Will a “multivitamin” a day keep the “MASLD doctor” away?
Abstract
This commentary discusses the results of a study that assessed the relationship between homocysteine metabolism and histological severity of metabolic dysfunction-associated steatotic liver disease (MASLD), and applied a mathematical model to examine how replacement with different cofactors (pyridoxine, cobalamin, betaine, and folate) may affect homocysteine levels in patients with MASLD. It highlights the clinical implications of the study and examines the pathophysiological support behind the detected associations. It also discusses its limitations, emphasizing the need for further longitudinal and interventional studies to confirm whether modulating homocysteine levels could be a viable therapeutic strategy for MASLD.
Keywords
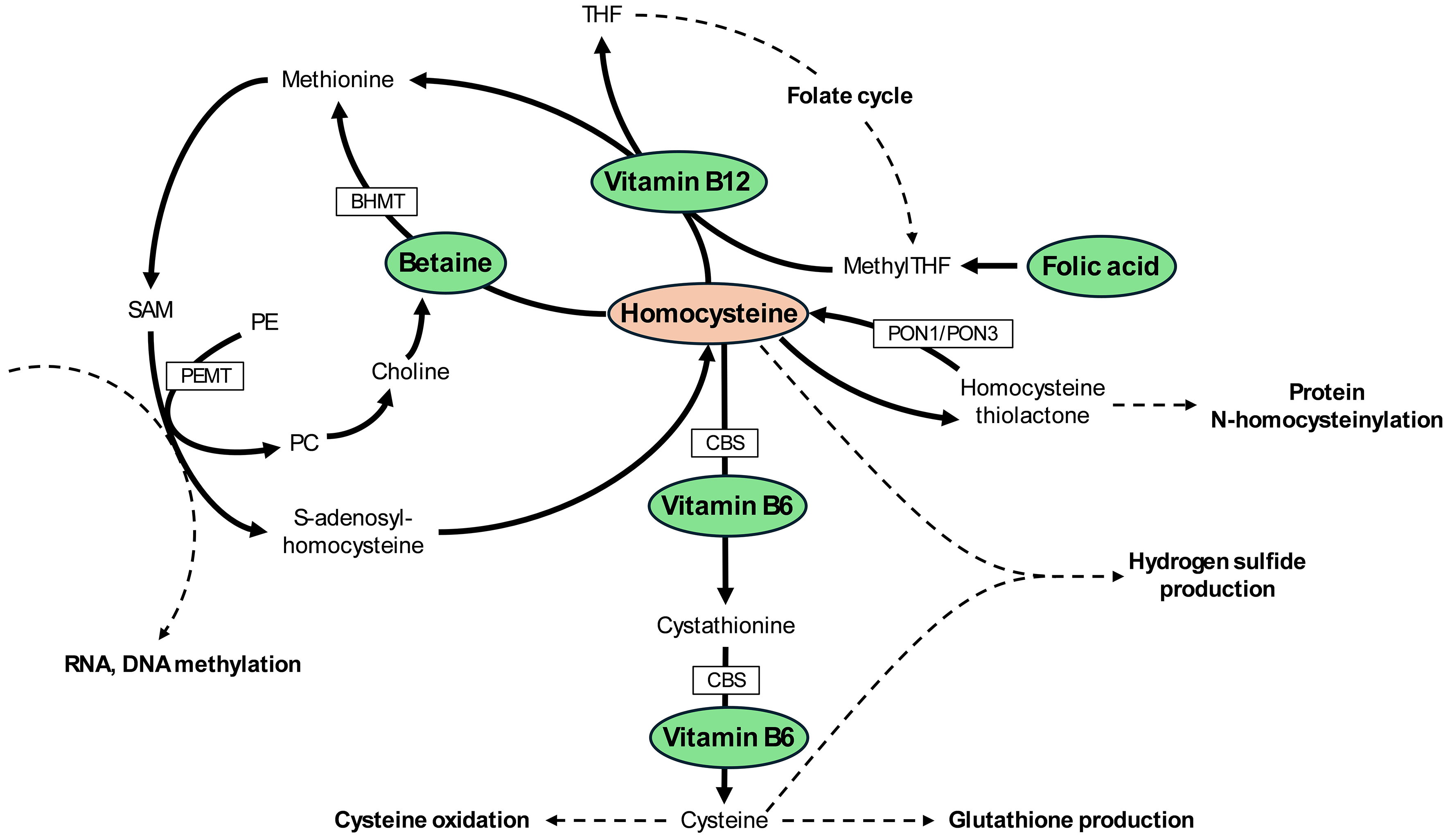
I read with interest the study by Suzuki et al.[1], which assessed the relationship between homocysteine metabolism and the histological severity of nonalcoholic fatty liver disease (NAFLD), and modeled how replacement with different cofactors, such as pyridoxine (vitamin B6), cobalamin (vitamin B12), betaine, and folic acid (vitamin B9), may affect hepatic homocysteine levels in patients with NAFLD. Not all NAFLD patients included in the study fulfilled the criteria for metabolic dysfunction-associated steatotic liver disease (MASLD); therefore, throughout this commentary, we will continue using the term NAFLD when referring specifically to the study population. We will use MASLD when referring globally to the liver condition. In Figure 1, we have summarized the main metabolic pathways that regulate homocysteine levels. As shown, vitamin B6, vitamin B12, betaine, and folic acid participate in distinct metabolic pathways that affect homocysteine levels, suggesting the potential for additive benefits when combined. It should also be noted that the transsulfuration pathway (leading to cysteine) occurs primarily in the liver, but also in the kidneys, pancreas, and small intestine[2]. Remethylation of homocysteine to methionine mediated by betaine occurs in the liver and kidney, whereas the vitamin B12-dependent pathway is present in most tissues[3]. While abnormalities in one-carbon metabolism have been associated with MASLD in dietary and genetic animal models of metabolic dysfunction-associated steatohepatitis (MASH)[4], their role in human MASH is less well established, making studies such as that by Suzuki et al. particularly valuable[1].
Figure 1. Pathways involved in the metabolism of homocysteine and role of cobalamin (vitamin B12), pyridoxine (vitamin B6), folic acid, and betaine. SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine; PE: phosphatidylethanolamine; PC: phosphatidylcholine; PEMT: phosphatidylethanolamine N-methyltransferase; BHMT: betaine-homocysteine methyltransferase; CBS: cystathionine β-synthase; THF: tetrahydrofolate; MethylTHF: 5-methyltetrahydrofolate; PON1: paraoxonase 1; PON3: paraoxonase 3.
To shed light on this issue, the authors analyzed data from 82 patients with biopsy-proven NAFLD. The study cohort consisted of middle-aged, mostly non-Hispanic White individuals, with a broad range of disease severity, including 79% with nonalcoholic steatohepatitis (NASH) and 46% with advanced fibrosis. However, given the relatively small sample size and the underrepresentation of racial/ethnic minorities, it remains unclear whether the findings can be generalized to other populations. In logistic regression analyses, lower gene expression of cystathionine beta-synthase (CBS) and phosphatidylethanolamine N-methyltransferase (PEMT) was associated with a higher risk of hepatic fibrosis. Furthermore, lower hepatic gene expression of paraoxonase (PON)1 and PON3 correlated with a higher risk of hepatic steatosis.
Given the cross-sectional nature of this part of the study, no causality can be determined, and the implications of these associations are unknown. However, it is interesting to note that all detected associations have a strong pathophysiological support, and they have previously been reported in the literature in other experimental conditions[5-9]. For example, lower expression of CBS, a key enzyme of the transsulfuration pathway, would result in elevated homocysteine and methionine levels, and a consequent reduction of glutathione production. In turn, high homocysteine and/or low glutathione have been shown to contribute to hepatic inflammation and liver fibrosis in prior reports[5-7]. By decreasing glutathione levels, impaired transsulfuration would increase susceptibility to oxidative stress, which in turn could lead to mitochondrial dysfunction and cellular damage[10]. Lower activity of PEMT would result in reduced levels of phosphatidylcholine, which is essential for very-low-density lipoprotein (VLDL) secretion, and its deficiency has been associated with MASLD[11]. Moreover, lower PEMT activity could lead to choline deficiency, particularly in choline-deficient diets, which has been associated with liver damage and other organ dysfunction[12]. Finally, lower PEMT activity can result in increased RNA and DNA methylation, affecting epigenetic (and epitranscriptomic) regulation[13]. This would occur as a consequence of increased S-adenosylmethionine (SAM) availability, with a consequent decrease in S-adenosylhomocysteine (SAH), thereby enhancing the activity of methyltransferases[14]. Paraoxonases participate in the metabolism of homocysteine-thiolactone, protecting against N-homocysteinylation of proteins (PON1 > > PON3). Both PON1 and PON3 have also been found to be reduced in patients with MASLD in prior reports[8,9], and PON3 was even reduced in isolated high-density lipoprotein (HDL) lipoproteins from patients with MASLD[15].
The authors went further by applying a mathematical model to predict hepatic homocysteine levels based on consumption of one-carbon metabolism cofactors, such as pyridoxine, cobalamin, betaine, folate, or the combination of the four, along with differences in men and women. Based on these models, they showed that the combination of the four cofactors was able to induce lower levels of hepatic homocysteine compared to each cofactor individually, with some interesting differences in men vs. premenopausal women vs. postmenopausal women. While we could hypothesize that this may also occur in patients without NAFLD, no controls were included in the study. Most of the evidence of the effects of supplements on homocysteine levels in healthy controls comes from studies looking at serum homocysteine levels, and not hepatic levels as modelled in the study by Suzuki et al.[1,16-20]. For example, a meta-analysis of individual data from 25 randomized, controlled trials showed a dose-dependent reduction in plasma homocysteine concentrations with increasing folic acid doses[16]. Moreover, combination with vitamin B12 was associated with an additional 7% reduction in homocysteine levels in that study. A network meta-analysis including 16 studies reported that combination of folate, vitamin B6, and vitamin B12 was the most favorable to reduce blood homocysteine levels[20]. Of note, serum homocysteine levels do not represent liver levels, as other organs (e.g., kidney and skeletal muscle) also play a key role in determining serum homocysteine levels[21]. While the findings reported by Suzuki et al. are encouraging, it remains to be determined whether reducing hepatic homocysteine or homocysteine-thiolactone would result in an improvement in MASLD/MASH[1]. Longitudinal and interventional studies are needed in order to appropriately answer that question.
The idea that nutritional deficiencies could be related to MASLD/MASH is not new[22]. Several cross-sectional studies have reported associations between nutritional deficiencies and MASLD/MASH[23-27], although results have not been consistent, and a causal relationship cannot be established. Moreover, different nutrients and vitamins, including some that participate in one-carbon metabolism, have been replaced in randomized, controlled trials in patients with MASH with inconsistent results[28-31].
In summary, the study by Suzuki et al. provides an interesting view regarding the potential role of one-carbon metabolism in the development and progression of MASLD in humans[1]. However, whether these findings translate into a potential therapeutic target by modulating hepatic homocysteine levels remains to be determined in prospective, interventional studies. Until then, it is unclear whether a ‘multivitamin’ every day would benefit patients with MASLD.
DECLARATIONS
Authors’ contributions
The author contributed solely to the article.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This research was supported by a pilot grant from the UAB Diabetes Research Center (NIDDK P30 DK079626), to University of Alabama at Birmingham.
Conflicts of interest
Bril F is an Editorial Board Member of the journal Metabolism and Target Organ Damage. Bril F was not involved in any steps of the editorial process, including reviewers’ selection, manuscript handling, or decision-making.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Suzuki A, Henao R, Reed MC, et al. Lower hepatic CBS and PEMT expression in advanced NAFLD: inferencing strategies to lower homocysteine with a mathematical model. Metab Target Organ Damage. 2024;4:21.
2. Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157:S40-4.
3. Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
4. da Silva RP, Eudy BJ, Deminice R. One-carbon metabolism in fatty liver disease and fibrosis: one-carbon to rule them all. J Nutr. 2020;150:994-1003.
5. Tripathi M, Singh BK, Zhou J, et al. Vitamin B12 and folate decrease inflammation and fibrosis in NASH by preventing syntaxin 17 homocysteinylation. J Hepatol. 2022;77:1246-55.
6. Yuan S, Chen J, Dan L, et al. Homocysteine, folate, and nonalcoholic fatty liver disease: a systematic review with meta-analysis and Mendelian randomization investigation. Am J Clin Nutr. 2022;116:1595-609.
7. Sarna LK, Siow YL, O K. The CBS/CSE system: a potential therapeutic target in NAFLD? Can J Physiol Pharmacol. 2015;93:1-11.
8. Kotani K, Watanabe J, Miura K, Gugliucci A. Paraoxonase 1 and non-alcoholic fatty liver disease: a meta-analysis. Molecules. 2021;26:2323.
9. Subudhi S, Drescher HK, Dichtel LE, et al. Distinct hepatic gene-expression patterns of NAFLD in patients with obesity. Hepatol Commun. 2022;6:77-89.
10. Balsa E, Perry EA, Bennett CF, et al. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat Commun. 2020;11:2714.
11. Li Z, Agellon LB, Allen TM, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321-31.
13. Nakatsuka A, Matsuyama M, Yamaguchi S, et al. Insufficiency of phosphatidylethanolamine N-methyltransferase is risk for lean non-alcoholic steatohepatitis. Sci Rep. 2016;6:21721.
14. Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318-23.
15. Bril F, Pearce RW, Collier TS, McPhaul MJ. Differences in HDL-bound apolipoproteins in patients with advanced liver fibrosis due to nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2022;108:42-51.
16. Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806-12.
17. Sohouli MH, Almuqayyid F, Alfardous Alazm A, et al. A comprehensive review and meta-regression analysis of randomized controlled trials examining the impact of vitamin B12 supplementation on homocysteine levels. Nutr Rev. 2024;82:726-37.
18. Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291-5.
19. Lu XT, Wang YN, Mo QW, et al. Effects of low-dose B vitamins plus betaine supplementation on lowering homocysteine concentrations among Chinese adults with hyperhomocysteinemia: a randomized, double-blind, controlled preliminary clinical trial. Eur J Nutr. 2023;62:1599-610.
20. Liu C, Yao H, Wang F. Effect of nutritional supplements for reducing homocysteine levels in healthy adults: a systematic review and network meta-analysis of randomized trials. Nutr Rev. 2025;83:e1533-43.
21. Sadre-Marandi F, Dahdoul T, Reed MC, Nijhout HF. Sex differences in hepatic one-carbon metabolism. BMC Syst Biol. 2018;12:89.
22. Pickett-Blakely O, Young K, Carr RM. Micronutrients in nonalcoholic fatty liver disease pathogenesis. Cell Mol Gastroenterol Hepatol. 2018;6:451-62.
23. Qi X, Guo J, Li Y, et al. Vitamin E intake is inversely associated with NAFLD measured by liver ultrasound transient elastography. Sci Rep. 2024;14:2592.
24. Yao B, Lu X, Xu L, Jiang Y. Association of serum folate with prevalence of non-alcoholic fatty liver disease among adults (NHANES 2011-2018). Front Nutr. 2023;10:1141156.
25. Li J, Huang J, Lv Y, Ji H. Association between dietary intakes of B vitamins and nonalcoholic fatty liver disease in postmenopausal women: a cross-sectional study. Front Nutr. 2023;10:1272321.
26. Pan J, Zhou Y, Pang N, Yang L. Dietary niacin intake and mortality among individuals with nonalcoholic fatty liver disease. JAMA Netw Open. 2024;7:e2354277.
27. Bril F, Lomonaco R, Orsak B, et al. Vitamin D deficiency and development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Hepatology. 2013;58:522a. Available from: https://journals.lww.com/hep/citation/2013/10001/vitamin_d_deficiency_and_development_of.657.aspx. [Last accessed on 10 Sep 2025].
28. Abdelmalek MF, Sanderson SO, Angulo P, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818-26.
29. Bril F, Biernacki DM, Kalavalapalli S, et al. Role of Vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481-8.
30. Kitson MT, Pham A, Gordon A, Kemp W, Roberts SK. High-dose vitamin D supplementation and liver histology in NASH. Gut. 2016;65:717-8.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].