High-precision electrohydrodynamic printing of EGaIn-AgNPs biphasic conductive ink for conformal and lightweight bioelectrodes
Abstract
Low-melting-point liquid metals (LMs), characterized by exceptional electrical conductivity, mechanical compliance, and eco-friendly, cost-effective processability, hold great promise as flexible conductors in human-machine interfaces, wearable bioelectronics, and emerging technologies. However, their intrinsic fluidity compromises device stability, while high surface tension and low viscosity present significant challenges for high-resolution patterning and scalable manufacturability. In this study, we develop a eutectic gallium indium-silver nanoparticles (EGaIn-AgNPs) biphasic conductive ink and employ electrohydrodynamic printing to achieve precise, high-resolution patterning of the EGaIn-AgNPs biphasic structure (~5 μm). This approach strategically embeds a solid phase within the LM matrix, effectively suppressing its inherent fluidity and substantially augmenting its mechanical stability and structural robustness. By leveraging the versatility and precision of electrohydrodynamic printing, we successfully fabricate lightweight, highly resolved conductive patterns that can conform seamlessly to complex and dynamic surfaces, such as human skin and plant leaves. This advancement addresses key challenges in LM-based flexible electronics, unlocking transformative opportunities in wearable electronics, implantable devices, next-generation consumer electronics, and smart agricultural systems.
Keywords
INTRODUCTION
Stretchable conductive patterns are crucial for the development of flexible electronic devices capable of maintaining strong conformity under mechanical deformation. These patterns demand exceptional electrical conductivity, high stretchability, long-term stability, and mechanical reliability[1-4]. Such materials have found extensive applications in human-machine interfaces[5-7], wearable bioelectronics[8-10], and soft actuators[11-13]. Unlike conventional rigid materials, emerging gallium-based liquid metals (LMs), celebrated for their extraordinary electrical conductivity and mechanical compliance, are increasingly recognized as ideal candidates for fabricating stretchable conductive patterns, drawing significant research attention[14,15].
Among these, eutectic gallium-indium (EGaIn) stands out due to its superior fluidity, low toxicity, and unique material properties, i.e., low melting point (below 20 °C), low viscosity (approximately 2 mPa·s), high electrical conductivity (3.4 × 106 S/m), and negligible toxicity. These characteristics position EGaIn as a promising material for wearable devices, soft robotics, and printed electronics. Encapsulating EGaIn within flexible elastomers enables the formation of stable electrical pathways between solid circuit components while accommodating substantial deformation[16,17]. However, in contrast to solid-state materials with fixed geometries, LMs are prone to delamination from flexible substrates due to their inherent fluidity and lack of stable mechanical properties. To overcome this limitation, researchers have leveraged the “reactive wetting” behavior of LMs, wherein gallium interacts with various metals to form intermetallic compounds (IMCs). By incorporating metallic phases into EGaIn, a biphasic liquid-solid system is achieved, offering an optimized balance between exceptional electrical conductivity and high mechanical ductility.
Recent advances have demonstrated that biphasic systems comprising LMs and intermetallic phases outperform pure metals or metal-elastomer composites in achieving a synergy between fluidity and structural stability, making them particularly suitable for stretchable conductive devices. For example, the introduction of Ag[18-20], Cu[21], or Ni[22] into EGaIn produces biphasic mixtures (AgIn2/EGaIn, CuGa/EGaIn, and Ga4Ni3/InNi3/EGaIn, respectively). These materials have been successfully applied in flexible light-emitting diode (LED) displays, wearable sensors, stretchable nanogenerators, and flexible circuits. In contrast, the incorporation of other metallic elements such as Fe[23,24] and Au[25] does not result in the formation of IMCs with EGaIn. Instead, these elements exist in a dispersed state within the EGaIn matrix, likewise contributing to the formation of a biphasic structure. They are often employed to meet specific requirements, for instance, in electromagnetic control or disease treatment. Among these candidates, silver nanoparticles (AgNPs) exhibit superior controllable reactivity, low interfacial energy, and the ability to rapidly form IMCs at room temperature[26,27]. Therefore, Ag is more suitable for achieving rapid and stable solid-liquid phase hybridization under printing conditions, better maintaining the continuity of the conductive network and enhancing the structural stability of the printed patterns. Despite these advancements, effectively controlling the phase behavior of LMs to achieve a combination of high electrical conductivity, exceptional stretchability, long-term stability, and reliable mechanical performance remains a critical challenge in the field.
In recent years, significant advancements have been made in the patterning technologies for EGaIn and its composite inks, leading to the exploration of various approaches[28]. For pristine LM, the most straightforward patterning strategy is the microchannel technique, which involves microchannel fabrication, vacuum injection, and sealing steps[29]. LM injection under high pressure or vacuum requires mechanically strong microchannels to prevent collapse, which necessitates the use of thick and rigid elastomers, limiting its application in flexible electronics[30,31]. Researchers have also developed various alternative methods, including direct-write printing[32], stencil printing[33], microchannel injection[34], microcontact printing[35], laser processing[36,37], and selective wetting[38,39]. However, the inherently high surface tension of EGaIn, approximately 624 mN/m, imposes substantial limitations on the achievable patterning resolution. To address this issue, previous studies have demonstrated that shear mixing[40] or ultrasonic treatment[41] of EGaIn in a carrier solvent can generate EGaIn particles, which can then be combined with other components to formulate composite inks. Owing to its high power density, ultrasonication typically produces LM particles with smaller size. This process reduces the impact of EGaIn’s high surface tension[42]. Notably, each resulting particle is encapsulated with a gallium oxide layer approximately 1~3 nm thick, which prevents particle coalescence and maintains stability within the ink[43]. These composite inks can be patterned using techniques such as inkjet printing[44], screen printing[45], transfer printing[46], or spray coating[47]. Despite these innovations, several technical challenges and resolution constraints persist, particularly for industrial applications. For instance, both screen printing and gravure printing require the fabrication of specialized stencil masks[48,49], while the resolution of inkjet printing remains limited to approximately 30 μm[50-52]. These limitations highlight the need for further advancements. Developing a printing technique that combines high efficiency, user-friendliness, and significantly enhanced resolution is therefore a critical research direction for optimizing the patterning and broadening the industrial applicability of LM composite inks.
Herein, we developed a EGaIn-AgNPs conductive ink specifically tailored for electrohydrodynamic (EHD) printing, enabling the fabrication of high-resolution EGaIn-AgNPs biphasic conductive structures. The EGaIn-AgNPs ink is synthesized through ultrasonic fragmentation of EGaIn to produce micro-nanoparticles, which are subsequently mixed with a AgNP conductive ink. The chemical interaction between Ag and EGaIn leads to the formation of IMCs (AgIn2 and Ag9In4), introducing a solid phase into the liquid EGaIn matrix. This biphasic composition effectively reduces the fluidity of the EGaIn while significantly improving its mechanical stability. Under tensile deformation, the liquid EGaIn within the biphasic structure exhibits a self-adaptive flow, forming new conductive pathways to maintain the overall electrical conductivity of the pattern. This innovative solid-liquid biphasic architecture mitigates the inherent fluidity of EGaIn while preserving its exceptional stretchability. The EGaIn-AgNPs conductive ink is patterned using advanced EHD printing technology, enabling the precise deposition of structures with line widths below 5 μm. By systematically optimizing key printing parameters, including applied voltage, nozzle-to-substrate distance, and printing speed, we achieve ultrahigh-resolution and geometrically accurate patterns. These lightweight, high-resolution electrode structures are further applied to monitor electrophysiological signals on both human skin and plant surfaces, demonstrating their versatility and functionality. High-resolution (~5 μm) and lightweight electrodes are highly effective in capturing localized, low-amplitude electrophysiological signals with minimal spatial averaging, enabling precise mapping of bioactivities in human tissues and delicate plant structures. Their small feature size and low mass minimize mechanical interference and physiological burden, ensuring negligible impact on natural behaviors. The biphasic EGaIn-AgNPs ink, through the formation of IMCs (AgIn2 and Ag9In4), enhances interfacial adhesion, prevents leakage, and maintains stable electrical performance. Meanwhile, EHD printing enables ultrahigh-resolution deposition with sub-5 μm line widths, allowing conformal electrodes to capture localized bioelectrical signals with minimal interference. Together, the ink-printing integration enables robust monitoring of human and plant electrophysiology. The integration of EGaIn-based biphasic inks with EHD printing techniques paves the way for next-generation high-precision, high-performance, and multifunctional applications in flexible electronics and beyond.
EXPERIMENTAL
Materials
All reagents were used as received without further purification. Ferrous sulfate (FeSO4), trisodium citrate (C6H5Na3O7), silver nitrate (AgNO3), and ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Gallium (Ga, 99.99%), indium (In, 99.99%), and ethylene glycol (EG) were supplied by Macklin Biochemical Co., Ltd. (Shanghai, China). PVP K30 was sourced from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). EGaIn was prepared by melting Ga and In (weight ratio 75.5:24.5) at 120 °C for 2 h. Ecoflex was procured from BASF Co., Ltd. (Ludwigshafen, Germany).
Preparation of AgNPs and AgNP-based conductive ink
AgNPs were synthesized by chemical reduction method. The synthesis process is outlined below. First,
Preparation of EGaIn particles and EGaIn-based conductive ink
A suspension of EGaIn particles was prepared by combining 1.6 g of EGaIn with 0.04 g of PVP in 8 mL of ethanol, followed by magnetic stirring at 300 rpm at room temperature for 5 min. The resulting solution was then subjected to ultrasonic treatment for 20 min using a 400 W cell disruptor probe to produce a uniform suspension of EGaIn particles. After ultrasonic processing, the suspension was allowed to settle for 10 min and was subsequently centrifuged at 4,000 rpm to remove the supernatant. The remaining sediment was dispersed into a deionized water and EG mixture, achieving a EGaIn mass fraction of 15 wt%.
Preparation of EGaIn-AgNPs conductive ink
Volumes of 1.8, 1.6, 1.4, and 1.2 mL of EGaIn conductive ink, along with 0.2, 0.4, 0.6, and 0.8 mL of AgNPs conductive ink, were measured separately and mixed pairwise. This process produced EGaIn-AgNPs conductive inks with varying ratios, specifically EGaIn:Ag = 90:10, 80:20, 70:30, and 60:20. The mixed solutions were then magnetically stirred at 300 rpm for 10 min at room temperature.
Fabrication of EGaIn-AgNPs conductive patterns by EHD printing
EGaIn-AgNPs conductive patterns were fabricated using an EHD printing platform (HEIJ-H, Wuhan Huaweike Intelligent Technology Co., Ltd.). The EGaIn-AgNPs ink was loaded into a 60 μm metal nozzle, which was connected to an air pump and a voltage source. The printing process started when a high voltage was applied between the nozzle and the substrate. After preparing the EGaIn-AgNPs conductive ink and conducting EHD printing, the patterns underwent mechanical sintering. A metal rod with rollers was used to apply pressure along the path of the EGaIn-AgNPs ink, activating the pattern and imparting electrical conductivity. Subsequently, 2 mL of Ecoflex prepolymer (1:1 ratio) was spin-coated over the printed pattern at 1,000 rpm for 60 s and then cured in an 80 °C oven for 2 h. Finally, the cured Ecoflex/EGaIn-AgNPs composite structure was peeled off from the glass substrate.
Fabrication of conformal devices for monitoring electrical signals on human and plant surfaces
The EGaIn-AgNPs electrodes used for monitoring electrical signals on human and plant surfaces were fabricated on Ecoflex substrates. Initially, grid or serpentine line electrodes were designed for testing, and the electrodes were subsequently fabricated by the EGaIn-AgNPs conductive patterning process. In this experiment, electrocardiogram (ECG) and electromyogram (EMG) signals were tested using a commercial platform (Run E2, Shenzhen Runyitaiyi Technology Co., Ltd., China). This system comprises working electrodes (EGaIn-AgNPs electrodes), reference electrodes, ground electrodes, a microcontroller for signal processing, a Bluetooth module, and a signal reception and plotting platform (laptop). Once the EGaIn-AgNPs electrodes are attached to the chest or the surface of the target muscle group, the test can be conducted. The experiment obtained consent from all participants.
Additionally, plant surface electrical signals were assessed using a commercial open-source platform (Plant Spikerbox, Backyard Brains, USA). This setup includes working electrodes (EGaIn-AgNPs electrodes), a signal reception main unit, conductive gel, a precision stimulator, USB data cables, ground wires, a 9 V battery, communication cables, and a signal reception/processing platform (laptop). By affixing the EGaIn-AgNPs electrodes to the surface of the plant under investigation and applying mechanical stimuli (touching with a metal rod) and trauma stimuli (cutting with scissors and burning with a flame), electrical signals were effectively recorded.
Materials characterization
Scanning electron microscopy (SEM, MERLIN Compact, ZEISS, Germany) integrated with energy-dispersive X-ray spectroscopy (EDS) was used to observe the EGaIn-AgNPs structure’s microstructural morphologies. X-ray diffraction (XRD) analysis was conducted with a D8-ADVANCE instrument (Bruker GmbH, Germany), and X-ray photoelectron spectroscopy (XPS) measurements were performed using Thermo Scientific K-Alpha (Thermo Fisher Scientific Inc., USA). The initial volume conductivity was measured using a surface resistivity meter (MCP-T370 with ASP probe, Mitsubishi Chemical Corporation, Japan). Stretchability was assessed using a universal tensile tester (FT2000, Shanghai Mifang Electronic Technology Co., Ltd., China), while electrical resistance was monitored in real time with a Keysight 34420A multimeter.
RESULTS AND DISCUSSION
Preparation and patterning process of EGaIn-AgNPs ink
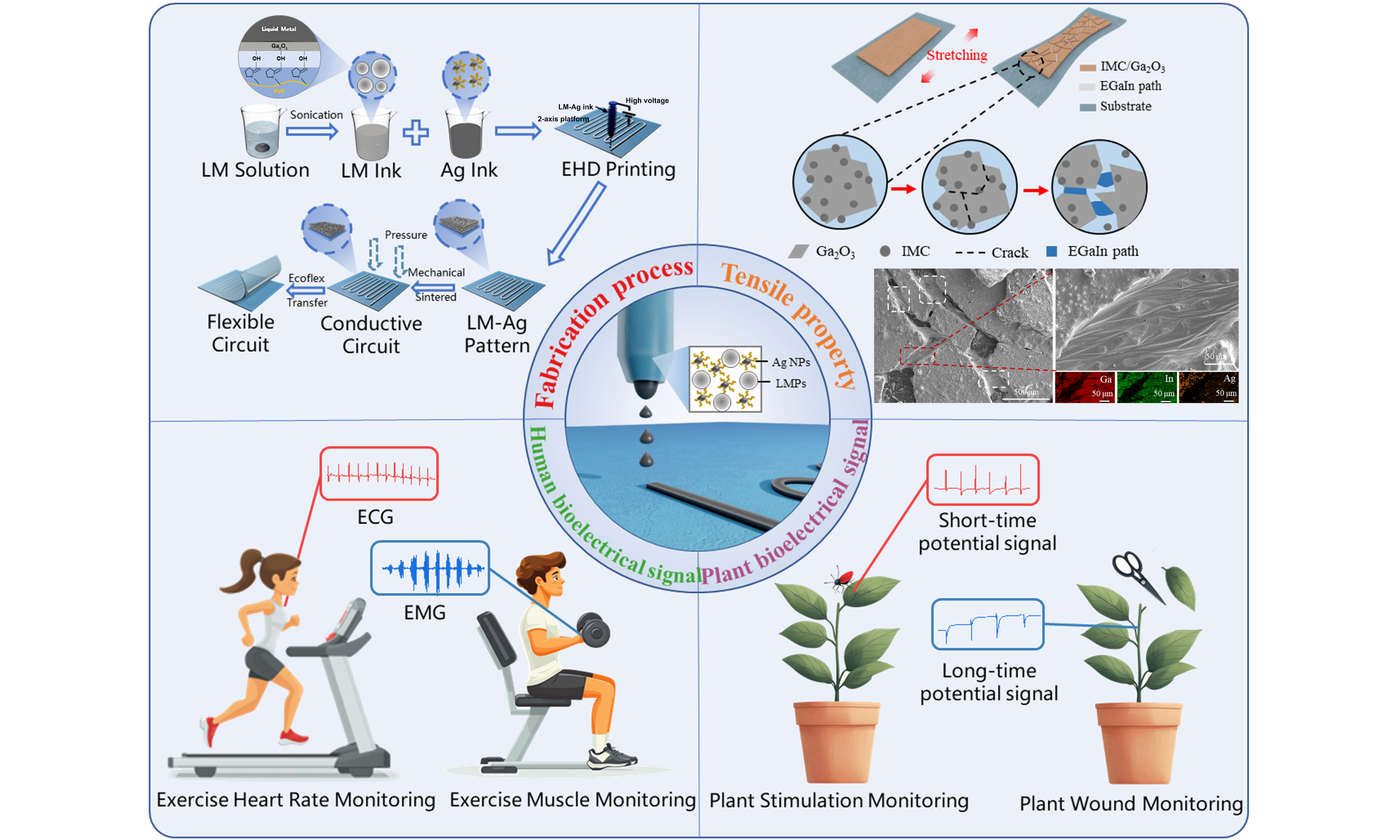
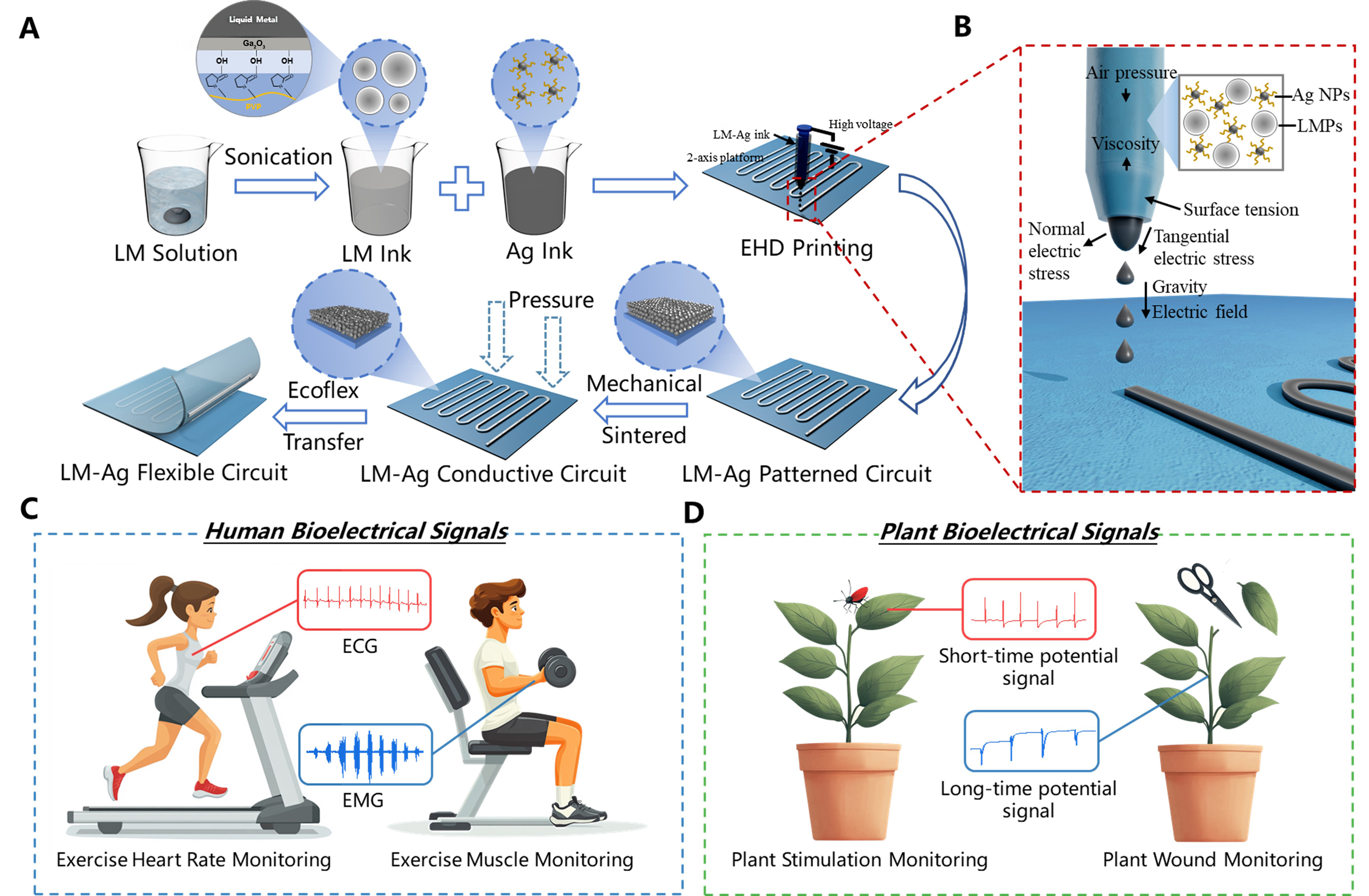
Figure 1A schematically illustrates the fabrication process of high-precision flexible devices utilizing EGaIn-AgNPs conductive ink. The process begins with the dispersion of bulk EGaIn into an ethanol solution containing polyvinylpyrrolidone (PVP). Ultrasonic treatment is applied to break the pure EGaIn into micron- and nanoscale particles. During this step, a Ga2O3 oxide layer forms on the particle surfaces, which interacts with PVP through hydrogen bonding. This interaction effectively prevents the reaggregation of the EGaIn particles into bulk form. Subsequently, the EGaIn particles are redispersed in a solution of water and EG and mechanically blended with AgNPs ink to produce the EGaIn-AgNPs conductive ink. Using EHD printing technology, high-precision patterns are formed from the ink. At this stage, the printed patterns consist of numerous discrete EGaIn particles encapsulated by oxide layers, rendering them initially non-conductive. To achieve conductivity, external pressure is applied, rupturing the oxide layers and allowing the internal liquid phase to flow outward and form continuous conductive pathways. Following this step, a thin layer of liquid Ecoflex is uniformly applied over the printed substrate. Once cured, the Ecoflex layer enables the EGaIn-AgNPs pattern to be peeled off the substrate using a scraper, yielding a freestanding, high-precision flexible device. This approach successfully fabricates advanced flexible devices based on the EGaIn-AgNPs biphasic conductive system.
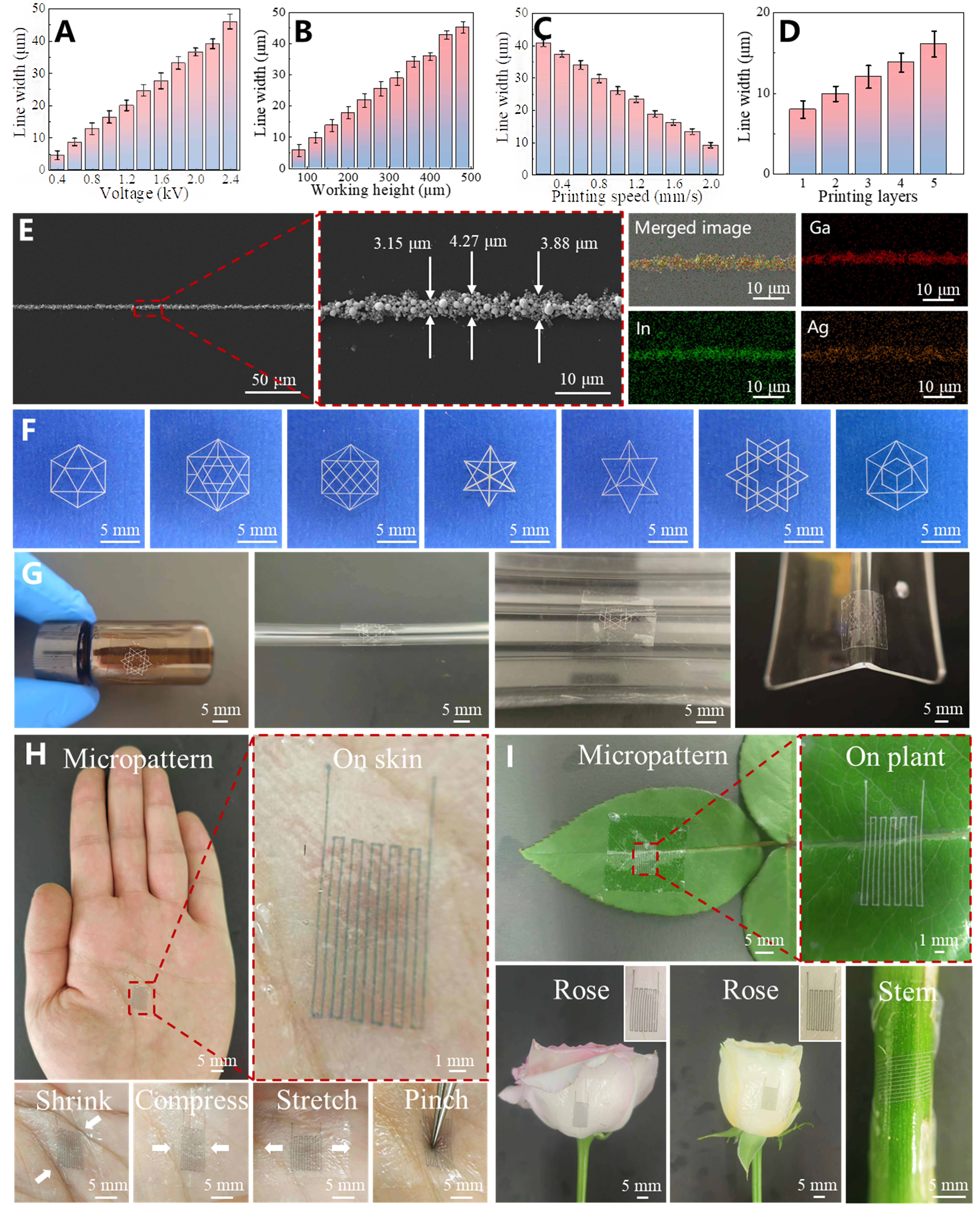
Figure 1. Patterning process and application of EGaIn-AgNPs biphasic conductive ink based on EHD printing. (A) Preparation and patterning process of EGaIn-AgNPs biphasic conductive ink. (B) Schematic diagram of the formation of a Taylor cone during the EHD printing process. Flexible electronic devices with EGaIn-AgNPs biphasic structures applied for (C) electrical signal monitoring on human skin and (D) electrical signal monitoring on plant surfaces. EHD: Electrohydrodynamic; ECG: electrocardiogram; EMG: electromyogram; LM: liquid metal; EGaIn-AgNPs: eutectic gallium indium-silver nanoparticles.
The fabrication of high-precision EGaIn-AgNPs patterns relies on the advanced capabilities of EHD printing technology [Figure 1B]. During the EHD printing process, the conductive ink at the nozzle is influenced by the interplay of air pressure, viscous forces, electric field forces, surface tension, and gravity. By precisely adjusting the voltage applied between the nozzle and the substrate, as well as fine-tuning the working distance, the strength of the electric field acting on the EGaIn-AgNPs conductive ink can be meticulously controlled. When the electric field force exceeds the combined resistances of surface tension and viscous forces, a Taylor cone forms at the nozzle tip, directing ink droplets onto the substrate with precision. The resulting line width of the pattern produced by the Taylor cone jet is significantly narrower than the nozzle diameter, enabling high-resolution patterning. EGaIn-AgNPs biphasic devices fabricated using EHD printing technology demonstrate not only high precision but also lightweight construction, making them ideal for applications in electrophysiological signal monitoring on both human and plant surfaces. When applied to humans, these electrodes are capable of measuring ECG signals under resting conditions or post-exercise, as well as EMG signals associated with muscle contraction and relaxation [Figure 1C]. In plants, these devices can detect short-term potential signals triggered by external mechanical stimuli and long-term potential signals generated in response to injury [Figure 1D].
Characterization and tensile testing of EGaIn-AgNPs ink
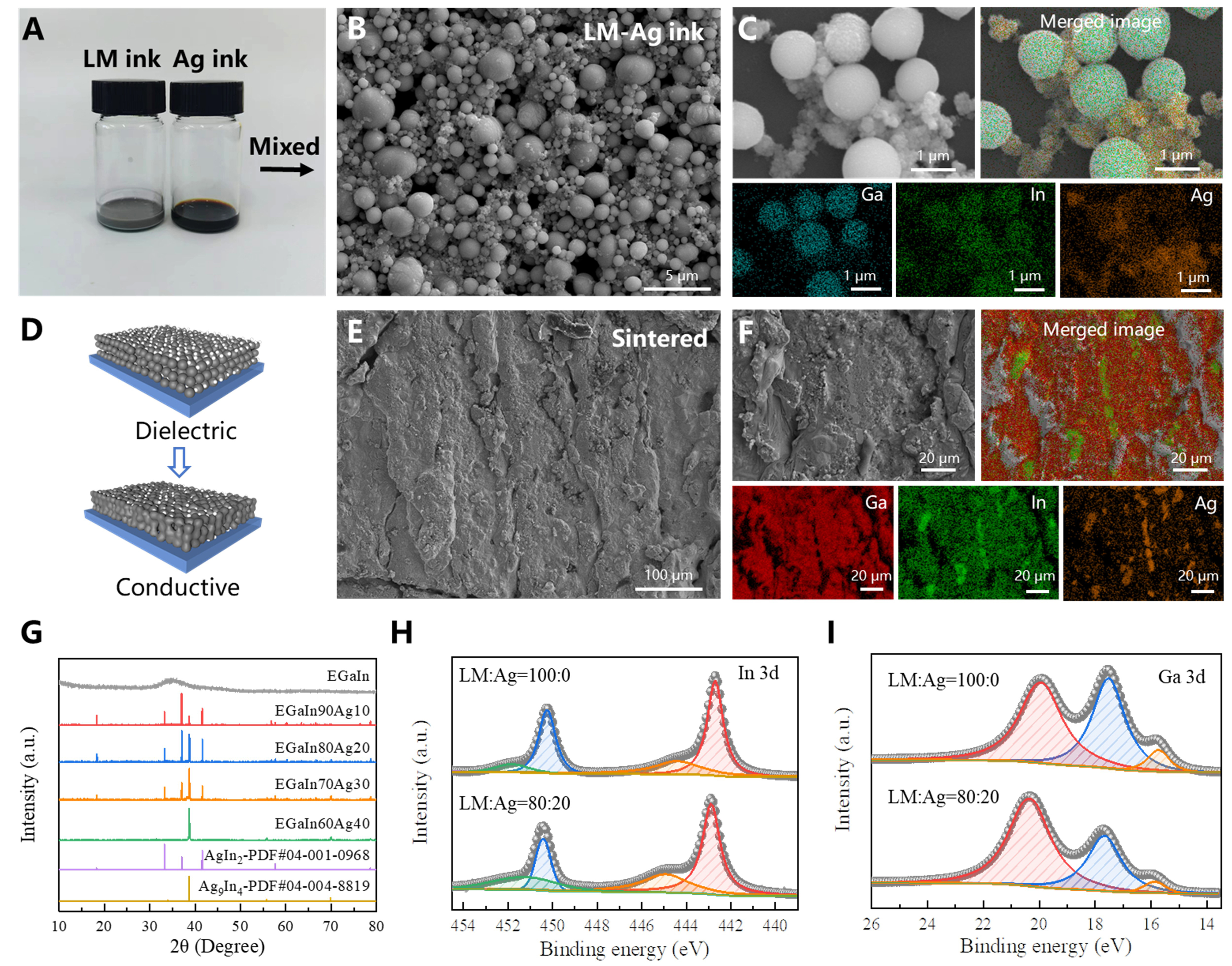
The EGaIn-AgNPs conductive ink used in this study was synthesized by mechanically mixing EGaIn ink with AgNPs ink. Specifically, the EGaIn ink was prepared through ultrasonic fragmentation of bulk EGaIn to generate EGaIn particles, as depicted on the left side of Figure 2A. The AgNPs ink, on the other hand, was synthesized via a chemical reduction method to produce AgNPs (right side of Figure 2A). By systematically increasing the ultrasonic treatment time from 1 to 60 min, the size of EGaIn particles was significantly reduced from the micron scale to approximately 300 nm [Supplementary Figure 1A-F]. Surface scanning analysis of EGaIn particles treated for 40 min revealed a uniform distribution of Ga and In without observable aggregation [Supplementary Figure 2]. This reduction in particle size and improvement in uniformity effectively minimized the risk of nozzle clogging during EHD printing, enabling stable and continuous deposition. Notably, when the ultrasonic treatment time exceeded 20 min, no nozzle clogging was observed throughout prolonged printing, as evidenced in Supplementary Figure 3. The microscopic morphology of the chemically synthesized AgNPs is shown in Supplementary Figure 1G and H. After mixing EGaIn ink and AgNPs ink, the morphology of the dried mixture at room temperature [Figure 2B], where smaller AgNPs are distributed among larger EGaIn particles. The viscosity of the ink obtained with different LM and Ag ratios is shown in Supplementary Figure 4. Surface scanning results [Figure 2C] indicate that some AgNPs react with In to form IMCs (AgIn2 and Ag9In4), while others remain isolated. Together, the AgNPs, EGaIn particles, and IMCs form a cohesive network structure. However, the presence of a Ga2O3 oxide layer on the EGaIn particle surface renders them non-conductive. External mechanical force can rupture the oxide layer, allowing the internal liquid phase to flow out and establish electrical conductivity across the pattern. This process, referred to as “mechanical sintering”, is schematically illustrated in Figure 2D. Supplementary Figure 5 highlights the boundary of mechanical sintering, showing that nanoparticles in the mechanically sintered region are flattened, and the internal liquid phase has flowed out. In contrast, the unsintered region remains unchanged and non-conductive.
Figure 2. Characterization of EGaIn-AgNPs biphasic conductive ink. (A) Optical images of EGaIn ink and AgNPs ink; (B) Microscopic morphology of EGaIn-AgNPs ink; (C) Elemental distribution of EGaIn-AgNPs ink; (D) Schematic diagram of mechanical sintering; (E) EGaIn-AgNPs structure after mechanical sintering; (F) Elemental distribution after mechanical sintering; (G) XRD results of EGaIn-AgNPs ink with different ratios; XPS results for (H) In and (I) Ga elements. XRD: X-ray diffraction; XPS: X-ray photoelectron spectroscopy; LM: liquid metal; EGaIn-AgNPs: eutectic gallium indium-silver nanoparticles.
SEM and surface scanning of mechanically sintered EGaIn-AgNPs patterns [Figure 2E and F] reveal that nearly all Ag has reacted with In to form IMCs, which anchor within the EGaIn matrix. Interestingly, Ag does not react with Ga, leaving Ga unaltered within the system. XRD analysis of EGaIn-AgNPs patterns with varying EGaIn-to-Ag ratios is shown in Figure 2G. Compared with the amorphous liquid phase of EGaIn, the incorporation of AgNPs results in the formation of two IMCs: AgIn2 and Ag9In4. Both IMCs are present when the EGaIn:Ag ratio is 90:10, 80:20, or 70:30. However, at a higher Ag content of 40% (EGaIn:Ag = 60:40), only Ag9In4 forms. This behavior occurs because, at lower Ag contents, the system contains sufficient In to support the formation of both IMCs. As the Ag content increases, the relative In concentration decreases, becoming insufficient to sustain the formation of the high-In-content IMC
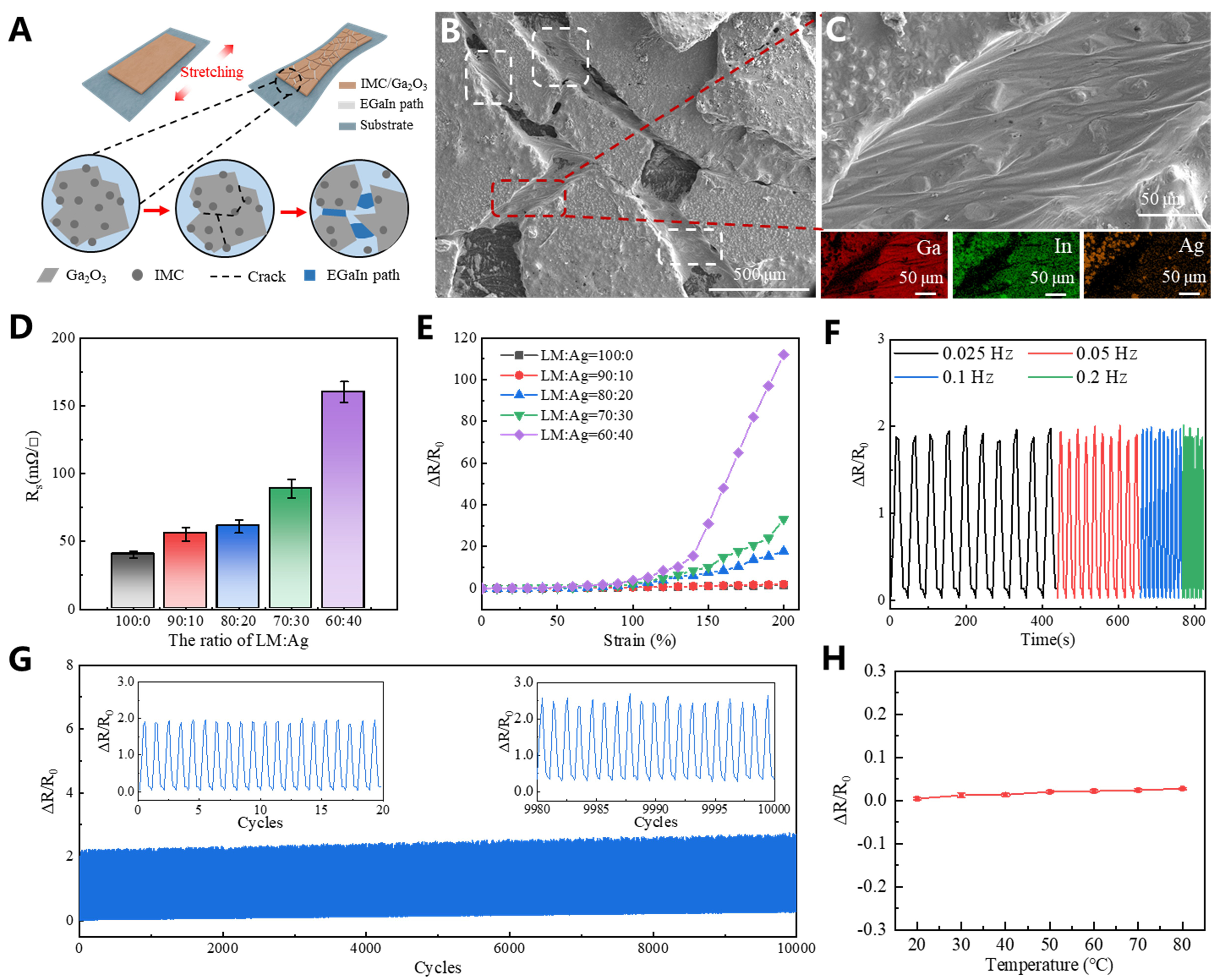
After mechanical sintering, Ag and In react to form an IMC layer that encapsulates the internal liquid EGaIn, enabling the structure to maintain excellent electrical conductivity even under tensile strain. The mechanism by which the EGaIn-AgNPs structure retains conductivity during stretching is illustrated in Figure 3A. Initially, the liquid EGaIn is fully encapsulated within the IMC layer formed by Ag and In. Upon stretching, cracks appear in the IMC layer, allowing the internal liquid EGaIn to flow outward and establish new conductive pathways. This dynamic behavior preserves the overall electrical conductivity of the structure. In a demonstration [Supplementary Figure 8], an EGaIn-AgNPs structure connected to a red LED maintains conductivity even under strains exceeding 200%, with the LED remaining lit. The corresponding demonstration video is provided as Supplementary Video 1. Figure 3B shows the microscopic structure of the IMC cracks and the conductive pathways formed by the liquid EGaIn. The red and white boxes highlight regions where the liquid EGaIn connects adjacent IMC areas. The localized surface scanning results in Figure 3C reveal the compositional distribution: Ga, In, and Ag are present in the upper-left and lower-right regions, while the central region contains only Ga and In, confirming that the conductive pathway is composed of liquid EGaIn.
Figure 3. Characterization of the tensile and electrical properties of the EGaIn-AgNPs biphasic structure. (A) Mechanism diagram of the EGaIn-AgNPs structure maintaining conductivity under stretching; (B) Microstructure of IMC cracks and EGaIn paths under stretching; (C) Local surface scanning results; (D) Effect of different Ag ratios on conductivity; (E) Resistance response of EGaIn-AgNPs structures with different Ag ratios under different strains; (F) Resistance response test of samples with 20% Ag content (EGaIn:Ag=80:20) under a frequency range of 0.025~0.2 Hz (0.025, 0.05, 0.1, 0.2 Hz); (G) Cyclic stability of the EGaIn-AgNPs structure within 10,000 cycles under 100% strain. The insets show the initial 20 cycles and the final 20 cycles, respectively; (H) Temperature stability test of EGaIn-AgNPs structure; EGaIn-AgNPs: eutectic gallium indium-silver nanoparticles.
The influence of Ag content on the conductivity of the EGaIn-AgNPs composite structure was systematically evaluated, as shown in Figure 3D. As the Ag content increased from 0 to 40 wt%, the sheet resistance rose from 40.21 ± 2.70 mΩ/□ to 160.13 ± 7.60 mΩ/□. Additionally, the electrical response of the EGaIn-AgNPs structure under tensile strain varied with Ag content, as shown in Figure 3E. Higher Ag content resulted in greater resistance changes at equivalent strain levels. When the Ag content was below
Further characterization was conducted on EGaIn-AgNPs samples with 20 wt% Ag (EGaIn:Ag = 80:20) subjected to tensile strains up to 100% at different frequencies (0.025, 0.05, 0.1, and 0.2 Hz), as shown in Figure 3F. The results indicate consistent relative resistance changes across all frequencies, demonstrating that the electrical response is largely unaffected by the applied frequency. Finally, the cyclic stability of the EGaIn-AgNPs samples was assessed under 100% strain over 10,000 cycles [Figure 3G]. Throughout the entire cyclic test, the waveform of the resistance response remained stable, indicating good cyclic stability. The peak value of ΔR/R0 increased from 1.927 to 2.504, corresponding to a 29.94% change. These findings highlight the exceptional performance of EGaIn-AgNPs composite structures in maintaining electrical and mechanical properties under prolonged cyclic loading conditions. Environmental stability tests show that EGaIn-AgNPs structures exhibit stable resistance (ΔR/R0 < 0.03) across 20~80 °C [Figure 3H] and 20%~80% RH [Supplementary Figure 10], confirming good environmental adaptability under variable ambient conditions.
Optimization of EHD printing process parameters for EGaIn-AgNPs ink
EHD printing technology facilitates the high-precision patterning of EGaIn-AgNPs conductive ink, achieving line widths as narrow as 5 µm. Critical parameters influencing the printing quality include voltage, working height (nozzle-to-substrate distance), printing speed (nozzle movement velocity), and the number of printed layers. Through systematic optimization of these parameters [Figure 4A-D], we found that reducing both voltage and working distance, while increasing printing speed, significantly improved printing resolution. Microstructural analysis of the printed lines was performed, as shown in Figure 4E. The printed lines exhibited a width of 4.51 ± 1.34 μm, with the minimum line width reaching 3.15 μm. The resulting lines demonstrated excellent continuity, structural integrity, and sharply defined edges. Elemental mapping confirmed the uniform distribution of Ga, In, and Ag across the printed lines. Additional examples of high-precision EGaIn-AgNPs line printing, along with demonstrations of adjustable line widths, are provided in Supplementary Figure 11. Notably, compared to other methods employing LM biphasic inks, our approach delivers the highest resolution, as summarized in Supplementary Figure 12 and Supplementary Table 1[18-22,54-61]. Leveraging the optimized EHD printing process, we successfully fabricated a variety of high-precision and intricate patterns, as depicted in Figure 4F. The fabrication process of representative patterns is shown in Supplementary Video 2, highlighting the exceptional capability of this technique for creating complex and detailed structures with unmatched precision.
Figure 4. EHD process parameter optimization and representative printed pattern examples. Process parameter optimization of (A) voltage; (B) working height (nozzle-substrate distance); (C) printing speed (nozzle moving speed) and (D) number of printed layers; (E) Microscopic characterization of high-precision lines printed by EHD; (F) Representative EGaIn-AgNPs patterns printed with optimized parameters; (G) Demonstration of EGaIn-AgNPs pattern conformality, attached to sample bottles, round tubes, wavy patterns, and rectangular corners; (H) EGaIn-AgNPs pattern attached to the center of the palm, which can withstand shrinking, compression, stretching, and pinching; (I) EGaIn-AgNPs pattern attached to plant surfaces such as leaves, petals and stems. EHD: Electrohydrodynamic; EGaIn-AgNPs: eutectic gallium indium-silver nanoparticles.
Moreover, EGaIn-AgNPs patterns transferred onto Ecoflex demonstrate excellent conformability, adhering well to complex curved surfaces such as bottles, tubes, and corrugated structures [Figure 4G]. The schematic diagram of the transfer process is shown in Supplementary Figure 13. Their lightweight nature and flexibility make them ideal for applications on human skin and plant leaves to monitor physiological signals. As shown in Figure 4H, serpentine electrodes conform to the palm and maintain integrity under shrinking, stretching, and pinching. Similarly, Figure 4I shows their adaptability to plant surfaces, conforming to leaves and petals. Additionally, their small size and lightweight design allow them to wrap around extremely thin plant stems, further showcasing their potential for applications requiring high flexibility and minimal interference.
Applications for monitoring physiological electrical signals in humans and plants
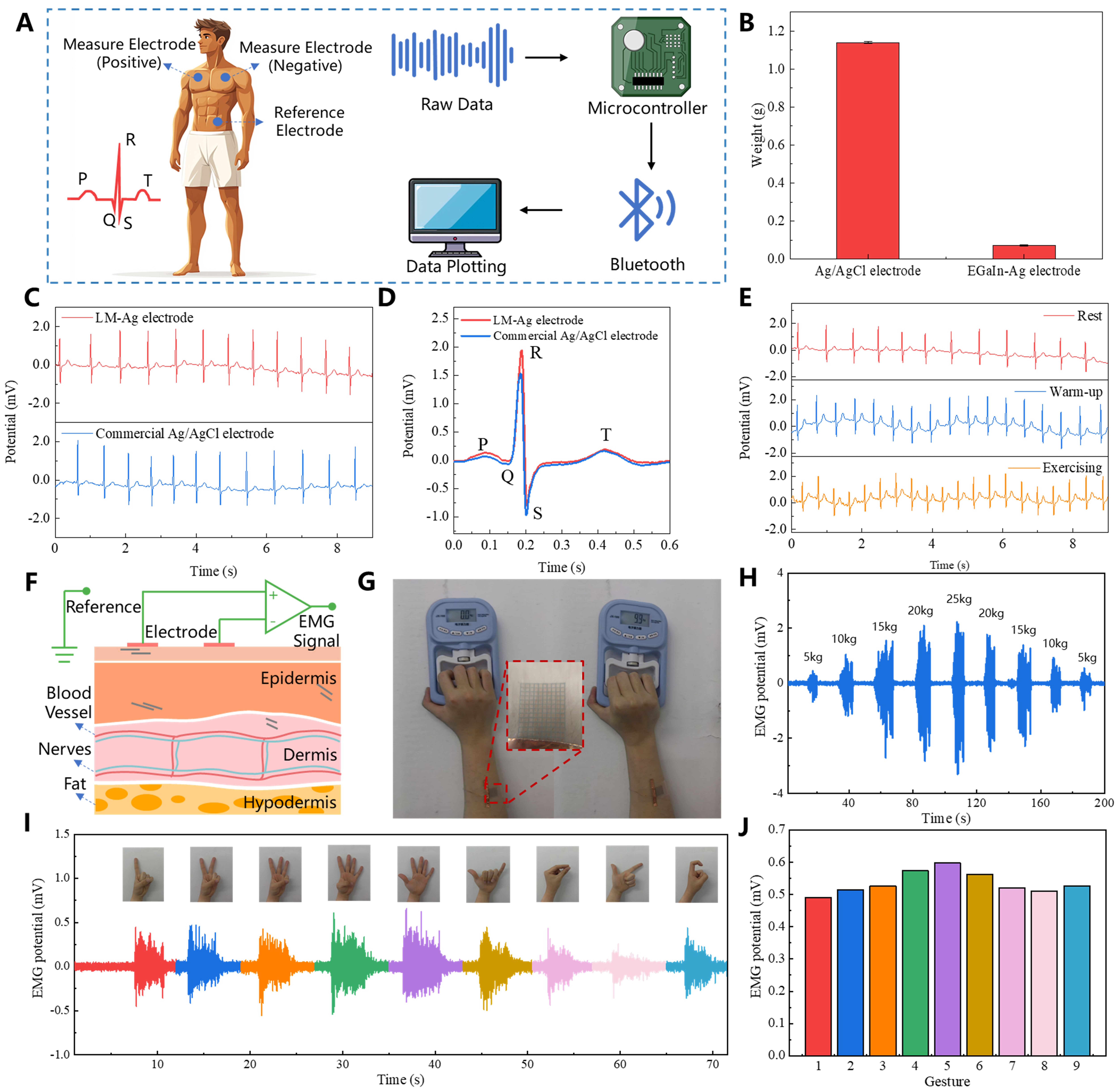
Leveraging the high conductivity and conformability of EGaIn-AgNPs electrodes, we applied them to ECG and EMG tests on human skin as a proof of concept. To fabricate the flexible bioelectrodes, the EGaIn-AgNPs patterns were transferred onto soft substrates using a flexible Ecoflex layer. Ecoflex is a widely used silicone elastomer that cures at 80 °C and remains thermally stable up to 230 °C after curing. With a tensile modulus of approximately 2.2 MPa, it provides excellent environmental stability and mechanical flexibility, making it suitable for constructing skin-conformal and stretchable electronic devices[62]. A fundamental characteristic of cardiac activity is the generation of electrical signals. These electrical currents propagate through various tissues to the skin surface, creating distinct potential changes at specific locations, which form the theoretical foundation for ECG measurement. The electrode patch arrangement for the three-lead ECG testing method is shown in Figure 5A. A real-time data acquisition system collects raw data, transmits it via Bluetooth, and displays it on a computer in real time. It is worth noting that compared with the commercial Ag/AgCl electrode (1.139 ± 0.005 g), the mass of the EGaIn-AgNPs electrode prepared by EHD printing is only 0.072 ± 0.003 g, a mass reduction of 93.68%, showing a huge advantage in lightweighting, as shown in Figure 5B. The electrode mass reduction arises from the combined effects of narrower printed lines enabled by EHD printing and the use of thinner substrates, both of which effectively limit the overall material volume, as shown in Supplementary Figure 14. In addition, Supplementary Figure 15 showed that the electrodes remained stable under weakly acidic and alkaline conditions, supporting their reliable use on human and plant surfaces for physiological signal monitoring. During resting-state ECG monitoring, both EGaIn-AgNPs electrodes and commercial Ag/AgCl electrodes captured stable, clear, and complete waveforms [Figure 5C]. Notably, compared to commercial electrodes, LM-Ag electrodes exhibited more pronounced P-wave and R-wave signals [Figure 5D]. Additionally, real-time ECG signals recorded from volunteers wearing EGaIn-AgNPs electrodes during rest, warm-up, and exercise states are shown in Figure 5E, highlighting the stable performance of EGaIn-AgNPs electrodes under varying exercise conditions. To further validate the versatility of EGaIn-AgNPs electrodes, ECG monitoring was conducted on three volunteers under different exercise states, with results shown in Supplementary Figure 16. Furthermore, for abnormal ECG conditions, such as right bundle branch block, the electrodes successfully detected irregular signals. As shown in Supplementary Figure 17, the ECG waveform exhibits a widened QRS complex compared to that of the normal subject, indicating conduction abnormalities.
Figure 5. Flexible EGaIn-AgNPs electrodes for human electrical signal monitoring. (A) Three-electrode connection positions for ECG monitoring; (B) Comparison of the weight of EGaIn-AgNPs electrode and commercial Ag/AgCl electrode; (C) Comparison of ECG monitoring effects of EGaIn-AgNPs electrodes and commercial Ag/AgCl; (D) Comparison of single ECG signal test results; (E) ECG monitoring signals of volunteers wearing EGaIn-AgNPs electrodes at rest, warm-up and exercise; (F) Schematic diagram of three-electrode EMG test; (G) Connection positions of EGaIn-AgNPs grid electrodes for testing EMG signals under different grip forces; (H) EMG signal test under different grip forces (5~25 kg); (I) Collection of EMG signals generated by different gestures (numbers 1 to 9); (J) Potential amplitudes of different gestures. ECG: Electrocardiogram; EMG: electromyogram; EGaIn-AgNPs: eutectic gallium indium-silver nanoparticles.
In addition to ECG monitoring, EGaIn-AgNPs electrodes were employed to collect EMG signals, another critical physiological indicator. Muscle activity is regulated by the nervous system, and the bioelectric currents generated during superficial muscle contractions form the basis of EMG signals. The nervous system coordinates contractions and relaxations, and the resulting diverse signals from motor units of different muscle fibers are detected on the skin surface. These potential differences are filtered and amplified before being recorded by surface electrodes, as schematically illustrated in Figure 5F. Figure 5G demonstrates the placement of a EGaIn-AgNPs grid electrode on a volunteer’s forearm. When the hand exerted varying grip strengths, the wrist flexor muscles contracted, producing corresponding EMG signals. Sequential grip forces (5, 10, 15, 20, and 25 kg) generated EMG signals shown in Figure 5H, where consistent potential amplitudes were observed for the same grip strength within each cycle. Additionally, by positioning EGaIn-AgNPs electrodes on the wrist flexors (inner forearm) of a volunteer, EMG signals corresponding to different hand gestures were monitored. The volunteer sequentially performed gestures numbered from 1 to 10, with the resulting EMG data displayed in Figure 5I. The amplitude of the EMG potentials corresponding to each gesture is summarized in Figure 5J, demonstrating the sensitivity of EGaIn-AgNPs electrodes to distinct muscle movements.
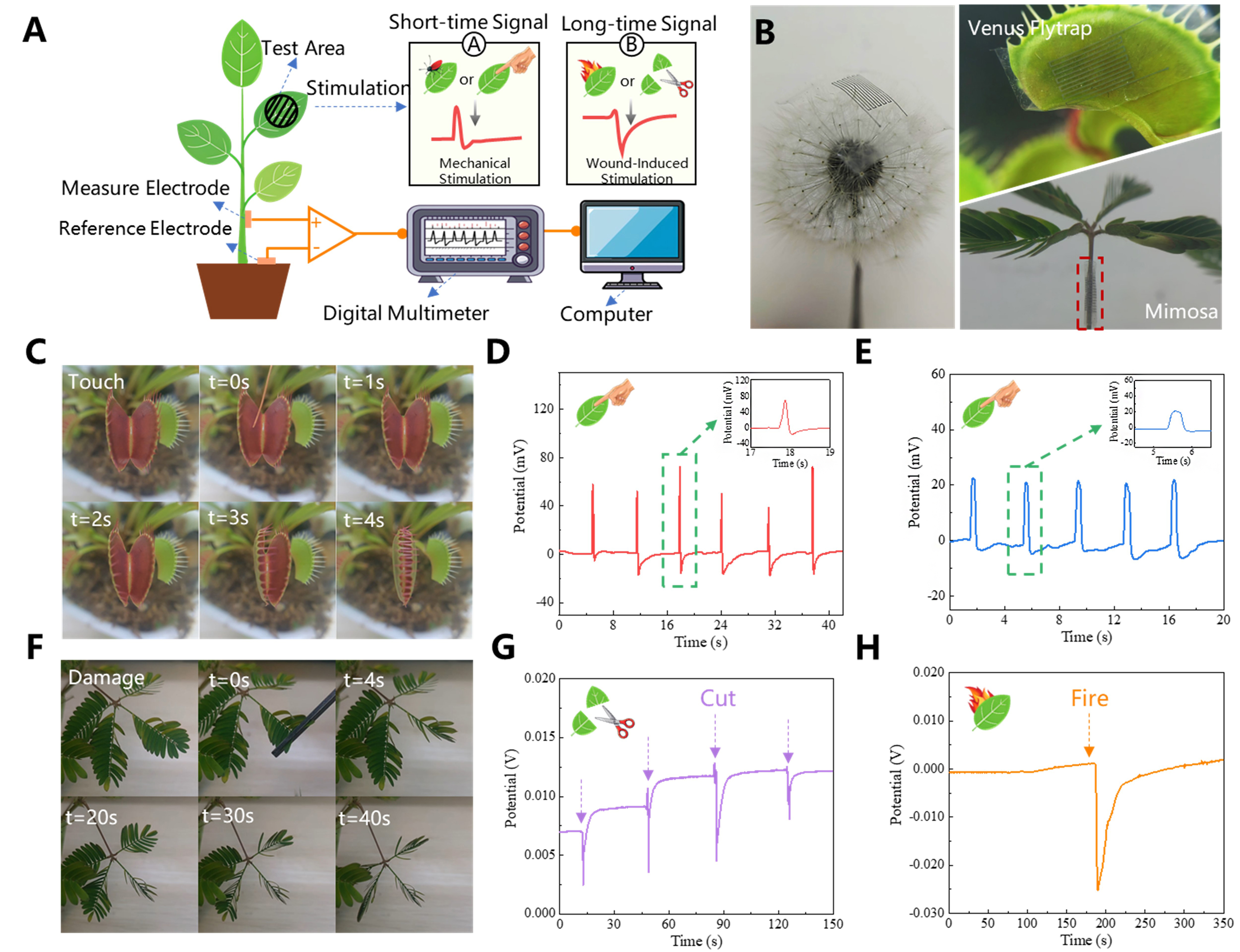
The mechanisms of electrical signal generation and conduction in plants are analogous to those observed in human physiological activities. Environmental factors such as temperature fluctuations, light intensity, and external mechanical stimuli induce widespread variations in membrane potential, which regulate plant growth and other physiological functions. These electrical signals serve as a rapid and long-distance signaling pathway, playing a vital role in plant survival and adaptability. To validate the applicability of EGaIn-AgNPs electrodes in plant electrophysiology, we utilized these electrodes to record the electrical responses of Dionaea muscipula (Venus flytrap) and Mimosa pudica under external stimuli, as shown in Figure 6A. Mechanical stimuli, such as touch, induce short-duration electrical signals in plants known as action potentials (APs), which facilitate the swift transmission of stimulus information. These APs trigger immediate responses, such as the rapid closure of Venus flytrap leaves or the folding of Mimosa leaves, enabling plants to quickly address external threats and protect themselves from harm. Conversely, traumatic events, such as cutting or burning, generate longer-duration electrical signals referred to as system potentials (SPs). SPs play a central role in regulating long-term defense mechanisms and physiological adjustments, coordinating internal resource allocation, promoting tissue repair and regeneration, and activating defensive responses that enhance the plant’s resilience to sustained stress or injury.
Figure 6. Flexible EGaIn-AgNPs electrodes for plant electrical signal monitoring. (A) Experimental setup for testing electrical signals of plants under external stimuli (mechanical stimulation and trauma); (B) Demonstration of the lightweight and conformal properties of EGaIn-AgNPs electrodes applied to plant surfaces; (C) The complete process of leaf closure caused by external stimulation of Venus flytrap; (D) The electrical signal response of Venus flytrap when touched by an external source. The inset shows a magnified image of one of the electrical signals; (E) The electrical signal response of Mimosa pudica when touched by an external source. The inset shows a magnified image of one of the electrical signals; (F) The process of leaf closure caused by trauma of Mimosa pudica and signal transmission to the rest of the body; (G) The electrical signal response of Mimosa pudica when it is injured by cutting; (H) The electrical signal response of Mimosa pudica when its leaves are burned. EGaIn-AgNPs: Eutectic gallium indium-silver nanoparticles.
Figure 6B highlights the lightweight and conformable advantages of EGaIn-AgNPs electrodes. Fabricated using EHD printing, these electrodes can adhere closely to delicate plant surfaces, such as dandelion structures, Venus flytrap leaves, and Mimosa stems. The entire process of Venus flytrap leaf closure under mechanical stimulation is depicted in Figure 6C. Upon stimulation, APs are generated within the leaves and propagate rapidly, causing changes in cell membrane potential and altering osmotic pressure. This triggers the coordinated contraction of muscle-like tissues on either side of the leaf, resulting in rapid closure. When the Venus flytrap was subjected to six consecutive mechanical stimulations, the recorded potential signals
This study employed EGaIn-AgNPs electrodes to capture the electrical responses of Dionaea muscipula and Mimosa pudica under various external stimuli, demonstrating the high reliability and applicability of these electrodes in plant electrophysiology. The lightweight and conformable properties of EGaIn-AgNPs electrodes ensure tight adhesion to plant surfaces, significantly enhancing the accuracy and sensitivity of signal acquisition while minimizing interference with the plant itself, thereby preserving the authenticity of experimental data. By distinguishing between AP and SP, this research provides deeper insights into plants’ rapid response and long-term defense mechanisms, revealing the intricate electrophysiological processes underlying their reactions to diverse stimuli. These findings not only enrich the theoretical understanding of plant electrophysiology but also introduce advanced tools and methodologies for exploring plant perception and response mechanisms. The demonstrated stability of EGaIn-AgNPs electrodes in capturing complex electrical signals highlights their broad application potential in agriculture, ecological monitoring, and plant growth regulation. For instance, EGaIn-AgNPs electrodes could be employed to monitor crop physiological states in real-time under pest infestations or environmental stress, enabling precision agricultural management and promoting ecological sustainability.
CONCLUSIONS
In this study, we developed a biphasic conductive ink composed of EGaIn and AgNPs and achieved high-resolution EGaIn-Ag structures via EHD printing. The incorporation of Ag into EGaIn initiates the formation of IMCs, specifically AgIn2 and Ag9In4, which serve as solid components within the EGaIn matrix. This integration effectively reduces the intrinsic fluidity of the EGaIn while significantly enhancing its mechanical strength. Furthermore, when subjected to mechanical stretching, the remaining EGaIn within the biphasic structure dynamically flows to form new conductive networks, thereby maintaining the overall electrical conductivity of the pattern. Using advanced EHD printing techniques, we precisely patterned the biphasic ink, achieving ultra-fine line widths below 5 µm by optimizing parameters such as applied voltage, nozzle height, and printing speed. These high-precision and lightweight electrodes were subsequently applied to monitor electrical signals on complex and dynamic surfaces, including human skin and plant leaves. The combination of EGaIn-based biphasic inks with high-resolution EHD printing provides innovative design concepts and scalable processing strategies for the development of next-generation high-precision, high-performance, and multifunctional commercial applications. While this work demonstrates promising performance in high-resolution patterning and biosignal monitoring, several challenges remain. The current fabrication process is conducted under laboratory-scale conditions, and its consistency and throughput in large-area, high-volume production still require improvement. Future efforts may focus on upgrading the printing system by designing multi-nozzle arrays to enable scalable, rapid, and uniform EHD printing for industrial applications in flexible electronics. In addition, integration with wireless communication platforms is essential to enable fully untethered, portable, and real-time monitoring systems. Furthermore, enhancing the accuracy and robustness of physiological signal acquisition may be achieved by developing low-impedance interface materials, optimizing electrode architectures, and incorporating intelligent algorithms for signal denoising and feature extraction.
DECLARATIONS
Authors’ contributions
Conceptualization, methodology, investigation and visualization: Ma, J.; Sa, Z.; Meng, F.; Feng, Z.; Sun, Y.
Data curation and resources: Feng, J.; Sa, Z.; Meng, F.
Data analysis and writing - original draft: Ma, J.; Sun, Q.
Project administration, funding acquisition and writing - review and editing: Tian, Y.; Feng, J.; Sun, Q.; Wang, S.; Wen, J.
Availability of data and materials
All data are included in the main text or Supplementary Materials. Additional details are available from the corresponding authors upon request.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Grant No. 52175300 and No. 52475336) and Heilongjiang Province Key Research and Development Program (Grant No. 2022XJ03C07).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
All physiological electrical signal tests involving human participants were performed with the consent of the volunteer (one author of the study). The experiments involved only non-invasive monitoring of physiological electrical signals on the surface of the human body, in full compliance with relevant ethical guidelines and local regulations and did not cause any risk or discomfort to the participants. This study was exempt from formal ethical review because it did not involve sensitive personal data or commercial interests.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Zhu, M.; Ji, S.; Luo, Y.; et al. A mechanically interlocking strategy based on conductive microbridges for stretchable electronics. Adv. Mater. 2022, 34, e2101339.
2. Liu, Y.; Zheng, M.; O’Connor, B.; Dong, J.; Zhu, Y. Curvilinear soft electronics by micromolding of metal nanowires in capillaries. Sci. Adv. 2022, 8, eadd6996.
3. Yoon, H.; Choi, J.; Kim, J.; et al. Adaptive epidermal bioelectronics by highly breathable and stretchable metal nanowire bioelectrodes on electrospun nanofiber membrane. Adv. Funct. Mater. 2024, 34, 2313504.
4. Xiong, W.; Zhang, F.; Qu, S.; Yin, L.; Li, K.; Huang, Y. Marangoni-driven deterministic formation of softer, hollow microstructures for sensitivity-enhanced tactile system. Nat. Commun. 2024, 15, 5596.
5. Heng, W.; Solomon, S.; Gao, W. Flexible electronics and devices as human-machine interfaces for medical robotics. Adv. Mater. 2022, 34, e2107902.
6. Choi, Y.; Kang, K.; Son, D.; Shin, M. Molecular rationale for the design of instantaneous, strain-tolerant polymeric adhesive in a stretchable underwater human-machine interface. ACS. Nano. 2022, 16, 1368-80.
7. Rahman, M. T.; Rahman, M. S.; Kumar, H.; Kim, K.; Kim, S. Metal-organic framework reinforced highly stretchable and durable conductive hydrogel-based triboelectric nanogenerator for biomotion sensing and wearable human‐machine interfaces. Adv. Funct. Mater. 2023, 33, 2303471.
8. Lu, Y.; Yang, G.; Wang, S.; et al. Stretchable graphene-hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2024, 7, 51-65.
9. Ershad, F.; Patel, S.; Yu, C. Wearable bioelectronics fabricated in situ on skins. Npj. Flex. Electron. 2023, 7, 32.
10. Bai, Y.; Zhou, Y.; Wu, X.; et al. Flexible strain sensors with ultra-high sensitivity and wide range enabled by crack-modulated electrical pathways. Nanomicro. Lett. 2024, 17, 64.
11. Shin, G.; Choi, Y.; Jeon, B.; Choi, I.; Song, S.; Park, Y. Soft electromagnetic artificial muscles using high-density liquid-metal solenoid coils and bistable stretchable magnetic housings. Adv. Funct. Mater. 2024, 34, 2302895.
12. Ma, S.; Xue, P.; Valenzuela, C.; et al. Highly stretchable and conductive MXene-encapsulated liquid metal hydrogels for bioinspired self-sensing soft actuators. Adv. Funct. Mater. 2024, 34, 2309899.
13. Ren, Z.; Zhang, M.; Song, S.; et al. Soft-robotic ciliated epidermis for reconfigurable coordinated fluid manipulation. Sci. Adv. 2022, 8, eabq2345.
14. Chen, S.; Wang, H.; Zhao, R.; Rao, W.; Liu, J. Liquid metal composites. Matter 2020, 2, 1446-80.
15. Markvicka, E. J.; Bartlett, M. D.; Huang, X.; Majidi, C. An autonomously electrically self-healing liquid metal-elastomer composite for robust soft-matter robotics and electronics. Nat. Mater. 2018, 17, 618-24.
16. Pan, C.; Markvicka, E. J.; Malakooti, M. H.; et al. A liquid-metal-elastomer nanocomposite for stretchable dielectric materials. Adv. Mater. 2019, 31, e1900663.
17. Zang, W.; Wang, Y.; Wu, W.; et al. Superstretchable liquid-metal electrodes for dielectric elastomer transducers and flexible circuits. ACS. Nano. 2024, 18, 1226-36.
18. Tavakoli, M.; Malakooti, M. H.; Paisana, H.; et al. EGaIn-assisted room-temperature sintering of silver nanoparticles for stretchable, inkjet-printed, thin-film electronics. Adv. Mater. 2018, Epub ahead of print.
19. Carneiro M, Majidi C, Tavakoli M. Multi-electrode printed bioelectronic patches for long‐term electrophysiological monitoring. Adv. Funct. Mater. 2022, 32, 2205956.
20. Lopes, P. A.; Santos, B. C.; de, Almeida. A. T.; Tavakoli, M. Reversible polymer-gel transition for ultra-stretchable chip-integrated circuits through self-soldering and self-coating and self-healing. Nat. Commun. 2021, 12, 4666.
21. Guo, R.; Li, T.; Wu, Z.; et al. Thermal transfer-enabled rapid printing of liquid metal circuits on multiple substrates. ACS. Appl. Mater. Interfaces. 2022, 14, 37028-38.
22. Wu, Y.; Deng, Z.; Peng, Z.; et al. A novel strategy for preparing stretchable and reliable biphasic liquid metal. Adv. Funct. Mater. 2019, 29, 1903840.
23. Guo, R.; Sun, X.; Yuan, B.; Wang, H.; Liu, J. Magnetic liquid metal (Fe-EGaIn) based multifunctional electronics for remote self-healing materials, degradable electronics, and thermal transfer printing. Adv. Sci. 2019, 6, 1901478.
24. Ku, H. H.; Wang, P. Y.; Huang, C. W. Remote control: electrochemically driving EGaIn@Fe liquid metal for application of soft robotics. Small 2024, 20, e2405279.
25. Guan, M.; Huang, Z.; Bao, Z.; Ou, Y.; Zou, S.; Liu, G. Gold nanoparticles incorporated liquid metal for wearable sensors and wound healing. Chem. Eng. J. 2025, 508, 161120.
26. Carneiro M, Majidi C, Tavakoli M. Gallium-based liquid-solid biphasic conductors for soft electronics. Adv. Funct. Mater. 2023, 33, 2306453.
27. Hajalilou, A.; Parvini, E.; Morgado, T. A.; et al. Replacing the gallium oxide shell with conductive Ag: toward a printable and recyclable composite for highly stretchable electronics, electromagnetic shielding, and thermal interfaces. ACS. Appl. Mater. Interfaces. 2024, 16, 61157-68.
28. Ma, J.; Krisnadi, F.; Vong, M. H.; Kong, M.; Awartani, O. M.; Dickey, M. D. Shaping a soft future: patterning liquid metals. Adv. Mater. 2023, 35, e2205196.
29. Zhu, J.; Li, J.; Tong, Y.; et al. Recent progress in multifunctional, reconfigurable, integrated liquid metal-based stretchable sensors and standalone systems. Prog. Mater. Sci. 2024, 142, 101228.
30. Kim, M.; Lim, H.; Ko, S. H. Liquid metal patterning and unique properties for next-generation soft electronics. Adv. Sci. 2023, 10, e2205795.
31. Tang, S. Y.; Khoshmanesh, K.; Sivan, V.; et al. Liquid metal enabled pump. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 3304-9.
32. Lin, Z.; Qiu, X.; Cai, Z.; et al. High internal phase emulsions gel ink for direct-ink-writing 3D printing of liquid metal. Nat. Commun. 2024, 15, 4806.
33. Wang, M.; Ma, C.; Uzabakiriho, P. C.; et al. Stencil printing of liquid metal upon electrospun nanofibers enables high-performance flexible electronics. ACS. Nano. 2021, 15, 19364-76.
34. Lin, Y.; Gordon, O.; Khan, M. R.; Vasquez, N.; Genzer, J.; Dickey, M. D. Vacuum filling of complex microchannels with liquid metal. Lab. Chip. 2017, 17, 3043-50.
35. Yalcintas, E. P.; Ozutemiz, K. B.; Cetinkaya, T.; Dalloro, L.; Majidi, C.; Ozdoganlar, O. B. Soft electronics manufacturing using microcontact printing. Adv. Funct. Mater. 2019, 29, 1906551.
36. Frey, E. J.; Im, S.; Bachmann, A. L.; Genzer, J.; Dickey, M. D. Patterning of a high surface area liquid metal-carbon composite film using laser processing. Adv. Funct. Mater. 2024, 34, 2308574.
37. Lu, T.; Markvicka, E. J.; Jin, Y.; Majidi, C. Soft-matter printed circuit board with UV laser micropatterning. ACS. Appl. Mater. Interfaces. 2017, 9, 22055-62.
38. Kim, J. H.; Kim, S.; Kim, H.; et al. Imbibition-induced selective wetting of liquid metal. Nat. Commun. 2022, 13, 4763.
39. Zhu, H.; Wang, S.; Zhang, M.; Li, T.; Hu, G.; Kong, D. Fully solution processed liquid metal features as highly conductive and ultrastretchable conductors. npj. Flex. Electron. 2021, 5, 123.
40. Shi, G.; Peng, X.; Zeng, J.; et al. A liquid metal microdroplets initialized hemicellulose composite for 3D printing anode host in Zn-Ion battery. Adv. Mater. 2023, 35, e2300109.
41. Yang, Y.; Yan, L.; Zheng, Y.; Dai, L.; Si, C. Lignin-based vitrimer for high-resolution and full-component rapidly recycled liquid metal printed circuit. Adv. Funct. Mater. 2025, 35, 2425780.
42. Lin, Y.; Genzer, J.; Dickey, M. D. Attributes, fabrication, and applications of gallium-based liquid metal particles. Adv. Sci. 2020, 7, 2000192.
43. Scharmann, F.; Cherkashinin, G.; Breternitz, V.; et al. Viscosity effect on GaInSn studied by XPS. Surf. Interface. Anal. 2004, 36, 981-5.
44. Ren, L.; Zhuang, J.; Casillas, G.; et al. Nanodroplets for stretchable superconducting circuits. Adv. Funct. Mater. 2016, 26, 8111-8.
45. Dong, R.; Wang, L.; Hang, C.; et al. Printed stretchable liquid metal electrode arrays for in vivo neural recording. Small 2021, 17, e2006612.
46. Tang, L.; Cheng, S.; Zhang, L.; et al. Printable metal-polymer conductors for highly stretchable bio-devices. iScience 2018, 4, 302-11.
47. Mohammed, M. G.; Kramer, R. All-printed flexible and stretchable electronics. Adv. Mater. 2017, 19, 1604965.
48. Liu, J.; Xiao, L.; Rao, Z.; Dong, B.; Yin, Z.; Huang, Y. High-performance, micrometer thick/conformal, transparent metal-network electrodes for flexible and curved electronic devices. Adv. Mater. Technol. 2018, 3, 1800155.
49. Wen, J.; Tian, Y.; Hao, C.; et al. Fabrication of high performance printed flexible conductors by doping of polyaniline nanomaterials into silver paste. J. Mater. Chem. C. 2019, 7, 1188-97.
50. Li, D.; Lai, W. Y.; Zhang, Y. Z.; Huang, W. Printable transparent conductive films for flexible electronics. Adv. Mater. 2018, 30, 1704738.
51. Wang, S.; Zeng, G.; Sun, Q.; et al. Flexible electronic systems via electrohydrodynamic Jet printing: a MnSe@rGO cathode for aqueous zinc-ion batteries. ACS. Nano. 2023, 17, 13256-68.
52. Zeng, G.; Sun, Q.; Horta, S.; et al. A layered Bi2Te3 @PPy cathode for aqueous zinc-ion batteries: mechanism and application in printed flexible batteries. Adv. Mater. 2024, 36, 2470004.
53. Delenne, J.; Soulié, F.; El, Youssoufi. M. S.; Radjai, F. From liquid to solid bonding in cohesive granular media. Mech. Mater. 2011, 43, 529-37.
54. Lee, G. H.; Woo, H.; Yoon, C.; et al. A personalized electronic tattoo for healthcare realized by on-the-spot assembly of an intrinsically conductive and durable liquid-metal composite. Adv. Mater. 2022, 34, e2204159.
55. Chen, W.; Tang, Q.; Zhong, W.; et al. Directly printable and adhesive liquid metal ink for wearable devices. Adv. Funct. Mater. 2025, 35, 2411647.
56. Qiu, Y.; Zou, Z.; Zou, Z.; et al. Deep-learning-assisted printed liquid metal sensory system for wearable applications and boxing training. npj. Flex. Electron. 2023, 7, 272.
57. Zhao, R.; Guo, R.; Xu, X.; Liu, J. A fast and cost-effective transfer printing of liquid metal inks for three-dimensional wiring in flexible electronics. ACS. Appl. Mater. Interfaces. 2020, 12, 36723-30.
58. Kim, M. S.; Kim, S.; Choi, J.; et al. Stretchable printed circuit board based on leak-free liquid metal interconnection and local strain control. ACS. Appl. Mater. Interfaces. 2022, 14, 1826-37.
59. Guo, R.; Cui, B.; Zhao, X.; et al. Cu-EGaIn enabled stretchable e-skin for interactive electronics and CT assistant localization. Mater. Horiz. 2020, 7, 1845-53.
60. Lopes, P. A.; Fernandes, D. F.; Silva, A. F.; et al. Bi-Phasic Ag-In-Ga-embedded elastomer inks for digitally printed, ultra-stretchable, multi-layer electronics. ACS. Appl. Mater. Interfaces. 2021, 13, 14552-61.
61. Carneiro M, de Almeida AT, Tavakoli M, Majidi C. Recyclable thin-film soft electronics for smart packaging and E-skins. Adv. Sci. 2023, 10, e2301673.
62. Siegenthaler, K. O.; Künkel, A.; Skupin, G.; Yamamoto, M. Ecoflex® and Ecovio®: biodegradable, performance-enabling plastics. In: Rieger, B.; Künkel, A.; Coates, G.W.; Reichardt, R.; Dinjus, E.; Zevaco, T.A.; Eds.; Synthetic biodegradable polymers. Berlin: Springer Berlin Heidelberg, 2012; pp 91-136.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].