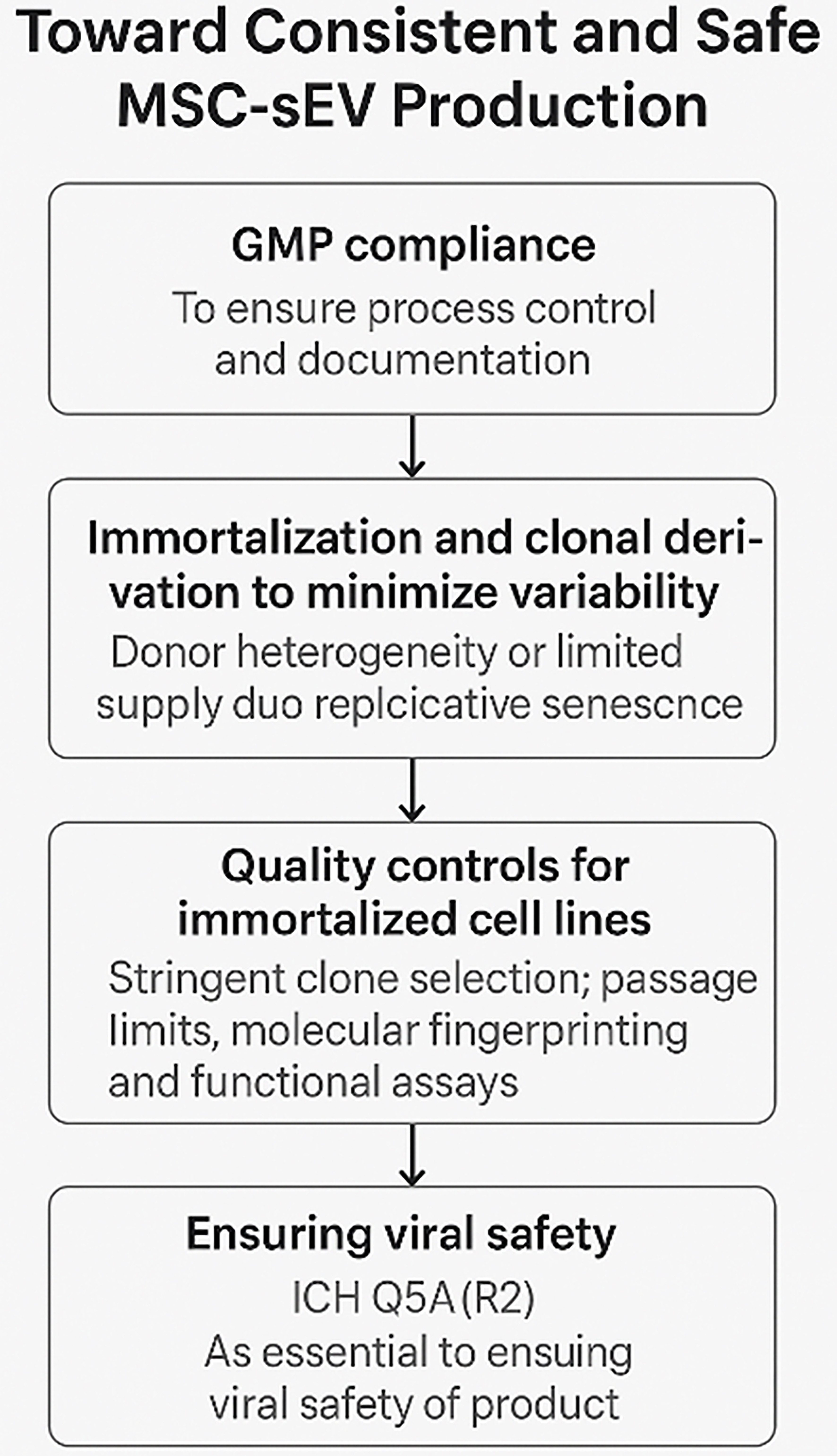
fig3
Figure 3. A critical path to consistent and safe MSC-sEV production. (Figure was created with AI-assisted technologies). MSC: Mesenchymal stem cell; sEV: small extracellular vesicle; GMP: Good Manufacturing Practice; ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.