Rethinking miRNAs in MSC-sEV therapeutics: implications for manufacture, mechanism of action, and development of robust potency CQAs
Abstract
Mesenchymal stromal cell-derived small extracellular vesicles (MSC-sEVs) have emerged as a promising cell-free alternative to MSC-based therapies, offering superior safety, scalability, and stability profiles. These nanosized vesicles are now widely regarded as the principal therapeutic effectors of MSCs, capable of recapitulating many of the benefits attributed to their parental cells. However, their successful clinical translation depends on overcoming key challenges, particularly those related to product variability, viral safety, and the definition of mechanistically relevant potency-associated critical quality attributes (CQAs). This review explores the sources of MSC-sEV variability, including MSC tissue origin, manufacturing parameters, and limitations associated with primary and pluripotent stem cell-derived MSCs. The use of immortalized monoclonal MSC lines is discussed as a potential solution to improve batch consistency. Regulatory frameworks such as the International Council for Harmonisation (ICH) guideline Q5A(R2) are also highlighted for ensuring viral safety in sEV manufacturing processes. A major focus is the critical evaluation of microRNAs (miRNAs) - long regarded as leading candidates for potency CQAs in MSC-sEV products. Despite their prevalence in the extracellular vesicle literature, mounting evidence challenges their functional relevance in therapeutic contexts. Studies consistently show that miRNAs are underrepresented in sEVs, occur at very low copy numbers, and lack essential components (e.g., Argonaute proteins) required for canonical RNA interference. Moreover, the efficiency of EV internalization and endosomal escape remains exceedingly low, rendering miRNA-based gene regulation mechanistically implausible at physiologically relevant doses. These findings call into question the widespread assumption that miRNAs are primary effectors of MSC-sEV activity.
Keywords
INTRODUCTION: FROM CELLS TO VESICLES - RETHINKING MSC THERAPEUTIC ACTIVITY
The discovery of mesenchymal stromal cell-derived small extracellular vesicles (MSC-sEVs) has fundamentally reshaped our understanding of MSC-based therapies. Initial studies revealed that the therapeutic benefits of MSCs were not dependent on their long-term engraftment or differentiation. A seminal 2002 review by Chopp and Li highlighted that both implanted and intravenously administered MSCs yielded similar functional recovery despite minimal in vivo persistence or differentiation[1]. These findings led to the hypothesis that MSCs mediate tissue repair predominantly through the secretion of bioactive factors - such as granulocyte colony-stimulating factor (G-CSF), stem cell factor (SCF), leukemia inhibitory factor (LIF), macrophage colony-stimulating factor (M-CSF), interleukin (IL)-6, and IL-11[2,3].
While initial efforts focused on soluble proteins and small molecules, a pivotal 2008 study revealed that the active fraction of the MSC secretome exceeded 1,000 kDa, suggesting the involvement of large macromolecular complexes[4]. In 2009, Bruno et al. reported that MSC-derived microvesicles (80-1,000 nm) could ameliorate acute kidney injury in mice[5]. Shortly thereafter, Lai et al. demonstrated that exosomes (~100-130 nm) - a subtype of small extracellular vesicles - conferred therapeutic benefit in a myocardial ischemia model[6]. In follow-up work, Bruno et al. further refined the active vesicle size to < 200 nm[7].
These insights catalyzed a paradigm shift, establishing MSC-sEVs within the 100-200 nm range - including exosomes and small microvesicles - as the principal effectors of MSC function[8]. Numerous preclinical studies have shown that MSC-sEVs can recapitulate or even exceed the therapeutic efficacy of their parental MSCs across diverse disease models[5,9-11], positioning them as a promising class of next-generation, cell-free regenerative therapeutics.
ADVANTAGES OVER CELL-BASED THERAPIES
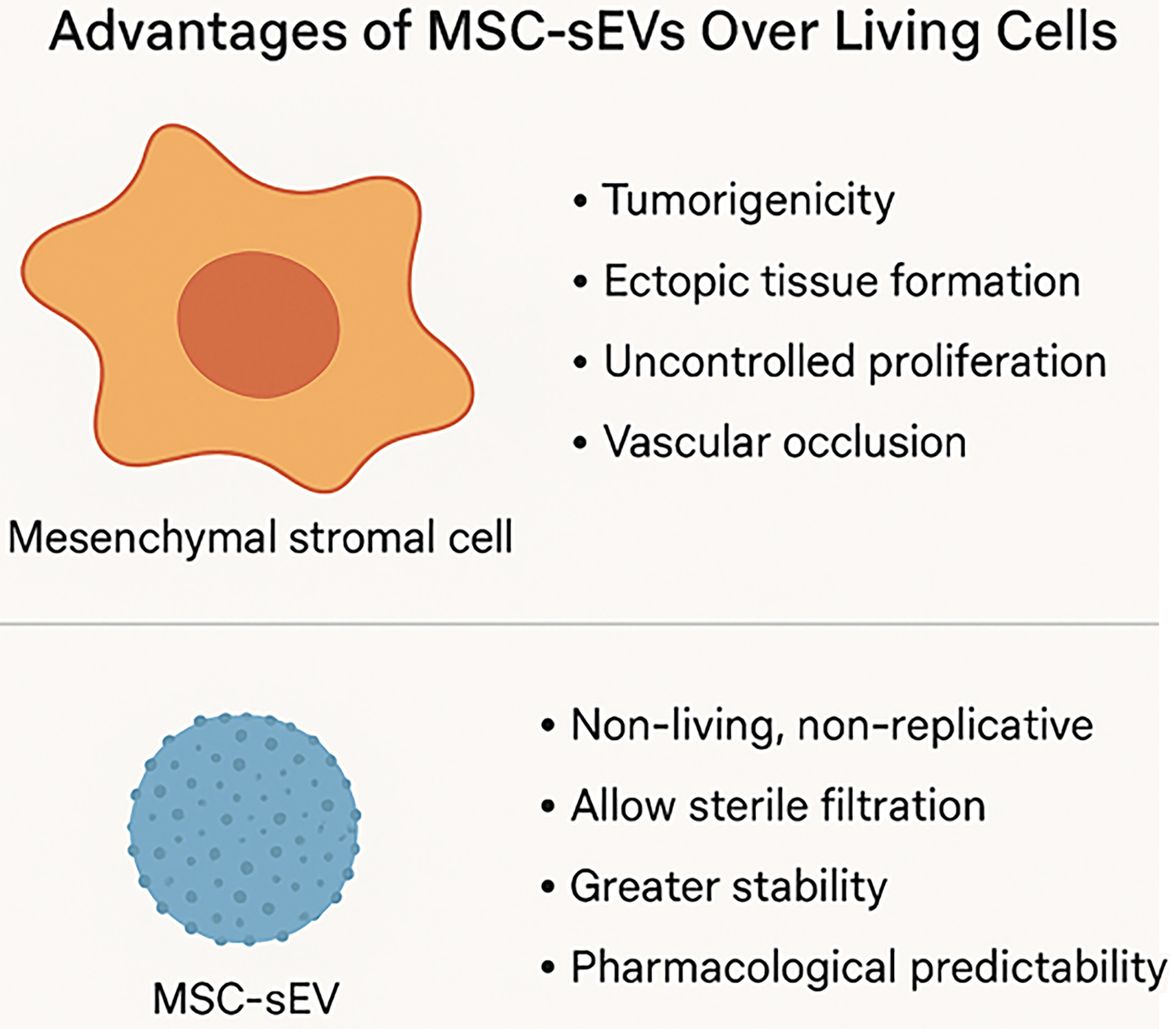
MSC-sEVs offer several distinct advantages over conventional cell-based therapies. As non-living, non-replicative biological entities, MSC-sEVs inherently avoid risks associated with viable cell administration, including tumorigenicity, ectopic tissue formation, and uncontrolled proliferation. Their nanoscale size minimizes the risk of vascular occlusion following systemic delivery and enables sterile filtration - an essential feature for meeting Good Manufacturing Practice (GMP) requirements in scalable, standardized bioproduction.
A major logistical advantage of MSC-sEVs lies in their stability. They can be lyophilized without compromising structural integrity or functional potency, facilitating long-term storage at ambient or refrigerated conditions. This dramatically reduces reliance on cold chain logistics, one of the major cost and infrastructure barriers limiting the widespread deployment of cell therapies. Such shelf stability supports broader geographic access, point-of-care administration, and the development of off-the-shelf formulations for both topical and parenteral applications.
Importantly, MSC-sEVs also exhibit greater pharmacological predictability. Unlike living MSCs - which dynamically respond to complex and often unpredictable in vivo cues - MSC-sEVs deliver a more consistent therapeutic profile, reflective of their defined in vitro characteristics. This reduced biological variability enhances their reproducibility in preclinical and clinical settings and facilitates more straightforward potency testing and regulatory evaluation [Figure 1].
Figure 1. Diagrammatic summary: advantages of MSC-sEVs over MSCs (Figure was created with AI-assisted technologies). MSC: Mesenchymal stem cell; sEV: small extracellular vesicle.
Taken together, these properties position MSC-sEVs as a practical, safe, and scalable alternative to living cell therapies across a wide range of clinical and therapeutic applications. Notably, MSC-sEVs have already entered clinical evaluation, marking a critical step toward their therapeutic translation [Table 1].
A list of MSC EV-based clinical trials registered at clinicaltrials.gov [Search terms: Intervention AND MSC AND (exosomes OR extracellular vesicles; date 19 August 2025)]
| NCT number | Study title | Study URL |
| NCT02138331 | Effect of microvesicles and exosomes therapy on Î2-cell Mass in T1DM | https://clinicaltrials.gov/study/NCT02138331 |
| NCT03384433 | Allogenic mesenchymal stem cell derived exosome in patients with acute ischemic stroke | https://clinicaltrials.gov/study/NCT03384433 |
| NCT03437759 | MSC-exos promote healing of macular holes | https://clinicaltrials.gov/study/NCT03437759 |
| NCT04173650 | MSC EVs in dystrophic epidermolysis bullosa | https://clinicaltrials.gov/study/NCT04173650 |
| NCT04356300 | Exosome of mesenchymal stem cells for multiple organ dysfuntion syndrome after surgical repaire of acute type a aortic dissection [Note: The title contains spelling errors (“Dysfuntion” and “Repaire”) in the original publication.] | https://clinicaltrials.gov/study/NCT04356300 |
| NCT04366063 | Mesenchymal stem cell therapy for SARS-CoV-2-related acute respiratory distress syndrome | https://clinicaltrials.gov/study/NCT04366063 |
| NCT04388982 | The safety and the efficacy evaluation of allogenic adipose MSC-exos in patients with alzheimer’s disease | https://clinicaltrials.gov/study/NCT04388982 |
| NCT04491240 | Evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2 associated pneumonia | https://clinicaltrials.gov/study/NCT04491240 |
| NCT04602442 | Safety and efficiency of method of exosome inhalation in COVID-19 associated pneumonia | https://clinicaltrials.gov/study/NCT04602442 |
| NCT04798716 | The use of exosomes for the treatment of acute respiratory distress syndrome or novel coronavirus pneumonia caused by COVID-19 | https://clinicaltrials.gov/study/NCT04798716 |
| NCT04850469 | Study of MSC-exo on the therapy for intensively ill children | https://clinicaltrials.gov/study/NCT04850469 |
| NCT05060107 | Intra-articular injection of MSC-derived exosomes in knee osteoarthritis (ExoOA-1) | https://clinicaltrials.gov/study/NCT05060107 |
| NCT05216562 | Efficacy and safety of EXOSOME-MSC therapy to reduce hyper-inflammation in moderate COVID-19 patients | https://clinicaltrials.gov/study/NCT05216562 |
| NCT05243368 | Evaluation of personalized nutritional intervention on wound healing of cutaneous ulcers in diabetics | https://clinicaltrials.gov/study/NCT05243368 |
| NCT05261360 | Clinical efficacy of exosome in degenerative meniscal injury | https://clinicaltrials.gov/study/NCT05261360 |
| NCT05402748 | Safety and efficacy of injection of human placenta mesenchymal stem cells derived exosomes for treatment of complex anal fistula | https://clinicaltrials.gov/study/NCT05402748 |
| NCT05499156 | Safety of injection of placental mesenchymal stem cell derived exosomes for treatment of resistant perianal fistula in crohn’s patients | https://clinicaltrials.gov/study/NCT05499156 |
| NCT05523011 | Safety and tolerability study of MSC exosome ointment | https://clinicaltrials.gov/study/NCT05523011 |
| NCT05669144 | Co-transplantation of mesenchymal stem cell derived exosomes and autologous mitochondria for patients candidate for CABG surgery | https://clinicaltrials.gov/study/NCT05669144 |
| NCT05738629 | Safety and efficacy of pluripotent stem cell-derived mesenchymal stem cell exosome (PSC-MSC-Exo) eye drops treatment for dry eye diseases post refractive surgery and associated with blepharospasm | https://clinicaltrials.gov/study/NCT05738629 |
| NCT05808400 | Safety and efficacy of umbilical cord mesenchymal stem cell exosomes in treating chronic cough after COVID-19 | https://clinicaltrials.gov/study/NCT05808400 |
| NCT05808400 | Safety and efficacy of umbilical cord mesenchymal stem cell exosomes in treating chronic cough after COVID-19 | https://clinicaltrials.gov/study/NCT05808400 |
| NCT05871463 | Effect of mesenchymal stem cells-derived exosomes in decompensated liver cirrhosis | https://clinicaltrials.gov/study/NCT05871463 |
| NCT05940610 | The safety and efficacy of MSC-EVs in acute/acute-on-chronic liver failure | https://clinicaltrials.gov/study/NCT05940610 |
| NCT06202547 | Intra-ovarian injection of MSC-EVs in idiopathic premature ovarian failure | https://clinicaltrials.gov/study/NCT06202547 |
| NCT06279741 | Safety and efficacy of MSC-EVs in the prevention of BPD in extremely preterm infants | https://clinicaltrials.gov/study/NCT06279741 |
| NCT06431152 | Intra-articular injection of UC-MSC exosome in knee osteoarthritis | https://clinicaltrials.gov/study/NCT06431152 |
| NCT06545175 | Intracochlear application of VSF1.01 for the reduction of cochlear implant surgery related trauma | https://clinicaltrials.gov/study/NCT06545175 |
| NCT06598202 | Exploring nasal drop therapy with small extracellular vesicles for ALS | https://clinicaltrials.gov/study/NCT06598202 |
| NCT06599346 | Effects of mesenchymal stem cell supernatant on prevention and treatment of skin/mucosal injury in hematology patients | https://clinicaltrials.gov/study/NCT06599346 |
| NCT06812637 | Efficacy and safety of wharton’s jelly-derived mesenchymal stem cell exosomes in the treatment of diabetic foot ulcers: a double-blinded randomized controlled clinical trial | https://clinicaltrials.gov/study/NCT06812637 |
| NCT06825572 | MSC-EVs in acute/ acute-on-chronic liver failure after liver transplantation | https://clinicaltrials.gov/study/NCT06825572 |
| NCT06866184 | Regenerative effects of birth material derived extracellular vesicles | https://clinicaltrials.gov/study/NCT06866184 |
| NCT06919380 | Nebulized MSC-exos for Anti-MDA5+ RP-ILD: safety and efficacy trial | https://clinicaltrials.gov/study/NCT06919380 |
| NCT06995625 | STEVIA | https://clinicaltrials.gov/study/NCT06995625 |
SOURCES OF VARIABILITY IN MSC-sEV PRODUCTS
Manufacturing variables
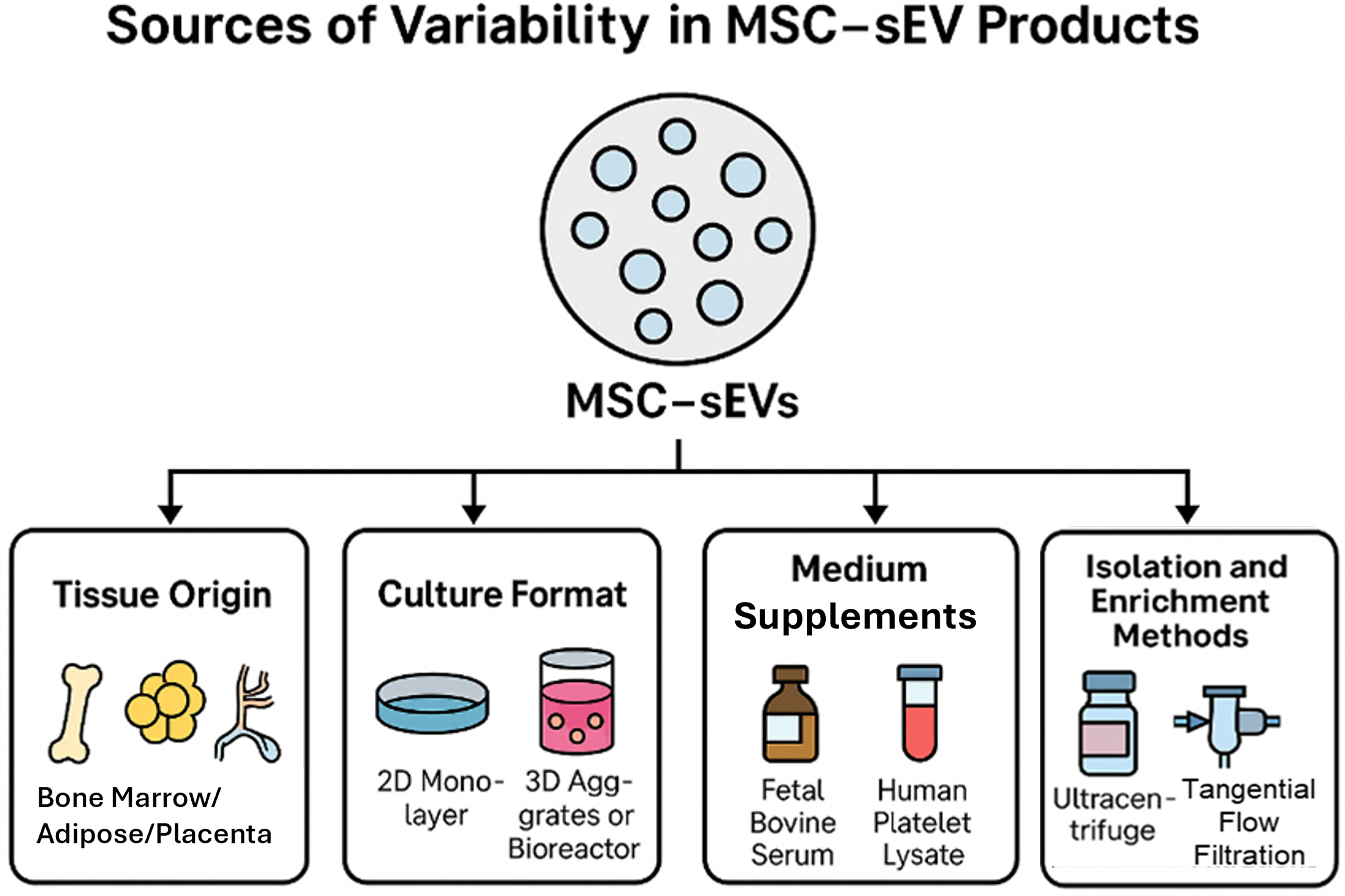
The composition and functional attributes of MSC-sEVs are highly sensitive to upstream manufacturing conditions[12,13]. Key sources of variability include:
· The tissue origin of MSCs [e.g., bone marrow[14], adipose tissue[15], placenta[16], umbilical cord[17], embryonic[18] or induced pluripotent stem cells (PSCs)[19]];
· Culture format (2D monolayer vs. 3D aggregates or bioreactors);
· Medium supplements (e.g., fetal bovine serum, human platelet lysate);
· Isolation and enrichment methods (e.g., ultracentrifugation, size-exclusion chromatography, tangential flow filtration).
Among these, the MSC tissue source is especially impactful, as it influences the baseline transcriptomic, proteomic, and secretory profiles of both cells and their derived sEVs.
Limitations of primary and PSC-derived MSCs
While primary MSCs are widely used and supported by existing clinical experience, they face inherent limitations such as donor-to-donor heterogeneity, finite expansion potential, and senescence-associated functional decline[12,13]. Similar challenges affect MSCs derived from PSCs, which - although scalable - may still exhibit batch-to-batch variability and developmental heterogeneity[18].
Impact of cellular heterogeneity on sEV consistency
A critical but unresolved issue is how variability at the cellular level translates to variability in sEV products. Although some early studies suggested general functional conservation among MSC-sEVs[20], more rigorous comparative studies contradict this view. For example, Madel et al. (2023) demonstrated significant differences in immunomodulatory activity among sEVs derived from MSCs of different donors - even when cultured and processed under identical conditions[21]. These findings emphasize that both intrinsic factors (e.g., donor genetics, cell age) and extrinsic factors (e.g., media, isolation method) contribute to variability in MSC-sEV profiles [Figure 2].
TOWARD CONSISTENT AND SAFE MSC-sEV PRODUCTION
Immortalized monoclonal MSC lines: a path to standardization
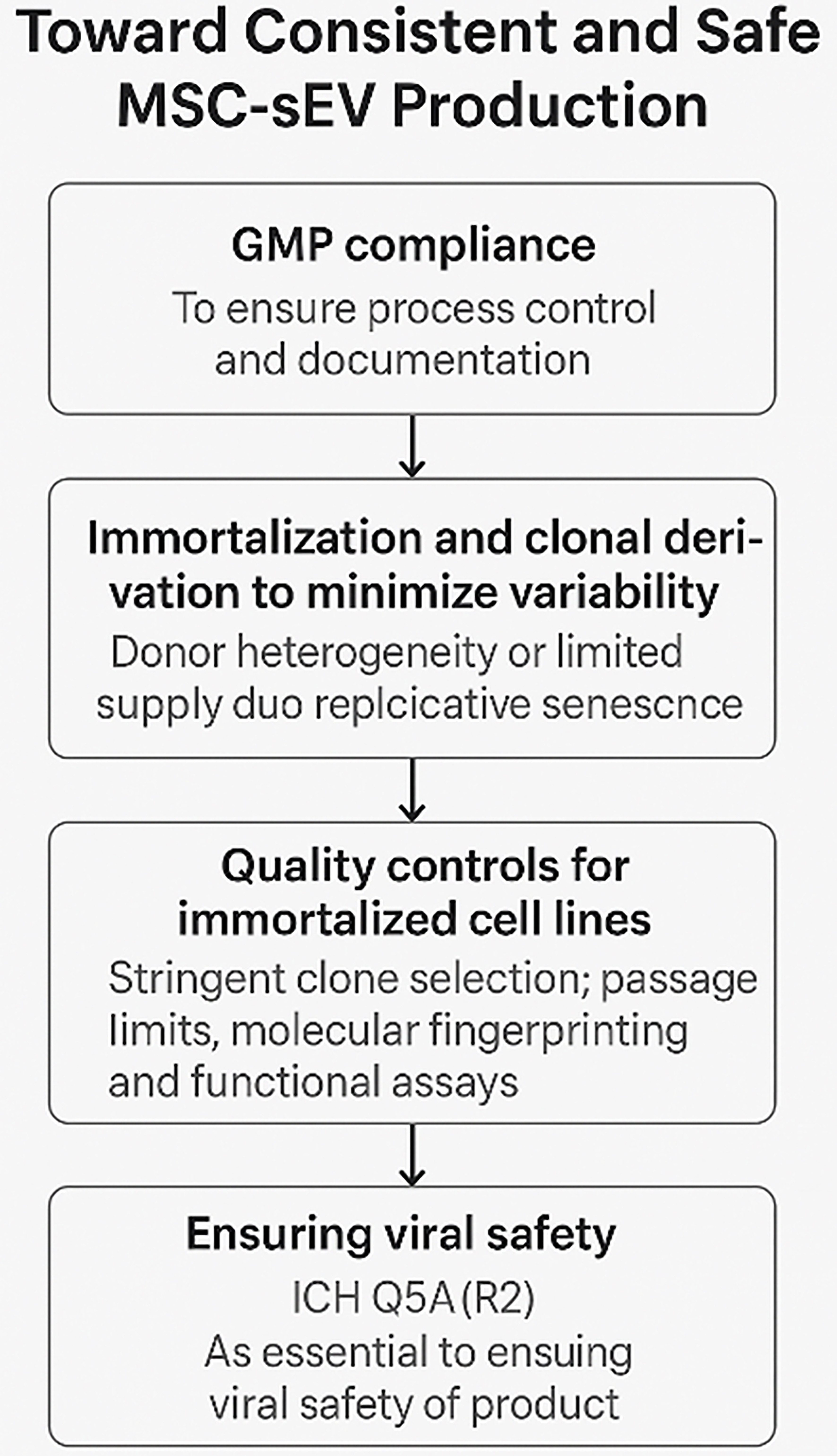
GMP compliance is necessary but insufficient to eliminate biological variability. GMP addresses process control and documentation but does not solve inherent issues related to donor sourcing, cellular heterogeneity, or replicative senescence[22,23]. Thus, even under GMP, consistent MSC-sEV production remains elusive unless variability at the source cell level is addressed. To mitigate these issues, Chen et al. proposed the use of immortalized, clonally selected MSC lines and this was subsequently adopted by others[24,25,26]. These cell lines offer theoretically unlimited expansion potential and allow for tighter control over input variability. When derived from a single MSC clone and appropriately characterized, such lines provide a more reproducible and scalable source of sEVs. Despite these advantages, immortalized monoclonal MSCs are not exempt from genetic and epigenetic drift. Asymmetric division, spontaneous mutations, or subtle transcriptomic shifts can occur over extended passaging as widely documented in other cell lines[13,21]. In the context of MSC-derived extracellular vesicles (MSC-EVs), passage number - and by extension, the cumulative impact of cellular drift - has been shown to affect both the potency and yield of secreted vesicles[27-29]. Consequently, rigorous clone selection, passage number control, and routine molecular fingerprinting are essential to preserve product consistency. Functional testing, including proteomic and RNA profiling of sEVs, remains critical, reinforcing the principle that “the process defines the product”.
Mitigating viral risk
The nanoscale dimensions of sEVs - typically 50 to 200 nm - place them in the same size range as many viruses, making viral safety a critical concern in MSC-sEV manufacturing. Unfortunately, conventional viral inactivation and removal methods - such as low pH exposure, heat treatment, and nanofiltration - are either ineffective or incompatible with sEVs. These procedures often disrupt membrane integrity, compromise bioactivity, or result in significant product loss. Given these limitations, compliance with International Council for Harmonisation (ICH) guideline Q5A(R2) (https://database.ich.org/sites/default/files/ICH_E6%28R3%29_DraftGuideline_2023_0519.pdf) is essential for ensuring viral safety in MSC-sEV products. This international guideline - titled “Viral Safety Evaluation of Biotechnology Products Derived From Cell Lines of Human or Animal Origin” - provides a harmonized framework for the detection, control, and risk mitigation of viral contaminants in biologics.
Under ICH Q5A(R2), manufacturers are required to:
· Characterize and qualify all source cell lines, including documentation of origin, history, and susceptibility to viral infection;
· Conduct extensive adventitious agent testing of cell banks and raw materials, using both in vitro and in vivo assays as appropriate;
· Implement rigorous aseptic processing controls, including closed systems and sterile filtration (where compatible);
· Evaluate the overall viral clearance capacity of the manufacturing process, even if traditional clearance steps (e.g., solvent/detergent treatment, chromatography) are inapplicable to sEVs.
For MSC-sEV platforms, adherence to ICH Q5A(R2) ensures that viral risk is mitigated through upstream controls, rather than relying on downstream inactivation. This places greater emphasis on:
· The use of thoroughly screened and qualified master cell banks;
· Serum-free or xeno-free media components;
· Aseptic bioreactor systems with validated cleaning procedures.
In summary, ICH Q5A(R2) provides the regulatory foundation for addressing the unique viral safety challenges of MSC-sEVs, especially when conventional purification techniques are not viable. Early integration of these principles into process development is essential for regulatory approval and safe clinical translation [Figure 3].
Figure 3. A critical path to consistent and safe MSC-sEV production. (Figure was created with AI-assisted technologies). MSC: Mesenchymal stem cell; sEV: small extracellular vesicle; GMP: Good Manufacturing Practice; ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
THE NEED FOR FUNCTIONAL POTENCY CRITICAL QUALITY ATTRIBUTES
Establishing functional potency critical quality attributes (CQAs) is essential for the development, manufacturing, and regulatory evaluation of MSC-sEV therapeutics. Potency CQAs serve as proxies for the biological activity of the final product, providing assurance of consistent efficacy across batches and aligning with regulatory expectations for biologics. According to the consensus roadmap published by SOCRATES, ISEV, and ISCT[30], the identification of potency CQAs must be rooted in a mechanistic understanding of how sEVs mediate therapeutic effects in vivo.
Traditionally, potency has been inferred from the molecular content of EVs - particularly microRNAs (miRNAs) - assumed to regulate gene expression in recipient cells through canonical RNA interference (RNAi) pathways. However, recent insights from pharmacokinetics, biodistribution, and mechanistic studies challenge this assumption. A comprehensive re-evaluation of the RNA-centric view of sEV function is warranted, particularly in light of the inefficiencies in EV uptake, endosomal escape, and the minimal stoichiometry of RNA cargo. This chapter outlines the current limitations of miRNAs as potency CQAs and highlights the need to reorient potency metrics toward more plausible mechanisms such as extracellular or receptor-mediated pathways.
Rethinking the miRNA-centric mechanism of action
The cargo delivery paradigm: a failing model
The canonical model suggests that MSC-sEVs deliver functional cargo - particularly miRNAs - into the cytoplasm of recipient cells to mediate therapeutic effects. However, recent in vivo studies using radiolabeled, fluorescently tagged, or barcoded EVs consistently show that fewer than 1% of intravenously administered EVs are internalized by target cells[31,32]. Moreover, within this internalized fraction, the vast majority of EVs are trafficked to lysosomes, where cargo is degraded, with minimal evidence of cytosolic release[33].
This internalization inefficiency presents a major barrier for functional RNA delivery. Engineering efforts to improve cellular uptake and endosomal escape, such as the incorporation of fusogenic proteins including VSV-G (vesicular stomatitis virus glycoprotein), have enhanced the functional delivery of catalytic proteins such as CRE recombinase into cells[34]. However, there is little evidence that VSV-G could facilitate delivery of miRNA to achieve functionally relevant miRNA concentrations in recipient cells at clinically feasible doses.
Extracellular mechanisms
The growing skepticism around the intracellular delivery hypothesis has prompted increasing attention to extracellular mechanisms of action as more biologically plausible alternatives. Rather than viewing EVs solely as cargo shuttles, these emerging models emphasize the biophysical and biochemical properties of EVs as active participants in cell communication.
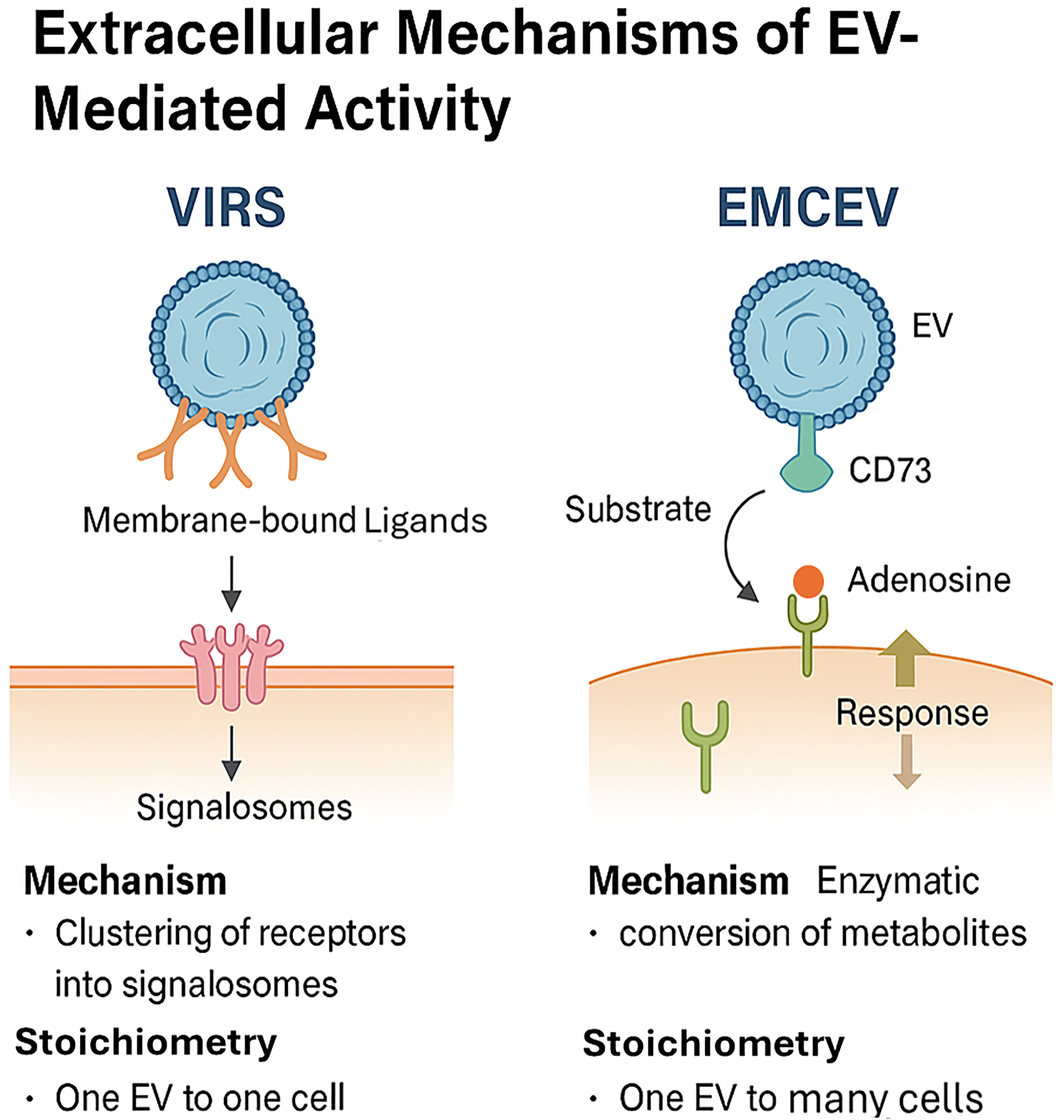
One such model is the Vesicle-Induced Receptor Sequestration (VIRS) hypothesis, proposed by Jahnke and Staufer[35]. VIRS represents a conceptual shift: EVs are envisioned not simply as carriers of ligands or nucleic acids but as nanoscale platforms that facilitate high-density, spatially organized presentation of membrane-bound ligands. By clustering target cell receptors into signaling complexes, or “signalosomes”, this configuration can amplify the potency of EV-presented ligands by 10- to 1,000-fold compared to their soluble forms. However, this mechanism depends on direct physical contact between EV and target cell, inherently restricting the interaction to a one-EV-to-one-cell stoichiometry. Such a constraint may limit therapeutic scalability and tissue distribution, especially in complex in vivo environments.
To address this limitation, Tan et al. proposed the External Modulation of Cell by EV (EMCEV) hypothesis[36]. This model posits that MSC-sEVs exert therapeutic effects through EV-associated enzymatic activity and microenvironmental modulation, rather than through vesicle internalization or receptor clustering alone. In the EMCEV framework, EVs act as extracellular catalytic agents - for example, via CD73-mediated conversion of extracellular adenosine monophosphate (AMP) to adenosine, which subsequently binds to purinergic receptors on nearby cells. Crucially, this mechanism allows a single EV to influence multiple surrounding cells, establishing a “one-to-many” stoichiometry and enabling broader paracrine effects without the need for EV uptake [Figure 4].
Figure 4. Comparison of the VIRS and EMCEV models, highlighting differences in mechanism and stoichiometry: (1) VIRS: Ligand clustering, signalosome formation, 1 EV : 1 cell; (2) EMCEV: Enzymatic conversion (e.g., CD73-mediated adenosine production), 1 EV : many cells. (Figure was created with AI-assisted technologies). VIRS: Vesicle-induced receptor signaling; EMCEV: enzymatic metabolite conversion by extracellular vesicles; EV: extracellular vesicle; CD73: ecto-5’-nucleotidase.
These extracellular models - particularly EMCEV - offer several advantages:
· Biological plausibility, grounded in known extracellular enzyme activity and receptor pharmacodynamics;
· Improved pharmacokinetic alignment, consistent with observed dose-response relationships at low vesicle concentrations;
· Direct linkage to therapeutic outcomes, through measurable surface protein activity or metabolite modulation.
Importantly, such mechanisms provide a rational basis for defining potency-related CQAs that do not depend on assumptions of intracellular delivery or gene silencing by miRNA cargo. As EV-based therapeutics advance toward clinical translation, mechanism-driven models such as VIRS and EMCEV will be pivotal in guiding potency assay development, regulatory classification, and product design.
Critical reassessment of miRNAs as potency-associated CQAs beyond cargo delivery
Overrepresentation of miRNA research
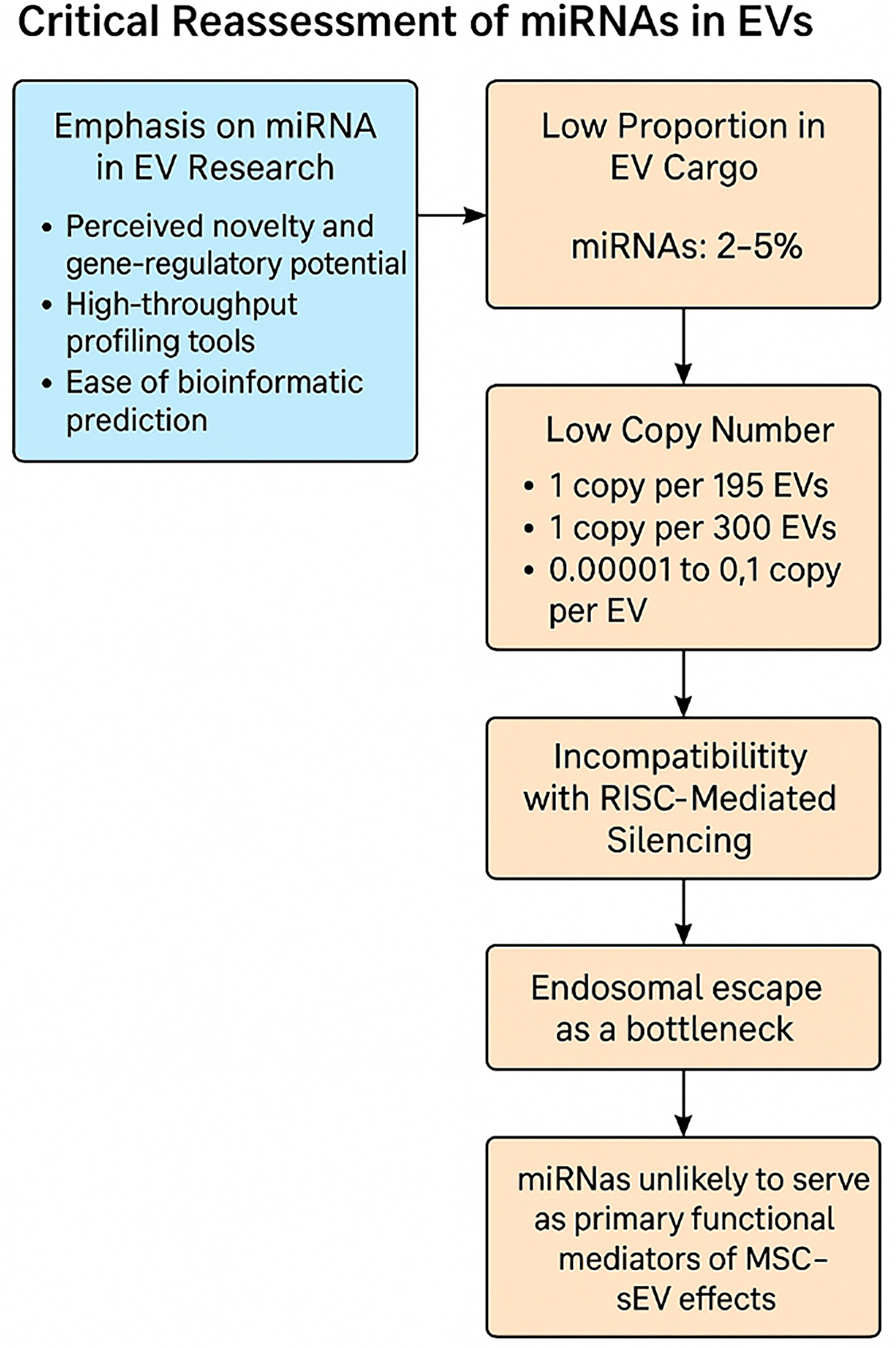
Despite limited functional evidence, miRNAs have dominated EV research. A 2024 bibliometric survey reported that over 30% of EV-related publications focused on miRNA content, profiling, or putative functions[37]. This emphasis stems from:
· The perceived novelty and gene-regulatory potential of miRNAs;
· The availability of high-throughput profiling tools [e.g., microarrays, quantitative polymerase chain reaction (qPCR), RNA sequencing (RNA-seq)];
· Ease of bioinformatic target prediction using seed region algorithms.
However, the prevalence of miRNA research has not translated into robust functional validation - particularly in the context of sEV-mediated delivery.
Misconceptions about miRNA enrichment
Multiple independent studies have shown that miRNAs represent only 2%-5% of small RNA cargo in MSC-sEVs[38-41] compared to 19%-49% in most cells including MSCs[38,42]. This underrepresentation is often masked by normalization practices that compare miRNA abundance to non-EV molecules such as glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or total RNA input, resulting in misleading conclusions about enrichment. Direct comparisons of small RNA profiles in the above studies reveal that miRNAs are selectively loaded in EVs relative to their cellular source.
Low copy number: a functional barrier
Despite widespread claims that MSC-EVs exert their therapeutic effects via intracellular delivery of miRNAs, there remains no direct evidence that the quantity of miRNAs contained within EVs is sufficient to account for the observed increases in cellular miRNA levels following EV exposure.
Indeed, the biological plausibility of EV-mediated gene regulation via miRNAs is significantly challenged by the exceedingly low copy numbers of these RNAs within extracellular vesicles. Even among the most abundant miRNAs detected in EVs, quantitative analyses reveal copy numbers that fall several orders of magnitude below those required to elicit functional gene silencing in recipient cells. For instance:
· miR-133a-3p, a highly abundant miRNA in EVs derived from C2C12 myoblasts, was detected at a frequency of only 1 copy per 195[43];
· EBV-miR-BHRF1-2, a highly abundant viral miRNA, was present at 1 copy per 300 EVs from Epstein-Barr virus-transformed lymphoblastoid cell lines[44];
· Many of the most abundant miRNAs in EVs from plasma, prostate cancer plasma, dendritic cells, mast cells, seminal fluid, and ovarian cancer cells are present at 0.00001 to 0.1 copies per EV[45].
In stark contrast, functionally active miRNAs within recipient cells typically exist at hundreds to thousands of copies per cell[46-50], a concentration necessary to saturate the RNA-induced silencing complex (RISC) and mediate measurable post-transcriptional repression. For example, miR-122, a liver-enriched miRNA, is estimated to exist at ~120,000 copies per hepatocyte[51].
Given this vast quantitative disparity, it is biologically implausible that the delivery of such miniscule miRNA doses via EVs - often below one copy per vesicle - could produce meaningful gene regulatory effects in recipient cells under physiological conditions. This strongly suggests that the reported elevation in cellular miRNA following EV treatment is more plausibly due to secondary regulatory effects rather than physical transfer of EV miRNAs. This raises serious doubts about the canonical RISC-dependent gene silencing model as the primary mechanism of EV-miRNA action, especially in vivo.
Incompatibility with RISC-mediated silencing
Canonical miRNA biogenesis involves Dicer processing of precursor stem-loop RNAs into mature miRNAs, which are then incorporated into Argonaute (AGO)-containing RISC to guide sequence-specific translational repression or, less commonly, mRNA cleavage. Proteomic analyses have shown that AGO proteins are either absent or present at extremely low levels in MSC-sEVs[52]. While precursor stem-loop RNAs have been reported to be enriched in MSC-EVs[53], most miRNAs in MSC-EVs are mature, single-stranded forms[38,39] and are not suited for RISC loading. Functional reporter assays have failed to show evidence of sEV-mediated gene silencing via canonical RNAi, even under optimized conditions with VSV-G engineered vesicles[44].
Endosomal escape: a major bottleneck
Even when EVs are internalized, most are sequestered in endolysosomal compartments. Only a small fraction escapes into the cytosol, where RNAi machinery resides. Albanese et al. (2021) used a dual-luciferase reporter system to test whether single miRNA species delivered via EVs could mediate gene silencing[44]. Despite rigorous experimental controls and enhancements, no functional activity was detected - highlighting endosomal escape as a critical bottleneck.
Summary for a miRNA-centric mechanism of action
The cumulative evidence strongly suggests that miRNAs are unlikely to serve as primary functional mediators of MSC-sEV therapeutic effects. Their low abundance, inefficient delivery, and lack of RISC compatibility make them implausible drivers of the observed regenerative, anti-inflammatory, and immunomodulatory outcomes [Figure 5].
Figure 5. Critical data-driven assessment of a miRNA-centric mechanism of action (Figure was created with AI-assisted technologies). EVs: Extracellular vesicles; MSC: mesenchymal stem cell; sEV: small extracellular vesicle; miRNA: microRNA.
Although numerous studies report increases in cellular miRNA content following EV exposure, the quantitative insufficiency of miRNAs within EVs, coupled with the inefficient uptake of EVs by recipient cells, makes it unlikely that these increases result from direct delivery of miRNA cargo. Instead, current evidence more convincingly supports an indirect regulatory mechanism, whereby cellular miRNA elevation arises as a secondary response to EV-induced cellular signaling, rather than from the physical transfer of miRNAs from EVs to cells. Accordingly, while EV-associated miRNAs may serve as useful biomarkers of manufacturing consistency or reflect the physiological state of the parent MSCs, they should not be relied upon as functional potency CQAs. The development of robust potency assays should instead focus on mechanistically relevant and functionally predictive attributes, particularly those linked to direct extracellular or surface-mediated mechanisms of action, such as enzymatic activity or receptor-level modulation.
CONCLUSION
While MSC-sEVs represent a transformative advancement in regenerative medicine, their path to clinical utility depends on the precise definition of identity and potency CQAs grounded in mechanistic reality. Among the proposed functional mediators, miRNAs have received disproportionate emphasis in literature and regulatory discourse. However, critical examination reveals that miRNAs are neither abundant nor reliably functional when delivered via native MSC-sEVs. Their low copy numbers, inefficient delivery, and lack of essential cofactors significantly limit their potential to act as meaningful therapeutic agents.
Instead of relying on miRNA-centric models, a shift toward extracellular or receptor-mediated mechanisms, such as ligand-receptor interactions or CD73-driven adenosine generation, may better reflect the true pharmacological action of MSC-sEVs. These mechanisms not only align with emerging evidence on sEV biodistribution and internalization dynamics but also offer more tractable endpoints for potency assay development. In this context, miRNAs may still serve as useful biomarkers of manufacturing consistency or cell state, but their utility as functional potency CQAs is highly questionable. Future development efforts should prioritize potency assays that reflect extracellular modes of action, integrate biodistribution and pharmacodynamic insights, and focus on protein- or activity-based metrics more directly tied to therapeutic outcomes. Reframing the discussion away from miRNAs and toward empirically grounded mechanisms will be essential for unlocking the full clinical potential of MSC-sEV-based therapies.
DECLARATIONS
Authors’ contributions
Reviewed and edited the paper: Tan TT
Conceptualized and drafted the paper: Lim SK
All authors approved the final submitted version of the manuscript.
Availability of data and materials
Not applicable.
AI and AI-assisted tools statement
AI and AI-assisted tools were used solely for grammar/spelling edits and for rendering assistance in figure preparation. The authors retain full responsibility for - and are the exclusive originators of - the manuscript’s concepts, structure, analyses, and conclusions.
Financial support and sponsorship
None.
Conflicts of interest
Tan TT and Lim SK are affiliated with Paracrine Therapeutics Pte. Ltd., and they declare that there are no potential conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92-100.
2. Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585-92.
3. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-84.
4. Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129-37.
5. Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-67.
6. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-22.
7. Bruno S, Tapparo M, Collino F, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23:1262-73.
8. Witwer KW, Van Balkom BWM, Bruno S, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206.
9. Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131-43.
10. Nawaz M, Fatima F, Vallabhaneni KC, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1073140.
11. Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8:880-6.
12. Tertel T, Dittrich R, Arsène P, Jensen A, Giebel B. EV products obtained from iPSC-derived MSCs show batch-to-batch variations in their ability to modulate allogeneic immune responses in vitro. Front Cell Dev Biol. 2023;11:1282860.
13. Hollmann J, Brecht J, Goetzke R, et al. Genetic barcoding reveals clonal dominance in iPSC-derived mesenchymal stromal cells. Stem Cell Res Ther. 2020;11:105.
14. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403.
15. Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138-43.
16. In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-45.
17. Kestendjieva S, Kyurkchiev D, Tsvetkova G, et al. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int. 2008;32:724-32.
18. Lian Q, Lye E, Suan Yeo K, et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425-36.
19. Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev. 2014;23:1084-96.
20. Allan D, Tieu A, Lalu M, Burger D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9:39-46.
21. Madel RJ, Börger V, Dittrich R, et al. Independent human mesenchymal stromal cell-derived extracellular vesicle preparations differentially attenuate symptoms in an advanced murine graft-versus-host disease model. Cytotherapy. 2023;25:821-36.
22. Selich A, Daudert J, Hass R, et al. Massive clonal selection and transiently contributing clones during expansion of mesenchymal stem cell cultures revealed by lentiviral RGB-barcode technology. Stem Cells Transl Med. 2016;5:591-601.
23. McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017;34:217-31.
24. Chen TS, Arslan F, Yin Y, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47.
25. Kraskiewicz H, Paprocka M, Bielawska-Pohl A, et al. Can supernatant from immortalized adipose tissue MSC replace cell therapy? Stem Cell Res Ther. 2020;11:29.
26. Labusek N, Mouloud Y, Köster C, et al. Extracellular vesicles from immortalized mesenchymal stromal cells protect against neonatal hypoxic-ischemic brain injury. Inflamm Regen. 2023;43:24.
27. Shekari F, Alibhai FJ, Baharvand H, et al. Cell culture-derived extracellular vesicles: considerations for reporting cell culturing parameters. J Extracell Biol. 2023;2:e115.
28. Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017;2:170-9.
29. Almeria C, Kreß S, Weber V, Egger D, Kasper C. Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell Biosci. 2022;12:51.
30. Gimona M, Brizzi MF, Choo ABH, et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy. 2021;23:373-80.
31. Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021;12:1864.
32. O'Brien K, Ughetto S, Mahjoum S, Nair AV, Breakefield XO. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022;39:110651.
33. Zickler AM, Liang X, Gupta D, et al. Novel endogenous engineering platform for robust loading and delivery of functional mRNA by extracellular vesicles. Adv Sci. 2024;11:e2407619.
34. Liang X, Gupta D, Xie J, et al. Multimodal engineering of extracellular vesicles for efficient intracellular protein delivery. bioRxiv. 2023; bioRxiv:2023.04.30.535834.
35. Jahnke K, Staufer O. Membranes on the move: the functional role of the extracellular vesicle membrane for contact-dependent cellular signalling. J Extracell Vesicles. 2024;13:e12436.
36. Tan TT, Lai RC, Sim WK, Zhang B, Lim SK. Enhancing EV-cell communication through “External Modulation of Cell by EV” (EMCEV). Cytotherapy. 2025;27:1-6.
37. Tan TT, Lim SK. Relevance of RNA to the therapeutic efficacy of mesenchymal stromal/stem cells extracellular vesicles. RNA Biol. 2025;22:1-7.
38. Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127.
39. De Luca L, Trino S, Laurenzana I, et al. MiRNAs and piRNAs from bone marrow mesenchymal stem cell extracellular vesicles induce cell survival and inhibit cell differentiation of cord blood hematopoietic stem cells: a new insight in transplantation. Oncotarget. 2016;7:6676-92.
40. Lai RC, Tan SS, Yeo RW, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828.
41. Kodali M, Madhu LN, Reger RL, et al. Intranasally administered human MSC-derived extracellular vesicles inhibit NLRP3-p38/MAPK signaling after TBI and prevent chronic brain dysfunction. Brain Behav Immun. 2023;108:118-34.
42. Kuchen S, Resch W, Yamane A, et al. Regulation of microRNA expression and abundance during lymphopoiesis. Immunity. 2010;32:828-39.
43. Hanson B, Vorobieva I, Zheng W, et al. EV-mediated promotion of myogenic differentiation is dependent on dose, collection medium, and isolation method. Mol Ther Nucleic Acids. 2023;33:511-28.
44. Albanese M, Chen YA, Hüls C, et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 2021;17:e1009951.
45. Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111:14888-93.
46. Bissels U, Wild S, Tomiuk S, et al. Absolute quantification of microRNAs by using a universal reference. RNA. 2009;15:2375-84.
47. Chang J, Nicolas E, Marks D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106-13.
48. Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell. 2014;54:766-76.
49. Ho V, Baker JR, Willison KR, Barnes PJ, Donnelly LE, Klug DR. Single cell quantification of microRNA from small numbers of non-invasively sampled primary human cells. Commun Biol. 2023;6:458.
50. Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166.
51. Valdmanis PN, Kim HK, Chu K, et al. miR-122 removal in the liver activates imprinted microRNAs and enables more effective microRNA-mediated gene repression. Nat Commun. 2018;9:5321.
52. van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic signature of mesenchymal stromal cell-derived small extracellular vesicles. Proteomics. 2019;19:e1800163.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].