Recent advances of conversion-type iron-based materials for sodium-ion batteries
Abstract
Lithium-ion batteries have come to dominate the secondary energy storage market; however, their broader application is limited by the scarcity of lithium resources and high production costs. As a promising alternative, sodium-ion batteries (SIBs) have attracted significant attention due to their similar electrochemical behavior and the abundant availability of sodium-based raw materials. It is well recognized that the electrode material plays a crucial role as it directly influences the overall cycle life of the battery. Iron-based materials are particularly attractive due to their abundant raw material availability, cost-effectiveness, safety profile, and environmental friendliness; thus, they represent one of the most suitable classes of electrode materials. Recently, many studies have focused on designing appropriate nanostructures and developing straightforward methods for improving the electrochemical features of conversion-type iron-based electrodes. This review summarizes recent advancements in iron-based electrodes for SIBs and outlines future directions for the advancement of conversion-type iron-based materials. It is expected to provide valuable insights for the design of high-performance iron-based electrodes for SIBs.
Keywords
INTRODUCTION
The growing popularity of low-carbon and environmentally friendly lifestyles has led to increasing demand for highly efficient energy storage devices. Currently, lithium-ion batteries (LIBs) are widely utilized in mobile phones, laptops, and other portable electronic devices due to their long service life, high operating voltage, and high energy density[1-3]. With the continuous enhancement of cycle performance and safety features, LIBs have demonstrated significant potential across various fields, including aerospace, electromobility, and large-scale electrochemical energy storage systems[1-3]. As a result, improving the electrochemical properties of rechargeable batteries remains a central focus of current research. In practical applications, consumers primarily seek cost-effective batteries with long service lives. However, it is noteworthy that most lithium salts required for LIB production are concentrated in plateau regions, where mining poses considerable challenges[4,5]. This reality makes it essential to identify alternative battery systems that offer abundant raw material availability, low cost, and favorable electrochemical properties. Among such candidates, sodium-ion batteries (SIBs) have emerged as a compelling option owing to the abundance of sodium resources, their lower cost, and electrochemical behavior similar to LIBs[6,7]. Research interest in SIBs has grown significantly in recent years. To optimize sodium utilization and further reduce battery costs, it is particularly crucial to identify low-cost electrode materials derived from abundant raw sources and enhance their electrochemical performance[8-10].
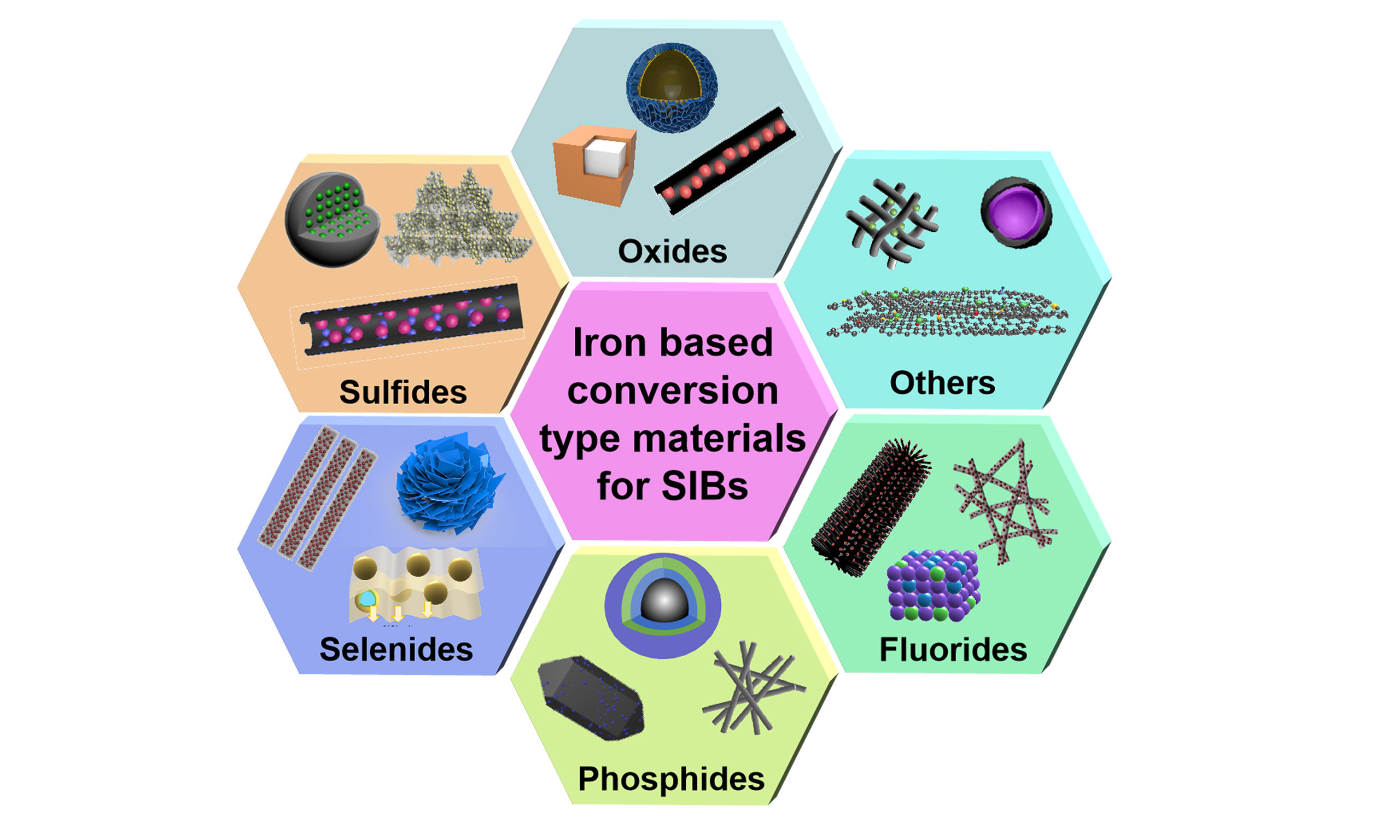
Iron is the second most abundant metal in the Earth's crust, second only to aluminum. Compared to currently commercialized anode and cathode materials, iron-based electrodes offer several advantages, including plentiful raw material availability, low environmental impact, cost-effectiveness, improved safety, and high theoretical specific capacity[11-14]. These characteristics make iron-based materials suitable for both anode and cathode use in secondary batteries. Most energy storage mechanisms in iron-based electrode materials are based on conversion reactions, which have garnered considerable attention among transition metal compounds[15-18]. To date, numerous iron-based materials have been explored, including oxides, sulfides, selenides, phosphides, and fluorides [Figure 1]. Most of them possess high theoretical capacities exceeding 600 mAh g-1[19-22]. Nevertheless, iron-based materials face inherent challenges, such as low electronic conductivity and sluggish ion diffusion, especially due to the large radius of Na+ ions, thus causing poor rate capability and pronounced voltage hysteresis. In addition, massive volume expansion and the agglomeration of Fe0 during charging/discharging compromise the structural integrity of the electrodes, resulting in poor cycle stability. To address these issues, researchers have developed various strategies, including nanostructure design, conductive coating or compositing, hierarchical porous structures, and electrolyte optimization[19-22]. For instance, Zhao et al. synthesized Fe3O4/PPy nanospheres via a hydrothermal method followed by ultrasonication-assisted polymerization[11]. The conductive polypyrrole (PPy) coating improved the conductivity, while the hollow porous structure enhanced structural stability[11]. Hou et al. In another example, FeP@C arrays supported on a conductive reduced graphene oxide network through a simple solution-phase reaction and gas-phase phosphidation[15]. These FeP@C arrays effectively mitigated volumetric strain, while the carbon coating enhanced electrical conductivity and prevented particle agglomeration during cycling[15]. Thus, the rational design of nanostructures and the development of straightforward synthesis methods are crucial to improving the electrochemical performance of conversion-type iron-based electrodes. This manuscript summarizes recent progress in iron-based electrodes for SIBs and outlines future research directions. It is hoped that this review could provide valuable insights for the development of high-performance iron-based electrodes in SIBs.
RECENT ADVANCES IN IRON-BASED ELECTRODES
Compared to the currently available cathodes/anodes for SIBs, iron-based electrodes offer several advantages: they are derived from plentiful resources, produce no pollution, are environmentally friendly, have low costs, ensure good safety profiles, and possess high theoretical specific capacity[11,15-17]. Consequently, these materials can be utilized as both cathodes/anodes in secondary batteries. The Na+ storage mechanism of iron-based electrodes is mostly classified as conversion-type.
Iron-based oxides
In recent years, iron-based oxides have gradually become the most promising electrodes due to their ultrahigh theoretical capacities and abundant natural availability[11,17-21]. These oxides include FeO, Fe3O4, and Fe2O3, with Fe2O3 (theoretical specific capacity ~1,008 mA h g-1) and Fe3O4 (926 mAh g-1) being the most extensively studied[11,17]. The sodium-ion storage mechanism of Fe2O3 in SIBs is as follows[21-24]:
However, iron oxide electrodes suffer from several inherent shortcomings: (1) the generation of electrochemically inactive Na2O during cycling severely degrades cycle stability; (2) the intrinsic low conductivity leads to sluggish ion diffusion, poor rate capability, and significant voltage hysteresis; (3) massive volume expansion and Fe0 nanoparticle agglomeration during cycling cause structural damage and rapid capacity decay[17,22-24].
To overcome these challenges, researchers have designed various nanostructures and incorporated conductive coatings on iron-based materials to enhance electronic conductivity and structural integrity[15,17]. For instance, Li et al. designed a hierarchical Fe2O3@MIL-101(Fe)/C anode via a MOF-derived method[18]. The hierarchical nanostructure increases the surface areas, facilitating electrolyte infiltration and shortening diffusion lengths for both Na+ ions and electrons[18]. Furthermore, the intrinsic hollow architecture helps mitigate volume changes during Na+ insertion/extraction. As a result, Fe2O3@MIL-101(Fe)/C exhibits excellent cycling stability, delivering a capacity of 662 mAh g-1 over 200 cycles at 200 mA g-1 with 93.2% capacity retention[18].
Graphene, known for its ultrahigh surface area and exceptional electronic conductivity, is also a highly promising component in electrode design. Li et al. synthesized Fe2O3 nanoparticles embedded in N-doped graphene with internal micro-channels (Fe2O3@N-GIMC)[19]. The interconnected porous structure offers abundant active sites and efficient ion pathways, while also mitigating nanoparticle aggregation and pulverization. Consequently, Fe2O3@N-GIMC exhibits outstanding Na+ storage performance, achieving a capacity of 308.9 mAh g-1 over 1,000 cycles and 200.8 mAh g-1 over 4,000 cycles at 1 A g-1[19].
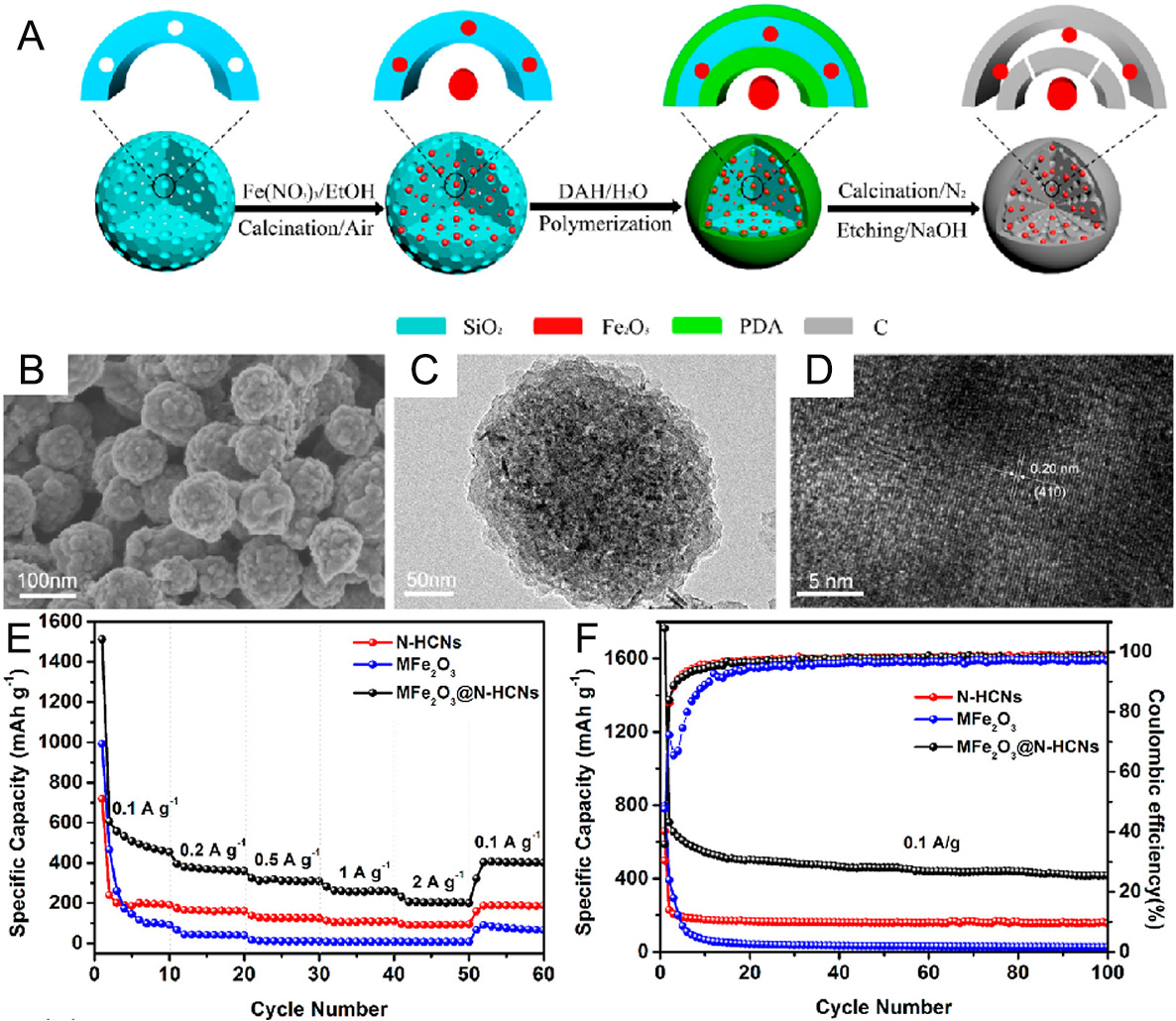
To alleviate the volumetric strain and enhance the electronic conductivity of Fe2O3 anodes, Chen et al. encapsulated a Fe2O3 core in N-doped carbon nanosphere shells (MFe2O3@N-HCNs) through a simple confined impregnation crystallization method [Figure 2A][20]. The as-obtained MFe2O3@N-HCNs not only possess a connected hierarchical structure that shortens the diffusion length, but also effectively accommodate the significant volumetric stress during Na+ insertion/extraction [Figure 2B and C]. Figure 2D shows a lattice spacing of 0.20 nm, which corresponds to the (410) crystal plane of Fe2O3. As a result, the
Figure 2. (A) Schematic diagram of the preparation process of MFe2O3@N-HCNs; (B) SEM, (C) TEM, and (D) HRTEM images of
Reduced graphene oxide (rGO) offers significant electronic conductivity and a high surface area, making it a promising composite material. Li et al. synthesized a composite of amorphous Fe2O3/graphene nanosheets (Fe2O3@GNS) via a simple procedure[21]. They found that amorphous ultrafine Fe2O3 particles (~5 nm) were uniformly riveted onto the GNS via strong oxygen-bridge (C-O-Fe) bonds. This composite anode exhibited superior Na+ storage performance compared to well-crystallized Fe2O3. Specifically, theFe2O3@GNS anode delivered a capacity of 440 mAh g-1 at 0.1 A g-1, yielding an initial Coulombic efficiency (ICE) of 81.2%. Even at a high current density of 2 A g-1, it retained a capacity of 219 mAh g-1. The strong interactions between GNS and Fe2O3 not only provided abundant active sites and facilitated Na+/electron diffusion but also mitigated volume changes during discharging/charging[21]. Furthermore, Li et al. prepared single-crystal
Except for Fe2O3, Fe3O4 possesses a high theoretical capacity of 926 mAh g-1, along with advantages such as safety, non-toxicity, environmental friendliness, abundant availability, and low cost. These properties have attracted significant attention to Fe3O4 as a promising anode material for SIBs[26-30]. The Na+ storage mechanism of Fe3O4 proceeds as follows[26-30]:
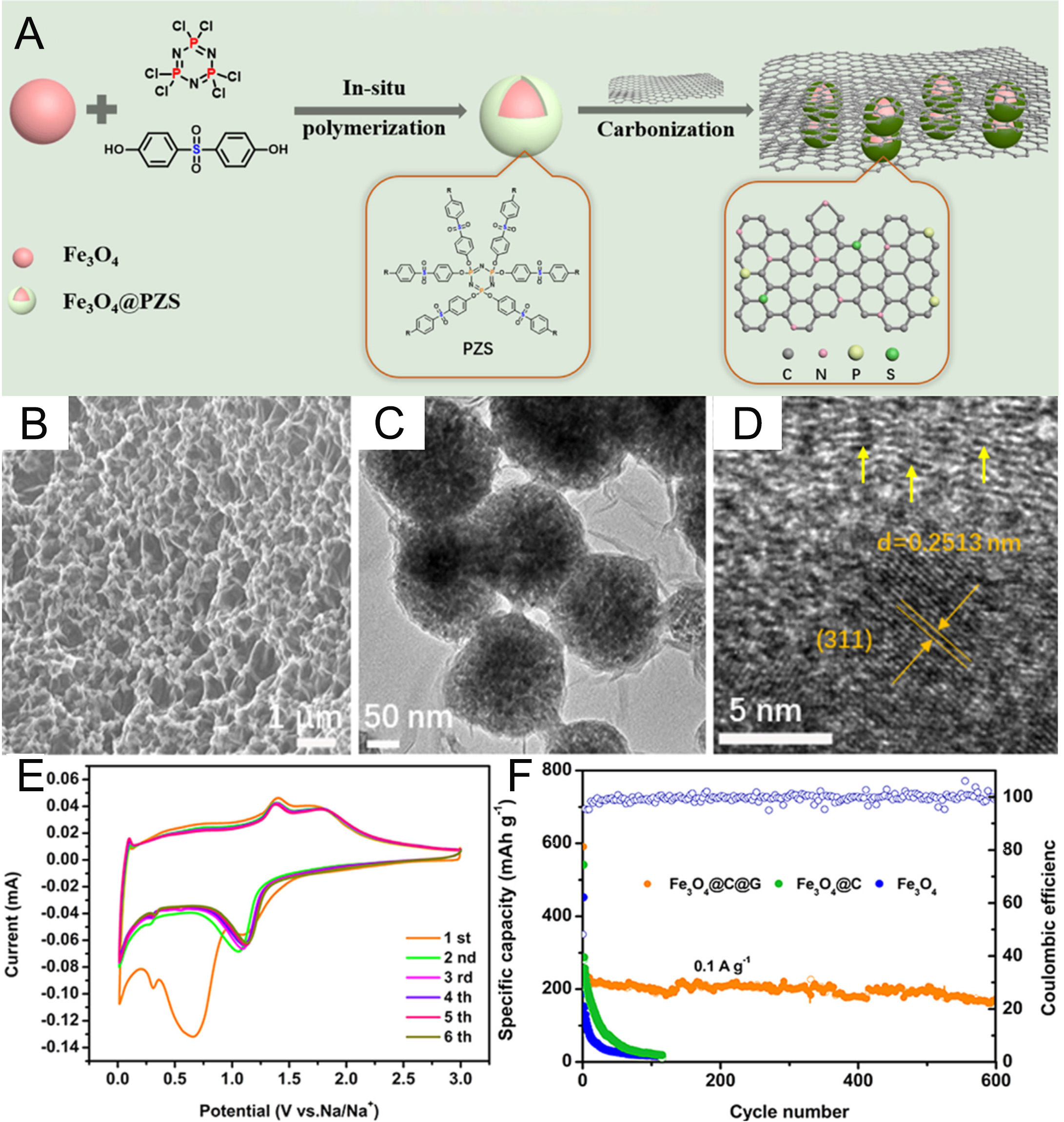
However, similar to other transition metal oxides, Fe3O4 suffers from substantial volume expansion during cycling. This volume change causes structural degradation and results in poor cycle stability, thereby limiting its practical application[27-31]. To address this challenge, Liu et al. synthesized a composite of graphene and Fe3O4 nanodots through a simple hydrothermal procedure and applied it as an anode material for SIBs[26]. This unique hierarchical structure provides a large solid electrolyte interphase (SEI) that facilitates rapid Na+/electron diffusion, mitigates volumetric strain, and prevents aggregation of nano-Fe3O4 during discharging/charging. Consequently, the graphene-Fe3O4 quantum dot composite achieved a high specific capacity of 525 mAh g-1 at 0.03 A g-1 and exhibited excellent cycling stability, retaining 312 mAh g-1 after 200 cycles at 50 mA g-1[26]. To further overcome the low conductivity of Fe3O4, Tao et al. developed an N/S/P-doped dual carbon-modified Fe3O4 nanoparticle (Fe3O4@C@G)[27]. As illustrated in Figure 3A, the
Figure 3. (A) Schematic illustration of the preparation of Fe3O4@C@G. (B) SEM, (C) TEM, and (D) HRTEM images of Fe3O4@C@G. (E) CV curves of Fe3O4@C@G; (F) Comparison of the cycling performance of Fe3O4, Fe3O4@C, and Fe3O4@C@G at 0.1 A g-1[27]. Copyright 2020, Elsevier.
Conventional carbon coating strategies primarily enhance electrical conductivity and mitigate volume expansion at the macro scale. In contrast, metal-organic framework (MOF)-derived approaches have emerged as effective nanoscale engineering methods, enabling the in situ encapsulation of metal oxide quantum dots during pyrolysis[29]. Qi et al. developed Fe3O4 quantum dots embedded in a raspberry-like carbon framework (Fe3O4 QD@C-GN) using iron-based MIL-88-Fe-NH2 as a template[29]. When tested within the voltage range of 0.05~3.0 V, Fe3O4 QD@C-GN exhibited capacities of 343, 234, and 149 mAh g-1 at 2, 5 and 10 A g-1, respectively, over 1,000 cycles[29].
To address the issues of poor electronic conductivity and structural degradation induced by the high volume stress during cycling, Qin et al. creatively introduced a magnetic stimulus to increase pressure within the sample chamber. This approach facilitated the uniform deposition of red P particles onto a chain-like Fe3O4/C composite, forming Fe3O4/C/P. When used as an anode material and cycled within a voltage range of 0.01~3 V, the Fe3O4/C/P structure exhibited excellent cycling stability and outstanding rate capability[30]. This magnetic field-assisted strategy effectively bridges the synthesis method with enhancements in electrochemical properties. Huang et al. encapsulated Fe3O4 in N-doped carbon nanofibers (CFs) through an electrospinning strategy followed by high-temperature calcination. The resulting three-dimensional (3D) interconnected CF network enhanced overall electronic conductivity, while N-doping introduced additional sites for Na+ storage. Hence, the optimized Fe3O4-CFs achieved a considerable capacity of 358.1 mAh g-1 over 200 cycles at 0.5 A g-1[31].
Although structural regulation, composite modification, and surface engineering have markedly improved the electrochemical performance of iron-based oxides for SIBs, further enhancements are still required to meet the demands of practical applications.
Iron-based sulfides
Compared to Na2O, Na2S exhibits higher electrochemical reactivity, and iron-based sulfide materials have also attracted considerable attention[20,31-34]. These materials offer relatively high theoretical capacities and are composed of naturally abundant, non-toxic and cost-effective elements (e.g., Fe and S)[35-39]. To date, a series of iron-based sulfides have been explored. Among them, FeS2 has received particular attention due to its ultrahigh capacity of 894 mAh g-1 resulting from a four-electron/Na+ transfer per FeS2 unit. Kitajou et al. investigated the discharge/charge mechanism of FeS2 using synchrotron-based X-ray adsorption near-edge structure (XANES), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD)[32]. The Na+ storage process in FeS2 proceeds as follows[32-35]:
The main contributors to the irreversible capacity are (i) the dissolution of S22- ions during charging/discharging; and (ii) the inevitable formation of a SEI layer, both of which result in low ICE[36-40]. To address these challenges, strategies such as nanochemistry, structural regulation, and surface modification have proven effective[39-42]. For instance, Li et al. demonstrated through ex situ TEM measurements that only a portion of FeS2 actively participates in the conversion reaction, while the formation of “inactive cores” leads to low capacity[33]. Moreover, the sluggish diffusion kinetics induced by the relatively larger Na+ ionic radius exacerbate electrode pulverization, thereby causing rapid capacity decay[33]. Zhang et al. developed mesoporous FeS2 nanorods via a scalable synthesis method[34]. Due to their unique one-dimensional porous structure and excellent strain relaxation capabilities, these nanorod anodes exhibited outstanding Na+ storage performance, maintaining a reversible capacity of 711.1 mAh g-1 over 450 cycles at 1 A g-1[34].
Despite the high theoretical capacity of FeS2, its practical application remains limited due to severe volume fluctuations during charging/discharging and the formation of soluble polysulfides at low voltages (< 0.8 V vs. Na/Na+). Kandula et al. designed N-doped carbon-coated FeS2 nanoparticles wrapped in N/S co-doped graphene/single-walled carbon nanotubes (FSCGS) via hydrothermal sulfuration[35]. When used as an anode for SIBs, the FSCGS composite achieved a superior rate capability (476 mAh g-1 at 10.0 A g-1). Furthermore, it demonstrated excellent stability with a capacity retention of 91.3% over 200 cycles at 0.5 A g-1[35]. The superior performance is attributed to the enhanced electron conductivity and electrochemical reactivity provided by the dual-carbon-modified hierarchical structure. Ma et al. designed a core-shell hollow sphere FeS2 (CHS-FeS2) using MIL-88B(Fe) as a template[36]. Its unique porous structure provides ample void space to buffer volume strain and promote Na+ diffusion. As a result, the CHS-FeS2 anode maintained a capacity of 546.5 mAh g-1 over 100 cycles at 0.5 A g-1 and retained 224 mAh g-1 even at 20 A g-1, reflecting its remarkable Na+ storage performance[36]. Lu et al. embedded ultrafine FeS2 particles into N/S co-doped CFs, along with FeS2 nanoflakes, forming a composite (FeS2@CF-NS) via electrospinning followed by annealing[37]. This unique architecture effectively reduces the Na+ diffusion path, accelerates
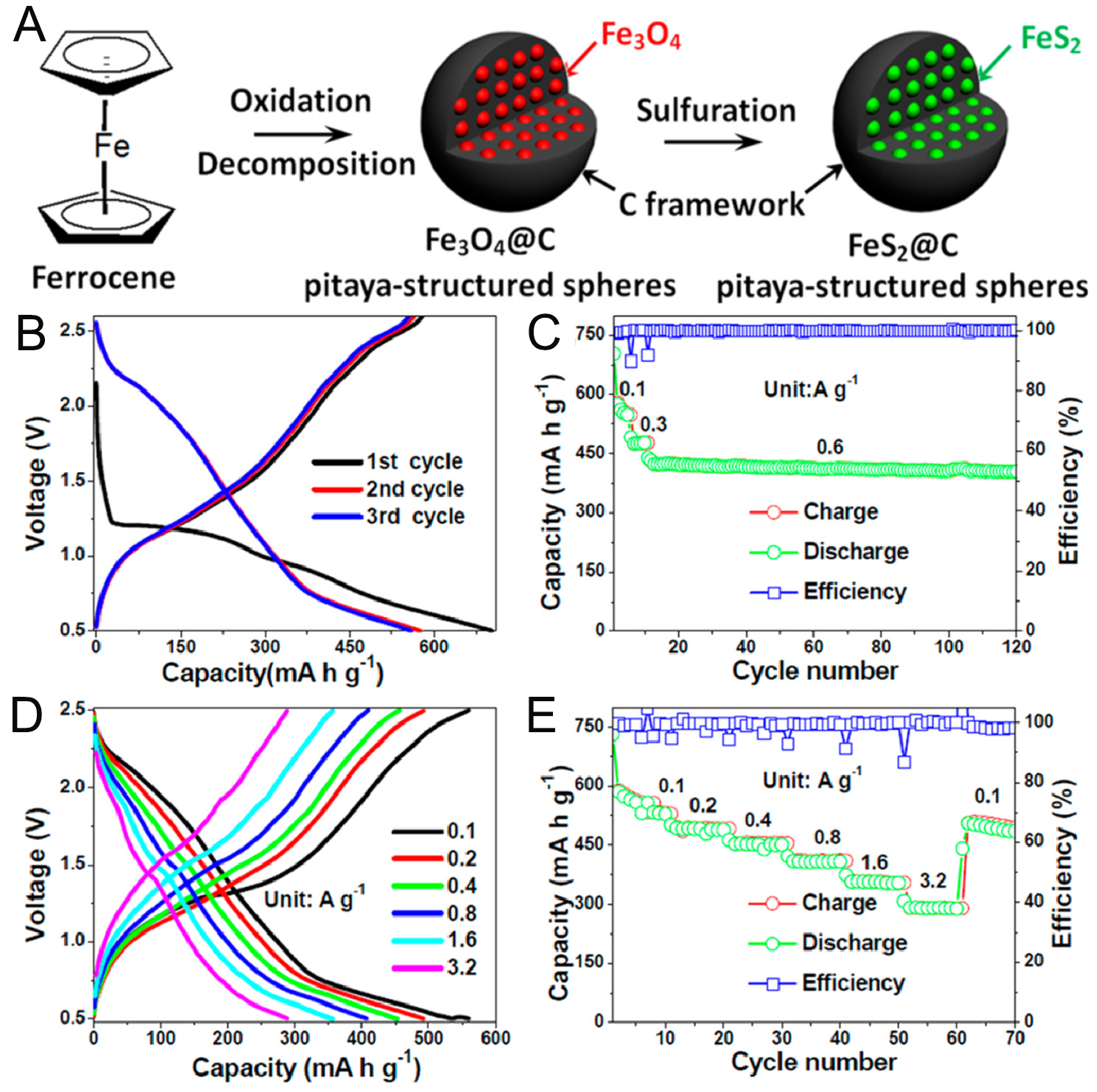
Furthermore, Xu’s group successfully prepared pitaya-like FeS2@C via a one-step in-situ encapsulation and hydrothermal sulfuration strategy, with ferrocene serving as both the iron and carbon source
Figure 4. (A) Schematic illustration of the preparation process for pitaya-structured FeS2@C. (B-E) electrochemical performance of FeS2@C: (B) initial three charge/discharge profiles, (C) cycling performance at 0.6 A g-1, (D) charge/discharge profiles from 0.1, 0.2, 0.4, 0.8, 1.6 to 3.2 A g-1, respectively, (E) rate performance[41]. Copyright 2017, American Chemical Society.
Chen et al. encapsulated graphene-coated FeS2 in carbon nanofibers using an electrospinning strategy and employed it as an anode for SIBs[42]. The double carbon modification improved electrical conductivity, thereby improving the structural reversibility of FeS2 during desodiation/sodiation; thus, the material maintained a high capacity of 305.5 mAh g-1 over 2,450 cycles at 3 A g-1. Owing to the markedly enhanced conductivity of the graphene-encapsulated FeS2 nanoparticles, the composite also exhibited excellent temperature tolerance, retaining stable capacity performance at 0 °C and -20 °C. When paired with
FeS also possesses a high theoretical specific capacity (~609 mAh g-1) and has been widely explored as an anode material for SIBs[43,44]. Its Na+ storage mechanism involves a two-step conversion reaction, as described below[45-49]:
FeS also suffers from unsatisfactory electrical conductivity and significant volumetric strain leading to rapid capacity decay and poor rate performance[43,49]. To address these issues, Cho et al. fabricated yolk-shell structured FeS/C composites via a vapor-pressure-induced synthesis route[43]. The ultrathin carbon coatings on the FeS nanosheets effectively improved their electrical conductivity and mitigated the volumetric strain during sodiation. As a result, the FeS/C yolk-shell nanosheets delivered a reversible capacity of
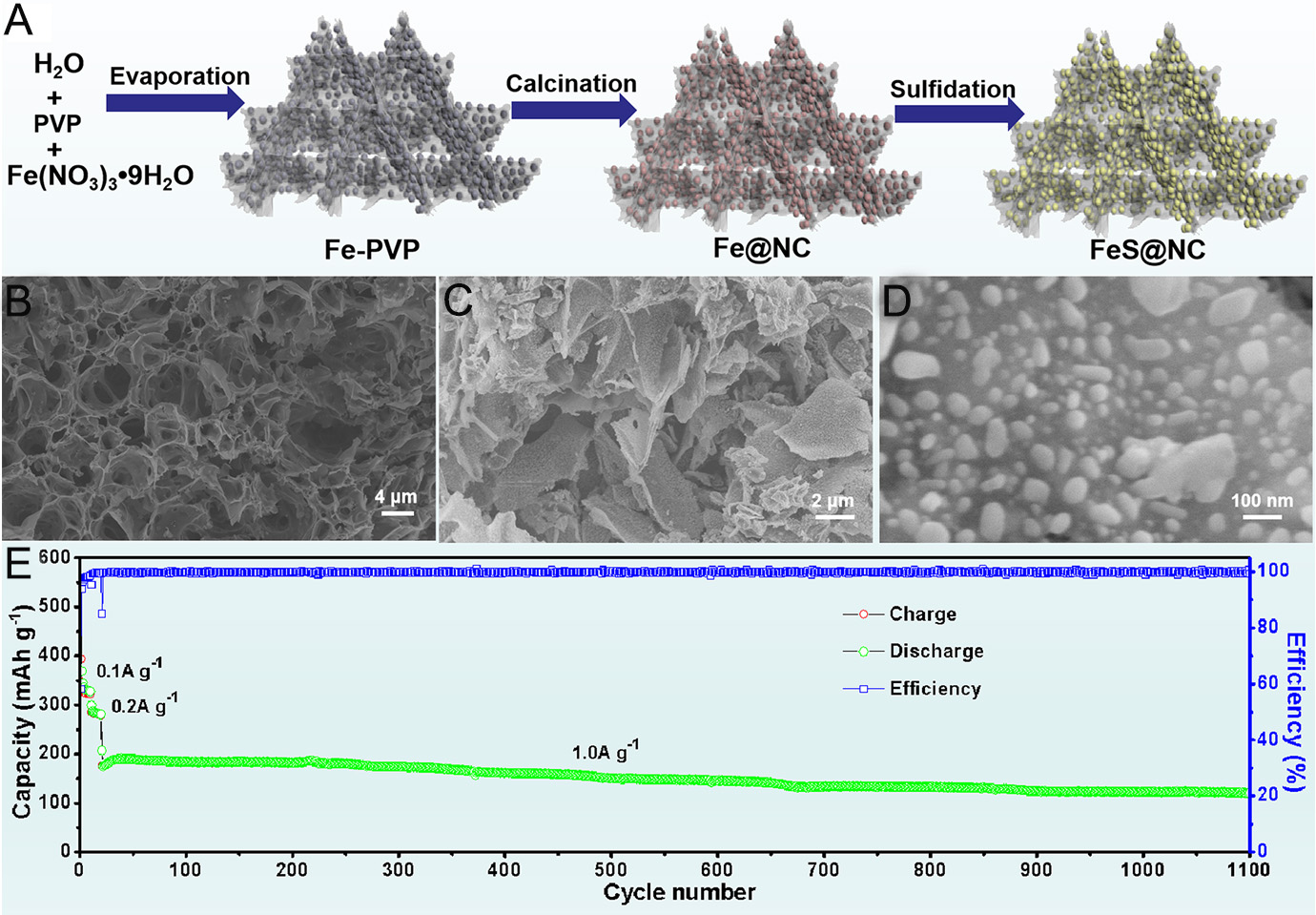
The practical application of FeS anodes is hindered by their poor electronic conductivity and significant volume changes during cycling. Yuan et al. synthesized ultrafine FeS nanoparticles encapsulated in three-dimensional (3D) NC nanosheets using a facile sol-gel approach followed by a solid-state sulfidation process [Figure 5A][47]. As displayed in Figure 5B, the sol-gel-derived precursor forms a 3D cross-linked carbon framework upon thermal treatment. After sulfidation, FeS nanoparticles are uniformly embedded in the carbon nanosheets [Figure 5C and D]. When used as an anode material for SIBs, the 3D FeS@NC composite delivered a stable capacity of 254 mAh g-1 over 1,100 cycles at 1.5 A g-1 [Figure 5E][47]. These remarkable electrochemical features are associated with the 3D porous nanosheet structure, which dramatically enhances electronic conductivity, suppresses the pulverization and aggregation of FeS particles, immobilizes dissolved polysulfides, increases the electrolyte-electrode contact area, and provides abundant active sites for Na+ storage.
Figure 5. (A) Schematic of the preparation of FeS@NC. (B) SEM image of Fe@NC; (C and D) SEM images of FeS@NC, (E) long-term cycling performance of FeS@NC at 1.0 A g-1[47]. Copyright 2022, Elsevier.
The incompatibility between electrodes and electrolytes significantly limits the electrochemical performance of iron-based sulfides. Poor interfacial compatibility leads to unstable solid electrolyte interphase (SEI), resulting in persistent irreversible side reactions that consume active Na+ ions. On account of these,
Considering the challenges posed by dissolved polysulfides and significant volume expansion, Huang et al. synthesized a bubble film-like FeS/C composite using Fe(NO3)3·9H2O, sulfur, and polyvinylpyrrolidone as precursors, followed by freeze-drying and carbonization[49]. The bubble film-like FeS/C exhibited reversible Na+ storage capacities of 455, 439, 405, 318, 256, and 219 mA h g-1 at current densities ranging from 0.1 to 5.0 A g-1, respectively[49]. The superior electrochemical performance is mainly attributed to the porous bubble film-like structure, which effectively suppresses the agglomeration of active nanoparticles, thereby enhancing cycling stability. Additionally, the porous carbon framework improves overall electrical conductivity, enabling excellent rate capability and high capacity retention. Moreover, the porous structure provides ample void space that mitigates volume expansion and maintains the structural integrity during charging/discharging.
Iron-based selenides
The SEI formed during electrochemical reactions can hinder the transport of Na+ ions, resulting in the low practical capacity of oxide-based anodes for SIBs[48-61]. As for sulfides, the formation of soluble polysulfide intermediates induces a “shuttle effect”, which aggravates capacity decay[50-54]. In contrast, metal selenides exhibit relatively high electronic conductivity and energy conversion efficiency, making them promising electrode materials for SIBs[55-59]. Among the various selenides studied to date, FeSe2 and Fe7Se8 are two of the most representative anode materials[58-61]. The Na-ion storage mechanism of FeSe2 is as follows[58-63]:
However, the sluggish diffusion kinetics and severe volume expansion during cycling lead to rapid capacity fading, hampering the practical application of FeSe2 in SIBs. Wei et al. synthesized nanorod-assembled FeSe2 clusters via a hydrothermal strategy and investigated their Na+ storage performance under various electrolyte conditions[50]. When paired with an ether-based electrolyte, the FeSe2 clusters demonstrated superior electrochemical properties, delivering a high capacity of 515 mAh g-1 over 400 cycles at 1 A g-1 and a high ICE of 97.4%. Remarkably, they retained 128 mAh g-1 even at an ultrahigh current density of
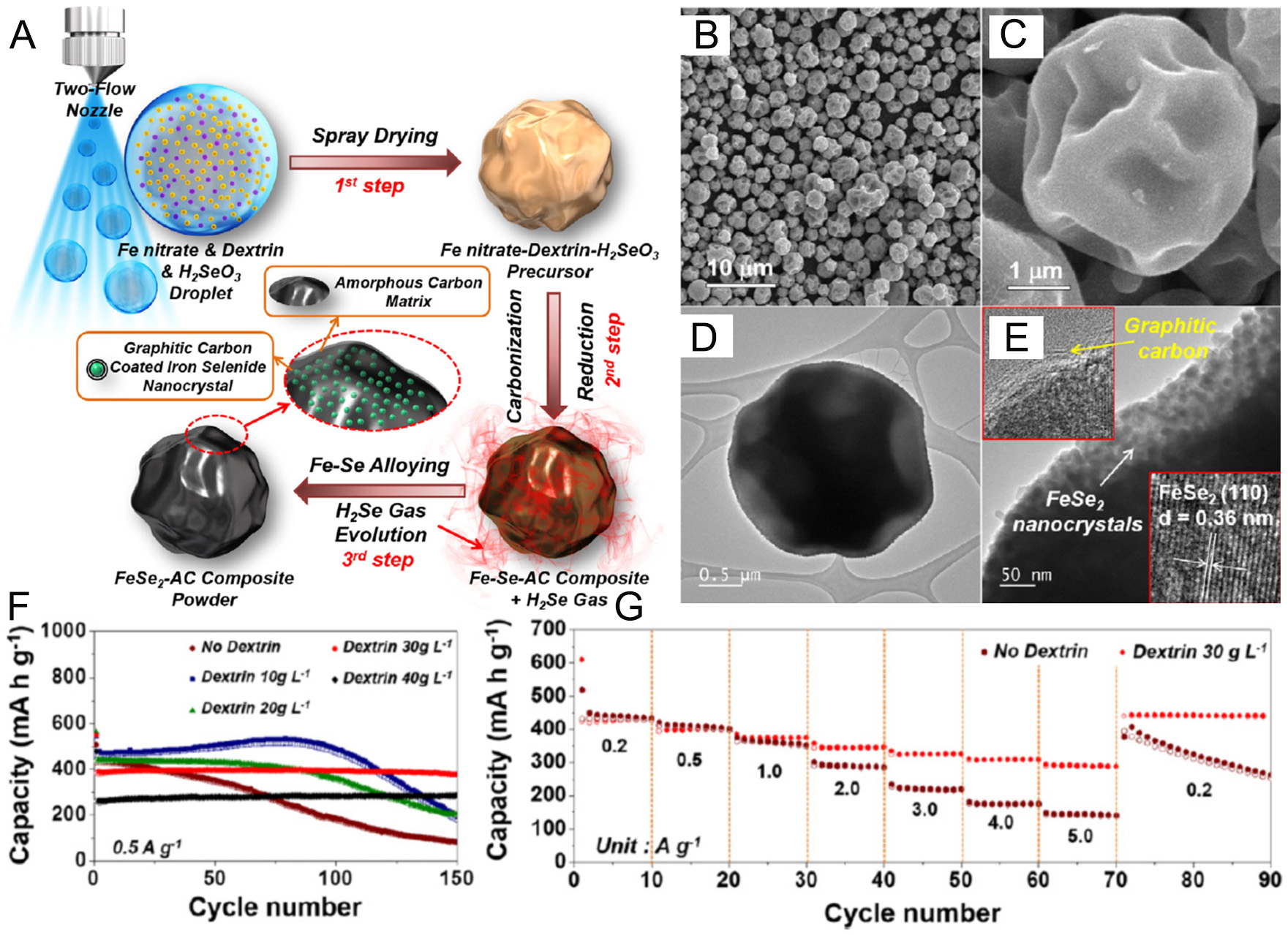
Figure 6. (A) Schematic illustration of the preparation process of the FeSe2-AC composite powder by the spray drying process,
Li et al. discovered that surface oxides significantly affect the electrochemical activity of FeSe2[53]. FeSe2 with a larger size and lower oxide content demonstrated superior properties compared to FeSe2/graphene composites with higher oxide content. By modulating the oxide content of the FeSe2/graphene anode, a specific capacity of 459 mAh g-1 was retained at 0.1 A g-1. This composite also displayed excellent rate capability, with only a 10% capacity loss when the current density increased from 0.1 to 5 A g-1. Remarkably, the FeSe2/graphene maintained a capacity of 227 mAh g-1 over 800 cycles even at 25 A g-1[53]. The surface oxide was found to transform into a sodiated shell with poor conductivity and high mechanical strength, which induced phase-transfer resistance. This resistance hindered the sodiation of the FeSe2 core and impeded the diffusion of Na+/electrons during subsequent sodiation processes. Zheng et al. reported that FeSe2@NC micro rods, consisting of a FeSe2 core and a NC shell with ample void space, were adopted as anodes for SIBs[53]. This ingeniously designed porous core-shell structure enhanced Na+/electron transport, prevented FeSe2 degradation from electrolyte exposure, and accommodated volume changes due to sufficient void space. As a result, the FeSe2@NC microrods achieved outstanding Na+ storage performance, maintaining a capacity of 411 mAh g-1 at 10.0 A g-1 and 401.3 mAh g-1 over 2,000 cycles at 5.0 A g-1[53].
To maintain structural integrity during cycling, Pan et al. proposed a FeSe2@NC composite anode. The strong interaction between FeSe2 nanorods and NC facilitates fast interfacial Na+/e- diffusion and enhances structural stability[54]. As a result, the FeSe2@NC electrode exhibited a capacity of 379.2 mA h g-1 at a high current density of 10 A g-1. When paired with a Na3V2(PO4)2F3@reduced graphene oxide cathode, the full cell achieved 167.2 mAh g-1 at 1 A g-1 over 1,000 cycles[54]. Zhou et al. synthesized a porous NC framework (NCF) embedded with FeSe2 (P-FeSe2/NCF) via a spray-drying and salt-template strategy[55]. This unique structure facilitates Na+/electron transport and alleviates large volume changes during cycling. As an anode for SIBs, P-FeSe2/NCF delivered 507 mAh g-1 over 200 cycles at 2 A g-1 and retained 386.7 mAh g-1 over 500 cycles at 10 A g-1[55]. Men et al. fabricated a CNT/FeSe2/C composite by encapsulating FeSe2/C within a carbon nanotube (CNT) framework through a facile wet-chemistry procedure followed by selenization[56]. The resulting three-dimensional network enhanced electronic/ionic transport while providing void space to accommodate volume expansion. The CNT/FeSe2/C composite exhibited excellent Na+ storage performance, delivering 546 mAh g-1 over 100 cycles at 0.1 A g-1. Even at a high mass loading of
The sodium storage mechanism of Fe7Se8 proceeds through the following electrochemical reactions during discharging[58-63]:
The corresponding charging reactions are as follows:
Tian et al. fabricated a monolithic architecture composed of radially aligned Fe7Se8 nanorod bundles (Fe7Se8 NRBs) using a facile self-template strategy followed by annealing[58]. The Fe7Se8 NRB anode delivered a specific capacity of 300 mAh g-1 at 0.5 A g-1 and retained 190 mAh g-1 over 8,000 cycles at a high current density of 20 A g-1, indicating prominent long-cycling stability. This feasible strategy highlights the potential of structural engineering to enhance the electrochemical performance of selenide-based materials[58].
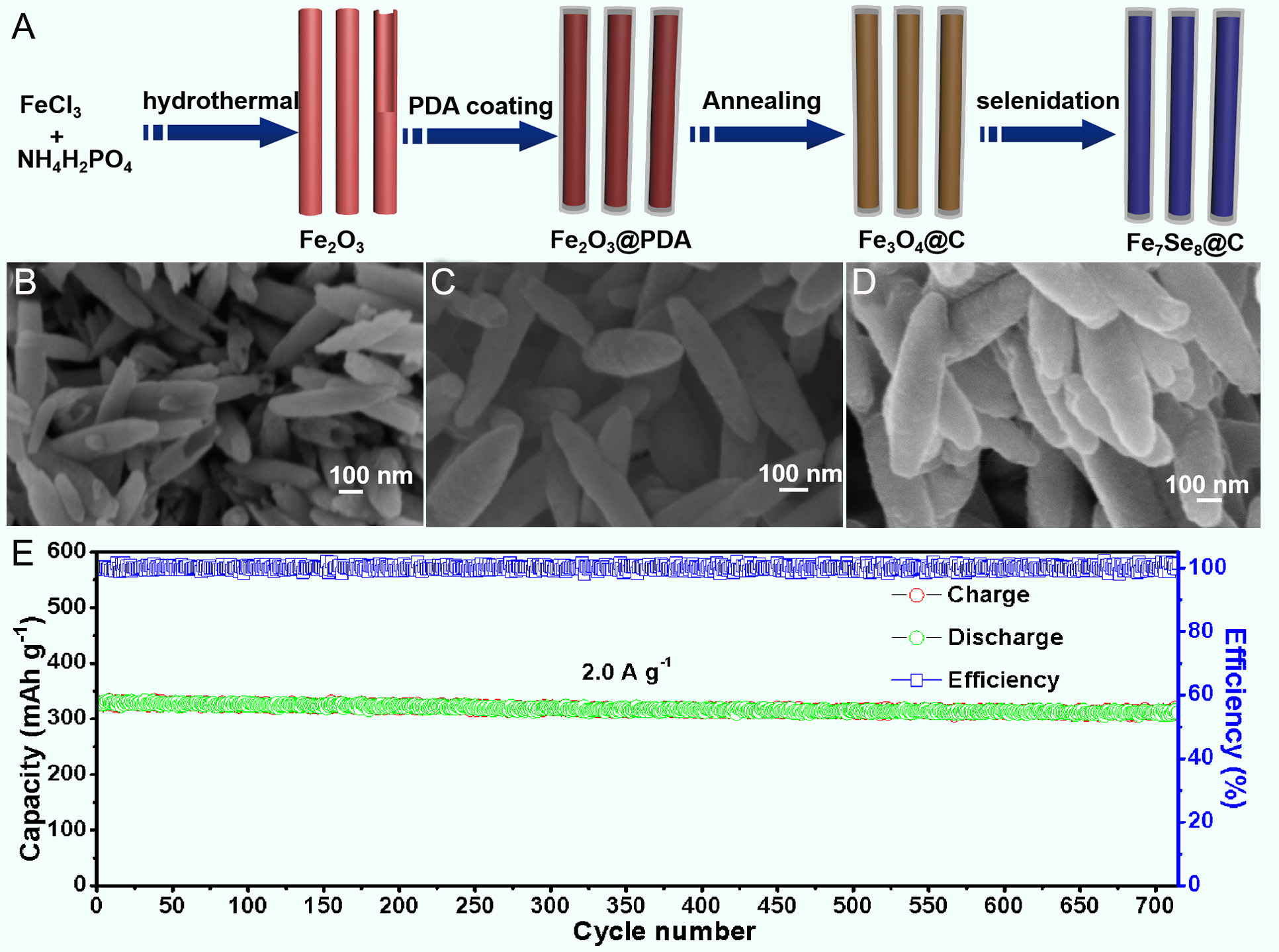
Figure 7. (A) Schematic illustration of the preparation process of Fe7Se8@C. SEM images of (B) Fe2O3, (C) Fe3O4@C, and (D) Fe7Se8@C. (E) Cycling performance of Fe7Se8@C at 2.0 A g-1[61]. Copyright 2022, Elsevier.
Yang et al. developed Fe7Se8@NC nanoboxes (Fe7Se8@NC NBs) by fully encapsulating Fe7Se8 within a robust NC shell via a facile etching and selenization process[63]. As an anode material for SIBs, the
Iron-based phosphides
Unlike iron oxides, which produce Na2O (433 K, 5 × 10-8 S cm-1) during Na+ insertion and exhibit low conductivity, iron phosphates react with Na+ to form Na3P (room temperature, > 1 × 10-5 S cm-1), thereby helping maintain electrochemical activity[66-70]. The Fe-P bond features unbound lone pairs of electrons in the 3p and 3d orbits, which significantly enhance the localized charge density, improving electronic conductivity[69-72]. Additionally, the Fe-P bond lengths, ranging from 2.186 to 2.447 Å, facilitate Na+ migration within the FeP crystal structure, improving ionic conductivity and partially mitigating volumetric strain. The Na+ storage process in FeP is as follows[66-70]:
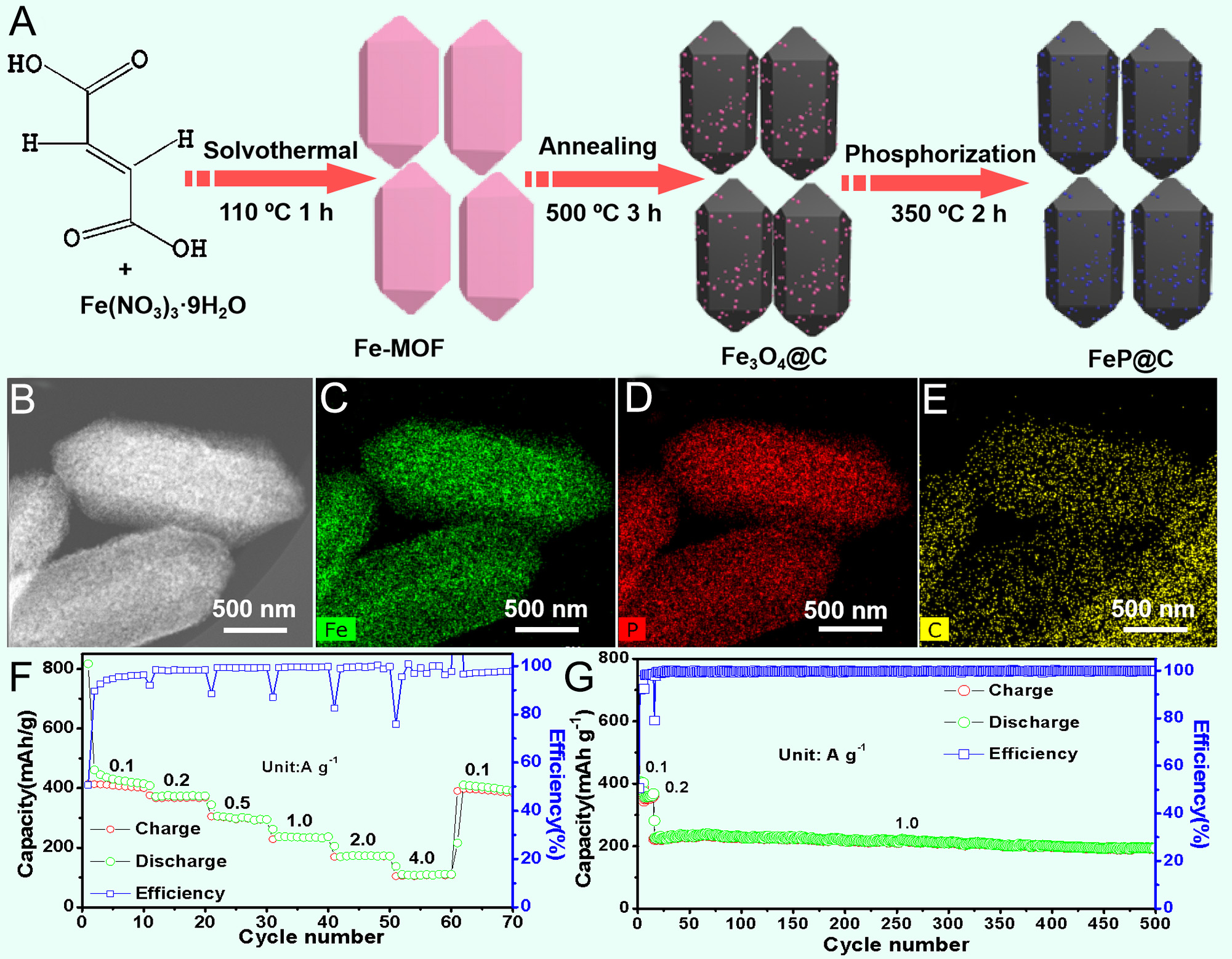
However, challenges such as volume expansion during charging/discharging and limited electronic conductivity hinder large-scale application. Therefore, Yang et al. designed a FeP/graphite composite anode through a straightforward ball milling strategy[66]. This composite significantly enhanced conductivity and achieved a capacity of 134 mAh g-1 at 0.5 A g-1[66]. To overcome Fe0 agglomeration and poor conductivity in FeP, Xu et al. in situ encapsulated ultrafine FeP particles in porous carbon (FeP@C) via a MOF-derived phosphorization strategy [Figure 8A][67]. Energy-dispersive X-ray (EDX) elemental mapping signals
Figure 8. (A) Schematic illustration of the preparation process of FeP@C. (B) Dark-field TEM image; (C-E) corresponding EDX mapping signals of Fe (C), P (D), and C (E) elements. (F) Rate performance of FeP@C measured from 0.1 to 4.0 A g-1 and (G) cycling performance of FeP@C at 2.0 A g-1[67]. Copyright 2019, Elsevier.
Compared to conventional power-based anodes, self-supported electrodes are typically grown on a conductive metal/carbon matrix, which can mitigate the side effects of binders and enhance the energy density of SIBs. Furthermore, such binder-free electrodes are intrinsically flexible, ultrathin, lightweight, and bendable, allowing them to maximize space utilization in electronic devices. For instance, Shi et al. reported a free-standing FeP@NPC film anode for SIBs, where FeP nanoparticles were embedded in a 3D cross-linked N,P-doped carbon (NPC) fiber network[71]. The FeP nanoparticles were in situ confined within the N,P-doped carbon fibers via thermal treatment of PAN nanofibers. The 3D-connected NPC framework offered efficient electron/ion transport pathways and featured void spaces to accommodate volumetric strain during Na+ extraction/insertion. This FeP@NPC anode achieved a capacity of 557 mAh g-1 at 0.1 A g-1 over 1,000 cycles. Their work broadens the approach to fabricating binder-free phosphide anodes for flexible SIBs[71]. Similarly, Park et al. developed a self-supported film composed of superfine FeP@C particles dispersed on P-doped graphene (FeP@C@PG) through solvent-free pyrogenic decomposition followed by roll pressing[72]. PA served as both the phosphorus and carbon source, enabling in-situ confinement of ultrafine FeP particles within the P-doped carbon matrix. This hierarchical structure accelerated diffusion kinetics, mitigated significant changes, and suppressed the aggregation/pulverization of FeP particles during charging/discharging. As a result, the FeP@C@PG film exhibited a capacity of 536.6 mAh g-1 over 1,000 cycles at 1 A g-1, and a high rate capability of 440.7 mAh g-1 at 5 A g-1.
Compared with other oxides and sulfides, iron-based phosphide materials have a lower charging/discharging potential, making them more suitable as SIB anodes. If their electrochemical properties can be further improved, they hold great promise for future applications.
Iron-based fluorides
Iron-based fluorides can support multi-electron transfer through multi-step electrochemical reactions, enabling them to reach high specific capacities[73-77]. Common iron-based fluorides include FeF2, FeF3, and hydrated forms. For instance, FeF3 exhibits an ultrahigh theoretical capacity of 712 mAh g-1 along with a relatively high operating voltage, which attracted considerable attention for its potential as a cathode material in SIBs[77-82]. The Na+ storage mechanism of FeF3 is as follows[77-82]:
At present, the electrochemical performance of fluoride materials remains unsatisfactory due to significant voltage hysteresis and sluggish reaction kinetics, resulting in rapid capacity decay[74,80-82]. Therefore, developing iron-based fluoride cathodes for SIBs and enhancing their electrochemical properties is of great importance.
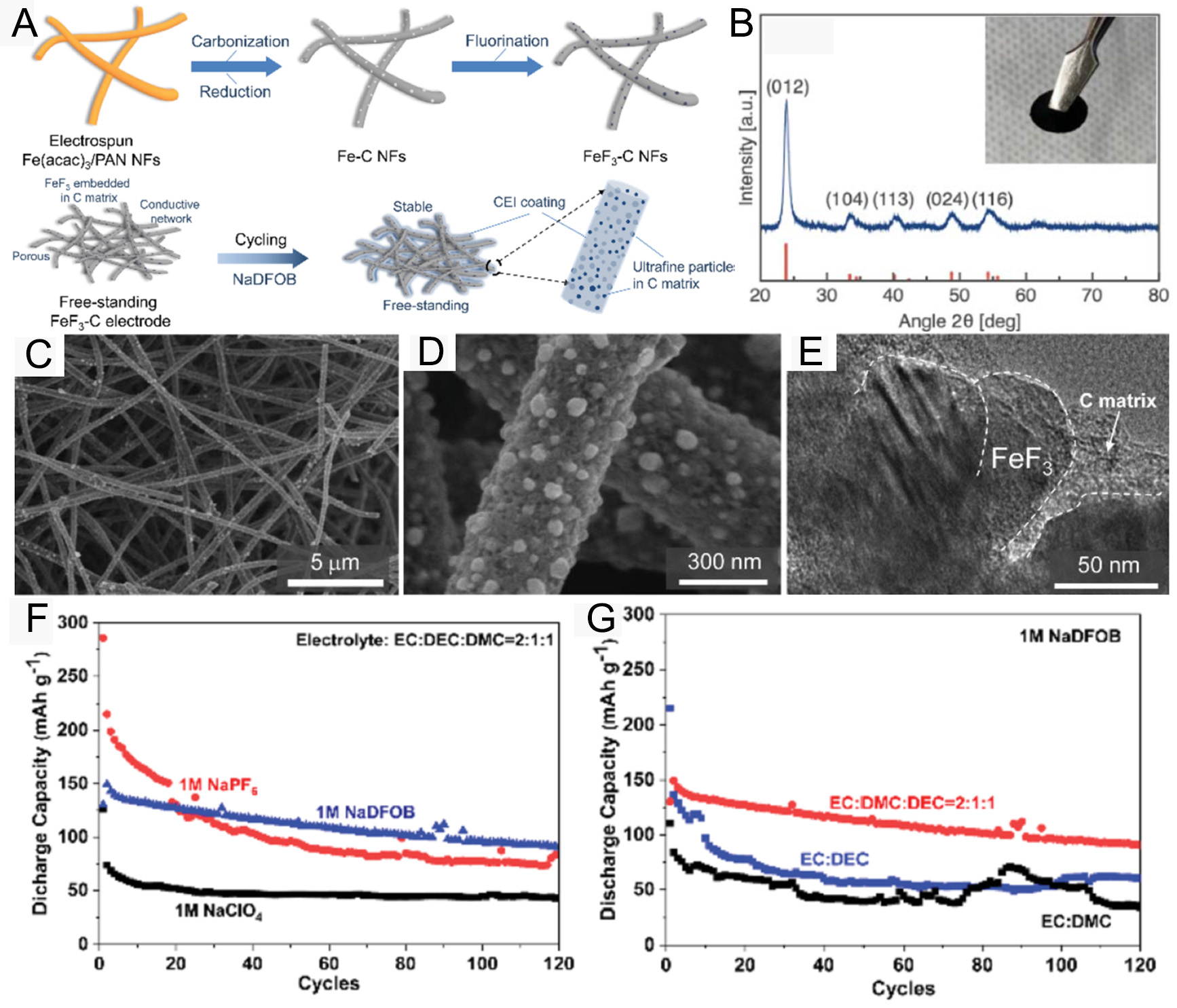
One major challenge is the severe pulverization of iron fluorides during cycling, which results in rapid structural degradation and capacity fading. To address this, Zhang et al. designed an amorphous FeF3/C nanocomposite cathode using Fe-MOF nanorods as precursors via high-temperature carbonization and fluorination[74]. This FeF3/C cathode demonstrated excellent cycling stability, retaining 126.7 mAh g-1 over 100 cycles at 75 mA g-1[74]. The improved performance is ascribed to the amorphous structure and the porous carbon backbone formed from the carbonized organic ligands of the Fe-MOF, which facilitate Na+/electron diffusion and enhance reaction kinetics. Sun et al. developed a free-standing FeF3 cathode and employed sodium-difluoro(oxalate)borate (NaDFOB) as the electrolyte salt for SIBs [Figure 9A][75]. XRD analysis [Figure 9B] confirmed that the synthesized material matched the hexagonal FeF3 phase. SEM images [Figure 9C and D] reveal that FeF3 nanoparticles were uniformly distributed within or on the surface of the NFs. TEM images [Figure 9E] further show that FeF3 crystals are well- encapsulated within the carbon matrix. As shown in Figure 9F, cells using NaDFOB demonstrated superior cycling stability compared to those using NaPF6 or NaClO4 over 120 cycles. Moreover, as illustrated in Figure 9G, cells using a DEC/EC/DMC solvent mixture achieved the highest capacity retention of 69.4%, significantly outperforming those using EC/DEC (28.17%) or EC/DMC (31.4%). This enhanced performance is associated with the synergistic effect between the nanoconfined FeF3 cathode and the NaDFOB electrolyte. Quantum mechanical calculations revealed that DFOB- facilitated the formation of a thin CEI layer on the FeF3-CNFs, improving stability[75]. Despite these advancements, the practical application of FeF3-based cathodes remains hindered by low capacity utilization and poor cycling performance. Sun et al. investigated various ionic liquid (IL) electrolytes to enhance the Na+ storage performance of FeF3 across a wide temperature range[76]. They found that the Pyr1,4FSI electrolyte exhibited an ignorable decay rate of ~0.023% per cycle over 1,000 cycles.
Figure 9. (A) Schematic of the preparation procedures of FeF3-C NFs and FeF3-C electrode cycled in the NaDFOB electrolyte; (B) XRD pattern of FeF3-C NFs cathode; (C and D) SEM images of FeF3-CNFs; (E) High-resolution TEM image of FeF3-CNFs. (F) Cycling performance of FeF3-CNFs with different sodium salts and (G) cycling performance of FeF3-CNFs in 1M NaDFOB electrolyte with different solvents[75]. Copyright 2020, Royal Society of Chemistry.
Ma et al. proposed a FeF3-Fe-RGO composite as a cathode for SIBs, in which a conductive matrix of metallic Fe and RGO was formed in situ from a single FeF2 grain during electrochemical cycling[77]. This FeF3-Fe-RGO cathode delivered a capacity of 150 mAh g-1 at 0.05 A g-1 and maintained a specific capacity of 60 mAh g-1 even at 2.0 A g-1[77]. The superior electrochemical performance is attributed to the enhanced electronic conductivity provided by the RGO and metallic Fe-based conductive framework. This design strategy could be extended to other electrode materials that offer high capacity but suffer from low electrical conductivity.
FeF2 has also emerged as a promising conversion-type cathode material for SIBs. Accordingly,
Despite these advancements, the poor electronic conductivity and side reactions of FeF3 cathodes result in sluggish reaction kinetics and rapid capacity decay during cycling, impeding their large-scale application. To address this, Liu et al. employed a surface modification method, synthesizing hollow FeF3·0.33H2O microspheres through a solvothermal approach followed by coating with AlPO4[81]. This surface treatment significantly reduced charge-transfer resistance and improved Na+ diffusion. Compared to pristine
In summary, coupling iron-based fluoride nanoparticles with conductive carbon materials can effectively improve electrical conductivity, alleviate volume expansion and particle agglomeration, and enhance the overall electrochemical properties of SIB cathodes.
Other iron-based materials
Other iron-based materials - such as FeTiO3, FeTe2, FePS3, FePO4, and Fe2(SO4)3 - have also shown promise for application in SIBs due to their relatively high theoretical specific capacities[82-87]. Researchers have explored strategies to control the growth and microstructure of these materials to improve their cycling stability and rate performance. For example, two-dimensional (2D) FePS3 nanosheets, a typical ternary metal phosphosulfide, were synthesized via ultrasonic exfoliation. A FePS3 nanosheets@MXene composite was subsequently fabricated by in situ mixing of the exfoliated nanosheets with ultrathin MXene layers[83]. This hybrid material improved electronic conductivity and increased the specific surface area, thus accelerating Na+ diffusion and alleviating volumetric strain during repeated cycling. As a result, the FePS3 nanosheets@MXene composite reached a reversible capacity of 676.1 mAh g-1 over 90 cycles at 0.1 A g-1[83]. This work provides a valuable pathway for the development of 2D/2D conversion-type electrode materials with enhanced performance for SIBs. In addition, tellurium-based materials have attracted attention due to tellurium’s inherently high electrical conductivity. Iron tellurides, in particular, have been investigated for Na+ storage applications. Recently, FeTe2 nanoparticles embedded in nitrogen-doped carbon nanofibers (FeTe2/CNFs) were synthesized via electrospinning[84]. The FeTe2/CNF anode demonstrated an initial capacity of 406.8 mAh g-1 and retained 221.3 mAh g-1 over 1,000 cycles at 1 A g-1. These outstanding electrochemical properties are attributed to the synergistic interaction between the FeTe2 particles and the carbon nanofiber matrix, which buffers volume changes and enhances structural stability during cycling[84]. FePO4, with a high theoretical capacity of 175 mAh g-1, can be easily synthesized on a large scale through a facile precipitation procedure at room temperature, making it a promising cathode candidate for SIBs[88]. Furthermore, monoclinic Fe2(SO4)3, when coupled with a Na anode, exhibits an average operating voltage of ~3.25 V and can store 1.8 Na+, delivering a specific capacity of ~120 mAh g-1, thus emerging as another potential cathode material[89].
Iron-based materials offer several advantages, including abundant raw resources and low production costs. A wide variety of iron-containing compounds and metal composites have been explored as electrode materials, leading to significant advancements in the development of both cathode and anode materials for SIBs.
SUMMARY AND OUTLOOK
This review summarizes recent advances in the design of tailored nanostructures and the development of straightforward methods to enhance the electrochemical properties of conversion-type iron-based electrodes. While numerous modification approaches have been employed to improve the long-cycling stability of these electrodes, conventional techniques still present certain limitations. As shown in Table 1, nanostructure engineering effectively enhances both the cycling stability and rate performance of iron-based materials. Additionally, novel MOF-derived methods have been applied in the synthesis of iron-based materials. During subsequent processing, organic functional groups form in situ coatings on the iron-based nanounits, which markedly enhance their cycling and rate capabilities.
Comparison of the electrochemical properties of conversion-type iron-based materials for SIBs
| Type | Electrode materials | Current density (mA g-1) | Specific capacity (mA h g-1) | Cycle number (cycles) | Voltage region (V) | Reference |
| Iron-based oxides | Fe2O3@MIL-101(Fe)/C | 200 | 662 | 200 | 0.01-3.0 | [18] |
| MFe2O3@N-HCNs | 2,000 | 363 | 4,500 | 0.01-3.0 | [20] | |
| Fe2O3/rGO | 50 | ~500 | 100 | 0.01-3.0 | [22] | |
| Fe2O3-C@CF | 30 | 530 | 100 | 0.01-3.0 | [23] | |
| Fe2O3 nanorods | 50 | 252 | 100 | 0.01-3.0 | [24] | |
| a-Fe2O3/pBC-N | 100 | 408 | 350 | 0.01-3.0 | [25] | |
| 3D-0D graphene-Fe3O4 | 50 | 312 | 200 | 0.01-3.0 | [26] | |
| Fe3O4@C@G | 100 | 180 | 600 | 0.01-3.0 | [27] | |
| Fe3O4@C@PPy | 1,000 | 640 | 100 | 0.01-2.5 | [28] | |
| Fe3O4 QD@C-GN | 2,000 | 343 | 1,000 | 0.01-3.0 | [29] | |
| Fe3O4/C/P | 200 | 1,390 | 200 | 0.01-3.0 | [30] | |
| Fe3O4-CNFs | 500 | 358.1 | 200 | 0.01-3.0 | [31] | |
| Iron-based sulfides | FeS2 | 200 | 94 | 100 | 0.01-3.0 | [33] |
| Mesoporous FeS2 | 1,000 | 711.1 | 450 | 0.01-2.8 | [34] | |
| FeS2-CGS | 1,000 | 855.9 | 200 | 0.01-3.0 | [35] | |
| CHS-FeS2 | 500 | 546.5 | 100 | 0.01-2.8 | [36] | |
| FeS2@CF-NS | 5,000 | 431.1 | 700 | 0.01-3.0 | [37] | |
| Co0.5Fe0.5S2 | 2,000 | 220 | 5,000 | 0.8-2.9 | [38] | |
| FeS2@C | 100 | 616 | 100 | 0.5-3.0 | [39] | |
| FeS2@C yolk-shell | 100 | 511 | 100 | 0.1-2.0 | [40] | |
| Pitaya-Structured FeS2@C | 600 | 415 | 120 | 0.5-2.5 | [41] | |
| FeS2@G@CNF | 3,000 | 305.5 | 2,450 | 0.5-2.5 | [42] | |
| Porous FeS nanofibers | 500 | 592 | 150 | 0.01-3.0 | [43] | |
| FeS/C | 10,000 | 300.4 | 10,000 | 0.01-3.0 | [44] | |
| FeS/NC | 1,000 | 326 | 500 | 0.01-3.0 | [45] | |
| Mn-doped FeS/NC | 8,000 | 206.2 | 8,000 | 0.4-2.8 | [46] | |
| 3D porous FeS@NC | 1,500 | 254 | 1,110 | 0.5-3 | [47] | |
| Iron-based selenides | FeSe2 clusters | 1,000 | 515 | 400 | 0.5-2.9 | [50] |
| Hierarchical porous FeSe2 | 10,000 | 333 | 1,800 | 0.01-3.0 | [51] | |
| FeSe2-AC | 500 | 379 | 150 | 0.01-3.0 | [52] | |
| FeSe2/GNS-400 | 25,000 | 227 | 800 | 0.01-2.8 | [53] | |
| FeSe2@C Microrods | 5,000 | 401.3 | 2,000 | 0.5-2.8 | [54] | |
| FeSe2@NC | 5,000 | 372.9 | 1,000 | 0.01-3.0 | [55] | |
| P-FeSe2/NCF | 2,000 | 507 | 200 | 0.01-3.0 | [56] | |
| CNT/FeSe2/C | 100 | 546 | 100 | 0.01-3.0 | [57] | |
| Fe7Se8 NRBs | 200 | 294 | 1,800 | 0.4-2.8 | [58] | |
| Fe7Se8@C@MoSe2 | 1,000 | 345 | 600 | 0.01-3.0 | [60] | |
| Fe7Se8@C nanotubes | 2,000 | 319 | 720 | 0.5-3.0 | [61] | |
| Fe7Se8@NC | 5,000 | 185.6 | 6,000 | 0.5-3.0 | [62] | |
| Fe7Se8@C/N nanoboxes | 5,000 | 316 | 1,000 | 0.5-2.5 | [63] | |
| Fe7Se8@C/CNT-EG | 1,000 | 336 | 1,000 | 0.01-3.0 | [64] | |
| Iron-based phosphides | FeP/graphite | 50 | 240 | 70 | 0.01-1.5 | [66] |
| MOF-derived FeP@C | 100 | 387 | 100 | 0.01-3.0 | [67] | |
| FeP@NC | 500 | 375 | 2,000 | 0.01-3.0 | [68] | |
| H-FeP@C@GR | 100 | 400 | 250 | 0.01-3.0 | [69] | |
| FeP@PNC | 500 | 224.5 | 4,500 | 0.01-3.0 | [70] | |
| FeP@NPC film | 1,000 | 253 | 300 | 0.01-3.0 | [71] | |
| FeP@C@PG film | 1,000 | 536.6 | 1,000 | 0.01-3.0 | [72] | |
| FeF3/CNF | 20 | ~161 | 100 | 1.2-4.2 | [75] | |
| FeF3/Fe/RGO | 100 | 70 | 1,000 | 0.8-4.5 V | [77] | |
| FeF2 | 300 | 214.2 | 500 | 1.2-4.2 | [78] | |
| FeF2@MHCS | 200 | 112 | 100 | 1.5-4.5 | [79] | |
| FeF2-rGO | 200 | 85 | 200 | 1.5-4.5 | [80] | |
| FeF3·0.33H2O/AlPO4 | 200 | 211 | 80 | 1.2-4.0 | [81] |
From an application standpoint, the initial Coulomb efficiency and overall electrochemical performance of certain iron-based electrodes still require significant improvement and further investigation. It is essential to explore in greater depth the relationship between crystal structure, electronic structure, and sodium storage performance. Through rational element doping and precise crystal structure engineering, the conversion reaction mechanisms of iron-based materials can be optimized, increasing the number of active sites involved in the reaction and thereby enhancing the specific capacity. To further improve the cycling stability of iron-based electrodes, several strategies can be employed. On the one hand, nanostructure design - such as the fabrication of nanoparticles, nanowires, and nanotubes - can shorten the Na+ diffusion paths and alleviate the volume changes during charging/discharging. On the other hand, surface coating and composite fabrication techniques are also effective. For example, coating iron-based materials with conductive carbon or other stable compounds can not only enhance electronic conductivity but also suppress side reactions between the active material and the electrolyte, thereby strengthening structural stability. Optimizing the electronic conductivity and Na+ diffusion rate is crucial to improving the rate capability of these materials. This can be achieved by incorporating highly conductive additives or constructing three-dimensional conductive networks to facilitate electron transfer. Additionally, designing materials with large transport channels or introducing structural defects can accelerate Na+ ion diffusion. Previous studies have also shown that some iron-based materials can help stabilize the SEI[90,91]. Thus, iron-based electrodes can be combined with other electrode materials to further enhance overall electrochemical performance. With ongoing technological advances and breakthroughs in synthesis techniques, the advantages of iron-based materials are expected to become increasingly evident.
For future development, attention should also be given to exploring functional electrolytes and additives that are compatible with the wide-temperature operational requirements of iron-based electrodes. Moreover, iron-based electrodes have been investigated for LIBs[92,93], indicating the potential for iron-based cathodes such as FeF3, FeF2, and Fe2(SO4)3 to be paired with solid-state electrolytes in solid-state sodium metal batteries, thereby further improving energy density.
Advanced characterization techniques are essential for probing the structural and interfacial stability of iron-based materials. In particular, in situ techniques such as XRD, TEM, and XPS enable real-time monitoring of structural and interfacial evolution during sodiation/desodiation. ToF-SIMS can offer detailed compositional analysis of the SEI through depth profiling. Nevertheless, the lack of a clear mechanistic understanding still hinders the widespread practical application of these materials. In conclusion, structural optimization, when combined with targeted surface engineering, can significantly improve the electrochemical properties of iron-based electrodes. It is hoped that this review will shed light on the rational design of conversion-type electrodes for SIBs.
DECLARATIONS
Authors’ contributions
Writing of the original draft: Chen, S.; Ye, S.
Review & editing: Xu, X.; Sun, H.
Conceptualization, review, and supervision: Fan, W.; Zhao, J.; Huo, Y.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (No. 52301266), Guangdong Basic and Applied Basic Research Foundation (No. 2025A1515012152), the Science and Technology Planning Project of Guangzhou (No. 2024A04J3332), and the Chenzhou National Sustainable Development Agenda Innovation Demonstration Zone Provincial Special Project (No. 2023sfq11).
Conflicts of interest
Fan, W. and Zhao, J. are affiliated with Guangzhou Tinci Materials Technology Co., Ltd., while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569-614.
2. Sun, Y.; Li, Y.; Gong, Y.; et al. Constructing three-dimensional architectures to design advanced anodes materials for sodium-ion batteries: from nanoscale to microscale. Energy. Mater. 2024, 4, 400002.
3. Liu, Z.; Song, Y.; Fu, S.; et al. Multiphase manganese-based layered oxide for sodium-ion batteries: structural change and phase transition. Microstructures 2024, 4, 2024036.
4. Huang, Y.; Zeng, W.; Li, K.; Zhu, X. Na-deficient P2-type layered oxide cathodes for practical sodium-ion batteries. Microstructures 2024, 4, 2024027.
5. Zhang, R.; Chen, H.; Yue, H. Room-temperature synthesis of layered open framework cathode for sodium-ion batteries. Chin. Chem. Lett. 2023, 34, 107580.
6. Yin, H.; Han, D.; Wang, W.; et al. Bimetallic sulfide anodes based on heterojunction structures for high-performance sodium-ion battery anodes. Chin. Chem. Lett. 2024, 110537.
7. Li, X.; Liu, X.; Xiang, Y.; Zheng, Q.; Wei, X.; Lin, D. Metal-organic frameworks derived carbon-coated ZnSe/Co0.85Se@N-doped carbon microcuboid as an advanced anode material for sodium-ion batteries. Chin. Chem. Lett. 2022, 33, 3197-202.
8. Wan, Y.; Liu, Y.; Chao, D.; Li, W.; Zhao, D. Recent advances in hard carbon anodes with high initial Coulombic efficiency for sodium-ion batteries. Nano. Mater. Sci. 2023, 5, 189-201.
9. Rehman, A.; Saleem, S.; Ali, S.; Abbas, S. M.; Choi, M.; Choi, W. Recent advances in alloying anode materials for sodium-ion batteries: material design and prospects. Energy. Mater. 2024, 4, 400068.
10. Wang, Y.; Kuang, Y.; Cui, J.; et al. Self-template construction of hierarchical Bi@C microspheres as competitive wide temperature-operating anodes for superior sodium-ion batteries. Nano. Lett. 2024, 24, 15242-51.
11. Zhao, J.; Zhang, S.; Liu, W.; Du, Z.; Fang, H. Fe3O4/PPy composite nanospheres as anode for lithium-ion batteries with superior cycling performance. Electrochim. Acta. 2014, 121, 428-33.
12. Zhao, Y.; Wang, F.; Wang, C.; et al. Encapsulating highly crystallized mesoporous Fe3O4 in hollow N-doped carbon nanospheres for high-capacity long-life sodium-ion batteries. Nano. Energy. 2019, 56, 426-33.
13. Wei, C.; Wang, R.; Wu, Z.; et al. Revealing the size effect of FeS2 on solid-state battery performances at different operating temperatures. Chin. Chem. Lett. 2024, 35, 108717.
14. Qiu, D.; Gao, A.; Zhao, W.; et al. Fast-charging degradation mechanism of two-dimensional FeSe anode in sodium-ion batteries. ACS. Energy. Lett. 2023, 8, 4052-60.
15. Hou, B. H.; Wang, Y. Y.; Ning, Q. L.; et al. An FeP@C nanoarray vertically grown on graphene nanosheets: an ultrastable Li-ion battery anode with pseudocapacitance-boosted electrochemical kinetics. Nanoscale 2019, 11, 1304-12.
16. Wu, F.; Srot, V.; Chen, S.; et al. 3D honeycomb architecture enables a high-rate and long-life iron (III) fluoride-lithium battery. Adv. Mater. 2019, 31, e1905146.
17. Philippe, B.; Valvo, M.; Lindgren, F.; Rensmo, H.; Edström, K. Investigation of the electrode/electrolyte interface of Fe2O3 composite electrodes: Li vs Na batteries. Chem. Mater. 2014, 26, 5028-41.
18. Li, C.; Hu, Q.; Li, Y.; et al. Hierarchical hollow Fe2O3@MIL-101(Fe)/C derived from metal-organic frameworks for superior sodium storage. Sci. Rep. 2016, 6, 25556.
19. Li, L.; Li, H.; Liu, L.; Yan, X.; Long, Y.; Han, W. Amorphous Fe2O3 anchored on N-doped graphene with internal micro-channels as an active and durable anode for sodium-ion batteries. Nanomaterials 2024, 14, 937.
20. Chen, M.; Niu, D.; Mao, J.; et al. A movable Fe2O3 core in connected hierarchical pores for ultrafast intercalation/deintercalation in sodium-ion batteries. ACS. Appl. Energy. Mater. 2021, 4, 5888-96.
21. Li, D.; Zhou, J.; Chen, X.; et al. Amorphous Fe2O3/graphene composite nanosheets with enhanced electrochemical performance for sodium-ion battery. ACS. Appl. Mater. Interfaces. 2016, 8, 30899-907.
22. Li, T.; Qin, A.; Yang, L.; et al. In situ grown Fe2O3 single crystallites on reduced graphene oxide nanosheets as high performance conversion anode for sodium-ion batteries. ACS. Appl. Mater. Interfaces. 2017, 9, 19900-7.
23. Bhar, M.; Ghosh, S.; Martha, S. K. Designing freestanding electrodes with Fe2O3-based conversion type anode material for sodium-ion batteries. J. Alloys. Compd. 2023, 948, 169670.
24. Zhao, D.; Xie, D.; Liu, H.; Hu, F.; Wu, X. Flexible α-Fe2O3 nanorod electrode materials for sodium-ion batteries with excellent cycle performance. Funct. Mater. Lett. 2018, 11, 1840002.
25. Shi, L.; Li, Y.; Zeng, F.; et al. In situ growth of amorphous Fe2O3 on 3D interconnected nitrogen-doped carbon nanofibers as high-performance anode materials for sodium-ion batteries. Chem. Eng. J. 2019, 356, 107-16.
26. Liu, H.; Jia, M.; Zhu, Q.; et al. 3D-0D graphene-Fe3O4 quantum dot hybrids as high-performance anode materials for sodium-ion batteries. ACS. Appl. Mater. Interfaces. 2016, 8, 26878-85.
27. Tao, X.; Li, Y.; Wang, H. G.; et al. Multi-heteroatom-doped dual carbon-confined Fe3O4 nanospheres as high-capacity and long-life anode materials for lithium/sodium ion batteries. J. Colloid. Interface. Sci. 2020, 565, 494-502.
28. Biswal, R.; Shukla, V.; Janakiraman, S.; Ghosh, S.; Adyam, V. Novel dual core@shell Fe3O4@C@polypyrrole (PPy) composite for sodium ion batteries. Mater. Today. Proc. 2020, 33, 5088-92.
29. Qi, L. Y.; Zhang, Y. W.; Zuo, Z. C.; et al.
30. Qin, G.; Duan, J.; Yang, Y.; Liu, F. Magnetic field facilitated resilient chain-like Fe3O4/C/red P with superior sodium storage performance. ACS. Appl. Mater. Interfaces. 2018, 10, 6441-52.
31. Huang, Y.; Zhou, J.; Zhang, Y.; et al. Encapsulating hollow Fe3O4 in intertwined N-doped carbon nanofibers for high-performance supercapacitors and sodium-ion batteries. J. Alloys. Compd. 2022, 918, 165672.
32. Kitajou, A.; Yamaguchi, J.; Hara, S.; Okada, S. Discharge/charge reaction mechanism of a pyrite-type FeS2 cathode for sodium secondary batteries. J. Power. Sources. 2014, 247, 391-5.
33. Li, Z.; Zhang, Y.; Li, X.; et al. Reacquainting the electrochemical conversion mechanism of FeS2 sodium-ion batteries by operando magnetometry. J. Am. Chem. Soc. 2021, 143, 12800-8.
34. Zhang, Z.; Zhong, X.; Zhang, Y.; et al. Scalable synthesis of mesoporous FeS2 nanorods as high-performance anode materials for sodium-ion batteries. Rare. Met. 2022, 41, 21-8.
35. Kandula, S.; Youn, B. S.; Cho, J.; Lim, H.; Gon Son, J. FeS2@N-C nanorattles encapsulated in N/S dual-doped graphene/carbon nanotube network composites for high performance and high rate capability anodes of sodium-ion batteries. Chem. Eng. J. 2022, 439, 135678.
36. Ma, L.; Hou, B.; Zhang, H.; et al. Regulation of MIL-88B(Fe) to design FeS2 core-shelled hollow sphere as high-rate anode for a full sodium-ion battery. Chem. Eng. J. 2023, 453, 139735.
37. Lu, Z.; Zhai, Y.; Wang, N.; et al. FeS2 nanoparticles embedded in N/S co-doped porous carbon fibers as anode for sodium-ion batteries. Chem. Eng. J. 2020, 380, 122455.
38. Zhang, K.; Park, M.; Zhou, L.; et al. Cobalt-doped FeS2 nanospheres with complete solid solubility as a high-performance anode material for sodium-ion batteries. Angew. Chem. Int. Ed. 2016, 55, 12822-6.
39. Man, Z.; Li, P.; Zhou, D.; et al. Two birds with one stone: FeS2@C yolk-shell composite for high-performance sodium-ion energy storage and electromagnetic wave absorption. Nano. Lett. 2020, 20, 3769-77.
40. Liu, Z.; Lu, T.; Song, T.; Yu, X.; Lou, X. W.; Paik, U. Structure-designed synthesis of FeS2@C yolk-shell nanoboxes as a high-performance anode for sodium-ion batteries. Energy. Environ. Sci. 2017, 10, 1576-80.
41. Xu, X.; Liu, J.; Liu, Z.; et al. Robust pitaya-structured pyrite as high energy density cathode for high-rate lithium batteries. ACS. Nano. 2017, 11, 9033-40.
42. Chen, C.; Yang, Y.; Tang, X.; et al. Graphene-encapsulated FeS2 in carbon fibers as high reversible anodes for Na+/K+ batteries in a wide temperature range. Small 2019, 15, e1804740.
43. Cho, J. S.; Park, J.; Kang, Y. C. Porous FeS nanofibers with numerous nanovoids obtained by Kirkendall diffusion effect for use as anode materials for sodium-ion batteries. Nano. Res. 2017, 10, 897-907.
44. Han, M.; Liu, J.; Deng, C.; et al. Yolk-shell structure and spin-polarized surface capacitance enable FeS stable and fast ion transport in sodium-ion batteries. Adv. Energy. Mater. 2024, 14, 2400246.
45. Liu, Y.; Zhong, W.; Yang, C.; et al. Direct synthesis of FeS/N-doped carbon composite for high-performance sodium-ion batteries. J. Mater. Chem. A. 2018, 6, 24702-8.
46. Chen, H.; Yang, X.; Lv, P.; Tian, P.; Wan, S.; Liu, Q. Mn-doped FeS with larger lattice spacing as advance anode for sodium ion half/full battery. Chem. Eng. J. 2022, 450, 137960.
47. Yuan, J.; Mu, M.; Xu, X.; et al. Three-dimensional porous FeS@N doped carbon nanosheets for high-rate and high-stable sodium/potassium storage. Compos. Part. B. Eng. 2022, 247, 110300.
48. Zhang, J.; Meng, Z.; Yang, D.; et al. Enhanced interfacial compatibility of FeS@N,S-C anode with ester-based electrolyte enables stable sodium-ion full cells. J. Energy. Chem. 2022, 68, 27-34.
49. Huang, X.; He, Q.; Xun, J.; et al. Constructing strain-alleviated structures in ultrathin FeS/C composites for durable lithium and sodium storage. Sci. China. Mater. 2023, 66, 2601-12.
50. Wei, X.; Tang, C.; An, Q.; et al. FeSe2 clusters with excellent cyclability and rate capability for sodium-ion batteries. Nano. Res. 2017, 10, 3202-11.
51. Wang, L.; Bai, K.; Lu, Y.; Mo, W.; Zhang, L. Controllable hierarchical porous FeSe2 with excellent long cycle lifespan as anode materials for sodium-ion battery. J. Power. Sources. 2024, 592, 233913.
52. Park, G. D.; Kim, J. H.; Kang, Y. C. Large-scale production of spherical FeSe2-amorphous carbon composite powders as anode materials for sodium-ion batteries. Mater. Charact. 2016, 120, 349-56.
53. Li, D.; Zhou, J.; Chen, X.; Song, H. Achieving ultrafast and stable Na-ion storage in FeSe2 nanorods/graphene anodes by controlling the surface oxide. ACS. Appl. Mater. Interfaces. 2018, 10, 22841-50.
54. Pan, Q.; Zhang, M.; Zhang, L.; et al. FeSe2@C microrods as a superior long-life and high-rate anode for sodium ion batteries. ACS. Nano. 2020, 14, 17683-92.
55. Zhou, S.; Jiang, R.; Wang, S.; et al. FeSe2 micro-nanorods confined in N-doped carbon as an advanced anode for fast sodium ion storage. J. Mater. Chem. A. 2024, 12, 11028-37.
56. Men, S.; Lin, J.; Zhou, Y.; Kang, X. N-doped porous carbon wrapped FeSe2 nanoframework prepared by spray drying: a potential large-scale production technique for high-performance anode materials of sodium ion batteries. J. Power. Sources. 2021, 485, 229310.
57. Yousaf, M.; Wang, Z.; Wang, Y.; et al. Core-shell FeSe2/C nanostructures embedded in a carbon framework as a free standing anode for a sodium ion battery. Small 2020, 16, e2002200.
58. Tian, W.; Ma, W.; Feng, Z.; et al. Formation of hierarchical Fe7Se8 nanorod bundles with enhanced sodium storage properties. J. Energy. Chem. 2020, 44, 97-105.
59. Liu, T.; Li, Y.; Zhao, L.; et al. In situ fabrication of carbon-encapsulated Fe7X8 (X = S, Se) for enhanced sodium storage. ACS. Appl. Mater. Interfaces. 2019, 11, 19040-7.
60. Chen, S.; Huang, S.; Zhang, Y. F.; et al. Constructing stress-release layer on Fe7Se8-based composite for highly stable sodium-storage. Nano. Energy. 2020, 69, 104389.
61. Yuan, J.; Gan, Y.; Xu, X.; et al. Construction of Fe7Se8@Carbon nanotubes with enhanced sodium/potassium storage. J. Colloid. Interface. Sci. 2022, 626, 355-63.
62. Yang, S.; Jiang, J.; He, W.; et al. Nitrogen-doped carbon encapsulating Fe7Se8 anode with core-shell structure enables high-performance sodium-ion capacitors. J. Colloid. Interface. Sci. 2023, 630, 144-54.
63. Sun, Z.; Wu, X.; Gu, Z.; et al. Rationally designed nitrogen-doped yolk-shell Fe7Se8/Carbon nanoboxes with enhanced sodium storage in half/full cells. Carbon 2020, 166, 175-82.
64. Wang, L.; Zhao, Y.; Chang, Y.; Zhu, S.; Qi, X. Carbon nanotube interweaved Fe7Se8 nanosheet/nanorod hybrids on expanded graphite for high-performance sodium storage. J. Energy. Storage. 2024, 95, 112593.
65. Zhang, D. M.; Jia, J. H.; Yang, C. C.; Jiang, Q. Fe7Se8 nanoparticles anchored on N-doped carbon nanofibers as high-rate anode for sodium-ion batteries. Energy. Storage. Mater. 2020, 24, 439-49.
66. Yang, Q.; Li, W.; Chou, S.; Wang, J.; Liu, H. Ball-milled FeP/graphite as a low-cost anode material for the sodium-ion battery. RSC. Adv. 2015, 5, 80536-41.
67. Xu, X.; Feng, J.; Liu, J.; et al. Robust spindle-structured FeP@C for high-performance alkali-ion batteries anode. Electrochim. Acta. 2019, 312, 224-33.
68. Li, Z.; Zhao, H.; Du, Z.; Zhao, L.; Wang, J.; Zhang, Z. Iron phosphide@N-doped carbon nanosheets with open-framework structure as an ultralong lifespan and outstanding rate performance electrode material for sodium-ion batteries. J. Power. Sources. 2020, 465, 228253.
69. Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational design of three-dimensional graphene encapsulated with hollow FeP@carbon nanocomposite as outstanding anode material for lithium ion and sodium ion batteries. ACS. Nano. 2017, 11, 11602-16.
70. Jiang, J.; Ma, C.; Zhang, W.; He, Y.; Li, X.; Yuan, X. Controlled design for integration of FeP into 3D carbon frameworks for superior Na storage. Chem. Eng. J. 2022, 429, 132271.
71. Shi, S.; Li, Z.; Shen, L.; et al. Electrospun free-standing FeP@NPC film for flexible sodium ion batteries with remarkable cycling stability. Energy. Storage. Mater. 2020, 29, 78-83.
72. Park, S.; Kim, C. W.; Lee, K. S.; Hwang, S. J.; Piao, Y. A densely packed air-stable free-standing film with FeP nanoparticles@C@P-doped reduced graphene oxide for sodium-ion batteries. Nanoscale 2023, 15, 14155-64.
73. Wang, Y.; Zhou, P.; Zhang, M.; et al. High-performance honeycombed FeF3@C cathodes enabling practical lithium pouch cells and silicon-metal fluoride batteries. Energy. Storage. Mater. 2023, 60, 102847.
74. Zhang, L.; Ji, S.; Yu, L.; Xu, X.; Liu, J. Amorphous FeF3/C nanocomposite cathode derived from metal-organic frameworks for sodium ion batteries. RSC. Adv. 2017, 7, 24004-10.
75. Sun, Z.; Fu, W.; Liu, M. Z.; et al. A nanoconfined iron (iii) fluoride cathode in a NaDFOB electrolyte: towards high-performance sodium-ion batteries. J. Mater. Chem. A. 2020, 8, 4091-8.
76. Sun, Z.; Wang, B.; Boebinger, M. G.; et al. Stability of FeF3-based sodium-ion batteries in nonflammable ionic liquid electrolytes at room and elevated temperatures. ACS. Appl. Mater. Interfaces. 2022, 29, 33447-56.
77. Ma, D.; Wang, H.; Li, Y.; et al. In situ generated FeF3 in homogeneous iron matrix toward high-performance cathode material for sodium-ion batteries. Nano. Energy. 2014, 10, 295-304.
78. Maulana, A. Y.; Song, J.; Futalan, C. M.; Kim, J. Improved reversibility of phase transformations using electron-rich graphitic carbon matrix in FeF2 cathode for sodium-ion batteries. Chem. Eng. J. 2022, 434, 134727.
79. Ni, D.; Fang, L.; Sun, W.; et al. FeF2@MHCS cathodes with high capacity and fast sodium storage based on nanostructure construction. ACS. Appl. Energy. Mater. 2020, 3, 10340-8.
80. Ni, D.; Sun, W.; Lu, C.; et al. Improved rate and cycling performance of FeF2-rGO hybrid cathode with poly (acrylic acid) binder for sodium ion batteries. J. Power. Sources. 2019, 413, 449-58.
81. Liu, M.; Wang, X.; Zhang, R.; et al. Hollow porous FeF3·0.33H2O microspheres by AlPO4 coating as a cathode material of Na-ion batteries. J. Energy. Storage. 2018, 18, 103-11.
82. Brugnetti, G.; Fiore, M.; Lorenzi, R.; Paleari, A.; Ferrara, C.; Ruffo, R. FeTiO3 as anode material for sodium-ion batteries: from morphology control to decomposition. ChemElectroChem 2020, 7, 1713-22.
83. Lin, Z.; Zhang, H.; Yang, C.; et al. Constructing FeTe2 nanoparticles embedded in N-doped carbon nanofiber composites as a long-life and high-rate anode material for sodium-ion batteries. Sustain. Energy. Fuels. 2024, 8, 934-41.
84. Ding, Y.; Chen, Y.; Xu, N.; et al. Facile synthesis of FePS3 nanosheets@MXene composite as a high-performance anode material for sodium storage. Nanomicro. Lett. 2020, 12, 54.
85. Kim, J.; Kim, H.; Kang, K. Conversion-based cathode materials for rechargeable sodium batteries. Adv. Energy. Mater. 2018, 8, 1702646.
86. Xu, S.; Dong, H.; Yang, D.; et al. Promising cathode materials for sodium-ion batteries from lab to application. ACS. Cent. Sci. 2023, 9, 2012-35.
87. Zheng, Y.; Zhang, Z.; Jiang, X.; et al. A comprehensive review on iron-based sulfate cathodes for sodium-ion batteries. Nanomaterials 2024, 14, 1915.
88. Zhang, Z.; Du, Y.; Wang, Q. C.; et al. A yolk-shell-structured FePO4 cathode for high-rate and long-cycling sodium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 17504-10.
89. Park, H.; Yoo, J. K.; Ko, W.; et al. Monoclinic Fe2(SO4)3: a new Fe-based cathode material with superior electrochemical performances for Na-ion batteries. J. Power. Sources. 2019, 434, 226750.
90. Hu, H.; Zhang, X.; Gao, Z.; et al. Boosting the cycle performance of iron trifluoride based solid state batteries at elevated temperatures by engineering the cathode solid electrolyte interface. Small 2024, 20, e2307116.
91. Chu, Y.; Mu, Y.; Zou, L.; et al. Thermodynamically stable dual-modified LiF&FeF3 layer empowering Ni-rich cathodes with superior cyclabilities. Adv. Mater. 2023, 35, e2212308.
92. Hu, J.; Lei, M.; Zhu, C.; Zhang, B.; Li, C. Highly conductive doped fluoride solid electrolytes with solidified ionic liquid to enable reversible FeF3 conversion solid state batteries. Adv. Funct. Mater. 2024, 34, 2314044.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].