Phosphorus-based anodes for fast-charging lithium-ion batteries: advances, challenges and prospects
Abstract
The electrification of transportation and the proliferation of portable electronics demand high-performance
Keywords
INTRODUCTION
Lithium-ion batteries (LIBs) have become the dominant energy storage technology for electric vehicles (EVs), portable electronics, and smart grids because of their high energy density and long cycle life, playing a pivotal role in advancing transportation electrification and facilitating the efficient integration of renewable energy resources[1-4]. In response to growing market demands for faster energy access and extended driving ranges, shortening charging time while maintaining high specific energy and long-term cycling stability has become a central objective in the development of next-generation fast-charging LIBs[5-8]. According to the technical specifications established by the United States Advanced Battery Consortium (USABC), fast charging is defined as achieving a state-of-charge of ≥ 80% within ≤ 15 min at a charging rate of ≥ 4C[9,10]. However, current LIBs in EVs typically require approximately 60 min to achieve full charge[11], which far exceeds the USABC fast-charging benchmark and remains significantly slower than the 3-5 min refueling time of conventional internal combustion engine vehicles[12,13]. This disparity represents a major technological bottleneck impeding the broader adoption of EVs. Driven by the rapid expansion of the EV market and rising concern over range anxiety, developing high-performance LIBs with superior fast-charging capability is now recognized as a critical prerequisite for enabling large-scale EV deployment and achieving carbon neutrality in future transportation systems[14,15].
A typical LIB consists of a cathode, an anode, an electrolyte, and a Li+-conducting separator[16]. LIBs are often referred to as “rocking-chair batteries” as their operation relies on the reversible intercalation and deintercalation of Li+ between the cathode and anode during charge and discharge processes[17]. Among these components, electrode materials play a decisive role in determining the battery performance[18]. Notably, current commercial LIBs based on LiCoO2 or LiFePO4 cathodes and graphite anodes still fail to meet the stringent requirements of fast-charging applications[19,20]. In particular, the anode has been identified as the primary limiting factor compared to the cathode[21]. The commonly used graphite anode suffers from sluggish Li+ diffusion kinetics (Li+ diffusion coefficient of ~10-11 cm2 s-1)[22] and a low lithiation potential (~0.1 V vs. Li+/Li)[23]. Under high charging rates, the large anode polarization can drive the local potential below the Li+/Li threshold, thereby triggering Li plating on the anode surface. This not only reduces Coulombic efficiency (CE) and accelerates capacity degradation, but also poses serious safety risks such as dendrite formation and thermal runaway[24,25]. Moreover, the relatively low theoretical specific capacity of graphite (372 mAh g-1) has already been nearly reached in commercial cells[26], leaving limited room for further improvement in energy density. As a consequence, it is imperative to develop alternative anode materials capable of supporting fast charging without compromising energy density, cycle life, and safety[27,28].
Over the past decade, extensive efforts have been devoted to exploring alternative anode materials for
Figure 1. (A) Comparison of representative anode materials in terms of theoretical specific capacity and Li+ diffusion barrier; (B) Schematic illustration of the structure and working principle of typical LIBs and key challenges and strategies for fast-charging P-based anodes applied in LIBs. LIBs: Lithium-ion batteries.
Comparison of representative anode materials in terms of electrochemical properties and cost in LIBs
| Material | Theoretical specific capacity (mAh g-1) | Li+ diffusion barrier (eV) | Lithiation potential (V vs. Li+/Li) | Electronic conductivity | High-rate performance (mAh g-1) | Volume change | Cost ($ kg-1) |
| Graphite[22,32] | 372 | 0.29 | ~0.1 | High | 180 at 18.6 A g-1 | ~10% | 7-12 |
| 266 | 0.241 | 0.6 | Low | 109 at 20 A g-1 | 5.5% | 50-100 (estimated based on raw materials) | |
| graphene[22,30] | 744 | 0.181 | ~0.1 | High (106 S cm-1) | 235 at 25 A g-1 | < 10% | 500-1,500 |
| Li4Ti5O12[22,31] | 175 | 0.3 | ~1.5 | Low (10-13 S cm-1) | 169 at 50C | ~0% | 30-70 |
| P[13,32] | 2,596 | 0.08 | ~0.7 | Low (BP higher than RP) | 700 at 13 A g-1 | ~200%-300% | 3-6.5 |
| Si[32,33] | 4,200 | 0.57 | ~0.2 | Low | 850 at 20C | ~300%-420% | 70-150 |
| Sn[22,34] | 994 | 0.39 | ~0.6 | High (106 S cm-1) | 377 at 5 A g-1 | ~260% | 25-60 |
Despite the above attractive properties, the practical application of P-based anodes is still constrained by several key challenges, including severe volume variation during lithiation/delithiation[9,47], intrinsically low electronic conductivity[48,49], interfacial instability and surface passivation[50,51], and the dissolution and shuttling of polyphosphide intermediates[47,52] [Figure 1B]. To address these issues, various material design and interfacial engineering strategies have been proposed, contributing to great progress toward
This review systematically discusses recent advances in the development of P-based anodes for fast-charging LIBs. It begins with an introduction of the fundamental physical and chemical properties and lithium storage mechanisms of both elemental P allotropes and TMPs. Then, the critical challenges that hinder the practical deployment of P-based anodes are summarized, followed by an in-depth analysis of major optimization strategies. Finally, future research directions are proposed to address the remaining bottlenecks, aiming at guiding the rational design of scalable and durable P-based anodes for
FUNDAMENTAL PROPERTIES AND LITHIUM STORAGE MECHANISMS OF P-BASED ANODES
The family of P-based materials includes elemental allotropes such as white phosphorus (WP), red phosphorus (RP), and black phosphorus (BP), along with a wide range of TMPs. Among them, RP, BP, and certain TMPs have shown considerable promise as anodes for fast-charging LIBs. Understanding their fundamental physical and chemical properties and their lithium storage mechanisms is essential to fully unlock their potential in high-rate energy storage applications. This section provides a comprehensive overview of the crystal structures, synthesis methods, electronic properties, and lithium storage behaviors of these representative P-based anodes. Particular emphasis is placed on the structure-property-performance relationships that govern their fast-charging capability and electrochemical stability.
RP
Structural characteristics and classification of RP
RP is commonly regarded as an amorphous allotrope of P, characterized by structurally complex, disordered polymeric networks with limited long-range order. However, RP is not a single-phase material but rather a family of thermodynamically distinct allotropes that differ in structural order and thermal stability. Based on these differences, early studies classified RP into five distinct types (Types I-V)[62]. These phases are predominantly determined by synthesis parameters, polymer chain dimensions, and the chemical composition of terminal functional groups[63]. RP is typically synthesized from WP using
The structure of RP has long been the subject of debate, with multiple allotropes encompassing both amorphous and crystalline forms. Among these allotropes, Type I is the only form unambiguously identified as amorphous and is the most commonly used in electrochemical energy storage due to its high reactivity and isotropic structure[64]. Despite its widespread application, the atomic structure of amorphous RP remains controversial. Two prevailing structural models have been proposed[65]. Model I suggests the presence of polymeric phosphorus nanotubes with pentagonal cross-sections, structurally analogous to Hittorf’s P (violet P, monoclinic system)[66,67]. On the contrary, Model II describes a disordered layered architecture resembling orthorhombic BP[67-70]. Shanabrook et al.[68] provided strong support for Model II by identifying intermediate-range order in amorphous RP through systematic X-ray diffraction (XRD) and Raman spectroscopy. However, Olego et al.[67] and Goodman et al.[71] observed features consistent with Model I, pentagonal nanotubes, via Raman and X-ray photoelectron spectroscopy (XPS), indicating that the local structure of Type I RP may be heterogeneous and sensitive to synthesis conditions.
The crystalline RP phases, particularly Types II, IV, and V, exhibit well-defined atomic arrangements. In 1947, Roth et al.[72] first identified Types II and III RP through differential thermal analysis, reporting three reproducible exothermic peaks corresponding to discrete phase transitions upon heating to 550 °C. More recently, Sun et al.[73] proposed a solution-phase solvothermal strategy for synthesizing Type II RP using
Figure 2. (A) Schematic diagram of the synthesis route of Type II RP through a solvothermal method; (B) The XRD pattern and (C) SAED pattern of the prepared Type II RP. Reproduced with permission[73]. Copyright 2022, Royal Society of Chemistry; (D) Crystallographic representation of the repeating [P9]P2[P8]P2 tubular unit in Type IV RP. Reproduced with permission[77]. Copyright 2017, American Chemical Society; Linkage of the P strands and stacking of the layers in (E) fibrous RP and (F) Hittorf’s P. The blue tubes are added to emphasize the fibrous nature. Reproduced with permission[75]. Copyright 2020, Royal Society of Chemistry; Band structure, total density of states, and partial density of states of (G) monolayer hittorfene and (H) bulk Hittorf’s P based on
Electronic properties of RP
The structural distinctions can strongly influence the electronic properties of RP. For example, exfoliating RP into few-layer or monolayer forms can shift its electronic band structure from an indirect to a direct bandgap[78]. Schusteritsch et al.[79] performed first-principles calculations to investigate the band structures of monolayer and bulk Hittorf’s P, respectively. The monolayer Hittorfene exhibits a direct bandgap of
Lithium storage mechanism of RP
The lithium storage mechanism of RP, generally referred to as Type I RP, is governed by an alloying reaction, wherein P forms lithium-rich phases, primarily Li3P, during lithiation. The lithiation proceeds through a stepwise conversion: P → LixP → Li3P, accompanied by substantial structural reorganization and phase evolution[81]. The amorphous nature of Type I RP facilitates relatively uniform Li+ diffusion and accommodates ionic transport in multiple directions[64]. This isotropic framework, combined with its thermodynamic stability and high reactivity, contributes to its favorable electrochemical activity under moderate current densities. In-situ and ex-situ studies have confirmed the reversible formation of Li3P and the structural adaptability of amorphous RP during charge/discharge cycles[81]. However, challenges such as large volume variation and low intrinsic electronic conductivity can influence its long-term cycling performance and rate response. These aspects, along with recent advances in material design to address them, will be discussed in detail in subsequent sections. In contrast to amorphous RP, the lithium storage behaviors of crystalline RP phases remain less extensively studied. Nonetheless, their well-defined atomic arrangements and intrinsic anisotropy are expected to significantly affect Li+ diffusion pathways and electrochemical response[82].
BP and phosphorene
Structural characteristics and electronic properties of BP and phosphorene
BP exhibits the highest thermodynamic stability and mass density among all known P allotropes under standard ambient conditions[62]. Traditional methods for synthesizing bulk BP crystals typically rely on high-pressure conditions (often exceeding 1 GPa) to induce phase transitions from other P allotropes. In a pioneering study, Bridgman et al.[83] successfully prepared bulk BP by heating molten purified WP sealed within a steel enclosure. This assembly was subjected to controlled pressure conditions of 1.2-1.3 GPa in a specialized apparatus, accompanied by thermal activation at 200 °C. The reaction system was maintained under these conditions for 5-30 min to complete the phase transformation. Despite its historical significance, this method requires expensive equipment and high energy consumption, restricting its economic viability for mass production.
Phosphorene, a single or few-layer derivative of BP, can be synthesized by using two principal strategies: top-down exfoliation and bottom-up growth. The top-down approach adapts techniques originally developed for graphene. In 2014, Liu et al.[84] first isolated monolayer and few-layer phosphorene from bulk BP through mechanical exfoliation. The fabrication process involved iterative delamination of bulk crystals using adhesive tape, followed by precise transfer onto SiO2/Si substrates (300 nm oxide thickness), yielding atomically thin phosphorene sheets with characteristic thicknesses approximating 0.85 nm. While this method provides high-quality flakes, it suffers from low throughput and poor thickness control. In comparison, bottom-up methods such as chemical vapor deposition (CVD) offer greater potential for scalable production[85]. In a representative study, Smith et al.[86] demonstrated the controlled growth of
Bulk BP exists in three distinct crystalline phases: orthorhombic, simple cubic, and rhombohedral structures[65]. Through systematic XRD analysis, Jamieson revealed that BP undergoes a reversible two-step phase transformation under elevated pressure conditions[87]. Specifically, the orthorhombic structure transforms into a rhombohedral phase at 8.3-10.2 GPa, followed by a transition to a simple cubic phase above 12.4 GPa. Upon decompression, the reverse sequence is observed: the simple cubic phase reverts to rhombohedral at 9.3-11.1 GPa, and full release of pressure restores the orthorhombic structure. These findings indicate that both simple cubic and rhombohedral phases are thermodynamically unstable under ambient conditions, thereby limiting their practical applicability. As a result, the orthorhombic phase remains the most extensively studied and technologically relevant form of BP, particularly for applications in electronics and electrochemical energy storage.
As displayed in Figure 3A and B, bulk BP exhibits a layered crystal structure analogous to graphene[88]. Each P atom is covalently bonded to three adjacent P atoms via sp3 hybridized orbitals, forming stable, interconnected hexagonal rings[89]. These rings collectively constitute an orthorhombic honeycomb lattice with a folded structure[65]. Through time-of-flight neutron powder diffraction analysis, Cartz et al.[90] found that BP crystallizes in the orthorhombic system (space group Cmca) with lattice parameters determined as a = 3.3133(9) Å, b = 10.473(5) Å, and c = 4.374(2) Å. The distinctive folded architecture of bulk BP arises from the non-coplanar arrangement of P atoms[91] [Figure 3C]. This feature was further elucidated by Hultgren et al.[92] via XRD coupled with Fourier analysis. The six-membered ring structure in monolayer BP exhibits evident non-planarity, with three P atoms residing within the upper lattice layer and the remaining three occupying a sublayer arrangement. This spatial organization generates a bilayer-mimetic folded structure featuring an internal corrugated structure with a 2.1 Å interlayer spacing, contrasting with the 5.25 Å spacing observed in van der Waals-stacked multilayered BP. Figure 3D demonstrates that covalent bonding within the phosphene unit cell exhibits a bond length of 2.18 Å and an average bond angle of 102°, forming folded chain structures along distinct crystallographic directions[93]. This anisotropic bonding arrangement defines two main in-plane crystallographic directions: the armchair direction (parallel to folded chain alignment) and the zigzag orientation (perpendicular to the folding axis). Owing to its distinctive lattice symmetry, BP is an intrinsic direct bandgap semiconductor, with highly anisotropic and layer-dependent electronic characteristics[88] [Figure 3E]. Combined with optical absorption spectroscopy and photoluminescence experiments, Li et al.[94] resolved the layer-number dependence of phosphorene’s bandgap while quantitatively correlating this structural parameter with electronic properties through
Figure 3. Crystal structures of (A) graphene (planar) and (B) BP (puckered orthorhombic). Reproduced with permission[88]. Copyright 2022, Elsevier; (C) Modeled mono-, bi-, and tri-layer BP structures, illustrating the stacking of puckered layers. Reproduced with permission[91]. Copyright 2015, Springer Nature; (D) Top view of single-layer BP (phosphorene), showing bond angles (96.3° and 102.1°) and the anisotropic lattice directions (zigzag vs. armchair). Reproduced with permission[93]. Copyright 2015, National Academy of Sciences; (E) Evolution of BP bandgap with sample thickness, showing the thickness-dependent bandgap change. Reproduced with permission[88]. Copyright 2022, Elsevier. BP: Black phosphorus.
Lithium storage mechanism of BP
The lithium storage mechanism of BP is governed by an intercalation-alloying reaction pathway, in which P sequentially reacts with lithium to form intermediate LixP species-specifically crystalline Li3P7, LiP, and
TMPs
Besides elemental P, TMPs have garnered significant attention as promising alternatives to conventional graphite anodes for fast-charging LIBs due to their moderate lithiation potentials and relatively high theoretical capacities. Based on the electrochemical reactivity of the metallic constituents with lithium, TMPs can be divided into two categories: inactive and active[9,58].
Inactive TMPs
In this class, the metal component is electrochemically inert toward lithium and mainly functions as a conductive matrix, while the P element actively participates in lithium storage. Representative materials include FeP, CoP, NixP, etc. The general lithium storage mechanism can be represented by the reversible conversion reaction[32]:
During lithiation, Li+ reacts with P to form lithium-rich phosphides (e.g., Li3P), accompanied by the reduction of the transition metal to its metallic state. The resultant metal nanoparticles, though electrochemically inactive, construct a percolating conductive framework that improves electronic conductivity and buffers structural stress from volume fluctuations. Upon charging, partial reconversion of Li3P and redistribution of the metal can occur, depending on particle size, crystallinity, and cycling conditions. Due to their chemical stability, metallic backbone, and structural robustness, inactive TMPs often exhibit enhanced rate capability and cycling durability, making them attractive for fast-charging scenarios[32,99].
FeP is a typical inactive TMP, possessing a high theoretical capacity (924 mAh g-1), moderate lithiation potential, abundant natural reserves, and low toxicity[40,41]. The lithium storage mechanism of FeP involves a two-step reversible lithiation/delithiation process[100-102]: FeP + Li ↔ LiFeP and LiFeP + 2Li ↔ Li3P + Fe. Generally, FeP is synthesized via a two-step approach. Initially, a Fe-based precursor with a tailored nanostructure is prepared, which is followed by phosphorization in an inert atmosphere using a phosphorus source, resulting in the formation of FeP. The morphology and crystallinity of the final product are predominantly dictated by the precursor architecture, phosphorization temperature, and reaction atmosphere. For instance, Veluri et al.[103] prepared FeP via a polyol-mediated route to fabricate porous
Figure 4. (A) Schematic illustration of the microstructure of FeP@C/Ti3C2 nanocomposite; (B) The cycling performance of the FeP@C/Ti3C2 electrode at a current density of 2 A g-1, maintaining a capacity retention of 93.1% over 1,500 cycles. Reproduced with permission[105]. Copyright 2022, Elsevier; (C) Schematic illustration of the fabrication process of CoP@CC; (D) The first-four charge/discharge profiles of the CoP@CC electrode at 0.1 A g-1. Reproduced with permission[107]. Copyright 2025, John Wiley and Sons; (E) The structure and the charge/discharge profiles of the GeP/SWCNTs electrode in the first two cycles at 0.1 A g-1 with
CoP is another common inactive TMP anode for LIBs due to its high theoretical capacity (894 mAh g-1) and relatively low redox potential (~0.6 V vs. Li+/Li)[42,43]. During lithiation, CoP reacts with Li+ to form Li3P and metallic Co nanoparticles according to CoP + 3Li+ + 3e- → Co + Li3P. Upon delithiation, the reverse transformation restores the initial CoP phase. The in-situ generated metallic Co nanoparticles contribute to improved electrode conductivity and provide a percolated electron transport network, while the Li3P phase serves as the active lithium host. In terms of synthesis, CoP is generally prepared via phosphorization of
In addition, NixP is a class of inactive TMPs that has garnered considerable interest as anode materials for LIBs due to their relatively high theoretical specific capacities compared to graphite (542 mAh g-1 for
Active TMPs
Unlike inactive TMPs, both the metal and P participate in reversible lithiation reactions for active TMPs. Representative materials include GeP, Sn4P3, ZnP2, etc. Their lithium storage mechanism can be expressed as:
Li+ ions insert into both the metal and P sites, forming lithium-rich alloys (e.g., LiyM and Li3P)[32]. The dual participation not only enhances theoretical capacity but also improves electronic conductivity compared to pure P-based systems.
GeP features a high theoretical specific capacity of 1,900 mAh g-1 and excellent electronic conductivity of
As an emerging anode candidate in the TMP family, Sn4P3 offers a synergistic combination of the high specific capacity of P with the superior electronic conductivity of Sn (1,255 mAh g-1 and 30.7 S cm-1)[113]. Its layered crystal structure supports dual lithium storage mechanisms, involving both the alloying of Sn (Sn ↔ LixSn) and the conversion of P to Li3P. Besides, the moderate lithiation potential (0.4 V vs. Li+/Li) helps reduce the risk of lithium plating during fast charging. However, the associated volume change (~200%) poses a critical challenge to structural integrity and cycling stability[114,115]. He et al.[116] designed a Sn4P3 encapsulated in P-doped porous carbon nanosheets (Sn4P3/P-C@CNs) composite anode through a one-step thermal treatment approach, producing ultrafine Sn4P3 nanoparticles uniformly embedded within the conductive P-doped carbon framework. The resulting architecture provides a mechanically robust and electrically continuous matrix that effectively buffers volumetric fluctuations, preserves electrode integrity, and accelerates charge transport, thereby enabling enhanced cycling stability and rate capability. Consequently, the Sn4P3/P-C@CNs composite electrode delivered a high reversible capacity of 770 mAh g-1 at 0.1 A g-1 upon 100 cycles and exhibited 717 mAh g-1 at 1 A g-1 after 1,000 cycles.
Recently, ZnP2 has emerged as a promising active TMP anode for fast-charging LIBs due to its high theoretical specific capacity (1,580 mAh g-1), superior electrical conductivity, and cost efficiency. The lithium storage mechanism of ZnP2 follows a conversion reaction of ZnP2 + 7Li+ + 7e- → LiZn + 2Li3P, wherein both Zn and P participate in lithiation via alloying and phosphide formation, respectively. Liu et al.[117] developed a multicomponent amorphous ZnP2-based hybrid [a-ZnP2/Zn3(PO4)2/P/C] via a one-step high-energy ball milling route, employing ZnO, RP, and carbon black as precursors. This strategy enabled the formation of a disordered composite matrix featuring homogeneously distributed Zn, P, and conductive carbon domains. The amorphous nature of the material not only facilitates isotropic Li+ diffusion and stress relaxation but also suppresses phase separation and mechanical failure during cycling. Benefiting from structural and interfacial merits, the optimized a-ZnP2/Zn3(PO4)2/P/C electrode delivered an impressive reversible capacity of 985 mAh g-1 at 5 A g-1 with 92.3% retention over 2,200 cycles. Even under ultrafast charging conditions (20 A g-1, 12C), it retained 734 mAh g-1 after 2,000 cycles and 592 mAh g-1 after
With regard to scalability and sustainability, TMP synthesis faces additional trade-offs. Conventional phosphorization approaches (using RP, PH3, or NH4H2PO4 as P sources) are effective in structural control but suffer from hazardous precursors, high temperatures, and complex reaction atmospheres, which increase both cost and environmental burden. MOF-derived routes and other nanostructuring strategies can deliver precise nanostructures with enhanced electrochemical performance, yet their reliance on costly precursors and multistep treatments challenges industrial feasibility. Ball-milling or one-step solid-state phosphorization methods provide more economical and scalable alternatives, though they often yield products with structural inhomogeneity. Moving forward, establishing low-cost, environmentally friendly, and scalable synthetic frameworks for TMPs will be vital to bridge the gap between laboratory-scale demonstrations and industrial adoption in fast-charging LIBs.
To sum up, the structural diversity and lithium storage mechanisms of P-based materials offer a rich foundation for designing high-performance anodes tailored for fast-charging LIBs. A simple comparison of these materials in terms of their structural characteristics and lithium storage properties has been summarized in Table 2. Amorphous RP features isotropic ionic pathways and structural adaptability, while crystalline RP variants provide insights into structure-dependent reactivity. BP and its few-layer derivative phosphorene exhibit anisotropic charge transport and tunable band structures governed by quantum confinement, enabling rapid kinetics and enhanced rate performance. Inactive TMPs primarily utilize the P component for reversible lithiation via conversion reactions, with the transition metal matrix acting as a conductive scaffold to buffer volume changes and maintain electrode integrity. In contrast, active TMPs leverage synergistic lithiation of both metal and phosphorus components, offering higher theoretical capacities and improved electrical conductivity. Detailed lithiation/delithiation mechanisms of various
Figure 5. Schematic illustrations of lithiation/delithiation mechanisms for P-based anodes: (A) RP, (B) BP, (C) inactive TMPs, and (D) active TMPs. RP: Red phosphorus; BP: black phosphorus; TMPs: transition metal phosphides; P: phosphorus.
Comparison of RP, BP, and TMPs in terms of their structural characteristics and lithium storage properties in LIBs
| Material | Sub-type | Crystal structure | Thermodynamic stability | Electronic conductivity | Volume expansion | Lithium storage mechanism |
| RP | Type I | Amorphous | Moderate | Extremely low | ~300% | Alloying reaction |
| BP | Bulk | Orthorhombic | Very High | Moderate (~102 S m-1) | ~200%-300% | Intercalation-alloying reaction |
| Phosphorene | Low (air-unstable) | High | ||||
| TMPs | Inactive | Variable (depending on material) | High | Moderate-high | < 100% | Conversion reaction |
| Active | ~150%-200% | Conversion + alloying reaction |
CHALLENGES OF P-BASED ANODES FOR FAST-CHARGING LIBS
Despite compelling advantages of P-based anodes for fast-charging LIBs, their practical application remains constrained by a series of intrinsic and interfacial challenges. These limitations arise from the fundamental structural, electrochemical, and transport characteristics of elemental P and TMPs, particularly under
Figure 6. Critical challenges of P-based anodes for fast-charging LIBs, including severe volume variation, low electronic conductivity, dissolution of intermediates, and surface passivation. LIBs: Lithium-ion batteries.
Severe volume variation
P-based anodes can undergo extreme volumetric expansion (up to ~300%) during lithiation. This pronounced volume fluctuation imposes substantial mechanical strain on the electrode architecture, leading to structural disruption and destabilization of the solid electrolyte interphase (SEI) layer. In essence, SEI is electronically insulating yet Li+-conductive. The mechanical integrity and continuity of SEI layers are critical for sustaining rapid Li+ transport and preventing continuous electrolyte decomposition, especially under fast-charging conditions. Operando electrochemical atomic force microscopy (EC-AFM), combined with ex-situ spectroscopic analyses, has revealed the dynamic fracture and reconstruction of the SEI layer during repeated lithiation/delithiation[32,118]. Such continuous rupture-reformation not only accelerates electrolyte decomposition but also causes persistent loss of active P species and progressive thickening of the interfacial layer, ultimately resulting in increased impedance, compromised rate capability, and shortened cycling life. Additionally, repeated expansion-contraction induces delamination between active materials and current collectors, causing electrical isolation and capacity decay[9,47]. In amorphous RP, the isotropic but substantial expansion (~300%) leads to structural pulverization and continuous disintegration due to the lack of
Intrinsically low electronic conductivity
Elemental P, particularly RP, suffers from extremely low electronic conductivity (~10-14 S cm-1 at room temperature), which severely limits electron transfer and active material utilization. This poor conductivity leads to high charge-transfer resistance, sluggish reaction kinetics, and large polarization under high current densities, ultimately degrading rate capability and power performance. While BP and some TMPs offer better conductivity, their performance still requires significant enhancement, especially under fast-charging conditions[48,49].
Surface passivation
Both few-layer BP flakes and phosphorene are highly susceptible to surface passivation arising from rapid chemical degradation upon exposure to ambient oxygen and moisture. Upon exposure to air, P atoms at the surface undergo spontaneous oxidation, forming PxOy species and phosphoric acid derivatives, which disrupt the lattice continuity and alter the local electronic environment. This degradation significantly elevates contact resistance, reduces carrier mobility, and impairs the overall charge transport capability of anode materials[50,51].
Dissolution of polyphosphide intermediates
During the lithiation and delithiation processes of P-based anodes, a series of lithium polyphosphide species is generated as metastable intermediates. These species exhibit high solubility in conventional
STRATEGIES FOR P-BASED ANODES ENABLING FAST-CHARGING LIBS
To address the aforementioned challenges associated with P-based anodes in LIBs, research efforts have concentrated on four principal optimization strategies: constructing C-P composites, surface modification, nanostructuring, and electrolyte optimization. Each is tailored to target specific challenges while exhibiting synergistic effects with others. Constructing C-P composites primarily mitigates volume variation and low conductivity by integrating conductive carbon matrices; surface modification focuses on alleviating surface passivation and stabilizing interfaces against volume-induced strain and polyphosphide dissolution; nanostructuring enhances Li+ transport kinetics and relieves volumetric stress through shortened diffusion pathways; electrolyte optimization primarily stabilizes the SEI layer and suppresses polyphosphide dissolution. Collectively, these strategies work in concert to address the multifaceted limitations of P-based anodes. In the following sections, key design principles and representative advances for each strategy, along with their specific correlations to the aforementioned challenges, are systematically discussed.
Constructing C-P composites
As a primary strategy targeting both severe volume variation and intrinsically low electronic conductivity of P-based anodes, the integration of P with highly conductive carbon matrices has become widely adopted. The conductive carbon frameworks [e.g., graphite, graphene, carbon nanotubes (CNTs), fullerenes, etc.][53] not only provide electron transport pathways to address low conductivity but also act as mechanical buffers to accommodate volumetric expansion during lithiation/delithiation. For instance, graphite, a conventional anode material for LIBs, features a layered crystal structure that facilitates reversible Li+ intercalation/deintercalation while offering structural support to mitigate the volume changes of P. Graphene is a type of two-dimensional, monoatomic-thick carbon material with excellent conductivity and ultrahigh specific surface area.
Although structurally analogous to graphite, graphene possesses superior electronic conductivity and lithium storage capacity due to its quantum confinement effects. CNTs are one-dimensional nanocarbon materials with high conductivity and mechanical strength, which synergistically enhance lithium storage performance when hybridized with graphene through the formation of three-dimensional conductive networks. Fullerenes, another class of nanocarbons distinguished by unique cage-like structures and strong electron affinity, can be incorporated into graphene composites to expand interlayer spacing, thereby optimizing Li+ storage capacity. These carbon matrices demonstrate critical applications in LIBs owing to their notable electron transport capability, structural stability, and chemical inertness[54].
P-C composites exhibit three key advantages as advanced anode materials: (1) The conductive carbon matrices form percolating networks that enhance bulk electronic conductivity, facilitating efficient electron transport to the active P-based material, which is essential for achieving high rate capability; (2) The strategic integration of carbon structures, particularly porous carbons (PC), CNTs, and graphene frameworks, enables effective buffering of volumetric variation during cycling, preserving electrode integrity and promoting the formation of stable SEI layer; (3) The hierarchical carbon matrix with optimized porosity and surface characteristics offers abundant electrolyte-permeable channels, significantly shortening ion diffusion pathways and enhancing ion transport kinetics through improved electrode-electrolyte interfacial contact improved electrode-electrolyte interfacial contact[55].
To improve the electrochemical performance of P-based anodes, Zhang et al.[48] adopted a vapor condensation strategy to infiltrate PC with RP (RP-PC). The abundant edge sites in the carbon matrix facilitated the physisorption and polymerization of P4 molecules, along with the formation of stable P-C bonds [Figure 7A]. This structural design enabled a high phosphorus loading capacity (approaching
Figure 7. (A) Schematic illustration of RP-PC with stable P-C bonds prepared by the vaporization-condensation process and corresponding transmission paths of electron and Li+; (B) The cycling performance of the RP-PC and RP-MWCNT electrodes for
These studies confirm the effectiveness of C-P composite strategies in simultaneously addressing the inherent low electrical conductivity and severe volume expansion of P-based anodes. Key design principles include maximizing interfacial contact (e.g., via P-C bonding), tailoring pore structure for P confinement and strain buffering, and constructing three-dimensional conductive networks to facilitate electron/ion transport. Furthermore, future efforts should focus on enhancing P loading capacity, exploring sustainable phosphorus sources, and integrating carbon compositing with complementary strategies to further promote the electrochemical performance of P-based anodes under fast-charging conditions.
Surface modification
Surface modification is mainly employed to suppress surface passivation and improve electrical conductivity and interfacial stability against the detrimental effects of volume variation and polyphosphide dissolution. In recent years, diverse surface modification strategies, such as coating with conductive polymers, surface functionalization, core-shell structure construction, etc., have been developed. These strategies not only effectively mitigate electrode pulverization caused by volume changes but also enhance the interfacial stability and inhibit the shuttle effect of polyphosphide intermediates, thereby substantially improving the electrochemical performance of P-based anodes[56,57,121,122].
Elastomeric conductive polymer coatings can effectively accommodate the volumetric expansion of active materials, enhance electrical conductivity, and prevent direct contact with electrolyte. This synergistic mechanism preserves the structural integrity of both the active material and the SEI layer, thereby markedly improving the cycling stability and rate performance of LIBs[121,122]. As a representative study, Sun et al.[121] fabricated a BP/CNT@polypyrrole (PPy) composite with a robust core-shell structure through a two-step synthesis strategy involving high-energy ball milling and subsequent liquid-phase [Figure 8A]. The core component consists of RP-derived BP, while the incorporated CNT network effectively mitigates volume expansion of BP during cycling. The conformal PPy shell exhibits multifunctional roles: its inherent conductivity accelerates charge transfer kinetics; the flexible polymeric matrix accommodates volume changes while physically isolating the active materials from direct electrolyte contact; and, importantly, the nitrogen functionalities in PPy strongly chemisorb polyphosphide intermediates, thereby effectively suppressing their dissolution and shuttle effect. As shown in Figure 8B, electrochemical testing demonstrated that the BP/CNT@PPy anode maintains a specific capacity of 1,376.3 mAh g-1 after 200 cycles at 260 mA g-1, representing a 2.6-fold enhancement compared to the BP/CNT control (530.1 mAh g-1). Similarly, Wang et al.[56] synthesized few-layer BP nanosheets through liquid-phase exfoliation, subsequently fabricating BP-graphene oxide-polyaniline (BP-GO-PANI) ternary hybrid anodes via a straightforward
Figure 8. (A) Schematic illustration of the synthesis process of the BP/CNT@PPy composite; (B) Comparison of the cycle performance between BP/CNT@PPy and BP/CNT composites at a current density of 260 mA g-1 within 0.01-3.0 V. Reproduced with permission[121]. Copyright 2021, American Chemical Society; (C) Sketch map of the whole compounding process of BP-GO-PANI via a solvothermal reaction between BP and GO and then coated in-situ by PANI; Cycling performance of the BP-GO-PANI, BP, and BP-GO electrodes at (D) 0.1 A g-1 and (E) 5 A g-1. Reproduced with permission[56]. Copyright 2022, Elsevier; (F) Schematic illustration of self-healing and delithiation accelerant effects of the LM-BP/G anode; (G) The cycling performance of LM-BP/G and BP/G anodes at 5 A g-1; (H) The rate performance of LM-BP/G and BP/G anodes at varied rates from 0.5 to 15 A g-1. Reproduced with permission[123]. Copyright 2025, Elsevier. BP: Black phosphorus; CNTs: carbon nanotubes; PPy: polypyrrole; BP-GO-PANI: BP-graphene oxide-polyaniline; LM-BP/G: liquid metal-BP-graphite; RP: red phosphorus.
Although the polymer coating strategy enhances the structural stability of P-based anodes by buffering mechanical stress, it may induce significant polarization under high-rate cycling. Additionally, conventional solid-state polymer coatings lack intrinsic self-healing capabilities. To address these limitations, Zhang et al.[123] fabricated a liquid metal-BP-graphite (LM-BP/G) composite anode by homogeneously dispersing liquid metal, BP, and graphite through ultrasonic treatment, followed by ball-milling. The incorporation of LM, with high conductivity and fluidity, serves as a dynamic self-healing agent that effectively preserves electrochemical connectivity between pulverized P and LixP particles during prolonged lithiation/delithiation cycles. Furthermore, LM synergistically facilitates Li+ transport and enhances the delithiation kinetics of Li3P [Figure 8F]. In addition, the strong affinity of LM toward soluble polyphosphide intermediates suppresses their dissolution and shuttle effect, thereby mitigating active-material loss and further stabilizing the electrode-electrolyte interface. Consequently, the LM-BP/G anode demonstrated superior rate capability and cyclability across a broad temperature range (20-50 °C). Specifically, it achieved a specific capacity of 1,123 mAh g-1 at 5 A g-1 (~4 C, 15 min charging) with 80% capacity retention after
Although the above studies demonstrate that surface modification can effectively enhance the conductivity, buffer the volume variation, and suppress polyphosphide dissolution in P-based anodes, their practical application still faces notable challenges. In particular, many reported strategies rely on complex synthesis and costly functional components, which hinder scalability and cost-effectiveness. Future research should therefore prioritize the development of low-cost, multifunctional, and self-healing surface modifiers, together with simplified and scalable fabrication processes, to accelerate the practical deployment of P-based anodes in fast-charging LIBs.
Nanostructuring
Nanostructuring is a versatile strategy to simultaneously alleviate volume variation and enhance Li+ transport kinetics in P-based anodes. Downsizing to the nanoscale shortens Li+ diffusion pathways, increases electrode-electrolyte contact area, and creates sufficient void space to buffer volumetric expansion, thereby suppressing mechanical degradation during repeated lithiation/delithiation cycles. These structural and kinetic advantages represent the dominant benefits of nanostructuring in most P-based systems. Notably, when the particle size approaches the quantum confinement, additional modulation of the electronic band structure may occur, further facilitating charge transfer and partially overcoming the intrinsically low conductivity[55,58,59]. Thus, nanostructuring provides both structural/kinetic improvements and, in certain cases, electronic structure tuning, establishing it as a fundamental strategy for enabling
Meng et al.[124] initially fabricated Ti3C2 nanosheets (TNS) through a two-step approach using Ti3AlC2 as the precursor. Subsequently, BP quantum dots (BPQDs) anchored on TNS (BPQD/TNS) were synthesized via mechanical grinding of bulk BP, followed by ultrasonic processing in 1-methyl-2-pyrrolidone (NMP).
Figure 9. (A) Rate performance of the BPQD/TNS composite electrode at varied from 50 mA g-1 to 2,000 mA g-1 followed with a cycling performance at 100 mA g-1; (B) The cycling performance of the BPQD/TNS composite electrode at a high current rate of 1,000 mA g-1 between 0.005-3.0 V. Reproduced with permission[124]. Copyright 2018, John Wiley and Sons; (C) Schematic illustration of the preparation procedure of L-BP-S by a liquid-phase laser-assisted exfoliation method with 8 ns pulse duration and a repetition rate of 10 Hz; (D) Cycling performance of the L-BP-Shexane,
Recently, Cui et al.[127] developed a universal “assembly-phosphorization” strategy to encapsulate monodispersed TMPs (MPx: Ni2P, FeP, CoP, and CuP2) within flexible, N-doped carbon multi-chambers with pre-reserved voids (MPx@NC). This architecture simultaneously enhances electronic conductivity, shortens Li+ diffusion pathways, and buffers conversion-induced volume change. As a representative, the Ni2P@NC anode delivered 613 mAh g-1 at 0.2 A g-1 after 200 cycles and retained 475 mAh g-1 even at a high rate of 2 A g-1 after 800 cycles. Furthermore, a full-cell based on the Ni2P@NC anode and the commercial LiFePO4 cathode achieved a high capacity of 150.1 mAh g-1 with 87.4% capacity retention after 100 cycles at 0.1 A g-1. In-situ XRD confirmed a reversible conversion reaction, while in-situ/ex-situ TEM revealed effective volume accommodation within the carbon chambers and preserved structural integrity without excessive SEI growth. Complementary DFT calculations further indicated strong MPx-C coupling, improved electronic density of states, and reduced Li+ diffusion barriers (0.16 eV vs. 0.44 eV for bare Ni2P), which collectively rationalize the superior rate capability and long-term cycling stability. Overall, this work highlights a synergistic C-P composite, nanostructuring, and interfacial engineering strategy that integrates mechanistic reversibility with device-level feasibility, providing valuable guidance for the multidimensional design of high-performance P-based anodes.
Electrolyte optimization
Electrolyte optimization is primarily focused on addressing the dissolution of polyphosphide intermediates and stabilizing the SEI layer, which are critical for mitigating capacity degradation caused by active material loss and interfacial instability. While P-C composites mitigate volumetric variation, the inherent instability of SEI (exacerbated by volume changes) and the dissolution of polyphosphide intermediates in conventional electrolytes remain unresolved. These issues trigger electrolyte consumption, capacity degradation, and decreased CE with excessive SEI accumulation, further elevating interfacial impedance and impairing
Lei et al.[60] developed a straightforward approach for synthesizing highly reversible P-based hybridized anode materials (P-SP) through high-energy ball-milling of RP and Super P carbon black (SP). This method yields nanoscale P-SP particles, which effectively shorten the electron/ion diffusion pathways and alleviate volume expansion during repeated lithiation/delithiation cycles. To further improve interfacial stability,
Figure 10. HRTEM images of P-SP hybrids without additive (A) and with 10 vol% FEC (B) after 100 cycles; (C) The corresponding interfacial resistance of P-SP hybrids without additive and with 10 vol% FEC after 100 cycles. Inset: equivalent circuit model; (D) Comparison of cycling performance of the P-SP hybrid anode with 10 vol% FEC to the P-SP hybrid anode and the P-SP mixture anode without additive at 0.5C. Reproduced with permission[60]. Copyright 2019, American Chemical Society; (E) Schematic illustration of the functions for each component and the synergy in the designed ternary LiFSI-TEP/FEC electrolyte for RP anode; (F) Cycling performance of RP/CNT electrodes at 3 A g-1 in LiPF6-based and LiFSI-TEP (7):FEC(3) electrolytes, respectively. Reproduced with permission[128]. Copyright 2020, Elsevier. HRTEM: High-resolution TEM; TEM: transmission electron microscopy; FEC: fluoroethylene carbonate; P: phosphorus; LiFSI-TEP/FEC: lithium bis(fluorosulfonyl)imide in triethyl phosphate/FEC; RP: red phosphorus; CNTs: carbon nanotubes;
Overall, electrolyte optimization has proven to be an effective strategy for stabilizing the SEI and suppressing polyphosphide dissolution, thereby mitigating active-material loss, reducing interfacial impedance, and enabling improved rate capability and cycling stability of P-based anodes. Nevertheless, the persistent instability of the SEI-exacerbated by repeated volume changes and uncontrolled interfacial reactions-remains a critical bottleneck. Future efforts should focus on developing multifunctional electrolyte systems that simultaneously regulate solvation structures, construct robust and self-healing SEI layers, and suppress the shuttle effect, which are essential for advancing P-based anodes toward practical fast-charging applications. Moreover, to enable a direct comparison across material classes and architectures, Table 3 summarizes representative electrochemical performance for various P-based anodes discussed above.
Summary of the electrochemical performance for representative P-based anodes
| Type | Electrode material | Current density (A g-1) | Specific capacity (mAh g-1) | Cycling number | Voltage region (V vs. Li+/Li) | Ref. |
| RP | RP-PC | 8.32 (16.7C) | 649.5 | 1,000 | 0.01-3.0 | [48] |
| N-doped carbon bubble-CNT@RP | 5 | 975.1 | 1,500 | 0.01-2.0 | [49] | |
| RP-CNT@tannic acid- pyrrole | 5.2 (12C) | 446 | 100@1 A g-1 | 0.01-3.0 | [57] | |
| RP/C | ~0.54 (0.2C) | 1,806 | 500 | 0.01-2.0 | [59] | |
| RP-SP | 0.5C | 1,783 | 300 | 0.01-2.0 | [60] | |
| RP/CNT | 3 | 1,390.2 | 300 | 0.01-3.0 | ||
| BP | (BP-G)/PANI | 13 | ~700 | 2,000 | 0.001-2.5 | [13] |
| BP-GO-PANI | 5 | 958 | 2,000 | 0.01-2.5 | [56] | |
| BP@CNTs | 0.5 | 800 | 650 | 0.01-2.0 | [119] | |
| BP/CNT@PPy | 0.05 | 2,378.4 | [email protected] A g-1 | 0.01-3.0 | [121] | |
| LM-BP/G | 5 (4C) | 1,404 | 200 | 0.01-2.5 | [123] | |
| BPQD/TNS | 1 | 280 | 2,400 | 0.005-3.0 | ||
| L-BP-Shexane | 1 | 441.1 | 200 | 0.001-3.0 | ||
| Fe-Co PBA@10BQ | 0.1C | 991 | 500 | 0.01-3.0 | ||
| Inactive TMP | Fe-CoP/CC | 0.2 | 1,320.7 | 140 | 0.01-3.0 | [43] |
| FeP@NC | 10 | 305.9 | 5,000 | 0.01-3.0 | ||
| FeP | 0.1 | 618 | 50 | 0.01-2.2 | ||
| FeP@C/Ti3C2 | 2 | 809.6 | 1,500 | 0.01-3.0 | ||
| Co-doped FeP@P-C | 1 | 555.6 | 700 | 0.01-3.0 | ||
| CoP@CC | 2 | 795.3 | 420 | 0.01-3.0 | ||
| NixP/C | 0.1 | 719.5 | 100 | 0.01-3.0 | ||
| Ni1.2Co0.8P | 1 | 228 | 3,000 | 0.01-3.0 | ||
| CoP@C | 1 | 500 | 800 | 0.01-2.0 | [120] | |
| Ni2P@NC | 2 | 411 | 800 | 0.01-3.0 | ||
| Active TMP | GeP/SWCNTs | 0.1 | 778 | 200 | 0.005-3.0 | [112] |
| graphene-Sn4P3 composite | 0.1C | 860 | 100 | 0.01-3.0 | [113] | |
| Sn4P3/P-C@CNs | 1 | 587 | 1,000 | 0.01-3.0 | [116] | |
| a-ZnP2/Zn3(PO4)2/P/C | 5 (3C) | 1,128 | 2,200 | 0.01-3.0 | [117] |
CONCLUSION AND OUTLOOK
The critical bottleneck in advancing fast-charging LIBs lies in the sluggish Li+ diffusion kinetics and structural degradation at the anode-electrolyte interface. To tackle these challenges, the development of advanced anode materials with fast charge-transfer kinetics and robust structural stability has emerged as a pivotal research direction. In recent years, P-based anode materials have demonstrated significant potential in emerging fast-charging energy storage systems, owing to their superior theoretical capacities, low Li+ diffusion energy barrier, and safe reaction potentials, offering a viable alternative beyond conventional graphite anodes. Over the past decade, persistent investigations have established a substantial theoretical foundation for P-based anodes in fast-charging LIBs. This review comprehensively presents some key achievements within the field. Nevertheless, critical scientific issues remain to be resolved to fully exploit the fast-charging potential of P-based anode materials. Several future directions regarding these issues are outlined below [Figure 11].
Figure 11. Future improvement directions for P-based anodes toward practical fast-charging LIBs. LIBs: Lithium-ion batteries; DFT: density functional theory; SEI: solid electrolyte interphase; AI: artificial intelligence.
In-situ multiscale characterization of Li+ transport and interfacial kinetics
The comprehensive elucidation of Li+ diffusion pathways and interfacial reaction mechanisms in P-based anodes under fast-charging conditions constitutes a pivotal challenge for optimizing their electrochemical performance. Conventional characterization methodologies (e.g., ex-situ XRD, SEM) encounter inherent limitations in dynamically tracking intricate phase transition behaviors and interfacial evolution during high-rate cycling. Recent breakthroughs in in-situ characterization techniques have enabled significant progress[9,32]. Atomic-scale visualization of structural deformations and dynamic SEI reconstruction during lithiation/delithiation can now be achieved through in-situ TEM coupled with electron energy loss spectroscopy. The spatially and temporally resolved analytical capabilities of synchrotron-based X-ray absorption spectroscopy (XAS) combined with in-situ Raman spectroscopy prove particularly effective in elucidating transient changes in local bonding structures and intermediate species within P-based materials. Furthermore, in-situ EC-AFM provides quantitative assessments of mechanical-electrochemical coupling behaviors, especially regarding the correlation between interfacial mechanics and volumetric expansion. And in-situ cryogenic electron microscopy (cryo-EM) can be applied to directly visualize SEI formation and evolution in working cells, revealing unprecedented nanoscale details of interfacial chemistry and decomposition pathways. However, fundamental gaps remain in correlating structural models with electrochemical behavior. For example, the local structure of amorphous RP remains debated (polymeric nanotube vs. layered model), and its correlation with Li+ transport has not been conclusively established. Similarly, the phase evolution of crystalline RP and TMPs during high-rate cycling is poorly understood. Addressing these controversies will require integrated in-situ multimodal probes coupled with atomistic simulations to provide unified mechanistic insights. Future research should prioritize the advancement of high-resolution, cross-scale, multimodal characterization platforms that integrate cryo-EM, XAS, EC-AFM, neutron imaging, and ab initio molecular dynamics simulations, etc., to establish quantitative
Precise design and dynamic regulation of SEI
The unstable SEI layer represents one of the primary bottlenecks limiting the fast-charging performance of P-based anodes. Current modification strategies primarily encompass two approaches: electrolyte optimization and surface engineering. The introduction of film-forming additives (e.g., FEC) facilitates the formation of inorganic-organic hybrid SEI layers (thickness < 5 nm) enriched with LiF, which notably reduce interfacial resistance. Pre-constructing artificial SEI layers through advanced deposition techniques (e.g., atomic layer deposition, CVD) on P surfaces lowers the diffusion energy barrier of Li+ ions, while simultaneously mitigating polyphosphide dissolution and enhancing mechanical resilience. Nevertheless, significant knowledge gaps remain. The chemical heterogeneity of SEI layers, their dynamic reconstruction under fast-charging, and the causal relationship between polyphosphide dissolution, SEI thickening, and electrolyte decomposition are not fully understood. To address these issues, future research should integrate advanced characterization methodologies such as cryogenic electron microscopy and time-of-flight secondary ion mass spectrometry to enable high-resolution mapping of SEI composition and spatial architecture. Concurrently, machine learning-driven predictions could streamline the development of optimal electrolyte formulations and interfacial modification protocols.
Constructing highly conductive channels for fast Li+ transport
Establishing continuous and stable rapid Li+ transport channels within solid-state electrode systems is critical for achieving high-rate charging/discharging performance, yet their practical implementation still faces multiple challenges. RP suffers from sluggish reaction kinetics due to its extremely low electronic conductivity and relatively high solid-state diffusion barriers. In BP and phosphorene, the strongly anisotropic lattice leads to directional dependence of Li+ transport, while surface passivation further compromises interfacial charge transfer. For TMPs, although the presence of metallic species provides enhanced conductivity, repeated cycling still induces severe volume expansion, structural pulverization, and redistribution of metallic phases, which together disrupt long-term ion/electron conduction pathways. To address these issues, researchers primarily employ three optimization strategies: (1) construction of
Full-cell integration and collaborative enhancement of safety and scalability
To translate the promising electrochemical performance of P-based anodes into practical fast-charging LIBs, it is imperative to address the compatibility of P-based anodes with commercially viable cathodes and electrolytes. Full-cell assembly poses critical challenges in balancing the capacity ratio, operating voltage window, and dynamic Li+ diffusion kinetics across electrodes. For instance, the large specific capacity and moderate potential of P-based anodes must be carefully matched with cathodes such as Ni-rich layered oxides, LiFePO4, or high-voltage spinel oxides to ensure optimal energy density, voltage stability, and safety. Mismatches in electrode kinetics and Li+ transport rates can lead to over-lithiation or Li plating, especially under high-rate cycling. Meanwhile, the selection of compatible electrolytes is critical. Fluorinated weakly solvating electrolytes, localized high-concentration formulations, ionic liquids, and emerging solid-state electrolytes have shown promise in forming robust SEI layers, suppressing polyphosphide dissolution, and maintaining interfacial stability. Recent full-cell demonstrations further highlight both the feasibility and the limitations of P-based anodes in practical fast-charging LIBs. For example, Sun et al.[59] reported nanoscale RP confined in conductive carbon frameworks, which in full-cells delivered much better fast-charge capability than graphite or Li4Ti5O12 at industrially relevant areal capacities, though with notable first-cycle irreversible loss due to SEI formation. Jin et al.[13] designed a BP-graphite/polyaniline composite that, when paired with LiCoO2 cathodes, enabled ~80% state-of-charge recovery within ~9 min and maintained 90% capacity retention over 2000 cycles, far outperforming graphite under identical conditions. TMPs such as AlP have also shown promise: AlP/CNT||LiFePO4 full-cells retained ~80% capacity after 85 cycles, though limited by moderate initial CE[129]. More recently, Xie et al.[130] demonstrated that fluorinated weakly solvating electrolytes combined with RP@carbon anodes achieved ~2,600 mAh g-1 in half-cells and 87.6% retention in LiFePO4 full-cells after 300 cycles at 4C, simultaneously improving safety and fast-charging tolerance. Collectively, these studies demonstrate that P-based anodes can surpass conventional graphite in terms of theoretical capacity, fast-charge capability, and long-term cycling stability. Nevertheless, unresolved issues, including large initial irreversible capacity, SEI instability under repeated expansion, and the need for scalable, low-cost synthesis routes, still hinder their competitiveness at the full-cell level. In terms of cost, the relatively high price of high-purity phosphorus precursors and the additional expenses associated with structural engineering (e.g., nanostructuring, carbon coating, and heteroatom doping) raise concerns regarding economic feasibility. From the perspective of scalability, most of the current fabrication strategies, such as high-energy ball-milling, vapor deposition, or solution-based processes, are difficult to translate into large-scale, continuous production, necessitating the development of more industrially compatible routes. Furthermore, the practical implementation of P-based anodes remains challenged by critical safety and environmental concerns: (1) Synthetic safety: the generation of WP (highly toxic and pyrophoric) must be rigorously suppressed during electrode fabrication, which requires the establishment of environmentally benign synthetic pathways; (2) Thermal stability: localized heat accumulation during
In conclusion, owing to their notable theoretical capacity and tunable electronic architecture, P-based anodes demonstrate high potential for fast-charging LIBs. Future research should synergistically integrate multiscale characterization, computational materials science, and AI to elucidate Li+ transport kinetics and interfacial degradation mechanisms at the atomic scale. Such multidisciplinary approaches will facilitate the rational design of integrated “structure-function” electrodes with simultaneously improved dynamic performance and long-term cyclability. Addressing these interdisciplinary challenges-spanning atomic-scale characterization, interface engineering, ion transport optimization, and scalable manufacturing-will be crucial for translating the high theoretical promise of P-based materials into practical, high-performance fast-charging LIBs. Concurrent systematic investigations into safety evaluation protocols and
DECLARATIONS
Authors’ contributions
Data sourcing, collection, and original draft writing: Tang, H.; Yue, L.
Supervision, original draft writing, and review: Mu, X.; Xia, H.; Zhou, H.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (22209070) and the Fundamental Research Funds for the Central Universities (30925010416).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Zheng, M.; You, Y.; Lu, J. Understanding materials failure mechanisms for the optimization of lithium-ion battery recycling. Nat. Rev. Mater. 2025, 10, 355-68.
2. Li, R.; Kirkaldy, N. D.; Oehler, F. F.; Marinescu, M.; Offer, G. J.; O’Kane, S. E. J. The importance of degradation mode analysis in parameterising lifetime prediction models of lithium-ion battery degradation. Nat. Commun. 2025, 16, 2776.
3. Machala, M. L.; Chen, X.; Bunke, S. P.; et al. Life cycle comparison of industrial-scale lithium-ion battery recycling and mining supply chains. Nat. Commun. 2025, 16, 988.
4. Istrate, R.; Mas-fons, A.; Beylot, A.; et al. Decarbonizing lithium-ion battery primary raw materials supply chain. Joule 2024, 8, 2992-3016.
5. Cho, T. H.; Chen, Y.; Liao, D. W.; et al. Enabling 6C fast charging of Li-ion batteries at sub-zero temperatures via interface engineering and 3D architectures. Joule 2025, 9, 101881.
6. Cui, H.; Song, Y.; Ren, D.; Wang, L.; He, X. Electrocapillary boosting electrode wetting for high-energy lithium-ion batteries. Joule 2024, 8, 29-44.
7. Guo, Y.; Guo, C.; Li, P.; et al. Improving the fast-charging capability of NbWO-based Li-ion batteries. Nat. Commun. 2025, 16, 2441.
8. Zeng, W.; Xia, F.; Wang, J.; et al. Entropy-increased LiMn2O4-based positive electrodes for fast-charging lithium metal batteries. Nat. Commun. 2024, 15, 7371.
9. Lan, X.; Li, Z.; Zeng, Y.; Han, C.; Peng, J.; Cheng, H. Phosphorus-based anodes for fast-charging alkali metal ion batteries. EcoMat 2024, 6, e12452.
10. Ye, Y.; Xu, R.; Huang, W.; et al. Quadruple the rate capability of high-energy batteries through a porous current collector design. Nat. Energy. 2024, 9, 643-53.
11. Jin, H.; Huang, Y.; Wang, C.; Ji, H. Phosphorus-based anodes for fast charging lithium-ion batteries: challenges and opportunities. Small. Sci. 2022, 2, 2200015.
13. Jin, H.; Xin, S.; Chuang, C.; et al. Black phosphorus composites with engineered interfaces for high-rate high-capacity lithium storage. Science 2020, 370, 192-7.
14. Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy. 2019, 4, 540-50.
15. Jin, S.; Gao, X.; Hong, S.; et al. Fast-charge, long-duration storage in lithium batteries. Joule 2024, 8, 746-63.
16. Xu, J.; Cai, X.; Cai, S.; et al. High-energy lithium-ion batteries: recent progress and a promising future in applications. Energy. Environ. Mater. 2023, 6, e12450.
17. Yan, X.; Zhao, G.; Wu, C.; et al. A biphase coupled cathode enables all-organic rocking-chair lithium ion batteries based on crystalline AB-stacked covalent triazine-based frameworks. Green. Chem. 2024, 26, 10593-603.
18. Du, W.; Du, X.; Ma, M.; Huang, S.; Sun, X.; Xiong, L. Polymer electrode materials for lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2110871.
19. Dong, W.; Ye, B.; Cai, M.; et al. Superwettable high-voltage LiCoO2 for low-temperature lithium ion batteries. ACS. Energy. Lett. 2023, 8, 881-8.
20. Huang, J.; Zhang, B.; Zhang, S.; et al. Ultra-fine Nano-Mg(OH)2 electrodeposited in flexible confined space and its enhancement of the performance of LiFePO4 lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2307215.
21. Meng, F.; Xiong, X.; Tan, L.; Yuan, B.; Hu, R. Strategies for improving electrochemical reaction kinetics of cathode materials for subzero-temperature Li-ion batteries: a review. Energy. Storage. Mater. 2022, 44, 390-407.
22. Wang, R.; Wang, L.; Liu, R.; Li, X.; Wu, Y.; Ran, F. “Fast-Charging” anode materials for lithium-ion batteries from perspective of ion diffusion in crystal structure. ACS. Nano. 2024, 18, 2611-48.
23. Weng, S.; Yang, G.; Zhang, S.; et al. Kinetic limits of graphite anode for fast-charging lithium-ion batteries. Nano. Micro. Lett. 2023, 15, 215.
24. Yue, X.; Zhang, J.; Dong, Y.; et al. Reversible Li plating on graphite anodes through electrolyte engineering for fast-charging batteries. Angew. Chem. 2023, 135, e202302285.
25. Zhang, Y.; Cheng, L.; Zhu, Y.; et al. Reversible Li plating regulation on graphite anode through a barium sulfate nanofibers-based dielectric separator for fast charging and high-safety lithium-ion battery. J. Energy. Chem. 2025, 101, 511-23.
26. Cai, W.; Yao, Y. X.; Zhu, G. L.; et al. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 2020, 49, 3806-33.
27. Lee, S. H.; Cho, Y.; Jeon, Y. P.; et al. Sustainable eco-friendly sub-micron NaCl crystal powder-assisted method to synthesize SiOx/C as anode materials originated from rice husk for lithium-ion batteries. EcoMat 2023, 5, e12401.
28. Xing, J.; Chen, T.; Yi, L.; et al. Endowing Cu foil self-wettable in molten lithium: a roll-to-roll wet coating strategy to fabricate high-performance ultrathin lithium metal anodes. Energy. Storage. Mater. 2023, 63, 103067.
29. Liu, H.; Zhu, Z.; Yan, Q.; et al. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63-7.
30. Wu, Z. S.; Ren, W.; Xu, L.; Li, F.; Cheng, H. M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS. Nano. 2011, 5, 5463-71.
31. Jin, X.; Han, Y.; Zhang, Z.; et al. Mesoporous single-crystal lithium titanate enabling fast-charging Li-ion batteries. Adv. Mater. 2022, 34, 2109356.
32. Han, X.; Gong, H.; Li, H.; Sun, J. Fast-charging phosphorus-based anodes: promises, challenges, and pathways for improvement. Chem. Rev. 2024, 124, 6903-51.
33. Wu, H.; Chan, G.; Choi, J. W.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310-5. https://www.nature.com/articles/nnano.2012.35#citeas (accessed 2025-09-28).
34. Zhou, X.; Yu, L.; Yu, X.; Lou, X. W. Encapsulating Sn nanoparticles in amorphous carbon nanotubes for enhanced lithium storage properties. Adv. Energy. Mater. 2016, 6, 1601177.
35. Wen, Y.; Yuan, B.; Peng, W.; Liu, Y.; Han, Q.; Hu, R. Enhanced diffusion kinetics in Y-doped SnO2 anodes for low-temperature lithium-ion batteries: a combined theoretical and experimental study. J. Alloys. Compd. 2024, 990, 174481.
36. Jiang, Y.; Song, D.; Wu, J.; et al. Sandwich-like SnS2/graphene/SnS2 with expanded interlayer distance as high-rate lithium/sodium-ion battery anode materials. ACS. Nano. 2019, 13, 9100-11.
37. Ning, H.; Liang, C.; Qiang, S.; et al. Gold-doped iron disulfide as cathode materials for enhanced electrochemical performance in thermal batteries. Rare. Met. 2025, 44, 1687-700.
38. Liang, X.; Li, X.; Xiang, Q.; et al. Surficial oxidation of phosphorus for strengthening interface interaction and enhancing lithium-storage performance. Nano. Lett. 2022, 22, 9335-42.
39. Zhang, W.; Pang, W. K.; Sencadas, V.; Guo, Z. Understanding high-energy-density Sn4P3 anodes for potassium-ion batteries. Joule 2018, 2, 1534-47.
40. Zheng, Z.; Wu, H. H.; Liu, H.; et al. Achieving fast and durable lithium storage through amorphous FeP nanoparticles encapsulated in ultrathin 3D P-doped porous carbon nanosheets. ACS. Nano. 2020, 14, 9545-61.
41. Zhang, X.; Ou-yang, W.; Zhu, G.; Lu, T.; Pan, L. Shuttle-like carbon-coated FeP derived from metal-organic frameworks for lithium-ion batteries with superior rate capability and long-life cycling performance. Carbon 2019, 143, 116-24.
42. Xu, X.; Liu, J.; Hu, R.; et al. Self-supported CoP nanorod arrays grafted on stainless steel as an advanced integrated anode for stable and long-life lithium-ion batteries. Chem. Eur. J. 2017, 23, 5198-204.
43. Ni, L.; Chen, G.; Liu, X.; et al. Self-supported fe-doped cop nanowire arrays grown on carbon cloth with enhanced properties in lithium-ion batteries. ACS. Appl. Energy. Mater. 2019, 2, 406-12.
44. Aso, K.; Hayashi, A.; Tatsumisago, M. Phase-selective synthesis of nickel phosphide in high-boiling solvent for all-solid-state lithium secondary batteries. Inorg. Chem. 2011, 50, 10820-4.
45. Xiang, J.; Wang, X.; Xia, X.; Zhong, J.; Tu, J. Fabrication of highly ordered porous nickel phosphide film and its electrochemical performances toward lithium storage. J. Alloys. Compd. 2011, 509, 157-60.
46. Liu, Y.; Zeng, C.; Liu, X.; et al. Rational Design of high-entropy phosphorus-based alloy anodes for fast-charging lithium-ion batteries. Adv. Funct. Mater. , 2025, e10753.
47. Liu, C.; Han, M.; Cao, Y.; et al. Unlocking the dissolution mechanism of phosphorus anode for lithium-ion batteries. Energy. Storage. Mater. 2021, 37, 417-23.
48. Zhang, S.; Liu, C.; Wang, H.; et al. A covalent P-C bond stabilizes red phosphorus in an engineered carbon host for high-performance lithium-ion battery anodes. ACS. Nano. 2021, 15, 3365-75.
49. He, S. A.; Liu, Q.; Cui, Z.; et al. Red phosphorus anchored on nitrogen-doped carbon bubble-carbon nanotube network for highly stable and fast-charging lithium-ion batteries. Small 2022, 18, 2105866.
50. Ryder, C. R.; Wood, J. D.; Wells, S. A.; et al. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016, 8, 597-602.
51. Tian, H.; Wang, H.; Wang, J.; Qu, G.; Yu, X.; Jiang, G. Understanding the intrinsic reactivity of black phosphorus. Acc. Mater. Res. 2024, 5, 1472-83.
52. Zhang, S.; Zhang, Y.; Zhang, Z.; et al. Bi Works as a Li reservoir for promoting the fast-charging performance of phosphorus anode for Li-ion batteries. Adv. Energy. Mater. 2022, 12, 2103888.
53. Chen, X.; Chen, X. R.; Hou, T. Z.; et al. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes. Sci. Adv. 2019, 5, eaau7728.
54. Yoo, E.; Kim, J.; Hosono, E.; Zhou, H. S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano. Lett. 2008, 8, 2277-82.
55. Liu, C.; Han, X.; Cao, Y.; Zhang, S.; Zhang, Y.; Sun, J. Topological construction of phosphorus and carbon composite and its application in energy storage. Energy. Storage. Mater. 2019, 20, 343-72.
56. Wang, J.; Liu, W.; Wang, C. Superior rate and long-lived performance of few-layered black phosphorus-based hybrid anode for lithium-ion batteries. Electrochim. Acta. 2022, 403, 139697.
57. Li, X.; Han, X.; Liu, R.; et al. Tannic acid-polypyrrole multifunctional coating layer enhancing the interface effect and efficient Li-ion transport of a phosphorus anode. Nanoscale 2022, 14, 3625-31.
58. Wu, Y.; Huang, H. B.; Feng, Y.; Wu, Z. S.; Yu, Y. The promise and challenge of phosphorus-based composites as anode materials for potassium-ion batteries. Adv. Mater. 2019, 31, 1901414.
59. Sun, Y.; Wang, L.; Li, Y.; et al. Design of red phosphorus nanostructured electrode for fast-charging lithium-ion batteries with high energy density. Joule 2019, 3, 1080-93.
60. Lei, W.; Liu, Y.; Jiao, X.; et al. Improvement of cycling phosphorus-based anode with LiF-rich solid electrolyte interphase for reversible lithium storage. ACS. Appl. Energy. Mater. 2019, 2, 2699-707.
61. Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820.
62. Cai, Y.; Zhang, G.; Zhang, Y. Phosphorene: physical properties, synthesis, and fabrication, 1th ed.; CRC Press, 2019.
63. Toy, A. D. F. The chemistry of phosphorus: pergamon texts in inorganic chemistry; Vol 3, Elsevier, 2016. https://books.google.com/books?id=sAJPDAAAQBAJ&hl=zh-CN&source=gbs_navlinks_s (accessed 2025-09-26).
64. Fung, C. M.; Er, C. C.; Tan, L. L.; Mohamed, A. R.; Chai, S. P. Red phosphorus: an up-and-coming photocatalyst on the horizon for sustainable energy development and environmental remediation. Chem. Rev. 2022, 122, 3879-965.
65. Tian, H.; Wang, J.; Lai, G.; et al. Renaissance of elemental phosphorus materials: properties, synthesis, and applications in sustainable energy and environment. Chem. Soc. Rev. 2023, 52, 5388-484.
66. Jones, R. O.; Hohl, D. Structure of phosphorus clusters using simulated annealing-P2 to P8. J. Chem. Phys. 1990, 92, 6710-21.
67. Olego, D.; Baumann, J.; Kuck, M.; Schachter, R.; Michel, C.; Raccah, P. The microscopic structure of bulk amorphous red phosphorus: a Raman scattering investigation. Solid. State. Commun. 1984, 52, 311-4.
68. Shanabrook, B. V.; Lannin, J. S. Structural and vibrational properties of amorphous phosphorus. Phys. Rev. B. 1981, 24, 4771.
70. Farman, H.; Dore, J.; Elliott, S. Intermediate-range order in amorphous phosphorus. Phys. Lett. A. 1994, 186, 410-4.
71. Goodman, N. B.; Ley, L.; Bullett, D. W. Valence-band structures of phosphorus allotropes. Phys. Rev. B. 1983, 27, 7440.
72. Roth, W. L.; DeWitt, T. W.; Smith, A. J. Polymorphism of red phosphorus. J. Am. Chem. Soc. 1947, 69, 2881-5.
73. Sun, Z.; Zhang, B.; Yan, Q. Solution phase synthesis of the less-known Form II crystalline red phosphorus. Inorg. Chem. Front. 2022, 9, 4385-93.
74. Yoon, J. Y.; Lee, Y.; Kim, D. G.; et al. Type-II red phosphorus: wavy packing of twisted pentagonal tubes. Angew. Chem. Int. Ed. 2023, 62, e202307102.
75. Zhu, Y.; Ren, J.; Zhang, X.; et al. Elemental red phosphorus-based materials for photocatalytic water purification and hydrogen production. Nanoscale 2020, 12, 13297-310.
76. Ruck, M.; Hoppe, D.; Wahl, B.; Simon, P.; Wang, Y.; Seifert, G. Fibrous red phosphorus. Angew. Chem. Int. Ed. 2005, 44, 7616-9.
77. Baumer, F.; Ma, Y.; Shen, C.; et al. Synthesis, characterization, and device application of antimony-substituted violet phosphorus: a layered material. ACS. Nano. 2017, 11, 4105-13.
78. Ding, K.; Wen, L.; Huang, S.; Li, Y.; Zhang, Y.; Lu, Y. Electronic properties of red and black phosphorous and their potential application as photocatalysts. RSC. Adv. 2016, 6, 80872-84.
79. Schusteritsch, G.; Uhrin, M.; Pickard, C. J. Single-layered hittorf’s phosphorus: a wide-bandgap high mobility 2D material. Nano. Lett. 2016, 16, 2975-80.
80. Hu, Z.; Yuan, L.; Liu, Z.; Shen, Z.; Yu, J. C. An elemental phosphorus photocatalyst with a record high hydrogen evolution efficiency. Angew. Chem. Int. Ed. 2016, 128, 9732-7.
81. Yu, Z.; Song, J.; Gordin, M. L.; Yi, R.; Tang, D.; Wang, D. Phosphorus-graphene nanosheet hybrids as lithium-ion anode with exceptional high-temperature cycling stability. Adv. Sci. 2015, 2, 1400020.
82. Sun, Z.; Chen, W.; Zhang, B.; et al. Polarization conversion in bottom-up grown quasi-1D fibrous red phosphorus flakes. Nat. Commun. 2023, 14, 4398.
84. Liu, H.; Neal, A. T.; Zhu, Z.; et al. Phosphorene: an unexplored 2D semiconductor with a high hole mobility. ACS. Nano. 2014, 8, 4033-41.
85. Boidi, G.; Ronai, B.; Heift, D.; et al. Tribology of 2D black phosphorus - current state-of-the-art and future potential. Adv. Colloid. Interface. Sci. 2024, 328, 103180.
86. Smith, J. B.; Hagaman, D.; Ji, H. F. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602.
87. Jamieson, J. C. Crystal structures adopted by black phosphorus at high pressures. Science 1963, 139, 1291-2.
88. Zhong, Q. Intrinsic and engineered properties of black phosphorus. Mater. Today. Phys. 2022, 28, 100895.
89. Xia, F.; Wang, H.; Jia, Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458.
90. Cartz, L.; Srinivasa, S. R.; Riedner, R. J.; Jorgensen, J. D.; Worlton, T. G. Effect of pressure on bonding in black phosphorus. J. Chem. Phys. 1979, 71, 1718-21.
91. Lee, H. U.; Lee, S. C.; Won, J.; et al. Stable semiconductor black phosphorus (BP)@titanium dioxide (TiO2) hybrid photocatalysts. Sci. Rep. 2015, 5, 8691.
92. Hultgren, R.; Gingrich, N. S.; Warren, B. E. The Atomic distribution in red and black phosphorus and the crystal structure of black phosphorus. J. Chem. Phys. 1935, 3, 351-5.
93. Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M. S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 4523-30.
94. Li, L.; Kim, J.; Jin, C.; et al. Direct observation of the layer-dependent electronic structure in phosphorene. Nat. Nanotechnol. 2017, 12, 21-5.
95. Kim, J.; Baik, S. S.; Ryu, S. H.; et al. Observation of tunable band gap and anisotropic Dirac semimetal state in black phosphorus. Science 2015, 349, 723-6.
96. Macdonald, T. J.; Clancy, A. J.; Shutt, R. R.; Howard, C. A. Phosphorene nanoribbons for next-generation energy devices. Joule 2022, 6, 2441-6.
97. Ma, R.; Xiong, L.; Jiao, P.; et al. Origins of severe structural changes during alloying-dealloying reactions in black phosphorus. J. Am. Chem. Soc. 2024, 146, 23044-53.
98. Boretti, A. Challenges in using phosphorene as electrode material in lithium-ion batteries. Energy. Storage. 2024, 6, e607.
99. Liang, W.; Chen, B.; Li, D.; et al. Understanding the structural relation and electrochemical evolution between ZnGeP2 and ZnSiP2 twin phosphides for advanced Li-ion batteries. Chem. Eng. J. 2024, 496, 154332.
100. Hou, B. H.; Wang, Y. Y.; Ning, Q. L.; et al. An FeP@C nanoarray vertically grown on graphene nanosheets: an ultrastable Li-ion battery anode with pseudocapacitance-boosted electrochemical kinetics. Nanoscale 2019, 11, 1304-12.
101. Lin, X.; Ke, Y.; Peng, X.; et al. Improving the rate capacity and cycle stability of FeP anodes for lithium-ion batteries via in situ carbon encapsulation and copper doping. J. Colloid. Interface. Sci. 2023, 634, 346-56.
102. Wang, C.; Yan, J.; Li, T.; et al. A Coral-like FeP@NC anode with increasing cycle capacity for sodium-ion and lithium-ion batteries induced by particle refinement. Angew. Chem. 2021, 133, 25217-23.
103. Veluri, P. S.; Mitra, S. Iron phosphide (FeP) synthesis, and full cell lithium-ion battery study with a [Li(NiMnCo)O2] cathode. RSC. Adv. 2016, 6, 87675-9.
104. Zheng, J.; Dong, R.; Liu, P.; et al. Interfacial engineered Fe2O3@FeP nanorod arrays as capacitive storage dominated and high charge transfer anode for high-rate lithium-ion batteries. Surf. Coat. Technol. 2021, 421, 127471.
105. Li, Z.; Zhang, Y.; Bai, J.; Wang, J.; Zhao, H. Well-dispersed FeP@C nanoparticles anchored on MXene conductive network as outstanding cyclic performance anode for Li/Na-ion batteries. Carbon 2025, 234, 120008.
106. Yu, J.; He, Y.; Li, J.; et al. In-situ rooting biconical-nanorods-like Co-doped FeP @carbon architectures toward enhanced lithium storage performance. Chem. Eng. J. 2023, 477, 146996.
107. Yang, Y.; Xia, J.; Guan, X.; et al. In situ growth of CoP nanosheet arrays on carbon cloth as binder-free electrode for high-performance flexible lithium-ion batteries. Small 2022, 18, 2204970.
108. Turarova, G.; Taniguchi, I.; Bakenov, Z.; Belgibayeva, A. In situ steam oxidation of nickel phosphide/carbon composite nanofibers as anode materials for lithium-ion batteries. J. Power. Sources. 2024, 613, 234933.
109. Li, F.; Gao, J.; He, Z.; Brandon, N.; Li, X.; Kong, L. Engineering novel Ni2-XCoxP structures for high performance lithium-ion storage. Energy. Storage. Materials. 2022, 48, 20-34.
110. Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy. Storage. Mater. 2019, 16, 290-322.
111. Barreteau, C.; Michon, B.; Besnard, C.; Giannini, E. High-pressure melt growth and transport properties of SiP, SiAs, GeP, and GeAs 2D layered semiconductors. J. Cryst. Growth. 2016, 443, 75-80.
112. Shen, H.; Shi, Y.; Bian, W.; et al. Revisiting the failure mechanism of layered germanium phosphide anode for lithium/sodium-ion batteries: decisive role of mechanical robustness. J. Power. Sources. 2025, 630, 236171.
113. Jiang, Y.; Wang, Y.; Jiang, J.; et al. In-situ solvothermal phosphorization from nano-sized tetragonal-Sn to rhombohedral-Sn4P3 embedded in hollow graphene sphere with high capacity and stability. Electrochim. Acta. 2019, 312, 263-71.
114. Zhang, Y.; Liu, L.; Zhao, L.; et al. Sandwich-like CoMoP2/MoP heterostructures coupling N, P co-doped carbon nanosheets as advanced anodes for high-performance lithium-ion batteries. Adv. Compos. Hybrid. Mater. 2022, 5, 2601-10.
115. Zhang, D.; Guo, X.; Tong, X.; et al. High-performance battery-type supercapacitor based on porous biocarbon and biocarbon supported Ni-Co layered double hydroxide. J. Alloys. Compd. 2020, 837, 155529.
116. He, R.; Wang, X.; Li, J.; Chang, L.; Wang, H.; Nie, P. Engineering ultra-small tin phosphide encapsulated in 3D phosphorous-doped porous carbon nanosheets as high-performance anodes for lithium-ion batteries. Appl. Surf. Sci. 2024, 654, 159532.
117. Liu, L.; Xie, H.; Zheng, Y.; et al. Multicomponent anodes based on amorphous ZnP2 for Fast-charging/discharging lithium-ion batteries. Adv. Energy. Mater. 2025, 15, 2404900.
118. Said, S.; Shutt, R. R. C.; Zhang, Z.; Lovett, A. J.; Howard, C. A.; Miller, T. S. Electrochemical atomic force microscopy of black phosphorus composite anodes: electrode destabilization and degradation mechanisms in alkali-ion batteries. ACS. Appl. Mater. Interfaces. 2024, 16, 43512-25.
119. Zhang, Y.; Wang, L.; Xu, H.; Cao, J.; Chen, D.; Han, W. 3D chemical cross-linking structure of black phosphorus@CNTs hybrid as a promising anode material for lithium ion batteries. Adv. Funct. Mater. 2020, 30, 1909372.
120. Liu, Z.; Yang, S.; Sun, B.; Chang, X.; Zheng, J.; Li, X. A Peapod-like CoP@C nanostructure from phosphorization in a low-temperature molten salt for high-performance lithium-ion batteries. Angew. Chem. Int. Ed. 2018, 57, 10187-91.
121. Sun, J.; Liu, C.; Wang, H.; et al. Core-shell structure of a polypyrrole-coated phosphorus/carbon nanotube anode for high-performance lithium-ion batteries. ACS. Appl. Energy. Mater. 2021, 4, 4112-8.
122. Liu, W.; Yuan, X.; Yu, X. A core-shell structure of polydopamine-coated phosphorus-carbon nanotube composite for high-performance sodium-ion batteries. Nanoscale 2018, 10, 16675-82.
123. Zhang, S.; Wan, Y.; Cao, Y.; et al. Delithiation-accelerating and self-healing strategies realizes high-capacity and high-rate black phosphorus anode in wide temperature range. eScience 2025, 5, 100328.
124. Meng, R.; Huang, J.; Feng, Y.; et al. Black phosphorus quantum Dot/Ti3C2 MXene nanosheet composites for efficient electrochemical lithium/sodium-ion storage. Adv. Energy. Mater. 2018, 8, 1801514.
125. Zheng, W.; Lee, J.; Gao, Z.; et al. Laser-assisted ultrafast exfoliation of black phosphorus in liquid with tunable thickness for Li-ion batteries. Adv. Energy. Mater. 2020, 10, 1903490.
126. Liu, X.; Yu, M.; Wu, S.; Gong, J. Composite nanoarchitectonics for efficient lithium storage by encapsulating black phosphorus quantum dots in cobalt/iron based Prussian blue analogues. J. Alloys. Compd. 2023, 969, 172291.
127. Cui, X.; Chen, J.; Sun, Z.; et al. A general route for encapsulating monodispersed transition metal phosphides into carbon multi-chambers toward high-efficient lithium-ion storage with underlying mechanism exploration. Adv. Funct. Mater. 2023, 33, 2212100.
128. Han, X.; Sun, J. Improved fast-charging performances of phosphorus electrodes using the intrinsically flame-retardant LiFSI based electrolyte. J. Power. Sources. 2020, 474, 228664.
129. Lin, H.; Chen, K.; Chang, C.; Tuan, H. Aluminum phosphide as a high-performance lithium-ion battery anode. J. Power. Sources. 2020, 465, 228262.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data





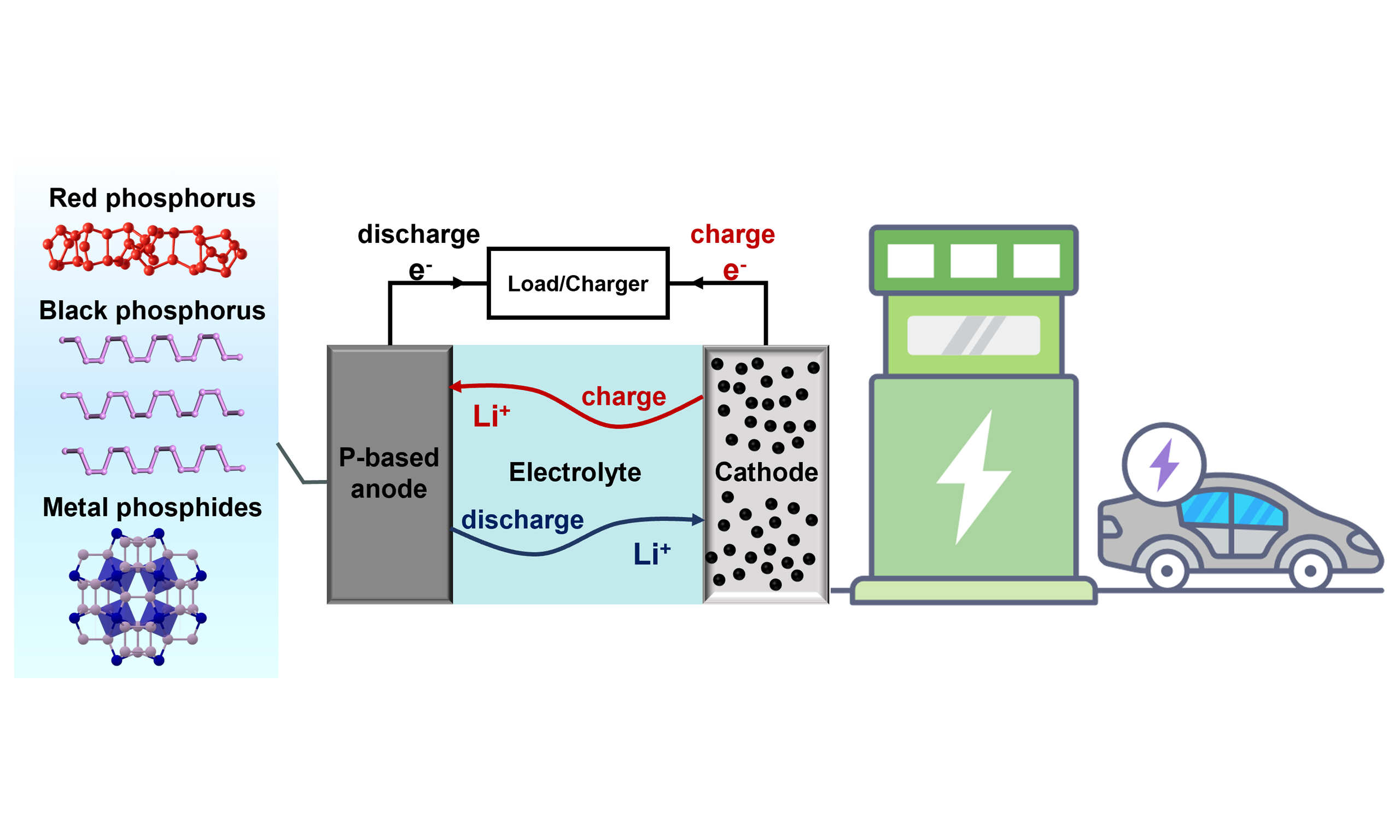
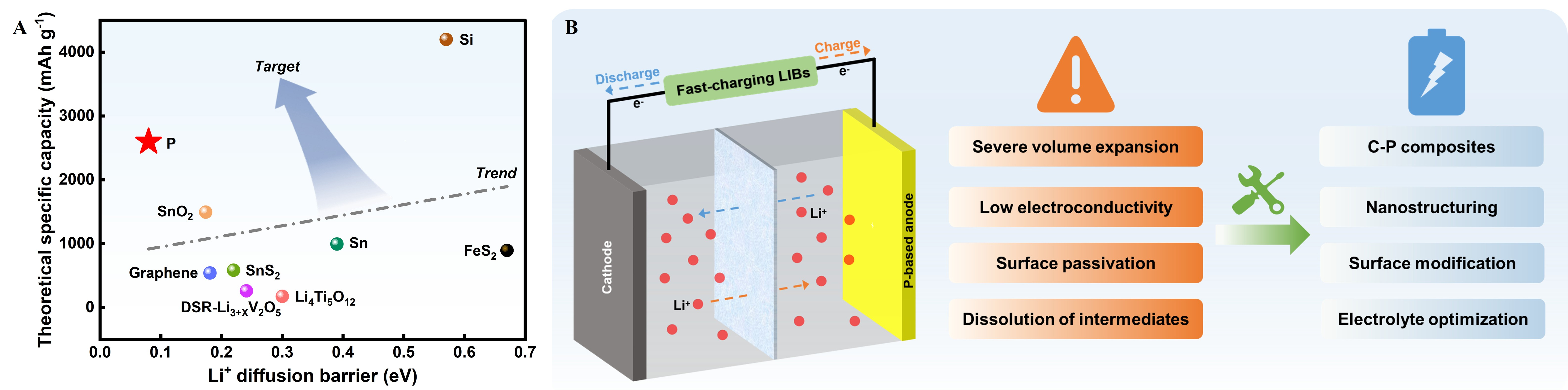
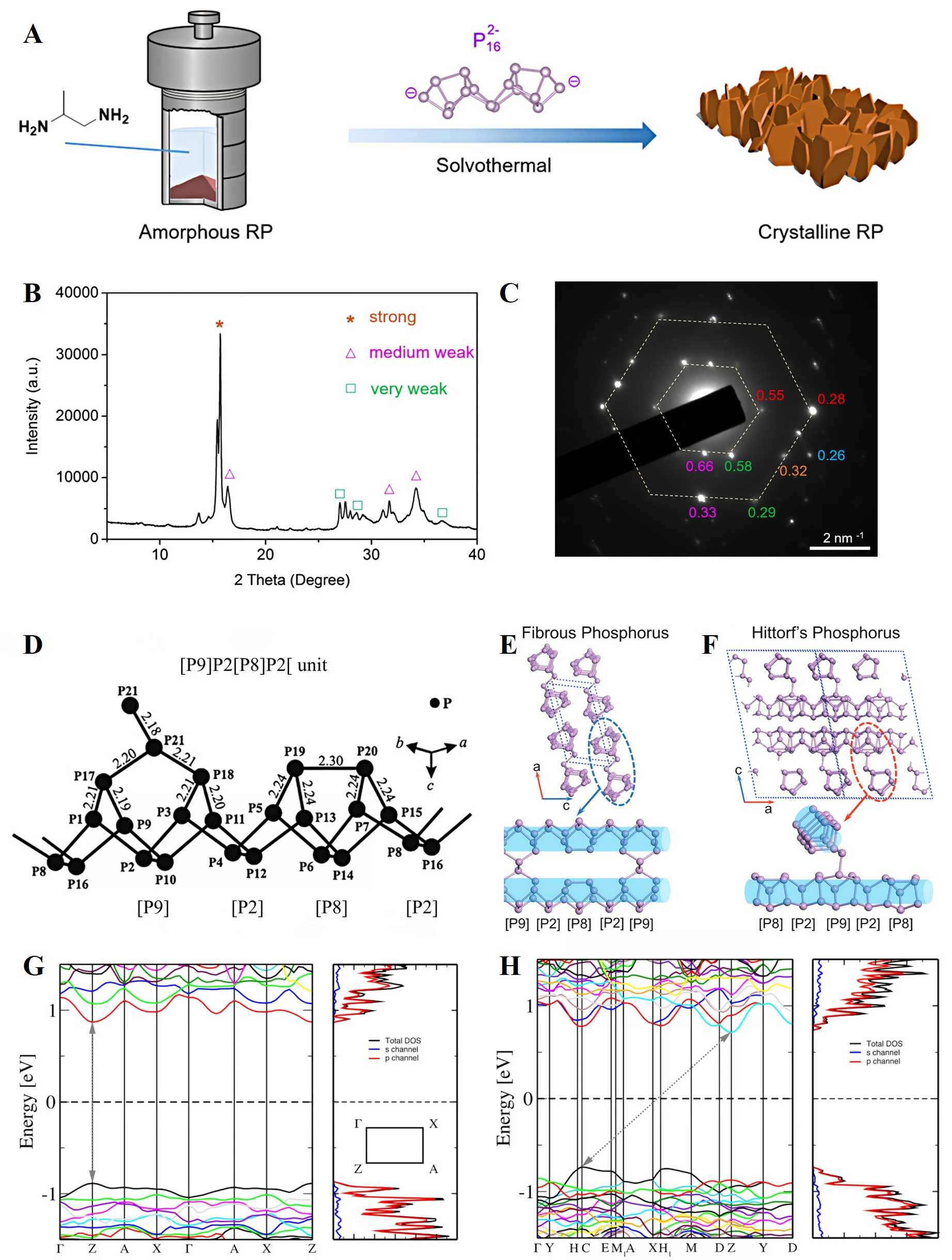
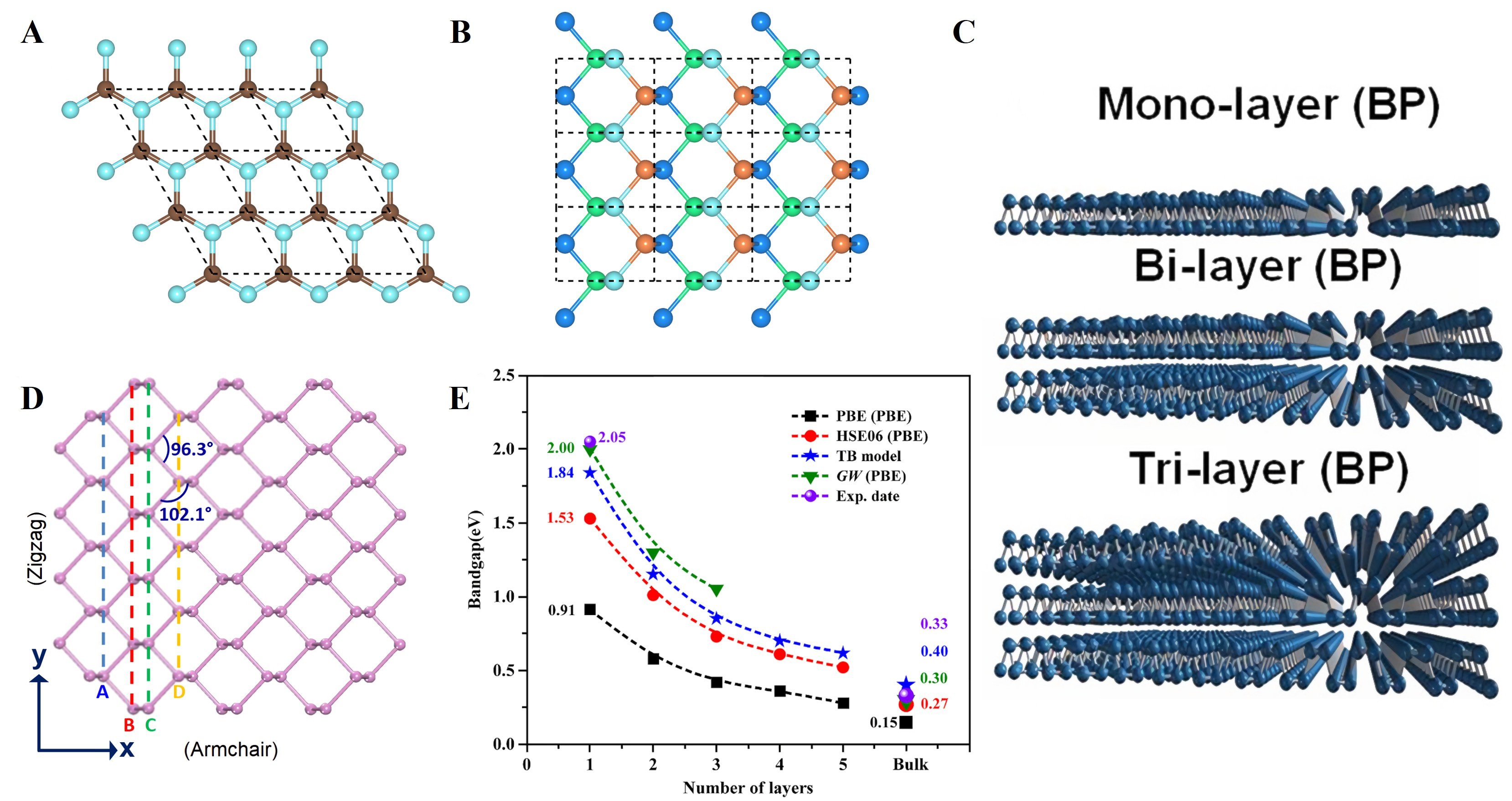
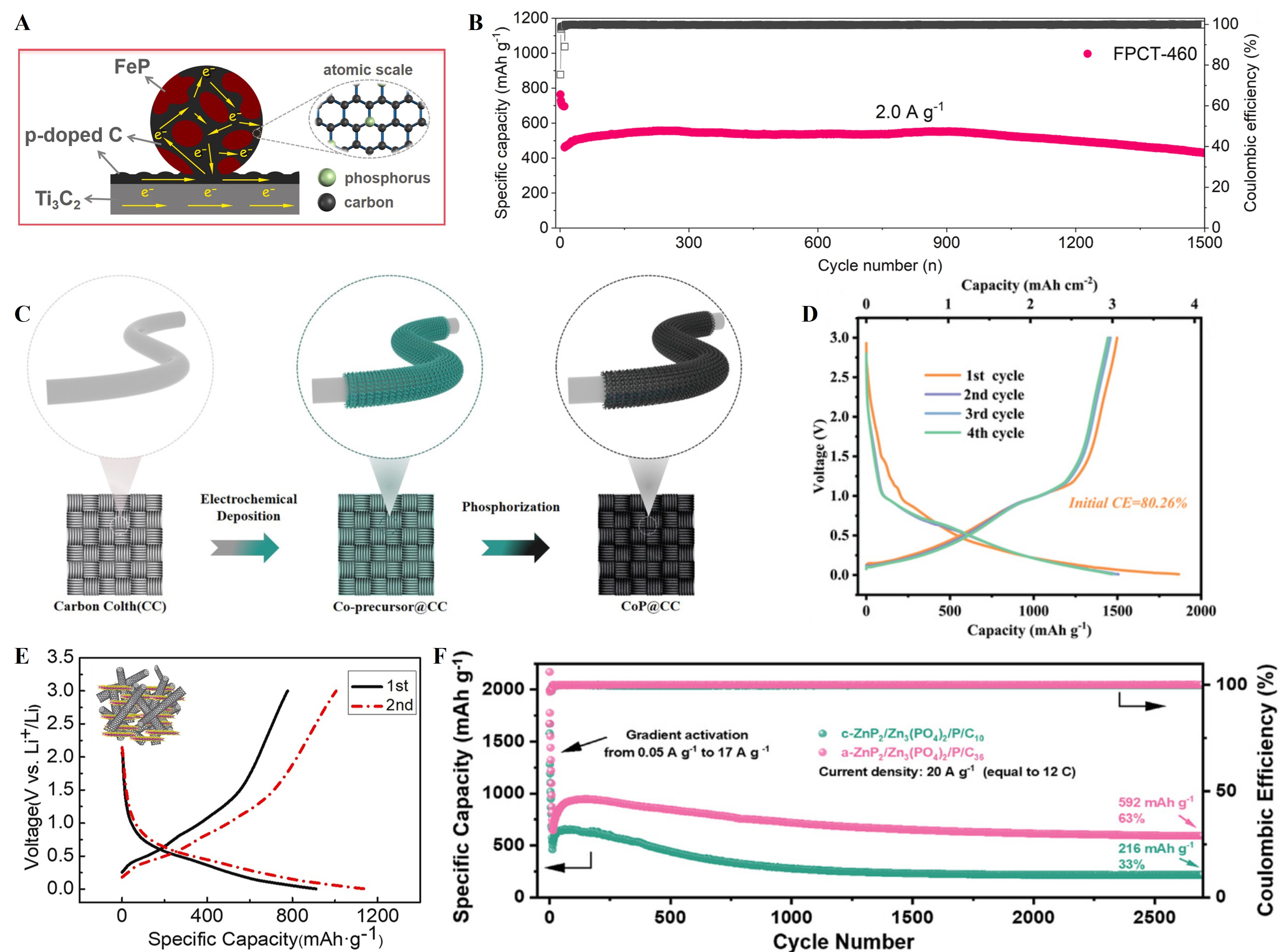
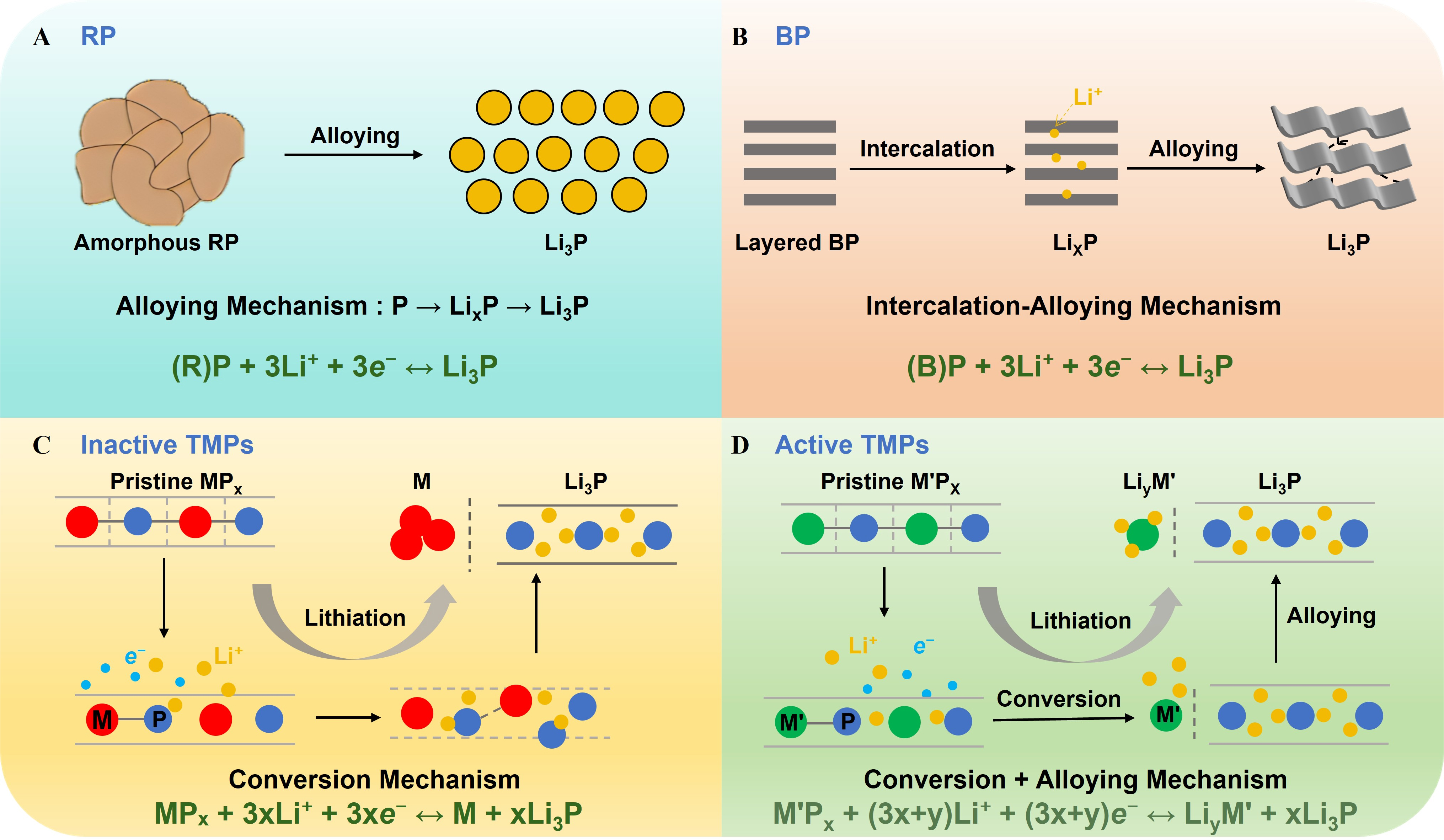
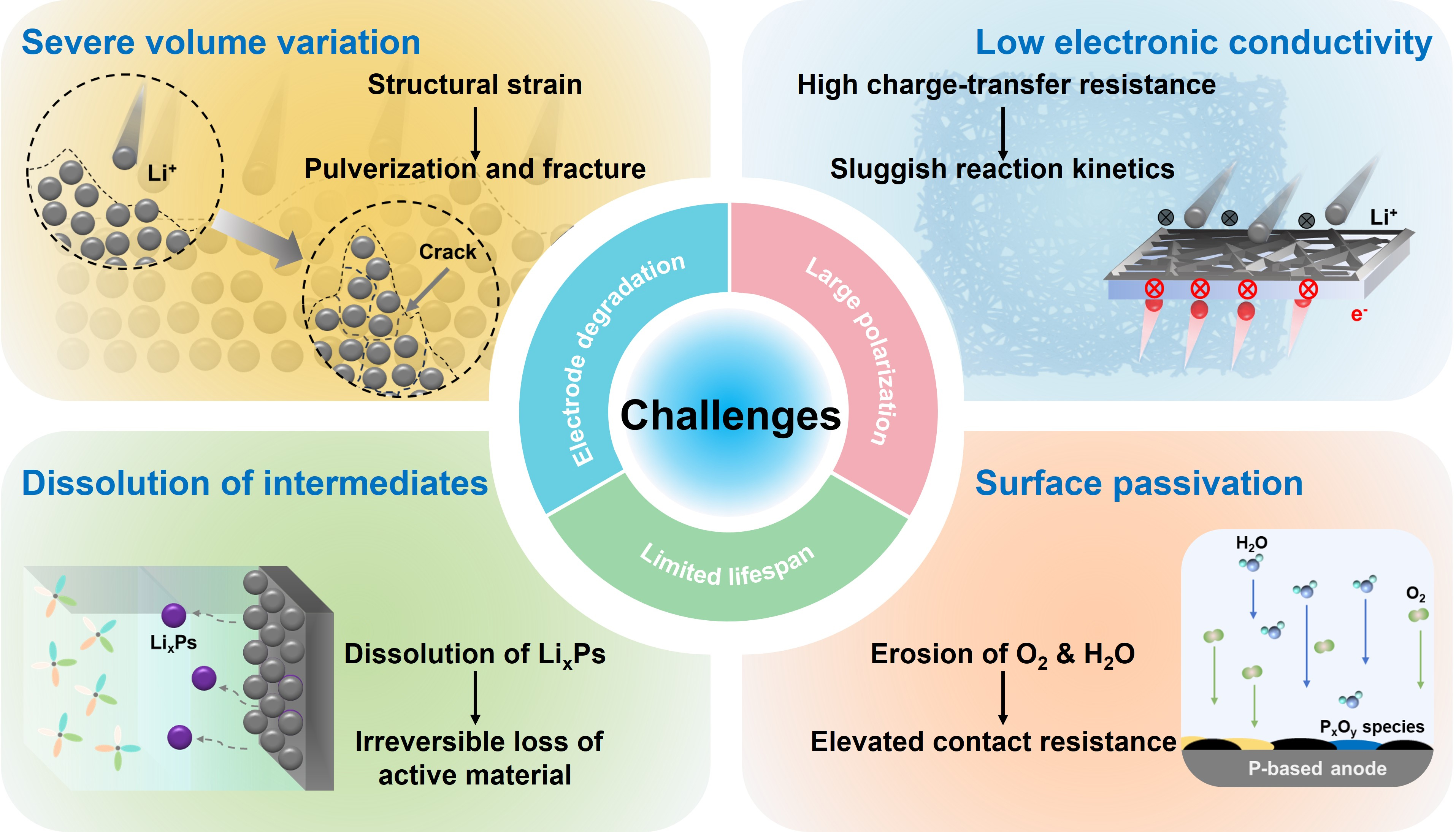
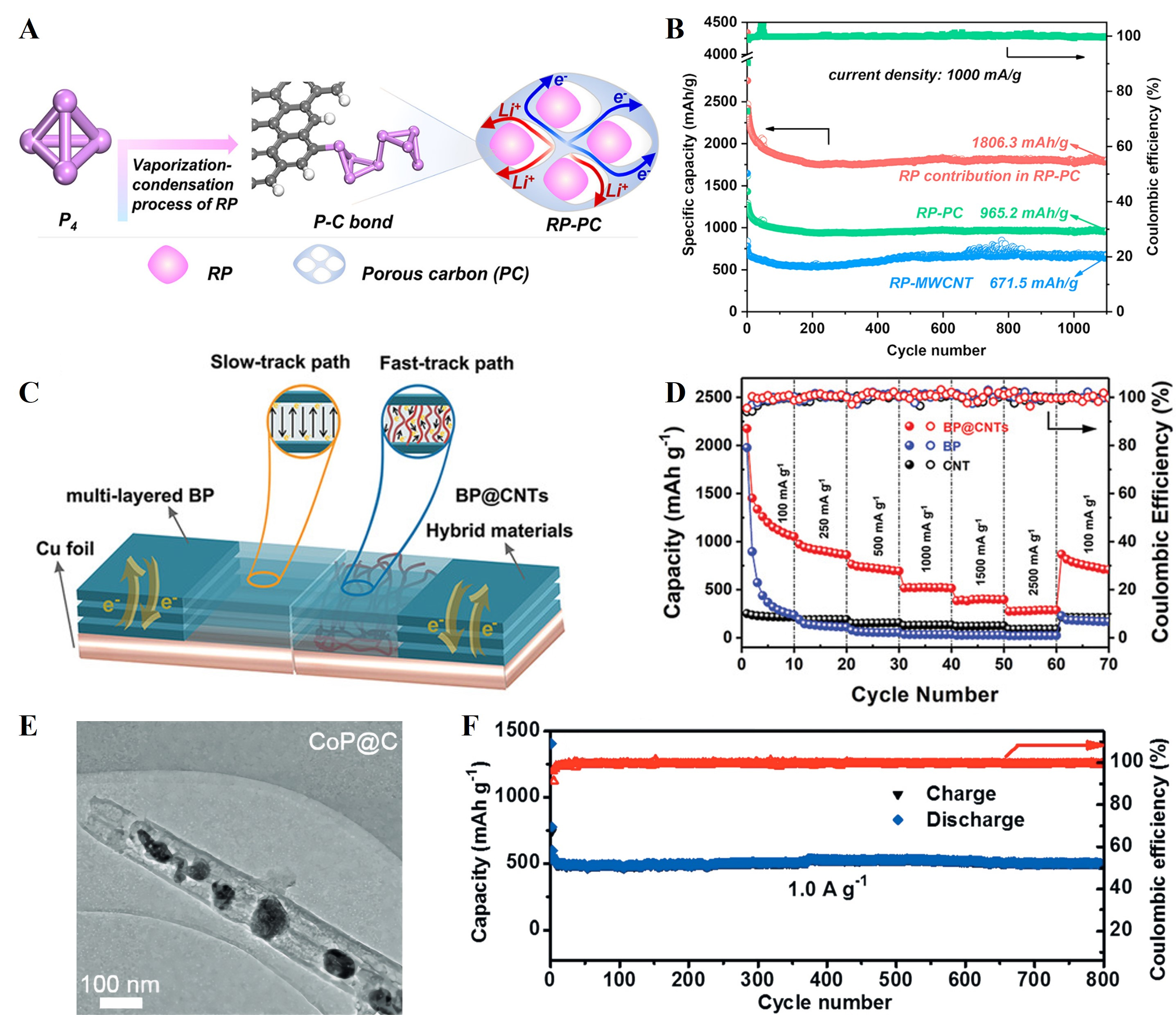
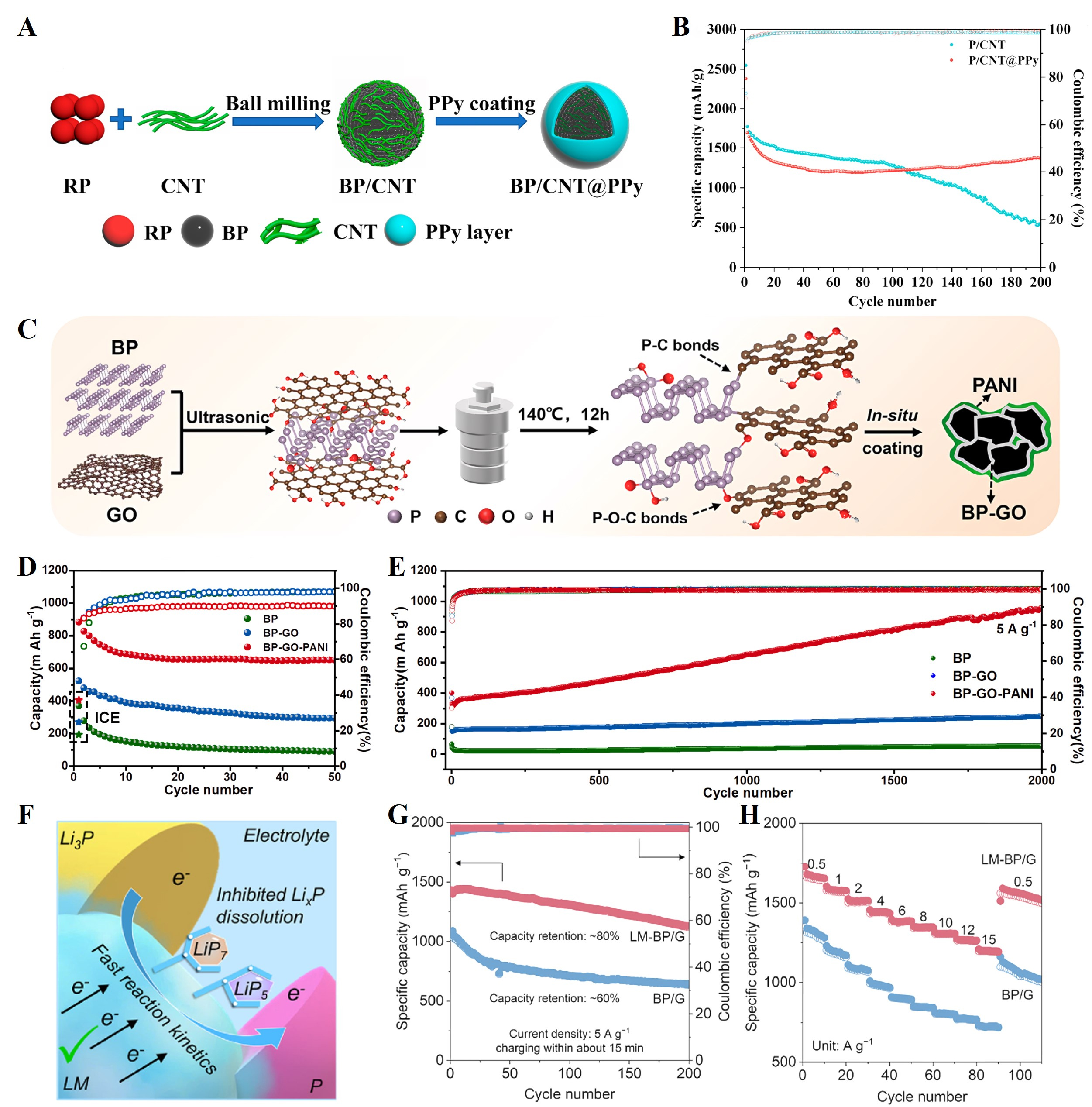
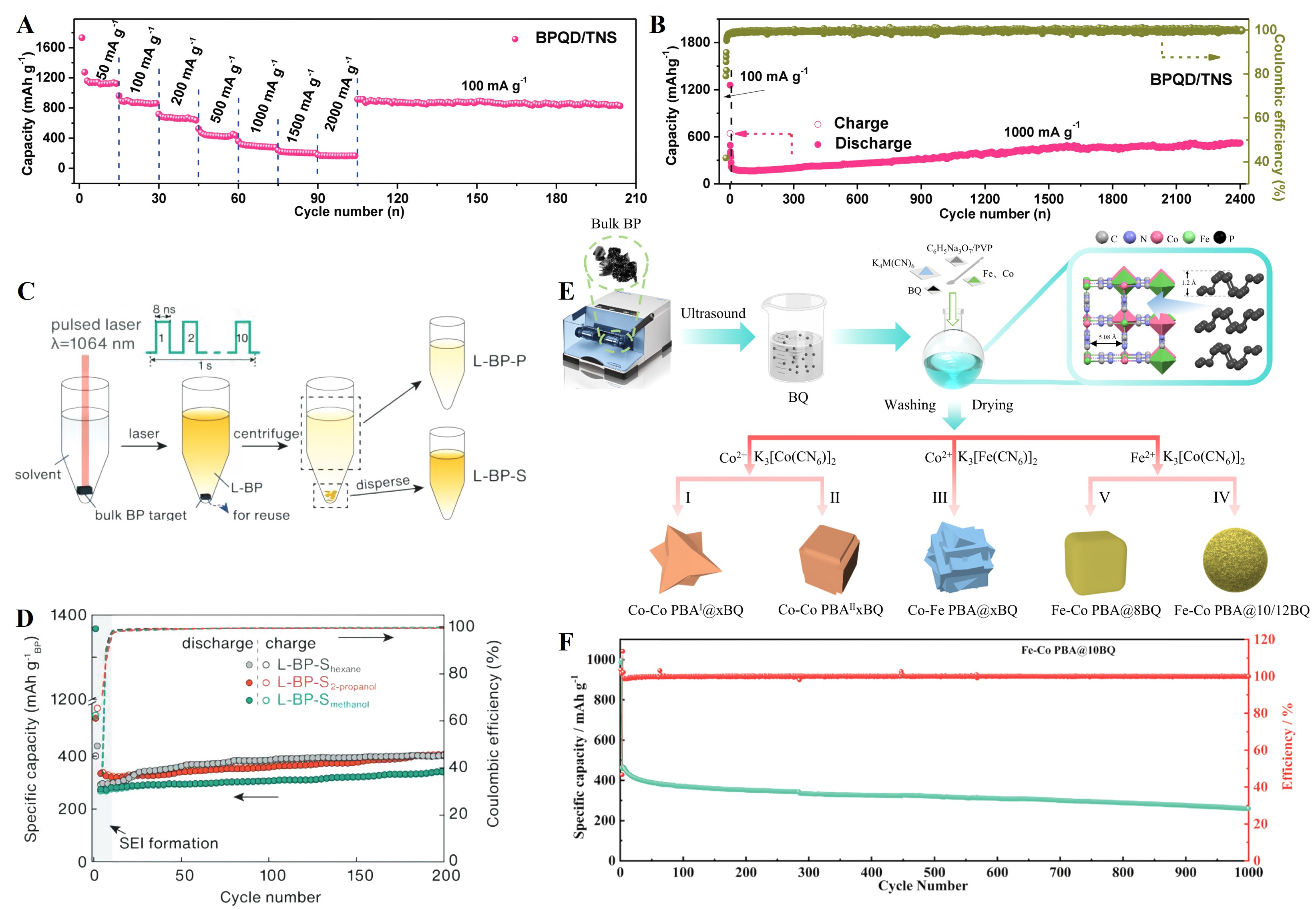
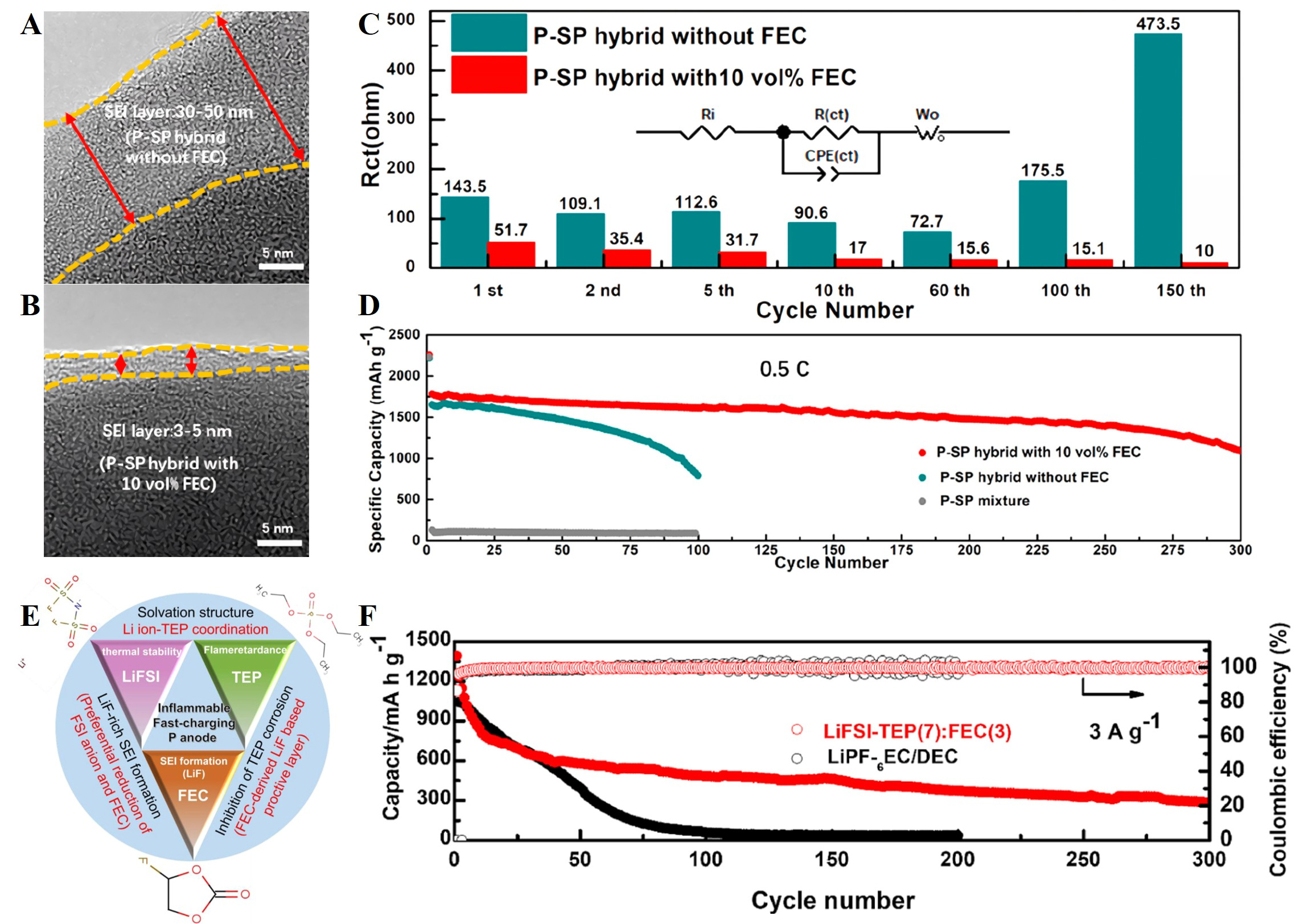












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].