Effects of steatotic liver disease and cardiometabolic risk factors in MASLD on adverse pregnancy outcomes
Abstract
Aim: A new definition of metabolic dysfunction-associated steatotic liver disease (MASLD) was proposed in 2023; however, its impact on adverse pregnancy outcomes (APOs) remains undetermined. This study aimed to comprehensively analyze the association between MASLD and the risk of APOs, and to evaluate the relative contributions of its components - steatotic liver disease (SLD) and cardiometabolic risk factors (CMRF).
Methods: This retrospective cohort study enrolled 4,118 pregnant women admitted to the hospital between March 2020 and December 2022. Participants were categorized into MASLD and non-MASLD groups based on MASLD status. The non-MASLD group was further divided into three subgroups according to the presence of SLD and CMRF. Adjusted relative risks (aRRs) for APOs were estimated using modified Poisson regression analysis, adjusting for relevant covariates.
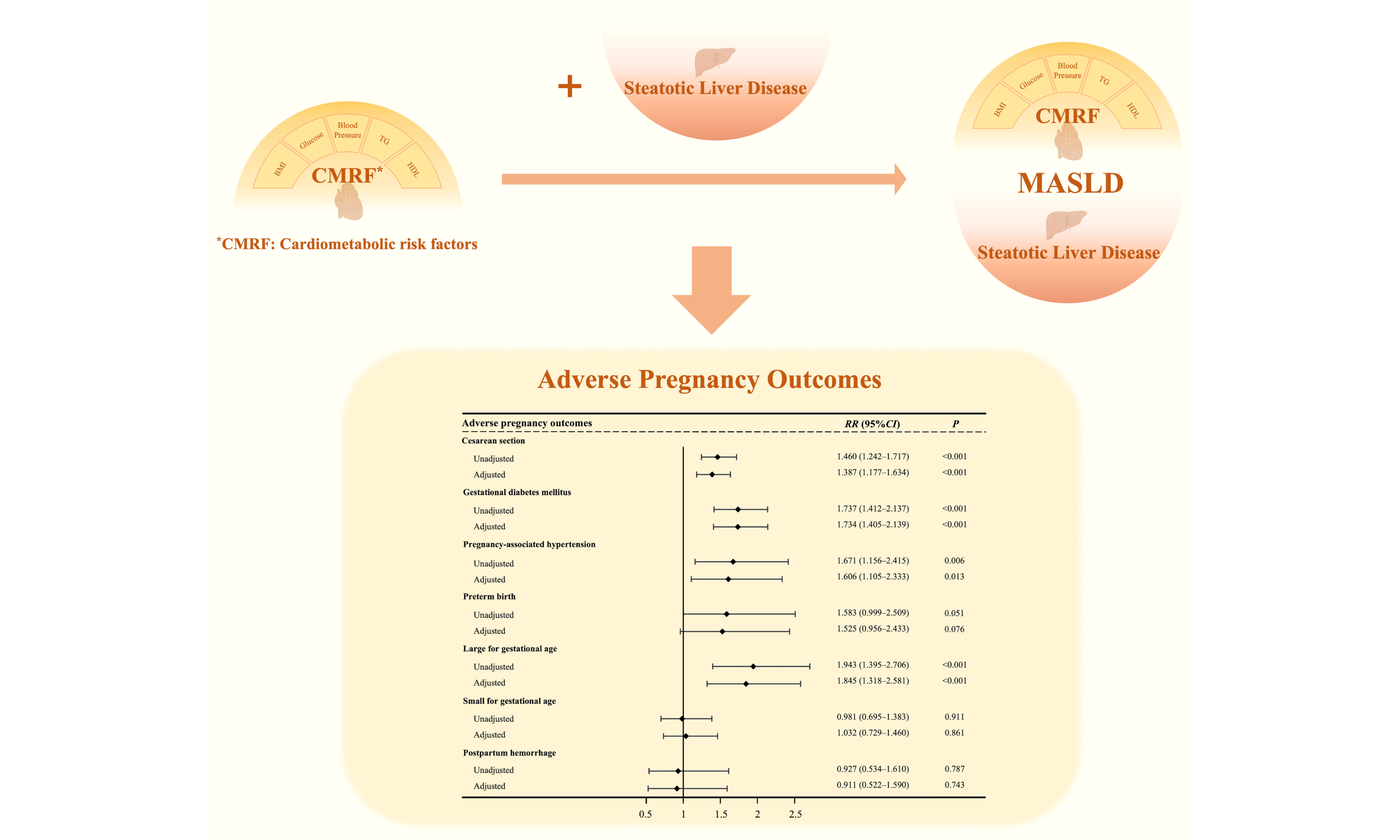
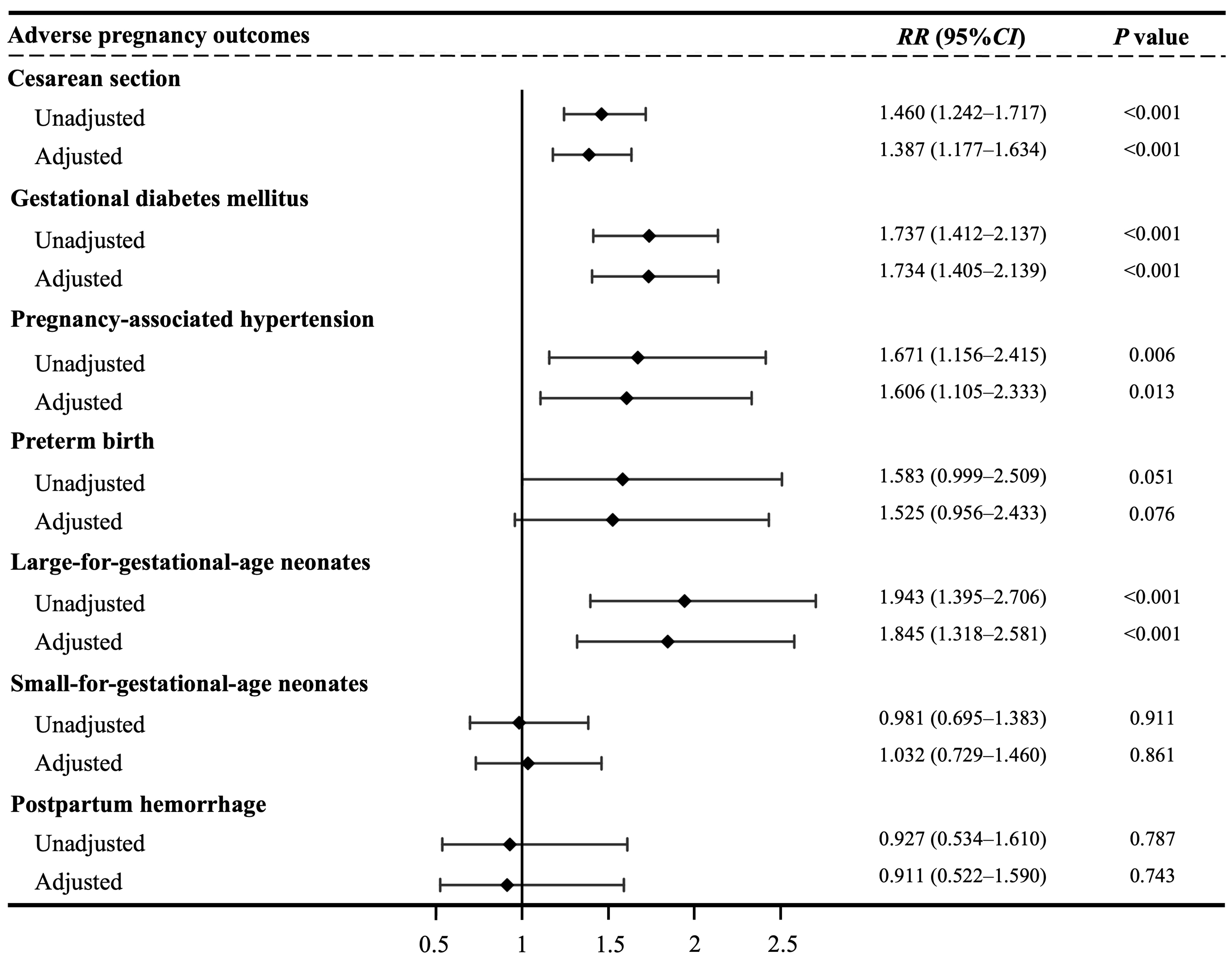
Results: The prevalence of MASLD among pregnant women was 7.3%. MASLD was associated with elevated risks of cesarean section (aRR = 1.459, 95%CI: 1.253-1.700, P < 0.001), gestational diabetes mellitus(aRR = 2.081, 95%CI: 1.711-2.530, P < 0.001), pregnancy-associated hypertension (aRR = 2.192, 95%CI: 1.541-3.118, P < 0.001), preterm birth (aRR = 1.826, 95%CI: 1.181-2.823, P = 0.001), and large-for gestational-age neonates (aRR = 2.024, 95%CI: 1.488-2.754, P < 0.001). Compared with the CMRF-only group, the MASLD group showed higher risks of cesarean section (aRR = 1.387, 95%CI: 1.177-1.634, P < 0.001), gestational diabetes mellitus (aRR = 1.734, 95%CI: 1.405-2.139, P < 0.001), pregnancy-associated hypertension (aRR = 1.606, 95%CI: 1.105-2.333, P = 0.013), and large-for-gestational-age neonates (aRR = 1.845, 95%CI: 1.318-2.581, P = 0.001). No significant differences in risk were observed between the MASLD and SLD-only groups.
Conclusion: MASLD during pregnancy is associated with an increased risk of several APOs, with SLD appearing to play a more critical role than CMRF.
Keywords
INTRODUCION
Nonalcoholic fatty liver disease (NAFLD) represents one of the most prevalent chronic liver diseases globally, affecting over 25% of adults worldwide, with prevalence exceeding 30% in China[1,2]. Notably, approximately 10% of women of reproductive age (20-40 years) are affected by NAFLD[3], despite the presumed protective role of estrogen[4]. NAFLD is now recognized as being associated with increased cardiometabolic risk[5,6] and is considered the hepatic manifestation of systemic metabolic dysfunction[7]. In response to this understanding, a new diagnostic term - metabolic dysfunction-associated steatotic liver disease (MASLD) - was proposed in 2023[8]. This definition requires both the presence of steatotic liver disease (SLD) and cardiometabolic risk factors (CMRF), while excluding other causes of hepatic steatosis. Evidence suggests that approximately 95% of individuals with NAFLD meet the diagnostic criteria for MASLD[2,9].
Emerging studies indicate that NAFLD elevates the risk of adverse maternal and neonatal outcomes. Associations have been reported between NAFLD and a range of adverse pregnancy outcomes (APOs), including cesarean section[10], gestational diabetes mellitus (GDM)[11,12], pregnancy-associated hypertension[10,13], preterm birth[10,12], and large-for-gestational-age (LGA) neonates[14], although some findings have been inconsistent[15,16]. However, due to the recent introduction of the MASLD terminology, few studies have specifically explored the relationship between MASLD and APOs. One study by Niu et al. reported an increased risk of gestational hypertensive complications in women with MASLD[17], but evidence regarding its association with other APOs remains limited.
SLD, a component of MASLD, contributes to systemic oxidative stress and heightened inflammatory responses via ectopic lipid accumulation in the liver[18,19]. It also promotes insulin resistance by disrupting intracellular signaling cascades and impairing metabolic homeostasis[20-22]. The other component, CMRF, also plays a critical role in metabolic dysfunction. Each of its five constituent risk factors independently contributes to cardiometabolic disease risk: elevated body mass index (BMI) and serum glucose are associated with insulin resistance[23,24]; reduced high-density lipoprotein cholesterol (HDL-C) is linked to increased inflammation and impaired cholesterol transport[25]; elevated triglycerides (TG) levels contribute to dyslipidemia and pancreatic dysfunction[26,27]; and elevated blood pressure increases the risk of left ventricular hypertrophy[28]. However, it remains unclear whether SLD or CMRF plays a more dominant role in the association between MASLD and APO risk.
Therefore, this study aims to investigate the association between MASLD during pregnancy and the risk of APOs, and to comparatively assess the relative contribution of its two components, SLD and CMRF, to this relationship.
METHODS
Study population
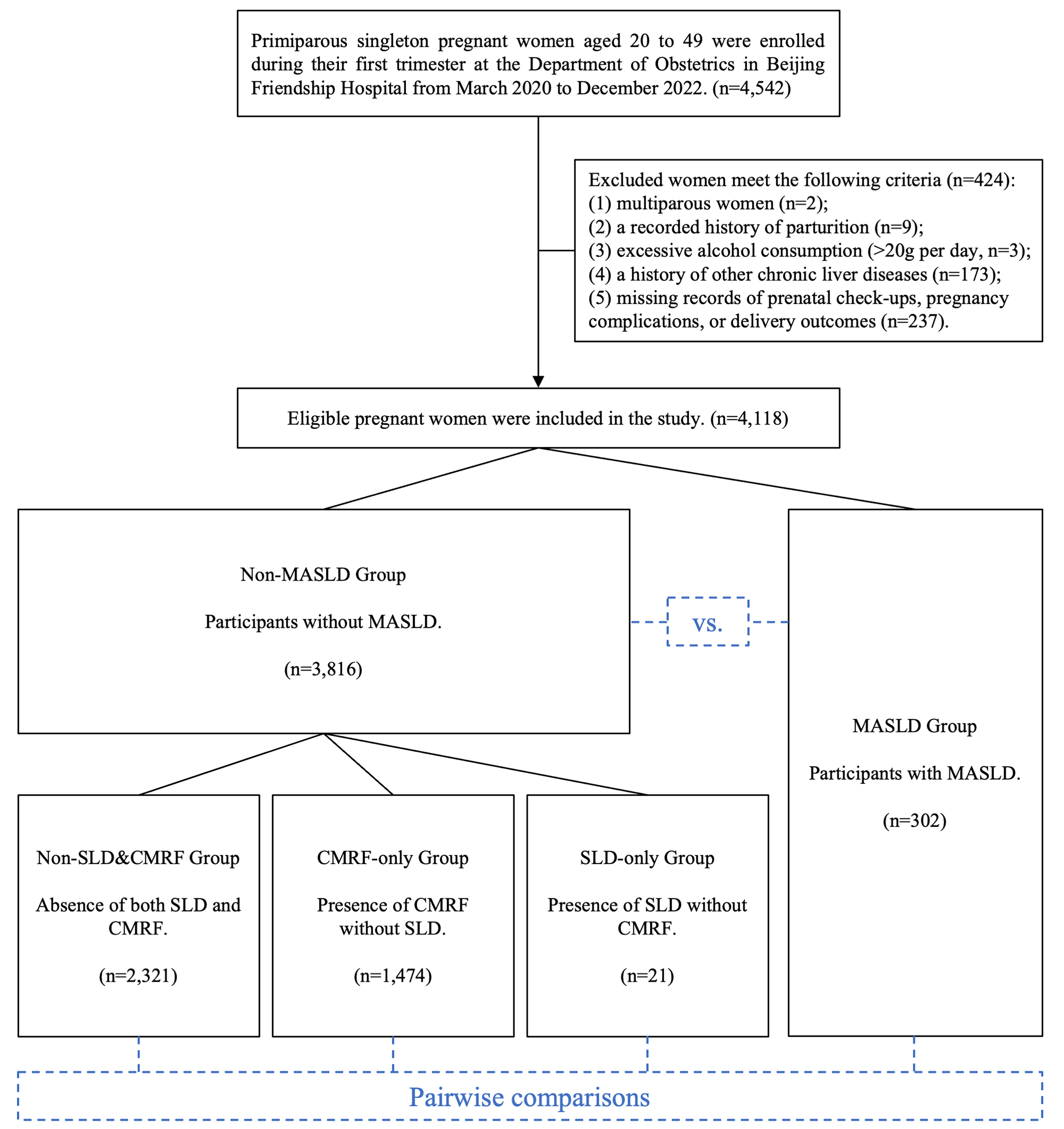
This retrospective cohort study was conducted in the Department of Obstetrics at Beijing Friendship Hospital and included 4,542 singleton, primiparous pregnancies in women aged 20-49 years who were registered during their first trimester between March 10, 2020, and December 31, 2022. Participants who met the following criteria were excluded: (1) multiple pregnancies (n = 2); (2) a recorded history of parturition (n = 9); (3) excessive alcohol consumption, defined as more than 20 g daily (n = 3); (4) a history of chronic liver diseases, including alcoholic liver disease, viral hepatitis, cholangitis, and autoimmune hepatitis (n = 173); or (5) incomplete records on prenatal checkups, pregnancy complications, or delivery outcomes (n = 237). Finally, a total of 4,118 pregnant women were included in the analysis. Under China’s residence-based healthcare policy, the majority of participants were recruited from Beijing. The hospital’s multiple campuses are located across various urban and suburban areas of the city, covering a broad geographic and demographic spectrum, which enhances the representativeness of the study cohort. Ethical approval was obtained from the Ethics Committee of Beijing Friendship Hospital (Approval Number: 2023-P2-175-02), and due to the registry-based nature of the study, informed consent was waived.
Baseline characteristics acquisition and management
From enrollment until the 14th week of gestation, trained health workers collected baseline demographic and prepregnancy data, including maternal age (years), education level (Bachelor’s degree or above vs. below), current smoking status (yes/no), alcohol consumption (g/day), and medical history. Standardized anthropometric measurements, including height (m), weight (kg), and systolic/diastolic blood pressure (SBP/DBP) (mmHg), were obtained by hospital health workers with standardized training during the initial prenatal record establishment and at each subsequent prenatal checkup. Given the minimal weight fluctuation in early gestation, the weight recorded at the time of prenatal record establishment (before the 14th week of gestation) was considered the prepregnancy weight.
BMI was calculated as weight (kg) divided by squared height (m2), and classified according to Chinese standards as underweight (BMI < 18.5 kg/m2), normal weight (18.5 ≤ BMI < 24.0 kg/m2), overweight (24.0 ≤ BMI < 28.0 kg/m2), or obesity (BMI ≥ 28.0 kg/m2)[29]. Total gestational weight gain was defined as the difference between the last measured weight before or at delivery and the prepregnancy weight. Gestational weight gain was then classified as insufficient, normal, or excessive based on the recommended weight gain ranges for different prepregnancy BMI categories[30]. Detailed ranges are provided in Supplementary Table 1.
Laboratory data measurement
Laboratory data were collected at the time of liver ultrasonography, primarily between the 9th and 11th weeks of gestation, and analyzed for blood chemistry. Standard screening for GDM was performed between the 24th and 28th weeks of gestation using a 75-g oral glucose tolerance test (OGTT), followed by laboratory analysis. Fasting blood samples were obtained after at least 8 h of fasting and analyzed at the laboratory of Beijing Friendship Hospital in accordance with standard operating procedures. Clinicians notified patients to repeat testing when abnormalities were detected. The blood biomarkers analyzed in this study included fasting blood glucose (FBG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), 1-h postload glucose, and 2-h postload glucose.
Definitions of SLD, CMRF, and MASLD
Liver ultrasonography was performed on all pregnant participants before 14 weeks of gestation, primarily between the 9th and 11th weeks. SLD was defined as lipid accumulation in more than 5% of hepatocytes, characterized by “diffuse enhancement of near-field echoes in the liver region (greater than those of the kidneys and spleen) with gradual attenuation of far-field echoes”, as assessed by experienced sonologists[31,32]. CMRF was defined by the presence of at least one of the following: (1) BMI ≥ 24 kg/m2 (following Chinese recommendations); (2) FBG ≥ 5.6 mmol/L, type 2 diabetes mellitus (T2DM), or treatment for T2DM; (3) blood pressure ≥ 130/85 mmHg or use of antihypertensive medication; (4) plasma TG ≥ 1.70 mmol/L or lipid-lowering treatment; or (5) plasma HDL-C ≤ 1.3 mmol/L (for females) or lipid-lowering treatment. MASLD was diagnosed based on the presence of both SLD and any CMRF, excluding cases of excessive alcohol consumption or a history of chronic liver disease[8]. These exclusion criteria were already applied during participant screening, eliminating the need for further exclusions.
Classification of participants
Participants were stratified into two main groups based on MASLD status: non-MASLD and MASLD. The non-MASLD group was further subdivided according to the presence or absence of SLD and CMRF, yielding three subgroups: (1) non-SLD&CMRF group (absence of both SLD and CMRF); (2) CMRF-only group (presence of CMRF without SLD); and (3) SLD-only group (presence of SLD without CMRF). Figure 1 illustrates the participant classification and analytical workflow.
Definition of APOs
The primary outcomes of this study were the occurrence of APOs, including cesarean section, GDM, pregnancy-associated hypertension, preterm birth, LGA neonates, small for gestational age (SGA) neonates, and postpartum hemorrhage. GDM was diagnosed between the 24th and 28th weeks of gestation based on a 75-g OGTT, with at least one abnormal plasma glucose value required for diagnosis: fasting ≥ 5.1 mmol/L, a 1-h ≥
Statistical analysis
Continuous variables with a normal distribution are presented as the mean and standard deviation (SD), while skewed variables are presented as the median and interquartile range (IQR). Comparisons were performed via Student’s t-test or the Kruskal-Wallis rank-sum test, as appropriate. Categorical variables are presented as frequencies (percentages) and compared using the chi-square test or Fisher’s exact test when applicable. Modified Poisson regression models were employed to estimate relative risks (RRs) of APOs. The non-MASLD group served as the reference group, and risks in the MASLD group were calculated both unadjusted and adjusted for prespecified covariates. Pairwise comparisons of risks were performed across the four participant groups. Adjusted models included the following covariates: maternal age (years), education level (Bachelor’s degree or above vs. below), gravidity (multigravidity/not), and gestational weight gain (insufficient/normal/excessive). A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.3.2.
RESULTS
Baseline characteristics of the study population
A total of 4,118 participants were included in the study, comprising 302 women with MASLD and 3,816 without, corresponding to a MASLD prevalence of 7.3%. Compared with those without MASLD, pregnant women with MASLD were older, had higher prepregnancy weight and BMI, but showed lower gestational weight gain and educational level (all P < 0.05). Among women with MASLD, 43.4% were classified as overweight and 45.4% as obese, compared to only 13.9% and 2.7%, respectively, in the non-MASLD group
Characteristics of the pregnant participants with or without MASLD
| Characteristics | Non-MASLD | MASLD | P value* |
| Number of participants | 3,816 (92.7%) | 302 (7.3%) | |
| Age (year) | 31.22 (29.04, 33.25) | 32.10 (30.22, 35.00) | < 0.001 |
| < 35 years | 3,353 (87.9%) | 226 (74.8%) | < 0.001 |
| ≥ 35 years | 463 (12.1%) | 76 (25.2%) | |
| Prepregnant weight (kg) | 55.00 (51.00, 61.00) | 73.00 (66.00, 80.00) | < 0.001 |
| Height (cm) | 163 (160, 166) | 163 (160, 166) | 0.566 |
| BMI (kg/m2) | 20.94 (19.47, 22.96) | 27.68 (25.59, 30.10) | < 0.001 |
| Underweight | 415 (10.9%) | 0 (0.0%) | < 0.001 |
| Normal weight | 2,768 (72.5%) | 34 (11.3%) | |
| Overweight | 531 (13.9%) | 131 (43.4%) | |
| Obesity | 102 (2.7%) | 137 (45.4%) | |
| History of chronic hypertension | 47 (1.2%) | 27 (8.9%) | < 0.001 |
| History of T2DM | 40 (1.0%) | 25 (8.3%) | < 0.001 |
| Gestational weight gain (kg) | 13.20 (10.00, 16.20) | 10.00 (5.85, 14.00) | < 0.001 |
| Insufficient | 452 (11.9%) | 79 (26.2%) | < 0.001 |
| Normal | 1,692 (44.4%) | 93 (30.8%) | |
| Excessive | 1,667 (43.7%) | 130 (43.0%) | |
| Multigravidity | 101 (2.6%) | 7 (2.3%) | 0.875 |
| SBP (mmHg) | 113 (106, 120) | 122 (115, 129) | < 0.001 |
| DBP (mmHg) | 67 (61, 72) | 74 (68, 80) | < 0.001 |
| Educational level | |||
| Below Bachelor’s degree | 794 (20.8%) | 86 (28.5%) | 0.002 |
| Bachelor’s degree or above | 3,022 (79.2%) | 216 (71.5%) | |
| Gestational age at diagnosis (wk) | 9+6 (9+0, 11+0) | 9+6 (9+0, 11+0) | 0.531 |
| AST (U/L) | 17.00 (15.00, 20.00) | 18.30 (15.30, 22.10) | < 0.001 |
| ALT (U/L) | 13.00 (10.00, 20.00) | 21.00 (14.25, 30.00) | < 0.001 |
| TC (mmol/L) | 4.35 (3.87, 4.84) | 4.70 (4.16, 5.26) | < 0.001 |
| TG (mmol/L) | 0.99 (0.79, 1.27) | 1.36 (1.10, 1.77) | < 0.001 |
| FBG (mmol/L) | 4.63 (4.39, 4.87) | 4.89 (4.63, 5.25) | < 0.001 |
| GGT (U/L) | 14.00 (11.00, 18.00) | 24.00 (18.00, 33.75) | < 0.001 |
| HDL-C (mmol/L) | 1.52 (1.34, 1.72) | 1.29 (1.10, 1.45) | < 0.001 |
| LDL-C (mmol/L) | 2.31 (2.01, 2.65) | 2.76 (2.36, 3.14) | < 0.001 |
The non-MASLD group was further subdivided into non-SLD&CMRF (n = 2,321), CMRF-only (n = 1,474), and SLD-only (n = 21) groups. Compared with the non-SLD&CMRF group, the CMRF-only and SLD-only groups had significantly higher prepregnancy weights and BMIs, with the CMRF-only group also exhibiting lower gestational weight gain and lower educational level. The MASLD group, in comparison with all other groups, showed higher age, prepregnancy weight, BMI, and blood pressure alongside lower educational level. There were no significant differences among the groups regarding gestational age at diagnosis
Characteristics of the pregnant participants
| Characteristics | Non-SLD&CMRF | CMRF-only | SLD-only | MASLD | P value* |
| Number of participants | 2,321 (56.36%) | 1,474 (35.79%) | 21 (0.51%) | 302 (7.33%) | |
| Age (year) | 31.15 (29.07, 33.13) | 31.39 (29.04, 33.54) | 30.11 (28.15, 32.82) | 32.10 (30.22, 35.00) | < 0.001 |
| < 35 years | 2,075 (89.4%) | 1,260 (85.5%) | 18 (85.7%) | 226 (74.8%) | < 0.001 |
| ≥ 35 years | 246 (10.6%) | 214 (14.5%) | 3 (14.3%) | 76 (25.2%) | |
| Prepregnant weight (kg) | 54.00 (50.00, 58.00) | 60.00 (54.12, 67.00) | 57.00 (54.00, 63.00) | 73.00 (66.00, 80.00) | < 0.001 |
| Height (cm) | 163 (160, 166) | 163 (160, 166) | 163 (159, 165) | 163 (160, 166) | 0.844 |
| BMI (kg/m2) | 20.31 (19.07, 21.63) | 23.05 (20.51, 25.13) | 22.43 (21.37, 23.43) | 27.68 (25.59, 30.10) | < 0.001 |
| Underweight | 329 (14.2%) | 86 (5.8%) | 0 (0.0%) | 0 (0.0%) | < 0.001 |
| Normal weight | 1,992 (85.8%) | 755 (51.2%) | 21 (100.0%) | 34 (11.3%) | |
| Overweight | 0 (0.0%) | 531 (36.0%) | 0 (0.0%) | 131 (43.4%) | |
| Obesity | 0 (0.0%) | 102 (6.9%) | 0 (0.0%) | 137 (45.4%) | |
| History of chronic hypertension | 0 (0.0%) | 47 (3.2%) | 0 (0.0%) | 27 (8.9%) | < 0.001 |
| History of T2DM | 0 (0.0%) | 40 (2.7%) | 0 (0.0%) | 25 (8.3%) | < 0.001 |
| Gestational weight gain (kg) | 13.70 (11.00, 16.60) | 13.00 (9.50, 16.00) | 14.00 (12.00, 15.70) | 10.00 (5.85, 14.00) | < 0.001 |
| Insufficient | 263 (11.3%) | 187 (12.7%) | 2 (9.5%) | 79 (26.2%) | < 0.001 |
| Normal | 1,096 (47.3%) | 587 (39.9%) | 9 (42.9%) | 93 (30.8%) | |
| Excessive | 959 (41.4%) | 698 (47.4%) | 10 (47.6%) | 130 (43.0%) | |
| Multigravidity | 58 (2.5%) | 43 (2.9%) | 0 (0.0%) | 7 (2.3%) | 0.724 |
| Educational level | |||||
| Below Bachelor’s degree | 439 (18.9%) | 351 (23.8%) | 4 (19.0%) | 86 (28.5%) | < 0.001 |
| Bachelor’s degree or above | 1,882 (81.1%) | 1,123 (76.2%) | 17 (81.0%) | 216 (71.5%) | |
| Gestational age at diagnosis (wk) | 9+6 (9+0, 11+0) | 9+6 (9+0, 11+1) | 10+1 (8+5, 10+4) | 9+6 (9+0, 11+0) | 0.604 |
| SBP (mmHg) | 112 (105, 118) | 116 (108, 122) | 115 (109, 123) | 122 (115, 129) | < 0.001 |
| DBP (mmHg) | 66 (61, 71) | 69 (63, 75) | 72 (61, 78) | 74 (68, 80) | < 0.001 |
| AST (U/L) | 16.80 (15.00, 19.70) | 17.30 (14.90, 20.30) | 17.20 (15.40, 20.80) | 18.30 (15.30, 22.10) | < 0.001 |
| ALT (U/L) | 13.00 (10.00, 18.00) | 15.00 (11.00, 22.00) | 18.00 (13.00, 25.00) | 21.00 (14.25, 30.00) | < 0.001 |
| TC (mmol/L) | 4.38 (3.96, 4.82) | 4.27 (3.72, 4.87) | 4.65 (4.34, 5.01) | 4.70 (4.16, 5.26) | < 0.001 |
| TG (mmol/L) | 0.94 (0.76, 1.15) | 1.13 (0.86, 1.53) | 1.22 (0.96, 1.42) | 1.36 (1.10, 1.77) | < 0.001 |
| FBG (mmol/L) | 4.59 (4.36, 4.83) | 4.69 (4.43, 4.94) | 4.64 (4.43, 4.79) | 4.89 (4.63, 5.25) | < 0.001 |
| GGT (U/L) | 13.00 (11.00, 17.00) | 15.00 (12.00, 21.00) | 20.00 (17.00, 24.00) | 24.00 (18.00, 33.75) | < 0.001 |
| HDL-C (mmol/L) | 1.60 (1.46, 1.78) | 1.29 (1.20, 1.55) | 1.44 (1.38, 1.65) | 1.29 (1.10, 1.45) | < 0.001 |
| LDL-C (mmol/L) | 2.28 (2.01, 2.59) | 2.38 (2.02, 2.75) | 2.58 (2.29, 2.79) | 2.76 (2.36, 3.14) | < 0.001 |
Regarding serum biomarkers, the MASLD group exhibited more pronounced deviations from normal ranges in liver enzymes, serum lipids, and FBG levels compared to the non-MASLD group. While the CMRF-only and SLD-only groups also showed some metabolic abnormalities, these were less extensive than those observed in the MASLD group [Tables 1 and 2, Supplementary Table 2].
Incidence of APOs
Compared with the non-MASLD group, women with MASLD delivered at earlier gestational ages (P = 0.001) and experienced markedly higher rates of cesarean section (62.9% vs. 39.5%), GDM (40.1% vs. 18.6%), pregnancy-associated hypertension (12.6% vs. 5.2%), preterm birth (7.9% vs. 4.2%), and LGA neonates (16.2% vs. 7.4%; all P < 0.05). Compared with the non-SLD&CMRF group, the CMRF-only and SLD-only groups also showed higher incidences of cesarean section, GDM, and preterm birth. Additionally, the CMRF-only group had a higher incidence of pregnancy-associated hypertension. Notably, no significant differences in the incidence of SGA neonates or postpartum hemorrhage were observed across any of the groups
Adverse pregnancy outcomes among pregnant women in different groups
| Outcomes | Non-SLD&CMRF (N = 2,321) | CMRF-only (N = 1,474) | SLD-only (N = 21) | MASLD (N = 302) | P value* |
| Gestational age at delivery (wk) | 39+6 (39+0, 40+4) | 39+6 (38+6, 40+4) | 39+4 (37+6, 41+0) | 39+3 (38+4, 40+3) | 0.015 |
| Cesarean section | 861 (37.1%) | 635 (43.1%) | 13 (61.9%) | 190 (62.9%) | < 0.001 |
| Gestational diabetes mellitus | 362 (15.6%) | 340 (23.1%) | 8 (38.1%) | 121 (40.1%) | < 0.001 |
| Pregnancy-associated hypertension | 88 (3.8%) | 111 (7.5%) | 1 (4.8%) | 38 (12.6%) | < 0.001 |
| Preterm birth | 82 (3.5%) | 74 (5.0%) | 3 (14.3%) | 24 (7.9%) | < 0.001 |
| Large-for-gestational-age neonates | 158 (6.8%) | 123 (8.4%) | 3 (14.3%) | 49 (16.2%) | < 0.001 |
| Small-for-gestational-age neonates | 336 (14.5%) | 194 (13.2%) | 3 (14.3%) | 39 (12.9%) | 0.665 |
| Postpartum hemorrhage | 149 (6.4%) | 79 (5.4%) | 1 (4.8%) | 15 (5.0%) | 0.491 |
Risks of APOs across groups
Compared with pregnant women without MASLD, those with MASLD had significantly higher adjusted risks for multiple APOs, including cesarean section [adjusted RR (aRR) = 1.459, 95%CI: 1.253-1.700, P < 0.001], GDM (aRR = 2.081, 95%CI: 1.711-2.530, P < 0.001), pregnancy-associated hypertension (aRR = 2.192, 95%CI: 1.541-3.118, P < 0.001), preterm birth (aRR = 1.826, 95%CI: 1.181-2.823, P = 0.001), and LGA neonates (aRR = 2.024, 95%CI: 1.488-2.754, P < 0.001). These elevated risks were consistent when comparing the MASLD group with the non-SLD&CMRF group [Tables 4 and 5, Supplementary Table 5].
Association between MASLD and the risk of adverse pregnancy outcomes
| Adverse pregnancy outcomes | Non-MASLD | MASLD | |
| RR (95%CI) | P value | ||
| Cesarean section | |||
| Unadjusted | Ref. | 1.591 (1.368, 1.850) | < 0.001 |
| Adjusted | Ref. | 1.459 (1.253, 1.700) | < 0.001 |
| Gestational diabetes mellitus | |||
| Unadjusted | Ref. | 2.153 (1.776, 2.611) | < 0.001 |
| Adjusted | Ref. | 2.081 (1.711, 2.530) | < 0.001 |
| Pregnancy-associated hypertension | |||
| Unadjusted | Ref. | 2.401 (1.697, 3.396) | < 0.001 |
| Adjusted | Ref. | 2.192 (1.541, 3.118) | < 0.001 |
| Preterm birth | |||
| Unadjusted | Ref. | 1.907 (1.242, 2.930) | 0.003 |
| Adjusted | Ref. | 1.826 (1.181, 2.823) | 0.007 |
| Large-for-gestational-age neonates | |||
| Unadjusted | Ref. | 2.178 (1.609, 2.950) | < 0.001 |
| Adjusted | Ref. | 2.024 (1.488, 2.754) | < 0.001 |
| Small-for-gestational-age neonates | |||
| Unadjusted | Ref. | 0.924 (0.667, 1.279) | 0.633 |
| Adjusted | Ref. | 0.968 (0.698, 1.344) | 0.847 |
| Postpartum hemorrhage | |||
| Unadjusted | Ref. | 0.828 (0.491, 1.395) | 0.478 |
| Adjusted | Ref. | 0.801 (0.473, 1.355) | 0.407 |
Association of different states of SLD and CMRF with the risk of adverse pregnancy outcomes after adjustment
| Adverse pregnancy outcomes | Non-SLD&CMRF | CMRF-only | SLD-only | MASLD | |||
| aRR (95%CI) | P value | aRR (95%CI) | P value | aRR (95%CI) | P value | ||
| Cesarean section | Ref. | 1.117 (1.007, 1.238) | 0.036 | 1.677 (0.970, 2.901) | 0.064 | 1.532 (1.304, 1.800) | < 0.001 |
| Ref. | 1.484 (0.857, 2.572) | 0.159 | 1.387 (1.177, 1.634) | < 0.001 | |||
| Ref. | 0.980 (0.555, 1.729) | 0.944 | |||||
| Gestational diabetes mellitus | Ref. | 1.487 (1.281, 1.726) | < 0.001 | 2.551 (1.266, 5.143) | 0.009 | 2.410 (1.948, 2.980) | < 0.001 |
| Ref. | 1.642 (0.814, 3.313) | 0.166 | 1.734 (1.405, 2.139) | < 0.001 | |||
| Ref. | 1.046 (0.507, 2.158) | 0.903 | |||||
| Pregnancy-associated hypertension | Ref. | 1.842 (1.390, 2.443) | < 0.001 | 1.190 (0.166, 8.544) | 0.863 | 3.050 (2.060, 4.515) | < 0.001 |
| Ref. | 0.662 (0.092, 4.748) | 0.682 | 1.606 (1.105, 2.333) | 0.013 | |||
| Ref. | 2.892 (0.394, 21.217) | 0.296 | |||||
| Preterm birth | Ref. | 1.427 (1.038, 1.961) | 0.029 | 4.530 (1.428, 14.375) | 0.010 | 2.146 (1.342, 3.430) | 0.001 |
| Ref. | 2.908 (0.915, 9.242) | 0.070 | 1.525 (0.956, 2.433) | 0.076 | |||
| Ref. | 0.531 (0.156, 1.805) | 0.311 | |||||
| Large-for-gestational-age neonates | Ref. | 1.180 (0.931, 1.496) | 0.172 | 2.097 (0.669, 6.577) | 0.204 | 2.224 (1.599, 3.095) | < 0.001 |
| Ref. | 1.712 (0.544, 5.383) | 0.358 | 1.845 (1.318, 2.581) | < 0.001 | |||
| Ref. | 1.037 (0.321, 3.351) | 0.952 | |||||
| Small-for-gestational-age neonates | Ref. | 0.930 (0.778, 1.111) | 0.421 | 0.996 (0.320, 3.106) | 0.995 | 0.929 (0.663, 1.300) | 0.666 |
| Ref. | 1.041 (0.332, 3.262) | 0.944 | 1.032 (0.729, 1.460) | 0.861 | |||
| Ref. | 0.915 (0.277, 3.017) | 0.883 | |||||
| Postpartum hemorrhage | Ref. | 0.828 (0.629, 1.089) | 0.177 | 0.774 (0.108, 5.536) | 0.799 | 0.733 (0.428, 1.257) | 0.259 |
| Ref. | 0.897 (0.125, 6.458) | 0.914 | 0.911 (0.522, 1.590) | 0.743 | |||
| Ref. | 1.031 (0.133, 8.007) | 0.977 | |||||
Women with isolated CMRF had elevated risks of cesarean section (aRR = 1.117, 95%CI: 1.007-1.238, P = 0.036), GDM (aRR = 1.487, 95%CI: 1.281-1.726, P < 0.001), pregnancy-associated hypertension (aRR = 1.842, 95%CI: 1.390-2.443, P < 0.001), and preterm birth (aRR = 1.427, 95%CI: 1.038-1.961, P = 0.029). The SLD-only group also presented increased risks of GDM (aRR = 2.551, 95%CI: 1.266-5.143, P = 0.009) and preterm birth (aRR = 4.530, 95%CI: 1.428-14.375, P = 0.010) [Table 5 and Supplementary Table 5].
When using the CMRF-only group as the reference, the MASLD group had significantly greater risks of cesarean section (aRR = 1.387, 95%CI: 1.177-1.634, P < 0.001), GDM (aRR = 1.734, 95%CI: 1.405-2.139, P < 0.001), pregnancy-associated hypertension (aRR = 1.606, 95%CI: 1.105-2.333, P = 0.013), and LGA neonates (aRR = 1.845, 95%CI: 1.318-2.581, P = 0.001) [Figure 2]. However, no significant differences were observed when MASLD was compared with the SLD-only group [Table 5 and Supplementary Table 5].
DISCUSSION
This study revealed a significant association between MASLD and elevated risks of APOs, including cesarean section, GDM, pregnancy-associated hypertension, preterm birth, and LGA neonates. Furthermore, the presence of either isolated CMRF or SLD alone was associated with elevated risks of specific APOs. Notably, compared to the CMRF-only group, the MASLD group - characterized by concomitant SLD superimposed on CMRF - demonstrated significantly increased risks for multiple APOs. In contrast, no additional risk increase was observed when comparing the MASLD group to the SLD-only group. These findings indicate that SLD may play a more critical pathogenetic role in the development of APOs.
Pregnancy is a period of profound physiological adaptation, during which the cardiovascular system is subjected to considerable strain to meet heightened metabolic demands, thereby increasing susceptibility to physiologic insulin resistance and cardiovascular burden[39,40]. The presence of MASLD exacerbates these challenges, potentially leading to a range of adverse maternal and neonatal outcomes. Our findings demonstrate that MASLD carries a risk profile for APOs comparable to that historically associated with NAFLD during pregnancy[10-12,14]. This similarity likely stems from the substantial diagnostic overlap between NAFLD and MASLD, with approximately 95% of NAFLD cases meeting the current MASLD criteria[2,9].
MASLD, CMRF, and SLD are all correlated with systemic metabolic dysfunctions[6,40]. Under such conditions, insulin resistance and disrupted glucose or lipid homeostasis increase the risk of GDM[40,41]. Additionally, hepatic lipid accumulation and hyperlipidemia drive excessive secretion of very-low-density lipoprotein (VLDL), contributing to lipoprotein abnormalities that activate Toll-like receptors, culminating in endothelial injury and a heightened risk of pregnancy-associated hypertension[19]. Elevated blood pressure further increases cardiac workload[28], compounding this risk. Dyslipidemia during gestation is also independently associated with an increased risk of preterm birth[42]. Moreover, maternal overweight or obesity disrupts metabolic homeostasis, induces insulin resistance[43,44], and exacerbates the aforementioned pathological cascades. Obesity-related complications, including preeclampsia and fetal growth abnormalities, are common indications for cesarean section[45]. Together, these interconnected mechanisms underlie the elevated risk of multiple APOs in pregnant women with MASLD.
Importantly, the manifestation of SLD may play a more substantial role in the relationship between MASLD and multiple APOs. Our findings align with those of Qian et al.[14], who stratified pregnant women by BMI - a key CMRF. They found that pregnancies with normal BMI but complicated by NAFLD were associated with an increased risk of APOs. Similarly, among overweight or obese gravidas, NAFLD was linked to a higher risk of GDM and preeclampsia. After adjusting for all CMRF, our analysis substantiates that isolated SLD is independently associated with specific APOs. Moreover, when SLD is superimposed on preexisting CMRF, it is associated with an increased risk of multiple APOs.
However, our findings differ from those of Lee et al., who concentrated on metabolic dysfunction-associated fatty liver disease (MAFLD) and identified metabolic dysfunction (MD) as the primary driver of APO risk[46]. This discrepancy may arise from several methodological differences. First, our reference group comprised women without SLD or CMRF, thereby excluding those with isolated CMRF, who themselves demonstrated elevated APO risk. Second, the definitions of CMRF and MD differ substantially. CMRF, as a predictor of future cardiometabolic disease, does not include insulin resistance biomarkers but does include individuals receiving antihypertensive and lipid-lowering treatments. An epidemiological survey corroborated this interpretation, showing that fewer NAFLD patients meet MAFLD criteria compared to MASLD due to diagnostic differences[2].
The elevated APO risk associated with SLD involves multiple mechanisms. As a central metabolic and immunological organ, the liver, when burdened with ectopic lipid accumulation, promotes chronic systemic oxidative stress, accelerating cellular damage and disease progression[18]. Hepatic steatosis also activates Kupffer cells, triggering the release of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukins(IL-1β, IL-6, IL-10, IL-12, and IL-18), all of which contribute to APO pathogenesis[47-49]. Additionally, elevated intrahepatic diacylglycerol levels induce insulin resistance[50,51], disrupt sympathetic tone, and impair vascular endothelial function, leading to vasomotor dysfunction and increased risk of pregnancy-associated hypertension and cardiovascular complications[22,52]. Furthermore, ectopic lipid deposition and insulin resistance exacerbate maternal hyperglycemia and elevate free fatty acid levels, promoting excessive fetal growth and LGA neonates[53]. Thus, SLD may exert a more detrimental impact on immune and metabolic homeostasis than isolated CMRF. Genetic variants, such as patatin-like phospholipase domain-containing protein 3 (PNPLA3) and transmembrane 6 superfamily member 2 (TM6SF2), further compound these effects by impairing hepatic triglyceride export and cholesterol metabolism[49], thereby worsening steatosis and related pathophysiological cascades. Nonetheless, while both SLD and CMRF are independently associated with an increased risk of preterm birth, their coexistence does not appear to confer additional risk. This may reflect the fact that neither SLD nor CMRF is a primary driver of the pathogenesis of preterm birth[35,54].
This study provides a comprehensive analysis of the associations between MASLD and APOs. To our knowledge, this is the first study with a moderately large sample size in a Chinese population to systematically evaluate the differential contributions of CMRF and SLD within MASLD to APO risk. Nevertheless, several limitations warrant consideration. First, despite a cohort size of over 4,000 participants, the number of women with isolated SLD was relatively small due to the frequent coexistence of CMRF in individuals with SLD[9], which may have limited statistical power to detect subtle associations. However, our findings suggest a potentially dominant role of SLD in MASLD, and further validation in larger cohorts, particularly among pregnant women with isolated SLD, is necessary. Second, ultrasonographic diagnosis of SLD involves multiple sonologists, potentially introducing interobserver variability. However, being a tertiary hospital in China, all sonologists at this institution receive standardized training and possess extensive clinical experience. Adherence to strict diagnostic guidelines ensures a high degree of reproducibility. Future studies would benefit from quality control measures such as periodic assessments of interobserver agreement to further enhance diagnostic consistency. Third, due to the retrospective nature of the study, some potential confounders - such as lifestyle factors, dietary patterns, family history, and medication records - were not available, which may introduce bias. Prospective cohort studies should incorporate systematic collection of these variables to strengthen future analyses. Finally, as this study focused on a Chinese population, validation in multiethnic cohorts is needed to establish broader applicability.
In conclusion, MASLD during pregnancy is associated with an increased risk of multiple APOs, including cesarean section, GDM, pregnancy-associated hypertension, preterm birth, and LGA neonates. Importantly, SLD appears to play a more critical role in driving these risks. These findings highlight the importance of early screening for SLD during pregnancy to identify women at elevated risk for APOs. Early identification may facilitate the implementation of targeted preventive interventions and improve both maternal and neonatal short- and long-term health outcomes.
DECLARATIONS
Acknowledgment
Image of liver in the graphic abstract by Jan-Clusmann (https://www.linkedin.com/in/jan-clusmann/) is licensed under CC0 (https://creativecommons.org/publicdomain/zero/1.0/). Image of heart in the graphic abstract by Servier (https://smart.servier.com/) is licensed under CC-BY 3.0 Unported (https://creativecommons.org/licenses/by/3.0/).
Authors’ contributions
Study design: Wang H, Yang L, Yang S
Data collection and organization: Yang S, Guan H, Yang L, Wang Y, Wang M, Wu Y, Yang Y
Data analysis: Yang S, Wang Y, Hebestreit A
Manuscript writing: Yang S, Guan H, Wang Y
All authors made manuscript revision, read and approved the submitted version.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request. Due to ethical and privacy restrictions, the data are not publicly available. Researchers interested in accessing the data may contact corresponding author to submit a formal request, which will be reviewed in accordance with institutional and ethical guidelines.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Ethical approval was obtained from the Ethics Committee of Beijing Friendship Hospital (Approval Number: 2023-P2-175-02). Due to the register-based nature of the study, informed consent was waived.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022;31:17-27.
2. Wu T, Ye J, Mo S, et al. Impact of nomenclature as metabolic associated steatotic liver disease on steatotic liver disease prevalence and screening: a prospective population survey in Asians. J Gastroenterol Hepatol. 2024;39:1636-47.
3. Hershman M, Mei R, Kushner T. Implications of nonalcoholic fatty liver disease on pregnancy and maternal and child outcomes. Gastroenterol Hepatol (N Y). 2019;15:221-8.
4. Zhang ZC, Liu Y, Xiao LL, et al. Upregulation of miR-125b by estrogen protects against non-alcoholic fatty liver in female mice. J Hepatol. 2015;63:1466-75.
5. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589-600.
6. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
7. Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-50.
8. Rinella ME, Lazarus JV, Ratziu V, et al; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-86.
9. Song R, Li Z, Zhang Y, Tan J, Chen Z. Comparison of NAFLD, MAFLD and MASLD characteristics and mortality outcomes in United States adults. Liver Int. 2024;44:1051-60.
10. Hagström H, Höijer J, Ludvigsson JF, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016;36:268-74.
11. Hong S, Lee SM, Kwak SH, et al. A comparison of predictive performances between old versus new criteria in a risk-based screening strategy for gestational diabetes mellitus. Diabetes Metab J. 2020;44:726-36.
12. Sarkar M, Grab J, Dodge JL, et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73:516-22.
13. Herath RP, Siriwardana SR, Ekanayake CD, Abeysekara V, Kodithuwakku SUA, Herath HP. Non-alcoholic fatty liver disease and pregnancy complications among Sri Lankan women: a cross sectional analytical study. PLoS One. 2019;14:e0215326.
14. Qian Y, Zhang Y, Fan X, et al. Nonalcoholic fatty liver disease and adverse pregnancy outcomes in women with normal prepregnant weight. J Clin Endocrinol Metab. 2023;108:463-71.
15. Chai TY, Byth K, George J, Pasupathy D, Cheung NW. Elevated hepatic steatosis index is associated with the development of adverse maternal, but not adverse neonatal, outcomes: a retrospective cohort study. Int J Womens Health. 2023;15:589-98.
16. Zhou BG, Xia JL, Jiang X, Ding YB, She Q. Non-alcoholic fatty liver disease and gestational diabetes mellitus: a bidirectional two-sample mendelian randomization study. BMC Endocr Disord. 2024;24:40.
17. Niu C, Zhang J, Khalid N, et al. Cardiovascular complications during delivery hospitalizations in patients with nonalcoholic fatty liver disease in pregnancy. Eur J Gastroenterol Hepatol. 2024;36:1141-8.
18. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313-27.
19. Lechner K, McKenzie AL, Kränkel N, et al. High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, atherogenic dyslipidemia, and inflammation. Metab Syndr Relat Disord. 2020;18:176-85.
20. Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713-23.
21. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173-94.
22. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509-20.
23. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1.
24. criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341-63.
25. Woollett LA, Catov JM, Jones HN. Roles of maternal HDL during pregnancy. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159106.
26. Liu PJ, Liu Y, Ma L, et al. The predictive ability of two triglyceride-associated indices for gestational diabetes mellitus and large for gestational age infant among chinese pregnancies: a preliminary cohort study. Diabetes Metab Syndr Obes. 2020;13:2025-35.
27. Brereton MF, Rohm M, Shimomura K, et al. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat Commun. 2016;7:13496.
29. National Health and Family Planning Commission of the People’s Republic of China. Criteria of weight for adults (WS/T 428-2013). Beijing: Standards Press of China; 2013. (In Chinese) Available from: https://www.nhc.gov.cn/wjw/yingyang/201308/a233d450fdbc47c5ad4f08b7e394d1e8.shtml. [Last accessed on 28 Jul 2025].
30. National Health Commission of the People’s Republic of China. Standard of recommendation for weight gain during pregnancy period (WS/T 801-2022). Beijing: Standards Press of China; 2022. (In Chinese) Available from: https://www.nhc.gov.cn/wjw/c100311/202208/deb61e5c2299451ea1b957b0672272b3.shtml.
31. Graif M, Yanuka M, Baraz M, et al. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol. 2000;35:319-24.
32. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-74.
33. Metzger BE, Gabbe SG, Persson B, et al; International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-82.
34. American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Hypertension in pregnancy: report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-31.
35. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75-84.
36. National Health Commission of the People’s Republic of China. Growth standard for newborns by gestational age (WS/T 800-2022). Beijing: Standards Press of China; 2022. (In Chinese) Available from: https://www.nhc.gov.cn/wjw/c100311/202208/07787ef64ba34fe1bc8bbdae9fd0d4e5.shtml. [Last accessed on 28 Jul 2025].
37. Borovac-Pinheiro A, Pacagnella RC, Cecatti JG, et al. Postpartum hemorrhage: new insights for definition and diagnosis. Am J Obstet Gynecol. 2018;219:162-8.
38. Committee on Practice Bulletins-Obstetrics. Practice bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-86.
39. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157-60.
40. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-41.
41. Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - a metabolic and reproductive disorder. Biomed Pharmacother. 2021;143:112183.
42. Nasioudis D, Doulaveris G, Kanninen TT. Dyslipidemia in pregnancy and maternal-fetal outcome. Minerva Ginecol. 2019;71:155-62.
43. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:1-10.
44. Richard AJ, White U, Elks CM, Stephens JM. Adipose tissue: physiology to metabolic dysfunction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555602/. [Last accessed on 21 Jul 2025].
45. Coates D, Homer C, Wilson A, et al. Indications for, and timing of, planned caesarean section: a systematic analysis of clinical guidelines. Women Birth. 2020;33:22-34.
46. Lee SM, Jung YM, Choi ES, et al. Metabolic dysfunction-associated fatty liver disease and subsequent development of adverse pregnancy outcomes. Clin Gastroenterol Hepatol. 2022;20:2542-50.e8.
48. Svenvik M, Raffetseder J, Brudin L, et al. Early prediction of spontaneous preterm birth before 34 gestational weeks based on a combination of inflammation-associated plasma proteins. Front Immunol. 2024;15:1415016.
49. Ismaiel A, Dumitraşcu DL. Cardiovascular risk in fatty liver disease: the liver-heart axis-literature review. Front Med. 2019;6:202.
50. Scifres CM, Davis EM, Orris S, et al. Metabolic factors and perinatal outcomes among pregnant individuals with mild glucose intolerance. Diabetes Res Clin Pract. 2024;216:111830.
51. Byrne CD. Ectopic fat, insulin resistance and non-alcoholic fatty liver disease. Proc Nutr Soc. 2013;72:412-9.
52. Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat Rev Cardiol. 2010;7:577-84.
53. Metzger BE, Lowe LP, Dyer AR, et al; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].