Recent advances in green synthesis of porous organic frameworks
Abstract
Recently, porous organic frameworks (POFs) have emerged as functional materials and have been widely used in various applications. Crystalline POFs include covalent organic frameworks (COFs) and partial crystalline covalent triazine frameworks (CTFs). Amorphous porous organic materials are mainly divided into porous aromatic frameworks (PAFs), conjugated microporous polymers (CMPs), and hypercrosslinked porous polymers (HCPs). Although POFs have many unique structural features and excellent performance, the harsh synthesis conditions and difficulty in large-scale production have always limited their widespread use. Therefore, more researchers are paying attention to developing green, energy-saving, and environmentally friendly synthesis processes for large-scale preparation of POFs. Herein, we provide a timely overview on green synthesis of POFs and critically discuss some typical research work in detail. Meanwhile, the green synthesis strategies of POFs are emphatically described to categorize relevant reports. Finally, the challenges and opportunities of green synthetic POFs in the future are proposed according to the above classification research.
Keywords
INTRODUCTION
Porous materials, as an important class of functional materials, are formed by two parts: the interconnected solid-state skeleton and the permanent pore generated by gas or liquid phases[1]. According to the International Union of Pure and Applied Chemistry (IUPAC), they are categorized into three types based on pore size: microporous materials (< 2 nm), mesoporous materials (2-50 nm), and macroporous materials (> 50 nm). Except for the commonly classic inorganic porous materials, such as zeolites[2,3], porous carbons[4], mesoporous silicon materials[5], porous metal materials[6], and metal-organic frameworks[7,8], porous organic frameworks (POFs) as a rising class of functional porous materials attract a huge amount of interest from many researchers because of their rich porous structures from micro to mesopores, high specific surface areas, atomic level controllability, and excellent stability[9]. Generally, POFs are mainly divided into two categories, crystalline and amorphous materials on the basis of their orderliness. The important crystalline materials are covalent organic frameworks (COFs) and partial crystalline covalent triazine frameworks (CTFs) with highly ordered structures[10,11]. Other classic amorphous POFs principally include three types: porous aromatic frameworks (PAFs)[12-14], conjugated microporous polymers (CMPs)[15,16], and hypercrosslinked porous polymers (HCPs)[17,18]. These porous materials have a certain degree of short-range ordering, but lack long-range ordered structures. Notably, there is no clear boundary for the classification of these amorphous POFs.
The pore structure plays the most important role in different applications of POFs. To construct permanent pore structures and avoid the nonporous phenomenon caused by the dense stacking of organic molecules, the rigid and non-planar organic molecular blocks are always designed and used to form POFs[19,20]. The pore environment of POFs can be precisely regulated by the molecular size, configuration, functional site, organic coupling method, and synthetic condition[21,22]. The synthetic reactions are selected according to diverse organic coupling functional groups. The commonly used approaches contain Suzuki coupling[23], Yamamoto coupling[24], Schiff-base condensation[25], Friedel-Crafts alkylation[26], Sonogashira-Hagihara cross-coupling[27], Knoevenagel condensation reactions[28], Ullmann coupling[29], and aldol condensations[30].
Although various types of POFs have been extensively prepared to form a large research system, the synthesis of porous materials still faces severe problems. The solvothermal method is the most classical synthesis approach for POFs, but this method has various disadvantages. (1) Harmful organic solvents, such as tritylene, 1,4-dioxane, N, N-dimethylformamide, dimethyl sulfoxide and dichlorobenzene, are usually used as reaction media in the reaction system; (2) The operation process is very complex and strict, including cyclic freezing, degassing protection, flame sealing, high temperature, and long reaction time; (3) The synthesis scale is relatively small, making it difficult to meet the needs of large-scale production. In general, the traditional synthesis method includes harsh reaction conditions, high energy consumption, heavy pollution, and small-scale preparation to greatly limit their widespread applications. Hence, it is necessary to develop green and environmentally friendly synthetic strategies for the large-scale preparation of functional POFs.
The concept of green chemistry is proposed in the fields of organic synthesis and industrial chemical processes. In recent years, different green synthesis processes have emerged indirectly in the preparation of POF materials. As an emerging field of importance, only a few reviews focus on green synthesis of COFs[31,32]. However, there is a lack of novel and systematic summary on the design and preparation of POFs via green synthesis technology. In this review, we summarize the recent advances in green synthesis of POFs and discuss some typical examples in detail [Scheme 1]. Furthermore, the challenges and opportunities of this field in the future are proposed.
POROUS ORGANIC MATERIALS
Porous organic materials are intrinsic porous materials formed by covalent bonding specific structural units with geometric shapes using a molecular design method[33]. POFs have the basic characteristics of light weight, abundant pore, good stability, high specific surface area, functional modifiability, etc., so they can be considered as a new class of functional porous materials with outstanding performance in various fields, such as gas separation[34,35], luminescence sensor[36,37], heterogeneous catalysis[38,39], and optoelectronics[40,41]. To rationally construct POFs with unique structures and excellent functions, it is very important to investigate topological structures and reasonably select appropriate organic monomers, polymerization reactions, and synthesis conditions. Different active sites can be introduced into the organic units to affect POFs and enhance their performance in multiple applications[42,43]. POFs can be mainly divided into crystalline materials (COFs and CTFs) and amorphous frameworks (PAFs, CMPs, and HCPs) based on the skeleton order degree.
Crystalline porous organic materials
Crystalline porous organic materials with long-range ordered structures and well-defined pores, such as COFs and crystalline CTFs, are widely used as functional materials. These materials attract lots of research interest in various application fields. In fact, crystalline CTFs with special covalent triazine functional groups also belong to COFs[44,45]. With a deeper understanding of the structures of porous crystalline materials, the suitable covalent bonds between rigid organic monomers play the most important role in forming the crystal skeletons of porous organic materials. Up to now, a growing number of COFs have been widely designed and prepared since the first reported COF in 2005[46]. Appropriate dynamic covalent bonds are key to preparing crystalline materials. The current main COF materials include four categories: B–O,
Although crystalline materials have good orderliness, there are still relatively few materials with complete single crystals to determine their fine structures[64-69]. In general, crystal structures of crystalline POFs are determined through simulation and comparison with powder X-ray diffraction (PXRD) characteristic peaks. This is also a thorny problem that most researchers are trying to solve in the research field of crystalline porous organic materials.
Amorphous porous organic materials
Currently, most of the reported porous COFs have a practical drawback, that is, relatively poor physicochemical stability as compared with amorphous POFs, to limit their use under extreme conditions, which is mainly attributed to the reversible covalent chemistry in crystalline COFs. An increasing number of amorphous porous organic materials, including PAFs, CMPs, and HCPs, have been designed and prepared because of their highly stable and irreversible covalent bonds through Friedel-Crafts alkylation, Suzuki coupling, Yamamoto coupling, Sonogashira-Hagihara cross-coupling, and so on[70-72]. The specific surface area of porous materials can be analyzed in detail by different adsorption models, including Langmuir theory based on the monomolecular adsorption layer and Brunauer-Emmett-Teller (BET) theory according to the multi-layer adsorption. For example, PAF-1, as a porous polyphenylene framework, was successfully prepared by nickel(0)-catalyzed Yamamoto-type Ullmann cross-coupling [Figure 2A]. PAF-1 not only exhibits excellent stability under extremely harsh conditions, but also has an unprecedented high surface area, including a BET surface area of 5,640 m2·g-1 and Langmuir surface area of 7,100 m2·g-1[73].
Figure 2. (A) The structure of PAF-1; (B-D) Synthesis schemes for PAF-1 with different functional groups. (A) Adapted with permission[73]. Copyright 2019, John Wiley and Sons. (B) Adapted with permission[76]. Copyright 2019, American Chemical Society. (C) Adapted with permission[77]. Copyright 2020, American Chemical Society. (D) Adapted with permission[78]. Copyright 2017, American Chemical Society. PAF: Porous aromatic framework.
Moreover, amorphous porous organic materials can be easily functionalized by the monomer design or post-modification, resulting in the superior targeting function of as-synthesized materials[74,75]. Recently, PAF-1, as a research mode, has been separately modified by different functional groups, resulting in its excellent performance in various applications [Figure 2B-D][76-78]. According to their excellent physicochemical stability, rich skeletal structures, and good controllability, amorphous POFs have been widely prepared as a potentially universal platform for numerous application fields.
GREEN SYNTHESIS
Multiple synthesis strategies have been developed to construct porous organic materials, but the commonly used solvent thermal method still needs high temperature and pressure which directly affect the cost, output, and industrial usability of POFs. In order to achieve energy-saving, environmentally friendly, and high-yield preparation, various green synthesis strategies for POFs have been successively explored and developed in recent years.
Room temperature synthesis
Synthesis at room temperature is convenient and economical because it does not require the use of special high or low temperature equipment. This method reduces energy consumption and avoids some temperature-induced side reactions. In addition, the mild synthetic approach can protect the high-temperature sensitive functional groups to construct POFs. Therefore, it offers both energy consumption and operational convenience, leading to its potential application in synthesizing functional POFs[79-84].
The solvent thermal condition for the preparation of POFs requires the employment of hazardous organic solvents, high reaction temperatures, and intricate systems. For instance, Guo et al. described a two-step dissolution precipitation (DP) technique for the synthesis of ketoimines- and imine-linked COFs in an aqueous phase [Figure 3A][85]. Using this DP approach, five ketoimine- and two imine-linked COFs were remarkably manufactured in one reaction at room temperature with a reaction time of 5 min. These COFs showed prospective uses in the adsorption of uranyl and iodine, displaying very high crystallinity and porosity. In 2024, Kong et al. reported a straightforward and eco-friendly process for room-temperature aqueous solution synthesis of imine bonded-COFs [Figure 3B][86]. It is very important to control the reaction rate for the synthesis of crystalline COFs. The reaction reactivity of aldehyde monomers in an aqueous solution was greatly increased by preactivating them with acetic acid (AcOH). The management of reaction equilibrium and product crystallization was made possible by the slightly lower imine production rate and higher imine cleavage rate in an aqueous solution compared to the traditional solvothermal synthesis. Consequently, the crystalline COFs with a large surface area and high yield can be generated in a very short space of time.
Figure 3. (A) Synthesis of model compounds and dpCOF-1; (B) Schematic depiction of imine-linked COFs and the possible formation mechanism; (C) Schematic diagram of a bio-friendly one-pot synthesis of different HRP-COFs. (A) Adapted with permission[85]. Copyright 2023, American Chemical Society. (B) Adapted with permission[86]. Copyright 2024, American Chemical Society. (C) Adapted with permission[87]. Copyright 2023, John Wiley and Sons. COF: Covalent organic framework; HRP: horseradish peroxidase.
In addition, the highly stable and non-metallic properties of COFs make them ideal carriers for enzyme immobilization. Liang et al. constructed three types of COFs [benzene-1,3,5-tricarboxaldehyde and p-phenylenediamine co-condensation framework (COF-LZU1), benzene-1,3,5-tricarboxaldehyde and 1,3,5-tris(4-aminophenyl)benzene co-condensation imine-linked framework (RT-COF-1), and benzene-1,3,5-tricarboxaldehyde and hydrazine hydrate co-condensation azine-linked framework (ACOF-1)] using a bio-friendly one-pot synthesis strategy in an aqueous solution at room temperature. COF-LZU1 bound to horseradish peroxidase (HRP) was found to maintain the highest activity [Figure 3C][87]. The mild room temperature reaction condition not only reduces the energy consumption and cost, but also can provide an effective synthesis strategy for in situ encapsulation of temperature-sensitive substances, such as biomolecules, enzymes, etc., into COFs.
The synthesis of POFs at the liquid-liquid interface at room temperature is a highly anticipated green synthesis method, which can easily and efficiently prepare POF films with adjustable thickness to bring new possibilities to the field of material science[88,89]. For example, He et al. proposed a liquid-liquid IP method to address the challenges in the field of molecular separation[Figure 4A][90]. This method restricts the polymerization reaction through the liquid-liquid interface and precisely controls the growth process of COF films, achieving adjustable COF film thickness and providing good flexibility for different applications. This work provides a feasible approach for preparing high-performance COF membranes. Meanwhile, Wu et al. adopted a green synthesis strategy of liquid-liquid interfacial polymerization (IP) and successfully synthesized the ethylene-bridged COF membranes with complete sp2 carbon skeletons at room temperature [Figure 4B][91]. The COF nanospheres formed in the initial stage gradually could evolve into self-supporting COF thin films with long-range ordered arrangements after covalent self-assembly at the liquid-liquid interface. In addition, Chen et al. proposed a room-temperature liquid-liquid interface polymerization method, namely surfactant-assisted IP (SAIP) [Figure 4C][92]. By adding surfactants and toluene solvents, the self-assembled chains of zwitterionic surfactants at the interface facilitated the monomer aggregation and pre-assembly to improve their transport at the interface and promote the complete topological growth of COF membranes. This method not only saves polymerization time during the catalytic process, but also enables the preparation of highly crystalline and uniformly porous COF membranes. The interface synthesis strategy at room temperature is applicable to other porous polymers. For instance, Li et al. studied a novel method for synthesizing CMPs at room temperature interfaces [Figure 4D][93]. Using a strategy based on the Schiff-base reaction, the in-situ formation of CMP nanofilms was achieved on porous polymer substrates. During this process, the AcOH migrated from the aqueous phase to the interface, accelerating the aldehyde amine condensation reaction in the oil phase, resulting in the formation of CMP films of terephthalaldehyde (PDA)-m-phenylenediamine (MPD) and 2,5-dihydroxyterephthalaldehyde (DHA)-MPD after 15 min. This strategy provides an enormous potential for the manufacturing of thin film-based electronic and optoelectronic devices.
Figure 4. (A) The fabricating process of COF membranes with the AA stacking mode; (B) The vinylene-bridged COF thin films via an interfacial synthesis; (C) SAIP and IP strategies for the synthesis of COF membranes; (D) Interface synthesis diagram and chemical structures of CMP membranes. (A) Adapted with permission[90]. Copyright 2023, Elsevier. (B) Adapted with permission[91]. Copyright 2023, American Chemical Society. (C) Adapted with permission[92]. Copyright 2024, Elsevier. (D) Adapted with permission[93]. Copyright 2021, American Chemical Society. COF: Covalent organic framework; SAIP: surfactant-assisted interfacial polymerization; IP: interfacial polymerization; CMP: conjugated microporous polymer.
The lotion polymerization at room temperature, as an important environmental protection synthesis method, has attracted much attention in recent years. This synthetic process was used to construct porous organic materials. For example, Zhang et al. proposed a green phase transfer catalyzed lotion polymerization method, which could efficiently and quickly synthesize β-ketoenamine COFs with the controllable morphology [Figure 5A][94]. This green method could achieve the fast synthesis of COFs under mild conditions, while avoiding the use of acid catalysts and a large number of organic solvents. The pyridine cationic surfactant not only acts as an emulsifier, but also could be considered an efficient catalyst and shape modifier. This synthetic strategy is fast, simple and green for fabricating COFs. In addition, under mild conditions, Shi et al. also conducted a similar work to synthesize porous conjugated polymers with the precise mesoporous size and tunable nanostructure. The synthetic strategy mainly contains the emulsion system of toluene oil/water mixture, the surfactant of triblock copolymer F127, and the precursor of 1,3,5-tris(4-aminophenyl) benzene (TAPB) [Figure 5B][95]. The TAPB monomers in an aqueous solution were prone to self-aggregation phenomena at room temperature by the oxidative polymerization of aromatic multi-amines catalyzed by the ammonium persulfate (APS) oxidant. Notably, the mild condition could offer good compatibility with the soft-template self-assembly strategy, which was easily removed while ensuring no damage to the targeted materials. Hence, a series of uniform π-CMPs with different pore diameters were obtained through this one-pot operation.
Figure 5. (A) The synthesis description of TpPa-COF; (B) Schematic illustration of π-conjugated porous nanospheres via an emulsion-induced interface assembly strategy. (A) Adapted with permission[94]. Copyright 2023, American Chemical Society. (B) Adapted with permission[95]. Copyright 2022, John Wiley and Sons. COF: Covalent organic framework; Tp: 1,3,5-triformylphloroglucinol; TAPB: 1,3,5-tris(4-aminophenyl) benzene; APS: ammonium persulfate.
According to the above reports, room temperature synthesis is considered as a green approach to construct porous organic materials. The mild reaction condition provides an available approach to control the morphology and size of porous materials. However, room temperature conditions may lead to poor solubility of organic monomers to limit their application in the preparation of POFs. In addition, most reports still focus on porous organic materials formed by reversible covalent bonds, and the applicability of non-reversible covalent bond-based porous materials needs further exploration and certification.
Green solvent synthesis
Except for some specific synthesis methods, most porous organic materials require solvents during the synthesis process. Organic solvents can dissolve various organic monomers to provide a molecular-level reaction environment. Meanwhile, the high boiling point of organic solvents can allow the synthetic reactions to occur at high temperatures. However, organic solvents are expensive and toxic, which also need to be solved from the perspective of green environmental protection. In the realm of green chemistry, numerous researchers have put forth a variety of creative techniques and strategies to prepare POFs via green solvents. For instance, Zhao et al. successfully created high crystallinity COF materials with rich pore structures in a short reaction time by utilizing the ultrasonic chemical synthesis method and green solvents
Figure 6. (A) The sonochemical synthesis for crystalline COFs; (B) The ATPS approach for COF membranes; (C) The “in-water” strategy is inspired by the “prodrug mimic” design methodology. (A) Adapted with permission[96]. Copyright 2022, Springer Nature. (B) Adapted with permission[97]. Copyright 2022, Springer Nature. (C) Adapted with permission[98]. Copyright 2023, American Chemical Society. COF: Covalent organic framework; ATPS: aqueous two-phase system; Dex: dextran; PEG: polyethylene glycol.
On the other hand, the aqueous reaction system is thought of as an available green synthesis method to construct POFs, especially two-dimensional thin films. Recently, Wang et al. made a breakthrough in the construction of membranes in non-organic solvent systems [Figure 6B][97]. The aqueous two-phase system (ATPS) approach was used to prepare COF membranes from a green chemistry perspective. In order to create a dual aqueous system, the organic monomers of COFs were dissolved in the lower aqueous phase of dextran (Dex) and the upper aqueous phase of polyethylene glycol (PEG), respectively. IP was a good platform to create COF membranes with varying levels of crystallinity. The significant result showed that the continuous COF membranes throughout the film-forming process depended critically on the proper water-water interface tension. This study could be applied to produce premium membrane materials, serving as an environmentally friendly membrane manufacturing technique. Additionally, Hu et al. were able to create COF colloidal dispersions by adding polar groups, which increased the water solubility of aldehydes. They demonstrated the overall procedure for synthesizing processable COFs in water via the sol-gel technique [Figure 6C][98]. In addition to offering significant insights for the sustainable preparation in the realm of green chemistry, this proposed sol-gel strategy could be applied for shaping and synthesizing β-ketoenamine-linked two-dimensional COFs.
Besides the common COFs formed by the dynamic aldehyde-amine condensation, ethylene-linked COFs have attracted lots of attention due to their outstanding stability. In recent years, some researchers have focused on the study of the crystallinity and ionic properties of olefin- or vinylene-linked COFs via green synthetic approaches. A typical example was reported by Zhang et al. in 2023. In this work, the authors utilized the DMAP catalyst and hydrothermal conditions to successfully synthesize highly crystalline and robust zwitterionic vinylene-linked COFs (ZVCOFs) from the pre-designed zwitterionic building blocks
Figure 7. (A) Schematic illustration for constructing ZVCOFs; (B) The synthetic process of V-C2DPs through the SMAIS method. (A) Adapted with permission[99]. Copyright 2023, American Chemical Society. (B) Adapted with permission[100]. Copyright 2024, John Wiley and Sons. COF: Covalent organic framework; DMAP: 4-dimethylaminopyridine; ZVCOF: zwitterionic vinylene-linked COF; V-C2DPs: vinylene-linked two-dimensional polymer; SMAIS: surfactant-monolayer assisted interfacial synthesis.
Furthermore, some reports have been focused on the synthesis of porous organic materials in water systems[101-104]. Many researches show that green solvent synthesis is considered as a feasible approach to construct porous materials. Obviously, water is the most commonly used and ideal green solvent. However, most organic molecules have low solubility in water, which causes significant challenges for the fabrication of POFs in aqueous systems. Meanwhile, the correlational research on porous materials linked by different covalent bonds still needs to be further investigated and explored.
Solvent-free synthesis
Solvent-free synthesis can avoid the use and removal of organic solvents during the preparation of porous organic materials. From the perspectives of energy conservation, environmental protection, ease of operation, and cost-effectiveness, the solvent-free strategy is highly appealing and offers potential application value for large-scale POF production.
Recently, the use of molten salts as the reaction solvent, structure-directing agent, and catalyst has been proven to be an efficient and green method to synthesize POFs. Especially, CTFs, as a class of porous organic materials, are commonly prepared by the cyclotrimerization of aromatic nitriles in the molten ZnCl2 salt at high temperatures[105-110]. Metal salts are considered as Lewis acid sites to catalyze the trimerization reaction, and the molten reaction system can accelerate the mass transfer process. For example, Rangaraj
Figure 8. (A) Schematic diagram of the preparation process of Pz-CTFs; (B) Ionothermal synthetic CTF-based photocatalysts via a ternary NaCl-KCl-ZnCl2 system; (C) The polymerization reaction for CTFs by the KCl-catalytic approach; (D) SEM and (E) EDS element mappings of KCl nanoparticles coated by CTF-DCB NSs; (F) SEM, (G) AFM, and (H) PXRD of CTF-DCB NSs; (I) Large-scale preparation of CTF-DCB NSs. (A) Adapted with permission[111]. Copyright 2022, Elsevier. (B) Adapted with permission[112]. Copyright 2022, John Wiley and Sons. (C-I) Adapted with permission[113]. Copyright 2023, American Chemical Society. Pz-CTF: Phosphonitrilic core covalent triazine framework; HCPz: hexakis(oxy)hexabenzonitrile phosphazene; SEM: scanning electron microscopy; EDS: energy-dispersive spectrometry; CTF-DCB NS: covalent triazine framework-dicyanobenzene nanosheet; AFM: atomic force microscopy; PXRD: powder X-ray diffraction.
To further overcome the shortcomings of molten metal salts, different catalysts have been used to prepare CTFs in recent years. One reported catalyst is P2O5, which can catalyze the trimerization reaction of aromatic primary amide groups to s-triazine rings. As seen in Figure 9A and B, a two-dimensional stacked covalent triazine-based framework (pCTF-1) with a large surface area was synthesized via the P2O5-catalyzed dehydration condensation of terephthalamide (TA) molecules[114]. This porous material exhibited high CO2 and H2 sorption abilities. Another research team prepared pCTF by P2O5 to detect NH3 gas at room temperature[115]. Both reports demonstrated that aromatic amide and nitrile compounds could be catalyzed by P2O5 at high temperatures to produce porous CTFs. Meanwhile, H6P4O13 as a commonly used polyphosphoric acid was applied to catalyze the nitrile trimerization reaction, leading to the formation of four two-dimensional crystalline CTFs as confirmed by PXRD patterns [Figure 9C-G][116]. Especially, the high crystallinity of as-synthesized CTFs endowed them with large surface areas in the range of 794-
Figure 9. (A) The P2O5-catalyzed dehydration condensation for the synthesis of pCTF-1; (B) The crystal structure of pCTF-1; (C) The synthetic strategy of crystalline CTFs by H6P4O13; (D-G) PXRD patterns of different as-synthesized CTFs; (H) N2 sorption isotherms at 77 K and (I) pore size distributions of four CTFs; (J) The large-scale preparation of CTF-DCB. (A and B) Adapted with permission[114]. Copyright 2018, John Wiley and Sons. (C-J) Adapted with permission[116]. Copyright 2022, John Wiley and Sons. TA: Terephthalamide; DCB: 1,4-dicyanobenzene; CTF: covalent triazine framework; TCB: 1,3,5-tris(N-carbazolyl)benzene; PXRD: powder X-ray diffraction.
Furthermore, it is still a formidable challenge for the large-scale and low-cost synthesis of POFs to meet the industrial demand. Recently, Zhang et al. have focused on the development of novel green strategies to prepare COFs in large quantities. One example was reported to produce COFs with highly ordered structures[117]. In this work, the authors attempted to efficiently prepare collidine-based COFs with olefin linkages via a green melt polymerization strategy of PDA and 2,4,6-collidine [Figure 10A]. Referring to the research from Wang et al., benzoic anhydride was considered as a potential molecule to balance crystallization and polymerization, leading to the generation of COFs with outstanding crystallinity. As a comparison, no product was generated using the traditional solvothermal method, and an amorphous polymer was found through the direct reaction without benzoic anhydride and its derivatives. It is worth noting that over one kilogram of COFs could be acquired at low costs, indicating the enormous potential application value in the industrial field. On the other hand, this research group further proposed an organic flux-mediated synthesis to construct highly crystalline irreversible-linked COFs, which showed more outstanding application potentials than the reversible-linked COFs due to the higher stability and structural robustness of the irreversible-linked COFs[118]. Nevertheless, feasible and green synthetic methods are lacking for the irreversible-linked COFs. As seen in Figure 10B, the acidic molecules were used as modulators and reactive fluxes to prepare imide-linked COFs. The possible catalytic mechanism mainly contains two steps. The amide precursors were obtained by the reaction between benzoic acid and triamine, leading to good solubility in the benzoic acid flux. Another organic monomer could slowly replace the previously formed precursors through the covalent bond conversion from amide to imide, resulting in the generation of the targeted imide-linked COFs. The same COFs were prepared by the traditional solvothermal approach, which were used as comparative materials. What is even more exciting is that the flu-synthesized COFs exhibited larger surface areas and higher crystallinity. The flux synthesis provides a convenient gram-scale synthetic strategy to obtain crystalline irreversible-linkage COFs.
Figure 10. (A) Synthetic strategies, optics pictures, and simulated structures of as-synthesized samples using different strategies; (B) Schematic diagram of reaction routes and possible mechanisms. (A) Adapted with permission[117]. Copyright 2022, John Wiley and Sons. (B) Adapted with permission[118]. Copyright 2023, Elsevier. COF: Covalent organic framework; TAPB: 1,3,5-tris(4-aminophenyl) benzene.
Solvent-free synthesis is an environmentally friendly method, which can utilize different catalytic systems, such as metal salts, P2O5, H6P4O13, and benzoic anhydride, to realize the control preparation of porous organic materials. Especially, the synthetic approach can enhance the stability and crystallinity of POFs, even for irreversible covalent bonds-based porous organic skeletons. However, this method is usually processed under high temperatures to trigger thermal decomposition or carbonization of the monomer. Developing more catalytic systems for the preparation of POFs under mild conditions remains a great challenge.
Mechanochemical synthesis
Mechanochemical synthesis is a simple, economical and environmentally friendly method. During this process, the reaction monomers are put into a mortar or machine at room or low temperature with a little or no solvent via different rotational speeds and reaction times[119-121]. The mechanical force can be utilized as the main energy source for producing POFs.
Recently, some research groups have focused on the design and synthesis of POFs via the mechanochemical approach[122-124]. The main type of COFs is almost prepared by the aldehyde-amine Schiff-base condensation. For example, Shinde et al. prepared bipyridine functionalized COFs with permanent porosity, crystallinity and strong acid-base stability by a liquid-assisted mechanochemical route [Figure 11A][122]. The mechanochemically prepared COFs possessed less porous and more compact pellets, which could be used as a solid-state electrolyte with higher conductivity and more stable open circuit voltage. Another luminescent COF was reported by Liu et al., who successfully prepared COF-TpMA (MC) via the low-cost mechanochemical synthesis of triformylphloroglucinol (Tp) and melamine (MA) monomers
Figure 11. (A) Schematic representation of the TpBpy COF as a solid-state electrolyte; (B) Chemical structures of monomers and COF-TpMA (MC); (C) The preparation process of Thdz@COF-F. (A) Adapted with permission[122]. Copyright 2016, Royal Society of Chemistry. (B) Adapted with permission[123]. Copyright 2019, Royal Society of Chemistry. (C) Adapted with permission[124]. Copyright 2024, American Chemical Society. COF: Covalent organic framework; Tp: 1,3,5-triformylphloroglucinol; MA: melamine; TTA: 4,4’,4”-(1,3,5-triazine-2,4,6-triyl)trianiline; TFTA: 2,3,5,6-tetrafluoroterephthalaldehyde.
Moreover, two porous organic materials [TMCPD (MC) and TMCBD (MC), with TMCPD (MC) synthesized from p-phenylenediamine and 1,3,5-benzenetricarbonyl chloride and TMCBD (MC) derived from benzidine and 1,3,5-benzenetricarbonyl chloride using mechanochemical methods] based on the secondary amide linkage were obtained by a solvent-free, fast, and room temperature mechanochemical synthesis [Figure 12A][125]. The organic coupling reaction of amine and acid chloride easily occurred in the presence of triethylamine as an acid binding agent. The flexible amide linkage made the dynamic skeleton of POFs with multiple hydrogen bond donor and acceptor sites. Another similar work was reported by Li
Figure 12. Mechanochemical synthesis of (A) amide-based TMCPD and TMCBD, (B) COF-TP and COF-TE, and (C) MHP-P5Q. (A) Adapted with permission[125]. Copyright 2014, American Chemical Society. (B) Adapted with permission[126]. Copyright 2019, Elsevier. (C) Adapted with permission[127]. Copyright 2020, Springer Nature. COF: Covalent organic framework; P5Q: pillar[5]quinone.
Mechanochemical Friedel-Crafts alkylation is also a commonly used polymerization coupling reaction for the fabrication of POFs. Troschke et al. synthesized a series of porous CTFs through the mechanochemical synthesis based on a Friedel-Crafts alkylation reaction mechanism[128]. The easily available MA chloride as a triazine node reacted with different electron-rich aromatic compounds [Figure 13A]. Carbazole and cyanuric chloride were mixed with each other in the presence of AlCl3 as an activating reagent and ZnCl2 as a bulking agent, leading to the synthesis of the porous carbazole-CTF with a large specific surface area after ball milling for 1 h. This study offers a solvent-free, time-efficient, and scalable production of CTFs via the mechanochemical synthesis. Another type of polymerization is monomer self-polymerization or cross-coupling in solvents. For example, Chen et al. synthesized multiple PAFs via the FeCl3-promoted mechanical coupling reaction using p-terphenyl and o-terphenyl as reaction monomers [Figure 13B][129]. The introduced formaldehyde dimethyl acetal (FDA) could form the C–O bond-linked PAFs. These as-synthesized porous PAFs could adsorb and separate C2 hydrocarbons. Another similar work was reported by Krusenbaum et al.[130]. As a commonly used organic aromatic monomer, 1,3,5-triphenylbenzene (TPB) could respectively react with two organochloride crosslinking agents, CH2Cl2 and CHCl3, via the mechanochemical Friedel-Crafts alkylation [Figure 13C]. The CH2Cl2-linked polymer was a flexible porous structure with low-degree polymerization, but the CHCl3-linked porous organic polymer showed a highly crosslinked rigid skeleton with a larger surface area. Other similar works were reported by other groups[131-133]. Hence, the mechanochemical Friedel-Crafts alkylation provides a versatile tool to generate porous organic materials.
Figure 13. (A) Mechanochemical synthesis of CTFs by melamine chloride and various aromatic monomers; (B) The mechanically synthetic processes of PAF-104 and PAF-105 via the FeCl3-promoted reaction; (C) The mechanochemical Friedel-Crafts alkylation of TPB and two organochloride crosslinking agents. (A) Adapted with permission[128]. Copyright 2017, John Wiley and Sons. (B) Adapted with permission[129]. Copyright 2022, Royal Society of Chemistry. (C) Adapted with permission[130]. Copyright 2022, John Wiley and Sons. CTFs: Covalent triazine frameworks; PAF: porous aromatic framework; TPB: 1,3,5-triphenylbenzene.
Furthermore, ionic porous organic polymers can be synthesized by the mechanochemical method. The targeted functional ionic POFs are prepared via the solid grinding coupling reaction of ionic organic blocks. For instance, Zhang et al. prepared cation-functionalized charged porous polymers (CPPs) by a dibenzodioxane-forming reaction between the contorted 5,5,6,6-tetrahydroxy-3,3,3,3-tetramethyl-1,1’-spirobisindane (TTSBI) and fluorine-activated ionic monomers. The mild, environmentally friendly, and highly efficient mechanochemical method was carried out to produce cationic CPPs [Figure 14A][134]. Compared with the traditional solvothermal synthesis method, the mechanochemical grinding approach can quickly complete the polymerization process after 60-90 min and reduce the condensation reaction temperature. Another class of porous ionic polymers (PIPs) was reported by Hou et al.[135]. As illustrated in Figure 14B, different neutral organic monomers containing pyridine or benzyl bromide fragments were used for the mechanical grinding synthesis of various cationic PIPs via the Menshutkin coupling reaction. NaBr, as a salt template, was rationally introduced in the reaction system, which was also easy to reuse for reducing the environmental pollution. The above two examples prove that the green mechanical synthesis is a useful method to construct ionic porous organic materials.
The mechanochemical process has many advantages, including being solvent-free, operating at low temperatures, enabling large-scale production, and allowing fast reactions, making it a highly promising method in the preparation of POFs. For different catalytic reactions, it takes considerable time to determine the mechanical synthetic conditions, such as the addition of catalysts and other excipients. Therefore, the use of mechanochemical construction of porous organic materials still needs a lot of research in the future.
Electrochemical synthesis
Electrochemical synthesis technology features environmental friendliness, high efficiency, low emissions, and precise controllability. The controllable energy input from the electrode offers an opportunity to manipulate the chemical reaction in a pre-designed manner and in a confined space. In recent years, various materials, such as COFs, CMPs and PAFs, have been successfully fabricated by the electrochemical synthesis, exhibiting high fabrication efficiency[136-138].
Electrochemical synthesis of COFs has been reported by different research groups. In 2023, Shirokura et al. synthesized a TAPB-PDA COF film by an electrochemical synthesis method [Figure 15A][139]. In this work, electrochemical oxidation of 1,2-diphenylhydrazine (DPH) produced electrogenerated acid (EGA) at an electrode surface, which could act as an effective Brønsted acid catalyst to improve imine bond formation from the corresponding amine and aldehyde monomers. Simultaneously, it provided the corresponding COF film deposited on the electrode surface. The as-synthesized COFs not only possessed high crystallinity and abundant pores, but also exhibited the controlled film thickness through using the potential-sweep method [Figure 15B]. In addition, Wang et al. created an electrochemical IP technique for three distinct ultrathin COF membranes in the same year. The aldehyde monomer (Tp) and the amine monomer (Pa) were dissolved in methanol, which could migrate to the cathode under the control of voltage and current
Figure 15. (A) The synthetic process of COFs on the electrode surface using EGA; (B) SEM image of the as-synthesized COF film; (C) The growth TpPa COF membrane on PAN via electrochemical polymerization; (D-K) SEM and (L-O) TEM images of TpPa membranes at different electropolymerization times; (P) Schematic representation for the electrochemical synthesis of COF membranes. (A and B) Adapted with permission[139]. Copyright 2023, John Wiley and Sons. (C-O) Adapted with permission[140]. Copyright 2023, John Wiley and Sons. (P) Adapted with permission[141]. Copyright 2022, American Chemical Society. COFs: Covalent organic frameworks; EGA: electrogenerated acid; DPH: 1,2-diphenylhydrazine; TAPB: 1,3,5-tris(4-aminophenyl) benzene; PDA: terephthalaldehyde; SEM: scanning electron microscopy; Tp: 1,3,5-triformylphloroglucinol; Pa: p-phenylenediamine; PAN: polyacrylonitrile; TEM: transmission electron microscopy.
Carbazole-based POFs are a major type of amorphous porous materials. The electroactive carbazole moiety can be utilized as a functional molecule to facilitate the electropolymerization coupling reaction[142-144]. Recently, Zhou et al. designed and synthesized different polycarbazole-type CMPs using a scale-up electrochemical approach. One CMP was successfully prepared by the electropolymerization of 1,3,5-tris(N-carbazolyl)benzene (TCB) monomers [Figure 16A][145]. The thickness of CMP membranes could be controlled via the cyclic voltammetry (CV) scanning from -0.8 to 1.23 V. The TEM image of the as-synthesized composite showed that CMP was tightly connected to the carbon nanotube (CNT) substrate [Figure 16B]. Notably, CMP membranes have many advantages, including outstanding stability, large surface area, abound pores, and uniform pore size, but their brittleness leads to extremely poor mechanical behavior. Hence, this research group further synthesized a flexible ionic CMP membrane with the precisely tailored pore architecture by a coelectropolymerization (COEP) strategy[146]. As shown in Figure 16C, the CMP structure was constructed by two compositions, including the rigid TCB monomer for the uniform porous structure and the flexible charged CbzC6-N monomer for the mechanical flexibility. Various functional sites were introduced into the organic building blocks via the rational monomer design, which could simultaneously introduce multifunctional sites into porous organic materials to achieve excellent performance. Moreover, electrochemical uranium extraction from seawater by PAFs also provided a new opportunity for the sustainable supply of nuclear fuel. In 2023, Chen et al. reported a facile electropolymerization and the following functionalization to grow PAF-144-AO (amidoxime group modified PAFs) with TCB and N-(2-cyanoethyl)-pyrrole (NCP) on carbon cloths. The self-standing and binder-free electrodes were successfully prepared for electrochemical uranium extraction [Figure 16D][147]. According to a series of comparative experiments, the amidoxime group, electroactive site, and porous framework synergistically improved uranium extraction performance via the adsorption-electro catalysis. This electrochemical process showed higher uptake and faster kinetics compared to the physicochemical adsorption.
Figure 16. (A) The electropolymerization approach of TCB; (B) The TEM image of as-synthesized polycarbazoles on CNTs; (C) Schematic illustration of the fabrication and possible mechanism of the flexible ionic CMP membrane; (D) The fabrication diagram of self-standing PAFs. (A and B) Adapted with permission[145]. Copyright 2021, American Chemical Society. (C) Adapted with permission[146]. Copyright 2022, John Wiley and Sons. (D) Adapted with permission[147]. Copyright 2023, American Chemical Society. TCB: 1,3,5-tris(N-carbazolyl)benzene; TEM: transmission electron microscopy; CNTs: carbon nanotubes; CMP: conjugated microporous polymer; COEP: coelectropolymerization; CbzC6-N: 6-(9H-carbazol-9-yl)-N,N,N-trimethylhexan-1-aminium bromide; PAFs: porous aromatic frameworks; NCP: N-(2-cyanoethyl)-pyrrole.
The morphology and size of POFs can be precisely controlled by changing monomer concentration, potential, sweep speed, and other parameters in electrochemical synthesis. However, compared to other green synthesis methods, there is relatively little research on the electrochemical synthesis strategy for synthesizing porous organic materials. The electrochemical synthesis strategy for POFs is still in the preliminary research stage. There are still many issues that require extensive research to determine, such as electroactive organic monomer, appropriate electrolyte, electropolymerization condition, and so on. In addition to the advantages of the electrochemical synthesis for preparing thin films, further exploration is needed on how to prepare high crystalline porous organic materials in large quantities.
Other green synthetic methods
Porous organic materials often need to be supplied with energy during the synthesis process. In addition to the above-mentioned green synthesis methods, some other environmentally friendly synthesis strategies with specific energy sources are summarized. In the field of green chemistry, some researchers have utilized diversified energy supply methods to controllably prepare porous organic materials, including microwave-assisted heating and photo-induced catalysis[148,149].
For the microwave-assisted heating method, microwave radiation can produce a better temperature distribution in the synthetic system to eliminate the characteristic temperature distribution from the outside to the inside produced by the conventional heating method. Hence, the microwave-assisted green synthesis provides a favorable platform to prepare COFs with homogeneous morphology. For example, three two-dimensional imine-conjugated COFs with different functional group modifications were successfully synthesized using a simple microwave-assisted method for 1 h by Alsudairy et al. in 2023 [Figure 17A][150]. Compared with those samples obtained by the solvothermal method, the microwave-assisted COFs showed more homogeneous morphology, higher crystallinity, and larger yield. Due to the homogeneous spherical morphology and the high chemical stability caused by the built-in electron-donating groups, the as-synthesized material was used as an iodine adsorbent with the advantages of fast kinetics, high capacity, and excellent reusability. In the same year, another important work was reported by Wang et al.
Figure 17. (A) The photopolymerization approach of Mw-TFB-BD-X COFs; (B) The synthesis diagram of CD-COF-Li for the solid-state Li-O2 battery; (C) The preparation procedure of LZU-191 and (D) its model reaction for synthesizing 2-phenylbenzo[d]oxazole; (E) Schematic diagram of the preparation process of polymeric porous microspheres. (A) Adapted with permission[150]. Copyright 2023, American Chemical Society. (B) Adapted with permission[151]. Copyright 2022, Elsevier. (C and D) Adapted with permission[152]. Copyright 2022, American Chemical Society. (E) Adapted with permission[155]. Copyright 2023, Royal Society of Chemistry. COFs: Covalent organic frameworks; DABD: 2,5-diamino-1,4-benzenediol dihydrochloride; TBA-eosin Y: tetrabutylammonium salt of Eosin Y; NMP: N-methyl-2-pyrrolidone; TFPT: 1,3,5-tris(4-formylphenyl)triazine; UV: ultraviolet; PVA: poly(vinyl alcohol); SSE: solid-state electrolyte.
The photocatalytic synthesis has attracted widespread attention due to its energy-saving, high efficiency, economic, and environmentally friendly features. In 2022, Wu et al. reported a photocatalytic method for the synthesis of COFs [Figure 17C][152]. Under room temperature conditions, a photocatalytic synthesis of COFs was achieved by a reaction of 2,5-diamino-1,4-benzenediol dihydrochloride (DABD) and tetrabutylammonium salt of Eosin Y (TBA-eosin Y) assisted by 1,3,5-tris(4-formylphenyl)triazine (TFPT). The as-synthesized benzoxazole-linked COF LZU-191 possessed good stability and high crystallinity. The reaction mechanism of this strategy is photocatalytic cyclization under sunlight, oxygen, TBA-eosin Y, and N-methyl-2-pyrrolidone (NMP) [Figure 17D]. In addition, the preparation of porous polymer microspheres by photopolymerization is an attractive method due to the ability of ultraviolet (UV) light to initiate free radical polymerization[153,154]. As seen in Figure 17E, the authors proposed an efficient, versatile, and tunable strategy for the preparation of porous polymer microspheres based on the UV photopolymerization[155]. The authors utilized poly(vinyl alcohol) (PVA) solution as the aqueous phase. The photopolymerization materials and photoinitiators in an organic solution were used as the oil phase, which was rapidly mixed and photopolymerized with UV-induced photopolymerization to obtain the polymers after removing the PVA template. During this synthetic process, the organic solvent was encapsulated in the prepared microspheres to act as a co-solvent and pore-forming agent, which could be removed ultimately to leave pores in the microspheres. This method is important for green synthesis of porous organic polymers in only a few minutes at room temperature.
Overall, these synthesis strategies can shorten reaction time, simplify experimental manipulation, and substantially lower energy consumption. Additionally, they enable the design and synthesis of new POFs or different morphologies that are difficult to achieve through the conventional solvothermal method. This provides new ideas for expanding the synthesis strategy of POFs.
CONCLUSION AND OUTLOOK
Porous organic materials exhibit excellent performance in enormous applications, leading to their huge potential in industrial use. Compared to other industrially applied porous materials, such as zeolite and activated carbon, the synthesis process of most POFs does not satisfy the industrial needs, including low-energy consumption, non-pollution, good repeatability, easy operation, and large-scale preparation. As described in this review, an increasing number of POFs have been prepared by various environmentally friendly green synthesis strategies in recent years. However, many problems need to be solved to meet practical industrial applications. Herein, we highlight the challenge and direction for the green synthesis of POFs in the near future.
(1) The current research mainly focuses on the synthetic strategy and design principle for synthesizing POFs, but it clearly lacks the exploration on intermediate transition states, as well as in-depth analysis of synthesis mechanisms and reaction pathways. Amorphous porous organic materials, including PAFs, CMPs and HCPs, have been synthesized mainly through Friedel-Crafts alkylation, Suzuki coupling, Yamamoto coupling, and Sonogashira-Hagihara cross-coupling reactions with clear catalytic mechanisms. However, the effects of new reaction media or reaction methods on the formation, growth and crystallization of COFs are not fully understood. The mechanism study is crucial for optimizing synthesis conditions, which plays a guiding role in the green synthetic process of POFs. The theoretical calculation is a useful method to investigate the possible reaction path. Meanwhile, the largest energy barrier between different intermediates can be determined as the rate-determining step by the calculated energy barrier, which is beneficial for preliminary determination of suitable reaction conditions, such as temperature, catalyst, potential, and so on. Furthermore, varieties of in-situ characterization techniques, including Fourier transform infrared spectra, Raman spectra and electron spin resonance spectroscopy, can be performed to verify intermediate products from an experimental perspective. On the other hand, time-resolved nuclear magnetic resonance, X-ray diffraction, and mass spectroscopy techniques can be further utilized to study the change of crystal phase, organic linker exchange, polymerization reaction, self-assembly, etc. Based on the above theoretical and experimental analysis, it is possible to preliminarily explore different green synthesis process conditions, monomers and polymerization reactions.
(2) For most organic coupling reactions in the POF synthesis, different types of catalysts are often required to promote the reaction occurrence. Catalysts can significantly reduce the reaction energy barrier, which is beneficial for achieving the efficient synthesis of POFs under green and mild conditions. However, most catalysts are not only expensive, but also difficult to separate and contaminate the targeted POFs. Especially, some catalysts are always sensitive to both air and water, leading to the harsh reaction conditions with a significant impact on the structure and crystallinity of POFs in the large-scale synthesis. Therefore, the development of high-performance and low-cost catalysts is crucial for the green synthesis of porous organic materials. The ideal catalyst should have the following characteristics: low cost, high catalytic activity, easy separation, good stability, and available reproducibility even in the large-scale preparation. In addition to the above characteristics, the low life-cycle cost, high security, fine reusability and sustainability of catalysts should be comprehensively evaluated and considered in the catalyst-assisted green and industrial synthesis system.
(3) It is urgently in need of extensive research on more efficient, green, scalable, and sustainable synthesis methods, leading to the transformation from the laboratory synthesis to the industrial large-scale preparation of POFs. Some synthetic technologies, such as extrusion or spray drying, may enhance the productivity of the large-scale synthesis, even exceeding the laboratory scale. Therefore, considering the practical requirements such as industrial equipment, energy conservation, environmental protection, product quality, separation and purification, the suitable green scale synthesis processes of the targeted POFs should be developed and investigated in detail.
In summary, porous organic materials exhibit great potential in various applications. POFs have attracted much attention and research, hoping to realize practical applications and breakthroughs in industrial fields. In fact, many aspects limit the industrial practical application of POFs from the laboratory level. One of the key factors is the development of green scale synthesis processes. Up to now, various green synthetic strategies, including room temperature, green solvent, solvent-free, mechanochemical and electrochemical synthesis, have been developed to prepare different POFs. Nevertheless, the current research on green synthesis processes is slightly immature and there is still a significant gap from the practical application. Further development in this field needs collaboration from multiple multidisciplinary experts. We believe that scientific researchers will achieve more excellent results and push the industrial application of POFs to a new level in the future.
DECLARATIONS
Authors’ contributions
Investigation, methodology, data curation, writing original draft: Wen, H. M.
Writing original draft: Zhang, S.
Methodology, conceptualization, formal analysis: Li, P.
Formal analysis: Kong, X.
Writing - review and editing: Yuan, R.
Supervision, writing - review and editing: He, H.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Zhao, D.; Zhao, T. Pore engineering for high performance porous materials. ACS. Cent. Sci. 2023, 9, 1499-503.
2. Wang, Y.; Tong, C.; Liu, Q.; Han, R.; Liu, C. Intergrowth zeolites, synthesis, characterization, and catalysis. Chem. Rev. 2023, 123, 11664-721.
3. Chen, L. H.; Sun, M. H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B. L. Hierarchically structured zeolites: from design to application. Chem. Rev. 2020, 120, 11194-294.
4. Yang, C.; Zhao, T.; Pan, H.; Liu, F.; Cao, J.; Lin, Q. Facile preparation of N-doped porous carbon from chitosan and NaNH2 for CO2 adsorption and conversion. Chem. Eng. J. 2022, 432, 134347.
5. Oliveira, A. D. N. D.; Cardoso, R. D. S.; Ferreira, I. M.; et al. Valorization of silica-based residues for the synthesis of ordered mesoporous silicas and their applications. Micropor. Mesopor. Mat. 2023, 354, 112520.
6. Su, Z.; Chen, T. Porous noble metal electrocatalysts: synthesis, performance, and development. Small 2021, 17, e2005354.
7. Hajivand, P.; Carolus, J. J.; Pardo, E.; Armentano, D.; Mastropietro, T. F.; Azadmehr, A. Application of metal-organic frameworks for sensing of VOCs and other volatile biomarkers. Coordin. Chem. Rev. 2024, 501, 215558.
8. Tian, Y.; Deng, C.; Peng, Y.; Zhang, X.; Zhang, Z.; Zaworotko, M. J. State of the art, challenges and prospects in metal–organic frameworks for the separation of binary propylene/propane mixtures. Coordin. Chem. Rev. 2024, 506, 215697.
9. Hao, Q.; Tao, Y.; Ding, X.; et al. Porous organic polymers: a progress report in China. Sci. China. Chem. 2023, 66, 620-82.
10. Diercks, C. S.; Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585.
11. Liu, M.; Huang, Q.; Wang, S.; et al. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. Engl. 2018, 57, 11968-72.
12. Yuan, Y.; Yang, Y.; Zhu, G. Molecularly imprinted porous aromatic frameworks for molecular recognition. ACS. Cent. Sci. 2020, 6, 1082-94.
13. Ma, Y.; Cui, F.; Rong, H.; et al. Continuous porous aromatic framework membranes with modifiable sites for optimized gas separation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202113682.
14. Shen, X.; Faheem, M.; Matsuo, Y.; et al. Polarity engineering of porous aromatic frameworks for specific water contaminant capture. J. Mater. Chem. A. 2019, 7, 2507-12.
15. Gong, J.; Lin, R.; Chen, B. Conjugated microporous polymers with rigid backbones for organic solvent nanofiltration. Chem 2018, 4, 2269-71.
16. Tantisriyanurak, S.; Duguid, H. N.; Peattie, L.; Dawson, R. Acid functionalized conjugated microporous polymers as a reusable catalyst for biodiesel production. ACS. Appl. Polym. Mater. 2020, 2, 3908-15.
17. Giri, A.; Biswas, S.; Hussain, M. W.; Dutta, T. K.; Patra, A. Nanostructured hypercrosslinked porous organic polymers: morphological evolution and rapid separation of polar organic micropollutants. ACS. Appl. Mater. Interfaces. 2022, 14, 7369-81.
18. Zhang, W.; Ma, F.; Ma, L.; Zhou, Y.; Wang, J. Imidazolium-functionalized ionic hypercrosslinked porous polymers for efficient synthesis of cyclic carbonates from simulated flue gas. ChemSusChem 2020, 13, 341-50.
19. Ren, H.; Zhu, G. Porous organic frameworks: synthetic strategy and their applications. Acta. Chim. Sinica. 2015, 73, 587.
20. Li, W.; Xiao, W.; Luo, Q.; et al. Ionic liquids promoted synthesis, enhanced functions, and expanded applications of porous organic frameworks. Coordin. Chem. Rev. 2023, 493, 215304.
21. He, C.; Wang, Y.; Chen, Y.; et al. Microregulation of pore channels in covalent-organic frameworks used for the selective and efficient separation of ethane. ACS. Appl. Mater. Interfaces. 2020, 12, 52819-25.
22. Liu, Y.; Wang, S.; Meng, X.; et al. Molecular expansion for constructing porous organic polymers with high surface areas and well-defined nanopores. Angew. Chem. Int. Ed. Engl. 2020, 59, 19487-93.
23. Cao, L.; Wang, C.; Wang, H.; et al. Rationally designed cyclooctatetrathiophene-based porous aromatic frameworks (COTh-PAFs) for efficient photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. Engl. 2024, 63, e202402095.
24. Hauser, B. G.; Farha, O. K.; Exley, J.; Hupp, J. T. Thermally enhancing the surface areas of Yamamoto-derived porous organic polymers. Chem. Mater. 2013, 25, 12-6.
25. Zhang, Y.; Li, Z.; Zhang, C.; et al. Multifunctional porous organic polymers as ideal platforms for gas uptake, metal-ions sensing, and cell imaging. Polym. Chem. 2023, 14, 4199-204.
26. Xue, Y.; Zhang, H.; Han, Z.; He, H. Electrochemical impedimetric aptasensors based on hyper-cross-linked porous organic frameworks for the determination of kanamycin. J. Mater. Chem. C. 2021, 9, 12566-72.
27. He, H.; Wen, H.; Li, P.; et al. Tailor-made yolk-shell nanocomposites of star-shape Au and porous organic polymer for nitrogen electroreduction to ammonia. Chem. Eng. J. 2023, 476, 146760.
28. Xu, Z.; Liu, K.; Wang, S.; et al. Viologen-based cationic radical porous organic polymers for visible-light-driven photocatalytic oxidation. ACS. Appl. Polym. Mater. 2024, 6, 701-11.
29. Huang, J.; Peng, Q.; Liu, C.; et al. Microporous nitrogen-rich polymers via ullmann coupling reaction for selective adsorption of C2H2 over CH4. Chin. J. Chem. 2023, 41, 514-20.
30. Jadhav, T.; Fang, Y.; Patterson, W.; Liu, C. H.; Hamzehpoor, E.; Perepichka, D. F. 2D poly(arylene vinylene) covalent organic frameworks via aldol condensation of trimethyltriazine. Angew. Chem. Int. Ed. Engl. 2019, 58, 13753-7.
31. Chen, Y.; Li, W.; Wang, X.; Gao, R.; Tang, A.; Kong, D. Green synthesis of covalent organic frameworks based on reaction media. Mater. Chem. Front. 2021, 5, 1253-67.
32. Azadi, E.; Dinari, M. Green and facile preparation of covalent organic frameworks based on reaction medium for advanced applications. Chemistry 2023, 29, e202301837.
33. Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: strategic design and structure-function correlation. Chem. Rev. 2017, 117, 1515-63.
34. Abdelnaby, M. M.; Cordova, K. E.; Abdulazeez, I.; et al. Novel porous organic polymer for the concurrent and selective removal of hydrogen sulfide and carbon dioxide from natural gas streams. ACS. Appl. Mater. Interfaces. 2020, 12, 47984-92.
35. Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. High-flux vertically aligned 2D covalent organic framework membrane with enhanced hydrogen separation. J. Am. Chem. Soc. 2020, 142, 6872-7.
36. Nailwal, Y.; Devi, M.; Pal, S. K. Luminescent conjugated microporous polymers for selective sensing and ultrafast detection of picric acid. ACS. Appl. Polym. Mater. 2022, 4, 2648-55.
37. Afshari, M.; Dinari, M.; Farrokhpour, H.; Zamora, F. Imine-linked covalent organic framework with a naphthalene moiety as a sensitive phosphate ion sensing. ACS. Appl. Mater. Interfaces. 2022, 14, 22398-406.
38. Zhang, Y.; Guo, J.; VanNatta, P.; et al. Metal-free heterogeneous asymmetric hydrogenation of olefins promoted by chiral frustrated lewis pair framework. J. Am. Chem. Soc. 2024, 146, 979-87.
39. Chen, M.; Xiong, J.; Shi, Q.; et al. How the π bridge in donor-π-acceptor type covalent triazine frameworks influenced their photocatalytic hydrogen evolution performance. Chem. Eng. J. 2023, 475, 146099.
40. Yang, S.; Chen, Z.; Zou, L.; Cao, R. Construction of thiadiazole-linked covalent organic frameworks via facile linkage conversion with superior photocatalytic properties. Adv. Sci. 2023, 10, e2304697.
41. Gu, C.; Huang, N.; Chen, Y.; et al. Porous organic polymer films with tunable work functions and selective hole and electron flows for energy conversions. Angew. Chem. Int. Ed. Engl. 2016, 55, 3049-53.
42. Liao, C.; Liu, S. Tuning the physicochemical properties of reticular covalent organic frameworks (COFs) for biomedical applications. J. Mater. Chem. B. 2021, 9, 6116-28.
43. Prakash, K.; Mishra, B.; Díaz, D. D.; Nagaraja, C. M.; Pachfule, P. Strategic design of covalent organic frameworks (COFs) for photocatalytic hydrogen generation. J. Mater. Chem. A. 2023, 11, 14489-538.
44. Cui, K.; Tang, X.; Xu, X.; Kou, M.; Lyu, P.; Xu, Y. Crystalline dual-porous covalent triazine frameworks as a new platform for efficient electrocatalysis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202317664.
45. Xiong, S.; Guo, J.; Lv, F.; et al. Solvothermal synthesis and supercapacitive properties of highly electrochemical stable covalent organic frameworks with triazine building block. J. Appl. Polym. Sci. 2023, 140, e54538.
46. Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O'Keeffe, M.; Matzger, A. J.; Yaghi, O. M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166-70.
47. Li, H.; Chavez, A. D.; Li, H.; Li, H.; Dichtel, W. R.; Bredas, J. L. Nucleation and growth of covalent organic frameworks from solution: the example of COF-5. J. Am. Chem. Soc. 2017, 139, 16310-8.
48. Li, X.; Jin, X.; Ma, L.; et al. Construction of borate-ester-based COFs with high specific surface area for the detection of H2O content in the food field. Microchem. J. 2024, 199, 109976.
49. Chang, P. H.; Sil, M. C.; Reddy, K. S. K.; Lin, C. H.; Chen, C. M. Polyimide-based covalent organic framework as a photocurrent enhancer for efficient dye-sensitized solar cells. ACS. Appl. Mater. Interfaces. 2022, 14, 25466-77.
50. DeBlase, C. R.; Silberstein, K. E.; Truong, T. T.; Abruña, H. D.; Dichtel, W. R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821-4.
51. Yu, H.; Wang, D. Metal-free magnetism in chemically doped covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 11013-21.
52. Yang, C.; Hu, H.; Qian, C.; Liao, Y. Hollow sp2 -conjugated covalent organic framework encapsulating thiophene-based photosensitizer for enhanced visible-light-driven hydrogen evolution. J. Mater. Chem. A. 2023, 11, 25899-909.
53. Yang, N.; Gu, Y.; Shan, Y.; et al. Dual rate-modulation approach for the preparation of crystalline covalent triazine frameworks displaying efficient sodium storage. ACS. Macro. Lett. 2022, 11, 60-5.
54. Martín-Illán, J. Á.; Suárez, J. A.; Gómez-Herrero, J.; et al. Ultralarge free-standing imine-based covalent organic framework membranes fabricated via compression. Adv. Sci. 2022, 9, e2104643.
55. Yao, B. J.; Li, J. T.; Huang, N.; et al. Pd NP-loaded and covalently cross-linked COF membrane microreactor for aqueous CBs dechlorination at room temperature. ACS. Appl. Mater. Interfaces. 2018, 10, 20448-57.
56. Parvatkar, P. T.; Kandambeth, S.; Shaikh, A. C.; et al. A tailored COF for visible-light photosynthesis of 2,3-dihydrobenzofurans. J. Am. Chem. Soc. 2023, 145, 5074-82.
57. Campbell, A.; Alsudairy, Z.; Dun, C.; et al. Dioxin-linked covalent organic framework-supported palladium complex for rapid room-temperature Suzuki–Miyaura coupling reaction. Crystals 2023, 13, 1268.
58. Luan, T. X.; Du, L.; Wang, J. R.; et al. Highly effective generation of singlet oxygen by an imidazole-linked robust photosensitizing covalent organic framework. ACS. Nano. 2022, 16, 21565-75.
59. Seo, J. M.; Noh, H. J.; Jeon, J. P.; et al. Conductive and ultrastable covalent organic framework/carbon hybrid as an ideal electrocatalytic platform. J. Am. Chem. Soc. 2022, 144, 19973-80.
60. Jaryal, R.; Khullar, S.; Kumar, R. Benzothiazole-derived covalent organic framework for multimedia iodine uptake. J. Clust. Sci. 2024, 35, 461-79.
61. Yang, Z.; Chen, H.; Wang, S.; et al. Transformation strategy for highly crystalline covalent triazine frameworks: from staggered AB to eclipsed AA stacking. J. Am. Chem. Soc. 2020, 142, 6856-60.
62. Guo, L.; Wang, X.; Zhan, Z.; et al. Crystallization of covalent triazine frameworks via a heterogeneous nucleation approach for efficient photocatalytic applications. Chem. Mater. 2021, 33, 1994-2003.
63. Wang, H.; Shi, L.; Qu, Z.; et al. Increasing donor-acceptor interactions and particle dispersibility of covalent triazine frameworks for higher crystallinity and enhanced photocatalytic activity. ACS. Appl. Mater. Interfaces. 2024, 16, 2296-308.
64. Ma, T.; Kapustin, E. A.; Yin, S. X.; et al. Single-crystal x-ray diffraction structures of covalent organic frameworks. Science 2018, 361, 48-52.
65. Xu, H. S.; Luo, Y.; Li, X.; et al. Single crystal of a one-dimensional metallo-covalent organic framework. Nat. Commun. 2020, 11, 1434.
66. Wang, X.; Enomoto, R.; Murakami, Y. Ionic additive strategy to control nucleation and generate larger single crystals of 3D covalent organic frameworks. Chem. Commun. 2021, 57, 6656-9.
67. Natraj, A.; Ji, W.; Xin, J.; et al. Single-crystalline imine-linked two-dimensional covalent organic frameworks separate benzene and cyclohexane efficiently. J. Am. Chem. Soc. 2022, 144, 19813-24.
68. Zhou, Z.; Xiong, X. H.; Zhang, L.; et al. Linker-guided growth of single-crystal covalent organic frameworks. J. Am. Chem. Soc. 2024, 146, 3449-57.
69. Zhou, Z.; Zhang, L.; Yang, Y.; et al. Growth of single-crystal imine-linked covalent organic frameworks using amphiphilic amino-acid derivatives in water. Nat. Chem. 2023, 15, 841-7.
70. Liu, T.; Liu, G. Porous organic materials offer vast future opportunities. Nat. Commun. 2020, 11, 4984.
71. Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A. 2017, 5, 1334-47.
72. Zhang, Y.; Riduan, S. N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083-94.
73. Ben, T.; Ren, H.; Ma, S.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. Engl. 2009, 48, 9457-60.
74. Kim, J. H.; Kang, D. W.; Yun, H.; et al. Post-synthetic modifications in porous organic polymers for biomedical and related applications. Chem. Soc. Rev. 2022, 51, 43-56.
75. Zhang, Z.; Liu, Z.; Xue, C.; Chen, H.; Han, X.; Ren, Y. Amorphous porous organic polymers containing main group elements. Commun. Chem. 2023, 6, 271.
76. Yuan, Y.; Meng, Q.; Faheem, M.; et al. A molecular coordination template strategy for designing selective porous aromatic framework materials for uranyl capture. ACS. Cent. Sci. 2019, 5, 1432-9.
77. Ma, T.; Zhao, R.; Li, Z.; et al. Efficient gold recovery from E-waste via a chelate-containing porous aromatic framework. ACS. Appl. Mater. Interfaces. 2020, 12, 30474-82.
78. Li, B.; Sun, Q.; Zhang, Y.; et al. Functionalized porous aromatic framework for efficient uranium adsorption from aqueous solutions. ACS. Appl. Mater. Interfaces. 2017, 9, 12511-7.
79. Luo, D.; Shi, T.; Li, Q. H.; et al. Green, general and low-cost synthesis of porous organic polymers in sub-kilogram scale for catalysis and CO2 capture. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305225.
80. Park, J. I.; Jang, J. Y.; Ko, Y. J.; et al. Room-temperature synthesis of a hollow microporous organic polymer bearing activated alkyne IR probes for nonradical thiol-yne click-based post-functionalization. Chem. Asian. J. 2021, 16, 1398-402.
81. Zhang, F.; Hong, M.; Liu, Z.; et al. Facile room-temperature synthesis of novel porous three-component hybrid covalent organic polymers and their applications towards sulfadiazine adsorption. ChemistrySelect 2019, 4, 12719-25.
82. Zhao, Y.; Lu, W.; Zhang, Y.; Liu, X.; Sun, B. Room temperature synthesis of piperazine-based nitrogen-rich porous organic polymers for efficient iodine adsorption. Micropor. Mesopor. Mat. 2024, 366, 112954.
83. Ma, W.; Zheng, Q.; He, Y.; et al. Size-controllable synthesis of uniform spherical covalent organic frameworks at room temperature for highly efficient and selective enrichment of hydrophobic peptides. J. Am. Chem. Soc. 2019, 141, 18271-7.
84. Ma, W.; Li, G.; Zhong, C.; et al. Room-temperature controllable synthesis of hierarchically flower-like hollow covalent organic frameworks for brain natriuretic peptide enrichment. Chem. Commun. 2021, 57, 7362-5.
85. Guo, L.; Zhang, Q. Y.; Yu, Z.; Krishna, R.; Luo, F. Minute and large-scale synthesis of covalent-organic frameworks in water at room temperature by a two-step dissolution–precipitation method. Chem. Mater. 2023, 35, 5648-56.
86. Kong, X.; Wu, Z.; Strømme, M.; Xu, C. Ambient aqueous synthesis of imine-linked covalent organic frameworks (COFs) and fabrication of freestanding cellulose nanofiber@COF nanopapers. J. Am. Chem. Soc. 2024, 146, 742-51.
87. Liang, J.; Ruan, J.; Njegic, B.; et al. Insight into bioactivity of in-situ trapped enzyme-covalent-organic frameworks. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303001.
88. Zhou, D.; Tan, X.; Wu, H.; Tian, L.; Li, M. Synthesis of C-C bonded two-dimensional conjugated covalent organic framework films by Suzuki polymerization on a liquid-liquid interface. Angew. Chem. Int. Ed. Engl. 2019, 58, 1376-81.
89. Sahabudeen, H.; Qi, H.; Ballabio, M.; et al. Highly crystalline and semiconducting imine-based two-dimensional polymers enabled by interfacial synthesis. Angew. Chem. Int. Ed. Engl. 2020, 59, 6028-36.
90. He, J.; Yu, L.; Li, Z.; Ba, S.; Lan, F.; Wu, Y. Catalyst regulated interfacial synthesis of self-standing covalent organic framework membranes at room temperature for molecular separation. J. Colloid. Interface. Sci. 2023, 629, 428-37.
91. Wu, D.; Che, Q.; He, H.; et al. Room-temperature interfacial synthesis of vinylene-bridged two-dimensional covalent organic framework thin film for nonvolatile memory. ACS. Mater. Lett. 2023, 5, 874-83.
92. Chen, J.; Li, R.; Liu, S.; et al. Surfactant-assisted interfacial polymerization towards high-crystallinity COF membranes for organic solvent nanofiltration. J. Membrane. Sci. 2024, 694, 122404.
93. Li, K.; Zhu, J.; Liu, D.; Zhang, Y.; Van, B. B. Controllable and rapid synthesis of conjugated microporous polymer membranes via interfacial polymerization for ultrafast molecular separation. Chem. Mater. 2021, 33, 7047-56.
94. Zhang, J.; Cheng, C.; Guan, L.; Jiang, H. L.; Jin, S. Rapid synthesis of covalent organic frameworks with a controlled morphology: an emulsion polymerization approach via the phase transfer catalysis mechanism. J. Am. Chem. Soc. 2023, 145, 21974-82.
95. Shi, L.; Li, W.; Wu, Y.; et al. Controlled synthesis of mesoporous π-conjugated polymer nanoarchitectures as anodes for lithium-ion batteries. Macromol. Rapid. Commun. 2022, 43, e2100897.
96. Zhao, W.; Yan, P.; Yang, H.; et al. Using sound to synthesize covalent organic frameworks in water. Nat. Synth. 2022, 1, 87-95.
97. Wang, H.; Zhao, J.; Li, Y.; et al. Aqueous two-phase interfacial assembly of COF membranes for water desalination. Nanomicro. Lett. 2022, 14, 216.
98. Hu, F.; Hu, Z.; Liu, Y.; et al. Aqueous sol-gel synthesis and shaping of covalent organic frameworks. J. Am. Chem. Soc. 2023, 145, 27718-27.
99. Zhang, Z.; Xu, Y. Hydrothermal synthesis of highly crystalline zwitterionic vinylene-linked covalent organic frameworks with exceptional photocatalytic properties. J. Am. Chem. Soc. 2023, 145, 25222-32.
100. Yang, Y.; Sabaghi, D.; Liu, C.; et al. On-water surface synthesis of vinylene-linked cationic two-dimensional polymer films as the anion-selective electrode coating. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316299.
101. Liu, Y.; Wang, Y.; Li, H.; et al. Ambient aqueous-phase synthesis of covalent organic frameworks for degradation of organic pollutants. Chem. Sci. 2019, 10, 10815-20.
102. Martín-Illán, J. Á.; Rodríguez-San-Miguel, D.; Franco, C.; et al. Green synthesis of imine-based covalent organic frameworks in water. Chem. Commun. 2020, 56, 6704-7.
103. Zhao, B.; He, M.; Chen, B.; Hu, B. Facile green synthesis of magnetic porous organic polymers for fast preconcentration of trace lead and mercury from environmental water followed by graphite furnace atomic absorption spectrometry detection. Spectrochim. Acta. B. 2022, 196, 106524.
104. Huang, L.; He, M.; Chen, B.; Cheng, Q.; Hu, B. Facile green synthesis of magnetic porous organic polymers for rapid removal and separation of methylene blue. ACS. Sustain. Chem. Eng. 2017, 5, 4050-5.
105. Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. Engl. 2008, 47, 3450-3.
106. Dong, B.; Wang, D.; Wang, W.; Tian, X.; Ren, G. Post synthesis of a glycine-functionalized covalent triazine framework with excellent CO2 capture performance. Micropor. Mesopor. Mat. 2020, 306, 110475.
107. Jiang, K.; Peng, P.; Tranca, D.; et al. Covalent triazine frameworks and porous carbons: perspective from an azulene-based case. Macromol. Rapid. Commun. 2022, 43, e2200392.
108. Mohamed, M. G.; Sharma, S. U.; Liu, N. Y.; et al. Ultrastable covalent triazine organic framework based on anthracene moiety as platform for high-performance carbon dioxide adsorption and supercapacitors. Int. J. Mol. Sci. 2022, 23, 3174.
109. Wang, G.; Leus, K.; Zhao, S.; Van, D. V. P. Newly designed covalent triazine framework based on novel N-heteroaromatic building blocks for efficient CO2 and H2 capture and storage. ACS. Appl. Mater. Interfaces. 2018, 10, 1244-9.
110. Mohamed, M. G.; El-mahdy, A. F. M.; Takashi, Y.; Kuo, S. Ultrastable conductive microporous covalent triazine frameworks based on pyrene moieties provide high-performance CO2 uptake and supercapacitance. New. J. Chem. 2020, 44, 8241-53.
111. Rangaraj, V. M.; Reddy, K. S. K.; Karanikolos, G. N. Ionothermal synthesis of phosphonitrilic-core covalent triazine frameworks for carbon dioxide capture. Chem. Eng. J. 2022, 429, 132160.
112. Lan, Z. A.; Wu, M.; Fang, Z.; et al. Ionothermal synthesis of covalent triazine frameworks in a NaCl-KCl-ZnCl2 eutectic salt for the hydrogen evolution reaction. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201482.
113. Wang, C.; Lyu, P.; Chen, Z.; Xu, Y. Green and scalable synthesis of atomic-thin crystalline two-dimensional triazine polymers with ultrahigh photocatalytic properties. J. Am. Chem. Soc. 2023, 145, 12745-54.
114. Yu, S. Y.; Mahmood, J.; Noh, H. J.; et al. Direct synthesis of a covalent triazine-based framework from aromatic amides. Angew. Chem. Int. Ed. Engl. 2018, 57, 8438-42.
115. Niu, F.; Shao, Z.; Tao, L.; Ding, Y. Covalent triazine-based frameworks for NH3 gas sensing at room temperature. Sensor. Actuat. B. Chem. 2020, 321, 128513.
116. Sun, T.; Liang, Y.; Luo, W.; Zhang, L.; Cao, X.; Xu, Y. A general strategy for kilogram-scale preparation of highly crystal-line covalent triazine frameworks. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203327.
117. Zhang, P.; Wang, Z.; Wang, S.; et al. Fabricating industry-compatible olefin-linked COF resins for oxoanion pollutant scavenging. Angew. Chem. Int. Ed. Engl. 2022, 61, e202213247.
118. Wang, Z.; Zhang, Y.; Wang, T.; et al. Organic flux synthesis of covalent organic frameworks. Chem 2023, 9, 2178-93.
119. Krusenbaum, A.; Grätz, S.; Tigineh, G. T.; Borchardt, L.; Kim, J. G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873-905.
121. Leonardi, M.; Villacampa, M.; Menéndez, J. C. Multicomponent mechanochemical synthesis. Chem. Sci. 2018, 9, 2042-64.
122. Shinde, D. B.; Aiyappa, H. B.; Bhadra, M.; et al. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A. 2016, 4, 2682-90.
123. Liu, W.; Cao, Y.; Wang, W.; et al. Mechanochromic luminescent covalent organic frameworks for highly selective hydroxyl radical detection. Chem. Commun. 2018, 55, 167-70.
124. Zhang, X.; Xue, S.; Yan, Y.; Liu, S.; Ye, Q.; Zhou, F. Mechanochemical synthesis of thiadiazole functionalized COF as oil-based lubricant additive for reducing friction and wear. Langmuir 2024, 40, 4373-81.
125. Rajput, L.; Banerjee, R. Mechanochemical synthesis of amide functionalized porous organic polymers. Cryst. Growth. Des. 2014, 14, 2729-32.
126. Li, G.; Ye, J.; Fang, Q.; Liu, F. Amide-based covalent organic frameworks materials for efficient and recyclable removal of heavy metal lead (II). Chem. Eng. J. 2019, 370, 822-30.
127. Jie, K.; Zhou, Y.; Sun, Q.; et al. Mechanochemical synthesis of pillar[5]quinone derived multi-microporous organic polymers for radioactive organic iodide capture and storage. Nat. Commun. 2020, 11, 1086.
128. Troschke, E.; Grätz, S.; Lübken, T.; Borchardt, L. Mechanochemical Friedel-Crafts alkylation-A sustainable pathway towards porous organic polymers. Angew. Chem. Int. Ed. Engl. 2017, 56, 6859-63.
129. Chen, X.; Yuan, Z.; Zhong, Y.; Sun, F.; Ren, H. Synthesis of a series of porous aromatic frameworks by mechanical ball milling. New. J. Chem. 2022, 46, 22504-8.
130. Krusenbaum, A.; Geisler, J.; Kraus, F. J. L.; et al. The mechanochemical Friedel-Crafts polymerization as a solvent-free cross-linking approach toward microporous polymers. J. Polym. Sci. 2022, 60, 62-71.
131. Yuan, R.; Yan, Z.; Shaga, A.; He, H. Solvent-free mechanochemical synthesis of a carbazole-based porous organic polymer with high CO2 capture and separation. J. Solid. State. Chem. 2020, 287, 121327.
132. Krusenbaum, A.; Kraus, F. J. L.; Hutsch, S.; et al. The rapid mechanochemical synthesis of microporous covalent triazine networks: elucidating the role of chlorinated linkers by a solvent-free approach. Adv. Sustain. Syst. 2023, 7, 2200477.
133. Pan, Q.; Xu, Z.; Deng, S.; et al. A mechanochemically synthesized porous organic polymer derived CQD/chitosan-graphene composite film electrode for electrochemiluminescence determination of dopamine. RSC. Adv. 2019, 9, 39332-7.
134. Zhang, P.; Jiang, X.; Wan, S.; Dai, S. Charged porous polymers using a solid C-O cross-coupling reaction. Chemistry 2015, 21, 12866-70.
135. Hou, S.; Meng, M.; Liu, D.; Zhang, P. Mechanochemical process to construct porous ionic polymers by menshutkin reaction. ChemSusChem 2021, 14, 3059-63.
136. Tao, Y.; Liu, H.; Kong, H. Y.; et al. Electrochemical preparation of porous organic polymer films for high-performance memristors. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205796.
137. Zhang, M.; Jing, X.; Zhao, S.; et al. Electropolymerization of molecular-sieving polythiophene membranes for H2 separation. Angew. Chem. Int. Ed. Engl. 2019, 58, 8768-72.
138. Gu, C.; Huang, N.; Chen, Y.; et al. π-Conjugated microporous polymer films: designed synthesis, conducting properties, and photoenergy conversions. Angew. Chem. Int. Ed. Engl. 2015, 54, 13594-8.
139. Shirokura, T.; Hirohata, T.; Sato, K.; et al. Site-selective synthesis and concurrent immobilization of imine-based covalent organic frameworks on electrodes using an electrogenerated acid. Angew. Chem. Int. Ed. Engl. 2023, 62, e202307343.
140. Wang, M.; Wang, Y.; Zhao, J.; et al. Electrochemical interfacial polymerization toward ultrathin COF membranes for brine desalination. Angew. Chem. Int. Ed. Engl. 2023, 62, e202219084.
141. Wang, L.; Xu, C.; Zhang, W.; et al. Electrocleavage synthesis of solution-processed, imine-linked, and crystalline covalent organic framework thin films. J. Am. Chem. Soc. 2022, 144, 8961-8.
142. Gu, C.; Huang, N.; Gao, J.; Xu, F.; Xu, Y.; Jiang, D. Controlled synthesis of conjugated microporous polymer films: versatile platforms for highly sensitive and label-free chemo- and biosensing. Angew. Chem. Int. Ed. Engl. 2014, 53, 4850-5.
143. Long, J.; Liu, Y.; Huang, Z.; et al. Electropolymerization of preferred-oriented conjugated microporous polymer films for enhanced fluorescent sensing. Chemistry 2024, 30, e202304268.
144. Gu, C.; Huang, N.; Wu, Y.; Xu, H.; Jiang, D. Design of highly photofunctional porous polymer films with controlled thickness and prominent microporosity. Angew. Chem. Int. Ed. Engl. 2015, 54, 11540-4.
145. Zhou, Z.; Guo, D.; Shinde, D. B.; et al. Precise sub-angstrom ion separation using conjugated microporous polymer membranes. ACS. Nano. 2021, 15, 11970-80.
146. Zhou, Z.; Shinde, D. B.; Guo, D.; et al. Flexible ionic conjugated microporous polymer membranes for fast and selective ion transport. Adv. Funct. Mater. 2022, 32, 2108672.
147. Chen, D.; Li, Y.; Zhao, X.; et al. Self-standing porous aromatic framework electrodes for efficient electrochemical uranium extraction. ACS. Cent. Sci. 2023, 9, 2326-32.
148. Kim, S.; Park, C.; Lee, M.; et al. Rapid photochemical synthesis of sea-urchin-shaped hierarchical porous COF-5 and its lithography-free patterned growth. Adv. Funct. Mater. 2017, 27, 1700925.
149. Wei, H.; Chai, S.; Hu, N.; Yang, Z.; Wei, L.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178-81.
150. Alsudairy, Z.; Brown, N.; Yang, C.; et al. Facile microwave-assisted synthesis of 2D imine-linked covalent organic frameworks for exceptional iodine capture. Precis. Chem. 2023, 1, 233-40.
151. Wang, X.; Chi, X.; Li, M.; Guan, D.; Miao, C.; Xu, J. An integrated solid-state lithium-oxygen battery with highly stable anionic covalent organic frameworks electrolyte. Chem 2023, 9, 394-410.
152. Wu, C. J.; Li, X. Y.; Li, T. R.; et al. Natural sunlight photocatalytic synthesis of benzoxazole-bridged covalent organic framework for photocatalysis. J. Am. Chem. Soc. 2022, 144, 18750-5.
153. Wang, F.; Qiu, Y.; Wang, B.; Wang, H.; Long, Y. Green method to fabricate porous microspheres for ultrasensitive SERS detection using UV light. RSC. Adv. 2016, 6, 100519-25.
154. Jaszcz, K. Highly porous crosslinked poly(ester-anhydride) microspheres with high loading efficiency. Chin. J. Polym. Sci. 2015, 33, 1271-82.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data





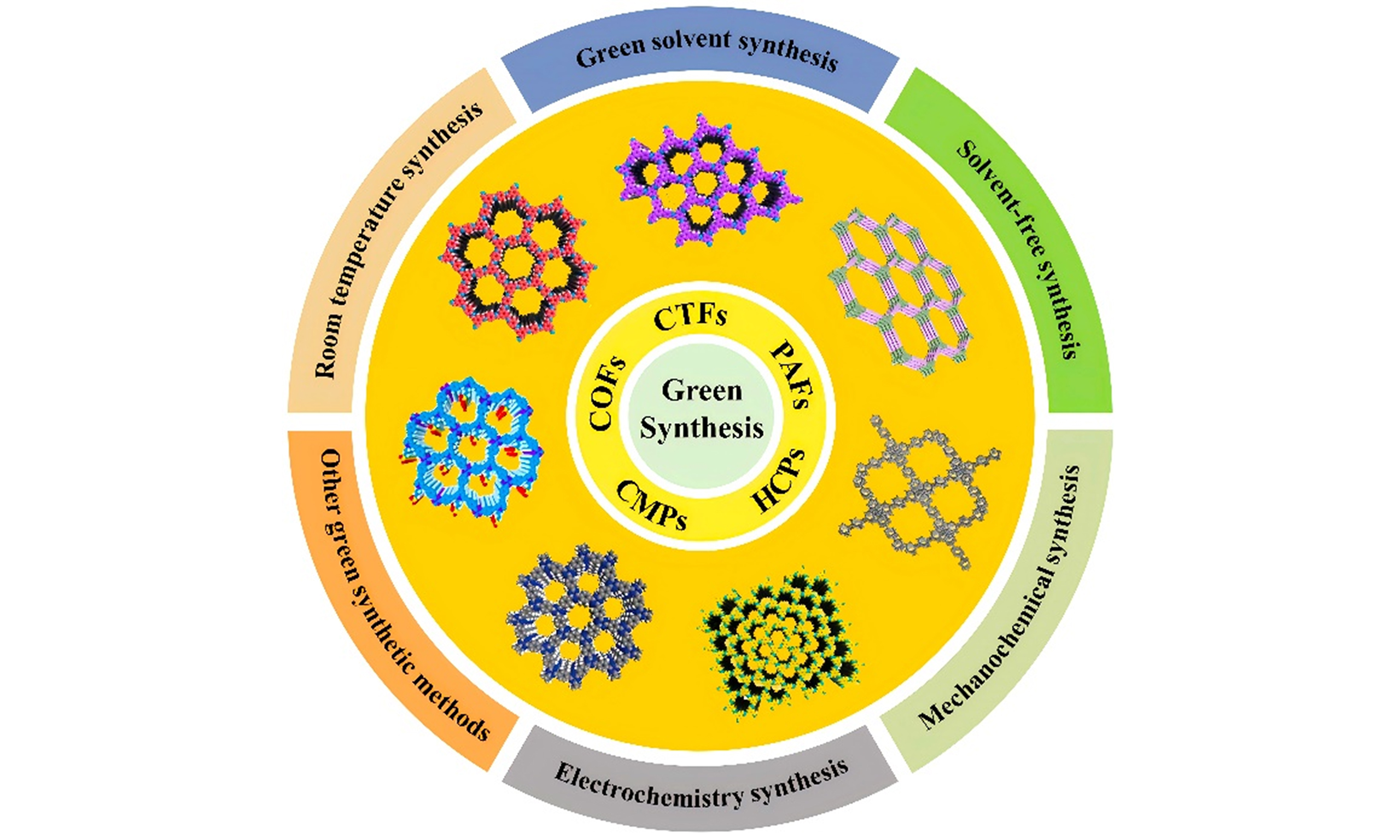
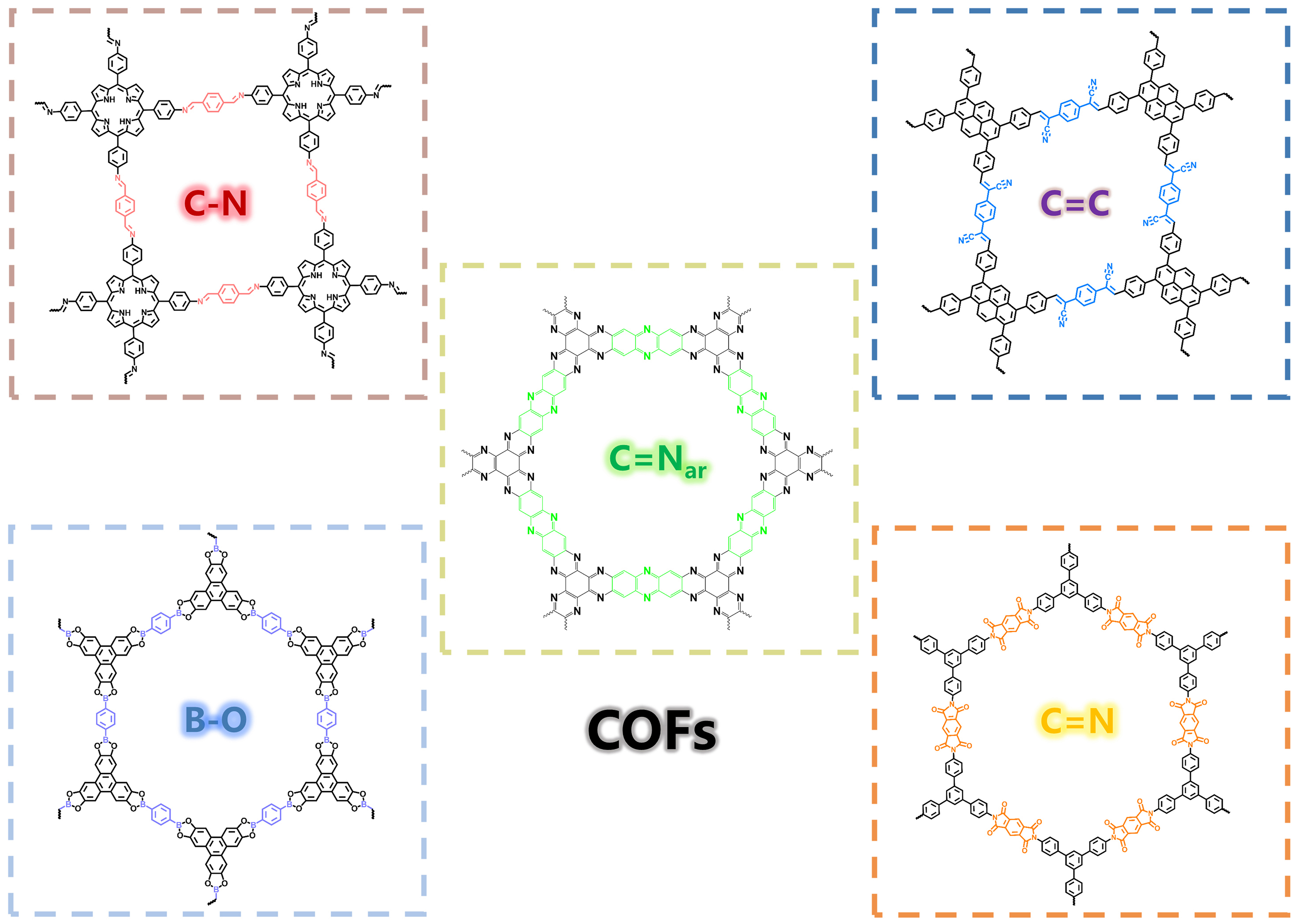
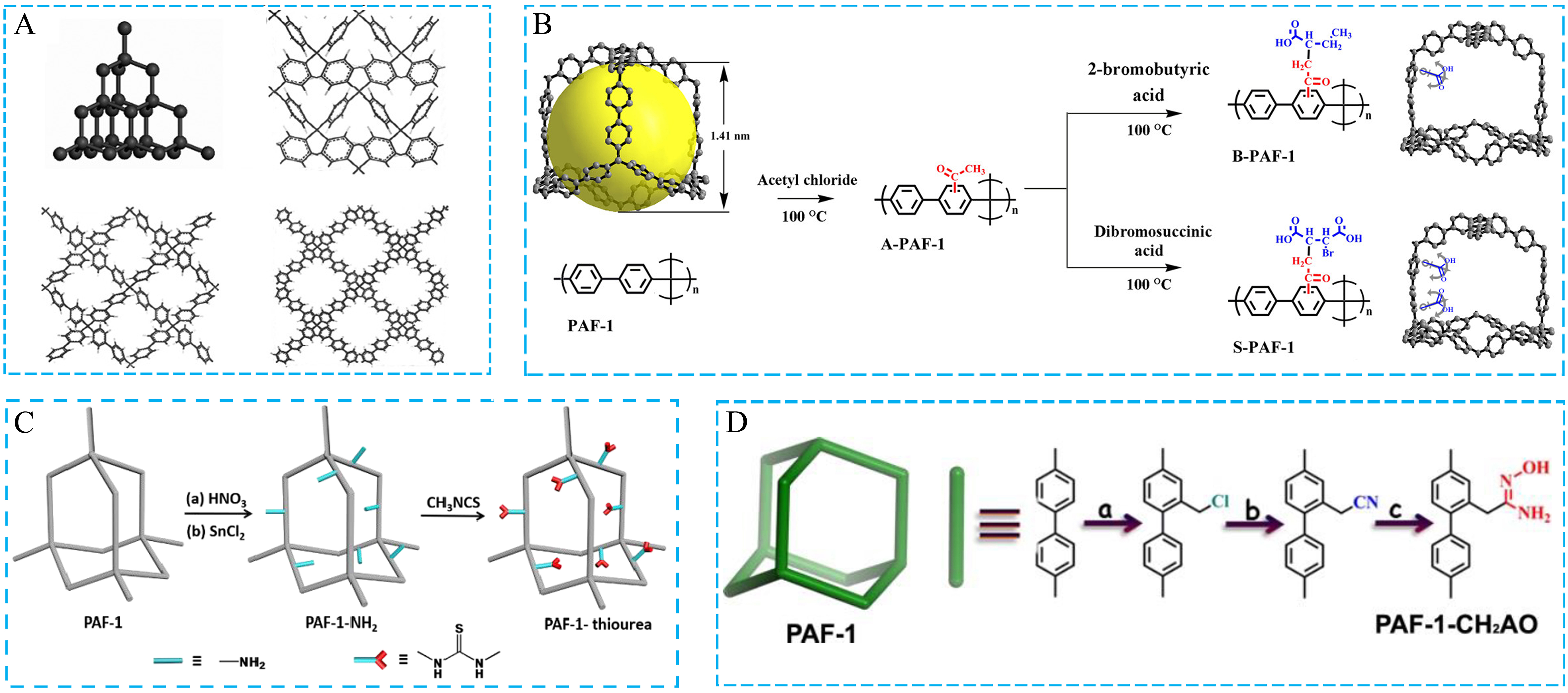
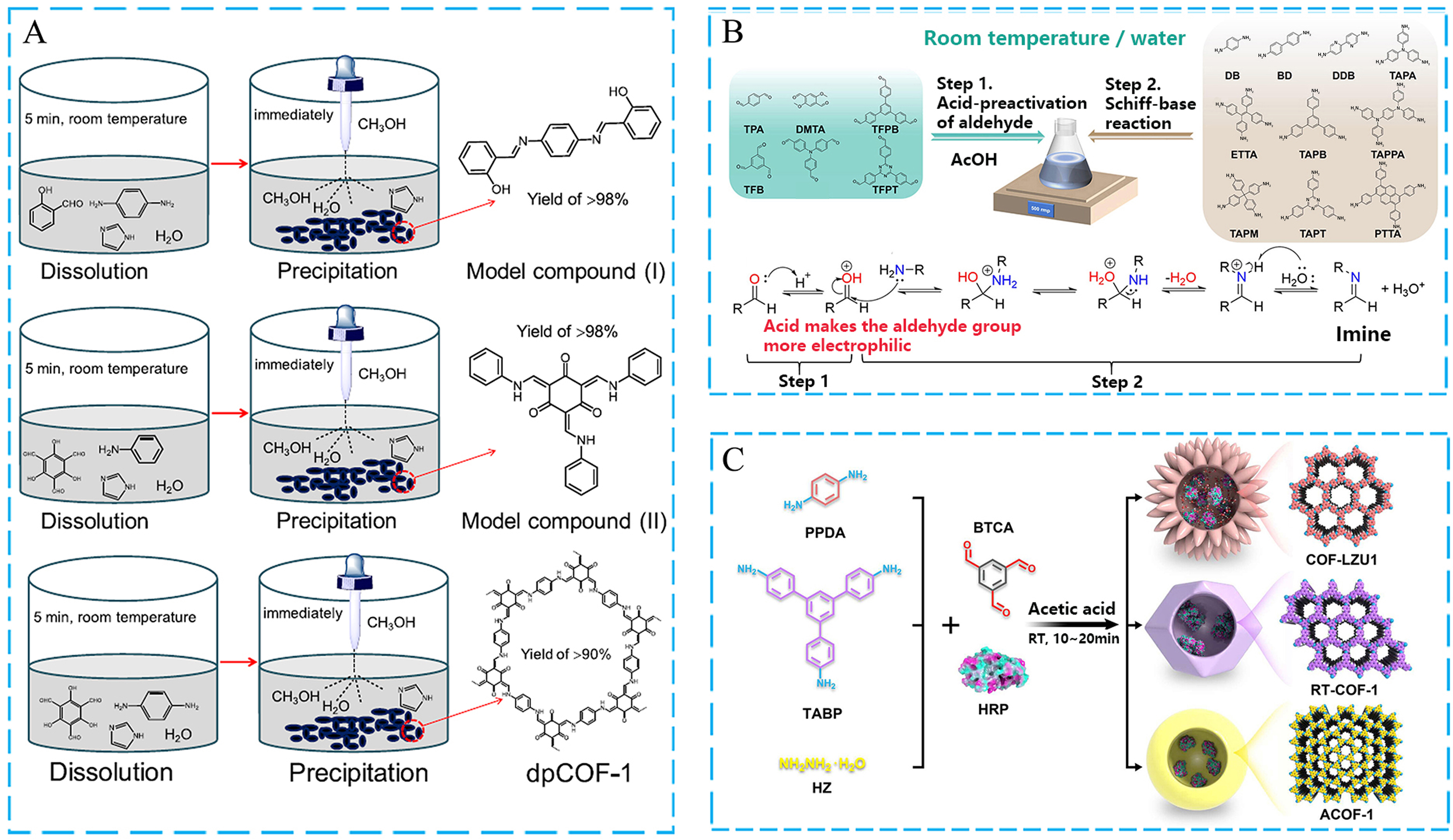
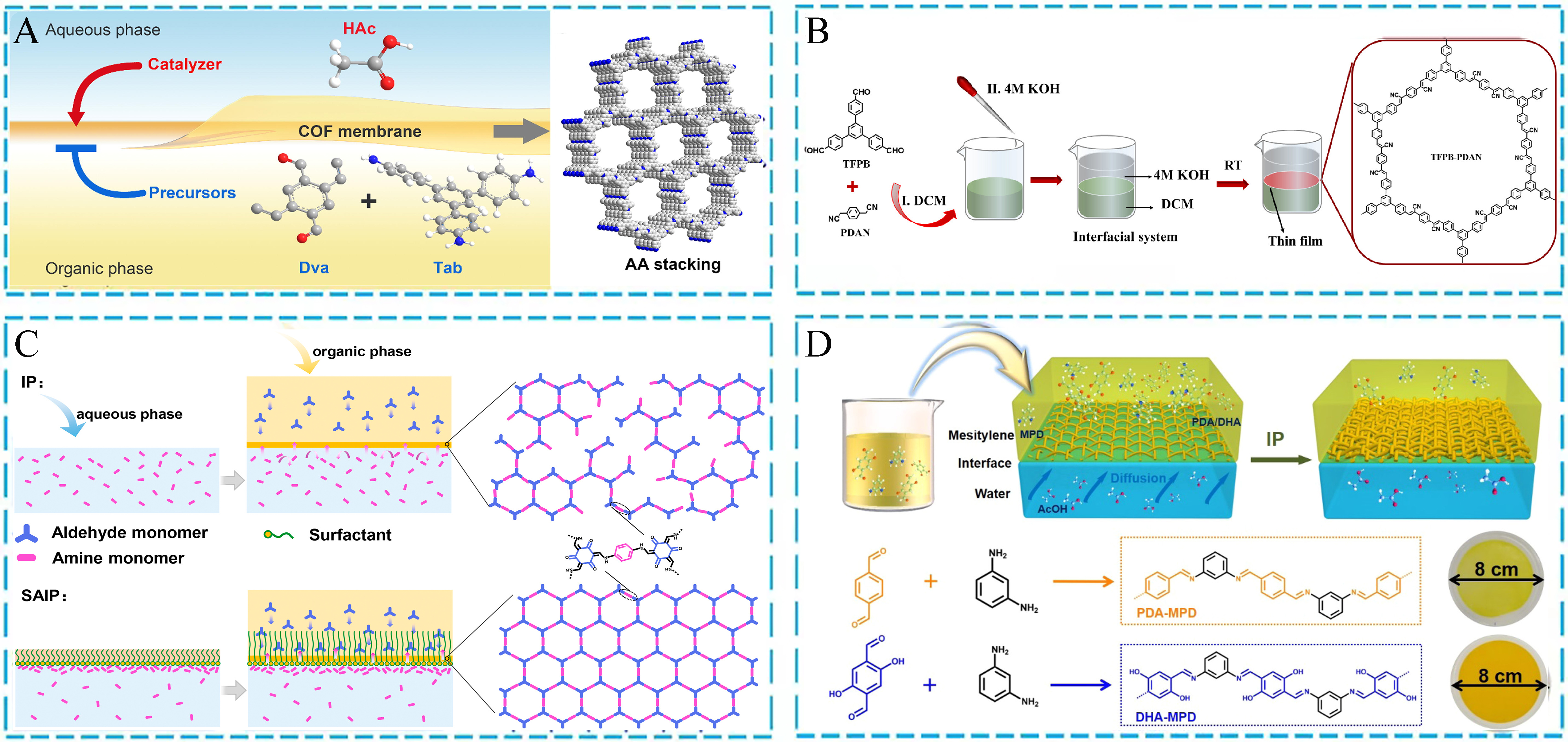
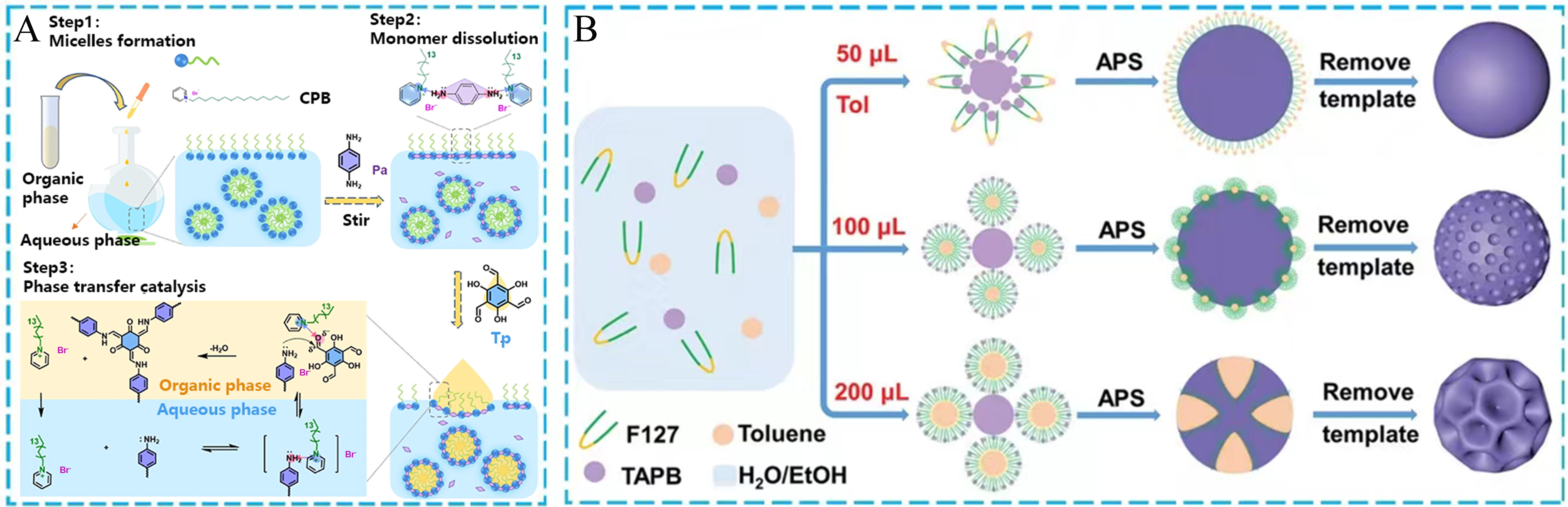
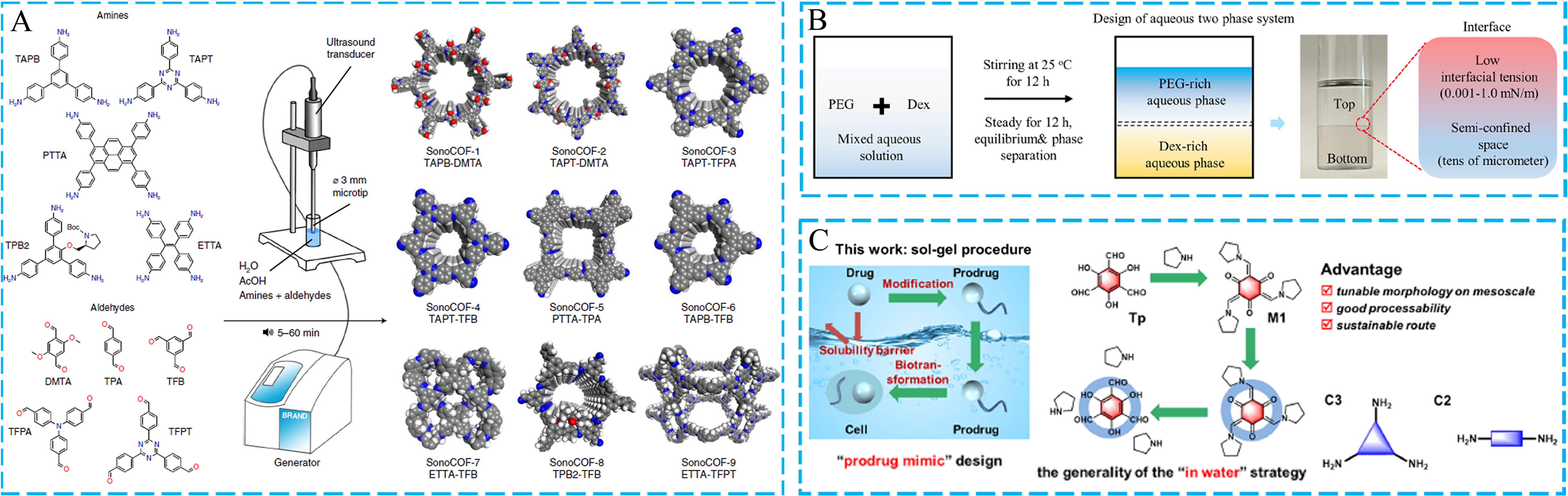
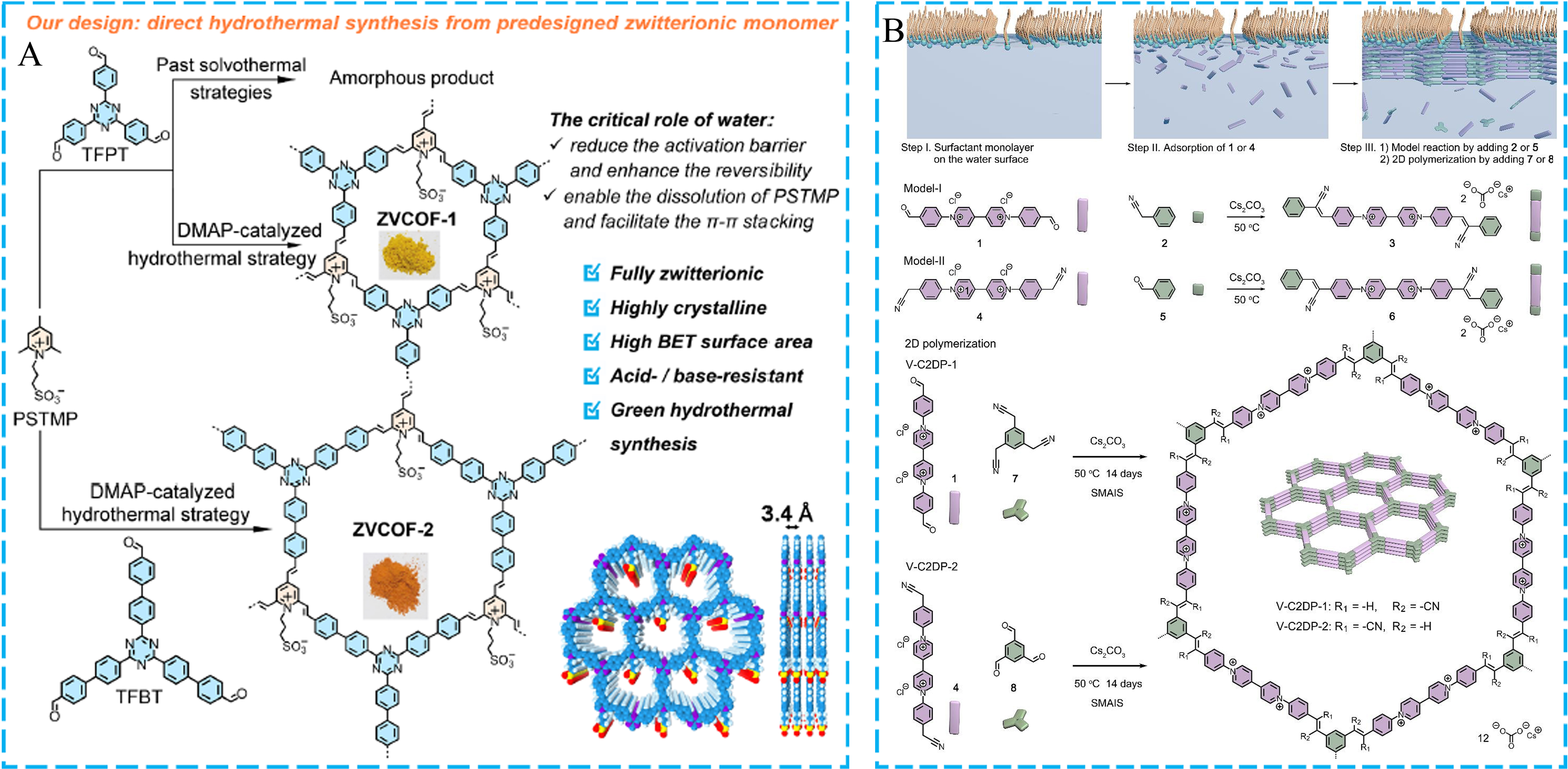
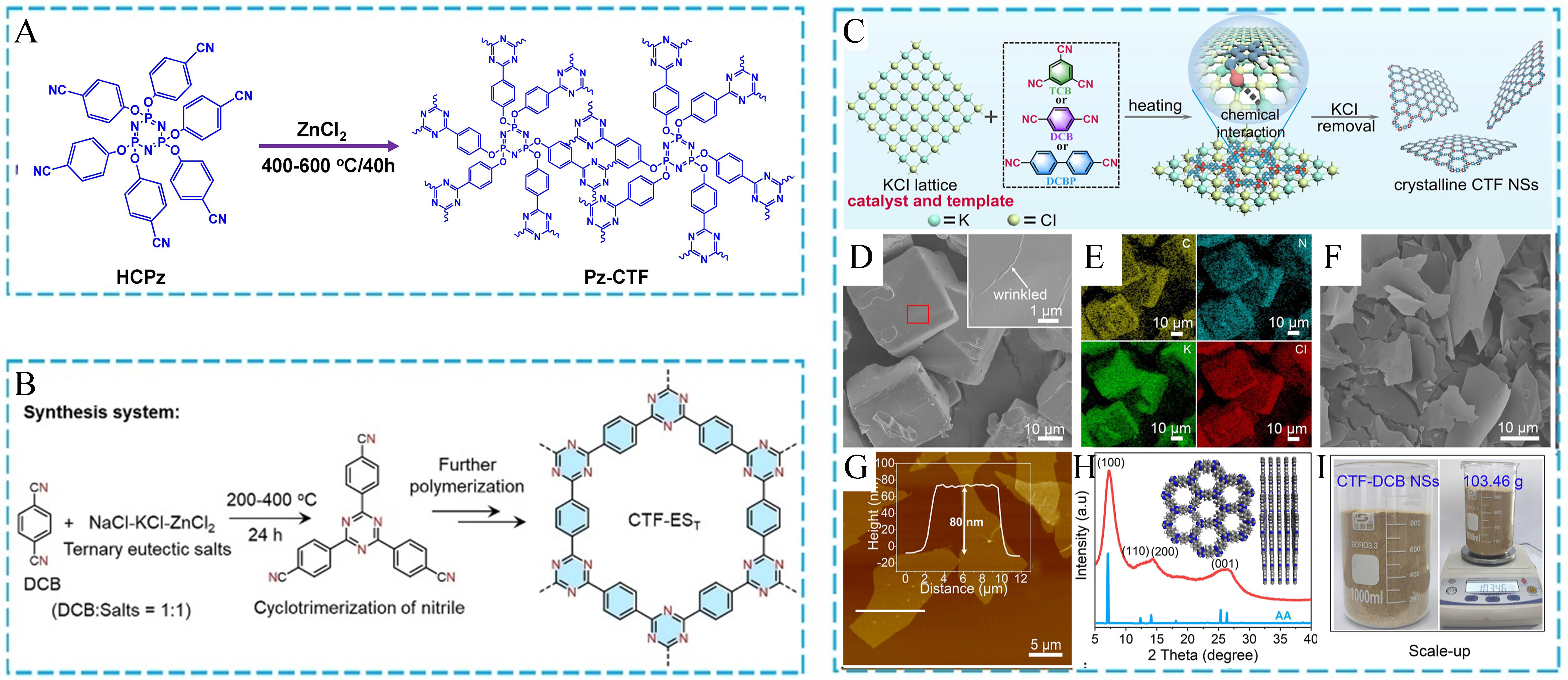
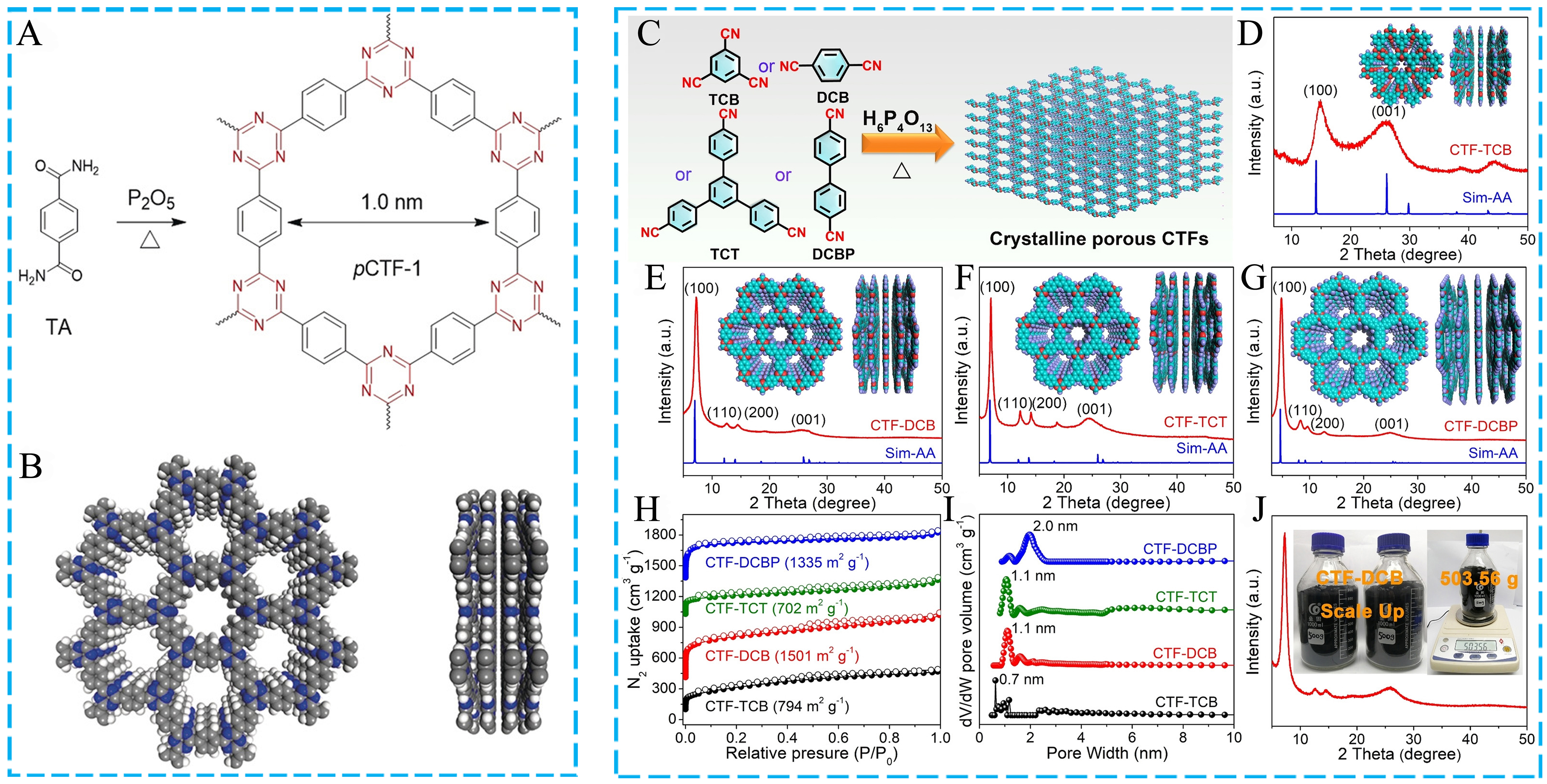
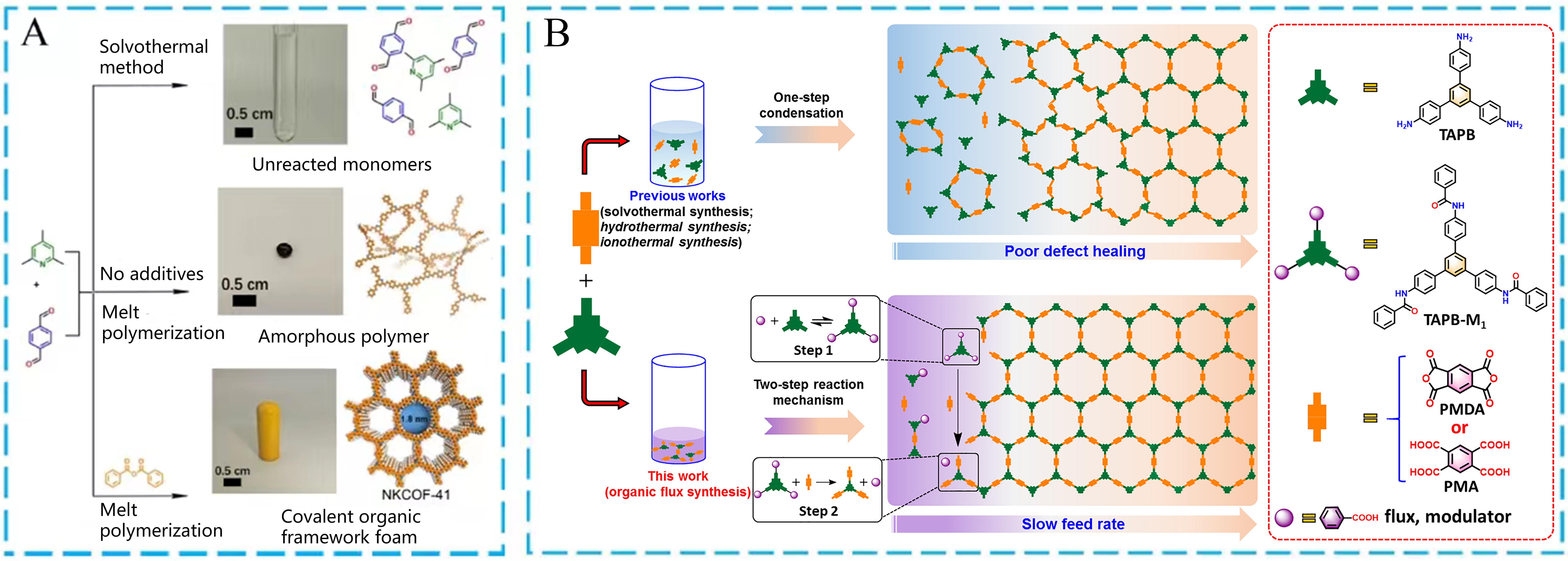
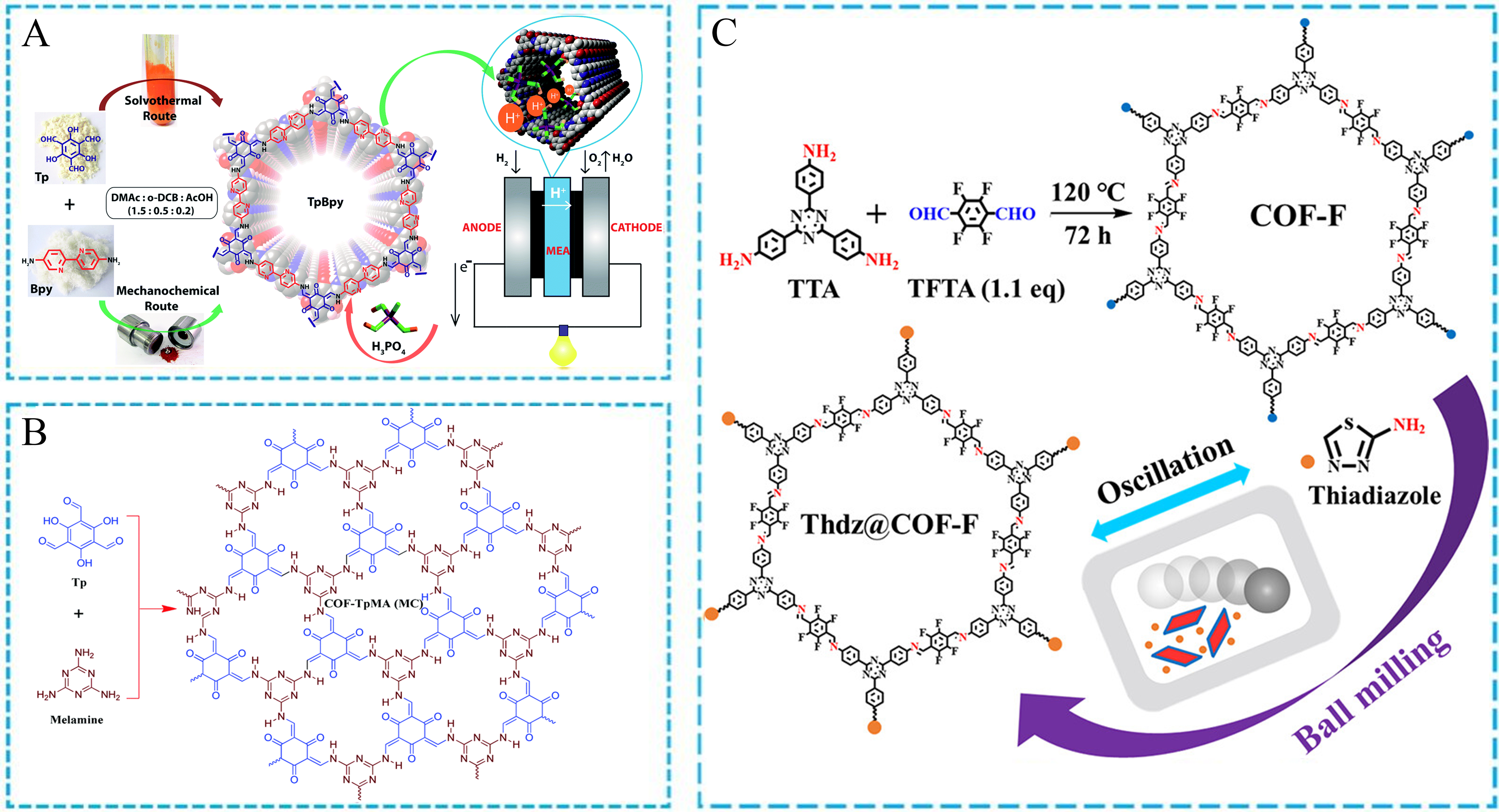
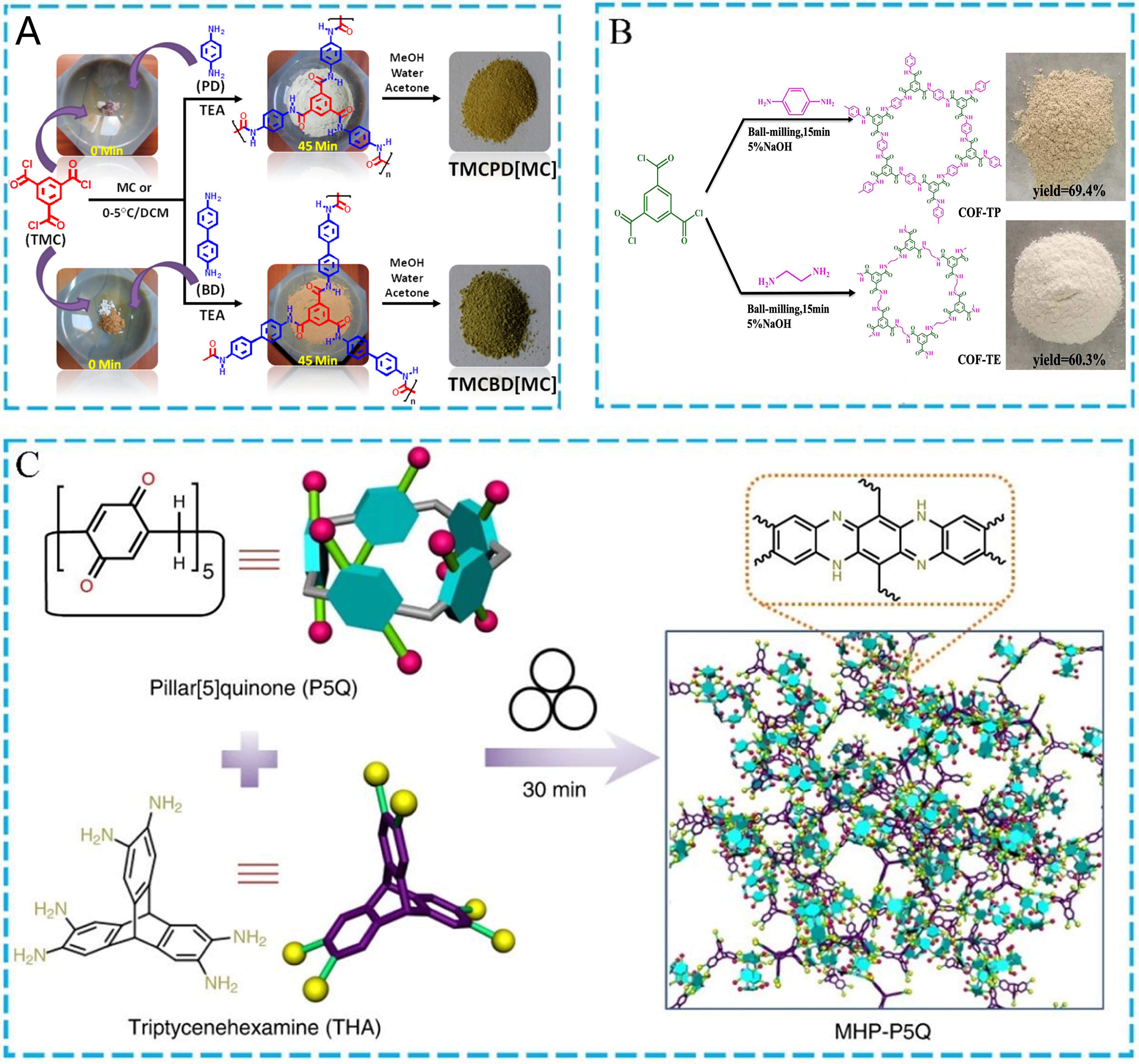
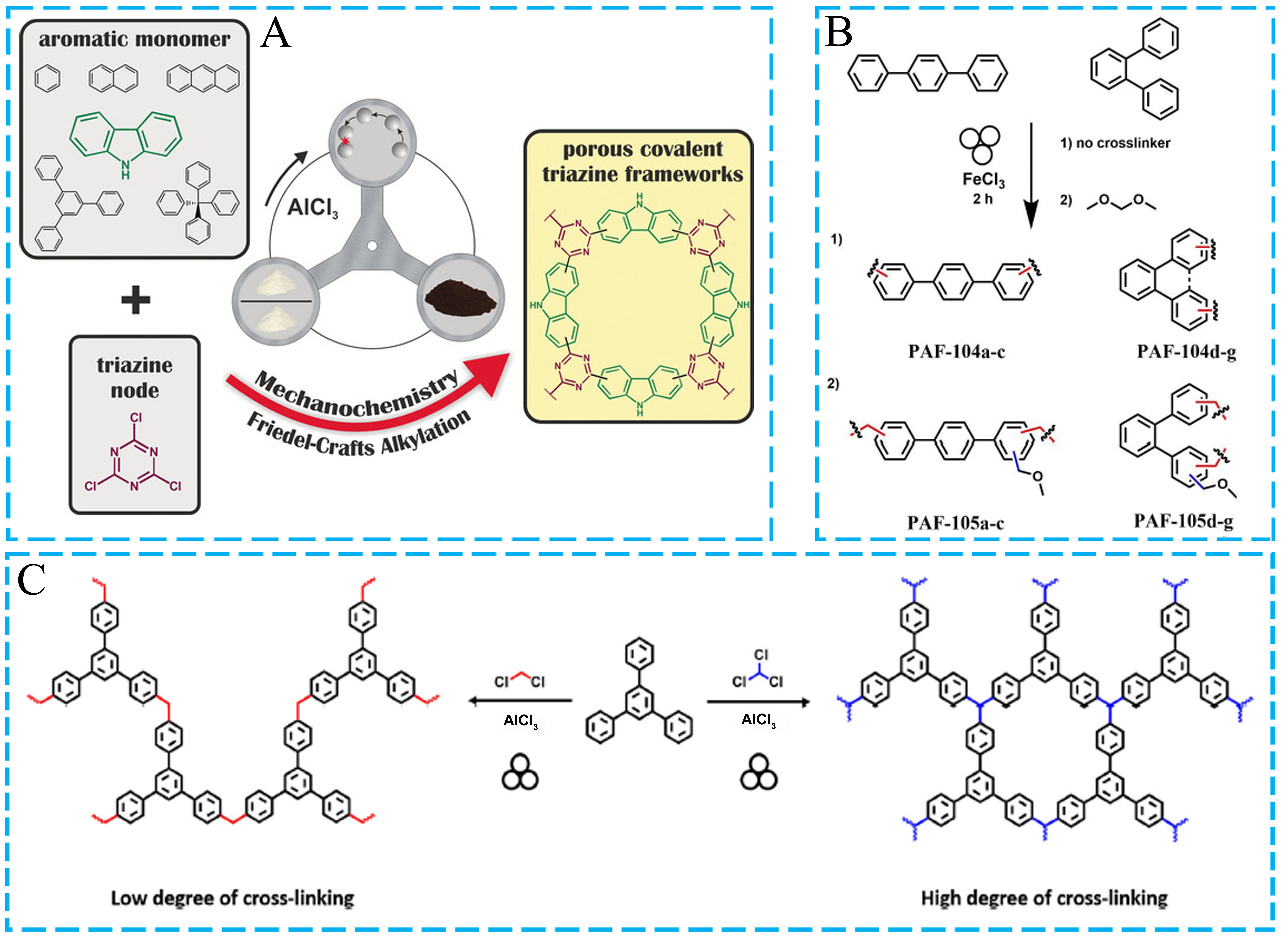
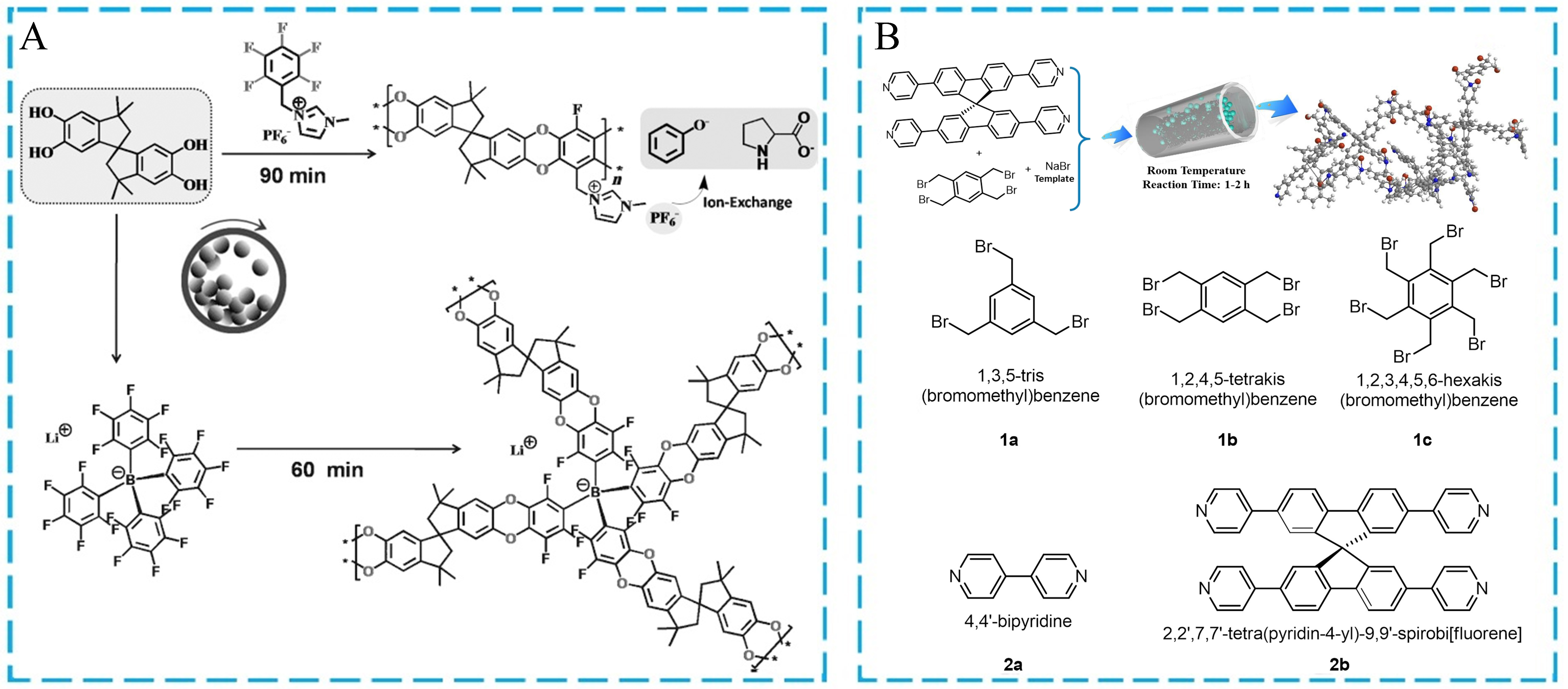
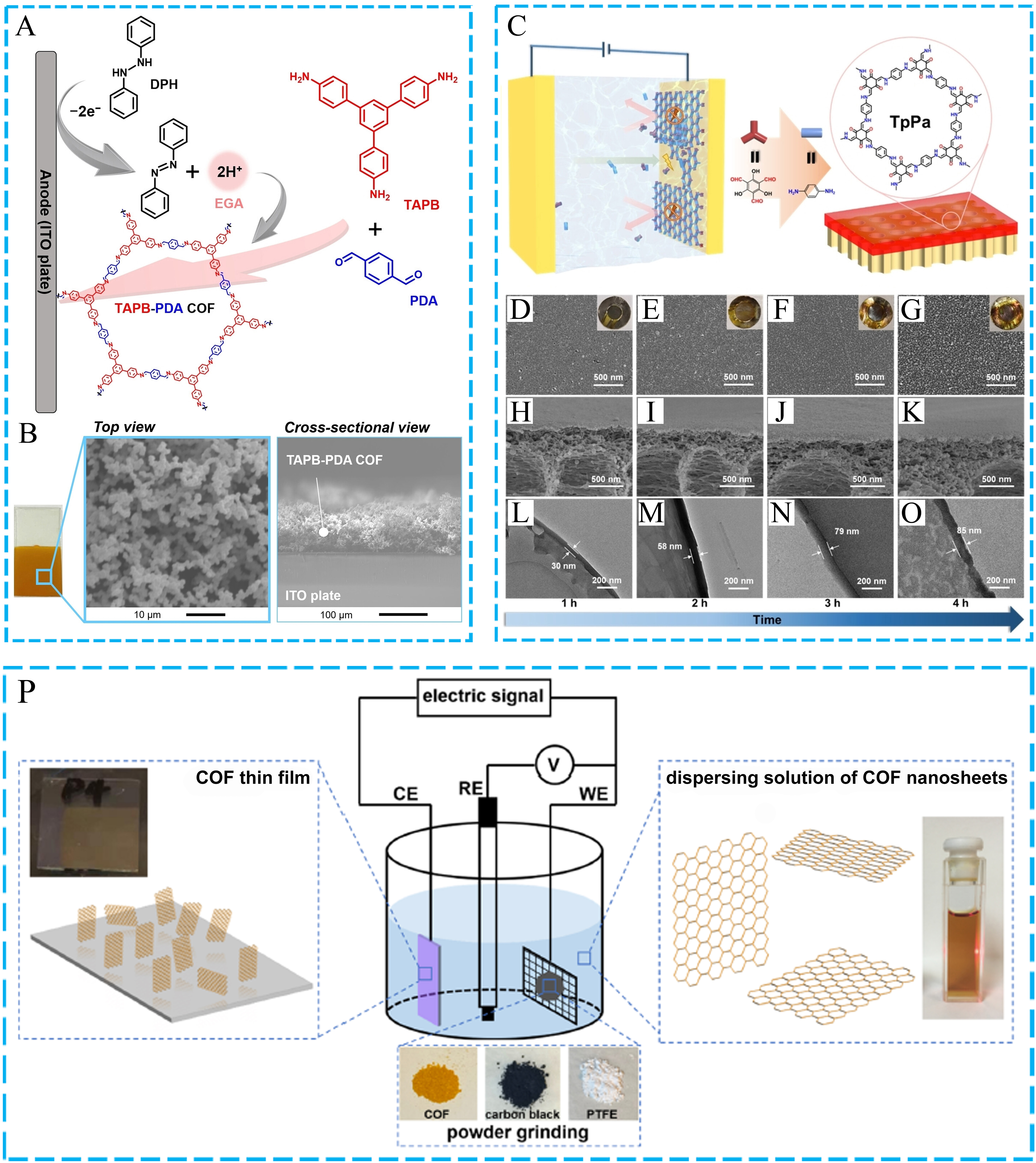
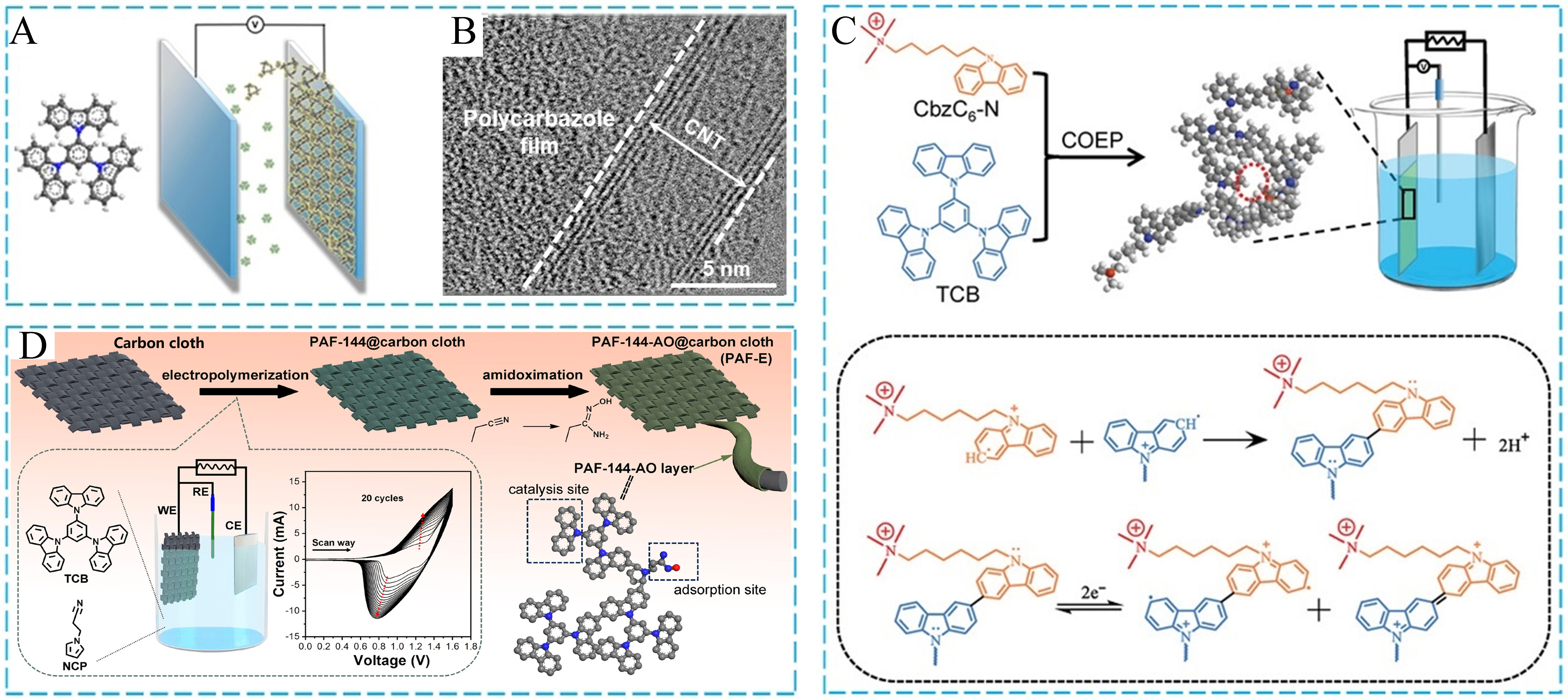
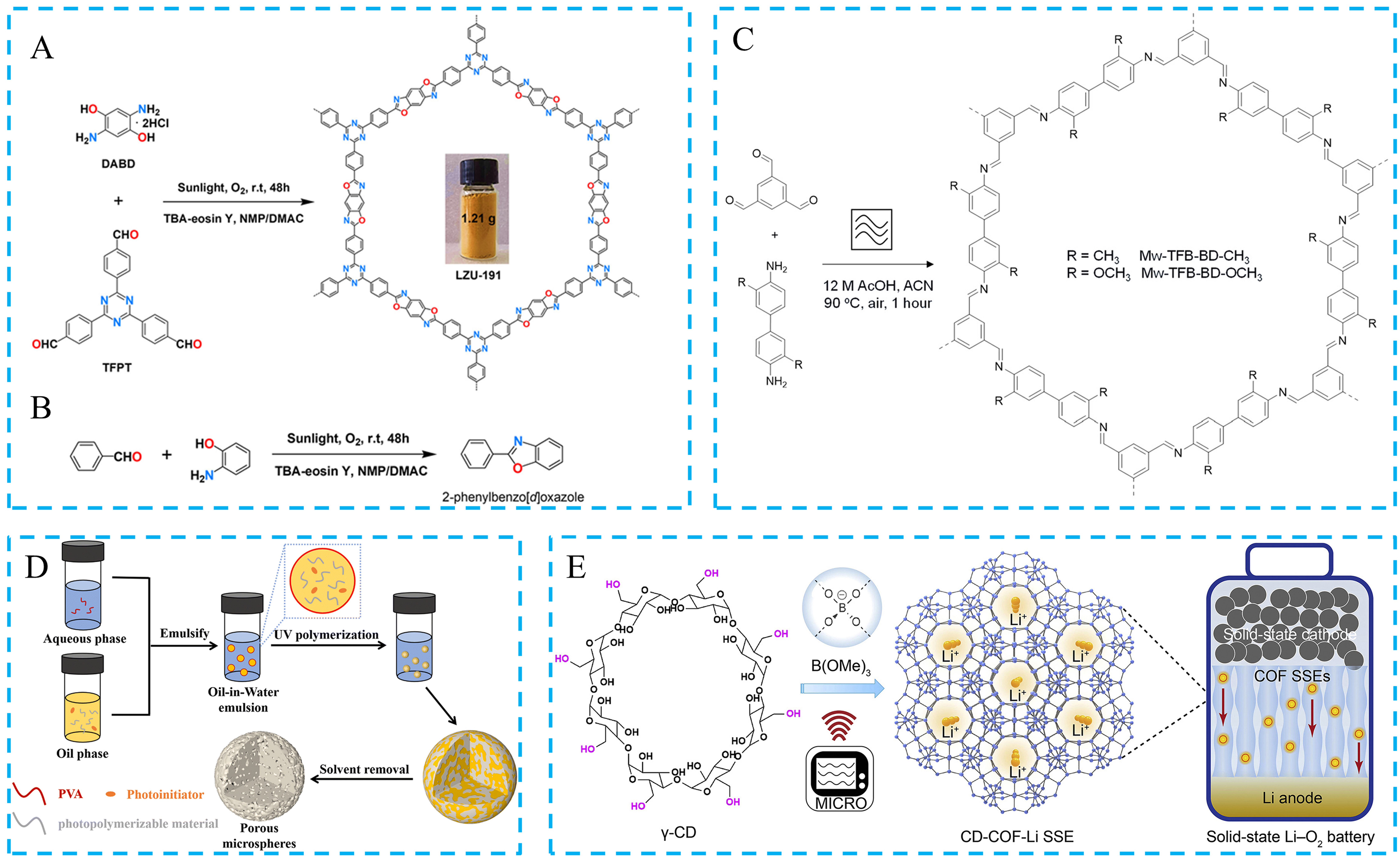













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].