Integrating omics to optimize precision cardiac rehabilitation
Abstract
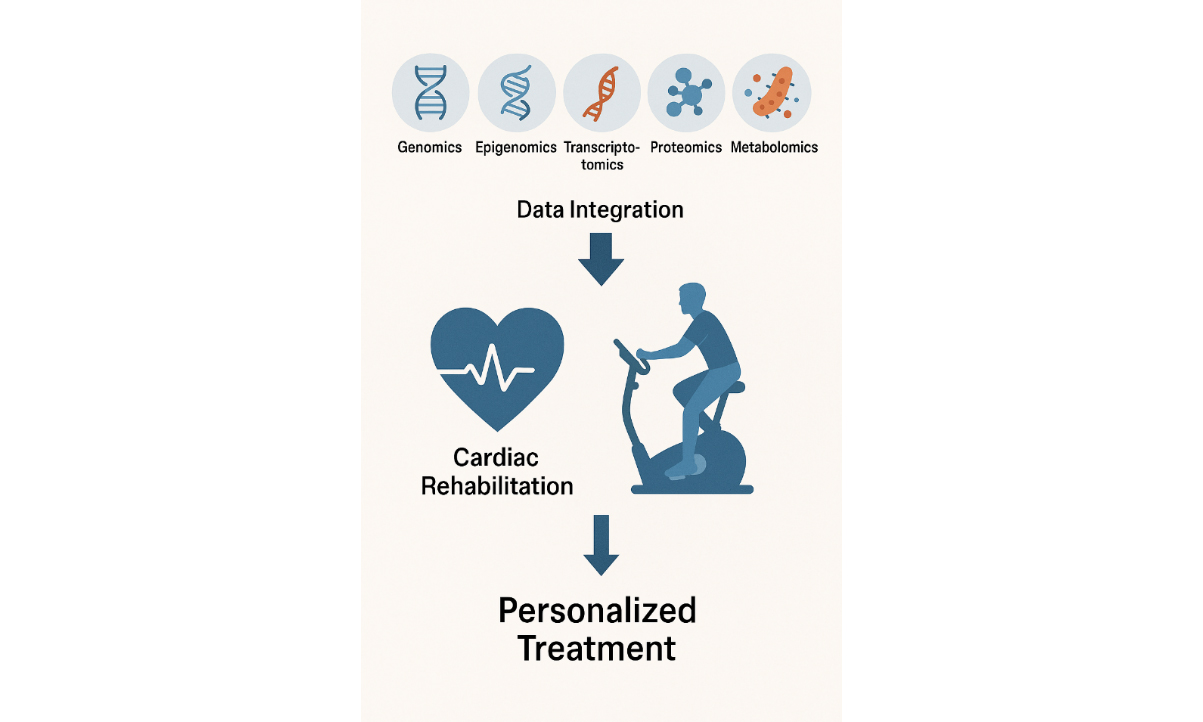
Cardiovascular diseases (CVD) remain the leading global cause of mortality with a complex etiology involving both genetic and environmental factors. Despite the identification of numerous genetic loci associated with CVD, the mechanisms underlying disease variability are incompletely understood. Recent advances in multi-omics technologies, including genomics, epigenomics, transcriptomics, proteomics, metabolomics, and microbiomics, offer a more comprehensive view of the molecular networks involved in disease progression and recovery. This commentary explores how multi-omics data could enhance cardiac rehabilitation (CR) by identifying novel biomarkers, revealing individualized responses to exercise, and informing personalized therapeutic strategies. We present specific use cases for omics technology in CR, highlight barriers such as cost and implementation feasibility, and propose future research directions, including the need for pilot studies and standardization protocols. Integrating omics technologies into CR has the potential to improve patient outcomes and promote precision cardiovascular care, provided that practical and ethical challenges are adequately addressed.
Keywords
INTRODUCTION
Cardiovascular diseases (CVD) are the primary global cause of death, affecting millions of individuals who often require interventions like cardiac rehabilitation (CR) to improve heart health and lower the likelihood of recurrence[1]. CR plays a pivotal role in improving patient outcomes, reducing hospital readmissions, and enhancing overall quality of life by promoting physical activity, stress reduction, and nutrition optimization[2]. Most of the profound improvements in outcomes following CR are at least explained statistically by improvements in cardiorespiratory fitness (CRF)[3]. While traditional CR programs focus on exercise, dietary changes, and behavioral modifications[4], they often fall short in addressing the underlying biological factors that contribute to CVD progression.
Current approaches tend to be broad and one-size-fits-all, lacking the precision necessary for patients with CVD. This has led to suboptimal outcomes for some, but almost 23% of our patients do not even improve CRF, at least as assessed with gas exchange via cardiopulmonary stress testing, highlighting the need for more targeted, individualized interventions[5]. While exercise provides numerous health benefits, significant gaps remain in our understanding of its molecular mechanisms and why individuals experience varying degrees of benefit[6].
Recent advancements in molecular biology have illuminated the role of omics technologies in understanding CVD[7]. Omics technologies, including genomics, epigenomics, transcriptomics, proteomics, metabolomics, and microbiomics, provide a comprehensive picture of the biological systems at play and powerful tools for understanding the complex pathophysiology of CVD[7]. Unlike traditional biomarkers that provide limited static snapshots of health, omics technologies could dynamically capture complex biological processes across multiple layers (e.g., gene expression, protein signaling, metabolic flux)[8]. This enables the identification of novel and individualized disease mechanisms, including gene-environment-exercise interactions, which cannot be detected using conventional clinical measures[8].
For instance, while high-sensitivity C-reactive protein (CRP) indicates systemic inflammation[9] and lipid profile reveals dyslipidemia[10], omics approaches can reveal pathway-specific dysregulation (e.g., mitochondrial diseases, epigenetic silencing of exercise-responsive genes)[11,12], enabling more precise risk stratification and therapeutic targeting. Thus, omics data could identify patient-specific biological barriers to the efficacy of CR that remain undetected by traditional clinical biomarkers[13], thereby enabling the development of more precise and individualized intervention strategies. These molecular-level insights have the potential to revolutionize CR by enabling more precise, patient-tailored interventions. By leveraging multi-omics, clinicians may identify predictive biomarkers of CR responsiveness, tailor exercise intensity or modality to molecular phenotypes, and monitor real-time physiological adaptations beyond what is possible with standard assessments. Together, these strategies collectively offer the potential to tailor CR programs, enhancing outcomes across varied patient groups. This commentary aims to explore how integrating omics technologies data into CR programs can offer a more nuanced approach to patient care. By focusing on these molecular pathways, we can enhance the precision of CR strategies, improve patient outcomes, and personalize treatment plans, ultimately paving the way for a new era of precision CR in the management of CVD. This commentary offers a concise perspective on the role of omics technologies in advancing CR [Table 1].
Summary of the integration of omics in cardiac rehabilitation
| Omics in cardiac rehabilitation | Omics biomarkers to enhance diagnosis, prognosis, and therapeutic decision making. Healthcare providers can refine effective and personalized cardiac rehabilitation protocols |
| Genomics | Genomic approach to design individualized exercise regimens and optimize cardiac rehabilitation outcomes. Understand a patient’s genomic profile in cardiac rehabilitation |
| Epigenomics | How environmental factors influence gene expression without altering the DNA sequence. MicroRNAs to repair heart tissue and improve overall cardiovascular health, heart function, resilience, and overall metabolic health |
| Transcriptomics | Enable a better understanding of cardiovascular diseases and the impact of exercise on heart health. Identify biomarkers and molecular pathways involved in cardiovascular diseases and develop targeted interventions for cardiac rehabilitation |
| Proteomics | Monitor patient progress in cardiac rehabilitation Manage elevated blood pressure and reduce long-term cardiovascular disease risks. Provide early indications of recovery or flag patients at risk for future major cardiovascular disease events |
| Metabolomics | Identify circulating metabolites that predict exercise capacity Biomarkers could serve as diagnostic tools in personalized healthcare disease screening, prognostication, and guiding therapeutic interventions |
| Microbiomics | Explore the role of the gut microbiome in cardiovascular health and its potential interactions with cardiac rehabilitation programs Help identify patients with unfavorable microbiome profiles and guide interventions to reduce these risks Monitor patients’ progress in cardiac rehabilitation and identify those who may benefit from additional interventions, such as probiotics or dietary changes to improve heart health |
| Challenges | The vast amounts of data generated necessitate robust storage solutions and sophisticated computational tools for analysis Handling and interpreting large datasets can be resource-intensive and may require advanced bioinformatics capabilities Variability in protocols and technologies can lead to inconsistent results |
OMICS BIOMARKERS IN PRECISION CR
In the context of CR, omics biomarkers have immense potential to complement traditional screening methods by enhancing diagnosis, prognosis, and therapeutic decision making. By integrating diverse data types, healthcare providers can refine CR protocols, ensuring that they are effective and personalized, and can better identify molecular signatures that are specific to CR outcomes. The continued development and implementation of omics-based biomarkers in CR hold the promise of improving patient outcomes, optimizing therapeutic success, and driving innovation in precision medicine. This could include biomarkers that track myocardial recovery, inflammation resolution, or metabolic improvements during therapy, as well as measures assessing improvements in CRF.
Genomics in CR: Genomics explores genetic predispositions to CVD by analyzing deoxyribonucleic acid (DNA) variations, including single-nucleotide polymorphisms (SNPs)[14]. By examining how specific genes and genetic variations influence cardiovascular health, exercise capacity, and disease progression, genomics can contribute to more individualized CR approaches[15,16]. In the context of CR, a genomic approach can also be applied to design individualized exercise regimens, optimizing CR outcomes by tailoring interventions to each patient's distinct genetic profile. Understanding a patient’s genomic profile in CR may aid in optimizing the selection and dosing of commonly used medications such as statins, anticoagulants, and antihypertensives, ultimately improving clinical outcomes and enhancing patient safety. Identifying these genetic factors may enable healthcare providers to predict how a patient’s potential benefits from exercise training in CR and potentially help better define exercise prescriptions[6].
Epigenomics in CR: Epigenomics is the study of the full spectrum of epigenetic modifications, including DNA methylation, histone modification, and non-coding RNA regulation, that influence the genome[7]. These modifications can aid in the early diagnosis of CVD and help predict treatment response[7,17]. Epigenetic mechanisms can offer insights into how environmental factors, such as exercise and diet, influence gene expression without altering the DNA sequence[7,18]. Exercise, a key component of CR, enables the identification of patient-specific risk profiles and modifiable factors, facilitating the development of personalized CR interventions that enhance cardiovascular health and reduce disease recurrence. Exercise has been found to decrease the hypermethylation of genes involved in nitric oxide production, thereby improving vascular function[19], influence the levels of circulating microRNAs, such as miR-126 and miR-133, which play key roles in promoting angiogenesis and preventing apoptosis in cardiomyocytes[20], and to enhance histone acetylation, which activates genes that are critical for mitochondrial biogenesis and energy metabolism[21]. These microRNAs could contribute to the repair and regeneration of heart tissue, enhancing overall cardiovascular health and improving heart function, resilience, and overall metabolic health.
Transcriptomics in CR: Transcriptomics investigates RNA expression to identify dysregulated pathways associated with conditions such as atherosclerosis and heart failure[22]. A comprehensive meta-analysis, known as MetaMEx, integrated data from 66 studies to examine gene expression changes in skeletal muscle following various exercise modalities. This analysis identified NR4A3 as one of the most responsive genes to both exercise and inactivity, underscoring its significant role in muscle adaptation[23]. Trained individuals had higher transcriptional activity in type I myofibers and mitochondria, while sedentary individuals showed elevated immune-related signatures, with both groups benefiting from endurance exercise regardless of prior activity levels[24].
Transcriptomics can advance our understanding of CVD and the impact of exercise on heart health. By analyzing the transcriptome, researchers can identify biomarkers and molecular pathways involved in CVD, leading to improved diagnostics and personalized treatments. However, to the best of the authors' knowledge, no prior study has assessed transcriptomic predictors following CR. In the realm of CR, exercise may induce beneficial transcriptomic changes. For instance, a machine learning-based study uncovered 18 transcriptomic biomarkers predictive of CVD outcomes with reported accuracy of up to 96%[25]. However, it is important to note that this study was not conducted among CR populations; therefore, its findings should be interpreted cautiously to avoid overgeneralization.
Moreover, the practical application of transcriptomic profiling in CR settings is currently limited by several logistical and clinical challenges.
The complexity of omics data may present difficulties for clinicians who are unfamiliar with molecular diagnostics, and existing multi-omics workflows may not align with the relatively short duration (typically 6-12 weeks) of standard CR programs. Future research should therefore prioritize validation of transcriptomic signatures in CR cohorts and develop simplified, clinically feasible approaches that can be readily integrated into routine practice.
Proteomics in CR: Proteomics examines protein expression and interactions, helping to identify specific biomarkers such as cardiac troponins (cTnl, cTnT) and natriuretic peptides (BNP, NT-proBNP), which are well-established for diagnosing myocardial injury and predicting outcomes in CVD[26]. Exercise-based CR has been associated with notable shifts in the proteome, including reductions in oxidative stress markers[27] and inflammatory cytokines[28,29], both of which play significant roles in left ventricular remodeling following myocardial infarction. Proteomics studies have also revealed favorable changes in the neurohormonal profile of patients with heart failure with preserved ejection fraction, evidenced by decreased levels of NT-proBNP and related neuropeptides[30].
Additionally, proteomics biomarkers may serve as valuable tools for monitoring patient response during CR, allowing clinicians to detect early signs of recovery or identify patients at higher risk for adverse outcomes. These molecular insights can guide personalized modifications to rehabilitation strategies. For example, exercise-induced changes in protein expression can objectively reflect improvements in myocardial function, vascular health, and skeletal muscle strength[31]. These biomarkers have been validated in multiple CR cohorts, demonstrating their utility in monitoring patient response to rehabilitation interventions. For example, decreases in IL-6 and CRP levels correlate with improved exercise tolerance and reduced cardiovascular risk after CR[32], while dynamic changes in lactate concentrations provide insights into skeletal muscle metabolic adaptations[33]. The clinical implementation of proteomics in traditional CR remains constrained by challenges including a lack of standardized assay protocols, high costs, and limited availability of simplified data interpretation tools for frontline clinicians. Future research aims to validate these biomarkers further and develop accessible platforms to enhance their practical utility in CR programs.
Metabolomics in CR: Metabolomics is the study of small-molecule metabolites involved in processes such as oxidative stress and energy production. Changes in metabolite profiles, such as a reduction in branched-chain amino acids, are associated with reduced exercise capacity in individuals with coronary artery disease[34]. Metabolomics in CR can identify circulating metabolites that predict exercise capacity, offering potential for individualized CR programs. Exercise-based CR may play a crucial role in enhancing the metabolic management of HFpEF[35] and improving exercise capacity by reducing oxidative stress and boosting the body's antioxidant systems in patients with CVD[36]. This understanding enables CR programs to be customized, ensuring treatment is optimized to suit each patient's unique metabolic needs. Candidate biomarkers identified through metabolomics could serve as valuable tools in personalized healthcare, with potential applications in disease screening, prognosis, and the tailoring of therapeutic strategies, including exercise regimens[37,38].
Microbiomics in CR: Microbiomics is the study of the microbiome, which refers to the collection of microorganisms, such as bacteria, viruses, fungi, and other microbes, that live in and on the human body[39]. The gut microbiome, plays a vital role in digestion, immune system regulation, vitamin production, and protection against harmful pathogens[40]. Emerging research has highlighted the significant role of gut microbiota-host interactions in maintaining human health and contributing to the development of various diseases, including inflammatory and cardiovascular conditions[41].
Although no interventional human studies have explored the impact of the gut microbiota or microbiome within CR programs, there is growing scientific interest in this domain. Recent review suggests that dysbiosis of the gut microbiota may contribute to the pathogenesis of cardiovascular diseases through mechanisms involving systemic inflammation, metabolic dysfunction[42], and increased production of harmful metabolites such as trimethylamine N-oxide[43]. Novakovic et al. further emphasize the role of gut microbial imbalance in promoting leaky gut syndrome and inflammation, proposing that restoring microbial integrity through targeted interventions may offer a novel strategy for cardiovascular prevention and management[44].
In this context, microbiomics in CR could provide valuable insights into individual variability in rehabilitation response. By identifying patients with unfavorable microbiome profiles, clinicians may be able to personalize CR programs using adjunctive interventions such as probiotics, prebiotics, or dietary modifications. Furthermore, monitoring microbiomic changes throughout CR may allow for the early detection of suboptimal responses and guide therapeutic adjustments. While this field is still in its early stages, integrating microbiome profiling into CR holds promise for enhancing clinical outcomes and advancing precision rehabilitation strategies. Future human studies are needed to validate these concepts and determine their practical applications.
CHALLENGES
High-throughput sequencing assays and other omics assays are powerful tools in molecular biology, but they present several challenges. These methods demand specialized expertise for implementation and interpretation. For instance, high-throughput sequencing involves intricate processes such as sample preparation, sequencing, and data analysis, each requiring specific skills and knowledge[45]. The vast amounts of data generated require robust storage solutions and sophisticated computational tools for analysis[46]. Handling and interpreting large datasets can be resource-intensive and may require advanced bioinformatics capabilities. Variability in protocols and technologies can lead to inconsistent results. Furthermore, variability in laboratory protocols could lead to inconsistent results. In addition to technical obstacles, cost remains a significant implementation barrier. Omics profiling, particularly multi-layered analyses, is currently expensive and may not be feasible in routine CR settings, especially in low- and middle-income countries. Without scalable or cost-effective models, widespread adoption could inadvertently exacerbate healthcare disparities, limiting access to those in more resource-rich environments. These challenges require technological and methodological innovations.
FUTURE DIRECTIONS
Identifying specific biomarkers that reflect the effectiveness of CR interventions can lead to more personalized and effective treatment strategies. Longitudinal studies tracking genetic and molecular modifications in response to exercise may provide insights into the mechanisms by which exercise influences cardiovascular health. Emerging tools such as single-cell omics can further refine this understanding by revealing cell type-specific adaptations to exercise, thereby offering higher-resolution targets for individualized interventions. In parallel, wearable biosensors that enable real-time tracking of circulating metabolites, such as glucose, lactate, or cortisol, present new opportunities for dynamic, feedback-driven CR protocols that adapt to patients’ physiological responses in real time. To translate these insights into clinical practice, future research must address key implementation challenges. Cost-effectiveness analyses are essential to evaluate whether omics-based interventions provide added value over standard care and to prevent exacerbating healthcare disparities. Feasibility studies should assess how omics profiling can be integrated into routine CR workflows without overburdening clinical teams or increasing patient dropout. In parallel, standardized implementation protocols must be developed to ensure reproducibility and consistency of omics assays across diverse healthcare settings. Simplified and scalable approaches for sample processing and data interpretation are needed to support wider adoption, particularly in low-resource environments. Interdisciplinary collaboration among clinicians, omics scientists, data analysts, and health economists is vital to ensure that these innovations are both clinically effective and practically sustainable. Finally, digital health tools, including wearables and remote sampling platforms, could democratize access by enabling individualized CR monitoring beyond the clinic, particularly benefiting underserved or geographically isolated populations. By addressing these future directions, omics technologies hold the potential to transform CR into a truly precision-based, patient-centered practice.
PRACTICAL APPLICATIONS
The integration of omics technologies in CR requires a clear rationale for sample collection, ensuring that timing, frequency, and clinical goals align with patient needs and healthcare system capacities. For instance, practitioners may aim to assess overall health or monitor CR progress using omics-derived biomarkers. Clinician and patient buy-in are vital to ensuring successful implementation. Healthcare providers should communicate the benefits of omics technologies, such as enhanced recovery tracking, reduced adverse events, and improved long-term outcomes, to foster CR adherence and patient outcomes. Ultimately, interdisciplinary collaboration among physicians, CR professionals, omics researchers, data scientists, and health economists is necessary to ensure these innovations are both clinically effective and practically sustainable. By adhering to these considerations, omics technologies could be seamlessly incorporated into CR, providing actionable insights to enhance precision medicine.
CONCLUSION
This commentary highlights the emerging potential of integrating multi-omics technologies into CR to better understand the biological mechanisms driving individual variability in response to treatment. By examining exercise-induced changes in genomic, proteomic, and metabolomic profiles, researchers can begin to unravel why some patients respond more favorably than others. These insights offer a foundation for stratifying patients based on molecular risk and tailoring interventions more precisely. However, current evidence supporting routine clinical integration remains preliminary. Before multi-omics could be translated into everyday CR practice, significant challenges, including biomarker validation, cost-effectiveness, and workflow feasibility, must be addressed. As the field evolves, leveraging interdisciplinary collaboration and emerging digital tools will be critical to ensure omics-guided CR becomes both scientifically sound and clinically viable.
DECLARATIONS
Authors’ contributions
Conceptualization, writing-original draft: Ghram A
Writing-review and editing: Lavie CJ
Final approval of the published version of the manuscript: Ghram A, Lavie CJ
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Lavie CJ is an Editorial Board member of the journal Metabolism and Target Organ Damage. Lavie CJ was not involved in any steps of the editorial process, including reviewer selection, manuscript handling, or decision making. The other author declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11:CD001800.
2. Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol. 2022;19:180-94.
3. Tutor A, Lavie CJ, Kachur S, Dinshaw H, Milani RV. Impact of cardiorespiratory fitness on outcomes in cardiac rehabilitation. Prog Cardiovasc Dis. 2022;70:2-7.
4. Brown TM, Pack QR, Aberegg E, et al; American Heart Association Exercise. Core components of cardiac rehabilitation programs: 2024 update: a scientific statement from the American heart association and the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2024;150:e328-47.
5. De Schutter A, Kachur S, Lavie CJ, et al. Cardiac rehabilitation fitness changes and subsequent survival. Eur Heart J Qual Care Clin Outcomes. 2018;4:173-9.
6. Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18:273-89.
7. Wang RS, Maron BA, Loscalzo J. Multiomics network medicine approaches to precision medicine and therapeutics in cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2023;43:493-503.
8. Alemu R, Sharew NT, Arsano YY, et al. Multi-omics approaches for understanding gene-environment interactions in noncommunicable diseases: techniques, translation, and equity issues. Hum Genomics. 2025;19:8.
9. Krishnamurthy HK, Reddy S, Jayaraman V, et al. Association between high-sensitivity C-reactive protein (hs-CRP) levels with lipids and micronutrients. Cureus. 2024;16:e67268.
10. Wazir M, Olanrewaju OA, Yahya M, et al. Lipid disorders and cardiovascular risk: a comprehensive analysis of current perspectives. Cureus. 2023;15:e51395.
11. Khan S, Ince-Dunn G, Suomalainen A, Elo LL. Integrative omics approaches provide biological and clinical insights: examples from mitochondrial diseases. J Clin Invest. 2020;130:20-8.
12. Nativio R, Lan Y, Donahue G, et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat Genet. 2020;52:1024-35.
13. Leopold JA, Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018;122:1302-15.
15. Zaree A, Dev S, Yaseen Khan I, et al. Cardiac rehabilitation in the modern era: optimizing recovery and reducing recurrence. Cureus. 2023;15:e46006.
16. Sabbahi A, Canada JM, Babu AS, Severin R, Arena R, Ozemek C. Exercise training in cardiac rehabilitation: setting the right intensity for optimal benefit. Prog Cardiovasc Dis. 2022;70:58-65.
17. Sarno F, Benincasa G, List M, et al; International Network Medicine Consortium. Clinical epigenetics settings for cancer and cardiovascular diseases: real-life applications of network medicine at the bedside. Clin Epigenetics. 2021;13:66.
18. Wu G, Zhang X, Gao F. The epigenetic landscape of exercise in cardiac health and disease. J Sport Health Sci. 2021;10:648-59.
19. Ferrari L, Vicenzi M, Tarantini L, et al. Effects of physical exercise on endothelial function and DNA methylation. Int J Environ Res Public Health. 2019;16:2530.
20. Ma Y, Liu H, Wang Y, et al. Roles of physical exercise-induced MiR-126 in cardiovascular health of type 2 diabetes. Diabetol Metab Syndr. 2022;14:169.
21. Li J, Wang Z, Li C, et al. Impact of exercise and aging on mitochondrial homeostasis in skeletal muscle: roles of ROS and epigenetics. Cells. 2022;11:2086.
22. Zhu M, Zhang C, Zhang Z, et al. Changes in transcriptomic landscape in human end-stage heart failure with distinct etiology. iScience. 2022;25:103935.
23. Pillon NJ, Gabriel BM, Dollet L, et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun. 2020;11:470.
24. Rubenstein AB, Hinkley JM, Nair VD, et al. Skeletal muscle transcriptome response to a bout of endurance exercise in physically active and sedentary older adults. Am J Physiol Endocrinol Metab. 2022;322:E260-77.
25. DeGroat W, Abdelhalim H, Patel K, Mendhe D, Zeeshan S, Ahmed Z. Discovering biomarkers associated and predicting cardiovascular disease with high accuracy using a novel nexus of machine learning techniques for precision medicine. Sci Rep. 2024;14:1.
26. Chandramouli K, Qian PY. Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Hum Genomics Proteomics. 2009;2009:239204.
27. Al-Menhali AS, Anderson C, Gourine AV, Abramov AY, D'Souza A, Jaganjac M. Proteomic analysis of cardiac adaptation to exercise by high resolution mass spectrometry. Front Mol Biosci. 2021;8:723858.
28. Kurgan N, Noaman N, Pergande MR, Cologna SM, Coorssen JR, Klentrou P. Changes to the human serum proteome in response to high intensity interval exercise: a sequential top-down proteomic analysis. Front Physiol. 2019;10:362.
29. Sim YJ, Yu S, Yoon KJ, Loiacono CM, Kohut ML. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200:1434-42.
30. Billebeau G, Vodovar N, Sadoune M, Launay JM, Beauvais F, Cohen-Solal A. Effects of a cardiac rehabilitation programme on plasma cardiac biomarkers in patients with chronic heart failure. Eur J Prev Cardiol. 2017;24:1127-35.
31. Mi S, Jiang H, Zhang L, et al. Regulation of cardiac-specific proteins expression by moderate-intensity aerobic exercise training in mice with myocardial infarction induced heart failure using MS-based proteomics. Front Cardiovasc Med. 2021;8:732076.
32. Luo C, Li L, Hou L, Shi F. Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease. Open Life Sci. 2025;20:20221040.
33. Fulghum K, Hill BG. Metabolic mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med. 2018;5:127.
34. Shah RV, Miller P, Colangelo LA, et al. Blood-based fingerprint of cardiorespiratory fitness and long-term health outcomes in young adulthood. J Am Heart Assoc. 2022;11:e026670.
35. Corbi G, Conti V, Troisi J, et al. Cardiac rehabilitation increases SIRT1 activity and β-hydroxybutyrate levels and decreases oxidative stress in patients with HF with preserved ejection fraction. Oxid Med Cell Longev. 2019;2019:7049237.
36. Fukuda T, Kurano M, Fukumura K, et al. Cardiac rehabilitation increases exercise capacity with a reduction of oxidative stress. Korean Circ J. 2013;43:481-7.
37. Griffiths WJ, Koal T, Wang Y, Kohl M, Enot DP, Deigner HP. Targeted metabolomics for biomarker discovery. Angew Chem Int Ed Engl. 2010;49:5426-45.
38. Zhang A, Sun H, Wang X. Power of metabolomics in biomarker discovery and mining mechanisms of obesity. Obes Rev. 2013;14:344-9.
39. Reynoso-García J, Miranda-Santiago AE, Meléndez-Vázquez NM, et al. A complete guide to human microbiomes: Body niches, transmission, development, dysbiosis, and restoration. Front Syst Biol. 2022;2:951403.
40. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4-14.
41. Nesci A, Carnuccio C, Ruggieri V, et al. Gut microbiota and cardiovascular disease: evidence on the metabolic and inflammatory background of a complex relationship. Int J Mol Sci. 2023;24:9087.
42. McIntyre CW, Harrison LE, Eldehni MT, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133-41.
43. Tang WH, Hazen SL. Probiotic therapy to attenuate weight gain and trimethylamine-N-Oxide generation: a cautionary tale. Obesity (Silver Spring). 2015;23:2321-2.
44. Novakovic M, Rout A, Kingsley T, et al. Role of gut microbiota in cardiovascular diseases. World J Cardiol. 2020;12:110-22.
45. Loeffelholz M, Fofanov Y. The main challenges that remain in applying high-throughput sequencing to clinical diagnostics. Expert Rev Mol Diagn. 2015;15:1405-8.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].