Exploring the value of immune-related gene set score in gastric cancer prognosis and immunotherapy efficacy
Abstract
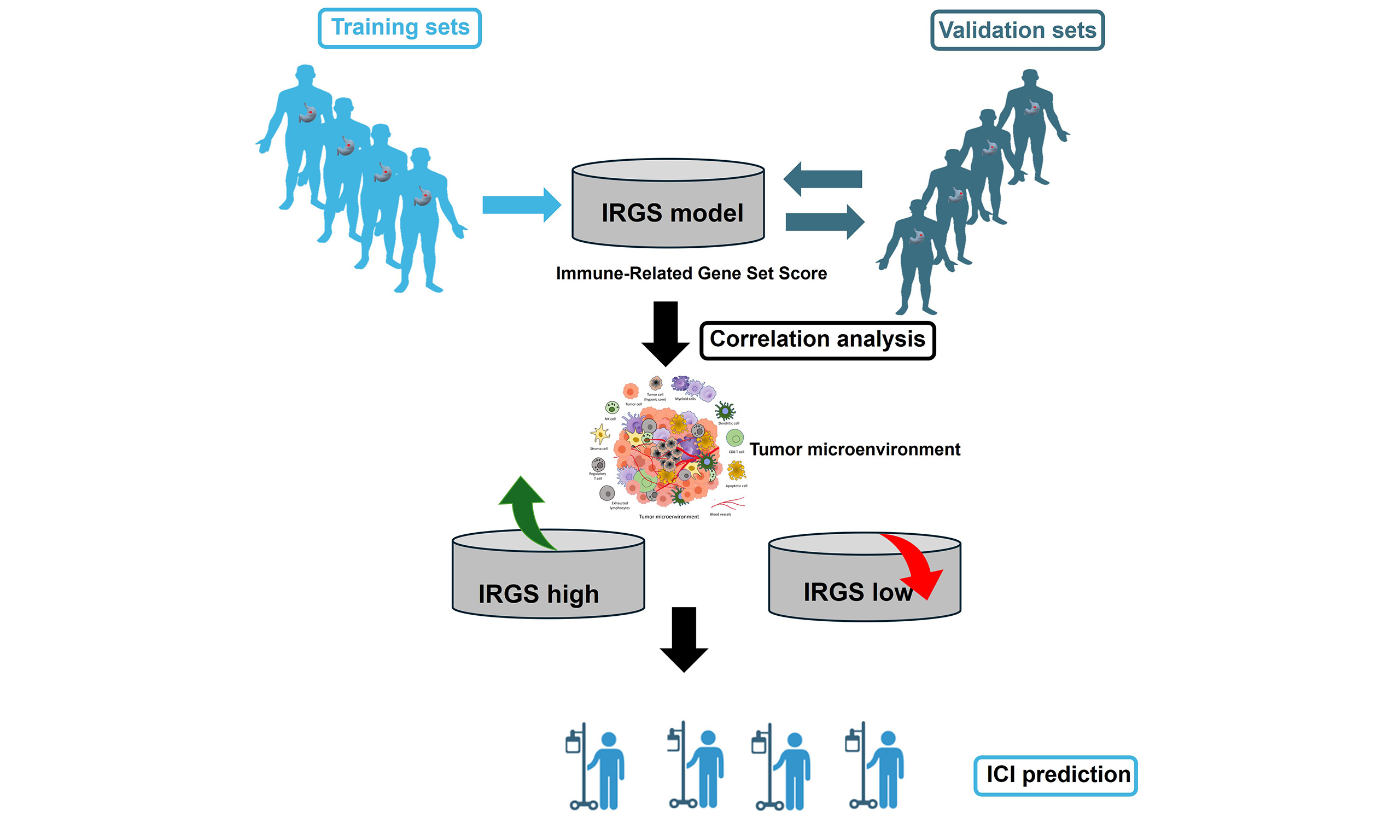
Aim: To characterize the gastric cancer immune landscape and evaluate its correlation with prognosis and immunotherapy efficacy.
Methods: Gene expression data from eight GEO datasets were preprocessed. The datasets were partitioned into training and validation subsets. Immune- and prognostic-related genes were identified from the training set to construct an Immune-Related Gene Set Score (IRGS) prediction model. The model underwent external validation in two independent cohorts, with further optimization incorporating clinical factors. Differences in immune biomarkers between IRGS groups were analyzed and their correlation with therapeutic response was assessed in an immune cohort.
Results: Elevated IRGS significantly correlated with prolonged overall survival (OS) in both training (HR 0.46,
Conclusions: The IRGS model is a robust predictor of prognosis and immunotherapy response in GC, underscoring its potential clinical utility.
Keywords
INTRODUCTION
Gastric cancer (GC) is a common malignancy worldwide. Global Cancer Observatory (GLOBOCAN) 2020 data show 1.089 million new cases of GC, with around 769,000 deaths[1]. According to the latest data from the Chinese Cancer Registry, in 2022, there were approximately 357,800 new cases of GC in China, and the death toll from GC was around 260,400. The incidence of GC ranked fifth among all malignancies, while its mortality rate ranks third[2]. The prognosis of GC is closely related to the timing of diagnosis and treatment. Despite multimodal therapy, a five-year survival rate below 30% is maintained for advanced-stage GC[3]. The influencing factors for the occurrence of GC are complex, and there is currently no optimal method to predict the occurrence and prognosis of GC. These could be contributing factors to the higher incidence and mortality rates of GC in China.
Immune components critically influence cancer onset and progression, with immune evasion now established as a cancer hallmark[4,5]. IRGS are involved in the process of tumor occurrence and development. Current studies on IRGS scoring in GC have underscored its capacity to delineate tumor immune microenvironment (TIME)[6] and forecast immunotherapy response. Li et al. correlated signatures exosome-related long non-coding RNAs (lncRNAs) and TIME; however, this association lacked mechanistic validation[7]. In contrast, Sui and Wu[8] utilized weighted gene co-expression network analysis (WGCNA) to reveal immune-associated gene modules, underscoring the necessity for clinical corroboration.
Through the accumulation of cytoplasmic DNA mediated by DNA damage, the function of Treg cells is negatively regulated by the inhibition of DNA Damage Repair (DDR), while the infiltration of T cells is positively promoted, thereby potentiating antitumor immunity. The tumor microenvironment (TME) exerts a substantial influence on the efficacy of antitumor therapies[10,11]. It can induce GC by suppressing T cell activation and enhancing Treg infiltration. Treg-mediated suppression of both CD8+ and CD4+ T cell populations within the TME is a significant impediment to the efficacy of antitumor immune responses. Those cells regulate the immune response by influencing tumor antigens and perform cytotoxic functions. DCs capture and process tumor antigens and present antigen peptides to CD4+ and CD8+ T cells, while NK cells play an immune surveillance role by directly killing tumor cells and producing cytokines. Meanwhile, GC-derived mesenchymal stem cells (GC-MSCs) migrate to cancer-associated fibroblasts (CAFs) upon IL-6/TNF-α stimulation, driving tumor progression[12]. This implies that immune characteristics may be correlated with the prognosis of the tumor. Additionally, in recent years, immunotherapy has flourished in GC, particularly with immune checkpoint-targeting therapies, such as those against programmed cell death 1 (PD-1) or programmed death-ligand 1 (PD-L1), demonstrating promising efficacy across various stages of the disease[13-16]. However, even in patients with MSI-H, the ORR did not exceed 60%, and the patients with MSI-H constitute less than 10% of GC cases[17]. Thus, profiling tumor immune characteristics may critically predict immunotherapy response, while targeting dysregulated immune genes represents an emerging therapeutic frontier.
This research utilizes bioinformatics approaches to analyze and evaluate the IRGS in GC patients. By identifying prognosis-associated immune-related genes and constructing a prognostic prediction model, it aims to assist in clinical diagnosis and therapy. Gaining a deeper understanding of the immune characteristics of GC and the expression of its regulatory genes is crucial for advancing future diagnostic and treatment methods.
METHODS
Data collection
RNAseq data from GC patients were downloaded from the Gene Expression Omnibus (GEO) database, including GSE13861, GSE14208, GSE15459, GSE26899, GSE26901, GSE28541, GSE34942, GSE62254, and GSE84437. Notably, the GSE84437 dataset was not included in the initial model training/validation but served solely for external validation. Raw RNA-seq data from the eight GEO cohorts were processed using the R package limma, which included log2 transformation and quantile normalization. ComBat from the sva package was applied to address batch effects across cohorts, ensuring harmonized gene expression profiles. The GC transcriptome data were downloaded from TCGA. The RNA-seq data of immunotherapy-treated GC patients were obtained from the European Nucleotide Archive (accession: PRJEB25780; Available from: https://www.ebi.ac.uk/ena/browser/view/PRJEB25780). The TCGA-STAD RNA-seq data were downloaded from the GDC Data Portal (https://xenabrowser.net/datapages/?dataset=TCGA-STAD.star_counts.tsv&host=https%3A%2F%2Fgdc.xenahubs.net&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443). Immune-related genes were collected from the literature entitled “The Immune Landscape of Cancer”[18,19].
Clarified criteria
GC patients were included if they had (1) histologically confirmed GC, (2) RNA-seq data, (3) survival data, and (4) no prior immunotherapy.
Gene selection
The eight GEO datasets were combined into one cohort, then randomly assigned to training and validation cohort according to 7:3. The association between each gene's expression and OS was assessed using univariable Cox regression. Genes with P < 0.05 in the subsequent meta-analysis were retained. The influencing genes were further selected by LASSO-Cox analysis. A 10-fold cross-validated LASSO-Cox model was applied to the candidate genes, with the optimal penalty (λ) selected by minimizing partial likelihood deviance.
IRGS model construction and validation
Model coefficients were calculated from gene meta HR using the formula HR/[1-se(HR)]. Individual gene scores were generated from z-score-normalized expression profiles, and the IRGS was obtained as their weighted sum.
Patients were grouped by the median IRGS value from the training set, and OS was compared between groups. The use of consistent cutoff values across all analyses further validates the findings in an independent GEO cohort (GSE84437) and the TCGA cohorts.
IRGS independence analysis and model optimization
Independent factors (including IRGS and clinical factors) were identified using univariate and multivariate Cox regression analyses.
Functional enrichment analysis
The clusterProfiler R package identified significantly enriched GO terms and KEGG pathways in each IRGS group.
TME and subtype analysis
Immune infiltration scores (ESTIMATE algorithm, https://bioinformatics.mdanderson.org/estimate/) were calculated in the GEO cohort and compared between high/low IRGS groups. Subgroup analysis of IRGS levels in patients with EBV, POLE positive, and different stages of disease was also performed.
Outcome analysis in immune checkpoint inhibitor cohort
Survival curve analysis was performed on the Kim2018 GC cohort that received immunotherapy, and the differences in the efficacy of immunotherapy in different IRGS groups were compared.
Statistical analysis
Statistical analyses were performed using R (v4.3.0). Kaplan-Meier curves with log-rank tests compared to OS between groups. Univariable and multivariable Cox regression analyses assessed the model's impact on survival. Continuous variables were compared using the Mann-Whitney U test, and categorical variables using the chi-square test. Spearman's correlation assessed associations between the IRGS and other factors. The model's predictive performance was evaluated using the area under the AUC and the C-index. The Benjamini-Hochberg false discovery rate (FDR) correction (Q < 0.05) was applied to immune checkpoint marker analyses. Statistical significance was defined as P < 0.05.
RESULTS
Key prognostic genes identified for OS prediction
To identify survival-associated predictors in GC patients, Cox analysis (across eight cohorts) followed by meta-analysis identified 689 candidate genes (meta P < 0.05). Subsequent LASSO analysis yielded 16 significant genes associated with OS [Supplementary Figure 1].
Robust prognostic value of the IRGS model for long-term survival prediction
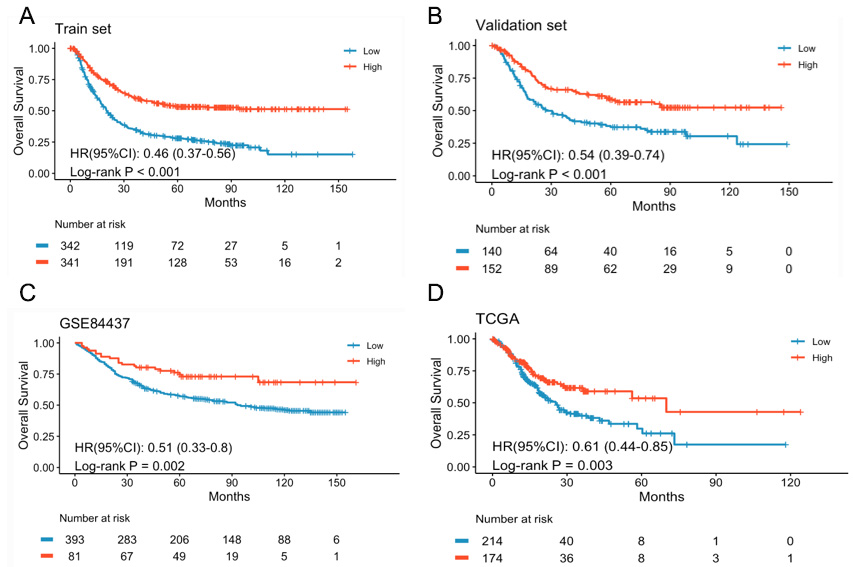
To develop trusted models to predict long-term survival, the IRGS was constructed based on the meta HR and expression levels of 16 genes. Based on the training set median IRGS value, patients were stratified into high/low-IRGS subgroups. Notably, the high-IRGS group exhibited significantly superior OS compared to the low-IRGS group (HR = 0.46, 95%CI: 0.37-0.56, P < 0.001) [Figure 1A]. Similarly, the validation set patients are also divided into two groups based on the same cutoff. The patients in the high-IRGS group exhibited significantly improved OS compared to the low-IRGS group (HR = 0.54, 95%CI: 0.39-0.74,
Figure 1. Kaplan-Meier survival curves comparing OS between high- and low-IRGS subgroups. (A) Training set, (B) Validation set, (C) GSE84437 set, (D) TCGA set. High IRGS: Better survival; Low IRGS: worse survival.
The IRGS model's prognostic performance was validated in two external cohorts. Significantly better OS was observed in high-IRGS versus low-IRGS patients in both the GSE84437 cohort (HR = 0.51, 95%CI: 0.33-0.8, P = 0.002) and the TCGA cohort (HR = 0.61,
IRGS correlates with clinical characteristics and enhances survival prediction
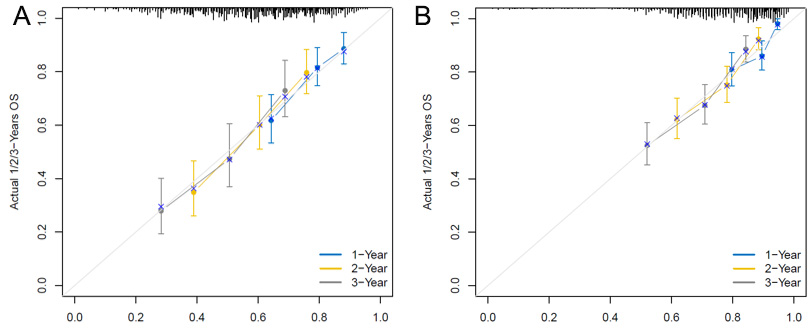
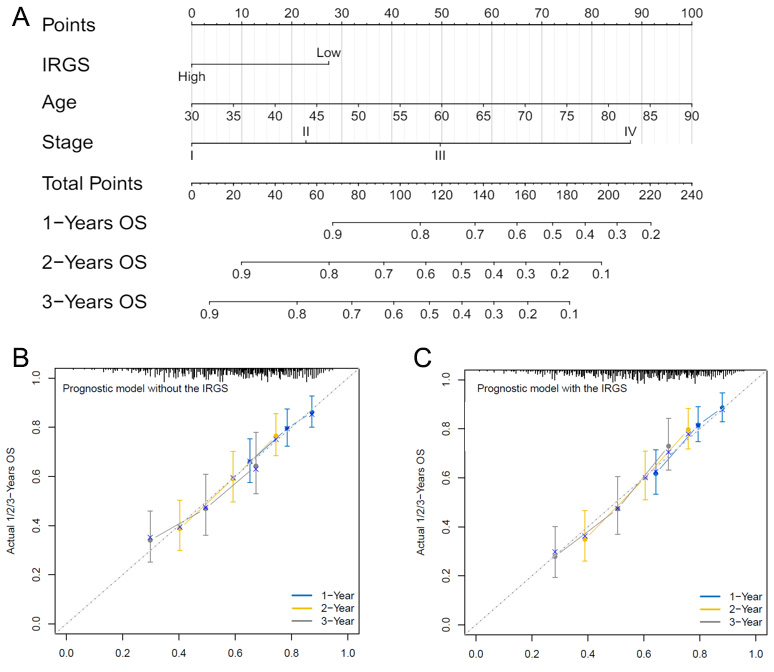
To clarify the relationship between the IRGS model and clinical features, univariate and multivariate analyses were performed in two external cohorts. Significant correlations were observed between IRGS and age, gender, and stage [Supplementary Tables 1 and 2]. Then, combine the above three clinical factors with the IRGS scores, optimize the prediction model, and present it with the Nomogram analysis [Figure 3A]. The Nomogram analysis demonstrates that the combined IRGS and clinical feature model has better predictive ability for 1-, 2-, and 3-year OS rates compared to either IRGS or clinical features alone
Figure 3. Optimization analysis of the IRGS model. (A) Nomogram analysis combining clinical factors with the IRGS model, (B) Calibration analysis of the model's predictive performance when including only clinical factors, (C) Calibration analysis of the optimized model's performance when combining clinical factors with the IRGS model. The total risk score is calculated by summing the points assigned to each variable, and the combined score is then projected onto the 1-, 2-, and 3-year survival axes. (e.g., Age = 60 years → 50 points; IRGS = High → 0 points; Stage = III → 50 points, Total score = 100 points, corresponding to a predicted 1-year OS probability of ~82%.)
IRGS identifies key carcinogenic pathways and DNA damage repair mechanisms
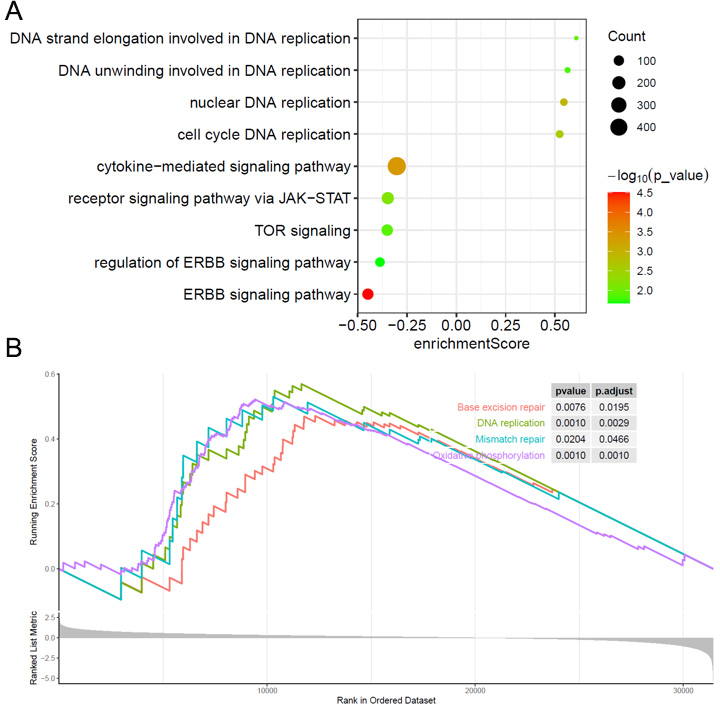
How do oncogenic pathways and DDR mechanisms affect IRGS? Differential gene expression analysis comparing high- and low-IRGS subgroups was conducted to explore the prognostic associations of IRGS. The up- and downregulated genes were subsequently analyzed through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. The results indicate that the high IRGS group is primarily associated with DDR pathways and functions, while the low IRGS group is linked to carcinogenic pathways [Figure 4A and B].
IRGS correlates with immune characteristics and tumor microenvironment features
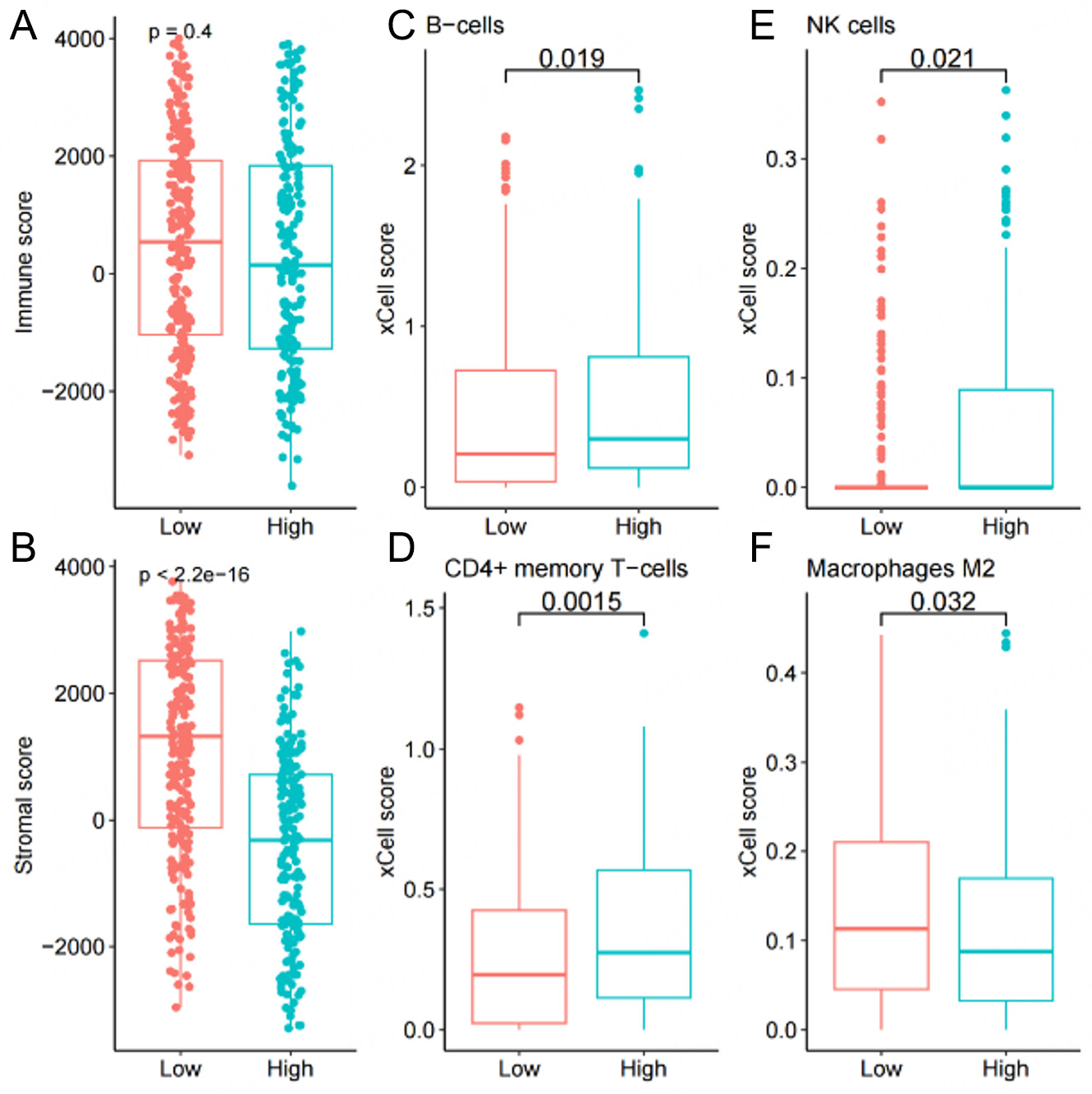
To determine whether IRGS can further predict immune characteristics, we analyzed its associations with tumor immunological features by comparing Tumor Mutational Burden (TMB), tumor purity, and immune checkpoint molecule expression across high- and low-IRGS subgroups. Results demonstrated significantly elevated TMB, tumor purity, and HAVCR2 expression in high-IRGS patients compared to low-IRGS patients, while TIGIT expression showed an inverse pattern [Supplementary Figure 2]. For the characteristics of the immune microenvironment, the analysis of ESTIMATE indicates that there is no difference in the immune component between high- and low-IRGS subgroups [Figure 5A]. Notably, the Low group exhibited a significantly higher stromal cell component than the High group [Figure 5B]. Subsequent assessment of IRGS-immune cell relationships using the TIMER algorithm revealed significant associations with B cells, CD4+ T cells, NK cells, M2 macrophages, and other immune subsets [Figure 5C-F]. Distinct patterns of immune cell composition emerged among the IRGS subgroups. Specifically, the high IRGS group exhibited significantly elevated levels of B cells, CD4+ T cells, and activated NK cells, along with a marked reduction in M2 macrophages. These disparities may be indicative of divergent immune microenvironmental states, wherein elevated IRGS has been linked to augmented adaptive immunity (B and CD4+ T cells) and cytotoxic activity (activated NK cells). Concurrently, the decline in immunosuppressive M2 macrophages observed in high IRGS groups indicates a plausible transition away from a state of tumor-promoting immune regulation.
Figure 5. Correlation analysis between immune infiltration levels and IRGS scores. Immune infiltration markers include (A) immune score, (B) stromal score, (C) B cells, (D) CD4+ memory T cells, (E) NK cells, and (F) M2 macrophages.
In addition, subgroup analysis reveals that patients with EBV (Epstein-Barr Virus) positivity and MSI-H exhibit higher IRGS scores, and patients in stage I show significantly higher IRGS scores compared to other stages [Supplementary Figure 3].
IRGS and immune response
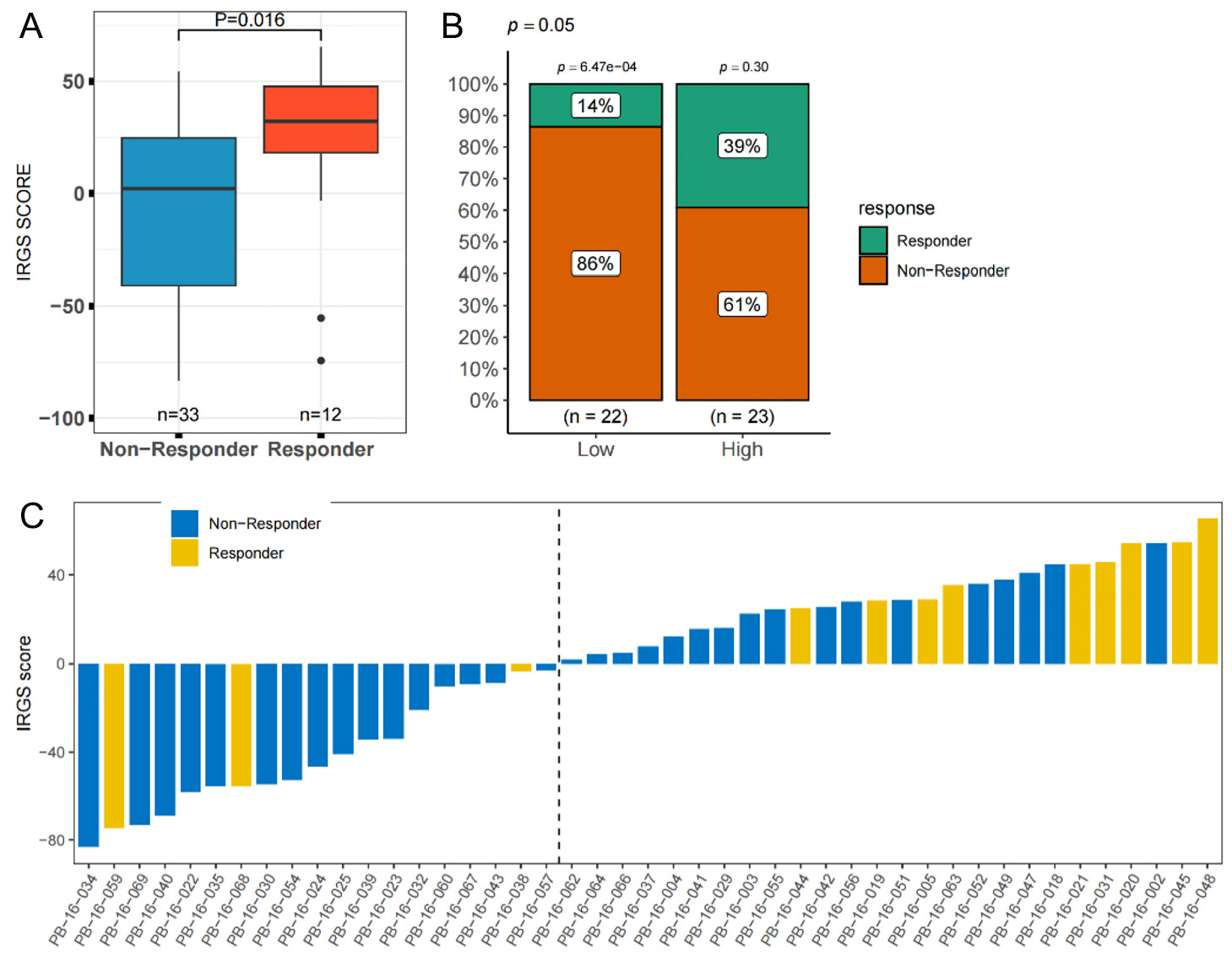
To investigate the relationship between IRGS and immune response after treatment, we analyzed the IRGS in the Kim2018 GC cohort, in which patients underwent immunotherapy. Responders to immunotherapy exhibited significantly higher IRGS scores compared to non-responders (P = 0.016, Figure 6A). Similarly, within the IRGS Low group, the number of responders was significantly lower than that of non-responders, whereas no significant difference was observed in the High group [Figure 6B and C].
Figure 6. Correlation analysis between IRGS scores and the efficacy of immunotherapy in the immunotherapy cohort. (A) differences in IRGS score levels between responders and non-responders, (B) differences in response rates between high- and low-IRGS score groups, (C) distribution of IRGS scores and treatment efficacy for each patient in the cohort.
DISCUSSION
GC remains one of the most common and deadly malignancies in China, often diagnosed at advanced stages with poor prognosis[20]. The immune system, a crucial defense against cancer, correlates with tumor development and prognosis[21]. The evolving era of cancer immunotherapy has brought attention to tumor immune characteristics. Exploring the correlation between tumor immune features, prognosis, and treatment is crucial. Establishing a predictive model based on IRGS may effectively forecast the prognosis and immunotherapy efficacy in GC patients, potentially aiding clinical treatment decisions.
Recent studies have begun to explore the correlation between prognosis risk and molecular features to investigate the significant clinical implications of predicting prognosis in GC patients[22-25]. Tumor immune characteristics are closely related to cancer development, with various solid tumor studies demonstrating a significant correlation between Immune-Related Genes (IRG) and tumor prognosis and treatment[26-28]. However, such research in GC is limited. Utilizing meta HR of prognosis-related IRG genes, our IRGS model accurately predicted patient outcomes in training/internal validation sets and maintained robust performance across two independent external cohorts. All cohort results show significantly better prognosis for patients with high IRGS, consistent with previous GC and other solid tumor-related studies[27-29]. Furthermore, combining clinically significant influencing factors with IRGS optimizes the predictive model, demonstrating good performance in external validation cohorts. Multimodal analysis of molecular and clinical features enhances prediction accuracy.
To explore the immune mechanism related to IRGS, both KEGG and GO were analyzed. Results revealed that the IRGS High group is primarily associated with DDR pathways and upregulated functions. Dysfunction in processes drives tumorigenesis, malignant progression, treatment resistance, and prognosis[30]. Notably, DDR deficiency can increase immunogenicity by raising the number of somatic mutations and the amount of intracellular DNA fragments, which leads to the formation of neoantigens and triggers antitumor immune responses[31,32]. Beyond boosting immunogenicity, DDR pathways in tumor cells influence immune surveillance and response mechanisms by promoting cytoplasmic DNA accumulation. This triggers DNA damage signaling and downstream interferon pathways. Ultimately, this influences T cell infiltration and PD-1/PD-L1 expression[33,34]. Furthermore, DDR inhibition has been demonstrated to suppress Treg function while enhancing the infiltration of cytotoxic T cells, thereby shaping the TME[35]. In GC, tumors with low DDR characteristic scores were independently associated with shorter overall survival, and such patients may not benefit from adjuvant chemotherapy and monoclonal antibodies targeting PD-1 treatment, indicating an upregulation of DDR pathway expression correlates with favorable immune characteristics, consistent with our research[36].
The literature has shown that IRGS are associated with immune molecules. TME is a network of immune cells, stromal cells, cancer cells, cytokines, chemokines, and others[37]. Immune-related cells and factors are not only involved in the whole process of tumor development, but also closely related to the ability of the immune system to suppress tumors, so they are particularly important[38,39].
Several studies have shown that high levels of CD4+ memory T cells and NK cells are associated with better immunotherapy efficacy[40,41]. On the contrary, high M2 macrophage infiltration correlates with poor prognosis in GC, and their combination with ICI may achieve better therapeutic effects[42]. T cell, B cell, NK cell, and macrophage infiltration levels collectively predict GC prognosis[43]. Consistent with our findings, CCL28 targeting in preclinical models boosts CD8+ T cell recruitment and activity in the TME via B cell and plasma cell modulation, suggesting a promising immunotherapeutic synergy[44]. In our analysis, the high-IRGS subgroup exhibited elevated infiltration of B cells, CD4+ T cells, and activated NK cells, whereas M2 macrophage abundance was significantly reduced. Furthermore, it has been validated in a clinical cohort of immunotherapy. A high IRGS score predicts better survival outcomes and greater benefits from immunotherapy. In the immunotherapy cohort analysis, the IRGS score of patients in the effective immunotherapy group was significantly higher than that in the ineffective immunotherapy group
The present study has the following limitations: First, the analysis and model validation were based on public datasets, and further validation in clinical patients from the Chinese population was not included. Second, mechanistically, only functional annotation, exploration of immunogenicity, and subgroup analysis were conducted, without delving into basic research exploration. The use of multiplex immunofluorescence is imperative to validate immune cell infiltration patterns. Therefore, these mechanisms remain validated.
Conclusion
In this study, we established an IRGS prognostic prediction model for GC patients based on immune-related genomic signatures and further explored its mechanisms and immunological characteristics. High-IRGS patients demonstrate significantly improved survival outcomes, enhanced antitumor immunity, and superior response to immunotherapy. This model may have promised predictive value for prognosis and immunotherapeutic outcomes in GC, offering valuable implications for clinical application.
DECLARATIONS
Authors' contributions
Involved in the conceptualization, data analysis, and original draft preparation: Chen W, Xu L, Zha J
Involved in the data analysis and methodology: Zhang F, Cao T, Li S, Shen X
Reviewing and editing the manuscript: Li H
All authors have read and approved the final version.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This project was supported by Shenzhen Key Medical Discipline Construction Fund (SZXK014), Shenzhen Science and Technology Program (KQTD20180411185028798, ZDSYS20210623091811035, JCYJ20220530142203008), Sanming Project of Medicine in Shenzhen (SZSM202211017), Guangdong Basic and Applied Basic Research Foundation (2022A1515110156), Futian Healthcare Research Program (FTWS2022051), and the Young Scientists Fund of the National Natural Science Foundation of China (82403259).
Conflict of interests
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study was approved by the Ethics Office of the University of Hong Kong-Shenzhen Hospital (HKU-SZH25061901). Since the data were collected retrospectively and deidentified, patient consent was not required.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338-49.
2. Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53.
3. Kakeji Y, Ishikawa T, Suzuki S, et al. A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001-2013). Gastric Cancer. 2022;25:1082-93.
4. Lan Q, Wang P, Tian S, Dong W. Mining TCGA database for genes of prognostic value in gastric cancer microenvironment. J Cell Mol Med. 2020;24:11120-32.
5. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-37.
6. Xu L, Zou C, Zhang S, et al. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol. 2022;15:87.
7. Li C, Zhang Z, Peng E, Peng J. Role of an exosomes-related lncRNAs signature in tumor immune microenvironment of gastric cancer. Front Cell Dev Biol. 2022;10:873319.
8. Sui X, Wu G. Immune landscape and prognostic gene signatures in gastric cancer: implications for cachexia and clinical outcomes. Front Immunol. 2023;14:1297363.
9. Miliotis C, Ma Y, Katopodi XL, et al. Determinants of gastric cancer immune escape identified from non-coding immune-landscape quantitative trait loci. Nat Commun. 2024;15:4319.
10. Xu L, Li B, Liu Y, et al. Unveiling KLHL23 as a key immune regulator in hepatocellular carcinoma through integrated analysis. Aging. 2024;16:13608-26.
11. Wu F, Xu L, Tu Y, et al. Sirtuin 7 super-enhancer drives epigenomic reprogramming in hepatocarcinogenesis. Cancer Lett. 2022;525:115-30.
12. Li B, Chen H, Yang S, et al. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol Cancer. 2023;22:71.
13. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-30.
14. Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402:2197-208.
15. Rha SY, Oh DY, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181-95.
16. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40.
17. Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7:895-902.
18. Galassi C, Chan TA, Vitale I, Galluzzi L. The hallmarks of cancer immune evasion. Cancer Cell. 2024;42:1825-63.
19. Germanà E, Pepe L, Pizzimenti C, et al. Programmed cell death ligand 1 (PD-L1) immunohistochemical expression in advanced urothelial bladder carcinoma: an updated review with clinical and pathological implications. Int J Mol Sci. 2024;25:6750.
20. Russo AE, Strong VE. Gastric cancer etiology and management in Asia and the West. Annu Rev Med. 2019;70:353-67.
21. Peña-Romero AC, Orenes-Piñero E. Dual effect of immune cells within tumour microenvironment: pro- and anti-tumour effects and their triggers. Cancers. 2022;14:1681.
22. Duan S, Wang P, Liu F, et al. Novel immune-risk score of gastric cancer: a molecular prediction model combining the value of immune-risk status and chemosensitivity. Cancer Med. 2019;8:2675-85.
23. Sun P, Lu Q, Li Z, et al. Assessment of prognostic prediction models for gastric cancer using genomic and transcriptomic profiles. Meta Gene. 2021;28:100890.
24. Qiu L, Qu X, He J, et al. Predictive model for risk of gastric cancer using genetic variants from genome-wide association studies and high-evidence meta-analysis. Cancer Med. 2020;9:7310-6.
25. Cheong JH, Wang SC, Park S, et al. Development and validation of a prognostic and predictive 32-gene signature for gastric cancer. Nat Commun. 2022;13:774.
26. Xu X, Lu Y, Wu Y, et al. A signature of seven immune-related genes predicts overall survival in male gastric cancer patients. Cancer Cell Int. 2021;21:117.
27. Jiang B, Sun Q, Tong Y, et al. An immune-related gene signature predicts prognosis of gastric cancer. Medicine. 2019;98:e16273.
28. Li C, Huang Y, Yi X, Tang Y, Okita R, He J. Pan-cancer prognostic model and immune microenvironment analysis of natural killer cell-related genes. Transl Cancer Res. 2024;13:1936-53.
29. Liu Y, Li D, Chen Y, et al. Integrated bioinformatics analysis for conducting a prognostic model and identifying immunotherapeutic targets in gastric cancer. BMC Bioinformatics. 2023;24:191.
30. Molinaro C, Martoriati A, Cailliau K. Proteins from the DNA damage response: regulation, dysfunction, and anticancer strategies. Cancers. 2021;13:3819.
31. Wang M, Chen S, Ao D. Targeting DNA repair pathway in cancer: mechanisms and clinical application. MedComm. 2021;2:654-91.
32. Chen M, Linstra R, van Vugt MATM. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2022;1877:188661.
33. Shi C, Qin K, Lin A, et al. The role of DNA damage repair (DDR) system in response to immune checkpoint inhibitor (ICI) therapy. J Exp Clin Cancer Res. 2022;41:268.
34. Ye Z, Shi Y, Lees-Miller SP, Tainer JA. Function and molecular mechanism of the DNA damage response in immunity and cancer immunotherapy. Front Immunol. 2021;12:797880.
35. Zhou C, Lin A, Cao M, et al. Activation of the DDR pathway leads to the down-regulation of the TGFβ pathway and a better response to ICIs in patients with metastatic urothelial carcinoma. Front Immunol. 2021;12:634741.
36. Lou S, Wang Y, Zhang J, et al. Patient-level DNA damage repair pathway profiles and anti-tumor immunity for gastric cancer. Front Immunol. 2021;12:806324.
37. Graves EE, Maity A, Le QT. The tumor microenvironment in non-small-cell lung cancer. Semin Radiat Oncol. 2010;20:156-63.
38. Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20:840.
39. Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403.
40. Sun Y, Liu L, Fu Y, et al. Metabolic reprogramming involves in transition of activated/resting CD4+ memory T cells and prognosis of gastric cancer. Front Immunol. 2023;14:1275461.
41. Wang F, Lau JKC, Yu J. The role of natural killer cell in gastrointestinal cancer: killer or helper. Oncogene. 2021;40:717-30.
42. Li J, Sun J, Zeng Z, et al. Tumour-associated macrophages in gastric cancer: from function and mechanism to application. Clin Transl Med. 2023;13:e1386.
43. Sukri A, Hanafiah A, Kosai NR. The roles of immune cells in gastric cancer: anti-cancer or pro-cancer? Cancers. 2022;14:3922.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].