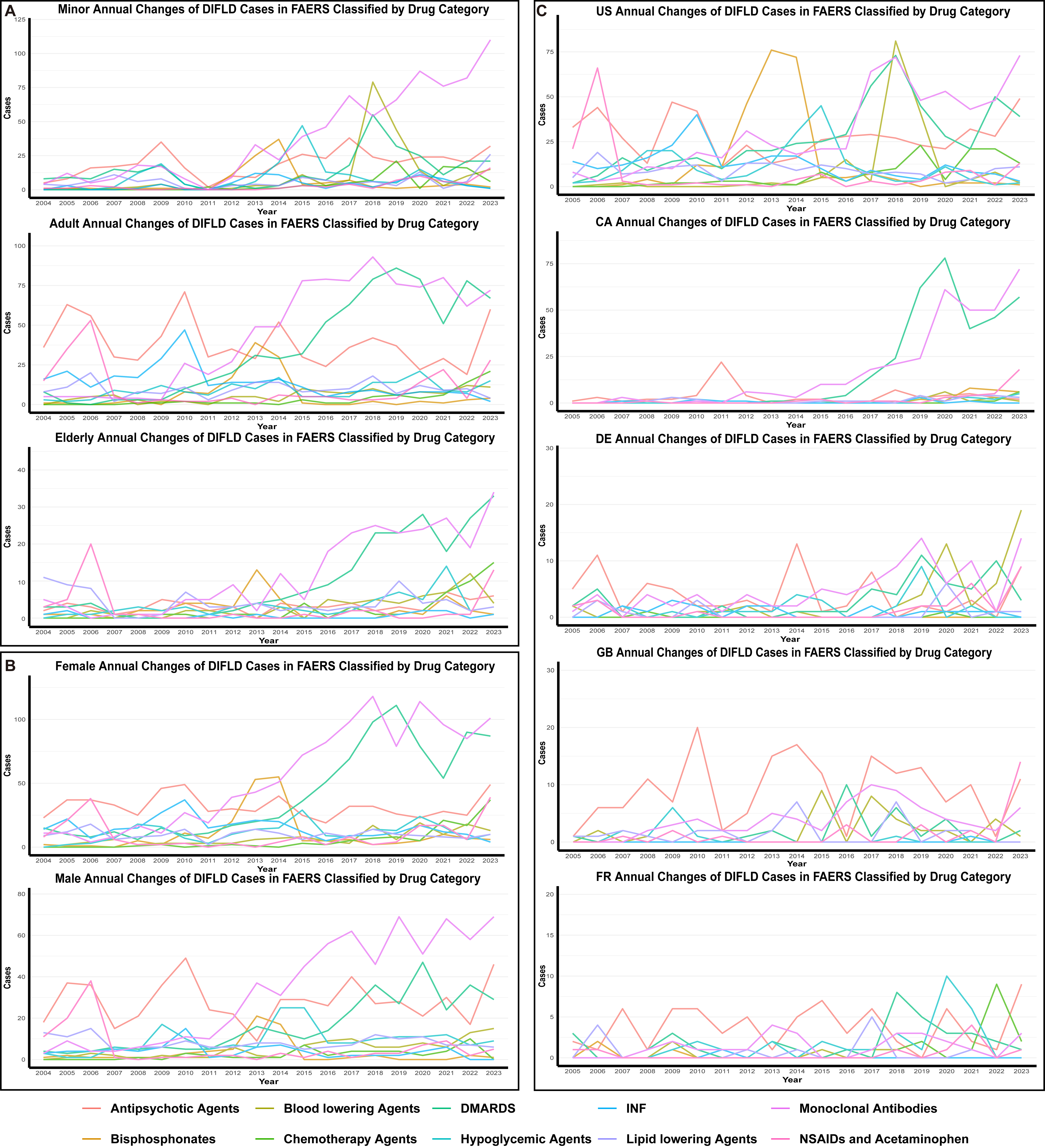
fig4
Figure 4. SLD reports linear regression analysis by drug classes in the country, age and gender group. (A) Trends in the DIFLD reports associated with various drug categories from 2004 to 2023 in age group; (B) Trends in the DIFLD reports associated with various drug categories from 2004 to 2023 in gender group; (C) Trends in the DIFLD reports associated with various drug categories from 2005 to 2023 in country group. Trends segmented by demographic groups, including males, females, elderly, and adults. Data represent annual case counts reported to the FAERS, illustrating temporal changes and potential impacts of different drug categories and patient populations on DIFLD reporting. We confirm that the figure is original and does not require external copyright permission. SLD: Steatotic liver disease; DIFLD: drug-induced fatty liver disease; FAERS: Food and Drug Administration’s Adverse Event Reporting System; US: United States; CA: Canada; DE: Germany; GB: Great Britain; FR: France; DMARDs: disease-modifying antirheumatic drugs; INF: interferon; NSAIDs: nonsteroidal anti-inflammatory drugs.