Sex and gender differences in MASLD: pathophysiological mechanisms, clinical implications, and future directions
Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a leading and increasingly prevalent cause of chronic liver disease worldwide, with significant hepatic and extrahepatic consequences. Sex and gender are major, yet underrecognized, determinants of MASLD risk, progression, and related clinical events. This narrative review synthesizes evidence on (1) biological sex effects - including sex chromosomes, genetic variants, and the modulatory roles of estrogens and androgens on lipid metabolism, mitochondrial function, immune signalling, and fibrogenesis - and (2) gendered socio-behavioral influences such as diet, physical activity, alcohol and tobacco use, health-seeking behavior, pregnancy and lactation, and gender-affirming hormone therapy. We highlight sex differences in metabolic phenotypes (fat distribution, insulin resistance, dyslipidemia, and hypertension), emerging sex-specific gene-microbiome interactions in the gut-liver axis, and sex-dependent patterns of extrahepatic comorbidity (cardiovascular disease, chronic kidney disease, and selected cancers). Evidence also suggests that commonly used diagnostic tools and predictive algorithms - including non-invasive fibrosis scores and machine-learning models - may misclassify risk if sex and hormonal status are not considered, and that pharmacologic, surgical, and hormone-based therapies exhibit preliminary sex-specific effects. Based on these observations, we propose a conceptual framework in which sex and gender, individually and interactively, shape the MASLD burden. Critical knowledge gaps remain, particularly for premenopausal women, transgender, and non-binary populations. Incorporating sex and gender into study design, diagnostics, risk stratification, and clinical trials is essential to advance precision prevention and equitable care for MASLD.
Keywords
INTRODUCTION
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is now recognized as the most prevalent chronic liver condition globally, affecting nearly one-third of the adult population[1,2]. MASLD is defined by the presence of ≥ 5% hepatic steatosis in the absence of competing causes such as chronic viral hepatitis, steatogenic medications, autoimmune or genetic liver diseases (e.g., autoimmune hepatitis, hemochromatosis, Wilson’s disease), or significant alcohol consumption[3].
Closely linked to obesity, insulin resistance (IR), type 2 diabetes (T2D), and dyslipidemia, MASLD spans a disease spectrum ranging from simple steatosis to steatohepatitis, advanced fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[3,4]. Given its strong association with metabolic syndrome, MASLD poses a significant public health challenge and contributes substantially to global liver-related morbidity and mortality[5].
Importantly, both sex and gender influence the epidemiology and clinical course of MASLD. Sex refers to biological attributes, including chromosomes, hormones, and reproductive organs, whereas gender reflects sociocultural roles, behaviors, and expectations that shape health outcomes[6]. Together, these dimensions contribute to variability in MASLD prevalence, pathophysiology, and clinical outcomes[7]. Men generally display higher rates of MASLD, yet postmenopausal women are at increased risk for advanced fibrosis[8]. This disparity is influenced not only by hormonal changes but also by sex-specific differences in obesity patterns, insulin sensitivity, inflammatory responses, and gut microbiome composition, all of which can differentially affect MASLD development and progression[7,9]. Moreover, emerging data suggest sex-based disparities in progression to MASLD-related HCC[9].
Understanding these sex and gender differences is essential to improve risk stratification, early diagnosis, and personalized therapeutic strategies[10]. This review provides a comprehensive overview of sex-related differences in MASLD, focusing on hormonal, genetic, metabolic, and gut microbial mechanisms, and their impact on disease progression and outcomes. We also examine the role of gender, including the impact of social norms and behavioral factors. Finally, the clinical implications of these findings are discussed.
To identify relevant studies for this review, we searched PubMed and Embase using controlled vocabulary terms (including MeSH and Emtree) and free-text keywords. The search was limited to publications in English and studies conducted in adult populations (≥ 18 years), up to 11 July 2025. Detailed keywords and the search syntax for each database are provided in Table 1. We additionally reviewed supplementary materials of studies reporting MASLD-related outcomes or clinical trial results to capture sex-specific data.
Search strategies used in PubMed and Embase combining controlled vocabularies (MeSH and Emtree terms) with free-text keywords for studies on sex, gender, and MASLD
| Database | Search syntax |
| PubMed | (“Sex Characteristics”[MeSH] OR “Gender Role”[MeSH] OR “Gender Equity”[MeSH] OR “Gender-Based Violence”[MeSH] OR “Gender Dysphoria”[MeSH] OR “Sexual and Gender Minorities”[MeSH] OR “Gender-Nonconforming Persons”[MeSH] OR “Gender-Affirming Care”[MeSH] OR “Gender-Affirming Procedures”[MeSH] OR “Sexual and Gender Disorders”[MeSH] OR “Transsexualism”[MeSH] OR “Sexism”[MeSH] OR “sex differences”[tiab] OR “sex-specific”[tiab] OR “sex dimorphism”[tiab] OR gender[tiab]) AND (“non-alcoholic fatty liver disease”[MeSh] OR “NAFLD”[tiab] OR “fatty liver”[tiab] OR “nonalcoholic steatohepatitis”[tiab] OR “non-alcoholic steatohepatitis”[tiab] OR “NASH”[tiab] OR “metabolic dysfunction-associated steatohepatitis”[tiab] OR “MASH”[tiab] OR “MASLD”[tiab] OR “steatotic liver”[tiab] OR “MAFLD”[tiab]) AND (“1900/01/01”[Date - Publication]: “2025/07/11”[Date - Publication]) AND (humans[Filter]) AND (english[Filter]) AND (alladult[Filter]) |
| Embase | (“sex difference”/de OR “sexual and gender minority”/de OR “gender identity”/de OR “transgenderism”/de OR “transgender and gender nonbinary”/de OR “sex difference*”:ab,ti OR “sex-specific”:ab,ti OR “sex dimorphism”:ab,ti OR “gender”:ab,ti) AND (“nonalcoholic fatty liver”/de OR “NAFLD”:ab,ti OR “fatty liver”:ab,ti OR “nonalcoholic steatohepatitis”:ab,ti OR “non-alcoholic steatohepatitis”:ab,ti OR “NASH”:ab,ti OR “metabolic dysfunction-associated steatohepatitis”:ab,ti OR “MASH”:ab,ti OR “MASLD”:ab,ti OR “steatotic liver”:ab,ti OR “MAFLD”:ab,ti) NOT [medline]/lim AND [01-01-1900]/sd NOT [1-07-2025]/sd AND ([adult]/lim OR [aged]/lim OR [very elderly]/lim) AND [humans]/lim AND [english]/lim AND [article]/lim |
| The search was limited to studies conducted in adult humans, published in English through 11 July 2025 | |
EPIDEMIOLOGY OF MASLD BY SEX
Epidemiological data reveal consistent sex-based differences in MASLD prevalence[3,11]. For instance, in Asia, incidence rates range from 19 to 44.5 per 1,000 person-years and are higher in males than in females[12]. The overall prevalence among men spans from 4.3% to 42%, while for women it ranges between 1.6% and 24% across various cohorts[12].
These wide ranges reflect not only true population variability but also differences in diagnostic approaches. MASLD diagnosis relies on multiple methods - including liver biopsy, ultrasonography, magnetic resonance spectroscopy (MRS), and biochemical markers - each with differing sensitivity and specificity. MRS is considered the non-invasive gold standard[12,13]. However, its limited availability and high cost restrict widespread use, leading to inconsistencies in prevalence estimates across studies. Additionally, methodological differences, such as the use of liver enzymes or administrative codes, may underestimate or overestimate MASLD prevalence depending on selection criteria and referral bias[14,15]. For example, studies based on biopsy samples may overrepresent individuals with more severe disease, while reliance on blood tests or coding data may miss asymptomatic or early-stage cases.
Geographic and demographic heterogeneity further contributes to inconsistent findings[16]. A Shanghai population study reported higher MASLD prevalence in men under 50 years of age, but a reversal of this trend in individuals over 50, with women showing higher rates[17]. Similarly, a Japanese study found MASLD prevalence increased from young adulthood to middle age in men, followed by a decline after age 50-60, while in women, rates rose sharply after menopause[18]. Another cohort revealed a MASLD prevalence of 6% in premenopausal women, compared to 24% in men and 15% in postmenopausal women[19].
HORMONAL INFLUENCES
Sex hormones play a central role in modulating susceptibility to MASLD [Figure 1] through their influence on lipid metabolism, mitochondrial function, oxidative stress, immune response, and tissue repair[20,21]. Estrogens regulate key hepatic metabolic pathways. They enhance fatty acid oxidation and suppress de novo lipogenesis, mechanisms that collectively reduce hepatic fat accumulation[22]. These protective effects are largely mediated via estrogen receptor alpha (ERα), which downregulates stress pathways such as JNK (c-Jun N-terminal kinase) and upregulates antioxidant enzymes[23,24]. Estrogens also mitigate mitochondrial oxidative stress and lipid droplet accumulation by increasing mitochondrial thioredoxin 2 (TRX2) expression[25]. Animal studies confirm that estrogen deficiency - through ovariectomy or ERα deletion - leads to hepatic lipid accumulation, mitochondrial dysfunction, and IR[26,27]. Restoration of estrogen improves these parameters only when liver ERα is functional[27]. Clinical data mirror these findings. In young women undergoing oophorectomy for endometrial cancer, MASLD risk significantly increased, highlighting the protective role of ovarian hormones[28]. In pediatric MASLD cohorts, higher estrone and estradiol levels were linked to lower portal inflammation in both sexes[29].
Figure 1. Hormonal and Metabolic Modulation of MASLD Risk Across Sex and Life Stages. This figure illustrates how sex hormones-estrogens, androgens, and related binding proteins-and metabolic factors collectively influence susceptibility to MASLD across life stages in both sexes. It integrates hormonal transitions (e.g., pregnancy, menopause, and aging) with sex-specific metabolic determinants such as insulin resistance, lipid metabolism, and body fat distribution, highlighting differences in pathophysiology and clinical presentation. PCOS: Polycystic ovary syndrome; SHBG: sex hormone-binding globulin; TRT: testosterone replacement therapy; GDM: gestational diabetes mellitus; MASLD: metabolic dysfunction-associated steatotic liver disease.
The menopausal transition, characterized by estrogen decline, marks a critical risk period for the development and progression of MASLD[30-32]. During this phase, estrogen levels drop rapidly, while androgen levels decline more gradually, leading to a relative hyperandrogenic state[32]. This hormonal shift is further compounded by a reduction in circulating sex hormone-binding globulin (SHBG), which increases the free androgen index (FAI) and thereby amplifies androgen bioavailability[33-35]. Postmenopausal women with high bioavailable testosterone or FAI show greater fatty liver burden and reduced insulin sensitivity[36-38]. In contrast, higher SHBG levels are inversely associated with MASLD severity and metabolic dysfunction in postmenopausal women[37,39-41], indicating SHBG’s potential protective role.
Androgens show contrasting effects in men and women. In men, low serum testosterone is associated with higher MASLD risk and IR. Some evidence supports testosterone replacement therapy for reversing hepatic steatosis in hypogonadal men, although results are inconsistent[42-44]. In contrast, elevated testosterone levels in women are associated with MASLD progression, especially in those with polycystic ovary syndrome (PCOS), where androgen excess is common[45]. In premenopausal women, higher testosterone levels correlate with increased steatosis, metabolic dysfunction-associated steatohepatitis (MASH) risk, and abdominal adiposity, but these associations diminish after menopause[46,47]. Pediatric data show that higher testosterone levels are linked to improved steatosis and fibrosis in boys, while the opposite is observed for girls-further underscoring sex-specific hormonal effects[29].
GENDER DIFFERENCES: LIFESTYLE AND BEHAVIORAL FACTORS
While biological sex governs certain physiological predispositions, gender reflects sociocultural norms, roles, and behaviors that influence an individual’s exposures and health choices. These factors shape dietary patterns, physical activity, alcohol and tobacco use, healthcare engagement, and reproductive behaviors such as pregnancy and breastfeeding, as well as decisions regarding gender-affirming hormone therapy (GAHT) in transgender individuals. Each of these gendered determinants can modulate the onset, trajectory, and outcomes of MASLD [Figure 2].
Figure 2. Gender Differences in Lifestyle and Behavioral Factors Contributing to MASLD. This figure illustrates gender-specific influences across dietary habits, physical activity patterns, alcohol and tobacco use, health-seeking behaviors, and considerations for gender-diverse individuals. ↑Indicates an increase or higher level of a variable; ↓indicates a decrease or lower level of a variable; →indicates a relationship or influence between variables (e.g., “leads to”, “is associated with”, or “results in”). MASLD: Metabolic dysfunction-associated steatotic liver disease; FFA: free fatty acids; MetALD: metabolic and alcohol-related liver disease.
Dietary habits and nutritional exposure
Sex and gender shape both nutrient metabolism and dietary behaviors[48]. Women tend to consume more fiber-rich, nutrient-dense foods, while men favor red/processed meats, saturated fats, and sugary beverages-patterns shaped by biological and sociocultural factors, including women’s greater adherence to dietary guidelines[49,50]. Men and postmenopausal women are more susceptible to overnutrition and central adiposity, increasing liver fat[51-55]. Nutritional epidemiology indicates that high-energy, low-fiber diets are more strongly associated with MASLD in men[56], whereas women show greater hepatic lipogenic responses to fructose and high-glycemic foods[57-59]. Supporting these observations, a cohort of 2,466 MASLD patients found that both low and high total energy intake significantly increased all-cause mortality in men, with no significant effect in women[60]. Gender-related barriers-including food insecurity, cultural dietary restrictions, and caregiving responsibilities-further affect women’s nutritional quality, especially in low-resource settings, and contribute to greater MASLD vulnerability[61,62]. Sex-specific responses to dietary interventions have also been reported[63,64]; Table 2 summarizes some of these findings. Men generally achieve greater metabolic improvements from low-calorie or Mediterranean diets, whereas premenopausal women may respond better to low-glycemic index strategies[65-69]. Ketogenic diets are effective for weight loss, but estrogens appear to blunt hepatic improvements in reproductive-age women, with postmenopausal women showing the least favorable responses[68].
Sex-specific effects of lifestyle modifications on MASLD outcomes
| Author, year[Ref.] | Lifestyle modifications | Design | Method | Sex-specific results | Comment |
| Trouwborst et al., 2021[65] | Low-Calorie diet | RCT (NCT00390637) | The DiOGenes trial enrolled 782 overweight or obese adults (65% women) who followed a LCD of approximately 800 kcal/day for 8 weeks. After the initial weight-loss phase, participants entered a 6-month weight-maintenance phase on ad libitum diets that varied in protein content and glycemic index. The study assessed body weight, insulin sensitivity (HOMA-IR), lipid profile, and other cardiometabolic biomarkers both after the LCD and at the end of follow-up. Results were adjusted for changes in body weight | Men lost more weight than women during the LCD phase (-12.8 ± 3.9 kg vs. -10.1 ± 2.8 kg; P < 0.001) but regained more during follow-up (1.5 ± 5.4 kg vs. -0.5 ± 5.5 kg; P < 0.001). Men had greater improvements in insulin sensitivity, triglycerides, HDL, LDL, total cholesterol, blood pressure, and adiponectin (std. β: 0.073-0.144; all q < 0.05), while women had more favorable HDL, triglyceride, and diacylglycerol rebound during follow-up (std. β: 0.114-0.164; all q < 0.05) | Men benefit more in short-term cardiometabolic improvement, while women-particularly premenopausal-appear more resilient in maintaining lipid-related benefits over time. Differences in fat distribution (visceral vs. subcutaneous), estrogen status, and metabolic adaptation likely drive this sexual dimorphism |
| Leblanc et al., 2014[67] | Mediterranean diet | Clinical trial (NCT01852721) | In a 12-week trial, 64 men and 59 premenopausal women participated in a Mediterranean diet-based nutritional intervention using motivational interviewing through individual and group sessions. Dietary intake (via food frequency questionnaire), eating behaviors (via the Three-Factor Eating Questionnaire), anthropometric, and metabolic measures were assessed at baseline, post-intervention, and 3- and 6-month follow-ups | Men showed greater reductions in red and processed meat intake (P = 0.03), greater increases in whole fruit intake (P = 0.04), and more reduction in disinhibition behavior (P = 0.03). Waist circumference decreased in both sexes during the intervention, but only remained reduced in men at follow-up (P = 0.05). Improvements in total cholesterol to HDL-C ratio, triglycerides, and TG/HDL-C ratio were more pronounced in men than women (P ≤ 0.03). Despite similar initial adherence to the Mediterranean diet, dietary improvements regressed in both sexes post-intervention (P < 0.0001), with men showing more lasting metabolic benefits | Men showed more sustained metabolic improvements and behavioral changes than women, despite similar initial adherence. This suggests sex-specific physiological and behavioral responses, underscoring the importance of tailored dietary interventions |
| D’Abbondanza et al., 2020[68] | Ketogenic diet | Clinical trial (NCT03564002) | Seventy participants with severe obesity (42 females and 28 males) underwent a 25-day VLCKD. Baseline and post-intervention assessments included anthropometric measurements, bioimpedance analysis, liver steatosis grading via ultrasonography, liver function tests (including γ-glutamyl transferase), and glucose homeostasis parameters. Female participants were also stratified by menopausal status for subgroup analysis | In this 25-day VLCKD trial including 42 women and 28 men with severe obesity, men had significantly greater EBWL and γGT reduction than women. Among women, premenopausal participants had the least favorable outcomes. No sex difference was found in steatosis grade or Edmonton stage improvement | Men benefit more from short-term VLCKD in terms of weight loss and liver enzyme reduction. Female response varies by menopausal status, suggesting estrogen plays a role in modulating diet efficacy |
| Vitale et al., 2023[69] | Low glycemic index diet | RCT (NCT03410719) | This multi-national, randomized, controlled, parallel-group trial enrolled 156 adults (82 women, 74 men) with at least two metabolic syndrome traits across centers in Italy, Sweden, and the United States. Participants were randomized to consume either a low- or high-GI Mediterranean diet for 12 weeks. Both diets were isoenergetic and matched for macronutrient composition, including 270 g/day of carbohydrates and 35 g/day of fiber. The only difference was the starch source. Glucose and insulin profiles were monitored for 8 h at baseline and post-intervention, with meals replicating the assigned dietary pattern | In women, the high-GI diet led to significantly higher 8 h average plasma glucose levels than the low-GI diet as early as Day 1 (+23%, P < 0.05), increasing to +37% by Week 12 (P < 0.05). In men, no significant difference was observed between high- and low-GI diets. A significant interaction between sex and dietary GI was confirmed by two-way ANOVA (F = 7.887, P = 0.006) | This trial highlights a compelling sex-specific metabolic sensitivity to dietary glycemic index, with women showing a significantly more adverse glycemic response to high-GI foods than men. These findings reinforce the importance of sex-specific dietary recommendations, particularly in individuals at high risk for type 2 diabetes |
| Curci et al., 2024[81] | Physical activity/Exercise | Prospective cohort | A population-based prospective cohort study was conducted in Castellana Grotte, Southern Italy, enrolling 1,826 adults (> 30 years) from an eligible pool of 2,970 starting in 1985. Participants underwent anthropometric assessment, liver ultrasonography for MASLD diagnosis, and completed validated questionnaires on diet and LTPA. Vital status was tracked through municipal records to assess all-cause mortality over time | Low LTPA in individuals with MASLD was associated with significantly increased all-cause mortality risk, especially in women. Women with MASLD and low LTPA had the highest mortality risk and were 19% less likely to survive to age 82 compared to active counterparts. Physical activity mitigated mortality risk regardless of MASLD status, but the protective effect was more pronounced in women | Postmenopausal women have a higher mortality risk related to low activity levels than men; early-life inactivity in women increases later risk due to hormonal protection loss |
| Ajmera et al., 2019[139] | Lactation | Multicenter community-based longitudinal cohort | Participants from the CARDIA cohort who delivered ≥ 1 child post-baseline (Y0: 1985-1986) and underwent CT quantification of hepatic steatosis 25 years later (Y25: 2010-2011, n = 844). Lactation duration was summed across all post-baseline births. NAFLD at Y25 was defined by liver attenuation ≤ 40 Hounsfield Units after exclusion of other causes. Logistic regression (unadjusted and multivariable) adjusted for age, race, education, and baseline BMI | Longer lactation was inversely associated with NAFLD. Women with > 6 months lactation had lower odds of NAFLD vs. 0-1 month: OR 0.46 (95%CI: 0.22-0.97, P = 0.04, adjusted) | Prolonged lactation may represent a modifiable lifestyle factor with long-term hepatoprotective effects in women |
Physical activity patterns
Regular physical activity protects against MASLD by improving insulin sensitivity, enhancing fat oxidation, and reducing hepatic steatosis[70]. Both biological sex and gender roles shape activity patterns and responses. Men generally engage in more vigorous and resistance-based exercise, whereas women prefer moderate aerobic activities such as walking or group classes[71-73]. Biological differences-such as greater muscle mass and cardiorespiratory capacity in men-may lead to larger reductions in hepatic fat from resistance training, while women often derive greater glycemic and metabolic benefits from aerobic exercise[74-76]. Gender-related factors, including caregiving responsibilities, cultural norms, limited access to exercise facilities, and time constraints, disproportionately restrict women’s activity, particularly after childbirth and during midlife[77-80]. Low levels of leisure-time physical activity are associated with higher mortality in postmenopausal women, and early-life inactivity imposes long-term risks[81,82]. Additionally, obesity-related pathophysiology varies by sex: men typically present with more severe baseline steatosis and fibrosis, which may explain their greater fibrosis regression following substantial weight loss[83,84]
Alcohol consumption and smoking
Although MASLD is distinct from alcohol-associated liver disease (ALD), even modest alcohol use can exacerbate steatosis, inflammation, and fibrosis in individuals with metabolic dysfunction, as reflected in the recently defined MetALD category[85-88]. Men generally consume more alcohol, yet women are more vulnerable to liver injury at lower doses due to differences in metabolism, body composition, and hormonal factors[89,90]. Smoking - still more common in men though narrowing globally - promotes MASLD through IR, oxidative stress, and inflammation, and may accelerate fibrosis when combined with steatosis[91-95]. Emerging evidence suggests that female smokers may face greater metabolic and liver-related harm than males[96]. Gender-related influences, including social norms, stress, and limited cessation resources, further shape alcohol and tobacco use[97-99].
Health-seeking behaviors
Timely MASLD diagnosis depends on health-seeking behavior, which varies by gender[100]. Women are generally more likely to engage with healthcare systems, attend regular check-ups, and follow preventive guidelines[100]. This pattern may lead to earlier detection of metabolic comorbidities and liver abnormalities in women. In contrast, men are less likely to seek care or follow treatment, often presenting with more advanced disease despite similar risk profiles[101]. Masculine norms (e.g., stoicism, self-reliance) contribute to delayed care and poorer outcomes in men[102]. Healthcare gender bias may downplay liver disease severity in women, especially when their metabolic profile seems less concerning[103]. Additionally, women often face competing demands related to caregiving or employment, which may hinder consistent follow-up and lifestyle modification[104].
Pregnancy and breastfeeding
Women of reproductive age experience a convergence of biological and gender-related factors that shape MASLD risk and outcomes[105]. Pregnancy introduces unique metabolic demands that, when combined with preexisting liver disease, heighten the risk of gestational diabetes, hypertensive disorders, preterm birth, and large-for-gestational-age infants[106-123]. Gendered behaviors-including diet, physical activity, healthcare use, and prenatal care-further influence these risks. Beyond maternal biology, pregnancy-related outcomes such as delivery mode, cesarean section rates, and breastfeeding practices are strongly shaped by cultural norms, healthcare access, and social support[124-128]. Maternal MASLD also affects long-term child health by programming susceptibility to obesity and MASLD, consistent with the developmental origins of health and disease hypothesis[129-137]. Conversely, breastfeeding provides protection by improving maternal metabolic recovery and infant metabolic programming, but uptake is often lower in women with obesity or MASLD due to social, behavioral, and clinical barriers[128,138-146]. Collectively, these insights highlight that MASLD in pregnancy is driven not only by biology but also by gendered behaviors and cultural context, underscoring the importance of tailored preconception counseling, culturally sensitive care, and targeted support for nutrition, physical activity, and lactation to optimize maternal and child metabolic health[147].
Transgender individuals
Transgender individuals often pursue GAHT to align physical appearance with gender identity, relieve dysphoria, and improve mental health[148,149]. GAHT alters body composition and metabolism in ways that may influence MASLD risk, although direct liver-specific data are scarce. Systematic reviews and prospective cohorts show feminizing therapy decreases lean mass, increases fat mass, and may worsen IR, whereas masculinizing therapy increases lean mass, lowers fat mass, and generally improves or maintains insulin sensitivity, though evidence on diabetes risk is mixed[150-156]. A large study of U.S. transgender veterans found that estradiol was associated with reduced metabolic syndrome risk, while testosterone increased risk, with transmasculine individuals showing the highest burden[157]. Complementing this, a case-control study in Chinese transwomen reported that GAHT increased total and regional body fat and redistributed fat toward a feminine pattern without raising obesity or dyslipidemia prevalence, though fasting glucose rose modestly[158]. Additional work shows that GAHT modulates vascular, inflammatory, and hemostatic markers[159] and modestly shifts blood pressure[160]. HIV coinfection further compounds steatosis and IR in transfeminine populations[161]. Beyond biological effects, transgender and gender-diverse individuals experience lower physical activity, stigma, discrimination, and barriers to care, which collectively worsen cardiometabolic health[162-165]. Overall, current evidence robustly supports GAHT-mediated shifts in body composition and metabolic risk but remains indirect regarding MASLD development or progression, highlighting the need for prospective studies with standardized GAHT definitions, liver-specific endpoints, and careful consideration of behavioral and structural determinants.
METABOLIC RISK FACTORS
Sex and gender significantly influence the development, clinical presentation, and progression of MASLD through their modulation of key metabolic risk factors[166]. These include obesity and body fat distribution, IR and T2D, dyslipidemia, and hypertension. Importantly, distinct sex-specific metabolic phenotypes emerge due to the interplay of sex hormones, genetics, and sociocultural factors, which may explain differences in MASLD prevalence, severity, and outcomes between women and men [Figure 1].
Men preferentially accumulate visceral fat, predisposing them to lipotoxicity, IR, and inflammation[167-170], whereas premenopausal women store fat subcutaneously, which lowers metabolic risk[171,172]. Estrogen contributes to this protective pattern by promoting subcutaneous lipid storage, improving insulin sensitivity, and enhancing lipid profiles, including higher high-density lipoprotein (HDL) and lower low-density lipoprotein (LDL) and triglycerides[173-177]. With menopause, declining estrogen drives fat redistribution toward the visceral compartment, worsens IR and dyslipidemia, and narrows the sex gap in MASLD prevalence[178-180]. Men with testosterone deficiency also develop visceral adiposity, IR, and accelerated fibrosis progression[180]. They generally exhibit greater hepatic IR even at lower adiposity, whereas premenopausal women remain relatively insulin sensitive[181-186]. However, once T2D develops, women face higher risks of fibrosis, MASH[8,187-189], cardiovascular[190] and renal complications[191].
Sex differences also extend to triglycerides and blood pressure. Men more often present with high triglycerides and low HDL, both linked to fibrosis[192], whereas hyperandrogenism in women with PCOS promotes atherogenic lipids and MASLD risk[177,193]. Menopause worsens lipid profiles, contributing to the midlife rise in MASLD[8,177]. Hypertension, strongly associated with fibrosis[194], is more prevalent in men at younger ages but rises sharply in women after menopause, often surpassing male rates[195-197]. Postmenopausal women with MASLD frequently exhibit worse cardiometabolic profiles and suboptimal blood pressure control[198-200]. Overall, men typically present earlier with a “classic” MASLD phenotype-central obesity, IR, dyslipidemia, and hypertension[166]-while women may develop MASLD with fewer overt metabolic abnormalities, influenced by menopause, PCOS, or lean MASLD phenotypes characterized by adipose dysfunction, sarcopenia, and altered bile acid signaling[193,201,202].
GENETIC AND EPIGENETIC MODIFIERS
Sex differences in MASLD susceptibility and progression are shaped not only by hormonal influences but also by genetic and epigenetic factors, including sex chromosome complement and autosomal gene variants[203]. These biological dimensions contribute to the observed sexual dimorphism in metabolic traits and liver outcomes [Figure 3].
Figure 3. Genetic, Epigenetic, and Microbiome Modifiers of MASLD: Sex-Linked Pathways and Gut-Liver Interactions. This figure integrates genetic, epigenetic, and microbiome-related mechanisms contributing to MASLD pathogenesis, emphasizing sex-specific influences. Left side of the figure (Genetic Modifiers in MASLD): ↑Indicates an increase or higher level of a variable; ↓indicates a decrease or lower level of a variable; →indicates a relationship or influence between variables (e.g., “leads to”, “is associated with”, or “results in”). Right side of the figure (Gut-Liver Axis in MASLD): ↓Indicates a relationship or influence between variables (e.g., “leads to,” “is associated with,” or “results in”); ↕ indicates a bidirectional relationship or influence between variables. MASLD: Metabolic dysfunction-associated steatotic liver disease; PNPLA3: patatin-like phospholipase domain-containing protein 3; HSD17B13: hydroxysteroid 17-beta dehydrogenase 13; Erα: estrogen receptor alpha; XX/XY: chromosomal sex complement; LPS: lipopolysaccharide; TLRs: Toll-like receptors.
Sex chromosomes and metabolic regulation
Beyond gonadal hormones, evidence from sex-chromosome aneuploidies suggests that chromosomal complement itself influences metabolic regulation and MASLD risk[204-211]. Turner syndrome (TS; 45,X) and Klinefelter syndrome (KF; 47,XXY) are both associated with increased obesity, IR, and metabolic syndrome[212-215]. While these conditions often involve hormonal abnormalities - such as hypoestrogenism in TS and hypogonadism in KF - their early metabolic manifestations, including features of metabolic syndrome in prepubertal boys with KF, support a potential hormone-independent role of sex chromosome complement[216-219].
Registry and cohort data reinforce this viewpoint. Large national registries report markedly increased liver disease in TS: for example, a Danish registry[210] identified a substantially higher incidence of fibrosis/cirrhosis in people with TS [incidence rate ratio 16.5, 95% confidence interval (CI) 2.2-122.1 vs. age-matched controls], and another registry[208] found a similarly elevated prevalence of liver fibrosis/cirrhosis in TS. Specific karyotypes (e.g., isochromosome Xq, ring X) have been linked to a greater risk of liver dysfunction (defined by elevated liver enzymes) in multicenter analyses[220]. Importantly, these excess risks are reported to be independent of estrogen replacement[207-211,221-223] and are not clearly associated with indices of hypogonadism, such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH)[222,224]. Data for KF are limited: a small study reported MASLD prevalence of up to 50% in individuals with KF[225], and a large population-based cohort found higher odds of liver dysfunction (OR 1.71, 95%CI: 1.30-2.25) compared with age-matched male controls, again independent of testosterone therapy[226]. Collectively, these findings support that sex chromosome complement may contribute to MASLD susceptibility through mechanisms that extend beyond gonadal hormone effects; mechanistic studies and larger, well-phenotyped cohorts are needed to disentangle chromosomal vs. hormonal drivers and to define underlying pathways.
Genetic variants influencing MASLD
The PNPLA3 (Patatin-Like Phospholipase Domain-Containing Protein 3) I148M variant (rs738409) is the strongest inherited risk factor for MASLD, with a greater effect on liver fat in women and an increased risk of liver-related events in older, non-obese women[227-229]. Estrogen receptor α (ERα) signaling may upregulate PNPLA3 expression, suggesting a sex hormone-genotype interaction in disease pathogenesis[227]. By contrast, the HSD17B13 (Hydroxysteroid 17-Beta Dehydrogenase 13) rs72613567 insertion appears protective against steatohepatitis and advanced fibrosis, with stronger effects reported in women, particularly after menopause[203,230]. This variant is also associated with lower circulating sex steroids, including 17-OH progesterone, testosterone, and androstenedione[166,203,230,231], reinforcing the interplay between genetic susceptibility and hormonal status. Emerging data further link these variants to distinct hepatic microbial DNA profiles, pointing to possible gene-microbiome interactions that may shape MASLD risk and progression[232]. Together, these findings highlight a complex network of genetic, hormonal, and microbial factors that interact in sex-specific ways to influence MASLD outcomes.
THE ROLE OF THE GUT-LIVER AXIS AND MICROBIOME
The gut-liver axis is a bidirectional communication network [Figure 3] in which microbial metabolites, bile acids, dietary components, and inflammatory mediators reach the liver through the portal circulation and influence hepatic and metabolic pathways[233]. Under physiological conditions, the gut microbiota supports nutrient metabolism, generates bioactive compounds, and maintains mucosal immune balance[234-238]. Conversely, disturbances such as dysbiosis or impaired barrier function are associated with greater translocation of microbial products [e.g., lipopolysaccharides (LPS)], which can activate hepatic toll-like receptor signaling and have been linked to inflammation, steatosis, IR, and fibrogenesis - processes central to MASLD pathogenesis[234-238].
Sex differences in gut microbial composition, diversity, and metabolic outputs have been described and appear to be shaped by a combination of genetic, hormonal, dietary, and environmental factors[239,240]. For example, premenopausal women often show greater microbial diversity and enrichment of taxa associated with metabolic resilience compared with men and postmenopausal women[241]. After menopause, microbiota profiles shift toward those observed in men, coinciding with estrogen decline and higher prevalence of IR, visceral adiposity, and MASLD in women[241]. Although these findings are observational, they suggest that endocrine-microbiome interactions may partly explain sex-specific metabolic outcomes[242].
Experimental data in animals provide additional support. In murine models, microbiota-mediated effects on steatosis and glucose metabolism vary by sex and have been shown to depend on nuclear receptor pathways such as the farnesoid X receptor (FXR), a regulator of bile acid signaling[243]. Whether similar mechanisms operate in humans remains uncertain, but these data raise the possibility that sex-specific host-microbe interactions contribute to differences in MASLD risk and therapeutic response. Given the current limitations, sex-stratified human studies are needed to determine how hormonal transitions across the lifespan influence gut microbial composition, and whether microbiome-targeted strategies-including diet, probiotics, or FXR agonists-offer differential benefit by sex or hormonal status.
CARDIO METABOLIC AND EXTRAHEPATIC COMORBIDITIES
MASLD is increasingly recognized as a multisystem disease with significant comorbidities [Figure 4], including cardiovascular disease (CVD), chronic kidney disease (CKD), and various malignancies[244]. Notably, sex differences influence the prevalence, clinical presentation, and prognosis of these comorbidities[198,244,245].
Figure 4. Overview of Cardio-Metabolic and Extrahepatic Comorbidities in MASLD: Sex-Specific Risk Patterns. This figure provides a concise summary of the major systemic comorbidities associated with MASLD, with an emphasis on sex-related differences in risk. ↑Indicates that the risk of the specified MASLD-related complication is higher in the mentioned sex compared with the other sex. MASLD: Metabolic dysfunction-associated steatotic liver disease; CVD: cardiovascular disease; CKD: chronic kidney disease; HCC: hepatocellular carcinoma.
CVD
CVD is the leading cause of mortality among individuals with MASLD. While MASLD is more prevalent in men, women with MASLD often carry a greater burden of cardiometabolic risk factors[198], which may diminish or even reverse the natural cardiovascular advantage typically observed in premenopausal women[246-249]. A meta-analysis of 36 cohorts, including over 18.5 million individuals, reported that MASLD was associated with an increased risk of cardiovascular events in both sexes[250]. However, the relative risk was higher in women [pooled hazard ratio (HR) 1.59, 95%CI: 1.44-1.75] than in men (pooled HR 1.37, 95%CI: 1.27-1.48), and MASLD severity was more strongly linked to CVD risk in women (pooled HR 2.40, 95%CI: 1.73-3.32) than in men (pooled HR 1.69, 95%CI: 1.21-2.35)[250]. Importantly, these findings were independent of shared cardiometabolic risk factors such as obesity, T2D, hypertension, and dyslipidemia, underscoring the role of sex as an additional modifier of cardiovascular risk in MASLD (250).
CKD
MASLD is independently associated with an increased risk of CKD, and sex differences influence this risk[251]. A large study of over 760,000 patients reported a 16% greater CKD risk in men (adjusted HR 1.16, 95%CI: 1.13-1.18)[252]. Similarly, a Korean cohort of nearly 41,400 individuals found MASLD predicted incident CKD in men (adjusted HR 1.26, 95%CI: 1.04-1.52) but not in women (adjusted HR 1.03, 95%CI: 0.74-1.44)[175], while a U.S. study of ~263,000 adults showed a stronger risk in men (adjusted HR 1.70, 95%CI: 1.59-1.81) than in women (HR 1.47, 95%CI: 1.38-1.58)[253,254]. Notably, these associations were independent of shared cardiometabolic risk factors[252-254]. In contrast, a German database analysis found no significant sex difference in incident CKD, although men exhibited a trend toward a higher risk of requiring dialysis therapy[255]. Taken together, accumulating evidence supports a greater MASLD-related CKD burden in men, yet further research is warranted to clarify the modifiers of this sex-specific risk.
Malignancies
Sex hormones, metabolic traits, and gene expression jointly shape cancer susceptibility in MASLD[256-260]. Men with MASLD show a markedly higher risk of HCC and other malignancies[252,261]. In a large cohort study of ~600,000 patients with MASLD - balanced by sex and matched for age and cardiometabolic risk factors - men demonstrated more than double the risk of HCC (adjusted HR 2.59, 95%CI: 2.39-2.80) and a 32% higher risk of non-specific cancers compared with women (adjusted HR 1.32, 95%CI: 1.27-1.37)[252]. Beyond HCC, sex-specific differences extend to extrahepatic cancers[262]. A meta-analysis of four studies found MASLD to be associated with female-specific cancers, including breast cancer (pooled HR 1.39, 95%CI: 1.13-1.71) and gynecological cancers (pooled HR 1.62, 95%CI: 1.13-2.32)[263]. These findings highlight sex-specific patterns of MASLD-related malignancies that warrant further investigation.
IMPLICATIONS FOR DIAGNOSIS AND RISK STRATIFICATION
Accurate diagnosis and risk stratification are central to the effective management of MASLD[264]. While non-invasive tests (NITs) such as the Fibrosis-4 index (FIB-4), controlled attenuation parameter (CAP), and liver stiffness measurement (LSM) using vibration-controlled transient elastography (VCTE) have largely replaced liver biopsy in clinical practice, most diagnostic algorithms still apply uniform thresholds across sexes[265]. This risks underdiagnosis or misclassification [Table 3], particularly in women, where hormonal status, body composition, and metabolic profiles differ from those of men[264,266]. For example, CAP values may underestimate steatosis severity in women, while indices such as the fatty liver index (FLI) and hepatic steatosis index (HSI) - which use waist circumference, HDL cholesterol, and liver enzyme cut-offs-often need sex-specific adjustments to improve diagnostic accuracy.[267-271].
Sex-specific considerations in the diagnostic evaluation of MASLD
| Diagnostic tool | Sex differences consideration | Reference(s) |
| Imaging | ||
| CAP | Women exhibit earlier metabolic and vascular alterations at lower CAP values; applying uniform CAP thresholds may underestimate disease severity in women | Ramírez-Vélez et al.[267] |
| Ultrasound (Sonography) | When combined with the FLI, lower FLI cut-offs are needed in women to improve diagnostic accuracy | Crudele et al.[272] |
| MRI/CT | Significant sex-based differences in liver attenuation exist, potentially driven by differences in visceral adiposity and triglyceride levels | North et al.[274] |
| Elastography (Transient/MR Elastography) | Postmenopausal women have higher fibrosis risk despite lower MASLD prevalence; sex-adjusted liver stiffness cut-offs may enhance diagnostic precision | Balakrishnan et al.[8] |
| Blood-based biomarkers/scores | ||
| FLI | Women require ~50% lower FLI cut-offs than men for accurate MASLD detection, reflecting sex-specific metabolic and hormonal differences | Crudele et al.[272] |
| HSI | Incorporates sex as a variable; women generally have higher ALT and distinct AST/ALT ratios, leading to detection of steatosis at lower HSI values | Xu et al.[271] |
| FIB-4 Index | Postmenopausal women are at greater risk for fibrosis; sex-adjusted cut-offs could improve diagnostic accuracy, although standardized thresholds are lacking | Feng et al.[280] |
| NFS | Lean women benefit from lower NFS cut-offs for accurate fibrosis risk stratification, indicating relevant sex-based differences | Park et al.[282] |
| ELF test | Postmenopausal women show higher fibrosis risk, though sex-specific ELF thresholds are not yet established | Hinkson et al.[283] |
| APRI | Currently lacks validated sex-specific thresholds; a single cut-off is applied across sexes despite potential for sex-related variability | Xiao et al.[284] |
Imaging-based modalities also reflect sex-specific variability. Women generally display higher liver attenuation values on CT and lower visceral adiposity for the same body mass index (BMI), which may mask MASLD if male-derived thresholds are applied[272-275]. Similarly, elastography studies indicate that postmenopausal women may be at higher risk of fibrosis despite lower MASLD prevalence, suggesting the need for revised stiffness cut-offs in this group[276,277]. These findings underscore that uniform imaging thresholds may fail to capture clinically relevant sex-related risk, particularly during menopause transition when liver fat accumulation and fibrosis accelerate.
Biomarker-based NITs reveal parallel challenges. The FLI performs best when cut-offs are reduced by nearly 50% in women[272,278], while HSI incorporates sex directly as a variable but may still underestimate disease risk in premenopausal women with lower ALT and AST/ALT ratios[271,279]. Similarly, fibrosis scores such as FIB-4 and the NAFLD fibrosis score (NFS) appear to overestimate risk in men and underestimate it in postmenopausal women, but sex-stratified thresholds have not been standardized[280-282]. The enhanced liver fibrosis (ELF) and AST to platelet ratio index (APRI) also show promising accuracy, yet neither has validated sex-specific ranges[283,284].
Going forward, practical implementation requires integrating sex-adjusted thresholds into clinical algorithms and validating them across diverse populations. Since many NITs incorporate metabolic parameters (e.g., waist circumference, HDL, triglycerides, liver enzymes), the interaction between sex-specific metabolic phenotypes and biomarker performance should be systematically addressed. For instance, women may develop MASLD and fibrosis at lower levels of adiposity or dyslipidemia than men, necessitating tailored diagnostic scoring. Another challenge is the risk of digital amplification of existing disparities. A recent analysis of machine learning models trained on the Indian Liver Patient Dataset (ILPD) revealed that widely used classifiers systematically underperform in women, with false negative rates up to 24% higher than in men[103]. In practice, this translates into disproportionately missed diagnoses in female patients, reinforcing inequities already present in traditional diagnostic pathways. The study highlights how algorithmic approaches, unless sex-stratified, can codify and perpetuate sex bias in liver disease diagnosis.
Collectively, these observations demonstrate that diagnostic performance improves when sex-related metabolic, hormonal, and algorithmic biases are addressed, yet most tools remain calibrated for “average” populations. Large-scale, sex-stratified validation studies, coupled with fairness audits of emerging AI-based models, are essential to move from recognition of sex differences to actionable and equitable diagnostic pathways.
THERAPEUTIC AND PREVENTIVE CONSIDERATIONS
The management of MASLD requires individualized strategies that reflect sex-related differences in pathogenesis, progression, and response to interventions. While lifestyle modification remains foundational, pharmacologic therapy, bariatric surgery, and hormone replacement therapy (HRT) are increasingly important[285,286], with accumulating evidence that sex-specific factors influence efficacy, safety, and long-term outcomes [Tables 4 and 5].
Sex-differentiated responses to medical interventions for MASLD
| Author, year[Ref.] | Therapeutic approach | Design | Method | Sex-specific results | comment |
| Harrison et al., 2024[290] | Thyroid hormone receptor agonists (e.g., Resmetirom) | RCT (NCT03900429) | This phase 3, randomized, double-blind, placebo-controlled trial enrolled adults with biopsy-confirmed NASH and fibrosis stages F1B to F3. Participants were randomized to receive resmetirom 80 mg, 100 mg, or placebo once daily. Primary endpoints at week 52 were: (1) NASH resolution (≥ 2-point reduction in NAFLD activity score with no fibrosis worsening); and (2) ≥ 1-stage fibrosis improvement with no worsening in activity score. Key secondary outcomes included changes in triglycerides (24 weeks), hepatic fat (16 and 52 weeks), and liver stiffness (52 weeks) | In the 100 mg group, fibrosis improvement had an EDP of 15.1% (95%CI: 7.2-23.1) in men (n = 141) and 9.6% (95%CI: 2.2-17.0) in women (n = 180). In the 80 mg group, EDP was 9.5% (95%CI: 1.9-17.1) in men (n = 137) and 10.6% (95%CI: 2.8-18.4) in women (n = 179), suggesting similar efficacy between sexes | Although efficacy was consistent in both sexes, subtle differences in drug response by dose may reflect underlying sex-based variations in lipid metabolism or thyroid hormone signaling. The slightly higher EDP in men at 100 mg and in women at 80 mg may warrant further investigation into optimal dosing strategies by sex to enhance personalized treatment for NASH |
| Loomba et al., 2023[300] | FGF21 analogue (e.g., Pegozafermin) | RCT (NCT04929483) | This multicenter, phase 2b, randomized, double-blind, placebo-controlled trial enrolled adults with biopsy-confirmed NASH and stage F2-F3 fibrosis. Participants were randomly assigned to receive subcutaneous pegozafermin at 15 mg or 30 mg weekly (QW), 44 mg once every 2 weeks (Q2W), or placebo for 24 weeks. The two co-primary endpoints were: (1) ≥ 1-stage fibrosis improvement without worsening of NASH; (2) NASH resolution without fibrosis worsening | In men, pegozafermin significantly improved fibrosis in both the 30 mg QW (EDP: 30.7%; 95%CI: 8.5-52.8) and 44 mg Q2W (EDP: 33.8%; 95%CI: 7.6-60.1) groups. In women, the corresponding EDPs were lower and not statistically significant: 11.46% (95%CI: -6.1 to 29.1) for 30 mg QW and 9.6% (95%CI: -8.9 to 28.2) for 44 mg Q2W. This suggests a differential response based on sex | These results highlight a potential sex-related disparity in response to FGF21 analogues, with men showing more robust fibrosis improvement than women at the same doses. The underlying mechanisms may involve sex-specific differences in FGF21 receptor sensitivity, hepatic lipid handling, or inflammation pathways |
| Sanyal et al., 2025[297] | GLP-1 receptor agonists (e.g., Semaglutide) | RCT (NCT04822181) | In this phase 3, multicenter, randomized, double-blind, placebo-controlled trial, 1197 patients with biopsy-confirmed MASH and fibrosis stage F2 or F3 were randomized in a 2:1 ratio to receive once-weekly subcutaneous semaglutide 2.4 mg or placebo for 240 weeks. An interim analysis conducted at week 72 (part 1) involved the first 800 patients. The primary endpoints were resolution of steatohepatitis without worsening of fibrosis and improvement in fibrosis without worsening of steatohepatitis | EDP for MASH resolution was higher in women (31.4%, 95%CI: 21.4-41.4) than in men (25.8%, 95%CI: 14.4-37.1), while for fibrosis improvement, EDP was higher in men (16.9%, 95%CI: 6.7-27.1) than in women (12.4%, 95%CI: 3.2-21.8) | This large RCT reveals intriguing sex-based divergences in semaglutide efficacy: while women experienced superior resolution of steatohepatitis, men showed greater improvement in fibrosis. These opposing trends may reflect sex-specific variations in GLP-1 receptor expression, metabolic-inflammatory responses, or adipose-liver crosstalk |
| Sanyal et al., 2010[304] | Thiazolidinediones (e.g. Pioglitazone) | RCT (NCT00063622) | In a multicenter, randomized, placebo-controlled trial (NCT00063622), 247 non-diabetic adults with biopsy-proven NASH were randomly assigned to receive either pioglitazone 30 mg daily (n = 80), vitamin E 800 IU daily (n = 84), or placebo (n = 83) for 96 weeks. The primary outcome was histological improvement of NASH, defined by a composite score including steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis | Histological improvement was greater in women than men, with an EDP of 20% for women vs. 4% for men | These findings suggest a sex-based difference in histological response to pioglitazone in NASH, with women demonstrating substantially greater benefit. Potential explanations may include differences in adipose tissue biology, insulin sensitivity, or drug metabolism between sexes |
| Lin et al., 2025[308] | SGLT2 Inhibitors (e.g., dapagliflozin) | RCT (NCT03723252) | This multicentre, double-blind, randomized, placebo-controlled trial was conducted across six tertiary hospitals in China between November 2018 and March 2023. A total of 154 adults with biopsy-confirmed MASH (with or without type 2 diabetes) were randomly assigned to receive either 10 mg dapagliflozin or a matching placebo once daily for 48 weeks. The primary endpoint was MASH improvement (≥ 2-point reduction in NAS or NAS ≤ 3) without fibrosis worsening. Secondary endpoints included MASH resolution without fibrosis worsening and fibrosis improvement without MASH worsening. Analyses were conducted on an intention-to-treat basis | Dapagliflozin showed a greater relative benefit for MASH resolution in women (RR: 5.1, 95%CI: 0.6-39.6) compared to men (RR: 2.2, 95%CI: 0.9-5.4). Although not statistically significant, women also demonstrated a higher relative rate of fibrosis improvement (RR: 3.2, 95%CI: 0.8-12.6) than men (RR: 2.1, 95%CI: 1.2-3.6) | This study suggests a potential sex-specific response to SGLT2 inhibition in MASH, with women showing a numerically greater benefit in both histological endpoints. While confidence intervals are wide, these findings raise important questions about sex-based differences in metabolic and fibrotic response mechanisms |
| Hider et al., 2024[286] | Bariatric surgery | Retrospective cohort study | Patients who underwent gastric bypass or sleeve gastrectomy (June 2006-June 2022) were identified from a state-wide bariatric registry. Primary outcomes: % excess body weight loss and BMI change at 1 year. Secondary outcome: 30-day risk-adjusted complications | Among 107,504 patients (79.2% female), males were older (47.6 vs. 44.8 y), had higher baseline weight (346.6 lbs vs. 279.9 lbs) and BMI (49.9 vs. 47.2 kg/m2), and more comorbidities. Males achieved slightly greater total weight loss (105.1 lbs vs. 84.9 lbs) and excess body weight loss (60.0% vs. 58.8%) but had higher BMI at 1 year (34.0 vs. 32.8 kg/m2). Males experienced higher rates of serious complications (2.5% vs. 1.9%), leak/perforation (0.5% vs. 0.4%), VTE (0.7% vs. 0.4%), and medical complications (1.5% vs. 1.0%) compared to females | Bariatric surgery induces substantial weight loss in both sexes, which is expected to reduce MASLD risk. Men achieve slightly greater weight reduction but experience higher complication rates, likely due to greater baseline comorbidities, highlighting the need for early referral and careful perioperative management |
Impact of Hormonal Replacement Therapies on MASLD
| Author, year[Ref.] | Therapeutic approach | Design | Method | Sex-specific results | comment |
| Kim et al., 2023[313] | Estrogen replacement therapy | Observational retrospective cohort | This retrospective observational cohort study evaluated 368 postmenopausal women who received MHT over a 12-month period. Participants were categorized into two groups based on the route of estrogen administration: transdermal (n = 75) and oral (n = 293). The primary objective was to compare changes in the prevalence of NAFLD before and after 12 months of MHT between these two groups. In the oral estrogen group, the study further examined whether NAFLD progression differed based on the dose of estrogen or the type of progestogen used | After 12 months, NAFLD prevalence decreased in the transdermal group (24% to 17.3%) but increased in the oral group (25.3% to 29.4%). While the transdermal group showed no major clinical changes, the oral group had improved lipid profiles. Estrogen dose and progestogen type did not significantly affect NAFLD outcomes in the oral group | The observed improvement in hepatic outcomes with transdermal estrogen supports its potential as a liver-friendly option in MHT, likely due to its avoidance of first-pass hepatic metabolism. Despite favorable changes in lipid profiles in the oral group, the increased NAFLD prevalence raises concerns about the hepatic implications of oral estrogen |
| Shigehara et al., 2025[317] | Testosterone replacement therapy | RCT | In this randomized controlled trial, 186 hypogonadal men were enrolled and assigned to receive either TRT (n = 88) or no treatment (control; n = 98) for 12 months. The TRT group received intramuscular testosterone enanthate 250 mg every four weeks. Baseline and 12-month assessments included anthropometric measures (waist circumference, BMI, body fat volume), metabolic parameters (fasting blood sugar, HbA1c, cholesterol, triglycerides, HDL), and liver fibrosis markers (FIB-4 index based on age, AST, ALT, and platelet count). A subgroup analysis focused on patients with a baseline FIB-4 index ≥ 1.30 | At 12 months, TRT led to significant reductions in waist circumference, body fat volume, and an increase in platelet count compared to the control group, though overall FIB-4 index changes were not significant. However, in patients with elevated baseline FIB-4 (≥ 1.30), TRT significantly improved FIB-4 scores (-0.10 vs. +0.04; P = 0.0311) along with favorable changes in triglycerides, body fat, and platelet counts. This suggests TRT may improve liver fibrosis markers in men at higher fibrotic risk | These findings suggest that testosterone therapy may offer antifibrotic benefits in metabolically vulnerable hypogonadal men. Given sex-related hormonal influences on liver disease, TRT could represent a targeted strategy to mitigate fibrosis progression in men with metabolic dysfunction |
Pharmacologic therapies
Pharmacological therapies targeting liver fat, inflammation, and fibrosis are increasingly used in MASLD management, with emerging evidence for benefits beyond the liver, including improvements in the cardiovascular-kidney-metabolic (CKM) profile[287]. Two Food and Drug Administration (FDA)-approved drugs-resmetirom and semaglutide-already provide insights into sex-specific effects. Resmetirom, a liver-specific thyroid hormone receptor-β agonist, has shown promise in reducing liver fat and fibrosis by promoting lipophagy and mitochondrial biogenesis while inhibiting fibrogenesis via transforming growth factor-β (TGF-β) blockade[288,289]. In a recent phase 3 trial, resmetirom demonstrated similar antifibrotic efficacy in both sexes, though subtle dose-related differences suggest possible sex-based variations in response[290]. GLP-1 receptor agonists (GLP-1RAs) such as liraglutide, semaglutide, and tirzepatide are effective for T2D and obesity, and may improve MASLD histology[291,292]. Several trials revealed that women achieved greater weight loss and glycemic responses, partly due to ~30% higher drug exposure compared to men[293-296]. In a phase 3 trial of semaglutide in biopsy-confirmed MASH, women experienced greater steatohepatitis resolution, whereas men showed more pronounced fibrosis improvement[297]. These divergent effects may reflect sex-specific differences in GLP-1 receptor expression, adipose tissue dynamics, or hepatic inflammatory signaling[296,298,299]. While these findings were encouraging, post-marketing surveillance remains essential to validate sex-specific treatment patterns and to clarify long-term effects on CKM outcomes.
Other pharmacologic agents remain investigational, and evidence is still preliminary. FGF21 (fibroblast growth factor 21) analogs such as pegozafermin have shown potential in improving liver fibrosis and resolving NASH by modulating lipid metabolism and reducing inflammation[300]. In a recent phase 2b trial, pegozafermin led to meaningful fibrosis improvement in men, while the benefits in women were less pronounced[300], potentially due to sex differences in receptor activity and hepatic lipid handling[301]. Thiazolidinediones, particularly pioglitazone, have shown benefits in steatohepatitis and insulin sensitivity, although fibrosis improvement is limited[302,303]. In a multicenter randomized trial, women demonstrated greater histological improvement than men[304], and in a Chinese cohort, pioglitazone combined with lifestyle intervention reduced liver fat more effectively in women[305]. Similarly, sodium-glucose cotransporter-2 (SGLT2) inhibitors such as empagliflozin and dapagliflozin modestly reduced hepatic steatosis and ALT levels in T2D patients[306,307]. In a recent randomized trial (RCT), dapagliflozin appeared to provide greater histological benefit in women, although the difference did not reach statistical significance[308,309] . Together, these findings highlighted the importance of designing future sex-stratified trials to better define differential efficacy and to determine whether these therapies improve CKM health in a sex-specific manner.
Bariatric surgery
Bariatric surgery is among the most effective interventions for severe obesity and MASLD, producing durable weight loss, improved insulin sensitivity, and marked reductions in hepatic steatosis and fibrosis[310]. Both men and women benefit, but sex-specific differences have been observed. Men often achieve slightly greater absolute weight loss, yet they experience higher perioperative complication rates, which are likely explained by older age at surgery, higher baseline BMI, and greater comorbidity burden[286]. Women, on the other hand, tend to undergo surgery at younger ages and may experience more favorable improvements in metabolic risk factors, including IR and dyslipidemia[286]. These differences suggest that earlier surgical referral for men could maximize benefits while reducing complication risk. Overall, bariatric surgery improves long-term MASLD outcomes across sexes, but tailoring timing and perioperative management to sex-specific risk profiles may optimize safety and efficacy.
HRT
HRT has been investigated as a potential modifier of MASLD risk, though most studies remain observational or exploratory. In postmenopausal women, low-dose estradiol or norethisterone was associated with improved liver enzymes and reduced steatosis compared with non-users[311,312], and transdermal estrogen therapy correlated with a lower prevalence of MASLD over 12 months, whereas oral estrogen showed a modest increase, likely due to first-pass hepatic metabolism[313]. However, these findings are preliminary and require confirmation in larger RCTs. Importantly, estrogen exposure can also be harmful under certain conditions: prolonged or high-dose use, especially with synthetic formulations, has been linked to hepatotoxicity, intrahepatic cholestasis, vascular lesions, adenomas, and, rarely, hepatic vein thrombosis[314,315]. In hypogonadal men, testosterone therapy [e.g., LPCN 1144 (an oral prodrug of bioidentical testosterone)] demonstrated improvements in body fat, waist circumference, and fibrosis markers in a recent trial[316,317], suggesting possible antifibrotic benefits in metabolically vulnerable men. Still, long-term safety, particularly regarding cardiovascular outcomes, is insufficiently characterized. Overall, while physiological sex hormone replacement shows potential for modifying liver outcomes, further well-powered RCTs are needed to establish sex-specific efficacy and safety.
SEX-GENDER CROSSTALK IN MASLD
Considering the differences outlined in previous sections, we propose a conceptual framework in which MASLD risk emerges from the dynamic interplay between biological sex and gendered exposures, rather than from either domain alone [Figure 5]. Biological sex determines genetic background, hormonal milieu, body composition, and microbiome architecture, while gender - encompassing sociocultural norms, behaviors, and roles - shapes diet, physical activity, stress, healthcare engagement, and social responsibilities[6]. These factors interact bidirectionally. For example, caregiving burdens, occupational constraints, or limited access to exercise can influence hormonal status, amplifying metabolic and hepatic risk[6]. Similarly, reproductive events such as pregnancy and breastfeeding affect MASLD risk, but their impact is strongly mediated by gendered supports, including cultural norms, workplace policies, and social networks[105]. In men, biological susceptibility to more severe steatosis and fibrosis is often compounded by gender norms that discourage timely healthcare use, leading to delayed diagnosis and worse outcomes[102].
Figure 5. Conceptual Framework of Sex-Gender Crosstalk in MASLD. This figure illustrates how MASLD risk and disease course emerge from the dynamic, bidirectional interaction between biological sex and gender-related factors. MASLD: Metabolic dysfunction-associated steatotic liver disease.
Gut dysbiosis - regulated in part by sex hormones - also interacts with gendered exposures, including diet, alcohol use, and stress, to influence both hepatic and extrahepatic events[239]. In transgender and gender-diverse populations, the inseparability of sex and gender is further illustrated by GAHT, which alters body composition and metabolic risk[157]. Meanwhile, stigma, discrimination, and structural barriers magnify vulnerability and reduce access to appropriate care[164]. Collectively, these interactions demonstrate that MASLD risk cannot be attributed to hormones alone but emerges from biology intersecting with gendered determinants. Effective risk stratification, diagnostics, and therapeutic approaches must therefore integrate biological mechanisms with social context to fully capture the complexity of sex-gender crosstalk in MASLD.
KNOWLEDGE GAPS AND FUTURE RESEARCH DIRECTIONS
Although significant progress has been made in understanding the pathophysiology of MASLD, substantial knowledge gaps persist regarding sex and gender differences across biological, behavioral, and clinical domains[6]. Hormonal regulation is widely recognized as a determinant of hepatic fat accumulation, inflammation, and fibrosis[180]. However, the precise molecular mechanisms through which these hormones interact with immune signaling, mitochondrial function, and fibrogenesis remain incompletely understood[180]. Moreover, while metabolic abnormalities are central to MASLD, their interplay with sex-specific fat distribution, lean mass, and metabolic flexibility is not yet well defined[318]. Lean women and older individuals with sarcopenic obesity may represent a particularly vulnerable group requiring focused investigation.
Certain populations remain markedly underrepresented in MASLD research. Premenopausal women, particularly regarding long-term maternal and offspring outcomes and the effects of breastfeeding, are understudied, despite evidence that sex-gender crosstalk significantly influences metabolic trajectories. Transgender and non-binary individuals, including those undergoing GAHT, are similarly underrepresented, despite being highly affected by the interplay of biological and gendered determinants.
Genetic and epigenetic modifiers have demonstrated variable expression and impact across sexes, yet sex-stratified genomic analyses are still rare[229,319]. Additionally, the gut-liver axis, a key modulator of MASLD, has shown sexually dimorphic microbial patterns in other diseases, but its role in MASLD remains largely speculative[242]. Future studies should integrate metagenomics and metabolomics with hormonal and immunologic profiling to better define these complex interactions.
From a clinical standpoint, cardiovascular and extrahepatic outcomes of MASLD show divergence between men and women, but current diagnostic tools and risk models do not reflect these differences[250,320]. Most clinical trials still lack sex-specific subgroup analyses, limiting the generalizability of findings[321]. Collectively, advancing MASLD care will require routine incorporation of sex and gender as biological and analytical variables in both mechanistic studies and therapeutic trials[322].
CONCLUSION
Sex and gender differences are increasingly recognized as fundamental determinants in the development, progression, and clinical expression of MASLD. This review has synthesized current knowledge across multiple domains - including hormonal regulation, metabolic risk factors, genetic and epigenetic influences, microbiome profiles, and behavioral determinants - highlighting how these sex- and gender-based factors shape MASLD phenotypes.
Women and men differ not only in the distribution of traditional metabolic risk factors such as adiposity, IR, and dyslipidemia, but also in how these factors interact with sex hormones, immune responses, and lifestyle behaviors. These biological and sociocultural influences are further reflected in the burden of extrahepatic complications and response to treatment. Importantly, differences are not static and can evolve with aging, menopause, and shifting social contexts, underscoring the need for dynamic clinical assessment.
Despite growing evidence, current clinical frameworks rarely consider sex or gender when evaluating MASLD risk or guiding treatment. Integrating this perspective into clinical reasoning will improve the precision of both diagnostic assessments and therapeutic strategies. By bringing together diverse lines of evidence, this review supports a sex- and gender-informed approach to MASLD and reinforces the importance of contextualizing liver disease within the broader framework of individualized, equitable care.
DECLARATIONS
Authors’ contributions
Conceptualized and designed the study: Jamalinia M, Saeian S, Lankarani KB
Performed the literature review and drafted the manuscript: Jamalinia M, Saeian S, Nikkhoo N, Nazerian A
Prepared the figures and tables: Jamalinia M, Nikkhoo N, Nazerian A
All authors critically revised the manuscript and approved the final version for submission.
Availability of data and materials
All data and materials presented in this paper were extracted from previously published studies, which are publicly available and can be accessed through the cited sources.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Since this study is a review of previously published papers, no new ethical approval was required. All original studies included in this review obtained appropriate ethical approval and informed consent from participants.
Consent for publication
This review does not include individual-level or identifiable data; therefore, consent for publication was not required.
Copyright
© The Author(s) 2025.
REFERENCES
1. Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707.
2. Rinella ME, Lazarus JV, Ratziu V, et al; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-86.
3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
4. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-57.
6. Jamalinia M, Lonardo A, Weiskirchen R. Sex and gender differences in liver fibrosis: pathomechanisms and clinical outcomes. Fibrosis. 2024;2:10006.
7. Joo SK, Kim W. Sex differences in metabolic dysfunction-associated steatotic liver disease: a narrative review. Ewha Med J. 2024;47:e17.
8. Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61-71.e15.
10. Krawczyk M, Bonfrate L, Portincasa P. Nonalcoholic fatty liver disease. Best Pract Res Clin Gastroenterol. 2010;24:695-708.
11. Moghaddasifar I, Lankarani KB, Moosazadeh M, et al. Prevalence of non-alcoholic fatty liver disease and its related factors in iran. Int J Organ Transplant Med. 2016;7:149-60.
12. Burra P, Bizzaro D, Gonta A, et al.; Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021;41:1713-33.
13. Alkaabi J, Afandi B, Alhaj O, Kanwal D, Agha A. Identifying metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes mellitus using clinic-based prediction tools. Front Med. 2024;11:1425145.
14. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-47.
15. Hayward KL, Johnson AL, Horsfall LU, Moser C, Valery PC, Powell EE. Detecting non-alcoholic fatty liver disease and risk factors in health databases: accuracy and limitations of the ICD-10-AM. BMJ Open Gastroenterol. 2021;8:e000572.
16. Pal P, Palui R, Ray S. Heterogeneity of non-alcoholic fatty liver disease: implications for clinical practice and research activity. World J Hepatol. 2021;13:1584-610.
17. Fan JG, Zhu J, Li XJ, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508-14.
18. Eguchi Y, Hyogo H, Ono M, et al.; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586-95.
19. Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J Gastroenterol. 2012;18:237-43.
20. Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406-14.
21. Shen M, Shi H. Sex hormones and their receptors regulate liver energy homeostasis. Int J Endocrinol. 2015;2015:294278.
22. Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227-56.
23. Galmés-Pascual BM, Martínez-Cignoni MR, Morán-Costoya A, et al. 17β-estradiol ameliorates lipotoxicity-induced hepatic mitochondrial oxidative stress and insulin resistance. Free Radic Biol Med. 2020;150:148-60.
24. Galmés-Pascual BM, Nadal-Casellas A, Bauza-Thorbrügge M, et al. 17β-estradiol improves hepatic mitochondrial biogenesis and function through PGC1B. J Endocrinol. 2017;232:297-308.
25. Smiriglia A, Lorito N, Bacci M, et al. Estrogen-dependent activation of TRX2 reverses oxidative stress and metabolic dysfunction associated with steatotic disease. Cell Death Dis. 2025;16:57.
26. Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304-19.
27. Zhu L, Brown WC, Cai Q, et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424-34.
28. Matsuo K, Gualtieri MR, Cahoon SS, et al. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause. 2016;23:189-96.
29. Mueller NT, Liu T, Mitchel EB, et al. Sex hormone relations to histologic severity of pediatric nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2020;105:3496-504.
30. Pafili K, Paschou SA, Armeni E, Polyzos SA, Goulis DG, Lambrinoudaki I. Non-alcoholic fatty liver disease through the female lifespan: the role of sex hormones. J Endocrinol Invest. 2022;45:1609-23.
31. Ryu S, Suh BS, Chang Y, et al. Menopausal stages and non-alcoholic fatty liver disease in middle-aged women. Eur J Obstet Gynecol Reprod Biol. 2015;190:65-70.
32. Meda C, Dolce A, Della Torre S. Metabolic dysfunction-associated steatotic liver disease across women’s reproductive lifespan and issues. Clin Mol Hepatol. 2025;31:327-32.
33. Markopoulos MC, Kassi E, Alexandraki KI, Mastorakos G, Kaltsas G. Hyperandrogenism after menopause. Eur J Endocrinol. 2015;172:R79-91.
34. Zaman A, Rothman MS. Postmenopausal hyperandrogenism: evaluation and treatment strategies. Endocrinol Metab Clin North Am. 2021;50:97-111.
35. Gershagen S, Doeberl A, Jeppsson S, Rannevik G. Decreasing serum levels of sex hormone-binding globulin around the menopause and temporary relation to changing levels of ovarian steroids, as demonstrated in a longitudinal study. Fertil Steril. 1989;51:616-21.
36. Lazo M, Zeb I, Nasir K, et al. Association between endogenous sex hormones and liver fat in a multiethnic study of atherosclerosis. Clin Gastroenterol Hepatol. 2015;13:1686-93.e2.
37. Polyzos SA, Kountouras J, Tsatsoulis A, et al. Sex steroids and sex hormone-binding globulin in postmenopausal women with nonalcoholic fatty liver disease. Hormones. 2013;12:405-16.
38. Larsson H, Ahrén B. Androgen activity as a risk factor for impaired glucose tolerance in postmenopausal women. Diabetes Care. 1996;19:1399-403.
39. Wang N, Zhai H, Zhu C, et al. Combined association of vitamin D and sex hormone binding globulin with nonalcoholic fatty liver disease in men and postmenopausal women: a cross-sectional study. Medicine. 2016;95:e2621.
40. Flechtner-Mors M, Schick A, Oeztuerk S, et al.; EMIL-Study Group. Associations of fatty liver disease and other factors affecting serum SHBG concentrations: a population based study on 1657 subjects. Horm Metab Res. 2014;46:287-93.
41. Wang X, Xie J, Pang J, et al. Serum SHBG is associated with the development and regression of nonalcoholic fatty liver disease: a prospective study. J Clin Endocrinol Metab. 2020;105:e791-804.
42. Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S. Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ann Hepatol. 2017;16:382-94.
43. Mahmoud M, Kawtharany H, Awali M, Mahmoud N, Mohamed I, Syn WK. The effects of testosterone replacement therapy in adult men with metabolic dysfunction-associated steatotic liver disease: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2025;16:e00787.
44. Lee HS, Han SH, Swerdloff R, Pak Y, Budoff M, Wang C. The effect of testosterone replacement therapy on nonalcoholic fatty liver disease in older hypogonadal men. J Clin Endocrinol Metab. 2024;109:e757-64.
45. Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018;15:e1002542.
46. Park JM, Lee HS, Oh J, Lee YJ. Serum testosterone level within normal range is positively associated with nonalcoholic fatty liver disease in premenopausal but not postmenopausal women. J Womens Health. 2019;28:1077-82.
47. Sarkar MA, Suzuki A, Abdelmalek MF, et al.; NASH Clinical Research Network. Testosterone is associated with nonalcoholic steatohepatitis and fibrosis in premenopausal women with NAFLD. Clin Gastroenterol Hepatol. 2021;19:1267-74.e1.
48. Sayas-Barberá E, Pérez-Álvarez JA, Navarro-Rodríguez de Vera C, Fernández-López M, Viuda-Martos M, Fernández-López J. Sustainability and gender perspective in food innovation: foods and food processing coproducts as source of macro- and micro-nutrients for woman-fortified foods. Foods. 2022;11:3661.
49. Nasreddine L, Chamieh MC, Ayoub J, Hwalla N, Sibai AM, Naja F. Sex disparities in dietary intake across the lifespan: the case of Lebanon. Nutr J. 2020;19:24.
50. Feraco A, Armani A, Amoah I, et al. Assessing gender differences in food preferences and physical activity: a population-based survey. Front Nutr. 2024;11:1348456.
51. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309-38.
52. Loeffelholz C, Coldewey SM, Birkenfeld AL. A narrative review on the role of AMPK on de novo lipogenesis in non-alcoholic fatty liver disease: evidence from human studies. Cells. 2021;10:1822.
53. Morán-Costoya A, Proenza AM, Gianotti M, Lladó I, Valle A. Sex differences in nonalcoholic fatty liver disease: estrogen influence on the liver-adipose tissue crosstalk. Antioxid Redox Signal. 2021;35:753-74.
54. Goul C, Peruzzo R, Zoncu R. The molecular basis of nutrient sensing and signalling by mTORC1 in metabolism regulation and disease. Nat Rev Mol Cell Biol. 2023;24:857-75.
55. Basil B, Myke-Mbata BK, Eze OE, Akubue AU. From adiposity to steatosis: metabolic dysfunction-associated steatotic liver disease, a hepatic expression of metabolic syndrome - current insights and future directions. Clin Diabetes Endocrinol. 2024;10:39.
56. Nemer M, Osman F, Said A. Dietary macro and micronutrients associated with MASLD: analysis of a national US cohort database. Ann Hepatol. 2024;29:101491.
57. Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453-61.
58. Low WS, Cornfield T, Charlton CA, Tomlinson JW, Hodson L. Sex differences in hepatic de novo lipogenesis with acute fructose feeding. Nutrients. 2018;10:1263.
59. Piras IS, Raju A, Don J, Schork NJ, Gerhard GS, DiStefano JK. Hepatic PEMT expression decreases with increasing NAFLD severity. Int J Mol Sci. 2022;23:9296.
60. Wang C, Ma H, Yang H, et al. Sex differences in the association between total energy intake and all-cause mortality among patients with metabolic dysfunction-associated steatotic liver disease. Sci Rep. 2025;15:19176.
61. O’Meara L, de Bruyn J, Hope T, et al. Conceptual framework of women’s food environments and determinants of food acquisition and dietary intake in low- and middle-income countries: a scoping review. Lancet Planet Health. 2025;9:101280.
62. Sinclair K, Thompson-colón T, Bastidas-granja AM, Del Castillo Matamoros SE, Olaya E, Melgar-quiñonez H. Women’s autonomy and food security: connecting the dots from the perspective of indigenous women in rural Colombia. SSM Qual Res Health. 2022;2:100078.
63. Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes Facts. 2024;17:374-444.
64. Chen Y, Kim M, Paye S, Benayoun BA. Sex as a biological variable in nutrition research: from human studies to animal models. Annu Rev Nutr. 2022;42:227-50.
65. Trouwborst I, Goossens GH, Astrup A, Saris WHM, Blaak EE. Sexual dimorphism in body weight loss, improvements in cardiometabolic risk factors and maintenance of beneficial effects 6 months after a low-calorie diet: results from the randomized controlled DiOGenes trial. Nutrients. 2021;13:1588.
66. Bédard A, Corneau L, Lamarche B, Dodin S, Lemieux S. Sex-related differences in the effects of the mediterranean diet on glucose and insulin homeostasis. J Nutr Metab. 2014;2014:424130.
67. Leblanc V, Bégin C, Hudon AM, et al. Gender differences in the long-term effects of a nutritional intervention program promoting the Mediterranean diet: changes in dietary intakes, eating behaviors, anthropometric and metabolic variables. Nutr J. 2014;13:107.
68. D’Abbondanza M, Ministrini S, Pucci G, et al. Very low-carbohydrate ketogenic diet for the treatment of severe obesity and associated non-alcoholic fatty liver disease: the role of sex differences. Nutrients. 2020;12:2748.
69. Vitale M, Costabile G, Bergia RE, et al. The effects of Mediterranean diets with low or high glycemic index on plasma glucose and insulin profiles are different in adult men and women: data from MEDGI-Carb randomized clinical trial. Clin Nutr. 2023;42:2022-8.
70. Razzak I, Fares A, Stine JG, Trivedi HD. The role of exercise in steatotic liver diseases: an updated perspective. Liver Int. 2025;45:e16220.
71. Kretschmer L, Salali GD, Andersen LB, et al.; International Children’s Accelerometry Database (ICAD) Collaborators. Gender differences in the distribution of children’s physical activity: evidence from nine countries. Int J Behav Nutr Phys Act. 2023;20:103.
72. Hurley KS, Flippin KJ, Blom LC, Bolin JE, Hoover DL, Judge LW. Practices, perceived benefits, and barriers to resistance training among women enrolled in college. Int J Exerc Sci. 2018;11:226-38.
73. Reading JM, LaRose JG. Exercise preferences among emerging adults: do men and women want different things? J Am Coll Health. 2022;70:1301-5.
74. Pataky MW, Dasari S, Michie KL, et al. Impact of biological sex and sex hormones on molecular signatures of skeletal muscle at rest and in response to distinct exercise training modes. Cell Metab. 2023;35:1996-2010.e6.
75. Freer CL, George ES, Tan SY, Abbott G, Dunstan DW, Daly RM. Effect of progressive resistance training with weight loss compared with weight loss alone on the fatty liver index in older adults with type 2 diabetes: secondary analysis of a 12-month randomized controlled trial. BMJ Open Diabetes Res Care. 2022;10:e002950.
76. Keating SE, Sabag A, Hallsworth K, et al. Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: a position statement from exercise and sport science Australia. Sports Med. 2023;53:2347-71.
77. Kling K, Margaryan L, Fuchs M. (In) equality in the outdoors: gender perspective on recreation and tourism media in the Swedish mountains. Current Issues in Tourism. 2020;23:233-47.
78. Merino M, Tornero-Aguilera JF, Rubio-Zarapuz A, Villanueva-Tobaldo CV, Martín-Rodríguez A, Clemente-Suárez VJ. Body perceptions and psychological well-being: a review of the impact of social media and physical measurements on self-esteem and mental health with a focus on body image satisfaction and its relationship with cultural and gender factors. Healthcare. 2024;12:1396.
79. Nournezhad H, Davar S, Vahabzadeh D, Mohaddesi H, Sahebazzamani Z, Yas A. Physical activity and food frequency in postmenopausal women: a cross-sectional study. J Midwifery Reprod Health. 2023;11:3734-43.
80. Zhang Z, He Z, Yang H, Li D, Duan P, Wei X. The accumulation of visceral fat in postmenopausal women: the combined impact of prenatal genetics, epigenetics, and fat depot heterogeneity - a descriptive review. Clin Exp Obstet Gynecol. 2025;52:26194.
81. Curci R, Bonfiglio C, Franco I, Bagnato CB, Verrelli N, Bianco A. Leisure-time physical activity in subjects with metabolic-dysfunction-associated steatotic liver disease: an all-cause mortality study. J Clin Med. 2024;13:3772.
82. Mosca L, Mochari H, Christian A, et al. National study of women’s awareness, preventive action, and barriers to cardiovascular health. Circulation. 2006;113:525-34.
83. Williams RL, Wood LG, Collins CE, Callister R. Effectiveness of weight loss interventions--is there a difference between men and women: a systematic review. Obes Rev. 2015;16:171-86.
84. Rinaldi R, De Nucci S, Donghia R, et al. Gender differences in liver steatosis and fibrosis in overweight and obese patients with metabolic dysfunction-associated steatotic liver disease before and after 8 weeks of very low-calorie ketogenic diet. Nutrients. 2024;16:1408.
85. Gratacós-Ginès J, Ariño S, Sancho-Bru P, Bataller R, Pose E. MetALD: clinical aspects, pathophysiology and treatment. JHEP Rep. 2025;7:101250.
86. Moon JH, Jeong S, Jang H, Koo BK, Kim W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: a nationwide cohort study. EClinicalMedicine. 2023;65:102292.
87. Israelsen M, Torp N, Johansen S, et al.; GALAXY consortium. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9:218-28.
88. Dunn N, Al-Khouri N, Abdellatif I, Singal AK. Metabolic dysfunction and alcohol-associated liver disease: a narrative review. Clin Transl Gastroenterol. 2025;16:e00828.
89. Bizzaro D, Becchetti C, Trapani S, et al.; AISF Special Interest Group on Gender in Hepatology. Influence of sex in alcohol-related liver disease: pre-clinical and clinical settings. United European Gastroenterol J. 2023;11:218-27.
90. White AM. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol Res. 2020;40:01.
91. Xu J, Li Y, Feng Z, Chen H. Cigarette smoke contributes to the progression of MASLD: from the molecular mechanisms to therapy. Cells. 2025;14:221.
92. Kopp W. Pathogenesis of (smoking-related) non-communicable diseases-evidence for a common underlying pathophysiological pattern. Front Physiol. 2022;13:1037750.
93. Alcaraz A, Lazo E, Casarini A, et al. Exploring gender disparities in the disease and economic tobacco-attributable burden in Latin America. Front Public Health. 2023;11:1321319.
94. Kim NH, Jung YS, Hong HP, et al. Association between cotinine-verified smoking status and risk of nonalcoholic fatty liver disease. Liver Int. 2018;38:1487-94.
96. Yoo JJ, Lee DH, Kim SG, Jang JY, Kim YS, Kim LY. Impacts of smoking on alcoholic liver disease: a nationwide cohort study. Front Public Health. 2024;12:1427131.
97. Aryee LNA, Flanagan SV, Trupe L, Yucel M, Smith J. Social norms and social opportunities: a qualitative study of influences on tobacco use among urban adolescent girls in Ghana. BMC Public Health. 2024;24:2978.
98. Gezinski LB, Gonzalez-Pons KM, Rogers MM. Substance use as a coping mechanism for survivors of intimate partner violence: implications for safety and service accessibility. Violence Against Women. 2021;27:108-23.
99. Fonseca F, Robles-Martínez M, Tirado-Muñoz J, et al. A gender perspective of addictive disorders. Curr Addict Rep. 2021;8:89-99.
100. Rata Mohan DS, Jawahir S, Manual A, et al. Gender differences in health-seeking behaviour: insights from the National Health and Morbidity Survey 2019. BMC Health Serv Res. 2025;25:900.
101. Schlichthorst M, Sanci LA, Pirkis J, Spittal MJ, Hocking JS. Why do men go to the doctor? BMC Public Health. 2016;16:1028.
102. Mokhwelepa LW, Sumbane GO. Men’s mental health matters: the impact of traditional masculinity norms on men’s willingness to seek mental health support; a systematic review of literature. Am J Mens Health. 2025;19:15579883251321670.
103. Straw I, Wu H. Investigating for bias in healthcare algorithms: a sex-stratified analysis of supervised machine learning models in liver disease prediction. BMJ Health Care Inform. 2022;29:e100457.
104. Stefanova V, Farrell L, Latu I. Gender and the pandemic: associations between caregiving, working from home, personal and career outcomes for women and men. Curr Psychol. 2023;42:17395-411.
105. Sarkar M, Kushner T. Metabolic dysfunction-associated steatotic liver disease and pregnancy. J Clin Invest. 2025;135:e186426.
106. Sarkar M, Grab J, Dodge JL, et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73:516-22.
107. Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025-36.
108. Kushner T, Lange M, Argiriadi PA, Meislin R, Sigel K, Terrault N; Fatty Liver In Pregnancy (FLIP) Study Group. Prevalence, risk profiles, and national implications of nonalcoholic fatty liver disease in pregnant individuals. Clin Gastroenterol Hepatol. 2024;22:194-6.e1.
109. Lee SM, Kwak SH, Koo JN, et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62:238-48.
110. Lee SM, Kim BJ, Koo JN, et al. Nonalcoholic fatty liver disease is a risk factor for large-for-gestational-age birthweight. PLoS One. 2019;14:e0221400.
111. Armistead B, Johnson E, VanderKamp R, et al. Placental regulation of energy homeostasis during human pregnancy. Endocrinology. 2020;161:bqaa076.
112. Hagström H, Höijer J, Ludvigsson JF, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016;36:268-74.
113. De Souza LR, Berger H, Retnakaran R, et al. Non-alcoholic fatty liver disease in early pregnancy predicts dysglycemia in mid-pregnancy: prospective study. Am J Gastroenterol. 2016;111:665-70.
114. Mousa N, Abdel-Razik A, Shams M, et al. Impact of non-alcoholic fatty liver disease on pregnancy. Br J Biomed Sci. 2018;75:197-9.
115. Herath RP, Siriwardana SR, Ekanayake CD, Abeysekara V, Kodithuwakku SUA, Herath HP. Non-alcoholic fatty liver disease and pregnancy complications among Sri Lankan women: a cross sectional analytical study. PLoS One. 2019;14:e0215326.
116. Sattari M, Bril F, Egerman R, Kalavalapalli S, Cusi K. Relationship between non-alcoholic fatty liver disease during pregnancy and abnormal glucose metabolism during and after pregnancy. J Investig Med. 2020;68:743-7.
117. Lee SM, Jung YM, Choi ES, et al. Metabolic dysfunction-associated fatty liver disease and subsequent development of adverse pregnancy outcomes. Clin Gastroenterol Hepatol. 2022;20:2542-50.e8.
118. Qian Y, Zhang Y, Fan X, et al. Nonalcoholic fatty liver disease and adverse pregnancy outcomes in women with normal prepregnant weight. J Clin Endocrinol Metab. 2023;108:463-71.
119. Jafari R, Karimi Moghaddam E, Ahmadzadeh A, Bahrami S, Alavinejad P, Manouchehri Zanjani S. Pregnancy outcome in patients with non-alcoholic fatty liver disease: a prospective cohort study. Gastroenterol Hepatol Bed Bench. 2024;17:180-6.
120. Koralegedara IS, Warnasekara JN, Dayaratne KG, De Silva FN, Premadasa JK, Agampodi SB. Non-alcoholic fatty liver disease (NAFLD): a significant predictor of gestational diabetes mellitus (GDM) and early pregnancy miscarriages-prospective study in Rajarata Pregnancy Cohort (RaPCo). BMJ Open Gastroenterol. 2022;9:e000831.
121. Amadou C, Nabi O, Serfaty L, et al. Association between birth weight, preterm birth, and nonalcoholic fatty liver disease in a community-based cohort. Hepatology. 2022;76:1438-51.
122. Jamaly H, Eslick GD, Weltman M. Systematic review with meta-analysis: non-alcoholic fatty liver disease and the association with pregnancy outcomes. Clin Mol Hepatol. 2022;28:52-66.
123. Lee SM, Hwangbo S, Norwitz ER, et al. Nonalcoholic fatty liver disease and early prediction of gestational diabetes mellitus using machine learning methods. Clin Mol Hepatol. 2022;28:105-16.
124. Sosseh SAL, Barrow A, Lu ZJ. Cultural beliefs, attitudes and perceptions of lactating mothers on exclusive breastfeeding in The Gambia: an ethnographic study. BMC Womens Health. 2023;23:18.
125. Cohen CC, Perng W, Sauder KA, et al. Maternal diet quality during pregnancy and offspring hepatic fat in early childhood: the healthy start study. J Nutr. 2023;153:1122-32.
126. Sarkar M, Brady CW, Fleckenstein J, et al. Reproductive health and liver disease: practice guidance by the american association for the study of liver diseases. Hepatology. 2021;73:318-65.
127. Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of liver diseases in pregnancy. J Hepatol. 2023;79:768-828.
128. Spatz DL, Rodríguez SÁ, Benjilany S, et al. Having enough milk to sustain a lactation journey: a call to action. Nurs Womens Health. 2024;28:256-63.
129. Ayonrinde OT, Oddy WH, Adams LA, et al. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol. 2017;67:568-76.
130. Soullane S, Willems P, Lee GE, Auger N. Early life programming of nonalcoholic fatty liver disease in children. Early Hum Dev. 2022;168:105578.
131. Leca BM, Lagojda L, Kite C, et al. Maternal obesity and metabolic (dysfunction) associated fatty liver disease in pregnancy: a comprehensive narrative review. Expert Rev Endocrinol Metab. 2024;19:335-48.
132. Hagström H, Simon TG, Roelstraete B, Stephansson O, Söderling J, Ludvigsson JF. Maternal obesity increases the risk and severity of NAFLD in offspring. J Hepatol. 2021;75:1042-8.
133. Goldner D, Lavine JE. Nonalcoholic fatty liver disease in children: unique considerations and challenges. Gastroenterology. 2020;158:1967-83.e1.
134. Zeng J, Shen F, Zou ZY, et al. Association of maternal obesity and gestational diabetes mellitus with overweight/obesity and fatty liver risk in offspring. World J Gastroenterol. 2022;28:1681-91.
135. Şanlı E, Kabaran S. Maternal obesity, maternal overnutrition and fetal programming: effects of epigenetic mechanisms on the development of metabolic disorders. Curr Genomics. 2019;20:419-27.
136. Forbes JD, Azad MB, Vehling L, et al.; Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018;172:e181161.
137. Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol. 2017;14:81-96.
138. Park Y, Sinn DH, Oh JH, et al. The association between breastfeeding and nonalcoholic fatty liver disease in parous women: a nation-wide cohort study. Hepatology. 2021;74:2988-97.
139. Ajmera VH, Terrault NA, Van Wagner LB, et al. Longer lactation duration is associated with decreased prevalence of non-alcoholic fatty liver disease in women. J Hepatol. 2019;70:126-32.
140. Marshall NE, Lau B, Purnell JQ, Thornburg KL. Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern Child Nutr. 2019;15:e12732.
141. Bish MR, Faulks F, Amir LH, et al. Relationship between obesity and lower rates of breast feeding initiation in regional Victoria, Australia: an 8-year retrospective panel study. BMJ Open. 2021;11:e044884.
142. Karachaliou GS, Suzuki A, Patel VA, Bastian LA, Diehl AM, Abdelmalek MF; Duke NAFLD Clinical Research Working Group. Longer breastfeeding duration is associated with decreased risk of hepatic fibrosis among young women with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2024;22:413-5.e3.
143. Lund-Blix NA, Dydensborg Sander S, Størdal K, et al. Infant feeding and risk of type 1 diabetes in two large scandinavian birth cohorts. Diabetes Care. 2017;40:920-7.
144. Abeysekera KW, Orr JG, Madley-Dowd P, et al. Association of maternal pre-pregnancy BMI and breastfeeding with NAFLD in young adults: a parental negative control study. Lancet Reg Health Eur. 2021;10:100206.
145. Yamakawa M, Yorifuji T, Inoue S, Kato T, Doi H. Breastfeeding and obesity among schoolchildren: a nationwide longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-25.
146. Tørris C, Bjørnnes AK. Duration of lactation and maternal risk of metabolic syndrome: a systematic review and meta-analysis. Nutrients. 2020;12:2718.
147. Kardashian A, Kushner T, Au JS, et al.; AASLD Women’s Initiative Committee. The key role of hepatology providers in optimizing reproductive care in patients with liver disease: a call to action. Hepatology. 2023;78:363-7.
148. Glintborg D, Møller JK, Rubin KH, et al. Gender-affirming treatment and mental health diagnoses in Danish transgender persons: a nationwide register-based cohort study. Eur J Endocrinol. 2023;189:336-45.
149. Hilden M, Glintborg D, Andersen MS, et al. Gender incongruence in Denmark, a quantitative assessment. Acta Obstet Gynecol Scand. 2021;100:1800-5.
150. Harper J, O’Donnell E, Sorouri Khorashad B, McDermott H, Witcomb GL. How does hormone transition in transgender women change body composition, muscle strength and haemoglobin? Br J Sports Med. 2021;55:865-72.
151. Spanos C, Bretherton I, Zajac JD, Cheung AS. Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: a systematic review. World J Diabetes. 2020;11:66-77.
152. Velho I, Fighera TM, Ziegelmann PK, Spritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology. 2017;5:881-8.
153. Klaver M, van Velzen D, de Blok C, et al. Change in visceral fat and total body fat and the effect on cardiometabolic risk factors during transgender hormone therapy. J Clin Endocrinol Metab. 2022;107:e153-64.
154. Shadid S, Abosi-Appeadu K, De Maertelaere AS, et al. Effects of gender-affirming hormone therapy on insulin sensitivity and incretin responses in transgender people. Diabetes Care. 2020;43:411-7.
155. Islam N, Nash R, Zhang Q, et al. Is there a link between hormone use and diabetes incidence in transgender people? J Clin Endocrinol Metab. 2022;107:e1549-57.
156. van Velzen D, Wiepjes C, Nota N, et al. Incident diabetes risk is not increased in transgender individuals using hormone therapy. J Clin Endocrinol Metab. 2022;107:e2000-7.
157. Hashemi L, Marijic Buljubasic A, Budoff MJ, et al. Gender-affirming hormone treatment and metabolic syndrome among transgender veterans. JAMA Netw Open. 2024;7:e2419696.
158. Pei Q, Song Y, Huang Z, et al. Effects of gender-affirming hormone therapy on body fat: a retrospective case-control study in Chinese transwomen. Lipids Health Dis. 2024;23:146.
159. Schutte MH, Kleemann R, Nota NM, et al. The effect of transdermal gender-affirming hormone therapy on markers of inflammation and hemostasis. PLoS One. 2022;17:e0261312.
160. Banks K, Kyinn M, Leemaqz SY, Sarkodie E, Goldstein D, Irwig MS. Blood pressure effects of gender-affirming hormone therapy in transgender and gender-diverse adults. Hypertension. 2021;77:2066-74.
161. Lake JE, Hyatt AN, Feng H, et al. Transgender women with HIV demonstrate unique non-alcoholic fatty liver disease profiles. Transgend Health. 2024;9:413-20.
162. Lightner JS, Schneider J, Grimes A, et al. Physical activity among transgender individuals: a systematic review of quantitative and qualitative studies. PLoS One. 2024;19:e0297571.
163. Jones BA, Haycraft E, Bouman WP, Arcelus J. The levels and predictors of physical activity engagement within the treatment-seeking transgender population: a matched control study. J Phys Act Health. 2018;15:99-107.
164. Goffnett J, Call J, Ciak J, et al. Conceptualizing trans-inclusive healthcare in the United States: environmental and interpersonal factors of healthcare provision. SSM - Health Systems. 2025;5:100112.
165. Waters J, Linsenmeyer W. The impact of gender-affirming hormone therapy on nutrition-relevant biochemical measures. Front Nutr. 2024;11:1339311.
166. Cherubini A, Della Torre S, Pelusi S, Valenti L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol Med. 2024;30:1126-36.
167. Kim H, Kim SE, Sung MK. Sex and gender differences in obesity: biological, sociocultural, and clinical perspectives. World J Mens Health. 2025;43:758-72.
168. Lee JH, Jeon S, Lee HS, Kwon YJ. Gender differences in the risk for incident non-alcoholic fatty liver disease according to the transition of abdominal obesity status: a 16-year cohort study. Nutrients. 2023;15:2880.
169. Lee E, Korf H, Vidal-Puig A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J Hepatol. 2023;78:1048-62.
170. Bansal S, Vachher M, Arora T, Kumar B, Burman A. Visceral fat: a key mediator of NAFLD development and progression. Hum Nutr Metab. 2023;33:200210.
171. Gavin KM, Bessesen DH. Sex differences in adipose tissue function. Endocrinol Metab Clin North Am. 2020;49:215-28.
172. Kuryłowicz A. Estrogens in adipose tissue physiology and obesity-related dysfunction. Biomedicines. 2023;11:690.
173. Holven KB, Roeters van Lennep J. Sex differences in lipids: a life course approach. Atherosclerosis. 2023;384:117270.
174. Tsutsumi T, Kawaguchi T, Fujii H, et al. Low HDL cholesterol levels in women and hypertriglyceridemia in men: predictors of MASLD onset in individuals without steatosis. J Gastroenterol. 2025;60:891-904.
175. Sun T, Liu J. Study on the correlation between triglyceride glucose index, triglyceride glucose index to high-density lipoprotein cholesterol ratio, and the risk of diabetes in nonalcoholic fatty liver disease. Front Endocrinol. 2025;16:1594548.
176. Chen H, Miao X, Hu M, et al. Associations between high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and their ratio with metabolic dysfunction-associated steatotic liver disease: a retrospective cohort study. Front Endocrinol. 2025;16:1585811.
177. Oortmerssen JAE, Mulder JWCM, Kavousi M, Roeters van Lennep JE. Lipid metabolism in women: a review. Atherosclerosis. 2025;405:119213.
178. Milani I, Chinucci M, Leonetti F, Capoccia D. MASLD: prevalence, mechanisms, and sex-based therapies in postmenopausal women. Biomedicines. 2025;13:855.
179. Ntikoudi A, Spyrou A, Evangelou E, Dokoutsidou E, Mastorakos G. The effect of menopausal status, insulin resistance and body mass index on the prevalence of non-alcoholic fatty liver disease. Healthcare. 2024;12:1081.
180. Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): beyond insulin resistance. J Hepatol. 2023;79:1524-41.
181. Kautzky-Willer A, Leutner M, Harreiter J. Sex differences in type 2 diabetes. Diabetologia. 2023;66:986-1002.
182. Huang C, Yang X, Liu J. Sex-based differences in the predictive significance of the waist circumference glucose index for future diabetes risk. Sci Rep. 2025;15:21477.
183. Manjarrés L, Xavier A, González L, et al. Sex differences in the relationship between body composition and MASLD progression in a murine model of metabolic syndrome. iScience. 2025;28:111863.
184. Bo T, Gao L, Yao Z, et al. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024;36:947-68.
185. Tao Z, Cheng Z. Hormonal regulation of metabolism-recent lessons learned from insulin and estrogen. Clin Sci. 2023;137:415-34.
186. Paoli M, Zakharia A, Werstuck GH. The role of estrogen in insulin resistance: a review of clinical and preclinical data. Am J Pathol. 2021;191:1490-8.
187. Younossi ZM, Golabi P, Price JK, et al. The global epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2024;22:1999-2010.e8.
188. de Abreu JDMF, Azulay RS, Rodrigues V, et al. Predictors of hepatic fibrosis in type 2 diabetes patients with metabolic-dysfunction-associated steatotic liver disease. Biomedicines. 2024;12:2542.
189. Melini S, Trinchese G, Lama A, et al. Sex differences in hepatic inflammation, lipid metabolism, and mitochondrial function following early lipopolysaccharide exposure in epileptic WAG/Rij rats. Antioxidants. 2024;13:957.
190. Chadalavada S, Jensen MT, Aung N, et al. Women with diabetes are at increased relative risk of heart failure compared to men: insights from UK biobank. Front Cardiovasc Med. 2021;8:658726.
191. Zhang F, Han Y, Zheng G, Li W. Gender differences in the incidence of nephropathy and changes in renal function in patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Metab Syndr Obes. 2024;17:943-57.
192. Gowda D, Shekhar C, B
193. Arvanitakis K, Chatzikalil E, Kalopitas G, et al. Metabolic dysfunction-associated steatotic liver disease and polycystic ovary syndrome: a complex interplay. J Clin Med. 2024;13:4243.
194. Gao Z, Deng H, Qin B, Bai L, Li J, Zhang J. Impact of hypertension on liver fibrosis in patients with metabolic dysfunction-associated fatty liver disease. Front Med. 2025;12:1539283.
195. Nakagami H. Mechanisms underlying the bidirectional association between nonalcoholic fatty liver disease and hypertension. Hypertens Res. 2023;46:539-41.
196. Yeo WJ, Abraham R, Surapaneni AL, et al. Sex differences in hypertension and its management throughout life. Hypertension. 2024;81:2263-74.
197. Chapman N, Ching SM, Konradi AO, et al. Arterial hypertension in women: state of the art and knowledge gaps. Hypertension. 2023;80:1140-9.
198. Burnside J, Cinque F, Sebastiani G, et al. Sex differences in the prevalence and cardiometabolic risk profiles of steatotic liver disease: a Canadian longitudinal study on aging analysis. Can J Public Health. 2025:Epub ahead of print.
199. Orhan İ, Koçak HS, Kaplan E. Determination of cardiometabolic risk in pre- and post-menopausal women. BMC Cardiovasc Disord. 2025;25:399.
200. Bager JE, Manhem K, Andersson T, et al. Hypertension: sex-related differences in drug treatment, prevalence and blood pressure control in primary care. J Hum Hypertens. 2023;37:662-70.
201. Eng PC, Forlano R, Tan T, Manousou P, Dhillo WS, Izzi-Engbeaya C. Non-alcoholic fatty liver disease in women - current knowledge and emerging concepts. JHEP Rep. 2023;5:100835.
202. Chen M, Cao Y, Ji G, Zhang L. Lean nonalcoholic fatty liver disease and sarcopenia. Front Endocrinol. 2023;14:1217249.
203. Sookoian S, Rotman Y, Valenti L. Genetics of metabolic dysfunction-associated steatotic liver disease: the state of the art update. Clin Gastroenterol Hepatol. 2024;22:2177-87.e3.
204. Link JC, Wiese CB, Chen X, et al. X chromosome dosage of histone demethylase KDM5C determines sex differences in adiposity. J Clin Invest. 2020;130:5688-702.
205. Chen X, McClusky R, Chen J, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709.
206. Reue K. Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities. Physiol Behav. 2017;176:174-82.
207. Mondal S, Gargari P, Bose C, Garg MK, Chowdhury S, Mukhopadhyay S. Abnormal body composition increases the cardiometabolic risk in adolescents and young adults with turner syndrome. Endocr Pract. 2024;30:259-69.
208. Singh I, Noel G, Barker JM, et al. Hepatic abnormalities in youth with Turner syndrome. Liver Int. 2022;42:2237-46.
209. Twohig P, Li L, Danford D, Craft M, Yetman AT. Prevalence of hepatic steatosis and fibrosis in Turner syndrome: a prospective case-control study. Liver Int. 2024;44:1309-15.
210. Viuff MH, Stochholm K, Grønbaek H, Berglund A, Juul S, Gravholt CH. Increased occurrence of liver and gastrointestinal diseases and anaemia in women with Turner syndrome - a nationwide cohort study. Aliment Pharmacol Ther. 2021;53:821-9.
211. Zaegel N, Brahimaj R, Battaglia-Hsu S, Lamiral Z, Feigerlova E. Systemic inflammatory indices and liver dysfunction in turner syndrome patients: a retrospective case-control study. J Endocr Soc. 2024;8:bvae099.
212. Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2:74-9.
213. Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225-45.
214. Calcaterra V, Brambilla P, Maffè GC, et al. Metabolic syndrome in Turner syndrome and relation between body composition and clinical, genetic, and ultrasonographic characteristics. Metab Syndr Relat Disord. 2014;12:159-64.
215. Bojesen A, Kristensen K, Birkebaek NH, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591-8.
216. Høst C, Skakkebæk A, Groth KA, Bojesen A. The role of hypogonadism in Klinefelter syndrome. Asian J Androl. 2014;16:185-91.
217. Gravholt CH. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur J Endocrinol. 2004;151:657-87.
218. Bardsley MZ, Falkner B, Kowal K, Ross JL. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatr. 2011;100:866-70.
219. Nagral A, Bangar M, Menezes S, et al. Gender differences in nonalcoholic fatty liver disease. Euroasian J Hepatogastroenterol. 2022;12:S19-25.
220. Fiot E, Zénaty D, Boizeau P, Haignere J, Dos Santos S, Léger J; French Turner Syndrome Study Group. X chromosome gene dosage as a determinant of congenital malformations and of age-related comorbidity risk in patients with Turner syndrome, from childhood to early adulthood. Eur J Endocrinol. 2019;180:397-406.
221. Bourcigaux N, Dubost E, Buzzi JC, et al. Focus on liver function abnormalities in patients with turner syndrome: risk factors and evaluation of fibrosis risk. J Clin Endocrinol Metab. 2023;108:2255-61.
222. Ridder LOR, Just J, Hvas CL, et al. Elevated liver enzymes in turner syndrome: the role of low-grade inflammation and hormonal imbalances. J Endocr Soc. 2025;9:bvaf059.
223. Wójcik M, Ruszała A, Januś D, Starzyk JB. Liver biochemical abnormalities in adolescent patients with turner syndrome. J Clin Res Pediatr Endocrinol. 2019;11:395-9.
224. El-Mansoury M, Berntorp K, Bryman I, et al. Elevated liver enzymes in Turner syndrome during a 5-year follow-up study. Clin Endocrinol. 2008;68:485-90.
225. Marrone A, Allosso F, Caturano A, et al. Metabolic dysfunction-associated steatotic liver disease in klinefelter syndrome: high prevalence uncovers an unmet need. J Clin Endocrinol Metab. 2025:Epub ahead of print.
226. Davis SM, Nokoff NJ, Furniss A, et al. Population-based assessment of cardiometabolic-related diagnoses in youth with klinefelter syndrome: a PEDSnet study. J Clin Endocrinol Metab. 2022;107:e1850-9.
227. Cherubini A, Ostadreza M, Jamialahmadi O, et al.; EPIDEMIC Study Investigators. Interaction between estrogen receptor-α and PNPLA3 p.I148M variant drives fatty liver disease susceptibility in women. Nat Med. 2023;29:2643-55.
228. Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-94.
229. Rosso C, Caviglia GP, Birolo G, et al. Impact of PNPLA3 rs738409 polymorphism on the development of liver-related events in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21:3314-21.e3.
230. Vilar-Gomez E, Pirola CJ, Sookoian S, Wilson LA, Liang T, Chalasani N. The protection conferred by HSD17B13 rs72613567 polymorphism on risk of steatohepatitis and fibrosis may be limited to selected subgroups of patients with NAFLD. Clin Transl Gastroenterol. 2021;12:e00400.
231. Smyk W, Papapostoli I, Żorniak M, et al. Liver phenotypes in PCOS: analysis of exogenous and inherited risk factors for liver injury in two European cohorts. Liver Int. 2023;43:1080-8.
232. Pirola CJ, Salatino A, Quintanilla MF, Castaño GO, Garaycoechea M, Sookoian S. The influence of host genetics on liver microbiome composition in patients with NAFLD. EBioMedicine. 2022;76:103858.
233. Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12:793.
234. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369-79.
235. Sharpton SR, Ajmera V, Loomba R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin Gastroenterol Hepatol. 2019;17:296-306.
236. Liu HX, Keane R, Sheng L, Wan YJ. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63:1502-10.
237. Zheng Z, Wang B. The gut-liver axis in health and disease: the role of gut microbiota-derived signals in liver injury and regeneration. Front Immunol. 2021;12:775526.
238. Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-75.
239. de la Cuesta-Zuluaga J, Kelley ST, Chen Y, et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems. 2019;4:e00261-19.
240. Dominianni C, Sinha R, Goedert JJ, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10:e0124599.
241. Mayneris-Perxachs J, Arnoriaga-Rodríguez M, Luque-Córdoba D, et al. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020;8:136.
242. Santos-Marcos JA, Mora-Ortiz M, Tena-Sempere M, Lopez-Miranda J, Camargo A. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol Sex Differ. 2023;14:4.
243. Sheng L, Jena PK, Liu HX, et al. Gender differences in bile acids and microbiota in relationship with gender dissimilarity in steatosis induced by diet and FXR inactivation. Sci Rep. 2017;7:1748.
244. Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691-702.
245. Provenzano PF, Caridi G, Parlongo G, et al. Are there sex differences in cardiovascular outcomes in non-dialysis CKD patients? Clin Kidney J. 2023;16:2141-6.
246. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457-69.
247. Lonardo A, Suzuki A. Sexual dimorphism of NAFLD in adults. focus on clinical aspects and implications for practice and translational research. J Clin Med. 2020;9:1278.
248. Pemmasani G, Yandrapalli S, Aronow W. Sex differences in cardiovascular diseases and associated risk factors in non-alcoholic steatohepatitis. Am J Cardiovasc Dis. 2020;10:362-6.
249. Khalid YS, Dasu NR, Suga H, et al. Increased cardiovascular events and mortality in females with NAFLD: a meta-analysis. Am J Cardiovasc Dis. 2020;10:258-71.
250. Jamalinia M, Zare F, Mantovani A, Targher G, Lonardo A. Metabolic dysfunction-associated steatotic liver disease and sex-specific risk of fatal and non-fatal cardiovascular events: a meta-analysis. Diabetes Obes Metab. 2025;27:5171-81.
251. García GG, Iyengar A, Kaze F, Kierans C, Padilla-Altamira C, Luyckx VA. Sex and gender differences in chronic kidney disease and access to care around the globe. Semin Nephrol. 2022;42:101-13.
252. Yan T, Zhang X, Wong T, Cheung R, Nguyen MH. Sex differences in adverse liver and nonliver outcomes in steatotic liver disease. JAMA Netw Open. 2024;7:e2448946.
253. Sinn DH, Kang D, Jang HR, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: a cohort study. J Hepatol. 2017;67:1274-80.
254. Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J Intern Med. 2019;286:711-22.
255. Kaps L, Labenz C, Galle PR, Weinmann-Menke J, Kostev K, Schattenberg JM. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United European Gastroenterol J. 2020;8:942-8.
256. Ho JK, Thurairajah PH, Leo J, Huang DQ, Fan KH. Sex differences in hepatocellular carcinoma. Hepatoma Res. 2024;10:53.
257. Nevola R, Tortorella G, Rosato V, et al. Gender differences in the pathogenesis and risk factors of hepatocellular carcinoma. Biology. 2023;12:984.
258. Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72-83.
259. Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4:66.
260. Zhang X, Nguyen MH. Metabolic dysfunction-associated steatotic liver disease: a sexually dimorphic disease and breast and gynecological cancer. Metabolism. 2025;167:156190.
261. Shi Y, Zhang X, Wong T, et al. Sex differences in risk of adverse liver events in patients with cirrhosis. JAMA Netw Open. 2025;8:e2523674.
262. Peng Y, Wang P, Liu F, et al. Metabolic dysfunction-associated steatotic liver disease and cancer risk: a cohort study. Diabetes Obes Metab. 2025;27:1940-9.
263. Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71:778-88.
264. Vali Y, van Dijk AM, Lee J, et al.; LITMUS investigators. Precision in liver diagnosis: varied accuracy across subgroups and the need for variable thresholds in diagnosis of MASLD. Liver Int. 2025;45:e16240.
265. Calès P, Canivet CM, Costentin C, et al. A new generation of non-invasive tests of liver fibrosis with improved accuracy in MASLD. J Hepatol. 2025;82:794-804.
266. Ji H, Cheng S; Heart-Liver Axis Research Collaboration. Sex differences in prevalence and prognosis of steatotic liver disease phenotypes: biological sex matters. J Hepatol. 2024;80:e68-9.
267. Ramírez-Vélez R, Izquierdo M, García-Hermoso A, Correa-Rodríguez M. Reference values and associated factors of controlled attenuation parameter and liver stiffness in adults: a cross-sectional study. Nutr Metab Cardiovasc Dis. 2024;34:1879-89.
268. Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542.
269. Yadav AK, MacNeill JJ, Krylov A, et al. Sex- and age-associated factors drive the pathophysiology of MASLD. Hepatol Commun. 2024;8:e0523.
270. Priego-parra BA, Triana-romero A, Martínez-pérez GP, et al. Hepatic steatosis index (HSI): a valuable biomarker in subjects with metabolic dysfunction-associated fatty liver disease (MAFLD). Annals of Hepatology. 2024;29:101391.
271. Xu B, Li C, Chen M, Zhang Y. Four hepatic steatosis indices in predicting quantitative computed tomography-based metabolic dysfunction-associated fatty liver disease. Explor Endocr Metab Dis. 2024;1:62-76.
272. Crudele L, De Matteis C, Novielli F, et al. Fatty liver index (FLI) is the best score to predict MASLD with 50% lower cut-off value in women than in men. Biol Sex Differ. 2024;15:43.
273. Westerbacka J, Cornér A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360-9.
274. North KE, Graff M, Franceschini N, et al. Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European American and African American participants of the NHLBI family heart study. Eur J Gastroenterol Hepatol. 2012;24:9-16.
275. van der Poorten D, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449-57.
276. Trembling PM, Apostolidou S, Gentry-Maharaj A, et al. The enhanced liver fibrosis test is associated with liver-related outcomes in postmenopausal women with risk factors for liver disease. BMC Gastroenterol. 2020;20:104.
277. Liguori A, Esposto G, Ainora ME, et al. Liver elastography for liver fibrosis stratification: a comparison of three techniques in a biopsy-controlled MASLD cohort. Biomedicines. 2025;13:138.
278. Cai X, Thorand B, Hohenester S, et al. Association of sex hormones and sex hormone-binding globulin with liver fat in men and women: an observational and Mendelian randomization study. Front Endocrinol. 2023;14:1223162.
279. Gong J, Chen A, Han Y, Xie L. Sex- and age-specific differences in the relationship between fatty liver index as well as fibrosis-4 score and arterial stiffness: results from fuzhou study. J Hypertens. 2025;43:e241.
280. Feng Q, Izzi-Engbeaya CN, Manousou P, Woodward M. Fibrosis status, extrahepatic multimorbidity and all-cause mortality in 53,093 women and 74,377 men with metabolic dysfunction associated steatotic liver disease (MASLD) in UK biobank. BMC Gastroenterol. 2025;25:546.
281. Gbadamosi SO, Nagelhout E, Sienko D, et al. Association of index and changes in fibrosis-4 score with outcomes in metabolic dysfunction-associated steatohepatitis. Gastro Hep Adv. 2025;4:100666.
282. Park H, Yoon EL, Ito T, et al. Diagnostic performance of the fibrosis-4 index and nonalcoholic fatty liver disease fibrosis score in lean adults with nonalcoholic fatty liver disease. JAMA Netw Open. 2023;6:e2329568.
283. Hinkson A, Lally H, Gibson H, et al. Meta-analysis: enhanced liver fibrosis test to identify hepatic fibrosis in chronic liver diseases. Aliment Pharmacol Ther. 2023;57:750-62.
284. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486-501.
285. Drygalski K. Pharmacological treatment of MASLD: contemporary treatment and future perspectives. Int J Mol Sci. 2025;26:6518.
286. Hider AM, Bonham A, Carlin A, et al. Association of sex differences on weight loss and complications following bariatric surgery. J Surg Res. 2024;299:359-65.
287. Targher G, Valenti L, Byrne CD. Metabolic dysfunction-associated steatotic liver disease. N Engl J Med. 2025;393:683-98.
288. Ritter MJ, Amano I, Hollenberg AN. Thyroid hormone signaling and the liver. Hepatology. 2020;72:742-52.
289. Kelly MJ, Pietranico-Cole S, Larigan JD, et al. Discovery of 2-[3, 5-dichloro-4-(5-isopropyl-6-oxo-1, 6-dihydropyridazin-3-yloxy) phenyl]-3, 5-dioxo-2, 3, 4, 5-tetrahydro [1, 2, 4] triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57:3912-23.
290. Harrison SA, Bedossa P, Guy CD, et al.; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:1632-3.
291. Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79:1557-65.
292. Armstrong MJ, Gaunt P, Aithal GP, et al.; LEAN trial team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-90.
293. Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20:409-18.
294. Onishi Y, Oura T, Nishiyama H, Ohyama S, Takeuchi M, Iwamoto N. Subgroup analysis of phase 3 studies of dulaglutide in Japanese patients with type 2 diabetes. Endocr J. 2016;63:263-73.
295. Quan H, Zhang H, Wei W, Fang T. Gender-related different effects of a combined therapy of Exenatide and Metformin on overweight or obesity patients with type 2 diabetes mellitus. J Diabetes Complications. 2016;30:686-92.
296. Overgaard RV, Petri KC, Jacobsen LV, Jensen CB. Liraglutide 3.0 mg for weight management: a population pharmacokinetic analysis. Clin Pharmacokinet. 2016;55:1413-22.
297. Sanyal AJ, Newsome PN, Kliers I, et al.; ESSENCE Study Group. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med. 2025;392:2089-99.
298. Yang Y, He L, Han S, et al. Sex differences in the efficacy of glucagon-like peptide-1 receptor agonists for weight reduction: a systematic review and meta-analysis. J Diabetes. 2025;17:e70063.
299. Mirabelli M, Chiefari E, Caroleo P, et al. Long-term effectiveness of liraglutide for weight management and glycemic control in type 2 diabetes. Int J Environ Res Public Health. 2019;17:207.
300. Loomba R, Sanyal AJ, Kowdley KV, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389:998-1008.
301. Bazhan NМ, Jakovleva TV, Kazantseva AY, et al. Studying sex differences in responses to fibroblast growth factor 21 administration in obese mice consuming a sweet-fat diet. Vavilovskii Zhurnal Genet Selektsii. 2023;27:333-41.
302. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305-15.
303. Dormandy JA, Charbonnel B, Eckland DJ, et al.; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macrovascular events): a randomised controlled trial. Lancet. 2005;366:1279-89.
304. Sanyal AJ, Chalasani N, Kowdley KV, et al.; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-85.
305. Yan H, Wu W, Chang X, et al. Gender differences in the efficacy of pioglitazone treatment in nonalcoholic fatty liver disease patients with abnormal glucose metabolism. Biol Sex Differ. 2021;12:1.
306. Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care. 2018;41:1801-8.
307. Harrison SA, Manghi FP, Smith WB, et al. Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study. Nat Med. 2022;28:1432-8.
308. Lin J, Huang Y, Xu B, et al. Effect of dapagliflozin on metabolic dysfunction-associated steatohepatitis: multicentre, double blind, randomised, placebo controlled trial. BMJ. 2025;389:e083735.
309. Sharma A, Wood S, Bell JS, De Blasio MJ, Ilomäki J, Ritchie RH. Sex differences in risk of cardiovascular events and mortality with sodium glucose co-transporter-2 inhibitors versus glucagon-like peptide 1 receptor agonists in Australians with type 2 diabetes: a population-based cohort study. Lancet Reg Health West Pac. 2023;33:100692.
310. Liu H, Lefere S, Guillot A, Zheng MH, Tacke F. Bariatric surgery for metabolic dysfunction-associated steatotic liver disease (MASLD): current knowledge of mechanisms. Hepatology. 2025:Epub ahead of print.
311. McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol. 2006;65:40-4.
312. Florentino GS, Cotrim HP, Vilar CP, Florentino AV, Guimarães GM, Barreto VS. Nonalcoholic fatty liver disease in menopausal women. Arq Gastroenterol. 2013;50:180-5.
313. Kim SE, Min JS, Lee S, Lee DY, Choi D. Different effects of menopausal hormone therapy on non-alcoholic fatty liver disease based on the route of estrogen administration. Sci Rep. 2023;13:15461.
314. Yang JD, Abdelmalek MF, Guy CD, et al.; Nonalcoholic Steatohepatitis Clinical Research Network. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1479.
315. LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
316. Albhaisi S, Kim K, Baker J, et al. LPCN 1144 resolves NAFLD in hypogonadal males. Hepatol Commun. 2020;4:1430-40.
317. Shigehara K, Kato Y, Shinzawa R, et al. Testosterone replacement therapy can improve a biomarker of liver fibrosis in hypogonadal men: a subanalysis of a prospective randomized controlled study in Japan (EARTH Study). World J Mens Health. 2025;43:661-8.
318. Alipour P, Azizi Z, Raparelli V, et al. Role of sex and gender-related variables in development of metabolic syndrome: a prospective cohort study. Eur J Intern Med. 2024;121:63-75.
319. Choudhary NS, Duseja A. Genetic and epigenetic disease modifiers: non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD). Transl Gastroenterol Hepatol. 2021;6:2.
320. Lankarani K, Jamalinia M, Zare F, Heydari ST, Ardekani A, Lonardo A. Liver-kidney-metabolic health, sex, and menopause impact total scores and monovessel vs. multivessel coronary artery calcification. Adv Ther. 2025;42:1729-44.
321. Kallen AN, Whirledge S, Goldman KN, Johnson J. Undermining women’s health research - gambling with the public’s health. N Engl J Med. 2025;392:2185-7.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data





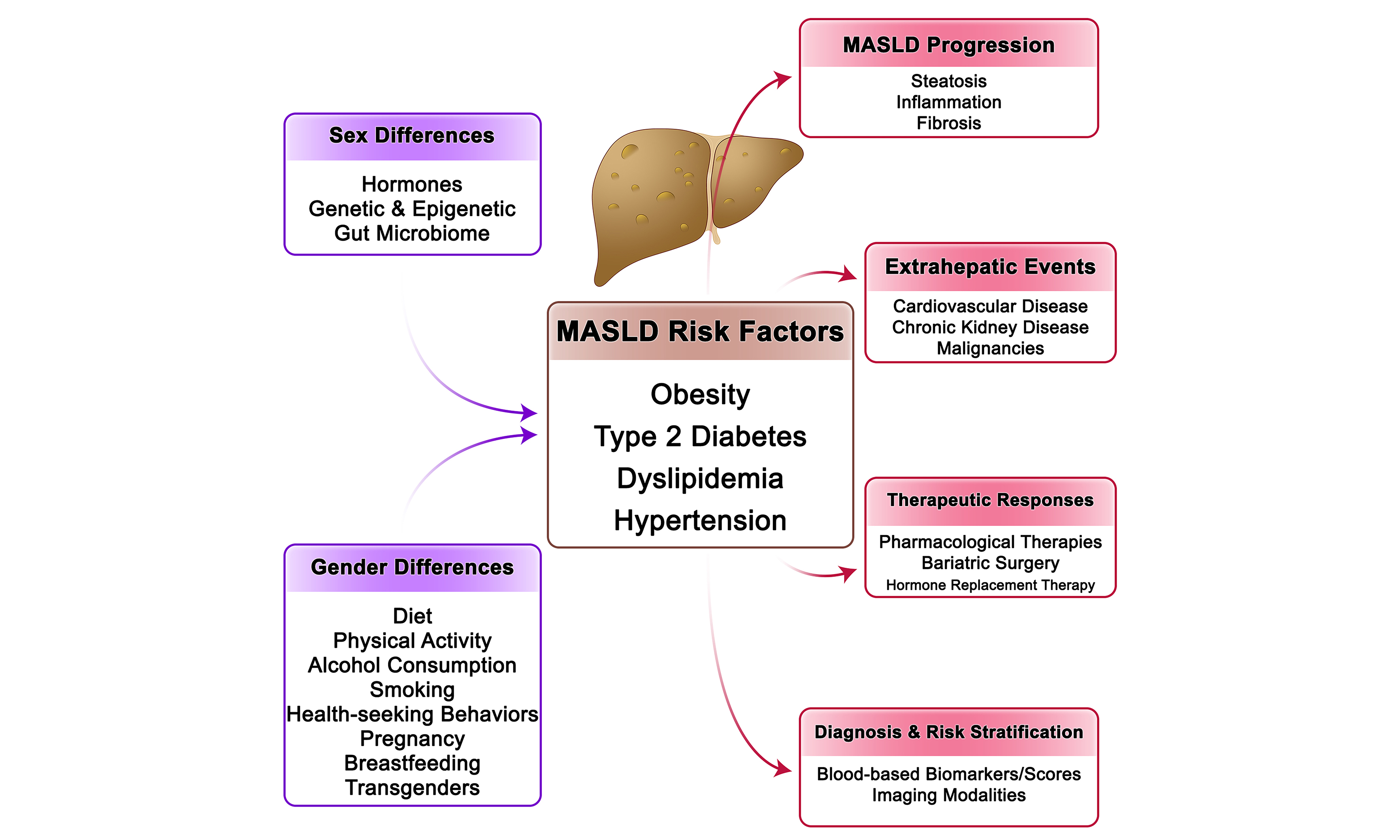
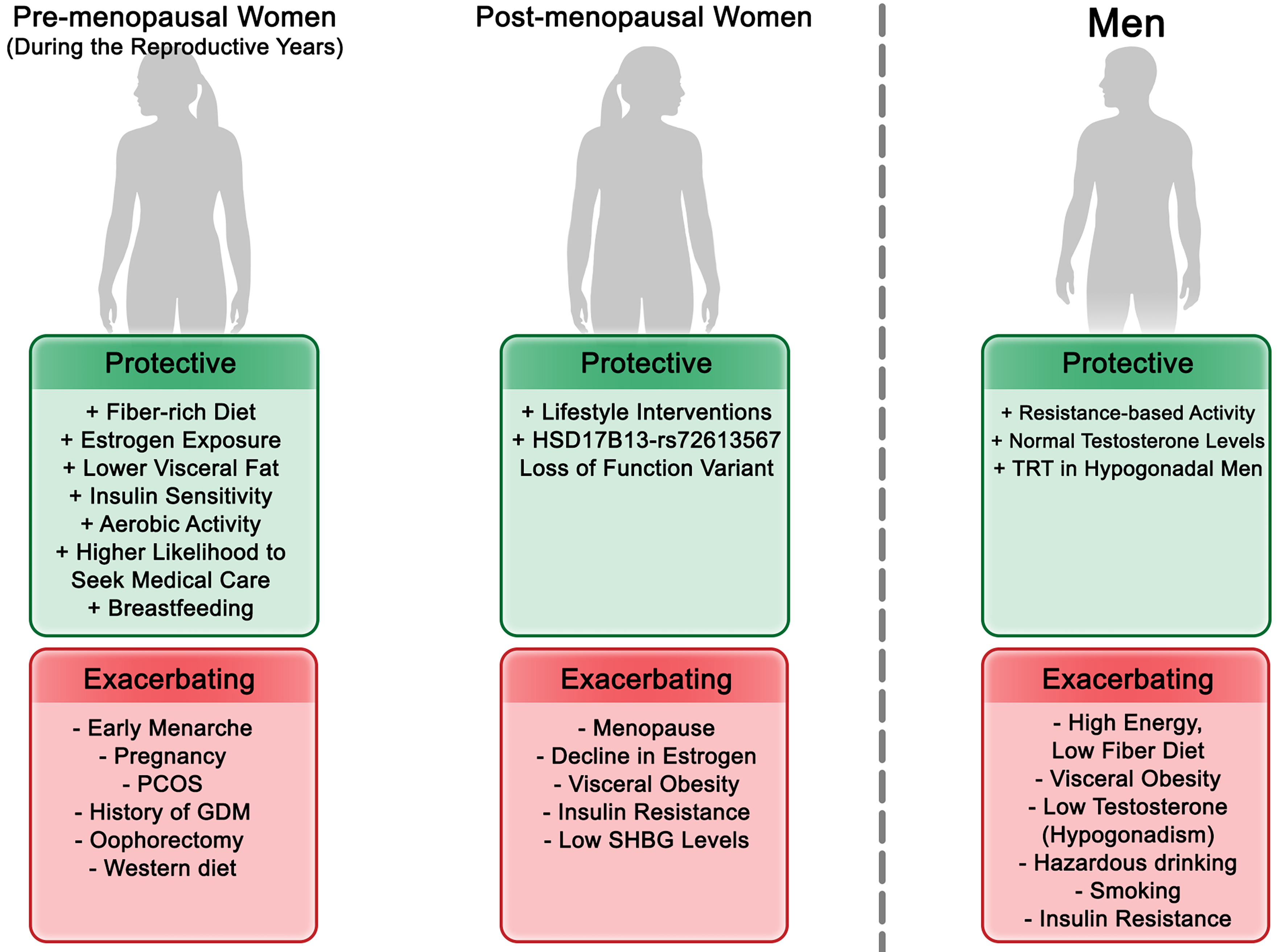
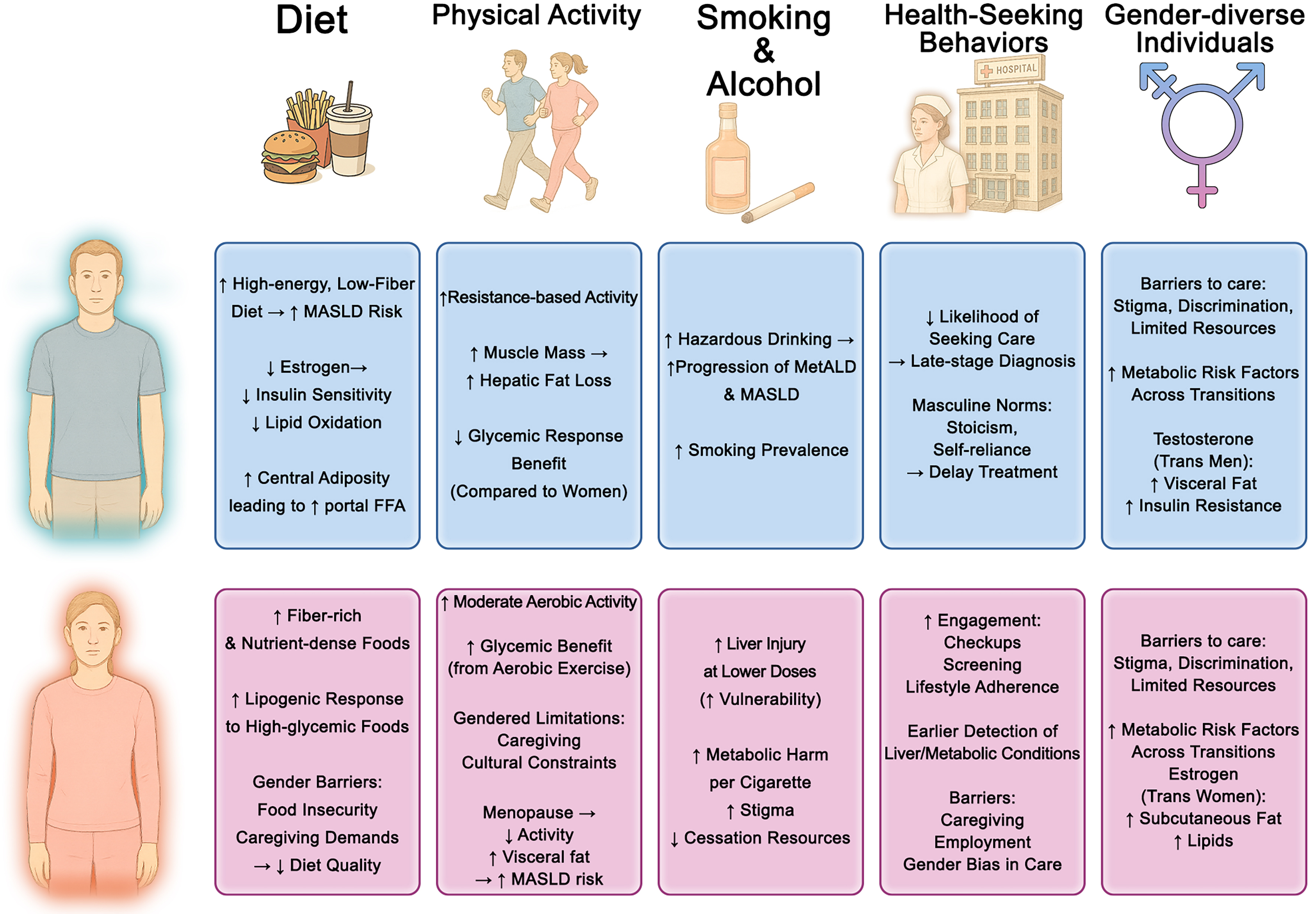
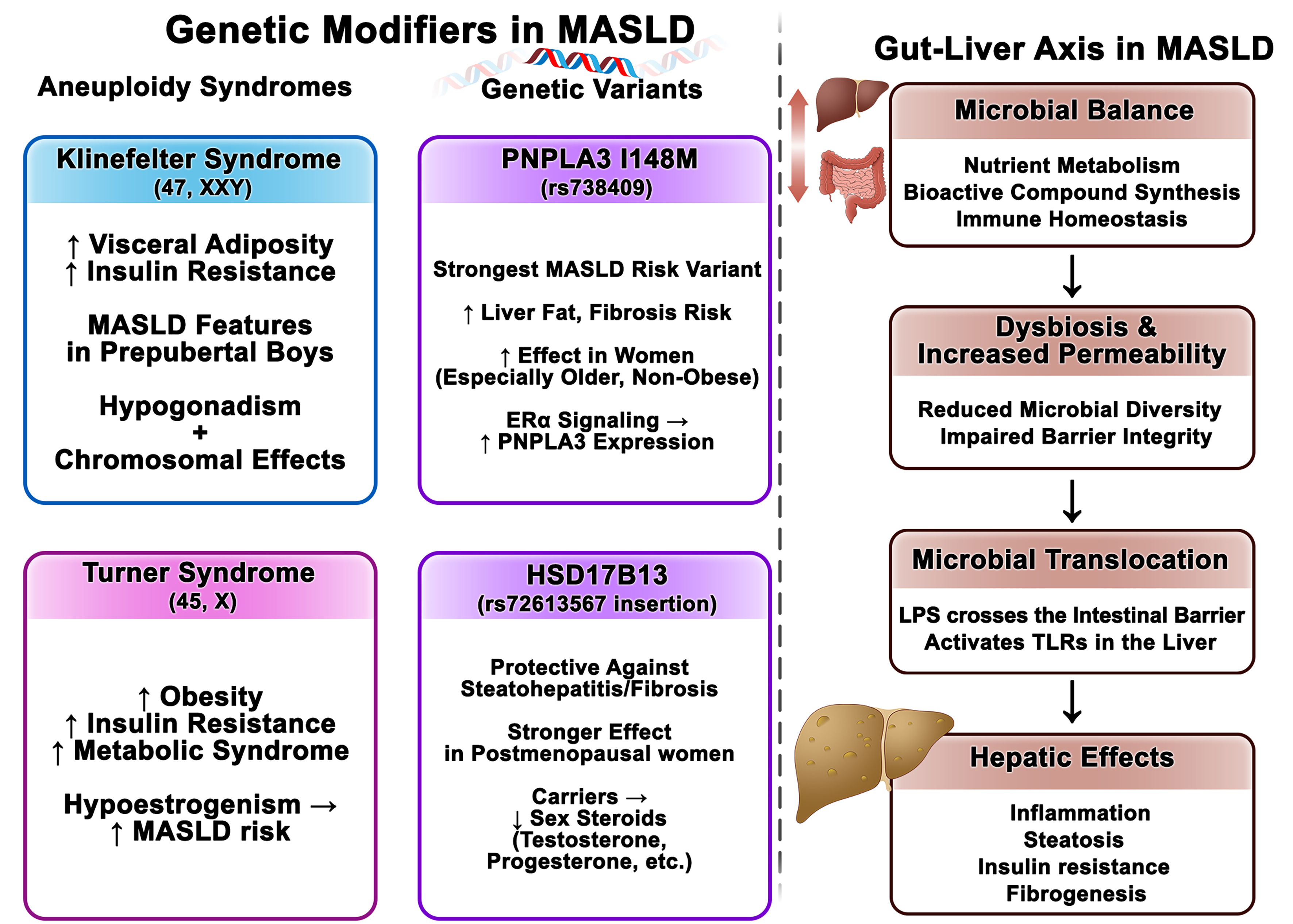
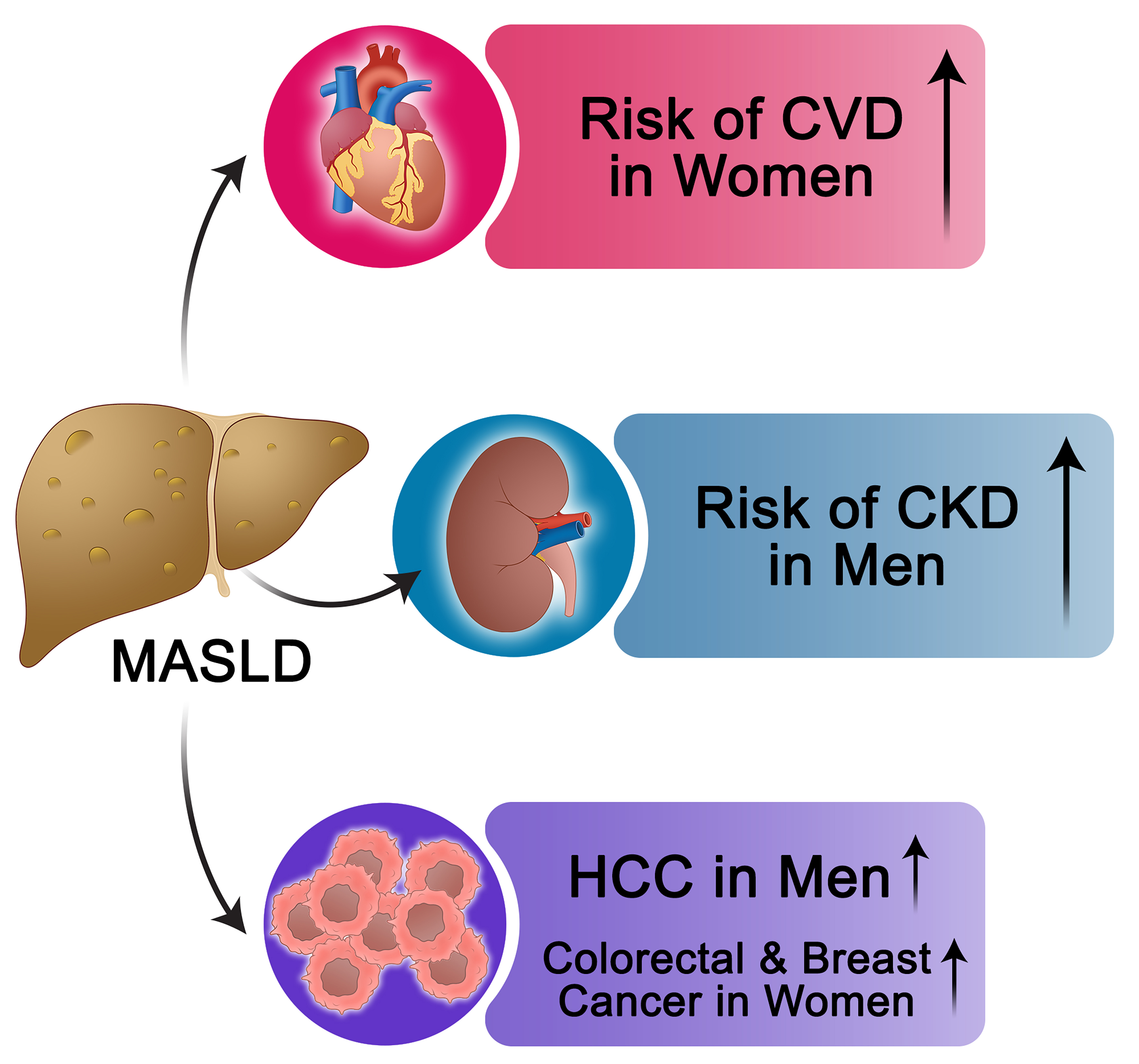
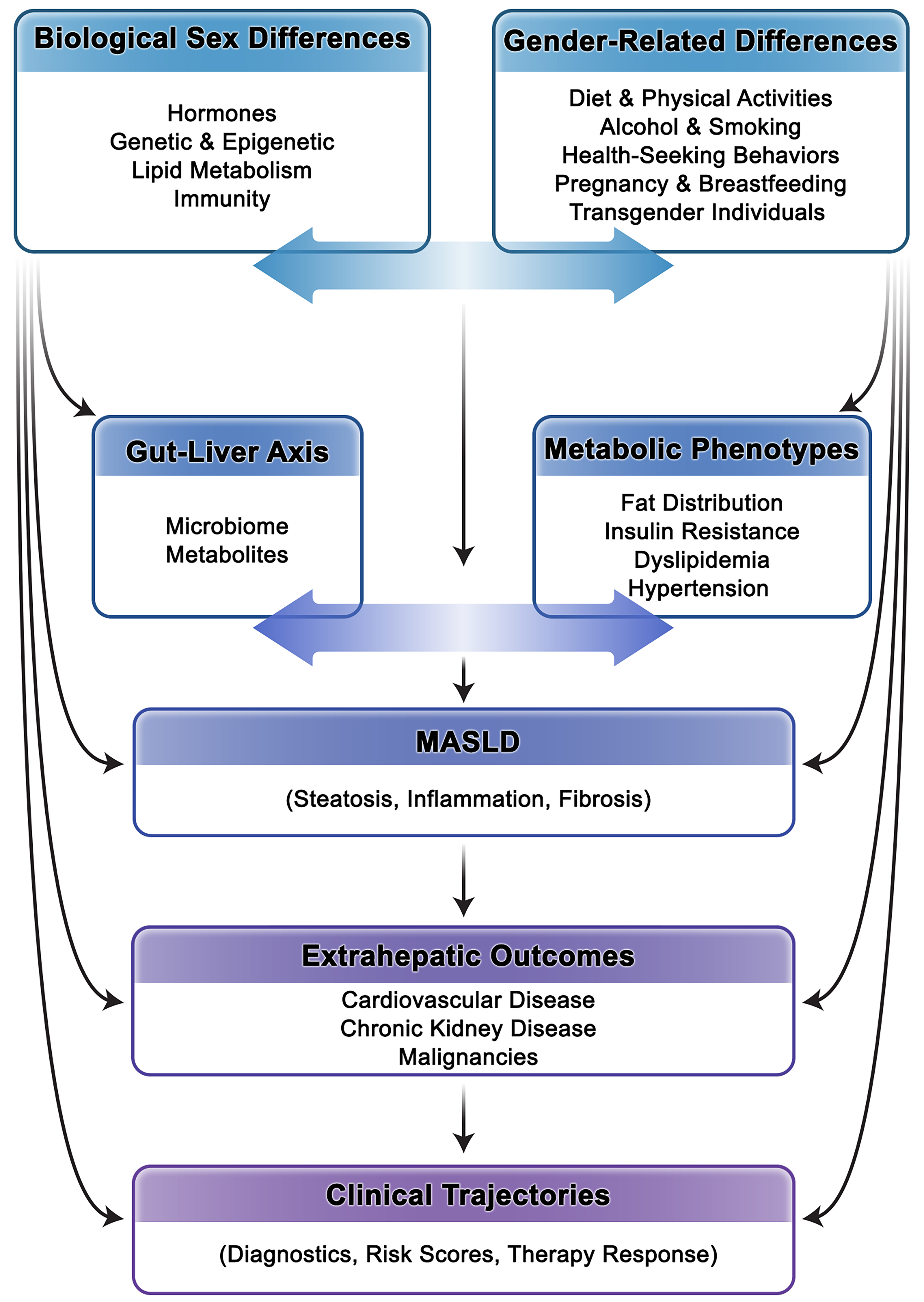












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].