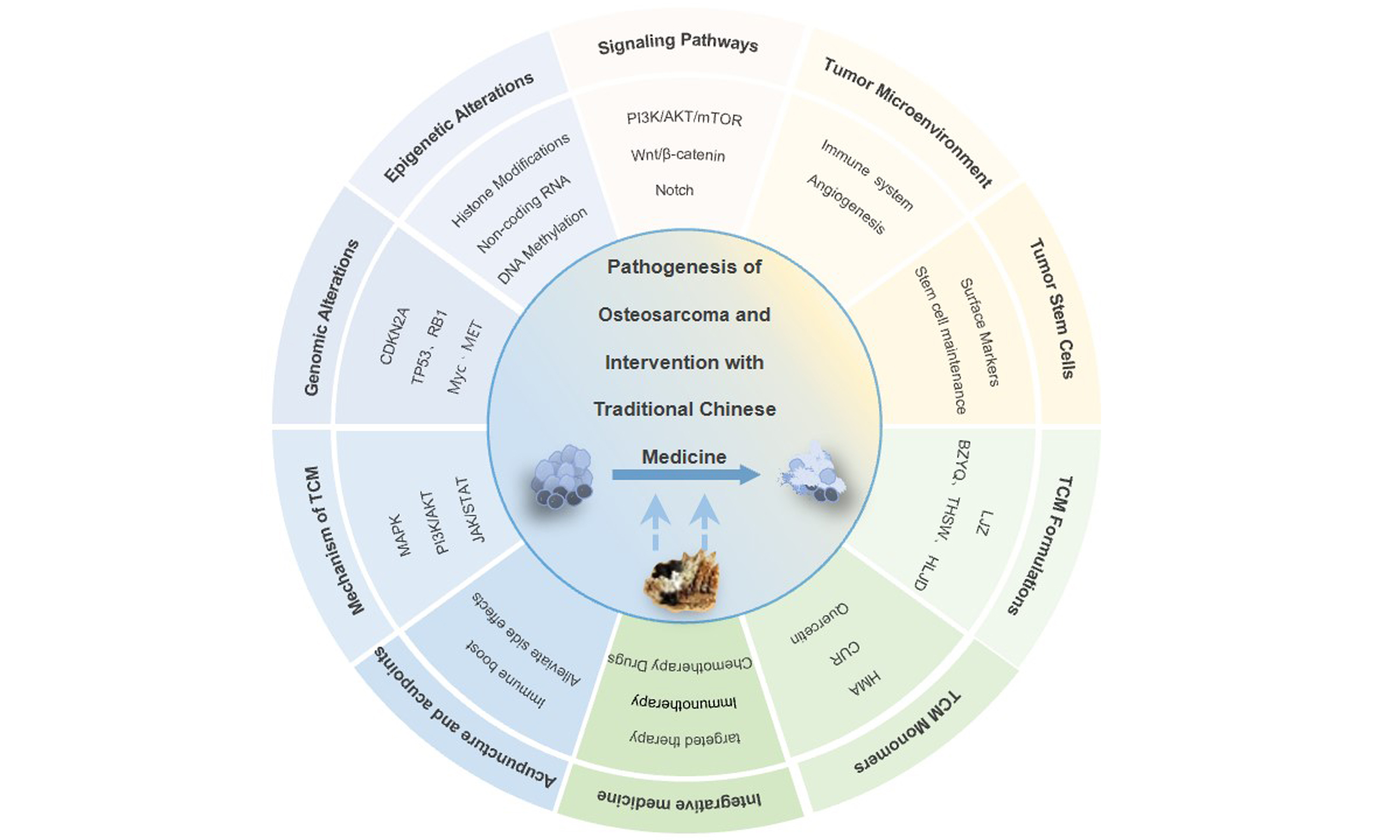
Pathogenesis of osteosarcoma and intervention with traditional chinese medicine
Abstract
Osteosarcoma (OS) is a malignant bone tumor characterized by rapid progression and a high propensity to metastasis. Elucidating the mechanisms underlying cell proliferation and metastasis is crucial to improving prognosis. Recent advances in OS research span multiple dimensions, such as genetic mutations, epigenetic alterations, and aberrant signaling pathways. Additionally, the roles of the tumor microenvironment and cancer stem cells are increasingly recognized. Furthermore, traditional Chinese medicine (TCM) has gained significant attention due to its ability to regulate OS through multiple targets and pathways. Specifically, TCM formulations combat tumor progression via holistic mechanisms. These include reinforcing healthy Qi, eliminating pathogenic factors, promoting blood circulation, resolving stasis, and clearing heat toxicity. The monomeric components of TCM exert antitumor effects by suppressing tumor growth, inducing apoptosis, modulating the immune microenvironment, and reversing drug resistance. Acupuncture has shown efficacy in alleviating
Keywords
INTRODUCTION
Osteosarcoma (OS) is a common primary malignant tumor of the bone tissue, characterized by high malignancy and rapid disease progression, often with lung metastasis13[1]. Its incidence is related to factors such as sex, height, weight, and lifestyle habits[2,3]. Currently, surgery combined with chemotherapy is the primary treatment for OS. With the development of neoadjuvant chemotherapy, the prognosis of patients with OS has improved significantly. However, the mechanisms underlying OS and related lung metastases have not been fully elucidated, which prevents control of OS prevalence and improvement in patient prognosis[4]. Traditional Chinese medicine (TCM) has attracted the attention of researchers due to its remarkable ability to inhibit tumor growth[5], showing great potential for OS treatment. This study reviews recent progress in the multidimensional mechanisms of OS and research on TCM interventions in OS, with the aim of providing constructive insights for the application of TCM in OS diagnosis and treatment.
GENOMIC ALTERATIONS
Inactivation of tumor suppressor genes
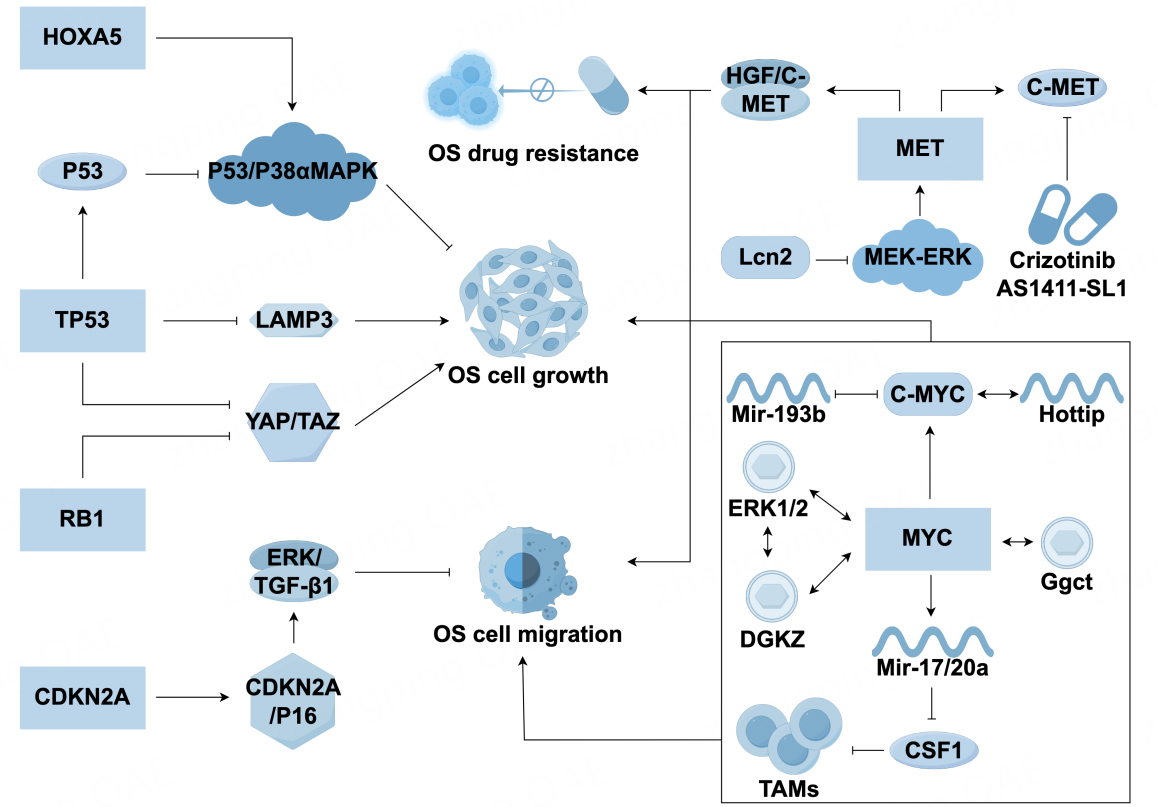
TP53
Early prognostic analysis of patients with OS indicated that patients with TP53 mutations have shorter survival times than those without mutations[6]. Several studies have highlighted a significant role for TP53 mutations in OS[7-9]. Mirabello et al. reported that 74% of adolescent patients with OS had structural variations (SVs) or somatic nucleotide variations in TP53[10]. Chen et al. further confirmed that SVs are common in adolescent OS and are likely to be caused by first intron breakpoint translocation[11]. Mechanisms by which TP53 mutations contribute to the development of OS are gradually being explored. Lysosomal-associated membrane protein 3 (LAMP3) promotes the survival of OS cells, and TP53 affects OS progression by regulating downstream targets such as LAMP3 and HOXA5: knocking out LAMP3 upregulates TP53 and inhibits OS cell survival[12], while abnormal expression of HOXA5 promotes OS apoptosis through the p53/p38α MAPK axis[13]. Additionally, JAB1 accelerates OS formation through p53 overexpression[14] and miRNA regulation (e.g., miR-122-5p[15] inhibits PI3K-Akt-mTOR activation, and miR-181b[16] forms a negative feedback loop with p53), which further influences proliferation and invasion. Other studies have shown that TP53 mutations can inhibit the osteogenic differentiation of mesenchymal stem cells (MSCs) by silencing transcriptional functions, increasing genomic instability, and promoting the secretion of proinflammatory factors, thus fostering tumor growth[17].
RB1
RB1 is the second most mutated gene in OS[18]. The RB1 mutation drives the cell cycle from the G1 to S, leading to genomic instability[19,20]. In mouse models, the Trp53/Rb1 double knockout upregulates YAP/TAZ expression and promotes glucose metabolic reprogramming, thus accelerating OS progression[21,22]. Clinical meta-analyses have shown that RB1 mutations are associated with a reduced histological response to chemotherapy and a high risk of metastasis, suggesting that RB1 may serve as a prognostic marker[23].
CDKN2A
CDKN2A (p16) inhibits tumor cell metastasis by targeting ERK/TGF-β1. CDKN2A mutations, particularly homozygous deletions at somatic mutation sites, are early events in OS. Escape from CDKN2A/p16 cell cycle control and deletion of CDKN2A/p14 disrupts p53 and prevents OS apoptosis[24]. Canine OS models have shown that deletions in the CDKN2A/B locus are associated with susceptibility[25], and artificial intelligence analysis has identified CDKN2A and PDHA1 as specific biomarkers of Cu cell proliferation in OS[26]. CDKN2A inactivation is closely related to the occurrence and development of OS, and further research on its underlying mechanisms is needed.
Activation of proto-oncogenes
Myc
The overexpression of the Myc gene promotes cell proliferation, metabolic reprogramming, and telomerase activity by activating the Myc-associated factor X (MAX) complex[27]. These transcription factors play a role in driving cell cycle progression in OS[28,29]. Myc interacts with extracellular signal-regulated kinase (ERK1/2) and diacylglycerol kinase zeta (DGKZ) to promote malignant phenotypes[30], and the HotTip/c-Myc positive feedback loop accelerates OS progression[31]. Furthermore, the MYC-miR-193b negative feedback loop leads to sustained MYC activation[32], and Myc overexpression downregulates CSF1 through miR-17/20a, thus reducing tumor-associated macrophages[33]. Myc promotes the development of p53-deficient OS by upregulating
Mesenchymal epithelial transition factor
Mesenchymal epithelial transition factor (MET) overexpression drives OS proliferation, metastasis, and drug resistance via the HGF/c-MET pathway[35-37]. Neutrophil-derived cytokine lipocalin-2 (LCN2) can inhibit OS cell metastasis by suppressing the mitogen activated extracellular signal regulated
Other gene mutations
Patients with Rothmund-Thomson syndrome with RecQ like helicase (RECQL) mutations are at a higher risk of OS[41], with mechanisms involving RECQL mutant allele stress[42]. Miller et al. reported that 62% of patients with OS had co-mutations in p53, Rb, and MDM2[43]. Wang et al. confirmed the coexpression of MDM2 and P53 mRNA in OS[44]. Sodium butyrate (SB) and indole-based MDM2 receptor blockers have been shown to inhibit OS cell proliferation by regulating the MDM2-p53 pathway[45,46], further confirming the link between MDM2 and P53. Furthermore, RUNX3 was abnormally upregulated in p53-deficient OS, forming a
EPIGENETIC ALTERATIONS
DNA methylation
Early studies have shown that the incidence of gene methylation in OS is significantly higher than in corresponding normal tissues[49]. The phosphatase and tensin homolog (PTEN), a key tumor suppressor gene, is closely associated with OS progression through methylation-mediated downregulation[50,51]. The methylation inhibitor 5-azacytidine (5-Aza-CdR) can reverse PTEN silencing and induce apoptosis in
Histone modifications
Histone modifications can influence the biological behavior of OS by regulating gene activation
Non-coding RNA
miRNAs regulate the expression of apoptosis-related genes and influence the cell cycle, thus altering proliferation and apoptosis levels. miRNA-296-5p targets Staphylococcal nuclease domain-containing protein 1, exerting tumor-suppressive effects[58]. Machine learning models suggest that miR-34c-3p and miR-154-3p have the potential to diagnose metastatic OS[59]. miRNAs, such as hsa-mir-23a-3p[60],
ABNORMAL SIGNALING PATHWAYS
Wnt/β-catenin pathway
Upregulation of the Wnt/β-catenin pathway is a hallmark of OS[72,73]. This pathway, often in conjunction with cell cycle and apoptosis regulator 2 (CCAR2), elevates levels of secreted protein acidic and rich in cysteine (SPARC), thereby promoting malignant phenotypes in OS[74]. Additionally, Mucin 15 (MUC15) drives OS invasion via Wnt/β-catenin signaling[75]. Biglycan, a class I small leucine-rich proteoglycan, contributes to chemotherapy resistance by activating this pathway and suppressing autophagy[76]. Activation of this pathway is related to the presence of tumor stem cell populations and to the phenotypic transformation of OS cells after chemotherapy, suggesting that this mechanism may confer drug resistance to OS cells. Further reinforcing this concept, further studies have shown that CD44 can influence OS cell biological behavior through the Wnt/β-catenin signaling pathway[77]. As experimental evidence supporting the pathway’s role in therapy resistance and stemness, inhibiting Wnt/β-catenin in MNNG-HOS cells, while combined with doxorubicin, can prevent the upregulation of factors associated with the transition to OS stem cell states[78]. Additionally, receptor tyrosine kinase-like orphan receptor 2 (ROR2), a receptor of the atypical Wnt signaling pathway, was shown to be activated by AKT, promoting OS lung metastasis through anoikis resistance[79].
Notch pathway
The Notch signaling pathway transduces signals only between cells in contact, requiring interaction with other pathways such as PI3K/AKT, NF-κB, integrins, and miRNAs to regulate OS metastasis[80]. Notch promotes OS metastasis by phosphorylating ERK (p-Erk)[81]. Cell migration-inducing protein (CEMIP) and spindle- and kinetochore-associated complex subunit 3 (SKA3) act as downstream effectors driving invasion[82,83]. Jagged1, a key Notch ligand, significantly inhibited OS proliferation and invasion when knocked out[84]. Therefore, the Notch signaling pathway promotes tumor proliferation, invasion, and metastasis by activating ERK phosphorylation and upregulating CEMIP/SKA3, depending on the Jagged1 ligand. Interactions between multiple pathways provide new directions for targeted therapies.
PI3K/AKT/mTOR pathway
PI3K/AKT/mTOR dysregulation promotes OS proliferation and regulates apoptosis and cell cycle[85,86]. The PI3K/Akt/mTOR pathway is involved in resistance to chemotherapy. Notably, lncRNAs are also implicated in stem cell maintenance within this context; specifically, the lncRNA DANCR enhances cancer stem cell (CSC) function by competitively binding miR-33a-5p to upregulate Axl, potentially via the PI3K-Akt pathway[87].
Studies have shown that rapamycin, an mTOR inhibitor, inhibits OS cell proliferation. However, dual inhibition of PI3K/mTOR was more effective than single-target inhibition in inducing apoptosis in primary mouse and human OS cell cultures[88]. Meng et al. confirmed that cisplatin induces autophagy by inhibiting this pathway[89]. Qiu et al.[90] and Chen et al.[91] further confirmed this view. Targeting the PI3K/Akt/mTOR pathway may have pro-apoptotic and antiproliferative effects on OS; however, further clinical studies are required to validate these findings. Beyond PI3K/AKT/mTOR, cross-talk with other pathways offers synergistic therapeutic potential. For example, Jing et al.[92] revealed that quercetin can inhibit OS cell proliferation and immune escape by non-covalently binding to the JH2 domain of JAK2, inhibiting the JAK2-STAT3-PD-L1 signaling axis, and improving its water solubility through folate-modified liposome encapsulation, thus providing a new molecular mechanism and drug delivery strategy for quercetin in OS treatment.
Ferroptosis-related pathways
Ferroptosis is a non-apoptotic form of cell death driven by lipid peroxidation[93]. Several studies have suggested that the promotion of ferroptosis is a promising method to inhibit the development of OS cells. Inhibition of the STAT3/Nrf2/GPX4 signaling pathway can induce ferroptosis, thus improving the sensitivity of OS cells to cisplatin and lipid oxidation[94]. Chen et al.[95] and Yuan et al.[96] further confirmed that the Nrf2/GPX4 signaling pathway could induce ferroptosis in OS cells. Furthermore, inhibition of the STAT3-MGST2 signaling pathway can achieve similar results[97], while inhibiting TFEB/FTH1[98] and activating PI3K/AKT/mTOR[86] have been observed to inhibit ferroptosis in OS cells.
TUMOR MICROENVIRONMENT
Immune cell regulatory network
Tumor-associated macrophages (TAMs) are primarily polarized into two types: classically activated M1 macrophages and alternatively activated M2 macrophages. M2-type TAMs have weaker antigen-presenting capabilities, promote angiogenesis, and help tumor cells achieve immune escape, which is highly correlated with OS invasiveness[99,100]. LncRNA RP11-361F15.2 can reduce the polarization of M2-type TAM by inhibiting cytoplasmic polyadenylation element binding protein 4[69]. After treatment with curcumin and cisplatin, M2-type TAMs are significantly reduced, confirming an association between TAMs and chemotherapy resistance[101]. In addition, physical stimuli such as ultrasound play an important role in immune activation, promoting the conversion of macrophages to the immune-stimulating M1 phenotype[102]. Moreover, TAM-secreted exosomes (e.g., C15orf41)[103] and interleukin-8 (IL-8)[104] have been found to promote OS metastasis. The PD-1/PD-L1 checkpoint is involved in the immune microenvironment of OS and influences tumor-related events[105]. Studies have shown that exosomal PD-L1 levels are significantly higher in OS patients than in healthy individuals and that extracellular PD-L1 levels are significantly higher in patients with OS with lung metastasis than in those without metastasis[106]. PD-L1 immune checkpoint inhibitors are beneficial for the treatment of patients with OS metastases[107], further confirming the importance of PD-L1 in OS. The detection of extracellular PD-L1 and N-cadherin in serum from patients with OS has been reported to predict the progression of lung metastasis[106]. The specific mechanisms of PD-L1 are not fully understood but may involve MSTO2P-mediated upregulation of
Angiogenesis
Vascular endothelial growth factor (VEGF) is an important prognostic factor in OS[111]. Ectopic expression of the RNA-binding protein AUF1 enhances proangiogenic effects in a VEGF-A-dependent manner[112], while overexpression of microRNA-638 can inhibit VEGF expression[113]. circ_001621 regulates VEGF expression by sponging miRNA-578[114]. Another study reported that α-ketoglutarate, a multifunctional intermediate in the Krebs cycle, can enhance the anti-OS effects in vitro by inducing apoptosis through JNK and caspase 9-dependent mechanisms and inhibiting VEGF and TGF-β production and metastatic potential[115].
CSCS
Surface markers
CSC is a promising target for the treatment of OS[116], and CD133 is a core marker of OS stem cells[117]. CD133+ cells downregulate P-gp-mediated resistance to CDDP through the Akt/NF-κB pathway[118].
Stem cell maintenance mechanisms
Activated MSCs maintain their stemness and stimulate cell migration by secreting IL-6[126]. B4GALT1-AS1 recruits HuR to enhance YAP and maintain OS stem cell characteristics[127]. The lncRNA WAC-AS1 can achieve similar effects by sponging miR-5047 to upregulate SOX2[128]. Another study reported that overexpression of small ubiquitin-like modifier-specific peptidase 1 (SENP1) in OS tissues and cell lines significantly reduced stem maintenance capacity and increased sensitivity to HSVtk/GCV[129]. Musculoaponeurotic fibrosarcoma oncogene homolog B can upregulate the stem cell regulator Sox9 at the transcriptional level, playing a key role in OS stem cell tumorsphere formation and tumorigenic capacity[130]. Dual-specificity phosphatase 3 inhibits stem cell differentiation by regulating the EGFR/STAT3/SOX2 axis[131], which could serve as an effective therapeutic target for OS. Table 1 summarizes the pathogenesis of osteosarcoma.
Comprehensive mechanisms of osteosarcoma pathogenesis
| Category | Key elements | Mechanism | Functional impact | References |
| Genomic alterations | TP53 | Somatic mutations/SVs disrupt TP53 function; regulate LAMP3, HOXA5, and miRNA feedback loops | Promotes OS cell survival, inhibits apoptosis, increases genomic instability | [6-17] |
| RB1 | Loss of function drives cell cycle via YAP/TAZ activation and glucose metabolism reprogramming | Enhances metastasis, reduces chemotherapy response, poor prognosis | [18-23] | |
| CDKN2A | Homozygous deletions disrupt p53 and cell cycle control | Early event in OS; linked to chemotherapy resistance and tumorigenesis | [24-26] | |
| Myc | Overexpression activates MAX complex, ERK/DGKZ, and miRNA networks (miR-17/20a) | Promotes OS progression; targeted by crizotinib and AS1411-SL1 | [27-34] | |
| MET | HGF/c-MET pathway activation drives proliferation, metastasis, and drug resistance | Increases OS susceptibility via mutant allele stress | [35-40] | |
| Epigenetic alterations | PTEN methylation | Hypermethylation silences PTEN expression | Promotes OS progression; reversed by 5-Aza-CdR | [50-53] |
| SENP3/FAS methylation | Abnormal methylation enhances OS invasiveness | Impairs DNA repair and apoptosis | [54,55] | |
| H3K27 acetylation | Enriched in the COL6A1 promoter; HDAC inhibitors suppress invasion | Enhances COL6A1 expression and metastasis | [56] | |
| Non-coding RNAs | - miRNA-296-5p targets SND1, miR-34c-3p/miR-154-3p predict metastasis. - lncRNAs (e.g., RP11-361F15.2, HCG18) regulate immune escape and glycolysis | Modulates apoptosis, immune microenvironment, and metabolic reprogramming | [58-71] | |
| Signaling pathway dysregulation | Wnt/β-catenin | Upregulation of SPARC and MUC15 via β-catenin activation | Promotes stemness, invasion, and chemotherapy resistance | [72-76] |
| Notch | Activates ERK phosphorylation and CEMIP/SKA3 to drive metastasis | Enhances invasion; Jagged1 knockout suppresses tumor growth | [80-84] | |
| PI3K/AKT/mTOR | Dysregulation promotes proliferation, inhibits autophagy, and induces cisplatin resistance | Dual PI3K/mTOR inhibition enhances apoptosis | [85-91] | |
| Ferroptosis | STAT3/Nrf2/GPX4 axis inhibition induces lipid peroxidation | Enhances cisplatin sensitivity; curcumin and baicalin promote ferroptosis | [93-98] | |
| Tumor microenvironment | Immune cell network | M2-TAMs secrete IL-8 and exosomal C15orf41; PD-L1 exosomes promote immune escape | Facilitates metastasis and chemotherapy resistance | [100-110] |
| Angiogenesis | VEGF-A/AUF1/miR-638/circ_001621 axis drives vascularization | Correlates with poor prognosis; AKG inhibits VEGF/TGF-β | [111-115] | |
| Cancer stem cells (CSCs) | CD133+/CD44+ cells activate PI3K/AKT, Wnt/β-catenin, and Rac pathways | Mediate lung metastasis, drug resistance, and stemness maintenance | [116-125] | |
| Stemness maintenance | lncRNAs (DANCR, B4GALT1-AS1) and IL-6/MSCs sustain CSC properties | Promotes tumor recurrence and therapy resistance | [126-131] |
RESEARCH PROGRESS ON TCM INTERVENTION IN OS
TCM formulations
Patients with OS often experience adverse reactions, such as bone marrow suppression, cancer-related fatigue, and gastrointestinal discomfort during treatment[132], which severely affect prognosis. TCM formulations based on the principle of holistic regulation have shown increasing influence and unique clinical value for various treatments. A meta-analysis and network pharmacology study suggested that Buzhong Yiqi Tang may alleviate CRF by regulating targets such as AKT1, IL-6, IL-1, PTGS2, CASP3, ESR1, and BCL2, as well as signaling pathways such as TNF, IL-17, TLR, and NF-κB[133]. Liu Jun Zi Tang relieved post-chemotherapy nausea and vomiting[134]. Duan et al.[135] analyzed the main chemical components of Taohong Siwu Tang (THSWD) using UPLC-Q-TOF-MS and confirmed that THSWD exerts antitumor effects by regulating the inflammatory microenvironment and tumor stem cell markers. Huang et al.[136] used network pharmacology to analyze whether Huanglian Jiedu Tang exerts anticancer effects by regulating inflammation-related pathways such as MAPK and TLR. The formulations of TCM are complex, making it difficult to identify the specific active components and elucidate their mechanisms. Current research often adopts an integrated approach involving network pharmacology and experimental validation to reveal the synergistic mechanisms of multiple components and targets in TCM. Future research should explore the synergistic effects of TCM with chemotherapy or targeted therapy and validate their safety and applicability through clinical translation, thus providing new strategies for comprehensive OS treatment.
TCM monomers
Research on the use of TCM monomers in the treatment of OS has gradually gained attention. Quercetin, an important member of the flavonoid family, exhibits antioxidant, anti-inflammatory, and antitumor effects[137,138]. However, its low solubility and poor bioavailability limit its applications[139]. Recent mechanistic studies reveal that Saikosaponin A enhances PD-1 inhibitors by downregulating PD-L1 via FASN inhibition. Its delivery in FA-modified thermosensitive nanohydrogel boosts tumor uptake by 40% while preserving > 90% normal cell viability[140]. Quercetin and saikosaponin A exhibit great clinical translational potential for synergistically enhancing the treatment of osteosarcoma. They achieve this by targeting the PD-L1-associated signaling pathway and surmounting their own limitations with the aid of an innovative delivery system.
Curcumin (CUR), a phenolic antioxidant, exerts anticancer effects by regulating signaling pathways[141]. CUR promotes the nuclear translocation of the transcription factor Nrf2, thereby activating its downstream antioxidant genes (e.g., HMOX1 and NQO1), while simultaneously upregulating the pro-oxidant gene KLF9. This dual action induces excessive production of reactive oxygen species (ROS), causing oxidative DNA damage and promoting apoptosis in OS cells. The low toxicity of CUR in normal cells[142] provides a theoretical basis for ROS-targeted combination therapy. CUR and its analogs, demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC), can induce apoptosis in HOS cells. CUR and DMC triggered
The extract of H. diffusa, 2-hydroxy-3-methylanthraquinone (HMA), inhibits OS cell activity and induces apoptosis[146]. Studies have shown that HMA downregulates MYC expression through the PI3K/AKT signaling pathway, thus inhibiting CHK1 and RAD51 activity and ultimately hindering homologous recombination repair. Astragaloside IV, a natural component extracted from astragalus, has antiproliferative and chemotherapy-sensitizing effects in various cancers. Hu et al. experimentally verified that astragaloside IV enhanced cisplatin-induced apoptosis and increased sensitivity to chemotherapy by activating the
Ginseng is known for its many pharmacological effects, including antitumor effects[149]. Ginsenoside Rg5, an extract of ginseng, was observed through network pharmacology and molecular docking to potentially regulate PI3K/Akt signaling and proteoglycan-related and VEGFA-related pathways, achieving similar effects[150]. Celastrol caused G2/M phase arrest in OS cells by regulating cell cycle-related proteins. It induces apoptosis through extrinsic and intrinsic apoptotic pathways, mitochondria-mediated apoptosis, and the ROS/JNK signaling pathway, achieving synergistic inhibition of OS cell growth with low toxicity to normal fibroblasts[151]. Additionally, in vivo and in vitro pharmacological studies have demonstrated the antitumor activity of Bee venom. The bee toxins in Bee venom have shown potential for the treatment of a wide range of tumors, including OS, while the specific mechanisms remain to be further explored[152]. The mechanisms involved in the treatment of OS with TCM monomers are summarized in Table 2.
Bioactive compounds from natural products demonstrate anti-osteosarcoma potential through elucidated mechanisms
| Compound name | Mechanism of action | Key technological breakthrough | References |
| Quercetin | Inhibits the JAK2-STAT3-PD-L1 axis, blocking tumor immune escape; folate liposomes enhance bioavailability | Development of nanodelivery systems | [92] |
| CUR | Activation of the Nrf2 and Smad2/3 apoptosis pathways; induction of ferroptosis | Synergistic enhancement mechanisms of analogs | [96,141,142] |
| Artemisinin | Inhibition of angiogenesis mediated by the p38 MAPK/CREB pathway | Elucidation of multiple anticancer mechanisms | [145] |
| HMA | Inhibition of CHK1 and RAD51 activity; disruption of homologous recombination repair | Targeting DNA repair pathways | [146] |
| Astragaloside IV | Activation of the Fas/FasL pathway; enhancement of cisplatin sensitivity | Validation of chemosensitization mechanisms | [147] |
| Resveratrol | Induction of ubiquitin degradation and ferroptosis | Research on ferroptosis mechanisms | [154] |
| G-Rg5 | PI3K/Akt signaling pathway; proteoglycan-related pathways; VEGFA-related pathways | Network pharmacology prediction combined with experimental validation | [150] |
| Celastrol | ROS/JNK-mediated apoptosis and autophagy synergy | Research on selective toxicity mechanisms | [151] |
TCM combined with chemotherapy drugs
Enhancing efficacy and reversing drug resistance: Cisplatin resistance poses a significant challenge to OS treatment. To overcome this and enhance chemotherapy effectiveness, diverse strategies leveraging TCM principles and components are being explored. For instance, Groenlandicine, extracted from Coptis, increases sensitivity to cisplatin in cisplatin-resistant OS cells through the BAX/Bcl-2/caspase-9/caspase-3 pathway and inhibits tumor growth by downregulating topoisomerase I (TOP1) levels[153]. Similarly, Polydatin, a precursor of resveratrol, enhances the antitumor effect of paclitaxel by regulating the Akt signaling pathway[154]. Icariin can reverse doxorubicin resistance in human OS cells by inhibiting the
Innovative delivery and synergistic mechanisms further expand the scope of TCM-chemotherapy combinations. Moving beyond traditional formulations, a copper and Tremella fuciformis polysaccharide-based tumor microenvironment-responsive injectable hydrogel (Cu-TFP-gel) demonstrates promise.
Reduction of side effects: chemotherapy can cause various adverse reactions. Danhong injection contains active components such as danshensu and salvianolic acid A/B, which target apoptosis-related proteins like CASP3 and BCL2, inhibit the mitochondrial apoptosis pathway (Bax/Bcl-2 imbalance and caspase-3 activation), and reverse oxidative stress (reducing MDA and increasing SOD/GSH-Px), effectively reducing doxorubicin-induced cardiotoxicity[160]. Angelica polysaccharide, a major active component of Angelica, can promote the proliferation and immunosuppressive function of myeloid-derived suppressor cells through STAT1 and STAT3 signaling pathways[161].
Optimizing OS-targeted therapy with TCM
Therefore, it is important to explore new therapeutic targets and efficient targeting strategies. Ginsenoside Rh2-loaded tumor cell membrane-camouflaged nanoparticles (Rh2@HMnO2-AM) modified with alendronate and K7M2 cell membranes efficiently targeted bone tumors. By responsively releasing Rh2 into the tumor microenvironment, it enhances the effects of immunotherapy[162] and can be used for MRI-guided immunochemodynamic therapy for orthotopic OS. Anti-angiogenesis is an effective antitumor strategy but is limited by drug resistance. Dihydroartemisinin (DHA)[163], a derivative of artemisinin, inhibits VEGFA secretion by downregulating LOXL2 expression and synergizing with anti-angiogenic drugs. Additionally, DHA can reverse anti-angiogenic drug resistance by interfering with lipid metabolic pathways, particularly fatty acid oxidation. Honokiol (HNK)[164], a small-molecule polyphenol, has limited applications due to its poor water solubility and lack of targeting. HA-DOPE@Lips/HNK efficiently targeted drug delivery to tumor cells by specifically binding to CD44 receptors and reducing toxicity to normal tissues. The application of nanomaterials in OS chemotherapy shows great potential[165], providing new strategies for TCM for OS treatment and demonstrating the potential of TCM-modified nanocarriers for targeted drug delivery. To overcome the challenging bone microenvironment in OS therapy, alendronate-based cationic platinum prodrug nanoparticles (Ale NP) employ a cascade-responsive strategy. This system leverages bone targeting and charge reversal for deep tissue penetration, achieves dual drug delivery through
TCM in OS immunotherapy
Tumor occurrence is closely related to individual immune functions. As immunotherapy has gained increasing attention, various components of TCM have been shown to inhibit tumors by regulating the immune system[168]. NGR1 inhibits OS cell proliferation and doxorubicin resistance by inhibiting IL-6 secretion from MSCs and blocking the activation of the JAK2/STAT3 signaling pathway[156]. Revealing the potential role of TCM in the regulation of the tumor microenvironment provides a theoretical basis for the development of TCM-based OS treatment strategies.
Application of acupuncture and acupoint therapy in tumors
Acupuncture is widely accepted among patients with cancer and can alleviate cancer pain[169,170], nausea, and vomiting[171] by stimulating specific acupoints. Acupuncture suppresses systemic inflammation by activating the vagal-adrenal reflex pathway to trigger catecholamine release, while simultaneously inhibiting NF-κB signaling in macrophages via the cholinergic anti-inflammatory pathway through α7 nicotinic acetylcholine receptors, thereby reducing proinflammatory cytokine levels. Furthermore, activation of the
Firstly, acupuncture modulates immune homeostasis by suppressing natural killer cell hyperactivation and attenuating cytotoxic T lymphocyte proliferation. This immunoregulation mitigates systemic stress responses. Secondly, it inhibits critical cytokine storms by significantly blunting pathological elevations in serum TNF-α and IL-6 while normalizing splenic IFN-γ levels toward physiological baselines. Finally, acupuncture restructures gut microbiota composition by reversing the pathological Firmicutes/Bacteroidetes ratio elevation, suppressing proinflammatory genus expansion, and modulating systemic immunity via the gut-immune axis. This multi-targeted regulation constitutes an integrated framework for delaying tumorigenesis through systemic network modulation[173]. Acupuncture can lower serum levels of proinflammatory factors (IL-6, TNF-α, and IFN-γ), partially reverse abnormal CD8+ elevation and CD4+ reduction, and exert systemic antitumor effects by improving gut microbiota imbalance through the “immune-microbiota axis”. Studies have shown that acupuncture can enhance the body’s immune response by regulating the activity of natural killer cells (NK cells), macrophages, mast cells, and microglia and can modulate T and B cell immune responses, enhancing antitumor immunity[174]. Acupuncture is widely accepted among patients with cancer worldwide due to its significant and rapid efficacy. Although physiological and pathological regulatory mechanisms are not fully understood, ongoing research will help to resolve these issues.
Mechanism research on TCM treatment of OS
The PI3K/AKT pathway plays a key role in OS[175]. Important monomers, such as HMA[143] and ginsenosides[149], can inhibit tumors by PI3K/AKT. BDMC, an extract of turmeric rhizomes[176], can significantly downregulate PI3K, p-Akt, NF-κB, and GSK3β expression, inhibiting OS cell migration. Cardamonin[177] inhibits OS through PI3K/AKT and ERK, but its effect is weaker than that of the P38-JNK pathway.
Casticin[178] can upregulate the expression of heme oxygenase 1 (HMOX1), microtubule-associated protein 1 light chain 3 (LC3), and nuclear receptor coactivator 4, thus activating the MAPK signaling pathway. MAPK amplifies ferroptosis by regulating oxidative stress and cell death signals, thus inhibiting tumor growth. Cryptotanshinone[179] and matrine[180] inhibit MAPK pathway activity and alter the expression of cell proliferation-related proteins, thus inhibiting the proliferation of OS cells. Berberine (BBR)[181] activates the p38 MAPK and JNK signaling pathways, inducing apoptosis in MG-63 and U2OS cells. BBR downregulates MMP-2 and MMP-9, inhibits cell migration and invasion, downregulates Bcl-2, upregulates Bax, and activates caspase-3 to promote apoptosis. Furthermore, BBR[182] combined with cisplatin improved the inhibitory effect in MG-63 cells by inhibiting the MAPK signaling pathway. In addition, BDMC and other TCM-active substances can inhibit MAPK signaling[176], affecting cell proliferation and invasion.
Tanshinone I, an extract from Salvia miltiorrhiza, inhibits IL-6-induced JAK1, 2, and STAT3 phosphorylation, blocks JAK/STAT3 signaling pathway activation, inhibits STAT3 binding to target genes (e.g., Bcl-2), reduces anti-apoptotic protein Bcl-2 expression, lowers matrix metalloproteinase MMP2 and MMP9 levels, and indirectly downregulates JAK/STAT3 to inhibit OS[183]. Andrographolide can inhibit the PI3K/AKT pathway, upregulate pro-apoptotic proteins (Bax and caspase-3), downregulate anti-apoptotic proteins (Bcl-2), and arrest the cell cycle (G0/G1 phase) to induce tumor cell apoptosis[184]. β-caryophyllene induces mitochondrial apoptosis by reducing the mitochondrial membrane potential and upregulates JAK1 and STAT3 protein expression[185]. Furthermore, curcumin[186], quercetin[92], and cryptotanshinone[179]can inhibit OS cell proliferation and invasion by inhibiting the JAK2/STAT3 signaling pathway. These findings provide strong evidence for the potential use of TCM monomers as OS therapeutic drugs and offer a theoretical basis for future clinical applications.
SUMMARY AND OUTLOOK
The pathogenesis of OS involves multiple dimensions, including genomics, epigenetics, aberrant signaling pathways, and the tumor microenvironment. Elucidating these mechanisms will lay the groundwork for developing novel therapeutic targets. TCM has demonstrated advantages in multi-target and multi-pathway intervention for OS treatment, particularly showing significant promise in combination with modern medical therapies. However, the clinical translation of current TCM research faces substantial challenges. Foremost is the lack of standardization, encompassing variations in the specific composition and dosage of different herbal formulations, as well as individualized differences in acupuncture point selection and manipulation techniques. These factors significantly compromise the reproducibility and reliability of TCM therapeutic strategies. Additionally, existing studies predominantly rely on in vitro models, which possess inherent limitations in replicating the complex human microenvironment. Furthermore, inconsistent nomenclature for TCM components or therapies across different studies impedes the comparison and integration of findings. Most critically, despite numerous preclinical studies revealing the mechanisms of TCM action, its clinical efficacy urgently requires validation through rigorously designed, large-scale randomized controlled trials.
Looking ahead, advancing the application of TCM in OS therapy necessitates addressing several key issues: First, establishing standardized protocols for TCM formulations and acupuncture practices is imperative to enhance research reproducibility and result comparability. Second, the low bioavailability of active constituents must be resolved, necessitating optimization through novel delivery systems such as nanocarriers. Third, accelerating clinical translation requires designing robust clinical trials, especially investigating synergistic effects when TCM is combined with chemotherapy, targeted therapy, or immunotherapy. Fourth, deepening mechanistic exploration involves actively integrating multi-omics technologies (genomics, proteomics, metabolomics) and incorporating artificial intelligence analysis to systematically dissect the multidimensional network mechanisms by which TCM modulates OS. The ultimate objective is to deeply integrate modern technology into TCM research - not only to clarify its specific targets and pathways in OS treatment but, crucially, to propel its clinical practice through
DECLARATIONS
Authors’ contributions
Conceptualization, investigation, visualization, writing - original draft, writing - review and editing: Liang M, Ni X
Investigation, data curation, writing - original draft: Dong Z
Investigation, validation, writing - original draft: Xue Q
Investigation, Formal Analysis, Writing - Original Draft: Li Z
Supervision, writing - review and editing, project administration: Xia P
Conceptualization, supervision, writing - review and editing, project administration, funding acquisition:
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Nos. 82274559 and 82474545), the China Postdoctoral Science Foundation (Nos. 2024T170247 and 2024M750820), the Natural Science Foundation of Hubei Province (No. 2024AFB1011), the Research Project of Traditional Chinese Medicine of Hubei Provincial Administration of Traditional Chinese Medicine (No. ZY2025Q039), and the Natural Science Foundation of Wuhan City (No. 2024040801020366).
Conflict of interest
Pu F is a Junior Editorial Board member of Journal of Cancer Metastasis and Treatment. Pu F was not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling and decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029-35.
2. Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480-91.
3. Valery PC, Laversanne M, Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control. 2015;26:1127-39.
5. Chang JL, Wang WY, Li YM, et al. Chinese herbal medicine for osteosarcoma in the mouse: a systematic review and meta-analysis. Chin J Integr Med. 2019;25:370-7.
6. Tsuchiya T, Sekine K, Hinohara S, Namiki T, Nobori T, Kaneko Y. Analysis of the p16INK4, p14ARF, p15, TP53, and MDM2 genes and their prognostic implications in osteosarcoma and Ewing sarcoma. Cancer Genet Cytogenet. 2000;120:91-8.
7. Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250-6.
8. Seidinger AL, Mastellaro MJ, Paschoal Fortes F, et al. Association of the highly prevalent TP53 R337H mutation with pediatric choroid plexus carcinoma and osteosarcoma in southeast Brazil. Cancer. 2011;117:2228-35.
9. Shimizu T, Sugihara E, Takeshima H, et al. Depletion of R270C mutant p53 in osteosarcoma attenuates cell growth but does not prevent invasion and metastasis in vivo. Cells. 2022;11:3614.
10. Mirabello L, Yeager M, Mai PL, et al. Germline TP53 variants and susceptibility to osteosarcoma. J Natl Cancer Inst. 2015;107:djv101.
11. Chen X, Bahrami A, Pappo A, et al; St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome Project. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7:104-12.
12. Liu S, Yue J, Du W, Han J, Zhang W. LAMP3 plays an oncogenic role in osteosarcoma cells partially by inhibiting TP53. Cell Mol Biol Lett. 2018;23:33.
13. Chen YQ, Yang TQ, Zhou B, Yang MX, Feng HJ, Wang YL. HOXA5 overexpression promotes osteosarcoma cell apoptosis through the p53 and p38α MAPK pathway. Gene. 2019;689:18-23.
14. Samsa WE, Mamidi MK, Bashur LA, et al. The crucial p53-dependent oncogenic role of JAB1 in osteosarcoma in vivo. Oncogene. 2020;39:4581-91.
15. Li KW, Wang SH, Wei X, Hou YZ, Li ZH. Mechanism of miR-122-5p regulating the activation of PI3K-Akt-mTOR signaling pathway on the cell proliferation and apoptosis of osteosarcoma cells through targeting TP53 gene. Eur Rev Med Pharmacol Sci. 2020;24:12655-66.
16. Wan J, Long F, Zhang C, Liu Y. miR181bp53 negative feedback axis regulates osteosarcoma cell proliferation and invasion. Int J Mol Med. 2020;45:1803-13.
17. Otani S, Date Y, Ueno T, et al. Runx3 is required for oncogenic Myc upregulation in p53-deficient osteosarcoma. Oncogene. 2022;41:683-91.
18. Zoumpoulidou G, Alvarez-Mendoza C, Mancusi C, et al. Therapeutic vulnerability to PARP1,2 inhibition in RB1-mutant osteosarcoma. Nat Commun. 2021;12:7064.
19. van Harn T, Foijer F, van Vugt M, et al. Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 2010;24:1377-88.
20. Ballatori SE, Hinds PW. Osteosarcoma: prognosis plateau warrants retinoblastoma pathway targeted therapy. Signal Transduct Target Ther. 2016;1:16001.
21. Li Y, Yang S, Liu Y, Yang S. Deletion of Trp53 and Rb1 in Ctsk-expressing cells drives osteosarcoma progression by activating glucose metabolism and YAP signaling. MedComm. 2022;3:e131.
22. Li Y, Yang S, Yang S. Verteporfin inhibits the progression of spontaneous osteosarcoma caused by Trp53 and Rb1 deficiency in ctsk-expressing cells via impeding hippo pathway. Cells. 2022;11:1361.
23. Ren W, Gu G. Prognostic implications of RB1 tumour suppressor gene alterations in the clinical outcome of human osteosarcoma: a meta-analysis. Eur J Cancer Care. 2017;26:e12401.
24. Mohseny AB, Tieken C, van der Velden PA, et al. Small deletions but not methylation underlie CDKN2A/p16 loss of expression in conventional osteosarcoma. Genes Chromosomes Cancer. 2010;49:1095-103.
25. Letko A, Minor KM, Norton EM, et al. Genome-wide analyses for osteosarcoma in leonberger dogs reveal the CDKN2A/B gene locus as a major risk locus. Genes. 2021;12:1964.
26. Jiang J, Zhan X, Wei J, et al. Artificial intelligence reveals dysregulation of osteosarcoma and cuproptosis-related biomarkers, PDHA1, CDKN2A and neutrophils. Sci Rep. 2023;13:4927.
27. Shaikh AB, Li F, Li M, et al. Present advances and future perspectives of molecular targeted therapy for osteosarcoma. Int J Mol Sci. 2016;17:506.
29. Walz S, Lorenzin F, Morton J, et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature. 2014;511:483-7.
30. Yu W, Tang L, Lin F, Yao Y, Shen Z. DGKZ acts as a potential oncogene in osteosarcoma proliferation through its possible interaction with ERK1/2 and MYC pathway. Front Oncol. 2018;8:655.
31. Tang Y, Ji F. lncRNA HOTTIP facilitates osteosarcoma cell migration, invasion and epithelial-mesenchymal transition by forming a positive feedback loop with c-Myc. Oncol Lett. 2019;18:1649-56.
32. Gao J, Ma S, Yang F, et al. miR193b exhibits mutual interaction with MYC, and suppresses growth and metastasis of osteosarcoma. Oncol Rep. 2020;44:139-55.
33. Nirala BK, Patel TD, Kurenbekova L, et al. MYC regulates CSF1 expression via microRNA 17/20a to modulate tumor-associated macrophages in osteosarcoma. JCI Insight. 2023;8:e164947.
34. Ueno T, Otani S, Date Y, et al. Myc upregulates Ggct, γ-glutamylcyclotransferase to promote development of p53-deficient osteosarcoma. Cancer Sci. 2024;115:2961-71.
35. Wen J, Xie Y, Zhang Y, et al. MACC1 Contributes to the development of osteosarcoma through regulation of the HGF/c-Met pathway and microtubule stability. Front Cell Dev Biol. 2020;8:825.
36. Kawano M, Tanaka K, Itonaga I, Iwasaki T, Kubota Y, Tsumura H. The anti-oncogenic effect of 17-DMAG via the inactivation of HSP90 and MET pathway in osteosarcoma cells. Oncol Res. 2023;31:631-43.
37. Patanè S, Avnet S, Coltella N, et al. MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res. 2006;66:4750-7.
38. Lu KH, Yang JS, Hsieh YH, et al. Lipocalin-2 inhibits osteosarcoma cell metastasis by suppressing MET expression via the MEK-ERK pathway. Cancers. 2021;13:3181.
39. Jia T, Cai M, Wang Z, Chen T. Anticancer effect of crizotinib on osteosarcoma cells by targeting c-Met signaling pathway. Cell Mol Biol. 2023;69:174-8.
40. Fu X, Huang J, Chen X, et al. Development of dual aptamers-functionalized c-MET PROTAC degraders for targeted therapy of osteosarcoma. Theranostics. 2025;15:103-21.
41. Wang LL, Gannavarapu A, Kozinetz CA, et al. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J Natl Cancer Inst. 2003;95:669-74.
42. Ng AJ, Walia MK, Smeets MF, et al. The DNA helicase recql4 is required for normal osteoblast expansion and osteosarcoma formation. PLoS Genet. 2015;11:e1005160.
43. Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koeffler HP. Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol. 1996;122:559-65.
44. Wang X, Wang S, Feng C. Detection of p53 and MDM2 gene expression in osteosarcoma with biotin-labelled in situ. Chin J Surg. 1997;35(3):178-180.
45. Xie C, Wu B, Chen B, et al. Histone deacetylase inhibitor sodium butyrate suppresses proliferation and promotes apoptosis in osteosarcoma cells by regulation of the MDM2-p53 signaling. Onco Targets Ther. 2016;9:4005-13.
46. Skalniak L, Twarda-Clapa A, Neochoritis CG, et al. A fluorinated indole-based MDM2 antagonist selectively inhibits the growth of p53wt osteosarcoma cells. FEBS J. 2019;286:1360-74.
48. Omori K, Otani S, Date Y, et al. C/ebpα represses the oncogenic Runx3-Myc axis in p53-deficient osteosarcoma development. Oncogene. 2023;42:2485-94.
49. Hou P, Ji M, Yang B, et al. Quantitative analysis of promoter hypermethylation in multiple genes in osteosarcoma. Cancer. 2006;106:1602-9.
50. Lopez C, Abuel-Haija M, Pena L, Coppola D. Novel germline PTEN mutation associated with cowden syndrome and osteosarcoma. Cancer Genomics Proteomics. 2018;15:115-20.
51. Zhou J, Xiao X, Wang W, Luo Y. Association between PTEN and clinical-pathological features of osteosarcoma. Biosci Rep. 2019;39:BSR20190954.
52. Song D, Ni J, Xie H, Ding M, Wang J. DNA demethylation in the PTEN gene promoter induced by 5-azacytidine activates PTEN expression in the MG-63 human osteosarcoma cell line. Exp Ther Med. 2014;7:1071-6.
53. Zhang Y, Liu Z, Yang X, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11:1473-92.
54. Yang P, Liu Y, Qi YC, Lian ZH. High SENP3 expression promotes cell migration, invasion, and proliferation by modulating DNA methylation of E-cadherin in osteosarcoma. Technol Cancer Res Treat. 2020;19:1533033820956988.
55. Sun JM, Chow WY, Xu G, et al. The role of FAS receptor methylation in osteosarcoma metastasis. Int J Mol Sci. 2023;24:12155.
56. Wang Y, Qin N, Zhao C, et al. The correlation between the methylation of PTEN gene and the apoptosis of osteosarcoma cells mediated by SeHA nanoparticles. Colloids Surf B Biointerfaces. 2019;184:110499.
57. Kong D, Ying B, Zhang J, Ying H. PCAF regulates H3 phosphorylation and promotes autophagy in osteosarcoma cells. Biomed Pharmacother. 2019;118:109395.
58. Huang YZ, Zhang J, Shen JJ, Zhao TX, Xu YJ. miRNA-296-5p functions as a potential tumor suppressor in human osteosarcoma by targeting SND1. Chin Med J. 2021;134:564-72.
59. Abedi S, Behmanesh A, Mazhar FN, et al. Machine learning and experimental analyses identified miRNA expression models associated with metastatic osteosarcoma. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167357.
60. Yang D, Chen Y, He ZNT, et al. Indoleamine 2,3-dioxygenase 1 promotes osteosarcoma progression by regulating tumor-derived exosomal miRNA hsa-miR-23a-3p. Front Pharmacol. 2023;14:1194094.
61. Shan HJ, Zhu LQ, Yao C, et al. MAFG-driven osteosarcoma cell progression is inhibited by a novel miRNA miR-4660. Mol Ther Nucleic Acids. 2021;24:385-402.
62. Luo P, Zhang YD, He F, et al. HIF-1α-mediated augmentation of miRNA-18b-5p facilitates proliferation and metastasis in osteosarcoma through attenuation PHF2. Sci Rep. 2022;12:10398.
63. Liu SH, Zhu JW, Xu HH, et al. A novel antisense long non-coding RNA SATB2-AS1 overexpresses in osteosarcoma and increases cell proliferation and growth. Mol Cell Biochem. 2017;430:47-56.
64. Li JP, Liu LH, Li J, et al. Microarray expression profile of long noncoding RNAs in human osteosarcoma. Biochem Biophys Res Commun. 2013;433:200-6.
65. Gong H, Tao Y, Xiao S, et al. LncRNA KIAA0087 suppresses the progression of osteosarcoma by mediating the SOCS1/JAK2/STAT3 signaling pathway. Exp Mol Med. 2023;55:831-43.
66. Pan X, Guo J, Liu C, et al. LncRNA HCG18 promotes osteosarcoma growth by enhanced aerobic glycolysis via the miR-365a-3p/PGK1 axis. Cell Mol Biol Lett. 2022;27:5.
67. Shen Y, Xu J, Pan X, et al. LncRNA KCNQ1OT1 sponges miR-34c-5p to promote osteosarcoma growth via ALDOA enhanced aerobic glycolysis. Cell Death Dis. 2020;11:278.
68. Yang D, Liu K, Fan L, et al. LncRNA RP11-361F15.2 promotes osteosarcoma tumorigenesis by inhibiting M2-Like polarization of tumor-associated macrophages of CPEB4. Cancer Lett. 2020;473:33-49.
69. Xie W, Ma F, Dou L, et al. Allicin affects immunoreactivity of osteosarcoma cells through lncRNA CBR3-AS1. Heliyon. 2024;10:e31971.
70. Tang N, Chen Y, Su Y, Zhang S, Huang T. The role of disulfidptosis-associated LncRNA-LINC01137 in osteosarcoma biology and its regulatory effects on macrophage polarization. Funct Integr Genomics. 2024;24:219.
71. Li R, Chen P, Zhou Y, et al. LncRNA HOXA-AS3 promotes cell proliferation and invasion via targeting miR-218-5p/FOXP1 axis in osteosarcoma. Sci Rep. 2024;14:16581.
72. Tao H, Chen F, Liu H, Hu Y, Wang Y, Li H. Wnt/β-catenin signaling pathway activation reverses gemcitabine resistance by attenuating Beclin1-mediated autophagy in the MG63 human osteosarcoma cell line. Mol Med Rep. 2017;16:1701-6.
73. Wang Q, Liu H, Wang Q, et al. Involvement of c-Fos in cell proliferation, migration, and invasion in osteosarcoma cells accompanied by altered expression of Wnt2 and Fzd9. PLoS One. 2017;12:e0180558.
74. Chen L, Zhou SJ, Xu Y, Liao QM, Zou YS, Pei H. CCAR2 promotes a malignant phenotype of osteosarcoma through Wnt/β-catenin-dependent transcriptional activation of SPARC. Biochem Biophys Res Commun. 2021;580:67-73.
75. Chen T, Chen Z, Lian X, et al. MUC 15 promotes osteosarcoma cell proliferation, migration and invasion through livin, MMP-2/MMP-9 and Wnt/β-catenin signal pathway. J Cancer. 2021;12:467-73.
76. Giatagana EM, Berdiaki A, Gaardløs M, Tsatsakis AM, Samsonov SA, Nikitovic D. Rapamycin-induced autophagy in osteosarcoma cells is mediated via the biglycan/Wnt/β-catenin signaling axis. Am J Physiol Cell Physiol. 2022;323:C1740-56.
77. Ji H, Kong L, Wang Y, et al. CD44 expression is correlated with osteosarcoma cell progression and immune infiltration and affects the Wnt/β-catenin signaling pathway. J Bone Oncol. 2023;41:100487.
78. Martins-Neves SR, Paiva-Oliveira DI, Wijers-Koster PM, et al. Chemotherapy induces stemness in osteosarcoma cells through activation of Wnt/β-catenin signaling. Cancer Lett. 2016;370:286-95.
79. Tran DTP, Kuchimaru T, Pongsuchart M, et al. ROR2 regulates the survival of murine osteosarcoma cells in lung capillaries. J Cancer Metastasis Treat. 2020:2020.
80. Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931-5.
81. Qin J, Wang R, Zhao C, et al. Notch signaling regulates osteosarcoma proliferation and migration through Erk phosphorylation. Tissue Cell. 2019;59:51-61.
82. Cheng J, Zhang Y, Wan R, et al. CEMIP promotes osteosarcoma progression and metastasis through activating notch signaling pathway. Front Oncol. 2022;12:919108.
83. Liang G, Duan C, He J, Shi L. Spindle and kinetochore-related complex subunit 3 has a protumour function in osteosarcoma by activating the Notch pathway. Toxicol Appl Pharmacol. 2024;483:116826.
84. Zhang J, Li N, Lu S, et al. The role of Notch ligand Jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J Orthop Surg Res. 2021;16:226.
85. Yun HM, Kim SH, Kwon YJ, Park KR. Effect of spicatoside a on anti-osteosarcoma MG63 cells through reactive oxygen species generation and the inhibition of the PI3K-AKT-mTOR pathway. Antioxidants. 2024;13:1162.
86. Huang X, Xia K, Wei Z, Liu W, Wei Z, Guo W. SLC38A5 suppresses ferroptosis through glutamine-mediated activation of the PI3K/AKT/mTOR signaling in osteosarcoma. J Transl Med. 2024;22:1004.
87. Jiang N, Wang X, Xie X, et al. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46-55.
88. Zhu J, Sun Y, Lu Y, et al. Glaucocalyxin a exerts anticancer effect on osteosarcoma by inhibiting GLI1 nuclear translocation via regulating PI3K/Akt pathway. Cell Death Dis. 2018;9:708.
89. Meng CY, Zhao ZQ, Bai R, et al. MicroRNA22 mediates the cisplatin resistance of osteosarcoma cells by inhibiting autophagy via the PI3K/Akt/mTOR pathway. Oncol Rep. 2020;43:1169-86.
90. Qiu C, Su W, Shen N, et al. MNAT1 promotes proliferation and the chemo-resistance of osteosarcoma cell to cisplatin through regulating PI3K/Akt/mTOR pathway. BMC Cancer. 2020;20:1187.
91. Chen Z, Ni R, Hu Y, Yang Y, Tian Y. Arnicolide D inhibits proliferation and induces apoptosis of osteosarcoma cells through PI3K/Akt/mTOR pathway. Anticancer Agents Med Chem. 2024;24:1288-94.
92. Jing D, Wu W, Chen X, et al. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 Via the JAK2-STAT3-PDL1. Pharmacol Res. 2022;182:106287.
93. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72.
94. Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43:1245-56.
95. Chen W, Li Z, Yu N, et al. Bone-targeting exosome nanoparticles activate Keap1 / Nrf2 / GPX4 signaling pathway to induce ferroptosis in osteosarcoma cells. J Nanobiotechnology. 2023;21:355.
96. Yuan C, Fan R, Zhu K, Wang Y, Xie W, Liang Y. Curcumin induces ferroptosis and apoptosis in osteosarcoma cells by regulating Nrf2/GPX4 signaling pathway. Exp Biol Med. 2023;248:2183-97.
97. Li Y, Bai X. Naringenin induces ferroptosis in osteosarcoma cells through the STAT3-MGST2 signaling pathway. J Bone Oncol. 2025;50:100657.
98. Shao Y, Zuo X. PTPRC inhibits ferroptosis of osteosarcoma cells via blocking TFEB/FTH1 signaling. Mol Biotechnol. 2024;66:2985-94.
99. Cersosimo F, Lonardi S, Bernardini G, et al. Tumor-associated macrophages in osteosarcoma: from mechanisms to therapy. Int J Mol Sci. 2020;21:5207.
100. Li Y, Li M, Wei R, Wu J. Identification and functional analysis of EPOR+ tumor-associated macrophages in human osteosarcoma lung metastasis. J Immunol Res 2020;2020:9374240.[PMID:32908942 DOI:10.1155/2020/9374240 PMCID:PMC7450330] Caution!.
101. Wang J, Jin J, Chen T, Zhou Q. Curcumol synergizes with cisplatin in osteosarcoma by inhibiting M2-like polarization of tumor-associated macrophages. Molecules. 2022;27:4345.
102. Guo Z, Saw PE, Jon S. Non-invasive physical stimulation to modulate the tumor microenvironment: unveiling a new frontier in cancer therapy. BIO Integr. 2024;5:1-14.
103. Yan CF, Xia J, Qun WS, et al. Tumor-associated macrophages-derived exo-let-7a promotes osteosarcoma metastasis via targeting C15orf41 in osteosarcoma. Environ Toxicol. 2023;38:1318-31.
104. Tatsuno R, Ichikawa J, Komohara Y, et al. Pivotal role of IL-8 derived from the interaction between osteosarcoma and tumor-associated macrophages in osteosarcoma growth and metastasis via the FAK pathway. Cell Death Dis. 2024;15:108.
105. Hashimoto K, Nishimura S, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in osteosarcoma. Diagnostics. 2020;10:528.
106. Wang J, Zhang H, Sun X, et al. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J Nanobiotechnology. 2020;18:151.
107. Toda Y, Kohashi K, Yamada Y, et al. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J Cancer Res Clin Oncol. 2020;146:2607-20.
108. Shi C, Huang CM, Wang B, Sun TF, Zhu AX, Zhu YC. Pseudogene MSTO2P enhances hypoxia-induced osteosarcoma malignancy by upregulating PD-L1. Biochem Biophys Res Commun. 2020;530:673-9.
109. Zheng S, Wei Y, Jiang Y, Hao Y. LRP8 activates STAT3 to induce PD-L1 expression in osteosarcoma. Tumori. 2021;107:238-46.
110. Lin J, Xu A, Jin J, et al. MerTK-mediated efferocytosis promotes immune tolerance and tumor progression in osteosarcoma through enhancing M2 polarization and PD-L1 expression. Oncoimmunology. 2022;11:2024941.
111. Wu H, Zhang J, Dai R, Xu J, Feng H. Transferrin receptor-1 and VEGF are prognostic factors for osteosarcoma. J Orthop Surg Res. 2019;14:296.
112. Al-Khalaf HH, Aboussekhra A. AUF1 positively controls angiogenesis through mRNA stabilization-dependent up-regulation of HIF-1α and VEGF-A in human osteosarcoma. Oncotarget. 2019;10:4868-79.
113. Xue M, Shen J, Cui J, et al. MicroRNA-638 expression change in osteosarcoma patients via PLD1 and VEGF expression. Exp Ther Med 2019;17:3899-906.[PMID:30988774 DOI:10.3892/etm.2019.7429 PMCID:PMC6447936] Caution!.
114. Ji X, Shan L, Shen P, He M. Circular RNA circ_001621 promotes osteosarcoma cells proliferation and migration by sponging miR-578 and regulating VEGF expression. Cell Death Dis. 2020;11:18.
115. Kaławaj K, Sławińska-Brych A, Mizerska-Kowalska M, et al. Alpha ketoglutarate exerts in vitro anti-osteosarcoma effects through inhibition of cell proliferation, induction of apoptosis via the JNK and caspase 9-dependent mechanism, and suppression of TGF-β and VEGF production and metastatic potential of cells. Int J Mol Sci. 2020;21:9406.
116. Jubelin C, Muñoz-Garcia J, Cochonneau D, Moranton E, Heymann MF, Heymann D. Biological evidence of cancer stem-like cells and recurrent disease in osteosarcoma. Cancer Drug Resist. 2022;5:184-98.
117. Li J, Zhong XY, Li ZY, et al. CD133 expression in osteosarcoma and derivation of CD133+ cells. Mol Med Rep. 2013;7:577-84.
118. Li K, Li X, Tian J, Wang H, Pan J, Li J. Downregulation of DNA-PKcs suppresses P-gp expression via inhibition of the Akt/NF-κB pathway in CD133-positive osteosarcoma MG-63 cells. Oncol Rep. 2016;36:1973-80.
119. Wang JH, Gong C, Guo FJ, et al. Knockdown of STIP1 inhibits the invasion of CD133positive cancer stemlike cells of the osteosarcoma MG63 cell line via the PI3K/Akt and ERK1/2 pathways. Int J Mol Med. 2020;46:2251-9.
120. Xu N, Kang Y, Wang W, Zhou J. The prognostic role of CD133 expression in patients with osteosarcoma. Clin Exp Med. 2020;20:261-7.
121. He A, Yang X, Huang Y, et al. CD133+ CD44+ cells mediate in the lung metastasis of osteosarcoma. J Cell Biochem. 2015;116:1719-29.
122. Shiratori H, Koshino T, Uesugi M, Nitto H, Saito T. Acceleration of lung metastasis by up-regulation of CD44 expression in osteosarcoma-derived cell transplanted mice. Cancer Lett. 2001;170:177-82.
123. Kim CK, Oh S, Kim SJ, Leem SH, Heo J, Chung SH. Correlation of IGF1R expression with ABCG2 and CD44 expressions in human osteosarcoma. Genes Genomics. 2018;40:381-8.
124. Gerardo-Ramírez M, Keggenhoff FL, Giam V, et al. CD44 contributes to the regulation of MDR1 protein and doxorubicin chemoresistance in osteosarcoma. Int J Mol Sci. 2022;23:8616.
125. Wang B, Hu H, Wang X, et al. POLE2 promotes osteosarcoma progression by enhancing the stability of CD44. Cell Death Discov. 2024;10:177.
126. Cortini M, Massa A, Avnet S, Bonuccelli G, Baldini N. Tumor-activated mesenchymal stromal cells promote osteosarcoma stemness and migratory potential via IL-6 secretion. PLoS One. 2016;11:e0166500.
127. Li Z, Wang Y, Hu R, Xu R, Xu W. LncRNA B4GALT1-AS1 recruits HuR to promote osteosarcoma cells stemness and migration via enhancing YAP transcriptional activity. Cell Prolif. 2018;51:e12504.
128. Yang Z, Liu Z, Lu W, Guo H, Chen J, Zhang Y. LncRNA WAC-AS1 promotes osteosarcoma Metastasis and stemness by sponging miR-5047 to upregulate SOX2. Biol Direct. 2023;18:74.
129. Liu F, Li L, Li Y, et al. Overexpression of SENP1 reduces the stemness capacity of osteosarcoma stem cells and increases their sensitivity to HSVtk/GCV. Int J Oncol. 2018;53:2010-20.
130. Chen Y, Wang T, Huang M, et al. MAFB promotes cancer stemness and tumorigenesis in osteosarcoma through a Sox9-mediated positive feedback loop. Cancer Res. 2020;80:2472-83.
131. Wei Z, Zheng D, Xia K, et al. DUSP3 restrains the progression and stemness property of osteosarcoma through regulating EGFR/STAT3/SOX2 axis. Int J Biol Sci. 2025;21:160-74.
132. Adel N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am J Manag Care. 2017;23(14 Suppl):S259-S265.
133. Zeng J, Wu Q, Meng XD, Wang J. Systematic review of Buzhong Yiqi method in alleviating cancer-related fatigue: a meta-analysis and exploratory network pharmacology approach. Front Pharmacol. 2024;15:1451773.
134. Morishige KI. Traditional herbal medicine, Rikkunshito, for chemotherapy-induced nausea and vomiting. J Gynecol Oncol. 2017;28:e57.
135. Duan X, Pan L, Bao Q, Peng D. UPLC-Q-TOF-MS study of the mechanism of THSWD for breast cancer treatment. Front Pharmacol. 2019;10:1625.
136. Huang J, Guo W, Cheung F, Tan HY, Wang N, Feng Y. Integrating network pharmacology and experimental models to investigate the efficacy of coptidis and scutellaria containing huanglian jiedu decoction on hepatocellular carcinoma. Am J Chin Med. 2020;48:161-82.
137. Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35:5352-64.
138. Georgiou N, Kakava MG, Routsi EA, et al. Quercetin: a potential polydynamic drug. Molecules. 2023;28:8141.
139. Alizadeh SR, Ebrahimzadeh MA. Quercetin derivatives: drug design, development, and biological activities, a review. Eur J Med Chem. 2022;229:114068.
140. Chen YQ, Yang D, Li K, Liu JS, Feng HJ, Zhou JW. Thermo-responsive nano-hydrogel-based delivery of Saikosaponin a to enhance anti-PD-1 therapy in osteosarcoma. Nanomedicine. 2025;20:1677-91.
141. Wang W, Li M, Wang L, Chen L, Goh BC. Curcumin in cancer therapy: exploring molecular mechanisms and overcoming clinical challenges. Cancer Lett. 2023;570:216332.
142. Xu C, Wang M, Zandieh Doulabi B, Sun Y, Liu Y. Paradox: curcumin, a natural antioxidant, suppresses osteosarcoma cells via excessive reactive oxygen species. Int J Mol Sci. 2023;24:11975.
143. Huang C, Lu HF, Chen YH, Chen JC, Chou WH, Huang HC. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin induced caspase-dependent and -independent apoptosis via Smad or Akt signaling pathways in HOS cells. BMC Complement Med Ther. 2020;20:68.
144. Wen L, Chan BC, Qiu MH, Leung PC, Wong CK. Artemisinin and its derivatives as potential anticancer agents. Molecules. 2024;29:3886.
145. Li Z, Ding X, Wu H, Liu C. Artemisinin inhibits angiogenesis by regulating p38 MAPK/CREB/TSP-1 signaling pathway in osteosarcoma. J Cell Biochem. 2019;120:11462-70.
146. Jing D, Chen X, Zhang Z, et al. 2-Hydroxy-3-methylanthraquinone inhibits homologous recombination repair in osteosarcoma through the MYC-CHK1-RAD51 axis. Mol Med. 2023;29:15.
147. Hu T, Fei Z, Wei N. Chemosensitive effects of Astragaloside IV in osteosarcoma cells via induction of apoptosis and regulation of caspase-dependent Fas/FasL signaling. Pharmacol Rep. 2017;69:1159-64.
148. Wen RJ, Dong X, Zhuang HW, et al. Baicalin induces ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4 regulatory axis. Phytomedicine. 2023;116:154881.
149. Zhang Z, Yu C, Wang H, et al. The history, beneficial ingredients, mechanism, processing, and products of Panax ginseng for medicinal and edible value. Food Med Homol. 2025.
150. Liu MY, Jiang DX, Zhao X, et al. Exploration in the mechanism of ginsenoside Rg5 for the treatment of osteosarcoma by network pharmacology and molecular docking. Orthop Surg. 2024;16:462-70.
151. Li HY, Zhang J, Sun LL, et al. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study. Cell Death Dis. 2015;6:e1604.
152. Ni Y, Zhu Y, Xu L, Duan J, Xiao P. Pharmacological activities and mechanisms of proteins and peptides derived from traditional Chinese medicine. Sci Tradit Chin Med. 2024;2:260-75.
153. Zhao Z, Wu Q, Xu Y, et al. Groenlandicine enhances cisplatin sensitivity in cisplatin-resistant osteosarcoma cells through the BAX/Bcl-2/Caspase-9/Caspase-3 pathway. J Bone Oncol. 2024;48:100631.
154. Zhao W, Chen Z, Guan M. Polydatin enhances the chemosensitivity of osteosarcoma cells to paclitaxel. J Cell Biochem. 2019;120:17481-90.
155. Wang ZD, Wang RZ, Xia YZ, Kong LY, Yang L. Reversal of multidrug resistance by icaritin in doxorubicin-resistant human osteosarcoma cells. Chin J Nat Med. 2018;16:20-8.
156. Lu M, Xie K, Lu X, Lu L, Shi Y, Tang Y. Notoginsenoside R1 counteracts mesenchymal stem cell-evoked oncogenesis and doxorubicin resistance in osteosarcoma cells by blocking IL-6 secretion-induced JAK2/STAT3 signaling. Invest New Drugs. 2021;39:416-25.
157. Xie C, Sun Q, Chen J, et al. Cu-Tremella fuciformis polysaccharide-based tumor microenvironment-responsive injectable gels for cuproptosis-based synergistic osteosarcoma therapy. Int J Biol Macromol. 2024;270:132029.
158. Lu S, Li Y, Yu Y. Glutathione-scavenging celastrol-Cu nanoparticles induce self-amplified cuproptosis for augmented cancer immunotherapy. Adv Mater. 2024;36:2404971.
159. Niu Y, Stadler FJ, He T, Zhang X, Yu Y, Chen S. Smart multifunctional polyurethane microcapsules for the quick release of anticancer drugs in BGC 823 and HeLa tumor cells. J Mater Chem B. 2017;5:9477-81.
160. Yi X, Wang F, Feng Y, Zhu J, Wu Y. Danhong injection attenuates doxorubicin-induced cardiotoxicity in rats via suppression of apoptosis: network pharmacology analysis and experimental validation. Front Pharmacol. 2022;13:929302.
161. Shen J, Zhang M, Zhang K, et al. Effect of angelica polysaccharide on mouse myeloid-derived suppressor cells. Front Immunol. 2022;13:989230.
162. Fu L, Zhang W, Zhou X, Fu J, He C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact Mater. 2022;17:221-33.
163. Ding X, Zhang Y, Liang J, et al. Dihydroartemisinin potentiates VEGFR-TKIs antitumorigenic effect on osteosarcoma by regulating Loxl2/VEGFA expression and lipid metabolism pathway. J Cancer. 2023;14:809-20.
164. Zhang X, Chen H, Zhang Y, et al. HA-DOPE-modified honokiol-loaded liposomes targeted therapy for osteosarcoma. Int J Nanomedicine. 2022;17:5137-51.
165. Yu T, Cai Z, Chang X, et al. Research progress of nanomaterials in chemotherapy of osteosarcoma. Orthop Surg. 2023;15:2244-59.
166. Shen M, Wang Y, Bing T, Tang Y, Liu X, Yu Y. Alendronate triggered dual-cascade targeting prodrug nanoparticles for enhanced tumor penetration and STING activation of osteosarcoma. Adv Funct Mater. 2023;33:2307013.
167. Zhang Y, You P, Liu R, et al. Artificial intelligence in clinical trials of lung cancer: current and future prospects. Intell Oncol. 2025;1:34-51.
168. Wang Y, Zhang Q, Chen Y, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570.
169. Yang J, Wahner-Roedler DL, Zhou X, et al. Acupuncture for palliative cancer pain management: systematic review. BMJ Support Palliat Care. 2021;11:264-70.
170. He Y, Guo X, May BH, et al. Clinical evidence for association of acupuncture and acupressure with improved cancer pain: a systematic review and meta-analysis. JAMA Oncol. 2020;6:271-8.
171. Yan Y, López-Alcalde J, Zhang L, Siebenhüner AR, Witt CM, Barth J. Acupuncture for the prevention of chemotherapy-induced nausea and vomiting in cancer patients: a systematic review and meta-analysis. Cancer Med. 2023;12:12504-17.
172. Wang M, Liu W, Ge J, Liu S. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. 2023;14:1147718.
173. Xu X, Feng X, He M, et al. The effect of acupuncture on tumor growth and gut microbiota in mice inoculated with osteosarcoma cells. Chin Med. 2020;15:33.
174. Wang N, Zhao L, Zhang D, Kong F. Research progress on the immunomodulatory mechanism of acupuncture in tumor immune microenvironment. Front Immunol. 2023;14:1092402.
175. Xiang Y, Yang Y, Liu J, Yang X. Functional role of MicroRNA/PI3K/AKT axis in osteosarcoma. Front Oncol. 2023;13:1219211.
176. Ma YS, Peng SF, Wu RS, et al. Bisdemethoxycurcumin suppresses human osteosarcoma U2 OS cell migration and invasion via affecting the PI3K/Akt/NFκB, PI3K/Akt/GSK3β and MAPK signaling pathways in vitro. Oncol Rep. 2022;48:210.
177. Zhang L, Yang C, Huang Y, et al. Cardamonin inhibits the growth of human osteosarcoma cells through activating P38 and JNK signaling pathway. Biomed Pharmacother. 2021;134:111155.
178. Jiwa H, Xie Z, Qu X, et al. Casticin induces ferroptosis in human osteosarcoma cells through Fe2+ overload and ROS production mediated by HMOX1 and LC3-NCOA4. Biochem Pharmacol. 2024;226:116346.
179. Vundavilli H, Datta A, Sima C, et al. Anti-tumor effects of cryptotanshinone (C19H20O3) in human osteosarcoma cell lines. Biomed Pharmacother. 2022;150:112993.
180. Huang X, Zeng J, Ruan S, Lei Z, Zhang J, Cao H. The use of matrine to inhibit osteosarcoma cell proliferation via the regulation of the MAPK/ERK signaling pathway. Front Oncol. 2024;14:1338811.
181. Xu X, Liu M, Wu H. Berberine inhibits the growth of osteosarcoma through modulating MMP/NM-23 and MAPK/JNK signal pathways. Am J Transl Res. 2023;15:729-44.
182. Gao X, Zhang C, Wang Y, Zhang P, Zhang J, Hong T. Berberine and cisplatin exhibit synergistic anticancer effects on osteosarcoma MG-63 cells by inhibiting the MAPK pathway. Molecules. 2021;26:1666.
183. Wang W, Li J, Ding Z, et al. Tanshinone I inhibits the growth and metastasis of osteosarcoma via suppressing JAK/STAT3 signalling pathway. J Cell Mol Med. 2019;23:6454-65.
184. Cai Q, Zhang W, Sun Y, et al. Study on the mechanism of andrographolide activation. Front Neurosci. 2022;16:977376.
185. Annamalai V, Kotakonda M, Periyannan V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J Biochem Mol Toxicol. 2020;34:e22514.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].