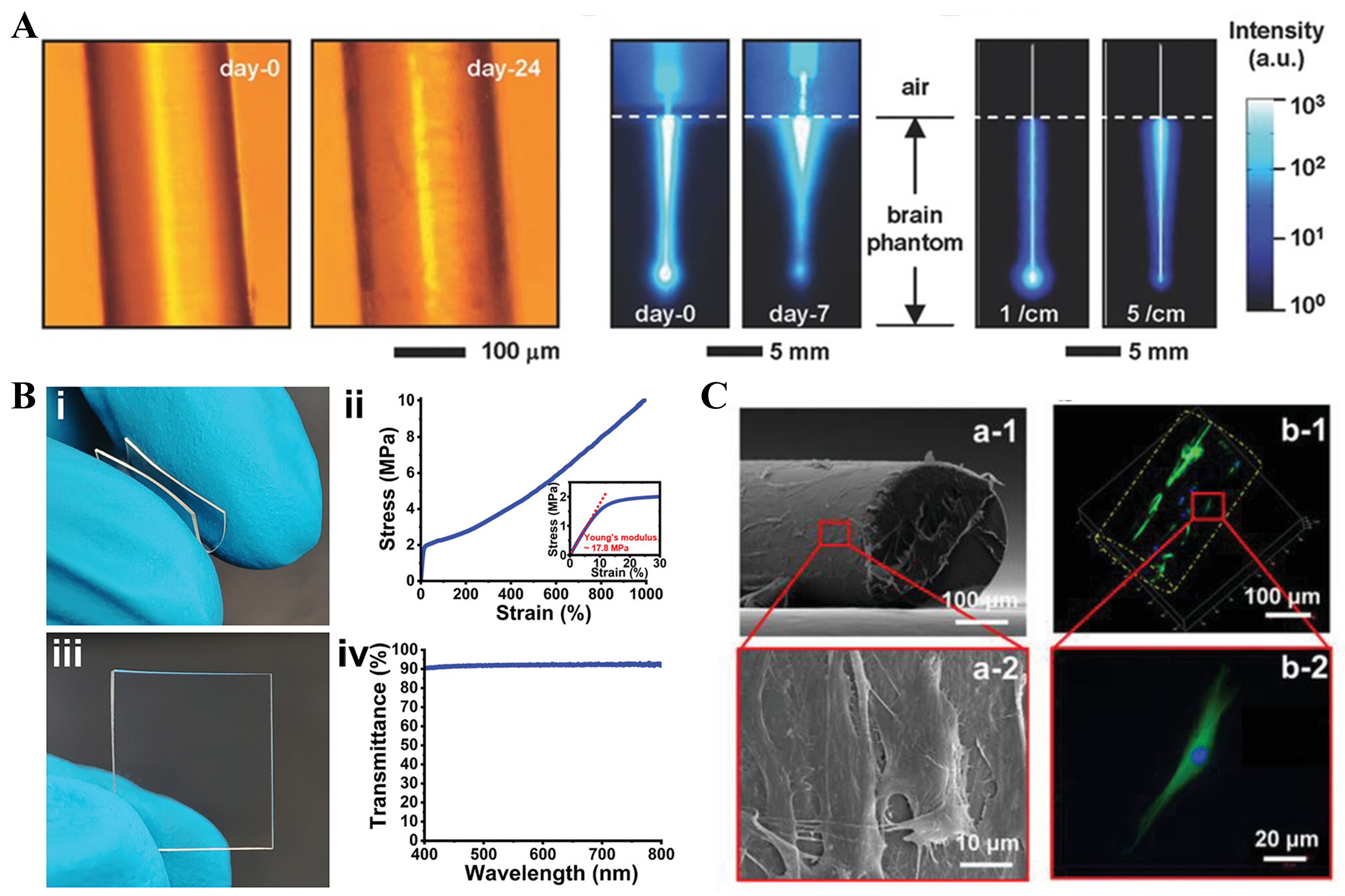
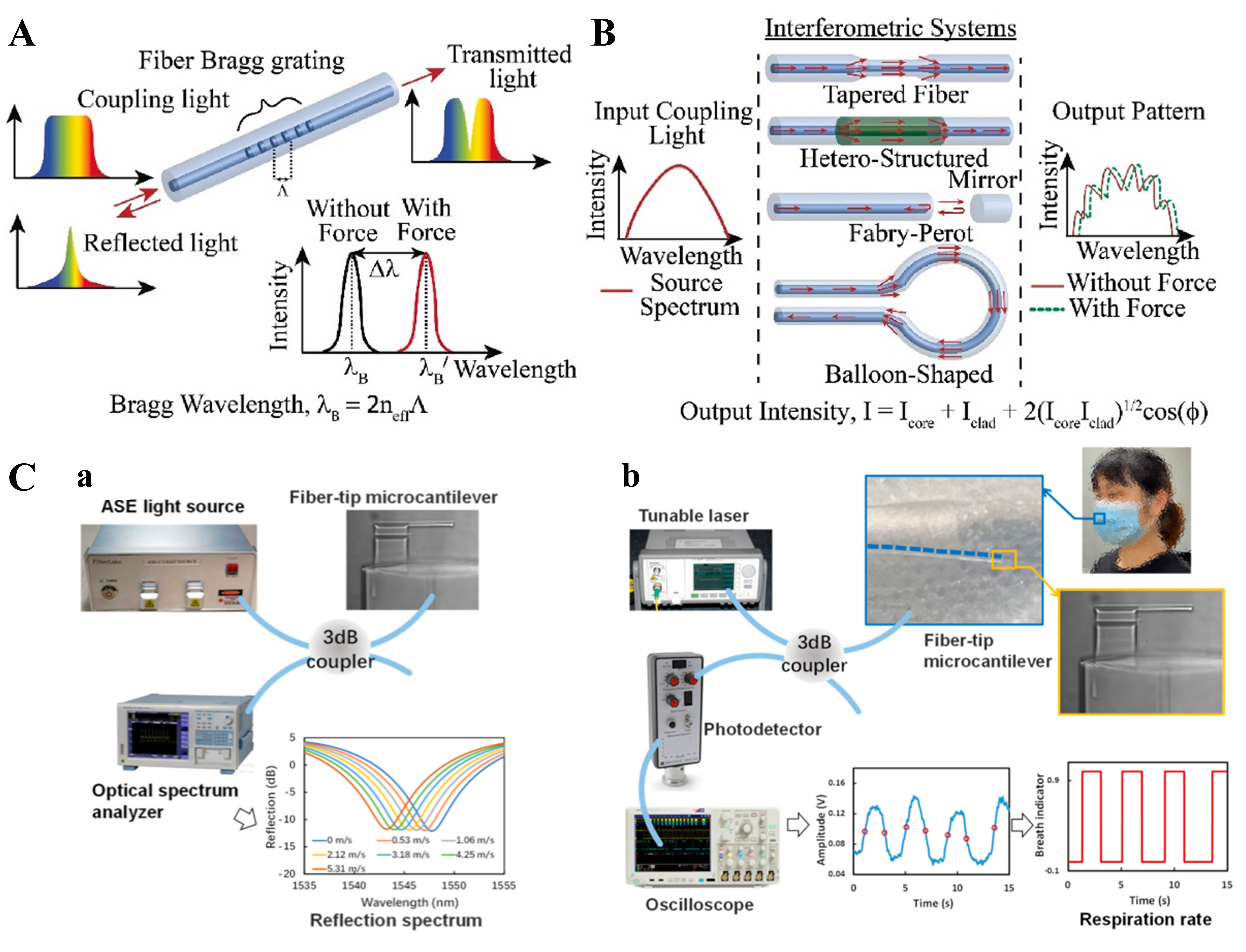
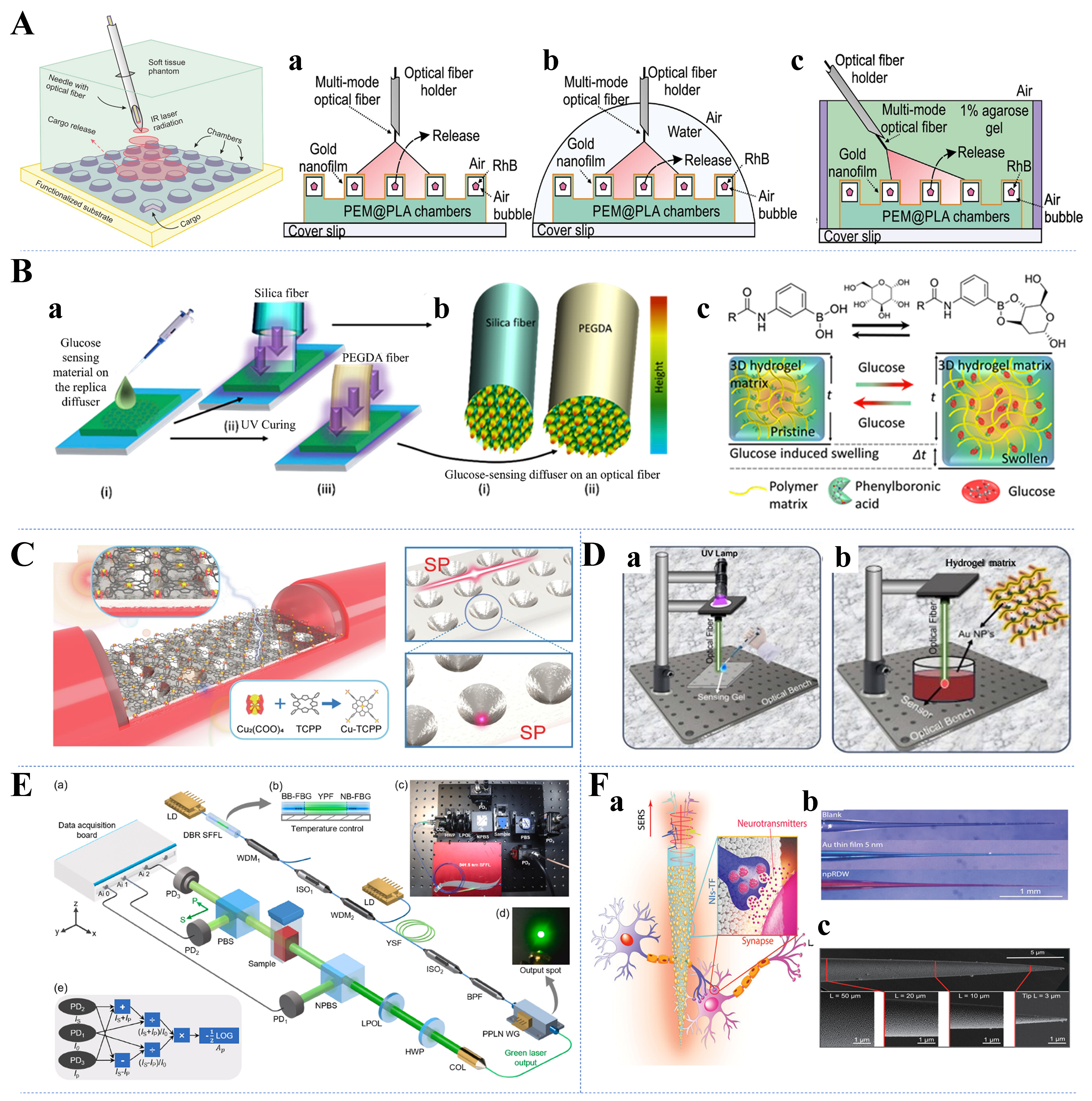
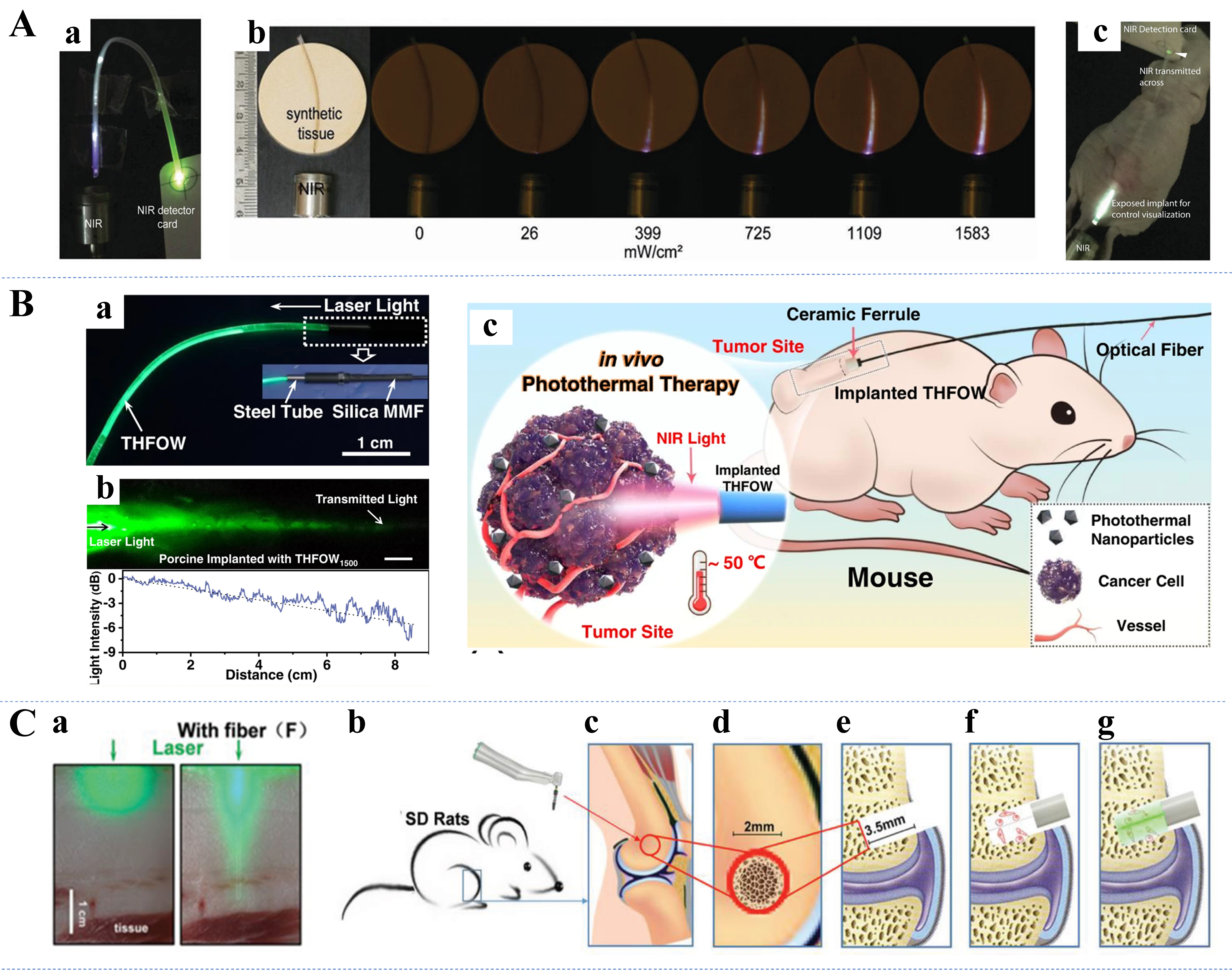
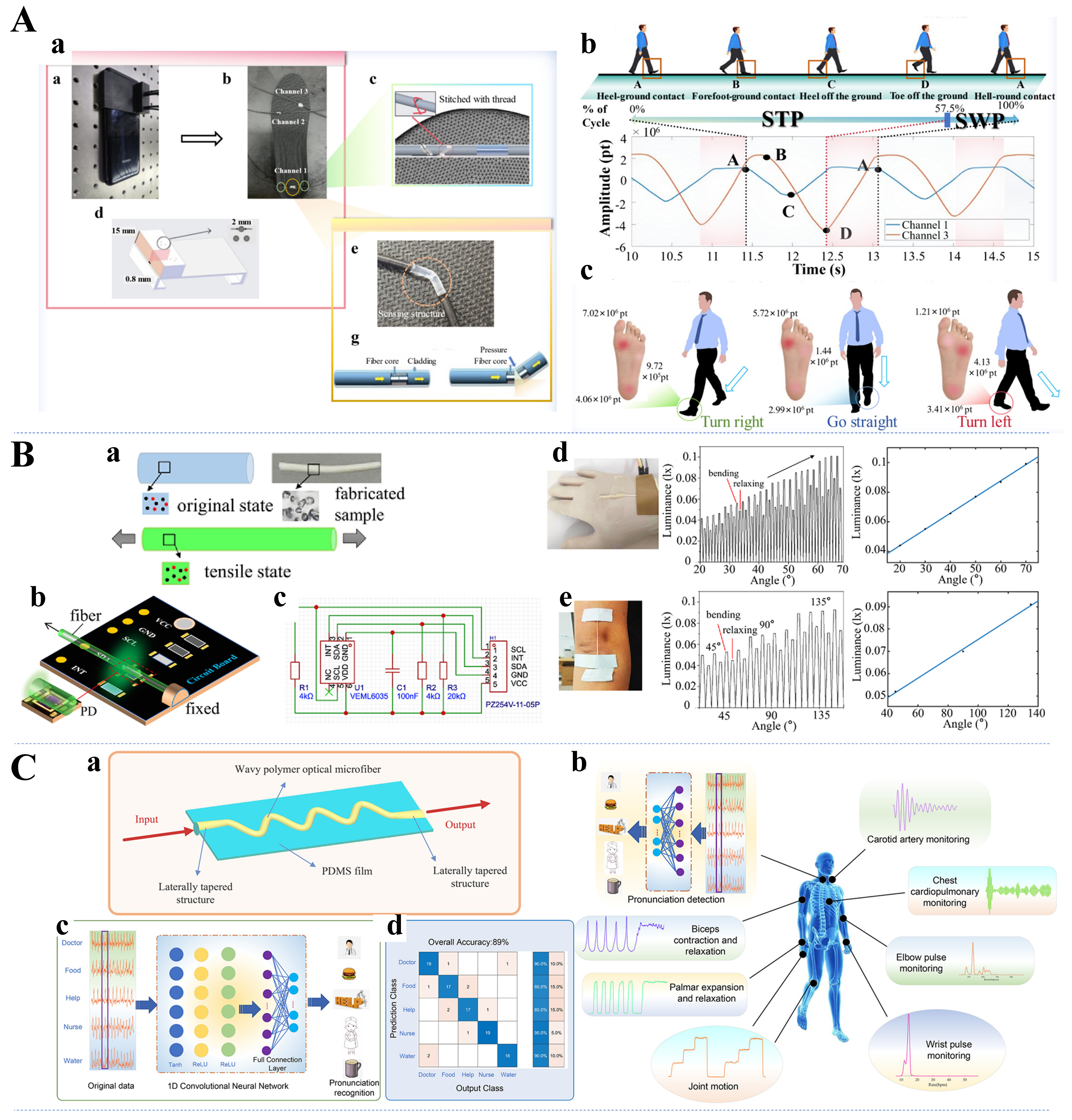
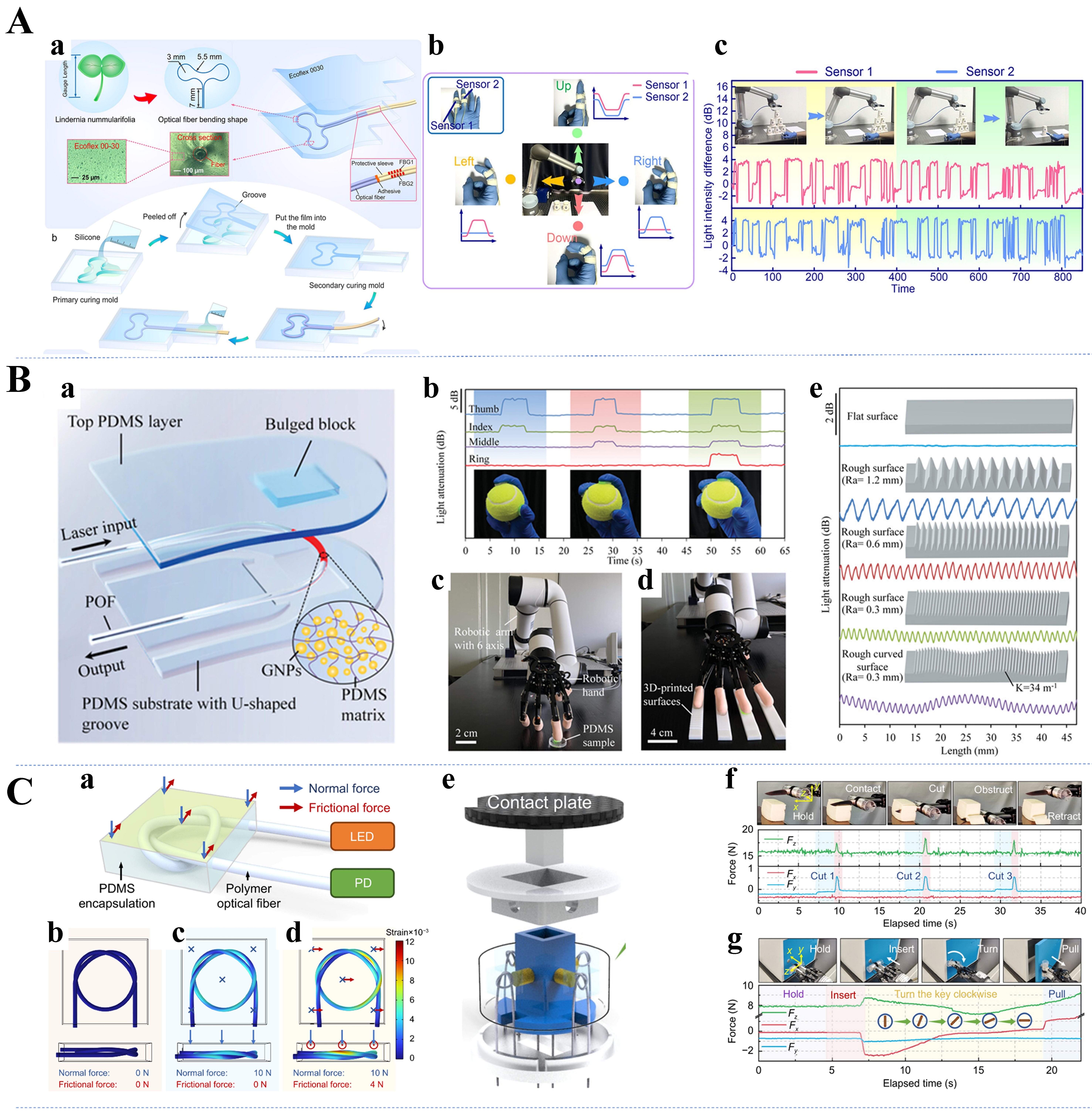
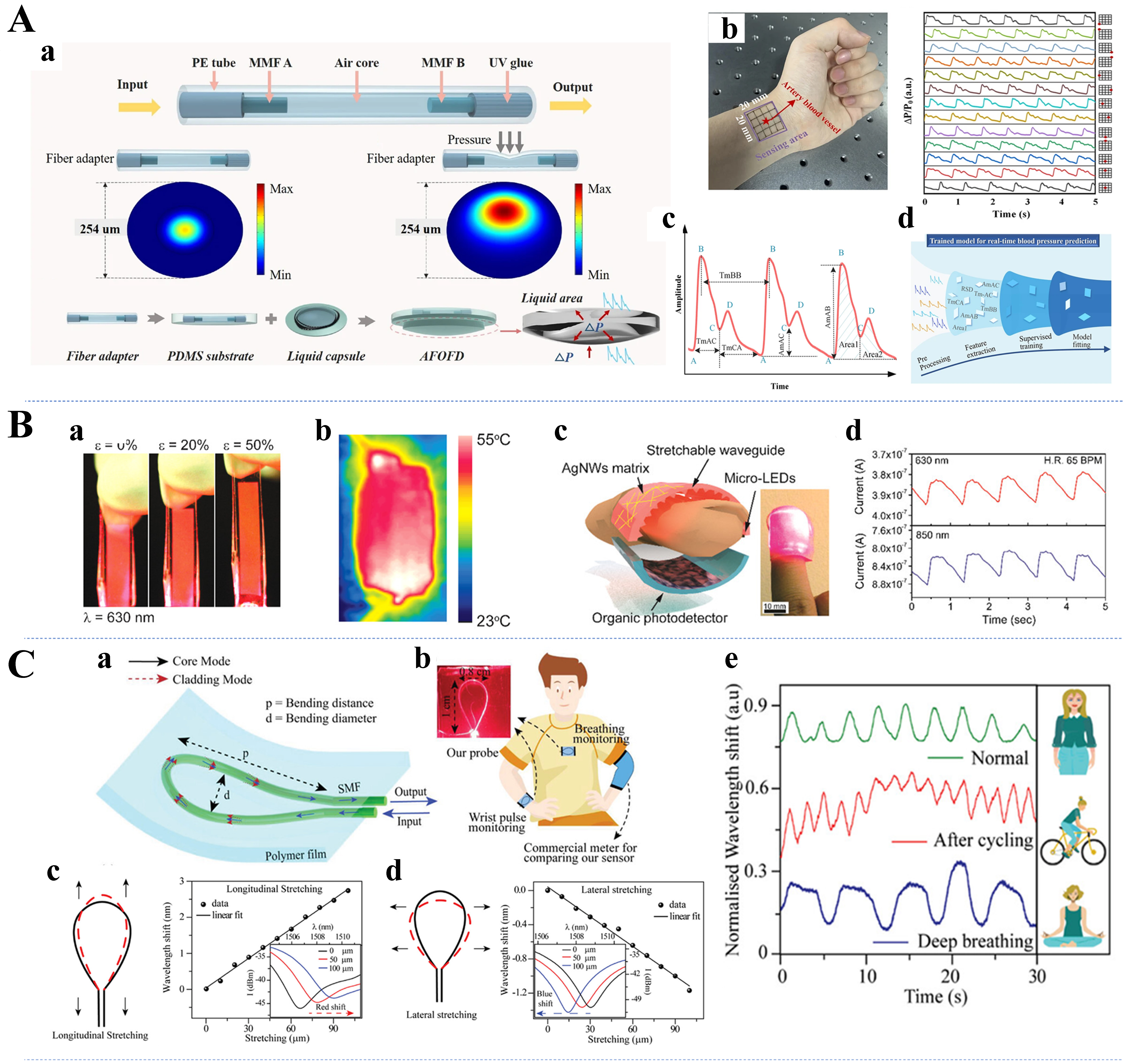












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].