Extrahepatic manifestation of metabolic dysfunction-associated steatotic liver disease
Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD), is a fast-growing global medical concern, affecting approximately one-third of the population, with numbers rising. Recognized as a multisystemic disease, MASLD extends beyond the liver, presenting extrahepatic manifestations such as cardiovascular disease, type 2 diabetes, endocrine disorders such as hypothyroidism and polycystic ovarian syndrome, chronic kidney disease, psoriasis, and extrahepatic malignancies. This review aims to summarize the systemic effects of MASLD/NAFLD and to highlight possible shared pathophysiological pathways, including insulin resistance, lipid metabolism dysregulation, inflammation, oxidative stress, and gut dysbiosis. In addition, we discuss emerging therapeutic strategies for MASLD/NAFLD and its associated comorbidities. By integrating current evidence, this review provides insights into the multisystemic nature of MASLD and underscores the need for comprehensive management approaches.
Keywords
INTRODUCTION
Recently, a multi-society Delphi consensus, with input from 236 experts across 56 countries, endorsed the redefinition of nonalcoholic fatty liver disease (NAFLD) as metabolic dysfunction-associated steatotic liver disease (MASLD) to reduce stigma and highlight the underlying pathophysiology[1]. Of note, the contextualization of fatty liver disease in a broader systemic setting was emphasized by the Asian Pacific Association for the Study of the Liver (APASL), which introduced the term metabolic-associated fatty liver disease (MAFLD)[2]. While NAFLD is a diagnosis of exclusion, MAFLD criteria acknowledge the coexistence of metabolic dysfunction with other liver diseases, such as chronic hepatitis B, with patients experiencing faster progression of fibrosis and increased hepatocellular carcinoma (HCC) risk[3,4]. Therefore, patients with viral hepatitis may be closely monitored for coexisting steatotic liver disease (SLD)[4]. The subsequent 2023 multi-society Delphi consensus, which introduced MASLD, reinforces this inclusion-based diagnosis. While diagnostic criteria for NAFLD and MASLD include hepatic fat accumulation and exclude significant alcohol consumption, MASLD diagnosis additionally requires at least one of the cardiometabolic criteria[5]. Globally, MASLD has a pressing prevalence, with the highest rates observed in the Middle East and South America, rising prevalence in Asia (attributed to lean MASLD), and lower but increasing rates in Africa associated with westernization of diets and lifestyle[6]. An up-to-date study in the United States (US) found the prevalence of MASLD to be 31.9% [95% confidence interval (CI): 30.4%-33.4%][7]. Notably, the prevalence of advanced fibrosis in SLD was significantly higher during the coronavirus disease 2019 (COVID-19) era[7]. Another study reported an increase in all-cause mortality from MASLD, particularly pronounced during the early COVID-19 pandemic[8]. In 2023, non-Hispanic White individuals experienced the highest mortality for MASLD, recorded at 1.03 per 100,000[9], compared with 0.94 among Hispanic individuals and 0.29 among non-Hispanic Asian individuals[9].
MASLD is characterized by the accumulation of fat in hepatocytes due to insulin resistance, as well as genetic and environmental factors, leading to metabolic dysfunction-associated steatohepatitis (MASH), fibrosis, and potential progression to cirrhosis, HCC, and systemic complications[10]. MASH and advanced fibrosis are associated with higher mortality and are risk factors for HCC[11]. MASH is the leading cause of HCC in individuals awaiting liver transplants in the US[12]. Mortality rates for MASH-related HCC steadily increased before and during the COVID-19 pandemic in the US[13]. By 2050, the incidence of MASLD-related HCC is expected to double, while the number of liver transplants is projected to increase nearly fourfold[14]. MASLD also imposes a substantial clinical and economic burden: in the US alone, direct annual medical costs are predicted to be $103 billion, with a 26% increase in healthcare costs at a five-year follow-up[15,16].
Recognized as a multisystemic disease, MASLD extends beyond the liver. Increasing studies suggest that MASLD plays a significant role in developing extrahepatic manifestations. The liver-related aspects of MASLD represent just one facet of a complex multi-organ disease that impacts the cardiovascular, endocrine, renal, skin, other organ systems, and extrahepatic cancers. The primary causes of death among individuals with MASLD are cardiovascular disease (CVD)-related deaths, followed by extrahepatic cancers and liver-related deaths[17,18]. Increasing research has suggested genetic predispositions to MASLD susceptibility and disease progression, including patatin-like phospholipase domain-containing protein 3 (PNPLA3)[19], transmembrane 6 superfamily member 2 (TM6SF2)[20], membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7)[21], and hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13)[22] polymorphisms. Consequently, physicians and patients need to recognize the multisystemic connections associated with MASLD to improve screening and management of these comorbid conditions.
While recent studies increasingly adopt the term MASLD in place of NAFLD, much of the existing literature on extrahepatic manifestations relies on NAFLD’s diagnostic criteria. This overlap exists because many studies were conducted before the terminology change. However, there is a significant diagnostic concordance, with 99.5% of individuals previously diagnosed with NAFLD also meeting the definition for MASLD[23]. Consequently, this review article will integrate recent research regarding extrahepatic manifestations of MASLD [Figure 1 and Table 1] by incorporating the term MASLD to refer to NAFLD.
Figure 1. Extrahepatic manifestations of MASLD. This figure was created in Biorender. Shenoy A. (2025) https://biorender.com/d9e8i5h. CAD: Coronary artery disease; MASLD: metabolic dysfunction-associated steatotic liver disease; PAD: peripheral artery disease.
Key extrahepatic manifestations of MASLD
| Extrahepatic manifestation | Key finding |
| Cardiovascular disease | MASLD is associated with an increased risk of CVE and mortality Chronic inflammation, impaired cholesterol efflux, oxidative stress, and insulin resistance are implicated |
| Endocrine | Endocrine disorders are associated with MASLD through common pathogenic mechanisms |
| Type 2 diabetes | MASLD and T2DM have a bidirectional risk relationship Insulin resistance, impaired lipogenesis, gut microbial dysbiosis, and chronic inflammation are common mechanisms |
| Hypothyroidism | Subclinical and clinical hypothyroidism are linked with MASLD and MASH/significant fibrosis Impaired thyroid hormones contribute to insulin resistance, dyslipidemia, chronic inflammation, and oxidative stress |
| PCOS | PCOS increases MASLD risk by ~2.5 fold Hyperandrogenism and insulin resistance in PCOS contribute to MASLD pathogenesis |
| Chronic kidney disease | MASLD is associated with a ~1.5-fold increased risk of CKD and worse outcomes Insulin resistance, pro-inflammatory mediators, and liver metabolites promote renal damage |
| Psoriasis | High prevalence of MASLD in psoriatic patients, conferring increased risk of CVE Shared inflammatory pathways implicated in MASLD/psoriasis progression |
| Extrahepatic cancer | MASLD is associated with an increased risk of gastrointestinal, endocrine, reproductive, and urinary system cancers Mechanisms are insulin resistance, chronic inflammation, gut microbial dysbiosis, and genetic polymorphisms |
EXTRAHEPATIC MANIFESTATIONS
Cardiovascular-liver axis
Epidemiology
We summarized essential studies regarding CVD in individuals with MASLD in Table 2. Growing evidence indicates that MASLD is a significant risk factor for CVD, which is the leading cause of mortality among individuals with MASLD - accounting for approximately one-third of deaths[17,24-26]. A meta-analysis reported that hospitalized CVD patients with MASLD exhibit a higher overall mortality compared to those without MASLD[27]. Furthermore, a nationwide Swedish cohort study of 10,422 adults found that individuals with MASLD experienced a notably higher rate of CVD events irrespective of traditional cardiometabolic risk factors. With an adjusted hazard ratio (HR) of 1.67 (95%CI: 1.47-1.89) for noncirrhotic fibrosis and 2.15 (95%CI: 1.77-2.61) for cirrhosis, this risk heightened with the severity of MASLD histology[28]. A meta-analysis of 11 longitudinal cohort studies comprising over 11 million individuals indicated that those with MASLD experienced a 1.5-fold increase in long-term risk of new-onset heart failure, with a pooled random-effects HR of 1.50 (95%CI: 1.34-1.67)[29]. Additionally, meta-analyses show that MASLD is associated with cardiac complications besides heart failure, including ischemic stroke (HR: 1.16, 95%CI: 1.07-1.26)[30], myocardial infarction [odds ratio (OR): 1.66, 95%CI: 1.39-1.99][31], coronary artery disease [relative risk (RR): 1.21, 95%CI: 1.07-1.38][32], and cardiac arrhythmias such as atrial fibrillation (HR: 1.12, 95%CI: 1.11-1.13; OR: 1.71, 95%CI: 1.14-2.57), heart block (OR: 2.65, 95%CI: 1.88-3.72), premature atrial/ventricular contraction (OR: 2.53, 95%CI: 1.70-3.78), and prolonged QT interval (OR: 2.86, 95%CI: 1.64-4.99)[33].
Essential studies evaluating cardiovascular disease in individuals with MASLD
| Study (year)2345678 | Country | Study design | Total population (Number of MASLD) | Mean age of participants (years) | Female (%) | Diagnostic method | Outcomes | Confounder adjustment |
| Hagström et al. (2019)[25] | Sweden | Matched-population cohort study | 6,872 (8.8%) | MASLD: 47.4 Total: 47.9 | MASLD: 37 Total: 36.7 | Liver-biopsy | MASLD patients were found to have a 50% increased risk of having a CVD event (HR: 1.54; 95%CI: 1.30-1.83) | Age, sex, hypertension, hyperlipidemia, T2DM, smoking, triglycerides |
| Saokaew et al. (2021)[27] | Turkey, Italy, Greece | Meta-analysis | Turkey: 415 (7.7%) Italy: 360 (53.1%) Greece: 264 (58.0%) | Turkey: Grade 0: 58 Grade 1: 59 Grade 2: 62 Grade 3: 63 Italy: Alive: 80 Deceased: 85 Greece: MASLD: 77.4 No MASLD: 78.8 | Turkey: Grade 0: 39 Grade 1: 27 Grade 2: 34 Grade 3: 23 Italy: Alive: 53.2 Deceased: 45.0 Greece: MASLD: 60% No MASLD: 62.9 | Ultrasonography | MASLD were at a significantly higher risk of all-cause mortality than non-MASLD patients (HR: 2.08; 95%CI: 1.56-2.59) | Age, sex, BMI, waist circumference, diabetes, chronic kidney disease, hypertension, CVD (others listed in paper) |
| Simon et al. (2022)[28] | Sweden | Population-based study | 56,939 (18%) | MASLD: 52.3 No MASLD: 51.2 | MASLD: 45.2 No MASLD: 46.0 | MASLD: Liver biopsy Fibrosis: liver biopsy | MASLD is associated with higher incidence of MACE (HR: 1.63; 95%CI: 1.56 to 1.70) Cirrhosis had a 27% higher incidence of MACE compared to simple steatosis (HR: 1.27; 95%CI: 1.08-1.49) | Age, sex, diabetes, obesity, hypertension, dyslipidemia, CKD, history of CVD, education level, hospitalizations within 1 year of biopsy, alcohol misuse, medications (lipid-lowering, antidiabetic, antihypertensive, anticoagulant agents), COPD (proxy for smoking) |
| Mantovani et al. (2022)[29] | Sweden, Finland, UK, USA, South Korea | Meta-analysis | 11,242,231 (26.2%) | 55 | 50.1 | Serum GGT levels, FLI score, ICD codes, CT, liver biopsy | Presence of NAFLD associated with increased risk of incident HF (n = 11 studies; HR: 1.50; 95%CI: 1.34-1.67) Risk of incident HF increases with higher levels of liver fibrosis (n = 2 studies; HR: 1.76; 95%CI: 0.75-4.36) | Age, sex, adiposity measures, diabetes, other common cardiovascular risk factors |
| Xu et al. (2021)[30] | China | Prospective cohort study | 79,905 (MASLD: 31%) | Severe MASLD: 52.88% Moderate MASLD: 52.65% Mild MASLD: 52.41% Nonfatty liver: 50.99% | Severe MASLD: 29.75% Moderate MASLD: 28.04% Mild MASLD: 26.81% Nonfatty liver: 25.68% | Ultrasonography | MASLD associated with 16% increased risk of ischemic stroke (HR: 1.16; 95%CI: 1.07-1.26) Risk increased with severity: mild (HR: 1.15; 95%CI: 1.05-1.25); moderate (HR: 1.19; 95%CI: 1.06-1.34); severe (HR: 1.21; 95%CI: 1.08-1.50) | Age, sex, physical activity, body mass index, smoker, history of hypertension, diabetes, hypercholesterolemia, lipid-lowering medication, high-density lipoprotein, triglyceride, hs-CRP, and fasting blood glucose |
Mechanism
MASLD enhances CVD outcomes via multiple mechanisms, encompassing inflammatory, metabolic, and coagulation pathways [Figure 2]. It causes persistent low-grade systemic inflammation by elevating levels of several pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1β, IL-17, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP)[24,34]. Increasing the delivery of microRNA-1 (miR-1) from hepatocyte-derived extracellular vesicles leads to endothelial cell dysfunction by the upregulation of adhesion molecules [E-selectin, intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1)] nuclear factor-kappa B (NF-KB) activation, and monocyte recruitment, thereby promoting atherogenesis[35,36]. Moreover, MASLD was identified as a disruptor of ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux, facilitating foam cell formation in macrophages and progression of atherosclerosis[37]. MASLD was found to alter hemostasis by fostering a procoagulant state through increased fibrinogen, prothrombin, factor VIII, von Willebrand Factor, Plasminogen Activator Inhibitor-1, D-dimer, and thrombin. Conversely, anti-coagulant factors, protein C and antithrombin levels, were reduced[38]. MASLD induces oxidative stress and the generation of reactive oxygen species (ROS), which disrupts the signaling pathways of AMP-activated kinase (AMPK), peroxisome proliferator-activated receptors (PPARs), sterol regulatory element-binding proteins (SREBPs), nuclear factor erythroid-2-related factor-2 (Nrf2), and NF-KB. This results in impaired β-oxidation while promoting lipogenesis and insulin resistance[39]. Altered hepatic lipid metabolism in MASLD leads to atherogenic dyslipidemia through elevated plasma triglycerides, lipoprotein cholesterol levels, and small dense LDL particles that contribute to plaque formation[40,41].
Figure 2. Putative mechanisms linking MASLD to cardiovascular disease. This figure was created in Biorender. Shenoy A. (2025) https://biorender.com/hovzrch. MASLD: Metabolic dysfunction-associated steatotic liver disease; MASH: metabolic dysfunction-associated steatohepatitis; HCC: hepatocellular carcinoma; IL: interleukin; TNF-α: tumor necrosis factor alpha; CRP: C-reactive protein; ICAM1: intercellular adhesion molecule 1; VCAM1: vascular cell adhesion molecule 1; NF-KB: nuclear factor kappa-light-chain-enhancer of activated B cells; ABCA1: ATP-binding cassette transporter A1; LDL: low-density lipoprotein; AMPK: AMP-activated protein kinase; PPARs: Peroxisome proliferator-activated receptors; SREBPs: sterol regulatory element-binding proteins; Nrf2: nuclear factor erythroid 2-related factor 2; CVD: cardiovascular disease; HF: heart failure; MI: myocardial infarction; CAD: coronary artery disease.
Treatment
Although there are no specific practice guidelines for the treatment of CVD risk factors in the presence of MASLD, the American Association for the Study of Liver Diseases (AASLD)[42] and the American Association for Clinical Endocrinology (AACE)[43] recommend regular screening for CVD risk factors, with treatment focusing on lipid-lowering agents, glycemic control, management of hypertension, smoking cessation, and lifestyle modifications, including weight control[44]. Dietary modification, such as the Mediterranean diet, has been shown to improve CVD risk profiles[45,46] and alleviate hepatic steatosis[47,48]. Additionally, research highlights the positive impact of physical activity on lowering CVD mortality rates[49]. Combining dietary changes with increased physical activity has demonstrated a substantial reduction in hepatic steatosis, with the degree of fat loss correlating with the intensity of the interventions undertaken[50-53]. The AASLD guidelines recommend several pharmacological treatments for MASH, such as glucagon-like peptide-1 (GLP-1) receptor agonists, vitamin E, pioglitazones, and sodium-glucose co-transporter 2 (SGLT2) inhibitors[42]. Recent studies suggest that GLP-1 analogues, semaglutide[54,55], and liraglutide[56,57], may improve CVD risk and reduce liver steatosis, possibly through mechanisms related to weight loss[44]. PPAR agonists including pioglitazone[58] and lanfibranor[59,60] have shown potential in improving CVD risk factors and liver histology, though further research is warranted. Statins are commonly prescribed to manage hyperlipidemia in individuals with type 2 diabetes mellitus (T2DM) and obesity and those at risk of CVD. Despite their proven benefits in treating CVD and associated risk factors, statins remain underprescribed, with 50% of patients with MASLD and 33% of those with both MASLD and clinical atherosclerotic CVD not receiving treatment[61,62]. Concerns regarding potential liver damage have contributed to this underutilization, yet research suggests that statins are not only safe for most patients but may also reduce overall disease burden and mortality[63-65]. Nonetheless, caution is advised in patients with decompensated cirrhosis[63]. Bariatric surgery has demonstrated effectiveness in improving hepatic fibrosis and achieving sustained weight loss[66,67], with one study finding that 80% of patients experienced resolution of MASH maintained over five years[68].
This review shows that MASLD is associated with increased risk of cardiovascular morbidity and mortality due to inflammatory, metabolic, and coagulation mechanisms. Future research is needed to focus on early detection and the therapeutic effects on both MASLD and CVD.
ENDOCRINE-LIVER AXIS
T2DM
Epidemiology
We summarized essential studies regarding endocrine abnormalities in individuals with MASLD in Table 3. T2DM and MASLD are closely interlinked, with increasing evidence indicating a bidirectional risk relationship. A retrospective cohort study of 15,039 individuals from Japan found a significantly elevated risk of T2DM in MASLD compared with the healthy population as the reference[69]. Additionally, a meta-analysis found that MASLD conferred a more than twofold greater risk of developing diabetes (HR: 2.22, 95%CI: 1.84-2.60)[70]. Prevalence of T2DM has been shown to have an escalating risk with the severity of MASLD. One retrospective study indicated that a higher percentage of individuals with fibrosis stages 3-4 (HR: 3.07, 95%CI: 1.35-6.96) developed T2DM than patients with fibrosis stages 0-2 (HR: 2.51, 95%CI: 1.07-5.88)[71]. Among individuals with MASLD and T2DM, nearly one-third exhibited significant fibrosis, and approximately one-tenth had cirrhosis[72]. Conversely, T2DM predisposes patients to MASLD and is acknowledged as an independent risk factor for advanced liver disease[73,74]. The worldwide pooled prevalence of MASLD in patients with T2DM has risen to 65.33% from 55.86% during the 1990-2004 period[75]. A recent population-based study in the US reported the prevalence of MASLD was notably high in individuals with diabetes, ranging from 68.6% to 77.7%, and in those with prediabetes, ranging from 42.4% to 56.5% during the 2017-2023 period[72]. Recent research suggests a majority of patients with T2DM have MASLD, with one study finding the prevalence of MASLD, advanced fibrosis, and cirrhosis among individuals with T2DM to be 65%, 14%, and 6%, respectively[76]. The strong bidirectional association between MASLD and T2DM highlights the need for routine screening for T2DM in patients with MASLD, and vice versa, to allow for earlier detection and intervention.
Essential studies evaluating endocrine and chronic kidney disease in individuals with MASLD
| Study (year)2345678 | Country | Study design | Total population (Number of MASLD) | Mean age of participants (years) | Female (%) | Diagnostic method | Outcomes | Confounder adjustment |
| Type 2 diabetes | ||||||||
| Sakai et al. (2024)[69] | Japan | Retrospective cohort study | 15,039 (MASLD: 18%) | 44 | 40.4 | Ultrasonography | MASLD significantly associated with increased risk of T2DM (OR: 127.0; 95%CI: 40.4-399.0) | Age, sex, smoking status, exercise habits, alcohol consumption |
| Björkström et al. (2017)[71] | Sweden | Retrospective study | 2,176 (MASLD: 23%) | 45.8 | 35.1 | Liver biopsy | Higher fibrosis stage associated with increased risk of T2DM (F3-4: HR: 3.07; 95%CI: 1.35-6.96 vs. F0-2: HR: 2.51; 95%CI: 1.07-5.88) | Age, sex, BMI, serum triglycerides |
| Ajmera et al. (2023)[76] | USA | Prospective cross-sectional study | 501 (MASLD: 65.3%) | 64.4 | 63.1 | MASLD: MRI-PDFF Fibrosis: MRE or VCTE | Prevalence of NAFLD, advanced fibrosis and cirrhosis was 65%, 14% and 6% Obesity and insulin use associated with increased odds of advanced fibrosis (OR: 2.50; 95%CI: 1.38-4.54 and OR: 2.71; 95%CI: 1.33-5.50, respectively) | Age, sex |
| Hypothyroidism | ||||||||
| Kim et al. (2020) [112] | United States | Population-based study | 7,259 (4.8% advanced fibrosis) | 46.0 | 49.2 | Presence of one of three noninvasive fibrosis markers | Hypothyroidism is associated with MASLD in a dose-dependent manner: Low normal TSH (2.5-4.5) (OR: 1.94; 95%CI: 1.10-3.44) Subclinical hypothyroidism (> 4.5) (OR: 2.05; 95%CI: 1.01-4.16) | Age, sex, race/ethnicity, education level, marital status, economic status, smoking status, waist circumference, hypertension, diabetes, total cholesterol, insulin resistance |
| Chronic kidney disease | ||||||||
| Mantovani et al. (2022)[133] | South Korea, Japan, China, Sweden, Finland, Germany, Italy, USA | Meta-analysis | 1,222,032 (28.1%) | 51.7 | 46.4 | Blood biomarkers/scores, ICD, imaging techniques or biopsy | MASLD associated with increased risk of incident CKD (n = 10 studies; HR: 1.43; 95%CI: 1.33-1.54) Advanced fibrosis confers greater risk for incident CKD (n = 1 study; HR: 1.59; 95%CI: 1.31-1.93) | Age, sex, BMI, smoking status, diabetes, hypertension, dyslipidemia, baseline estimated glomerular filtration rate |
| Paik et al. (2019)[134] | United States | Population-based study | 11,695 (18.6%) | 43.3 | 51.6 | Ultrasonography | CKD is associated with advanced fibrosis (HR: 2.51; 95%CI: 1.98-3.18) CKD and advanced fibrosis is associated with a higher risk of overall mortality (HR: 2.16; 95%CI: 1.29-3.63) | Age, sex, race, smoking status, presence of metabolic syndrome |
| Hashim et al. (2023)[136] | United States | Retrospective cohort study | 420,893 (0.19%) | 70.1 | 41.3 | ICD-10 code (K75.81) | MASLD is associated with an increased risk of 6-month readmission with acute kidney injury (26.8% vs. 16.6%, HR: 1.44; 95%CI: 1.14-1.82) | Age, sex, hypertension, diabetes, peripheral vascular disease, obesity, COPD, smoking status |
Mechanism
The pathophysiological mechanisms linking MASLD and T2DM are complex and mutually exacerbating. Insulin resistance has been implicated in both hepatic lipid accumulation and systemic glucose dysregulation[77]. In MASLD, hepatic insulin resistance diminishes the ability of insulin to inhibit gluconeogenesis, leading to increased glucose production. Recent research utilizing isotope tracers found that individuals with MASLD had increased glucose production associated with a greater uptake of gluconeogenic substrates, such as lactate, glycerol, and free fatty acids, and reduced expression of insulin signaling genes, insulin receptor substrate 1 (IRS1), insulin receptor substrate 2 (IRS2), and AKT serine/threonine-protein kinase 2 (AKT2)[78]. In parallel, insulin resistance in adipose tissue promotes lipolysis and the release of free fatty acids, which are absorbed by the liver and are esterified to triglycerides that exacerbate hepatic steatosis[79]. In patients with T2DM, upregulation of carbohydrate response element binding protein (ChREBP) and sterol regulatory element-binding protein 1 (SREBP-1c) has been found to contribute to de novo hepatic lipogenesis and fatty acid synthesis[80]. Additionally, chronic inflammation due to hepatocyte stress has been found to activate inflammatory pathways, including NF-KB and c-Jun N-terminal kinase (JNK) signaling[81], leading to the release of pro-inflammatory cytokines such as CRP, which is implicated in leptin signaling, insulin signaling, and mitochondrial dysfunction, further driving MASLD progression[82]. Of interest is the growing body of evidence suggesting the gut microbiota dysbiosis contributes to pathophysiology of both MASLD and T2DM[83]. One proposed mechanism suggests that increased Bacteroides abundance is correlated with increased deoxycholic acid, D-pinitol, choline, raffinose, and stachyose, which have been shown to increase liver apoptosis, fibrosis, and inflammation in MASLD patients[82]. However, the mechanisms that underlie this association remain to be fully elucidated. The complex interaction of MASLD and T2DM highlights the role each has in the progression of the other, supporting that MASLD is both a cause and consequence of T2DM.
Treatment
Treatment of T2DM has shown promising results in improving MASLD by addressing common metabolic dysfunctions. Evidence highlights the benefits of dietary intervention, pharmacological therapies, weight loss, and lifestyle modifications on both T2D and MASLD. Dietary changes, including increased protein intake[84,85], decreased fructose and sucrose consumption[86,87], and the Mediterranean diet[88], have demonstrated efficacy in improving liver fat content and insulin resistance. Various medications for T2DM have shown potential in improving MASLD. Metformin has been shown to improve liver enzyme levels and hepatic fat content by decreasing inflammation and oxidative stress[89]. Additionally, insulin therapy significantly improved hepatic steatosis and insulin secretion in patients with T2DM and MASLD[90]. One study found that insulin-metformin combination therapy reduced hepatic steatosis by 45% (P < 0.001) within a 3-month period[91]. Thiazolidinediones, specifically pioglitazone[92,93] have been found to improve insulin sensitivity, systemic inflammation, liver enzyme levels, and steatosis; however, findings on rosiglitazone’s effect on liver histology remain inconsistent[93,94]. Various studies have reported a positive effect of GLP-1 receptor agonists such as liraglutide[95-98] and the novel GLP-1/glucose-dependent insulinotropic polypeptide (GIP) receptor agonist tirzepatide[99,100] on reducing liver fat, improving insulin sensitivity, and suppressing lipolysis. Semaglutide, a GLP-1 receptor agonist, with established anti-obesity effects, demonstrates benefits in MASLD by not only promoting weight loss and improving glycemia but also decreasing hepatic steatosis, inflammation, and fibrosis, with higher resolution rates[101]. Emerging evidence also suggests that SGLT2 inhibitors such as canagliflozin[102-104], empagliflozin[105,106], and dapagliflozin[107,108] have a dual effect on T2DM and MASLD, increasing urinary glucose excretion, promoting weight loss, and improving liver enzymes and histological features. Bariatric surgery has been shown to improve hyperlipidemia and liver enzyme levels in patients with MASLD and T2DM[109].
In summary, the bidirectional relationship between MASLD and T2DM is attributed to shared insulin resistance, dyslipidemia, inflammation, and gut microbiota dysbiosis. However, more research is needed to standardize pharmaceutical options for patients with coexisting MASLD and T2DM.
Hypothyroidism
Epidemiology
The prevalence of hypothyroidism in the US has increased from 9.5% to 11.7% in the last decade[110]. A meta-analysis of 28 observational studies on 76.5 million individuals found that primary hypothyroidism was associated with an increased risk of MASLD (OR: 1.43, 95%CI: 1.23-1.66) and an even greater risk of MASH or advanced fibrosis (OR: 2.84, 95%CI: 2.07-3.90)[111]. Regarding mortality, over a median follow-up of 23 years, low thyroid function was connected to a heightened risk of all-cause and cardiovascular mortality among individuals with MASLD[112].
Mechanism
Hypothyroidism has been recognized as a driver in the pathogenesis of MASLD through both direct hepatic and systemic metabolic effects. Thyroid hormones play a crucial role in hepatic function. T3 and T4 decrease liver fat accumulation by promoting hepatic lipophagy and mitochondrial biogenesis and inhibiting hepatic lipogenesis through downregulation of SREBP-1c and acetyl-CoA carboxylase[113]. They can also disrupt the process of liver fibrosis by inhibiting transforming growth factor- β (TGF-β)/Sma and Mad Related Protein (SMAD) signaling[113]. Thyroid-stimulating hormone (TSH), which is elevated in subclinical and clinical hypothyroidism, has been found to upregulate SREBP-1c, causing hepatic lipogenesis[114]. TSH has also been implicated in insulin resistance through reduced GLUT2 expression[115]. Subclinical and clinical hypothyroidism causes dyslipidemia, low-grade chronic inflammation, and oxidative stress, all of which drive MASLD pathogenesis[116]. In addition, triiodothyronine (T3) plays a key role in glucose regulation, hepatic lipid metabolism, and mitochondrial function. Reduced levels of T3 found in hypothyroidism lead to impaired β-oxidation, increased hepatic lipogenesis, and decreased lipid clearance, all contributing to liver steatosis[116,117].
Treatment
Current pharmacological treatment for hypothyroidism focuses on hormone supplementation. Levothyroxine, a synthetic form of thyroxine T4, can improve hepatic steatosis. A post hoc analysis of a randomized controlled trial found that among 363 Chinese adults with significant hypothyroidism (TSH ≥ 10 mIU/L), levothyroxine treatment reduced the prevalence of MASLD from 48.5% to 24.2% (P = 0.041)[118]. While there is not sufficient evidence to treat MASLD with levothyroxine alone, levothyroxine may be considered in patients with both clinical hypothyroidism and MASLD.
Resmetirom, an orally administered thyroid hormone receptor beta-1 agonist, has been shown to have a dual effect on hypothyroidism and MASLD. A double-blind, randomized, placebo-controlled study of 348 patients found resmetirom reduced hepatic fat by 37.3% after 36 weeks (-37.3% resmetirom vs. -8.5% placebo)[119]. Resmetirom showed superior performance by improving disease activity, including the resolution of MASH, and reducing fibrosis compared to placebo[120]. These findings resulted in the expedited approval of resmetirom by the US Food and Drug Administration (FDA) in March 2024.
This evidence highlights the role of hypothyroidism in MASLD pathogenesis through hepatic and systemic effects. While standard hormone replacement therapy, levothyroxine, improves hepatic steatosis, the novel thyroid hormone receptor agonist resmetirom shows promise in addressing both diseases.
Polycystic ovary syndrome
Epidemiology
Polycystic ovary syndrome (PCOS) is a prevalent endocrinopathy in premenopausal women, defined by hyperandrogenism and ovulatory dysfunction, and is frequently accompanied by insulin resistance and metabolic abnormalities. MASLD is highly prevalent (pooled prevalence of 43%) among patients with PCOS despite their relatively young age[121]. Several metabolic and PCOS-specific factors appear to contribute to this increased risk[121]. Growing data suggests a strong association between PCOS and MASLD, with one meta-analysis finding women with PCOS face an almost two-and-a-half-fold increase in risk of MASLD than controls (OR: 2.49, 95%CI: 2.20-2.82)[122].
Mechanism
Hyperandrogenism and insulin resistance, central features of PCOS, are responsible for the development and progression of MASLD. A UK-based longitudinal study involving 63,120 women with PCOS found that elevated serum testosterone levels correlated with a significantly increased risk of MASLD (HR: 2.30, 95%CI: 1.16-4.53 for 3-3.49 nmol/L and HR:2.40, 95%CI: 1.24-4.66, for > 3.5 nmol/L)[123]. A proposed mechanism linking increased testosterone to MASLD comes from Andrisse et al., who reported that dihydrotestosterone (DHT) administration in female mice blunts insulin signaling, contributing to metabolic dysfunction and, ultimately, hepatic fat accumulation[124]. Another study found that impaired IRS-PI3K-Akt insulin signaling, along with increased IL-6, monocyte chemoattractant protein 1 (Mcp1) mRNA, and TNF-α protein, exacerbates hepatic fat accumulation and inflammation[125]. Additionally, a considerable percentage of women with PCOS are affected by obesity and metabolic syndrome. Approximately 60% are obese, and 50% exhibit characteristics of metabolic syndrome[126].
Treatment
Therapeutic strategies for PCOS addressing weight loss, improving insulin sensitivity, and hyperandrogenism have demonstrated efficacy in improving liver health in MASLD. Lifestyle modifications, including dietary restriction and physical activity, remain the first-line management for PCOS, improving insulin sensitivity, reducing hyperandrogenism, and restoring ovulatory function[127]. Pharmacological treatments targeting insulin resistance, such as metformin[128] and GLP-1 receptor agonists[129], have demonstrated effectiveness in managing both PCOS and MASLD. However, oral contraceptive pills - regularly prescribed to patients with PCOS to regulate menstrual cycles and reduce androgen levels - could have adverse effects on liver function[130]. Therefore, liver function should be closely monitored for women receiving oral contraceptive pill therapy.
Current treatment options focus on alleviating the prevalent insulin resistance linked to MASLD and PCOS; however, future research should investigate the targeting of hyperandrogenism and its influence on the progression of MASLD/PCOS.
RENAL-LIVER AXIS
Epidemiology
We summarized essential studies regarding chronic kidney disease (CKD) in individuals with MASLD in Table 3. CKD is the gradual deterioration of renal function, leading to the accumulation of waste products and fluid and electrolyte imbalances[131]. Individuals needing renal replacement therapy are expected to surge 5-fold by 2030, highlighting the growing burden of CKD[132]. Several studies demonstrated that individuals with MASLD have an increased prevalence of CKD. A meta-analysis of 13 studies involving over 1 million individuals found that MASLD is associated with a 1.45-fold increase in long-term risk of developing CKD (HR: 1.43, 95%CI: 1.22-1.54)[133]. Additionally, patients with CKD and MASLD have a greater risk of mortality as opposed to patients with CKD alone[134]. One study found that the severity of MASLD correlated with a 1.34-fold increased risk of prevalent CKD[135]. Furthermore, a nationwide analysis of acute kidney readmissions in heart failure patients found that MASLD conferred an increased risk of 6-month hospital readmission due to acute kidney injury (HR: 1.44, 95%CI: 1.14-1.82) and reduced mean time to readmission (145 ± 45 days vs. 155 ± 42 days, β = -10 days, P = 0.044)[136].
Mechanism
MASLD has been identified as a predictor of both the development and progression of CKD, suggesting a link between hepatic steatosis and renal dysfunction. Insulin resistance, a hallmark of MASLD, has been shown to promote sodium retention, glomerular filtration, and renin-angiotensin-aldosterone system activation, all contributing to glomerular injury[137]. Additionally, pro-inflammatory, pro-fibrogenic, and anti-fibrinolytic mediators triggered by MASLD, such as fibroblast growth factor 21 (FGF-21), TNF-α,
Treatment
Lifestyle modifications have been shown to improve both MASLD and CKD. A 6-month randomized controlled trial involving 128 patients with MASLD found that diet modification and physical activity improved liver fat content and glomerular filtration rate[140]. Additionally, pharmacological therapies such as GLP-1 agonists and SGLT2 inhibitors are considered to be first-line therapies for managing coexisting MASLD and CKD[141,142]. GLP-1 agonists are nephroprotective by reducing sodium reabsorption in the proximal tubules, mitigating renal damage and CKD progression[143]. Similarly, SGLT2 inhibitors have been shown to induce natriuresis by decreasing sodium reabsorption. Multiple trials have shown promising results demonstrating that SGLT2 inhibitors reduce incident nephropathy and slow the decline of kidney function[144].
This research highlights the role of MASLD in the risk and progression of CKD through insulin resistance, inflammation, and increased uremic toxins. Therapeutic strategies involving lifestyle modification, GLP-1 agonists, and SGLT2 inhibitors show promise; however, more research is required to standardize care for patients with MASLD-CKD burden.
SKIN-LIVER AXIS
Epidemiology
Recent research suggests a bidirectional relationship between MASLD and psoriasis, a chronic systemic inflammatory skin disease. Patients with psoriasis exhibit a significantly higher prevalence of MASLD than the general population[145]. A US population-based study revealed that individuals with psoriasis exhibit a greater prevalence of MASLD (32.7% vs. 26.6%) than those without psoriasis[145]. Psoriasis was statistically significantly associated with MASLD (OR: 1.67; 95%CI: 1.03-2.70)[145]. A meta-analysis reported that psoriatic patients have a 4-fold increased risk of incident MASLD (OR: 4.01, 95%CI: 2.17-7.77)[146]. Interestingly, a cross-sectional study found that MASLD is significantly associated with increased the 10-year risk of CVD events in individuals with psoriasis as compared to those without MASLD (71.5% vs. 29.2%; OR: 6.0, 95%CI: 3.3-11.1)[147].
Mechanism
Psoriasis and MASLD share common immunological pathways that could result in both cutaneous and hepatic inflammation. Both are associated with increased pro-inflammatory cytokines contributing to their disease progression. In psoriasis, release of TNF-α, IL-6, and IL-17 into systemic circulation contributes to liver inflammation while the release of similar cytokines by hepatic macrophages contributes to keratinocyte proliferation. This immune pathway crosstalk highlights a possible mechanism underlying the co-occurrence of psoriasis and MASLD[148,149]. Pro-inflammatory adipokines, IL-1, IL-6, TNF-α, and leptin, have been shown to play a role in keratinocyte proliferation in psoriasis and liver fibrogenesis, leading to insulin resistance[149].
Treatment
Lifestyle modification, such as the Mediterranean diet, has effectively reduced systemic inflammation, improving both hepatic and dermatologic outcomes[150,151]. Despite overlapping mechanisms, several anti-psoriatric medications have been associated with hepatotoxicity and should be used cautiously in individuals with MASLD. Specifically, methotrexate[152], cyclosporine[152], and acitretin[153] were found to have adverse effects on the liver. Conversely, secukinumab, ixekizumab, adalimumab, and apremilast might not confer this same risk[154]. Nonetheless, further studies are needed to confirm the hepatic safety of these treatments, and patients on anti-psoriatic medications should have their liver function monitored.
Psoriasis and MASLD are interconnected, mutually exacerbating diseases that require further research into therapeutics targeting systemic inflammation while mitigating liver damage.
EXTRA ORGAN-LIVER AXIS
In addition to the previously described cardiovascular, endocrine, renal, and dermatologic systems, recent research suggests potential effects of MASLD on the pulmonary, intestinal, and musculoskeletal systems; nevertheless, the associated risks and causative relationships remain unclear.
Pulmonary-liver axis
Recent research indicates that MASLD may be linked to pulmonary dysfunction, encompassing obstructive sleep apnea (OSA) and diminished lung function. Research involving individuals with T2DM revealed a substantial correlation between MASLD and OSA, with 100% of patients with severe MASLD exhibiting OSA and 91.5% of patients with moderate-to-severe OSA presenting with moderate-to-severe MASLD[155]. Emerging research suggests that it activates the hypoxia-inducible factor 1-alpha (HIF-1α) pathway, facilitating lipogenesis and oxidative stress, while also contributing to systemic inflammation and insulin resistance, all of which are associated with the pathogenesis of MASLD[156]. Moreover, several studies have examined the impacts of MASLD on pulmonary function. Miao et al. found that increasing fibrosis severity was inversely correlated to forced expiratory volume (FEV1), forced vital capacity (FVC), and predicted FVC, after adjusting for confounders[157]. However, another study enrolled 3,462 individuals and found no significant difference in FEV1, FVC, FEV1/FVC ratio, and FEV1/predicted FEV1 ratio in patients with MASLD. Additionally, there was no correlation between MASLD and chronic obstructive pulmonary disease (P = 0.407) or asthma (P = 0.808)[158]. Given these inconclusive results, the association between MASLD and pulmonary function needs to be studied for confirmation.
Intestinal-liver axis
Recent research has shown that intestinal dysbiosis significantly contributes to MASLD. Composition’s variations of gut bacteria have been extensively documented, suggesting a decrease in beneficial microorganisms, including F. prausnitzii and A. muciniphila, which are critical for the production of anti-inflammatory short-chain fatty acids[159]. Conversely, an overgrowth of pathobionts, including Enterobacteriaceae and B. vulgatus, results in inflammation, insulin resistance, liver fat accumulation, and hepatic fibrosis[159]. A panel of 37 bacterial strains accurately predicted the existence of fibrosis among patients with MASLD[160], suggesting a potential application of microbiota-based markers to project the progression of MASLD. In addition, intestinal diseases, such as inflammatory bowel disease[161], celiac disease[162], and irritable bowel syndrome[163], have been strongly associated with MASLD, potentially due to impaired gut barrier function that fosters systemic inflammation in MASLD[164-166]. Despite strong associations between intestinal dysfunction and MASLD pathogenesis, further research is needed to establish causality better.
Musculoskeletal-liver axis
MASLD is associated with adverse alterations in muscle composition, including sarcopenia, which is characterized by the reduction of muscular mass and strength. A meta-analysis based on 29 studies and 63,330 patients reported that sarcopenia was associated with MASLD (adjusted OR: 2.08, 95%CI: 1.58-2.74, I2 = 93.6%)[167]. Additionally, sarcopenia was correlated to a higher 10-year major osteoporotic and hip fracture probability[167], atherosclerotic CVD[168], and increased risk of all-cause mortality in individuals with MASLD[169]. Petta et al. found a linear relationship between the prevalence of sarcopenia and the severity of fibrosis and steatosis, independent of other hepatic and metabolic risk factors[170]. MASLD and sarcopenia share pathophysiological links, including insulin resistance, which plays a role in both diseases. In addition to the role that insulin resistance plays in MASLD, in the setting of sarcopenia, it decreases protein synthesis and increases protein catabolism in skeletal muscles[171]. Hepatokines may facilitate the liver-muscle communication in MASLD, wherein modified hepatokine concentrations exacerbate muscle atrophy and inflammation, perpetuating a cycle of increasing insulin resistance, fat deposition, and sarcopenia[171,172]. Likewise, MASLD has been linked to myosteatosis[173]. Despite strong correlative data, more research is needed to investigate the causal link between MASLD and sarcopenia and myosteatosis.
EXTRAHEPATIC CANCER
Epidemiology
We summarized essential studies regarding extrahepatic cancer in individuals with MASLD in Table 4. Increasing evidence suggests that MASLD not only elevates the risk of HCC but is also linked to several extrahepatic malignancies. A meta-analysis including 16.7 million individuals from 18 cohort studies found that MASLD was linked to a higher risk of gastric (HR: 1.47, 95%CI: 1.07-2.01), colorectal (HR: 1.33, 95%CI: 1.16-1.53), pancreatic (HR: 1.41, 95%CI: 1.11-1.79), biliary tract (HR: 1.27, 95%CI: 1.18-1.37), thyroid (HR: 1.46, 95%CI: 1.02-2.09), urinary system (HR: 1.45, 95%CI: 1.25-1.69), breast (HR: 1.17, 95%CI: 1.08-1.26), and female genital organ cancers (HR: 1.36, 95%CI: 1.11-1.66)[174]. A significant likelihood of advanced fibrosis has been linked to developing HCC and extrahepatic cancers[175]. A cohort study conducted in the US involving age- and sex-matched individuals with and without MASLD found that MASLD was associated with a 90% higher risk of cancer[176]. Additionally, extrahepatic cancer and cardiovascular mortality rates in MASLD-related cirrhosis were more pronounced than in MASLD without cirrhosis[177].
Essential studies evaluating extrahepatic cancers in individuals with MASLD
| Study (year)2345678 | Country | Study design | Total population (Number of MASLD) | Mean age of participants (years) | Female (%) | Diagnostic method | Outcomes | Confounder adjustment |
| Zhou et al. (2024)[174] | South Korea, China, Japan, Sweden, US, Germany, UK | Meta-analysis | 1,023-8,120,674 | 44-61 | 19-100 | Ultrasonography, ICD codes, CT, FLI, liver biopsy | MASLD linked to higher risk of: Gastric cancer (HR: 1.47, 95%CI: 1.07-2.01) Colorectal cancer (HR: 1.33, 95%CI: 1.16-1.53) Pancreatic cancer (HR: 1.41, 95%CI: 1.11-1.79) Biliary tract cancer (HR: 1.27, 95%CI: 1.18-1.37) Thyroid cancer (HR: 1.46, 95%CI: 1.02-2.09) Urinary system cancer (HR: 1.45, 95%CI: 1.25-1.69) Breast cancer (HR: 1.17, 95%CI: 1.08-1.26) Female genital organ cancers (HR: 1.36, 95%CI: 1.11-1.66) | Age, sex, BMI, smoking, alcohol consumption, hypertension, dyslipidemia, diabetes, physical activity (others listed in paper) |
| Kim et al. (2017)[175] | South Korea | Cohort-based study | 25,947 (33.6%) | 48.0 | 46.2 | Ultrasonography | Cancer incident rate higher in MASLD population vs. non-MASLD population (782.9 vs. 592.8 per 100,000 person-years; HR: 1.32; 95%CI: 1.17-1.49 MASLD has a strong association with: hepatocellular carcinoma (HR: 16.73; 95%CI: 2.09-133.85); colorectal cancer in males (HR: 2.01; 95%CI: 1.10-3.68; P = 0.02) breast cancer in females (HR: 1.92; 95%CI: 1.15-3.20) | Age, sex, smoking status, diabetes, hypertension, serum levels of GGT, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides |
| Allen et al. (2019)[176] | US | Population-based study | 19,163 (24.6%) | 53.2 | 53.2 | ICD codes | MASLD is associated with 90% higher overall risk of malignancy (IRR: 1.9; 95%CI: 1.3-2.7) The highest increase in risk: liver cancer (IRR: 2.8; 95%CI 1.6-5.1); uterine cancer (IRR, 2.3; 95%CI: 1.4-4.1); stomach cancer (IRR: 2.3; 95%CI: 1.3-4.1); pancreas cancer (IRR: 2.0; 95%CI: 1.2-3.3); colon cancer (IRR: 1.8; 95%CI: 1.1-2.8) | Age, sex, diabetes, cirrhosis |
Mechanism
While more research into the mechanism driving malignancies in MASLD patients is needed, several theories have been proposed. Hyperinsulinemia, a key feature of insulin resistance, can act as a growth factor and increase the risk of cancer by activating PI3K-AKT-mTOR and RAS-MAPK proliferative pathways[178]. Additionally, the pro-inflammatory environment found in the setting of MASLD can contribute to the development of malignancies[179]. Gut microbiota dysbiosis could also be a significant player in the pathogenesis of cancers in those with MASLD through lipopolysaccharide (LPS), toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MYD88), and NF-KB, which are intimately linked to inflammation and gastrointestinal cancers[180,181]. Genetic polymorphisms of PNPLA3[182], TM6SF2[20], and MBOAT7[183] genes have been dually implicated in MASLD and related cancers.
Treatment
These findings highlight the need for increased cancer screening in patients with MASLD, and patients should be counseled on how to minimize their risk. Further studies are needed to investigate the effects of therapeutic interventions for MASLD on mitigating the risk of extrahepatic cancers.
Individuals with MASLD have a higher risk of developing both liver and various extrahepatic cancers, including those of the digestive, urinary, hormonal, and reproductive systems, compared to the general population.
Future directions
Although we have acquired substantial insights into the extrahepatic manifestations of MASLD over the past few decades, there are still notable gaps in our understanding that need future research. Although insulin resistance, inflammation, dysregulation of lipid metabolism, and oxidative stress are acknowledged mechanistic pathways, the precise molecular linkages between MASLD and extrahepatic manifestations remain unclear. Although we have commented on other organ-liver axes, major knowledge gaps warrant further research regarding this topic, such as the gut, pulmonary, and musculoskeletal liver axes. Additionally, recent studies suggest an association between MASLD and neurodegenerative diseases, including dementia[184] and Parkinson’s disease[185], although the results are mostly observational. Furthermore, in-depth research on pathophysiological mechanisms is still required, and the development of comprehensive therapeutic strategies remains unclear. Current therapeutic options for extrahepatic manifestations still include mainstay lifestyle modification with weight loss, including diet and physical activity. However, due to the advantageous synergistic effects of GLP-1 receptor agonists, SGLT2 inhibitors, and statins, these treatment strategies require further assessment of the impact on extrahepatic manifestations of MASLD.
CONCLUSION
MASLD is a multisystemic disease implicated in various extrahepatic manifestations. It significantly increases the risk of CVD, T2DM, CKD, endocrine disorders such as PCOS and hyperthyroidism, dermatologic conditions such as psoriasis, other extrahepatic manifestations such as pulmonary disease, sarcopenia, and intestinal diseases, and multiple extrahepatic malignancies. Insulin resistance, chronic inflammation, dysregulation of lipid metabolism, oxidative stress, and dysbiosis of gut microbiota were the common pathophysiological mechanisms of MASLD and extrahepatic manifestations. MASLD may be both a cause and a consequence of broader extrahepatic manifestations. Given the current burden of MASLD and extrahepatic manifestations, early screening and comprehensive management are essential. While lifestyle modifications, including weight loss through diet and physical activity, remain the main treatment options, emerging evidence suggests medications, such as GLP-1 receptor agonists, SGLT-2 inhibitors, and resmetirom, could be used for the management of both MASLD and its extrahepatic manifestations.
Given the significantly growing healthcare and economic burden of MASLD, physicians and policymakers should prioritize early screening and interventions for extrahepatic manifestations among individuals with MASLD. Future studies should focus on further elucidating mechanistic links and developing a standardized approach to the treatment of MASLD and its extrahepatic manifestations.
DECLARATIONS
Acknowledgments
The graphic abstract was created in BioRender. Shenoy A. (2025). (https://BioRender.com/4s42itr).
Authors’ contributions
Conceptualization: Kim D, Ahmed A, Shenoy A
Data collection and literature review: Shenoy A, Kim D
Writing, original draft: Shenoy A, Kim D
Writing, review, and editing: Kim D, Ahmed A
All authors have read and approved the final version of the manuscript for submission.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Kim D is an Editorial Board Member of of the journal Metabolism and Target Organ Damage. Kim D was not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling and decision making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Kim GA, Moon JH, Kim W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: implication of Janus-faced modernity. Clin Mol Hepatol. 2023;29:831-43.
2. Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919.
3. van Kleef LA, Choi HSJ, Brouwer WP, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3:100350.
4. Wang X, Xie Q. Metabolic dysfunction-associated fatty liver disease (MAFLD) and viral hepatitis. J Clin Transl Hepatol. 2022;10:128-33.
5. Rinella ME, Lazarus JV, Ratziu V, et al; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-86.
6. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
7. Kim D, Danpanichkul P, Wijarnpreecha K, Cholankeril G, Loomba R, Ahmed A. Current burden of steatotic liver disease and fibrosis among adults in the United States, 2017-2023. Clin Mol Hepatol. 2025;31:382-93.
8. Kim D, Danpanichkul P, Wijarnpreecha K, Cholankeril G, Ahmed A. Trends in mortality from chronic liver disease before, during, and after the COVID-19 pandemic, 2015 to 2023. Ann Intern Med. 2025;178:1054-7.
9. Kim D, Danpanichkul P, Wijarnpreecha K, Cholankeril G, Ahmed A. Contemporary burden of mortality from chronic liver disease by sex and race/ethnicity in the United States. Clin Mol Hepatol. 2025;31:e268-72.
10. Sohn W, Lee YS, Kim SS, et al; Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for the management of metabolic dysfunction-associated steatotic liver disease 2025. Clin Mol Hepatol. 2025;31:S1-31.
11. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-53.
12. Koh JH, Ng CH, Nah B, Tan DJH, Loomba R, Huang DQ; Nash HCC Transplant Collaborative. NASH is the leading cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2024;22:197-9.e3.
13. Kim D, Manikat R, Wijarnpreecha K, Cholankeril G, Ahmed A. Burden of mortality from hepatocellular carcinoma and biliary tract cancers by race and ethnicity and sex in US, 2018-2023. Clin Mol Hepatol. 2024;30:756-70.
14. Le P, Tatar M, Dasarathy S, et al. Estimated burden of metabolic dysfunction-associated steatotic liver disease in US adults, 2020 to 2050. JAMA Netw Open. 2025;8:e2454707.
15. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-86.
16. Baumeister SE, Völzke H, Marschall P, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134:85-94.
17. Konyn P, Ahmed A, Kim D. Causes and risk profiles of mortality among individuals with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S43-57.
18. Danpanichkul P, Suparan K, Prasitsumrit V, Ahmed A, Wijarnpreecha K, Kim D. Long-term outcomes and risk modifiers of metabolic dysfunction-associated steatotic liver disease between lean and non-lean populations. Clin Mol Hepatol. 2025;31:74-89.
19. Lindén D, Tesz G, Loomba R. Targeting PNPLA3 to treat MASH and MASH related fibrosis and cirrhosis. Liver Int. 2025;45:e16186.
20. Luo F, Oldoni F, Das A. TM6SF2: a novel genetic player in nonalcoholic fatty liver and cardiovascular disease. Hepatol Commun. 2022;6:448-60.
21. Varadharajan V, Ramachandiran I, Massey WJ, et al. Membrane-bound O-acyltransferase 7 (MBOAT7) shapes lysosomal lipid homeostasis and function to control alcohol-associated liver injury. Elife. 2024;12:RP92243.
22. Mahmood S, Morrice N, Thompson D, et al. Hydroxysteroid 17β-dehydrogenase 13 (Hsd17b13) knockdown attenuates liver steatosis in high-fat diet obese mice. Exp Physiol. 2025;110:1071-86.
23. Hagström H, Vessby J, Ekstedt M, Shang Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol. 2024;80:e76-7.
24. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691-705.
25. Hagström H, Nasr P, Ekstedt M, et al. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197-204.
26. Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013;1281:106-22.
27. Saokaew S, Kanchanasurakit S, Thawichai K, et al. Association of non-alcoholic fatty liver disease and all-cause mortality in hospitalized cardiovascular disease patients: a systematic review and meta-analysis. Medicine. 2021;100:e24557.
28. Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2022;71:1867-75.
29. Mantovani A, Petracca G, Csermely A, et al. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022;72:372-80.
30. Xu J, Dai L, Zhang Y, et al. Severity of nonalcoholic fatty liver disease and risk of future ischemic stroke events. Stroke. 2021;52:103-10.
31. Alon L, Corica B, Raparelli V, et al. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29:938-46.
32. Abosheaishaa H, Hussein M, Ghallab M, et al. Association between non-alcoholic fatty liver disease and coronary artery disease outcomes: a systematic review and meta-analysis. Diabetes Metab Syndr. 2024;18:102938.
33. Gong H, Liu X, Cheng F. Relationship between non-alcoholic fatty liver disease and cardiac arrhythmia: a systematic review and meta-analysis. J Int Med Res. 2021;49:3000605211047074.
34. Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:948-63.
35. Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72:156-66.
36. Badmus OO, Hinds TD Jr, Stec DE. Mechanisms linking metabolic-associated fatty liver disease (MAFLD) to cardiovascular disease. Curr Hypertens Rep. 2023;25:151-62.
37. Chen X, Chen S, Pang J, et al. Hepatic steatosis aggravates atherosclerosis via small extracellular vesicle-mediated inhibition of cellular cholesterol efflux. J Hepatol. 2023;79:1491-501.
38. Pezzino S, Luca T, Castorina M, Puleo S, Latteri S, Castorina S. Role of perturbated hemostasis in MASLD and its correlation with adipokines. Life. 2024;14:93.
39. Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116-41.
40. Westcott F, Dearlove DJ, Hodson L. Hepatic fatty acid and glucose handling in metabolic disease: potential impact on cardiovascular disease risk. Atherosclerosis. 2024;394:117237.
41. Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. 2020;42:101092.
42. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-835.
43. Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: Co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-62.
44. Mellemkjær A, Kjær MB, Haldrup D, Grønbæk H, Thomsen KL. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:28-34.
45. Correia LCL. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;379:1387.
46. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189-96.
47. Kawaguchi T, Charlton M, Kawaguchi A, et al. Effects of Mediterranean diet in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression analysis of randomized controlled trials. Semin Liver Dis. 2021;41:225-34.
48. Haigh L, Kirk C, El Gendy K, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin Nutr. 2022;41:1913-31.
49. Kim D, Murag S, Cholankeril G, et al. Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;19:1240-7.e5.
50. Tsunoda K, Kitano N, Kai Y, Jindo T, Uchida K, Arao T. Dose-response relationships of accelerometer-measured sedentary behaviour and physical activity with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1330-9.
51. van Kleef LA, Hofman A, Voortman T, de Knegt RJ. Objectively measured physical activity is inversely associated with nonalcoholic fatty liver disease: the rotterdam study. Am J Gastroenterol. 2022;117:311-8.
52. Franco I, Bianco A, Mirizzi A, et al. Physical activity and low glycemic index Mediterranean diet: main and modification effects on NAFLD score. Results from a randomized clinical trial. Nutrients. 2020;13:66.
53. Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine. 2019;98:e14918.
54. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1002.
55. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-44.
56. Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
57. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-90.
58. Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:367-78.
59. Majzoub AM, Nayfeh T, Barnard A, et al. Systematic review with network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment Pharmacol Ther. 2021;54:880-9.
60. Francque SM, Bedossa P, Ratziu V, et al; NATIVE Study Group. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547-58.
61. Thomson MJ, Serper M, Khungar V, et al. Prevalence and factors associated with statin use among patients with nonalcoholic fatty liver disease in the TARGET-NASH study. Clin Gastroenterol Hepatol. 2022;20:458-60.e4.
62. Shahab O, Biswas R, Paik J, Bush H, Golabi P, Younossi ZM. Among patients with NAFLD, treatment of dyslipidemia does not reduce cardiovascular mortality. Hepatol Commun. 2018;2:1227-34.
63. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-22.
64. Ayada I, van Kleef LA, Zhang H, et al. Dissecting the multifaceted impact of statin use on fatty liver disease: a multidimensional study. EBioMedicine. 2023;87:104392.
65. Kaplan DE, Serper MA, Mehta R, et al; VOCAL Study Group. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology. 2019;156:1693-706.e12.
66. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-601.
67. Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173:20-8.
68. Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159:1290-301.e5.
69. Sakai K, Okamura T, Toyokuni E, et al. Metabolic dysfunction-associated steatotic liver disease: a superior predictor for incident type 2 diabetes over traditional criteria - NAGALA study. J Diabetes Investig. 2024;15:1788-96.
70. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41:372-82.
71. Björkström K, Stål P, Hultcrantz R, Hagström H. Histologic scores for fat and fibrosis associate with development of type 2 diabetes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:1461-8.
72. Kim D, Loomba R, Ahmed A. Current burden of MASLD, MetALD, and hepatic fibrosis among US adults with prediabetes and diabetes, 2017-2023. Clin Mol Hepatol. 2025;31:e235-8.
73. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793-801.
74. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-47.
75. Younossi ZM, Golabi P, Price JK, et al. The global epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2024;22:1999-2010.e8.
76. Ajmera V, Cepin S, Tesfai K, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. 2023;78:471-8.
77. Marušić M, Paić M, Knobloch M, Liberati Pršo AM. NAFLD, insulin resistance, and diabetes mellitus type 2. Can J Gastroenterol Hepatol. 2021;2021:6613827.
78. Sabatini S, Sen P, Carli F, et al. Hepatic glucose production rises with the histological severity of metabolic dysfunction-associated steatohepatitis. Cell Rep Med. 2024;5:101820.
79. Pal SC, Méndez-Sánchez N. Insulin resistance and adipose tissue interactions as the cornerstone of metabolic (dysfunction)-associated fatty liver disease pathogenesis. World J Gastroenterol. 2023;29:3999-4008.
80. Zhang C, Sui Y, Liu S, Yang M. Molecular mechanisms of metabolic disease-associated hepatic inflammation in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Explor Dig Dis. 2023;2:246-75.
81. Yu L, Gao F, Li Y, et al. Role of pattern recognition receptors in the development of MASLD and potential therapeutic applications. Biomed Pharmacother. 2024;175:116724.
82. Ding Z, Wei Y, Peng J, Wang S, Chen G, Sun J. The potential role of C-reactive protein in metabolic-dysfunction-associated fatty liver disease and aging. Biomedicines. 2023;11:2711.
83. Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-75.
84. Drummen M, Dorenbos E, Vreugdenhil ACE, et al. Long-term effects of increased protein intake after weight loss on intrahepatic lipid content and implications for insulin sensitivity: a PREVIEW study. Am J Physiol Endocrinol Metab. 2018;315:E885-91.
85. Skytte MJ, Samkani A, Petersen AD, et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62:2066-78.
86. Cho Y-E, Kim D-K, Seo W, Gao B, Yoo S-H, Song B-J. Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450-2E1-mediated oxidative and nitrative stress. Hepatology. 2021;73:2180-95.
87. Souza Cruz EM, de Morais Juliana M, Dalto da Rosa CVi, et al. Long-term sucrose solution consumption causes metabolic alterations and affects hepatic oxidative stress in Wistar rats. Biol Open. 2020;9:bio047282.
88. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083-94.
89. Pinyopornpanish K, Leerapun A, Pinyopornpanish K, Chattipakorn N. Effects of metformin on hepatic steatosis in adults with nonalcoholic fatty liver disease and diabetes: insights from the cellular to patient levels. Gut Liver. 2021;15:827-40.
90. Diabetes A. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes - 2018. Diabetes Care. 2018;41:S73-85.
91. Lingvay I, Raskin P, Szczepaniak LS. Effect of insulin-metformin combination on hepatic steatosis in patients with type 2 diabetes. J Diabetes Complications. 2007;21:137-42.
92. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305-15.
93. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633-40.
94. Ratziu V, Giral P, Jacqueminet S, et al; LIDO Study Group. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) trial. Gastroenterology. 2008;135:100-10.
95. He Y, Ao N, Yang J, Wang X, Jin S, Du J. The preventive effect of liraglutide on the lipotoxic liver injury via increasing autophagy. Ann Hepatol. 2020;19:44-52.
96. Bizino MB, Jazet IM, de Heer P, et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia. 2020;63:65-74.
97. Eguchi Y, Kitajima Y, Hyogo H, et al; Japan Study Group for NAFLD (JSG-NAFLD). Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res. 2015;45:269-78.
98. Petit JM, Cercueil JP, Loffroy R, et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the lira-NAFLD study. J Clin Endocrinol Metab. 2017;102:407-15.
99. Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352-5.
100. Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10:393-406.
101. Sanyal AJ, Newsome PN, Kliers I, et al; ESSENCE Study Group. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med. 2025;392:2089-99.
102. Akuta N, Kawamura Y, Watanabe C, et al. Impact of sodium glucose cotransporter 2 inhibitor on histological features and glucose metabolism of non-alcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Res. 2019;49:531-9.
103. Seko Y, Sumida Y, Tanaka S, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1072-8.
104. Gautam A, Agrawal PK, Doneria J, Nigam A. Effects of canagliflozin on abnormal liver function tests in patients of type 2 diabetes with non-alcoholic fatty liver disease. J Assoc Physicians India. 2018;66:62-6.
105. Lai L-L, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan W-K. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci. 2020;65:623-31.
106. Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial). Diabetes Care. 2018;41:1801-8.
107. Eriksson JW, Lundkvist P, Jansson PA, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923-34.
108. Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285-92.
109. Bharatselvam S, Schwenger KJP, Ghorbani Y, et al. Assessing clinical and metabolic responses related to hyperlipidemia, MASLD and type 2 diabetes: sleeve versus RYGB. Nutrition. 2024;126:112530.
110. Wyne KL, Nair L, Schneiderman CP, et al. Hypothyroidism prevalence in the United States: a retrospective study combining national health and nutrition examination survey and claims data, 2009-2019. J Endocr Soc. 2022;7:bvac172.
111. Mantovani A, Csermely A, Bilson J, et al. Association between primary hypothyroidism and metabolic dysfunction-associated steatotic liver disease: an updated meta-analysis. Gut. 2024;73:1554-61.
112. Kim D, Vazquez-Montesino LM, Escober JA, et al. Low thyroid function in nonalcoholic fatty liver disease is an independent predictor of all-cause and cardiovascular mortality. Am J Gastroenterol. 2020;115:1496-504.
113. Ritter MJ, Amano I, Hollenberg AN. Thyroid hormone signaling and the liver. Hepatology. 2020;72:742-52.
114. Yan F, Wang Q, Lu M, et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol. 2014;61:1358-64.
115. Bao S, Li F, Duan L, Li J, Jiang X. Thyroid-stimulating hormone may participate in insulin resistance by activating toll-like receptor 4 in liver tissues of subclinical hypothyroid rats. Mol Biol Rep. 2023;50:10637-50.
116. Kizivat T, Maric I, Mudri D, Curcic IB, Primorac D, Smolic M. Hypothyroidism and nonalcoholic fatty liver disease: pathophysiological associations and therapeutic implications. J Clin Transl Hepatol. 2020;8:347-53.
117. Liu Y, Wang W, Yu X, Qi X. Thyroid function and risk of non-alcoholic fatty liver disease in euthyroid subjects. Ann Hepatol. 2018;17:779-88.
118. Liu L, Yu Y, Zhao M, et al. Benefits of levothyroxine replacement therapy on nonalcoholic fatty liver disease in subclinical hypothyroidism patients. Int J Endocrinol. 2017;2017:5753039.
119. Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012-24.
120. Harrison SA, Bedossa P, Guy CD, et al; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497-509.
121. Manzano-Nunez R, Santana-Dominguez M, Rivera-Esteban J, et al. Non-alcoholic fatty liver disease in patients with polycystic ovary syndrome: a systematic review, meta-analysis, and meta-regression. J Clin Med. 2023;12:856.
122. Shengir M, Chen T, Guadagno E, et al. Non-alcoholic fatty liver disease in premenopausal women with polycystic ovary syndrome: a systematic review and meta-analysis. JGH Open. 2021;5:434-45.
123. Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018;15:e1002542.
124. Andrisse S, Childress S, Ma Y, et al. Low-dose dihydrotestosterone drives metabolic dysfunction via cytosolic and nuclear hepatic androgen receptor mechanisms. Endocrinology. 2017;158:531-44.
125. Zhang Y, Meng F, Sun X, et al. Hyperandrogenism and insulin resistance contribute to hepatic steatosis and inflammation in female rat liver. Oncotarget. 2018;9:18180-97.
126. Kelley CE, Brown AJ, Diehl AM, Setji TL. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J Gastroenterol. 2014;20:14172-84.
128. Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-74.
129. Austregésilo de Athayde De Hollanda Morais B, Martins Prizão V, de Moura de Souza M, et al. The efficacy and safety of GLP-1 agonists in PCOS women living with obesity in promoting weight loss and hormonal regulation: a meta-analysis of randomized controlled trials. J Diabetes Complications. 2024;38:108834.
130. Chane E, Wondifraw H, Hadgu R, Fasil A. Assessment of liver function tests of women taking hormonal contraceptives at University of Gondar comprehensive specialized hospital and Family Guidance Association of Gondar (FGAE), 2022; a comparative cross-sectional study. PLoS One. 2023;18:e0289746.
133. Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2022;71:156-62.
134. Paik J, Golabi P, Younoszai Z, Mishra A, Trimble G, Younossi ZM. Chronic kidney disease is independently associated with increased mortality in patients with nonalcoholic fatty liver disease. Liver Int. 2019;39:342-52.
136. Hashim A, Maraey A, Elzanaty A, et al. Nonalcoholic fatty liver disease predicts acute kidney injury readmission in heart failure hospitalizations: a nationwide analysis. Curr Probl Cardiol. 2023;48:101816.
137. Bansal A, Chonchol M. Metabolic dysfunction-associated kidney disease: pathogenesis and clinical manifestations. Kidney Int. 2025;108:194-200.
138. Zhen J, Zhou Z, He M, et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol. 2023;14:1085041.
139. Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, et al. Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins. 2018;10:300.
140. Abbate M, Mascaró CM, Montemayor S, et al. Energy expenditure improved risk factors associated with renal function loss in NAFLD and mets patients. Nutrients. 2021;13:629.
141. Truong E, Noureddin M. The interplay between nonalcoholic fatty liver disease and kidney disease. Clin Liver Dis. 2022;26:213-27.
142. Sumida Y, Yoneda M, Toyoda H, et al. Common drug pipelines for the treatment of diabetic nephropathy and hepatopathy: can we kill two birds with one stone? Int J Mol Sci. 2020;21:4939.
143. Skov J, Dejgaard A, Frøkiær J, et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab. 2013;98:E664-71.
144. Mima A. Sodium-glucose cotransporter 2 inhibitors in patients with non-diabetic chronic kidney disease. Adv Ther. 2021;38:2201-12.
145. Ruan Z, Lu T, Chen Y, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol. 2022;158:745-53.
146. Untaaveesup S, Kantagowit P, Ungprasert P, et al. The risk of metabolic dysfunction-associated steatotic liver disease in moderate-to-severe psoriasis: a systematic review and meta-analysis. J Clin Med. 2025;14:1374.
147. Romero-Pérez D, Belinchón-Romero I, Bellot P, Francés R, Marco F, Ramos-Rincón JM. Nonalcoholic fatty liver disease puts patients with psoriasis at greater cardiovascular risk. Australas J Dermatol. 2019;60:e304-10.
148. Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between non-alcoholic fatty liver disease and psoriasis: a novel hepato-dermal axis? Int J Mol Sci. 2016;17:217.
149. Costache DO, Blejan H, Cojocaru DL, et al. Intersecting pathways: nonalcoholic fatty liver disease and psoriasis duet-A comprehensive review. Int J Mol Sci. 2024;25:2660.
150. Aryanian Z, Asghari M, Zanousi PP, Ghadimi R, Kebria AS, Hatami P. Adherence to the Mediterranean diet in patients with psoriasis and its relationship with the severity of the disease: a case-control study. Health Sci Rep. 2024;7:e70049.
151. Sualeheen A, Tan SY, Georgousopoulou E, et al. Mediterranean diet for the management of metabolic dysfunction-associated steatotic liver disease in non-Mediterranean, Western countries: What’s known and what’s needed? Nutr Bull. 2024;49:444-62.
152. Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30:282-7.
153. Silva FS, Ribeiro MP, Santos MS, Rocha-Pereira P, Santos-Silva A, Custódio JB. Acitretin affects bioenergetics of liver mitochondria and promotes mitochondrial permeability transition: potential mechanisms of hepatotoxicity. Toxicology. 2013;306:93-100.
154. Krefting F, Scheib C, Benson S, et al. Baseline pathological liver function tests in patients with psoriasis support the indication for systemic therapy rather than being a reason against it: a real-world analysis. Psoriasis. 2025;15:29-44.
155. Jia X, Zou C, Lu N, Lu Q, Xie C. Obstructive sleep apnea is ralated to metabolic dysfunction associated steatotic liver disease in type 2 diabetes mellitus. Sci Rep. 2025;15:24627.
156. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-35.
157. Miao L, Yang L, Guo LS, et al. Metabolic dysfunction-associated fatty liver disease is associated with greater impairment of lung function than nonalcoholic fatty liver disease. J Clin Transl Hepatol. 2022;10:230-7.
158. Feng T, Li J, Wu L, He X, Ye J. Association between metabolic dysfunction-associated steatotic liver disease and pulmonary function: a population-based and two-sample mendelian randomization study. BMC Pulm Med. 2024;24:368.
159. Abdelhameed F, Mustafa A, Kite C, et al. Gut microbiota and metabolic dysfunction-associated steatotic liver disease (MASLD): emerging pathogenic mechanisms and therapeutic implications. Livers. 2025;5:11.
160. Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054-62.e5.
161. Martínez-Domínguez SJ, García-Mateo S, Gargallo-Puyuelo CJ, et al. Inflammatory bowel disease is an independent risk factor for metabolic dysfunction-associated steatotic liver disease in lean individuals. Inflamm Bowel Dis. 2024;30:1274-83.
162. Yao J, Sun J, Ebrahimi F, et al. Long-term risk of chronic liver disease in patients with celiac disease: a nationwide population-based, sibling-controlled cohort study. Lancet Reg Health Eur. 2025;50:101201.
163. Wu S, Yuan C, Yang Z, et al. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: a prospective cohort study. BMC Med. 2022;20:262.
164. Sartini A, Gitto S, Bianchini M, et al. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018;9:87.
165. Wei Z, Wang J. Exploration of the core pathway of inflammatory bowel disease complicated with metabolic fatty liver and two-sample Mendelian randomization study of the causal relationships behind the disease. Front Immunol. 2024;15:1375654.
166. Ng JJJ, Loo WM, Siah KTH. Associations between irritable bowel syndrome and non-alcoholic fatty liver disease: a systematic review. World J Hepatol. 2023;15:925-38.
167. Lee HJ, Lee DC, Kim CO. Association between 10-year fracture probability and nonalcoholic fatty liver disease with or without sarcopenia in Korean men: a nationwide population-based cross-sectional study. Front Endocrinol. 2021;12:599339.
168. Han E, Lee YH, Kim YD, et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. 2020;115:584-95.
169. Li X, He J, Sun Q. The prevalence and effects of sarcopenia in patients with metabolic dysfunction-associated steatotic liver disease (MASLD): a systematic review and meta-analysis. Clin Nutr. 2024;43:2005-16.
170. Petta S, Ciminnisi S, Di Marco V, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510-8.
171. Wong R, Yuan LY. Sarcopenia and metabolic dysfunction associated steatotic liver disease: time to address both. World J Hepatol. 2024;16:871-7.
172. Stefan N, Schick F, Birkenfeld AL, Häring HU, White MF. The role of hepatokines in NAFLD. Cell Metab. 2023;35:236-52.
173. Kim MJ, Cho YK, Kim EH, et al. Association between metabolic dysfunction-associated steatotic liver disease and myosteatosis measured by computed tomography. J Cachexia Sarcopenia Muscle. 2024;15:1942-52.
174. Zhou BG, Jiang X, She Q, Ding YB. Association of MASLD with the risk of extrahepatic cancers: a systematic review and meta-analysis of 18 cohort studies. Eur J Clin Invest. 2024;54:e14276.
175. Kim GA, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017;68:140-6.
176. Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - a longitudinal cohort study. J Hepatol. 2019;71:1229-36.
177. Kim D, Adejumo AC, Yoo ER, et al. Trends in mortality from extrahepatic complications in patients with chronic liver disease, from 2007 through 2017. Gastroenterology. 2019;157:1055-66.e11.
179. Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab J. 2021;45:622.
180. Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20:40-54.
181. Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15:671-82.
182. Tai J, Hsu CW, Chen WT, et al. Association of liver fibrosis with extrahepatic cancer in steatotic liver disease patients with PNPLA3 I148M GG genotype. Cancer Sci. 2024;115:564-74.
183. Longo M, Meroni M, Paolini E, et al. TM6SF2/PNPLA3/MBOAT7 loss-of-function genetic variants impact on NAFLD development and progression both in patients and in in vitro models. Cell Mol Gastroenterol Hepatol. 2022;13:759-88.
184. Medina-Julio D, Ramírez-Mejía MM, Cordova-Gallardo J, Peniche-Luna E, Cantú-Brito C, Mendez-Sanchez N. From liver to brain: how MAFLD/MASLD impacts cognitive function. Med Sci Monit. 2024;30:e943417.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data






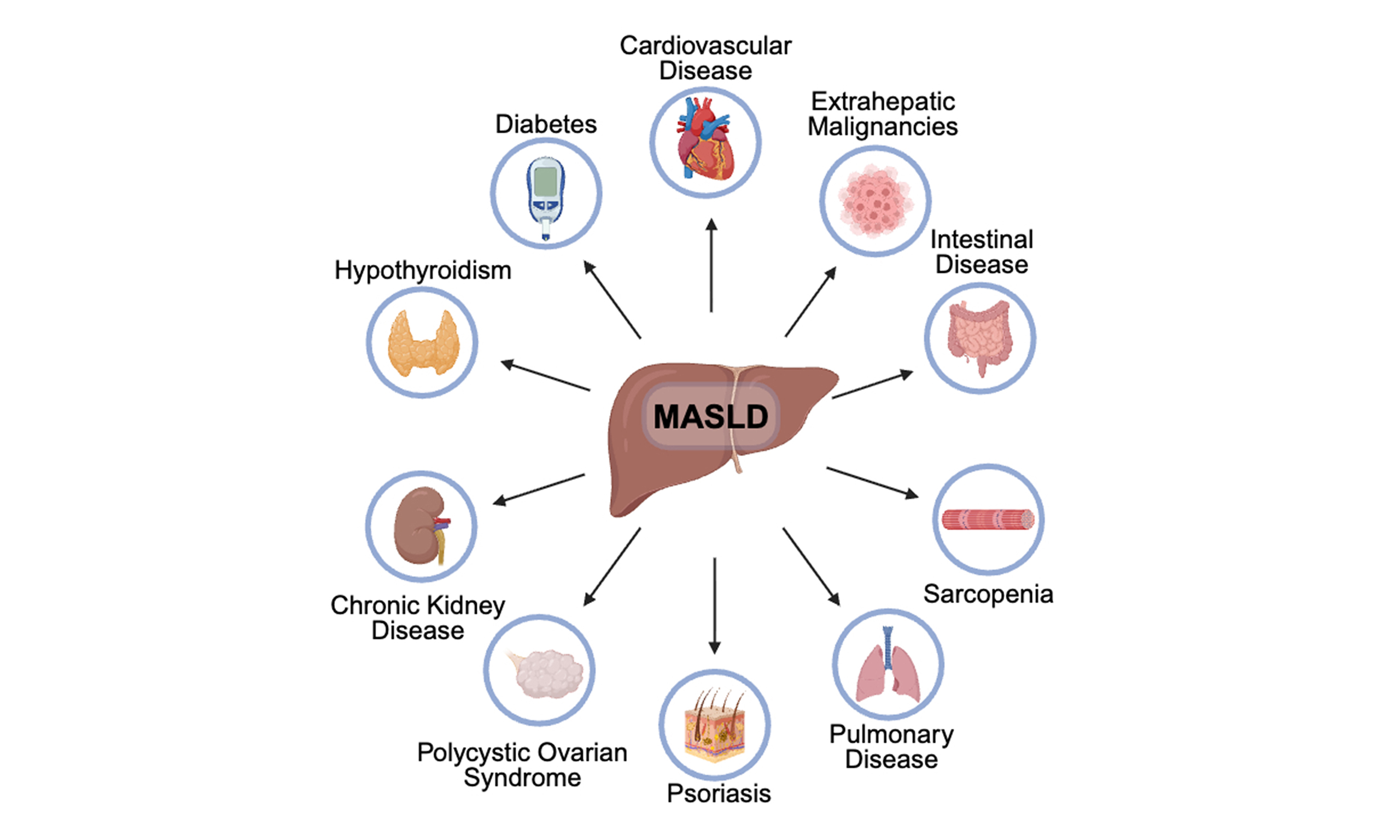
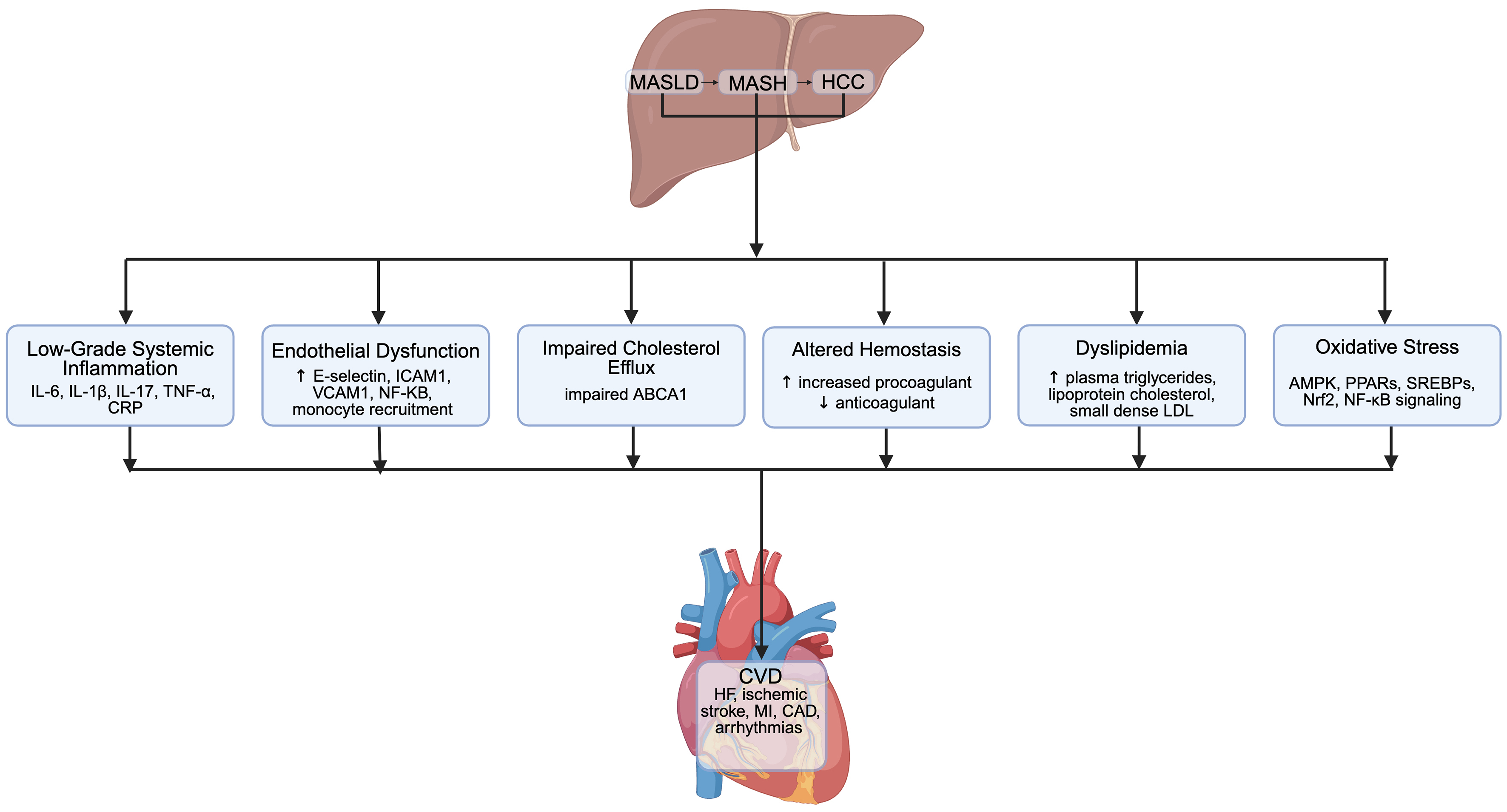












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].