fig2
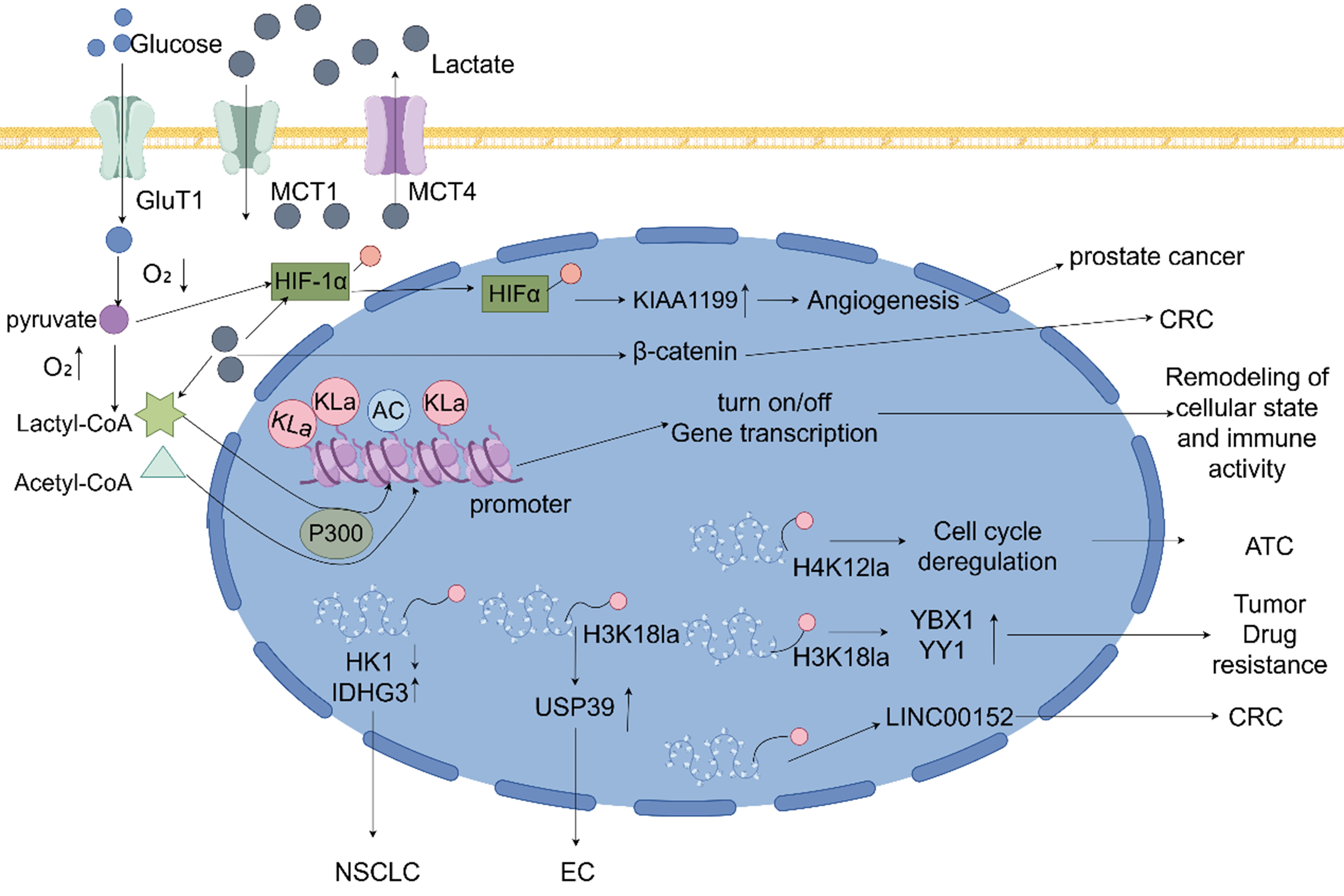
Figure 2. Lactate as a signaling molecule influencing gene transcription and immune evasion through histone and non-histone lysine lactylation, promoting cancer progression. Glycolysis breaks down glucose into pyruvate. Under hypoxic conditions, lactylation of HIFα is activated, which subsequently promotes the expression of KIAA1199, angiogenesis, and the progression of prostate cancer. Under aerobic conditions, lactyl-CoA and acetyl-CoA, driven by P300, catalyze lactylation and acetylation of promoters, thereby reshaping cellular states and immune activity by upregulating or downregulating gene transcription. Elevated levels of H4K12la disrupt the cell cycle and accelerate the progression of ATC. H3K18 lactylation not only enhances the expression of transcription factors YBX1 and YY1 to promote tumor drug resistance but also upregulates USP39 to facilitate the progression of EC. Histone lactylation at the LINC00152 promoter, combined with β-catenin lactylation, jointly drives CRC progression. Moreover, lactylation promotes NSCLC progression by downregulating HK1 expression and upregulating IDHG3 expression. GluT1: Glucose transporter 1; MCT1/4: monocarboxylate transporter 1/4; Kla: histone lysine lactylation; AC: acetylation; HIF: hypoxia-inducible factor; HK1: hexokinase 1; IDHG3: isocitrate dehydrogenase 3; USP39: ubiquitin-specific protease 39; ATC: anaplastic thyroid cancer; NSCLC: non-small cell lung cancer; CRC: colorectal cancer; EC: endometrial cancer.