Supported noble metal catalysts for complete propane oxidation: structure-activity relationships and reaction mechanisms
Abstract
Volatile organic compounds are important precursors of air pollution and photochemical smog, and they pose potential risks to human health and the ecological environment. Among them, propane is particularly challenging to eliminate due to its stable chemical nature. The catalytic oxidation of propane offers a promising strategy to tackle this pressing environmental issue and serves as an excellent model reaction for investigating the C–H and C–C bond activation and oxygen redox processes on supported noble metal catalysts. In this review, we focus on commonly used supported noble metal catalysts and systematically discuss how the chemical state, particle size, support type, addition of promoters, and metal-support interactions influence the catalytic performance in complete propane oxidation. Subsequently, the thermal stability of different noble metal catalysts is summarized. We then provide an overview of the common propane oxidation mechanisms reported in the literature, including Langmuir-Hinshelwood, Eley-Rideal, and Mars-van Krevelen mechanisms. Finally, we summarize and prospect the precise regulation of noble metal-support interface and the application of newly developed electrothermal catalytic technologies for highly efficient propane oxidation, guiding the design of future high-performance catalysts. This review aims to provide mechanistic insights and design principles bridging fundamental catalysis and practical oxidation applications.
Keywords
INTRODUCTION
With the rapid development of industrialization and urbanization, volatile organic compounds (VOCs) have become a major class of air pollutants due to their significant environmental and health impacts[1-4]. VOCs are organic chemicals with high vapor pressure and low boiling point at room temperature and atmospheric pressure, and therefore volatile and participate in complex atmospheric photochemical reactions to form ground-level ozone (O3) and respirable particulate matter (PM2.5), which contribute to photochemical smog, ozone layer depletion, and exacerbate global warming problems[5-10]. In addition, many VOCs are toxic, mutagenic, or carcinogenic, such as aromatic VOCs [triphenyls, polycyclic aromatic hydrocarbons (PAHs), etc.], oxygenated VOCs (alcohols, ketones, phenols, esters, etc.), hydrocarbons (alkanes, olefins, etc.), and heteroatom-containing VOCs (halogenated hydrocarbons, etc.)[11,12]. Exposure to high concentrations of VOCs may cause direct damage to organs such as the eyes, nasal passages, and respiratory tract, and may even be life-threatening in severe cases[13]. Prolonged exposure to low solubility VOCs (even at concentrations below 1 ppm) may cause symptoms such as confusion, fatigue, nausea, irritation of mucous membranes and eyes, shortness of breath, and chest tightness[14,15].
At present, existing VOC abatement technologies can be broadly classified into physical and chemical approaches. Physical methods include absorption, adsorption, membrane separation, and condensation[16,17], while chemical techniques encompass biodegradation[18-20], direct thermal combustion, and catalytic oxidation[21,22]. Among these, catalytic complete oxidation (combustion) possesses remarkable advantages, including a wide applicable concentration range (mg/m3~g/m3) and a broad treatment air volume range (102-106 m3/h). This method enables flameless combustion at relatively low temperatures, effectively avoiding the secondary pollution associated with direct combustion, while ensuring safety and stability in treating flammable and explosive organic gases. Therefore, catalytic complete oxidation is regarded as one of the most promising technologies for industrial VOC purification, with its core lying in the design and optimization of cost-effective and efficient catalysts[2,23-26].
Propane (C3H8), a low-carbon alkane and one of the most common VOCs, is primarily derived from natural gas processing, petroleum refining, and emissions from the petrochemical industry[27-29]. Compared with aromatic hydrocarbons, lipids, acids, and oxygenated VOCs, light alkanes such as propane are chemically inert and possess stable C–H bonds, making them difficult to adsorb and activate on catalyst surfaces[30-32]. Consequently, the rapid and effective reduction of emissions from short-chain alkanes remains a pressing challenge in the field of atmospheric pollution control. Therefore, there is an urgent need to develop efficient, safe, and environmentally friendly catalyst systems for propane abatement[33-35].
Supported noble metal catalysts, owing to their excellent activity and stability, have demonstrated great potential in propane catalytic oxidation. In addition, propane oxidation is also an ideal model to probe the C–H and C–C bond activation and oxygen redox processes on supported noble metal catalysts. This review first systematically elucidates how multiple factors, including the chemical states, dispersion levels, metal-support interactions (MSIs), and promoter modifications of noble metals (Pt, Pd, Ru), affect the catalytic activity, revealing the intrinsic correlations and regulatory mechanisms between these structural features and catalytic performance [Figure 1].
Secondly, from a practical application perspective, this review focuses on the deactivation mechanisms of catalysts during propane oxidation, including the aggregation of active metal particles caused by sintering or the structural reconstruction via redispersion. It also systematically summarizes effective anti-deactivation strategies, providing theoretical and practical guidance for improving catalyst stability. In addition, this review provides a detailed introduction to the main reaction mechanisms involved in propane oxidation, including Langmuir-Hinshelwood (L-H), Eley-Rideal (E-R), and Mars-van Krevelen (MvK) mechanisms. The applicability and differences in reaction pathways of these mechanisms in various catalytic systems are comparatively analyzed. Such discussions contribute to a deeper understanding of the fundamental nature of propane oxidation processes and offer important theoretical guidance for the design and optimization of high-performance catalysts. Finally, this review offers an in-depth outlook on precise regulation of noble metal-support interfaces and emerging electrothermal synergistic catalysis strategies, highlighting their broad prospects for enhancing catalytic activity, lowering reaction temperatures, and improving stability of catalysts. Through systematic summarization and forward-looking analysis, this review provides valuable theoretical foundations and technical references for achieving efficient and stable propane oxidation and VOC abatement, thereby promoting the further development and industrial application of related catalytic technologies.
SUPPORTED NOBLE METAL CATALYSTS FOR CATALYTIC PROPANE OXIDATION
Noble metals, owing to their unique electronic structure and strong adsorption capacity, can effectively promote the activation of O2 and propane, accelerate C–H bond cleavage, and drive the further oxidation of intermediate products, thereby significantly enhancing the efficiency of propane complete oxidation. Among these, precious metal active components such as palladium (Pd), platinum (Pt), and ruthenium (Ru) have been extensively studied in catalytic propane oxidation, particularly in low-temperature applications, demonstrating great potential for practical use[28,36-38]. Table 1 systematically summarizes recent Pt-, Pd-, and Ru-based catalysts supported on commonly used oxide supports (Al2O3, TiO2, and CeO2) for the complete oxidation of propane, together with the corresponding reaction conditions and catalytic performances. The catalytic activity for the complete oxidation of propane is strongly affected by the nature of the active phase, the support, promoters/additives, and operating conditions.
Recent Pt-, Pd-, and Ru-based catalysts supported on Al2O3, TiO2, and CeO2 for the complete oxidation of propane (2019-2025)
| Support type | Catalyst | Metal loading (wt%) | Reactant | WHSV (mL·g-1·h-1) | T90 | TOF (s-1) | Reaction rate (μmol·gmetal-1·s-1) | Ref. |
| Al2O3 | Pt/ST-Al2O3 | 0.80 | 0.2% C3H8 + 1% O2 | 30,000 | ~250 | 0.0233 (220 °C) | 8.0 (220 °C) | [39] |
| Pt/Al2O3 | 1.98 | 0.2% C3H8 + 2% O2 | 80,000 | ~350 | 0.0022 (220 °C) | 11.4 (220 °C) | [40] | |
| Pt-15Nb/Al2 O3 | 1.80 | 0.2% C3H8 + 2% O2 | 80,000 | ~260 | 0.0213 (220 °C) | 62.2 (220 °C) | [40] | |
| 1Pt/Al2O3 | 0.98 | 0.2% C3H8 + 2% O2 | 80,000 | ~350 | 0.0022 (220 °C) | 8.7 (220 °C) | [41] | |
| Pt/Al2O3-500 | 1.99 | 0.2% C3H8 + 2% O2 | 20,000 | ~295 | - | - | [42] | |
| Pt-SO42-/Al2O3-400 | 2.01 | 0.2% C3H8 + 2% O2 | 20,000 | ~195 | - | - | [42] | |
| Pt/Al2O3 | - | 1.0% C3H8 + 5.2% O2 | 30,000 | ~255 | 0.039 (170 °C) | - | [43] | |
| Pt/W1/Al2O3 | - | 1.0% C3H8 + 5.2% O2 | 30,000 | ~220 | 0.156 (170 °C) | - | [43] | |
| Pd/Al2O3 | 1.00 | 0.1% C3H8 + 10% O2 | 50,000 | ~365 | 0.0014 (250 °C) | 4.03 (250 °C) | [44] | |
| Pd/Al2O3 | 2.00 | 0.1% C3H8 + 10% O2 | 50,000 | ~350 | 0.0015 (250 °C) | 3.92 (250 °C) | [44] | |
| Pd/Al2O3 | 0.60 | 0.1% C3H8 + 8% O2 | 100,000 | 473 | [45] | |||
| Ru/Al2O3-Con | 2.11 | 0.2% C3H8 + 10% O2 | 36,000 | 269 | 0.0020 (175 °C) | 5.0 (175 °C) | [46] | |
| Ru/NF-Al2O3 | 2.05 | 0.2% C3H8 + 10% O2 | 36,000 | 242 | 0.0025 (175 °C) | 8.4 (175 °C) | [46] | |
| Ru-Ce7/NF-Al2O3 | 1.91 | 0.2% C3H8 + 10% O2 | 36,000 | 207 | 0.0058 (175 °C) | 19.0 (175 °C) | [46] | |
| 5%Ru/γ-Al2O3 | 5.00 | 0.08% C3H8 + air | 60,000 | ~235 | 0.0029 (175 °C) | 0.74 (165 °C) | [47] | |
| 5% Ru-1.6%Mo/γ-Al2O3 | 5.00 | 0.08% C3H8 + air | 60,000 | ~220 | 0.0034 (175 °C) | 0.93 (165 °C) | [47] | |
| TiO2 | Pt/TiO2 | 0.20 | 0.2% C3H8 + 1% O2 | 60,000 | 290 | - | 10 (200 °C) | [48] |
| Pt@SiOx/TiO2 | 0.24 | 0.2% C3H8 + 1% O2 | 60,000 | 210 | - | 269 (200 °C) | [48] | |
| Pt/TiO2 | 0.52 | 1.0% C3H8 + 20% O2 | 60,000 | 280 | - | 75 (200 °C) | [49] | |
| Pt/F-TiO2 | 0.48 | 0.2% C3H8 + 10% O2 | - | 320 | - | ~0.54 (210 °C) | [50] | |
| Pt/TiO2 | 1.0 | 0.2% C3H8 + 2% O2 | 36,000 | ~290 | 0.0136 (200 °C) | 13.7 (200 °C) | [51] | |
| Pt-TiO2(R) | 0.34 | 0.1% C3H8 + 5% O2 | 120,000 | ~500 | 0.013 (300 °C) | - | [52] | |
| Pt-TiO2(A) | 0.35 | 0.1% C3H8 + 5% O2 | 120,000 | ~340 | 0.061 (300 °C) | - | [52] | |
| Pd/TiO2 | 0.60 | 0.1% C3H8 + 8% O2 | 100,000 | 420 | - | - | [45] | |
| Pd/AT | 0.60 | 0.1% C3H8 + 8% O2 | 100,000 | 440 | ~0.060 (-) | - | [45] | |
| Pd/3APT | 0.60 | 0.1% C3H8 + 8% O2 | 100,000 | 367 | ~0.039 (-) | - | [45] | |
| 2Pd/TiO2 | 2.0 | 0.5% C3H8 + 2% O2 | 44,000 | ~480 | 0.0004 (300 °C) | 0.2 (300 °C) | [53] | |
| 2Pd-MnOx/TiO2 | 2.0 | 0.5% C3H8 + 2% O2 | 44,000 | ~425 | 0.0084 (300 °C) | 3.1 (300 °C) | [53] | |
| 1Ru/TiO2 | 1.0 | 0.5% C3H8 + 2% O2 | 45,000 | 190 | 0.052 (110 °C) | 240 (110 °C) | [54] | |
| 2Ru/TiO2 | 2.0 | 0.5% C3H8 + 2% O2 | 45,000 | 185 | 0.065 (110 °C) | 330 (110 °C) | [54] | |
| CeO2 | Pt/CeO2 | 0.94 | 0.2% C3H8 + 2% O2 | 30,000 | 410 | - | 1.3 (230 °C) | [55] |
| Pt/9Nb-CeO2 | 0.93 | 0.2% C3H8 + 2% O2 | 30,000 | 300 | - | 47.9 (230 °C) | [55] | |
| 1.3PtCe | 0.80 | 0.2% C3H8 + 2% O2 | 30,000 | 307 | 0.0016 (210 °C) | 7.3 (210 °C) | [56] | |
| 5.0PtCe | 0.80 | 0.2% C3H8 + 2% O2 | 30,000 | 273 | 0.0329 (210 °C) | 37.9 (210 °C) | [56] | |
| Pt/CeO2 | 0.88 | 0.2% C3H8 + 2% O2 | 30,000 | 379 | 0.0013 (220 °C) | 3.6 (220 °C) | [57] | |
| Pt/CeO2-0.3POx | 0.81 | 0.2% C3H8 + 2% O2 | 30,000 | 287 | 0.0239 (220 °C) | 42.9 (220 °C) | [57] | |
| Pt/CeO2-r | 1.00 | 0.2% C3H8 + 2% O2 | 30,000 | ~295 | 0.0074 (200 °C) | 12.7 (200 °C) | [58] | |
| Pt/CeO2-o | 0.38 | 0.2% C3H8 + 2% O2 | 30,000 | ~330 | 0.0134 (200 °C) | 28.6 (200 °C) | [58] | |
| 0.02Pt/CeO2 | 0.014 | 0.2% C3H8 + 2% O2 | 24,000 | - | 0.0041 (220 °C) | 20.8 (220 °C) | [59] | |
| 2Pt/CeO2 | 2.1 | 0.2% C3H8 + 2% O2 | 24,000 | ~400 | 0.0039 (220 °C) | 12.2 (220 °C) | [59] | |
| Pd/CeO2 | 1.99 | 0.1% C3H8 + 20% O2 | 20,000 | 290 | - | 0.09 (240 °C) | [60] | |
| PdCo/CeO2 | 1.48 | 0.1% C3H8 + 20% O2 | 20,000 | 260 | - | 0.18 (240 °C) | [60] | |
| Pd/CeO2(500)-IWI | 1.1 | 0.1% C3H8 + 0.95% O2 | 90,000 | 373 | - | - | [21] | |
| Pd/CeO2(1000)-EG | 0.8 | 0.1% C3H8 + 0.95% O2 | 90,000 | 309 | - | - | [21] | |
| Pd/Ce(500) | 1.1 | 0.2% C3H8 + 0.96% O2 + 1% CO + 0.08% NO | 90,000 | ~420 | 0.0207 (250 °C) | 16.6 (250 °C) | [61] | |
| Pd/Ce(1000) | 1.1 | 0.2% C3H8 + 0.96% O2 + 1% CO + 0.08% NO | 90,000 | ~290 | 0.0039 (250 °C) | 50.1 (250 °C) | [61] | |
| Ru/CeO2 | 1.7 | 0.2% C3H8 + 2% O2 | 30,000 | 310 | - | 7.9 (200 °C) | [62] | |
| Ru/CeO2-2 | 1.6 | 0.2% C3H8 + 2% O2 | 30,000 | 265 | - | 16.8 (200 °C) | [63] | |
| Ru-CeO2-2 | 1.4 | 0.2% C3H8 + 2% O2 | 30,000 | 220 | - | 42.0 (200 °C) | [63] | |
| Ru/CeO2-IM | 0.92 | 0.2% C3H8 + 2% O2 | 30,000 | 234 | - | 22.6 (200 °C) | [64] | |
| Ru/CeO2-CS | 1.05 | 0.2% C3H8 + 2% O2 | 30,000 | 170 | - | 62.3 (200 °C) | [64] | |
| Ru/CeO2-O | 1.79 | 0.2% C3H8 + 2% O2 | 30,000 | ~225 | 0.026 (155 °C) | 1.3 (155 °C) | [65] | |
| Ru/CeO2-C | 1.94 | 0.2% C3H8 + 2% O2 | 30,000 | ~205 | 0.073 (155 °C) | 7.8 (155 °C) | [65] | |
| Ru/CeO2-R | 1.91 | 0.2% C3H8 + 2% O2 | 30,000 | ~180 | 0.344 (155 °C) | 21.3 (155 °C) | [65] |
Supported Pt-based catalysts
Pt-based catalysts are widely considered the most effective catalytic systems for propane oxidation, primarily due to their outstanding C–H bond cleavage capability. The unique properties of platinum enable it to effectively activate propane molecules during the catalytic process, thereby promoting their complete oxidation. The catalytic performance of Pt-based catalysts is closely related to their structure, with key factors including the chemical state of platinum, particle size, dispersion, interactions with the support, and the addition of promoters.
The effect of the chemical state of Pt
Studies have shown that the chemical state of platinum species (such as Pt0, Pt2+, etc.) is one of the most important factors influencing the activity and stability of catalysts in propane catalytic oxidation[42,43,50,55]. However, the true active species of Pt for propane oxidation remains a subject of extensive debate. Most studies propose that metallic Pt (Pt0) is the primary active center, while some emphasize the role of oxidized Pt (PtOx), or suggest a synergistic effect between Pt0 and PtOx. Li et al. proposed a cooperative mechanism in which C–H bond activation and cleavage primarily occur at Pt0 sites, while subsequent oxidation steps are facilitated by PtOx[41]. Thus, optimal catalytic performance is achieved when the Pt0/PtOx ratio approaches 1:1. Similarly, Enterkin et al. deposited Pt onto SrTiO3 perovskite supports using atomic layer deposition[66]. The results show that the strong epitaxial interaction between SrTiO3 and Pt enables Pt to exist in a stable metallic state, and under oxidative conditions, a Pt/PtO core-shell structure is formed, thereby significantly enhancing the catalytic oxidation performance of propane [Figure 2A]. Bal’zhinimaev et al. found that Pt catalyst prepared by ion-exchange method on silicate glass fibers (FG) [Pt(v)/FG] and Pt catalyst prepared by ion-exchange method on N-modified silicate glass fibers (FG) [Pt(v)/N-FG] catalysts containing both Pt2+ and Pt0 exhibit higher activity than the Pt catalyst prepared by incipient wetness impregnation on silicate glass fibers (FG) [Pt(s)/FG] catalyst with only Pt0[67]. Shan et al. achieved a controllable surface transformation from PtNiCo alloy to PtNiCo-PtNiOCoO structure by adjusting the calcination conditions[68]. The result revealed that the synergistic effect at the alloy-oxide interface stabilized Ptδ+, significantly improving propane oxidation performance and thermal stability (with unchanged activity after aging at
Figure 2. (A) Transmission electron microscopy images of Pt/SrTiO3 after five ALD cycles and propane oxidation light-off curves over two temperature cycles (25-400 °C) for 1c, 3c, and 5c Pt/SrTiO3, 4.7%Pt/γ-Al2O3, and platinum-free STO nanocuboids, with C3H8/O2 = 1:16 and C3H8 WHSV = 4 h-1. Reproduced with permission from Ref.[66]; (B) Relationship between TOFs at 220 °C and Pt/Ptn+ ratios for various catalysts. Reproduced with permission from Ref.[70]; (C) Evolution of the chemical state of Pt species on a Pt/BN catalyst during propane oxidation. Reproduced with permission from Ref.[70]. ALD: atomic layer deposition; STO: SrTiO3; WHSV: weight hourly space velocity; TOFs: turnover frequencies; BN: boron nitride.
The effect of support
Different types of supports significantly influence the catalytic performance of Pt by regulating its electronic structure, dispersion, and redox properties. Avila et al. prepared supported Pt catalysts using CeO2, TiO2, and Al2O3 as supports via the impregnation method[73]. They found that the turnover frequency (TOF) for propane catalytic oxidation followed the order Pt/TiO2 > Pt/CeO2 > Pt/Al2O3. It was concluded that using reducible oxides (such as CeO2 and TiO2) as supports enhances the interaction with Pt active sites, thereby improving the catalytic activity for propane oxidation. In addition, the acid-base properties of the support can also influence the chemical state of the supported Pt. Increasing the surface acidity can promote the presence of Pt species in the metallic state (Pt0), thereby significantly affecting the catalytic performance of the catalyst. Yazawa et al. synthesized a series of supported Pt catalysts, including Pt/MgO, Pt/La2O3, Pt/ZrO2, Pt/Al2O3, Pt/SiO2, Pt/SiO2-Al2O3, and Pt/SO42--ZrO2[74,75]. The results showed that the catalytic activity for propane oxidation is closely related to the acidity of the support surface, with the stronger the acidity, the higher the catalytic activity. Further research revealed that the acid-base properties of the supports directly regulate the chemical state of Pt. On acidic supports, part of the Pt exists in the metallic state (Pt0), whereas on basic supports, Pt mainly exists in an oxidized form[75]. Huang et al. and Wang et al. directly compared the performance differences of Pt catalysts supported on redox oxide CeO2 and acidic oxide Nb2O5 in propane oxidation [Figure 3A and B][30,76]. Their studies showed that the Pt/Nb2O5 catalyst surface contains a higher proportion of metallic Pt (Pt0) species, which facilitates the activation of C–H bonds and significantly enhances the catalytic oxidation performance of propane. In contrast, the Pt-CeO2 interface in Pt/CeO2 catalysts more effectively activates oxygen species and promotes CO oxidation [Figure 3C-E]. Furthermore, the type of support strongly influences the reaction pathway of propane oxidation. On Pt/CeO2 catalysts, propane primarily forms propionate intermediates, which are then oxidized to formate and acetate species, whereas on Pt/Nb2O5 catalysts, propane tends to undergo dehydrogenation to propylene first, followed by oxidation to acrylate species, demonstrating a more efficient oxidation pathway. Xian et al. pointed out that, in addition to serving as supports, acidic carriers can also activate saturated alkanes, allowing propane and oxygen to avoid competing for Pt sites[77]. Consequently, a synergistic catalytic effect arises between the Pt surface and the acidic sites, thereby enhancing the performance of propane oxidation. Wen et al. also reported that the physical mixture of acidic Mo2O3 with Pt/CeO2 exhibits superior propane oxidation performance compared to catalysts possessing solely acidic (Pt/Mo2O3) or redox properties (Pt/CeO2)[78].
Figure 3. (A) Pt 4f XPS spectra and (B) catalytic activity of propane oxidation of Pt/CeO2, Pt/SnO2, Pt/ZrO2, and Pt/Nb2O5 catalysts. Reproduced with permission from Ref.[30]; XPS spectra of (C) Pt 4f and (D) O 1s of Pt/CeO2 and Pt/Nb2O5 catalysts. Reproduced with permission from Ref.[76]; (E) Catalytic activity of propane oxidation over Pt/CeO2 and Pt/Nb2O5 catalysts. Reproduced with permission from Ref.[76]. XPS: X-ray photoelectron spectroscopy.
The effect of the size of Pt nanoparticles
Extensive research indicates that as the size of noble metal particles shrinks from the nanoscale to the single-atom scale, their geometric structure and chemical state undergo significant changes, which profoundly influence their catalytic performance. As a structure-sensitive reaction, propane oxidation exhibits high sensitivity to the size of Pt particles, and the catalytic performance can be effectively enhanced by precisely tuning the particle size. Luo et al. prepared Pt/Al2O3 catalysts with average Pt particle sizes of 1.5, 2.5, 3.1, and 11.0 nm, respectively, and applied them to propane oxidation[79]. The result showed that the catalyst with a 2.5 nm particle size exhibited optimal performance. Park et al. pointed out that on hydrogen-type Zeolite Socony Mobil-5 (H-ZSM-5, SiO2/Al2O3 = 23), the specific reaction rate for propane oxidation decreases with increasing Pt particle size[80]. For active oxide supports such as CeO2, the regulation of electronic structure by Pt particle size is particularly important. Lykhach et al. combined density functional theory (DFT) calculations with synchrotron radiation experiments to quantitatively study the relationship between electron transfer at the Pt/CeO2 interface and Pt particle size[81]. The results revealed that when the Pt particle contains approximately 50 atoms, electron transfer from Pt to CeO2 reaches a maximum, about one electron transferred per ten Pt atoms. For larger Pt particles, the CeO2 support itself imposes stronger limitations on electron transfer, while for smaller, even atomic-scale Pt particles, the concentration of oxygen vacancies on the CeO2 surface becomes the dominant factor, and the reduced CeO2 has limited capacity to accept additional electrons.
In our recent study[56], we systematically investigated the effect of Pt size on the efficiency of complete propane oxidation by synthesizing nanocubes of CeO2 with well-defined (100) facets to support Pt nanoparticles of various sizes, ranging from 1.3 to 7 nm [Figure 4A-C]. We found that for Pt/CeO2(100) catalysts, when the Pt particle size is in the range of 1.3-4.0 nm, a strong MSI between Pt and the CeO2(100) support leads to partial oxidation of Pt to Ptδ+. In addition, the catalytic activity is mainly influenced by the chemical state of Pt. As the Pt particle size exceeds 4.0 nm, the ability of the CeO2(100) support to regulate electron transfer weakens, and the chemical state of Pt stabilizes, with the catalytic activity then depending more on the geometric structure of the Pt particles. Therefore, both the chemical state and geometric structure of Pt jointly determine the catalytic performance, resulting in a volcano-shaped relationship between propane oxidation activity and Pt particle size. Additionally, the TOF increases consistently as the particle size grows within this range. The study shows that the apparent performance for propane oxidation peaks when the Pt particle size is approximately 5 nm, while the intrinsic activity continues to rise with increasing Pt particle size. Gao et al. conducted a similar study, loading Pt single atoms, clusters, and nanoparticles separately onto ceria to systematically investigate the effect of Pt particle size on propane oxidation catalytic performance[59]. However, their results showed that despite differences in Pt morphology and size, these catalysts exhibited similar trends in apparent activation energy (Ea) and TOF. The discrepancy between the two studies mainly arises from the different Pt particle size ranges investigated: our work focused on different uniform sizes within the 1-7 nm range, while the other study examined smaller sizes (from single atoms to about 2.5 nm Pt nanoparticles) and mixed-size species. This indicates that the effect of Pt particle size on catalytic performance in propane oxidation exhibits a distinct nonlinear behavior, which is fundamentally governed by multiple factors, including the strength of the MSI, electronic structure modulation, and the exposure of geometric structures. Although reducing the size of Pt nanoparticles to the single-atom level can maximize noble metal atom utilization and reduce material consumption, for propane oxidation, single-atom Pt lacks metal ensemble sites that are essential for the simultaneous activation of oxygen molecules and cleavage of C–H bonds. As a result, single-atom Pt exhibits relatively poor catalytic activity. Jeong et al. also confirmed this finding, showing that Pt with metal ensemble structures demonstrates superior catalytic performance in propane oxidation compared with fully dispersed single-atom Pt[82].
Figure 4. (A) Transmission electron microscopy images and particle size distributions of Pt particles with different sizes. Reproduced with permission from Ref.[56]; (B) Propane oxidation activity of Pt nanoparticles with different particle sizes. Reproduced with permission from Ref.[56]; (C) Correlation between the reaction rate and TOF for complete propane oxidation at 210 °C and the Pt particle size. Reproduced with permission from Ref.[56]. TOF: Turnover frequency.
The influence of promoters
To enhance the catalytic performance and stability of supported Pt catalysts, promoters are often introduced to modify the catalyst structure and surface properties. Yoshida et al. investigated the effects of various additives, including alkali metals, alkaline earth metals, and fifth-period elements, on the propane oxidation activity of Pt/Al2O3 catalysts[29,75]. The results showed that additives such as niobium (Nb), tungsten (W), and molybdenum (Mo), which possess strong electron-withdrawing abilities and electronegativities greater than 2.7, effectively suppressed the oxidation of Pt species under oxygen-rich conditions, stabilizing Pt in its metallic state and thereby improving propane oxidation activity. Based on this understanding, many Pt/MOx (MOx = Al2O3, CeO2, TiO2, ZrO2, etc.) catalytic systems have been developed by introducing strongly acidic transition metal oxides (such as NbOx, MoOx, WOx, VOx, etc.), significantly enhancing the catalytic performance in propane oxidation. Shao et al. reported that modifying 0.5% Pt/ZrO2 with WOx led to the incorporation of WOx into the ZrO2 lattice, forming a highly dispersed Zr1-xWxO2+x/2 structure[83]. This structure facilitates electron transfer from W to Pt, generating strong acid sites and Pt0 species that accelerate the reaction process [Figure 5A]. Similarly, Liao et al. synthesized a series of 1 wt% Pt and different W contents (x wt%) supported on hexagonal boron nitride (Pt-xW/BN) catalysts with varying tungsten contents via a secondary impregnation method[84]. They found that the propane catalytic oxidation activity was highest when the tungsten content was 7 wt%, and the activity decreased when the tungsten content exceeded this value. The results of the characterization demonstrated that the Pt-WOx interface acts as a novel active site, effectively promoting the heterolytic cleavage of C–H bonds in propane and exhibiting higher propane oxidation activity than the conventional Pt0-Ptδ+ dual active sites. Zhao et al. also reported that NbOx modification of Pt/Al2O3 promoted the formation of Pt0 species, significantly enhancing the catalytic performance of Pt/Al2O3[40]. Moreover, the catalyst exhibited the highest activity when the Nb content was 10 wt% [Figure 5B]. Wen et al. found that introducing an ultralow loading of Mo (0.1 wt%) as a promoter in Pt/SiO2 catalysts not only effectively reduced the Pt particle size but also donated electrons to Pt particles, significantly facilitating propane activation[85].
Figure 5. (A) Reaction mechanism and rate of propane oxidation over Pt/WOx/t-ZrO2 and Pt/WOx/m-ZrO2 catalysts. Reproduced with permission from Ref.[83]; (B) Relationship between the propane conversion rate and reaction temperature of Pt-Nb/Al2O3 catalysts. Reproduced with permission from Ref.[40]; (C) Light-off curves for propane oxidation over Pt/CeO2-yPOx catalysts. Reproduced with permission from Ref.[57]; (D and E) Light-off curves (D) and reaction rates (E) for C3H8 oxidation over Pt/F-TiO2 and Pt/TiO2. Reproduced with permission from Ref.[50].
In addition to doping with metal elements, introducing non-metal elements such as phosphorus (P), sulfur (S), or fluorine (F) to regulate the acidity of the support and the electronic structure of Pt is also an effective strategy to enhance the catalytic performance for C3H8 oxidation. Huang et al. modified the Pt/CeO2 via phosphorus doping (Pt/CeO2-yPOx) and found that both the reaction rate and TOF increased progressively with increasing P content[57]. When y = 0.3, the Pt/CeO2-0.3POx catalyst exhibited the highest reaction rate and TOF, indicating optimal catalytic performance. Further investigations revealed that the surface phosphate (POx) species significantly enhanced the acidity and redox properties of the support, promoting the formation of Ptδ+-(CeO2-POx)δ- dipolar active sites. These sites effectively facilitated C–H bond activation, thereby significantly improving the catalytic performance for propane oxidation [Figure 5C]. Yu et al. doped fluorine into the TiO2 support, successfully tuning the electronic structure of the supported Pt to generate electron-rich Ptδ+ active sites[50]. These electron-rich Ptδ+ sites not only promoted propane adsorption and oxygen activation, alleviated the competitive adsorption between them, but also enhanced C–H bond cleavage efficiency, significantly improving the propane oxidation activity of the catalyst at low temperatures [Figure 5D and E]. Zhou et al. also observed a similar phenomenon on P-modified Pt/Al2O3 catalysts, finding a significant synergistic effect between Pt active sites and acidic sites[86]. This synergy effectively suppressed the competitive adsorption of oxygen and propane on the Pt sites, resulting in a notable decrease in the apparent Ea for propane oxidation, from 88.2 ± 4.7 to 59.2 ± 3.3 kJ·mol-1, thereby significantly enhancing the catalytic reaction efficiency.
Sulfation of metal oxides is a commonly used and effective method for preparing solid superacids. Introducing sulfate groups onto the surface of a metal oxide support not only significantly enhances the surface acidity but also greatly improves their catalytic performance. Pt catalysts supported on such sulfated metal oxide supports exhibit excellent activity in the oxidation of propane, primarily due to the promotional effect of the strong acidity on the support surface. Specifically, the acidic sites on the support surface can efficiently adsorb and activate propane molecules, thereby lowering the Ea, increasing the reaction rate, and facilitating the efficient oxidative conversion of alkanes[42,87]. Xu et al. conducted a systematic comparison of sulfated Zeolite Socony Mobil-5 (ZSM-5), Al2O3, ZrO2, and CeO2 supports, and the results demonstrated a significant synergistic catalytic effect between surface sulfate species and platinum species, which effectively promoted propane oxidation[88]. Among the catalysts studied, the sulfated Pt/ZSM-5 catalyst exhibited the highest catalytic activity. Further analysis revealed that the 1 g 2Pt/ZSM-5 catalyst treated by 0.05 g (NH4)2SO4 (5S-2Pt/ZSM-5) with Pt/S atomic ratio of 0.5 regulated the electron transfer and interaction strength between platinum and sulfate species, thereby optimizing their synergistic catalytic effect and significantly enhancing the catalytic performance for propane oxidation. Similarly, Shao et al. synthesized nanoflower-like Pt/ZrOSO4 catalysts via a solvothermal method and found that the SO42- and adsorbed SO3 [(SO3)ad] species in the support promoted the formation of Pt0 and Pt+ species through Pt0-SO42- and Pt+-(SO3)ad interactions, respectively[87]. This synergistically enhanced the cleavage of C–C bonds and the heterolytic dissociation of C–H bonds in propane, thereby significantly improving catalytic oxidation performance. Gawthrope et al. loaded Pt onto pre-sulfated Al2O3 and found that propane combustion activity was positively correlated with the surface coverage of aluminum sulfate sites, with the best propane combustion performance observed for alumina impregnated with 0.1 M sulfuric acid[89]. Wang et al. investigated the propane oxidation mechanism over Pt/SO42-/CeO2-ZrO2 catalysts using in situ infrared spectroscopy, confirming that sulfation not only enhanced the catalyst’s ability to adsorb propane but also that the Pt-SO42- interfacial active sites favored efficient C–H bond cleavage[90]. Huang et al. introduced SO2 directly into the reaction gas and found that while the addition of SO2 only slightly increased propane adsorption, it significantly suppressed oxygen adsorption on Pt sites, thereby promoting C–C bond cleavage[91].
In summary, both metallic and non-metallic promoters typically show an optimal loading window. At insufficient loadings, the promotional effect is not fully manifested, whereas excessive amounts can deteriorate catalytic performance by blocking active sites, inducing overly strong or weak interactions, and/or altering the support structure, resulting in decreased catalytic performance. Moreover, when multiple promoters are introduced simultaneously, synergistic effects may emerge from cooperative tuning of acidity and redox properties and/or strengthened MSIs. Conversely, antagonistic effects may occur due to competitive adsorption or phase separation.
The effect of MSIs
Classical MSIs are typically achieved by reducing metal/oxide catalysts under a high-temperature (> 450 °C) hydrogen atmosphere. During this process, metal nanoparticles are partially encapsulated by a defective layer derived from the support oxide, forming structures rich in interfacial sites that significantly affect the catalytic performance. For example, in the Pt@TiOx/TiO2 catalyst constructed by Hao et al., Pt is encapsulated by an amorphous TiOx layer under reductive conditions[92]. The TiOx layer not only stabilizes metallic Pt0 through electronic interactions, preventing its transformation into inactive PtOx under high-temperature oxidative conditions, but also significantly enhances the activity and stability for propane oxidation [Figure 6A]. The strength of the MSI in catalysts typically depends on the physicochemical properties of the support. For other redox-type oxide supports, such as CeO2, the MSI can also be directly regulated by adjusting the number of metal-oxygen bonds through other strategies. Tan et al. successfully modulated the interfacial strength of Pt1-Ce0.9Zr0.1O2 single-atom catalysts by varying the calcination temperature (350-750 °C)[93]. Higher temperatures facilitated the incorporation of Pt atoms into the support lattice, creating stronger interactions and simultaneously increasing the concentration of Ce3+ species and oxygen vacancies. This enhanced O2 activation and promoted the rapid conversion of reaction intermediates (such as carbonates/carboxylates), thereby boosting catalytic performance [Figure 6B]. Zhang et al. reported a Pt single-atom catalyst (PtSA/CeZrO2) in which isolated Pt atoms are anchored on an ordered macroporous (OM) Ce0.8Zr0.2O2 support[94]. The improved high-temperature activity and durability were attributed to Zr-stabilized, dynamically low-coordinated Pt sites that weaken Pt–O bond occupation and increase free d-electron availability, thereby sustaining peroxide-related reactivity and promoting propane C–H activation. Consequently, the catalyst maintained 92% conversion at 450 °C after 50 h aging at 800 °C with 10 vol.% H2O, and was further demonstrated in a 3.4-L commercial cordierite monolith for scalable converter applications. Liu et al. used a photo-assisted deposition method to load Pt nanoparticles onto CeO2 nanorods with abundant oxygen defects, significantly enhancing the strong interaction between Pt and CeO2, thereby improving the catalytic performance of the catalyst[95].
Figure 6. (A) Schematic illustration of the effect of SMSI between Pt and TiOx on the chemical state of Pt. Reproduced with permission from Ref.[92]; (B) Effect of different Pt-CeO2 interaction strengths and coordination environments on propane combustion performance. Reproduced with permission from Ref.[93]; (C and D) Temperature-dependent C3H8 oxidation reaction of Pt/CeO2 (C), and Pt/9Nb-CeO2 (D). Reproduced with permission from Ref.[55]; (E) The schematic of the evolution of Pt on Pt/CeO2 and Pt/9Nb-CeO2. Reproduced with permission from Ref.[55]; (F) Correlation between the TOF for propane oxidation at 220 °C and the Pt-MgAl2O4 interaction strength. Reproduced with permission from Ref.[97]. SMSI: Strong metal-support interaction; TOF: turnover frequency; MSI: metal-support interaction.
However, such strong interactions often induce the formation of partially oxidized Pt species (e.g., Ptδ+ or Pt2+) at the metal-support interface, which can inhibit the adsorption and activation of propane, ultimately deteriorating the efficiency of propane oxidation. In contrast, inert supports exhibit weaker interactions with noble metals, favoring the preservation of metallic Pt0, though they provide limited stabilization against particle agglomeration. Although redox-type supports can provide active oxygen species, their more significant impact lies in regulating the valence state of Pt, thereby influencing the overall catalytic performance. It should be noted that enhancing the MSI mainly benefits reactions where the active sites are located at the interface, such as CO oxidation, whereas its effect is relatively limited for propane oxidation, where metallic Pt0 serves as the primary active center. Therefore, the key to enhancing propane oxidation performance lies in regulating the MSI to effectively prevent metal particle aggregation while maintaining an adequate amount of metallic Pt0 active sites. In our recent study[58], we found that on octahedral CeO2 (CeO2-o), where the MSI is relatively weak, Pt remained predominantly in the metallic state, resulting in superior propane oxidation activity. In contrast, on rod-shaped CeO2 (CeO2-r), stronger interfacial interactions induced partial oxidation of Pt, which hindered the decomposition and removal of intermediate species, thereby lowering the catalytic efficiency.
Regarding the excessively strong interaction between Pt and CeO2-r[55], we found that this leads to the redispersion of metallic Pt ensembles and the formation of oxidized Pt single-atom species under propane oxidation reaction conditions, driven by the promotion of the reaction product H2O. This phenomenon ultimately causes the deactivation of the Pt/CeO2 catalyst during the complete oxidation of propane [Figure 6C]. To address this issue, we introduced NbOx as a decorator to effectively block the strong interaction sites on CeO2 that anchor Pt, the Pt-CeO2 interaction is weakened, preventing the redispersion of Pt nanoclusters during the reaction and maintaining Pt in metallic ensembles form before and after reaction [Figure 6D], thus enhancing complete propane oxidation performance and preventing catalyst deactivation [Figure 6E]. Another study[48,96] also revealed a strong MSI between Pt and rutile TiO2, which leads to the epitaxial growth of PtO2 species on the (101) facet of rutile TiO2, forming atomic-layer structures. This phenomenon limits the exposure of metallic Pt and results in lower propane oxidation performance. After introducing a SiOx coating layer (i.e., Pt@SiOx/TiO2), the interfacial interaction between Pt and TiO2 is effectively weakened, suppressing the epitaxial growth of PtO2. Consequently, Pt species are dispersed as metallic nanoparticles across the entire TiO2 support surface. Simultaneously, the engineered Pt-SiOx interface creates new active sites that facilitate the intermediate C–C bond cleavage, synergistically enhancing the catalytic activity along with the active metallic Pt species for propane oxidation. Remarkably, T90 is achieved at just 210 °C with a low Pt loading of only 0.2 wt%.
For irreducible oxide supports, however, which cannot be easily regulated in terms of MSI due to their stable O2- ions, the formation of metal-metal bonds is uncommon. We adopted a method to modify the irreducible MgAl2O4 (MAO) support via H2O2 treatment, reducing the density of Brønsted acid sites on MAO responsible for anchoring Pt, thereby weakening the MSI[97]. This allowed Pt to be more readily reduced by propane into active metallic Pt0, enhancing the activation of C–H bonds in propane and facilitating the rapid consumption of intermediate species. As a result, catalytic activity significantly improved, exhibiting an impressive increase in intrinsic activity of over 32-fold compared to traditional Pt/MAO [Figure 6F].
In summary, by regulating macroscopic factors such as Pt particle size, morphology, MSIs, and the rational introduction of promoters, it is possible to precisely tune microscopic characteristics, including the electronic state, coordination environment, geometric structure, and dispersion of Pt. The synergistic effect of these factors significantly optimizes the surface properties of supported Pt catalysts, thereby effectively enhancing their catalytic activity and structural stability, and improving the overall performance and lifespan of the catalysts.
Supported Pd-based catalysts
Compared to Pt, research on Pd in the field of propane catalytic oxidation is relatively limited. Existing literature shows that Pd-based catalysts are more commonly applied in the oxidation of methane and other small hydrocarbons. Compared with its outstanding performance in methane oxidation, Pd-based catalysts are less frequently employed for propane oxidation, mainly due to differences in the rate-controlling step and surface chemistry. In methane oxidation, the surface PdO phase provides highly active lattice oxygen that effectively participates in the initial C–H bond activation, leading to a lower activation barrier and high catalytic activity[98-100]. However, in propane oxidation, the overall reaction rate is governed not only by the initial C–H cleavage but by C–C cleavage and the oxidation and desorption of surface intermediates such as propene, CO, and carbonate species. Nevertheless, the potential of Pd in propane oxidation has been gaining increasing attention. This section focuses on the chemical state and particle size of Pd, as well as the effect of promoter doping on the propane oxidation performance of Pd-based catalysts.
The effect of chemical state of Pd
Studies have shown that, unlike Pt, high-valent Pd is generally regarded as the key active species in propane oxidation[101-103]. Hu et al. prepared Pd catalysts supported on CeO2 nanomaterials with different morphologies and applied them to propane oxidation[104]. They found that when Pd was loaded on octahedral CeO2 exposing mainly the (111) crystal facets, Pd predominantly existed in the form of PdOx nanoparticles, exhibiting superior catalytic activity compared with those supported on rod-like and cubic CeO2 [Figure 7A and B]. Subsequently, Liu et al. regulated the oxygen vacancy concentration of CeO2 by varying its calcination temperature, thereby tuning the chemical state of Pd[61]. The results indicated that high-temperature calcined CeO2 possessed fewer oxygen vacancies, leading to a weaker Pd-CeO2 interaction and promoting the aggregation of Pd in the form of PdO. These PdO species effectively facilitated the decomposition of acetate intermediates, thus enhancing propane oxidation activity. In contrast, low-temperature calcined CeO2 contained more oxygen vacancies, resulting in a stronger Pd-CeO2 interaction, where Pd mainly existed in a solid-solution state, corresponding to lower catalytic activity. Furthermore, Li et al. revealed that in the Pd/Al2O3 and Pd/Ce0.33Zr0.67O2 system, unencapsulated Pd species formed a tight interface with the alumina support[105]. Due to the higher oxidation state of Pd, abundant surface PdO species were generated at the Pd-Al2O3 interface. These PdO species were identified as the main active sites for propane oxidation, leading to significantly enhanced catalytic performance. In addition to the support effect, the synthetic method also plays a crucial role in determining the dispersion and chemical state of Pd species. Peng et al. prepared a single-crystalline zeolite silicalite-1 (S-1) with intra-mesopores encapsulating palladium (Pd) NPs (Pd@IM-S-1) catalyst via an in situ dry-gel conversion method[106]. Compared with conventionally prepared Pd@SiO2 and Pd/S-1 catalysts, Pd@IM-S-1 exhibited superior propane oxidation activity, thermal stability, cycling durability, and water resistance. Further characterization revealed that during propane oxidation, metallic Pd particles were partially oxidized to form PdOx species, and the Pd-PdO interface provided abundant active oxygen species, which facilitated C–H bond cleavage in propane molecules [Figure 7C and D].
Figure 7. (A and B) Reaction rate in propane oxidation (A) and Pd 3d XPS spectra (B) of Pd/CeO2 catalysts. Reproduced with permission from Ref.[104]; (C and D) Schematic illustration of the synthesis (C) and catalytic performance in propane oxidation over Pd@IM-S-1 and Pd@S-1 (D). Reproduced with permission from Ref.[106]. XPS: X-ray photoelectron spectroscopy.
The effect of the size of Pd nanoparticles
Besides the chemical state of Pd, the particle size of Pd also plays a crucial role in determining the catalytic performance of supported Pd-based catalysts for propane oxidation. Khudorozhkov et al. deposited Pd particles with sizes ranging from 3 to 6 nm on Al2O3 by adjusting the acidity of the Pd precursor solution[107]. The results demonstrated that the TOF for propane oxidation increased monotonically with the Pd particle size and exhibited a clear linear correlation with the Pd2+/Pd0 ratio.
Similarly, Zhou et al. prepared a series of 0.5 wt% Pd/La-Al2O3 (PLA) catalysts using the impregnation method and found that the TOF for propane oxidation was jointly influenced by the redox properties of the catalyst and the Pd particle size[108]. Larger Pd particles generally showed higher catalytic activity for propane oxidation, among which the aged catalyst supported on La-Al2O3 calcined at 500 °C exhibited the highest TOF value (0.125 s-1). In addition, Bailey et al. prepared Pd particles of different sizes on Al2O3 by varying the Pd loading[44]. The study revealed that the overall catalytic activity was primarily determined by the number of active Pd sites. As the Pd loading increased, the Pd particle size also grew; however, the total number of active sites increased correspondingly, leading to a significant enhancement in catalytic performance.
The influence of promoters
To further enhance the activity and stability of supported Pd-based catalysts, researchers commonly employ support doping and modification strategies. Li et al. reported that introducing La into Pd/Al2O3 catalysts leads to a strong interaction between Pd and La, which alters the oxidation state of Pd[109]. The incorporation of La creates basic sites on the support surface, promoting the formation of more oxidized Pd species and significantly enhancing the catalytic activity of Pd/Al2O3 in propane oxidation. Yan et al. indicated that doping Co into Pd/Al2O3 catalysts can also markedly improve propane oxidation activity, primarily due to the enhanced redox properties of the catalyst and its increased ability to activate gaseous oxygen[110]. In addition, acidic elements can be introduced to modify the support, thereby tuning the surface properties of the catalyst. Taylor et al. found that both Pd0 and Pd2+ species coexist in Pd/TiO2 catalysts[111]. Upon the introduction of W, a Pd-WOx interface forms between PdOx and TiO2, causing Pd species to predominantly exist in the Pd2+ state, which significantly promotes propane catalytic oxidation. Liang et al. prepared Pd-based catalysts supported on Al2O3-TiO2 composite oxides by combining mechanical ball milling with wet impregnation, followed by surface etching with phosphoric acid at various concentrations[45]. The results showed that when the Al/P molar ratio was 3:1, the phosphoric acid-modified Pd/Al2O3-TiO2 catalyst exhibited the best performance in propane catalytic oxidation.
Supported Ru-based catalysts
In recent years, supported Ru catalysts have attracted considerable attention in the field of complete propane oxidation, among which Ru/CeO2 catalysts stand out due to their remarkable catalytic activity, excellent thermal stability, and outstanding redox properties. These catalysts can efficiently facilitate the activation of propane and oxygen molecules, exhibiting superior catalytic performance for propane oxidation even at relatively low temperatures.
The effect of chemical state of Ru
Unlike the conventional view that the primary active sites for propane catalytic oxidation are metallic Pt, Ru-based catalysts exhibit a significantly different reaction mechanism. Because Ru has a relatively weak ability to activate molecular oxygen, its catalytic activity does not mainly rely on isolated metal centers, but is more likely derived from RuOx or the interface regions between Ru and the support[46]. Therefore, constructing highly dispersed RuOx and highly active interfacial sites is crucial for enhancing catalytic performance. Choosing a support that can form a strong MSI with Ru and possesses excellent redox properties (such as CeO2) not only helps stabilize Ru species and inhibit sintering but also provides active oxygen species, thereby significantly improving the activity and stability of Ru-based catalysts in propane oxidation reactions. Okal et al. systematically studied the effect of CeO2, Al2O3, and ZnAl2O4 supports on the propane oxidation performance of Ru-based catalysts[112]. The results showed that CeO2 can supply reactive oxygen species involved in the reaction, resulting in excellent propane oxidation activity. Xia et al. further pointed out that Ru-O-M interfaces or highly dispersed RuOx are the main active sites, with the activity order as follows: Ru-O-Ce interface > Ru-O-Co interface ≈ Co3O4 > highly dispersed RuOx >> CeO2[113]. Hu et al. demonstrated that the Ru-CeO2 interface can provide additional propane adsorption and activation sites, thereby broadening the reaction pathway for propane oxidation [Figure 8A][114]. Building on this, Wang et al. found that the morphology of the CeO2 support significantly affects the number of surface oxygen vacancies near the Ru-CeO2 interface by modulating the interaction between Ru and different CeO2 crystal planes[65]. Among them, the Ru/CeO2-R catalyst exhibited a higher concentration of surface oxygen vacancies, thereby showing stronger adsorption and activation abilities for both oxygen and propane [Figure 8B]. Wu et al. indicated that a dense and uniformly distributed Ru-O-Ce interface is a key active site for propane oxidation, which may be related to asymmetric bridging adsorption and activation[115]. However, the presence of chlorine can inhibit the formation of highly dispersed RuOx and Ru-O-Ce interfaces, and accelerate CeO2 sintering and interface degradation at high temperatures. To further increase the number of Ru-O-Ce interfacial sites and RuOx species, Yan et al. prepared a Ru/CeO2-CS catalyst with more RuOx and oxygen vacancies via a colloidal chemistry method[64]. Compared with Ru/CeO2-IM (IM: Impregnation Method) catalysts prepared by conventional impregnation, Ru/CeO2-CS possesses more Ru-O-Ce interfacial sites, oxygen vacancies, and Lewis acid sites, which help maintain Ru in a low-valence state and significantly enhance propane
Figure 8. (A) Catalytic activity for the total oxidation of propane on Ru/Al2O3 and Ru/CeO2 catalysts. Reproduced with permission from Ref.[114]; (B) Correlation between TOF (at 155 °C) and oxygen vacancy concentration of catalysts for propane oxidation. Reproduced with permission from Ref.[65]; (C) Schematic illustration of the chemical states of Ru active sites on catalysts Ru/Ce@Co and Ru/CeO2. Reproduced with permission from Ref.[62]; (D) Illustration of the preparation procedures for the Ru-CeO2-x and Ru/CeO2-x catalysts. Reproduced with permission from Ref.[63]. TOF: Turnover frequency; TPD: temperature-programmed desorption; XPS: X-ray photoelectron spectroscopy.
The effect of the size of Ru nanoparticles
The particle size of Ru also has a significant impact on the propane catalytic oxidation performance of supported Ru-based catalysts. Okal et al. found that treating Ru/γ-Al2O3 catalysts under different atmospheres led to variations in particle size and oxidation state: samples treated with H2 at low temperature (250 °C) formed non-stoichiometric RuOx structures encapsulating metallic Ru with smaller particle sizes, exhibiting the highest propane oxidation activity, in contrast, O2 treatment at 600 °C caused severe sintering of RuO2 nanoparticles (9-25 nm), resulting in the lowest catalytic activity[116]. The treatment with O2 at 250 °C produced well-crystallized RuO2 nanoparticles (approximately 4 nm) with moderate activity. Subsequently, they synthesized Ru nanoparticles supported on γ-Al2O3 via a microwave-polyol method and found that catalysts with an average particle size of 1.6 nm exhibited excellent activity, whereas those with an average particle size of 6 nm showed significantly lower activity, further indicating that high dispersion and small particle size of Ru nanoparticles are key factors for their superior catalytic performance[117]. Camposeco et al. prepared Ru/TiO2 catalysts with different Ru loadings using a urea deposition method and found that hydrogen pre-treatment effectively reduced the Ru particle size, thereby significantly enhancing propane oxidation activity[54]. In addition, Zhang et al. compared single-atom (Ru/CeO2-SA), diatomic (Ru2/CeO2), and tetra-atomic cluster (Ru4/CeO2) catalysts, revealing the significant effect of ultra-low Ru loading on propane oxidation performance[118]. The study showed that single-atom Ru catalysts excelled at suppressing propane adsorption, regulating oxygen activation, and promoting the formation of acrylic acid intermediates, thereby markedly improving catalytic efficiency.
The influence of promoters
To further enhance the propane oxidation performance of Ru/CeO2 catalysts, the introduction of promoters to precisely regulate the concentration of oxygen vacancies in CeO2 and the interfacial interaction between Ru and CeO2 has been proven to be an effective strategy. This is because metal doping can not only modify the crystal structure and electronic properties of CeO2, but also adjust its surface redox ability and oxygen mobility, thereby optimizing the dispersion of Ru species and promoting the formation of active RuOx, ultimately enhancing the overall catalytic performance in propane oxidation and related reactions. Deng
In summary, the rational design of supports and the regulation of Ru structure and dispersion, which promote the formation of metal-support interfaces, enhance the activation of reactive oxygen species, and optimize reaction pathways, constitute an effective strategy for improving both the catalytic activity and long-term stability of Ru-based catalysts in complete propane oxidation.
Supported bimetallic catalysts
Compared with monometallic catalysts, multimetallic catalysts have attracted increasing attention in recent years due to their superior catalytic activity, selectivity, and thermal stability. In the catalytic oxidation of propane, bimetallic catalysts exhibit significantly better performance than their monometallic counterparts[121]. This enhancement is mainly attributed to the ability of bimetallic systems to provide multiple types of active sites simultaneously, thereby meeting the complex requirements of multi-step reactions such as C–H and C–C bond activation, oxygen adsorption, and intermediate transformation. In addition, electronic interactions between different metals can induce electron redistribution and valence state modulation, leading to the formation of interfacial regions with unique electronic structures, such as electron-rich or electron-deficient metal sites, which effectively promote key steps in the reaction process. Huang et al. prepared bimetallic PtFe catalysts and conducted pre-treatment in an oxygen-rich atmosphere[122]. The resulting PtxFey@FeOx core-shell-like structure not only provided excellent stability but also enhanced activity due to the appropriate surface Pt0 sites and a high concentration of oxygen vacancies. Huang et al. reported that the introduction of Ir into Pt/TiO2 significantly enhanced the catalytic activity for propane deep oxidation, with the TOF reaching 0.031 s-1, twice that of the monometallic Pt catalyst[51]. The promotional role of Ir was attributed to its ability to stabilize metallic Pt0 species under oxidative conditions and enhance oxygen adsorption capacity. More importantly, the reaction pathway shifted from competitive adsorption of propane and oxygen on Pt sites to non-competitive adsorption, with propane on Pt and oxygen on Ir, resulting in a lower apparent Ea and improved catalytic efficiency. Tsui et al. reported that in the Pt-Ru bimetallic catalyst, electron transfer from Ru to Pt forms a synergistic interfacial structure composed of electron-rich Ptδ- and electron-deficient Ruδ+, resulting in catalytic performance significantly superior to that of monometallic catalysts[123]. The electron-rich Pt sites promote the activation and cleavage of C–H bonds in propane, while the electron-deficient Ru sites facilitate the dissociation of H2O to generate Ru-OH species, which rapidly oxidize CO intermediates adsorbed on Pt sites, effectively alleviating CO poisoning. This electronic coupling enables cooperative bifunctional catalysis, and even after high-temperature aging, the catalyst still exhibits excellent performance, achieving 90% propane conversion (T90) at merely 247 °C and full CO conversion at only 201 °C. Chen et al. employed Cl- ions to coordinate and complex Ru and Ag cations, enabling self-assembly into a RuAg alloy catalyst, which demonstrated good activity and stability for propane oxidation[124]. Further studies revealed that after high-temperature sintering and deactivation, the RuAg alloy particles could be redispersed via redox processes assisted by Cl- ions. Adamska and Baranowska et al. introduced Mo and rhenium (Re) into Ru/Al2O3 materials to prepare bimetallic Ru-based catalysts[47,125,126]. The addition of Mo and Re enhanced the interaction between the highly dispersed, partially oxidized RuOx species and the alumina support, significantly improving the resistance of the bimetallic Ru-Mo catalysts to deactivation under oxygen-rich conditions. Among them, the Ru-Mo/γ-Al2O3 catalyst (5 wt% Ru - 1.6 wt% Mo) achieved 95% propane conversion at 200 °C and exhibited a TOF of 0.034 s-1 at 165 °C, demonstrating excellent catalytic activity.
Thermal stability
In general, the thermal stability of catalysts encompasses two primary aspects: one is the stability of the catalyst structure (sintering resistance), and the other is the stability of the active phase (phase transformation resistance)[127-129]. Regarding the sintering mechanisms of supported noble metal catalysts, there are primarily two types: migration-aggregation and Ostwald ripening, with the latter being more widely applied in recent years. It is generally believed that the driving force for Ostwald ripening originates from the surface energy differences between particles. When the particle size distribution is wide and the size differences are large, the surface energy differences are also significant, making sintering more likely to occur[130-132]. Conversely, when the particle size distribution is narrow and the particle size differences are small, sintering is more difficult. Wettergren et al. studied the sintering behavior of Pt clusters with different particle sizes and found that when the particle size distribution was broad, heating led to noticeable cluster aggregation, whereas when the size distribution was more uniform, heating did not cause significant agglomeration[128].
However, in the catalytic oxidation of propane and other alkanes, the redispersion of noble metals can also lead to catalyst deactivation. Goodman et al. found that Pd on Al2O3 forms single atoms due to redispersion, resulting in deactivation of methane oxidation catalysts[133]. They suggested that this deactivation is related to the particle size and loading of Pd, and that increasing the Pd particle size and loading can mitigate the deactivation effect caused by this process. As we previously mentioned in our recent study[55], it was found that during propane oxidation, the excessively strong interaction between Pt and CeO2 caused Pt to undergo a structural evolution from atomically dispersed → Pt nanoclusters → atomically dispersed after reduction and propane catalytic oxidation, leading to a decline in catalytic activity. Introducing NbOx effectively occupies the strong binding step sites for Pt on CeO2, leaving the weaker terrace sites exposed. This guides Pt species to these weaker binding sites and prevents fragmentation into single atoms caused by water generated during the reaction. Thereby, Pt species remain stable throughout the propane reaction processes, always existing in the form of metallic Pt nanoclusters, thereby enhancing the catalyst’s performance. In a complementary approach, Zhang et al. utilized the Zr atoms in the OM structure of Ce0.8Zr0.2O2 to precisely control the coordination structure of Pt single-atom sites, stabilizing dynamic low-coordinated Pt single atoms[94]. This reduces the occupation of Pt–O bonds, releasing more free d-electrons, which maintains peroxide activity at high temperatures and enhances the activation of propane C–H bonds. By integrating the optimized design of OM CeZrO2, the three major deactivation issues of Pt catalysts, Pt sintering, overoxidation, and Pt loss, were successfully addressed[94] [Figure 9A-C].
Figure 9. (A) Photograph of the OM catalyst integrated into a commercial cordierite honeycomb monolith. Reproduced with permission from Ref.[94]; (B) Changes in Pt loading of PtSA/CeZrO2 catalysts after aging at 800 and 1,100 °C. Reproduced with permission from Ref.[94]; (C) Schematic diagram illustrating the inhibitory effect of the OM structure on Pt loss during propane oxidation. Reproduced with permission from Ref.[94]. OM: Ordered macroporous; DOC: diesel oxidation catalyst.
Although Ru metal exhibits higher activity and superior performance in propane catalytic oxidation compared to Pt- and Pd-based catalysts, its stability still faces significant challenges in practical applications. In addition to particle aggregation, under oxygen-rich oxidative conditions, the highly active RuOx species tend to be further oxidized, forming higher-valence and more stable RuO2 particles, which leads to a significant decline in catalytic activity and adversely affects the overall efficiency of propane oxidation. Moreover, the formation of high-valence RuO4 can cause volatilization and loss of ruthenium, accelerating catalyst deactivation and limiting its long-term stability and practical viability[134,135]. Therefore, compared with Pt- and Pd-based catalysts, the poor stability of Ru species in propane oxidation reactions limits its application, leading to greater attention and more extensive study of Ru in this context. Literature reports indicate that strategies to stabilize Ru-based catalysts mainly include: encapsulation to protect the active metal, constructing stable oxides with specific structures, enhancing MSIs, utilizing lattice-matching effects between the support and specific metal crystal facets, and tuning the properties of the metal through alloying.
To prevent Ru aggregation, Wang et al. utilized K+ to repair silanol defects, constructing confined ≡Si-Ruδ+-O-K anchoring sites within sinusoidal 10-membered ring channels, enabling the precise fixation of sub-nanometer Ru clusters[136]. Local strain induced electron transfer from Ru to the support, forming electron-deficient Ruδ+ active centers. The synergistic effect of geometric confinement and electronic regulation allowed the catalyst to remain highly dispersed without noticeable aggregation even after aging at 1,000 °C, while maintaining its catalytic activity. Tao et al. designed and prepared a series of ultrafine Ru nanoclusters encapsulated within S-1 zeolite shells using a one-pot method[137]. The Ru1@S-1 catalyst exhibited excellent performance in propane oxidation, achieving a T95 as low as 294 °C. Moreover, due to the protective and shielding effect of the zeolite shell, the Ru1@S-1 catalyst demonstrated outstanding thermal stability, hydrothermal stability, and recyclability in water-containing environments.
Regarding the further oxidation of Ru species, Okal et al. found that after treatment in an oxidizing atmosphere at 600 °C, the highly active defective amorphous RuOx phase transforms into crystalline RuO2, resulting in a decrease in catalytic activity[138]. Dinhová et al., through in-situ near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) studies, found that during propane oxidation, Ru nanoparticles supported on cerium dioxide (CeO2) are easily oxidized to RuO2 in an oxygen-containing atmosphere at temperatures below 200 °C[139,140]. As the temperature increases, RuO2 may further transform into higher-valence ruthenium oxides (RuOx, x > 2), which are volatile and tend to slowly migrate or evaporate from the catalyst surface [Figure 10A]. Therefore, to prevent the volatilization of Ru, Wang et al. prepared Ru-doped perovskite-type oxides LaFe0.9Ru0.1O3 via a sol-gel method, which maintained good propane evolution performance even after continuous oxidation and reduction treatments at 800 °C [Figure 10B][141,142]. Gao et al. introduced La into the Ru/Co3O4 catalytic system, inducing the formation of a structurally stable LaRuO3 perovskite phase under high-temperature conditions[143]. This effectively suppressed the volatilization of Ru at elevated temperatures and significantly enhanced the catalytic activity and thermal stability of the catalyst in propane oxidation. In addition, rutile TiO2 and RuO2 have the same crystal structure and similar lattice constants. Therefore, RuO2 supported on the surface of rutile TiO2 forms an epitaxial layer on the surface due to lattice matching. This epitaxial growth of the RuO2 film, along with the strong bonding between the RuO2 and TiO2 substrate, effectively prevents the migration and agglomeration of RuO2 at high temperatures, thereby improving the thermal stability of the catalyst [Figure 10C and D]. Debecker et al. loaded RuO2 nanoparticles with an average size of 2 nm onto commercial P25 TiO2 (with an anatase-to-rutile ratio of approximately 8:2) and found that during calcination, RuO2 migrated to the rutile TiO2 and stabilized on the TiO2 due to the formation of an epitaxial structure between RuO2 and the support[144,145]. Ledwa et al. first synthesized RuxCe1-xO2-γ particles using a reverse microemulsion method and then supported them on γ-Al2O3 to obtain the final catalyst[146,147]. Studies showed that, due to the strong interaction between Ru and CeO2, this catalyst could effectively stabilize RuOx active species at 800 °C and achieve a 50% propane conversion rate at 225 °C. Chen et al. coordinated Ru and Ag cations with chloride ions to form a RuAg alloy catalyst through self-assembly, which exhibited good activity and stability in propane oxidation reactions[124]. In addition to the aforementioned methods, high-entropy alloys are considered an effective approach to enhancing the thermal stability of precious metals. Zhang et al. found that the IrPdRhMoW and RuFeCoNiCu high-entropy alloys exhibit excellent long-term stability under harsh reaction conditions[148,149]. These findings provide valuable material design insights for developing thermally stable noble-metal-based, particularly Ru-containing, propane oxidation catalysts.
Figure 10. (A) Schematic illustration of the changes in the chemical states of Ru species in Ru/CeO₂ under oxidizing conditions. Reproduced with permission from Ref.[139]; (B) Schematic illustration of the Ru precipitation in Ru-substituted LaFeO3 perovskite oxides during the redox process. Reproduced with permission from Ref.[142]; (C) Catalytic combustion of LHs (ΔTC3-90) on the aged Co3O4-based catalysts at 750 °C for 100 or 200 h. Reproduced with permission from Ref.[143]; (D) The T90 of fresh and aged Ru/La-Co3O4, RuLa/La-Co3O4 for C3H8 oxidation. Reproduced with permission from Ref.[143]. LHs: Light hydrocarbons; LFR3: LaFeO3.
REACTION MECHANISM
Literature reports that propane oxidation reactions typically follow three mechanisms: the L-H mechanism, the MvK mechanism, and the E-R mechanism. Specifically, the L-H and E-R mechanisms are generally classified as surface reaction pathways, in which the reaction process mainly relies on the participation of surface-adsorbed oxygen species[46,150,151]. In contrast, the MvK mechanism is regarded as an interfacial reaction pathway, where the reaction primarily proceeds through the involvement and migration of lattice oxygen. The reaction mechanisms and pathways of catalytic propane oxidation vary depending on the catalyst used. The E-R mechanism can be considered a single-site reaction, in which a gas-phase molecule reacts directly with an adsorbed species. In the catalytic degradation of propane, gas-phase propane molecules either react directly with adsorbed active oxygen species or undergo a reaction between chemisorbed pollutants and gas-phase oxygen, producing H2O and CO2. In contrast, the L-H mechanism is a dual-site reaction mechanism, where chemical reactions occur between adsorbed species. In this case, gas-phase propane and oxygen are first adsorbed and dissociated on the catalyst surface to form active adsorbed intermediates, which then react with each other to ultimately produce CO2 and H2O.
Ma et al. investigated the reaction mechanism of propane over Co3O4 nanorods and Pt/Al2O3 using Fourier transform infrared spectroscopy (FTIR)[152]. They found that, on both catalysts, propane is first converted into a C3H7O* intermediate, which further dissociates and is oxidized into carboxylate species, mainly acetates and/or formates, before being ultimately oxidized to CO2 and H2O. However, the specific oxidation pathways differ between the two catalysts. On Co3O4 nanorods, propane oxidation proceeds via the L-H mechanism, with the rate-determining step being the dissociation of the C–H bond in adsorbed propane molecules. In contrast, on Pt/Al2O3 catalysts, the propane oxidation reaction follows the E-R mechanism, where the activation of gaseous C3H8 is the rate-determining step. Liu et al. proposed that the catalytic reaction over Pt/Ti0.1AlOy follows the L-H mechanism, in which propane adsorbed on the catalyst surface reacts with adsorbed oxygen[153]. Based on in situ DRIFTS, the possible reaction pathway is speculated to be: propane → isopropanol → propylene → acetone → carboxylate → CO2 + H2O.
Compared with the previous two mechanisms, the MvK mechanism is the most common reaction pathway in the catalytic oxidation of propane. In the MvK mechanism, the interface between the noble metal and the support, as well as the lattice oxygen of the support, plays a crucial role. Specifically, propane is first adsorbed and activated on the catalyst surface. The activated propane molecules are then attacked by the surface lattice oxygen, and deeply oxidized into CO2 and H2O. At this stage, the catalyst becomes reduced, accompanied by the formation of a large number of oxygen vacancies. Subsequently, oxygen molecules from the gas phase preferentially adsorb at these oxygen vacancies and, through a series of transformations among intermediate oxygen species, replenish the consumed lattice oxygen by incorporating into the lattice of the support. The catalyst is thereby re-oxidized and continues to catalytically degrade VOC pollutants, with the entire catalytic process proceeding in a continuous redox cycle. In general, the oxidation of propane pollutants on the surface of metal oxides with excellent redox properties, such as CeO2, Co3O4, and MnOx, typically follows the MvK mechanism. Therefore, when reducible metal oxides are used as supports, they not only enhance the dispersion of noble metals and inhibit their sintering under high-temperature reaction conditions through MSI but also provide active oxygen species that directly participate in the oxidation reactions. Dong et al. proposed that the oxidation of propane over the the hierarchical silicalite-1 (S-1) zeolite enveloping Pd-CeO2 nanowires (Pd-CeO2NW@S-1) catalyst follows the MvK mechanism[154]. Propane is first adsorbed by the silicalite-1 shell and transferred to the active sites, where it is oxidized by lattice oxygen from CeO2 to form enolate or acetone intermediates. These intermediates are then converted into acetate, bicarbonate, and formate species, and ultimately transformed into H2O and CO2, while gaseous oxygen replenishes the CeO2 lattice, completing the redox cycle. Hu et al. found that the oxidation of propane over the Ru/CeO2 catalyst not only follows a reaction pathway similar to that on Ru/Al2O3, but also proceeds via an additional route[114]. Specifically, C3H8 is first adsorbed and oxidized on the CeO2 surface to form propionate species (CH3CH2COO-). Subsequently, the propionate species undergo dehydrogenation at the Ru-Ce interface to generate acrylate species (CH2CHCOO-). These acrylate species are then further decomposed at the Ru-Ce interface into CO2 and H2O. Finally, gaseous O2 replenishes the oxygen vacancies in the CeO2 lattice, restoring its oxidized state and completing the redox cycle [Figure 11A and B]. Wang et al. also reported that the oxidation of propane over nanorod-like TiO2-supported Ru catalysts follows the MvK mechanism, in which the surface lattice oxygen species at the Ru-TiO2 interface directly participate in the propane oxidation process and serve as the key factor responsible for the enhanced oxidation performance of the catalyst[155]. Our previous studies indicate that for Pt catalysts supported on rod-shaped ceria, the propane oxidation reaction follows the MvK mechanism, in which the lattice oxygen at the Pt-CeO2 interface can participate in the propane oxidation process[58] [Figure 11C and D].
Figure 11. (A and B) Schematic illustration of the propane oxidation mechanism over Ru/Al2O3 (A) and Ru/CeO2 (B) catalysts. Reproduced with permission from Ref.[114]; (C and D) Schematic illustration of the propane oxidation pathway over Pt/CeO2-r (C) and Pt/CeO2-o (D) catalysts. Reproduced with permission from Ref.[58].
In addition to reducible supports where the MvK mechanism can occur, catalysts with strong oxygen activation abilities, such as those containing transition metals or transition metal oxides, can also participate in reactions through surface active oxygen species, thereby achieving a similar redox cycle. Chen et al. reported that propane oxidation catalyzed by Ru-Ag alloys follows the MvK mechanism[124]. Ru is primarily responsible for the activation of hydrocarbon molecules, and Ag facilitates oxygen activation; their synergy significantly promotes low-temperature propane oxidation. In situ DRIFTS characterization revealed that propane is first converted into reactive hydrocarbon intermediates ([CHx]) on Ru, which then react with active oxygen provided by Ag, followed by further oxidation to intermediates such as carbonates and CO, and ultimately fully mineralized to CO2. Liu et al. pointed out that the deep oxidation of propane over the Rh-MnOx@S-1 catalyst follows the MvK mechanism, in which the adsorbed C3H8 molecules react with active oxygen species from the Rh-MnOx interface[156]. Tao et al. prepared a core-shell Ru1@S-1 catalyst for propane oxidation[137]. In situ DRIFTS confirms that propane adsorbed on Ru directly abstracts oxygen from zeolite framework hydroxyls, undergoing efficient degradation via the MvK pathway.
CONCLUSION AND OUTLOOK
This review summarizes progress in complete propane oxidation over supported noble-metal catalysts, covering Pt-, Pd-, Ru-based and multimetallic systems. Across these catalyst families, the reported activity and durability are primarily controlled by the identity and chemical state of noble-metal sites, their size/dispersion. Meanwhile, the support and promoter further optimize the catalytic performance by regulating the chemical properties of the noble metals, the strength and nature of the MSIs, as well as the oxygen availability and stability. The literature collectively indicates that deactivation remains closely linked to structural and interfacial evolution under oxidative and hydrothermal environments, making stability as critical as intrinsic activity for practical deployment.
Despite notable advances, achieving low-temperature activity, long-term durability, and cost-effectiveness simultaneously remains a central challenge. Future research should emphasize catalyst design and evaluation protocols under realistic exhaust conditions, while exploring electro-thermal catalysis[157,158] as a complementary route toward robust low-temperature propane abatement under increasingly stringent emission standards.
DECLARATIONS
Authors’ contributions
Topic proposal: Ge, S.; Tang, X.
Manuscript preparation: Ge, S.
Collective discussion and revision: Wang, A.; Guo, Y. (Yanglong Guo); Zhan, W.; Wang, L.
Review and editing, methodology, supervision, project administration, funding acquisition: Tang, X.; Guo, T. (Yun Guo)
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Key Research and Development Program of China (2022YFB3504200), the National Natural Science Foundation of China (22306063), and the Young Elite Scientists Sponsorship Program by the China Association for Science and Technology (CAST; YESS20230258).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Wu, S.; Liu, H.; Huang, Z.; Xu, H.; Shen, W. O-vacancy-rich porous MnO2 nanosheets as highly efficient catalysts for propane catalytic oxidation. Appl. Catal. B. Environ. 2022, 312, 121387.
2. Li, Y. Y.; Ren, Y.; He, J.; Xiao, H.; Li, J. R. Recent advances of the effect of H2O on VOC oxidation over catalysts: influencing factors, inhibition/promotion mechanisms, and water resistance strategies. Environ. Sci. Technol. 2025, 59, 1034-59.
3. Wang, L.; Lin, B.; Gong, Z.; et al. Boosting low-temperature activity and high water-tolerance of CoxCey catalysts on propane catalytic oxidation: insights of the Ce corporation. Appl. Surf. Sci. 2025, 714, 164386.
4. Guo, X.; Sun, S.; Gao, M.; et al. Highly efficient Ag/Ce–Zr catalyst for catalytic oxidation of NVOCs: balance of redox ability and acidity. Rare. Met. 2024, 43, 6473-85.
5. Zhang, H.; Wang, Z.; Wei, L.; Liu, Y.; Dai, H.; Deng, J. Recent progress on VOC pollution control via the catalytic method. Chin. J. Catal. 2024, 61, 71-96.
6. Lou, B.; Shakoor, N.; Adeel, M.; et al. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523.
7. Zhao, J.; Shi, L.; Duan, W.; et al. Emission factors of environmentally persistent free radicals in PM2.5 from rural residential solid fuels combusted in a traditional stove. Sci. Total. Environ. 2021, 773, 145151.
8. Lewis, A. C.; Carslaw, N.; Marriott, P. J.; et al. A larger pool of ozone-forming carbon compounds in urban atmospheres. Nature 2000, 405, 778-81.
9. Ge, S.; Chen, Y.; Guo, Y.; Llorca, J.; Soler, L. Mechanochemically activated Au/CeO2 for enhanced CO oxidation and COPrOX reaction. Appl. Mater. Today. 2023, 33, 101857.
10. Pei, J.; Ge, S.; Xu, G.; et al. Nanocatalyst engineering via metal ion doping: regulating rate-determining steps for stable ozone decomposition. ACS. Appl. Nano. Mater. 2025, 8, 21902-11.
11. Liang, B.; Bai, H.; Bai, D.; Liu, X. Emissions of non-methane hydrocarbons and typical volatile organic compounds from various grate-firing coal furnaces. Atmos. Pollut. Res. 2022, 13, 101380.
12. Sun, J.; Shen, Z.; Zhang, L.; et al. Volatile organic compounds emissions from traditional and clean domestic heating appliances in Guanzhong Plain, China: emission factors, source profiles, and effects on regional air quality. Environ. Int. 2019, 133, 105252.
13. Hussain, M. S.; Gupta, G.; Mishra, R.; et al. Unlocking the secrets: volatile organic compounds (VOCs) and their devastating effects on lung cancer. Pathol. Res. Pract. 2024, 255, 155157.
14. Yang, C.; Miao, G.; Pi, Y.; et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: a review. Chem. Eng. J. 2019, 370, 1128-53.
15. Ren, Y.; Guan, X.; Peng, Y.; et al. Characterization of VOC emissions and health risk assessment in the plastic manufacturing industry. J. Environ. Manage. 2024, 357, 120730.
16. Sadegh, F.; Sadegh, N.; Wongniramaikul, W.; Apiratikul, R.; Choodum, A. Adsorption of volatile organic compounds on biochar: a review. Process. Saf. Environ. Prot. 2024, 182, 559-78.
17. Moreira, M. T.; Noya, I.; Feijoo, G. The prospective use of biochar as adsorption matrix - a review from a lifecycle perspective. Bioresour. Technol. 2017, 246, 135-41.
18. Wu, Z.; Meng, X.; Zhao, Z. Efficient removal of VOCs enabled by triboelectric-photocatalytic coupling effect. Nano. Energy. 2024, 132, 110364.
19. Yang, Y.; Zhao, S.; Cui, L.; et al. Recent advancement and future challenges of photothermal catalysis for VOCs elimination: From catalyst design to applications. Green. Energy. Environ. 2023, 8, 654-72.
20. Li, J.; Yu, E.; Cai, S.; et al. Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light. Appl. Catal. B. Environ. 2019, 240, 141-52.
21. Liu, Y.; Cai, Y.; Tang, X.; et al. Insight into the roles of Pd state and CeO2 property in C3H8 catalytic oxidation on Pd/CeO2. Appl. Surf. Sci. 2022, 605, 154675.
22. Zhao, Q.; Zheng, Y.; Song, C.; et al. Novel monolithic catalysts derived from in-situ decoration of Co3O4 and hierarchical Co3O4@MnOx on Ni foam for VOC oxidation. Appl. Catal. B. Environ. 2020, 265, 118552.
23. Li, G.; Li, N.; Sun, Y.; et al. Efficient defect engineering in Co-Mn binary oxides for low-temperature propane oxidation. Appl. Catal. B. Environ. 2021, 282, 119512.
24. Zhang, K.; Ding, H.; Pan, W.; et al. Research progress of a composite metal oxide catalyst for VOC degradation. Environ. Sci. Technol. 2022, 56, 9220-36.
25. Xu, L.; Zhou, Q.; Wen, C.; et al. Mechanistic understanding of oxygen spillover enables efficient propane combustion over Pt/AlSOx catalyst. Sep. Purif. Technol. 2025, 354, 129193.
26. Mu, Y.; Wang, X.; Yang, Y.; et al. Synthesis, characterization and application of copper-ceria catalysts for catalytic elimination of air pollutants: a review. J. Rare. Earths. 2025, In Press.
27. Zhang, W.; Valverde, J. L.; Giroir-fendler, A. Co3O4-based catalysts for propane total oxidation: a state-of-the-art minireview. Appl. Catal. B. Environ. 2023, 337, 122908.
28. Liotta, L. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B. Environ. 2010, 100, 403-12.
29. Yoshida, H.; Yazawa, Y.; Hattori, T. Effects of support and additive on oxidation state and activity of Pt catalyst in propane combustion. Catal. Today. 2003, 87, 19-28.
30. Huang, Z.; Ding, J.; Yang, X.; et al. Highly efficient oxidation of propane at low temperature over a Pt-based catalyst by optimization support. Environ. Sci. Technol. 2022, 56, 17278-87.
31. Ma, X.; Tang, Y.; Liu, Y.; et al. A-site cation exfoliation of amorphous SmMnxOy oxides for low temperature propane oxidation. J. Catal. 2022, 409, 59-69.
32. Dong, J.; Li, D.; Zhang, Y.; Chang, P.; Jin, Q. Insights into the CeO2 facet-depended performance of propane oxidation over Pt-CeO2 catalysts. J. Catal. 2022, 407, 174-85.
33. Wang, Y.; Wang, C.; Zeng, K.; et al. Revealing the strong interaction effect of MnO nanoparticles and Nb2O5 supports with variable morphologies on catalytic propane oxidation. Appl. Surf. Sci. 2022, 576, 151797.
34. Tan, B.; Huo, Z.; Sun, L.; et al. Ionic liquid-modulated synthesis of MnO2 nanowires for promoting propane combustion: microstructure engineering and regulation mechanism. Colloids. Surf. A. Physicochem. Eng. Asp. 2023, 656, 130431.
35. Song, X.; Sun, L.; Gao, P.; et al. Unveiling the remarkable catalytic performance of Al2O3@Cu‐Ce core–shell nanofiber catalyst for carbonyl sulfide hydrolysis at low temperature. EcoEnergy 2025, 3, e70011.
36. Chu, S.; Wang, E.; Feng, F.; et al. A review of noble metal catalysts for catalytic removal of VOCs. Catalysts 2022, 12, 1543.
37. Meng, F.; Tang, X.; Kadja, G. T.; et al. A systematic review with improving activity and stability in VOCs elimination by oxidation of noble metals: starting from active sites. Sep. Purif. Technol. 2025, 354, 129222.
38. Liu, Y.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts. Chin. J. Catal. 2016, 37, 1193-205.
39. You, Y.; Xu, A.; Lv, Y.; et al. Constructing tri-coordinated Al (AlIII) sites to boost complete propane oxidation of the Pt/Al2O3 catalyst. Catal. Sci. Technol. 2024, 14, 4058-67.
40. Zhao, P.; Li, X.; Liao, W.; et al. Understanding the role of NbOx on Pt/Al2O3 for effective catalytic propane oxidation. Ind. Eng. Chem. Res. 2019, 58, 21945-52.
41. Li, X.; Liu, Y.; Liao, W.; et al. Synergistic roles of Pt0 and Pt2+ species in propane combustion over high-performance Pt/AlF3 catalysts. Appl. Surf. Sci. 2019, 475, 524-31.
42. Cen, B.; Wang, W.; Zhao, P.; et al. Revealing the different roles of sulfates on Pt/Al2O3 catalyst for methane and propane combustion. Catal. Lett. 2022, 152, 863-71.
43. Wang, H.; Rao, C.; Liu, K.; et al. W single-atom-engineered acid sites over Pt/Al2O3 catalysts to boost propane oxidation activity. ACS. Sustainable. Chem. Eng. 2025, 13, 15050-61.
44. Bailey, L. A.; Douthwaite, M.; Davies, T. E.; Morgan, D. J.; Taylor, S. H. Controlling palladium particle size and dispersion as a function of loading by chemical vapour impregnation: an investigation using propane total oxidation as a model reaction. Catal. Sci. Technol. 2024, 14, 5045-53.
45. Liang, P.; Wang, L.; Wang, Q.; et al. Improved catalytic oxidation of propane over phosphate-modified Pd/Al2O3-TiO2 catalyst. J. Environ. Chem. Eng. 2023, 11, 109569.
46. Liu, W.; Yang, S.; Zhang, Q.; et al. Insights into flower-like Al2O3 spheres with rich unsaturated pentacoordinate Al3+ sites stabilizing Ru-CeOx for propane total oxidation. Appl. Catal. B. Environ. 2021, 292, 120171.
47. Adamska, K.; Okal, J.; Tylus, W. Stable bimetallic Ru-Mo/Al2O3 catalysts for the light alkane combustion: effect of the Mo addition. Appl. Catal. B. Environ. 2019, 246, 180-94.
48. Xu, A.; Ge, S.; Yu, A.; et al. Breaking the trade-off between Pt chemical state and active site density by SiOx engineering on Pt/TiO2 for complete propane oxidation. Appl. Catal. B. Environ. Energy. 2026, 382, 125958.
49. Fang, Y.; Li, H.; Zhang, Q.; et al. Oxygen vacancy-governed opposite catalytic performance for C3H6 and C3H8 combustion: the effect of the Pt electronic structure and chemisorbed oxygen species. Environ. Sci. Technol. 2022, 56, 3245-57.
50. Yu, Z.; Fang, Y.; Pan, C.; et al. Construction of electron-enriched Ptδ+ with reactive oxygen species for enhanced propane catalytic combustion. ACS. Appl. Mater. Interfaces. 2025, 17, 21246-56.
51. Huang, L.; Xu, L.; Gao, B.; et al. Deep oxidation of propane over PtIr/TiO2 bimetallic catalysts: mechanistic investigation of promoting roles of Ir species. Appl. Surf. Sci. 2023, 638, 158149.
52. Dong, J.; Li, T.; Li, S.; et al. Local environment and electronic structure of Pt-TiO2 catalysts define the reactivity of CO oxidation and C3H8 combustion: the crystal phase of TiO2 determining. ACS. Catal. 2025, 15, 15794-807.
53. Camposeco, R.; Castillo, S.; Zanella, R. Catalytic oxidation of propane and carbon monoxide by Pd nanoparticles on Mn/TiO2 catalysts. Catal. Lett. 2024, 154, 155-69.
54. Camposeco, R.; Miguel, O.; Torres, A. E.; Armas, D. E.; Zanella, R. Highly active Ru/TiO2 nanostructures for total catalytic oxidation of propane. Environ. Sci. Pollut. Res. Int. 2023, 30, 98076-90.
55. Tang, X.; Ge, S.; Lv, Y.; et al. Blocking the operando formation of single-atom spectators by interfacial engineering. Angew. Chem. Int. Ed. Engl. 2025, 64, e202505507.
56. Ge, S.; Fan, W.; Tang, X.; et al. Revealing the size effect of ceria nanocube-supported platinum nanoparticles in complete propane oxidation. ACS. Catal. 2024, 14, 2532-44.
57. Huang, Z.; Cao, S.; Yu, J.; et al. Total oxidation of light alkane over phosphate-modified Pt/CeO2 catalysts. Environ. Sci. Technol. 2022, 56, 9661-71.
58. Ge, S.; Chen, Y.; Tang, X.; et al. Preformed Pt nanoparticles supported on nanoshaped CeO2 for total propane oxidation. ACS. Appl. Nano. Mater. 2023, 6, 15073-84.
59. Gao, B.; Zhang, K.; Wang, Y.; Lu, J.; Liu, P. On the structure insensitivity of propane total oxidation over Pt/CeO2: a comparison between single atoms, clusters and nanoparticles. ChemCatChem 2023, 15, e202301160.
60. Xia, L.; Jian, Y.; Liu, Q.; et al. Boosted light alkane deep oxidation via metal bond length modulation-induced C-C bond preferential activation. Environ. Sci. Technol. 2024, 58, 3472-82.
61. Liu, Y.; Yang, J.; Yang, J.; et al. Understanding the three-way catalytic reaction on Pd/CeO2 by tuning the chemical state of Pd. Appl. Surf. Sci. 2021, 556, 149766.
62. Wang, A.; Ding, J.; Li, M.; et al. Robust Ru/Ce@Co catalyst with an optimized support structure for propane oxidation. Environ. Sci. Technol. 2024, 58, 12742-53.
63. Sun, Y.; Ye, F.; Ding, J.; et al. Regulating the spatial distribution of Ru nanoparticles on CeO2 support for enhanced propane oxidation. ACS. Appl. Nano. Mater. 2022, 5, 3937-45.
64. Yan, J.; Wang, Y.; Ding, M.; et al. Highly efficient Ru/CeO2 catalyst using colloidal chemical method for propane oxidation. Chem. Eng. J. 2024, 500, 156936.
65. Wang, Z.; Huang, Z.; Brosnahan, J. T.; et al. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion. Environ. Sci. Technol. 2019, 53, 5349-58.
66. Enterkin, J. A.; Setthapun, W.; Elam, J. W.; et al. Propane oxidation over Pt/SrTiO3 nanocuboids. ACS. Catal. 2011, 1, 629-35.
67. Bal’zhinimaev, B. S.; Kovalyov, E. V.; Kaichev, V. V.; Suknev, A. P.; Zaikovskii, V. I. Catalytic abatement of VOC over novel Pt fiberglass catalysts. Top. Catal. 2017, 60, 73-82.
68. Shan, S.; Li, J.; Maswadeh, Y.; et al. Surface oxygenation of multicomponent nanoparticles toward active and stable oxidation catalysts. Nat. Commun. 2020, 11, 4201.
69. O’brien, C. P.; Jenness, G. R.; Dong, H.; Vlachos, D. G.; Lee, I. C. Deactivation of Pt/Al2O3 during propane oxidation at low temperatures: kinetic regimes and platinum oxide formation. J. Catal. 2016, 337, 122-32.
70. Liu, Y.; Li, X.; Liao, W.; et al. Highly active Pt/BN catalysts for propane combustion: the roles of support and reactant-induced evolution of active sites. ACS. Catal. 2019, 9, 1472-81.
71. Peng, Q.; Han, W.; Han, W.; Dong, F.; Tang, Z.; Zhou, Z. Tailored Pt/NiaCobAlOx catalysts derived from LDH structure for efficient catalytic combustion of propane. Chem. Eng. J. 2024, 500, 157181.
72. Dun, Y.; Liu, Y.; Xu, J.; et al. Pt0-MnSO4 active centers on modified SmMn2O5 mullite oxides for efficient propane oxidation. Appl. Catal. B. Environ. Energy. 2025, 371, 125223.
73. Avila, M.; Vignatti, C.; Apesteguía, C.; Garetto, T. Effect of support on the deep oxidation of propane and propylene on Pt-based catalysts. Chem. Eng. J. 2014, 241, 52-9.
74. Yazawa, Y.; Yoshida, H.; Hattori, T. The support effect on platinum catalyst under oxidizing atmosphere: improvement in the oxidation-resistance of platinum by the electrophilic property of support materials. Appl. Catal. A. Gen. 2002, 237, 139-48.
75. Yazawa, Y.; Yoshida, H.; Komai, S.; Hattori, T. The additive effect on propane combustion over platinum catalyst: control of the oxidation-resistance of platinum by the electronegativity of additives. Appl. Catal. A. Gen. 2002, 233, 113-24.
76. Wang, W.; Li, D.; Yu, H.; et al. Insights into different reaction behaviors of propane and CO oxidation over Pt/CeO2 and Pt/Nb2O5: the crucial roles of support properties. J. Phys. Chem. C. 2021, 125, 19301-10.
77. Xian, Y.; Li, B.; Wen, C.; et al. Boosting propane combustion on dual active sites of Pt/WO3 through regulating Pt sites and activating propane on WO3 surface. Fuel 2025, 385, 134172.
78. Wen, C.; Xu, L.; Hao, Y.; et al. Individual functionality and synergistic effects of redox site–acid site in propane oxidation. ACS. Catal. 2025, 15, 10746-57.
79. Luo, H.; Wu, X.; Weng, D.; Liu, S.; Ran, R. A novel insight into enhanced propane combustion performance on PtUSY catalyst. Rare. Met. 2017, 36, 1-9.
80. Park, J. E.; Kim, K. B.; Kim, Y.; Song, K. S.; Park, E. D. Effect of Pt particle size on propane combustion over Pt/ZSM-5. Catal. Lett. 2013, 143, 1132-8.
81. Lykhach, Y.; Kozlov, S. M.; Skála, T.; et al. Counting electrons on supported nanoparticles. Nat. Mater. 2016, 15, 284-8.
82. Jeong, H.; Kwon, O.; Kim, B.; et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 2020, 3, 368-75.
83. Shao, C.; Yang, J.; You, Y.; et al. Regulating the selective dispersion of tungsten oxide to promote propane combustion on Pt-nanoparticle catalysts supported on WOx/ZrO2 by tuning the zirconia crystal phase. ACS. Appl. Nano. Mater. 2022, 5, 13482-97.
84. Liao, W.; Fang, X.; Cen, B.; et al. Deep oxidation of propane over WO3-promoted Pt/BN catalysts: the critical role of Pt-WO3 interface. Appl. Catal. B. Environ. 2020, 272, 118858.
85. Wen, C.; Xu, L.; Zhao, W.; et al. Novel strategy to regulate the geometric and electronic structure of Pt catalyst for efficient propane combustion. Appl. Catal. A. Gen. 2024, 680, 119778.
86. Zhou, Q.; Liu, L.; Xian, Y.; et al. Synergistic catalysis of Pt-acid sites on Al2O3-AlPO4 hybrids for enhanced propane combustion. Fuel 2026, 405, 136540.
87. Shao, C.; Cui, Y.; Zhang, L.; et al. Boosting propane purification on Pt/ZrOSO4 nanoflowers: insight into the roles of different sulfate species in synergy with Pt. Sep. Purif. Technol. 2023, 304, 122367.
88. Xu, L.; Wen, C.; Luo, X.; et al. Regulating the synergy of sulfate and Pt species in Pt/ZSM-5 for propane complete oxidation. Appl. Catal. B. Environ. Energy. 2024, 354, 124135.
89. Gawthrope, D. E.; Lee, A. F.; Wilson, K. Support-mediated alkane activation over Pt–SO4/Al2O3 Catalysts. Catal. Lett. 2004, 94, 25-9.
90. Wang, B.; Wu, X.; Ran, R.; Si, Z.; Weng, D. Participation of sulfates in propane oxidation on Pt/SO42-/CeO2–ZrO2 catalyst. J. Mol. Catal. A. Chem. 2012, 361-362, 98-103.
91. Huang, L.; Zhu, X.; Xu, L.; Wang, Y.; Lu, J. Towards the promoting roles of SO2 in total oxidation of propane over Pt catalysts. Sep. Purif. Technol. 2025, 355, 129759.
92. Hao, H.; Jin, B.; Liu, W.; Wu, X.; Yin, F.; Liu, S. Robust Pt@TiOx/TiO2 catalysts for hydrocarbon combustion: effects of Pt-TiOx interaction and sulfates. ACS. Catal. 2020, 10, 13543-8.
93. Tan, W.; Xie, S.; Cai, Y.; et al. Surface lattice-embedded Pt single-atom catalyst on ceria-zirconia with superior catalytic performance for propane oxidation. Environ. Sci. Technol. 2023, 57, 12501-12.
94. Zhang, B.; Liu, R.; Li, L.; et al. Ultra-stable low-coordinated PtSA/CeZrO2 ordered macroporous structure integrated industrial-scale monolithic catalysts for high-temperature oxidation. Nat. Commun. 2025, 16, 7847.
95. Liu, Y.; Zou, Y.; Wang, Y.; Ma, Y.; Zhang, S.; Qu, Y. Strong metal-support interactions between Pt and CeO2 for efficient methanol decomposition. Chem. Eng. J. 2023, 475, 146219.
96. Tang, X.; Yu, A.; Yang, Q.; et al. Significance of epitaxial growth of PtO2 on rutile TiO2 for Pt/TiO2 catalysts. J. Am. Chem. Soc. 2024, 146, 3764-72.
97. You, Y.; Xu, A.; Tang, X.; et al. Refining metal–support interactions via surface modification of irreducible oxide support for enhanced complete propane oxidation. ACS. Catal. 2024, 14, 11457-67.
98. Duan, H.; Kong, F.; Bi, X.; et al. Catalytic combustion of methane over noble metal catalysts. ACS. Catal. 2024, 14, 17972-92.
99. Van den Bossche, M.; Grönbeck, H. Methane oxidation over PdO(101) revealed by first-principles kinetic modeling. J. Am. Chem. Soc. 2015, 137, 12035-44.
100. Chin, Y. H.; Buda, C.; Neurock, M.; Iglesia, E. Consequences of metal-oxide interconversion for C-H bond activation during CH4 reactions on Pd catalysts. J. Am. Chem. Soc. 2013, 135, 15425-42.
101. Kim, K. B.; Kim, M. K.; Kim, Y. H.; Song, K. S.; Park, E. D. Propane combustion over supported Pd catalysts. Res. Chem. Intermed. 2010, 36, 603-11.
102. You, R.; Li, Z.; Cao, T.; Nan, B.; Si, R.; Huang, W. Synthesis in a glovebox: utilizing surface oxygen vacancies to enhance the atomic dispersion of palladium on ceria for carbon monoxide oxidation and propane combustion. ACS. Appl. Nano. Mater. 2018, 1, 4988-97.
103. Khan, H. A.; Abou-Daher, M.; de Freitas, A. S.; Subburaj, J.; Tall, O. E.; Farooq, A. Performance studies of Pt, Pd and PtPd supported on SBA-15 for wet CO and hydrocarbon oxidation. Catal. Today. 2024, 426, 114370.
104. Hu, Z.; Liu, X.; Meng, D.; Guo, Y.; Guo, Y.; Lu, G. Effect of ceria crystal plane on the physicochemical and catalytic properties of Pd/ceria for CO and propane oxidation. ACS. Catal. 2016, 6, 2265-79.
105. Li, M.; Wu, X.; Wan, J.; Liu, S.; Ran, R.; Weng, D. Pd–Ce0.33Zr0.67O2–Al2O3 catalyst for propane oxidation: interactions between the precious metal and support under the hydrothermal ageing. Catal. Today. 2015, 242, 322-8.
106. Peng, H.; Dong, T.; Yang, S.; et al. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes. Nat. Commun. 2022, 13, 295.
107. Khudorozhkov, A. K.; Chetyrin, I. A.; Bukhtiyarov, A. V.; Prosvirin, I. P.; Bukhtiyarov, V. I. Propane oxidation over Pd/Al2O3: kinetic and in situ XPS study. Top. Catal. 2017, 60, 190-7.
108. Zhou, R.; Xing, F.; Wang, S.; Lu, J.; Jin, L.; Luo, M. CO and C3H8 total oxidation over Pd/La-Al2O3 catalysts: effect of calcination temperature and hydrothermal treatment. J. Rare. Earths. 2014, 32, 621-7.
109. Li, M.; Weng, D.; Wu, X.; Wan, J.; Wang, B. Importance of re-oxidation of palladium by interaction with lanthana for propane combustion over Pd/Al2O3 catalyst. Catal. Today. 2013, 201, 19-24.
110. Yan, J.; Wang, L.; Guo, Y.; Guo, Y.; Dai, Q.; Zhan, W. Comparisons on thermal and water-resistance of Ru and Pd supported on cobalt-doped alumina nanosheets for catalytic combustion of propane. Appl. Catal. A. Gen. 2021, 628, 118398.
111. Taylor, M. N.; Zhou, W.; Garcia, T.; et al. Synergy between tungsten and palladium supported on titania for the catalytic total oxidation of propane. J. Catal. 2012, 285, 103-14.
112. Okal, J.; Zawadzki, M.; Krajczyk, L. Light alkane oxidation over Ru supported on ZnAl2O4, CeO2 and Al2O3. Catal. Today. 2011, 176, 173-6.
113. Xia, H.; Bai, Y.; Niu, Q.; et al. Support-dependent activity and thermal stability of Ru-based catalysts for catalytic combustion of light hydrocarbons. Ind. Eng. Chem. Res. 2023, 62, 1826-38.
114. Hu, Z.; Wang, Z.; Guo, Y.; et al. Total oxidation of propane over a Ru/CeO2 catalyst at low temperature. Environ. Sci. Technol. 2018, 52, 9531-41.
115. Wu, J.; Chen, B.; Yan, J.; et al. Ultra-active Ru supported on CeO2 nanosheets for catalytic combustion of propane: experimental insights into interfacial active sites. Chem. Eng. J. 2022, 438, 135501.
116. Okal, J. The interaction of oxygen with high loaded Ru/γ-Al2O3 catalyst. Mater. Res. Bull. 2009, 44, 318-23.
117. Okal, J.; Zawadzki, M.; Tylus, W. Microstructure characterization and propane oxidation over supported Ru nanoparticles synthesized by the microwave-polyol method. Appl. Catal. B. Environ. 2011, 101, 548-59.
118. Zhang, Q.; Song, Y.; Xu, A.; et al. C3H8 oxidation on atomic-scale catalysts: insights into active oxygen species and reaction pathways. J. Hazard. Mater. 2025, 494, 138716.
119. Deng, W.; Gao, B.; Huang, J.; et al. Combustion of propane over various metals doped CeO2 nanosheet supported ruthenium catalysts. J. Environ. Chem. Eng. 2025, 13, 116264.
120. Deng, W.; She, X.; Chen, B.; et al. Catalytic combustion of propane over second metal-modified Ru supported on CeO2 nanosheet. Sep. Purif. Technol. 2025, 356, 129874.
121. Ge, H.; Yao, J.; Fan, J.; Zeng, J.; Li, R.; Qin, Y. Pt–Co separation for enhancing bimetallic catalysis in selective hydrogenation reaction. ACS. Catal. 2025, 15, 16740-7.
122. Huang, Z.; Yu, J.; Li, W.; et al. Optimising PtFe nanoparticle structure to enhance catalytic activity and stability for propane oxidation. Appl. Catal. B. Environ. 2024, 340, 123198.
123. Tsui, C. J.; Leung, K.; Tay, Y.; et al. Highly durable Pt–Ru-doped Ce0.9Zr0.1O2 as an effective dual catalyst for low-temperature simultaneous propane and carbon monoxide oxidation. J. Phys. Chem. C. 2022, 126, 9334-51.
124. Chen, J.; Lv, X.; Xu, W.; Li, X.; Chen, J.; Jia, H. Utilizing Cl coordination to facilitate Ru-Ag self-assembling into alloy and recover thermally-inactivated catalyst for propane combustion. Appl. Catal. B. Environ. 2021, 290, 119989.
125. Baranowska, K.; Okal, J. Bimetallic Ru-Re/γ-Al2O3 catalysts for the catalytic combustion of propane: effect of the Re addition. Appl. Catal. A. Gen. 2015, 499, 158-67.
126. Baranowska, K.; Okal, J. Performance and stability of the Ru–Re/γ-Al2O3 catalyst in the total oxidation of propane: influence of the order of impregnation. Catal. Lett. 2016, 146, 72-81.
127. Yazawa, Y.; Yoshida, H.; Takagi, N.; et al. Acid strength of support materials as a factor controlling catalytic activity of noble metal catalysts for catalytic combustion. Stud. Surf. Sci. Catal. 2000, 130, 2189-94.
128. Wettergren, K.; Schweinberger, F. F.; Deiana, D.; et al. High sintering resistance of size-selected platinum cluster catalysts by suppressed Ostwald ripening. Nano. Lett. 2014, 14, 5803-9.
129. Yuan, W.; Zhang, D.; Ou, Y.; et al. Direct in situ TEM visualization and insight into the facet-dependent sintering behaviors of gold on TiO2. Angew. Chem. Int. Ed. Engl. 2018, 57, 16827-31.
130. Hu, S.; Li, W. X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360-5.
131. Xiong, H.; Lin, S.; Goetze, J.; et al. Thermally stable and regenerable platinum-tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. Engl. 2017, 56, 8986-91.
132. Cargnello, M.; Delgado Jaén, J. J.; Hernández Garrido, J. C.; et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 2012, 337, 713-7.
133. Goodman, E. D.; Johnston-Peck, A. C.; Dietze, E. M.; et al. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2019, 2, 748-55.
134. Okal, J.; Zawadzki, M. Influence of catalyst pretreatments on propane oxidation over Ru/γ-Al2O3. Catal. Lett. 2009, 132, 225-34.
135. Okal, J.; Zawadzki, M. Combustion of propane over novel zinc aluminate-supported ruthenium catalysts. Appl. Catal. B. Environ. 2011, 105, 182-90.
136. Wang, J.; Jiang, Z.; Xu, H.; et al. Elucidating confinement and microenvironment of Ru clusters stably confined in MFI zeolite for efficient propane oxidation. Angew. Chem. Int. Ed. Engl. 2025, 64, e202417618.
137. Tao, J.; Zhang, Q.; Zhao, Y.; et al. Elucidating the role of confinement and shielding effect over zeolite enveloped Ru catalysts for propane low temperature degradation. Chemosphere 2022, 302, 134884.
138. Okal, J.; Zawadzki, M.; Kraszkiewicz, P.; Adamska, K. Ru/CeO2 catalysts for combustion of mixture of light hydrocarbons: effect of preparation method and metal salt precursors. Appl. Catal. A. Gen. 2018, 549, 161-9.
139. Dinhová, T. N.; Bezkrovnyi, O.; Piliai, L.; et al. Unraveling the effects of reducing and oxidizing pretreatments and humidity on the surface chemistry of the Ru/CeO2 catalyst during propane oxidation. J. Phys. Chem. C. Nanomater. Interfaces. 2025, 129, 1746-57.
140. Bezkrovnyi, O.; Vorokhta, M.; Pawlyta, M.; et al. In situ observation of highly oxidized Ru species in Ru/CeO2 catalyst under propane oxidation. J. Mater. Chem. A. 2022, 10, 16675-84.
141. Wang, Y.; Gallego, J.; Wang, W.; et al. Unveiling the self-activation of exsolved LaFe0.9Ru0.1O3 perovskite during the catalytic total oxidation of propane. Chin. J. Catal. 2023, 54, 250-64.
142. Wang, Y.; Paciok, P.; Pielsticker, L.; et al. Boosting Ru atomic efficiency of LaFe0.97Ru0.03O3 via knowledge-driven synthesis design. Chem. Sci. 2025, 16, 7739-50.
143. Gao, B.; Deng, W.; Xia, H.; et al. Thermally reconstructed Ru/La-Co3O4 nanosheets with super thermal stability for catalytic combustion of light hydrocarbons: induced surface LaRuO3 active phase. Adv. Sci. 2025, 12, e2414919.
144. Debecker, D. P.; Farin, B.; Gaigneaux, E. M.; Sanchez, C.; Sassoye, C. Total oxidation of propane with a nano-RuO2/TiO2 catalyst. Appl. Catal. A. Gen. 2014, 481, 11-8.
145. Kim, A.; Debecker, D. P.; Devred, F.; Dubois, V.; Sanchez, C.; Sassoye, C. CO2 methanation on Ru/TiO2 catalysts: on the effect of mixing anatase and rutile TiO2 supports. Appl. Catal. B. Environ. 2018, 220, 615-25.
146. Ledwa, K. A.; Kępiński, L.; Ptak, M.; Szukiewicz, R. Ru0.05Ce0.95O2-y deposited on functionalized alumina as a smart catalyst for propane oxidation. Appl. Catal. B. Environ. 2020, 274, 119090.
147. Ledwa, K. A.; Pawlyta, M.; Kępiński, L. RuxCe1-xO2-y nanoparticles deposited on functionalized γ-Al2O3 as a thermally stable oxidation catalyst. Appl. Catal. B. Environ. 2018, 230, 135-44.
148. Zhang, D.; Shi, Y.; Chen, X.; Lai, J.; Huang, B.; Wang, L. High-entropy alloy metallene for highly efficient overall water splitting in acidic media. Chin. J. Catal. 2023, 45, 174-83.
149. Zhang, D.; Zhao, H.; Wu, X.; et al. Multi‐site electrocatalysts boost pH‐universal nitrogen reduction by high‐entropy alloys. Adv. Funct. Mater. 2021, 31, 2006939.
150. Gao, Y.; Jiang, M.; Yang, L.; Li, Z.; Tian, F. X.; He, Y. Recent progress of catalytic methane combustion over transition metal oxide catalysts. Front. Chem. 2022, 10, 959422.
151. Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review. Appl. Catal. B. Environ. 2021, 281, 119447.
152. Ma, L.; Geng, Y.; Chen, X.; Yan, N.; Li, J.; Schwank, J. W. Reaction mechanism of propane oxidation over Co3O4 nanorods as rivals of platinum catalysts. Chem. Eng. J. 2020, 402, 125911.
153. Liu, L.; Han, W.; Dong, F.; Feng, H.; Tang, Z. Designing ordered mesoporous confined Pt/Ti0.1AlOy catalysts for the catalytic combustion of propane. New. J. Chem. 2023, 47, 5519-33.
154. Dong, T.; Liu, W.; Ma, M.; et al. Hierarchical zeolite enveloping Pd-CeO2 nanowires: an efficient adsorption/catalysis bifunctional catalyst for low temperature propane total degradation. Chem. Eng. J. 2020, 393, 124717.
155. Wang, M.; Li, G.; Wang, S.; et al. Catalytic oxidation of propane over nanorod-like TiO2 supported Ru catalysts: structure-activity dependence and mechanistic insights. Chem. Eng. J. 2024, 481, 148344.
156. Liu, W.; Tao, J.; Zhao, Y.; et al. Boosting the deep oxidation of propane over zeolite encapsulated Rh-Mn bimetallic nanoclusters: elucidating the role of confinement and synergy effects. J. Catal. 2022, 413, 201-13.
157. Mei, X.; Zhu, X.; Zhang, Y.; et al. Decreasing the catalytic ignition temperature of diesel soot using electrified conductive oxide catalysts. Nat. Catal. 2021, 4, 1002-11.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data





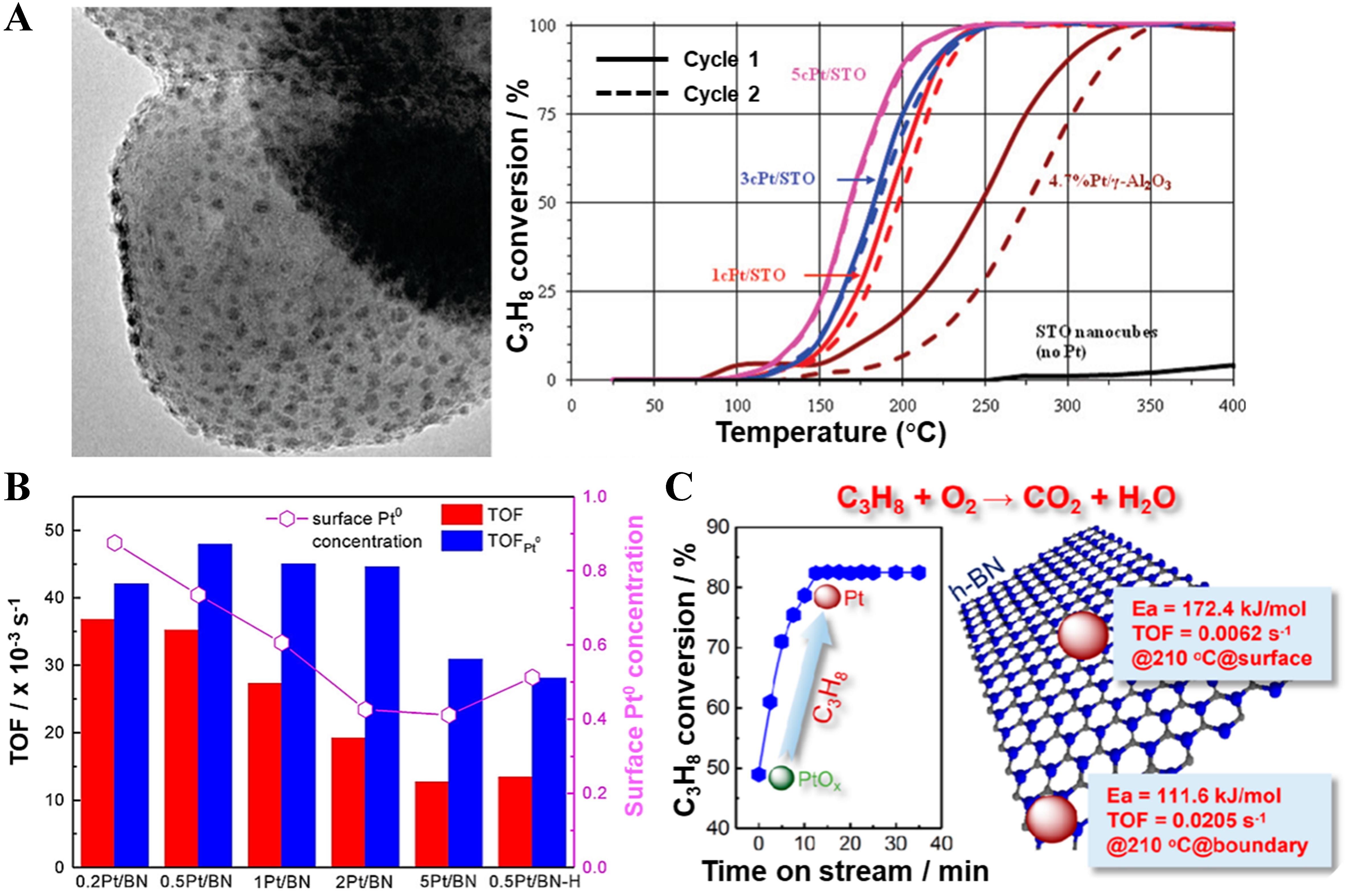
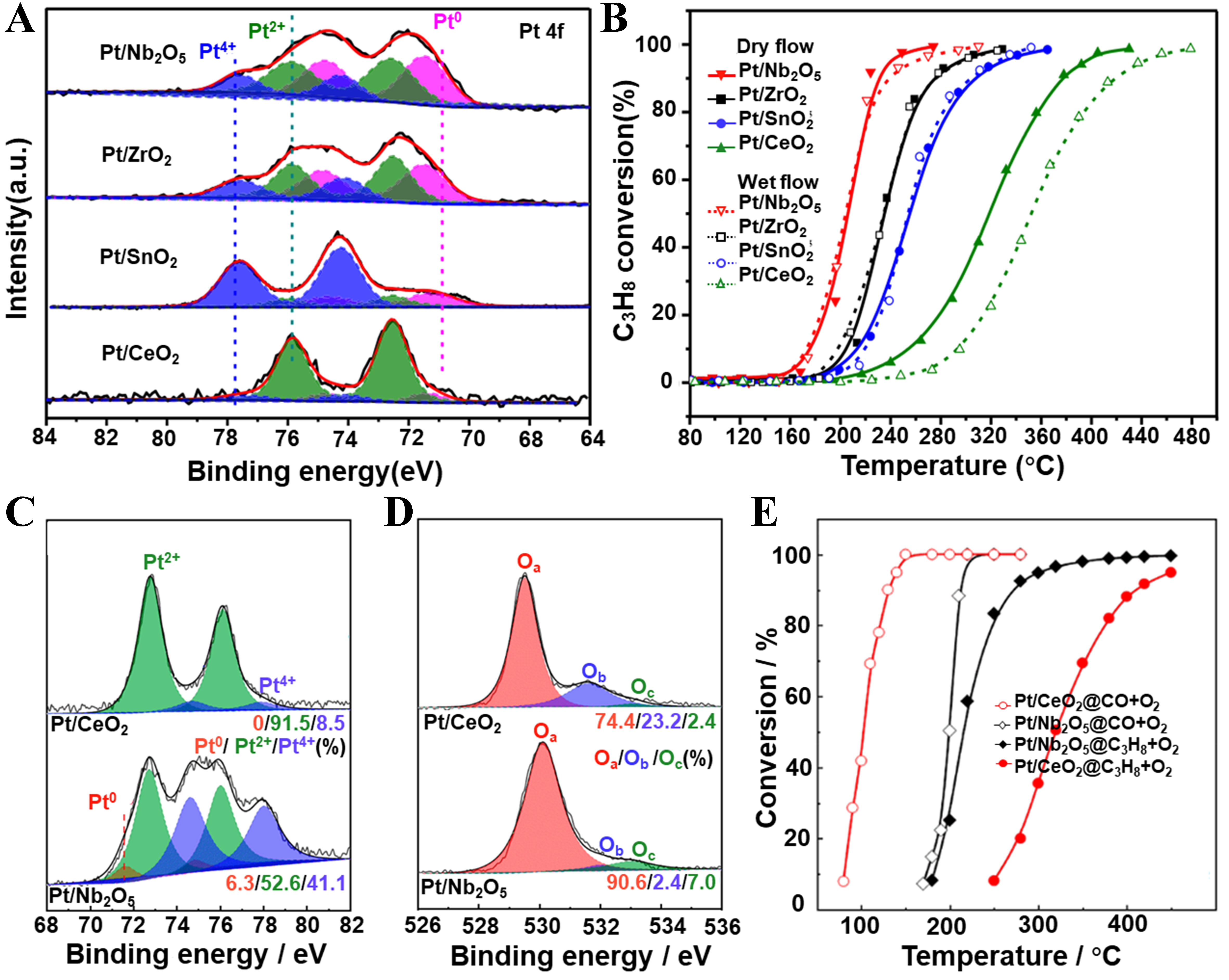
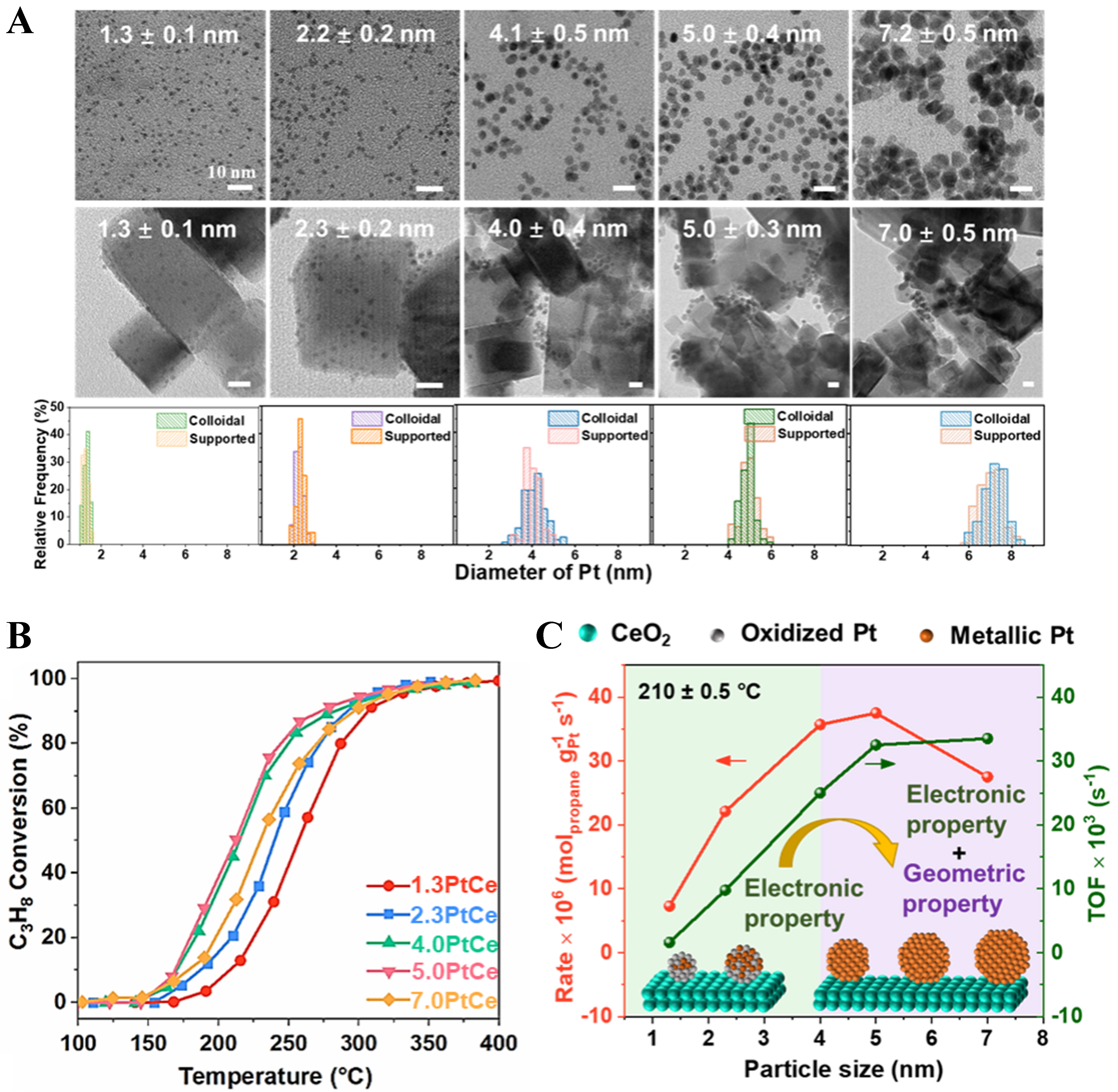
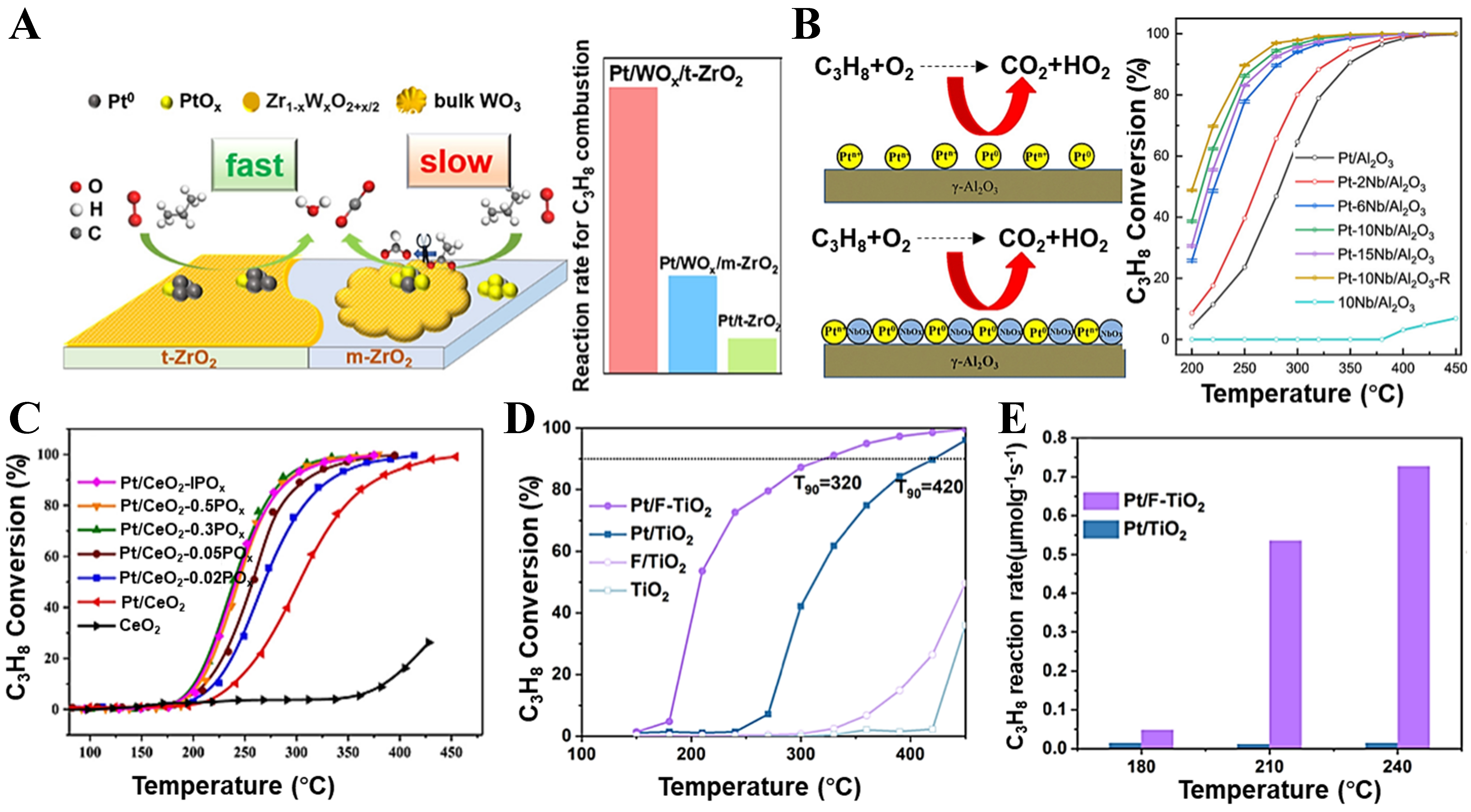
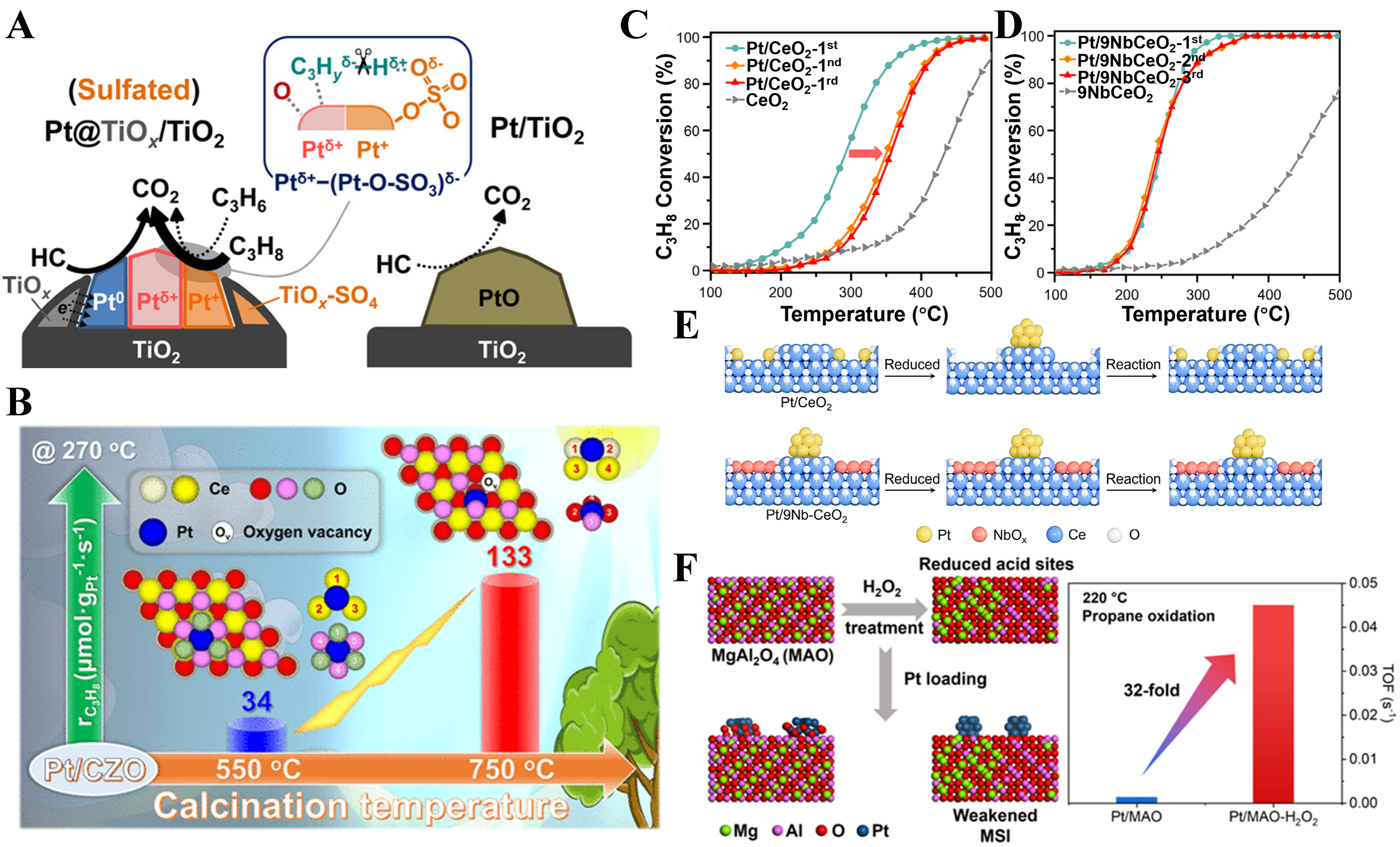
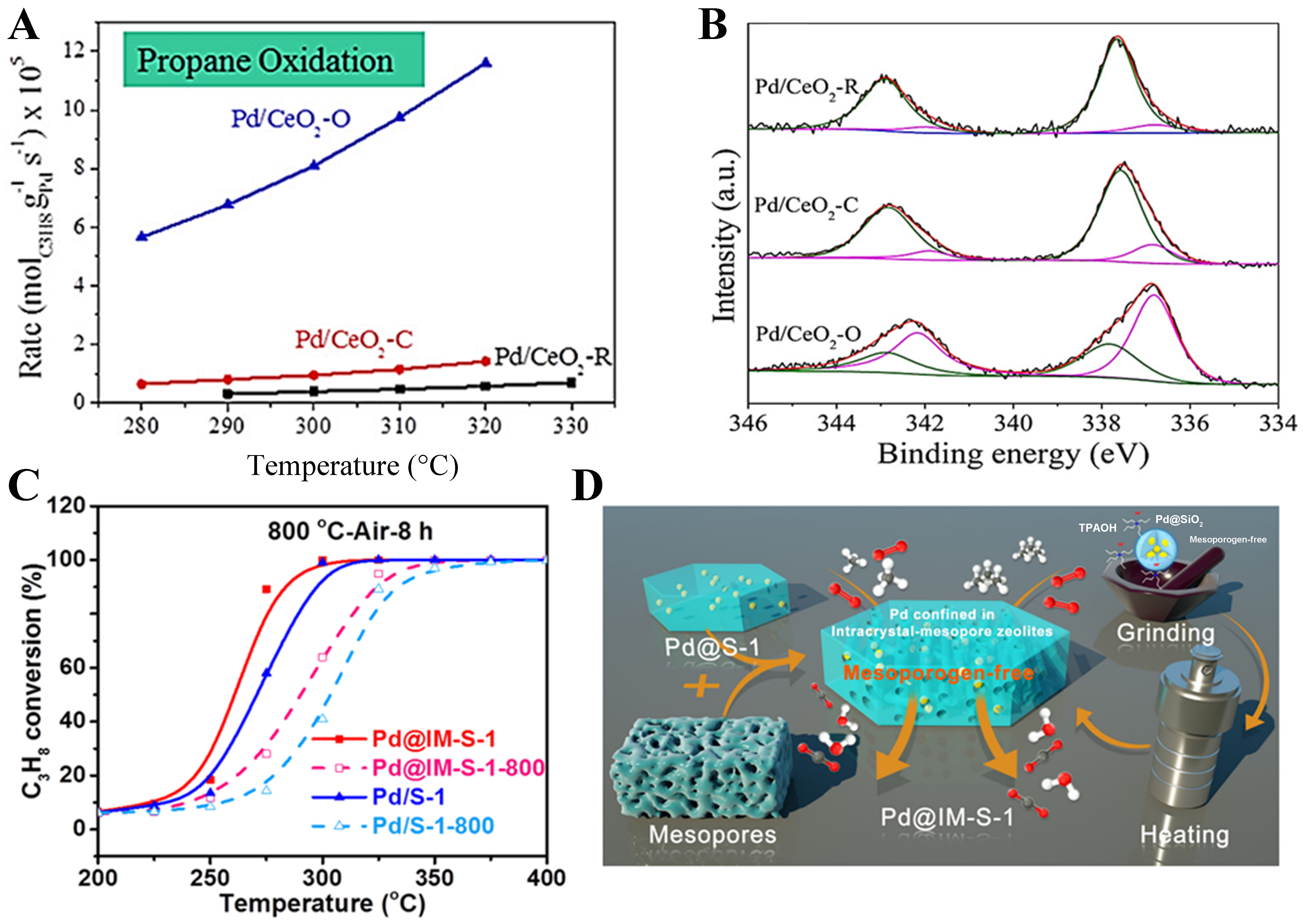
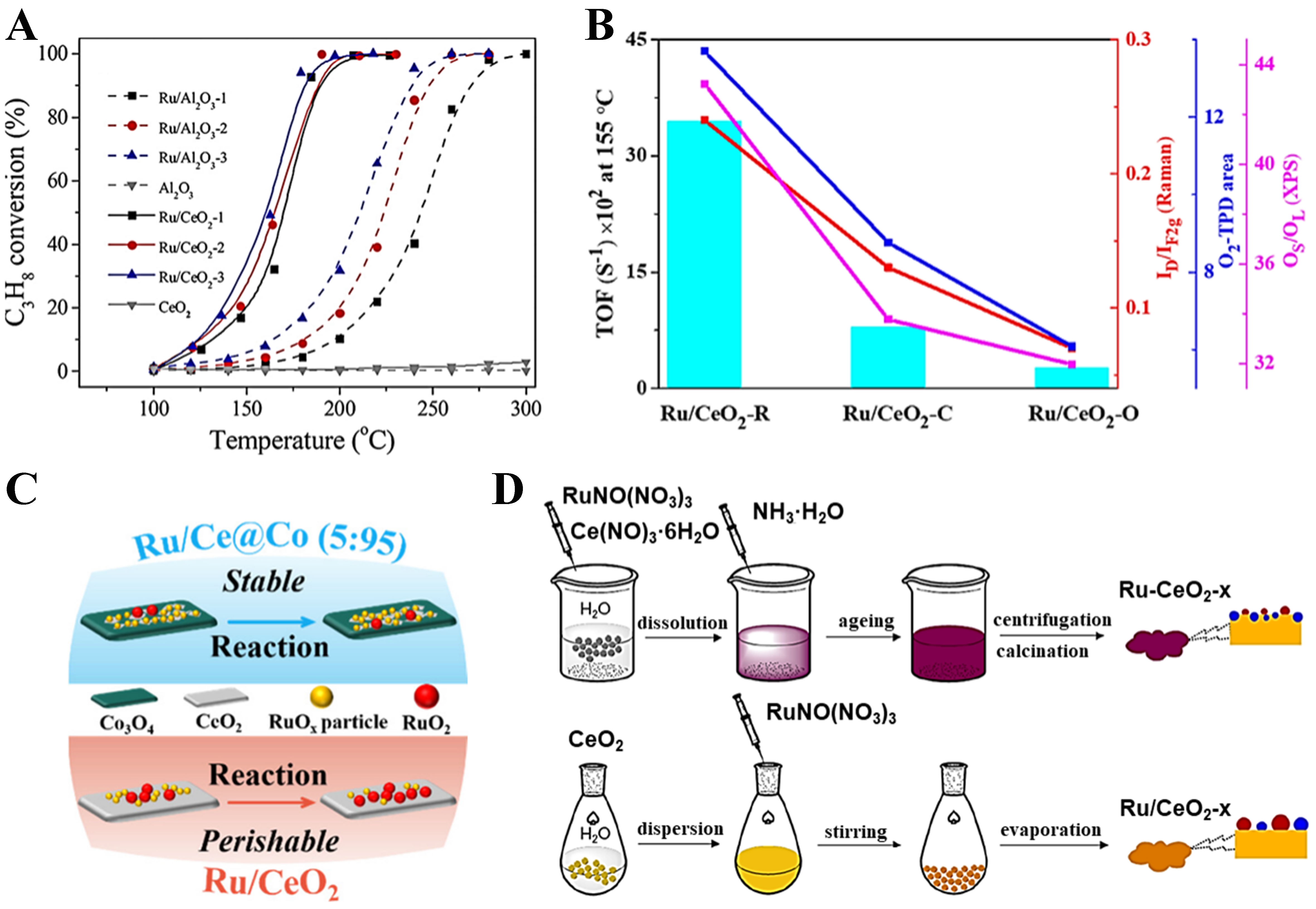
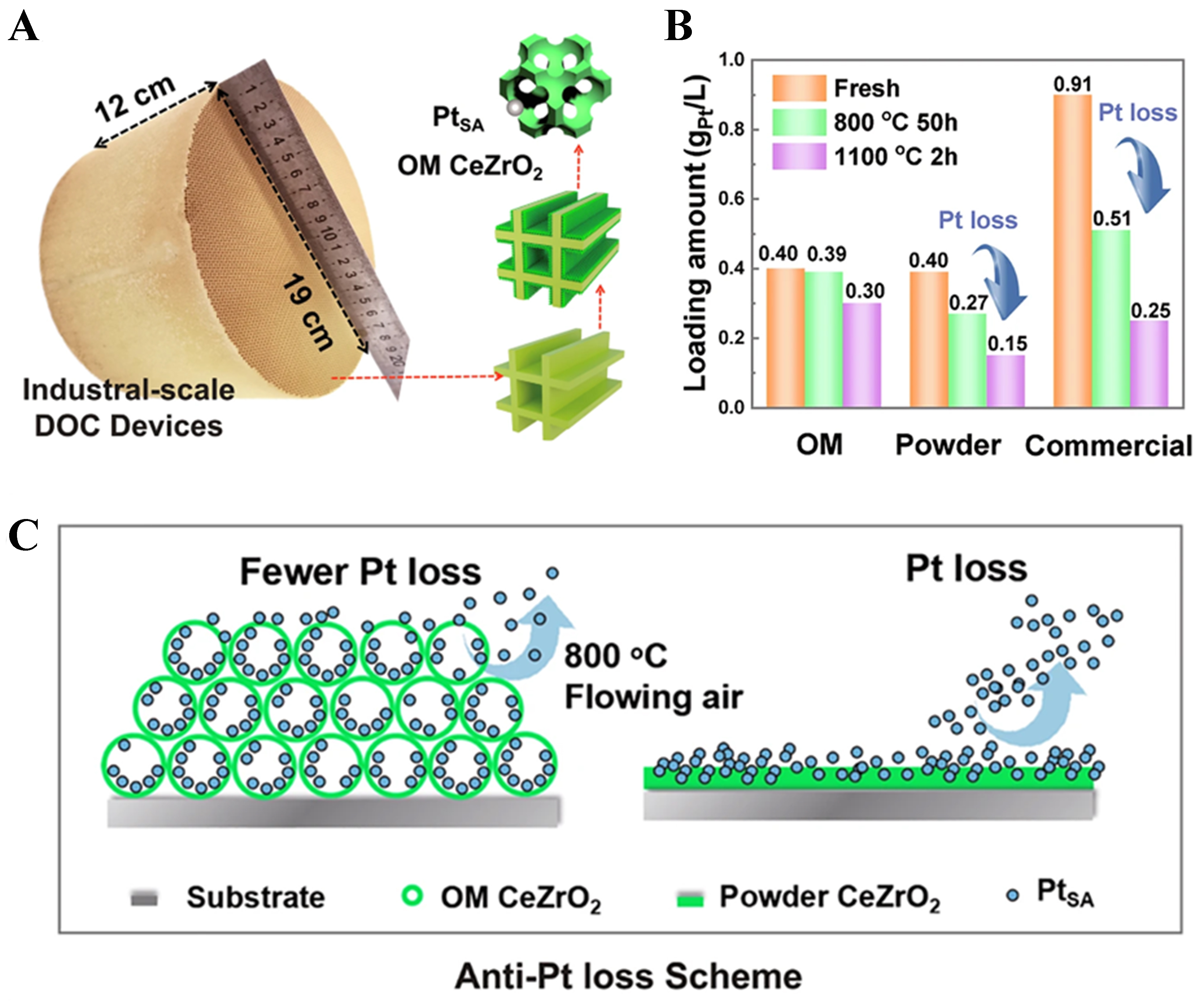
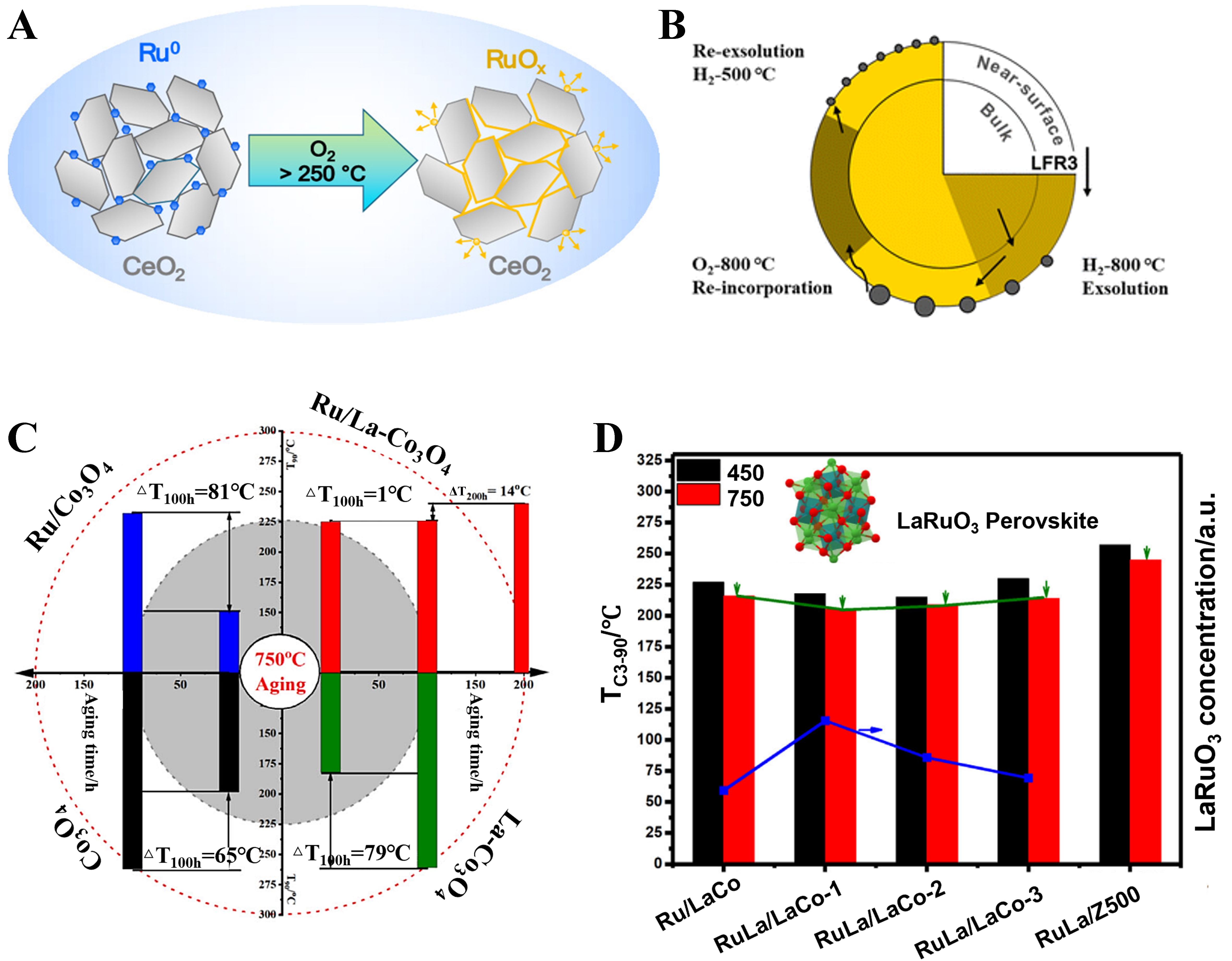
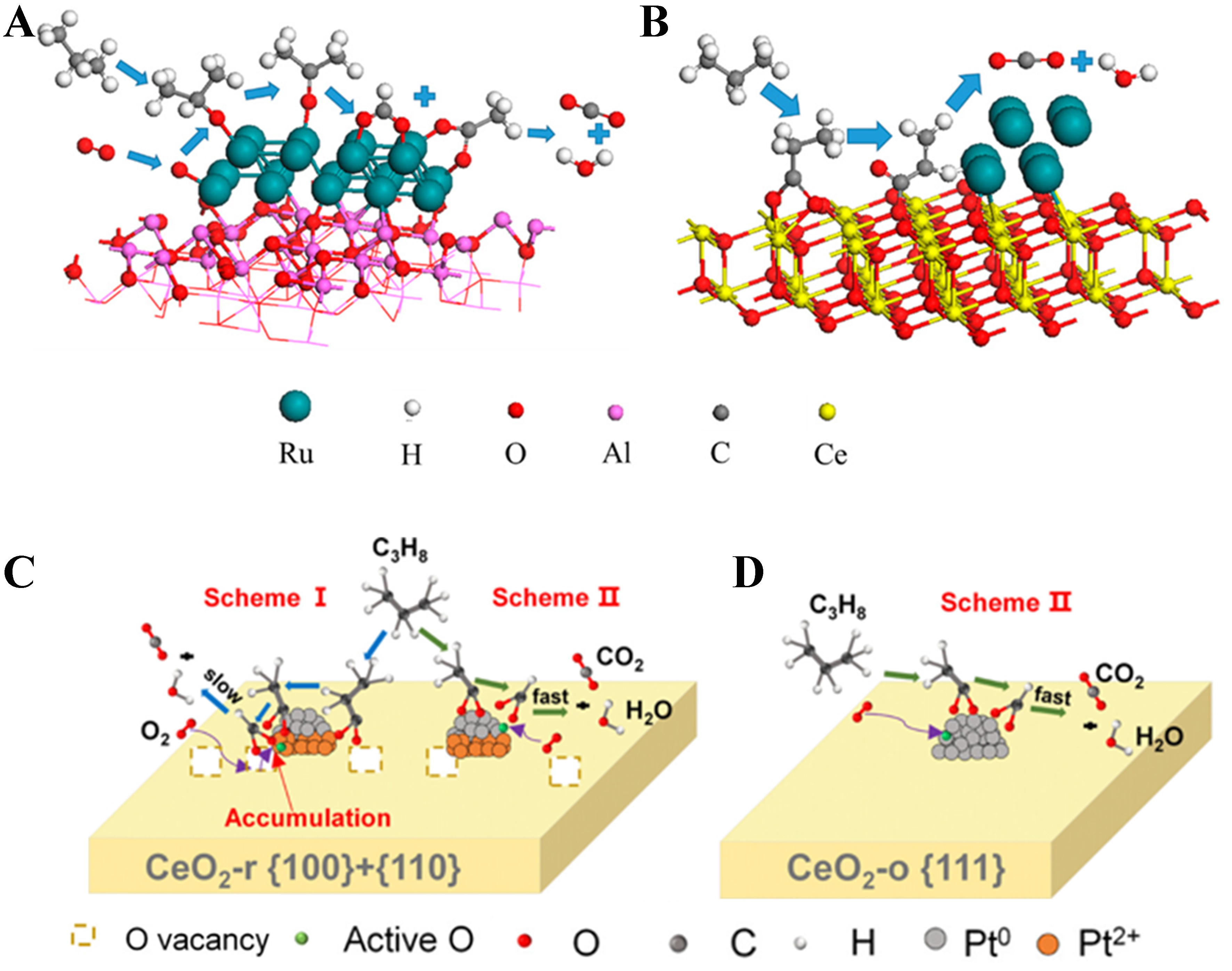












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].