Stage dependent management of sleeve gastrectomy leaks - a systematic review with proposed classification and management algorithm
Abstract
Aim: Gastric leak following foregut surgery remains a major challenge to clinicians. Treatment algorithms may vary between institutions often depending on clinician preference. The objective of this review is to assess the efficacy of different treatment strategies (i.e., endoscopic and surgical) for sleeve gastrectomy leaks across the literature, share our own centre’s experiences and attempt to implement an algorithm in managing sleeve leaks according to their severity as classified by a computed tomography-based staging system.
Methods: A comprehensive search of existing literature over the last decade was conducted using pre-defined criteria in accordance with preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Sleeve gastrectomy leaks in the included studies were further categorized according to severity, prior to analysing the efficacy of treatment methods.
Results: Following review of 1,109 potential articles, 36 studies were included, involving a total of 1,246 sleeve leak patients. The mean age and body mass index of patients ranged from 33 to 46 years of age and 37 to 48 kg/m2, respectively. In type 1-2 leaks, surgical or radiological drainage followed by primary endoscopic therapy (i.e., stenting, internal drainage, over the scope clips, fibrin glue and/or E-VAC) was effective (leak resolution rates - 50%-100% between reporting papers). Endoscopic therapy remains a viable treatment option in treating type 3-4 leaks with success rates ranging from 33%-95%, although surgery (i.e., fistulo-jejunostomy, Roux-en-Y gastric bypass or total gastrectomy) may be required in chronic leaks where all other modalities have failed.
Conclusion: Management of sleeve leaks should be driven by the underlying leak pathophysiology. Defining variables such as the size of the defect, size of any abscess/collection and presence of a stenosis can allow differing options to be applied. Patients who fail to respond appropriately can be escalated to alternate therapies with the aim of resuming per oral nutrition and minimizing inpatient stay.
Keywords
INTRODUCTION
The last two decades have seen an explosion in the caseload of complex foregut surgery, especially bariatric surgery. While the field of bariatric surgery has evolved and matured with commensurate reductions in the frequency of major complications, staple-line leaks - or, more correctly, deep/organ-space surgical site infections (SSIs) - remain a burden on patients, treating clinicians, hospitals, and payers, both in terms of hospital care and the payment of sometimes inevitable medico-legal claims[1,2]. Although several strategies have been developed, tested, and widely implemented, these methods have not been compared for safety and efficacy.
The emergency nature of presentations with deep/organ SSIs and their relative infrequency mean that treatment offered in various units is generally binary, with patients receiving either surgical therapy or endoscopic therapy, depending on the experience of the unit where they present[3,4]. In these circumstances, clinicians may select a therapy based on prior use rather than considering which option from a suite of therapies may best suit the patient. If the initial therapy is not immediately successful, patients may be at risk of prolonged treatment that encourages the development of chronic fistulizing disease or of being moved toward complex resectional therapy, which further raises the stakes. Consequently, inherent to this clinical quandary is a general lack of high-quality, evidence-based guidance on how to optimally and systematically classify the wide variety of leaks to better guide the implementation of effective solutions. The purpose of this review is to address this gap in knowledge.
In addition to our own centre’s data, we conducted a systematic review of the current literature on the management of laparoscopic sleeve gastrectomy (LSG) deep/organ-space SSIs, with the aim of proposing a staging system for LSG infectious complications that may help guide management according to the underlying pathophysiology expressed in the patient’s illness.
METHODS
Literature search strategy
Published studies reporting the treatment and management of deep/organ-space SSI associated with LSG were identified through searches of MEDLINE (EBSCO), PubMed, EMBASE, Scopus, CRANE Central Registry of Controlled Trials, CINAHL (EBSCO), and Web of Science. Combinations of medical subject headings and the following keywords were used: LSG, laparoscopic gastric sleeve, bariatric surgery, leak, abscess, fistula, stenosis, collection, treatment, and management. The search was limited to 2010-2023. The PubMed search strategy results are provided in Supplementary Materials. Relevant articles were retrieved and assessed according to the inclusion and exclusion criteria, and reference lists were manually searched to identify additional studies. The review protocol was pre-registered in PROSPERO (registration No. 196778) in September 2019 and adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines[5].
Selection criteria
Selection criteria were: (i) studies on LSG; (ii) studies reporting deep/organ-space SSI treatment; (iii) studies reporting treatment or management of these complications; and (iv) studies providing sufficient tabulated or descriptive data for inclusion in the analysis. Where complications other than deep/organ-space SSI were reported, outcomes had to be separable. When multiple publications from the same institution were identified, the most recent study with the largest cohort was included. Non-English studies, non-human studies, studies involving patients < 18 years of age, studies with < 10 patients, case reports, letters, and editorials were excluded.
Data extraction and critical appraisal
Initial exclusion from the combined database search involved removal of duplicate studies and abstract screening. Articles deemed relevant from the initial appraisal underwent full-text evaluation by two independent authors (QC and DLC), and data points were tabulated [Figure 1]. Extracted data included study characteristics, patient demographics, deep/organ-space SSI characteristics, and treatment and management modalities. Discrepancies were resolved with a third author (MLT). Meta-analysis was not performed due to heterogeneity, lack of comparison arms, and the retrospective case series design of most studies. A PRISMA flowchart of the search strategy is provided in Figure 1.
Deep/organ-space SSI staging
Deep/organ-space SSI following LSG was categorized according to a computed tomography (CT)-based staging system[6]. CT was chosen for its objective nature, high sensitivity and specificity compared with other diagnostic tests, and its ability to evaluate deep/organ-space SSI. This method was favored over endoscopy- and clinical-based alternatives.
Our proposed CT-based staging system for LSG leaks is as follows [Figure 2]:
Figure 2. Sleeve gastrectomy leak staging. The characteristics of leak types are as follows: I: CT evidence of phlegmon; IIa: small abscess at staple line (< 5cm); IIb: large abscess extending lateral to staple line (≥ 5cm); III: uncontained leak or perforation (generalized peritonitis); IVa: chronic fistula with abscess cavity close to staple line (< 5cm); IVb: chronic fistula with abscess cavity lateral to staple line (≥ 5cm). Reproduced with permission from: Cheng Q, Beitner J, Talbot M, “Stage-dependent management of sleeve gastrectomy leaks - asystematic review with proposed ct-based classification and management algorithm” 25th IFSO World Congress Silver Anniversary. Obes Surg 2022;32(Suppl 2):39-1044. https://doi.org/10.1007/s11695-022-06204-8.
Type 1 - CT evidence of an inflamed staple line without intra-abdominal abscess or contrast extravasation, with or without a small volume of extraluminal gas or free fluid. This presentation is analogous to a simple wound infection and is often described on CT imaging as a phlegmon.
Type 2 - Abscess cavity attached to the staple line with or without extravasation of oral contrast. With subdivision:
· Type 2a - small abscess (< 5 cm in any dimension) at the staple line.
· Type 2b - larger abscess (≥ 5 cm) extends laterally to the staple line.
Type 3 - Uncontained leak or perforation.
Type 4 - Chronic fistula with subdivision:
· Type 4a - Chronic fistula (persisting for > 12 weeks) with abscess cavity closely opposed to staple-line.
· Type 4b - Chronic fistula (persisting for > 12 weeks) with an abscess cavity in a space lateral to the staple-line.
An additional important element in assessing the underlying drivers of a leak is the presence or absence of a pressure gradient that favors preferential external drainage of enteric content. This may result from ileus, obstruction, or an anatomic or functional stenosis along the gastric staple line defect, i.e., gastric sleeve stenosis.
RESULTS
The initial search identified 1,109 potential articles. After excluding duplicates and unrelated studies, 805 abstracts were reviewed, with a further 704 deemed non-relevant. The remaining 101 full-text articles were assessed, of which 36 studies were included in the analysis, comprising a dataset of 1,246 LSG leak patients [Figure 1].
Of the 36 included studies, only seven were prospective trials[3,7,8]. The mean age of included patients ranged from 33 to 49 years, while mean pre-operative body mass index (BMI) ranged from 27 to 48 kg/m2. The breakdown of leak types amongst each study’s treated patient population is documented in Table 1. Leak categorization was not always possible with some studies either providing no description of leak severity or not including sufficiently exploitable data. The variations in treatment modalities and the focus of individual studies are also detailed. A separate literature review was performed to investigate gastric sleeve stenosis management with a total of 9 studies [Table 2].
Included study characteristics
| Study | ST | RP | Sleeve No. | Age | Gender (%F) | BMI | Leak No. | Leak type | Stenosis No. | Treatment modality discussed in detail | ||||
| 1 | 2a | 2b | 3 | 4 | ||||||||||
| Kelogrigoris et al.[14] | R | 2007-2009 | NR | 39 (26-52) | 57.1 | NR | 21 | - | 21 | - | - | - | Interventional radiology | |
| Sakran et al.[9] | R | 2006-2010 | 2834 | 42 ± 10 | 68.2 | 45 ± 9 | 44 | 4 | 40 | - | - | Medical mgx/Endoscopy/Interventional radiology/Surgery | ||
| Corona et al.[11] | R | 2004-2010 | NR | 43 ± 7 | 81.2 | 48 ± 6 | 16 | 3 | 12 | - | 1 | - | Medical mgx/Endoscopy - Stenting/Interventional radiology | |
| Van De Vrande et al.[23] | R | 2002-2011 | 812 | 12 | - | - | - | - | 12 | - | Surgery - RY fistula-jejunostomy | |||
| Thompson et al.[22] | R | NR | NR | 40 ± 15 | 66.7 | NR | 15 | - | - | - | - | 15 | - | Surgery - PGEJ |
| Christophorou et al.[10] | R | 2007-2012 | NR | 40 ± 12 | 83.6 | 40 ± 12 | 110 | NR | NR | NR | NR | NR | - | Medical mgx/Endoscopy/Interventional radiology/Surgery |
| Chouillard et al.[24] | P | 2007-2012 | - | 47 (22-59) | 76.2 | 44 (36-54) | 21 | - | - | - | - | 21 | - | Surgery - RY fistula-jejunostomy |
| Keren et al.[55] | R | 2010-2014 | NR | 39 (26-60) | 53.8 | 42.9 | 26 | 16 | 10 | - | - | - | Endoscopy - OTSC | |
| Southwell et al.[18] | R | 2007-2014 | 1050 | 45 (32-58) | 66.7 | 44 (31-58) | 21 | 5 | 16 (3 fistulae) | - | Endoscopy - Stenting | |||
| Nedelcu et al.[7] | P | 2007-2013 | NR | 33.2 | 68.4 | 46 (35-62) | 19 | - | 19 | - | 3 | Endoscopy - Stenting; internal drainage | ||
| Nimeri et al.[3] | P | 2010-2014 | 250 | 36 ± 12 | 50 | 37 ± 12 | 14 | - | 11 | 3 | - | 4 | Endoscopy - Stenting/Surgery | |
| Donatelli et al.[8] | P | 2013-2014 | NR | 43 (23-70) | 57/10 | NR | 67 | - | 23 | 42 | 1 | 6 | Endoscopy - internal drainage | |
| Rebibo et al.[13] | R | 2004-2014 | 1205 | 40 ± 11 | 85.1 | 45 ± 8 | 86 | - | 27 | 38 | 13 | - | - | Endoscopy - Stenting; internal drainage |
| Bruzzi et al.[25] | R | 2004-2012 | NR | 39 ± 9 | 75 | 41 ± 8 | 57 | - | - | - | - | 12 | - | Surgery - TGEJ |
| Klimczak et al.[19] | R | 2013-2016 | NR | 42 ± 7 | 70.8 | 46 ± 3 | 14 | 11 | 3 | - | - | Endoscopy - Stenting | ||
| Martin Del Campo et al.[17] | R | 2010-2016 | NR | 46 ± 12 | 70.8 | NR | 24 | - | 11 | 13 | - | - | Endoscopy - Stenting | |
| Almadi et al.[15] | R | 2011-2016 | NR | 36 ± 11 | 45.3 | 40 ± 8 | 64 | 46 | 18 | - | - | Endoscopy - Stenting | ||
| Al Hajj et al.[12] | R | 2004-2014 | 510 | 33 ± 11 | 75 | 43 ± 10 | 20 | 3 | 11 | 6 | - | Medical mgx/Endoscopy/Interventional radiology/Surgery | ||
| Assalia et al.[56] | R | 2013-2017 | 992 | 42 (32-53) | 58.3 | 42 (32-53) | 24 | NR | NR | NR | NR | - | Endoscopy - fibrin glue | |
| Benosman et al.[21] | R | 2010-2015 | NR | 46 ± 9 | 73.1 | 42 ± 7 | 26 | - | - | - | - | 26 | - | Endoscopy |
| De Moura et al.[16] | R | 2016-2018 | NR | 36 ± 10 | 70.3 | NR | 37 | 37 | - | - | - | Endoscopy - Stenting | ||
| Dammaro et al.[20] | R | 2013-2019 | NR | 38 (28-69)* | 92.9 | 40 (35-44)* | 14 | - | - | - | 14 | - | 3 | Endoscopy - internal drainage |
| Ferraz et al.[57] | R | 2011-2019 | 2374 | 39.5 | 76.2 | 39.6 | 21 | 21 | - | - | 15 | Endoscopy-Stenting; Internal drainage. Surgery-washout only | ||
| Bashah et al.[58] | R | 2012-2017 | 4250 | 36.3 ± 10.6 | 56.2 | 42 ± 8.8 | 73 | NR | NR | NR | NR | NR | 12 | Endoscopy-Stenting; Surgery-washout; fistula-jejunostomy |
| Olmi et al.[59] | R | 2010-2018 | 4294 | 41.5 (35-47) | 69.7 | 41.1 (37.9-47.6) | 66 | 63 | - | 3 | 3 | Medical mgx. Endoscopy-Stenting. Interventional radiology. Surgery-washout; suture repair | ||
| Rayman et al.[60] | R | 2011-2020 | NR | 36.8 | 70.6 | 29 (21-36) | 17 | - | - | - | - | 17 | NR | Surgery-Fistulo-jejunostomy; total gastrectomy |
| Montana et al.[61] | R | 2013-2018 | NR | 36.2 ± 10.7 | 84.6 | 36 ± 5 | 13 | - | - | - | - | 13 | NR | Surgery-Total gastrectomy |
| Manos et al.[26] | P | 2018-2020 | NR | 41.7 ± 11.5 | 77.3 | NR | 53 | - | 32 | 19 | 2 | 10 | Endoscopy-Internal drainage; dilatation; naso-cavitary drainage. Surgery-washout/drainage; fistula-jejunostomy | |
| Taleb et al.[62] | R | 2013-2019 | NR | 42.76 | 81 | 27.27 | 21 | - | - | - | - | 21 | NR | Surgery-Fistulo-jejunostomy; RYGB; total gastrectomy |
| Degrandi et al.[63] | R | 2013-2019 | NR | 39 (24-67) | 76.5 | 40 (30-52) | 17 | - | - | - | - | 17 | NR | Surgery-RYGB |
| Parmer et al.[64] | R | 2010-2019 | 1116 | 39.4 (24-56) | 70 | 43 (36-52) | 10 | 10 | - | 1 | Medical mgx. Endoscopy-Stenting; internal drainage; Endo-VACC. Interventional radiology. Surgery-washout | |||
| Kiriakopoulos et al.[65] | R | 2007-2020 | 1371 | 44.78 (36-58) | 70.4 | 43.5 (37-48.7) | 27 | 2 | 25 | - | NR | Endoscopy-Stenting, glue; internal drainage. Interventional radiology. Surgery-completion gastrectomy. Secondary medical mgx | ||
| D’Alessandro et al.[66] | R | 2013-2020 | NR | 37.5 ± 9 | 75 | 40.4 ± 6.1 | 40 | - | - | - | - | 40 | - | Endoscopy-Internal drainage |
| Billmann et al.[67] | R | 2007-2019 | 619 | 43.9 ± 10.5 | 52.2 | 52 ± 10.5 | 23 | 23 | 0 | 3 | Endoscopy-Stenting. Interventional radiology. Surgery-washout; subtotal gastrectomy; RYGB | |||
| Li et al.[68] | R | 2010-2020 | 15721 | 33.1 ± 8.5 | 65.4 | 39.9 ± 9.1 | 78 | 70 | 8 | NR | Endoscopy-Stenting; clipping; internal drainage. Surgery-washout | |||
| Lainas et al.[69] | P | 2017-2019 | NR | 49.2 (36-63) | 85.7 | 41.7 (35.9-52) | 14 | - | 14 | 3 | Endoscopy-stent. Surgery-Fistulo-jejunostomy | |||
Studies on the management of gastric sleeve stenosis
| Study | Type of study | Stenosis No. | Age | Sex (F/M) | BMI | Treatment algorithm | Max PD Pressure/mmHg | PD success rate | Overall endoscopic success rate | Surgery required and No. |
| Parikh et al.[28] | R | 10 | 42 | 8/2 | 42.6 | PD → Stent → Surgery | 20 | 80% | 80% | RYGB - 2 |
| Vilallonga et al.[38] | R | 16 | 41 (28-62) | 8/8 | 31 (18-43) | Surgery | Seromyotomy or RYGB - 14 wedge resections - 2 | |||
| Shnell et al.[70] | R | 16 | 44 | 13/3 | 39.7 | PD or CD → Surgery | 30 | 44% | 44% | RYGB - 5 |
| Ogra et al.[27] | R | 26 | 45 ± 9 | 15/11 | ND | PD or CD → Surgery | ND | SS - 86% LS - 48% | 100% | ND |
| Rebibo et al.[13] | R | 16 | ND | 19/1 | 42.2 | PD → Surgery | 40 | 87% | 88% | RYGB - 2 |
| Manos et al.[71] | R | 18 | 37 ± 8 | 13/5 | 41.6 | PD or Stent → Surgery | 30 | ND | 93% | RYGB - 1 |
| Al Sabah et al.[72] | R | 26 | 35 ± 11 | 22/4 | 43 ± 1.6 | PD → Surgery | ND | 89% | 89% | ND |
| Agnihotri et al.[29] | R | 17 | 43 ± 13 | 16/1 | 32.9 ± 7.7 | PD → Stent → Surgery | ND | 70.5% | 88% | RYGB - 1 |
| Dhorepatil et al.[54] | R | 33 | 46 ± 10 | 27/6 | 43.7 ± 6.4 | PD → Stent → Surgery | 40 | 94% | 97% | Partial Gastrectomy + Stricturoplasty - 1 |
The treatment of LSG leaks is non-standardized amongst different institutions which is reflected in the treatment methods reported in the included studies. In general, a combination of drainage (surgical and/or radiological) and endoscopic therapy was utilized to heal leaks. Treatment methods varied according to the severity, size, or chronicity of the leak, as well as institutional protocol preferences.
Management of type 1 and type 2 leaks
Fourteen of the included studies described their management for type 1 and 2 LSG leaks and reported on their outcomes [Table 3]. Five studies[3,9-12] reported outcomes of managing type 1 and 2 leaks with medical therapy alone [Table 4]. All five studies used medical management in clinically stable patients with early or small radiologically detected leaks or para-staple-line inflammation. Medical treatment involved intravenous antibiotics while maintaining nil-by-mouth and nutrition through total parenteral nutrition (TPN). Al Hajj et al. were the only authors to report outcomes of type 1 leaks managed with medical therapy alone, showing a 100% success rate (n = 3)[12]. When combining outcomes of type 1 and 2 leaks, the success rates were much lower, ranging from 9% to 29% across the five studies[3,9-12].
Studies describing type 1 and 2 leak management
| Study | Type 1 and 2 leak No. | Management algorithm | Conser-vative mgx | Perc drain | Management/Outcomes | Mortality | Overall healing rates | ||||||
| Endoscopic | Surgery | ||||||||||||
| Stenting (Success%) | Internal drainage (Success%) | Stent + internal drainage | Other (Success%) | Endoscopic success rate % | Surgical washout/Drainage | Definitive surgery | |||||||
| Al Hajj et al.[12] | 3/11* | Abx → drainage → endoscopic mgx +/- sx | 4 | 7 - type II | - | - | - | - Fibrin glue 1 Coiling 2 -E-VAC 2 | (2/4) 50% | 3 | RNY TG 1 RNY fistula- jejunostomy 1 RYGB 4 | - | (14/14) 100% |
| Almadi et al.[15] | 64* | Abx → drainage → endoscopic stenting | - | 46 | 64 (94%) | - | - | - | (60/64) 93.8% | 9 | RNY TG 1 lap stent removal for perforation 2 | 1 | (63/64) 98% |
| Corona et al.[11] | 3/12 | Abx → drainage +/- endoscopic stenting | 3 | 12 | 7 (100%) | - | - | - | (7/7) 100% | - | NR | - | (7/7) 100% |
| De Moura et al.[16] | 37 | Abx → endoscopic mgx +/- sx | - | 37 (78%) | 2 | - | - Septotomy 2 | (35/37) 94.6% | NR | RYGB 1 | 1 | (36/37) 97% | |
| Donatelli et al.[8] | 23 - 2a/42 - 2b/3 | Abx → drainage → endoscopic mgx | - | ND | 12 | 64 (78%) | - | - Pneumatic dilatation 6 - Fibrin glue 2 | 78.2% | 42 | RNY TG 3 | - | 90% |
| Kelogrigoris et al.[14] | 0/21 | Abx → CT drainage → surgery and/or stent for fistula | - | 21 (18/21) | 2 (100%) | - | - | NR | NR | 3 (2/3) | - | 1 | (20/21) 95% |
| Keren et al.[55] | 16/10 | Abx → drainage → endoscopic management with OTSC | - | 6 | 6 | - | - | OTSC 26 (85%) Fibrin glue 1 | (21/26) 80.8% | - | NR | NR | NR |
| Klimczak et al.[19] | 11 | Abx → if stable → stenting | - | - | 11 (82%) | - | - | - OTSC 1 - Fibrin glue 1 | (10/11) 90.9% | 1 | NR | - | (11/11) 100% |
| Martin Del Campo et al.[17] | 11 - 2a/ 13 - 2b/3 | Abx → drainage → endoscopic mgx +/- sx | - | 6 | 24 (79%) | - | - | - OTSC 2 - Fibrin glue 11 | (19/24) 70.9% | 12 | STG + RYGB 5 | 1 | (23/24) 96% |
| Nedelcu et al.[7] | 0/19* | Abx → internal drainage vs. stenting depending on abscess opening size | - | - | - | 9 (89%) | 10 (100%) | - | (18/19) 94.7% | 1 | NR | - | (18/19) 95% |
| Nimeri et al.[3] | 0/11 | Abx → peritonitis - Surgical washout or drainage/ early leak → stenting +/- Sx/ late leak → Sx | - | - | 4 (75%) | - | - | - | (3/4) 75% | 5 | Fistula Resection 1 T tube 2 RYGB 3 | - | NR |
| Rebibo et al.[73] | 0/27/50 | Abx → early leak - Sx +/- endoscopy/ late leak -endoscopy +/- Sx | - | 7 | 12 (83%) | 47 (91%) | 18 (83%) | - E-VAC 1 | (68/77) 88.3% | 56; 19 primary suture | RYGB 4 | 2 | (72/77) 94% |
| Sakran et al.[9] | 4/40* | Abx → drainage for unstable pt or endoscopy for stable pt | 4 (4/17) | - | 9 | - | - | - Endoclip 1 - Fibrin glue 1 | ND | 27; 9 primary suture | RNY TG 4 | 4 | NR |
| Southwell et al.[18] | 5/16* | Abx → drainage or endoscopic mgx | - | 6 | 21 (95%) | NR | - | - OTSC 4 - Pneumatic dilation 13 | (20/21) 95.2% | 9 | RYGB 1 | - | (20/21) 95% |
| Ferraz et al.[57] | 21 | Abx → Surgical washout → Stenting | - | - | 20 | 6 | - | -Pneumatic dilatation | NR | 14 | - | - | (21/21) 100% |
| Olmi et al.[59] | 66 | Abx → Surgery for unstable pt or endoscopy for stable pt | 8 | 9 | 59 | 1 | - | -Endoclip 2 | NR | 24 | - | - | 63/66 (95.5%) |
| Manos et al.[26] | 0/32 | Abx → internal drainage vs. septotomy/dilatation OR naso-cavitary drain depending on acuity and leak orifice size | - | 2 | - | 30 | - | -Naso-cavitary 19 -Septotomy 16 -Pneumatic dilatation | NR | 9 | Fistulo-jejunostomy 1 | - | 32/32 (100%) |
| Parmer et al.[64] | 10* | Abx → Surgery for unstable pt or endoscopy/radiological drainage for stable pt | 1 | 1 | 9 | 2 | 2 | -Endoclip 4 | NR | 4 | - | - | 10/10 (100%) |
| Kiriakopoulos et al.[65] | 27* | Abx → Endoscopic mgx or medical mgx | 19 | NR | 8 | - | - | - | NR | 9 | - | - | 27/27 (100%) |
| Billmann et al.[67] | 23* | Abx → stenting or surgery/radiological drainage | - | 8 | 23 | - | - | - | NR | 18 | Subtotal gastrectomy 1 RYGB 1 | - | 23/23 (100%) |
| Li et al.[68] | 78 | No algorithm stated | 33 | 34 | - | 4 | - | Endoclips 10 | NR | 41 | NR | - | 64/72 (88.9%) |
Studies describing medical management of sleeve leaks
| Studies | Medical management + nutrition | Reason for medical mgx | Success rate/% | Duration of healing/wks |
| Al Hajj et al.[12] | · IV Abx and anti-fungal · NBM/TPN | Clinically stable, small radiological leak or abscess | · 100% - type 1 leak (3/3) · 9% - type 2 leak (1/11) · 0% - type 3 and 4 leaks (0/6) | 3 |
| Corona et al.[11] | · IVAbx + TPN/NBM | Clinically stable, small early radiological leaks or inflammation | · 18.8% - type 1 and 2 leaks (3/16) | NR |
| Christophorou et al.[10] | · IVAbx + TPN/ NBM | Clinically stable, small radiological leaks or inflammation | · 5.5% - all leaks (6/110) | NR |
| Nimeri et al.[3] | · IVAbx + TPN/ NBM | Clinically stable, small radiological leaks or inflammation | · 9.1% - type 1 and 2 leaks (1/11) | 4.2 (3-6) |
| Sakran et al.[9] | · IVAbx + NBM | Clinically stable, early leak | · 23.5% - type 1 and 2 leaks (4/17) | NR |
| Olmi et al.[60] | · IVAbx + TPN/NBM | Clinically stable, smaller collection and leak diameter | · 87.5% | 3.3 |
| Parmer et al.[64] | · IVAbx + TPN | Clinically stable | · 100% | 2.1 |
When medical therapy was inadequate, other modalities such as radiological or surgical drainage, endoscopic therapy [i.e., stenting, internal drainage, over the scope clips (OTSC), fibrin glue, and endoscopic vacuum-assisted closure (E-VAC)] and surgery were used to heal leaks. In clinically unstable patients and/or those with drainable collections, most groups achieved sepsis control by first performing percutaneous drainage or surgical washout and drainage. Surgical washout and drainage were invariably performed laparoscopically with Sakran et al. and Rebibo et al., further describing attempts to perform primary suturing and closure of early detected leaks with described primary closure success rates of 44% (n = 9) and 21% (n = 19), respectively[9,13]. Both studies suggested higher success rates if performed within 2-3 days of leak development.
In clinically stable patients, all 14 studies described the use of therapeutic endoscopic methods for managing type 1 and 2 LSG leaks, with considerable variation in modalities utilized. Thirteen studies employed stenting, while four reported internal drainage, with closure success rates ranging from 75%-100% and 78%-91%, respectively. Most studies used multiple endoscopic modalities in patient management. Rebibo et al. and Nedelcu et al. described using stenting for larger internal leak openings before switching to internal drainage once the staple-line defect was reduced in size (success rates of 83% and 100%, respectively)[7,13]. Other modalities used independently or in combination with stenting or internal drainage included OTSC clips, fibrin glue, E-VAC, coiling, and pneumatic dilatations for post-LSG angular strictures. Overall, endoscopic treatment success rates for type 1 and 2 leaks ranged from 50% to 100% [Table 3].
In cases of failed endoscopic management, surgery was required in 2%-27% of patients, with overall healing rates ranging from 90% to 100%[7,8,11-19]. Ten studies described various surgical procedures, including subtotal or total gastrectomy, or fistula-jejunostomy with Roux-en-Y reconstruction [Table 3].
Management of type 3 leaks
Ten of the included studies reported on the management of type 3 leaks [Table 5]. Given the severity of peritonitis, none of the studies used a non-interventional approach.
Studies describing type 3 leak management
| Study | Type 3 and 4 leak No. | Management algorithm | Conser-vative mgx | Perc drain | Management/Outcomes | Mortality | Overall healing rates | ||||||
| Endoscopic | Surgery | ||||||||||||
| Stenting (Success%) | Internal drainage (Success%) | Stent + internal drainage | Other (Success%) | Endoscopic Success rate %** | Surgical washout/drainage | Definitive surgery | |||||||
| Al Hajj et al.[12] | 11/6 | Abx + TPN → drainage → endo-VACC +/- surgery | - | 11 (27%) | - | - | - | Endo VACC - 4 (25%) | NR | 6 (33%) | RYGB 9 RNY TG 3 | - | 100% |
| Almadi et al.[15] | 18/0 | Abx → drainage + stent → repeat stenting if required | - | 9 | 18 (89%) | - | - | - | 16/18 (89%) | 9 | RYGB 1 | 1 | 95% |
| Dammaro et al.[20] | 14/0 | Abx → Sx drainage/ washout + EID +/- stent | - | - | 3 (NR) | 14 (NR) | -3 | - | 13/14 (93%) | 14 | RNY + TG 1 | - | 100% |
| Donatelli et al.[8] | 42/1 | Abx → drainage/washout all → EID +/- stent | - | - | - | 42 (NR) | - | Balloon dilatation - 6 | NR | NR | RNY + TG 3 | - | NR |
| Klimczak et al.[19] | 3/0 | Abx → peritonitis - lap washout + stent | - | - | 3 (33%) | - | - | Fibrin glue -1 | 1/3 (33%) | 3 | - | 2^ | 33% |
| Martin Del Campo et al.[17] | 13/0# | Abx → drainage → endoscopy +/- surgery | - | - | 13 (NR) | - | - | Septectomy - 2 Fibrin glue - 6 OTSC - 2 | 10/13 (77%) | 13 | RNY + STG 2 | 1 | 92% |
| Nedelcu et al.[7] | 19/0# | Abx → endoscopic mgx +/- surgery | - | - | - | 9 (89%) | 10 (100%) | 18/19 (95%) | 1 | - | 95% | ||
| Nimeri et al.[3] | 3/0 | Abx → peritonitis → lap washout/drainage + feeding jej | - | - | - | - | - | - | - | 3 | T tube - 1 Fistulo-jejunostomy 1 | - | 100% |
| Rebibo et al.[13] | 13/0 | Abx → early leak: re-operation+/-endoscopy; late leak → endoscopy+/-surgery | - | - | NR | NR | NR | NR | NR | 13 | RYGB 4 | 2 | NR |
| Southwell et al.[18] | 13/3# | Abx → drainage → endoscopy +/- surgery | - | 7 | 16 (NR) | - | - | Dilatation OTSC | 15/16 (95%) | 10 | RYGB 1 | - | 100% |
Patients in the ten studies were all treated with intravenous antibiotics, with most achieving initial control of peritoneal sepsis through surgical washout and drainage, either laparoscopically or via laparotomy. Only three studies reported the use of percutaneously placed external drains for managing large abscess cavities[12,15,18]. Al Hajj et al. achieved resolution of the leak with surgical washout and drainage alone in 33% of patients (n = 6)[12].
Once sepsis was controlled, all studies except those by Nimeri et al. described the use of endoscopic modalities for managing type 3 leaks[3]. Stenting and/or internal drainage were the most commonly used endoscopic approaches [Table 5]. Almadi et al. and Klimczak et al. reported stenting success rates of 89% and 33%, respectively[15,19]. Donatelli et al. and Nedelcu et al. described sequential use of internal drainage following initial sepsis control, although only Nedelcu et al. provided extractable data, showing success rates of 89% with internal drainage alone and 100% when stents were used interchangeably with internal drainage[7,8]. Al Hajj et al. reported an E-VAC success rate of 25% (n = 4)[12]. Donatelli and Southwell et al. described balloon dilatation for patients with distal gastric sleeve strictures[8,18]. Other endoscopic modalities employed included septotomy, fibrin glue, and OTSC for smaller defects. Overall endoscopic success rates following initial drainage ranged from 33% to 95%.
Various forms of surgery were used more frequently for chronic type 3 leaks than for type 1-2 leaks, particularly when all other interventions had failed. The most common procedures were Roux-en-Y gastric bypass (RYGB) or total gastrectomy. Across the studies, 6%-55% of patients with type 3 leaks required surgery[3,8,12,13,15,17,18,20]. After excluding a single outlying study with a 33% success rate[19], the remaining studies reported overall healing rates of 92%-100% for type 3 leaks.
Management of type 4 leaks
Six of the included studies reported on the management of fistulae[12,21-25]. Initial management of fistulae was similar to that of other leak types, with early initiation of medical therapy and sepsis control via radiological or surgical drainage. In all studies, drainage alone was insufficient, necessitating endoscopic and/or surgical interventions for fistula resolution [Table 6].
Studies describing type 4 leak management
| Study | Type 4 leak No. | Management algorithm | Conser-vative mgx | Perc drain | Management/Outcomes | Mortality | Overall healing rates | ||||||
| Endoscopic | Surgery | ||||||||||||
| Stenting (Success%) | Internal drainage (Success%) | Stent + internal drainage | Other (Success%) | Endoscopic Success rate %** | Surgical washout/Drainage | Definitive surgery (success %) | |||||||
| Al Hajj et al.[12] | 6 | Abx + TPN → drainage → endo-VACC +/- surgery | - | 4 | - | - | - | E-VACC - 1 | NR | 3 | RYGB - 5 TG - 1 | - | 100% |
| Benosman et al.[21] | 26 | Abx + NBM/ drainage (radiological or surgical)/ endoscopic - stent or OTSC or pigtail drain | - | 10/22 (46%) | 7 (86%) | 0 (NA) | 2 (50%) | OTSC - 7 (100%) OTSC + drainage - 3 (100%) OTSC + stenting - 5 (80%) | 23/26 (88%) | 12/22 | NR | - | NR |
| Bruzzi et al.[25] | 12 | Abx + NBM/ surgery washout +/- drainage or T tube +/- CT draiange → endoscopic stenting +/- OTSC | - | 7 | 10 | NR | NR | OTSC - 4 | NR | 11 - laparotomy washout + T tube | Open RNY + TG (75%) | - | 100% |
| Chouillard et al.[24] | 21 | Abx + NBM/ early re-operation or drainage/Endoscopic means +/- surgery | - | NR | NR | NR | NR | NR | NR | NR | Fistulo-jejunostomy - 21 (100%) | - | NR |
| Thompson et al.[22] | 15 | Abx + NBM/ prox leak. → surgery or drainage (lap vs. perc), distal leak → T tube | - | NR | - | - | - | - | - | NR | RNY + STG - 15 (100%) | - | 100% |
| Van de Vrande et al.[23] | 13 | Abx + NBM/ drainage/ endoscopic means +/- surgery | - | NR | 3 (67%) | NR | NR | NR | NR | NR | Fistulo-jejunostomy - 11 (91%) | - | 100% |
| Rayman et al.[60] | 17 | Chronic gastric fistula → definitive surgery | - | 7 | 8 | 4 | 2 | OTSC-7 Endoscopic glue-3 | NR | - | Fistulo-jejunostomy - 9 Subtotal/Total gastrectomy - 8 | - | 100% |
| Montana et al.[61] | 13 | Chronic gastric fistula did not heal following radiological or endoscopic treatment → definitive surgery | - | 6 | - | NR | NR | NR | NR | NR | RNY TG - 13 | - | 100% |
| Taleb et al.[62] | 21 | Chronic gastric fistula did not heal following radiological or endoscopic treatment → definitive surgery | - | 1 | 9 | 9 | NR | OTSC-8 Endoscopic glue-8 | NR | 13 | Fistulo-jejunostomy - 14 RYGB - 5 TG - 2 | - | NR |
| Degrandi et al.[63] | 17 | Chronic gastric fistula did not heal following endoscopic treatment → definitive surgery | - | 1 | 6 | 11 | NR | - | NR | - | RYGB | - | NR |
| D’Alessandro et al.[66] | 40 | Abx + enteral feeding → endoscopic mgx for stable or surgical mgx for unstable patients; definitive surgery for refractory fistula | - | - | 21 | 40 | NR | - | 47.5% | 21 | Total gastrectomy - NR Thoracoscopy - NR Fistulo-jejunostomy - NR | - | NR |
| Lainas et al.[69] | 14 | Abx + drainage (radiological or endoscopic) → fistula-jejunostomy for refractory cases | - | 6 | 2 | 12 | 1 | OTSC 2 Dilatation | 0% | - | Fistulo-jejunostomy 14 | - | 100% |
Endoscopic management of staple-line leak fistulae can be effective, with Benosman et al. and Van De Vrande et al.[21,23] reporting favorable success rates using endoscopic methods alone. The authors employed individual (stenting, internal drainage, or OTSC) and combined endoscopic approaches, achieving overall success rates of 67% (n = 3) and 88% (n = 26), respectively [Table 6]. However, when considering the chronicity of fistulae, Benosman et al. reported that the efficacy of leak closure decreased from 100% to 70% for fistulae present for more than three weeks (P = 0.046)[21].
Reviewing the treatment algorithms in the studies, definitive surgical management was often reserved as a last resort for persistent fistulae when all other measures had failed [Table 6]. Five of the six included papers on type 4 leaks specifically focused on surgical treatment for persistent fistulae[12,22-25]. The two most common surgical options were total gastrectomy with Roux-en-Y oesophago-jejunostomy (TGEJ) and fistulo-jejunostomy, with both approaches achieving comparable initial fistula closure rates of 75%-100% and 91%-100%, respectively. Regarding revisional surgery, both Thompson et al. and Van De Vrande et al. emphasized the importance of optimizing nutrition and waiting 3-4 months before proceeding, allowing time for inflammatory adhesions to resolve and for non-surgical treatments to take effect[22,23].
Management of sleeve stenosis in the context of leak and on its own
Our review of the current literature on gastric sleeve stenosis management identified nine relevant studies [Table 2], including a total of 188 patients. The rate of gastric stenosis complications ranged from 1% to 4.3%, with the majority occurring at the gastric incisura. In addition to the rare occurrence of sleeve leaks, affected patients presented with obstructive symptoms, reflux, or non-specific abdominal pain.
Despite being a contributing factor to sleeve leaks, robust data on the management of gastric stenosis in conjunction with sleeve leaks remain limited. Only four of the 22 included sleeve leak studies reported data on stenosis within their populations, and only two studies - by Nedelcu et al. and Nimeri et al. - described treatment algorithms for sleeve leaks with concurrent stenosis. Both studies included patient populations with type 2 or 3 sleeve leaks, with all stenoses occurring at the gastric incisura[3,7]. Neither study reported the length of the strictures. In both algorithms, gastric sleeve stenosis was treated simultaneously with endoscopic leak management to facilitate healing and improve sleeve transit. Following initial resuscitation and sepsis localization, both studies favored endoscopic stenting (20-23 cm, 24 F Hanarostent). Nimeri et al. described concurrent management using an additional proximal covered stent, while Nedelcu et al. alternated between a pigtail drain or covered stent depending on leak orifice size (< 10 mm - pigtail; ≥ 10 mm - covered stent)[3,7]. Overall endoscopic success rates in this subgroup were 50% and 100%.
When assessing the management of gastric sleeve stenosis alone, most studies favored an initial endoscopic dilatation approach, except Manos et al., who preferred pneumatic dilatation for functional stenosis and stenting for mechanical stenosis[26]. The majority of studies (7 of 9) used pneumatic achalasia balloon dilatation rather than circumferential radial expansion (CRE) balloon dilatation, regardless of stenosis length. The mean number of dilatations ranged from 1.6 to 2.1 per patient, with sequential increases in pneumatic dilatation pressures up to 30-40 mmHg. Treatment success rates for pneumatic balloon dilatation ranged from 44% to 93.9%. Ogra et al. was the only study to report outcomes for CRE balloon dilatation, with success rates of 86% and 11% for short- and long-segment stenoses, respectively[27]. The only two studies by Parikh et al. and Agnihotri et al. incorporated stenting in their treatment algorithms (using 18 mm × 100 mm or 18 mm × 70 mm fully covered stents), achieving overall endoscopic success rates of 80% and 97%, respectively[28,29]. In cases of failed endoscopic management, surgical correction was required, with RYGB or stricturoplasty/seromyotomy being performed.
Our centre’s management experience of sleeve leaks and fistulae
Since 2004, the senior author of our unit has managed 22 cases of sleeve leaks or fistulae, predominantly using an endoscopic approach.
Within this series, 14 patients had type 2 or 3 leaks. The remaining eight patients had type 4 leaks, including four type 4b leaks referred from other centres.
We used fully covered self-expanding metal stents (FCSEMS) as the initial treatment in 12 patients, achieving an overall success rate of 83% (10/12). Ten of these patients had type 2-3 leaks, with a stent resolution rate of 90% (9/10), while the two patients with type 4 leaks/fistulae both failed stent therapy. One of these underwent fistula-jejunostomy, and the other required Roux-en-Y bypass followed by total gastrectomy after failing multiple endotherapies. One type 2 leak patient was switched to internal drainage due to stent intolerance. At our centre, stent therapy often involves simultaneous gastroscopy and laparoscopy during the initial management phase to facilitate washout and laparoscopic placement of an intra-abdominal drain adjacent to the leak site. In eight of the 12 cases, the stent was sutured laparoscopically to prevent migration. Most patients experienced stent-related discomfort and varying degrees of intolerance to oral fluids, necessitating enteral feeding via a nasojejunal tube.
Six patients with type 2-3 and type 4 sleeve leaks/fistulae were successfully managed with internal drainage using pigtail stents (100% success). The two type 4 leak patients in this group also underwent pneumatic dilatation of their sleeves due to concurrent gastric sleeve stenosis at the incisura. These patients were able to resume a fluid diet shortly after drain placement and had an uncomplicated recovery without recurrence of their leak or abscess.
We were able to avoid salvage surgery in all four referred type 4b low-volume fistula patients using sequential pneumatic dilatation up to 30 mm. All four patients had distal sleeve stenosis and had previously failed initial endoscopic management with stents or internal drainage at their respective centres.
DISCUSSION
Problems with current classifications
Our review of the literature highlighted the significant heterogeneity within the available evidence, including the lack of standardization in LSG leak classification. Most studies categorized leaks based on chronicity, location, and/or size of the staple-line or internal opening. Of the 13 studies defining leaks according to chronicity, only six followed the International Sleeve Gastrectomy Expert Panel Consensus Statement (Rosenthal’s classification), which categorizes leaks as acute (within 7 days), early (1-6 weeks), late (after 6 weeks), and chronic (after 12 weeks) based on the timing of presentation following the primary procedure[8,10,15,18,19,30].
Classifying leaks according to chronicity alone potentially limits discussion of the various drivers of infection, such as ileus, abscess size, leak location, and the presence or absence of stenosis or functional obstruction. For this reason, such classification does not adequately reflect the patient’s clinical picture or the pathophysiology of the leak, which dictates appropriate management options. Documentation of leak location is important, given the differing pathophysiology of proximal versus distal staple-line leaks. The size of internal defects is also relevant when considering external versus internal drainage, as well as other endoscopic options, such as covered stents or E-VAC[7,10]. Through our proposed classification system for LSG leaks [Figure 2], we aim to achieve two objectives. First, to standardize LSG leak classification within the literature by providing a more robust staging system that reflects clinical manifestations. Second, the system allows comparative evaluation of available radiologic, endoscopic, and surgical therapies, creating an algorithm of care that encourages treatment tailored to patient parameters, with the option to escalate or de-escalate therapy depending on response.
Stage dependent management of deep SSIs after sleeve gastrectomy
The nature of the abscess cavity and the presence or absence of a gastric sleeve stenosis distal to the internal defect are the main drivers of the illness[1,4,31-33]. While appropriate resuscitation, intravenous broad-spectrum antibiotics, and nutritional support are important, many patients require more than this basic level of care. Assessment of clinical signs in a gastric sleeve leak should prioritize identifying systemic inflammatory responses (e.g., fever, tachycardia, leukocytosis) and localized abdominal signs (e.g., rigidity, rebound tenderness) to promptly diagnose concurrent sepsis or acute peritonitis. Early recognition of these signs is critical, as they often dictate the urgency of surgical intervention and antibiotic therapy. We propose that both the stage of the abscess and the patient’s response to therapy should guide management. Clinicians should be prepared to escalate or switch treatment modalities promptly if a patient fails to show clear improvement. Prolonging an incompletely successful therapy is neither efficient nor effective. While traditional management of chronic fistulae can be successful for many foregut leaks, sleeve-related fistulae are often driven by distal sleeve stenosis. Prolonged external drainage in these cases may encourage lateralization of sepsis, leading to chronic gastrocutaneous fistulae and a higher likelihood of requiring complex reconstructive surgery. These patients are at risk of infections with multi-resistant organisms (MROs), fistulation to the skin, lungs, or other organs, and further prolongation of illness, potentially resulting in permanent disability.
Stage 1 - phlegmon
Our proposed management for patients with staple-line inflammation or phlegmon is to keep the patient nil by mouth and commence intravenous antibiotics, multivitamins, and thiamine therapy. Endoscopy at or soon after admission can confirm the diagnosis, assess sleeve anatomy, and allow for a 20 mm through-the-scope dilation to evaluate for actual or functional stenosis. Patients can be monitored clinically and with serial markers such as white cell count or C-reactive protein. Since patients continue to swallow saliva, prolonged nil by mouth is counterproductive; a high-protein fluid diet can be initiated once clinical improvement is evident. If patients fail to improve, reassessment with further CT ± endoscopy is warranted to determine progression to Stage 2 or the presence of gut lumen stenosis that may impede healing. Several studies have reported acceptable outcomes for patients with type 1 or 2 leaks treated conservatively[3,9-12].
(2a) Contained abscess, stable patient
The traditional surgical management of sleeve gastrectomy with an abscess on the staple line has either been laparoscopy with debridement and placement of external drain or placement of a CT-guided percutaneous drain[14,30,34]. This method is broadly available to many surgeons due to the promulgation of interventional radiology and therapeutic laparoscopy; however, these methods also encourage the creation of an external fistula through external drainage. Equally, some abscesses are not accessible to radiologic drainage which then leads surgeons to undertake laparoscopic debridement which will often de-roof a small contained abscess, potentially making it larger and less well contained. Talbot et al. and others have used endoscopic internal drainage with the placement of internal drains as a suitable and successful alternative and would use this method as the index therapy for all patients presenting with a suspected Type 2a sleeve leak[32]. Endoscopic treatment also allows assessment of the gastric tube for the presence of a stenosis and the placement of naso-enteric feeding if required. Several papers discuss this modality[8,10,17,20]; however, reasons for failure of this approach are not well described in the current literature. We encourage clinicians to consider endoscopic internal drainage at the time of admission, as it requires minimal complex equipment to perform while offering directed assessment of the patient's abscess and anatomy. Adequate internal drainage will allow patients to restart a per-oral diet quickly. In circumstances where a patient presents to a hospital without access to this therapy, they can often be stabilized with radiologic drainage before referral to a unit with endoscopic expertise, where external drainage can be converted to internal drainage.
(2b) Contained but large staple line abscess (2b) and (3) uncontrolled leak/peritonitis
These patients may either present with a large abscess and appear clinically unstable at the time of diagnosis or could be patients who have persisting symptoms with an abscess cavity despite percutaneous external drainage or endoscopic internal drainage. In a clinical scenario of a potentially unstable patient (i.e. type 3 leaks) or a patient who has not recovered quickly following initial therapy, the options could include external drainage/washout (radiological or laparoscopic) and placement of a FCSEMS or placement of an Endo-SPONGE (B-Braun Medical®) E-VAC. Several publications detail these modalities, with their outcomes summarized in Table 5[3,7-9,11-19]. Laparoscopy with debridement, lavage, and external drainage is an option where endoscopic therapy is not available; however, this approach often necessitates prolonged NBM and increases the risk of external fistula formation. In patients managed with this method, endoscopic therapy can still be considered, although the likelihood of success decreases if prolonged internal drainage is allowed and a chronic fistula develops. In our experience, it is usually feasible to transition to endoscopic therapy and remove external drains within 1-2 weeks of laparoscopy and drain placement.
The use of E-VAC in upper gastrointestinal and bariatric surgical leaks was first described five years ago[35,36]. Concerning controlled gastric sleeve leaks, studies by Leeds et al. and Morell et al. both reported reasonable healing rates of 77% (n = 9) and 83% (n = 6)[37-39], respectively, when used interchangeably with stenting. Of note, however, E-VAC may not be a feasible option in patients who have already developed gastrocutaneous fistula or are unable to return at regular intervals for endoscopic exchange[40]. The success rate of E-VAC has also been shown to diminish with the chronicity of the leak, with best results achieved when used within 4-6 weeks of the initial insult[41,42]. In our experience, E-VAC is easier to apply to larger staple-line defects, while FCSEMS placement combined with temporary external drainage may be more suitable in cases of gastric sleeve stenosis and/or small staple-line defects. In either situation, our approach is to transition to internal drainage once the cavity has reduced in size (typically after 2-3 weeks), thereby facilitating early oral intake and, when possible, discharge from hospital.
Limiting management to a binary approach risks unnecessary prolongation of therapy. The E-VAC method requires endoscopies every 3-4 days, with patients maintained on TPN and a nasogastric tube connected to suction, which significantly impacts quality of life and mobility[37,39,40,43,44]. In our observation, after one to two weeks of E-VAC application, most cavities shrink sufficiently to allow transition to an alternative method, such as internal drainage combined with pneumatic balloon dilatation.
Placement of a stent with external drainage is probably the method used by the majority of therapeutic endoscopists over the last 15 years[16,37,40]. Self-expanding metal stents (SEMs) can be used as a singular intervention or in conjunction with other modalities. A range of SEMs have been employed in this setting, including both covered (Taewong©; Hanarostent©) and partially covered (e.g. Wallstent©) stents with varying dimensions of luminal diameter (range 15-24 mm) and length (150-240 mm)[7,16-19,21]. Our systematic review and unit results show stenting to be effective, with sleeve leak resolution rates of 75%-100% for type 1/2 leaks and 33%-88% for type 3/4 leaks [Tables 3-5]. When considering the chronicity of leaks, the existing literature seems to suggest that stent success rates for leak resolution decrease in proportion to leak duration[45-48] which adds credence to the concept that stenting should be considered as a potential option as an index therapy to prevent fistula formation rather than as a treatment for persisting fistula after prolonged failure of initial therapy. Christophorou et al. demonstrated a diminishing stenting success rate over time (76.4% at 1 month vs. 41.7% at 12 months)[10]. This reduced success rate at 1 year is similar to that reported by another center (54.5%)[17].
Despite the evolution of stent technology, the fundamental problem of placing rigid straight tubes in a contracting, non-linear GI tract remains. Among the included studies that reported stent-related morbidity, stent migration - resulting in luminal obstruction and/or requiring repositioning - was commonly noted, with rates ranging from 9% to 61%[7,10,11,13,15-19,21]. Within our unit, we have elected to suture stents in place to mitigate migration when concurrent laparoscopic washout is performed. Results from included studies, as well as our own experience, have also highlighted significant disruptive symptoms associated with stents, including pain, regurgitation, salivation, and inability to lie flat (3%-23%)[10,15,16,18,21]. A minority of patients tolerate stents well and can be advanced to a liquid diet quickly; however, in most patients, this is not the case. Our approach is to remove the stent within one to two weeks if evidence of persistent fistula drainage is noted, and after two weeks if the stent has cured the fistula. There appears to be no logical reason to leave a stent in place beyond this period. A short duration of stent placement, even if insufficient to cure the fistula completely, is typically enough to create a small cavity close to the staple line, which can then be managed with internal drainage ± pneumatic dilatation[20,49].
Chronic fistula - 4a/b
Chronic gastric fistulas are usually associated with lateralisation of sepsis, often resulting from prolonged external drainage and/or undiagnosed or partially treated sleeve stenosis[6,31,35,38]. Stenosis promotes preferential drainage of saliva into the abscess cavity, while incomplete drainage encourages lateralisation of sepsis as it seeks an alternative epithelial surface for discharge. The importance of addressing stenosis in chronic fistula was first highlighted by Campos et al., who demonstrated fistula resolution in bariatric patients using pneumatic dilation to ablate the distal stenosis[50]. This aligns with our unit’s experience, where pneumatic dilatation has been effective in managing type 4 sleeve leaks/fistulae. Chronic fistulae are often associated with patient malnutrition, psychological maladaptation, and potential medicolegal risks for treating clinicians and institutions[13,23]. Bacteria colonising chronic abscesses and fistulae are frequently multidrug-resistant organisms (MROs), while fungal infections may persist through biofilm formation and inherent antimicrobial resistance.
(4a) Abscess close to the staple line
Patients with a chronic abscess typically have an associated stenosis[1,13,23]. Such stenoses may not always be apparent on endoscopy but can be identified using a functional or 3D CT scan, or during pneumatic dilatation when a non-dilating segment or “waist” is observed on the inflated balloon [Figure 3]. These patients are often managed with a liquid diet or post-pyloric enteral feeding. As proposed by Campos et al., a sequential series of pneumatic dilatations (30-40 mm), with or without internal drainage and/or septotomy/marsupialisation, represents an appropriate non-escalatory therapeutic approach[29,50]. Surgical intervention should be reserved for patients in whom the stenosis cannot be successfully ablated.
Figure 3. Sleeve gastrectomy leak endoscopic and surgical treatments. Reproduced with permission from: Cheng Q, Beitner J, Talbot M, “Stage-dependent management of sleeve gastrectomy leaks - a systematic review with proposed ct-based classification and management algorithm” 25th IFSO World Congress Silver Anniversary. Obes Surg 2022;32(Suppl 2):39-1044. https://doi.org/10.1007/s11695-022-06204-8.
(4b) Lateral/distant abscess connected to the stomach via fistula tract
In our experience, therapy for these patients is unlikely to succeed unless both the fistula opening and the lateral abscess are addressed. A fistula without an associated abscess cavity - often resulting from prolonged external drainage - will frequently respond to pneumatic dilatation alone. However, therapies directed solely at the staple line, such as pneumatic dilatation or septotomy, are rarely effective in managing lateral sepsis. When a lateral abscess cavity is present, it is often filled with dense biofilm, rendering simple external drainage inadequate. Endoscopic strategies may include dilatation of abscess tracts to permit improved internal drainage, with or without adjunctive treatment of the fistula tract using embolization or clipping[32]. Although these approaches are reasonable to attempt, the published literature and our clinical experience indicate that their success is largely anecdotal[21,23,25]. Our preferred management is fistula-jejunostomy combined with surgical debridement and drainage of the lateral abscess cavity, with the fistula-jejunostomy performed either concurrently or sequentially following cavity debridement.
The literature review shows initial fistula resolution rates of fistula-jejunostomy versus total gastrectomy to be comparable (refer to Table 6 - 91%-100% vs. 75%-100%, respectively)[12,22-25]. Fistula-jejunostomies work by promoting healing through offsetting the high-pressure system of the distal sleeve, which is often the cause of persisting fistulisation[48,51]. Fistula-jejunostomy preserves the patient’s original sleeve gastrectomy profile while avoiding the complications associated with an oesophagojejunal anastomosis in a poorly vascularized oesophagus or inflamed tissue[23,24]. It also maintains access to the distal stomach and biliary tree while keeping surgical options available for future conversion to either a gastric bypass or total/near-total gastrectomy if required as a last resort[23,24,47]. A follow-up study by Chouillard et al., shows that fistula-jejunostomy patients all remained healed at 2 years with adequate weight-loss results (> 80% excess weight loss) and uncompromised vitamin/nutritional levels[24]. Our experience is in concordance with
Management of sleeve stenosis in the context of sleeve leak
The incidence of gastric stenosis following sleeve gastrectomy ranges from 0.5%-1% (Manos et al.)[26]. Sleeve stenosis occurs most commonly at the level of the incisura and is often a consequence of inadequate surgical technique in sleeve formation, resulting in sleeve ischemia, kinking/torsion, or scarring along the staple line (Zundel et al. and Levy et al.)[52,53]. The presence of stenosis can be a significant factor in the propagation and potentiation of sleeve leaks (Levy et al.)[53]. In the setting of a sleeve leak, gastric stenosis should always be aggressively investigated and adequately managed, irrespective of leak type. In our experience, effective stenosis treatment helps reduce sleeve pressures and improve gastric drainage, ultimately increasing the chance of success with the intended sleeve leak treatment. As previously discussed, this is particularly relevant in chronic type 4A/B leaks (i.e., fistulae), where effective internal drainage through the sleeve becomes crucial for fistula closure.
Similar to the management of sleeve leaks, we advocate an escalatory approach in treating stenosis. Our review highlights the effectiveness of endoscopic therapy in managing sleeve stenosis [Table 3]. Within the existing literature, endoscopic dilatation (i.e., CRE balloon or pneumatic dilatation) has been shown to be effective, particularly in short-segment stenosis, with reported treatment success rates ranging from 44%-94%. Although the majority of included studies preferred pneumatic dilatation over CRE balloon dilatation, in our experience, CRE balloon dilatation can still be a useful tool in managing short-segment stenosis, especially in the setting of poor tissue quality and proximal leaks, with therapy escalated to pneumatic dilatation if unsuccessful. Pneumatic dilatation may be commenced at 20 mmHg, with incremental increases to 30-40 mmHg to achieve favorable outcomes, bearing in mind the risk-benefit profile of more aggressive dilatation.
Endoscopic stenting is another option, with Parikh et al., Agnihotri et al. and Dhorepatil et al. all utilizing stents in their treatment algorithms when dilatation was ineffective, particularly in cases of long-segment stenosis. In the setting of concurrent proximal leak and distal stenosis, long fully covered stents can be placed across the sleeve to simultaneously treat both complications[28,29,54]. When endoscopic options have been exhausted, salvage surgery may be necessary. For refractory gastric stenosis alone, the majority of included studies preferred conversion of the sleeve gastrectomy to a RYGB, with only Vilallonga et al. and Dhorepatil et al. attempting stricturoplasty/seromyotomy[38,54]. Although the surgical management of concurrent sleeve leak and stenosis may initially appear daunting, in practice, the options remain similar to those for managing refractory sleeve leak alone. Where gastric stenosis has been identified as the primary driver behind early or acute sleeve leaks (type 2-3), RYGB may be a good option to reduce proximal gastric pressure and promote healing at the leak site. In more complex chronic leaks/fistulae (type 4a/b), our preference is to perform a Roux-en-Y gastric fistulotomy with a long gastro-jejunal anastomosis to facilitate effective gastric drainage and reduce intraluminal pressure. Nevertheless, total gastrectomy with oesophago-jejunal reconstruction can still be considered when Roux-en-Y gastric fistulotomy fails.
Strengths
This systematic review offers several key strengths, including the development of a novel, pathophysiology-driven staging system for sleeve gastrectomy leaks, addressing the current lack of standardized classification. By synthesizing data from 1,109 potential articles - of which 36 studies (encompassing 1,246 patients) met inclusion criteria - we provide a comprehensive, evidence-based analysis spanning a 14-year period up to 2023. The proposed algorithm delivers a structured, stage-appropriate framework to optimize decision-making, reducing reliance on institutional bias or anecdotal experience. The inclusion of both surgical and endoscopic therapies ensures a balanced perspective that reflects the heterogeneity of clinical presentations. Furthermore, the emphasis on early recognition of inflammatory and abdominal signs aligns management with disease severity, potentially mitigating progression to chronic fistulas or high-risk salvage surgeries. Finally, the integration of the authors’ institutional experience with a rigorous systematic review enhances the practical applicability of the recommendations, while underscoring the need for future prospective validation.
Limitations
Even though this systematic review covers a wide range of treatment options for sleeve gastrectomy leaks, the findings are limited by the overall quality of the available literature. Despite the large number of gastric sleeve procedures performed globally, the incidence of leaks remains proportionally low, which is reflected in the relatively small cohorts or case series reported.
Significant heterogeneity also existed in both the characteristics of study populations and in how leaks were categorized and treatments prioritized across studies. As a result, direct comparisons between studies were challenging, and a meta-analysis could not be performed. Nonetheless, given the breadth of studies included in this review, we were able to adequately extract and synthesize data to support a robust discussion. Moreover, sleeve gastrectomy remains the best “model” for considering foregut leaks, as the majority occur at a predictable location, often in association with stenosis.
Summary
The management of post-sleeve gastrectomy organ-space SSI can be complex and challenging. The authors believe that the available endoscopic and laparoscopic therapies can be tailored to individual patient needs. Patients can initially be treated with minimally invasive therapies, and if they fail to respond promptly, therapy can be escalated to more complex interventions without delaying new treatments for more than a week or two. Resectional surgery should not be required for the majority of these patients, and most should be managed with the goal of early oral nutrition and outpatient care. The endoscopic and surgical therapies discussed in this paper have also been successfully applied to other foregut perforations and fistulae, including those following oesophagectomy, total gastrectomy, gastric bypass, and perforated duodenal diverticula.
To our knowledge, this represents the first systematic review to propose a pathophysiology-driven staging system for sleeve gastrectomy leaks that integrates both clinical presentation and radiologic findings to guide stage-adapted minimally invasive management, with a key emphasis on avoiding resectional revisional surgery whenever possible. Our classification system fills a critical gap in current practice, where treatment decisions remain largely institution-dependent rather than evidence-based. While previous studies have described individual management strategies for sleeve leaks, our work uniquely synthesizes 14 years of global data (n = 1,246 patients) to: (1) demonstrate that most leaks can be managed without resectional surgery when treated with timely, stage-appropriate interventions; and (2) provide an actionable algorithm that correlates leak progression (from acute sepsis to chronic fistulas) with optimal therapeutic approaches across endoscopic, radiologic, and surgical modalities.
While this systematic review demonstrates that stage-adapted endoscopic and laparoscopic therapies can successfully manage most sleeve gastrectomy leaks, future multicenter prospective studies are needed to validate our proposed classification system and to compare outcomes between different treatment strategies at each stage, particularly given the heterogeneity of existing literature. The predictable nature of sleeve leak locations makes them an ideal model for studying foregut perforations. We encourage focused research on standardized definitions (e.g., leak size, timing, sepsis criteria) and randomized trials comparing early minimally invasive therapies versus upfront complex interventions in high-risk subgroups. Given the limitations of current evidence, international registries tracking leak management outcomes - with granular data on inflammatory markers, stenosis severity, and treatment escalation timelines - could refine decision-making and help address the challenge of low case volumes at individual centers.
DECLARATIONS
Acknowledgements
The abstract of this article was previously published in: Guirgis M, Cheng Q, Chan D, Fisher O, Talbot M, “Stage Dependent Management of Sleeve Gastrectomy Leaks - A Systematic Review with Proposed Classification and Management Algorithm” 26th IFSO World Congress. Obesity Surgery, 33(Suppl 2), 147-1118 (2023), Springer Nature. Reproduced with permission. Available at https://doi.org/10.1007/s11695-023-06727-8.
Authors’ contributions
Writing of manuscript, data correlation and analysis: Cheng Q
Writing of manuscript, data correlation and analysis: Guirgis M
Data correlation and analysis: Chan DL
Formulation of tables and figures: Opperman TJ
Review and revision of the manuscript: Fisher OM
Review and revision of the manuscript: Talbot ML
Availability of data and materials
This paper is planned for open access and all relevant studies have been correlated from Medline, PubMed and the World Wide Web.
Financial support and sponsorship
This study was supported by a grant from the Upper Gastrointestinal and Metabolic Research Foundation.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. El-Sayes IA, Frenken M, Weiner RA. Management of leakage and stenosis after sleeve gastrectomy. Surgery. 2017;162:652-61.
2. Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20:403-9.
3. Nimeri A, Ibrahim M, Maasher A, Al Hadad M. Management algorithm for leaks following laparoscopic sleeve gastrectomy. Obes Surg. 2016;26:21-5.
4. Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20:13904-10.
5. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34.
6. Hassan TA, Mohey N, Kamar WH. Clinical-radiologic evaluation of the complications of laparoscopic sleeve gastrectomy: value of multidetector CT. Egypt J Radiol Nucl Med. 2015;46:879-84.
7. Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg. 2015;25:559-63.
8. Donatelli G, Dumont JL, Cereatti F, et al. Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID). Obes Surg. 2015;25:1293-301.
9. Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-5.
10. Christophorou D, Valats JC, Funakoshi N, et al. Endoscopic treatment of fistula after sleeve gastrectomy: results of a multicenter retrospective study. Endoscopy. 2015;47:988-96.
11. Corona M, Zini C, Allegritti M, et al. Minimally invasive treatment of gastric leak after sleeve gastrectomy. Radiol Med. 2013;118:962-70.
12. Hajj G, Chemaly R. Fistula following laparoscopic sleeve gastrectomy: a proposed classification and algorithm for optimal management. Obes Surg. 2018;28:656-64.
13. Rebibo L, Bartoli E, Dhahri A, et al. Persistent gastric fistula after sleeve gastrectomy: an analysis of the time between discovery and reoperation. Surg Obes Relat Dis. 2016;12:84-93.
14. Kelogrigoris M, Sotiropoulou E, Stathopoulos K, Georgiadou V, Philippousis P, Thanos L. CT-guided percutaneous drainage of infected collections due to gastric leak after sleeve gastrectomy for morbid obesity: initial experience. Cardiovasc Intervent Radiol. 2011;34:585-9.
15. Almadi MA, Bamihriz F, Alharbi O, et al. Use of self-expandable metal stents in the treatment of leaks complicating laparoscopic sleeve gastrectomy: a cohort study. Obes Surg. 2018;28:1562-70.
16. De Moura DTH, Moura EGH de, Neto MG, et al. Outcomes of a novel bariatric stent in the management of sleeve gastrectomy leaks: a multicenter study. Surg Obes Relat Dis. 2019;15:1241-51.
17. Del Campo SE, Mikami DJ, Needleman BJ, Noria SF. Endoscopic stent placement for treatment of sleeve gastrectomy leak: a single institution experience with fully covered stents. Surg Obes Relat Dis. 2018;14:453-61.
18. Southwell T, Lim TH, Ogra R. Endoscopic therapy for treatment of staple line leaks post-laparoscopic sleeve gastrectomy (LSG): experience from a large bariatric surgery centre in New Zealand. Obes Surg. 2016;26:1155-62.
19. Klimczak T, Klimczak J, Szewczyk T, Janczak P, Jurałowicz P. Endoscopic treatment of leaks after laparoscopic sleeve gastrectomy using MEGA esophageal covered stents. Surg Endosc. 2018;32:2038-45.
20. Dammaro C, Lainas P, Dumont JL, Tranchart H, Donatelli G, Dagher I. Endoscopic internal drainage coupled to prompt external drainage mobilization is an effective approach for the treatment of complicated cases of sleeve gastrectomy. Obes Surg. 2019;29:2929-35.
21. Benosman H, Rahmi G, Perrod G, et al. Endoscopic management of post-bariatric surgery fistula: a tertiary care center experience. Obes Surg. 2018;28:3910-5.
22. Thompson CE 3rd, Ahmad H, Lo Menzo E, Szomstein S, Rosenthal RJ. Outcomes of laparoscopic proximal gastrectomy with esophagojejunal reconstruction for chronic staple line disruption after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10:455-9.
23. van de Vrande S, Himpens J, El Mourad H, Debaerdemaeker R, Leman G. Management of chronic proximal fistulas after sleeve gastrectomy by laparoscopic Roux-limb placement. Surg Obes Relat Dis. 2013;9:856-61.
24. Chouillard E, Chahine E, Schoucair N, et al. Roux-En-Y Fistulo-Jejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula. Surg Endosc. 2014;28:1954-60.
25. Bruzzi M, Douard R, Voron T, Berger A, Zinzindohoue F, Chevallier JM. Open total gastrectomy with Roux-en-Y reconstruction for a chronic fistula after sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:1803-8.
26. Manos T, Nedelcu M, Nedelcu A, et al. Leak after sleeve gastrectomy: updated algorithm of treatment. Obes Surg. 2021;31:4861-7.
27. Ogra R, Kini GP. Evolving endoscopic management options for symptomatic stenosis post-laparoscopic sleeve gastrectomy for morbid obesity: experience at a large bariatric surgery unit in New Zealand. Obes Surg. 2015;25:242-8.
28. Parikh A, Alley JB, Peterson RM, et al. Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc. 2012;26:738-46.
29. Agnihotri A, Barola S, Hill C, et al. An algorithmic approach to the management of gastric stenosis following laparoscopic sleeve gastrectomy. Obes Surg. 2017;27:2628-36.
30. Rosenthal RJ, Diaz AA, Arvidsson D, et al; International Sleeve Gastrectomy Expert Panel. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
31. Sarkhosh K, Birch DW, Sharma A, Karmali S. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg. 2013;56:347-52.
32. Talbot M, Yee G, Saxena P. Endoscopic modalities for upper gastrointestinal leaks, fistulae and perforations. Anz J Surg. 2017;87:171-6.
33. Witte SR, Pauli EM. Management of gastrointestinal tract defects. Ann Laparosc Endosc Surg. 2019;4:67-67.
34. Palermo M, Davrieux CF, Acquafresca PA, et al. Percutaneous image-guided abdominal interventions for leaks and fistulas following sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Surg. 2019;29:2051-8.
35. Périssé LG, Périssé PC, Bernardo Júnior C. Endoscopic treatment of the fistulas after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Rev Col Bras Cir. 2015;42:159-64.
36. Guillaud A, Moszkowicz D, Nedelcu M, et al. Gastrobronchial fistula: a serious complication of sleeve gastrectomy. Results of a french multicentric study. Obes Surg. 2015;25:2352-9.
37. Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12:1278-85.
38. Vilallonga R, Himpens J, van de Vrande S. Laparoscopic Roux limb placement for the management of chronic proximal fistulas after sleeve gastrectomy: technical aspects. Surg Endosc. 2015;29:414-6.
39. Morell B, Murray F, Vetter D, Bueter M, Gubler C. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch Surg. 2019;404:115-21.
40. Smallwood NR, Fleshman JW, Leeds SG, Burdick JS. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc. 2016;30:2473-80.
41. Gagner M. Leaks after sleeve gastrectomy are associated with smaller bougies: prevention and treatment strategies. Surg Laparosc Endosc Percutan Tech. 2010;20:166-9.
42. Chouillard E, Younan A, Alkandari M, et al. Roux-en-Y fistulo-jejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula: mid-term results. Surg Endosc. 2016;10:4200-4.
43. van Koperen PJ, van Berge Henegouwen MI, Rosman C, et al. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc. 2009;23:1379-83.
44. Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818-25.
45. Murino A, Arvanitakis M, Le Moine O, Blero D, Devière J, Eisendrath P. Effectiveness of endoscopic management using self-expandable metal stents in a large cohort of patients with post-bariatric leaks. Obes Surg. 2015;25:1569-76.
46. Chang J, Sharma G, Boules M, Brethauer S, Rodriguez J, Kroh MD. Endoscopic stents in the management of anastomotic complications after foregut surgery: new applications and techniques. Surg Obes Relat Dis. 2016;12:1373-81.
47. Alazmi W, Al-Sabah S, Ali DA, Almazeedi S. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28:3425-8.
48. Bège T, Emungania O, Vitton V, et al. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc. 2011;73:238-44.
49. Pequignot A, Fuks D, Verhaeghe P, et al. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22:712-20.
50. Campos JM, Ferreira FC, Teixeira AF, et al. Septotomy and balloon dilation to treat chronic leak after sleeve gastrectomy: technical principles. Obes Surg. 2016;26:1992-3.
51. Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy--volume and pressure assessment. Obes Surg. 2008;18:1083-8.
52. Zundel N, Hernandez JD, Galvao Neto M, Campos J. Strictures after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2010;20:154-8.
53. Levy JL, Levine MS, Rubesin SE, Williams NN, Dumon KR. Stenosis of gastric sleeve after laparoscopic sleeve gastrectomy: clinical, radiographic and endoscopic findings. Br J Radiol. 2018;91:20170702.
54. Dhorepatil AS, Cottam D, Surve A, et al. Is pneumatic balloon dilation safe and effective primary modality of treatment for post-sleeve gastrectomy strictures? A retrospective study. BMC Surg. 2018;18:52.
55. Keren D, Eyal O, Sroka G, et al. Over-the-Scope Clip (OTSC) system for sleeve gastrectomy leaks. Obes Surg. 2015;25:1358-63.
56. Assalia A, Ilivitzki A, Ofer A, et al. Management of gastric fistula complicating laparoscopic sleeve gastrectomy with biological glue in a combined percutaneous and endoscopic approach. Surg Obes Relat Dis. 2018;14:1093-8.
57. Ferraz ÁAB, Feitosa PHF, Santa-Cruz F, et al. Gastric fistula after sleeve gastrectomy: clinical features and treatment options. Obes Surg. 2021;31:1196-203.
58. Bashah M, Khidir N, El-Matbouly M. Management of leak after sleeve gastrectomy: outcomes of 73 cases, treatment algorithm and predictors of resolution. Obes Surg. 2020;30:515-20.
59. Olmi S, Cesana G, Rubicondo C, et al. Management of 69 gastric leakages after 4294 consecutive sleeve: the experience of a high volume bariatric center. Obes Surg. 2020;30:3084-92.
60. Rayman S, Staierman M, Ben-David M, et al. Laparoscopic revision to total gastrectomy or fistulo-jejunostomy as a definitive surgical procedure for chronic gastric fistula after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2020;16:1893-900.
61. Montana L, Frosio F, Polliand C, Tresallet C, Rivkine E, Carandina S. Total gastrectomy with Roux-en-Y limb reconstruction for complex and chronic fistulas after laparoscopic sleeve gastrectomy: single-center experience. Obes Surg. 2021;31:5260-6.
62. Taleb S, Nedelcu M, Skalli M, Loureiro M, Nedelcu A, Nocca D. The evolution of surgical treatment for chronic leak following sleeve. Surg Obes Relat Dis. 2021;17:278-83.
63. Degrandi O, Nedelcu A, Nedelcu M, Simon A, Collet D, Gronnier C. Roux-en-Y gastric bypass for the treatment of leak following sleeve gastrectomy. Obes Surg. 2021;31:79-83.
64. Parmer M, Wang YHW, Hersh EH, Zhang L, Chin E, Nguyen SQ. Management of staple line leaks after laparoscopic sleeve gastrectomy. JSLS. 2022;26:e2022.00029.
65. Kiriakopoulos A, Kounatidis N, Menenakos I, Kostrova M, Zografos K, Menenakos E. Non-stenting treatment versus endoscopic stent placement in staple line leaks after laparoscopic sleeve gastrectomy. Langenbecks Arch Surg. 2022;407:1863-72.
66. D’Alessandro A, Galasso G, Zito FP, et al. Role of endoscopic internal drainage in treating gastro-bronchial and gastro-colic fistula after sleeve gastrectomy. Obes Surg. 2022;32:342-8.
67. Billmann F, Pfeiffer A, Sauer P, et al. Endoscopic stent placement can successfully treat gastric leak following laparoscopic sleeve gastrectomy if and only if an esophagoduodenal megastent is used. Obes Surg. 2022;32:64-73.
68. Li M, Zeng N, Liu Y, et al; Greater China Metabolic and Bariatric Surgery Database (GC-MBD) study group. Management and outcomes of gastric leak after sleeve gastrectomy: results from the 2010-2020 national registry. Chin Med J. 2023;136:1967-76.
69. Lainas P, Triantafyllou E, Ben Amor V, et al. Laparoscopic Roux-en-Y fistulojejunostomy as a salvage procedure in patients with chronic gastric leak after sleeve gastrectomy. Surg Obes Relat Dis. 2023;19:585-92.
70. Shnell M, Fishman S, Eldar S, Goitein D, Santo E. Balloon dilatation for symptomatic gastric sleeve stricture. Gastrointest Endosc. 2014;79:521-4.
71. Manos T, Nedelcu M, Cotirlet A, Eddbali I, Gagner M, Noel P. How to treat stenosis after sleeve gastrectomy? Surg Obes Relat Dis. 2017;13:150-4.
72. Al Sabah S, Al Haddad E, Siddique I. Endoscopic management of post-laparoscopic sleeve gastrectomy stenosis. Surg Endosc. 2017;31:3559-63.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data






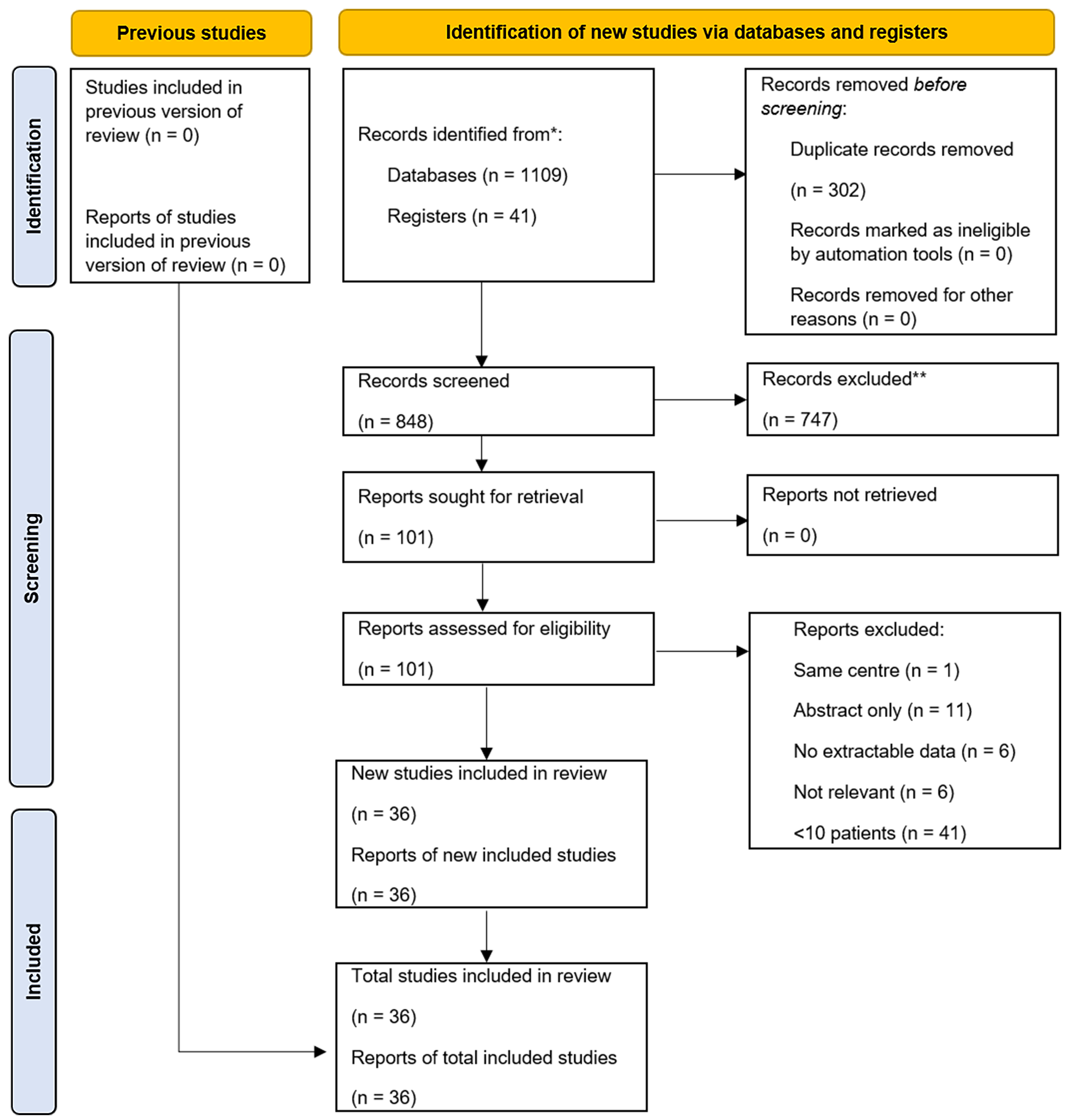
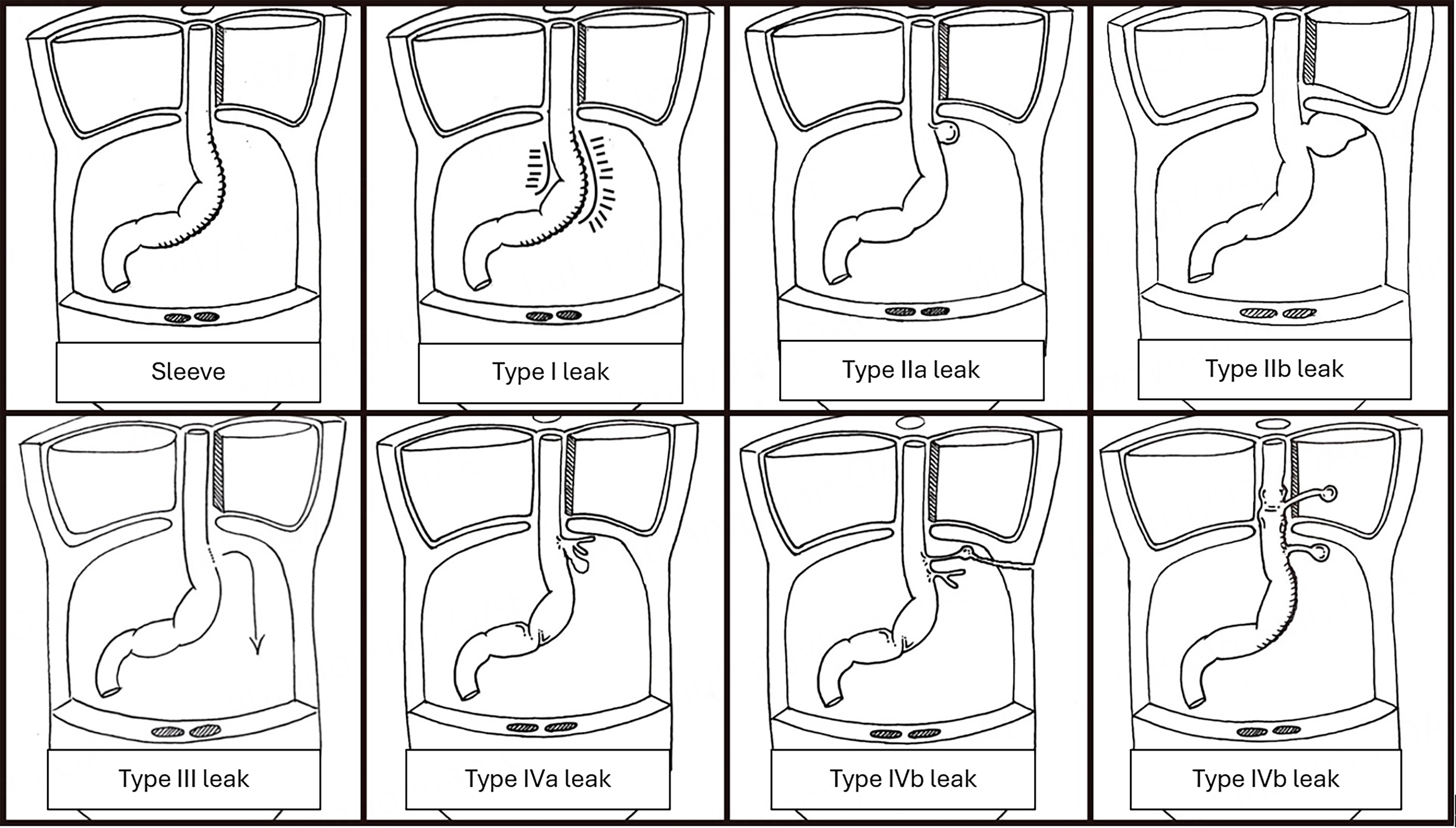
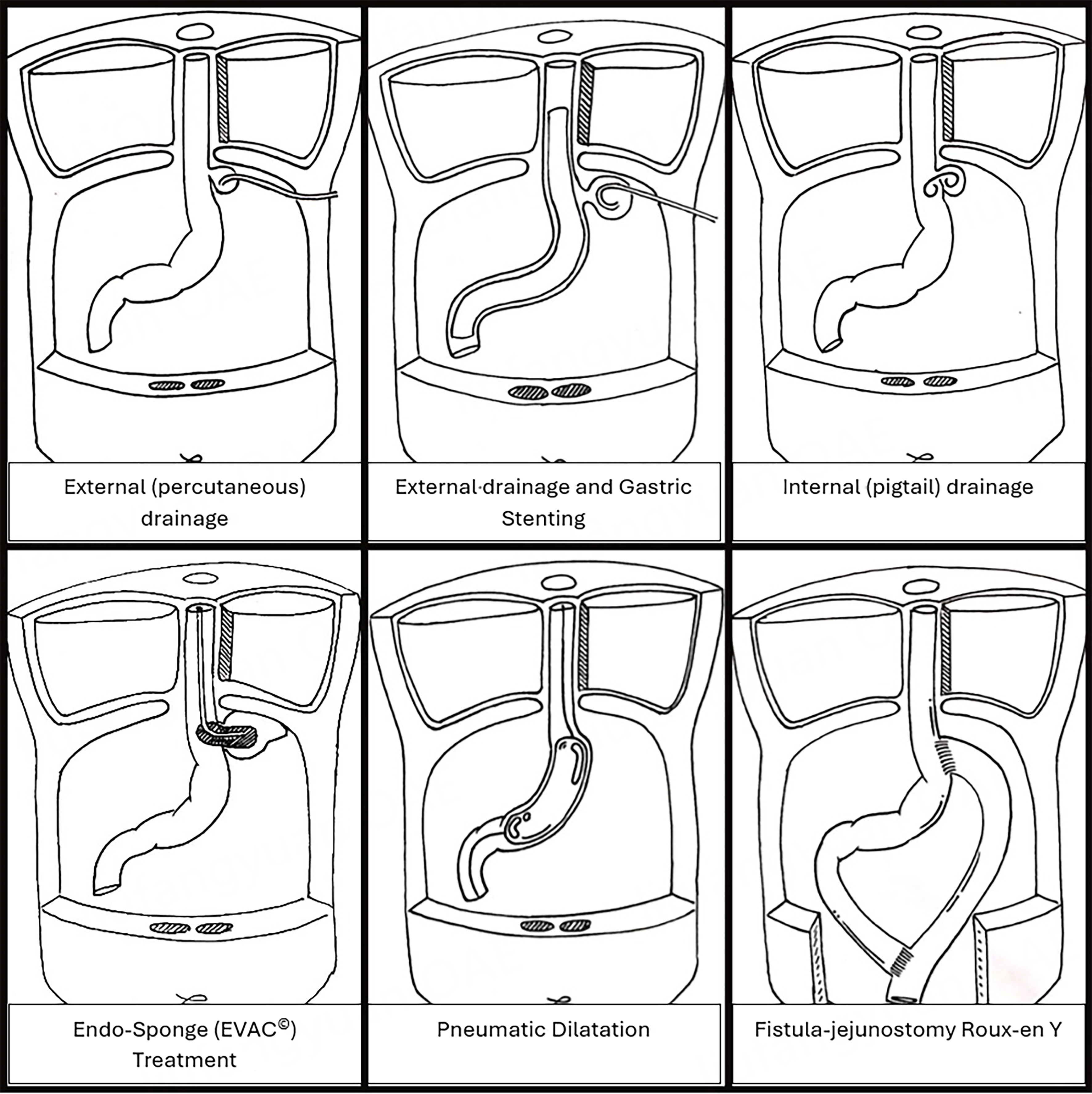












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].