Bioelectromagnetic fields in prostate cancer: molecular mechanisms and therapeutic implications
Abstract
Prostate cancer (PCa) remains a global health challenge, with emerging evidence implicating environmental electromagnetic fields (EMFs) as dual regulators of tumor progression and therapeutic innovation. This perspective synthesizes mechanistic insights into EMFs bioeffects through three interconnected axes: (1) Calcium signaling as a central biosensor, translating EMFs stimuli into oncogenic processes via piezoelectric and ionic mechanisms; (2) Extracellular vesicles (EVs)-mediated metastatic reprogramming through cargo remodeling (e.g., pro-inflammatory proteins) and dysregulation of oncogenic miRNAs; and (3) Ferroptosis, a frequency-modulated cell death pathway driven by calcium-iron-reactive oxygen species (ROS) crosstalk. Paradoxically, EMFs exhibit context-dependent duality: low-intensity fields exacerbate malignancy by rewiring calcium/EVs-driven tumor-microenvironment interactions, while high-intensity fields achieve tumor ablation via calcium overload, ferroptosis, hyperthermia, or electroporation. Novel strategies, such as PEGylated nanocrystals combined with intratumoral micromagnets, synergize ferroptosis with immunogenic cell death, demonstrating therapeutic potential with minimal toxicity. Our analysis underscores the critical need for parameter optimization (frequency, intensity, duration) to balance oncogenic risks against therapeutic precision. We propose that integrating biophysical targeting with electromagnetic engineering holds promise to redefine PCa management through mechanism-driven precision. This perspective aims to frame these insights and highlight their implications for future research and therapeutic development.
Keywords
INTRODUCTION
Prostate cancer (PCa) remains a major global health challenge, accounting for the second-highest cancer incidence and mortality among men worldwide[1,2]. While established risk factors such as aging, genetic predisposition, and lifestyle variables (e.g., dietary patterns, obesity and tobacco/alcohol use) significantly contribute to its pathogenesis[3], emerging evidence implicates environmental electromagnetic fields (EMFs) as critical epigenetic regulators of PCa progression[4,5]. While epidemiological studies remain inconclusive regarding EMFs’ carcinogenic potential[6], mechanistic research increasingly reveals their capacity to bidirectionally reprogram tumor biology through multifaceted interactions with cellular pathways and highlights their potential as therapeutic instruments.
Notably, the dual role of EMFs manifests in their ability to induce pro-metastatic phenotypes under certain parameters[7,8] while exhibiting therapeutic potential under others[9-12] [Table 1]. Low-frequency fields induce apoptosis via reactive oxygen species (ROS)-dependent caspase-3 activation, aligning with observed cell growth inhibition despite unspecified intensity parameters[9]. In contrast, high-frequency nanosecond pulses impair cell viability and mobility through membrane permeabilization[10]. Paradoxically, while some rodent models show no tumorigenic initiation from EMF exposure[17], preclinical PCa studies reveal
EMF interventions and therapeutic applications in prostate cancer
| Intervention method | Category | Treatment parameters | Efficacy measures | Effect classification | Ref. |
| 60-Hz sinusoidal magnetic field | Extremely low | Frequency: 60 Hz | Cell growth inhibition, apoptosis | Potential adjuvant for radiation therapy | [9] |
| PEGylated nanocrystals + internal magnetic field | Extremely low | - | Ferroptosis, immunogenic cell death | Synergistic tumoricidal efficacy with low systemic toxicity | [12] |
| Pulsed EMFs (nanosecond pulses) | High | Frequency: 1 Hz-1 MHz; Intensity: 7,000 V/cm; Duration: 200 ns/pulse (23 pulses total) | Cell permeabilization, death, mobility loss | Applicable to nanosecond electroporation protocols | [10] |
| Microwave ablation + pulsed EMFs | High | Intensity: 72,800/56,600/1,450 V/m | Reduced cell viability, apoptosis, migration inhibition | Therapeutic intervention for PCa | [13] |
| Alternating magnetic field + radiation therapy | Low | Frequency: 160 ± 5 kHz; Intensity: 24 kA/m; Duration: 20 min | Tumor growth delay | Improved radiation therapy efficacy | [14] |
| Low-dose alternating electric current | Low | Frequency: 100 kHz; Intensity: 15/25 mA; Duration: 15/60 min | Tumor growth inhibition, PSA reduction | Minimally invasive treatment option | [11] |
| Static magnetic field (medicinal biomagnetism) | Extremely low | Intensity: > 1000 Gauss | PSA reduction, adenocarcinoma regression | Alternative treatment for PCa | [15] |
| Low magnetic field exposure | Extremely low | Intensity: 20 nT; Duration: 4 h | Increased cancer aggressiveness and metastasis | Pro-carcinogenic | [7] |
| Terahertz wave | High | Frequency: 34.5 THz (wavelength: 8.7 μm) | Suppresses ferroptosis | Pro-carcinogenic (context-dependent) | [16] |
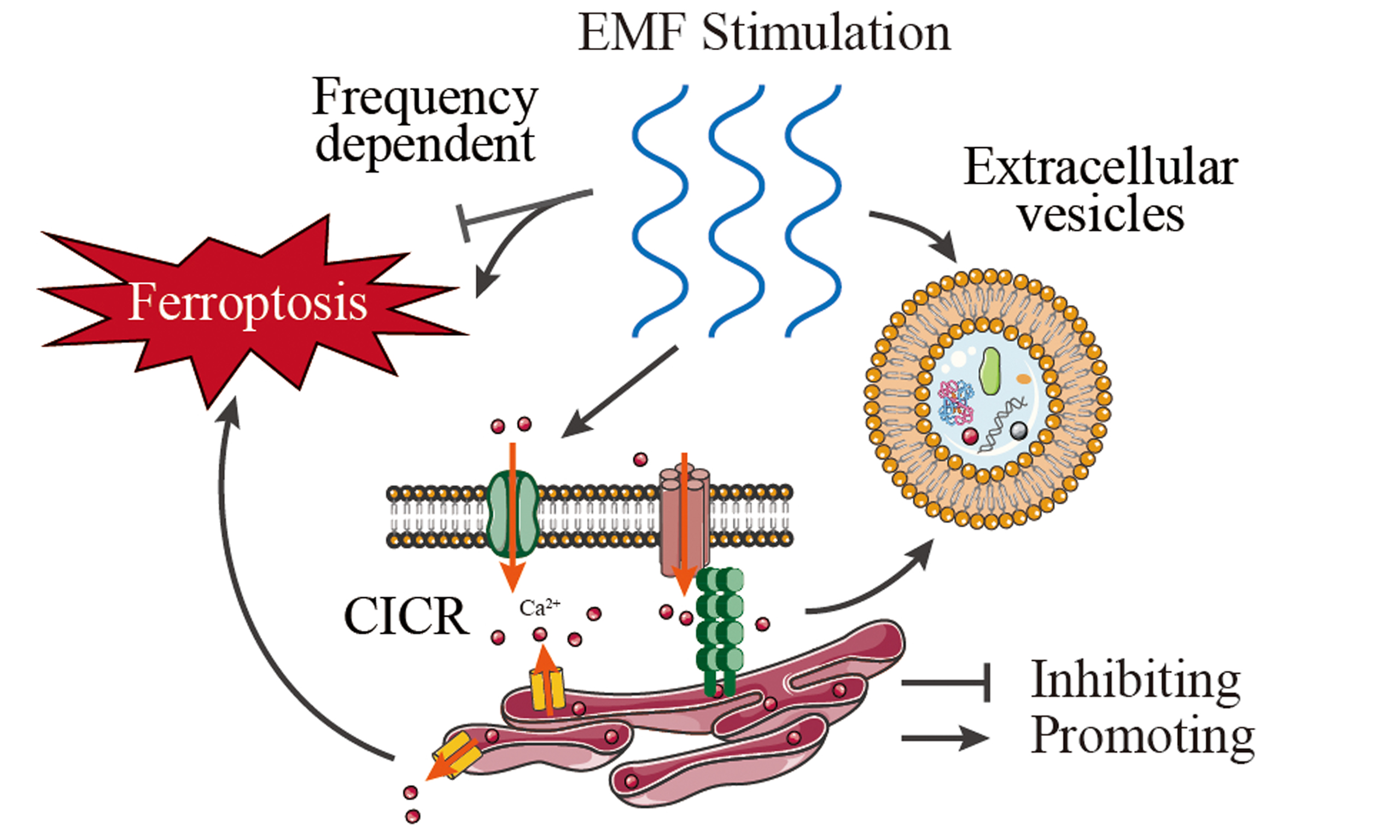
In this perspective, we integrate recent advances through three interconnected axes that bridge biophysical mechanisms with oncological paradigms [Figure 1] and propose a reconciling framework for EMFs’ duality: (1) Calcium signaling as the central electromagnetic biosensor, where EMFs modulate ion flux to drive tumor aggressiveness or therapeutic vulnerability; (2) EV-mediated metastatic reprogramming, involving electromagnetic-sensitive cargo sorting and miRNA dysregulation; (3) Frequency-modulated ferroptosis, a therapeutic frontier leveraging EMFs-iron-calcium crosstalk. By integrating these dimensions, we propose an integrative framework that reconciles EMFs’ dual roles in PCa pathobiology. In this paper, we not only elucidate how electromagnetic parameters dictate pro-tumorigenic versus tumor-suppressive outcomes but also establish calcium signaling as the nodal hub coordinating these effects. This perspective aims to guide the development of electromagnetic-based precision therapies while providing a mechanistic basis to mitigate potential microenvironmental risks in advanced PCa management.
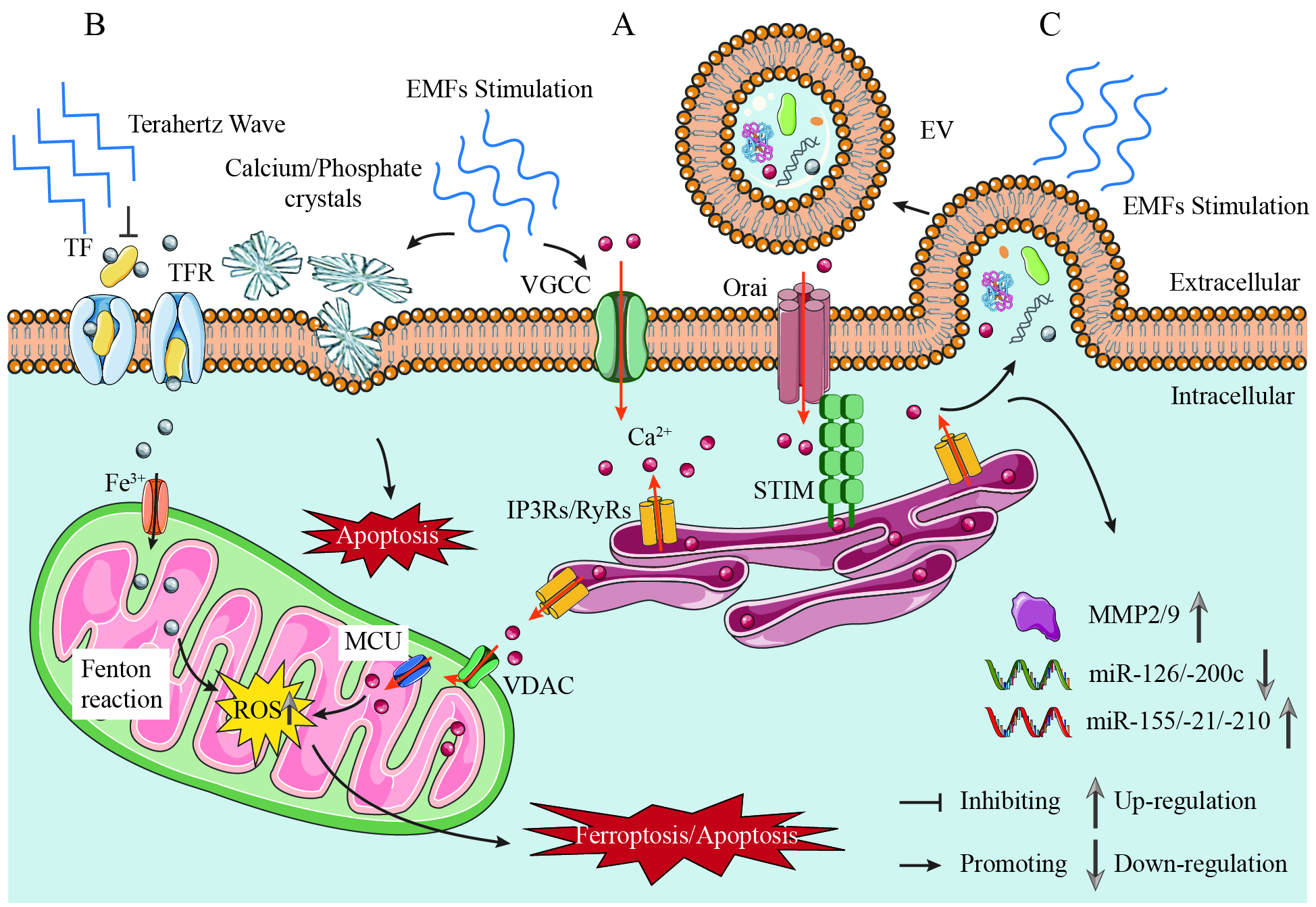
Figure 1. Mechanisms of EMF Regulation in Prostate Cancer: Calcium Signaling, Extracellular Vesicle Reprogramming, and Ferroptosis Modulation. (A) Calcium signaling as the central nodal effector. EMFs induce piezoelectric effects in prostatic calcium/phosphate crystals, generating mechanical stress that activates VGCCs, thereby initiating extracellular Ca2+ influx and intracellular signaling cascades, such as calcium-induced calcium release (CICR). EMFs (Low-Frequency) amplify this process, driving mitochondrial membrane depolarization and ROS overproduction, which synergizes with iron-dependent lipid peroxidation to potentiate ferroptosis; (B) Frequency-dependent ferroptosis modulation. EMFs (Low-Frequency) promote ferroptosis via the Ca2+-ROS-Fe2+ axis, inducing lipid peroxidation and oxidative damage. Terahertz Waves (34.5 THz) suppress ferroptosis by inhibiting iron-transferrin binding; (C)
ELECTROMAGNETIC-CA2+ CROSSTALK IN PROSTATE CANCER PATHOBIOLOGY
Calcium signaling drives the oncogenic progression of PCa through multifaceted mechanisms[23]. Dysregulated calcium fluxes promote malignant progression by disrupting cell cycle regulation, inhibiting apoptosis, and facilitating epithelial-mesenchymal transition (EMT) and cytoskeletal remodeling[23-27]. These processes are mediated by the compartment-specific dysregulation of calcium channel families.
Regulation of calcium channel families in PCa
Overexpression of ORAI calcium release-activated calcium modulator 3 (ORAI3) and its heteromerization with ORAI1 promote PCa cell proliferation by accelerating the cell cycle and inhibiting apoptosis[28]. Elevated extracellular calcium activates the calcium-sensing receptor (CaSR), triggering Akt signaling to stimulate proliferation in skeletal metastatic PCa cells[29]. TRP channels mediate PCa progression through the regulation of apoptosis and transient calcium accumulation. This facilitates metastasis by enhancing cytoskeletal dynamics and cellular invasiveness, particularly promoting bone metastases-a lethal characteristic of advanced PCa[24,30]. The piezo-type mechanosensitive ion channel component 1 (Piezo1) regulates calcium flux, driving EMT and increasing metastatic potential[31]. Furthermore, upregulation of voltage-gated Ca2+ channels (VGCCs), particularly CaV1.3, promotes resistance to androgen deprivation therapy (ADT) and the progression of castration-resistant prostate cancer (CRPC)[32].
Remodeling of calcium signaling is closely associated with the development of hormone-sensitive, castration-resistant, and highly aggressive PCa subtypes, directly regulating the responsiveness of PCa to anti-androgen therapies.
Electromagnetic influence on calcium channels in PCa
Electric fields influence cellular behavior through three primary mechanisms[33]: modifying plasma membrane potentials, redistributing extracellular ions and ionic currents, and generating mechanical forces transmitted intracellularly via the cytoskeleton. VGCCs act as the primary electromagnetic effector, directly converting field-induced membrane depolarization into shifts in the extracellular Ca2+ gradient that initiate intracellular signaling cascades, such as calcium-induced calcium release (CICR)[34] [Figure 1]. These mechanisms converge on calcium signaling as their nodal effector.
Mechanistic studies reveal that low-intensity magnetic fields (LMFs) regulate bioelectric properties through calcium ion flux modulation, altering intracellular calcium distribution to influence membrane polarization states and cell cycle regulation-key drivers of tumor aggressiveness[35-37]. This calcium-electromagnetic interplay further coordinates tumor-microenvironment communication through surface biomarker remodeling[36,38]. This calcium flux may not only mediate immediate bioelectric responses but also underpin EMFs’ dual regulation of stemness, promoting normal stem cell differentiation while inhibiting tumor stemness via cell cycle control[19,39,40].
The interplay between EMFs and calcium signaling constitutes a biomechanical axis in prostate carcinogenesis. Emerging evidence reveals that EMFs may induce piezoelectric effects within prostatic calcifications[41], where mechanical deformation of calcium/phosphate crystals under EMF exposure could alter cellular behavior and potentially enhance expression of apoptosis-inhibiting genes [Figure 1].
Notably, calcium electroporation-a non-thermal ablation method-has shown efficacy in localized PCa treatment. This approach significantly reduces cancer cell viability by inducing calcium overload to promote apoptosis in vitro, while pulsed electric fields concurrently disrupt actin cytoskeletal integrity and impair cellular motility[42]. Collectively, the electromagnetic-calcium crosstalk highlights calcium’s dual role as both mediator and therapeutic target in PCa pathobiology. EMFs may drive malignancy through piezoelectric/ionic calcium dysregulation, while targeted electromagnetic interventions such as electroporation, VGCC channel modulation, and magnetic nanoparticle-mediated modulation[43] can reverse this interplay to disrupt tumor progression. Adjunctive ER stress modulation (e.g., salubrinal) may reduce neuroinflammatory cascades exacerbated by EMF-driven calcium dysregulation[44]. This central axis underscores the critical importance of electromagnetic parameter control to exploit calcium signaling therapeutically while mitigating its oncogenic potential.
ELECTROMAGNETIC REPROGRAMMING OF EXTRACELLULAR VESICLES AND MIRNA NETWORKS
Extracellular vesicles (EVs) are increasingly recognized as key mediators in the development and progression of PCa[45]. They facilitate communication between cancer cells and their environment by transferring bioactive molecules (proteins, microRNAs, mRNAs, DNA, lipids), promoting tumor progression, angiogenesis, immune evasion, and metastasis[46-48]. Notably, microRNAs (miRNAs) transferred via EVs orchestrate critical oncogenic programs. These small non-coding RNAs regulate approximately 30% of protein-coding genes involved in cell differentiation, growth, and apoptosis during PCa pathogenesis[49-51]. By targeting key signaling pathways, including AR signaling, p53 networks, PTEN/PI3K/AKT, and Wnt/β-catenin cascades, miRNAs modulate cancer stemness, cell cycle progression, apoptotic resistance, and metastatic competence. Recent advances in EV engineering highlight their potential as precision nanocarriers for miRNA delivery in oncology, underscoring the clinical relevance of EV-mediated miRNA networks in pathological reprogramming[52]. Their stability in biofluids positions EVs and miRNAs as promising non-invasive biomarkers for PCa diagnosis stratification and treatment response prediction.
Experimental studies demonstrate that metastatic PCa-derived EVs enhance malignant phenotypes by stimulating cancer cell proliferation, migration, and invasion while suppressing apoptosis[53]. They can also induce EMT via TGFβ/androgen receptor signaling to promote invasion and drug resistance[54,55]. These findings position EVs as multiparametric targets for precision diagnostics and therapy. Current therapeutic strategies focus on blocking EV production/uptake[53], intercepting EV-activated pathways (MEK/ERK, TGFβ)[56], and disrupting EV-mediated microenvironmental reprogramming[57]. Notably, engineered EVs show promise as biocompatible drug carriers to overcome therapeutic resistance[53,54], with strategies like peptide-functionalized bacterial EVs demonstrating targeted miRNA delivery efficacy in bone diseases[58], reinforcing the potential of engineered EVs to overcome therapeutic resistance. Combinatorial approaches targeting both EV signaling and conventional therapies may enhance treatment efficacy.
Recent studies reveal that short-term exposure (4 h) to LMFs (20 nT) significantly reduced cellular EV release and modified EV cargoes in PCa cells (specifically PC-3 cells), inducing pro-tumorigenic transformations and significantly enhanced invasiveness[7]. Notably, LMF-induced calcium influx through voltage-gated channels may act as a trigger for EV biogenesis and cargo sorting, as calcium signaling is known to regulate vesicle trafficking and the matrix metalloproteinase (MMP) activation[59-61] [Figure 1]. In LMF-exposed PC-3 cells, EVs exhibit enriched inflammatory and metastatic protein profiles, associated with 16 disease pathways and 95 human phenotypes linked to cancer aggression. This oncogenic reprogramming extends to miRNA dysregulation: LMF exposure markedly upregulates three critical oncogenic miRNAs (miR-155, miR-21, miR-210) while suppressing two tumor-suppressive miRNAs (miR-126, miR-200c), thereby promoting a metastatic profile. In contrast, the normal prostate epithelial cell line PNT2 exhibited reduced EV protein cargo following identical LMF exposure, with fewer associated functional enrichment pathways and minimal alterations in miRNA expression or invasion.
The pro-metastatic effects are further validated by LMF-induced upregulation of zinc-dependent matrix metalloproteinases (MMP2/MMP9)[7] in PC-3 cells compared to PNT2 cells. These enzymes, which are critical for cancer invasion, demonstrate significantly elevated activity in patients with metastatic PCa and are associated with poor prognosis[62,63]. Elevated MMP levels correlate with enhanced basement membrane penetration and metastatic dissemination, while the coordinated regulation of EV-mediated signaling and miRNA networks provides mechanistic evidence for LMF-driven metastatic progression specifically in malignant cells. In contrast, non-ionizing EMFs may impede metastasis by modulating the EMT pathway (VEGFR/PI3K/AKT/MAPK) and by inducing cadherin switching. Notably, amplitude-modulated radiofrequency EMFs have been shown to selectively suppress brain metastasis[21,64,65]. Collectively, these findings establish electromagnetic fields as potent modulators of tumor microenvironment crosstalk, where reprogrammed EV release dynamics synergize with miRNA-driven epigenetic reprogramming to accelerate metastatic cascades in malignant cells like PC-3, while sparing normal counterparts such as PNT2. This electromagnetic-sensitive axis provides a compelling rationale for developing novel therapeutic strategies that specifically target these pathways in advanced PCa and highlights the critical influence of field parameters on metastatic reprogramming, demonstrating how changes in magnetic field exposures modulate EV-mediated and miR-regulatory processes in PCa metastasis.
ELECTROMAGNETIC FIELDS REGULATION OF FERROPTOSIS
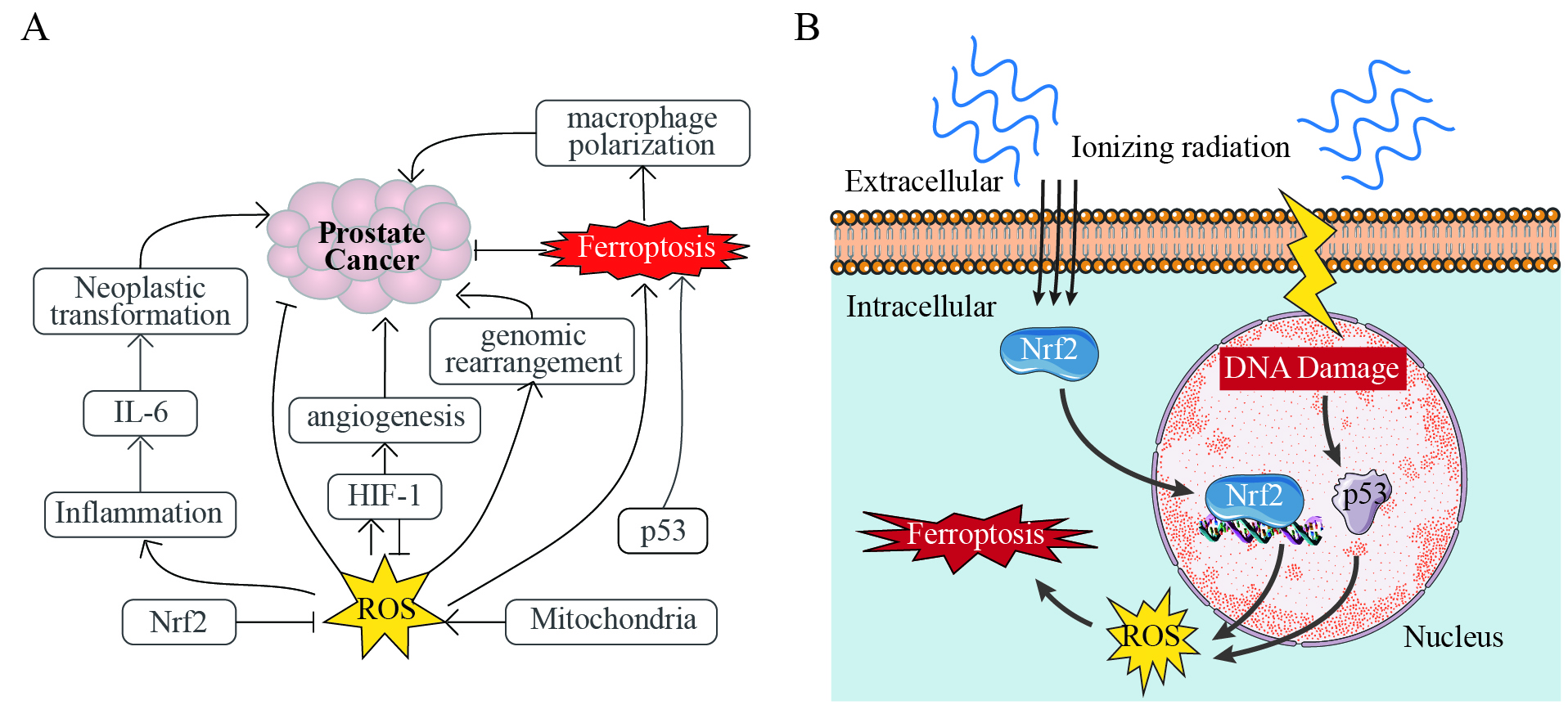
Ferroptosis, a form of programmed cell death characterized by iron overload and lipid peroxidation, has emerged as a promising therapeutic strategy for PCa[66]. Its induction exerts direct tumor-suppressive effects through iron-mediated oxidative damage, yet paradoxically drives pro-tumorigenic adaptations in the tumor microenvironment (TME), particularly in advanced PCa with dysregulated iron metabolism[66,67]. Recent studies highlight the potential of ferroptosis in suppressing PCa metastasis and overcoming drug resistance[66,68], while ferritin degradation may potentiate neurotoxicity through iron-mediated oxidative stress[69]. ROS acts as a central hub connecting ferroptosis to PCa pathogenesis, amplified by inflammatory signals and redox-sensitive transcription factors (e.g., Nrf2, HIF-1α) that either directly enhance oxidative stress or indirectly modulate ferroptosis susceptibility [Figure 2A]. Therapeutically, p53 activation dualistically regulates apoptosis and ferroptosis to suppress PCa growth, but ferroptosis-driven macrophage polarization in the TME may inadvertently promote tumor metastasis [Figure 2A], emphasizing the need for context-specific modulation to balance efficacy and paradoxical outcomes.
Figure 2. Dual Roles of Ferroptosis in Prostate Cancer. (A) Ferroptosis directly suppresses prostate cancer (PCa) through
The regulatory role of EMFs in ferroptosis is increasingly recognized as a critical axis in PCa biology, with distinct effects dictated by frequency, intensity, and waveform parameters. This duality-where specific EMF parameters promote ferroptosis while others inhibit it-underscores the need for precise parameter control. Terahertz waves (ferroptosis-inhibiting EMFs, 34.5 THz) suppress ferroptosis through a direct mechanism targeting iron metabolism: they inhibit iron-transferrin binding, thereby reducing intracellular Fe2+ accumulation, lipid peroxidation, and malondialdehyde (MDA) levels[16] [Figure 1]. This effect is frequency-specific. The high-energy terahertz range disrupts iron-transport protein interactions, reducing intracellular Fe2+ availability. By limiting the iron source for the subsequent lipid peroxidation reaction, it suppresses ferroptosis without relying on significant ROS production, thus highlighting a non-oxidative pathway for ferroptosis inhibition.
In contrast, extremely low frequency electromagnetic fields (ELF-EMFs, ferroptosis-promoting EMFs, operating at 50 Hz and with magnetic field density between 1 μT and 1 mT ± 2%) act as potent ferroptosis promoters by triggering calcium-dependent oxidative stress. ELF-EMFs induce calcium influx and mitochondrial membrane depolarization, synergizing with iron-dependent lipid peroxidation through ROS overproduction[38,70] [Figure 1]. Additionally, electromagnetic exposure-induced calcium influx may destabilize membranes and promote nanopore formation, potentially exacerbating iron-dependent oxidative damage[60,71]. This calcium-ROS interplay disrupts mitochondrial-endoplasmic reticulum coupling, impairing metabolic adaptation and amplifying ferroptosis vulnerability.
Radiotherapy resistance in advanced PCa arises from multifactorial mechanisms, including enhanced DNA repair, cancer stem cell (CSC) persistence, immunosuppressive TME, and metabolic reprogramming[72,73]. These converge to promote ferroptosis suppression (e.g., GPX4/SLC7A11 upregulation) and apoptotic evasion. EMF-based strategies, such as combining ionizing radiation (IR) with ferroptosis inducers[74] or magnetic nanoparticle-guided iron delivery[12,75], utilize electromagnetic bioeffects to selectively sensitize resistant PCa. They achieve this by synchronizing ferroptosis activation with tumor biology while minimizing off-target toxicity.
IR is another electromagnetic stimulus that promotes ferroptosis through ROS-mediated MAPK/Nrf2 signaling and p53-dependent regulation of iron metabolism[74] [Figure 2B]. Unlike terahertz waves, ionizing radiation generates ROS directly. EMF-based therapeutic strategies leverage these mechanisms to enhance PCa treatment. Specifically, IR induces ferroptosis through ROS generation and ACSL4 (a key enzyme in lipid peroxidation) upregulation. When combined with inhibitors of ferroptosis suppressors (SLC7A11/GPX4), synergistic effects are observed, enabling reduced radiation doses[76]. In addition, magnetic fields improve the precision of targeting tumors by directing iron-based nanoparticles to them. These nanoparticles release Fe2+/Fe3+ in acidic lysosomes, thereby inducing ferroptosis[77]. When combined with magnetic field-driven ROS or ELF-EMFs-mediated calcium signaling, this approach enhances tumor suppression in preclinical models. For resistant PCa, PEGylated manganese-zinc ferrite nanocrystals combined with intratumoral micromagnets can enhance drug accumulation and induce ferroptosis. Under external magnetic guidance, they synergize with cGAS-STING-mediated immunogenic cell death to achieve tumoricidal efficacy[12,75]. The efficacy of this strategy has been validated in PCa mouse models fed a high-fat diet[75], highlighting its translational potential.
However, challenges persist. These include addressing resistance mechanisms, optimizing EMF parameters (for example, determining the appropriate ELF frequency and intensity for safe ferroptosis induction, as well as the proper terahertz dosing to avoid unwanted inhibition), and validating combinations with
SAFETY CONSIDERATIONS
Although emerging studies highlight the therapeutic potential of EMF–based interventions, their safety profile requires careful consideration, particularly regarding normal tissue toxicity and potential carcinogenic risks from chronic exposure. Strategies including microwave ablation with pulsed EMFs[13], static high-intensity magnetic fields[15], low-dose alternating electric currents[11], and focused
Beyond therapeutic settings, concerns persist regarding the potential carcinogenic risk of prolonged EMF exposure, especially at certain frequencies and intensities. While the International Agency for Research on Cancer has classified some EMFs as possibly carcinogenic in certain contexts, epidemiological studies remain inconclusive or show mixed results[6,17,78]. Nonetheless, it is prudent to recognize the necessity of long-term monitoring and well-designed cohort studies to clarify any causal relationship, particularly given the latency periods of cancers. A nuanced understanding of safe exposure thresholds and patient-specific susceptibility factors will be critical to ensure that EMF-based modalities do not inadvertently heighten the risk of secondary malignancies or other adverse outcomes.
Furthermore, balancing the therapeutic window requires integrating pharmacologic co-therapies, carefully calibrated frequency-intensity matrices, and longitudinal safety surveillance, particularly in advanced cases such as castration-resistant disease. Through systematic in vitro, in vivo, and clinical investigations that document both efficacy and normal tissue responses, EMF-based therapies may be refined to maximize anti-tumor activity while minimizing unintended toxicity.
CONCLUSIONS
This perspective delineates the parameter-dependent dualistic role of EMFs in PCa pathobiology and therapy through three interconnected axes [Table 2]. First, calcium signaling acts as the central orchestrator, converting electromagnetic stimuli into oncogenic signaling via piezoelectric and ionic mechanisms. Second, EV-mediated communication serves as an electromagnetic amplifier, coupling cargo remodeling with miRNA network dysregulation to drive metastatic progression. Third, the EMFs-ferroptosis axis reveals frequency-dependent therapeutic potential, where calcium signaling may bridge electromagnetic exposure to iron-mediated cell death. Innovatively, this paper provides the first integrative framework bridging calcium signaling, EV, and ferroptosis under electromagnetic regulation. By revealing how EMFs spatiotemporally coordinate these axes [Figure 1], we redefine a new therapeutic paradigm: exploiting electromagnetic bioeffects to simultaneously disrupt oncogenic circuits (e.g., Ca2+-driven stemness,
EMF-mediated mechanisms in cancer pathobiology
| Axis | Key molecules /pathways | Effects of EMFs | Pathological outcome | Ref. |
| Calcium signaling | - VGCC - Orai/STIM - Piezo1 - TRPV6 | - Induce Ca2+ influx - Modulate membrane polarization and EMT - Generate piezoelectric effects | ↑ Proliferation ↑ Therapy resistance ↑ Metastatic | [19-22] [23-28] [34-37] |
| EVs-mediated metastasis | - EVs release and cargo - miR-155/21/210 - miR-126/200c - MMP2/MMP9 | - Alter EVs release patterns and cargo - Upregulate oncogenic miRNAs - Suppress tumor-suppressive miRNAs - Activate MMPs | ↑ Inflammatory signaling ↑ Basement membrane invasion | [7] [38-46] [50-51] |
| Ferroptosis regulation | - Fe2+/Fe3+ - ACSL4/GPX4 - MAPK/Nrf2 - p53 | - Promote ferroptosis via ROS -Inhibit iron-transferrin binding (Terahertz) -Enhance lipid peroxidation | Context-dependent: ↑ Ferroptosis and tumor suppression (ELF-EMFs/IR) ↓ Ferroptosis (Terahertz) | [16] [52-62] |
Additionally, sex hormones (particularly androgens) drive PCa progression by activating oncogenic signaling pathways like AR-dependent transcription. Emerging evidence suggests that EMFs may indirectly modulate PCa biology through sex hormone fluctuations, though effects are context-dependent. In male workers, chronic low-frequency EMF exposure correlates with declining testosterone trends[79,80], while pulsed EMFs elevate testosterone in male mice[81]. EMFs also inhibit testosterone release in porcine endometrial models[82] and suppress testosterone in male rats[83]. Critically, no direct evidence links EMFs to prostaglandin alteration, which is a key mediator of PCa-associated inflammation and angiogenesis. Thus, EMFs’ hormonal influence remains an underexplored dimension in PCa pathobiology. Notably, there has been no research directly focusing on how EMFs regulate the androgen receptor (AR) signaling pathway in PCa. Considering the pivotal role of androgens in driving AR-mediated oncogenesis, it is plausible that EMFs could also exert significant effects on AR signaling. However, elucidation of the underlying mechanisms requires further experimental validation.
Future progress will require: (1) Parameter optimization studies: Systematic in vitro screening of
DECLARATIONS
Authors’ contribution
Writing - original draft, investigation, conceptualization, and funding acquisition: Wang LY
Writing - review and editing: Fan MY, Jiang XY, Bing KJ, Wang YJ, Zhang H
Writing - review and editing, supervision: Wang KS, Huang YM
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Natural Science Basic Research Program of Shaanxi Province (#2023-JC-QN-0239), the National Department of Education Central Universities Research Fund (#GK202207004), the University-Industry Collaborative Education Program (#231005940204433) to Wang LY, and the National Natural Science Foundation of China (#82000512) to Huang YM.
Conflicts of interest
Wang KH serves as a Junior Editorial Board member of Journal of Cancer Metastasis and Treatment. Wang KH was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63.
2. Bergengren O, Pekala KR, Matsoukas K, et al. 2022 update on prostate cancer epidemiology and risk factors-a systematic review. Eur Urol. 2023;84:191-206.
3. Berenguer CV, Pereira F, Câmara JS, Pereira JAM. Underlying features of prostate cancer-statistics, risk factors, and emerging methods for its diagnosis. Curr Oncol. 2023;30:2300-21.
4. Charles LE, Loomis D, Shy CM, et al. Electromagnetic fields, polychlorinated biphenyls, and prostate cancer mortality in electric utility workers. Am J Epidemiol. 2003;157:683-91.
5. Dart D, Koushyar S, Uysal-onganer P. Exploring the potential link between prostate cancer and magnetic fields. Med Hypotheses. 2024;189:111384.
6. Baan R, Grosse Y, Lauby-Secretan B, et al; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12:624-6.
7. Lange S, Inal JM, Kraev I, Dart DA, Uysal-onganer p. low magnetic field exposure alters prostate cancer cell properties. Biology. 2024;13:734.
8. Watson JM, Parrish EA, Rinehart CA. Selective potentiation of gynecologic cancer cell growth in vitro by electromagnetic fields. Gynecol Oncol. 1998;71:64-71.
9. Koh EK, Ryu BK, Jeong DY, Bang IS, Nam MH, Chae KS. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int J Radiat Biol. 2008;84:945-55.
10. Kiełbik A, Szlasa W, Novickij V, et al. Effects of high-frequency nanosecond pulses on prostate cancer cells. Sci Rep. 2021;11:15835.
11. Koreckij TD, Hill C, Azure L, et al. Low dose, alternating electric current inhibits growth of prostate cancer. Prostate. 2010;70:529-39.
12. Wang H, Guan Y, Li C, et al. PEGylated manganese-zinc ferrite nanocrystals combined with intratumoral implantation of micromagnets enabled synergetic prostate cancer therapy via ferroptotic and immunogenic cell death. Small. 2023;19:2207077.
13. Murat C, Kaya A, Kaya D, Erdoğan MA. Experimental study for in vitro prostate cancer treatment with microwave ablation and pulsed electromagnetic field. Electromagn Biol Med. 2024;43:135-44.
14. Attaluri A, Kandala SK, Wabler M, et al. Magnetic nanoparticle hyperthermia enhances radiation therapy: a study in mouse models of human prostate cancer. Int J Hyperthermia. 2015;31:359-74.
15. Rambo Martini A, Neris Cazella L, Martini Y, Viapiana Bossa A, Souza Santos J. Medicinal biomagnetismo in the treatment of prostate cancer: a case study. Health Soc. 2023;3:438-64.
16. Li X, Li Y, Xu J, et al. Terahertz wave desensitizes ferroptosis by inhibiting the binding of ferric ions to the transferrin. ACS Nano. 2025;19:6876-89.
17. Brabant C, Honvo G, Demonceau C, Tirelli E, Léonard F, Bruyère O. Effects of extremely low frequency magnetic fields on animal cancer and DNA damage: a systematic review and meta-analysis. Prog Biophys Mol Biol. 2025;195:137-56.
18. Murad HY, Yu H, Luo D, et al. Mechanochemical disruption suppresses metastatic phenotype and pushes prostate cancer cells toward apoptosis. Mol Cancer Res. 2019;17:1087-101.
19. Ma T, Ding Q, Liu C, Wu H. Electromagnetic fields regulate calcium-mediated cell fate of stem cells: osteogenesis, chondrogenesis and apoptosis. Stem Cell Res Ther. 2023;14:133.
20. Kurup R, Oakes EK, Manning AC, Mukherjee P, Vadlamani P, Hundley HA. RNA binding by ADAR3 inhibits adenosine-to-inosine editing and promotes expression of immune response protein MAVS. J Biol Chem. 2022;298:102267.
21. Sharma S, Wu SY, Jimenez H, et al. Ca2+ and CACNA1H mediate targeted suppression of breast cancer brain metastasis by AM RF EMF. EBioMedicine. 2019;44:194-208.
22. Szasz A. Bioelectromagnetism for cancer treatment-modulated electro-hyperthermia. Curr Oncol. 2025;32:158.
23. Silvestri R, Nicolì V, Gangadharannambiar P, Crea F, Bootman MD. Calcium signalling pathways in prostate cancer initiation and progression. Nat Rev Urol. 2023;20:524-43.
24. Ardura JA, Álvarez-Carrión L, Gutiérrez-Rojas I, Alonso V. Role of calcium signaling in prostate cancer progression: effects on cancer hallmarks and bone metastatic mechanisms. Cancers. 2020;12:1071.
25. Daba MY, Fan Z, Li Q, Yuan X, Liu B. The role of calcium channels in prostate cancer progression and potential as a druggable target for prostate cancer treatment. Crit Rev Oncol Hematol. 2023;186:104014.
26. Marchetti C. Calcium signaling in prostate cancer cells of increasing malignancy. Biomol Concepts. 2022;13:156-63.
27. Srivastava M, Bera A, Eidelman O, et al. A dominant-negative mutant of ANXA7 impairs calcium signaling and enhances the proliferation of prostate cancer cells by downregulating the IP3 receptor and the PI3K/mTOR pathway. Int J Mol Sci. 2023;24:8818.
28. Dubois C, Vanden Abeele F, Lehen'kyi V, et al. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26:19-32.
29. Liao J, Schneider A, Datta NS, McCauley LK. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66:9065-73.
30. Yang D, Kim J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep. 2020;53:125-32.
31. Lopez-Cavestany M, Hahn SB, Hope JM, et al. Matrix stiffness induces epithelial-to-mesenchymal transition via Piezo1-regulated calcium flux in prostate cancer cells. iScience. 2023;26:106275.
32. O’reilly D, Downing T, Kouba S, et al. CaV1. 3 enhanced store operated calcium promotes resistance to androgen deprivation in prostate cancer. bioRxiv 2021. https://www.biorxiv.org/content/10.1101/2021.09.03.458558v1.full (accessed 2025-08-08).
33. McLeod KJ. Microelectrode measurements of low frequency electric field effects in cells and tissues. Bioelectromagnetics. 1992;Suppl 1:161-78.
34. Bo W, Tang J, Ma J, Gong Y. Numerical study on calcium transport through voltage-gated calcium channels in response to nanosecond pulsed electric field. IEEE Trans Plasma Sci. 2018;46:2562-72.
35. Langthaler S, Zumpf C, Rienmüller T, et al. The bioelectric mechanisms of local calcium dynamics in cancer cell proliferation: an extension of the A549 in silico cell model. Front Mol Biosci. 2024;11:1394398.
36. Kaynak A, N'Guessan KF, Patel PH, et al. Electric fields regulate in vitro surface phosphatidylserine exposure of cancer cells via a calcium-dependent pathway. Biomedicines. 2023;11:466.
37. Kim YV, Conover DL, Lotz WG, Cleary SF. Electric field-induced changes in agonist-stimulated calcium fluxes of human HL-60 leukemia cells. Bioelectromagnetics. 1998;19:366-76.
38. Morabito C, Rovetta F, Bizzarri M, Mazzoleni G, Fanò G, Mariggiò MA. Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: a real-time, single-cell approach. Free Radic Biol Med. 2010;48:579-89.
39. Van Huizen AV, Morton JM, Kinsey LJ, et al. Weak magnetic fields alter stem cell-mediated growth. Sci Adv. 2019;5:eaau7201.
40. Alipour M, Hajipour-Verdom B, Javan M, Abdolmaleki P. Static and electromagnetic fields differently affect proliferation and cell death through acid enhancement of ROS generation in mesenchymal stem cells. Radiat Res. 2022;198:384-95.
41. Ghabili K, Shoja MM, Agutter PS. Piezoelectricity and prostate cancer: proposed interaction between electromagnetic field and prostatic crystalloids. Cell Biol Int. 2008;32:688-91.
42. Kiełbik A, Szlasa W, Michel O, et al. In vitro study of calcium microsecond electroporation of prostate adenocarcinoma cells. Molecules. 2020;25:5406.
43. Gorobets O, Gorobets S, Polyakova T, et al. Modulation of calcium signaling and metabolic pathways in endothelial cells with magnetic fields. Nanoscale Adv. 2024;6:1163-82.
44. Lucke-Wold BP, Logsdon AF, Turner RC, Huber JD, Rosen CL. Endoplasmic reticulum stress modulation as a target for ameliorating effects of blast induced traumatic brain injury. J Neurotrauma. 2017;34:S62-70.
45. Hu C, Chen Q, Wu T, et al. The role of extracellular vesicles in the treatment of prostate cancer. Small. 2024;20:2311071.
46. Tai YL, Lin CJ, Li TK, Shen TL, Hsieh JT, Chen BPC. The role of extracellular vesicles in prostate cancer with clinical applications. Endocr Relat Cancer. 2020;27:R133-44.
47. Ludwig M, Rajvansh R, Drake JM. Emerging role of extracellular vesicles in prostate cancer. Endocrinology. 2021:162.
48. Jain DP, Dinakar YH, Kumar H, Jain R, Jain V. The multifaceted role of extracellular vesicles in prostate cancer-a review. Cancer Drug Resist. 2023;6:481-98.
49. Ghafouri-Fard S, Shoorei H, Taheri M. Role of microRNAs in the development, prognosis and therapeutic response of patients with prostate cancer. Gene. 2020;759:144995.
50. Doghish AS, Ismail A, El-Mahdy HA, Elkady MA, Elrebehy MA, Sallam AM. A review of the biological role of miRNAs in prostate cancer suppression and progression. Int J Biol Macromol. 2022;197:141-56.
51. Gujrati H, Ha S, Wang BD. Deregulated microRNAs involved in prostate cancer aggressiveness and treatment resistance mechanisms. Cancers. 2023;15:3140.
52. Liu H, Geng Z, Su J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Extracell Vesicles Circ Nucl Acids. 2022;3:63-86.
53. Lázaro-Ibáñez E, Neuvonen M, Takatalo M, et al. Metastatic state of parent cells influences the uptake and functionality of prostate cancer cell-derived extracellular vesicles. J Extracell Vesicles. 2017;6:1354645.
54. El-Sayed IY, Daher A, Destouches D, et al. Extracellular vesicles released by mesenchymal-like prostate carcinoma cells modulate EMT state of recipient epithelial-like carcinoma cells through regulation of AR signaling. Cancer Lett. 2017;410:100-11.
55. Souza AG, B Silva IB, Campos-Fernández E, et al. Extracellular vesicles as drivers of epithelial-mesenchymal transition and carcinogenic characteristics in normal prostate cells. Mol Carcinog. 2018;57:503-11.
56. Hosseini-Beheshti E, Choi W, Weiswald LB, et al. Exosomes confer pro-survival signals to alter the phenotype of prostate cells in their surrounding environment. Oncotarget. 2016;7:14639-58.
57. Probert C, Dottorini T, Speakman A, et al. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene. 2019;38:1751-63.
58. Liu H, Wu Y, Wang F, et al. Bone-targeted engineered bacterial extracellular vesicles delivering miRNA to treat osteoporosis. Compos Part B Eng. 2023;267:111047.
59. Sun ZC, Ge JL, Guo B, et al. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci Rep. 2016;6:21774.
60. Zhang X, Liu X, Pan L, Lee I. Magnetic fields at extremely low-frequency (50 Hz, 0.8 mT) can induce the uptake of intracellular calcium levels in osteoblasts. Biochem Biophys Res Commun. 2010;396:662-6.
61. Karabakhtsian R, Broude N, Shalts N, Kochlatyi S, Goodman R, Henderson AS. Calcium is necessary in the cell response to EM fields. FEBS Lett. 1994;349:1-6.
62. Gohji K, Fujimoto N, Hara I, et al. Serum matrix metalloproteinase-2 and its density in men with prostate cancer as a new predictor of disease extension. Int J Cancer. 1998;79:96-101.
63. Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998;58:1395-9.
64. Mehdizadeh R, Madjid Ansari A, Forouzesh F, et al. Cross-talk between non-ionizing electromagnetic fields and metastasis; EMT and hybrid E/M may explain the anticancer role of EMFs. Prog Biophys Mol Biol. 2023;182:49-58.
65. Moori M, Norouzian D, Yaghmaei P, Farahmand L. Electromagnetic field as a possible inhibitor of tumor invasion by declining E-cadherin/N-cadherin switching in triple negative breast cancer. Electromagn Biol Med. 2024;43:236-45.
66. Wang S, Wei W, Ma N, Qu Y, Liu Q. Molecular mechanisms of ferroptosis and its role in prostate cancer therapy. Crit Rev Oncol Hematol. 2022;176:103732.
67. Huang M, Teng Q, Cao F, Huang J, Pang J. Ferroptosis and ferroptosis-inducing nanomedicine as a promising weapon in combination therapy of prostate cancer. Biomater Sci. 2024;12:1617-29.
68. Liang J, Liao Y, Wang P, et al. Ferroptosis landscape in prostate cancer from molecular and metabolic perspective. Cell Death Discov. 2023;9:128.
69. Panther EJ, Zelmanovich R, Hernandez J, Dioso ER, Foster D, Lucke-Wold B. Ferritin and Neurotoxicity: a contributor to deleterious outcomes for subarachnoid hemorrhage. Eur Neurol. 2022;85:415-23.
70. Franco-Obregón A. Harmonizing magnetic mitohormetic regenerative strategies: developmental implications of a calcium-mitochondrial axis invoked by magnetic field exposure. Bioengineering. 2023;10:1176.
71. Stejerean-Todoran I, Gibhardt CS, Bogeski I. Calcium signals as regulators of ferroptosis in cancer. Cell Calcium. 2024;124:102966.
72. Wu Y, Song Y, Wang R, Wang T. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer. 2023;22:96.
73. Wisdom AJ, Hong CS, Lin AJ, et al. Neutrophils promote tumor resistance to radiation therapy. Proc Natl Acad Sci U S A. 2019;116:18584-9.
74. Wang F, Dai Q, Xu L, et al. Advances on the role of ferroptosis in ionizing radiation response. Curr Pharm Biotechnol. 2024;25:396-410.
75. Chen J, Wang Y, Han L, et al. A ferroptosis-inducing biomimetic nanocomposite for the treatment of drug-resistant prostate cancer. Mater Today Bio. 2022;17:100484.
76. Lei G, Zhang Y, Koppula P, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146-62.
77. Wang S, Luo J, Zhang Z, et al. Iron and magnetic: new research direction of the ferroptosis-based cancer therapy. Am J Cancer Res. 2018;8 10:1933-46.
78. Brain JD, Kavet R, McCormick DL, et al. Childhood leukemia: electric and magnetic fields as possible risk factors. Environ Health Perspect. 2003;111:962-70.
79. Mohammadi H, Dehghan SF, Moradi N, et al. Assessment of sexual hormones in foundry workers exposed to heat stress and electromagnetic fields. Reprod Toxicol. 2021;101:115-23.
80. Kuzmina LP, Kisljakova AA, Bezrukavnikova LM, Khotuleva AG, Varakuta AL. The influence of electromagnetic fields of industrial frequency on the male reproductive system. Russ J Occup Health Ind Ecol. 2022;62:397-402.
81. Li JH, Jiang DP, Wang YF, et al. Influence of electromagnetic pulse on the offspring sex ratio of male BALB/c mice. Environ Toxicol Pharmacol. 2017;54:155-61.
82. Kozlowska W, Drzewiecka EM, Zmijewska A, Koziorowska A, Franczak A. Effects of electromagnetic field (EMF) radiation on androgen synthesis and release from the pig endometrium during the fetal peri-implantation period. Anim Reprod Sci. 2021;226:106694.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].