Bidirectional Mendelian randomization study of brain imaging-derived phenotypes unveils causal associations between thalamic nuclei volume and stroke risk
Abstract
Aim: Recent advances in brain-imaging techniques have enabled the identification of brain imaging-derived phenotypes (IDPs), representing physiological brain structure. Observational studies have suggested a correlation between these IDPs and stroke, which is confounded and based on limited samples. To investigate the causal relationship between IDPs and stroke and its subtypes for an in-depth mechanistic comprehension of their interplay, we conducted a bidirectional two-sample Mendelian Randomization (MR) study leveraging the
Methods: We utilized GWAS summary statistics from the BIG40 dataset, which included nearly 3,935 IDPs among 33,224 individuals, and GIGASTROKE, which included three etiological ischemia subtypes, as well as cerebral ischemia, intracerebral hemorrhage stroke, and overall stroke among 73,652 stroke cases and 1,234,808 controls.
Results: In the forward MR analysis, we identified eight significant IDPs influencing the risk of stroke and its subtypes after Bonferroni correction. Notably, the volume of the lateral posterior thalamus in the right hemisphere exhibited a significant negative association with all ischemic stroke (OR = 0.79; 95%CI: 0.74 to 0.84; P = 1.21e-13), all stroke (OR = 0.84; 95%CI: 0.79 to 0.89; P = 2.45e-9), and large vessel stroke (OR = 0.54; 95%CI: 0.43 to 0.69; P = 3.01e-7). Conversely, no significant causal association was observed in the reverse MR analysis.
Conclusion: This study enhances our understanding of causality between IDPs and stroke by pinpointing specific causal associations. These findings provide valuable insights into the etiology of stroke, offering potential strategies for predicting and intervening in stroke risk at the level of brain imaging.
Keywords
INTRODUCTION
Stroke is a prevalent clinical manifestation of cerebrovascular disease and a substantial global public health concern. It encompasses both ischemic and hemorrhagic stroke, characterized by the sudden onset of brain dysfunction[1]. The Global Burden of Disease study reported stroke as the second leading cause of death and the third leading cause of death and disability combined in 2019, with ischemic stroke accounting for 62.4% of all cases[2]. Acute ischemic stroke is a highly heterogeneous condition encompassing multiple subtypes, each with distinct pathophysiological mechanisms, risk factor profiles, and clinical outcomes[3]. The heterogeneity of acute ischemic stroke significantly influences stroke severity, recurrence risk, and functional outcomes, underscoring the importance of subtype-specific analysis in genetic and epidemiological studies[3]. Although reperfusion therapy has shown efficacy in reducing disability, its practice is constrained by the therapeutic time window[4]. In the absence of effective early prevention strategies, stroke continues to exert a substantial toll on lives and property[2]. Consequently, it is crucial to investigate the risk factors contributing to stroke development and devise new preventive strategies.
Brain imaging-derived phenotypes (IDPs) are digital characteristics of brain tissue obtained through the analysis of brain imaging data, offering precise, reliable, and quantitative information for neuroimaging research[5]. Advanced imaging techniques enable the prediction of diseases before symptoms manifest, particularly with risk-stratified cohorts[6]. Numerous observational studies have identified several associations between IDPs and stroke. For instance, periventricular white matter hyperintensity volume is positively associated with cardioembolic ischemic stroke[7]. Significant changes in cortical thickness, surface area, and gray matter volume have been observed during stroke recovery[8]. Patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy exhibit reduced fractional anisotropy (FA), altered mode of anisotropy, and increased mean diffusivity[9]. Stroke has also been linked to changes in intracellular volume fraction (ICVF), orientation dispersion index (OD), and quantitative T2 values[10,11]. Different IDPs may contribute to stroke subtypes, and conversely, stroke and its subtypes can induce alterations in IDPs. However, existing observational studies often suffer from small sample sizes, and some lack specific detection methods, leaving many aspects of the relationship between stroke and specific brain anatomy or connectivity structures unexplored.
Mendelian randomization (MR) is a statistical approach that utilizes genetic variation as an instrumental variable to assess and quantify causality[12]. Genome-wide association studies (GWAS) identify genetic variants associated with disease risk or traits, enabling the exploration of causality through MR. Previous studies have partially unraveled the causal relationship between brain IDPs and stroke, with a focus on brain connectivity[13,14]. However, these studies did not investigate the causal link between IDPs related to brain anatomy structures and stroke. Furthermore, these studies have primarily relied on smaller sample sizes derived from the Megastroke study (40,585 cases; 446,696 controls), while the recent Gigastroke has doubled the sample size with 73,652 cases and 1,234,808 controls and unraveled more association signals[15]. To obtain the most statistically robust understanding of the causalities between IDPs and stroke risk, we employed a two-sample Mendelian randomization (TSMR) method strategy, complemented by comprehensive sensitive analyses, on the largest scale GWAS summary data available to date. A preliminary version of this study has been published as a preprint[16].
METHODS
Data sources
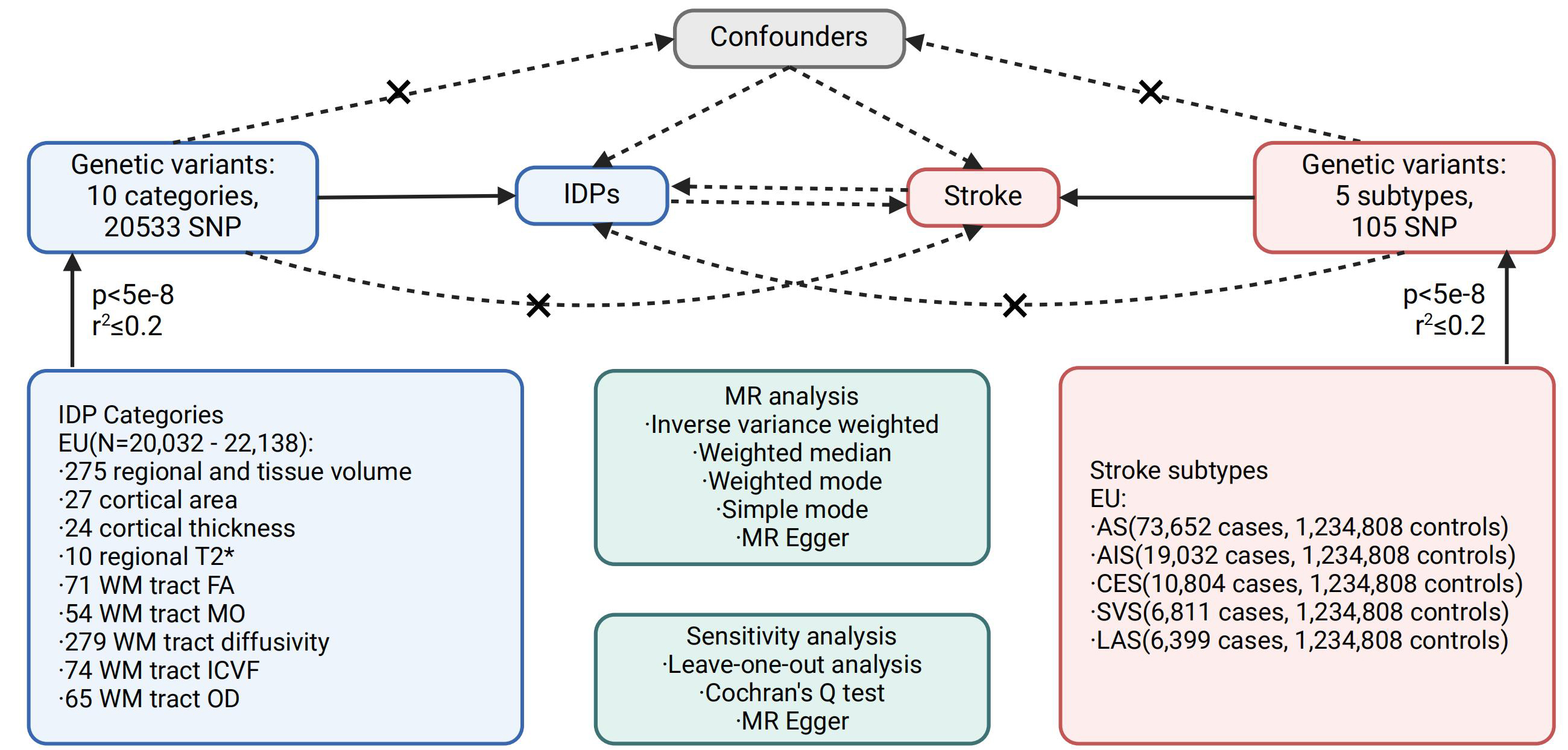
We employed the summary statistics of brain IDPs from Smith’s GWAS meta-analysis study, which encompassed 3,935 IDPs and 17,103,079 genome-wide Single Nucleotide Polymorphisms (SNPs) on autosomes 1-22 and the X chromosome. The study comprised a discovery cohort of 22,138 individuals and a replication cohort of 11,086 individuals, all of European ancestry[17]. Detailed data are available from the Oxford Brain Imaging Genetics (BIG40) web server (https://open.win.ox.ac.uk/ukbiobank/big40/). Based on previously reported observational studies, we selected 10 categories comprising a total of 2,010 IDPs: 647 regional and tissue volume, 371 cortical area, 303 cortical thickness, 14 regional T2*, 75 white matter (WM) tract FA, 75 WM tract diffusion tensor mode (MO), 300 WM tract diffusivity, 75 WM tract ICVF, 75 WM tract OD, 75 WM tract isotropic or free water volume fraction (ISOVF). Supplementary Table 1 provides additional information about the data sources for the IDPs.
In our study, we utilized the largest GWAS meta-analysis conducted by the GIGASTROKE consortium to investigate stroke. This analysis included over one million participants, with approximately 70% of European ancestry[15]. Comprehensive data can be accessed through the GWAS catalog (https://www.ebi.ac.uk/gwas/). Stroke was categorized into five groups: All Stroke (AS), All Ischemic Stroke (AIS), CardioEmbolic Stroke (CES), Small Vessel Stroke (SVS), and Large Artery Stroke (LAS). The GWAS data in the European population consisted of 1,234,808 controls and varying numbers of cases for each stroke subtype[15]. Supplementary Table 1 provides additional information about the data sources for stroke and its subtypes.
Study design
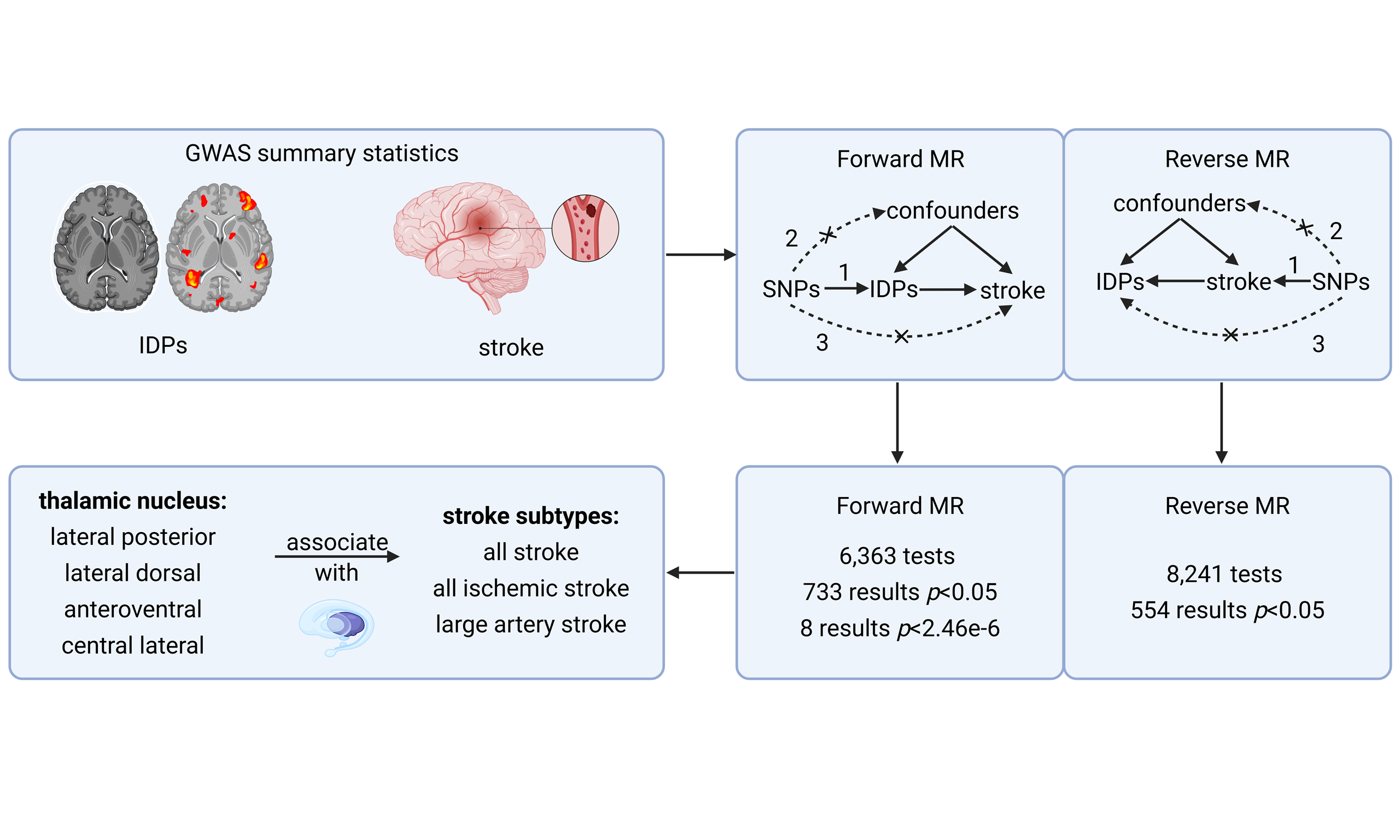
To investigate the causality between IDPs and stroke, we followed a four-step process based on Burgess’s study for Bidirectional Two-Sample Mendelian Randomization[12]. First, we collected GWAS summary data from public studies or websites mentioned earlier and organized the data into categories. Second, we selected SNPs as instrumental variables (IVs) for each IDP and stroke type based on three IV hypotheses. Third, we conducted a bidirectional two-sample MR using five methods separately for the causal directions of IDPs to stroke and stroke to IDPs. Lastly, we performed a sensitivity analysis to validate the IVs and ensure the reliability of the MR results. The workflow and a brief overview are presented in Figure 1.
Figure 1. Workflow of the causal inference between IDPs and stroke. Genome-wide significant SNPs (P < 5e-8, r2 ≤ 0.2) were used to assess associations from IDPs to stroke (forward MR) and from stroke to IDPs (reverse MR). IDPs are divided into 10 categories and stroke is divided into 5 subtypes. MR methods include inverse variance weighted, weighted median, weighted mode, simple mode, and MR Egger, and sensitivity analyses include leave-one-out, Cochran’s Q, and MR Egger. IDPs: Imaging-derived phentypes; WM: white matter; FA: fractional anisotropy; MO: diffusion tensor mode; ICVF: intra-cellular volume fraction; OD: orientation dispersion index; AS: all stroke; AIS: all ischemic stroke; CES: CardioEmbolic stroke; SVS: small vessel stroke; LAS: large artery stroke; MR: mendelian randomization.
Instrumental variable selection
The three IV assumptions, as outlined by MR guidelines[12], are as follows: (1) The variants exhibit a strong association with the exposure, (2) the variants are not associated with the outcome through any confounding factors, and (3) the variants do not directly impact the outcome, except via the exposure-outcome pathway. To select independent SNPs as IVs, we employed genome-wide conditional & joint association analysis (GCTA-COJO)[18]. This approach evaluates the variance explained by all SNPs on a chromosome or genome for a specific disease or trait to ensure the independence of multiple variants at a given locus. We applied a p-value threshold of 5e-8 and a linkage disequilibrium r-square less than 0.2 (gcta64 -bfile reference_panel_file -cojo-file gwas_file -cojo-slct -cojo-p 5e-8 -cojo-collinear 0.2) to select satisfactory SNPs, which were then included as IVs in our subsequent study. Supplementary Table 2 provides further information on the IVs for IDPs, while Supplementary Table 3 contains information on the IVs for stroke. The source code and data are openly accessible in the online GitHub repository (https://github.com/liusylab/IDP_Stroke_2SMR).
Statistical analysis
Specifically, in the forward MR, IDPs were treated as the exposure and stroke as the outcome; in the reverse MR, stroke was the exposure and IDPs were the outcome. In both analyses, we selected independent genetic variants associated with the exposure at genome-wide significance (P < 5e-8) with LD clumping (r2 ≤ 0.2). These instruments fulfill the IV assumptions: strongly associated with the exposure, not associated with confounders, and influence the outcome only through the exposure. At first, we used the harmonise_data function from the TwoSampleMR package to harmonize the exposure and outcome data. Then, we conducted bidirectional TSMR using five methods, including inverse variance weighted (IVW), MR Egger, weighted mode, simple mode, and weighted median. IVW was chosen as the primary method to explore the causal relationships between IDPs and stroke due to its robustness[19], while the other four methods were used to further validate the results. Sensitivity analysis was performed using MR Egger to assess horizontal pleiotropy[20], and leave-one-out analysis and Cochran’s Q test were also conducted. Leave-one-out analysis evaluated the reliability of the MR model, visually represented by a forest plot. Cochran’s Q test was employed to identify directional heterogeneity among SNPs. Additionally, MR Steiger analysis was performed to estimate the sensitivity ratio and assess the correctness of the causal direction. All statistical analyses were conducted using the TwoSampleMR packages in R version 4.2.2. To account for multiple testing, we applied Bonferroni correction across the entire MR analysis, setting the significance threshold at P < 2.46e-6 [0.05/ (2010*5*2)], where 2010*5 represents the number of all IDP-stroke pairs and 2 denotes forward and reverse MR tests.
RESULTS
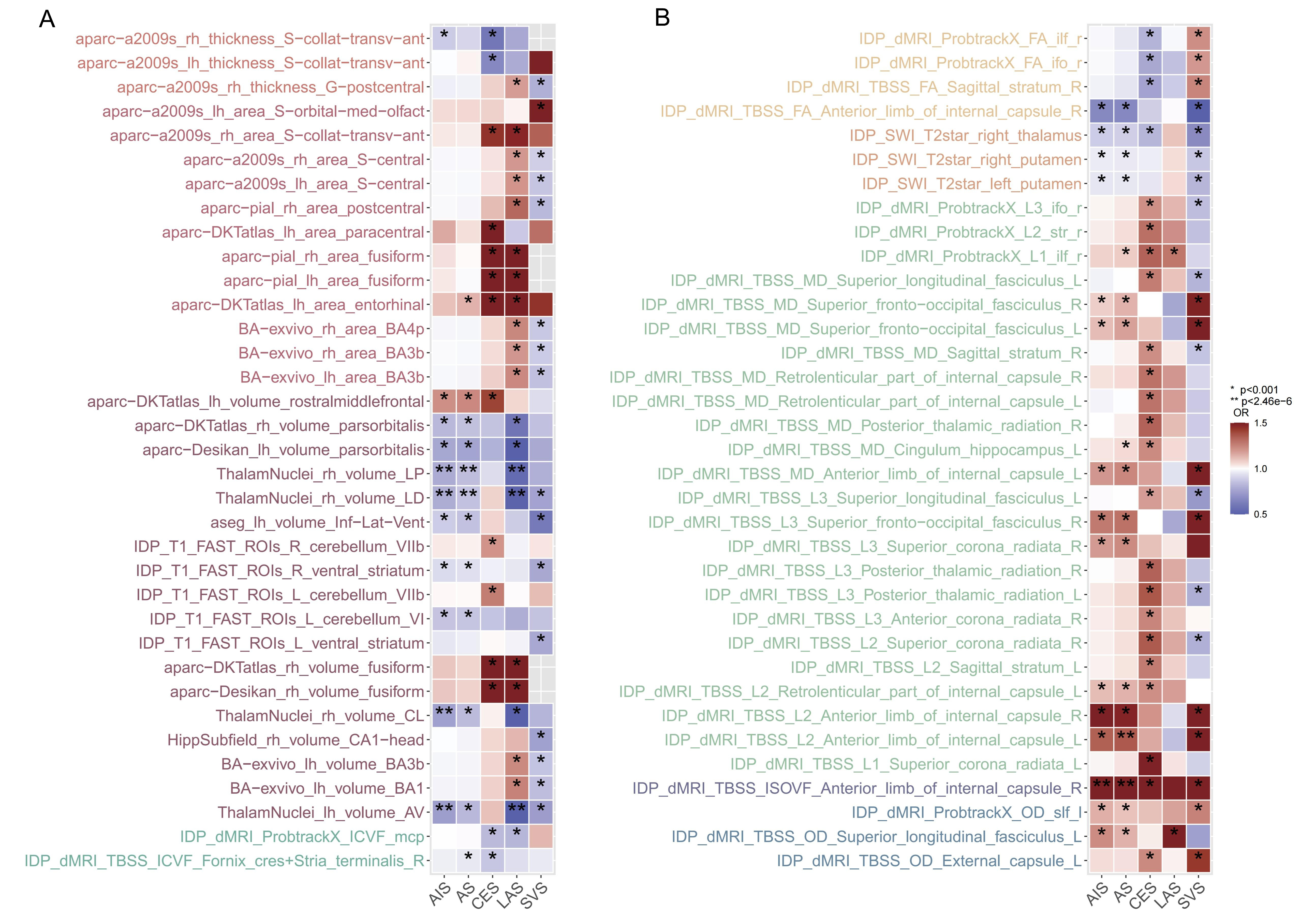
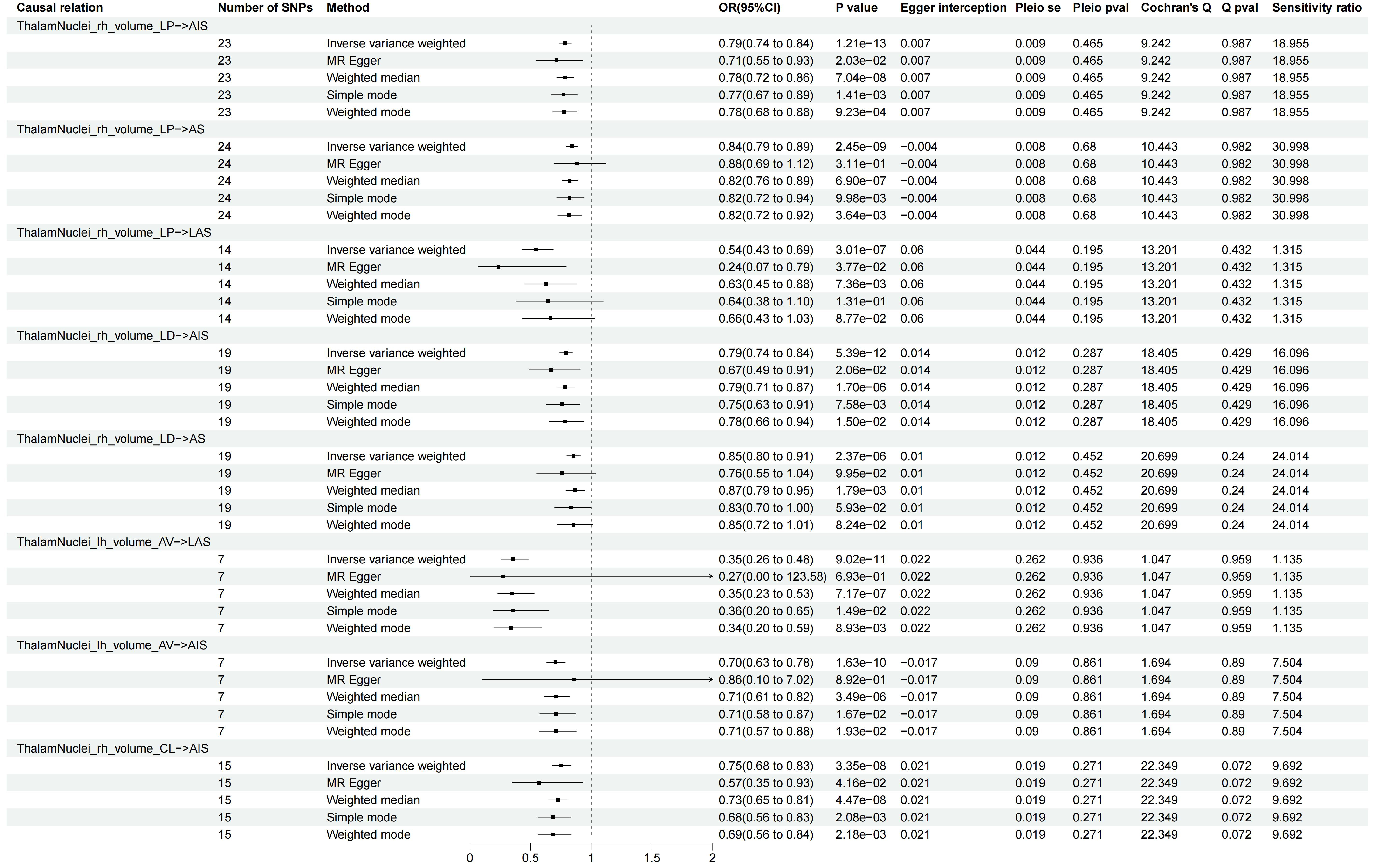
In the forward MR analysis, a total of 6,363 TSMR tests were conducted to assess the causal relationship between 1,289 IDPs and 5 stroke types. Using the robust IVW method, 733 results reached nominal significance (P < 0.05), involving 517 IDPs [Figure 2 and Supplementary Table 4]. Following the Bonferroni correction for multiple testing, we identified eight significant causal relationships between four IDPs and three stroke types. These associations were all within the same brain anatomy categories and specifically related to the volume of thalamic nuclei. All the results from the five MR methods, under the multiple testing correction, are provided in Figure 3 and visualized with the location and structure of the thalamus in Supplementary Figure 1.
Figure 2. Overview of the forward MR based on IVW. (A) Associations from IDPs to stroke, including regional and tissue volume, cortical area, cortical thickness, and WM tract ICVF. (B) Associations from stroke to IDPs, including regional T2*, WM tract FA, WM tract diffusivity, WM tract OD, and WM tract ISOVF. A Bonferroni-corrected significance threshold was set at P < 0.001 {0.05/[10 IDP categories × 5 stroke types]}. Only significant associations observed in both directions are presented. IDPs: Imaging-derived phentypes; WM: white matter; ICVF: intra-cellular volume fraction; FA: fractional anisotropy; OD: orientation dispersion index; ISOVF: isotropic or free water volume fraction; MR: mendelian randomization; IVW: including inverse variance weighted.
Figure 3. Significant associations in the forward MR analysis. Only associations that remained significant after Bonferroni correction (P < 2.46e-6) are shown. Arrows indicate that the confidence interval extends beyond the axis limit. LP: Lateral posterior; LD: lateral dorsal; AV: anteroventral; CL: central lateral; AS: all stroke; AIS: all ischemic stroke; CES: CardioEmbolic stroke; SVS: small vessel stroke; LAS: large artery stroke; MR: mendelian randomization.
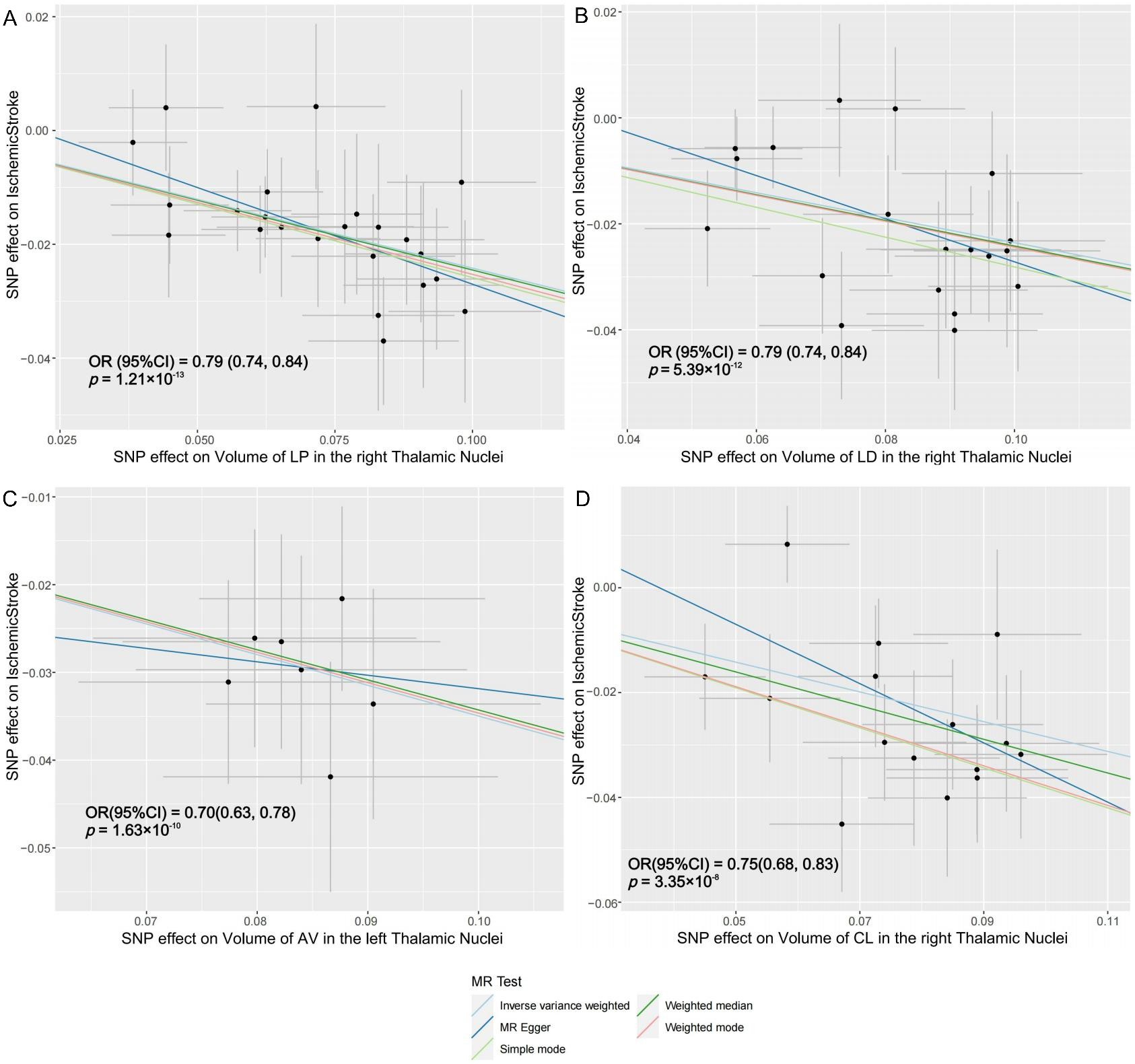
The lateral posterior (LP) thalamic nucleus receives extensive afferent fibers from the cerebral cortex. An increase of 1 s.d. in the volume of LP nucleus in the right hemisphere was associated with a 21% lower risk of AIS (OR = 0.79; 95%CI: 0.74 to 0.84; P = 1.21e-13), a 16% lower risk of AS (OR = 0.84; 95%CI: 0.79 to 0.89; P = 2.45e-9), and a 46% lower risk of LAS (OR = 0.54; 95%CI: 0.43 to 0.69; P = 3.01e-7). The lateral dorsal (LD) thalamic nucleus showed that an increase of 1 s.d. in its volume in the right hemisphere was associated with a 21% lower risk of AIS (OR = 0.79; 95%CI: 0.74 to 0.84; P = 5.39e-12) and a 15% lower risk of AS (OR = 0.85; 95%CI: 0.80 to 0.91; P = 2.37e-6). The anteroventral (AV) thalamic nucleus demonstrated that an increase of 1 s.d. in its volume in the left hemisphere was associated with a 65% lower risk of LAS (OR = 0.35; 95%CI: 0.26 to 0.48; P = 9.02e-11) and a 30% lower risk of AIS (OR = 0.70; 95%CI: 0.63 to 0.78; P = 1.63e-10). Similarly, the central lateral (CL) thalamic nucleus displayed that an increase of 1 s.d. in its volume in the right hemisphere was associated with a 25% lower risk of AIS (OR = 0.75; 95%CI: 0.68 to 0.83; P = 3.35e-8). Four significant IDPs are causally linked to AIS, with corresponding scatter plots presented in Figure 4. Additional scatter plots for other pairs can be found in Supplementary Figures 2-5.
Figure 4. Scatter plots of individual SNP effects and estimates from different MR methods. (A) Volume of the right LP thalamic nucleus and risk of ischemic stroke; (B) Volume of the right LD thalamic nucleus and risk of ischemic stroke; (C) Volume of the left AV thalamic nucleus and risk of ischemic stroke; (D) Volume of the left CL thalamic nucleus and risk of ischemic stroke. LP: Lateral posterior; LD: lateral dorsal; AV: anteroventral; CL: central lateral; MR: mendelian randomization.
Horizontal pleiotropy was assessed using the intercept term of MR Egger regression and funnel plots, which showed no evidence of its presence [Figure 3 and Supplementary Figures 6-13]. Directional heterogeneity was evaluated through Cochran’s Q test, which revealed no evidence of such heterogeneity [Figure 3]. The leave-one-out analysis demonstrated minimal change in the results after removing any individual SNP, indicating the high reliability of the MR models [Supplementary Figures 14-21]. In summary, the sensitivity analyses confirmed the reliability of our forward MR results.
In the reverse MR analysis, we performed 8,241 tests to examine the causal relationship between stroke and IDPs [Supplementary Table 5]. Using IVW, we identified 554 nominally significant results (P < 0.05), involving 464 IDPs [Supplementary Figure 22]. However, after applying the Bonferroni correction, none of the results remained significant.
DISCUSSION
Our study conducted bidirectional TSMR analyses to comprehensively investigate the causal relationship between brain IDPs and stroke or its subtypes in the European population. To ensure statistical validity, we utilized the largest available cohorts with genetic information for IDPs and stroke. We identified several IDPs that showed nominal significance with stroke and its subtypes in both causal directions (P < 0.05). After correcting for multiple tests, eight associations remained significant in the forward MR analysis, while no results remained significant in the reverse MR analysis.
In the forward MR analysis, we identified four IDPs causally associated with three stroke types, all related to the volume of thalamic nuclei. The LP thalamic nucleus - a thalamic subregion involved in visual processing[21] and rotation response[22]- showed negative associations with AIS, AS, and LAS. Stroke involving the LP nucleus often manifests as hemihypesthesia, hemiataxia, and executive dysfunction, typically resulting from arterial embolism and microangiopathy[23]. Notably, a pure sensory stroke, presenting either as complete hemisensory syndrome with a facio-brachio-crural distribution or as partial sensory deficits, is often attributable to a lacunar infarct involving the ventral posterolateral nucleus of the thalamus[24]. This well-established clinical-anatomical correlation underscores the potential relevance of thalamic subregion volume alterations in stroke pathophysiology and symptom presentation. The LD nucleus, a thalamic region connected to the posterior parietal cortex and cingulate gyrus[25], which play roles in spatial learning and memory processing[26], demonstrated negative associations with AIS and AS. However, no studies to date have explored the relationship between LD volume and stroke. The AV nucleus - a thalamic region with connections to the frontal cortex[27] and cingulate gyrus[28], implicated in behavioral activation[29] and learning functions[30] - exhibited a negative correlation between left AV volume and LAS risk. Although reduced AV volume has been reported in psychiatric disorders such as schizophrenia[31], no studies have investigated the association between the AV nucleus and stroke. The CL nucleus, part of the rostral intralaminar nuclei, receives inputs from limbic, sensorimotor, and cerebellar regions and projects to the frontal cortex and dorsal striatum[32]. It is thought to contribute to arousal[33] and motor control[34]. Our results indicated that a smaller right CL volume was associated with a higher risk of AIS. However, no studies have measured CL volume or examined its relationship with stroke. Previous research suggests that white matter loss following thalamic infarction may drive the cortical reshaping[35]. Stroke can result from cerebral vessels with different anatomical structures through various mechanisms[36]. Based on these findings, we hypothesize that genetically predicted reductions in thalamic nucleus volume may serve as early indicators of stroke susceptibility. Such structural alterations could reflect underlying microvascular or neuroanatomical vulnerabilities, such as increased tortuosity of nerve fibers or blood vessels, and may function as diagnostic or prognostic biomarkers.
While the evidence regarding specific thalamic nuclei and stroke types is limited, the thalamus has been consistently associated with stroke. Children with perinatal AIS exhibit smaller volumes of both thalami compared to control groups[37]. Similarly, non-thalamic stroke patients also display smaller thalamic volume on both sides[38]. Most observational studies have reported smaller volumes in the ipsilateral or bilateral thalamus, which may partially align with our findings but requires further investigation for confirmation. Assuming that the subcortical grey matter remains similar before and after a stroke within six weeks[39], the smaller thalamic volume before the stroke likely has an adverse impact by limiting resources[40]. Building on the association between lacunar infarct and the thalamus nucleus[24], a valuable direction for future research would be to explore how imaging-derived thalamic features differ in their associations with lacunar versus non-lacunar acute ischemic stroke. Given the unique pathophysiology, prognosis, and clinical manifestations of lacunar infarcts relative to other ischemic stroke subtypes, such analyses could yield more refined insights into the structural underpinnings of stroke subtype vulnerability.
Though clinical and observational studies have indicated that stroke can lead to various changes in IDPs, we did not establish a causal relationship between stroke and IDPs in our reverse MR analyses. Since the imaging data were obtained from a healthy population, it is expected that no reverse causality will be observed. On the other hand, this may suggest that the intricate mechanisms of neural connections account for the absence of causality, or the effects are too subtle to detect in our study.
Our findings differ significantly from previous bidirectional TSMR findings by Yu et al., published in BMC Medicine[14], and Jia et al., in Cerebral Cortex[13]. Since Jia’s work is similar to that of BMC, but with a narrower range, we primarily compare and discuss our results with those from Yu. Yu identified potential causal effects between decreased FA, increased MD, and ISOVF on stroke in the forward MR analysis, as well as the causal effect of stroke on ISOVF in the reverse MR analysis. However, these associations were not observed in our study. A comparison of the two studies is presented in Supplementary Table 6. The disparity in results could be attributed to two main reasons. First, we used the largest stroke GWAS dataset from GIGASTROKE[15], while Yu’s study used data from MEGASTROKE[14]. A larger GWAS dataset enhances the statistical power to assess causal relationships while reducing the likelihood of false positives. Second, the criteria for selecting IVs differed between the two studies, likely leading to variations in the IVs included. We were unable to replicate Yu’s tests using their IVs because the IV data they provided contained repeated entries and errors. Additionally, the significant IDP-stroke pairs identified in our study were not included in Yu’s study because they preselected IDPs prior to conducting the MR analysis.
Our study employed bidirectional TSMR using SNPs as instrumental variables to investigate the causal relationships between IDPs and stroke. This approach mitigated environmental or lifestyle confounding factors and demonstrated greater effectiveness compared to observational case-control studies. Additionally, we utilized the largest available GWAS summary data for both exposure and outcome, enhancing statistical power. Our findings identified a robust association between the volume of specific thalamic nuclei and stroke risk, suggesting that these brain imaging features may serve as early indicators, intermediate phenotypes, or potential biomarkers for stroke susceptibility. This previously underexplored relationship warrants further investigation through longitudinal and mechanistic studies.
Despite the advantages, there are a few limitations of our study. The selection of traits for bidirectional TSMR analysis was not based on careful consideration of existing observational studies, highlighting the need for further research to determine the exact nature of these relationships. However, screening of the IDPs without selection of the traits also ensures a comprehensive understanding of the causality relationships between IDPs and stroke. The population under investigation in this study is limited to individuals of European descent due to data availability constraints. Nevertheless, the insights gained and the methodologies employed in this research can serve as a valuable reference for future investigations among non-European populations. Additionally, we applied a conservative multiple-testing correction, which may have led to the exclusion of previously reported or potentially significant associations, but it guaranteed the robustness of our results. For instance, our study did not find a significant causal association between MD in the superior fronto-occipital fasciculus and stroke, despite its reported significance in Yu’s work[14]. This discrepancy could be attributed to differences in IV screening conditions and/or the utilization of a larger database. Further validation on the reasons for the discrepancy will require a complete release of the IVs in Yu’s work. We have made our IVs completely publicly available to facilitate the reproducibility of our study.
CONCLUSION
In summary, our study utilized bidirectional TSMR analysis with the largest available GWAS summary data to investigate the causal relationship between brain IDPs and stroke or its subtypes. We identified causal associations between the volume of thalamic nuclei and stroke, contributing to our understanding of the link between brain anatomy structure and stroke. These findings may have implications for potential strategies in predicting and intervening in stroke risk at the brain imaging level. By identifying individuals with specific brain IDPs that are genetically associated with an increased risk of stroke, clinicians may be able to recommend appropriate lifestyle modifications or initiate closer monitoring. This could facilitate the implementation of targeted prevention strategies in high-risk populations. Future research integrating multimodal imaging, longitudinal follow-up, and functional validation may help clarify how thalamic features evolve over time in individuals at risk for stroke. Moreover, examining gene-environment interactions could further illuminate the biological pathways contributing to stroke susceptibility.
DECLARATIONS
Authors’ contributions
Conceived and designed the study: Liu S, Cheng S (Shiyao Cheng), Cheng S (Si Cheng)
Collected GWAS summary data: Cheng S (Shiyao Cheng), Lin X, Yang X, Lin S, Su P, Tang X, Wu J
Conducted IV selection, MR analysis, and drafted the manuscript: Yang X
Revised the manuscript: Liu S, Cheng S (Shiyao Cheng), Yang X
Availability of data and materials
The GWAS summary statistics utilized in our study were sourced from the largest-scale published GWA studies on both IDPs and stroke. Further information about our study is provided in Supplementary Tables and Supplementary Figures. All supplementary data for this study are accessible in the online open-access repository (https://github.com/liusylab/IDP_Stroke_2SMR).
Financial support and sponsorship
This study was supported by grants from the National Key R&D Program of China (2022YFC2502400, 2022YFC2502402, 2022YFE0209600), the National Natural Science Foundation of China (82101359), and the Young Elite Scientists Sponsorship Program by CAST (2023QNRC001).
Conflicts of interest
Liu S (Siyang Liu) is a Junior Editorial Board member of Journal of Translational Genetics and Genomics. Liu S (Siyang Liu) was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study was based entirely on publicly available summary-level data from previously published GWAS. No individual-level or identifiable human data were used. Therefore, ethical approval and informed consent were not required.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
2. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820.
3. Gasull T, Arboix A. Molecular mechanisms and pathophysiology of acute stroke: emphasis on biomarkers in the different stroke subtypes. Int J Mol Sci. 2022;23:9476.
5. Gong W, Beckmann CF, Smith SM. Phenotype discovery from population brain imaging. Med Image Anal. 2021;71:102050.
6. Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523-36.
7. Kaffashian S, Tzourio C, Zhu YC, Mazoyer B, Debette S. Differential effect of white-matter lesions and covert brain infarcts on the risk of ischemic stroke and intracerebral hemorrhage. Stroke. 2016;47:1923-5.
8. Liu J, Wang C, Qin W, et al. Cortical structural changes after subcortical stroke: patterns and correlates. Hum Brain Mapp. 2023;44:727-43.
9. Zhang Q, Wang D, Wu S, et al. Diffuse tract damage correlates with global cognitive impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a tract-based spatial statistics study. J Comput Assist Tomogr. 2021;45:285-93.
10. Siemonsen S, Mouridsen K, Holst B, et al. Quantitative t2 values predict time from symptom onset in acute stroke patients. Stroke. 2009;40:1612-6.
11. Wang Z, Zhang S, Liu C, et al. A study of neurite orientation dispersion and density imaging in ischemic stroke. Magn Reson Imaging. 2019;57:28-33.
12. Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35:1880-906.
13. Jia Y, Sun H, Sun L, et al. Mendelian randomization analysis implicates bidirectional associations between brain imaging-derived phenotypes and ischemic stroke. Cereb Cortex. 2023;33:10848-57.
14. Yu K, Chen XF, Guo J, et al. Assessment of bidirectional relationships between brain imaging-derived phenotypes and stroke: a Mendelian randomization study. BMC Med. 2023;21:271.
15. Mishra A, Malik R, Hachiya T, et al; COMPASS Consortium. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. 2022;611:115-23.
16. Yang X, Cheng S, Lin S, et al. Brain imaging-derived phenotypes and stroke: a bidirectional Mendelian randomization study unveils causal links between thalamic nuclei volume and stroke risk in the European population. medRxiv. 2025:2025.03.22.25324441.
17. Smith SM, Douaud G, Chen W, et al. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat Neurosci. 2021;24:737-45.
18. Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76-82.
19. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985-98.
20. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512-25.
21. Casanova C, Nordmann JP, Molotchnikoff S. Pulvina-lateralis posterior nucleus complex of mammals and the visual function. J Physiol. 1991;85:44-57.
22. Motles E, Infante C, González M. Role of the pulvinar lateralis posterior complex on the rotation response. Relation with other cerebral structures. Pharmacological systems involved in this behavior. Acta Physiol Pharmacol Latinoam. 1985;35:237-49.
23. Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke. 2004;35:2826-31.
24. Arboix A, García-Plata C, García-Eroles L, et al. Clinical study of 99 patients with pure sensory stroke. J Neurol. 2005;252:156-62.
25. Shibata H, Naito J. Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Res. 2005;1059:93-103.
26. Bezdudnaya T, Keller A. Laterodorsal nucleus of the thalamus: a processor of somatosensory inputs. J Comp Neurol. 2008;507:1979-89.
27. Yeterian EH, Pandya DN. Corticothalamic connections of paralimbic regions in the rhesus monkey. J Comp Neurol. 1988;269:130-46.
28. Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain. 1980;103:525-54.
29. Luppi M, Cerri M, Di Cristoforo A, et al. c-Fos expression in the limbic thalamus following thermoregulatory and wake-sleep changes in the rat. Exp Brain Res. 2019;237:1397-407.
30. Shibata H, Yoshiko H. Thalamocortical projections of the anteroventral thalamic nucleus in the rabbit. J Comp Neurol. 2015;523:726-41.
31. Forno G, Saranathan M, Contador J, et al. Thalamic nuclei changes in early and late onset Alzheimer’s disease. Curr Res Neurobiol. 2023;4:100084.
32. Vertes RP, Linley SB, Rojas AKP. Structural and functional organization of the midline and intralaminar nuclei of the thalamus. Front Behav Neurosci. 2022;16:964644.
33. Xu J, Galardi MM, Pok B, et al. Thalamic stimulation improves postictal cortical arousal and behavior. J Neurosci. 2020;40:7343-54.
34. Sakayori N, Kato S, Sugawara M, et al. Motor skills mediated through cerebellothalamic tracts projecting to the central lateral nucleus. Mol Brain. 2019;12:13.
35. Conrad J, Habs M, Ruehl RM, et al. White matter volume loss drives cortical reshaping after thalamic infarcts. Neuroimage Clin. 2022;33:102953.
36. Kim YS, Kim BJ, Noh KC, et al. Distal versus proximal middle cerebral artery occlusion: different mechanisms. Cerebrovasc Dis. 2019;47:238-44.
37. Ilves N, Lõo S, Ilves N, et al. Ipsilesional volume loss of basal ganglia and thalamus is associated with poor hand function after ischemic perinatal stroke. BMC Neurol. 2022;22:23.
38. Geng J, Gao F, Ramirez J, et al. Secondary thalamic atrophy related to brain infarction may contribute to post-stroke cognitive impairment. J Stroke Cerebrovasc Dis. 2023;32:106895.
39. Egorova N, Liem F, Hachinski V, Brodtmann A. Predicted brain age after stroke. Front Aging Neurosci. 2019;11:348.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].