Engineering architectures of mesoporous polydopamine and derived carbon: from soft template synthesis to energy applications
Abstract
The mesoporous polydopamine and its derived carbon (MPDC) exhibit considerable potential for applications in separation, adsorption, sensing, energy storage, catalysis, and biomedicine. The development of flexible synthesis strategies for MPDC and the exploration of precise control of their morphology can further stimulate their potential for application. This paper reviews the advancements made in the synthesis of MPDC utilizing the soft template self-assembly technique over the past decade, with a particular focus on the fine control of its morphology. Furthermore, the potential applications of MPDC in energy-related fields, such as energy storage and electrocatalysis, are discussed. Additionally, the current challenges and future development directions of MPDC are outlined, providing a reference point for researchers in related fields.
Keywords
INTRODUCTION
In light of the mounting concern over global warming, the reduction of carbon dioxide (CO2) emissions has emerged as a pressing challenge[1]. The reduction of CO2 and the development of a low-carbon economy are currently of significant concern to governments and scientists alike[2]. Amidst the escalating demand for energy within the context of a transitioning low-carbon economy, it is of paramount importance to develop new materials with high energy conversion efficiency and excellent storage properties[3]. Since the pioneering discoveries of mesoporous silica in the 1990s[4,5], a diverse array of mesoporous materials had been developed. Among them, mesoporous carbon materials, which possess a high specific surface area and rich pore structure, coupled with their high electrical conductivity and excellent chemical/thermal stability, are widely utilized as electrode materials in advanced energy conversion and storage devices[6,7]. For instance, their substantial surface area facilitates abundant exposure of active sites at the interface, enabling efficient electrochemical reactions in areas such as catalysis and energy storage. The large pore volume can provide a certain buffer for the expansion and strain relaxation of the active material during the repetitive electrochemical energy storage process. The inherent high electrical conductivity of the carbon materials can accelerate the transfer of electrons. Furthermore, the thin pore walls and short channels can shorten the ion transfer path, which is highly advantageous for electrochemical energy applications[8,9].
Despite the aforementioned advantages, refining the mesoporous carbon materials for advanced energy applications still necessitates the fine-tuning of the pore size/volume, topological morphology, and surface properties of mesoporous carbon in order to maximize its application potential[10]. In 1999, Ryoo et al. pioneered the successful fabrication of mesoporous carbon materials through hard templating or nanocasting method, which employed mesoporous silica as a hard template for reverse replication[11]. Subsequently, Meng et al. groundbreakingly obtained mesoporous phenol-formaldehyde resin-based polymers and their derived carbon materials via an organic-organic soft templating self-assembly method[12]. Currently, reported mesoporous carbon materials are commonly prepared through the carbonization process of mesoporous polymers, often synthesized using either hard template or soft template self-assembly routes[6,13]. Additionally, template-free and non-solution-based synthesis methods have also been reported [Table 1]. The hard template method typically involves four steps: (1) preparation of the template; (2) infiltration of the precursor into the template pores; (3) conversion of the precursor into the product; and (4) removal of the template to yield the mesoporous product. However, the ultimate morphology and mesoporous structure are inherently constrained by the type of hard template used, and the intricate processes, including arduous operations and rigorous template removal steps, hinder the widespread adoption of this approach. In comparison to the hard template nanocasting method, the soft template method based on organic-organic self-assembly of polymer precursors and surfactant templates circumvents the complex and time-consuming synthesis process and high cost, while offering greater flexibility in regulating the pore structure and topological morphology of mesoporous carbon[14]. This provides a powerful route for the construction and regulation of the fine structure of mesoporous carbon materials. The preparation of mesoporous carbon materials typically involves the carbonization process of mesoporous polymers, which are formed by the synergistic assembly of organic precursors with soft template molecules[15]. However, the limited availability of organic precursors, the complex polymerization process, and the high reaction rate have hindered the advancement of research in this field, particularly in comparison to the extensive development of mesoporous inorganics, such as zeolites, silica, metals, and metal oxides[16]. For a long time, the precursors of mesoporous carbon were concentrated in phenolic resin-based polymers, which limited the widespread application of mesoporous carbon materials in the energy field[17].
Diverse techniques for synthesizing mesoporous materials
| Method | Morphology control | Pore size distribution | Surface area | Advantages | Limitations |
| Template-free methods | Limited | Difficult to control | Varies | Simple process, no additional templates required | Limited control over pore structure and morphology |
| Soft template self-assembly | Moderate to good | Tunable | High | Control over pore size and morphology, flexibility in design | May require complex synthesis conditions and optimization, incomplete template removal, costly |
| Hard template method | Excellent | Well-defined | High | Precise control over pore size and morphology, reproducibility, versatility in terms of the types of materials | Complex and time-consuming process, requires template removal, costly |
| Non-solution based method | Limited | Varies | Varies | Unique morphologies, direct synthesis or scalable production | Process-dependent, limited control |
Polydopamine (PDA), a biologically sourced polymer based on catechol, is renowned for its non-toxic nature, widespread availability, offering significant advantages as a sustainable carbon source[18]. Moreover, PDA exhibits a high carbon yield upon carbonization and, due to its abundant nitrogen content, yields nitrogen (N)-doped carbon materials[19]. These N-doped carbon materials possess superior conductivity, and enhance the electrochemical properties of carbon materials through the introduction of nitrogen atoms. PDA is typically synthesized through the polymerization of dopamine (DA) molecules, whose monomers contain diverse hydrophilic functional groups, conferring several advantages: (1) it is soluble in water and various polar organic solvents, allowing DA-based carbon materials to exhibit greater flexibility and adaptability during preparation, easily accommodating diverse chemical and physical environments; (2) it can interact with substrates through various forces, including hydrogen bonds, covalent bonds, coordination bonds, and other non-covalent interactions, enabling surface modification[20]. This surface modification further enhances the properties of mesoporous carbon materials, such as improving their adsorption capacity for specific substances and catalytic activity; (3) The catechol-based functional groups can form stable coordination bonds with metal ions, resulting in the production of hybrid materials uniformly doped with metal elements after carbonization, leading to the formation of metal-doped hybrid materials with unique properties and mechanical stability that may be tailored for specific applications, such as catalysis or energy storage[21]; (4) Its amino and catechol groups can form hydrogen bond interactions with the hydrophilic domains of amphipathic templates, binding them into composite micelles. By adjusting the reaction solution to a weakly alkaline pH, DA spontaneously polymerizes around these micelles to form PDA/soft template supramolecular assemblies. After removing the soft template, PDA with a mesoporous structure is obtained, which can be further carbonized to yield its derived mesoporous carbon material. Since its initial description in 2015[22], the solution-based soft template self-assembly method has enabled the bottom-up and scalable structural modulation of mesoporous PDA (mPDA) and its derived carbon (MPDC) at the nano/molecular scale. This provides opportunities for the development of advanced energy devices.
The advantages of large pore volume, easy modification, high photothermal conversion efficiency, and good biocompatibility have led to the publication of reviews of mPDA nanospheres/nanoparticles prepared based on the soft template method for direct use in biomedical applications[23-25]. Furthermore, although there are already some reviews on mesoporous carbons and their applications in the energy sector[26-29], our review stands out by providing a dedicated and comprehensive overview of the engineering architectures of mPDA and its derived carbon materials, from their soft template synthesis to their diverse energy applications. By doing so, we aim to fill a gap in previous reviews and offer valuable insights for researchers working in this rapidly evolving field. To be specific, this review examines the effects of various preparation strategies and synthesis conditions on the morphology of MPDC, with the objective of achieving controllable tuning of morphologies, including nanospheres/nanoparticles, nanotubes/nanorods/nanowires, nanosheets/nanofilms, and other complex morphologies. Furthermore, the potential applications of electrochemical energy, including batteries, supercapacitors, and electrocatalysis, will also be discussed. The advantages of mesoporous structures and topological morphologies in improving energy conversion efficiency and storage performance will be explored. Finally, the remaining challenges and potential research directions of MPDC will be summarized in order to provide constructive references and insights for future research.
MORPHOLOGICAL CONTROL OF MPDC
The morphological control is of critical importance with regard to their electrochemical performance and potential applications in the energy field[30-32]. By finely regulating the topological morphology, it is possible to modify the pore size distribution, specific surface area, mass transfer efficiency, and processability of MPDC, thereby enhancing their electrochemical activity and energy storage efficiency[33]. Consequently, a comprehensive investigation of the morphological control techniques employed in MPDC materials is crucial for the advancement of their utilization in the energy field. Due to the strong adhesion and interaction of DA, the precursors are able to co-assemble with spatially confined mono-micelles, or form a homogeneous coverage on the surface of microscopic templates or even on macroscopic solid-liquid interfaces, yielding MPDC with a desirable pore structure and specific morphology[34]. Over the past decade, the remarkable advancements in materials characterization techniques, notably scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy, have revolutionized our ability to precisely manipulate and control the morphology of MPDC materials[35]. Accordingly, various MPDC materials with different topological morphologies, including nanospheres/nanoparticles, nanorods/nanotubes/nanowires, nanosheets/nanofilms, and other complex morphologies, have been prepared by solution-based soft template-directed self-assembly route.
Nanospheres/nanoparticles
DA and templating agent molecules can be linked together by hydrogen bonding interactions to form spherical composite mono-micelles[36]. In a single-phase synthesis system, they tend to nucleate uniformly and assemble homogeneously into symmetrical assemblies, and the structure of the resulting mesoporous nanomaterials is usually nanospheres or nanoparticles[37]. As a first example, Tang et al. used polystyrene-block-poly(ethylene oxide) (PS-b-PEO) micelles as a soft template, where the catechol and N-H groups of the DA form hydrogen bonds with the -OH groups in the poly(ethylene oxide) blocks, driving the migration of precursors to the micelles surface to form larger-sized, spherical composite micelles DA/PS-b-PEO[22]. After the addition of ammonia solution, the DA spontaneously polymerizes under alkaline conditions to form PDA/PS-b-PEO supramolecular assemblies. Further calcination under an N2 atmosphere at 800 oC removes the template of the product, and simultaneously converts PDA into N-doped carbon nanoparticles with uniform internal and external pore size [Figure 1]. By using template molecules with different hydrophobic polystyrene chains, the pore size of the product could be adjusted (5-16 nm), while the particle size could be increased to 378 nm by controlling the reaction time. Furthermore, nanospheres[38], hollow spheres[39,40] or hollow bowl-shaped nanoparticles[41] and other MPDC with different particle sizes and pore structures can also be obtained using similar methods[42-44], which opens up a new way to construct MPDCs with broad applications.
Figure 1. Schematic diagram of mPDA and derived N-doped carbon nanospheres. This figure is quoted with permission from Tang et al.[22]. DA: Dopamine; PS-b-PEO: polystyrene-block-poly(ethylene oxide); NMCS: N-doped mesoporous carbon spheres; mPDA: mesoporous polydopamine.
In addition to high molecular weight PS-b-PEO, commercial amphiphilic surfactants are more readily available and less costly, which is conducive to the expanded manufacture and use of MPDC[45-47]. Guan
Microemulsions represent an optimal platform for the formation of anisotropic mesoporous nanoparticles[55,56]. In addition to homogeneous nucleation and homogeneous assembly into symmetric structures, the introduction of the TMB oil phase also alters the assembly of the composite micelles, yielding nanoparticles with distinctive asymmetric structures[57]. An emulsion-induced interfacial assembly method was developed by Guan et al. to obtain asymmetric bowl-shaped PDA particles with controllable radially oriented mesopores and large mesopores (~11 nm)[58]. The core of this method lies in the sequential assembly and anisotropically oriented growth of F127/TMB/PDA composite micellar seeds at the TMB/water interface along the inner diameter direction of the particles. This yields bowl-shaped mesoporous MPDC particles with radially oriented mesopores. Moreover, by selecting different amphiphilic triblock copolymers (P84, P105, P123, F127) as templates and finely manipulating the micellar structures as well as anisotropic self-assembly behaviors, Peng et al. prepared MPDC hemispheres with jellyfish-like shapes and radially multi-housed mesoscopic structures[59]. Additionally, a series of asymmetric nanostructures were created, including lotus-shaped, eggshell-shaped, mushroom-like, and jellyfish-like structures. The authors propose that the formation of those structures follows an interfacial energy-mediated nucleation and growth mechanism. The work provides a reference for tailoring various asymmetric polymer/carbon particles. Recently, Guo et al. reported a droplet-oriented anisotropic assembly method for the fabrication of semi-football-like asymmetric carbon nanoparticles with tailored hierarchical macro/mesopores[60]. In a pentanol-water emulsion system, F127/PDA composite micelles were assembled at the emulsion nanodroplet interface to form island-like fine-structured PDA seeds. Subsequent addition of TMB initiated the anisotropic growth of macroporous/mesoporous polymer nanoparticles. The morphology and pore structure of the resulting nanoparticles can be modified by adjusting the length of the oil-phase alcohol chain, resulting in the formation of non-porous spheres, hemispheres, and walnut-like particles with open channels. The above novel nanostructures offer new avenues for the bespoke application of MPDC.
Nanofibers/nanotubes/nanorods
One-dimensional (1D) nanomaterials have received considerable attention due to their high aspect ratio and unique properties[61,62]. DA has strong adhesive properties and can form a homogeneous coverage on a variety of surfaces[63], which makes it possible to prepare MPDC in a way that ensures a uniform distribution of precursors on the template and a tight bonding during the process of calcination and activation, preventing detachment or deformation. The introduction of 1D templates into the synthesis system can restrict the growth of precursors or provide support for them. Composite micelles will preferentially nucleate and assemble on the surface of the 1D template, yielding nanofibers, nanotubes, and nanorods with large aspect ratios and unique mesoporous structure[64].
Carbon nanotubes (CNTs) are attractive 1D carbon materials with low weight and excellent electrical, mechanical, and chemical properties[65,66]. The construction of mesoporous layers on their surfaces can increase their specific surface area and change their surface properties. Zhu et al. developed a molecular-mediated interface-directed co-assembly strategy using hydroxyl-functionalized CNTs as 1D templates[67]. By regulating the co-assembly of F127-DA-TMB composite micelles on the CNTs surface, a core-shell structure of CNTs@mPDA with a uniformly coated mPDA layer (thickness of ~35 nm) was achieved, which retained a uniform nanofiber morphology with an outer diameter of ~100 nm. The application of high-temperature treatment under an inert atmosphere yielded CNTs@mesoNC nanofibers with an ultrathin shell thickness of ~28 nm, and vertical mesopores in the carbon shell layer of ~6.9 nm. Furthermore, this group introduced metal ion precursors into the reaction process, utilizing the inherent strong chelating effect of DA on metal ions[68]. The strong coordination can strengthen the cross-linked structure, subsequent calcination under an inert atmosphere is employed to remove the template, resulting in the direct transformation of the product into metal-nitrogen-doped carbon. As shown in Figure 2, the product maintains the 1D morphology and mesoporous structure with even-distributed metal nanoparticles (size of ~5.3 nm). The above one-pot fabrication process ensures sufficient contact between metal ions and binding sites by introducing metal ions during the assembly process, and the 1D morphology and mesoporous structure effectively hinder the clustering of metal atoms during the thermal pyrolysis process. This versatile process is compatible with a diverse array of metal precursors, including Fe, Cu, Ni, and Pd.
Figure 2. (A and B) SEM images of CNTs@mesoC@CuPd nanostructures at different magnifications; (C) Histogram of statistical distribution of CuPd nanoparticle size; (D) Synthesis scheme of CNTs@mesoC@CuPd. This figure is quoted with permission from Xu et al.[68]. CNTs: Carbon nanotubes; mesoPDA: mesoporous polydopamine; mesoC: mesoporous carbon; SEM: scanning electron microscopy.
In addition to CNTs, Jiang et al. used P123 as a templating agent to grow an mPDA layer on the surface of MnO2 nanowires, and the thickness of the coating could be tailored by adjusting either the concentration of DA or the duration of the polymerization process[69]. The subsequent carbonization under an inert atmosphere yielded 1D MnO2-based hybrid nanowires with a mesoporous carbon layer, endowed with high specific surface area and excellent electrical conductivity. More than obtaining the above conventional mesoporous structures, Chen et al. used the irregular bicontinuous F127/P123-PDA mesostructure as a unit, which was assembled in a loosely packed manner at the interface of the MnOx nanowires, to hierarchically grow the MPDC shells with pore channels ranging from ~10 to 200 nm[70]. Jiang et al. used silver-vanadium oxide (Ag-V2O5) nanowires (diameter of ~13 nm) as a 1D template and P123 as a pore-forming agent to uniformly cover the nanowire surface with a PDA layer to obtain Ag-V2O5/PDA core/shell nanowires[71]. Further high-temperature annealing removed the V2O5 (melting point of 690 oC) template and carbonization to obtain Ag-CNTs with a hollow mesoporous structure. In addition, Han et al. also obtained nanorods with unique core-shell architectures by regulating the rapid adsorption and polymerization of F127/TMB/H2O/ethanol tetrameric microemulsion on the surface of Au nanorods[72]. The above studies not only underscore a promising avenue for the development of high-performance 1D mesoporous MPDC materials, but also can be extended to construct mesoporous core-shell materials with various 1D morphologies.
Nanosheets/nanofilms
Two-dimensional (2D) materials have recently emerged as a subject of considerable interest in a number of fields, including chemistry, materials science, nanotechnology, and energy[73,74]. This is due to their fascinating optical, electrical, and magnetic properties, which result from their atomic-level thickness[75]. The construction of mesoporous heterogeneous functional materials on the surface of 2D nanosheets [e.g., graphene oxide (GO), molybdenum disulfide, etc.] can simultaneously combine the advantages of 2D, mesoporous, and functional materials, such as ultrathin thickness, periodic ordered mesoscopic pores, high surface area, and multifunctionality[76,77]. This offers a novel avenue for the advancement of high-performance energy storage and catalyst materials. The majority of 2D mesoporous materials are currently prepared by self-assembly of composite micelles on 2D substrate materials[78]. Subsequent in situ polymerization of precursors results in the formation of a sandwich-structured MPDC[79].
The use of F127 as a soft template has been demonstrated by Guan et al. to result in the formation of composite micelles that are heterogeneously assembled on the surface of GO to form a tightly arranged composite[80]. The composite micelles were then polymerized and cross-linked to form a solid colloidal shell, which grew on the GO surface, and formed an mPDA layer. Subsequently, the products were subjected to heat treatment and carbonization in a nitrogen atmosphere, resulting in the formation of a mesoporous N-doped carbon wrapped with GO. Furthermore, Peng et al. introduced cysteine as a source of N and S dopants in the oil-water emulsion system previously described[81]. Cysteine binds to the hydroxyl group of DA via the amino or carboxyl domains, and mercaptan group can be adsorbed as a bidentate ligand. Moreover, the functional groups present in cysteine, DA, and GO can form hydrogen bonds with O. Freeze-drying and high-temperature carbonization in a nitrogen atmosphere can result in the formation of N, S, and O-doped carbon-based aerogel. The aerogel comprises a network of nanosheets with uniformly dispersed mesopores on the surface, which can be utilized as self-supporting electrodes directly.
The aforementioned hemi-spherically porous polymer layer, when employed as an electrode material, is not effective in avoiding channel blockage caused by nanosheet stacking. Tian et al. employed a strategy of interfacial self-assembly engineering to grow mPDA with columnar mesopores on reduced GO (rGO) nanosheets using cylindrical micelles formed by P123[82]. Hydrogen bonding interactions drove the tight alignment of the cylindrical micelles onto the GO surface, which was followed by the aggregation of monomers at the poly(ethylene oxide) domains and the polymerization of the wrapped micelles to form a polymer network. Upon removal of the P123 template and application of hydrothermal reduction, hybrid structures comprising cylindrical mesoporous polymer monolayers on the rGO surface were yielded. Furthermore, a similar approach was employed for the growth of cylindrical mPDAs on MXenes[83]. As shown in Figure 3, these hybrids exhibited an average mesopore diameter of 8 nm and a remarkable specific surface area reaching as high as 89 m2·g-1.
Figure 3. (A and B) SEM images, (C) TEM image, (D) schematic diagram of mPDA/MXene heterogeneous nanosheets. This figure is quoted with permission from Li et al.[83]. DA: dopamine; PDA: polydopamine; SEM: scanning electron microscopy; TEM: transmission electron microscopy; mPDA: mesoporous polydopamine.
In addition to the GO and MXene, a variety of sandwich-structured 2D mesoporous hybrids have been prepared by similar micro-interfacial self-assembly methods. In a related study, Lan et al. employed mesoporous TiO2 nanosheets as a platform, upon which they grew F127/DA composite micellar monolayers[84]. Subsequent annealing in nitrogen yielded heterostructured nanosheets of double mesoporous hybrid C@TiO2@C, comprising a monolayer of mesoporous TiO2 and a vertical mesoporous carbon monolayer encapsulated on both sides. Moreover, Qiu et al. coated an mPDA layer on a 2D WS2 through interfacial molecular interactions using PS-b-PEO as a pore-forming agent[85]. Due to the strong chelating effect of catechol groups, a series of monometallic, bimetallic, and even trimetallic metals could be successfully dispersed in the polymer framework. This was followed by conversion into N-doped carbon with a uniform distribution of metal nanoparticles simultaneously after annealing under inert atmosphere. Wen et al. employed readily accessible cobalt-based metal-organic framework (MOF) (Co-MOF) nanosheets as 2D templates, and realized the growth of mPDA layers on nanosheets via bottom-up micelle assembly[86]. Furthermore, the growth of mPDA layers on leaf-shaped Zn, Co-ZIF nanosheets was achieved by Li et al. using a similar approach[87].
The integration of a macroscopic functional planar substrate within the solution system allows for the easy modification, high specific surface area, and multifunctional properties of mPDA to be transferred onto the functional substrate in one step. Accordingly, large-area MPDC ultrathin films that can be firmly loaded onto the surface of the functional planar substrate can be obtained. Wang et al. accomplished the precise fabrication of vertically aligned MPDC films spanning the centimeter scale by introducing a macroscopic planar substrate into the system[88]. TMB not only acts as a swelling agent to regulate the pore size, but also mediates the directional fusion of micelles into columnar micelles and their subsequent vertical assembly at the solid-liquid interface. The films exhibit an ordered vertical mesoporous structure with a tunable mesopore size ranging from 8.4 to 13.5 nm. Notably, the method can be extended to grow monolayer or multilayer ordered mPDA films on a variety of substrates. On this basis, through the interfacial self-assembly, our group[89] constructed mPDA nanofilms with uniform thickness (30 nm) and a dense and uniform distribution of mesopores (average pore size of 16 nm) on different types of planar substrate surfaces [silicon wafers, glass plates, zinc foils, poly(ethylene terephthalate) sheets, and flexible poly(dimethylsiloxane) membranes]. This robust and adaptable approach for synthesizing mPDA under mild and non-toxic conditions, irrespective of substrate surface properties, configurations, and materials, is anticipated to facilitate the development of novel applications.
Other complex morphologies
In addition to the aforementioned low-dimensional mesoporous structures, three-dimensional (3D) mesoporous nanomaterials with complex structures have been widely reported[53]. Following the introduction of the 3D interface, the nearby composite micelles migrate to the 3D solid-liquid interface and undergo random nucleation. The composite micelles in solution exhibit a tendency to occupy the empty spaces on the solid surface, thereby minimizing the overall surface energy[90]. This process ultimately results in the formation of a monolayer composite micellar layer. The polymer with mesoscopic structure is then formed through polymerization at the solid interface, and 3D MPDC nanoparticles are obtained in the final stage. To date, researchers have successfully obtained a range of functional materials with varying compositions, mesoporous structures and morphologies, including core-shell structures, asymmetric mesoporous nano-heterogeneous materials and so forth, through the formation of heterogeneous assemblies on various 3D polymers or inorganic particles at the solid-liquid interface.
As with the synthesis of 1D or 2D mesoporous materials, various 3D matrices (e.g., inorganic particles, polymers, etc.) can also be employed to construct hierarchical porous materials. Furthermore, Guan et al. achieved the polymerization of mPDA layers onto diverse functional particles with different compositions, geometries, and sizes[80]. These included silica spheres, Fe2O3 quasi-cubes, Fe2O3 ellipsoids, polymer spheres, and MOF nanocrystals. Zhao et al. employed colloidal silica nanospheres with a uniform size of ~300 nm as a hard substrate and polystyrene-block-poly(4-vinylpyrrole)-block-poly(ethylene oxide) (PS-PVP-PEO) micelles as a soft template[91]. These were guided by a mono-micelle interfacial confinement assembly strategy to the polymerization of DA into 3D hierarchical mesoporous polymer superstructures. This methodology facilitated the accurate fabrication of mesoporous superparticles, showcasing adaptable structures such as 3D nanospheres, nanovesicles, and 2D nanosheets. Additionally, similar techniques have been utilized by other scientists to construct MPDC layers on various surfaces, including CdS nanorods,
Janus nanoparticles have attracted great attention for their unique structure and surface chemistry in many fields[92,93]. By modulating the interfacial assembly behavior and growth of composite micelles, asymmetric mesoporous nanohybrid materials can be prepared. Using surface-charge-mediated selective encapsulation of DA on the surface of magnetic Fe3O4, Zhao et al. prepared Fe3O4@mC&mSiO2 with core-shell structured hydrophobic mesoporous carbon nanorods at one end and Janus nanoparticles with 1D mesoporous silica at the other end[94]. The mesoporous SiO2 nanorods were ~100 nm in diameter and adjustable in length from 50 to 400 nm with highly ordered mesopore (pore size of ~2.7 nm), whereas they were connected by core-shell-structured Fe3O4@mC nanorods with a particle size of ~150 nm and radial mesopores of ~10 nm. Further, by introducing oil droplets interacting with mesoporous silica nanoparticles (MSNs) to form a bispherical structure, followed by manipulation of mPDA to encapsulate selectively on the oil droplets, Zhao et al. proposed an emulsion-directed self-assembly strategy, and homogeneous Janus bispherical MSN & mPDA nanoparticles with large mesopores were obtained[95]. As shown in Figure 4, the Janus nanoparticles consist of spherical MSNs, and mPDA hemispheres. In their other work[96], they successfully synthesized Fe3O4 and mPDA Janus nanoparticles featuring a unique badminton-shaped morphology and asymmetric mass distribution based on spatially controlled anisotropic assembly of layered mPDA onto magnetite Fe3O4 nanoparticles. The resulting badminton-like nanoparticles comprise a dense Fe3O4 “head” and a less dense mPDA polymer. The effects of the unique morphology and asymmetric mass distribution of the nanoparticles on their hydrodynamic properties were also computationally modeled. Subsequently, they formed Fe3O4@DMS with core@shell structure by encapsulating dendritic mesoporous silica (DMS) in Fe3O4 nanoparticles and further formed Fe3O4@DMS and PDA Janus nanostructures with a similar emulsion-directed assembly pathway[97]. These above functionalized Janus nanoparticles with tunable structural properties and surface properties, possess great potential for customized applications.
Figure 4. (A) Synthesis diagram; (B and D) TEM images with different magnifications; (C) SEM images; (E) dark-field TEM images and corresponding element maps; and (F) pore size distribution diagram of Janus MSN & mPDA nanoparticles; High-resolution TEM images of (G) MSN, and (H) mPDA. Scale bar: (B) 50 nm, (C) 100 nm, (D) 200 nm, (E, G, H) 20 nm. This figure is quoted with permission from Zhao et al.[95]. MSN: Mesoporous silica nanoparticle; PDA: polydopamine; TMB: 1,3,5-trimethylbenzene; CTAB: cetyltrimethylammonium bromide; TEM: transmission electron microscopy; SEM: scanning electron microscopy; mPDA: mesoporous polydopamine.
Building upon a similar selective anisotropic nucleation strategy, Liu et al. extended their research by devising a region-specific occupation self-assembly technique, enabling the targeted and anisotropic growth of amorphous mPDA at designated surface locations on MOF (ZIF-8) nanoparticles[98]. The controlled aggregation epitaxial growth of DA-F127 composite micellar subunits on the <100>, <110> surfaces of crystalline ZIF-8 was achieved through different crystal surface exposure ratios of truncated rhombic dodecahedral ZIF-8 with different zinc ion coordination environments. The adjustment of the ratio between TMB and DA monomers provides a means to manipulate the pore dimensions and thickness of the mesoporous polymer layer. Moreover, Chen et al. achieved anisotropic functionalization of mPDA layers on the microporous zeolite (S-1) surface, thereby obtaining hierarchical porous heterostructures with unique physical properties and performance[99]. The selective deposition and subsequent growth of mPDA on distinct facets of S-1 nanocrystals were achieved by strategically manipulating the specific packing density arrangement of F127/P123 mesoscale assembly elements across varied surface areas (including curved/edges surfaces, and flat planes) of the anisotropic zeolite nanocrystal S-1 polyhedra. By modifying the macroscopic shape of S-1 nuclei and fine-tuning the mass proportion of soft templates P123 to F127 within the reaction environment, researchers have demonstrated precise control over nucleation energy barriers. This advancement paves the path for the deliberate design of hierarchical porous heterostructures, endowed with tailored physical and chemical attributes, thereby broadening their applicability across various fields.
MPDC IN ENERGY APPLICATIONS
Due to the structural advantages and the possibility of realizing customizable designs, various forms of MPDC exhibit immense promise for use in the construction of electrochemical energy devices characterized by exceptional performance[47,49]. Its unique mesoporous structure endows MPDC with extremely high porosity and specific surface area, which not only promotes the rapid diffusion of ions and reactants but also enhances the interfacial interactions of the material, significantly improving the charge-discharge rate and energy density of energy storage devices. Furthermore, MPDC forms a highly conductive carbon skeleton during carbonization, ensuring efficient electron transfer within the material, which is crucial for accelerating electrocatalytic reaction rates and efficiencies. Moreover, MPDC exhibits excellent chemical stability and tunable surface chemistry. This stability allows MPDC to maintain stable performance in various harsh operating environments, extending the lifespan of energy storage and electrocatalytic devices. The tunable surface chemistry, on the other hand, offers the possibility for further functionalization of MPDC. By introducing different functional groups or catalysts, the properties of MPDC can be precisely tuned to meet the demands of diverse application scenarios. Compared to other materials widely used in energy storage and electrocatalysis, MPDC combines the high conductivity of traditional carbon materials with the rich porous structure of porous materials, while overcoming their shortcomings including chemical stability and surface chemistry tunability[14]. This comprehensive advantage of MPDC has led to its broad application prospects in fields such as supercapacitors, lithium-ion batteries (LIBs), fuel cells, and electrocatalytic water splitting. In order to fully explore the intrinsic structure-electrochemical performance relationship, it is necessary to summarize the recent progress of MPDC in energy-related applications, mainly including various types of rechargeable batteries, supercapacitors, and electrocatalytic reactions [Table 2].
The applications of MPDC
| Categories | Subcategories | Sample | Morphology | Electrochemical performance | Others | Ref. |
| Rechargeable battery | LIBs | Mesoporous carbon nanobowl | Nanoparticle | 300 mA·h·g-1 after 1,000 cycles | [100] | |
| Conductive porous Ag-CNTs | Nanotube | 1,637 mAh·g-1 at 100 mA·g-1 for 400 discharge/charge cycles | [71] | |||
| mPDA@rGO | Nanosheet | 151 mA·h·g-1 at 50 mA·g-1, 89% capacity retention after 1,000 charging/discharging cycles | Organic cathodes | [101] | ||
| mPDA spheres | Nanosphere | Cycling stability and rate performance are improved compared to the commercial Celgard 2400 separator | Separator | [102] | ||
| SIBs | Gradient porous MCSs | Nanoparticle | capacity of 392 mAh·g-1 at 0.1 A·g-1,up to 2,000 cycles stability (capacity of 183 mAh·g-1 maintained at 4.0 A·g-1) | [50] | ||
| Jellyfish-like mPDA-derived carbon | Nanoparticle | 124 mAh·g-1 at 5.0 A·g-1 after 1,000 cycles, 59 mAh·g-1 at 2.0 A·g-1 | [78] | |||
| Hollow porous carbon spheres | Nanosphere | ~101 mAh·g-1 at 20 A·g-1, ~120 mAh·g-1 after 5,000 cycles at 5 A·g-1) | [103] | |||
| MoC@MCNs | Nanosphere | Slope-dominated rate capability (125 mAh·g-1 at 50 A·g-1) | [104] | |||
| KIBs | MCNs | Nanoparticle | 134 mAh·g-1 at 5 A·g-1; 112 mAh·g-1 at 2 A·g-1 after 500 cycles | [81] | ||
| Li-S batteries | N-doped mesoporous carbon on GO | Nanosheet | Initial discharge capacity of 1,535.9 mAh·g-1), ~990 mAh·g-1 after 100 cycles at 0.2 A·g-1, more than 250 cycles of stability (2 A·g-1) | [105] | ||
| Zinc ion batteries | mPDA@MXene | Nanosheet | Cycle life of more than 1,000 h, capacity of 368 mAh·g-1 | Protective layer on Zn foil | [106] | |
| Capacitors | Supercapacitor | N-doped mesoporous carbon nanospheres | Nanosphere | 350 F·g-1 at 0.1 A·g-1 | [38] | |
| Hollow particles with a bowl-shaped N-doped carbon | Nanoparticle | 385 F·g-1 at 0.1 A·g-1, 410 F·cm-3 at 0.1 A·g-1 | [41] | |||
| Semi-football-shaped mesoporous carbon | Nanoparticle | 215 F·g-1 at 0.05 A·g-1, 143 F·g-1 at 20 A·g-1, energy density of 53.4 Wh·kg-1 | [60] | |||
| Ionic capacitors | mPDA/rGO heterostructure | Nanosheet | 133 mAh·g-1, 94 mAh·g-1 at 5 A·g-1 | Cathode | [107] | |
| N-doped carbon nanosheets (mNC/rGO) | Nanosheet | 550 mAh·g-1at 0.1 A·g-1, 225 mAh·g-1 at 5 A·g-1 | Anode | [107] | ||
| Electrocatalysis | HER | Single-atom Pt on the surface of the mesoporous carbon matrix | Nanosphere | Onset potential of ~0 V | [108] | |
| Mesoporous N-doped carbon nanorods | Nanorod | Overpotential of 270 mV at 10 mA·cm-2 | [109] | |||
| ORR | Mesoporous carbon nanospheres co-doped with atomic Fe and N | Nanosphere | Half-wave potential of 0.846 V | [110] | ||
| Cobalt embedded in N-doped carbon | Nanosphere | Half-wave potential of 0.93 V | [111] | |||
| 2D rGO-based heterogeneous nanosheets | Nanosheet | ORR half-wave potentials of ~0.82 V, high methanol concentration tolerance and stability | [85] | |||
| N-doped mesoporous defective carbon | Nanosheet | Onset potential of 0.90 V; limiting density current of 5.50 mA·cm-2 | [79] | |||
| Co@EMPC | 3D | Half-wave potential of 0.874 V and high stability | [112] | |||
| NO3-RR | CNTs@mesoC@CuPd | Nanotube | conversion of 100%, N2 selectivity (98%), cycling stability (> 30 days) | [68] | ||
| Co/Cu/Cu2O-MesoC | 3D | Faraday efficiency: 100% ± 1% at -0.25 V, maximum yield of NH3 was as high as 6.416 ± 0.78 mmol·mg·cat-1·h-1 at -0.45 V | [113] |
Rechargeable battery
LIBs
LIBs represent the most prevalent type of energy storage device currently available on the market, which are employed in a multitude of applications, including electric vehicles, portable electronics, and numerous others[114,115]. The mesoporous architecture facilitates the efficient transportation of lithium ions and electrons within the electrode by reducing their diffusion path, ultimately leading to an accelerated diffusion rate of the electrolyte and improved electrochemical performance[116]. This has led to the MPDC material garnering significant interest as an anode for LIBs. Qian et al. investigated the impact of MPDC morphology and porosity on lithium storage[100]. Compared with the other two PDA-derived carbon nanospheres with different morphologies and porosities, the bowl-like morphology has been demonstrated to effectively relieve the stress of the charging and discharging process, and to significantly shorten the transport paths of lithium ions and electrons[100]. Consequently, the mesoporous carbon nanobowl electrode exhibits a high reversible capacity of
Eco-friendly organic cathodes derived from abundant and non-toxic sources have garnered significant interest in the realm of environmentally conscious, high-performance LIBs[117]. PDA, which is inherently redox-active, has been demonstrated to be an optimal lithium-ion cathode material, comprising 5,6-indole quinone units that can facilitate the storage of lithium ions through the provision of multiple redox-active carbonyl groups [Figure 5][118]. Wang et al. grew mPDA on conductive rGO nanosheets to obtain a heterogeneous nanosheet with a unique sandwich structure[101]. As an innovative organic cathode material for LIBs, the nanosheets showcase remarkable performance, including a high specific capacity, superior rate capability, and robust cycling stability. These attributes are attributed to the unique 2D porous structure, which enhances exposure of the active sites and fast lithium-ion transport. Subsequently, Li et al. demonstrated that the ordered mPDA/Ti3C2Tx sandwich-like composites also showed excellent electrochemical performance for LIBs[119]. Collectively, these studies highlight the promising potential of nanosheets and sandwich-like composites, featuring 2D porous structures, as innovative organic cathodes for LIBs, exhibiting exceptional capacity, rate performance, and cycling stability, thereby offering new avenues for advancing the development of advanced energy storage systems.
Figure 5. (A) Schematic diagram of pore channels of mPDA/rGO nanosheets and their interaction mechanism with lithium ions; (B) Cyclic voltammetry curves at a scanning rate of 0.5 mV·s-1; (C) charge/discharge curves at a current density of 50 mA·g-1; and (D) rate performance at a current density of 50 to 2,000 mA·g-1 of mPDA/rGO nanosheets and the comparison samples; (E) Stability test diagram of mPDA/rGO-2 for 1,000 cycles at a current density of 2,000 mA·g-1. This figure is quoted with permission from Wang et al.[101]. mPDA: Mesopore polydopamine; rGO: reduced graphene oxide; PDA: polydopamine.
In addition to its direct use as an electrode, the catechol functional group of PDA imparts strong adhesion and moisture resistance, suggesting its use as a separator for LIBs[120]. Zhu et al. composed mPDA spheres and environmentally friendly soybean isolate protein nanofibres into a sandwich-structured composite separator[102]. Highly porous mPDA nanospheres, enriched with polar functional groups, efficiently absorb significant amounts of liquid electrolyte, thereby enhancing electrochemical performance. Consequently, the composite separator manifests superior thermal stability and a strong affinity for the electrolyte. This design effectively mitigates Mn ion dissolution in the cathode, chelates escaping Mn ions, and inhibits the proliferation of Li dendrites, contributing to overall improved battery performance and safety. As a result, the rate performance and cycling stability are significantly improved compared to the commercial Celgard 2400 separator. The aforementioned research offers a fresh perspective, underscoring the immense potential of MPDC in high-performance LIBs.
Sodium-ion batteries
Sodium is more abundant in resources than lithium, and as a result, sodium-ion batteries (SIBs) are considered prime contenders for large-scale electrochemical energy storage systems[121]. The use of mesoporous carbon materials confers a number of advantages, including the facilitation of ion transfer, the mitigation of volume expansion and the exposure of active sites, which are conducive to the rapid storage of sodium ions during high-rate charging/discharging processes[122]. Peng et al. employed programmed shear force-assembled gradient porous MCSs as the electrodes of the SIBs[50]. Due to the structural advantages of the radially oriented 3D open-pore structure and the doping of N atoms to increase the electron delocalization [Figure 6], they provided excellent sodium ion storage capacity and up to 2,000 cycles stability. Similarly, when used as electrode materials in sodium-ion half-cell, the jellyfish-like mPDA-derived carbon provided an excellent cycling performance, and the full-cells assembled based on them exhibit superior rate capacity[78]. Lan et al. used 2D dual mesoporous carbon@TiO2@carbon vertically heterogeneous nanosheets as anodes for SIBs[84]. This composite mesostructure design boasts a vastly accessible surface area, enabling efficient electrolyte penetration, and offers a good buffer space for volumetric strain, and the corresponding SIBs exhibit ultra-high-rate capability and recyclability. Further, Liu et al. utilized a synergistic sequential assembly strategy of “soft” micellar subunit (F127/PDA composites) combined with “hard” rigid particles (colloidal SiO2 nanospheres) to obtain MPDC with highly tailored pore sizes and thicknesses[123]. This hierarchical porous (macropore and mesopore) carbon allows for shorter diffusion lengths and full access to the electrolyte for fast redox reactions, thus exhibiting excellent sodium storage rates and cycling properties, as well as fast pseudocapacitive charge transfer kinetics. This work enables the tailoring of porous carbon structures with different diameters and thicknesses, providing an ideal model system for fast sodium storage.
Figure 6. (A) Charge/discharge curve at a current density of 0.1 A·g-1; and (B and C) rate performance test diagram at a current density of 0.2-10.0 A·g-1 of jellyfish-like mesoporous carbon hemisphere-base sodium ion battery; (D) Charge/discharge curve at a current density of 0.1 A·g-1; (E) cyclic stability test diagram at a current density 0.1 A·g-1; and (F) photo of a practical demonstration for powering an electric fan of jellyfish-like mesoporous carbon hemisphere| |Na3V2(PO4)2F3 full battery. This figure is quoted with permission from Peng et al.[59]. FDU: Fudan University.
High-level heteroatom doping has been shown to be one of the candidate routes for the preparation of high-performance porous sodium carbon battery anodes[124]. Wang et al. achieved the construction of hollow porous carbon spheres with high N (6.05 at%) and P (5.19 at%) co-doping by chemically grafting hexachlorocyclotriphosphononitrile on PDA shells[103]. This composite exhibits exceptional high-rate capability and remarkable long-term cycling stability. Recently, by carbonization of ZIF-8@mPDA, Yu et al. obtained bilayer shell-layered porous N-doped carbon nanocages, and systematically investigated the impact of carbonization temperature on various structural properties, including surface area, pore volume, interlayer spacing, defect density, and nitrogen content, aiming to optimize their electrochemical properties[125]. They found that the products treated at 900 oC exhibited superior performance, attributed to the harmonious interplay of the double-shell, layered porous, N-doped, and hollow structural features. In response to the unavoidable severe skeleton shrinkage of mPDA carbonized at high temperatures and the ion transport hindered by narrow graphite domains, Liu et al. introduced molybdate during the synthesis process[104]. The strong coordination effect inhibited the skeleton shrinkage and graphitization during the calcination process; thus, derived porous carbon (MoC@MCNs) with highly disordered graphite domains and MoC-nanodot modifications was obtained. The incorporation of highly conductive MoC nanodots within disordered carbon matrices enhances electron transfer, mitigating the drawbacks of highly disordered graphite domains. As an anode for SIBs, MoC@MCNs exhibit remarkable rate capability. The full cell, further assembled with Na3V2(PO4)3 cathode, provided a high energy density of 178 Wh·kg-1 at
Other-type batteries
In recent years, potassium-ion batteries (KIBs) have garnered significant interest owing to their low cost, abundant resources, and relatively low potassium reduction potential[126]. The incorporation of nitrogen into carbon materials, coupled with a high specific surface area, has been reported to produce a large number of external defects and enhance electrode/electrolyte interactions to show good electrochemical performance[127]. Multi-shell hollow N-doped carbon nanospheres (MCNs) with unique chiral structures were applied as anode materials for KIBs by Peng et al.[51]. As shown in Figure 7, the helical MCNs exhibited outstanding rate capability, and remarkable cycling stability. This can be attributed to their unique spiral hollow nanostructure shortens the diffusion length of K ions and provides a larger mechanical stress/strain buffer space, as well as the high N doping content, which can be attributed to their unique spiral hollow nanostructure, enhances the adsorption capacity of K ions.
Figure 7. (A) CV curve with a scanning rate of 0.1 mV·s-1; (B) charge/discharge curve with a current density of 0.1 A·g-1; (C) capacity test diagram at a current density of 0.1 A·g-1; (D and E) rate performance test diagram a current density from 0.2 to 5.0 A·g-1; and (F) stability test diagram with 500 cycles a current density of 2.0 A·g-1 for helical multi-shelled carbon nanospheres-based KIBs anode. This figure is quoted with permission from Peng et al.[51]. CV: Cyclic voltammetry; KIBs: potassium-ion batteries.
Lithium-sulfur (Li-S) batteries have garnered significant attention due to their exceptional theoretical specific capacity of 1,675 mAh·g-1 and cost-effectiveness. Embedding sulfur in mesoporous carbon is a common method to solve the problems of poor conductivity of sulfur and volume expansion during the charging/discharging process[128]. To address the problem that the physical limitation of nonpolar carbon still makes it difficult to prevent polysulfide dissolution/diffusion, which leads to serious degradation of the electrochemical performance of Li-S batteries, Qiu et al. introduced a manganese source into the synthesis of 2D mesoporous heterogeneous nanosheets, and prepared functional sandwich nanosheets with sub-nanometer manganese oxide clusters (MOCs) homogeneously dispersed in N-doped mesoporous carbon on both sides of the GO[105]. The composite (S/rGO@mC-MnO-800), serving as the cathode host in Li-S batteries, exhibited impressive initial discharge capacity, high reversible capacity, and more than 250 cycles of stability (2 A·g-1). Notably, even with a high sulfur loading of 4.7 mg·cm-2, the corresponding batteries still achieve a significant area capacity of 2.5 mAh·cm-2 at 0.1 A·g-1. In addition to the unique mesoporous carbon nanosheets with high electrical conductivity and efficient buffering, experimental results and theoretical calculations demonstrate the strong chemisorption of polysulfides by sub-nanometre-scale MOCs, which significantly suppresses the shuttle effect of polysulfides, achieving fast kinetics and high capacity.
Aqueous zinc-ion batteries are considered as promising generation battery systems, primarily owing to their enhanced safety features and cost-effectiveness[129]. However, dendrite growth and hydrogen precipitation side reactions of Zn metal anodes in aqueous solution can compromise cycle life, reduce coulombic efficiency, and potentially cause battery failure[130]. Given that mPDA possesses abundant hydrophilic functional groups, it effectively sequesters water molecules, thereby mitigating their corrosive effects on Zn metal. Yu et al. constructed an mPDA protective layer on the surface of Zn foil[106]. Experiments combined with simulations show that the mPDA layer can reduce the nucleation overpotential, and the mesopores in the material facilitate the redistribution of ion circulation, leading to a more uniform electric field and Zn ion flux, thus inhibiting the deposition and growth of Zn metal dendrites. Consequently, the corresponding symmetric batteries exhibit remarkable ultra-long cycle life exceeding 1,000 h (a tenfold improvement over bare Zn) and exceptional rate capability. In addition, Zn-metal batteries integrated with NH4V4O10 cathode exhibit a notable capacity of 368 mAh·g-1, accompanied by minimal capacity degradation over 300 cycles, which provides guidance for its practical promotion[106]. This advancement, coupled with the demonstrated high capacity and stable cycling performance of Zn-metal batteries with NH4V4O10 cathodes, underscores the potential of aqueous zinc-ion batteries as a viable next-generation energy storage technology.
Capacitors
Supercapacitor
Supercapacitors are highly regarded as a promising energy storage solution, renowned for their high-power output and long cyclability[131]. Mesoporous carbon materials are typically more effective than microporous materials; the larger channels of mesopores facilitate full electrolyte infiltration, exposing more surface area for charge storage in supercapacitors[132]. Tian et al. were the first to utilize N-doped mesoporous carbon nanospheres as electrode materials for supercapacitors[38]. These carbon nanospheres exhibited a high specific capacity, superior rate capacity and cycling stability. The bowl-shaped hollow particles are expected to increase the filling density, which should contribute to a higher volumetric capacitance. Subsequently, the team obtained hollow particles with a bowl-shaped N-doped carbon structure through the use of kippah-vesicles formed by mono-micelles as templates[41]. Compared with spherical particles, the hollow particles exhibited a high specific capacitance (385 F·g-1 at 0.1 A·g-1), a volumetric capacitance of 410 F·cm-3 at the same current density, excellent capacitance retention, and remarkable cycling stability with no discernible loss in capacitance after 1,000 cycles. The pore structure was further optimized by ZnCl2 activation of semi-football-shaped mesoporous carbon nanoparticles by Guo et al.[60]. As depicted in Figure 8, the semi-football-shaped nanoparticles demonstrate exceptional reversibility, robust rate capability, and a high energy density. The simulation outcomes clarify that the hierarchical pore structure enhances the ion diffusion flux, while the large mesoporous structure favors the high-rate performance. In addition, various MPDC nanospheres are demonstrated to show enhanced electrochemical charge storage performance[42,43,45].
Figure 8. (A) Schematic diagram of the action mechanism of the pore structure of the MPDC electrode material in the double-layer supercapacitor; (B) CV curve at a scanning rate of 400 mV·s-1; (C) Galvanostatic charge/discharge curve at a current density of 20 A·g-1; (D) specific capacitance at a scanning rate of 400 mV·s-1; (E) the corresponding capacitance reduction rate in organic electrolyte at a charge/discharge current density from 0.05 to 20 A·g-1; (F) electrochemical impedance spectrum; and (G) energy and power density curves at different current densities for different electrode. This figure is quoted with permission from Guo et al.[60]. MCN1: Micro-/small mesopores; MCN2: meso-/micropores; MCN3: mesopore channels-micropores; HMC: hierarchical macro-/meso-/micropores; MPDC: mesoporous polydopamine and its derived carbon; CV: cyclic voltammetry.
With their tunable dimensions and chemical compositions, 1D nanostructures facilitate increased specific surface areas and shortened electron/ion diffusion pathways. Pioneeringly, Jiang et al. used 1D carbon-tipped MnO2/mesoporous carbon/MnO2 hybrid nanowire as electrodes, and the assembled supercapacitors exhibited excellent capacitive performance, presenting a high specific capacitance, excellent multiplicative performance, and impressive cycling stability (1,200 cycles)[69]. In addition, coating porous electrochemically active electrode materials on 2D conductive nanomaterials to produce sandwich-like high specific surface area heterostructured hybrids has been shown to be a promising strategy to efficiently prevent nanosheet stacking and improve capacitive performance[78]. Unique hierarchical macroporous-mesoporous-microporous N, O, S-doped carbon aerogel foams were used for supercapacitors by Peng et al.[81]. The great specific surface area of 2,685 m2·g-1 enabled the device to display outstanding capacitance and a superior rate capacity. The advantages of this hierarchical porous carbon structure were further demonstrated. Kim et al. grew mPDA on the ZIF-8 surface and used it as a carbon precursor to obtain hierarchical porous double-shell carbon electrodes with good electrolyte ion diffusion[133]. It maintained a high initial capacitance (76.7%) in the range of 1-10 A·g-1 with a maximum specific capacitance of 344.7 F-1. Future research may focus on further optimizing the pore structure and topological morphology to enhance the overall performance and practical applicability of these supercapacitors.
Ionic capacitors
The incorporation of battery-type electrodes into capacitor systems can enhance energy density and mitigate self-discharge, thereby bridging the gap between supercapacitors and alkali metal ion batteries. The use of 2D mesoporous heterogeneous nanosheets can be employed to optimize the energy storage capabilities of 2D materials, while also ensuring the rapid diffusion of ions and the kinetic process between adsorption and desorption[134]. Jiang et al. constructed a lithium-ion capacitor comprising a 2D ordered in-plane columnar mPDA/rGO heterostructure as the cathode and mPDA/rGO-derived N-doped carbon nanosheets (mNC/rGO) as the anode[107]. Accordingly, mPDA/rGO contributed a high specific capacity and an excellent rate capability, and the assembled device delivers a high energy density. This excellent performance is attributed to the 2D in-plane columnar mesoporous structure that favors smooth diffusion and kinetic equilibrium of lithium ions.
Electrocatalysis
Hydrogen evolution reaction
Hydrogen, with its high enthalpy of combustion and non-polluting nature, is one of the most promising sources of clean and sustainable energy. The electrochemically driven splitting of water by highly efficient electrocatalysts represents an attractive approach to the production of hydrogen fuel[135]. The development of electrochemical catalysts for electrolytic hydrogen production is pivotal in maximizing catalytic efficiency while minimizing the reliance on scarce precious or transition metal elements, thereby lowering production costs[136]. PDA-derived N-doped carbon usually exhibits limited HER activity. However, isolated Pt centers possess the ability to activate adjacent C/N atoms, thereby transforming these non-metallic elements into electrocatalytically active sites for HER. Zhang et al. employed the uniform dispersion of single-atom Pt on mesoporous carbon matrix surface and within the pores to tractor and stabilize the isolated Pt atoms within the carbon matrix[137]. The internal kinetic effect of high-temperature pyrolysis led to the gradual fusion of Pt ions into the lattice of PCM. Isolated Pt atoms were then coordinated with the surrounding C/N atoms in the lattice confinement geometry, resulting in the formation of a Pt@PCM electrocatalyst with highly dispersed active centers. The onset potential of this catalyst is ~0 V in comparison to the reversible hydrogen electrode (RHE). Furthermore, they combined PDA particles with a large mesoporous walnut multi-edge morphology with ruthenium, and one-step pyrolysis yielded isolated Ru sites decorated with edge-rich mesoporous carbon[108]. The catalyst exhibits good catalytic performance [Figure 9]. In addition to the above noble metals, Park et al. prepared mesoporous N-doped carbon nanorods with uniform and dense distribution of Ni single atoms by chelation of metals[109]. The metal content of this catalyst could be reduced to less than 1 wt%, and its overpotential was 270 mV at 10 mA·cm-2, which was reduced to 190 mV after 2,000 cycles, which was superior to most Ni-based catalysts. The distinctive structural features and electronic properties of these catalysts offer promising avenues for further optimization in energy conversion and storage systems, highlighting the importance of material engineering at the nanoscale.
Figure 9. (A) LSV curve and (B) Tafel plot, (C) corresponding overpotentials at J = 10 and 50 mA·cm-2; (D) comparison of mass activity under different overpotentials of the ECM@Ru and compared samples in 0.5 M H2SO4 acid electrolyte; (E) Corresponding capacitive
Oxygen reduction reaction
The oxygen reduction reaction (ORR) is a pivotal half-reaction in fuel cells and rechargeable metal-air battery energy conversion systems[138]. The development of non-platinum electrocatalysts with high ORR activity, such as N-doped carbon (N-C) or metal-N-carbon (M-N-C), has the potential to replace noble-metal-based catalysts in economic applications[139]. The mesoporous structure provides channels for the electrolyte to wet the surface, ensuring sufficient contact between the reactant domains and active sites, and can significantly reduce the mass transfer resistance[140]. In 2015, Tang et al. employed MPDC nanospheres with large-size mesopores and highly N-doped carbon nanorods as ORR electrocatalysts, which exhibited comparable electrocatalytic activity to Pt/C catalysts and excellent long-term stability[22]. Subsequently, various MPDC nanospheres/nanoparticles have been demonstrated to have enhanced electrocatalytic performance for the ORR. These include walnut-like macro/medium porous carbon particles, bowl-shaped carbon particles with radial mesoporous channels, dendritic ultra-large pore size mesoporous carbon nanospheres, and so on[48,49,58]. Furthermore, the geometrical configurations of the materials can also affect the electrocatalytic mass transfer by modulating the activation of the reactants, the diffusion of the intermediates and the separation of the reducing molecules[141]. Zhu et al. demonstrated that N-doped carbon CNTs@mesoNC nanofibers wrapped with vertical pores of CNTs exhibited higher ORR current density and electrochemical activity compared to domain-less porous fiber samples and mesoporous N-doped carbon samples[67]. This evidence suggests that both mesoporous structure and 1D nanomorphology can enhance electrocatalytic performance.
In addition to N-doped carbon, the introduction of metal atoms can bind to the N atoms in the carbon matrix to optimize the adsorption/desorption of intermediates and dramatically improve the ORR catalytic activity. Zhou et al. synthesized ordered mesoporous carbon nanospheres co-doped with atomic Fe and N by introducing an iron source during the assembly process[110]. They exhibited excellent activity and durability in ORR, which were superior to the Pt/C catalysts [Figure 10]. Wang et al. alloyed Pt with a mesoporous Fe-N-C carrier to obtain a catalyst with more than a 3.1-fold increase in mass activity and good long-term stability[139]. Zhao et al. demonstrated that the catalysts prepared by cobalt embedded in N-doped carbon materials with uniform nanospherical morphology displayed a half-wave potential of 0.93 V (RHE)[111]. And the mesoporous structure endowed it with greater limiting current density than Pt/C and demonstrated superior activity and persistent stability in rechargeable zinc-air batteries.
Figure 10. (A) ORR curves in 0.1 M KOH alkaline electrolyte; (B) half-wave potential E1/2 and dynamic current density jk of different samples; (C) ORR curves of Meso-Fe-N-C before and after 5,000 cycles; (D) The polarization curve and power density diagram of Meso-Fe-N-C based H2-O2 fuel cell. This figure is quoted with permission from Zhou et al.[110]. meso-Fe-N-C: Atomic Fe and N co-doped ordered mesoporous carbon nanosphere; ORR: oxygen reduction reaction.
The construction of 2D nanostructures allows for the creation of a greater number of active sites and an enhanced electrolyte-electrode accessibility area, which greatly reduces the electron-ion diffusion pathway[142]. Qiu et al. employed the strong chelating effect of catecholamine groups to introduce a series of mono-, bimetallic and trimetallic metals in situ into a polymer framework, which was then converted into 2D mesoporous rGO-based heterogeneous nanosheets with uniformly dispersed metal nanoparticles after annealing in an inert atmosphere[85]. Among the catalysts, those comprising cobalt nanoparticles and mesoporous carbon exhibited high ORR half-wave potentials of ~0.82 V (RHE). Furthermore, the incorporation of nanosheets into a 3D structure prevents the re-stacking of nanosheets, thereby reducing the electrical conductivity and accessible contact interface. Guo et al. prepared highly porous hollow 3D spheres consisting of Mo2C/N-doped carbon nanosheets by introducing molybdate during the assembly process with one-step carbonization[143]. Thanks to the good electronic structure of Mo2C, the composite can significantly enhance the ORR electrochemical performance. This was further demonstrated by Zhang et al.[144]. They also assembled zinc-air batteries that provided high power density and excellent battery stability.
The growth of mPDA layers on metal-based nanosurfaces can eliminate the tedious doping step, and the metal substrate can form a heterojunction in good contact with the mesoporous carbon coating, which improves the electron transfer and can change the activation energy of the reaction intermediates to enhance the catalytic activity of the electrocatalyst[145]. Chen et al. grew irregular-type macroporous/mPDA nanoshells on Fe2O3 rods and obtained Fe and N-doped porous carbon, which exhibited structural and compositional advantages that ensured excellent electrocatalytic performance for ORR in alkaline electrolytes[70]. Gou et al. successfully introduced elemental N into the Ti3C2 structure through the introduction of ammonia during heat treatment and introduced more defect sites in the N-doped mesoporous carbon[79]. ORR tests combined with theoretical calculations demonstrated the electronically coupled interactions between the N-doped mesoporous defective carbon and the N-modified Ti3C2 to improve the electronic structure of the whole heterostructure. The catalyst exhibited an onset potential of 0.90 V (RHE) and a limiting density current of 5.50 mA·cm-2, demonstrating good methanol tolerance and cycling durability. Zhao et al. grew mPDA layers on the ZnCo-ZIF MOF surface and produced a roof-like mesoporous hollow carbon-based catalyst Co@EMPC by controlling the thickness[112]. The optimized heterojunction interface, mesoporous hollow structure, overhanging eaves design and shortened mesoporous channels have the advantage of promoting rapid charge transfer and mass transfer. The integration of macroporous/mesoporous carbon nanostructures with metal oxides, MXenes, and MOF-derived materials, coupled with strategic doping and defect engineering, has emerged as a promising strategy to enhance ORR performance in alkaline electrolytes.
Nitrate reduction reaction
The conversion of nitrate pollutants in industrial wastewater to ammonia via a mild electroreduction process represents a promising pathway for the treatment of industrial wastewater and the green and sustainable synthesis of ammonia[146]. For instance, Xu et al. incorporated metal ions into the synthesis process, resulting in the dispersion and confinement of a multitude of ultra-small metal nanoparticles within the mesoporous channels of a core-shell structured CNT matrix[68]. Among them, CNTs@mesoC@CuPd exhibited good conversion, N2 selectivity, cycling stability, and removal rate, which was much better than that of previous reports, due to the alloying of Cu and Pd sites to precisely tune the internal electronic structure. The uniformly distributed CuPd alloy nanoparticles on the mesoporous channels provided sufficient active sites for electrochemical nitrate reduction reaction (NO3-RR), while the 1D morphology could provide a large number of fast electron transfer pathways, expanding the full contact between electrolyte and electrode. In addition, Zhao et al. introduced Co and Cu sources into a hollow mesoporous carbon matrix (MesoC) obtained by carbonization of ZIF-8 nanoparticles encapsulated in mPDA layers and synthesized a Co-modified Cu/Cu2O catalyst (Co/Cu/Cu2O-MesoC) loaded on a hollow MesoC by using a microwave-assisted one-step reduction method[113]. Thanks to the high specific surface area of the hollow mesoporous carbon matrix and the accelerated water electrolysis by Co doping, the Faraday efficiency of Co/Cu/Cu2O- MesoC was 100% ± 1% at -0.25 V. This confinement strategy of dispersing nanoparticles on a mesoporous carbon substrate opens up an effective route for the synthesis of NO3-RR catalysts with high selectivity, and operational stability.
CONCLUSION AND OUTLOOK
This paper reviews the major achievements of the past decade, focusing on the preparation of MPDC based on the soft template self-assembly route. MPDC with varying compositions, structures, morphologies, and surface properties has been constructed by controlling the homogeneous assembly process of soft templates with DA precursors in homogeneous solutions or by controlling their heterogeneous growth on functional surfaces with different morphologies. Additionally, we present a summary of the research progress made in their applications in the energy field, including but not limited to supercapacitors, rechargeable batteries, and electrocatalytic reactions. As the field of MPDC continues to evolve, these materials are being explored for increasingly impressive functions and applications. These results represent the latest breakthroughs in the field of mesopores, providing researchers with a deeper understanding of the synthetic concepts of MPDC and the relationships between synthesis, design, structure, and properties.
Despite notable advancements in MPDC synthesis and utilization in recent times compared to mesoporous silicon oxide and metal oxides, the rational design and sophisticated constructive synthesis of MPDC with tailored properties and functions will require further efforts from researchers in the future. There are still several unresolved challenges and research directions for the future of this field, as follows:
(1) The process of soft template-directed self-assembly is typically driven by precursors with well-matched interactions with the soft templates. The templates that have been reported thus far center around a limited selection of costly nonionic surfactants: diblock copolymers, triblock copolymers, and so on. The low-cost anionic/cationic surfactants and nonionic small molecule surfactants that are often employed in other instances of mesoporous material synthesis have not yet been reported. It would be beneficial to pursue this avenue further.
(2) The currently established general paradigms and theoretical mechanisms for the synthesis of inorganic functional mesoporous materials are difficult to apply due to the significant differences in the complex polymerization and co-assembly processes of organic precursors. Future research should concentrate on the identification of suitable research models and the expansion of synthesis cases. This should be carried out in conjunction with theoretical calculations and in situ characterization techniques. The objective is to gain a deeper understanding of the self-assembly behavior at multiple scales, ranging from the atomic to the nano- and even microscale. This comprehension will pave the way for the precise, manageable, and tailored synthesis of MPDC materials.
(3) The DA is readily oxidized in air and is only soluble in certain polar solvents. Consequently, the well-established preparation methods of conventional mesoporous materials, such as solvent evaporation induced self-assembly, are not applicable, and their synthesis remains highly dependent on and constrained by the solution-based self-assembly pathway. It is of the utmost importance that innovative synthetic systems be continuously explored in order to enhance the reproducibility and scalability of synthetic systems. One promising avenue is to focus on non-solution-based synthesis systems that have the potential for scalable production of bulk MPDC. This endeavor necessitates relentless efforts from researchers to develop and refine these systems, overcoming the inherent challenges associated with non-solution processing.
(4) The accurate synthesis of MPDC materials with controllable topological morphology, tunable pore structure, and oriented polymer chain structure at the nanoscale level remains a significant challenge. Previous studies have demonstrated that the incorporation of heterogeneous interfaces can result in the formation of mesoporous MPDC materials that are distinct from those produced by the homogeneous assembly of single-phase solutions. In addition to the liquid-solid interface previously discussed, combining the synthesis of mesoporous MPDC with macroscopic liquid/gas-liquid interfaces is also beneficial for the macroscopic and large-area preparation of 2D-ordered MPDC ultrathin nanofilms. It is anticipated that the slow growth of organic precursors at domain-limited and mild interfaces will result in the formation of structurally regular, chain-arranged MPDC with fewer defects.
(5) One critical avenue for future exploration involves delving into newer and more promising applications beyond the currently focused areas of energy storage and conversion, electrocatalysis, and biomedicine. A plethora of emerging directions warrant investigation, such as harnessing MPDC as “nanoreactors” for energy storage in the form of fuel gases[147]. The tunable mesoporous structural features and adjustable surface wettability of MPDC nanostructures render them promising candidates for these applications[148]. By exploring these untapped potentials, researchers can unlock the full potential of MPDC and contribute to the development of more advanced and versatile materials for a wide range of industries.
(6) Currently, only a small fraction of ordered mesoporous materials (e.g., silica) have been prepared on a large scale. In comparison to the high cost of DA precursors and soft templates, as well as the low reproducibility of the harsh self-assembly conditions, most laboratories are currently only able to produce MPDC at the gram level. Nevertheless, the preparation of MPDC at the kilogram level remains a significant challenge. From the perspective of synthetic strategies, further research is required to improve the polymerization efficiency and utilization of DA precursors, as well as to identify more cost-effective template substitutes, in order to facilitate the large-scale, low-cost preparation and commercial application of MPDC.
It is our contention that the research presented in this thesis will have a profound impact on the development of porous materials science, nanomaterials science, supramolecular chemistry, and other related disciplines and cross-cutting fields. Furthermore, we believe that the research will facilitate the exploration of functional mesoporous materials with polyphenol molecules as key building blocks, thereby making it more accessible to a wider audience.
DECLARATIONS
Authors’ contributions
Prepared and revised the manuscript: Wei, F.; Fu, J.
Designed and revised the manuscript: Cheng, J.; Jing, C.; Liu, S.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 52373208 and 61831021).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Nguyen, N. N. Prospect and challenges of hydrate-based hydrogen storage in the low-carbon future. Energy. Fuels. 2023, 37, 9771-89.
2. Guerra, K.; Gutiérrez-alvarez, R.; Guerra, O. J.; Haro, P. Opportunities for low-carbon generation and storage technologies to decarbonise the future power system. Appl. Energy. 2023, 336, 120828.
3. Lan, K.; Zhao, D. Functional ordered mesoporous materials: present and future. Nano. Lett. 2022, 22, 3177-9.
4. Kresge, C. T.; Leonowicz, M. E.; Roth, W. J.; Vartuli, J. C.; Beck, J. S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710-2.
5. Zhao, D.; Feng, J.; Huo, Q.; et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548-52.
6. Benzigar, M. R.; Talapaneni, S. N.; Joseph, S.; et al. Recent advances in functionalized micro and mesoporous carbon materials: synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680-721.
7. Gang, D.; Uddin, A. Z.; Lian, Q.; Yao, L.; Zappi, M. E. A review of adsorptive remediation of environmental pollutants from aqueous phase by ordered mesoporous carbon. Chem. Eng. J. 2021, 403, 126286.
8. Mehdipour-Ataei, S.; Aram, E. Mesoporous carbon-based materials: a review of synthesis, modification, and applications. Catalsts 2023, 13, 2.
9. Gao, M.; Wang, L.; Yang, Y.; Sun, Y.; Zhao, X.; Wan, Y. Metal and metal oxide supported on ordered mesoporous carbon as heterogeneous catalysts. ACS. Catal. 2023, 13, 4060-90.
10. Saleem, A.; Zhang, Y.; Usman, M.; Haris, M.; Li, P. Tailored architectures of mesoporous carbon nanostructures: from synthesis to applications. Nano. Today. 2022, 46, 101607.
11. Ryoo, R.; Joo, S. H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B. 1999, 103, 7743-6.
12. Meng, Y.; Gu, D.; Zhang, F.; et al. A family of highly ordered mesoporous polymer resin and carbon structures from organic−organic self-assembly. Chem. Mater. 2006, 18, 4447-64.
13. Ma, T. Y.; Liu, L.; Yuan, Z. Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977-4003.
14. Yan, Y.; Chen, G.; She, P.; et al. Mesoporous nanoarchitectures for electrochemical energy conversion and storage. Adv. Mater. 2020, 32, e2004654.
15. Chauhan, S. Synthesis of ordered mesoporous carbon by soft template method. Mater. Today. 2023, 81, 842-7.
16. Wu, Z.; Zhang, K.; Sun, J.; et al. Kinetics-controlled synthesis of ordered mesoporous carbon single crystals from liquefied wood. Adv. Funct. Mater. 2023, 33, 2213852.
17. Li, W.; Wang, G.; Sui, W.; et al. Facile and scalable preparation of cage-like mesoporous carbon from lignin-based phenolic resin and its application in supercapacitor electrodes. Carbon 2022, 196, 819-27.
18. Lee, H. A.; Park, E.; Lee, H. Polydopamine and its derivative surface chemistry in material science: a focused review for studies at KAIST. Adv. Mater. 2020, 32, e1907505.
19. Qu, K.; Wang, Y.; Vasileff, A.; Jiao, Y.; Chen, H.; Zheng, Y. Polydopamine-inspired nanomaterials for energy conversion and storage. J. Mater. Chem. A. 2018, 6, 21827-46.
20. Xu, Y.; Hu, J.; Hu, J.; et al. Bioinspired polydopamine hydrogels: strategies and applications. Prog. Polym. Sci. 2023, 146, 101740.
21. Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-containing polydopamine nanomaterials: catalysis, energy, and theranostics. Small 2020, 16, e1907042.
22. Tang, J.; Liu, J.; Li, C.; et al. Synthesis of nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles. Angew. Chem. Int. Ed. Engl. 2015, 54, 588-93.
23. Lin, K.; Gan, Y.; Zhu, P.; et al. Hollow mesoporous polydopamine nanospheres: synthesis, biocompatibility and drug delivery. Nanotechnology 2021, 32, 285602.
24. Zhu, M.; Shi, Y.; Shan, Y.; et al. Recent developments in mesoporous polydopamine-derived nanoplatforms for cancer theranostics. J. Nanobiotechnology. 2021, 19, 387.
25. Ma, H.; Peng, J.; Zhang, J.; et al. Frontiers in preparations and promising applications of mesoporous polydopamine for cancer diagnosis and treatment. Pharmaceutics 2022, 15, 15.
26. Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, BFnatrevmats201623.
27. Yin, J.; Zhang, W.; Alhebshi, N. A.; Salah, N.; Alshareef, H. N. Synthesis strategies of porous carbon for supercapacitor applications. Small. Methods. 2020, 4, 1900853.
28. Feng, Y.; Li, P.; Wei, J. Engineering functional mesoporous materials from plant polyphenol based coordination polymers. Coordin. Chem. Rev. 2022, 468, 214649.
29. Li, Z.; Li, B.; Yu, C.; Wang, H.; Li, Q. Recent progress of hollow carbon nanocages: general design fundamentals and diversified electrochemical applications. Adv. Sci. 2023, 10, e2206605.
30. Luo, H.; Kaneti, Y. V.; Ai, Y.; et al. Nanoarchitectured porous conducting polymers: from controlled synthesis to advanced applications. Adv. Mater. 2021, 33, e2007318.
31. Wei, F.; Zhang, T.; Xu, H.; et al. 2D mesoporous naphthalene-based conductive heteroarchitectures toward long-life, high-capacity zinc-iodine batteries. Adv. Funct. Mater. 2024, 34, 2310693.
32. Wei, F.; Xu, H.; Zhang, T.; et al. Mesoporous poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) as efficient iodine host for high-performance zinc-iodine batteries. ACS. Nano. 2023, 17, 20643-53.
33. Peng, L.; Peng, H.; Li, W.; Zhao, D. Monomicellar assembly to synthesize structured and functional mesoporous carbonaceous nanomaterials. Nat. Protoc. 2023, 18, 1155-78.
34. Pan, P.; Zhang, T.; Yue, Q.; et al. Interface coassembly and polymerization on magnetic colloids: toward core-shell functional mesoporous polymer microspheres and their carbon derivatives. Adv. Sci. 2020, 7, 2000443.
35. Khan, Y.; Sadia, H.; Ali, S. S. Z.; et al. Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: a review. Catalysts 2022, 12, 1386.
36. Feng, Y.; Qin, J.; Zhou, Y.; Yue, Q.; Wei, J. Spherical mesoporous Fe-N-C single-atom nanozyme for photothermal and catalytic synergistic antibacterial therapy. J. Colloid. Interface. Sci. 2022, 606, 826-36.
37. Duan, L.; Wang, C.; Zhang, W.; et al. Interfacial assembly and applications of functional mesoporous materials. Chem. Rev. 2021, 121, 14349-429.
38. Tian, H.; Lin, Z.; Xu, F.; et al. Quantitative control of pore size of mesoporous carbon nanospheres through the self-assembly of diblock copolymer micelles in solution. Small 2016, 12, 3155-63.
39. Qian, H.; Tang, J.; Hossain, M. S. A.; Bando, Y.; Wang, X.; Yamauchi, Y. Localization of platinum nanoparticles on inner walls of mesoporous hollow carbon spheres for improvement of electrochemical stability. Nanoscale 2017, 9, 16264-72.
40. Tang, J.; Liu, J.; Salunkhe, R. R.; Wang, T.; Yamauchi, Y. Nitrogen-doped hollow carbon spheres with large mesoporous shells engineered from diblock copolymer micelles. Chem. Commun. 2016, 52, 505-8.
41. Lin, Z.; Tian, H.; Xu, F.; Yang, X.; Mai, Y.; Feng, X. Facile synthesis of bowl-shaped nitrogen-doped carbon hollow particles templated by block copolymer “kippah vesicles” for high performance supercapacitors. Polym. Chem. 2016, 7, 2092-8.
42. Du, G.; Wang, H.; Liu, J.; Sun, P.; Chen, T. Hierarchically porous mesostructured polydopamine nanospheres and derived carbon for supercapacitors. Langmuir 2022, 38, 8964-74.
43. Tang, J.; Wang, J.; Shrestha, L. K.; et al. Activated porous carbon spheres with customized mesopores through assembly of diblock copolymers for electrochemical capacitor. ACS. Appl. Mater. Interfaces. 2017, 9, 18986-93.
44. Xiang, L.; Yuan, S.; Wang, F.; et al. Porous polymer cubosomes with ordered single primitive bicontinuous architecture and their sodium-iodine batteries. J. Am. Chem. Soc. 2022, 144, 15497-508.
45. Xiong, S.; Fan, J.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. A facile template approach to nitrogen-doped hierarchical porous carbon nanospheres from polydopamine for high-performance supercapacitors. J. Mater. Chem. A. 2017, 5, 18242-52.
46. Jung, H. S.; Cho, K. J.; Joo, S.; et al. Mesoporous polydopamine-encapsulated fluorescent nanodiamonds: a versatile platform for biomedical applications. ACS. Appl. Mater. Interfaces. 2023, 15, 33425-36.
47. Wu, D.; Duan, X.; Guan, Q.; et al. Mesoporous polydopamine carrying manganese carbonyl responds to tumor microenvironment for multimodal imaging-guided cancer therapy. Adv. Funct. Mater. 2019, 29, 1900095.
48. Guan, B. Y.; Zhang, S. L.; Lou, X. W. D. Realization of walnut-shaped particles with macro-/mesoporous open channels through pore architecture manipulation and their use in electrocatalytic oxygen reduction. Angew. Chem. Int. Ed. Engl. 2018, 130, 6284-8.
49. Peng, L.; Hung, C. T.; Wang, S.; et al. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 2019, 141, 7073-80.
50. Peng, L.; Peng, H.; Hung, C.; et al. Programmable synthesis of radially gradient-structured mesoporous carbon nanospheres with tunable core-shell architectures. Chem 2021, 7, 1020-32.
51. Peng, L.; Peng, H.; Liu, Y.; et al. Spiral self-assembly of lamellar micelles into multi-shelled hollow nanospheres with unique chiral architecture. Sci. Adv. 2021, 7, eabi7403.
52. Yang, X.; Lu, P.; Yu, L.; et al. An efficient emulsion-induced interface assembly approach for rational synthesis of mesoporous carbon spheres with versatile architectures. Adv. Funct. Mater. 2020, 30, 2002488.
53. Pan, P.; Liu, Q.; Hu, L.; et al. Dual-template induced interfacial assembly of yolk-shell magnetic mesoporous polydopamine vesicles with tunable cavity for enhanced photothermal antibacterial. Chem. Eng. J. 2023, 472, 144972.
54. Acter, S.; Vidallon, M. L. P.; Crawford, S.; Tabor, R. F.; Teo, B. M. Bowl-shaped mesoporous polydopamine nanoparticles for size-dependent endocytosis into HeLa cells. ACS. Appl. Nano. Mater. 2021, 4, 9536-46.
55. Huang, A.; Dai, H.; Wu, X.; Zhao, Z.; Wu, Y. Synthesis and characterization of mesoporous hydroxyapatite powder by microemulsion technique. J. Mater. Res. Technol. 2019, 8, 3158-66.
56. Dong, L.; Liu, M.; Fang, M.; et al. Nucleation-inhibited emulsion interfacial assembled polydopamine microvesicles as artificial antigen-presenting cells. Small 2024, 20, e2400714.
57. Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4, 775-91.
58. Guan, B. Y.; Yu, L.; Lou, X. W. Formation of asymmetric bowl-like mesoporous particles via emulsion-induced interface anisotropic assembly. J. Am. Chem. Soc. 2016, 138, 11306-11.
59. Peng, L.; Peng, H.; Xu, L.; et al. Anisotropic self-assembly of asymmetric mesoporous hemispheres with tunable pore structures at liquid-liquid interfaces. J. Am. Chem. Soc. 2022, 144, 15754-63.
60. Guo, P.; Zhao, R.; Zhang, Z.; et al. Droplet-directed anisotropic assembly of semifootball-like carbon nanoparticles with multimodal pore architectures. Adv. Funct. Mater. 2024, 34, 2400503.
61. Fan, L.; Xia, Z.; Xu, M.; Lu, Y.; Li, Z. 1D SnO2 with wire-in-tube architectures for highly selective electrochemical reduction of CO2 to C1 products. Adv. Funct. Mater. 2018, 28, 1706289.
62. Meng, Y.; Wang, W.; Ho, J. C. One-dimensional atomic chains for ultimate-scaled electronics. ACS. Nano. 2022, 16, 13314-22.
63. Wei, F.; Chen, B.; Fu, J.; et al. A universal strategy for large-scale and controlled fabrication of conductive mesoporous polymer monolayers. Chem. Eng. J. 2023, 460, 141504.
64. Liu, S.; Gordiichuk, P.; Wu, Z. S.; et al. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 2015, 6, 8817.
65. Lima, R. M. A. P.; Alcaraz-Espinoza, J. J.; da, S. F. A. G. J.; de, O. H. P. Multifunctional wearable electronic textiles using cotton fibers with polypyrrole and carbon nanotubes. ACS. Appl. Mater. Interfaces. 2018, 10, 13783-95.
66. Zhu, M.; Chen, J.; Huang, L.; Ye, R.; Xu, J.; Han, Y. F. Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction. Angew. Chem. Int. Ed. Engl. 2019, 58, 6595-9.
67. Zhu, X.; Xia, Y.; Zhang, X.; et al. Synthesis of carbon nanotubes@mesoporous carbon core–shell structured electrocatalysts via a molecule-mediated interfacial co-assembly strategy. J. Mater. Chem. A. 2019, 7, 8975-83.
68. Xu, H.; Chen, J.; Zhang, Z.; Hung, C. T.; Yang, J.; Li, W. In situ confinement of ultrasmall metal nanoparticles in short mesochannels for durable electrocatalytic nitrate reduction with high efficiency and selectivity. Adv. Mater. 2023, 35, e2207522.
69. Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P. S.; Ma, J. High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires. Energy. Environ. Sci. 2011, 4, 1813.
70. Chen, G.; Yan, Y.; Wang, J.; et al. General formation of macro-/mesoporous nanoshells from interfacial assembly of irregular mesostructured nanounits. Angew. Chem. Int. Ed. Engl. 2020, 59, 19663-8.
71. Jiang, H.; Zhang, H.; Fu, Y.; et al. Self-volatilization approach to mesoporous carbon nanotube/silver nanoparticle hybrids: the role of silver in boosting Li ion storage. ACS. Nano. 2016, 10, 1648-54.
72. Han, Z.; Gao, M.; Wang, Z.; Peng, L.; Zhao, Y.; Sun, L. pH/NIR-responsive nanocarriers based on mesoporous polydopamine encapsulated gold nanorods for drug delivery and thermo-chemotherapy. J. Drug. Deliv. Sci. Tec. 2022, 75, 103610.
73. Liu, J.; Yang, F.; Cao, L.; et al. A robust nonvolatile resistive memory device based on a freestanding ultrathin 2D imine polymer film. Adv. Mater. 2019, 31, e1902264.
74. Liu, C.; Chen, H.; Wang, S.; et al. Two-dimensional materials for next-generation computing technologies. Nat. Nanotechnol. 2020, 15, 545-57.
75. Khan, K.; Tareen, A. K.; Aslam, M.; et al. Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C. 2020, 8, 387-440.
76. Liu, S.; Wang, F.; Dong, R.; et al. Dual-template synthesis of 2D mesoporous polypyrrole nanosheets with controlled pore size. Adv. Mater. 2016, 28, 8365-70.
77. Wei, F.; Zhang, T.; Dong, R.; et al. Solution-based self-assembly synthesis of two-dimensional-ordered mesoporous conducting polymer nanosheets with versatile properties. Nat. Protoc. 2023, 18, 2459-84.
78. Wang, J.; Malgras, V.; Sugahara, Y.; Yamauchi, Y. Electrochemical energy storage performance of 2D nanoarchitectured hybrid materials. Nat. Commun. 2021, 12, 3563.
79. Gou, Z.; Qu, H.; Liu, H.; et al. Coupling of N-doped mesoporous carbon and N-Ti3C2 in 2D sandwiched heterostructure for enhanced oxygen electroreduction. Small 2022, 18, e2106581.
80. Guan, B. Y.; Yu, L.; Lou, X. W. Chemically assisted formation of monolayer colloidosomes on functional particles. Adv. Mater. 2016, 28, 9596-601.
81. Peng, H.; Yao, B.; Wei, X.; et al. Pore and heteroatom engineered carbon foams for supercapacitors. Adv. Energy. Mater. 2019, 9, 1803665.
82. Tian, H.; Qin, J.; Hou, D.; et al. General interfacial self-assembly engineering for patterning two-dimensional polymers with cylindrical mesopores on graphene. Angew. Chem. Int. Ed. Engl. 2019, 131, 10279-84.
83. Li, Q.; Xu, X.; Guo, J.; et al. Two-dimensional MXene-polymer heterostructure with ordered in-plane mesochannels for high-performance capacitive deionization. Angew. Chem. Int. Ed. Engl. 2021, 133, 26732-8.
84. Lan, K.; Wei, Q.; Wang, R.; et al. Two-dimensional mesoporous heterostructure delivering superior pseudocapacitive sodium storage via bottom-up monomicelle assembly. J. Am. Chem. Soc. 2019, 141, 16755-62.
85. Qiu, P.; Zhang, X.; Ai, Y.; Luo, W.; Li, W.; Zhao, D. Modular assembly of metal nanoparticles/mesoporous carbon two-dimensional nanosheets. NPG. Asia. Mater. 2023, 15, 482.
86. Wen, B.; Yang, H.; Lin, Y.; et al. Synthesis of core–shell Co@S-doped carbon@ mesoporous N-doped carbon nanosheets with a hierarchically porous structure for strong electromagnetic wave absorption. J. Mater. Chem. A. 2021, 9, 3567-75.
87. Li, X.; Zhang, H.; Yang, X.; et al. Mesoporous dopamine-modified leaf-like zeolitic imidazolate frameworks derived carbon for efficient capacitive deionization. J. Colloid. Interface. Sci. 2024, 654, 559-67.
88. Wang, R.; Lan, K.; Lin, R.; et al. Precisely controlled vertical alignment in mesostructured carbon thin films for efficient electrochemical sensing. ACS. Nano. 2021, 15, 7713-21.
89. Chen, B.; Wei, F.; Ma, Z.; et al. Interfacial self-assembly growth of mesoporous polydopamine nanofilms for formaldehyde sensing. J. Polym. Sci. 2024, 62, 1588-96.
90. Zhao, T.; Chen, L.; Lin, R.; et al. Interfacial assembly directed unique mesoporous architectures: from symmetric to asymmetric. Acc. Mater. Res. 2020, 1, 100-14.
91. Zhao, Z.; Duan, L.; Zhao, Y.; et al. Constructing unique mesoporous carbon superstructures via monomicelle interface confined assembly. J. Am. Chem. Soc. 2022, 144, 11767-77.
93. Li, H.; Liu, J. Janus mesoporous nanoparticles enable building biological logic systems. Sci. China. Chem. 2024, 67, 316-8.
94. Zhao, T.; Zhu, X.; Hung, C. T.; et al. Spatial isolation of carbon and silica in a single Janus mesoporous nanoparticle with tunable amphiphilicity. J. Am. Chem. Soc. 2018, 140, 10009-15.
95. Zhao, T.; Chen, L.; Liu, M.; et al. Emulsion-oriented assembly for Janus double-spherical mesoporous nanoparticles as biological logic gates. Nat. Chem. 2023, 15, 832-40.
96. Zhao, T.; Lin, R.; Xu, B.; et al. Mesoporous nano-badminton with asymmetric mass distribution: how nanoscale architecture affects the blood flow dynamics. J. Am. Chem. Soc. 2023, 145, 21454-64.
97. Hou, M.; Liu, M.; Yu, H.; et al. Spatially asymmetric nanoparticles for boosting ferroptosis in tumor therapy. Nano. Lett. 2024, 24, 1284-93.
98. Liu, M.; Shang, C.; Zhao, T.; et al. Site-specific anisotropic assembly of amorphous mesoporous subunits on crystalline metal-organic framework. Nat. Commun. 2023, 14, 1211.
99. Chen, G.; Han, J.; Niu, Z.; et al. Regioselective surface assembly of mesoporous carbon on zeolites creating anisotropic wettability for biphasic interface catalysis. J. Am. Chem. Soc. 2023, 145, 9021-8.
100. Qian, X.; Zhang, F.; Zhao, Y.; Liang, K.; Luo, W.; Yang, J. Polydopamine-derived carbon: what a critical role for lithium storage? Front. Energy. Res. 2020, 8, 140.
101. Wang, N.; Hou, D.; Li, Q.; Zhang, P.; Wei, H.; Mai, Y. Two-dimensional interface engineering of mesoporous polydopamine on graphene for novel organic cathodes. ACS. Appl. Energy. Mater. 2019, 2, 5816-23.
102. Zhu, M.; Wu, J.; Zhong, W.; Lan, J.; Sui, G.; Yang, X. A biobased composite gel polymer electrolyte with functions of lithium dendrites suppressing and manganese ions trapping. Adv. Energy. Mater. 2018, 8, 1702561.
103. Wang, H.; Lan, J.; Yuan, H.; et al. Chemical grafting-derived N, P co-doped hollow microporous carbon spheres for high-performance sodium-ion battery anodes. Appl. Surf. Sci. 2020, 518, 146221.
104. Liu, Y.; Wan, Y.; Zhang, J. Y.; et al. Surface stretching enables highly disordered graphitic domains for ultrahigh rate sodium storage. Small 2023, 19, e2301203.
105. Qiu, P.; Yao, Y.; Li, W.; et al. Sub-nanometric manganous oxide clusters in nitrogen doped mesoporous carbon nanosheets for high-performance lithium-sulfur batteries. Nano. Lett. 2021, 21, 700-8.
106. Yu, J.; Chen, C.; Shi, F.; et al. A multifunctional MXene-porous polydopamine interface for stable and dendrite-free zinc metal batteries. Energy. Storage. Mater. 2023, 63, 102966.
107. Jiang, S.; Xing, F.; Zhang, J.; et al. Two-dimensional redox polydopamine with in-plane cylindrical mesochannels on graphene for high-energy and high-power lithium-ion capacitors. Chem. Eng. J. 2023, 452, 139095.
108. Zhang, H.; Zhou, W.; Lu, X. F.; Chen, T.; Lou, X. W. D. Implanting isolated Ru atoms into edge-rich carbon matrix for efficient electrocatalytic hydrogen evolution. Adv. Energy. Mater. 2020, 10, 2000882.
109. Park, J. W.; Park, G.; Kim, M.; et al. Ni-single atom decorated mesoporous carbon electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2023, 468, 143733.
110. Zhou, Y.; Yu, Y.; Ma, D.; et al. Atomic Fe dispersed hierarchical mesoporous Fe–N–C nanostructures for an efficient oxygen reduction reaction. ACS. Catal. 2021, 11, 74-81.
111. Zhao, S.; Ban, L.; Zhang, J.; Yi, W.; Sun, W.; Zhu, Z. Cobalt and nitrogen co-doping of porous carbon nanosphere as highly effective catalysts for oxygen reduction reaction and Zn-air battery. Chem. Eng. J. 2021, 409, 128171.
112. Zhao, Y.; Zhu, L.; Tang, J.; et al. Enhancing electrocatalytic performance via thickness-tuned hollow N-doped mesoporous carbon with embedded Co nanoparticles for oxygen reduction reaction. ACS. Nano. 2024, 18, 373-82.
113. Zhao, Y.; Liang, S.; Zhao, Y.; et al. Hollow mesoporous carbon supported Co-modified Cu/Cu2O electrocatalyst for nitrate reduction reaction. J. Colloid. Interface. Sci. 2024, 655, 208-16.
114. Zuo, D.; Song, S.; An, C.; Tang, L.; He, Z.; Zheng, J. Synthesis of sandwich-like structured Sn/SnOx@MXene composite through in-situ growth for highly reversible lithium storage. Nano. Energy. 2019, 62, 401-9.
115. Gutsch, M.; Leker, J. Global warming potential of lithium-ion battery energy storage systems: a review. J. Energy. Storage. 2022, 52, 105030.
116. Wang, J.; Xia, Y.; Liu, Y.; Li, W.; Zhao, D. Mass production of large-pore phosphorus-doped mesoporous carbon for fast-rechargeable lithium-ion batteries. Energy. Storage. Mater. 2019, 22, 147-53.
117. Lu, Y.; Zhang, Q.; Li, F.; Chen, J. Emerging lithiated organic cathode materials for lithium-ion full batteries. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216047.
118. Jiang, W.; Yang, X.; Deng, J.; Zhang, J.; Zhang, G. Polydopamine-based materials applied in Li-ion batteries: a review. J. Mater. Sci. 2021, 56, 19359-82.
119. Li, T.; Ding, B.; Wang, J.; et al. Sandwich-structured ordered mesoporous polydopamine/MXene hybrids as high-performance anodes for lithium-ion batteries. ACS. Appl. Mater. Interfaces. 2020, 12, 14993-5001.
120. Dai, J.; Shi, C.; Li, C.; et al. A rational design of separator with substantially enhanced thermal features for lithium-ion batteries by the polydopamine–ceramic composite modification of polyolefin membranes. Energy. Environ. Sci. 2016, 9, 3252-61.
121. Jin, T.; Han, Q.; Jiao, L. Binder-free electrodes for advanced sodium-ion batteries. Adv. Mater. 2020, 32, e1806304.
122. Xue, P.; Zhai, Y.; Wang, N.; et al. Selenium@Hollow mesoporous carbon composites for high-rate and long-cycling lithium/sodium-ion batteries. Chem. Eng. J. 2020, 392, 123676.
123. Liu, L.; He, Y.; Yin, S.; et al. Bimodal ordered porous hierarchies from cooperative soft-hard template pairs. Matter 2023, 6, 3099-111.
124. Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y. Heteroatom-doped carbon materials: synthesis, mechanism, and application for sodium-ion batteries. Small. Methods. 2019, 3, 1800323.
125. Yu, M.; Sun, M.; Zhu, L.; et al. Double-shell and hierarchical porous nitrogen-doped carbon nanocages as superior anode material for advanced sodium-ion batteries. J. Energy. Storage. 2024, 86, 111211.
126. Min, X.; Xiao, J.; Fang, M.; et al. Potassium-ion batteries: outlook on present and future technologies. Energy. Environ. Sci. 2021, 14, 2186-243.
127. Xu, Y.; Zhang, C.; Zhou, M.; et al. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat. Commun. 2018, 9, 1720.
128. Li, X.; Guan, Q.; Zhuang, Z.; et al. Ordered mesoporous carbon grafted MXene catalytic heterostructure as Li-ion kinetic pump toward high-efficient sulfur/sulfide conversions for Li-S battery. ACS. Nano. 2023, 17, 1653-62.
129. Li, W.; Xu, H.; Zhang, H.; et al. Tuning electron delocalization of hydrogen-bonded organic framework cathode for high-performance zinc-organic batteries. Nat. Commun. 2023, 14, 5235.
130. Du, H.; Zhao, R.; Yang, Y.; Liu, Z.; Qie, L.; Huang, Y. High-capacity and long-life zinc electrodeposition enabled by a self-healable and desolvation shield for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114789.
131. Zhao, J.; Burke, A. F. Review on supercapacitors: technologies and performance evaluation. J. Energy. Chem. 2021, 59, 276-91.
132. Zhang, Q.; Deng, C.; Huang, Z.; et al. Dual-silica template-mediated synthesis of nitrogen-doped mesoporous carbon nanotubes for supercapacitor applications. Small 2023, 19, e2205725.
133. Kim, M.; Park, T.; Wang, C.; et al. Tailored nanoarchitecturing of microporous ZIF-8 to hierarchically porous double-shell carbons and their intrinsic electrochemical property. ACS. Appl. Mater. Interfaces. 2020, 12, 34065-73.
135. Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: from material to interfacial descriptors. Chem. Sci. 2019, 10, 9165-81.
136. Zhai, W.; Ma, Y.; Chen, D.; Ho, J. C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357.
137. Zhang, H.; An, P.; Zhou, W.; et al. Dynamic traction of lattice-confined platinum atoms into mesoporous carbon matrix for hydrogen evolution reaction. Sci. Adv. 2018, 4, eaao6657.
138. Chen, C.; Tang, Z. J.; Li, J. Y.; et al. MnO enabling highly efficient and stable Co-Nx/C for oxygen reduction reaction in both acidic and alkaline media. Adv. Funct. Mater. 2023, 33, 2210143.
139. Wang, X.; Zhang, Q.; Jiang, H.; et al. In situ alloying with hybrid mesoporous Fe–N–C to accelerate the catalysis efficiency of Pt for the oxygen reduction reaction. ACS. Sustainable. Chem. Eng. 2023, 11, 10051-60.
140. Lee, S. H.; Kim, J.; Chung, D. Y.; et al. Design principle of Fe-N-C electrocatalysts: how to optimize multimodal porous structures? J. Am. Chem. Soc. 2019, 141, 2035-45.
141. Zhang, Q.; Xiao, W.; Guo, W. H.; et al. Macroporous array induced multiscale modulation at the surface/interface of Co(OH)2/NiMo self-supporting electrode for effective overall water splitting. Adv. Funct. Mater. 2021, 31, 2102117.
142. Li, Z.; Zhang, X.; Cheng, H.; et al. Confined synthesis of 2D nanostructured materials toward electrocatalysis. Adv. Energy. Mater. 2020, 10, 1900486.
143. Guo, Y.; Tang, J.; Henzie, J.; et al. Assembly of hollow mesoporous nanoarchitectures composed of ultrafine Mo2C nanoparticles on N-doped carbon nanosheets for efficient electrocatalytic reduction of oxygen. Mater. Horiz. 2017, 4, 1171-7.
144. Zhang, J. Y.; Xia, C.; Su, Y.; et al. Boosted oxygen kinetics of hierarchically mesoporous Mo2C/C for high-current-density Zn-air battery. Small 2024, 20, e2307378.
145. Li, W.; Liu, J.; Guo, P.; et al. Co/CoP heterojunction on hierarchically ordered porous carbon as a highly efficient electrocatalyst for hydrogen and oxygen evolution. Adv. Energy. Mater. 2021, 11, 2102134.
146. Langevelde PH, Katsounaros I, Koper MT. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 2021, 5, 290-4.
147. Nguyen, N. N.; Nguyen, A. V. “Nanoreactors” for boosting gas hydrate formation toward energy storage applications. ACS. Nano. 2022, 16, 11504-15.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data





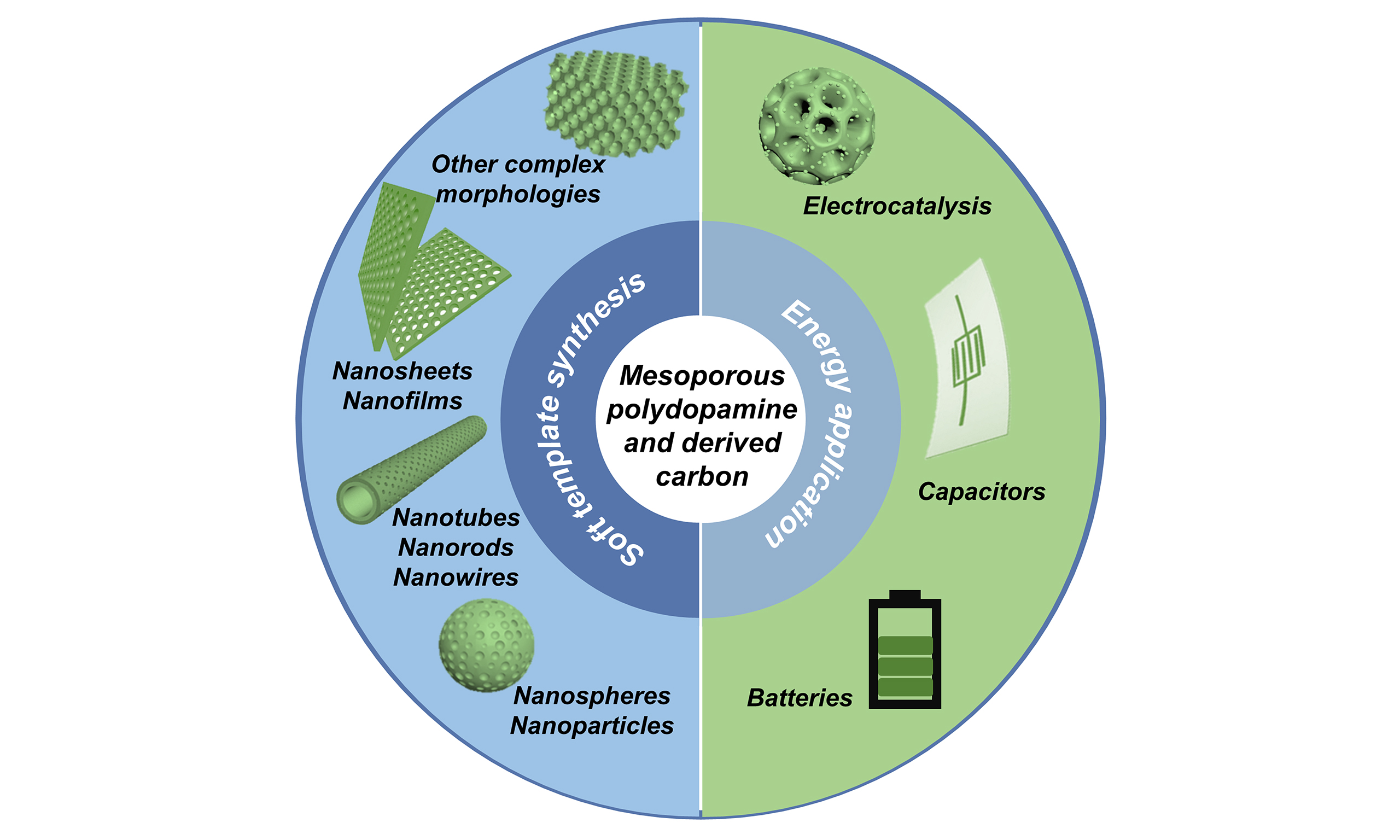
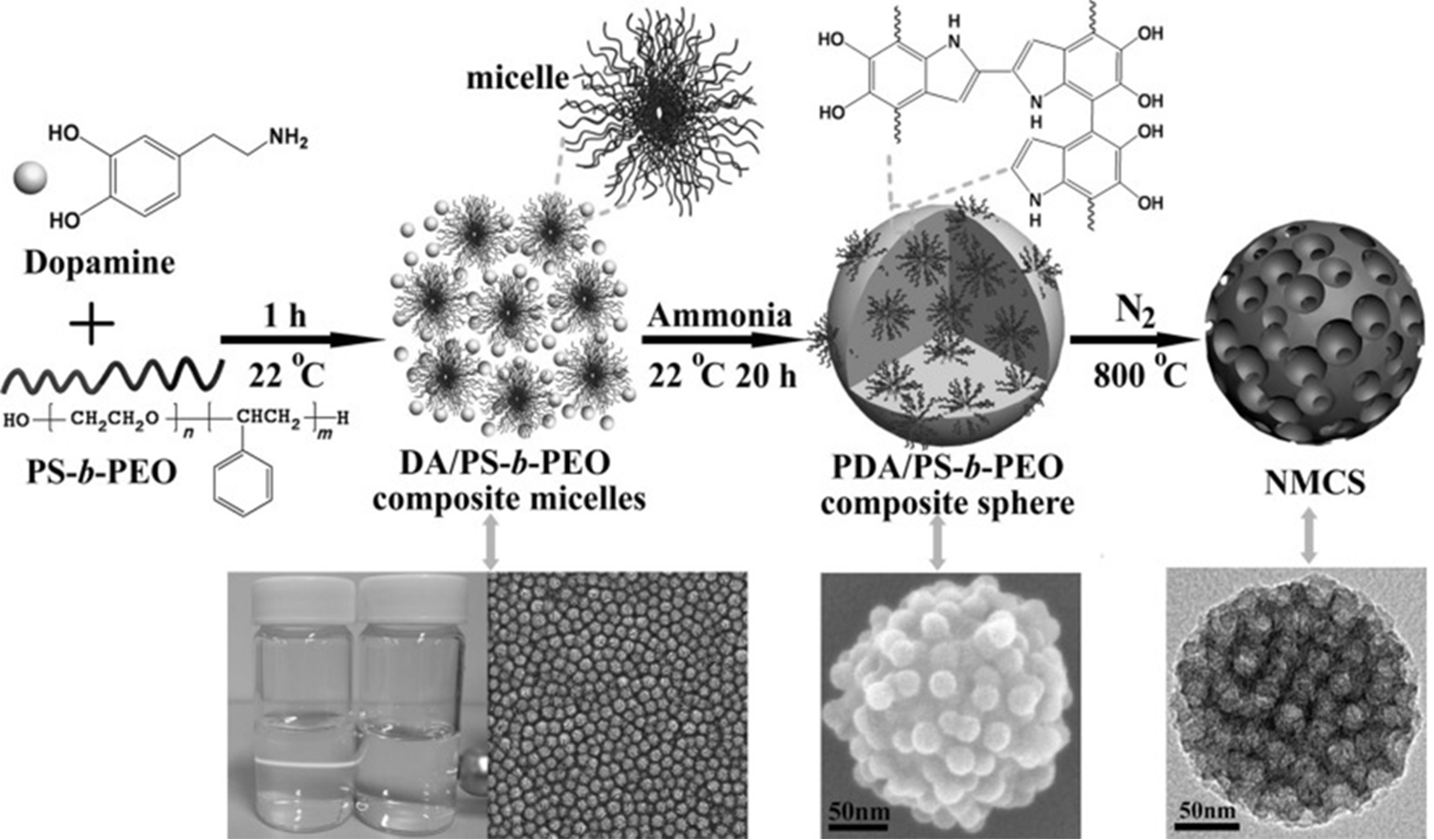
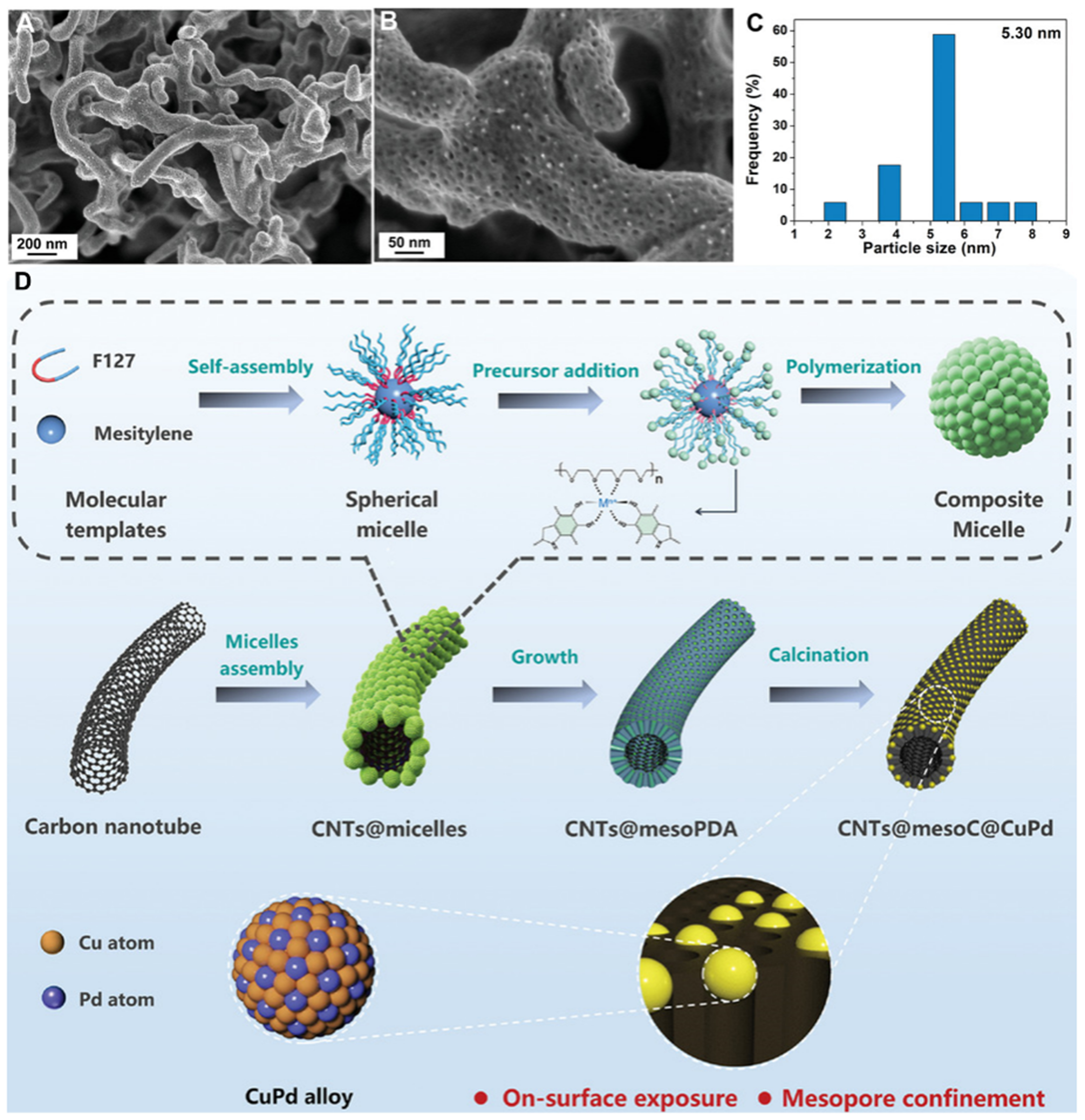
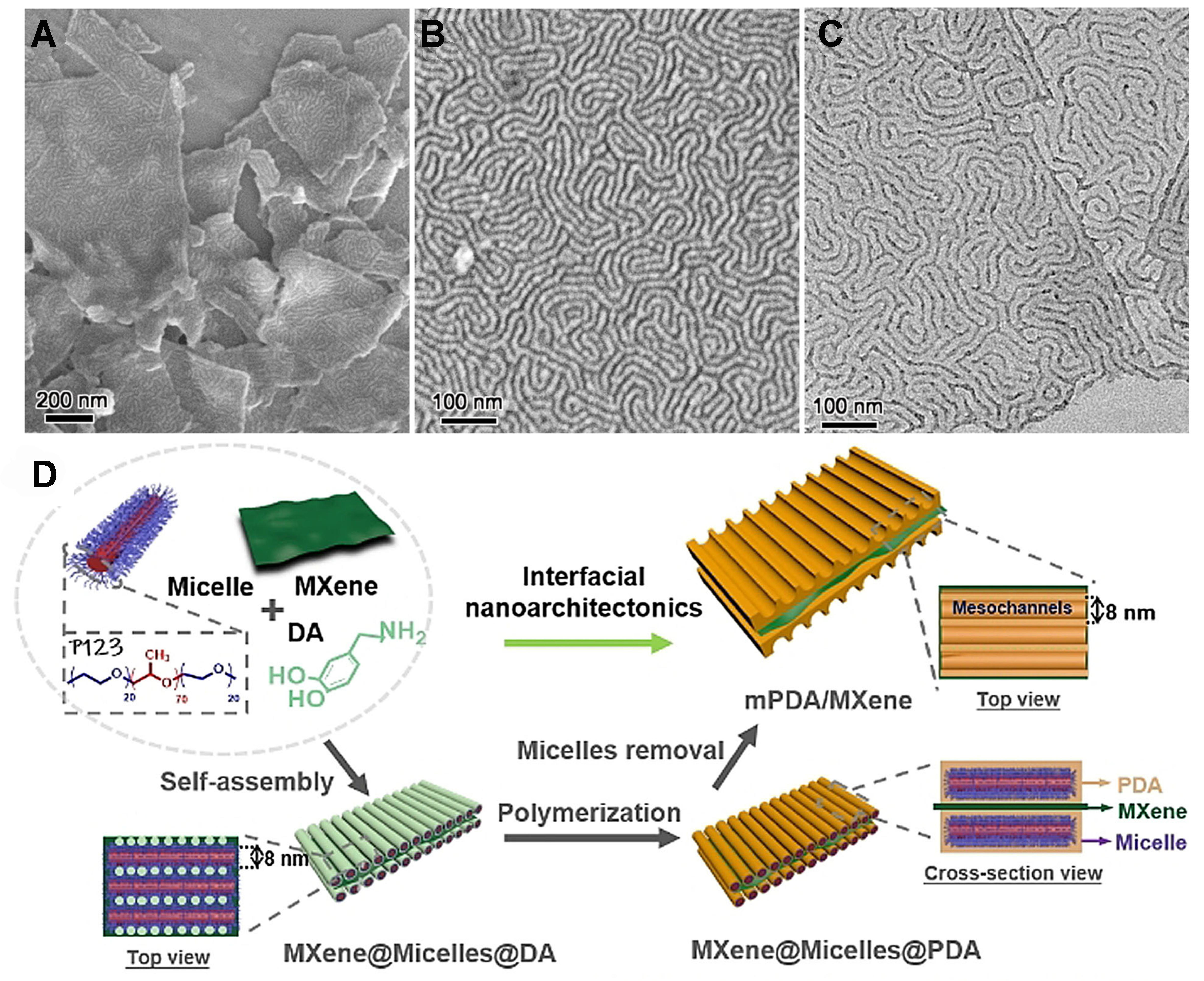
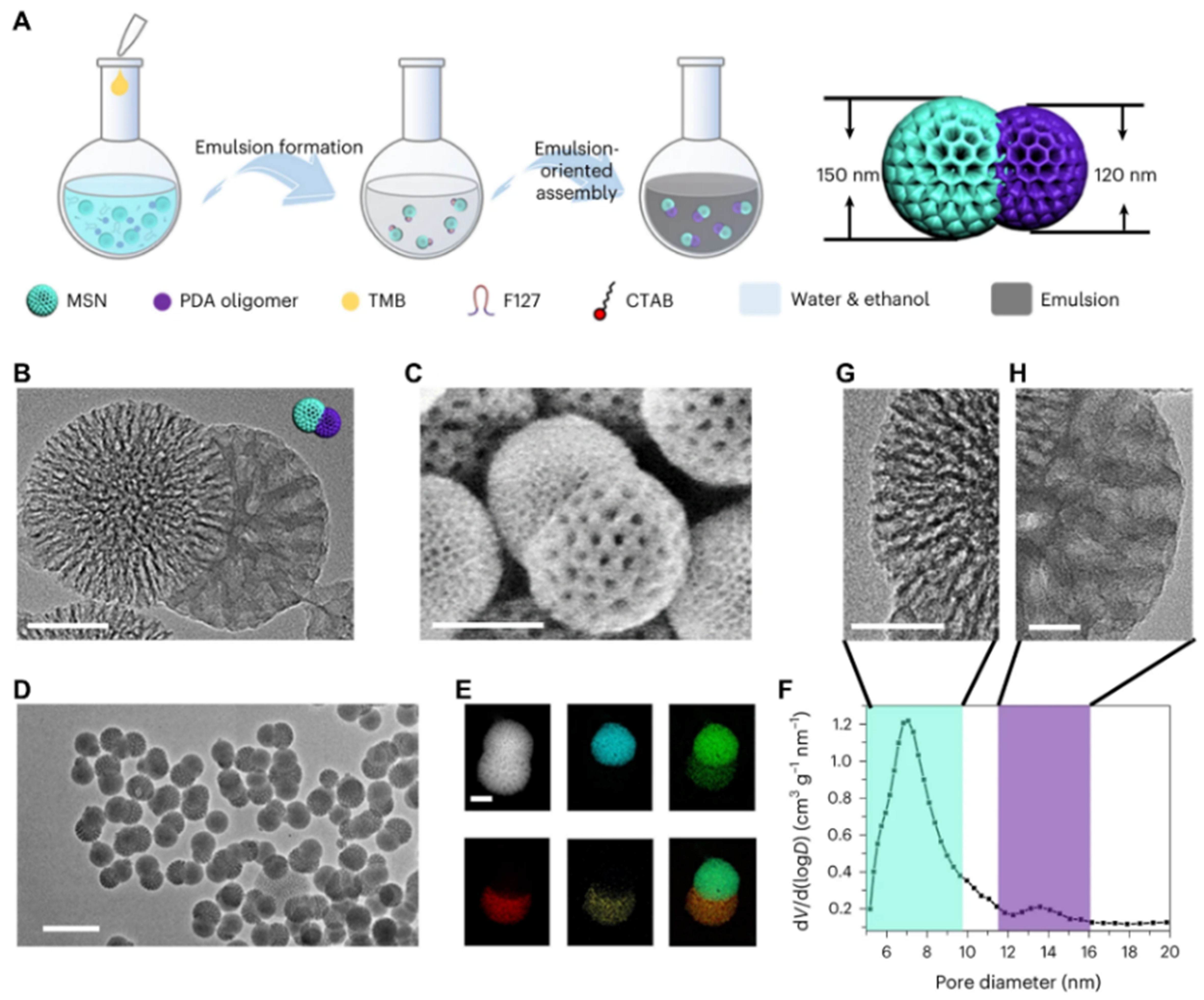
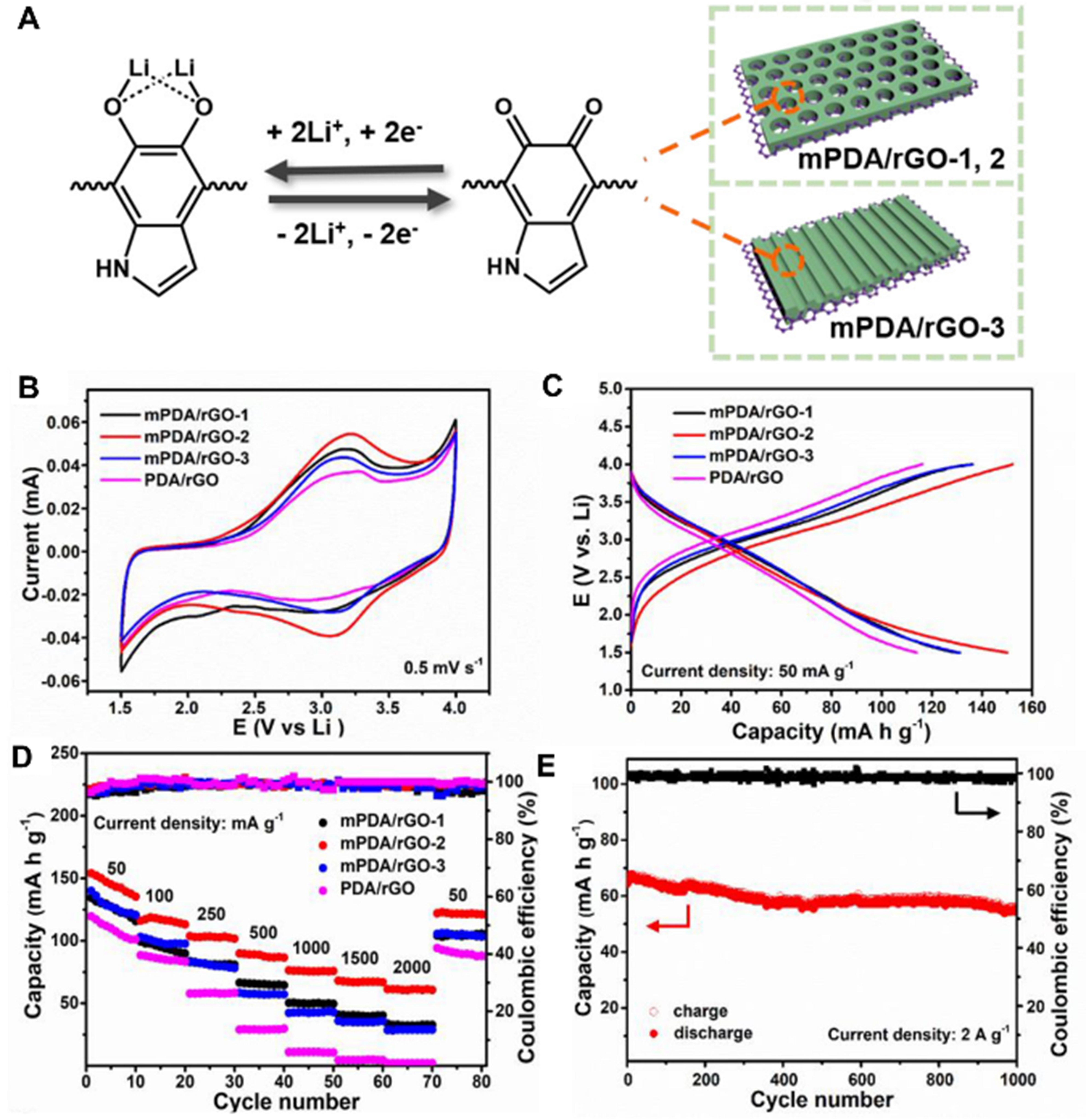
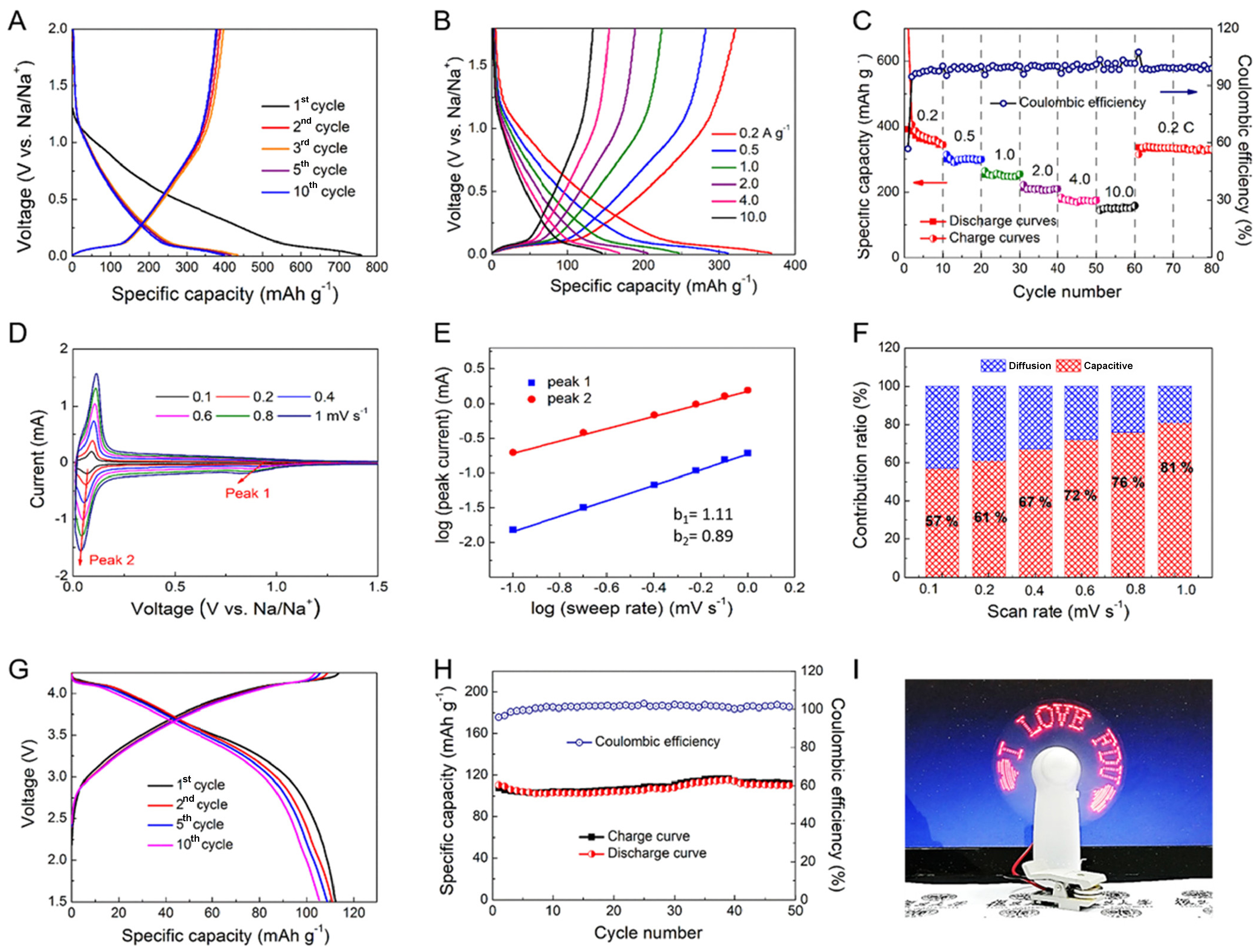
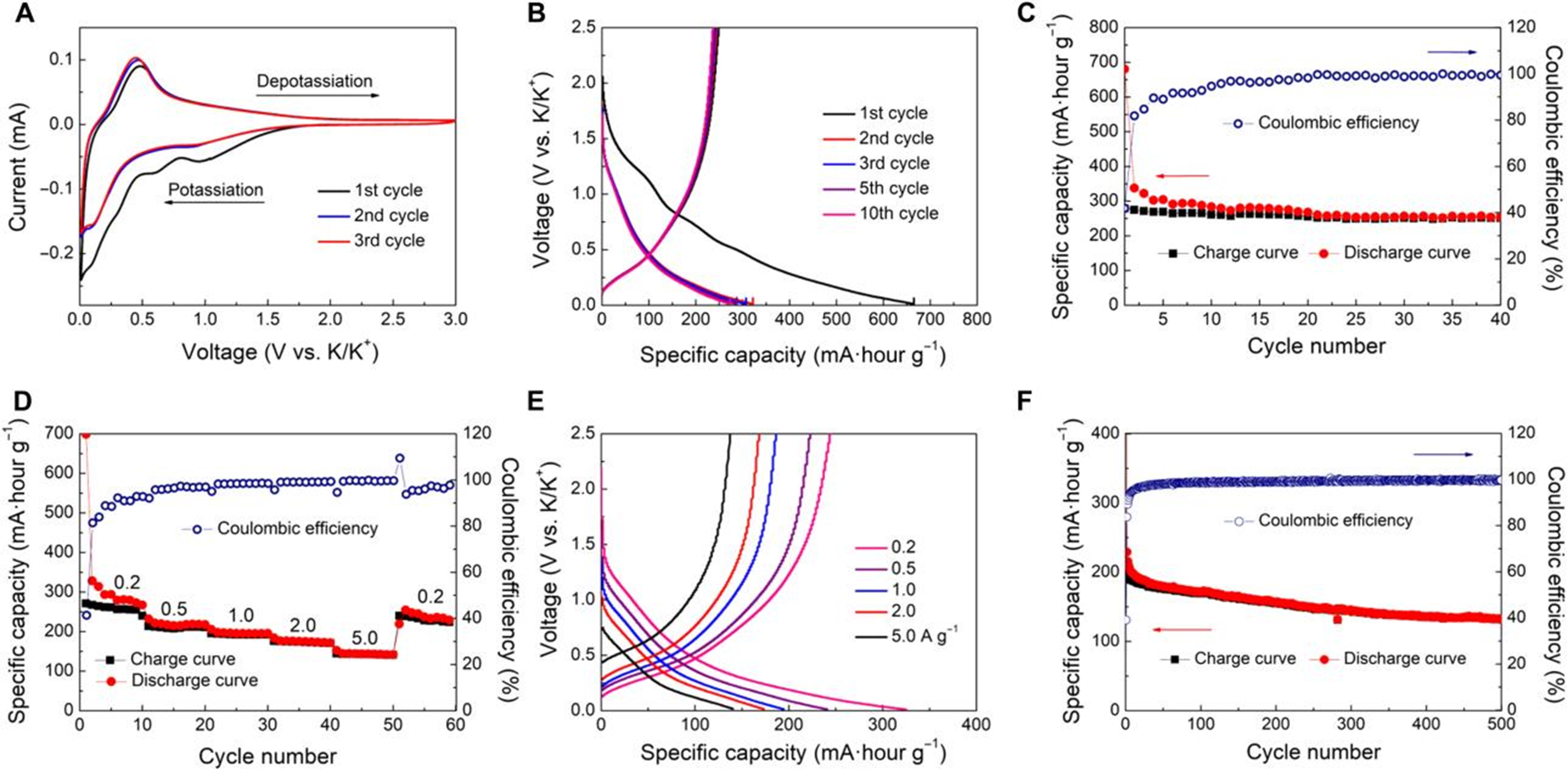
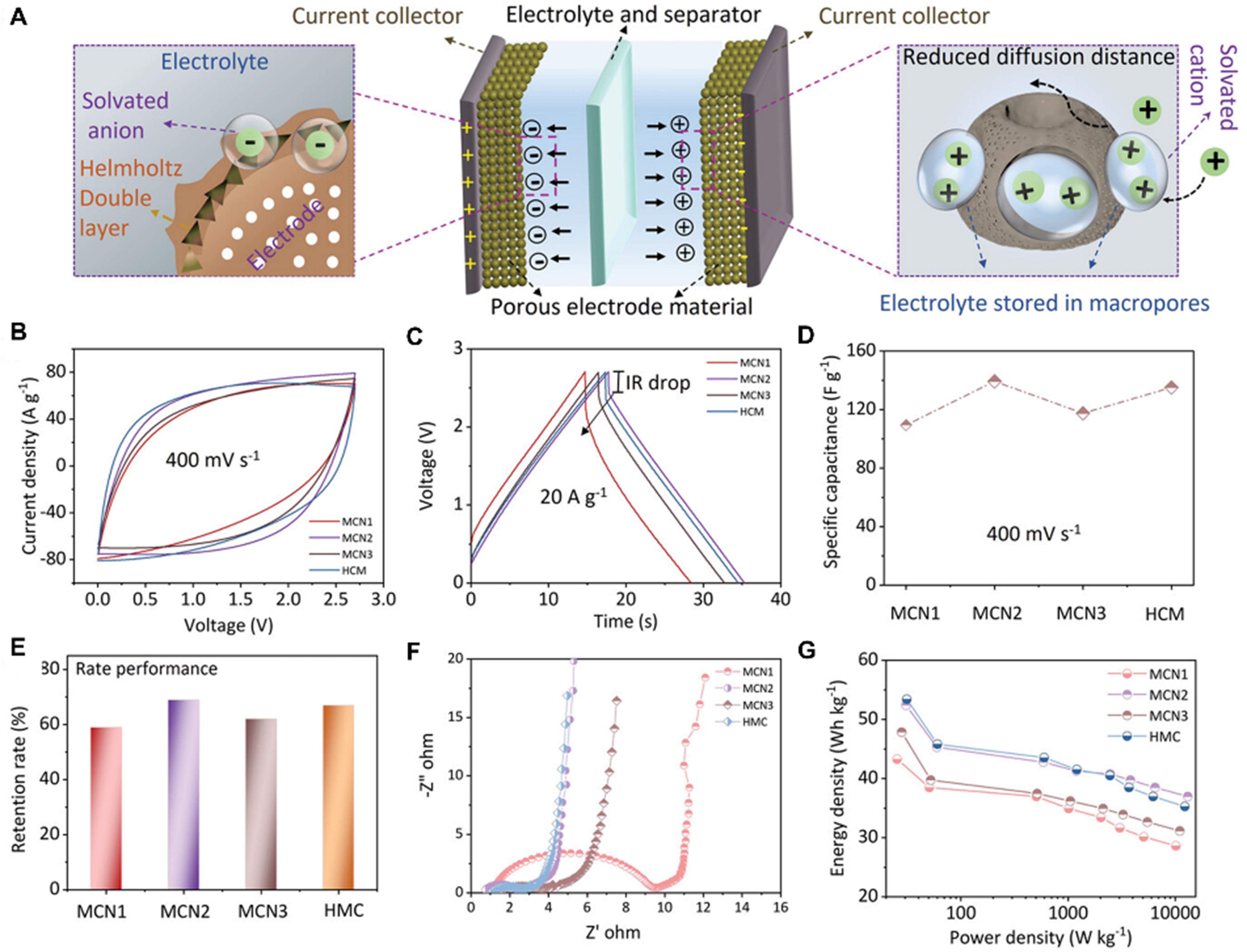
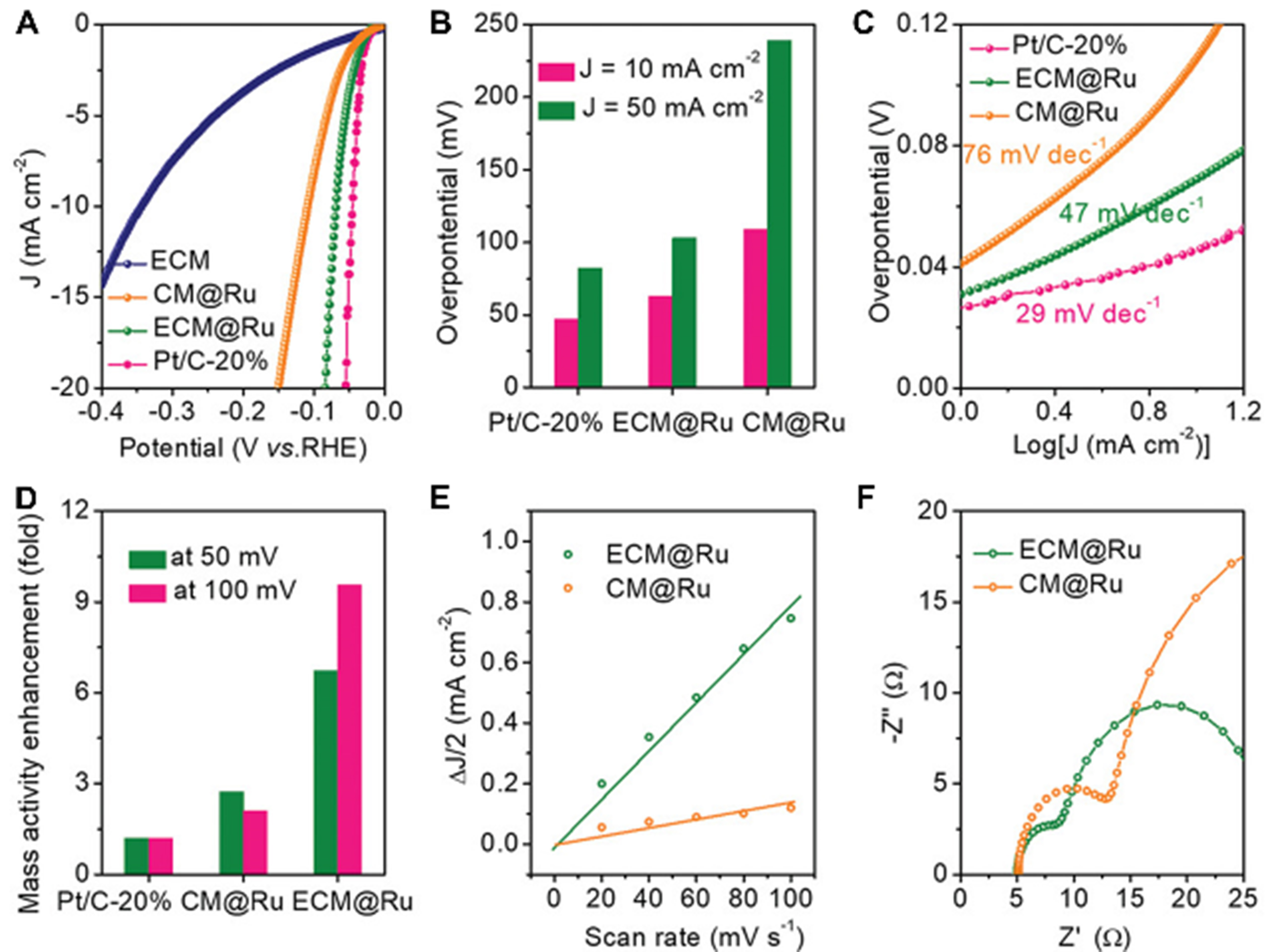
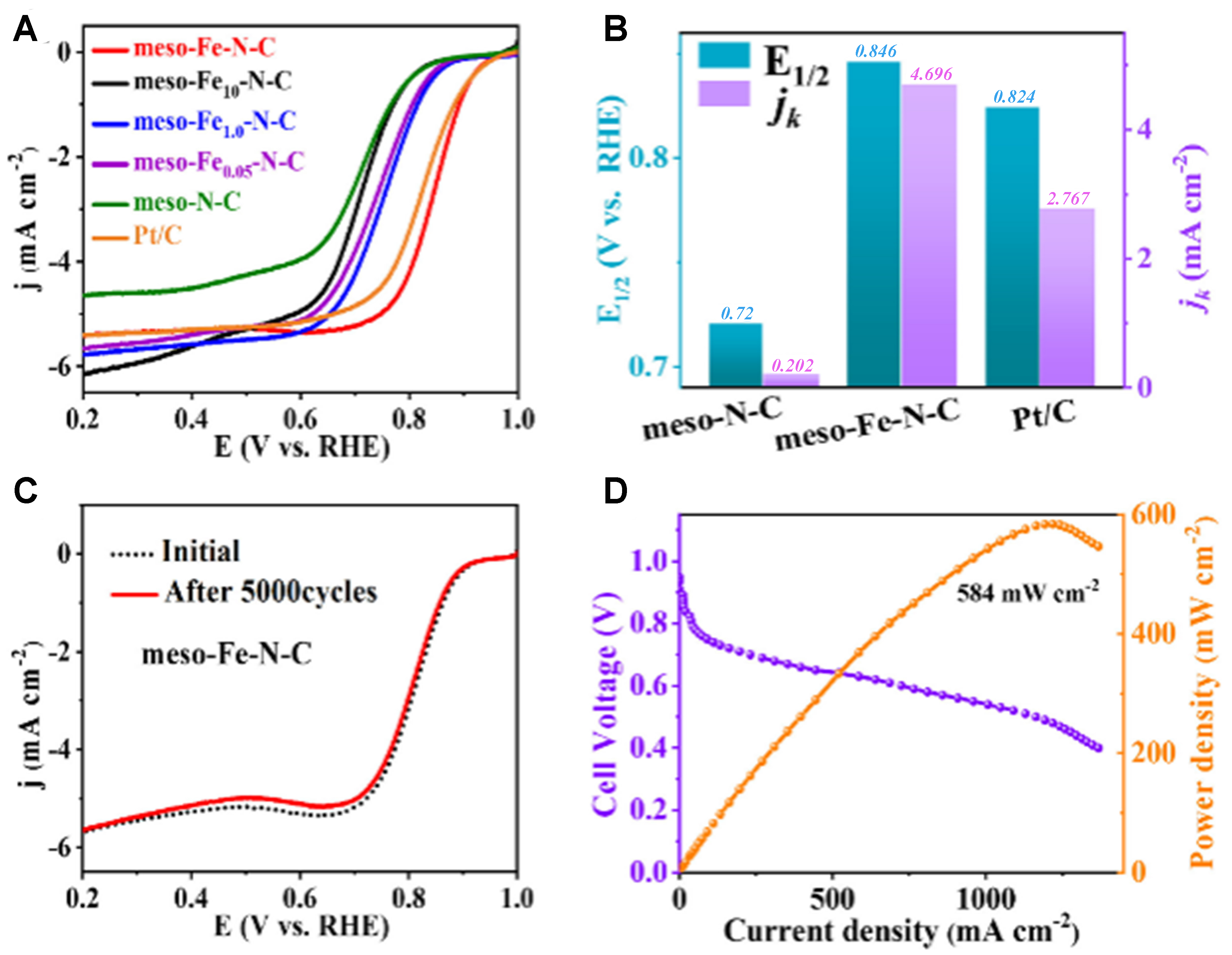













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].