Thermal ablation therapy for hepatocellular carcinoma: recurrence mechanisms and intervention strategies
Abstract
Thermal ablation, including radiofrequency ablation and microwave ablation, is a critical treatment modality for early-stage hepatocellular carcinoma (HCC); however, the high postoperative recurrence rate remains a significant challenge. Underlying mechanisms of post-ablation recurrence - including autophagy, epithelial-mesenchymal transition, hypoxic microenvironment formation, and immune suppression - are being progressively elucidated. Insufficient ablation is considered a key initiating factor for these processes. Advanced imaging techniques have thus been developed to improve intraprocedural monitoring and margin assessment, aiming to prevent insufficient ablation. For cases with confirmed residual or recurrent tumors, integrated therapies such as transarterial chemoembolization, radiotherapy, immunotherapy, and nanomedicine offer promising salvage options to mitigate further recurrence and improve survival. This review summarizes the mechanisms of tumor recurrence following HCC ablation and discusses potential countermeasures, aiming to provide valuable insights for clinical practice.
Keywords
INTRODUCTION
Locoregional therapies, particularly imaging-guided liver tumor-directed procedures, are crucial in managing 50%-60% of hepatocellular carcinoma (HCC) cases[1,2]. Thermal ablation, such as radiofrequency ablation (RFA) and microwave ablation (MWA), has become a standard treatment for early-stage HCC, especially in patients with compromised liver function, due to its repeatability, simplicity, and low complication rates[3,4]. However, the 5-year recurrence rate remains above 50%, often due to insufficient ablation[5]. Residual tumors frequently exhibit rapid post-treatment growth[6].
Studies indicate that sublethal thermal exposure from insufficient ablation triggers autophagy and epithelial-mesenchymal transition (EMT), thereby accelerating tumor progression[7,8]. Additionally, the hypoxic and immunosuppressive microenvironment that follows supports the survival and proliferation of residual tumor cells, promoting recurrence[9,10]. In this context, accurate tumor evaluation is essential. Imaging techniques such as contrast-enhanced ultrasound (CEUS) and computed tomography (CT) hepatic arteriography (CTHA) play a critical role in assessing tumor infiltration and detecting satellite lesions, facilitating more precise treatment planning[11,12]. Moreover, adjuvant therapies that combine ablation with modalities such as radiotherapy, targeted therapy, or immunotherapy show promise in reducing post-ablation recurrence[13]. However, many HCC patients with underlying liver dysfunction exhibit limited tolerance for aggressive adjuvant regimens. In addition, key challenges - including optimal regimen design, efficacy prediction, and understanding mechanisms of resistance - require further validation through large-scale, multicenter clinical trials.
In recent years, the emergence of nanomedicine has offered new opportunities for enhancing the efficacy of thermal ablation-based combination treatments. Nanocarriers enable multifunctional and precise drug delivery, potentially amplifying local therapeutic effects[14,15]. Our team previously developed a “macrophage hitchhiking” system (MAMH) for delivering drug-loaded macrophages to ablated HCC tissue. This method leverages the natural tropism of macrophages for post-ablation inflammatory gradients, increasing local drug concentration nearly 10-fold, with significant tumor suppression observed in mouse and rabbit models and favorable safety profiles[16]. However, clinical translation of nanomedicine still necessitates extensive systematic preclinical investigations and rigorous clinical trials for validation and optimization.
Considering these advances, we provide the first comprehensive review of the mechanisms behind tumor progression following thermal ablation for HCC and discuss effective countermeasures, with the aim of providing valuable insights for clinical practice.
OVERVIEW OF INSUFFICIENT ABLATION IN HCC
Thermal ablation, guided by imaging modalities such as ultrasound or CT, employs electrode needles to emit radiofrequency currents or microwaves, generating high temperatures between 60 and 100 °C within the tumor tissue, thereby inducing tumor necrosis[17]. Narrowly defined, insufficient ablation is identified as arterial-phase enhancement within the ablation zone one month post-procedure, detected by dynamic contrast-enhanced magnetic resonance imaging (MRI) or CEUS, suggesting residual tumor tissue[5]. More broadly, insufficient ablation encompasses any residual tumor cells after ablation, including microsatellite lesions and vascular infiltration, which are key contributors to tumor recurrence[18].
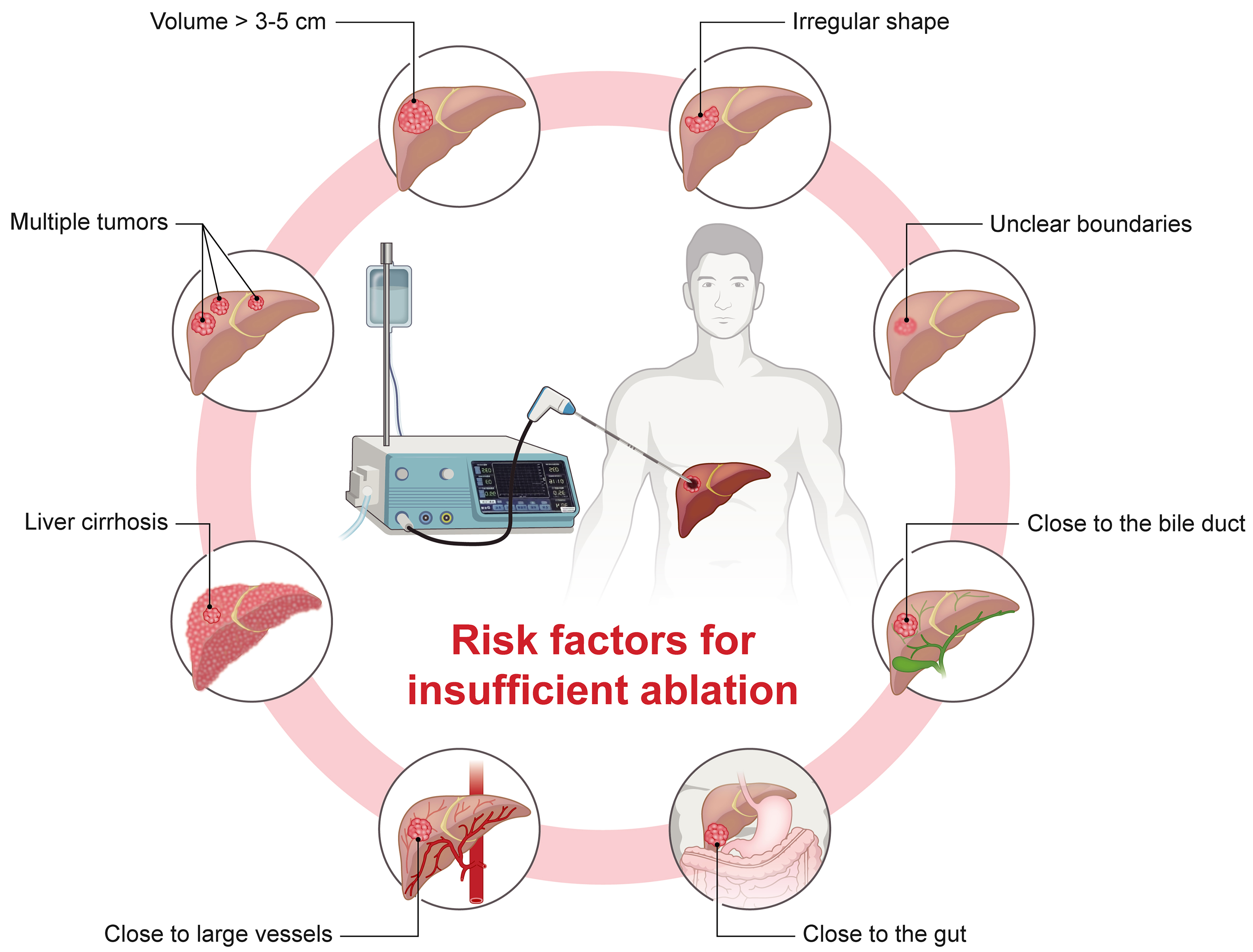
Key risk factors for insufficient ablation include large or multifocal tumors, irregular shapes, unclear boundaries, and proximity to vital organs and major blood vessels[19] [Figure 1]. For larger tumors, multi-point thermal field ablation may leave blind spots due to vaporization during tissue carbonization, increasing the risk of incomplete treatment[20]. Irregularly shaped or poorly defined tumors are also challenging to fully cover with the typically ellipsoidal ablation zone[20]. Tumors near vital organs (e.g., bile duct or gut) may require reduced ablation power to avoid damage, limiting the energy needed for complete ablation[21]. Distant regions from the heat source may also fail to reach ablative temperatures[22]. Additionally, tumors near major vessels may experience a “heat-sink effect”, where blood flow dissipates heat, preventing sufficient marginal temperatures for full ablation. Heating-induced pressure may also spread tumor cells via vascular pathways within the ablation zone[23]. Finally, liver cirrhosis and poor liver function are associated with satellite nodules and vascular invasion, further increasing the risk of insufficient ablation[5].
Figure 1. Risk factors for insufficient ablation in HCC. Key risk factors contributing to insufficient ablation include large tumors (> 3-
RECURRENCE MECHANISMS FROM INSUFFICIENT ABLATION
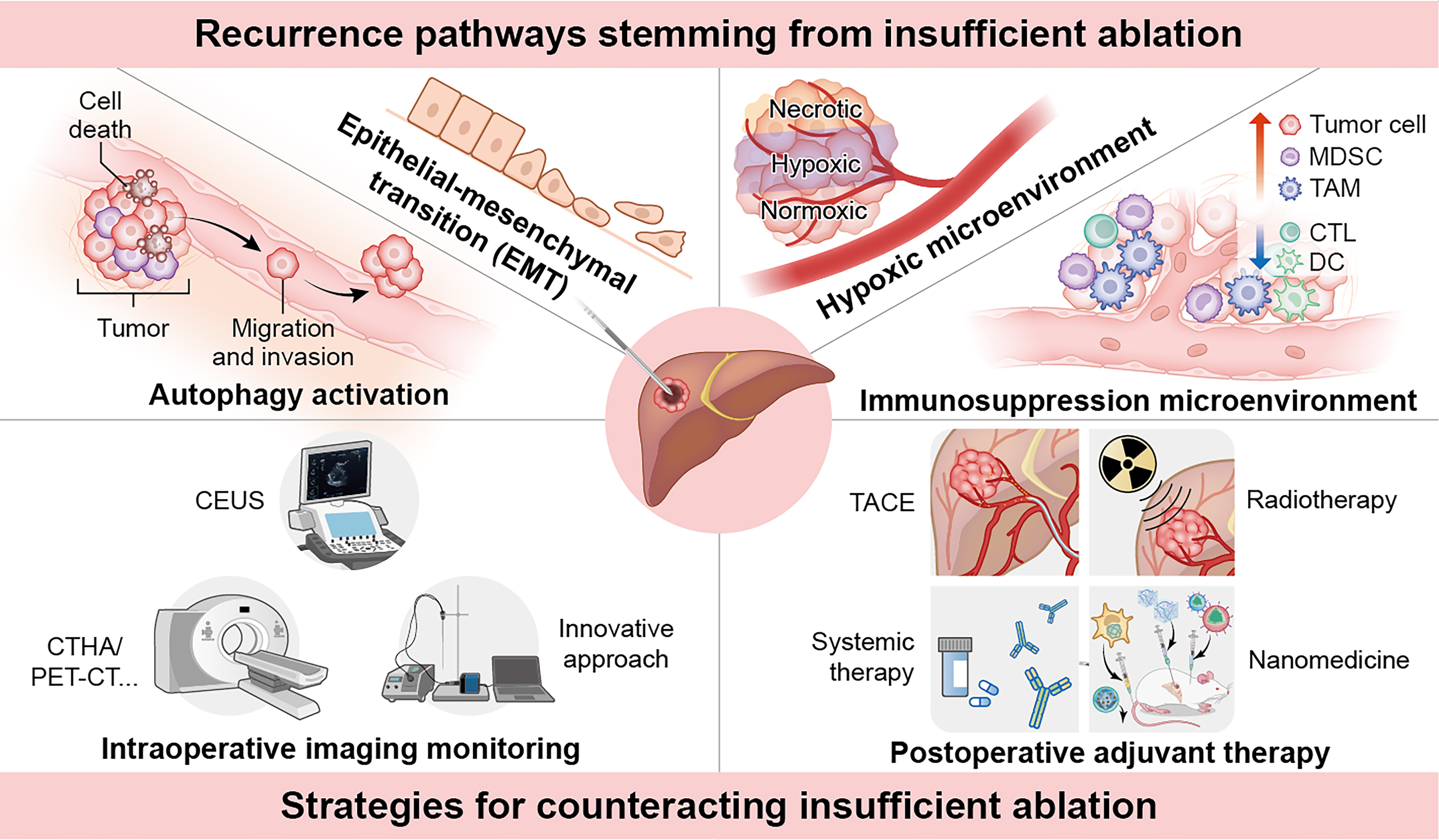
While initial ablation effectively eliminates most tumor tissue, residual cells often recur through mechanisms such as autophagy activation, EMT, hypoxic microenvironment formation, and immunosuppressive changes. These processes create favorable conditions for the survival, proliferation, and spread of residual cells in challenging environments [Figure 2]. Understanding these mechanisms is essential for developing combination therapies that reduce recurrence and enhance long-term survival in HCC patients.
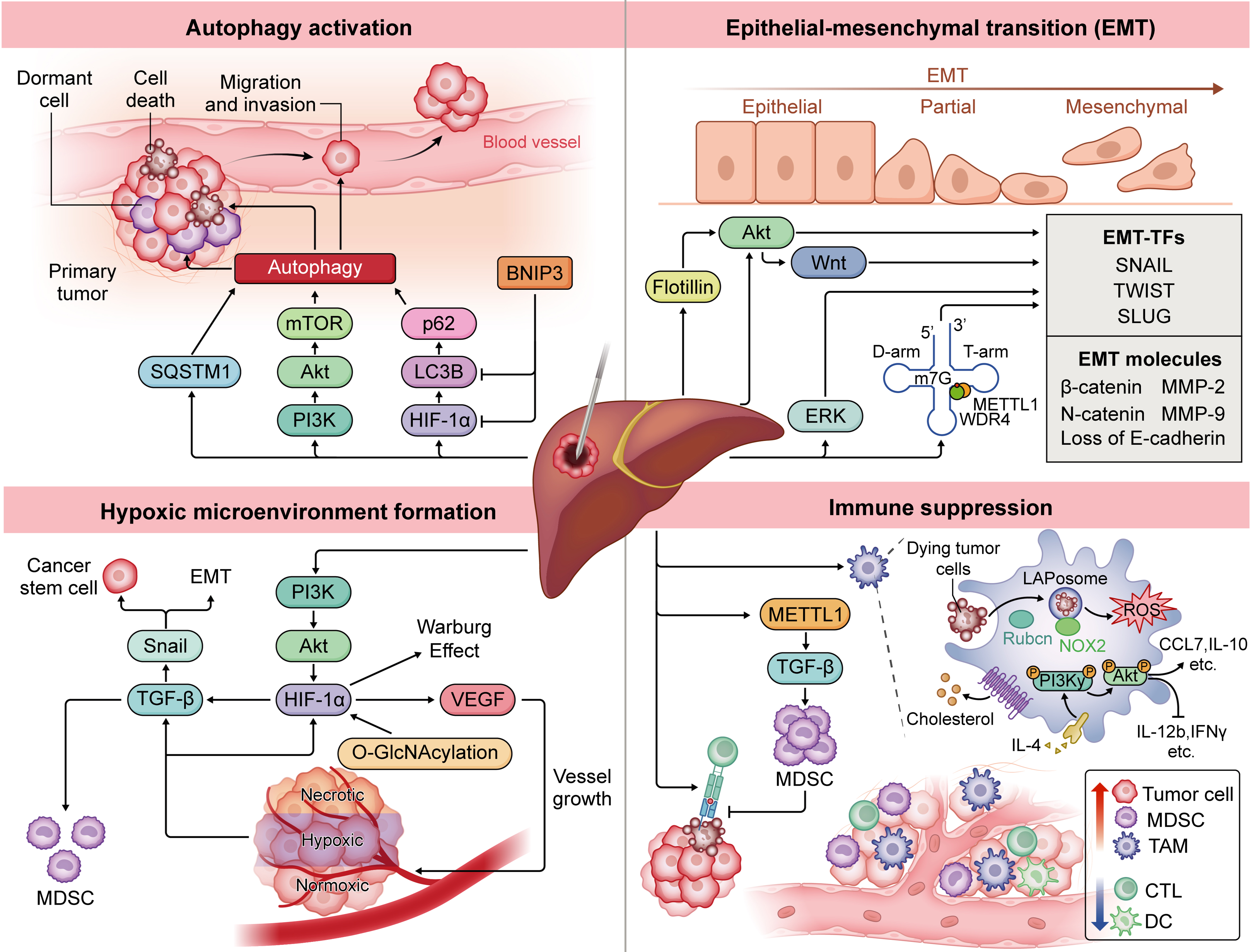
Figure 2. Mechanisms of recurrence after HCC ablation involve multiple biological processes. Autophagy activation supports cellular homeostasis[24], promoting invasion and metastasis, while EMT enhances tumor cell plasticity, fostering invasiveness and stress resistance[8]. The hypoxic microenvironment from heat-induced damage and vascular occlusion activates HIF-1α signaling, driving metabolic reprogramming, EMT, and angiogenesis[35]. Immune suppression is intensified via upregulated MDSCs[3] and TAMs[41], which inhibit effective anti-tumor immune responses and foster tumor growth. Collectively, these mechanisms allow residual tumor cells to survive, proliferate, and metastasize. Software: Adobe Illustrator (version 27.0; Adobe Inc.). HCC: Hepatocellular carcinoma; EMT: epithelial-mesenchymal transition; EMT-TFs: epithelial-mesenchymal transition-related transcription factors; Akt: protein kinase B; Wnt: Wingless/Integrated signaling pathway; ERK: extracellular signal-regulated kinase; SQSTM1: sequestosome 1; mTOR: mechanistic target of rapamycin; PI3K: phosphatidylinositol 3-kinase; p62: sequestosome 1/p62 protein; LC3B: microtubule-associated protein 1 light chain 3 beta; HIF-1α: hypoxia-inducible factor 1 alpha; BNIP3: BCL2 interacting protein 3; SNAIL: Snail family transcriptional repressor 1; TWIST: Twist family bHLH transcription factor; SLUG: Snail family transcriptional repressor 2; β-catenin: beta-catenin; MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9; METTL1: methyltransferase-like 1; WDR4: WD repeat domain 4; m7G: N7-methylguanosine; TGF-β: transforming growth factor beta; VEGF: vascular endothelial growth factor; O-GlcNAcylation: O-linked β-N-acetylglucosaminylation; MDSC: myeloid-derived suppressor cell; TAM: tumor-associated macrophage; LAPosome: LC3-associated phagosome; ROS: reactive oxygen species; NOX2: NADPH oxidase 2; CCL7: C-C motif chemokine ligand 7; IL-10: interleukin-10; IL-4: interleukin-4; IL-12b: interleukin-12 subunit beta; IFNγ: interferon gamma; CTL: cytotoxic T lymphocyte; DC: dendritic cell.
Autophagy activation
Autophagy, a cellular defense mechanism, sustains homeostasis by degrading damaged organelles and proteins. In the tumor microenvironment, however, autophagy enables tumor cells to adapt to stress, promoting survival and malignant progression[24]. Sublethal thermal stress has been shown to induce autophagy in cancer cell lines such as human cervical adenocarcinoma cell line (HeLa), human hepatocellular carcinoma cell line (Huh-7), and human hepatocellular carcinoma cell line (SMMC7721), highlighting its role in survival under stress[25]. This stress activates the phosphatidylinositol 3-kinase (PI3K)/mechanistic target of rapamycin (mTOR)/protein kinase B (AKT) pathway (regulates cell growth and survival), enhancing HCC cell proliferation and invasion, whereas autophagy inhibitors can reduce residual tumor growth after thermal stress[26]. Hypoxia may synergize with autophagy; high microtubule-associated protein 1A/1B-light chain 3B (LC3B; autophagosome marker) protein levels correlate with elevated hypoxia-inducible factor 1α (HIF-1α) in tumor regions[27]. Xu et al. found that silencing BCL2 interacting protein 3 (BNIP3; mediates mitophagy under hypoxia), a HIF-1α target, decreased heat-induced LC3B and HIF-1α, supporting this link[28]. Additionally, Yu et al. revealed that residual tumor cells post-insufficient RFA (iRFA) recur through the HIF-1α/LC3B/sequestosome 1/p62 protein (P62) autophagy pathway[29]. Another key autophagy-related molecule, Sequestosome 1 (SQSTM1; encodes p62, a selective autophagy receptor), has also been shown to be significantly associated with tumor recurrence after RFA[7]. Targeting the autophagy pathway may be an effective strategy to reduce recurrence.
EMT
EMT is essential for embryogenesis, wound healing, and malignant progression. In the context of neoplasia, EMT enhances tumor-initiating and metastatic potential, as well as therapy resistance. Yoshida et al. demonstrated that sublethal thermal treatment induces EMT in HCC cells, promoting a stem cell-like phenotype with high proliferation[8]. EMT transcription factors, including Snail (Snail family zinc finger transcription factor - a master regulator that initiates EMT by repressing epithelial genes such as E-cadherin), were notably upregulated by day 5 post-heat exposure[8]. In post-ablation recurrent HCC specimens, cluster of differentiation 44 (CD44), transforming growth factor-beta (TGF-β), twist family bHLH transcription factor (TWIST), and Snail expression levels increased, strongly correlating with EMT activation. EMT driven by protein kinase B (PKB, also known as Akt) and Extracellular signal-Regulated Kinase (ERK) signaling reduces E-cadherin and increases Neuronal-cadherin (N-cadherin), matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9), heightening lung metastasis risk[30].
Accumulation of β-catenin, a key wingless-related integration site (Wnt) factor, is a major mechanism driving EMT after ablation. EMT correlates with nuclear β-catenin buildup, and its inhibition can reduce invasiveness[31]. Elevated Flotillin expression further enhances invasiveness via the Akt/Wnt/β-catenin pathway[32]. Additionally, under sublethal thermal stress, methyltransferase 1 (METTL1) and N7-methylguanosine-modified transfer RNA (m7G tRNA) modifications enhance SLUG (Snail family transcriptional repressor 2, encoded by the SNAI2 gene)/Snail translation in a codon frequency-dependent manner[33]. Blocking EMT-related proteins and pathways thus presents a promising strategy to reduce recurrence and invasiveness of residual HCC cells post-ablation.
Hypoxic microenvironment formation
Thermal ablation induces tumor necrosis through high temperatures, but the resulting heat damage and vascular occlusion create a hypoxic environment that supports residual tumor cell survival[34]. Hypoxia-inducible factors (HIFs), key transcription factors in heat and hypoxia response, accelerate residual cell growth through mechanisms such as metabolic reprogramming, EMT, and angiogenesis[35]. Studies show that sublethal thermal stress-induced O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) stabilizes HIF-1α, promoting the Warburg effect and tumor progression in HCC. Hypoxia also elevates TGF-β expression, linked to malignant progression; in post-RFA tumor tissue, TGF-β levels are notably higher, enhancing cancer stem cell (CSC) invasiveness via the HIF-1α/TGF-β1/Snail pathway[30,36]. Increased TGF-β also induces myeloid-derived suppressor cells (MDSCs), fostering immunosuppression[37]. Blocking TGF-β signaling inhibits iRFA-induced progression and enhances anti-programmed death-1 (PD-1) antibody efficacy[38]. Hypoxia post-ablation raises vascular endothelial growth factor (VEGF) expression and microvessel density. Kong et al. found that thermal treatment in human hepatocellular carcinoma cell line (HepG2) cells upregulated pro-angiogenic PI3K/Akt/HIF-1α/vascular endothelial growth factor A (VEGFA) signaling, with VEGF overexpression driving proliferation, migration, and CSC characteristics in residual cells[37]. Anti-angiogenic agents such as bevacizumab and sorafenib counter this effect. Targeting hypoxia-related pathways may thus help mitigate recurrence risks associated with incomplete ablation. However, tumor heterogeneity and the spatiotemporal dynamics of hypoxia complicate treatment efficacy. Furthermore, clinical responses to anti-hypoxia and anti-angiogenic agents vary, with some patients developing resistance, underscoring the need for further exploration of combination therapies and biomarkers to precisely identify those who may benefit.
Immune suppression
Locoregional therapies can significantly influence the treatment outcomes of HCC by modulating the immune microenvironment[2]. Thermal ablation induces anti-tumor immunity by exposing antigens and triggering immunogenic cell death. However, residual tumors from incomplete ablation create an immunosuppressive state, accelerating progression and reducing immune checkpoint blockade (ICB) efficacy[39]. A major mechanism is MDSC upregulation, which is linked to postoperative recurrence[3]. Though ablation enhances T-cell responses to tumor-associated antigens (TAAs), these cells are suppressed by MDSCs, with insufficient memory to prevent recurrence[40]. Studies reveal that heat-induced METTL1 upregulates TGF-β2 translation, increasing MDSC counts, while pro-inflammatory factors from thermal stress enhance MDSC infiltration, promoting malignancy in residual tumors[3,10].
Tumor-associated macrophages (TAMs) also play a role; they accumulate in transitional zones post-ablation, suggesting involvement in residual tumor progression[41]. Mechanistically, TAMs undergo microtubule-associated protein 1A/1B-light chain 3 (LC3)-associated phagocytosis (LAP), enabling interleukin-4 (IL-4)-mediated reprogramming that activates the phosphoinositide 3-kinase gamma (PI3Kγ)/AKT pathway, releasing anti-inflammatory cytokines to induce immunosuppression[42]. Additionally, incomplete ablation reduces antigen presentation by dendritic cells (DCs) and diminishes cytotoxic T lymphocyte (CTL) infiltration, reinforcing the immunosuppressive microenvironment[15]. In summary, although incomplete ablation triggers an anti-tumor response, it also creates an immunosuppressive environment that promotes recurrence. Overcoming this immunosuppressive microenvironment through rational combination therapies is essential to improving ablation efficacy and reducing tumor relapse in HCC.
STRATEGIES FOR COUNTERACTING INSUFFICIENT ABLATION
Imaging monitoring
Ablation requires complete inactivation of the tumor with adequate safety margins while minimizing damage to normal liver tissue, demanding precise assessment of tumor infiltration and satellite lesions. Currently guided by ultrasound and CT, thermal ablation faces limitations in intraoperative real-time assessment. New methods aim to improve margin identification accuracy [Table 1]. Accurate imaging evaluation enables clinicians to promptly adjust treatment strategies, thereby reducing the occurrence of residual lesions.
Summary of ablation efficacy evaluation methods
| Method | Characteristics | Drawbacks |
| Ultrasound | Cost-effective and convenient[50] | Low resolution and poor sensitivity The imaging effect is affected by the operator |
| CEUS | Cost-effective and efficient, with sensitivity comparable to contrast-enhanced CT[70] | Difficult to accurately assess lesions located deep, above the hepatic dome, or in patients with limited positioning[70] Not suitable for patients with allergic tendencies |
| Ultrasound Fusion Imaging | Allows integration of information from different imaging modalities, leveraging the advantages of each to comprehensively evaluate, eliminate or minimize the drawbacks of each modality Aids in intraoperative assessment of ablation margins, detection of residual tumors, and supplementary ablation[70] | Complex operation and registration, time-consuming, highly dependent on experience[50] High equipment requirements, many influencing factors, image registration deformation |
| CT | Fast scanning speed, wide applicability Immediate intraoperative evaluation is primarily used to determine the presence of complications such as pneumothorax, bleeding, or injury to adjacent organs, and to make timely interventions[71-73] | Due to bleeding, edema, exudation, and infiltration of inflammatory cells around the ablation zone, accurate tumor evaluation requires several weeks post-ablation |
| MRI | No radiation exposure, high resolution, excellent for vascular imaging, can display temperature in real-time[74-76] | Slow imaging speed Ablation equipment must be compatible with MRI to ensure real-time imaging Operation is more complex |
| CTHA | Higher sensitivity for detecting small hepatic lesions both preoperatively and intraoperatively[11,12] | Requires catheter placement and specialized technical expertise CTHA-guided MWA takes longer than conventional CT |
| PET/CT | Can clearly delineate tumors before, during, and after ablation[49] | Expensive, patients are subjected to higher radiation doses, higher false-positive rates |
| Novel imaging techniques | Capable of clearly identifying tumor margins, and some methods can distinguish between residual active tumors and ablated dead tumors[47] | Still some time required before clinical translation and application |
CEUS reduces residual lesion incidence. In a study involving 93 patients, intraoperative CEUS identified insufficient ablation in 34 patients (36.5%), who subsequently received additional treatment within the same session. Twenty-four hours later, complete ablation was achieved in 88 patients (94.6%), thereby obviating the need for secondary treatments in 29 patients (31.1%)[43]. Due to vapor-induced artifacts, a 10-15 min wait before CEUS assessment post-ablation is recommended[44]. Additionally, hyperemia in ablated tissue can complicate CEUS interpretation, which is operator-dependent. Combining CT/MRI-CEUS fusion imaging has shown promise in detecting and assessing liver tumors[12]. Owing to its real-time capability and operational convenience, CEUS provides valuable intraoperative guidance, particularly in resource-limited settings.
Initially developed as a diagnostic tool, CTHA has demonstrated additional advantages in accurately delineating tumor and vascular anatomy, improving puncture guidance, and enabling real-time monitoring of the ablation zone[11]. CTHA can detect small lesions that are often missed by conventional contrast-enhanced CT or MRI, thereby reducing the need for repeat scans and minimizing radiation exposure[11]. Although its application is limited by higher costs and the requirement for catheter insertion, CTHA holds significant promise for optimizing ablation procedures. With its superior spatial resolution and vascular imaging capabilities, CTHA offers more precise treatment navigation, particularly in complex cases involving ill-defined tumor margins or lesions adjacent to critical structures.
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) has demonstrated significant potential for real-time evaluation of ablation efficacy. By administering 18F-FDG tracers both before and after ablation, the immediate assessment of treatment response can be achieved[45]. Furthermore, the combination of 13N-ammonia-based metabolic and perfusion imaging allows for accurate delineation of tumor margins within minutes post-ablation[46]. This approach highlights the feasibility of PET/CT as an integrated imaging modality that supports pre-ablation path planning, intraoperative needle positioning, immediate post-ablation assessment, and long-term follow-up. By providing dynamic insights into tumor metabolic activity, PET/CT facilitates early detection of residual or recurrent lesions and aids in the development of personalized therapeutic strategies.
Recent advances in imaging technologies have led to the development of novel modalities that improve the precision of ablation and the assessment of therapeutic outcomes. For instance, CEUS based on colony-stimulating factor 1 receptor (CSF-1R)-targeted nanobubbles has shown promise in accurately delineating ablation margins and detecting residual HCC lesions[47]. Another novel approach is indocyanine green (ICG)-guided optical imaging (OI), which allows for real-time visualization of tumor boundaries and differentiation between viable and necrotic tumor tissue[48]. Collectively, these technological advancements offer clinicians high-resolution, real-time insights that can significantly improve the safety and efficacy of ablation therapy. However, the clinical utility and translational potential of these methods remain to be rigorously validated in large-scale, multicenter studies.
In summary, the continuous advancement of imaging technologies has significantly enhanced the precision and dynamic monitoring capacity of ablation therapy for HCC, providing robust support for intraoperative decision-making and postoperative management. Notably, some advanced imaging modalities have already been standardized in clinical practice at select centers - for example, intraoperative CEUS combined with CT/MRI fusion imaging and real-time efficacy evaluation using PET/CT - demonstrating promising clinical feasibility and adaptability[12,49,50]. However, reliance on imaging alone remains insufficient to completely prevent tumor recurrence. Given the complex biological characteristics of residual lesions and the adaptive mechanisms within the tumor microenvironment, the integration of postoperative adjuvant therapies has become increasingly essential. Based on these considerations, the following sections will focus on recent advances in combining ablation with other local treatments, chemotherapy, targeted therapy, and immunotherapy, aiming to provide both theoretical rationale and practical guidance for developing a multidimensional, integrated treatment paradigm.
Postoperative adjuvant therapy
With the advancement of imaging technologies, the ability to detect residual lesions during and after ablation has been significantly improved, providing critical guidance for precise ablation procedures and individualized patient management. Imaging surveillance has become an essential reference for formulating postoperative treatment strategies, particularly in patients with a high risk of recurrence or suspected insufficient ablation. Imaging-based monitoring and postoperative adjuvant therapies should be regarded as an integrated interventional approach to collectively enhance overall treatment efficacy. Although ablation achieves a relatively high rate of local tumor control, recurrence remains frequent - especially in patients with minimal residual disease or highly aggressive tumors. Therefore, there is an urgent need to explore more effective adjuvant strategies to delay recurrence and improve long-term survival outcomes.
For local therapies, several studies have investigated combining ablation with transarterial chemoembolization (TACE), showing promise for tumor control, though large-scale randomized controlled trials (RCTs) are still needed[51]. According to current studies, TACE is potentially beneficial for reducing early recurrence in patients who have high-risk features, such as microvascular invasion (MVI), a tumor diameter ≥ 5 cm, multiple tumors, or suspected residual lesions[52-54]. Combining ablation with radiotherapy has demonstrated efficacy in preclinical models[55]. However, due to the limited tolerance of the liver to external beam radiotherapy, in patients with sufficient liver reserve who are suitable for arterial-based therapies, pairing ablation with transarterial radioembolization (TARE) may offer a more viable option[56]. In a multicenter, prospective trial, early-stage HCC patients with 2-5 cm lesions received holmium-166 microspheres (166Ho-MS) infusions 5-10 days post-RFA. Among 12 patients followed for one year, no local recurrence was observed[57].
Thermal ablation-induced tumor necrosis generates antigens that stimulate anti-tumor immunity; however, the resulting immunosuppressive microenvironment following insufficient ablation often drives rapid tumor progression. As a result, combination immunotherapy has emerged as a promising strategy. A multicenter Phase III trial (NCT00699816) found that postoperative cytokine-induced killer (CIK) cell therapy significantly extended recurrence-free survival (RFS), with medians of 44 months in the CIK group vs. 30 months in the control group. The CIK group also demonstrated reduced overall mortality [hazard ratio (HR) 0.21; 95% confidence interval (CI): 0.06-0.75; P = 0.008] and cancer-related mortality (HR 0.19; 95%CI: 0.04-0.87; P = 0.02)[58]. Emerging evidence indicates that adjuvant therapy with PD-1 inhibitors can activate anti-tumor immune responses within the HCC microenvironment and has shown promise in improving RFS[59]. A retrospective study found that combining PD-1 inhibitors with RFA yielded a 1-year RFS rate of 32.5%, compared to 10.0% for RFA alone[60]. The IMbrave050 study (NCT04102098) randomized high-risk HCC patients after resection or ablation into adjuvant atezolizumab-bevacizumab (atezo-bev) and active surveillance groups, enrolling 668 patients. After a median 17.4 month follow-up, the 12 month RFS rate was 78% in the atezo-bev group vs. 65% in the surveillance group. This is the first Phase III trial showing improved RFS with adjuvant therapy post-curative resection or ablation, underscoring its potential to enhance prognosis following insufficient ablation[61]. Numerous trials exploring ablation-combination therapies are ongoing, aiming to advance these strategies from preclinical studies into clinical practice [Table 2].
Summary of clinical trials for combination therapy with ablation
| Identifier | Study title | Interventions | Primary outcome | Secondary outcome | Phases | Locations |
| NCT00699816 | Efficacy and safety of immuncell-LC group and non-treatment group in hepatocellular carcinoma patients | Curative treatment combined with CIK cells | RFS | OS, cancer-specific survivals | III | Korea |
| NCT01853618 | Tremelimumab with chemoembolization or ablation for liver cancer | Tremelimumab + RFA or TACE | AEs | PFS, OS | I/II | United States |
| NCT02851784 | Combination therapy of microwave ablation and cellular immunotherapy for hepatocellular carcinoma | Radical MWA combined with CIK cells | Cumulative survival rates | DFS | II/III | China |
| NCT03864211 | Thermal ablation followed by immunotherapy for HCC | RFA/MWA followed by toripalimab | PFS | ORR, OS, DCR, TMR, etc. | I/II | China |
| NCT03867084 | Safety and efficacy of pembrolizumab (MK-3475) vs. placebo as adjuvant therapy in participants with HCC and complete radiological response after surgical resection or local ablation (MK-3475-937/KEYNOTE-937) | Complete surgical resection or local ablation combined with pembrolizumab | RFS, OS | AEs, HRQoL | III | United States, Argentina, Australia, Belgium, Brazil, Canada, etc. |
| NCT03939975 | Anti-PD-1 therapy combined with thermal ablation for advanced HCC | Pembrolizumab or nivolumab or JS001 combined with RFA/MWA | AEs, ORR, DoR, DCR | TTP, PFS, OS, etc. | II | China |
| NCT04102098 | A study of atezolizumab plus bevacizumab vs. active surveillance as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after surgical resection or ablation | Curative resection or ablation (RFA or MWA) combined with atezolizumab and bevacizumab or not | RFS | OS, TTR, time to extrahepatic spread or macrovascular invasion, AEs, etc. | III | United States, Australia, Austria, Brazil, Canada, China, Costa Rica, Czechia, France, Germany, etc. |
| NCT04204577 | The safety and efficacy of thermal ablation combined with apatinib and carilimub for advanced liver cancer | Local ablation combined with apatinib and PD-1 antibody SHR-1210 or not | PFS | ORR, DCR, OS, AEs, SAEs, HRQoL | II | China |
| NCT04220944 | Combined locoregional treatment with immunotherapy for unresectable HCC | MWA combined with simultaneous TACE plus PD-1 inhibitor | PFS | ORR, TTP, OS, TEAEs | I | China |
| NCT04517227 | The combination therapy of TACE and ablation with durvalumab in HCC at intermediate stage | TACE and ablation combined with durvalumab | AEs | PFS, TTP, OS | NA | China |
| NCT04652440 | Ablation combined with PD-1 in HCC: phase II study | PD-1 antibody and RFA or MWA | TRAEs, SAEs | CR, TFR, local recurrence rate, distant metastasis rate, DFS, OS | I/II | China |
| NCT04665609 | Thermal ablation combined with anlotinib and TQB2450 solution for HCC | MWA plus TQB2450 solution (anti-PD-L1) combined with anlotinib or not | ORR | OS, PFS, DCR, HRQoL, AEs, SAEs | II/I | China |
| NCT04864379 | Clinical study of a personalized neoantigen cancer vaccine combined with anti-PD-1 and RFA in patients with solid tumors | Neoantigen cancer vaccine (iNeo-Vac-P01) combined with anti-PD-1 antibody and RFA | AEs, ORR, measurement of CD4/CD8+T lymphocyte subsets | OS, PFS, the diversity and clonability of T cells | I | China |
| NCT06537505 | A Study of MWA combined with immunotherapy ± anti-angiogenic drugs in the treatment of solid tumors | MWA and adebrelimab combined with apatinib or not | ORR | DCR, PFS, OS, AEs | II | China |
The use of appropriate biomarkers for patient stratification is key to further improving the efficacy of immune checkpoint inhibitors (ICIs) in HCC. However, predicting response to ICIs in HCC is highly complex. The value of programmed death-ligand 1 (PD-L1) expression (≥ 1%) or tumor mutational burden (TMB) as predictive biomarkers has not been clearly established[62]. However, current research consensus suggests that “inflamed” HCCs - which are characterized by the enrichment of three distinct gene signatures: inflammatory, interferon-related antigen presentation, and T-cell-inflamed - are associated with a more favorable response to ICIs. These features are posited to be predictive of ICI responsiveness[63]. In clinical practice, caution is warranted when considering immunotherapy for patients with chronic hepatitis B virus (HBV) infection. HBV infection is closely linked to persistent liver inflammation and immune exhaustion. In particular, patients who are hepatitis B surface antigen (HBsAg)-positive or have a high viral load often exhibit severe T-cell dysfunction, which may impair their overall response to immunotherapy. Moreover, ICI therapy itself carries a significant risk of HBV reactivation[64]. While high-quality RCTs on adjuvant antiviral therapy for early-stage HCC patients’ post-ablation are lacking, retrospective studies suggest that antiviral therapy significantly reduces recurrence risk and prolongs overall survival (OS) in HBV-related HCC patients after RFA[65]. Future studies should incorporate HBV status into biomarker stratification to better guide personalized treatment strategies.
Inflammatory cytokines such as interleukin (IL)-1β, IL-6, and growth factors including TGF-β and VEGF play critical roles in tumor initiation and progression. Molecular targeted therapies aimed at these pro-tumorigenic signaling pathways may improve patient outcomes[36]. A multicenter retrospective study demonstrated that adjuvant sorafenib after RFA in early-stage HCC patients significantly reduced recurrence and prolonged OS[66]. However, the STORM trial (NCT00692770) failed to show a significant reduction in recurrence following curative surgery or ablation, possibly due to the lack of stratification based on inflammatory status and the limited proportion of ablation cases included[67]. Therefore, stratifying patients based on inflammatory biomarkers - such as IL-6, C-reactive Protein (CRP), and VEGFA - may enhance the efficacy of targeted adjuvant therapies. For patients with high systemic inflammation and poor hepatic reserve, locally delivered targeted drug systems may also offer a promising alternative by minimizing systemic toxicity.
Nanomedicine shows significant potential in preventing tumor recurrence post-ablation by enhancing immune responses and anti-tumor effects through various mechanisms. As an emerging field, its clinical indications have not yet been standardized but may include patients with insufficient ablation, poor hepatic reserve, or intolerance to systemic therapy. In these cases, nanoplatforms can leverage the enhanced permeability and retention (EPR) effect for tumor accumulation and remodel the immunosuppressive microenvironment, thereby inhibiting the progression of residual tumors[14,16,68]. Patient selection might prioritize individuals with an immunosuppressive tumor microenvironment or high risk of residual disease, though this requires further validation in clinical translation. Several preclinical studies have demonstrated its promise. For example, iRFA has been shown to enhance vascular permeability and facilitate the accumulation of arsenic trioxide (ATO)-loaded zeolitic imidazolate framework-8 (ZIF-8) nanoparticles in residual tumors, thereby reversing EMT and improving local control[68]. Ao et al. designed an injectable hydrogel containing stimulator of interferon genes (STING) agonists that effectively activated the STING pathway, increased M1 (classically activated, pro-inflammatory) macrophage polarization and cytotoxic T cell infiltration, and reversed local immunosuppression[15]. Xiao et al. created an antigen-capturing nanoplatform that co-delivers TAA and N6-methyladenosine (m6A) demethylase inhibitors to tumor-infiltrating DCs (TIDCs), enhancing DC maturation and, in combination with ICB therapy, inhibiting distant tumor growth and lung metastasis[69]. Moreover, a Trinity nanovaccine with spatiotemporal immune effects has shown improved immune activation as an adjuvant to RFA[14]. Cell-based micro/nanorobots with targeted penetration, immune modulation, and precise therapy are gaining prominence in biomedical research. Our team previously developed a “hitchhiking” technology that uses drug-loaded macrophages (MAMH) directed by ablation-induced inflammatory gradients. In this approach, macrophage robots carrying lenvatinib accumulate in tumor tissue under these gradients, enhancing drug concentration by nearly 10-fold within lesions. This method has shown significant tumor inhibition and favorable biosafety in mouse and rabbit models[16]. Nanomedicine offers a multifunctional platform that not only enables precise targeting of residual tumors but also enhances post-ablation anti-tumor responses by reshaping the immune microenvironment. However, despite their encouraging preclinical results, most nanomedicine strategies remain far from clinical translation. Major challenges include the lack of standardized manufacturing and characterization protocols, unpredictable biodistribution and long-term biosafety, and substantial differences between animal models and human pathophysiology. Furthermore, immune responses to nanomaterials and scalability of production under good manufacturing practice (GMP) conditions remain critical barriers. Future efforts should prioritize large-animal validation, comprehensive pharmacokinetic and toxicity evaluation, and early-phase clinical trials to bridge the gap between laboratory innovation and real-world application.
Conclusion and future perspective
Thermal ablation has become a crucial therapeutic modality for HCC, particularly in patients with impaired liver function or those unsuitable for surgical resection. Despite its minimally invasive nature and relatively high local control rates, postoperative tumor recurrence remains a significant clinical challenge[2,3]. The primary cause of recurrence is insufficient ablation leading to rapid progression of residual tumor cells, driven by key mechanisms such as autophagy activation, EMT, hypoxia-induced signaling, and tumor immune evasion. These biological processes collectively establish a tumor-promoting microenvironment around residual lesions, representing a major barrier to therapeutic efficacy[3,10,33].
To address these challenges, current research efforts are increasingly focused on multidimensional and multimodal comprehensive interventions. Foremost, achieving complete ablation under precise imaging guidance remains the cornerstone of successful treatment. Advanced imaging modalities - including CEUS, CTHA, PET/CT, and emerging intraoperative OI techniques - hold promise for improving the accuracy of ablation margin delineation and enabling real-time monitoring[12,18,43]. Furthermore, postoperative adjuvant therapies, particularly combinations involving interventional, molecular targeted, and immunotherapeutic approaches, have demonstrated potential in delaying tumor recurrence[38,67].
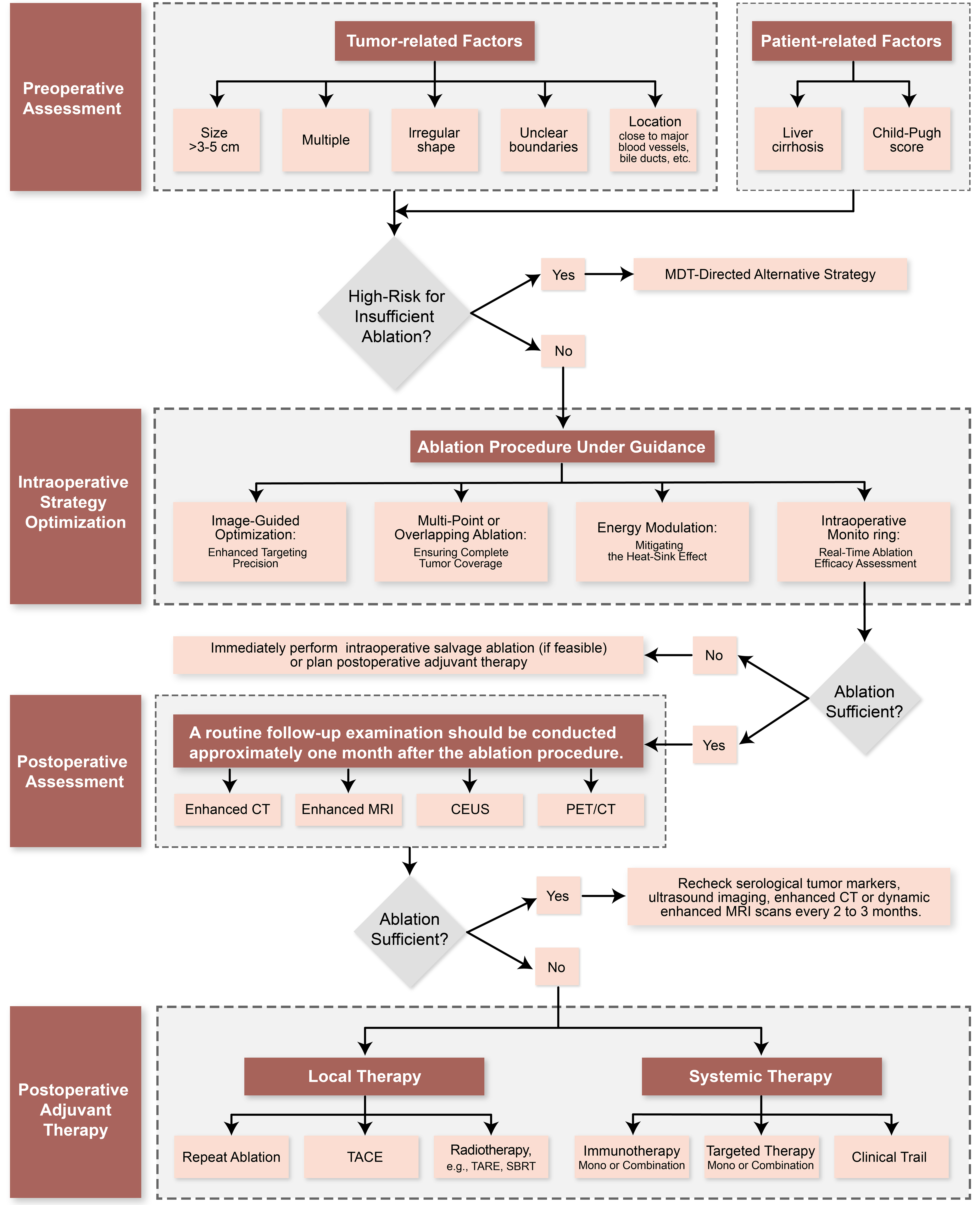
Based on these considerations, we propose an integrated management framework and decision algorithm for HCC thermal ablation [Figure 3]. This framework begins with preoperative risk stratification and multidisciplinary team (MDT) evaluation to identify high-risk features - including large or irregular tumors and those adjacent to critical structures - enabling consideration of alternative approaches such as surgical resection or combined TACE-ablation. The intraoperative phase emphasizes real-time imaging guidance with continuous assessment of the ablation zone and safety margins, incorporating technical adjustments such as multi-point ablation when needed, and allowing for immediate salvage ablation if margins appear insufficient[12,43,44]. Postoperative management is guided by one-month definitive imaging assessment. Patients are stratified according to clinical and pathological findings. For patients with confirmed macroscopic residual lesions in anatomically suitable locations and no distant metastases, local treatment options such as repeat ablation, TACE, or radiotherapy should be prioritized. For patients without macroscopic residual disease but exhibiting high-risk features (e.g., MVI, multiple tumors, or large tumor size), local regional therapies such as TACE may be considered to reduce the risk of early recurrence[52-54]. Concurrently, immunotherapy and/or targeted therapy serve as viable adjunctive strategies, demonstrating favorable recurrence control in multiple studies. Patients exhibiting tumor “inflamed” characteristics may derive greater benefit from immunotherapy[62,63,66]. Furthermore, novel therapies including nanomedicine and CIK cell therapy remain exploratory and should only be administered within rigorously designed clinical trials[14,16,68]. For patients achieving complete remission without high-risk features, standardized follow-up protocols are recommended, encompassing regular imaging studies and serological assessments.
Figure 3. Integrated management framework and decision algorithm for HCC thermal ablation. This comprehensive chart delineates the four-phase clinical management pathway, aligning procedural stages (left) with corresponding decision-making algorithms (right). Software: Adobe Illustrator (version 27.0; Adobe Inc.). HCC: Hepatocellular carcinoma; MDT: multidisciplinary team; CEUS: contrast-enhanced ultrasound; MRI: magnetic resonance imaging; PET/CT: positron emission tomography/computed tomography; TACE: transarterial chemoembolization; TARE: transarterial radioembolization; SBRT: stereotactic body radiation therapy.
Looking forward, advancements in HCC thermal ablation will inevitably depend on the deep integration of imaging-guided precision ablation and mechanism-driven combination therapies. The convergence of multidisciplinary approaches and innovative technologies is anticipated to overcome current therapeutic limitations, facilitating the development of personalized and precision medicine strategies that significantly improve long-term patient outcomes.
DECLARATIONS
Authors’ contributions
Provided advice and supervised the work: Zheng T, Li X (Xianjun Li)
Drafted the manuscript: Li X (Xuehan Li), Li S, Li Y
Polished the manuscript: Yuan G, Zhang Y
Revised the figures: Shi J, Liu C
All authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China [grants U20A20377 and 81872435 to Zheng T, and 82403891 to Li X (Xianjun Li).]; the Tou-Yan Innovation Team Program of Heilongjiang Province (grant 2019-15); the HMU Marshal Initiative Funding (grant HMUMIF-21004 to Zheng T.); the National Key Research and Development Program of China (grant 2023YFC3504600 to Zheng T.); the National Postdoctoral Program for Innovative Talents [grant BX20240098 to Li X (Xianjun Li).]; and the China Postdoctoral Science Foundation [grant 2023MD744211 to Li X (Xianjun Li).].
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203-22.
2. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313.
3. Zeng X, Liao G, Li S, et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents hepatocellular carcinoma recurrence after radiofrequency ablation. Hepatology. 2023;77:1122-38.
4. Wang Z, Liu M, Zhang DZ, et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5-cm HCC. Hepatology. 2022;76:66-77.
5. Huang Z, Guo Z, Ni J, et al. Four types of tumor progression after microwave ablation of single hepatocellular carcinoma of ≤ 5 cm: incidence, risk factors and clinical significance. Int J Hyperthermia. 2021;38:1164-73.
6. Shi ZR, Duan YX, Cui F, et al. Integrated proteogenomic characterization reveals an imbalanced hepatocellular carcinoma microenvironment after incomplete radiofrequency ablation. J Exp Clin Cancer Res. 2023;42:133.
7. Abdel-Moety A, Baddour N, Salem P, El-Tobgy H, El-Shendidi A. SQSTM1 expression in hepatocellular carcinoma and relation to tumor recurrence after radiofrequency ablation. J Clin Exp Hepatol. 2022;12:774-84.
8. Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58:1667-80.
9. Cui R, Wang L, Zhang D, et al. Combination therapy using microwave ablation and d-mannose-chelated iron oxide nanoparticles inhibits hepatocellular carcinoma progression. Acta Pharm Sin B. 2022;12:3475-85.
10. Shi L, Wang J, Ding N, et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun. 2019;10:5421.
11. Puijk RS, Dijkstra M, van der Lei S, et al. The added value of transcatheter CT hepatic angiography (CTHA) image guidance in percutaneous thermal liver ablation: an experts’ opinion pictorial essay. Cancers. 2024;16:1193.
12. Huang Q, Zeng Q, Long Y, et al. Fusion imaging techniques and contrast-enhanced ultrasound for thermal ablation of hepatocellular carcinoma - a prospective randomized controlled trial. Int J Hyperthermia. 2019;36:1207-15.
13. Qi X, Yang M, Ma L, et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J Immunother Cancer. 2020;8:e001038.
14. Li M, Jiang A, Han H, et al. A trinity nano-vaccine system with spatiotemporal immune effect for the adjuvant cancer therapy after radiofrequency ablation. ACS Nano. 2024;18:4590-612.
15. Ao F, Li X, Tan Y, et al. STING agonist-based hydrogel enhances immune activation in synergy with radiofrequency ablation for hepatocellular carcinoma treatment. J Control Release. 2024;369:296-308.
16. Li X, Zhang Y, Li S, et al. Macrophage hitchhiking for systematic suppression in postablative multifocal HCC. Hepatology. 2025;81:44-59.
17. Wu CY, Lin LY, Lee TY, et al. Clinical guidelines for early hepatocellular carcinoma treatment options: a systematic review and bibliometric analysis. Int J Surg. 2024;110:7234-44.
18. Minami Y, Nishida N, Kudo M. Therapeutic response assessment of RFA for HCC: contrast-enhanced US, CT and MRI. World J Gastroenterol. 2014;20:4160-6.
19. Yang Y, Chen Y, Ye F, et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol. 2021;31:3053-64.
20. Ke S, Ding XM, Kong J, et al. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J Transl Med. 2010;8:73.
21. Imajo K, Ogawa Y, Yoneda M, Saito S, Nakajima A. A review of conventional and newer generation microwave ablation systems for hepatocellular carcinoma. J Med Ultrason. 2020;47:265-77.
22. Tan J, Tang T, Zhao W, Zhang ZS, Xiao YD. Initial incomplete thermal ablation is associated with a high risk of tumor progression in patients with hepatocellular carcinoma. Front Oncol. 2021;11:760173.
23. Bigos KJ, Quiles CG, Lunj S, et al. Tumour response to hypoxia: understanding the hypoxic tumour microenvironment to improve treatment outcome in solid tumours. Front Oncol. 2024;14:1331355.
24. Hernandez GA, Perera RM. Autophagy in cancer cell remodeling and quality control. Mol Cell. 2022;82:1514-27.
25. Wang X, Deng Q, Feng K, et al. Insufficient radiofrequency ablation promotes hepatocellular carcinoma cell progression via autophagy and the CD133 feedback loop. Oncol Rep. 2018;40:241-51.
26. Jondal DE, Thompson SM, Butters KA, et al. Heat stress and hepatic laser thermal ablation induce hepatocellular carcinoma growth: role of PI3K/mTOR/AKT signaling. Radiology. 2018;288:730-8.
27. Jiang J, Chen S, Li K, et al. Targeting autophagy enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis for hepatocellular carcinoma. Int J Hyperthermia. 2019;36:499-510.
28. Xu WL, Wang SH, Sun WB, et al. Insufficient radiofrequency ablation-induced autophagy contributes to the rapid progression of residual hepatocellular carcinoma through the HIF-1α/BNIP3 signaling pathway. BMB Rep. 2019;52:277-82.
29. Yu ZJ, Guo SW, Wang BS, et al. Ruthenium complex suppresses proliferation of residual hepatocellular carcinoma after incomplete radiofrequency ablation therapy. Recent Pat Anticancer Drug Discov. 2024;Epub ahead of print.
30. Iwahashi S, Shimada M, Utsunomiya T, et al. Epithelial-mesenchymal transition-related genes are linked to aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation. Cancer Lett. 2016;375:47-50.
31. Zhang N, Wang L, Chai ZT, et al. Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-catenin signaling. PLoS One. 2014;9:e115949.
32. Zhang N, Li H, Qin C, et al. Insufficient radiofrequency ablation promotes the metastasis of residual hepatocellular carcinoma cells via upregulating flotillin proteins. J Cancer Res Clin Oncol. 2019;145:895-907.
33. Zhu S, Wu Y, Zhang X, et al. Targeting N7-methylguanosine tRNA modification blocks hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Mol Ther. 2023;31:1596-614.
34. Tong Y, Yang H, Xu X, et al. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci. 2017;108:753-62.
35. Ortmann BM, Taylor CT, Rocha S. Hypoxia research, where to now? Trends Biochem Sci. 2024;49:573-82.
36. Li K, Niu Y, Yuan Y, et al. Insufficient ablation induces E3-ligase Nedd4 to promote hepatocellular carcinoma progression by tuning TGF-β signaling. Oncogene. 2022;41:3197-209.
37. Kong J, Kong J, Pan B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS One. 2012;7:e37266.
38. Xiang S, Li J, Zhang M. TGF-β1 inhibitor enhances the therapeutic effect of microwave ablation on hepatocellular carcinoma. Int J Hyperthermia. 2024;41:2359496.
39. Chen Y, Bei J, Chen M, et al. Intratumoral lactate depletion based on injectable nanoparticles-hydrogel composite system synergizes with immunotherapy against postablative hepatocellular carcinoma recurrence. Adv Healthc Mater. 2024;13:e2303031.
40. Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448-57.
41. Collettini F, Brangsch J, Reimann C, et al. Hepatic radiofrequency ablation: monitoring of ablation-induced macrophage recruitment in the periablational rim using SPION-enhanced macrophage-specific magnetic resonance imaging. Invest Radiol. 2021;56:591-8.
42. Liu X, Zhang W, Xu Y, et al. Targeting PI3Kγ/AKT pathway remodels LC3-associated phagocytosis induced immunosuppression after radiofrequency ablation. Adv Sci. 2022;9:e2102182.
43. Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209-16.
44. Bansal S, Gui J, Merrill C, Wong JK, Burak KW, Wilson SR. Contrast-enhanced US in local ablative therapy and secondary surveillance for hepatocellular carcinoma. Radiographics. 2019;39:1302-22.
45. Fite BZ, Wang J, Ghanouni P, Ferrara KW. A review of imaging methods to assess ultrasound-mediated ablation. BME Front. 2022;2022:9758652.
46. Shyn PB, Casadaban LC, Sainani NI, et al. Intraprocedural ablation margin assessment by using ammonia perfusion PET during FDG PET/CT-guided liver tumor ablation: a pilot study. Radiology. 2018;288:138-45.
47. Jiang Q, Zeng Y, Xu Y, et al. Ultrasound molecular imaging as a potential non-invasive diagnosis to detect the margin of hepatocarcinoma via CSF-1R targeting. Front Bioeng Biotechnol. 2020;8:783.
48. Kan X, Zhou G, Zhang F, et al. Interventional optical imaging permits instant visualization of pathological zones of ablated tumor periphery and residual tumor detection. Cancer Res. 2021;81:4594-602.
49. Mateva G, Handzhiev S, Kostadinova I. The role of 18F-FDG PET/CT in evaluating the efficacy of radiofrequency ablation in metastatic and primary liver tumors: preliminary results. Mol Imaging Radionucl Ther. 2021;30:1-7.
50. Li K, Su ZZ, Xu EJ, Ju JX, Meng XC, Zheng RQ. Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer. 2016;16:277.
51. Liu B, Zhang Y, Chen H, Li W, Tsochatzis E. The combination of transcatheter arterial chemoembolisation (TACE) and thermal ablation versus TACE alone for hepatocellular carcinoma. Cochrane Database Syst Rev. 2022;1:CD013345.
52. Wei W, Jian PE, Li SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun. 2018;38:61.
53. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24:2074-81.
54. Guo B, Chen Q, Liu Z, Chen X, Zhu P. Adjuvant therapy following curative treatments for hepatocellular carcinoma: current dilemmas and prospects. Front Oncol. 2023;13:1098958.
55. Horkan C, Dalal K, Coderre JA, et al. Reduced tumor growth with combined radiofrequency ablation and radiation therapy in a rat breast tumor model. Radiology. 2005;235:81-8.
56. Young S, Golzarian J. Locoregional therapies in the treatment of 3- to 5-cm hepatocellular carcinoma: critical review of the literature. AJR Am J Roentgenol. 2020;215:223-34.
57. Hendriks P, Rietbergen DDD, van Erkel AR, et al; Dutch Hepatocellular and Cholangiocarcinoma Group. Adjuvant holmium-166 radioembolization after radiofrequency ablation in early-stage hepatocellular carcinoma patients: a dose-finding study (HORA EST HCC trial). Eur J Nucl Med Mol Imaging. 2024;51:2085-97.
58. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6.
59. Su JY, Li JR, Pan LX, et al. Tertiary lymphoid structures in HCC: influence on immune cell profiles in tumors and on efficacy of adjuvant PD-1 inhibitor therapy after hepatectomy. Hepatology. 2025;Epub ahead of print.
60. Wang X, Liu G, Chen S, et al. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: a propensity score matching analysis. Int J Hyperthermia. 2021;38:1519-28.
61. Chow P, Chen M, Cheng A, et al. Abstract CT003: IMbrave050: phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. Cancer Research. 2023;83:CT003.
62. Zhu AX, Abbas AR, de Galarreta MR, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28:1599-611.
63. Llovet JM, Pinyol R, Yarchoan M, et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol. 2024;21:294-311.
64. Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7:322.
65. Chen S, Shen B, Wu Y, et al. The relationship between the efficacy of thermal ablation and inflammatory response and immune status in early hepatocellular carcinoma and the progress of postoperative adjuvant therapy. Int Immunopharmacol. 2023;119:110228.
66. Feng X, Xu R, Du X, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol. 2014;109:1891-9.
67. Bruix J, Takayama T, Mazzaferro V, et al; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-54.
68. Chen X, Huang Y, Chen H, et al. Augmented EPR effect post IRFA to enhance the therapeutic efficacy of arsenic loaded ZIF-8 nanoparticles on residual HCC progression. J Nanobiotechnology. 2022;20:34.
69. Xiao Z, Li T, Zheng X, et al. Nanodrug enhances post-ablation immunotherapy of hepatocellular carcinoma via promoting dendritic cell maturation and antigen presentation. Bioact Mater. 2023;21:57-68.
70. Minami Y, Kudo M. Image guidance in ablation for hepatocellular carcinoma: contrast-enhanced ultrasound and fusion imaging. Front Oncol. 2021;11:593636.
71. Cao SE, Zhang LQ, Kuang SC, et al. Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrast-enhanced computed tomography. World J Gastroenterol. 2020;26:3660-72.
72. Grazioli L, Ambrosini R, Frittoli B, Grazioli M, Morone M. Primary benign liver lesions. Eur J Radiol. 2017;95:378-98.
73. Bottari A, Silipigni S, Carerj ML, et al. Dual-source dual-energy CT in the evaluation of hepatic fractional extracellular space in cirrhosis. Radiol Med. 2020;125:7-14.
74. Granata V, Grassi R, Fusco R, et al. Diagnostic evaluation and ablation treatments assessment in hepatocellular carcinoma. Infect Agent Cancer. 2021;16:53.
75. Mathew RP, Sam M, Raubenheimer M, Patel V, Low G. Hepatic hemangiomas: the various imaging avatars and its mimickers. Radiol Med. 2020;125:801-15.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].