Histopathological characteristics in livers of deceased patients with COVID-19
Abstract
Aim: The research aims to describe histopathological characteristics in the livers of deceased patients with COVID-19 and to identify possible associations with clinical and epidemiological variables.
Methods: An observational, descriptive, cross-sectional study was conducted with 559 deceased individuals over 19 years of age who underwent minimally invasive autopsies in various Cuban hospitals between October 2021 and December 2022. Epidemiological and liver histopathological data were analyzed. A logistic regression model was used to evaluate the variables that influence the likelihood of hepatocellular necrosis.
Results: The deceased were predominantly male (n = 323, 58.0%), with a mean age of 69.4 ± 15.6 years. An acute necroinflammatory pattern was predominant, with hepatocellular necrosis observed in 486 cases (87.0%), particularly confluent necrosis in 285 cases (51.0%). Necrosis was associated with multimorbidity odds ratio (OR): 1,986 (1,055-3,737), microvacuolar steatosis (> 5%) OR = 3,348 (1,188-9,439) and steatotic liver disease OR = 8,625 (3,093-24,050). Multivariate analysis confirmed steatotic liver disease adjusted OR = 4.657; (95% confidence interval [CI]: 1.872-14.985) and cirrhosis adjusted OR = 8.294; (95% CI: 3.18-18.762) as significant predictors.
Conclusion: Hepatocellular necrosis, particularly confluent necrosis, was the predominant histopathological lesion in COVID-19 deaths in Cuba and was associated with steatotic liver disease and cirrhosis. These findings highlight the importance of monitoring liver function in patients with pre-existing liver conditions during and after SARS-CoV-2 infection.
Keywords
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is a systemic illness with a broad spectrum of clinical manifestations. While respiratory complications have been most prominent, hepatic involvement has emerged as a significant feature in both clinical and pathological studies[1,2]. Liver injury in patients with COVID-19 is frequently observed and has been associated with increased disease severity and mortality, particularly among individuals with pre-existing hepatic conditions such as cirrhosis or metabolic dysfunction-associated steatotic liver disease (MASLD)[3].
Autopsy-based investigations have documented a range of hepatic histopathological findings in COVID-19 fatalities, including steatosis, lobular and portal inflammation, Küpffer cell activation, hepatocellular necrosis, and vascular alterations such as sinusoidal congestion and venous thrombosis[4-7].
Cuba reported its first cases of COVID-19 in March 2020, and subsequently implemented a national autopsy protocol utilizing minimally invasive autopsy (MIA) techniques. This initiative aimed to enhance understanding of COVID-19 pathophysiology through systematic postmortem tissue analysis, yet comprehensive reporting of liver-specific findings remains limited[8,9].
The present study aims to describe the histopathological features of the liver in a cohort of Cuban patients who died with confirmed COVID-19, and to identify clinical and epidemiological factors associated with hepatocellular necrosis. By characterizing the hepatic pathology in this setting, we seek to contribute to a more globally representative understanding of SARS-CoV-2-related liver injury and its clinical implications.
METHODS
Study design and setting
An observational, descriptive, cross-sectional study was conducted from October 2021 to December 2022 in several Cuban hospitals located in Havana and neighboring provinces. The study population included adult patients (≥ 19 years) with confirmed COVID-19 who died during hospitalization and who underwent MIA as part of the Cuban National Action Protocol for COVID-19[9].
Participants
Inclusion criteria were: (1) confirmed SARS-CoV-2 infection by reverse transcription-polymerase chain reaction (RT-PCR) prior to death, (2) age ≥ 19 years, and (3) availability of liver tissue obtained through MIA. Exclusion criteria included insufficient clinical data and poor-quality histological samples (e.g., improperly fixed, sectioned, or stained).
Between 2020 and 2022, a total of 1,290 COVID-19-related deaths were recorded in Havana[8]. Clinical data and histological liver samples were obtained for 800 of these cases and subsequently sent to a team of expert pathologists for evaluation. In October 2021, liver pathology specialists were formally incorporated into the research team, which had been established specifically for the purposes of this study. From that point onward, 563 of the collected samples underwent detailed histopathological analysis. Four cases were excluded due to inadequate sample quality or incomplete clinical documentation, resulting in a final analytical cohort comprising 559 cases [Figure 1].
Data sources and sample processing
Clinical and demographic data were extracted from standardized medical records using a preapproved case report form. Minimally invasive autopsies were performed at two designated centers: the “Dr. Luis Díaz Soto” Clinical and Surgical Hospital and the “Salvador Allende” Clinical and Surgical Hospital. One-cm liver samples were taken, fixed in 10% formalin for ≥ 48 h, and processed. The data collection form, paraffin blocks, and histological sections were sent to the central pathology laboratory of the Gastroenterology Institute of Havana.
Paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) for gross histological evaluation and with Masson's trichrome stain for fibrosis detection. All specimens were reviewed by a panel of five experienced pathologists blinded to clinical data. Diagnostic discrepancies were resolved by consensus or evaluated by an experienced pathologist.
Variables
Hepatocellular necrosis was considered as the dependent variable, defined as histological evidence of hepatocyte death accompanied by infiltration of inflammatory cells, activation of Küpffer cells and tissue disintegration, categorized as focal or confluent[10].
Independent variables were grouped into three domains:
Epidemiological: age (dichotomized at ≤ 69 vs. > 69 years based on the sample mean), sex, length of hospital stay (≤ 10 vs. > 10 days), and multimorbidity (defined as the presence of ≥ 2 chronic conditions including hypertension, diabetes mellitus, obesity, cardiovascular disease, chronic kidney disease, or malignancy)[11].
Acute histopathological findings: focal/confluent necrosis, apoptosis, Küpffer cell hyperplasia and hypertrophy, sinusoidal congestion, portal inflammation, periportal ductular reaction, venous thrombus, microvacuolar steatosis (> 5%), cholestasis, and endothelitis[12].
Chronic histopathological findings: liver fibrosis, steatotic liver disease (SLD), chronic liver congestion, chronic hepatitis, and cirrhosis[12].
Variable selection was informed by a systematic literature review across major databases (PubMed, Scopus, SciELO, Dialnet), supplemented with snowball sampling of references. Expert consensus was reached through a nominal group technique involving specialists in hepatology, pathology, and biostatistics.
Image analysis
The images are original, captured using an Olympus DP21 digital camera coupled to a Nikon Eclipse Model microscope and controlled by cellSens Standard v3.1 software (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics summarized patient characteristics and histopathological findings. Normality of continuous variables (age and hospital stay) was assessed using the Kolmogorov-Smirnov test, and variables were dichotomized based on mean values.
Associations between categorical variables and hepatocellular necrosis were evaluated using Chi-square or Fisher’s exact test, as appropriate. Variables with a P-value < 0.20 in univariate analyses were included in multivariate logistic regression models to identify independent predictors of hepatocellular necrosis, alongside clinically relevant covariates identified a priori (e.g., cirrhosis, venous thrombosis). Variance inflation factors (VIFs) were used to assess multicollinearity; variables with VIF > 10 were excluded from the final model to preserve model stability[13-15].
Model performance was assessed using the Hosmer–Lemeshow goodness-of-fit test for calibration and receiver operating characteristic (ROC) curve analysis to evaluate discrimination, with the area under the curve (AUC) reported alongside 95% confidence intervals (CIs).
RESULTS
Study population
The final cohort consisted of 559 deceased patients, with a mean age of 69.4 ± 15.6 years. Males constituted 58.0% of the sample (n = 323) and were significantly older than females (mean age: 70.6 ± 13.6 vs. 67.5 ± 17.8 years, P = 0.028). Multimorbidity was present in 87.3% of the patients, most commonly involving hypertension, diabetes mellitus, and cardiovascular disease [Table 1].
Distribution of variables in the study population
| Epidemiological variables | n (559) | Percentage |
| Age, years (half ± SD) | 69.4 ± 15.6 | |
| Hospital stay, days (mean ± SD) | 10.61 ± 7.1 | |
| Sex | ||
| Female | 236 | 42 |
| Male | 323 | 58 |
| Multimorbidity | ||
| No | 71 | 12.7 |
| Yes | 488 | 87.3 |
| Acute histopathological findings | ||
| Hepatocellular necrosis | 486 | 87.0 |
| Focal necrosis | 201 | 36.0 |
| Confluent necrosis | 285 | 51.0 |
| Apoptosis | 428 | 76.6 |
| Küpffer cell hyperplasia and hypertrophy | 389 | 69.6 |
| Sinusoidal congestion | 351 | 62.8 |
| Portal inflammation | 161 | 28.8 |
| Periportal ductular reaction | 138 | 24.7 |
| Venous thrombus | 111 | 19.9 |
| Microvacuoles (> 5%) | 83 | 15.0 |
| Cholestasis | 52 | 9.3 |
| Endothelitis | 26 | 4.7 |
| Chronic histopathological findings | ||
| Hepatic fibrosis | 271 | 48.5 |
| Steatotic liver disease | 237 | 42.4 |
| Chronic stasis liver | 211 | 37.7 |
| Chronic hepatitis | 35 | 6.3 |
| Cirrhosis | 29 | 5.2 |
Histopathological findings
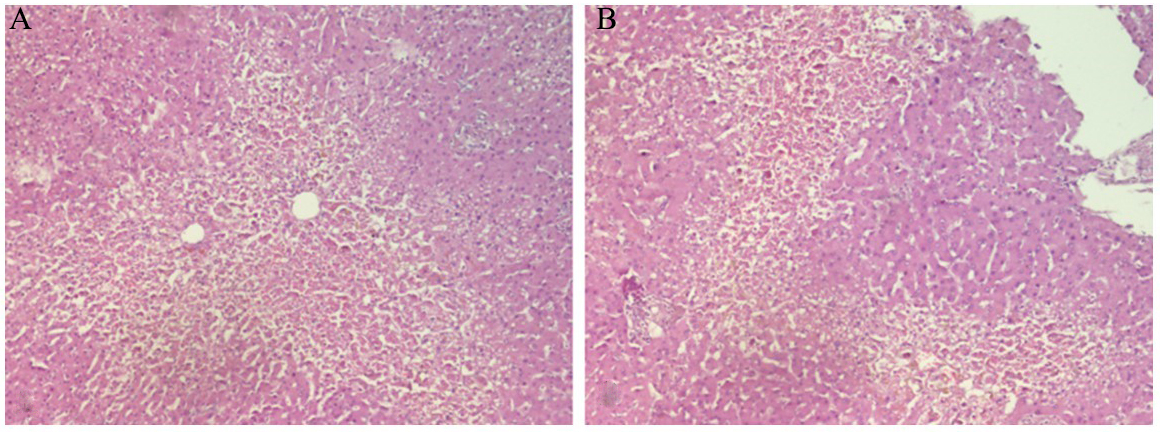
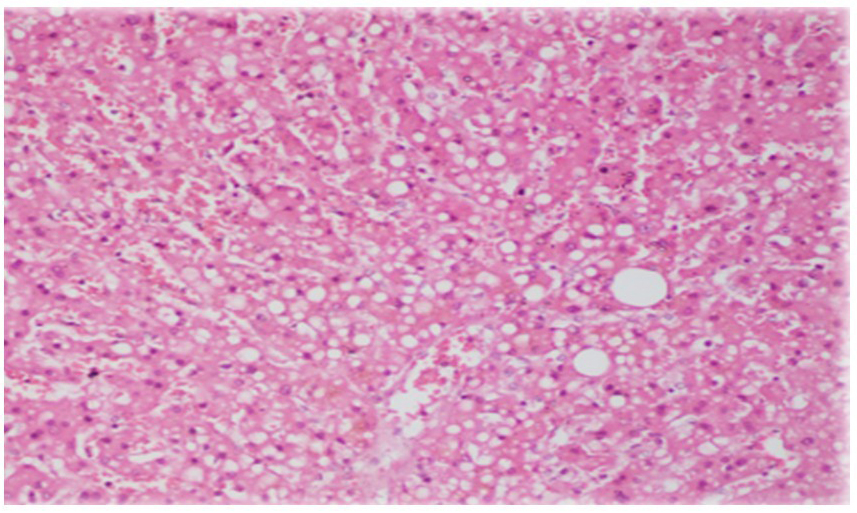
An acute necroinflammatory pattern was the predominant hepatic lesion, observed in 87.0% (n = 486) of cases [Figure 2A and B]. Microvacuolar steatosis (> 5%) was noted in 27.2%, while cholestasis was identified in only 9.3% of cases [Figure 3]. Chronic liver alterations were also frequent: liver fibrosis in 48.5%, SLD in 42.4%, and cirrhosis in 5.2% [Table 1].
Figure 2. Hepatocellular lobular necrosis. (A) Confluent necrosis around centrolobulillary veins; (B) Bridging necrosis (Haematoxylin/Eosin 10×).
Associations with hepatocellular necrosis
Bivariate analysis showed significant associations between hepatocellular necrosis and several variables, including multimorbidity, apoptosis, Küpffer cell hyperplasia and hypertrophy, sinusoidal congestion, periportal ductular reaction, microvacuolar steatosis, fibrosis, SLD, chronic hepatitis, and cirrhosis (all P < 0.05) [Table 2].
Distribution of variables according to the presence of hepatocellular necrosis in deceased patients with COVID-19
| Epidemiological variables | Hepatocellular necrosis | Total | P-value | ||
| Yes | No | ||||
| No.; (Percentage) | No.; (Percentage) | No.; (Percentage) | |||
| Age categories | ≤ 69 | 278; (57.2) | 38; (52.1) | 316; (56.5) | 0.408 |
| > 69 | 208; (42.8) | 35; (47.9) | 243; (43.5) | ||
| Hospital stay categories | ≤ 10 | 210; (43.2) | 30; (41.1) | 240; (42.9) | 0.831 |
| > 10 | 276; (56.8) | 43; (58.9) | 319; (57.1) | ||
| Sex | Female | 198; (40.7) | 38; (52.1) | 236; (42.0) | 0.090 |
| Male | 288; (59.3) | 35; (47.9) | 323; (58.0) | ||
| Multimorbidity | No | 56; (11.5) | 15; (20.5) | 71; (12.7) | 0.049 |
| Yes | 430; (88.5) | 58; (79.5) | 488; (87.3) | ||
| Acute histopathological findings | |||||
| Apoptosis | Yes | 408; (84.0) | 20; (27.4) | 428;(76.6) | < 0.001 |
| No | 78; (16.0) | 53; (72.6) | 131; (23.4) | ||
| Küpffer cell hyperplasia and hypertrophy | Yes | 379; (78.0) | 10; (13.7) | 389;(69.6) | < 0.001 |
| No | 107; (22.0) | 63; (86.3) | 170; (30.4) | ||
| Sinusoidal congestion | Yes | 314; (64.6) | 37; (50.7) | 351; (62.8) | 0,030 |
| No | 172; (35.4) | 36; (49.3) | 208; (37.2) | ||
| Portal inflammation | Yes | 149; (30.7) | 12; (16.4) | 161; (28.8) | 0,046 |
| No | 337; (69.3) | 61; (83.6) | 398; (71.2) | ||
| Periportal ductular reaction | Yes | 130; (26.7) | 8; (11.0) | 138; (24.7) | 0.004 |
| No | 356; (73.3) | 65; (89.0) | 421; (75.3) | ||
| Venous thrombus | Yes | 93; (19.1) | 18; (24.7) | 111; (19.9) | 0.452 |
| No | 393; (80.9) | 55; (75.3) | 448; (80.1) | ||
| Microvacuoles (> 5%) | Yes | 79; (16.3) | 4; (5.5) | 83; (14.8) | 0.025 |
| No | 407; (83.7) | 69; (94.5) | 476; (83.7) | ||
| Cholestasis | Yes | 50; (10.3) | 2; (2.7) | 52; (9.3) | 0.064 |
| No | 436; (89.7) | 71;(97.3) | 507; (90.7) | ||
| Endothelitis | Yes | 25; (5.1) | 1; (1.4) | 26; (4.7) | 0.232* |
| No | 461; (94.9) | 72; (98.6) | 533; (95.3) | ||
| Chronic histopathological findings | |||||
| Hepatic fibrosis | Yes | 252; (51.9) | 19; (26.0) | 271; (48.5) | < 0.001 |
| No | 234; (48.1) | 54; (74.0) | 288; (51.5) | ||
| Steatotic liver disease | Yes | 218; (44.9) | 19; (26.0) | 237; (42.4) | 0.002 |
| No | 268; (55.1) | 54; (74.0) | 322; (57.6) | ||
| Chronic liver congestion | Yes | 181; (37.2) | 30; (41.1) | 211; (37.7) | 0.614 |
| No | 305; (62.8) | 43; (58.9) | 348; (62.3) | ||
| Chronic hepatitis | Yes | 23; (4.7) | 12; (16.4) | 35; (6.3) | 0.001 |
| No | 463; (95.3) | 61; (83.6) | 524; (93.7) | ||
| Cirrhosis | Yes | 15; (3.1) | 14; (19.2) | 29; (5.2) | < 0.001 |
| No | 471; (96.9) | 59; (80.8) | 530; (94.8) | ||
In univariate logistic regression, hepatocellular necrosis was associated with: multimorbidity (odds ratio [OR] 1.98; 95% CI: 1.05-3.73), microvacuolar steatosis (> 5%) (OR = 3.34; 95% CI: 1.18-9.43), hepatic fibrosis (OR = 3.06; 95% CI: 1.76-5.31) and SLD (OR 8.62; 95% CI: 3.09-24.05) [Table 3].
Univariate analysis of patients with hepatocellular necrosis
| Selected variables | Hepatocellular necrosis | |||
| P value | Unadjusted OR | 95% CI | ||
| CI lower | CI upper | |||
| Age groups, years (≥ 69) | 0.409 | 0.812 | 0.496 | 1.330 |
| Hospital stay, days (≥ 10) | 0.734 | 0.917 | 0.556 | 1.511 |
| Sex (Male) | 0.070 | 1.579 | 0.964 | 2.587 |
| Multimorbidity | 0.033 | 1.986 | 1.055 | 3.737 |
| Küpffer cell hyperplasia and hypertrophy | < 0.001 | 22.315 | 11.072 | 44.974 |
| Sinusoidal congestion | 0.023 | 1.776 | 1.083 | 2.914 |
| Portal inflammation | 0.103 | 1.364 | 0.939 | 1.982 |
| Periportal ductular reaction | 0.005 | 2.967 | 1.386 | 6.353 |
| Venous thrombus | 0.377 | 0.796 | 0.479 | 1.321 |
| Microvacuoles (> 5%) | 0.022 | 3.348 | 1.188 | 9.439 |
| Cholestasis | 0.055 | 4.071 | 0.969 | 17.105 |
| Endothelitis | 0.185 | 3.905 | 0.521 | 29.262 |
| Hepatic fibrosis | < 0.001 | 3.061 | 1.762 | 5.317 |
| Steatotic liver disease | < 0.001 | 8.625 | 3.093 | 24.050 |
| Chronic liver congestion | 0.527 | 0.851 | 0.515 | 1.404 |
| Chronic hepatitis | < 0.001 | 0.253 | 0.120 | 0.533 |
| Liver cirrhosis | 0.419 | 0.519 | 0.106 | 2.547 |
Multicollinearity assessment led to the exclusion of Küpffer cell hyperplasia (VIF > 10) from the multivariate model. In the adjusted model, variables independently associated with hepatocellular necrosis were liver cirrhosis (adjusted OR: 8.29; 95% CI: 3.18-18.76), steatotic liver disease (adjusted OR: 4.66; 95% CI: 1.87-14.98), and microvesicular steatosis (> 5%) (Adjusted OR: 1.08; 95% CI: 1.01-11.16) [Table 4].
Results of binary logistic regression for hepatocellular necrosis in deceased patients with COVID-19
| Selected variables | P-value | Adjusted OR | 95% CI | |
| CI lower | CI upper | |||
| Sex (Male) | 0.090 | 1.062 | 0.993 | 1.137 |
| Multimorbidity | 0.916 | 0.956 | 0.056 | 3.621 |
| Sinusoidal congestion | 0.720 | 0.973 | 0.238 | 1.659 |
| Portal inflammation | 0.575 | 0.267 | 0.057 | 3.865 |
| Periportal ductular reaction | 0.753 | 0.826 | 0.252 | 2.712 |
| Microvacuoles (> 5%) | 0.030 | 1.082 | 1.006 | 11.162 |
| Cholestasis | 0.064 | 1.118 | 0.048 | 1.192 |
| Endothelitis | 0.058 | 1.083 | 0.950 | 1.235 |
| Hepatic fibrosis | 0.614 | 0.978 | 0.914 | 1.047 |
| Chronic hepatitis | 0.178 | 2.214 | 0.697 | 7.035 |
| Steatotic liver disease | 0.003 | 4.657 | 1.872 | 14.985 |
| Liver cirrhosis | 0.001 | 8.294 | 3.187 | 18.762 |
| Venous thrombus | 0.470 | 0.657 | 0.579 | 1.745 |
| Constante | 0.012 | 286.664 | ||
The area under the ROC curve was 0.900 (95% CI: 0.861-0.939; P < 0.001), which is consistent with what is represented in Figure 2.
DISCUSSION
This study presents a comprehensive histopathological analysis of hepatic alterations in a large cohort of Cuban patients who died with COVID-19. The predominant finding of confluent hepatocellular necrosis was significant, particularly among individuals with pre-existing metabolic and chronic liver conditions.
The high prevalence of necroinflammatory patterns observed aligns with prior autopsy-based investigations, which have reported hepatocellular necrosis, steatosis, and lobular inflammation as hallmark features of liver injury in COVID-19 patients[5-7]. In our cohort, SLD and cirrhosis were associated with hepatocellular necrosis. These findings are consistent with global evidence linking SLD with adverse COVID-19 outcomes, including elevated risk of mortality and multiorgan damage[16,17].
The presence of microvacuoles in the hepatocyte cytoplasm may reflect mitochondrial dysfunction secondary to hypoxia, the use of hepatotoxic drugs or anesthetic agents during intensive care unit (ICU) care, and/or a direct cytopathic effect of the virus[18,19].
Although a definitive diagnosis of MASLD could not be established in this study, the high prevalence of SLD and its association with necrosis support the hypothesis that underlying metabolic dysfunction plays a key role in COVID-19-associated liver injury[16].
Interestingly, neither age nor sex was associated with hepatocellular necrosis in our multivariate model, despite the predominance of older men in the cohort. This may reflect collinearity with metabolic comorbidities, which are known to be more prevalent in older male populations[18]. It also highlights the potential importance of metabolic status over chronological age in determining hepatic vulnerability in COVID-19[20-23].
The periportal ductular reaction observed in one-third of cases suggests an active regenerative response to extensive hepatic injury. Prior studies have proposed that ductular reactions contribute to fibrosis via cytokine-mediated recruitment of inflammatory cells and myofibroblast activation[24]. Its presence in this cohort may therefore signal both acute and evolving chronic injury pathways that warrant long-term surveillance in survivors of severe COVID-19.
Additionally, vascular alterations, such as sinusoidal congestion and occasional venous thrombi, were observed, reflecting the well-documented endothelial tropism of SARS-CoV-2 and the hypercoagulable state induced by severe infection[22,23]. These microvascular changes may contribute to hepatic ischemia, potentiating necrosis and apoptosis. In this regard, the study by Abenavoli et al. offers clear evidence of this pathological interaction: in a case series of patients who died from COVID-19, a progressive deterioration of coagulation markers was observed along with increasing liver damage, reflected in elevated Aspartate aminotransferase (AST), Alanine Transaminase (ALT), gamma glutamyl transpeptidase (GGT), and alkaline phosphatase (ALP), as well as decreased albumin[25]. The work by Abenavoli et al. also reported postmortem histopathological findings that included focal hepatocellular necrosis, predominantly in zone three of the liver lobe, supporting the hypothesis of a hypoxic and ischemic injury secondary to microthrombosis[25]. These findings are consistent with those observed in our cohort and reinforce the idea that liver damage in COVID-19 is a manifestation of systemic dysfunction involving the interaction between coagulopathy, inflammation, and metabolic stress.
Küpffer cell hyperplasia and hypertrophy were common in this cohort, highlighting the activation of the hepatic innate immune system. Although excluded from the final regression model due to multicollinearity, this marker remains biologically relevant, particularly in the context of MASLD and hypoxia-driven cytokine release. Elevated Küpffer cell activity may also amplify systemic inflammation, contributing to multiorgan dysfunction[16,20]. This systemic inflammation can affect the liver, disrupting hepatocyte function and bile transport mechanisms, contributing to cholestasis[26].
The association between hepatocellular necrosis and chronic hepatitis further underscores the vulnerability of patients with underlying liver disease. Prior meta-analyses have shown that individuals with chronic liver disease are at higher risk of progression to severe COVID-19 and liver-related complications[22,27]. These findings support current recommendations for prioritizing vaccination, close monitoring, and early intervention in patients with chronic liver disease.
While some studies have questioned the clinical relevance of liver injury in COVID-19[28-30], our data and others suggest that hepatic involvement is not only common but may reflect broader systemic pathology with prognostic implications. Given that many hepatic changes in COVID-19 are histologically subtle and may be underrecognized without systematic autopsy evaluation, our study adds important insight from a real-world setting.
It is important to emphasize that, due to the cross-sectional and postmortem nature of this study, no causal inferences or temporal relationships can be established between pre-existing liver comorbidities and the observed hepatocellular necrosis.
Future studies should investigate the temporal evolution of liver biomarkers - such as transaminases, bilirubin, international normalized ratio (INR), and inflammatory markers - during hospitalization in relation to postmortem histopathological findings. Integrating clinical, laboratory, and histological data could enhance our understanding of the underlying pathophysiology of liver injury in COVID-19. Moreover, longitudinal studies in survivors of severe disease are needed to determine whether acute histological changes are associated with long-term hepatic dysfunction, particularly in individuals with pre-existing liver disease or cirrhosis.
Limitations
This study has several limitations that merit consideration. First, its retrospective design and reliance on medical record abstraction may introduce information bias. Second, the lack of comprehensive clinical and metabolic data prevented a definitive diagnosis of MASLD, limiting the ability to assess the role of underlying liver disease. Furthermore, the use of logistic regression in a cross-sectional descriptive design, to adjust for confounders and estimate independent associations, rather than to build a predictive model, in the context of a high prevalence of hepatocellular necrosis (87%), may result in an overestimation of effect sizes, as odds ratios may inflate associations relative to prevalence ratios; future studies should consider alternative approaches such as Poisson regression with robust error variance. Finally, the absence of longitudinal data on liver function and drug exposure hampers the ability to distinguish between direct viral damage, drug-induced injury, and secondary effects of multiorgan failure. Despite these limitations, this study provides important histopathological evidence of liver injury in a high-risk cohort from a middle-income country, providing valuable insight into the hepatic manifestations of COVID-19 in diverse healthcare settings.
Conclusions
Our findings demonstrate that the liver is a target organ in severe COVID-19, predominantly exhibiting a confluent necrosis pattern. The association of necrosis with fatty liver disease and cirrhosis suggests that the liver's metabolic status and functional reserve are crucial determinants of susceptibility to SARS-CoV-2-induced liver injury. This reinforces the concept that liver injury in COVID-19 is likely multifactorial, where an underlying condition predisposes to more severe damage. Future research should determine whether this acute histological damage translates into long-term liver dysfunction in survivors, thus supporting the need for specialized hepatological follow-up after severe COVID-19.
DECLARATIONS
Acknowledgments
We thank Markos Kalligeros, MD, for assistance with the graphical abstract creation. (Created in BioRender. Kalligeros, M. (2025) https://BioRender.com/ewyfsft).
We are also deeply grateful to Dr. Justo González, a pharmacovigilance specialist, for his invaluable assistance with the translation of the document and with methodological aspects.
Authors’ contributions
Conceptualization: González Fabián L, Montero González TJ, Virginia Capó de Paz, Hurtado de Mendoza Amat J, López Marín L, Arús Soler E, Gra Oramas B, Díaz Machado A
Data Curation: González Fabián L, Díaz Machado A, Galban García E, Castellanos Fernández MI
Formal Analysis: González Fabián L, Díaz Machado A, Galban García E, Castellanos Fernández MI, Henry L
Research: González Fabián L, Díaz Machado A, Montero González TJ, Capó de Paz V, Hurtado de Mendoza Amat J, López Marín L, Cayetano Maldonado EP, Younossi ZM, Henry L
Methodology: González Fabián L, Díaz Machado A, Galban García E, Castellanos Fernández MI, Henry L
Project administration: González Fabián L
Supervision: González Fabián L, Montero González TJ, Arús Soler E, Díaz Machado A, Henry L
Visualization: González Fabián L, Díaz Machado A, Montero González TJ, Capó de Paz V, Hurtado de Mendoza Amat J, López Marín L, Cayetano Maldonado EP, Arús Soler E, Castellanos Fernández MI, Henry L
Writing - preparation of the original draft: González Fabián L, Díaz Machado A, Castellanos Fernández MI, Younossi ZM, Henry L
Writing - proofreading and editing: González Fabián L, Díaz Machado A, Montero González TJ, Capó de Paz V, Hurtado de Mendoza Amat J, López Marín L, Arús Soler E, Younossi ZM, Castellanos Fernández MI, Henry L
All authors read and approved the final version of the submitted manuscript.
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author upon reasonable request and approval by the ethics committee of the institution that approved the study.
Financial support and sponsorship
Partial funding was received from the Global NASH/MASH Council and the Center for Outcomes Research in Liver Disease.
Conflicts of interest
Linda Henry is an Editorial Board Member of Metabolism and Target Organ Damage but is not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision-making, while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
The study protocol was approved by the Institute of Gastroenterology (Havana, Cuba) ethics committee (Aval-2022-29a). All procedures adhered to national and international ethical standards for postmortem research. All data were anonymized to ensure confidentiality and were used solely for scientific purposes. The committee confirmed that informed consent was not required (Not Applicable) for this research.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.
2. Righi FA, Vander Heide RS, Graham RP, et al. A case-control autopsy series of liver pathology associated with novel coronavirus disease (COVID-19). Ann Diagn Pathol. 2024;68:152240.
3. Sarin SK, Choudhury A, Lau GK, et al.; APASL COVID Task Force. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700.
4. Sonzogni A, Previtali G, Seghezzi M, Alessio MG, Gianatti A, Licini L. Liver histopathology in severe COVID-19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-6.
5. Chornenkyy Y, Mejia-Bautista M, Bruca M, Blanke T, Dittmann D, Yeldandi A. Liver pathology and SARS-CoV-2 detection in formalin-fixed tissue of patients with COVID-19. Am J Clin Pathol. 2021;155:802-14.
6. Farrokhpour M, Rezaie N, Moradi N, et al. Infliximab and intravenous gammaglobulin in hospitalized severe COVID-19 Patients in intensive care unit. Arch Iran Med. 2021;24:139-43.
7. Sekhawat V, Green A, Mahadeva U. COVID-19 autopsies: conclusions from international studies. Diagn Histopathol. 2021;27:103-7.
8. Ministerio de Salud Pública de Cuba. Anuario Estadístico de Salud. Available from: https://temas.sld.cu/estadisticassalud/publicaciones-2/anuario-estadistico-de-salud/ [accessed 27 October 2025].
9. Mejías Sánchez Y, Morales Suárez I, Arteaga García A, Alfonso Sánchez I. Estructuración del Protocolo Cubano de Actuación para la Atención de Casos COVID-19. Rev Cub Salud Publica. 2021;47(3). Available from: https://revsaludpublica.sld.cu/index.php/spu/article/view/2922 [accessed 27 October 2025].
10. Bruguera M. El examen metódico de la biopsia hepática. In: Bosch J, Rodés J, editors. Enfermedades del hígado. 3rd ed. Barcelona: Elsevier; 2006. p. 1-15. Available from: https://www.elsevier.es/libros/enfermedades-del-higado/9788445815770.
12. Barreto IDJ, Múnera Contreras MN, Suárez Causado A. Relación entre el factor de crecimiento hepático y el estadio de la cirrosis. Rev Colomb Gastroenterol. 2017;32:24. (in Spanish).
13. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
14. Barnes GD. Predicting the quality of warfarin therapy: reframing the question. Thromb Haemost. 2019;119:509-11.
15. Heinze G, Wallisch C, Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom J. 2018;60:431-49.
16. Yoneda M, Kobayashi T, Honda Y, et al. Combination of tofogliflozin and pioglitazone for NAFLD: Extension to the ToPiND randomized controlled trial. Hepatol Commun. 2022;6:2273-85.
17. Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020;5:80.
18. Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32.
19. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-2.
20. Liu Q, Wang RS, Qu GQ, et al. Gross examination report of a COVID-19 death autopsy. J Forensic Med. 2020;36:21-3.
21. Marjot T, Webb GJ, Barritt AS, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-64.
22. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:768-771.e3.
23. Moreira JLS, Barbosa SMB, Vieira JG, Chaves NCB, Gonçalves Júnior J. Liver histopathological changes and COVID-19: what does literature have to tell us? Dig Liver Dis. 2022;54:296-8.
24. Ji D, Qin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-3.
25. Abenavoli L, Aquila I, Sacco MA, et al. Liver damage and impaired coagulation in COVID-19 patients: a case series. Diseases. 2023;11:141.
26. Mao R, Liang Y, Shen J, et al. Liver injury in COVID-19: mechanisms and potential therapeutic targets. Lancet Gastroenterol Hepatol. 2020;5:683-690.
27. Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2020;44:653-61.
28. Tazarghi A, Bazoq S, Taziki Balajelini MH, Ebrahimi M, Hosseini SM, Razavi Nikoo H. Liver injury in COVID-19: an insight into pathobiology and roles of risk factors. Virol J. 2024;21:65.
29. Cai Y, Ye LP, Song YQ, et al. Liver injury in COVID-19: detection, pathogenesis, and treatment. World J Gastroenterol. 2021;27:3022-36.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].