Calcified coronary nodules: pathophysiology, diagnosis, and interventional challenges
Abstract
Calcified coronary nodules (CCNs) represent a distinct and under-recognized form of coronary artery calcification with significant implications for percutaneous coronary intervention (PCI). Unlike superficial or sheet-like calcifications, CCNs are characterized by protruding, irregular calcium deposits that disrupt luminal integrity, promote thrombus formation, and hinder optimal stent expansion. They have been implicated in acute coronary syndromes (ACS), in-stent restenosis (ISR), and PCI failure, yet they are underappreciated on angiography. This review provides a comprehensive overview of the pathophysiology, diagnostic modalities, and interventional challenges associated with CCNs. Intravascular imaging, particularly optical coherence tomography (OCT) and intravascular ultrasound (IVUS), plays a crucial role in distinguishing nodular from concentric or superficial calcium, thereby guiding appropriate lesion preparation strategies. It also facilitates differentiation between eruptive and non-eruptive nodules, which exhibit distinct prognostic and therapeutic characteristics. Various calcium modification techniques-including rotational atherectomy, orbital atherectomy, intravascular lithotripsy, and scoring/cutting balloons-offer specific advantages and limitations in treating nodular calcium. We also discuss evolving interventional strategies to optimize PCI outcomes and note that restenosis rates with covered stents are unacceptably high, making them unsuitable as a treatment option. Finally, we highlight the need for further research into hybrid calcium modification techniques and long-term PCI outcomes in patients with CCNs. Coronary artery bypass grafting remains a viable alternative.
Keywords
INTRODUCTION
Calcified coronary nodules (CCNs) represent a distinct form of coronary artery calcification, differing from both superficial and deep calcific deposits[1-3]. Initially identified as a cause of acute coronary syndromes (ACS), with reported prevalence in culprit lesions ranging from 2%-8% in the literature[4-7]. CCNs are now increasingly recognized as contributors to percutaneous coronary intervention (PCI) failure, including suboptimal stent expansion and In-Stent Restenosis (ISR)[5]. The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial reported a CNN prevalence of 17% per artery and 30% per patient among non-culprit lesions in ACS patients[8]. CCNs are often underdiagnosed or misdiagnosed as thrombus on angiography. Accurate identification and classification require
PATHOPHYSIOLOGY OF CALCIFIED CORONARY NODULES
CCNs arise from chronic mechanical stress, microfractures, and inflammation-mediated calcification. Unlike traditional calcium deposits, they form as discrete nodules that can erode through a thin fibrous cap, leading to luminal fibrin deposition and thrombus formation[9-10]. Their pathological features are distinguished by specific formation sites, histopathologic characteristics, cellular mechanisms, and clinical risk factors.
CCNs frequently arise in regions subjected to high shear stress, such as vessel bifurcations. These areas, including the proximal-to-mid sections and ostial right coronary artery (RCA), as well as the left main coronary artery bifurcation, are prone to larger hinge motion of the coronary artery[1,10]. This vulnerability may be explained by the interplay of torsional stress with variations in the cardiac cycle and higher lipid burden at these sites[5,10,11]. Additionally, eccentric, less calcified, and more flexible calcium segments located between heavily calcified concentric regions may be more vulnerable to external mechanical forces due to greater movements during the cardiac cycle. At such sites, there appears to be a relative absence of collagen matrix that might increase susceptibility to torsional stress, facilitating protrusion of fragmented calcium sheets through fibrous caps into the lumen[5,7,10]. This process can induce intraplaque haemorrhage consequential to injury of adjacent capillaries or arterioles, resulting in intraluminal narrowing[10]. Acutely, this narrowing can resemble ACS. Over time, however, intraplaque haemorrhage can resolve with osteogenic transformation, resulting in a non-eruptive CCN. Notably, hinge motion fracturing underlying calcified sheets has also been observed in association with stent fracture[4,5,12,13].
The histopathological characteristics of CCNs have been well described[1,4]. They are morphologically distinct from other coronary occlusion lesions, including fibrocalcific plaques and fibrous cap atheromas. Although CCNs belong to the spectrum of lesions associated with thrombosis, they should be distinguished from plaque rupture and plaque erosion. Plaque rupture involves fibrous cap disruption, exposure of the necrotic core, and infiltration by macrophages and other inflammatory cells. Plaque erosion refers to thrombus formation overlying an intact plaque, or irregular luminal surfaces without thrombus but with diminished underlying plaque, without localized calcification[4]. In this process, exposed intima is characterized by smooth muscle cells and proteoglycans[1]. In contrast, CCNs cause endothelial denudation and fibrous cap disruption over irregular plaques with protruding calcification, often accompanied by substantial calcium deposits proximal or distal to the lesion[10,14]. CCNs are inherently thrombogenic, typically initiating fibrin deposition over disrupted nodules, followed by platelet aggregation[9]. This underlies their high thrombogenic potential[14], a mechanism also relevant following PCI when CCNs protrude into the lumen through stent struts[15]. For example, delayed strut coverage and suboptimal stent apposition have been frequently observed in lesions where CCNs are exposed to blood flow, predisposing to ACS recurrence[6].
CCNs are part of the broader spectrum of coronary calcification patterns. Variants include microcalcification, punctuate calcification, fragmented calcification, dense sheets of calcification, and nodular calcification, classified primarily by size[10]. Microcalcification, the earliest form, is thought to originate from smooth muscle cell apoptosis and macrophage accumulation, leading to punctuate deposits. These deposits may coalesce into larger deposits and extend from the necrotic core into the adjacent collagenous matrix[16]. Progressively, calcified sheets can fracture, producing nodular calcifications and protruding nodules that disrupt the endothelium and trigger acute thrombosis[16]. The mechanisms of calcium deposition are incompletely understood, but osteogenesis likely contributes to both atherosclerotic lesions and coronary calcification. Calcium regulatory proteins - including matrix G1a protein (MGP), osteocalcin, and bone morphogenetic protein-2 (BMP-2) - may facilitate calcium and hydroxyapatite deposition within the arterial extracellular matrix[17]. Another theory involves medial calcification in peripheral arteries of patients with long-standing diabetes, attributed to disrupted intracellular signaling pathways that give rise to osteoblast-like cells and osteoid metaplasia[18]. Additional contributors include calcium precipitation driven by chondrocyte-like cells, activated macrophages, local cytokine release, apoptosis of smooth muscle cells, macrophage and cholesterol accumulation, and bone deposition - all of which promote oxidative stress and calcification[18]. Interestingly, patients with either early or advanced coronary disease may exhibit elevated levels of osteocalcin-expressing endothelial progenitor cells, which may accelerate ossification[18].
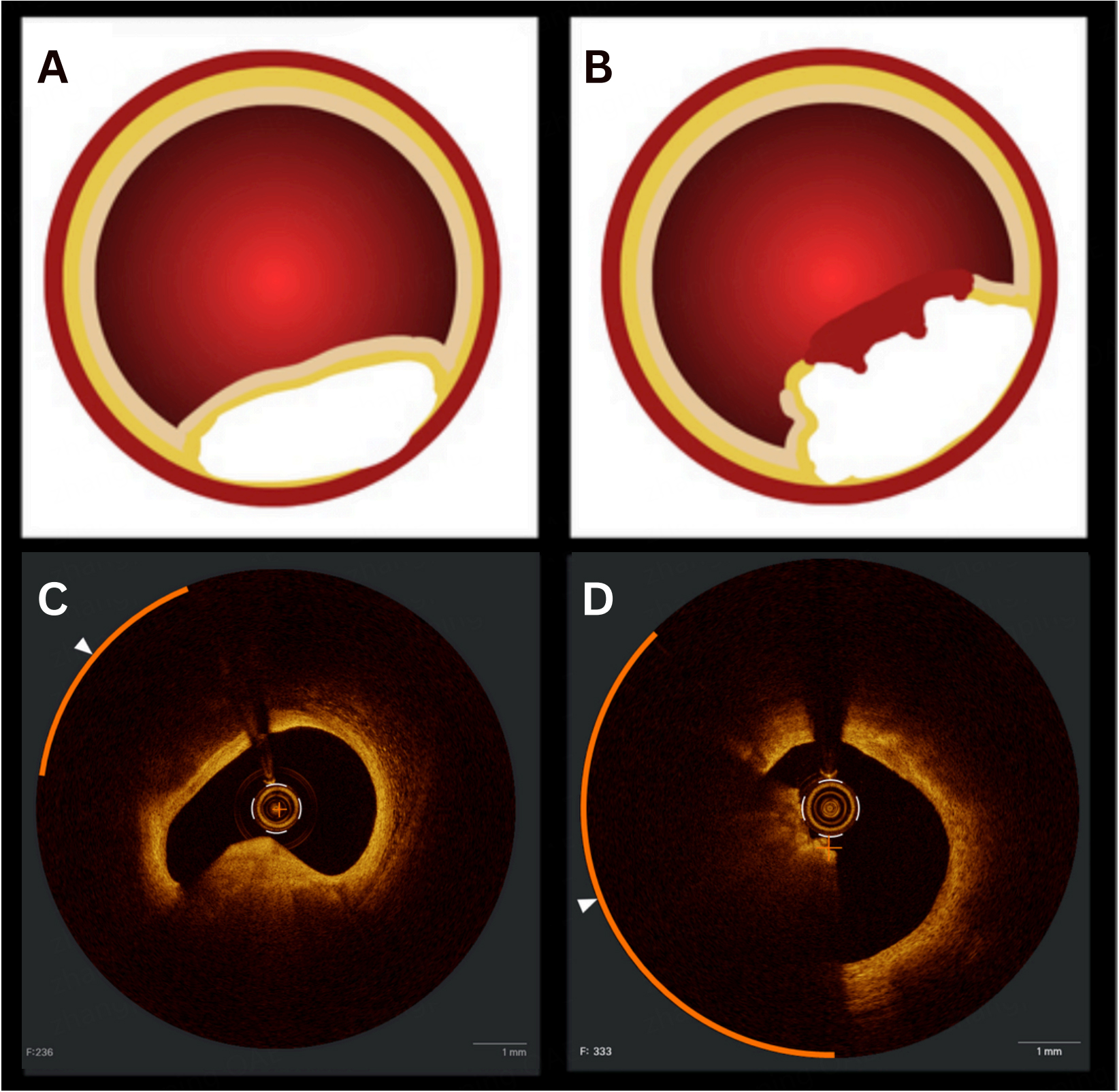
CCNs can be phenotypically classified as eruptive or non-eruptive [Figure 1]. These subtypes differ in both prognosis and histopathology. Eruptive CCNs consist of fragmented calcium protruding through the fibrous cap, often with overlying thrombus[11]. They are commonly associated with healed plaques and intraplaque hemorrhage. Non-eruptive CCNs, or nodular calcifications, typically display smooth, intact fibrous caps without overlying thrombus and may represent a healed eruptive CCN[11]. The CLIMA (Relationship between Coronary pLaque morphology of the left anterIor descending artery and long terM clinicAl outcome) registry demonstrated that eruptive CCNs exhibit thinner fibrous caps and smaller luminal diameters compared with non-eruptive CCNs[14].
Figure 1. Key morphological differences between eruptive and non-eruptive CCNs. (A) Non-eruptive nodules typically have smooth surfaces without overlying thrombus; (B) Eruptive nodules demonstrate irregular surfaces protruding into the vessel lumen, often with superficial thrombus; (C) OCT image of a non-eruptive CCN with a smooth fibrous cap; (D) OCT image of an eruptive CCN with fragmented, protruding calcium extending into the lumen. CCNs: Calcified coronary nodules; OCT: optical coherence tomography.
The clinical significance of this classification is paradoxical. Although eruptive CCNs are associated with better stent expansion, they generally predict worse long-term prognosis, though data on acute events remain inconsistent[7,11]. Eruptive CCNs more frequently occur in the mid-RCA, while non-eruptive CCNs are less common in the left main or distal branches of the left anterior descending, left circumflex, and right coronary arteries[11]. Eruptive nodules tend to be more mobile in the absence of collagen matrix, intraplaque hemorrhage, and fractured calcium. Target lesion revascularization (TLR) occurs twice as often in lesions with eruptive CCNs, yet no association has been found between calcium modification strategies (e.g., orbital atherectomy, rotational atherectomy, or intracoronary lithotripsy) and two-year TLR outcomes[11]. Mortality rates are also higher among patients with eruptive CCNs[8]. This aligns with their higher prevalence in ACS compared with non-eruptive CCNs, suggesting that eruptive CCNs may be predictive of future PCI complications.
Risk factors for CCNs largely overlap with those for atherosclerosis and thrombosis. Patients with CCNs are typically older and more likely to have hypertension, diabetes, and chronic kidney disease, as well as a history of coronary artery bypass grafting (CABG)[4,5,10]. The influence of sex remains inconsistent in the literature[2,10]. While the connection between renal dysfunction and coronary calcification is well established, the specific impact of hemodialysis on CCN lesions is unknown. Interestingly, non-eruptive CCNs are more frequently observed in native coronary segments proximal to anastomoses of grafted vessels and in patients with triple-vessel disease. Although chronic kidney disease prevalence is high in both groups, no differences have been observed between eruptive and non-eruptive CCNs[14].
DIAGNOSTIC MODALITIES
Accurate identification and differentiation of coronary atherosclerotic plaque are clinically important for determining optimal intravascular lesion preparation, guiding percutaneous therapy, and supporting ongoing patient management. Diagnostic modalities with varying sensitivity and specificity include angiography and intravascular coronary imaging, such as coronary computed tomography angiography (CCTA), intravascular ultrasound (IVUS), and optical coherence tomography (OCT).
CCTA
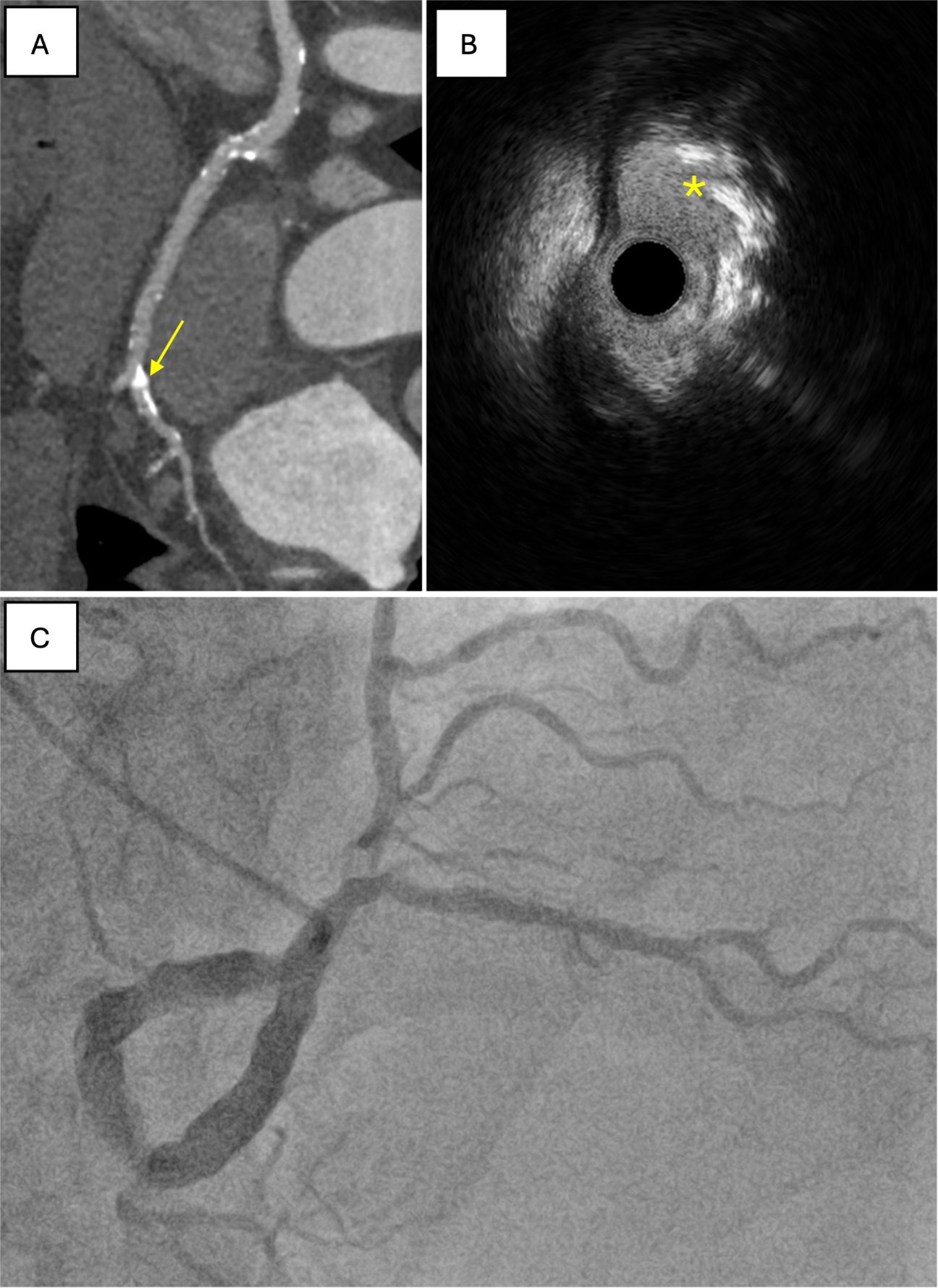
CCTA is a highly sensitive, non-invasive method for assessing coronary calcium burden and is less invasive than IVUS or OCT. It allows for morphological characterization of calcific plaques (both longitudinally and circumferentially) and enables assessment of calcium density and resistance to fracture [Figure 2][19]. However, CCTA lacks the spatial resolution needed to distinguish nodular or sheet-like calcium, which can only be evaluated with intracoronary imaging modalities, and its accuracy may be affected by blooming artifacts[20]. Emerging evidence suggests that CCTA-derived metrics, particularly Maximum Calcified Plaque Area (MCPA), are strongly associated with the presence of CCNs identified on OCT. This indicates a potential role for CCTA as a non-invasive predictor of CCNs[20].
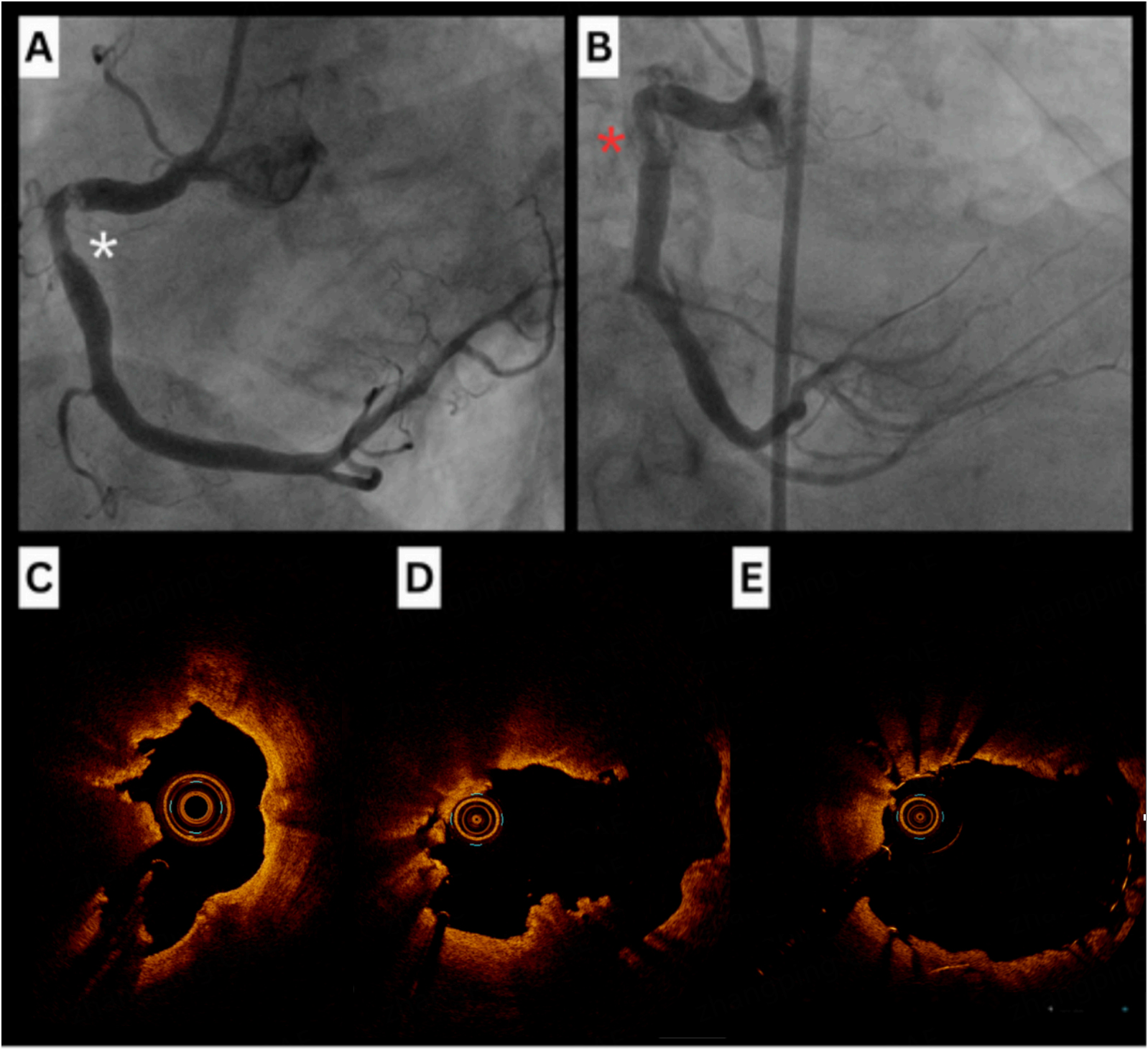
Figure 2. Features of CCNs identifiable on ultra-low dose, 640-slice volumetric CCTA. (A) prospective ECG-gated imaging with intravenous contrast following pre-treatment with sublingual nitrates. The effective dose for the angiographic run was 10.6 mSv/758 DLP. Extensive coronary calcification is visible, indicated by yellow arrows; This is compared with IVUS, where the nodule is marked by a yellow asterisk (B), and with angiography (C). CCNs: Calcified coronary nodules; CCTA: coronary CT angiography; ECG: electrocardiogram; DLP: dose-length product.
Angiography
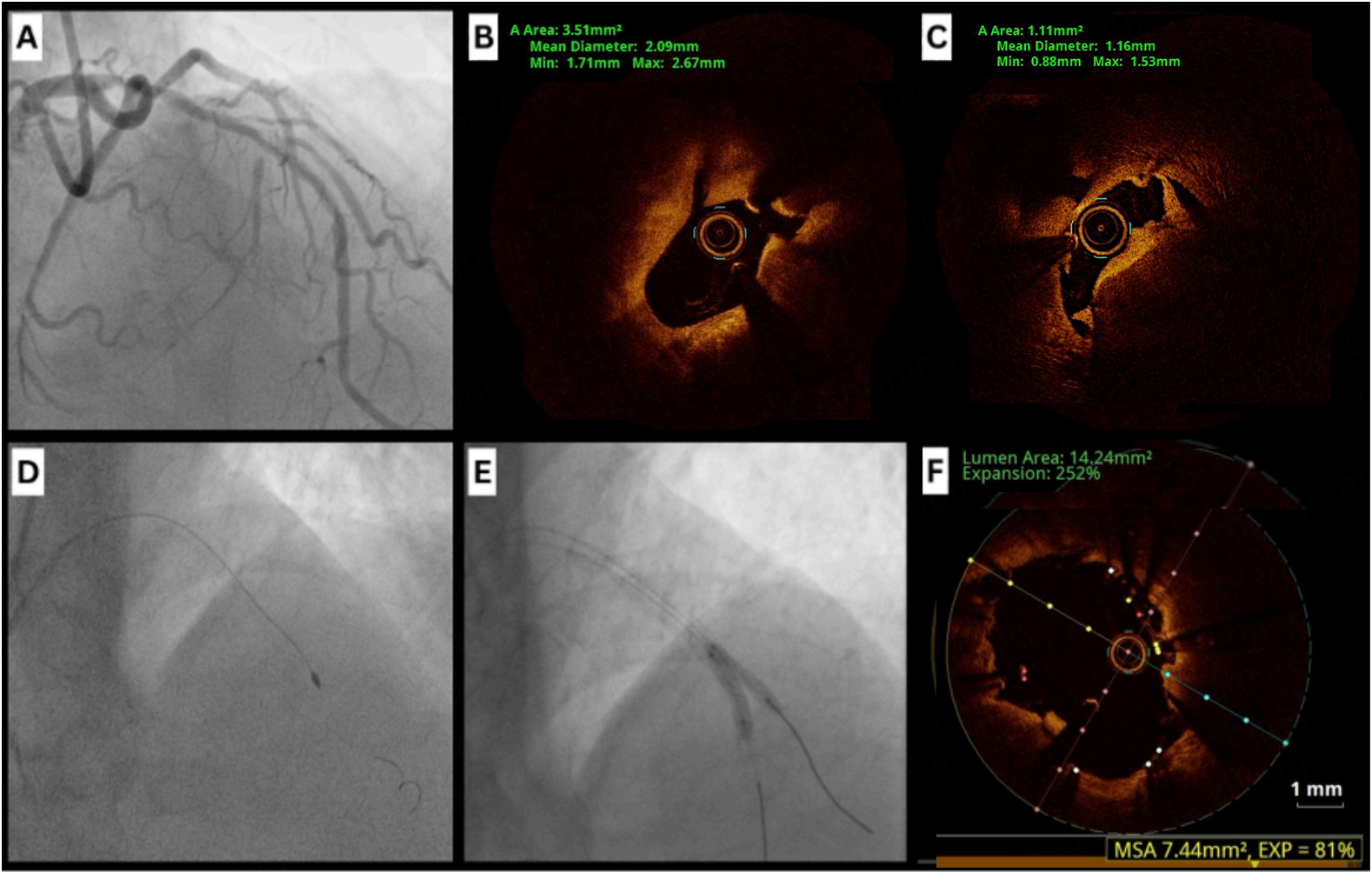
Angiography can reveal baseline characteristics of coronary calcification, including reference diameter, minimal lumen diameter, percentage of diameter stenosis, and lesion length[4]. Common angiographic features of CCNs include luminal haziness, abrupt vessel tapering, and step-like lesions. However, its ability to identify CCNs is limited by their irregular morphology, as previously documented[5,21]. A case series published in 1996 reported three patients whose calcified nodules were initially misdiagnosed on angiography as thrombi but were later correctly identified as nodules using IVUS[21]. We present a case that demonstrates some of these features [Figure 3].
IVUS
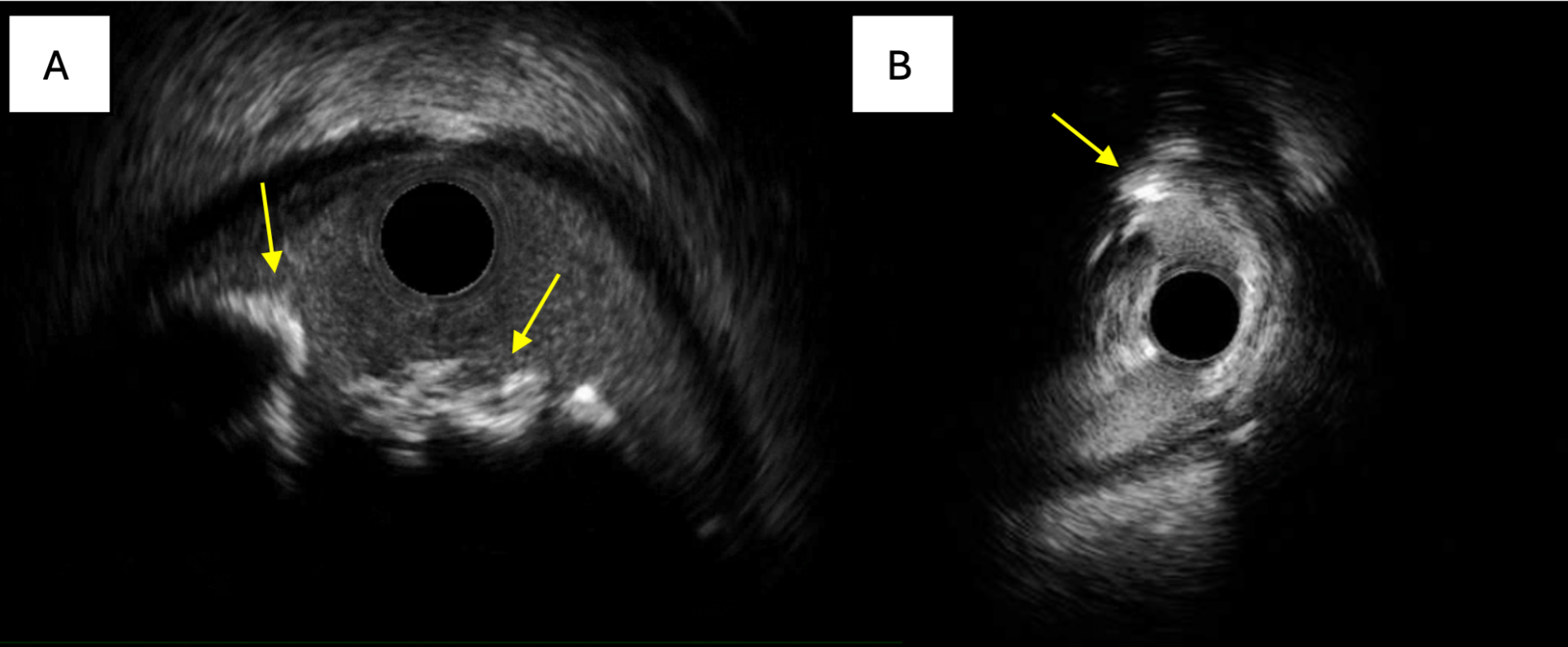
IVUS, which uses highly penetrable ultrasound waves, generates sequential cross-sectional images of the coronary vessel and lumen[22]. It is effective in detecting calcific lesions and shows a strong correlation with histological findings, with a sensitivity of 90% and a specificity of 100%[23]. On IVUS, CCNs typically appear as eccentric, hyperechoic, calcified lesions with acoustic shadowing that protrude into the lumen[21]. Other CCN features include a convex luminal surface (non-eruptive), an irregular luminal surface (eruptive), an irregular leading edge of calcium, and negative remodeling [Figure 4][4]. However, IVUS is limited in its ability to detect microcalcifications smaller than 0.05 mm[23]. As novel IVUS techniques with higher transducer frequencies emerge, the detection rate of calcified nodules is expected to increase.
Figure 4. Hallmark features of CCNs on IVUS. (A) IVUS shows an eccentric, hyperechoic lesion with acoustic shadowing irregularly protruding into the vessel lumen, characteristic of an eruptive CCN; (B) IVUS shows a severe nodule, indicated by the yellow arrow, with an irregular leading edge of calcium. CCNs: Calcified coronary nodules; IVUS: intravascular ultrasound.
OCT
OCT is considered the gold standard for CCN identification and has substantially improved the understanding of post-PCI outcomes of the two subtypes of CCNs. Unlike other modalities, OCT can distinguish between plaque rupture and coronary nodules, and it uses infrared light to penetrate calcium, enabling precise characterization of the morphology and thickness of calcified lesions[11].
OCT enables accurate identification of calcific nodules by differentiating eruptive CCNs, which have irregular surfaces, from non-eruptive CCNs, which appear smooth and lack overlying thrombus. Its
A major clinical advantage of OCT is its ability to assess stent expansion during PCI. CCNs tend to restrict stent expansion and are associated with higher rates of in-stent restenosis[6]. In the recent Calcified Lesion Intervention Planning Steered by OCT (CALIPSO) trial, patients with calcified lesions were randomized to undergo PCI guided by either OCT or conventional angiography. OCT guidance resulted in a significantly larger minimal stent area (MSA) compared with angiography alone (6.5 mm2 vs. 5.0 mm2, P < 0.001)[24]. It also improved stent expansion and reduced malapposition without increasing procedural complications. Similarly, a subgroup analysis from the ILUMIEN IV Study: Optical Coherence Tomography (OCT) Guided Coronary Stent Implantation Compred to Angiography: A Multicenter Randomized trial (OPTIMAL) in PCI demonstrated that, in complex angiographic lesions (including severely calcified lesions), OCT-guided intervention achieved a larger MSA and a lower risk of the composite endpoint of serious major adverse cardiovascular events (MACE) - cardiac death, target-vessel myocardial infarction, or stent thrombosis - compared with angiography-guided PCI at 2 years post intervention[25]. Moreover, definite or probable stent thrombosis at 2 years occurred in six patients (0.6%) in the OCT-guided group compared with 15 patients (1.6%) in the angiography-guided group. However, there was no difference in target vessel failure between the groups. Notably, the proportion of patients with severely calcified lesions was modest in both groups (34% in the OCT-guided arm and 32.2% in the angiography-guided arm)[25], and no subgroup data were available specifically for calcified coronary nodules.
Acutely, stent expansion is greater in eruptive CCNs than in non-eruptive CCNs. This distinction is clinically important, as stent underexpansion is a key predictor of long-term stent patency and clinical outcomes. OCT can therefore help identify lesions that may require more aggressive preparation before stent implantation[6].
Despite its advantages, OCT has limitations. Infrared light cannot penetrate fibrin-rich thrombus, which obscures visualization of the underlying lesion. Moreover, fibrin-rich thrombus appears bright, irregular, and protruding on OCT, making it difficult to distinguish from calcified spicules in eruptive CCNs[26].
Summary
In detecting calcified lesions, OCT is more sensitive than angiography but less sensitive than IVUS[27]. Due to its limited penetration, OCT provides a less reliable assessment of deep calcium and can be challenging to use in large-diameter vessels or ostial lesions. However, its superior image resolution enables earlier detection of microcalcifications compared with IVUS, and it is particularly effective in differentiating eruptive from non-eruptive calcium nodules[22]. While OCT excels in characterizing calcium morphology and identifying calcium nodules, IVUS may be preferable in larger vessels or ostial lesions. CCTA, although less effective in detailing lesion characteristics, can better estimate calcium burden and guide the selection of an appropriate invasive intracoronary imaging modality. Ultimately, the choice of imaging should be tailored to lesion characteristics and patient risk factors.
TREATMENT
Calcium modification strategies
CCNs present several challenges, including reduced vessel compliance, high mechanical resistance, heavy calcification, and eccentric morphology, all of which contribute to suboptimal stent apposition. Inadequate lesion preparation may lead to insufficient stent expansion and an increased risk of stent failure. To address this, various calcium modification strategies, such as atherectomy, intravascular lithotripsy (IVL), and specialized balloon technologies, have been explored to optimize PCI outcomes in CCNs.
Rotational atherectomy
Rotational atherectomy (RA) utilizes a high-speed, diamond-tipped burr to modify calcified plaques through antegrade “cutting” of the calcium surface[28]. The principle of differential cutting involves selectively ablating inelastic material while displacing elastic tissue away from the burr. While effective in lesions with concentric and sheet-like calcium, RA has shown limited efficacy in treating protruding calcific nodules[29,30].
Clinical evidence regarding RA in CCNs has been largely inconclusive. A subgroup analysis from the ROTA-Shock trial demonstrated that both RA and IVL reduced CCN thickness and plaque angle but did not significantly decrease plaque volume. Stent eccentricity remained high in both groups[31]. However, as there was no control group without RA or IVL, it was unclear whether the observed CCN displacement resulted from calcium modification or stent deployment. Other studies have reported no significant differences in stent expansion between RA, modified balloons (MB), and high-pressure non-compliant (NC) balloons[32]. Furthermore, RA did not significantly reduce ischemia-driven target vessel revascularization (TVR) compared to NC balloons, likely due to persistent stent malapposition[33]. To our knowledge, there are no published studies directly comparing RA outcomes in eruptive versus non-eruptive CCNs; thus, existing findings can only be generalized to CCNs overall.
Several factors must be considered when evaluating the limitations of RA in CCNs. Guidewire bias - the relative position of the guidewire to the calcified nodule and vessel wall- plays a critical role. Suboptimal wire bias can prevent the burr from adequately engaging the calcified nodule, particularly in cases of eccentric calcification or vessel tortuosity, leading to inadequate plaque modification and an increased risk of vessel dissection[34].
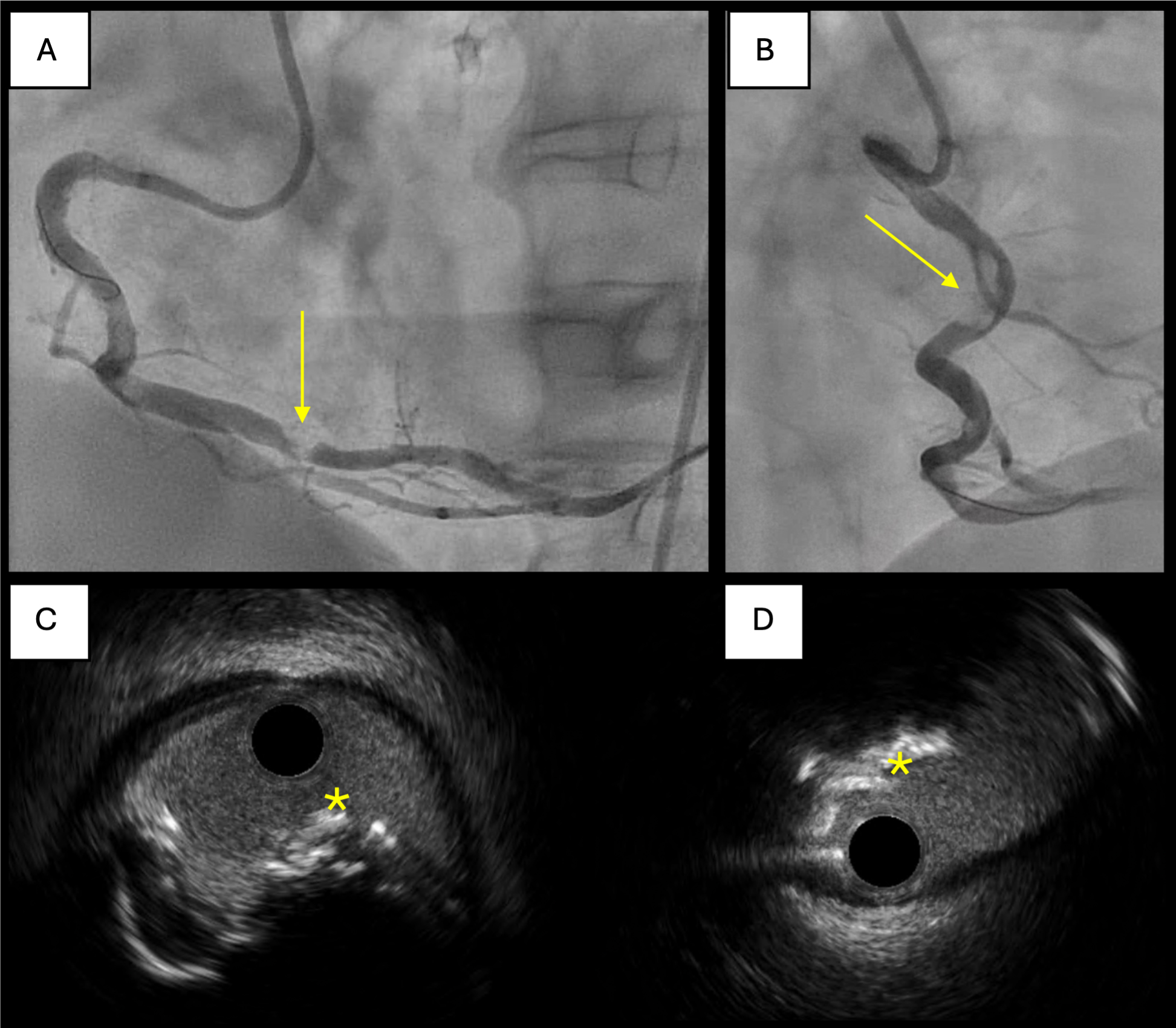
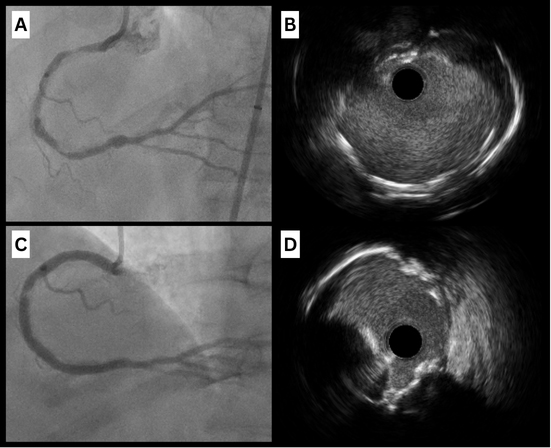
In some cases, multiple burr sizes and favorable wire bias may be necessary to achieve adequate calcium modification [Figure 5]. Expert consensus guidelines recommend strategic burr manipulation and, when necessary, upsizing to a larger burr to enhance calcium modification[35].
Figure 5. Case of successful rotablation of an eruptive nodule with favorable wire bias. (A and B) Angiographic views of severe
Orbital atherectomy
Orbital atherectomy (OA) employs an eccentrically mounted, diamond-coated crown that expands laterally. Through differential sanding and centrifugal forces, it enables bidirectional plaque modification, which is particularly effective for nodular calcium.
The Pivotal Trial to Evaluate the Safety and Efficacy of the Diamondback 360°® Orbital Atherectomy System in Treating De Novo, Severely Calcified Coronary Lesions (ORBIT) trial established the role of OA in treating severely calcified coronary lesions[36]. However, other evidence suggests that OA may be inferior to RA for treating sheet-like or concentric calcification. In the Direct Comparison of Rotational vs. Orbital Atherectomy for Calcified Lesions Guided by Optical Coherence Tomography (DIRO) trial, which enrolled 100 patients assessed by OCT and randomized 1:1 to OA or RA before PCI, RA resulted in a greater increase in lumen area (72.2% vs. 39.2%, P < 0.01) and superior mean stent expansion (72.2% vs. 64.1%, P = 0.05)[37]. Similarly, the Eclipse trial did not show superiority of OA over conventional balloon angioplasty before stenting in calcified lesions[38]. Although the MSA was slightly larger in the OA group (7.67 mm2 vs. 7.42 mm2, P = 0.08), clinical outcomes were comparable, with similar TVR rates at 1 year (11.5% vs. 10%, P = 0.28)[38]. These findings suggest that angiographic evidence of calcification may not be a reliable criterion for patient selection.
In our clinical practice, OA has been more effective than RA for eccentric or nodular calcification due to its differential sanding mechanism. It also performs better in large vessels at high speeds and, while still influenced by wire bias, is less affected than RA. OA improves vessel compliance and is associated with low rates of vascular injury, making it a promising alternative to RA in specific cases.
IVL
IVL delivers acoustic shockwaves that fracture both superficial and deep calcium, thereby facilitating stent expansion. A recent multicenter systematic review and meta-analysis of 38 studies involving 2,977 patients reported a procedural success rate of 98% and a clinical success rate of 96%. The analysis also showed significantly reduced residual area stenosis following IVL and PCI, with low incidences of MACEs, myocardial infarction (MI), and death at 30 days[39].
While highly effective and safe for concentric calcification, the role of IVL in treating protruding calcified nodules remains less well defined. A pooled OCT analysis from the Disrupt-CAD I-IV trials demonstrated that IVL effectively reduces residual area stenosis of CCNs and achieves long-term outcomes comparable to non-CCNs[40]. Additionally, in the ROTA-Shock trial, RA and IVL yielded similar outcomes in reducing CCN thickness and plaque angle, although neither strategy significantly reduced plaque volume[31].
In severely stenotic or tortuous vessels, where catheter delivery is challenging, upfront atherectomy (RA or OA) may be necessary to facilitate IVL insertion. In such cases, the cost implications of combining IVL with atherectomy should be considered. NODULE-SHOCK, an upcoming prospective randomized controlled trial, is designed to evaluate the efficacy of IVL with or without RA in patients with CCNs[41], using minimal stent area as the primary endpoint.
Although current evidence remains limited, existing data suggest that IVL holds promise in the modification of CCNs, offering favorable procedural success and clinical outcomes.
Modified balloons (Cutting or scoring balloons)
Cutting and scoring balloons are specialized angioplasty devices equipped with microblades or scoring elements designed to produce controlled plaque fractures (e.g., Scoreflex NC and Wolverine balloons).
The Cutting Balloon to Optimize Predilatation for Stenting (COPS) trial, which randomized patients with calcified lesions to cutting balloon (CB) or non-compliant balloon (NCB) inflation before PCI, demonstrated significantly larger minimal stent areas in the CB group compared to the NCB group
Overall, RA, OA, IVL, and MB have all demonstrated comparable clinical outcomes in small-scale studies[43]. However, their relative efficacy in treating CCNs remains uncertain. Hybrid strategies combining multiple calcium modification techniques have shown promise in case studies, but robust clinical validation is lacking[44-46]. Further randomized trials are needed to determine optimal lesion preparation strategies in CCNs.
Optimizing PCI outcomes
CCNs pose a higher risk of procedural complications during PCI because they hinder stent delivery and expansion. Effective management, therefore, requires a nuanced understanding of nodule characteristics and tailored interventional approaches.
Characteristics of calcific nodules
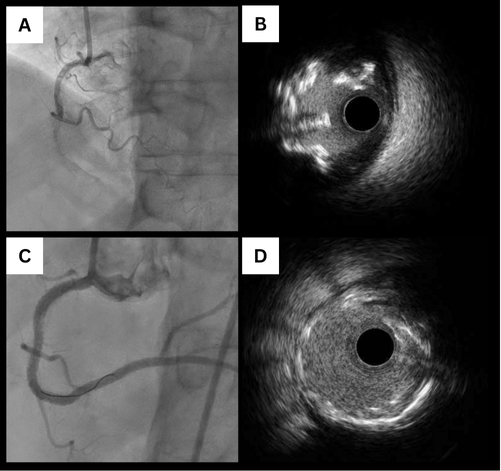
The choice of a calcium modification strategy in PCI is largely determined by the morphological characteristics of CCNs. As previously described, eruptive CCNs, typically marked by fibrous cap disruption and a more deformable structure, often respond well to NC balloon angioplasty and IVL, enabling satisfactory stent expansion[47]. However, despite their deformability, eruptive nodules are associated with poorer clinical outcomes, primarily due to early reprotrusion of calcium into the stented segment[48,49] [Figure 6].
Figure 6. Eruptive nodule reprotrusion post-PCI. (A) Coronary angiogram showing severe calcified RCA nodules. OA was performed, followed by stenting of proximal, mid, and distal lesions under IVUS guidance; (B) Post-stent IVUS showed good stent apposition and lumen expansion; (C) Repeat coronary angiogram 6 months later revealed recurrent nodule in the mid-RCA; (D) IVUS showed nodule reprotrusion through the stent. PCI: Percutaneous coronary intervention; RCA: right coronary artery; OA: orbital atherectomy; IVUS: intravascular ultrasound.
In contrast, non-eruptive CCNs, though linked to a more favorable clinical prognosis, pose greater procedural challenges due to their dense, rigid architecture and resistance to deformation. In one study evaluating the use of IVL in CCNs, all eruptive nodules (100%) were deformable, compared to 65% of
High-pressure post-dilatation with non-compliant balloons
Following initial lesion preparation, high-pressure post-dilatation using NC balloons is essential to ensure optimal stent expansion [Figure 7]. This approach aids in achieving maximal luminal gain and reduces the risk of stent underexpansion, a known predictor of adverse cardiac events.
Figure 7. Combination of RA and high-pressure balloon dilatation for eruptive CCNs. (A) Severe mid-RCA stenosis resulting in distal vessel CTO; (B) IVUS showing extensive long-segment calcification with eruptive CCNs; (C) Rotational atherectomy performed with a 1.5mm burr, followed by pre-dilatation with 2.75 and 3.0 mm NC balloons to high pressure, then PCI with overlapping drug-eluting stents (DESs); (D) Post-treatment IVUS showing good stent apposition. CCNs: Calcified coronary nodules; RA: rotational atherectomy; IVUS: intravascular ultrasound; RCA: RIGHT coronary artery; NC: non-compliant; PCI: percutaneous coronary intervention; CTO: chronic total occlusion.
While adequate stent expansion is vital, caution must be exercised to avoid excessive post-dilatation, which can cause vessel perforation, particularly on the side opposite the calcified nodule. Achieving the right balance requires careful, incremental balloon inflation. It is also important to note that eccentric expansion is frequently observed, but this is of less concern as long as the MSA is adequate.
Managing procedural complications
Stent underexpansion
Coronary calcification is the strongest predictor of stent underexpansion[51], which is closely linked to TVR. Adequate lesion preparation is essential to prevent this complication and should include intravascular imaging for accurate lesion sizing and characterization, along with tailored calcium modification strategies. When underexpansion occurs despite appropriate lesion preparation, evidence supports the use of post-PCI IVL. IVL delivers acoustic pressure waves that fracture underlying calcium, thereby facilitating subsequent stent expansion and apposition. In a multicenter registry of patients with stent underexpansion, post-PCI IVL significantly increased the minimum lumen diameter (from 1.49 mm to 2.41 mm, P < 0.001) and improved stent expansion by 125% (P = 0.016)[52].
Distal embolization
Eruptive calcific nodules are associated with a higher risk of distal embolization due to the fragmentation of calcific material during intervention. This can lead to downstream microvascular obstruction, increasing the risk of periprocedural myocardial infarction. In particular, RA and OA may exacerbate this issue by generating particulate debris.
Mitigation strategies again emphasize optimal lesion preparation. Intravascular imaging is critical for lesion characterization, enabling accurate vessel sizing and selection of appropriate calcium modification tools while avoiding overdilation. IVL may offer an alternative to atherectomy with a potentially lower risk of embolization. The use of embolic protection devices is generally limited in coronary interventions compared to peripheral vascular procedures.
Edge dissections and vessel perforation
The selection of atherectomy devices must be tailored to lesion morphology to minimize the risk of edge dissections and vessel perforation. RA is suitable for concentric, heavily calcified lesions or nodular lesions with favorable wire bias, whereas OA may be preferred for eccentric or nodular calcifications particularly in larger vessels. When feasible, intravascular imaging before atherectomy is valuable, as it demonstrates wire bias. Both rotational burrs and orbital atherectomy crowns follow the course of the guidewire and imaging catheter. If areas of contact with normal vessel segments or minor plaque are identified, atherectomy in these regions should be avoided or minimized. Careful device sizing and adherence to recommended operational speeds are critical to prevent vascular injury.
Plaque shift in bifurcation lesions
Calcified plaques in bifurcation lesions carry a risk of plaque shift, potentially compromising side branch patency. This occurs when plaque in the proximal main vessel segment or at the bifurcation carina shifts into the side branch ostium, impeding blood flow. Carina shift into the side branch has also been proposed as a mechanism of compromise[53]. Intracoronary imaging with IVUS is an effective method for evaluating plaque burden and predicting ostial side branch involvement. In a retrospective observational study, RA of the main vessel in calcified bifurcation lesions was associated with a significantly lower incidence of side branch compromise and occlusion during PCI[54]. Strategies such as side branch protection or bifurcation PCI may be needed to maintain side branch patency and optimize outcomes [Figure 8].
Figure 8. Staged PCI for a severely calcified LAD-diagonal bifurcation. (A) Angiographic views showing diseased LAD and diagonal vessels; (B) OCT of the LAD showing a non-eruptive nodule. The diagonal was pre-dilated to facilitate OCT; (C) OCT of the diagonal showing non-eruptive nodules at the ostium; (D) RA of the diagonal with 1.5 mm and 1.75 mm burrs. The diagonal was then post-dilated with a 2.5 mm NC balloon, followed by deployment of a 2.5 × 18 mm DES; (E) DK Crush was performed; (F) Final OCT post-PCI. PCI: percutaneous coronary intervention; DES: drug-eluting stent; OCT: optical coherence tomography; RA: rotational atherectomy; NC:
Overall, the management of coronary calcific nodules requires a comprehensive approach that includes accurate lesion characterization, meticulous lesion preparation, and vigilant management of potential complications. Advanced imaging techniques and individualized interventional strategies are essential to improve PCI outcomes in patients with calcified coronary lesions.
CLINICAL OUTCOMES AND FUTURE DIRECTIONS
CCNs may be considered harbingers of increased risk associated with standard PCI strategies. Patients with CCNs generally experience worse outcomes after PCI compared to those without, necessitating careful CCN classification, lesion preparation, calcium modification, and tailored interventional strategies.
Refining imaging modalities will be critical for accurate identification and phenotypic classification of CCNs, which have both prognostic and therapeutic implications. Hybrid imaging and artificial intelligence (AI)-driven techniques, such as optical coherence tomography-near infrared spectroscopy (OCT-NIRS), may enhance the precision of targeted interventions[55]. NIRS, for example, has previously been combined with IVUS to detect lipid content within plaques and arterial walls [56]. Ongoing studies are exploring the role of CCTA and AI in periprocedural planning. One such study, the P4 (Precise Procedural and PCI Plan; NCT05253677) trial, is a multicenter, non-inferiority randomized controlled trial (RCT) comparing
In lesions with CCNs requiring PCI, stenting is particularly challenging due to factors such as hinge locations, complex geometry, intraluminal hemorrhage, absent collagen matrix, and calcium fragmentation, all of which increase the risk of lesion deformity. Re-protrusion of calcific nodules through stent struts is associated with worse clinical outcomes compared to other mechanisms of ISR[7]. Drug-eluting balloons (DEBs) alone have been proposed to reduce this risk, but clinical data remain limited; further studies are warranted to validate their theoretical benefit and confirm safety regarding the risk of recoil[57]. Similarly, covered coronary stents have been suggested as a potential future strategy to mitigate reprotrusion, though evidence is sparse[7]. Bioresorbable vascular scaffolds (BRSs) offer another theoretical advantage by providing initial mechanical support and drug delivery similar to drug-eluting stents, while gradually dissolving over time and potentially reducing long-term ISR risk. Although no studies have specifically examined BRS use in CCNs, extrapolation from broader research on calcified lesions suggests comparable angiographic success. Nonetheless, these procedures often require longer procedural times and carry more than a twofold increase in periprocedural myocardial infarction risk-likely due to lesion complexity and the need for extensive lesion preparation[58]. Moreover, the nodular and protrusive morphology of CCNs can complicate BRS deployment, particularly impacting scaffold expansion and recoil.
Sequential lesion preparation techniques, along with advancements in bioresorbable scaffolds and covered stents, may provide future opportunities for targeted interventions to reduce re-protrusion and mitigate ISR[55]. For example, one case report described a patient who experienced coronary perforation post
CONCLUSION
Calcified coronary nodules present unique challenges in coronary intervention due to their irregular morphology and resistance to conventional PCI techniques. Advances in imaging and lesion modification have improved outcomes, but further studies are needed to refine treatment algorithms regarding safety, efficacy, and long-term prognosis. A tailored approach incorporating imaging guidance, calcium modification, and optimal stenting techniques is essential for improving patient outcomes.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study: Chiswell K, Abdo RM, Khialani B, Fairley S, Harding S, Ada C
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Authors 2025
REFERENCES
1. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-75.
2. Sugane H, Kataoka Y, Otsuka F, et al. Cardiac outcomes in patients with acute coronary syndrome attributable to calcified nodule. Atherosclerosis. 2021;318:70-5.
3. Pengchata P, Pongakasira R, Wongsawangkit N, Phichaphop A, Wongpraparut N. Characteristics and pattern of calcified nodule and/or nodular calcification detected by intravascular ultrasound on the device-oriented composite endpoint (DoCE) in patients with heavily calcified lesions who underwent rotational atherectomy-assisted percutaneous coronary intervention. J Interv Cardiol. 2023;2023:6456695.
4. Higuma T, Soeda T, Abe N, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:1166-76.
5. Lee T, Mintz GS, Matsumura M, et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imaging. 2017;10:883-91.
6. Khalifa AKM, Kubo T, Ino Y, et al. Optical coherence tomography comparison of percutaneous coronary intervention among plaque rupture, erosion, and calcified nodule in acute myocardial infarction. Circ J. 2020;84:911-6.
7. Shin D, Karimi Galougahi K, Spratt JC, et al. Calcified nodule in percutaneous coronary intervention: therapeutic challenges. JACC Cardiovasc Interv. 2024;17:1187-99.
8. Xu Y, Mintz GS, Tam A, et al. Prevalence, distribution, predictors, and outcomes of patients with calcified nodules in native coronary arteries: a 3-vessel intravascular ultrasound analysis from providing regional observations to study predictors of events in the coronary tree (PROSPECT). Circulation. 2012;126:537-45.
9. Alfonso F, Joner M. Untangling the diagnosis and clinical implications of calcified coronary nodules. JACC Cardiovasc Imaging. 2017;10:892-6.
10. Torii S, Sato Y, Otsuka F, et al. Eruptive calcified nodules as a potential mechanism of acute coronary thrombosis and sudden death. J Am Coll Cardiol. 2021;77:1599-611.
11. Sato T, Matsumura M, Yamamoto K, et al. Impact of eruptive vs noneruptive calcified nodule morphology on acute and long-term outcomes after stenting. JACC Cardiovasc Interv. 2023;16:1024-35.
12. Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22:399-411.
13. Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11:379-89.
14. Prati F, Gatto L, Fabbiocchi F, et al. Clinical outcomes of calcified nodules detected by optical coherence tomography: a sub-analysis of the CLIMA study. EuroIntervention. 2020;16:380-6.
15. Nakamura N, Torii S, Tsuchiya H, et al. Formation of calcified nodule as a cause of early in-stent restenosis in patients undergoing dialysis. J Am Heart Assoc. 2020;9:e016595.
16. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11:127-42.
17. Roijers RB, Debernardi N, Cleutjens JP, Schurgers LJ, Mutsaers PH, van der Vusse GJ. Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am J Pathol. 2011;178:2879-87.
18. Onnis C, Virmani R, Kawai K, et al. Coronary artery calcification: current concepts and clinical implications. Circulation. 2024;149:251-66.
19. Tajima A, Bouisset F, Ohashi H, et al. Advanced CT imaging for the assessment of calcific coronary artery disease and PCI planning. J Soc Cardiovasc Angiogr Interv. 2024;3:101299.
20. Sugiura J, Watanabe M, Nobuta S, et al. Prediction of optical coherence tomography-detected calcified nodules using coronary computed tomography angiography. Sci Rep. 2022;12:22296.
21. Duissaillant GR, Mintz GS, Pichard AD, et al. Intravascular ultrasound identification of calcified intraluminal lesions misdiagnosed as thrombi by coronary angiography. Am Heart J. 1996;132:687-9.
22. Petousis S, Skalidis E, Zacharis E, Kochiadakis G, Hamilos M. The role of intracoronary imaging for the management of calcified lesions. J Clin Med. 2023;12:4622.
23. Sharma SK, Vengrenyuk Y, Kini AS. IVUS, OCT, and coronary artery calcification: is there a bone of contention? JACC Cardiovasc Imaging. 2017;10:880-2.
24. DOCTORS-LM trial: breaking news from EuroPCR 2024. EMJ. 2024. Available from: https://www.emjreviews.com/interventional-cardiology/news/doctors-lm-trial-breaking-news-from-europcr-2024/ [Last accessed on 26 Sep 2025].
25. Ali ZA, Landmesser U, Maehara A, Shin D, Sakai K, et al. OCT-guided vs. angiography-guided coronary stent implantation in complex lesions: an ILUMIEN-IV substudy. J Am Coll Cardiol. 2024;84:368-78.
26. Lee JB, Mintz GS, Lisauskas JB, et al. Histopathologic validation of the intravascular ultrasound diagnosis of calcified coronary artery nodules. Am J Cardiol. 2011;108:1547-51.
27. Ijichi T, Nakazawa G, Torii S, et al. Evaluation of coronary arterial calcification-ex-vivo assessment by optical frequency domain imaging. Atherosclerosis. 2015;243:242-7.
28. Riley R, Patel M, Abbott D, Bangalore S, Brilakis E, et al. SCAI expert consensus statement on the management of calcified coronary lesions. J Soc Cardiovasc Angiogr Interv. 2024;3:101259.
29. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. 2018;11:e007415.
30. Abdel-Wahab M, Richardt G, Joachim Büttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (rotational atherectomy prior to taxus stent treatment for complex native coronary artery disease) trial. JACC Cardiovasc Interv. 2013;6:10-9.
31. Blachutzik F, Meier S, Blachutzik M, et al; ROTA. shock Investigators. Comparison of interventional treatment options for coronary calcified nodules: a sub-analysis of the ROTA.shock trial. Cardiovasc Revasc Med. 2024;68:37-42.
32. Rheude T, Fitzgerald S, Allali A, et al. Rotational atherectomy or balloon-based techniques to prepare severely calcified coronary lesions. JACC Cardiovasc Interv. 2022;15:1864-74.
33. Watanabe Y, Sakakura K, Taniguchi Y, et al. Comparison of clinical outcomes of intravascular ultrasound-calcified nodule between percutaneous coronary intervention with versus without rotational atherectomy in a propensity-score matched analysis. PLoS One. 2020;15:e0241836.
34. Morofuji T, Kuramitsu S, Shinozaki T, et al. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter Cardiovasc Interv. 2021;97:10-9.
35. Sakakura K, Ito Y, Shibata Y, et al. Clinical expert consensus document on rotational atherectomy from the Japanese association of cardiovascular intervention and therapeutics: update 2023. Cardiovasc Interv Ther. 2023;38:141-62.
36. Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-8.
37. Okamoto N, Egami Y, Nohara H, et al. Direct comparison of rotational vs orbital atherectomy for calcified lesions guided by optical coherence tomography. JACC Cardiovasc Interv. 2023;16:2125-36.
38. Kirtane AJ, Généreux P, Lewis B, et al; ECLIPSE Investigators. Orbital atherectomy versus balloon angioplasty before drug-eluting stent implantation in severely calcified lesions eligible for both treatment strategies (ECLIPSE): a multicentre, open-label, randomised trial. Lancet. 2025;405:1240-51.
39. Sagris M, Ktenopoulos N, Dimitriadis K, et al. Efficacy of intravascular lithotripsy (IVL) in coronary stenosis with severe calcification: a multicenter systematic review and meta-analysis. Catheter Cardiovasc Interv. 2024;103:710-21.
40. Ali ZA, Shin D, Singh M, et al. Outcomes of coronary intravascular lithotripsy for the treatment of calcified nodules: a pooled analysis of the disrupt CAD studies. EuroIntervention. 2024;20:e1454-64.
41. Intravascular lithotripsy with or without rotational atherectomy for coronary calcified nodule treatment (NODULE-SHOCK). ClinicalTrials.gov Identifier: NCT06327641. Available from: https://clinicaltrials.gov/study/NCT06327641 [Last accessed on 16 Sep 2025].
42. Mangieri A, Nerla R, Castriota F, et al. Cutting balloon to optimize predilation for stent implantation: the COPS randomized trial. Catheter Cardiovasc Interv. 2023;101:798-805.
43. Yasumura K, Koshy AN, Vinayak M, et al. Rotational, orbital atherectomy and intravascular lithotripsy for coronary calcified nodules: insights from optical coherence tomography. Catheter Cardiovasc Interv. 2024;104:1373-86.
44. Oka T, Sanada R, Urabe Y, Mitsuba N, Fukuda Y, Ueda H. Effectiveness of using both rotational atherectomy with smallest burr and orbital atherectomy system for stentless treatment of calcified nodules. Cardiovasc Interv Ther. 2023;38:424-6.
45. Adachi Y, Kinoshita Y, Suzuki T. Efficacy of combination atherectomy of orbital atherectomy system and rotational atherectomy for severely calcified nodule. Cardiovasc Revasc Med. 2021;28:100-1.
46. Nooryani AA, Sianos G, Abdelrahman N. Successful calcium modification of a large calcified nodule using shockwave intravascular lithotripsy in the setting of acute coronary syndrome: a case report. Eur Heart J Case Rep. 2024;8:ytae517.
47. Ali ZA, Kereiakes D, Hill J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of calcified nodules. JACC Cardiovasc Interv. 2023;16:1122-4.
48. Matsuhiro Y, Nakamura D, Dohi T, et al. Impact of calcified nodule on target lesion failure after stent implantation in hemodialysis patients. Catheter Cardiovasc Interv. 2023;101:701-12.
49. Hamana T, Kawamori H, Toba T, et al. Predictors of target lesion revascularisation after drug-eluting stent implantation for calcified nodules: an optical coherence tomography study. EuroIntervention. 2023;19:e123-33.
50. Galougahi KK, Shin D, Dakroub A, et al. Distinct challenges of eruptive and non-eruptive calcified nodules in percutaneous coronary intervention. Curr Cardiol Rep. 2024;26:757-65.
51. Sato T, Matsumura M, Yamamoto K, et al. A revised optical coherence tomography-derived calcium score to predict stent underexpansion in severely calcified lesions. JACC Cardiovasc Interv. 2025;18:622-33.
52. Tovar Forero MN, Sardella G, Salvi N, et al. Coronary lithotripsy for the treatment of underexpanded stents: the international & multicentre CRUNCH registry. EuroIntervention. 2022;18:574-81.
53. Xu J, Hahn JY, Song YB, et al. Carina shift versus plaque shift for aggravation of side branch ostial stenosis in bifurcation lesions: volumetric intravascular ultrasound analysis of both branches. Circ Cardiovasc Interv. 2012;5:657-62.
54. Mizuno Y, Sakakura K, Jinnouchi H, et al. Impact of rotational atherectomy on the incidence of side branch compromise in calcified bifurcation lesions undergoing elective percutaneous coronary intervention. J Cardiol. 2022;80:518-24.
55. Katsaros O, Sagris M, Karakasis P, et al. The role of calcified nodules in acute coronary syndrome: diagnosis and management. Int J Mol Sci. 2025;26:2581.
56. Negi SI, Didier R, Ota H, et al. Role of near-infrared spectroscopy in intravascular coronary imaging. Cardiovasc Revasc Med. 2015;16:299-305.
57. Masuda H, Kuramitsu S, Ito T, et al. Outcomes of paclitaxel-coated balloon angioplasty for in-stent calcified nodule: an optical coherence tomography study. Catheter Cardiovasc Interv. 2022;100:990-9.
58. Panoulas VF, Miyazaki T, Sato K, et al. Procedural outcomes of patients with calcified lesions treated with bioresorbable vascular scaffolds. EuroIntervention. 2016;11:1355-62.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].