Functional hydrogels for dental disease treatment
Abstract
Dental disease treatment has achieved significant progress, yet challenges remain due to limitations of conventional dental materials, including suboptimal biocompatibility and biodegradability. Functional hydrogels, as one kind of versatile biomaterial, have gained widespread attention in biomedical applications, offering notable advantages and promising potential for dental therapies. This paper summarizes the basic properties of functional hydrogels and recent advancements in treating various dental diseases. It emphasizes the working mechanisms and therapeutic effects. Lastly, the challenges and issues encountered by hydrogels in dental treatments are discussed, along with future development prospects. With ongoing advances in hydrogel design and fabrication, their performance is expected to improve further, expanding their role and potential in managing dental diseases, oral health, and related medical conditions.
Keywords
INTRODUCTION
Oral health is fundamental to overall human well-being, with dental health serving as a critical component[1-3]. Due to variations in diet and lifestyle, individuals may encounter a range of dental issues, including tooth discoloration[4-6], cavities[7-9], pulp diseases[10-13], and periapical/root canal diseases[14-17]. If not treated in time, the tooth may need to be extracted and there is a risk of infection in the extraction socket[18,19]. Among these, tooth discoloration and cavities are the most widespread oral concerns[20,21]. Surveys indicate that nearly half of Chinese adults have experienced some degree of tooth discoloration[20]; meanwhile, cavities rank alongside cardiovascular diseases and cancer as one of the three most common global health concerns[22,23]. Maintaining dental health is vital, yet current oral biomaterials face safety and reliability challenges, which emphasizes the urgent need for more effective and safe dental materials[24,25].
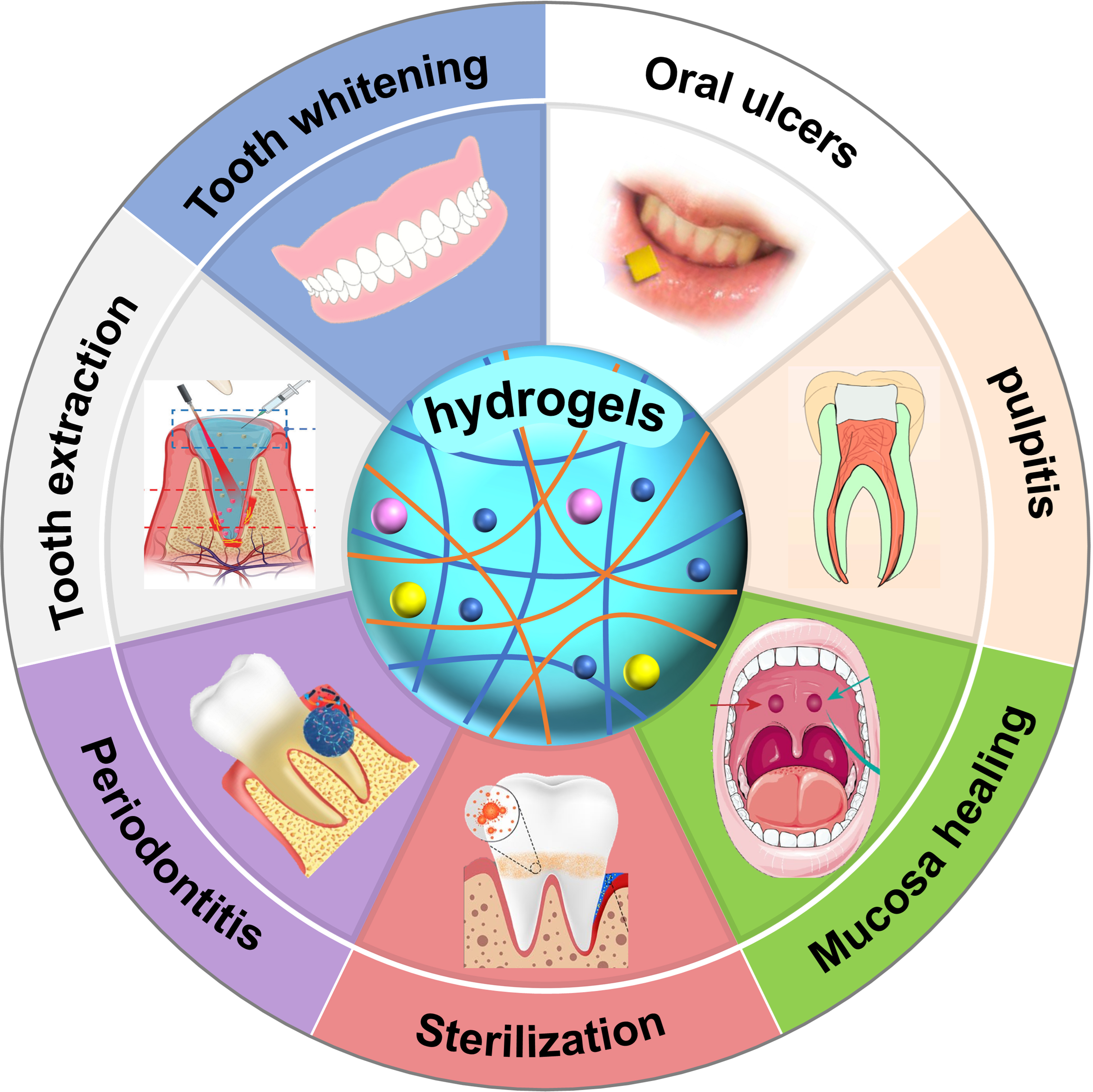
Functional hydrogels are emerging polymer materials that resemble natural soft tissues[26-29], due to their flexibility and elasticity. Their three-dimensional network structure endows functional hydrogels with high water absorption and retention capacities[30,31]. Importantly, they are highly biocompatible and biodegradable, meaning they can avoid immune rejection and gradually degrade within the body or the environment[32-35]. These properties make hydrogels ideal for biomedical applications such as wound healing, hemostasis, disinfection, and drug delivery[36-39]. Additionally, their adjustable wet adhesion, mechanical strength, and antifatigue properties make them suitable for the moist and dynamic environment of the oral cavity[40,41]. For example, the excellent wet adhesion and drug release properties facilitate the application of hydrogels in the wound healing of tooth extraction sockets[18,19], and the good injectability and temperature-sensitive properties make hydrogels suitable for root canal treatment and dental pulp regeneration[42-44]. In recent years, continuous advancements in hydrogel performance have led to their widespread use in the treatment of dental diseases, particularly for addressing common oral issues [Figure 1], such as oral ulcers, oral cancer, periodontitis, etc.[5,42,45-49].
Figure 1. Functional hydrogels for treating different dental and oral diseases, including tooth whitening, pulpitis, mucosa healing, etc.[5,42,45-49]. Reproduced with permission[42]. Copyright©2021, American Chemical Society. Reproduced with permission[5]. Copyright©2022, American Chemical Society. Reproduced with permission[45]. Copyright©2023, Wiley-VCH. Reproduced with permission[46]. Copyright©2023, American Chemical Society. Reproduced with permission[47]. Copyright©2023, Elsevier. Reproduced with permission[48]. Copyright©2023, MDPI. Reproduced with permission[49]. Copyright©2022, Springer.
This paper primarily discusses the research progress of functional hydrogels in the treatment of dental diseases, focusing on areas such as teeth whitening, caries treatment, post-extraction wound healing, and pulpitis treatment. It aims to analyze their mechanisms and therapeutic effects, while also discussing the challenges and opportunities associated with hydrogel-based dental therapies, and outlining future development trends.
RECENT PROGRESS IN FUNCTIONAL HYDROGELS FOR DENTAL DISEASE TREATMENT
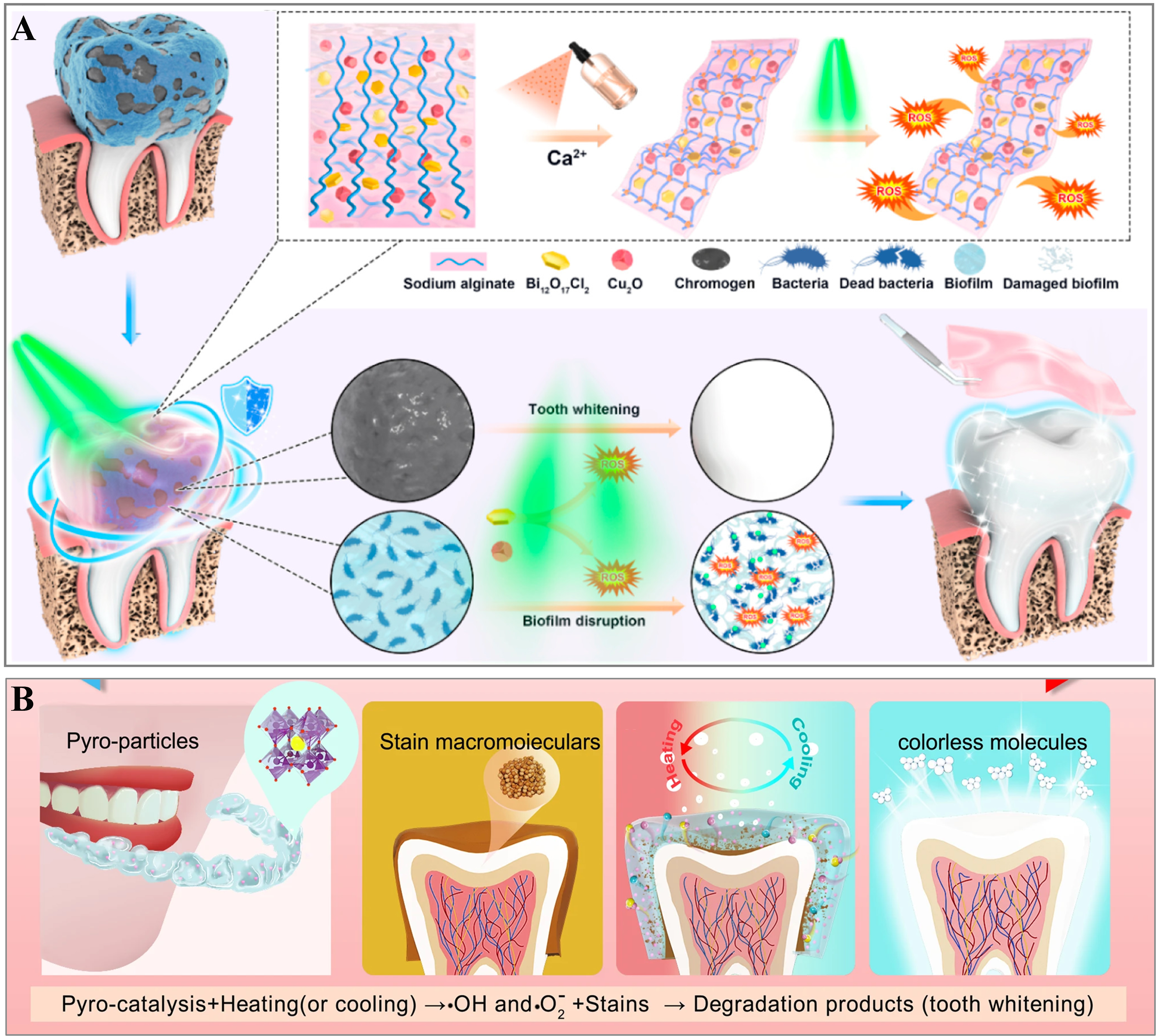
With the growing emphasis on beauty in modern society, white, bright, and healthy teeth have become key beauty standards[1,50]. Due to the limitations of current whitening technologies, various safe and effective methods have been developed, such as piezoelectric[51,52], photodynamic[4,53], and pyroelectric[54] catalytic whitening. These techniques rely on the generation of reactive oxygen species (ROS), which break down organic pigments into colorless molecules, producing a whitening effect[51,53].
Figure 2A illustrates the schematic diagram of photodynamic teeth whitening with an injectable hydrogel[6], and this hydrogel consists of copper(I) oxide (Cu2O) as an antibacterial agent and bismuth chloride
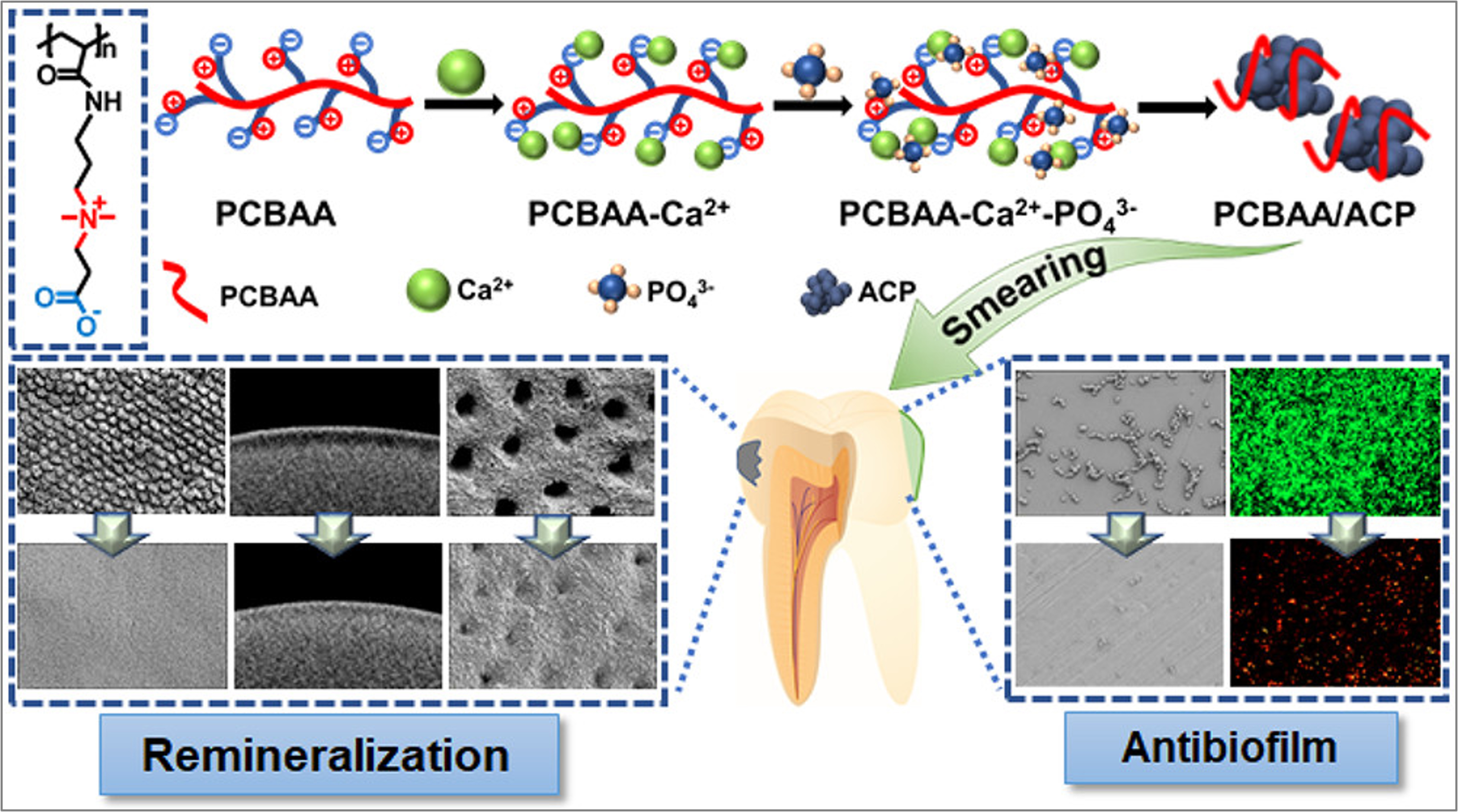
Cavities are among the most common human diseases and, if left untreated, can progress to pulpitis and periodontitis[55,56]. Biomineralization technology[56-58], which facilitates the remineralization of dental hard tissues by releasing Ca2+ and PO43- ions to convert amorphous calcium phosphate (ACP) into more stable forms, showing huge potential in caries treatment. Researchers have integrated ACP into amphoteric ion polycarboxylate betaine acrylamide (PCBAA), resulting in development of PCBAA/ACP nanocomposite material that exhibits both remineralization and antibiofilm properties[9]. Due to strong electrostatic interactions, the negatively charged carboxyl (-COO-) and positively charged quaternary ammonium
Figure 3. PCBAA/ACP polyzwitterion hydrogel with remineralization and antibiofilm functions for dental demineralization. Reproduced with permission[9]. Copyright©2022, American Chemical Society. PCBAA: Polycarboxylate betaine acrylamide; ACP: amorphous calcium phosphate.
As the only soft tissue within the tooth, when the dental pulp becomes inflamed, it will seriously endanger the safety of the tooth[13,59]. Pulp regeneration offers an effective approach for treating pulpitis and restoring tooth vitality by replacing damaged pulp tissue[60,61]. Injectable thermosensitive hydrogels, with excellent injectable molding capabilities, have emerged as ideal materials for biological scaffolds[42-44]. Researchers have created a thermosensitive hydrogel (HPCH/CW/Exo) by incorporating exosomes (Exo) from human dental pulp stem cells (hDPSCs) into a hydrogel made of hydroxypropyl chitosan (HPCH) and chitosan whiskers (CW)[62]. This hydrogel can easily penetrate irregular root canal spaces and solidify in place. When tested for dental tissue regeneration by injecting it into a human root model and implanting it in mice for 8 weeks, for the hDPSC-laden HPCH/CW/Exo hydrogel group, the pulp-like tissues with extracellular matrix and cells formed in the endodontic space, due to the exceptional delivery effect of the Exo that promotes odontogenesis and angiogenesis[62]. However, the endodontic space almost remained empty in the comparative experiments, which confirms the good pulp regeneration ability of the hDPSC-laden HPCH/CW/Exo hydrogels. Furthermore, its flowability enables precise drug delivery and sustained antibacterial release for root canal disinfection[63,64], and it demonstrates favorable biocompatibility with hDPSCs, highlighting its dual role in pulp regeneration and sterilization[65-67].
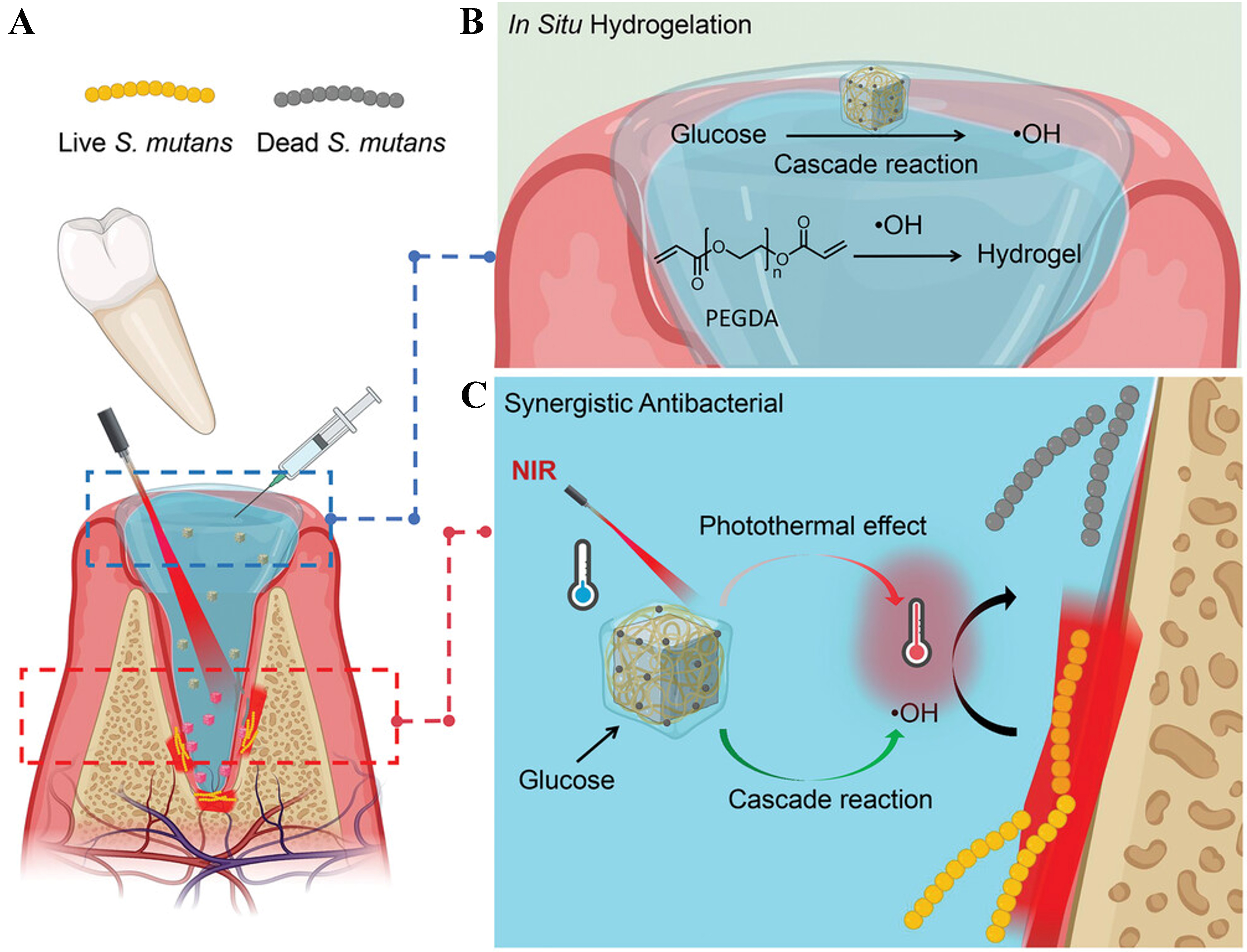
When a tooth is damaged to the extent that extraction is necessary, the healing of the extraction socket becomes critically important as well[37,39]. Injectable and moldable hydrogels, with their excellent wet adhesion capabilities, are ideal materials for promoting tooth-extraction socket healing. By loading drugs, hydrogels can further achieve antibacterial, disinfectant, and hemostatic effects[68,69]. Researchers developed a polyethylene glycol diacrylate (PEGDA) hydrogel containing chitosan-modified palladium nano-cube and glucose oxidase (GOx)-Fe2+ through step-by-step assembly[45]. This hydrogel can promote healing of tooth-extraction wounds [Figure 4A]. The hydrogel combines GOx and Fe2+, which generate toxic hydroxyl radicals (·OH) to enhance enzyme activity [Figure 4B]. Under 808 nm light, combined with photothermal effect from the palladium nano-cubes, the hydrogel’s photothermal effect and cascade reaction system help fight Streptococcus mutans that can cause infection [Figure 4C]. The ·OH radicals also trigger the gelation of PEGDA monomers, creating a moist and sterile environment for healing. The hydrogel is biodegradable, ensuring it degrades as tissue regenerates for effective wound healing.
Figure 4. (A) Schematic illustration of PEGDA-based hydrogel for bacteria-induced tooth-extraction healing; (B) The hydroxyl radicals induce in situ hydrogelation of PEGDA; (C) Photothermal effect and chemodynamic cascade reaction for eradicating bacteria. Reproduced with permission[45]. Copyright©2023, Wiley-VCH. PEGDA: Polyethylene glycol diacrylate.
SUMMARY AND OUTLOOK
This paper concludes the basic properties, advantages, and future prospects of functional hydrogels in dental disease treatment, highlighting the working mechanisms and corresponding therapeutic effects. For instance, hydrogels loaded with photosensitive or pyroelectric materials can generate ROS induced by light or temperature stimulation, enabling teeth whitening and bacterial disinfection. As drug carriers, hydrogels can release Ca2+ and PO43- ions to promote enamel remineralization, effectively treating cavities. Their injectability, moldability, and wet adhesion also make them suitable for pulp regeneration, post-extraction disinfection, and healing.
Hydrogels show great potential in dental disease treatment, but they still face several challenges. The most important factor is biocompatibility, which ensures the safety and reliability of dental treatments. However, prioritizing biocompatibility can reduce the mechanical strength of hydrogels. The mechanical properties can be improved by adding metal ions or nanoparticles, but this may reduce biocompatibility. For the hydrogels containing nanoparticles (e.g., Cu2O, BaTiO3), there is a lack of in-depth research and analysis on their degradation kinetics and degradation products, thus making it impossible to assess whether their long-term safety meets the standards set by the US Food and Drug Administration (FDA) and International Organization for Standardization (ISO). Additionally, the moist and dynamic nature of the oral environment presents challenges for hydrogels in clinical application, and the wet adhesive property can weaken due to tooth occlusion, saliva secretion, and swallowing over time. In future clinical applications, it is also necessary to consider the time cost of hydrogel preparation and preservation, as well as the chairside preparation work and patient compliance for light/temperature-activated systems. Based on all these considerations, the clinical application of safe, reliable and efficient hydrogels in treating dental diseases still has a long way to go.
Despite certain challenges, hydrogels offer significant advantages due to their simple preparation processes, low cost, and large-scale production. The diverse strategies for structural regulation and performance optimization also allow for the creation of functional hydrogels tailored to meet the specific requirements of disease treatment. Moreover, the rapidly developing artificial intelligence (AI) technology may be utilized to assess and optimize the properties of functional hydrogels, while providing feedback on the drug loading and release characteristics of these hydrogels. Overall, functional hydrogels still hold vast potential and significant advantages for future applications in the dental and oral field.
DECLARATIONS
Authors’ contributions
Literature review, the outline of the manuscript structure, and writing of manuscript draft: Li, H. (Huixu Li); Xu, X.
Revised the manuscript: Li, H. (Huixu Li); Xu, X.; Zhang, D.
Supervision, writing - review and editing: Zhang, D.; Li, H. (Haihui Li); Bao, P.
All authors have read the manuscript and approved the final version.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was funded and sponsored by Tianjin Health Research Project (Grant No. TJWJ2025QN072) and National Natural Science Foundation of China (52303238).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Li, H.; Zhang, D.; Bao, P.; et al. Recent advances in functional hydrogels for treating dental hard tissue and endodontic diseases. ACS. Nano. 2024, 18, 16395-412.
2. Xu, S.; Hu, B.; Dong, T.; et al. Alleviate periodontitis and its comorbidity hypertension using a nanoparticle-embedded functional hydrogel system. Adv. Healthc. Mater. 2023, 12, e2203337.
3. Gong, J.; Wang, S.; Liu, J.; et al.
4. Zhang, F.; Wu, C.; Zhou, Z.; et al. Blue-light-activated nano-TiO2@PDA for highly effective and nondestructive tooth whitening. ACS. Biomater. Sci. Eng. 2018, 4, 3072-7.
5. Gao, J.; Wang, J.; Yue, X.; et al. Photostable aggregation-induced emission photosensitizer nanoparticle/hyaluronic acid hydrogel for efficient photodynamic tooth bleaching. ACS. Appl. Nano. Mater. 2022, 5, 5944-51.
6. Li, Q.; Liu, J.; Xu, Y.; et al. Fast cross-linked hydrogel as a green light-activated photocatalyst for localized biofilm disruption and brush-free tooth whitening. ACS. Appl. Mater. Interfaces. 2022, 14, 28427-38.
7. Cheng, K.; She, P.; Wang, H.; et al. A bio-inspired versatile free-standing membrane for oral cavity microenvironmental monitoring and remineralization to prevent dental caries. Mater. Horiz. 2023, 10, 512-23.
8. Wang, D.; Deng, J.; Deng, X.; Fang, C.; Zhang, X.; Yang, P. Controlling enamel remineralization by amyloid-like amelogenin mimics. Adv. Mater. 2020, 32, e2002080.
9. He, J.; Yang, J.; Li, M.; et al. Polyzwitterion manipulates remineralization and antibiofilm functions against dental demineralization. ACS. Nano. 2022, 16, 3119-34.
10. Siddiqui, Z.; Sarkar, B.; Kim, K. K.; et al. Angiogenic hydrogels for dental pulp revascularization. Acta. Biomater. 2021, 126, 109-18.
11. Zheng, L.; Liu, Y.; Jiang, L.; et al. Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres for endodontic regeneration. Acta. Biomater. 2023, 156, 37-48.
12. Li, M.; Tian, J.; Yu, K.; et al. A ROS-responsive hydrogel incorporated with dental follicle stem cell-derived small extracellular vesicles promotes dental pulp repair by ameliorating oxidative stress. Bioact. Mater. 2024, 36, 524-40.
13. Liang, Z.; Li, J.; Lin, H.; et al. Understanding the multi-functionality and tissue-specificity of decellularized dental pulp matrix hydrogels for endodontic regeneration. Acta. Biomater. 2024, 181, 202-21.
14. Chung, Y. L.; Lee, J. J.; Chien, H. H.; Chang, M. C.; Jeng, J. H. Interplay between diabetes mellitus and periodontal/pulpal-periapical diseases. J. Dent. Sci. 2024, 19, 1338-47.
15. Wang, Y.; Zhu, J.; Zhou, Y.; Tang, Y.; Huang, C. Exploring relationships between circulating interleukins and pulp and periapical diseases: a bidirectional mendelian randomization study. J. Endod. 2025, 51, 132-9.
16. Ribeiro, J. S.; Bordini, E. A. F.; Ferreira, J. A.; et al. Injectable MMP-responsive nanotube-modified gelatin hydrogel for dental infection ablation. ACS. Appl. Mater. Interfaces. 2020, 12, 16006-17.
17. Robberecht, L.; Delattre, J.; Meire, M. Isthmus morphology influences debridement efficacy of activated irrigation: a laboratory study involving biofilm mimicking hydrogel removal and high-speed imaging. Int. Endod. J. 2023, 56, 118-27.
18. Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992-3034.
19. Yin, Y.; Shuai, F.; Liu, X.; Zhao, Y.; Han, X.; Zhao, H. Biomaterials and therapeutic strategies designed for tooth extraction socket healing. Biomaterials 2025, 316, 122975.
20. Reis, A.; Tay, L. Y.; Herrera, D. R.; Kossatz, S.; Loguercio, A. D. Clinical effects of prolonged application time of an in-office bleaching gel. Oper. Dent. 2011, 36, 590-6.
21. Kielbassa, A. M.; Maier, M.; Gieren, A. K.; Eliav, E. Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence. Int. 2015, 46, 881-97.
22. Huang, Y.; Liu, Y.; Shah, S.; et al. Precision targeting of bacterial pathogen via bi-functional nanozyme activated by biofilm microenvironment. Biomaterials 2021, 268, 120581.
23. Naha, P. C.; Liu, Y.; Hwang, G.; et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS. Nano. 2019, 13, 4960-71.
24. Jia, B.; Zhang, B.; Li, J.; et al. Emerging polymeric materials for treatment of oral diseases: design strategy towards a unique oral environment. Chem. Soc. Rev. 2024, 53, 3273-301.
25. Chen, X.; Huang, N.; Wang, D.; et al. Sulfated chitosan-modified CuS nanocluster: a versatile nanoformulation for simultaneous antibacterial and bone regenerative therapy in periodontitis. ACS. Nano. 2024, 18, 14312-26.
26. Xiao, M.; Zhang, X.; Luo, Y.; Xie, R.; Tao, K.; Wu, J. Engineering hydrogel-based conformal epidermal electrodes for human-machine interaction. Soft. Sci. 2025, 5, 40.
27. You, M.; Guo, Y.; Yu, H.; et al. Polyphenol-enhanced wet adhesive hydrogel with synergistic mechanical activation and ROS scavenging for accelerating diabetic wound healing. Chem. Eng. J. 2024, 500, 157103.
28. Deng, D.; Liang, L.; Su, K.; et al. Smart hydrogel dressing for machine learning-enabled visual monitoring and promote diabetic wound healing. Nano. Today. 2025, 60, 102559.
29. Wen, N.; Li, S.; Jiang, H.; et al. Bio-inspired self-healing hydrogel for fast hemostasis and accelerated wound healing of gastric ulcers. Adv. Funct. Mater. 2025, 35, 2411959.
30. Zhang, D.; Zhou, Y.; Mao, Y.; et al. Highly antifreezing thermogalvanic hydrogels for human heat harvesting in ultralow temperature environments. Nano. Lett. 2023, 23, 11272-9.
31. Liu, L.; Zhang, D.; Bai, P.; et al. Fatigue-resistant and super-tough thermocells. Nat. Commun. 2025, 16, 1963.
32. Yang, Q.; Sun, X.; Ding, Q.; et al. An ATP-responsive metal-organic framework against periodontitis via synergistic ion-interference-mediated pyroptosis. Natl. Sci. Rev. 2024, 11, nwae225.
33. Fang, X.; Wang, J.; Ye, C.; et al. Polyphenol-mediated redox-active hydrogel with H2S gaseous-bioelectric coupling for periodontal bone healing in diabetes. Nat. Commun. 2024, 15, 9071.
34. Dos Santos, D. M.; Moon, J. I.; Kim, D. S.; et al. Hierarchical chitin nanocrystal-based 3D printed dual-layer membranes hydrogels: a dual drug delivery nano-platform for periodontal tissue regeneration. ACS. Nano. 2024, 18, 24182-203.
35. Ma, Y.; Yang, X.; Chen, Y.; et al. Biomimetic peridontium patches for functional periodontal regeneration. Adv. Healthc. Mater. 2023, 12, e2202169.
36. Bai, L.; Yang, M.; Wu, J.; et al. An injectable adhesive hydrogel for photothermal ablation and antitumor immune activation against bacteria-associated oral squamous cell carcinoma. Acta. Biomater. 2024, 186, 229-45.
37. Chen, L.; Tang, S.; Zhang, J.; et al. Prussian blue nanohybridized multicellular spheroids as composite engraftment for antioxidant bone regeneration and photoacoustic tomography. ACS. Nano. 2024, 18, 24770-83.
38. He, G.; Xian, Y.; Lin, H.; et al. An injectable and coagulation-independent Tetra-PEG hydrogel bioadhesive for post-extraction hemostasis and alveolar bone regeneration. Bioact. Mater. 2024, 37, 106-18.
39. Ma, S.; Li, Y.; Yao, S.; et al. A deformable SIS/HA composite hydrogel coaxial scaffold promotes alveolar bone regeneration after tooth extraction. Bioact. Mater. 2025, 46, 97-117.
40. Xing, Q.; Zhen, L.; Zhou, X.; et al. Cohesion regulation of polyphenol cross-linked hydrogel adhesives: from intrinsic cross-link to designs of temporal responsiveness. Adv. Funct. Mater. 2025, 35, 2414294.
41. Li, M.; Su, Z.; Zhu, J.; et al. Clinically oriented oral environment-triggered underwater adhesives for root caries treatment through dentinal tubule occlusion and remineralization. ACS. Appl. Mater. Interfaces. 2025, 17, 16576-89.
42. Liu, S.; Wang, Y. N.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-responsive thermosensitive hydrogel loaded with SDF-1 facilitates in situ periodontal tissue regeneration. ACS. Appl. Mater. Interfaces. 2021, 13, 36880-93.
43. Wu, D.; Wang, P.; Wu, Q.; et al. Preparation and characterization of Bomidin-loaded thermosensitive hydrogel for periodontal application. J. Mater. Res. 2022, 37, 3021-32.
44. Hao, M.; Zhang, D.; Wang, W.; et al. HAp thermosensitive nanohydrogel cavities act as brood pouches to incubate and control-release NSCs for rapid spinal cord injury therapy. Adv. Funct. Mater. 2022, 32, 2203492.
45. Chen, L.; Peng, M.; Zhou, J.; et al. Supramolecular photothermal cascade nano-reactor enables photothermal effect, cascade reaction, and in situ hydrogelation for biofilm-associated tooth-extraction wound healing. Adv. Mater. 2023, 35, e2301664.
46. Sun, J.; Chen, T.; Zhao, B.; et al. Acceleration of oral wound healing under diabetes mellitus conditions using bioadhesive hydrogel. ACS. Appl. Mater. Interfaces. 2023, 15, 416-31.
47. Zhang, Z.; Zhang, Q.; Gao, S.; Xu, H.; Guo, J.; Yan, F. Antibacterial, anti-inflammatory and wet-adhesive poly(ionic liquid)-based oral patch for the treatment of oral ulcers with bacterial infection. Acta. Biomater. 2023, 166, 254-65.
48. Chen, A.; Deng, S.; Lai, J.; et al. Hydrogels for oral tissue engineering: challenges and opportunities. Molecules 2023, 28, 3946.
49. Qi, Y.; Yang, J.; Chi, Y.; et al. Natural polyphenol self-assembled pH-responsive nanoparticles loaded into reversible hydrogel to inhibit oral bacterial activity. Mol. Biomed. 2022, 3, 28.
50. Liu, M.; Huang, L.; Xu, X.; et al. Copper doped carbon dots for addressing bacterial biofilm formation, wound infection, and tooth staining. ACS. Nano. 2022, 16, 9479-97.
51. Dai, C.; Shi, Z.; Xu, Y.; et al. Wearable multifunctional hydrogel for oral microenvironment visualized sensing coupled with sonodynamic bacterial elimination and tooth whitening. Adv. Healthc. Mater. 2025, 14, e2401269.
52. Deng, S.; Zhang, Y.; Qiao, Z.; et al. Hierarchically designed biodegradable polylactide particles with unprecedented piezocatalytic activity and biosafety for tooth whitening. Biomacromolecules 2023, 24, 797-806.
53. Zhang, H.; Zhu, Y.; Li, Y.; et al. A bifunctional zwitterion-modified porphyrin for photodynamic nondestructive tooth whitening and biofilm eradication. Adv. Funct. Mater. 2021, 31, 2104799.
54. Wang, Y.; Wang, S.; Meng, Y.; et al. Pyro-catalysis for tooth whitening via oral temperature fluctuation. Nat. Commun. 2022, 13, 4419.
55. Gao, W.; Liu, Y.; Li, M.; et al. A drop-by-drop self-assembled all-natural hydrogel as a desensitizer for rapid and enduring management of dentin hypersensitivity. Adv. Healthc. Mater. 2024, 13, e2303153.
56. Zhu, J.; Zhang, M.; Qiu, R.; et al. Hagfish-inspired hydrogel for root caries: a multifunctional approach including immediate protection, antimicrobial phototherapy, and remineralization. Acta. Biomater. 2024, 188, 117-37.
57. Muşat, V.; Anghel, E. M.; Zaharia, A.; et al. A chitosan-agarose polysaccharide-based hydrogel for biomimetic remineralization of dental enamel. Biomolecules 2021, 11, 1137.
58. Zhu, J.; Zhen, L.; Gao, Y.; et al. Bionic wet-adhesive tooth band-aid with bidirectional anchoring to promote dentin remineralization. Chem. Eng. J. 2025, 514, 163314.
59. Soares, D. G.; Bordini, E. A. F.; Swanson, W. B.; de Souza Costa, C. A.; Bottino, M. C. Platform technologies for regenerative endodontics from multifunctional biomaterials to tooth-on-a-chip strategies. Clin. Oral. Investig. 2021, 25, 4749-79.
60. Xie, Z.; Shen, Z.; Zhan, P.; et al. Functional dental pulp regeneration: basic research and clinical translation. Int. J. Mol. Sci. 2021, 22, 8991.
61. Eramo, S.; Natali, A.; Pinna, R.; Milia, E. Dental pulp regeneration via cell homing. Int. Endod. J. 2018, 51, 405-19.
62. Wang, S.; Xing, X.; Peng, W.; et al. Fabrication of an exosome-loaded thermosensitive chitin-based hydrogel for dental pulp regeneration. J. Mater. Chem. B. 2023, 11, 1580-90.
63. Park, E. H.; Park, R.; Seo, J.; Kim, W.; Kim, H. Y.; Shon, W. J. Efficacy of a novel remotely-generated ultrasonic root canal irrigation system for removing biofilm-mimicking hydrogel from a simulated isthmus model. Int. Endod. J. 2023, 56, 765-74.
64. Li, T.; Luo, Y.; Wu, S.; et al. Super-rapid in situ formation of a silver ion-induced supramolecular hydrogel with efficient antibacterial activity for root canal disinfection. ACS. Appl. Mater. Interfaces. 2023, 15, 29854-65.
65. Wang, L.; Sun, L.; Bian, F.; Wang, Y.; Zhao, Y. Self-bonded hydrogel inverse opal particles as sprayed flexible patch for wound healing. ACS. Nano. 2022, 16, 2640-50.
66. Wang, J.; Ren, H.; Liu, Y.; et al. Bioinspired artificial liver system with hiPSC-derived hepatocytes for acute liver failure treatment. Adv. Healthc. Mater. 2021, 10, e2101580.
67. Monteiro, N.; Thrivikraman, G.; Athirasala, A.; et al. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389-99.
68. Xu, F.; Zhang, Q.; Liu, S.; Zhao, Y. Injectable, ROS-scavenging, drug-loaded hydrogel dressings of natural origin for oral postoperative care. Mater. Today. Commun. 2023, 35, 105634.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].