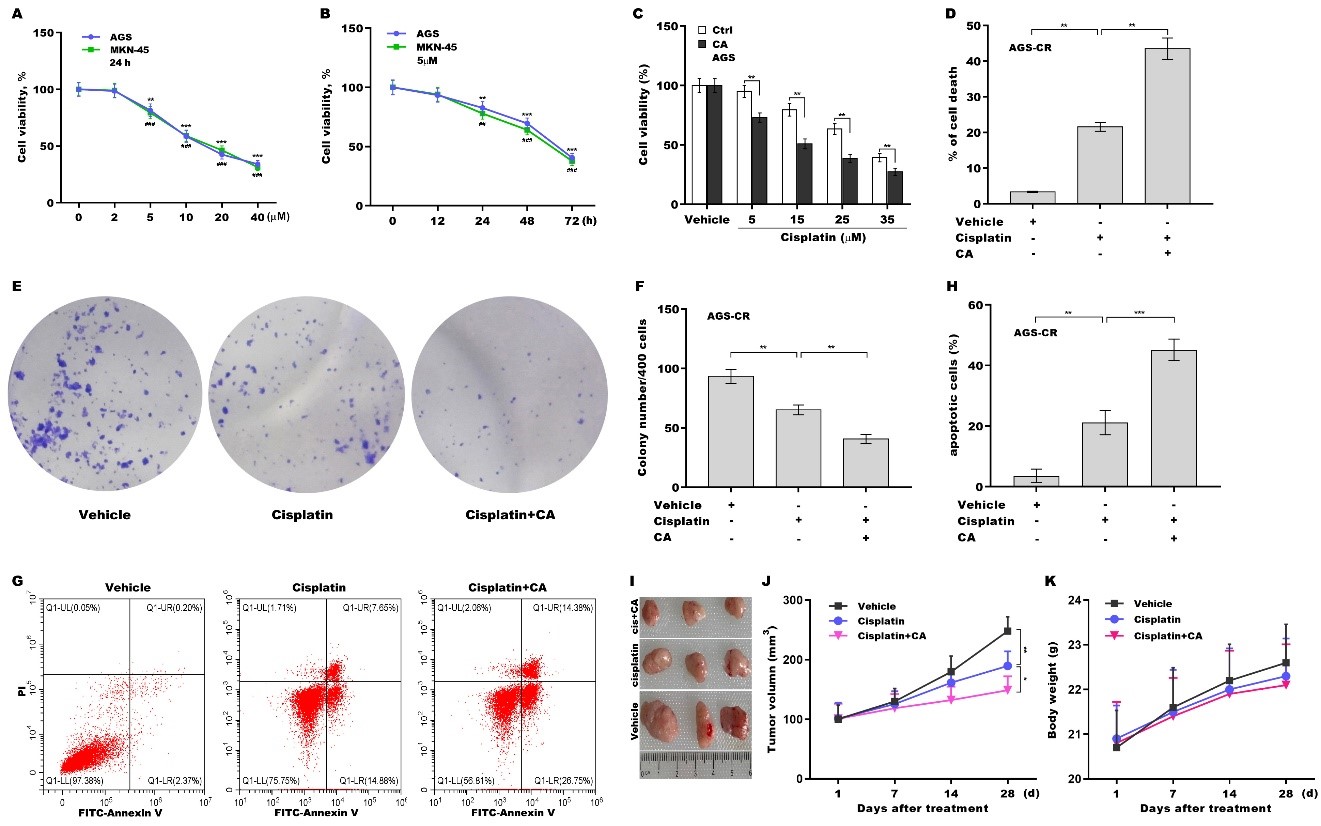
fig1
Figure 1. CA sensitized GC cells to cisplatin treatment. (A) AGS and MKN-45 cells were exposed to different concentrations of CA (0, 2, 5, 10, 20, and 40 μm) for 24 h, and cell viability was measured using CCK-8 (n = 3). Statistical significance was assessed using one-way ANOVA followed by Dunnett’s post-hoc test (AGS: **P < 0.01, ***P < 0.001. MKN-45: ##P < 0.01, ###P < 0.001); (B) AGS and MKN-45 cells were exposed to CA (5 µm) for different times (0, 12, 24, 48, and 72 h), and cell viability was assessed using CCK-8 (n = 3). Statistical significance was assessed using one-way ANOVA followed by Dunnett’s post-hoc test (AGS: **P < 0.01, ***P < 0.001. MKN-45: ##P < 0.01, ###P < 0.001); (C) AGS cells were exposed to CA (20 µm) and different concentrations of cisplatin (0, 5, 15, 25, and 35 µm) for 24 h, and cell viability was assessed using the CCK-8 assay (n = 3). Statistical significance was assessed using multiple t-tests; (D) AGS-CR cells were exposed to 5 µm of CA and 35 µm of cisplatin for 24 h, after which cell death was assessed using the CCK-8 assay (n = 3). Statistical significance was assessed using one-way ANOVA followed by Dunnett’s post-hoc test. AGS-CR cells were treated with 5 µm of CA and 35 µm of cisplatin for 3 days, and then clone formation assay (E) and quantitative analysis (F) were carried out (n = 3). Statistical significance was assessed using one-way ANOVA followed by Dunnett’s post-hoc test. AGS-CR cells were treated with 5 µm of CA and 35 µm of cisplatin for 24 h, after which flow cytometry (G) and quantitative analysis (H) were carried out (n = 3). Statistical significance was assessed using one-way ANOVA followed by Dunnett’s post-hoc test; (I-K) To assess the effect of CA on cisplatin resistance and its safety in vivo, a mouse xenograft model was developed by subcutaneously injecting AGS cells into nude mice. Once tumor volume approached 100 mm3, the mice with tumors were administered either vehicle, cisplatin, or combination treatment with cisplatin and CA, and tumor growth was monitored (I and J) and body weight was measured (K) (n = 3). Statistical significance was assessed using one-way ANOVA followed by Dunnett’s post-hoc test. *p < 0.05, **P < 0.01, ***P < 0.001. CA: Corosolic acid; GC: gastric cancer; CCK-8: cell counting kit-8; ANOVA: analysis of variance; CR: cisplatin-resistant.