Research and clinical trial design in patients with metabolic dysfunction and alcohol-associated steatotic liver disease (MetALD): a narrative review
Abstract
This narrative review examines current trial design strategies and highlights the limitations of traditional models in capturing the heterogeneity and dynamic nature of metabolic dysfunction and alcohol-associated liver disease (MetALD). MetALD represents an increasingly prevalent and clinically distinct phenotype that reflects the convergence of metabolic risk factors and alcohol-related liver injury. Designing effective clinical and translational research in MetALD presents unique challenges as its burden continues to rise. Novel frameworks, such as adaptive and enrichment designs, offer improved pathways for patient stratification and intervention testing. The integration of molecular and translational biomarkers to inform diagnosis, prognosis, and therapeutic responsiveness is central to this evolution. This review also addresses critical methodological issues, including the need for harmonized definitions, careful endpoint selection, and real-world applicability of findings. Emerging therapies targeting steatosis, inflammation, fibrosis, gut-liver axis dysfunction, and metabolic pathways are now entering clinical development and require trial designs tailored to the multifaceted biology of MetALD. In this context, the article also discusses the importance of early-phase proof-of-concept studies and innovative approaches for combination therapies. Ethical and operational considerations, such as alcohol use thresholds, stigma, and disparities in access to care, further influence trial feasibility and generalizability. Finally, multidisciplinary collaboration across hepatology, addiction medicine, endocrinology, and trial methodology is essential to advance this field.
Keywords
INTRODUCTION
Steatotic liver disease (SLD) is one of the leading causes of cirrhosis worldwide, with a major global impact, affecting approximately one in three adults[1,2]. In 2023, an international panel of experts recommended new terminology for non-alcoholic fatty liver disease (NAFLD) to avoid the stigmatizing terms “alcoholic” and “fatty” and to reflect its well-established pathophysiology, despite NAFLD being historically defined by exclusion[3]. The term SLD was proposed as an overarching category for all conditions associated with hepatic steatosis, and “metabolic dysfunction-associated steatotic liver disease” (MASLD) was introduced to replace NAFLD in patients with hepatic steatosis and cardiometabolic risk factors (CMRFs)[4]. For patients who meet the MASLD criteria and also have moderate alcohol consumption, the term “metabolic dysfunction and alcohol-associated liver disease” (MetALD) was created to differentiate them from alcohol-related liver disease (ALD)[3,5].
The term MetALD refers to patients with metabolic risk factors who consume at least 20 g but less than 50 g of alcohol per day for women, and at least 30 g but less than 60 g per day for men. This new definition encompasses the cumulative effects of alcohol consumption and metabolic factors on disease progression and prognosis[3]. Since the 2023 definition, several studies have estimated the prevalence of MetALD to range from 2.5% to 10%[6-9]. For example, in the United States (US), the prevalence of MetALD is estimated to be 1.7%-2.6%[6]. In Korea, a study of 2,535 previously undiagnosed patients estimated a MetALD prevalence of 7.8%[7]. Another study based on data from the United Kingdom Biobank showed an SLD prevalence of 27%, with 7.9% meeting the MetALD criteria[8]. In addition, a recent systematic review estimated a global MetALD prevalence of 4.1% in the adult general population and 9.4% in individuals with SLD, with MetALD being more prevalent in the European Region (8.6%) than in the Western Pacific (3.9%) and the Americas (2.7%)[10].
MetALD has been linked to increased all-cause, cancer-related, and liver-related mortality, especially in those with advanced fibrosis[11]. A recent systematic review also reported higher cardiovascular and cancer-related mortality in MetALD compared to non-SLD individuals[9]. Furthermore, nationwide and population-based cohort studies confirm that MetALD is associated with a higher risk of liver and gastrointestinal cancers and overall worse outcomes than MASLD in those who do not consume alcohol[12,13], prompting several ongoing clinical trials.
Designing studies on MetALD presents several challenges. One major limitation is the difficulty in replicating MetALD in animal models, which restricts the translation of preclinical findings to human studies. Additionally, the overlapping pathophysiology and clinical features of MASLD, ALD, and MetALD hinder accurate differential diagnosis[14]. Even histological findings are inconclusive in clearly distinguishing these entities, and the lack of disease-specific biomarkers further hinders their differentiation[12]. Another key challenge is the quantification of alcohol consumption, which is frequently underreported and difficult to estimate[15]. This review aims to discuss the diagnostic and therapeutic challenges in MetALD, providing a foundation for future translational and applied research in clinical trials. It critically evaluates current diagnostic approaches, including emerging biomarkers, and explores patient stratification strategies to optimize the study methodology.
METHODS
We conducted a narrative review following standardized recommendations for expert consensus and literature synthesis in emerging hepatological conditions. A comprehensive literature search was performed using PubMed, EMBASE, and Scopus from inception through April 30, 2025, with a combination of Medical Subject Headings (MeSH) and free-text terms related to MASLD, ALD, and MetALD. The following search terms were used: “MetALD” OR “metabolic dysfunction and alcohol-associated liver disease” OR “MASLD and alcohol” OR “alcohol-related liver disease” OR “steatohepatitis” OR “clinical trials” OR “biomarkers” OR “endpoint” OR “trial design” OR “alcohol biomarkers”.
We included original research articles, systematic reviews, meta-analyses, expert consensus statements, and regulatory documents focused on MetALD or relevant to the design of clinical trials in MASLD and ALD. No language restrictions were applied. Studies were included if they addressed any of the following: epidemiology, diagnosis, risk stratification, biomarkers, regulatory aspects, trial methodology, or emerging therapies related to MetALD. References were also identified by manual review of bibliographies from key articles and guidelines. Although this is not a formal systematic review, we adopted structured approaches to improve transparency and reproducibility. Quality assessment of included studies was based on relevance, peer-review status, and methodological rigor, including sample size, design, and clarity of reported outcomes. Where applicable, we prioritized high-quality randomized controlled trials, longitudinal cohort studies, and meta-analyses.
Barriers in the design of clinical trials in MetALD
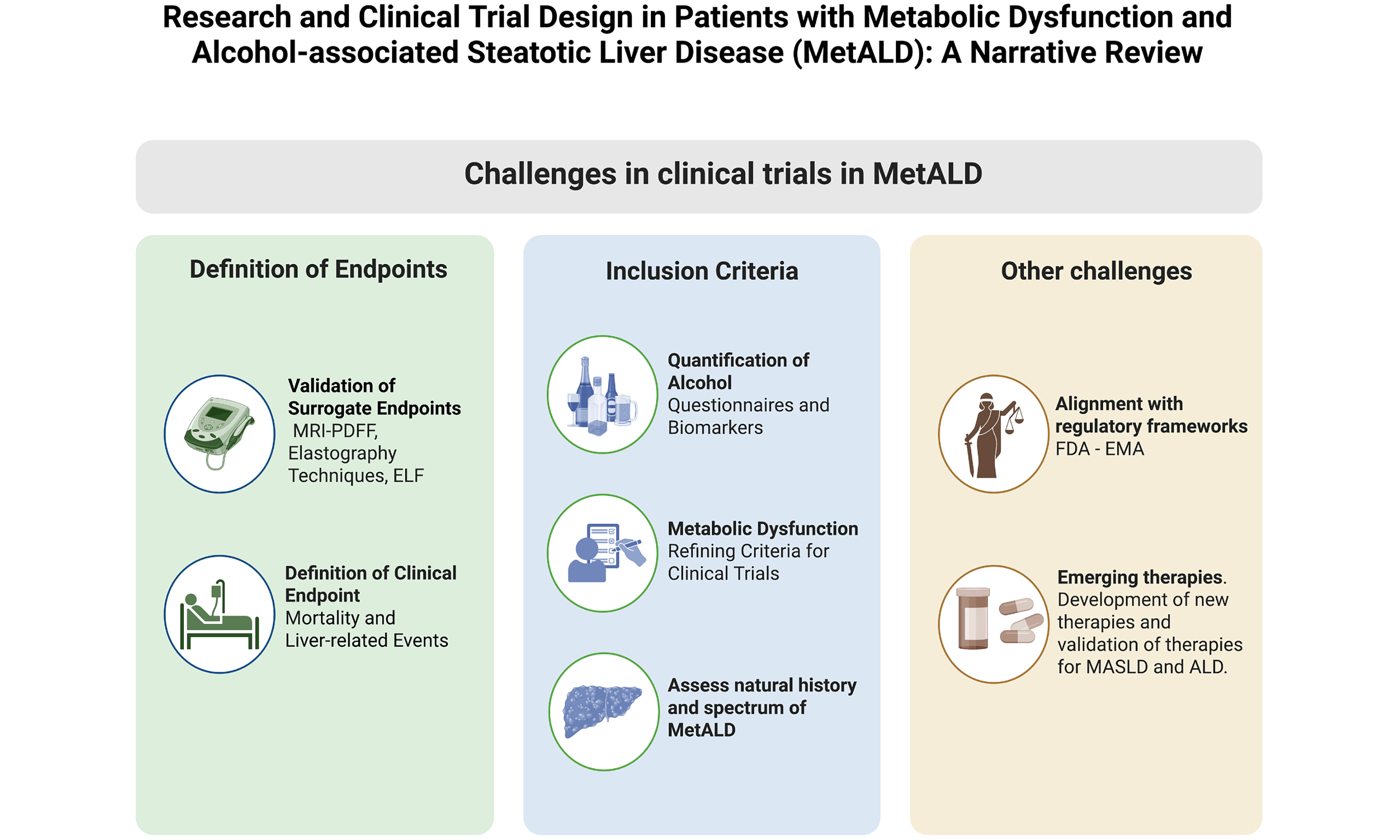
The term “MetALD” represents a conceptual advance, allowing for a more precise recognition of the coexistence of metabolic dysfunction and alcohol consumption[11]. This facilitates patient stratification and the design of studies more aligned with real-world hepatology clinics[16]. A key strength is its acknowledgment of the overlap between metabolic syndrome and alcohol consumption [Figure 1] allowing for the inclusion of a previously excluded patient population and potentially optimizing patient selection for future interventions[15]. The high global prevalence of MetALD highlights its epidemiological and clinical relevance[16].
Figure 1. Key Challenges in Designing Clinical Trials for MetALD. Trial design should consider the natural history and full spectrum of the disease, include assessment of cardiometabolic risk factors and alcohol use, address relevant legal regulations, clearly define clinical endpoints (e.g., mortality, liver-related events), and validate surrogate endpoints. Created in BioRender. Arab J. (2025) https://BioRender.com/dkn1f9j. FDA: Food and Drug Administration; EMA: European Medicines Agency; MRI-PDFF: magnetic resonance imaging-proton density fat fraction; ELF: enhanced liver fibrosis; MetALD: metabolic dysfunction-associated alcohol-related liver disease.
However, methodological challenges persist. The heterogeneity of this population, with combined variables such as insulin resistance/diabetes, obesity, dyslipidemia, and diverse alcohol consumption patterns, complicates standardization of inclusion criteria and results interpretation[17]. The additive interaction between metabolic factors and alcohol in the progression of liver fibrosis further complicates causal attribution and therapeutic designs[16].
While fibrosis stage and cardiovascular risk are the strongest predictors of MASLD-related mortality[18], moderate alcohol use in MetALD increases risks for malignancy[19,20] and results in higher mortality than MASLD alone[12-14,21]. Another barrier is the inter-individual variability in response to alcohol consumption, which prevents the establishment of safe drinking thresholds and limits the ability to define reproducible exposure criteria for clinical trials[12].
Although alcohol biomarkers, including phosphatidylethanol (PEth) and ethyl glucuronide (EtG)/ethyl sulfate (EtS), can improve subtype classification, their validation and standardization remain a challenge[22]. Additionally, factors such as the stigma associated with alcohol and self-reported underreporting can affect the accuracy of clinical phenotyping and data validity[23].
Despite these difficulties, the conceptualization of MetALD presents an opportunity to design more representative clinical studies that better reflect daily practice[24]. We propose that overcoming these challenges will require a multidisciplinary approach, innovative methodologies, and international collaborations to advance the understanding and treatment of this emerging entity.
Future research must extend beyond therapeutic targets to incorporate economic, social, and implementation dimensions[23,25]. Given the rising global burden, trials should include economic evaluations to inform policy and optimize resource utilization[26,27]. Patient-reported outcomes and quality of life measures are also critical, considering the psychosocial burden and stigma linked to alcohol use and metabolic disorders. Validated tools such as the Chronic Liver Disease Questionnaire or Patient-Reported Outcomes Measurement Information System (PROMIS) modules can provide insight into patient experiences[28,29].
Ethical and structural challenges must also be addressed as stigma can hinder enrollment, particularly among vulnerable populations with coexisting mental health conditions or socioeconomic disadvantages[30-32]. These issues, combined with care disparities, necessitate culturally sensitive and flexible trial designs. Choices such as interim analyses, response-adaptive randomization, and enrichment by PEth band are expert recommendations, as no MetALD-specific design standard exists.
Due to the likely amplification of extrahepatic complications - including renal and cardiovascular disease - by dual metabolic and alcohol injury, trials must assess multisystem outcomes rather than focusing exclusively on hepatic endpoints[22]. This broader approach would improve the real-world applicability of findings.
Finally, we propose that researchers proceed with caution. A risk of methodological errors exists if foundational elements are not fully established. A thorough understanding of the natural history of MetALD, validated diagnostic criteria, and patient stratification strategies is crucial before advancing to large-scale research[16]. Without this foundation, trial reliability and the interpretation of results may be compromised.
Regulatory considerations for MetALD clinical trials
The U.S. Food and Drug Administration (FDA) requires substantial evidence from well-designed clinical trials to ensure the safety and efficacy of new drugs[33]. Approval is based on substantial evidence from well-designed, controlled clinical trials. These trials must be grounded in a clear definition of the target pathology, including its pathophysiological, epidemiological, and clinical characteristics.
The FDA offers two primary pathways for drug approval: traditional (or standard) and accelerated. The latter, outlined under Subpart H of 21 CFR Part 314, is designed for drugs that address serious or life-threatening conditions and offer a meaningful therapeutic advantage over existing treatments[34]. Under this pathway, the FDA may grant conditional approval based on surrogate endpoints that are reasonably likely to predict a clinical benefit, such as biomarker improvements or imaging changes, rather than direct evidence of clinical efficacy. This allows for earlier access to promising therapies while confirmatory studies (post-marketing requirements) are being conducted. However, for a product to ultimately receive full (traditional) approval, the FDA requires robust clinical evidence demonstrating a meaningful effect on a clinically relevant endpoint, often referred to as a major adverse clinical outcome, such as mortality reduction, decreased hospitalization, or improved quality of life. Thus, accelerated approval serves as a provisional step, contingent on subsequent verification of benefits through well-validated outcomes[35].
Since MetALD is a newly defined entity, it faces various challenges in meeting standard regulatory requirements. The FDA has not yet recognized MetALD as an independent pathology, and a lack of consensus regarding its epidemiology and validated biomarkers complicates trial design. Given the shared pathogenic and clinical features of MetALD and MASLD, a similar regulatory approach is reasonable.
For MASLD, the FDA has accepted outcomes such as a reduction in steatohepatitis or a decrease in liver fibrosis, even in the absence of specific biomarkers[36]. Furthermore, the accelerated approval for resmetirom for MASLD management[37,38] and the recent approval of semaglutide for the treatment of MASH in adults with advanced liver fibrosis could serve as valuable precedents for MetALD.
Defining the study population
Accurate patient selection is essential for the validity of MetALD clinical trials. Studies must strictly adhere to the 2023 multi-society consensus criteria, which require evidence of metabolic dysfunction (≥ 1 CMRF) and validated moderate alcohol intake (20-50 g/day for women; 30-60 g/day for men)[3,22]. The combination of these factors significantly accelerates liver disease progression. An epidemiological study, for example, reported a 7.69-fold increased hazard of hepatic decompensation and a 15.04-fold increase in liver-related mortality among patients with fibrosis and alcohol use compared to those with SLD without alcohol use[11]. This underscores the importance of accurately assessing both metabolic dysfunction and alcohol intake.
Reliable alcohol assessment is equally critical. Biomarker-based studies show that 10%-55% of patients underreport their alcohol intake. For example, the MAESTRO-NASH trial detected undisclosed alcohol use in 10% of participants, despite strict exclusion criteria[39]. Without validated tools to detect such misclassification, studies risk enrolling inappropriate subjects and generating biased results.
Assessment of CMRFs
Accurate identification of CMRFs is crucial for defining metabolic dysfunction in MetALD[3]. These include central obesity, hypertension, dyslipidemia, and impaired glucose metabolism, all of which are common in the general population. When risk factors coexist with alcohol use, liver injury appears additive or even synergistic[40]. Histological studies have demonstrated that steatosis, inflammation, fibrosis, and cirrhosis occur more frequently in individuals who are both obese and consume alcohol excessively[16,41]. The interaction is likely multiplicative, with alcohol as the primary disease driver and metabolic dysfunction as a modifier[32,33]. For instance, type 2 diabetes mellitus (T2DM) triples the risk of cirrhosis among excessive alcohol users[42], and the combination of alcohol and metabolic syndrome is linked to an increased risk of hepatocellular carcinoma (HCC)[43].
While routine measurements are standard, they can be confounded by alcohol use, which may alter blood pressure, glycemic control, and triglyceride levels[44,45]. Therefore, a comprehensive assessment integrating clinical history, anthropometric measures, and laboratory values is essential for accurate identification of individuals meeting metabolic dysfunction criteria[17].
Standardized and rigorous CMRF assessments are necessary to prevent the misclassification of individuals with either isolated MASLD or ALD, which would threaten the internal validity of trials.
Alcohol use quantification
A major challenge in selecting patients with MetALD for clinical studies is the accurate assessment of alcohol consumption, as both the quantity and pattern of intake are essential for differentiating MASLD, MetALD, and ALD. Several methods exist to evaluate alcohol consumption, including standardized questionnaires and both indirect and direct biomarkers[22,46-49] [Table 1]. While self-reported surveys are widely used in research and clinical practice, they have substantial limitations[50]. Underreporting of alcohol intake is common and is often influenced by social stigma and perceptions surrounding alcohol use[30,51]. In populations initially diagnosed with MASLD, up to 29% of patients have tested positive for alcohol biomarkers, suggesting misclassification due to unreported intake[52]. Underreporting is especially common among vulnerable populations, including younger individuals and irregular or non-patterned drinkers[53].
Main questionnaires and alcohol biomarkers to detect and quantify alcohol use in steatotic liver disease
| Type | Methods | Characteristics/Performance |
| Screening questionnaires | AUDIT[46] | 10-item self-applied questionnaire about consumption (quantity, frequency, and measures), symptoms, and consequences of drinking AUDIT > 8 can identify AUD with a sensitivity of 64%-86% and specificity of 74%-94%. |
| AUDIT-C[56] | 3-item survey. Useful to identify hazardous drinking. A score ≥ 4 is related to alcohol use disorder in men and ≥ 3 in women In men, sensitivity is 76.5% and specificity is 85.3%. In women, 91% sensitivity and 68% specificity | |
| CAGE Questionnaire[47] | 4-item survey. Individuals with a score ≥ 2 should receive further evaluation Sensitivity is 75%-95%, and specificity is 90% | |
| Biomarkers | PEth[48,57] | High sensitivity and specificity as biomarkers for chronic alcohol consumption (up to 4 weeks). Not significantly influenced by sex or BMI Levels ≥ 72 ng/mL have 90% sensitivity and 66% specificity |
| EtG[57] | Detectable in blood or urine 24 to 72 h after consumption, useful to identify recent alcohol consumption Sensitivity 70%-89% and 93%-99% specificity | |
| EtS[49] | Used in association with EtG. Found in blood or urine Sensitivity 73%-82% and specificity 86%-89% | |
| CDT[57] | A metabolite resulting from chronic alcohol consumption. Detection window of 12 to 17 days Sensitivity 40%-79% and specificity 57%-99% | |
| FAEE[57] | Detectable in blood, urine, and hair weeks after consumption. Used to monitor chronic drinking patterns Sensitivity 84% and specificity 83.3% if FAEE ≥ 32 ng/g |
Among standardized screening tools, the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) is a widely used 3-item instrument designed to identify risky alcohol use[54]. A rapid alternative is a single-question screen: “How many times in the past year have you had five or more drinks in a day (for men) or four or more drinks (for women)?” The full AUDIT, a 10-item questionnaire validated in populations with liver disease, assesses both the frequency and quantity of alcohol consumption. A total score greater than 8 suggests probable alcohol use disorder (AUD), while thresholds above 15 for men and 13 for women demonstrate high sensitivity (100%) but low specificity (approximately 20%) for diagnosing alcohol dependence[55,56].
Currently, there are no specific biomarkers validated for the clinical diagnosis of MetALD, which poses a significant challenge for accurate patient identification and classification in clinical trials[57]. However, several direct biomarkers - byproducts of non-oxidative alcohol metabolism - are available. PEth is considered the most sensitive and specific direct blood biomarker for chronic alcohol consumption, with a detection window of approximately two to four weeks. EtG and EtS are detectable in blood and urine for up to 72 h and serve as sensitive markers of recent intake, but their detection window is much narrower than that of Peth[54]. Fatty acid ethyl esters (FAEEs), detectable in blood, urine, or hair, can reflect longer-term alcohol use[57]. The PEth 16:0/18:1 homologue appears most clinically useful for MetALD[58]; values
In a randomized trial of individuals with prior excessive use, PEth correlated with self-reported weekly intake and better predicted decompensation[64]. A recent study suggests 35-210 ng/mL adequately identifies the MetALD population[65], though alternative cut points of 25 or 80 ng/mL have been proposed; optimal thresholds require further validation[59,63,66].
In addition, exposure to alcohol can substantially vary over time, affecting the adequate SLD subtype classification and clinical trial eligibility[67]. Incorporating retrospective assessments, whether through validated recall instruments or longitudinal records, may enhance diagnostic accuracy and improve the validity of patient selection for clinical trials[30,50,55]. Given these limitations, a combined strategy incorporating validated questionnaires and sensitive biomarkers currently offers the most reliable method for comprehensive assessment of alcohol consumption in patients being evaluated for inclusion in MetALD studies.
We emphasize the need for future studies to assess whether racial and ethnic differences, along with variations in body composition, affect the natural history, biomarker performance, or treatment response in MetALD[10]. Data from MASLD and ALD suggest that genetic ancestry, socioeconomic factors, and visceral adiposity may drive disparities in disease outcomes, yet these factors remain largely unexamined in MetALD[26]. Defining race- and weight-specific thresholds for diagnosis and risk stratification will be essential to support precision medicine and promote equity in care.
Assessment of stages of liver disease
Patients with MetALD encompass a wide spectrum of liver disease, from steatosis and steatohepatitis to fibrosis and cirrhosis[68]. Individuals who have metabolic dysfunction-associated steatohepatitis (MASH), particularly those who have obesity and type 2 diabetes, are at an increased risk of developing liver fibrosis, HCC, and cardiovascular disease[69,70]. Notably, compared to those without SLD, patients with MetALD and advanced fibrosis (FIB-4 Index) have higher mortality rates from all-cause, cancer, and liver-related causes[11]. Therefore, precise staging is essential when selecting participants for clinical trials. Although liver biopsy is the gold standard for fibrosis assessment, its invasiveness, sampling variability, and procedural risks limit its utility in large-scale trials[71]. Noninvasive tests (NITs), including imaging modalities such as magnetic resonance imaging-proton density fat fraction (MRI-PDFF), magnetic resonance elastography (MRE)[72-74], and vibration-controlled transient elastography (VCTE)[75,76], offer reliable quantitative measures of steatosis and fibrosis, with strong correlations to clinical outcomes. Serum-based biomarkers, such as the Enhanced Liver Fibrosis (ELF) score, have demonstrated utility in identifying advanced fibrosis and predicting liver-related events[66].
We recommend using a combined approach of imaging and serum biomarkers to enhance diagnostic precision and enable longitudinal monitoring in MetALD clinical trials, providing a practical and scalable alternative to biopsy.
Endpoint selection for clinical trials in MetALD
To rigorously evaluate the efficacy of interventions and conduct clinical trials in MetALD, careful consideration of endpoint selection is essential. Endpoint determination must account for the natural history of the disease, its biological variability, and complex pathophysiology[77]. The use of surrogate endpoints facilitates the execution of clinical studies with greater efficiency and reduced costs while eliminating the need for prolonged follow-up periods. Importantly, surrogate endpoints allow for the early assessment of therapeutic efficacy when a validated correlation between the intervention and clinical outcome has been established[78].
In MASLD and ALD, universally accepted endpoints have been defined that may be extrapolated to MetALD. In this context, the reduction in liver-related mortality, overall mortality, hepatic decompensation, HCC, and liver-related events may represent clinical outcomes relevant to clinical trials targeting MetALD[71,73]. Liver histology has long been considered the gold standard; however, it is associated with complications and is a more invasive procedure, which limits its use in clinical trials[75,76]. In MASLD and ALD, endpoints primarily involving assessments of fibrosis, steatosis, and hepatic inflammation should also be considered and are applicable to patients with MetALD[77]. However, measures of steatosis and hepatic inflammation are less reliable predictors of disease trajectory because of their dynamic nature and susceptibility to external influences, particularly alcohol intake[79].
Noninvasive tools for evaluating fibrosis have largely replaced liver histology as the primary endpoint, supported by robust evidence linking advanced fibrosis to adverse clinical outcomes, including liver-related mortality. Thus, improvement in the fibrosis stage or stabilization without progression are considered clinically meaningful endpoints[80]. Multiple noninvasive methodologies are available for fibrosis assessment, including serum biomarkers such as the ELF test and FIB-4, techniques for measuring liver stiffness such as VCTE, and imaging modalities such as ultrasound elastography and MRE. Although MRE has a high sensitivity for the early detection of fibrosis, its widespread clinical application remains limited. While VCTE and MRE are well-validated in MASLD, data on their performance in MetALD are scarce[78]. In this regard, and given the good performance of noninvasive tools for fibrosis evaluation, improvement in fibrosis and avoidance of liver disease progression could be considered candidate surrogate endpoints in patients with MetALD.
In trials enrolling participants with ongoing alcohol consumption, the incorporation of biomarkers to objectively assess alcohol intake may be critical for endpoint definitions. For instance, PEth levels provide a sensitive and specific measure of recent alcohol exposure and could aid in distinguishing alcohol-related from non-alcohol-related liver injury, thus offering potential value in MetALD clinical studies[72]. Nonetheless, the identification of biomarkers suitable for clinical trial endpoints requires careful consideration of factors, including availability, cost, and interactions with alcohol metabolism and metabolic comorbidities. Given the accelerated progression to fibrosis characteristic of MetALD, biomarkers selected for study endpoints should demonstrate sensitivity for early fibrosis detection and responsiveness to change, enabling timely, meaningful intervention. Details such as how often PEth is measured, which PEth thresholds define adherence, and how we resolve conflicting PEth/EtG results are based on our expert recommendations, because standardized rules for MetALD are not yet established.
Given that MetALD encompasses a spectrum ranging from steatosis to cirrhosis, it is also necessary to consider the outcomes of patients with compensated advanced chronic liver disease (cACLD) within MetALD. In this context, we suggest that surrogate endpoints proposed in other settings, such as the hepatic venous pressure gradient (HVPG), criteria for clinically significant portal hypertension, and changes in the Model for End-Stage Liver Disease (MELD) or MELD 3.0 scores, could also be considered relevant outcomes in MetALD[74,81].
Emerging therapies
While various treatments are being developed for MASLD and ALD, with varying levels of success, this review focuses on a few significant therapies relevant to MetALD to address metabolic dysfunction and alcohol-induced injury. For clinical researchers, these therapies present unique challenges in terms of endpoint selection and patient stratification. Although no treatments have been officially approved specifically for MetALD, several drugs are under investigation for their potential benefits in this context.
Incretin-based therapies, particularly GLP-1 receptor agonists such as semaglutide and liraglutide, target multiple aspects of MetALD by improving metabolic parameters and reducing alcohol cravings[82-84]. Early studies with liraglutide, in a 48-week phase 2 trial including 52 UK patients, achieved histological resolution of non-alcoholic steatohepatitis (NASH) without fibrosis worsening in 39% of participants[84]. Subsequently, semaglutide, in a phase 3 trial, demonstrated significant improvement in NASH-related fibrosis, with 59% of patients with F2-F3 fibrosis achieving NASH resolution without fibrosis progression[82,83]. Furthermore, semaglutide provides cardiovascular benefits, reducing major adverse cardiovascular events by 20% in non-diabetic individuals with obesity (SELECT trial) and by 26% in patients with type 2 diabetes (SUSTAIN-6 trial)[85,86]. Nevertheless, its anti-fibrotic efficacy remains uncertain, as a 48-week trial including 71 patients with compensated cirrhosis did not significantly improve fibrosis or achieve NASH resolution[87].
Other dual or triple agonists, including tirzepatide, survodutide, pemvidutide, and retatrutide, have shown promising results in resolving MASH and promoting weight loss, making them compelling candidates for MetALD[88-91].
Beyond incretin therapies, other emerging treatments include fibroblast growth factor 21 (FGF-21) analogs, resmetirom, fecal microbiota transplantation (FMT), and spironolactone[92-94]. FGF-21 analogs, such as pegbelfermin, efruxifermin, and pegozafermin modulate metabolic pathways, improve insulin sensitivity, and exert anti-inflammatory and anti-fibrotic effects[95]. They may also reduce e alcohol-seeking behavior through hypothalamic melanocortin signaling, supporting their potential in MetALD; circulating FGF-21 could serve as a biomarker of hepatic and behavioral response[96], Clinically, FGF-21 analogs have produced histological and biochemical improvements[90,97-99] in the Phase 2b FALCON 1 trial, pegbelfermin did not meet the primary fibrosis endpoint but showed favorable trends in liver stiffness and aminotransferases with a strong safety profile[99]. In the BALANCED trial, efruxifermin significantly reduced hepatic fat fraction, ALT, and triglyceride levels, demonstrating both metabolic and hepatic benefits[90]. Similarly, in the ENLIVEN Phase 2b trial, pegozafermin achieved statistically significant improvements in fibrosis (≥ 1 stage) and NASH resolution at 24 weeks, with a mean fibrosis improvement of approximately 1.5 stages, positioning pegozafermin as one of the most promising FGF-21-based agents[98]. Together, these findings suggest FGF-21 analogs can address hepatic injury and, potentially, alcohol-related behaviors in patients with MASLD/MASH and MetALD.
Resmetirom, an oral thyroid hormone receptor beta agonist, has also shown promise. In a phase 3 trial, both 80 and 100 mg doses outperformed placebo in resolving MASH (29% vs. 9.7%) and ≥ 1-stage fibrosis improvement, while also lowering Low-Density Lipoprotein (LDL) cholesterol (16.3% vs. 0.1%)[92]. Although this trial used previous NAFLD definitions, a post hoc analysis identified potential MetALD (CDT > 2.5% and/or PEth > 20 ng/mL) who responded similarly to those without alcohol-use markers.
The gut-brain axis regulates human behavior and is disrupted by chronic liver disease[100-104] making the microbiota a plausible therapeutic target. In a phase 1 placebo-controlled trial, a single FMT enema in cirrhosis was safe over six months and reduced alcohol craving and consumption (confirmed by objective biomarkers within 15 days post-FMT). and increased short-chain fatty acids and beneficial microbiota composition[93]. Spironolactone, a mineralocorticoid receptor antagonist, is another candidate. In a U.S. observational cohort of 2,828 participants, use was linked to a reduction of 0.76 alcoholic drinks per week overall and 4.18 fewer drinks among heavy drinkers (> 7 drinks/week)[94]. In a translational work spanning rodent models and a human group with risky consumption (AUDIT-C ≥ 8), spironolactone led to dose-dependent decreases in alcohol consumption, particularly at doses ≥ 50 mg/day, without altering the responsiveness to non-alcohol rewards[105].
We advocate that future research should prioritize combinatorial strategies, including the potential pairing of recently approved therapies. For example, semaglutide and resmetirom - now both FDA-approved for MASH (FDA, 2024; FDA, 2025) - could be tested together, since their different mechanisms may work better in combination than alone. Additional efforts should also explore other potential targets, such as anti-inflammatory agents that block IL-23/IL-17 signaling and agents targeting high-risk genetic variants, but also exploring therapies aimed at IL-22 pathways[106]. Additional data are needed to validate biomarkers that optimize clinical outcomes, particularly in populations with progressive fibrosis. Collectively, these innovations support a precision medicine toMetALD; key therapies are summarized in Table 2.
Potential mechanisms of action and pharmacological agents to use in MetALD
| Mechanism of action | Drug | Supporting evidence |
| GLP-1 agonist | Semaglutide[82,83] | Study: Phase 2 trial Patients: 320 patients with biopsy-confirmed MASH and liver fibrosis of stage F1, F2, or F3 Results: Enhances metabolic parameters with 59% MASH resolution and 43% fibrosis improvement |
| Study: Phase 3 Patients: 1,197 patients with biopsy-defined MASH and fibrosis stage 2 or 3 Results: Reduction in liver fibrosis without worsening of steatohepatitis was reported in 36.8%, and combined resolution of steatohepatitis and reduction in liver fibrosis was reported in 32.7% | ||
| Liraglutide[84] | Study: Phase 2 trial Patients: 52 patients with non-alcoholic steatohepatitis Results: MASH Resolution (No Worsening Fibrosis) - 39% vs. 9% placebo (P = 0.019) and Fibrosis Progression 9% vs. 36% placebo (P = 0.04) | |
| Tirzepatide[88] | Study: Phase 2 trial Patients: 190 patients with biopsy-defined MASH and fibrosis stage 2 or 3 Results: MASH resolution without fibrosis worsening 62% (15 mg tirzepatide group), ≥ 1 fibrosis stage improvement (no MASH worsening), 51% weight reduction, and cardiometabolic measures improvement | |
| Survodutide[91] | Study: Phase 2 trial Patients: 293 patients with biopsy-defined MASH and fibrosis stage 1, 2, or 3 Results: A decrease in liver fat content by at least 30% occurred in 63% of the participants, and improvement in fibrosis by at least one stage in 34%-36% | |
| Pemvidutide[90] | Study: Phase 1 trial Patients: 94 patients with a BMI ≥ 28.0 kg/m2 and LFC ≥ 10% by magnetic resonance imaging-proton density fat fraction Results: Liver fat content reduced in 68.5%, maximum weight loss was 4.3%, alanine aminotransferase reduced by 13.8 IU/L, corrected cT1 reduced by 75.9 ms | |
| Retatrutide[89] | Study: Phase 2 trial Patients: 92 patients with obesity or overweight with weight-related complications other than T2D Results: Liver fat was reduced in 82.4% of participants on 12 mg. LF reductions were significantly related to changes in body weight, abdominal fat, and metabolic measures associated with improved insulin sensitivity and lipid metabolism | |
| FGF-21 analogs | Efruxifermin[97] | Study: Phase 2b trial Patients: 128 patients with biopsy-confirmed MASH with histological stage F2 or F3 fibrosis Results: ≥ 1 stage fibrosis improvement with no worsening of MASH at 24 weeks in 41% of patients (Efruxifermin 50 mg) |
| Pegozafermin[98] | Study: Phase 2b trial Patients: 222 patients with biopsy-confirmed MASH and stage F2 or F3 Results: MASH resolution in 26% and fibrosis improvement in 27% of the patients (44 mg pegozafermin group) | |
| Pegbelfermin[99] | Study: Phase 2b trial Patients: 197 patients with biopsy-confirmed MASH and stage 3 fibrosis Results: Relative reductions in hepatic fat fraction (magnetic resonance imaging-proton density fat fraction), although no differences reached statistical significance. Improvements in liver fibrosis (magnetic resonance elastography and N-terminal type III collagen propeptide) and liver injury/inflammation (alanine aminotransferase, aspartate aminotransferase) | |
| THR-β agonist | Resmetirom[92] | Study: Phase 3 trial Patients: 966 patients with biopsy-confirmed MASH and a fibrosis stage of F1B, F2, or F3 Results: MASH resolution with no worsening of fibrosis in 29.9% and fibrosis improvement by at least one stage with no worsening of the MASLD activity score in 25.9% of the patients. LDL cholesterol decreased 16.3% (100 mg resmetirom group) |
| Gut microbiota modulation | FMT[93] | Study: Phase 1 trial Patients: 20 patients with AUD-related cirrhosis with problem drinking (AUDIT-10 > 8) Results: Significant short-term reductions in alcohol craving and consumption in 90% of patients, along with favorable changes in microbial composition. At 6 months, FMT-treated patients experienced fewer AUD-related adverse events compared to placebo |
| Aldosterone antagonist | Spironolactone[94] | Study: Retrospective cohort study Patients: 523 spironolactone-treated adults and 2305 untreated adults Results: Reduced alcohol intake by up to 3.5 drinks/week, with greater effects in individuals with hazardous drinking (AUDIT-C ≥ 8); strongest results observed at doses ≥ 50 mg/day, showing a dose-response relationship |
CONCLUSION
MetALD is a new concept that defines a large, previously under-recognized population with dual metabolic and alcohol-related injury. To advance the field, clinical trials should prioritize (1) harmonized definitions and eligibility grounded in rigorous alcohol-exposure measurement (serial PEth with EtG/EtS); (2) careful endpoint selection spanning hepatic, cardiovascular/renal, and patient-reported outcomes; (3) adaptive, enrichment-ready designs that accommodate disease heterogeneity; and (4) validation of MetALD-specific biomarker strategies. Standardized diagnostic criteria, accurate exposure quantification, and stratification by alcohol pattern and metabolic risk are essential for comparability and generalizability. Multidisciplinary collaboration across hepatology, addiction medicine, endocrinology, and trial methodology supported by international collaboration and innovative study designs is essential to deliver generalizable, patient-centered evidence and accelerate the path to effective therapies.
DECLARATIONS
Acknowledgments
The graphical abstract was created with BioRender.com [Created in BioRender. Arab J. (2025) https://BioRender.com/t62vwd5].
Authors’ contributions
Writing - original draft: Acuña P, Albhaisi O, Idalsoaga F
Writing - review and editing: Acuña P, Albhaisi O, Idalsoaga F, Díaz LA
Review and editing: Alkhouri N, Noureddin M, Arab JP
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Arab JP is an associate editor of the journal Metabolism and Target Organ Damage, and Díaz LA is a Youth Editorial Board Member of the journal. Díaz LA is also a guest editor of the special issue Topic: Metabolic and Alcohol-Related Dual Liver Injury: Exploring MetALD. They were not involved in any steps of editorial processing, including reviewer selection, manuscript handling, or decision making. Alkhouri N is affiliated with the Clinical Research Institute of Ohio and Summit Clinical Research and declares that there are no conflicts of interest. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Feng G, Targher G, Byrne CD, et al. Global burden of metabolic dysfunction-associated steatotic liver disease, 2010 to 2021. JHEP Rep. 2025;7:101271.
2. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-47.
3. Rinella ME, Lazarus JV, Ratziu V, et al; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-86.
4. Kanwal F, Neuschwander-Tetri BA, Loomba R, Rinella ME. Metabolic dysfunction-associated steatotic liver disease: update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology. 2024;79:1212-9.
5. Hagström H, Adams LA, Allen AM, et al. The future of international classification of diseases coding in steatotic liver disease: an expert panel Delphi consensus statement. Hepatol Commun. 2024;8:e0386.
6. Kalligeros M, Vassilopoulos A, Vassilopoulos S, Victor DW, Mylonakis E, Noureddin M. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017-2020. Clin Gastroenterol Hepatol. 2024;22:1330-2.e4.
7. Lee CM, Yoon EL, Kim M, et al. Prevalence, distribution, and hepatic fibrosis burden of the different subtypes of steatotic liver disease in primary care settings. Hepatology. 2024;79:1393-400.
8. Schneider CV, Schneider KM, Raptis A, Huang H, Trautwein C, Loomba R. Prevalence of at-risk MASH, MetALD and alcohol-associated steatotic liver disease in the general population. Aliment Pharmacol Ther. 2024;59:1271-81.
9. Tampaki M, Tsochatzis E, Lekakis V, Cholongitas E. Prevalence, characteristics and outcomes of patients with metabolic and alcohol related/associated liver disease (MetALD): a systematic review and meta-analysis. Metabolism. 2025;163:156101.
10. Ho GJK, Tan FXN, Sasikumar NA, et al. High global prevalence of steatotic liver disease and associated subtypes: a meta-analysis. Clin Gastroenterol Hepatol. 2025;Epub ahead of print.
11. Kwak M, Kim HS, Jiang ZG, et al. MASLD/MetALD and mortality in individuals with any cardio-metabolic risk factor: A population-based study with 26.7 years of follow-up. Hepatology. 2025;81:228-37.
12. Wu Y, Dong P, Wu Q, et al. Insights into clinical trials for drugs targeting MASLD: progress, challenges, and future directions. Clin Pharmacol Ther. 2025;117:1614-26.
13. Park Y, Jung J, Han S, Kim GA. Metabolic dysfunction-associated steatotic liver disease and MetALD increases the risk of liver cancer and gastrointestinal cancer: a nationwide cohort study. Aliment Pharmacol Ther. 2024;60:1599-608.
14. Díaz LA, Arab JP, Louvet A, Bataller R, Arrese M. The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20:764-83.
15. Kim D, Wijarnpreecha K, Cholankeril G, Ahmed A. Steatotic liver disease-associated all-cause/cause-specific mortality in the United States. Aliment Pharmacol Ther. 2024;60:33-42.
16. Li M, Chen W, Deng Y, Xie W. Impacts of cardiometabolic risk factors and alcohol consumption on all-cause mortality among MASLD and its subgroups. Nutr Metab Cardiovasc Dis. 2024;34:2085-94.
17. Diaz LA, Ajmera V, Arab JP, et al. An expert consensus delphi panel in metabolic dysfunction- and alcohol-associated liver disease: opportunities and challenges in clinical practice. Clin Gastroenterol Hepatol. 2025;Epub ahead of print.
18. Sanyal AJ, Van Natta ML, Clark J, et al; NASH Clinical Research Network (CRN). Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559-69.
19. Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22:1071-80.
20. Anderson BO, Berdzuli N, Ilbawi A, et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health. 2023;8:e6-7.
21. Israelsen M, Torp N, Johansen S, et al; GALAXY consortium. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9:218-28.
22. Arab JP, Díaz LA, Rehm J, et al. Metabolic dysfunction and alcohol-related liver disease (MetALD): position statement by an expert panel on alcohol-related liver disease. J Hepatol. 2025;82:744-56.
23. Kulkarni AV, Singal AK, Kamath PS. Research priorities and future landscape of clinical trials in alcohol-associated liver disease. Clin Liver Dis. 2024;28:831-51.
24. Ayares G, Diaz LA, Idalsoaga F, et al. MetALD: new perspectives on an old overlooked disease. Liver Int. 2025;45:e70017.
25. Bae JH. Racial and ethnic disparities in metabolic dysfunction-associated steatotic liver disease outcomes: a call for culturally sensitive interventions: editorial on “Differences in liver and mortality outcomes of non-alcoholic fatty liver disease by race and ethnicity: a longitudinal real-world study”. Clin Mol Hepatol. 2024;30:665-8.
26. Díaz Carnicero J, Saurí-Ferrer I, Redon J, et al. Clinical and economic burden of metabolic dysfunction-associated steatotic liver disease (MASLD) in a Spanish Mediterranean region: a population-based study. J Clin Med. 2025;14:2441.
27. Zhao Y, Bo Y, Zu J, et al. Global burden of chronic liver disease and temporal trends: a population-based analysis from 1990 to 2021 with projections to 2050. Liver Int. 2025;45:e70155.
28. Yamamura S, Nakano D, Hashida R, et al. Patient-reported outcomes in patients with non-alcoholic fatty liver disease: a narrative review of Chronic Liver Disease Questionnaire-non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2021;36:629-36.
29. Younossi Z, Aggarwal P, Shrestha I, et al. The burden of non-alcoholic steatohepatitis: A systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 2022;4:100525.
30. Schomerus G, Leonhard A, Manthey J, et al. The stigma of alcohol-related liver disease and its impact on healthcare. J Hepatol. 2022;77:516-24.
31. Lazarus JV, Miralles-Sanchez JE, Agirre-Garrido L, et al. A call to action to address the steatotic liver disease public health threat in Barcelona. Lancet Reg Health Eur. 2025;52:101272.
32. Kardashian A, Serper M, Terrault N, Nephew LD. Health disparities in chronic liver disease. Hepatology. 2023;77:1382-403.
33. Loomba R, Ratziu V, Harrison SA; NASH Clinical Trial Design International Working Group. Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology. 2022;162:680-8.
34. Code of Federal Regulations. Subpart H -- accelerated approval of new drugs for serious or life-threatening illnesses. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-H. [Last accessed on 13 Oct 2025].
35. Harvey BE. How improvements in US FDA regulatory process and procedures led to the drug approval for first ever treatment of a common liver disease. Acta Pharmacol Sin. 2025;46:515-24.
36. Harvey BE. NASH: regulatory considerations for clinical drug development and U.S. FDA approval. Acta Pharmacol Sin. 2022;43:1210-4.
37. Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases, United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392-405.
39. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
40. Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18:157-63.
41. Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306-17.
42. Whitfield JB, Schwantes-An TH, Darlay R, et al; GenomALC Consortium. A genetic risk score and diabetes predict development of alcohol-related cirrhosis in drinkers. J Hepatol. 2022;76:275-82.
43. Ganne-Carrié N, Chaffaut C, Bourcier V, et al; for CIRRAL Group. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274-83.
44. De Oliveira E Silva ER, Foster D, McGee Harper M, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347-52.
45. Cecchini M, Filippini T, Whelton PK, et al. Alcohol intake and risk of hypertension: a systematic review and dose-response meta-analysis of nonexperimental cohort studies. Hypertension. 2024;81:1701-15.
46. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791-804.
47. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300:2054-6.
48. Jørgenrud BM, Bråthen CC, Steinson Stenehjem J, Kristiansen T, Rosseland LA, Bogstrand ST. Identifying excessive chronic alcohol use with phosphatidylethanol in patients with suspected severe injury-results from the IDART study. Alcohol Alcohol. 2024;59:agae014.
49. Stewart SH, Koch DG, Burgess DM, Willner IR, Reuben A. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-5.
50. McKnight-Eily LR, Okoro CA, Turay K, Acero C, Hungerford D. Screening for alcohol use and brief counseling of adults - 13 states and the district of Columbia, 2017. MMWR Morb Mortal Wkly Rep. 2020;69:265-70.
51. Krag A, Torp N, Younossi ZM, Israelsen M. Reporting discrepancy of alcohol intake affecting estimated prevalence of MetALD and ALD. Lancet Gastroenterol Hepatol. 2025;10:282-4.
52. Staufer K, Huber-Schönauer U, Strebinger G, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77:918-30.
53. Livingston M, Callinan S. Underreporting in alcohol surveys: whose drinking is underestimated? J Stud Alcohol Drugs. 2015;76:158-64.
54. Díaz LA, König D, Weber S, et al. Management of alcohol use disorder: a gastroenterology and hepatology-focused perspective. Lancet Gastroenterol Hepatol. 2025;10:475-90.
55. Tevik K, Bergh S, Selbæk G, Johannessen A, Helvik AS. A systematic review of self-report measures used in epidemiological studies to assess alcohol consumption among older adults. PLoS One. 2021;16:e0261292.
56. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208-17.
57. Harris JC, Leggio L, Farokhnia M. Blood biomarkers of alcohol use: a scoping review. Curr Addict Rep. 2021;8:500-8.
58. Aboutara N, Szewczyk A, Jungen H, et al. Phosphatidylethanol in patients with liver diseases of different etiologies: analysis of six homologues and comparison with other alcohol markers. Clin Chim Acta. 2022;524:171-8.
59. Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-11.
60. Hahn JA, Fatch R, Barnett NP, Marcus GM. Phosphatidylethanol vs transdermal alcohol monitoring for detecting alcohol consumption among adults. JAMA Netw Open. 2023;6:e2333182.
61. Helander A, Hansson T. The alcohol biomarker phosphatidylethanol (PEth) - test performance and experiences from routine analysis and external quality assessment. Scand J Clin Lab Invest. 2023;83:424-31.
62. Finanger T, Melby K, Spigset O, Andreassen TN, Lydersen S, Skråstad RB. Relationship between alcohol intake based on daily smartphone-reported consumption and PEth concentrations in healthy volunteers. Alcohol Alcohol. 2024;59:agae040.
63. Tavaglione F, Amangurbanova M, Yang AH, et al. Head-to-head comparison between phosphatidylethanol versus indirect alcohol biomarkers for diagnosis of MetALD versus MASLD: a prospective study. Aliment Pharmacol Ther. 2025;61:1043-54.
64. Hansen ED, Torp N, Johansen S, et al; GALAXY and MicrobLiver consortia. Quantification of alcohol intake in patients with steatotic liver disease and excessive alcohol intake. JHEP Rep. 2025;7:101200.
65. Vaz J, Nasr P, Helander A, et al. Phosphatidylethanol levels distinguish steatotic liver disease subgroups and are associated with risk of major liver outcomes. J Hepatol. 2025;Epub ahead of print.
66. Skråstad RB, Aamo TO, Andreassen TN, et al. Quantifying alcohol consumption in the general population by analysing phosphatidylethanol concentrations in whole blood: results from 24,574 subjects included in the HUNT4 study. Alcohol Alcohol. 2023;58:258-65.
67. Israelsen M, Thorhauge KH, Andersen P, et al. Steatotic liver disease classification is dynamic, affecting clinical trial eligibility and subclass-specific treatments. Clin Gastroenterol Hepatol. 2025;Epub ahead of print.
68. Allen AM, Therneau TM, Ahmed OT, et al. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J Hepatol. 2022;77:1237-45.
69. Cusi K, Budd J, Johnson E, Shubrook J. Making sense of the nonalcoholic fatty liver disease clinical practice guidelines: what clinicians need to know. Diabetes Spectr. 2024;37:29-38.
70. Lindén D, Romeo S. Therapeutic opportunities for the treatment of NASH with genetically validated targets. J Hepatol. 2023;79:1056-64.
72. Middleton MS, Heba ER, Hooker CA, et al; NASH Clinical Research Network. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153:753-61.
73. Qu Y, Li M, Hamilton G, Zhang YN, Song B. Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: a meta-analysis. Eur Radiol. 2019;29:5180-9.
74. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Corrigendum to ‘Baveno VII - Renewing consensus in portal hypertension’ [J Hepatol (2022) 959-974]. J Hepatol. 2022;77:271.
75. Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598-607.e2.
76. Hudson D, Afzaal T, Bualbanat H, et al. Modernizing metabolic dysfunction-associated steatotic liver disease diagnostics: the progressive shift from liver biopsy to noninvasive techniques. Therap Adv Gastroenterol. 2024;17:17562848241276334.
77. Rinella ME, Tacke F, Sanyal AJ, Anstee QM; participants of the AASLD/EASL Workshop. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. Hepatology. 2019;70:1424-36.
78. Ratziu V. A critical review of endpoints for non-cirrhotic NASH therapeutic trials. J Hepatol. 2018;68:353-61.
79. Roth NC, Qin J. Histopathology of alcohol-related liver diseases. Clin Liver Dis. 2019;23:11-23.
80. Gratacós-Ginès J, Ariño S, Sancho-Bru P, Bataller R, Pose E. MetALD: clinical aspects, pathophysiology and treatment. JHEP Rep. 2025;7:101250.
81. Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: the model for end-stage liver disease updated for the modern era. Gastroenterology. 2021;161:1887-95.e4.
82. Newsome PN, Buchholtz K, Cusi K, et al; NN9931-4296 Investigators. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113-24.
83. Sanyal AJ, Newsome PN, Kliers I, et al; ESSENCE Study Group. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med. 2025;392:2089-99.
84. Armstrong MJ, Gaunt P, Aithal GP, et al; LEAN trial team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-90.
85. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al; SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221-32.
86. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-44.
87. Loomba R, Abdelmalek MF, Armstrong MJ, et al; NN9931-4492 investigators. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511-22.
88. Loomba R, Hartman ML, Lawitz EJ, et al; SYNERGY-NASH Investigators. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391:299-310.
89. Sanyal AJ, Kaplan LM, Frias JP, et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30:2037-48.
90. Harrison SA, Browne SK, Suschak JJ, et al. Effect of pemvidutide, a GLP-1/glucagon dual receptor agonist, on MASLD: a randomized, double-blind, placebo-controlled study. J Hepatol. 2025;82:7-17.
91. Sanyal AJ, Bedossa P, Fraessdorf M, et al; 1404-0043 Trial Investigators. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391:311-9.
92. Harrison SA, Bedossa P, Guy CD, et al; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497-509.
93. Bajaj JS, Gavis EA, Fagan A, et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology. 2021;73:1688-700.
94. Palzes VA, Farokhnia M, Kline-Simon AH, et al. Effectiveness of spironolactone dispensation in reducing weekly alcohol use: a retrospective high-dimensional propensity score-matched cohort study. Neuropsychopharmacology. 2021;46:2140-7.
95. Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654-67.
96. Flippo KH, Trammell SAJ, Gillum MP, et al. FGF21 suppresses alcohol consumption through an amygdalo-striatal circuit. Cell Metab. 2022;34:317-328.e6.
97. Harrison SA, Frias JP, Neff G, et al; HARMONY Study Group. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8:1080-93.
98. Loomba R, Sanyal AJ, Kowdley KV, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389:998-1008.
99. Loomba R, Sanyal AJ, Nakajima A, et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and stage 3 fibrosis (FALCON 1): a randomized phase 2b study. Clin Gastroenterol Hepatol. 2024;22:102-12.e9.
100. Aghara H, Patel M, Chadha P, Parwani K, Chaturvedi R, Mandal P. Unraveling the gut-liver-brain axis: microbiome, inflammation, and emerging therapeutic approaches. Mediators Inflamm. 2025;2025:6733477.
101. Afzaal M, Saeed F, Shah YA, et al. Human gut microbiota in health and disease: unveiling the relationship. Front Microbiol. 2022;13:999001.
102. Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? Microorganisms. 2019;7:14.
103. Yan M, Man S, Sun B, et al. Gut liver brain axis in diseases: the implications for therapeutic interventions. Signal Transduct Target Ther. 2023;8:443.
104. Li X, Chen LM, Kumar G, et al. Therapeutic interventions of gut-brain axis as novel strategies for treatment of alcohol use disorder associated cognitive and mood dysfunction. Front Neurosci. 2022;16:820106.
105. Farokhnia M, Rentsch CT, Chuong V, et al. Spironolactone as a potential new pharmacotherapy for alcohol use disorder: convergent evidence from rodent and human studies. Mol Psychiatry. 2022;27:4642-52.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].