Probiotic applications of bifidobacteria in poultry: administration methods and microencapsulation techniques
Abstract
The search for sustainable alternatives to antibiotic growth promoters in poultry production has intensified in recent years, driven by global concerns over antimicrobial resistance and consumer demand for safer food systems. Among the probiotic candidates investigated, Bifidobacterium spp. stand out for their well-documented safety, immunomodulatory properties, and ability to enhance gut health. This review provides a comprehensive analysis of the biological roles, delivery strategies, and microencapsulation techniques for Bifidobacterium spp. as probiotics in poultry. Bifidobacteria contribute to poultry health by modulating the gut microbiota, improving intestinal morphology and digestive enzyme activity, and regulating immune responses through cytokine balance and epithelial barrier reinforcement. However, their strict anaerobic metabolism and sensitivity to gastric acid and processing conditions limit their viability during conventional administration. To address these challenges, we examine various administration routes, including oral, in ovo, spray/litter, and cloacal methods, highlighting their practical advantages and constraints. Special attention is given to microencapsulation technologies, such as spray drying, freeze drying, spray chilling, extrusion, and emulsion, which protect bifidobacteria from environmental stress and enhance their delivery to target intestinal sites. By integrating recent advances in biotechnology and delivery systems, this review underscores the potential of Bifidobacterium spp. as functional feed additives in antibiotic-free poultry production. Tailoring encapsulation materials and administration routes to match specific production goals is key to maximizing probiotic efficacy. Continued research on strain performance under commercial conditions will be essential to facilitate their large-scale application in sustainable poultry farming.
Keywords
INTRODUCTION
The poultry industry is a cornerstone of global food production, contributing significantly to protein supply and agricultural economies worldwide[1]. As demand for poultry meat and eggs continues to rise, particularly in low- and middle-income countries, maintaining the health and productivity of flocks has become more critical than ever. Traditionally, using antibiotics as growth promoters and disease prevention tools was a widespread and effective strategy[2]. However, mounting concerns about antimicrobial resistance, driven by the overuse and misuse of antibiotics in animal agriculture, have prompted regulatory restrictions and consumer-driven shifts toward more sustainable, antibiotic-free production systems[3]. In this context, the use of probiotics - defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”[4] - has emerged as a promising alternative to antibiotics in poultry farming. Among the diverse genera explored as probiotics, such as Lactobacillus, Enterococcus, Streptococcus, Propionibacterium, Bacillus, and Saccharomyces, Bifidobacterium stands out for its long-standing history of safe use in humans and animals, its extensive documentation in functional food applications, and its proven efficacy in modulating gut microbiota and host physiology[5,6]. Bifidobacteria are Gram-positive, non-motile, anaerobic microorganisms that naturally inhabit the gastrointestinal tract (GIT) of humans and animals, including poultry[7], particularly in early life stages[8]. Their ability to adhere to the intestinal mucosa, produce organic acids, compete with pathogens, and modulate immune responses makes them suitable candidates for improving intestinal health and general well-being in poultry[9]. These attributes are especially valuable during critical periods such as early chick development, feed transitions, and stress or immunosuppression, when the intestinal microbiota is most vulnerable to disruption.
The safety of bifidobacteria is well established, with several species classified as GRAS (generally regarded as safe) by the Food and Drug Administration (FDA) and QPS (qualified presumption of safety) by the European Food Safety Authority (EFSA)[10,11], supporting their use as probiotics in poultry production. While short-term trials (e.g., up to 42 days in Ross or Cobb broilers) consistently show that the administration of Bifidobacterium spp. improves performance and gut morphology with no adverse histological or immune effects[12,13], comprehensive long-term safety data across full production cycles of laying hens remain limited. Multi-strain formulations containing Bifidobacterium spp. administered to broilers and layers have passed EFSA assessment for safety, with no observed negative effects on organ health or evidence of antimicrobial resistance gene transfer when used within recommended doses[13-16]. However, broader reviews caution that high-dose or prolonged probiotic use may, in rare cases, alter immune parameters, particularly cytokine expression or inflammatory markers [e.g., interleukin-6 (IL-6), IL-8], underlining the necessity of monitoring immune homeostasis over the full lifespan in diverse breeds[17,18]. No breed-specific histopathological abnormalities have been noted to date across quail, broilers, or laying hens.
Despite the promising potential of bifidobacteria, their application in poultry systems has been less explored than that of Lactobacillus and other species because they face substantial obstacles when implemented as probiotics in poultry production systems[17]. A primary challenge arises from their stringent anaerobic growth requirements. Unlike lactic acid bacteria (LAB), Bacillus subtilis, or yeast, bifidobacteria cannot tolerate oxygen exposure without significant loss of viability because they lack robust oxidative stress defense systems[19]. This sensitivity imposes strict conditions during industrial-scale fermentation, downstream processing, and handling stages, thereby increasing complexity and production costs. The inability to survive in the presence of oxygen also exponentially magnifies difficulties during feed formulation and storage, where oxygen exposure is inevitable. Consequently, bifidobacteria require specialized anaerobic fermenters and oxygen-impermeable packaging, driving up manufacturing expenses and limiting their widespread commercial use compared to more aerotolerant strains[20]. In addition to oxygen sensitivity, bifidobacteria generally have slower growth rates and lower biomass yields than Bacillus spp. and many LAB strains. This directly impacts production throughput and scalability, as achieving economically viable cell counts demands longer fermentation times and more controlled conditions[20].
To overcome these challenges, recent advances in biotechnology have led to the development of microencapsulation techniques that protect probiotic bacteria during processing, storage, and passage through the harsh upper GIT. Microencapsulation involves the entrapment of live microorganisms within a matrix or coating material, such as alginate, chitosan, starch, or lipid-based systems, which shield the cells from environmental stressors and enable controlled release in the target intestinal site[24]. When tailored appropriately, microencapsulation not only enhances the viability of bifidobacteria but also improves their colonization efficiency and functional performance in the gut[25].
The purpose of this review is to provide an updated and comprehensive overview of the probiotic applications of Bifidobacterium spp. in poultry production, with special emphasis on administration strategies and encapsulation technologies. We discuss the biological roles of bifidobacteria in poultry health, evaluate the current literature on their efficacy as dietary supplements, and examine the most recent innovations in encapsulation methods aimed at preserving their functional integrity. By bridging the gap between experimental findings and practical implementation, this review aims to support the integration of Bifidobacterium-based probiotics into sustainable poultry production systems.
BIOLOGICAL ROLES OF BIFIDOBACTERIA IN POULTRY HEALTH
Bifidobacteria have been increasingly recognized for their probiotic potential in poultry by improving the overall health of the birds[26]. As shown in Figure 1, their roles encompass modulation of gut microbiota, enhancement of intestinal morphology, immune system regulation, and protection against pathogenic infections. Altogether, these effects synergistically contribute to enhanced poultry productivity.
Modulation of gut microbiota
The GIT of poultry hosts a complex and dynamic microbial community that plays a pivotal role in digestion, nutrient absorption, immune system development, and protection against pathogens. Among the beneficial microbes, bifidobacteria have garnered attention for their probiotic potential in modulating the gut microbiota to enhance poultry health and performance. Bifidobacteria can inhibit pathogenic bacteria through competitive exclusion, whereby they compete for adhesion sites and nutrients, effectively preventing colonization by harmful microbes. Additionally, bifidobacteria produce bacteriocins and organic acids like acetate and lactate, which lower the pH of the gut environment, creating unfavorable conditions for pathogenic bacteria[27]. In a recent study, Dixon et al. (2022) tested the inhibition of diverse poultry pathogens by B. longum ATCC 15708 in vitro[28]. This strain was able to inhibit the growth of common poultry pathogens, such as Escherichia coli, Salmonella pullorum, Salmonella enterica serovar Enteritidis,
Besides inhibiting pathogenic bacteria, the supplementation with bifidobacteria has been shown to increase the abundance of beneficial microbial populations in the poultry gut. This shift in microbial composition contributes to improved gut health and function[31], since gastrointestinal microorganisms hydrolyze dietary components to produce relevant metabolites[32]. For instance, dietary inclusion of a probiotic supplement composed of E. faecium, Bifidobacterium, and Pediococcus acidilactici (DSM Singapore Industrial Pte. Ltd.) enhanced the abundance of beneficial bacteria such as Lactobacillus and Faecalibacterium, while modulating the cecal microbiota structure of broilers[31]. The birds fed with the probiotic supplement presented an increased ratio of Firmicutes to Bacteroidota, which could improve the energy extraction from the feed. In another study, Liu et al. (2023) reported that the administration of a supplement composed of strains of Bifidobacterium, L. casei, and L. acidophilus improved the intestinal health of broilers by reducing the relative abundance of harmful bacteria, such as Proteobacteria, while increasing beneficial Firmicutes[33]. Furthermore, the introduction of bifidobacteria into the poultry diet has been linked to increased microbial diversity and stability within the gut ecosystem[34]. A diverse and stable microbiota is crucial for resilience against pathogenic invasions and for maintaining optimal digestive functions.
Enhancement of intestinal morphology and function
The structural integrity and functional efficiency of the intestinal mucosa are critical for optimal nutrient absorption, immune defense, and overall health in poultry. Supplementation with bifidobacteria has been shown to positively influence intestinal morphology and function, leading to improved growth performance and disease resistance. A supplement containing Bifidobacterium infantis CRL1395, a strain with the ability to bind soybean agglutinin (SBA) in its surface, Propionibacterium acidipropionici LET 103, Lactobacillus salivarius LET 201, L. reuteri LET 210, and E. faecium LET 301 effectively protected the jejunal microvilli of broilers from damage and shortening caused by a diet rich in SBA[35]. Feeding B. lactis JYBR-190 to chicks infected with S. pullorum significantly increased duodenal and jejunal villus height and the ratio between villus height and crypt depth, indicating improved mucosal recovery and function[36]. These morphological changes, also observed by other authors on birds not challenged with pathogens[37,38], expand the absorptive surface area, facilitating better nutrient uptake.
The intestinal barrier is essential for preventing pathogen translocation and maintaining gut homeostasis. The administration of a Bifidobacterium strain along with Saccharomyces cerevisiae Hansen and Rhodopseudomonas sphaeroides contributed to the reinforcement of this barrier by upregulating tight junction proteins such as zona occludens-1, claudin-1, and occludin in the jejunum of broilers[38]. Hu et al. (2024) observed increased expression of these proteins and junctional adhesion molecule-2 in the ileum of broilers fed with a combination of probiotics, including Bifidobacterium B8101, and betaine[37].
The digestive efficiency of poultry is closely linked to the activity of intestinal enzymes such as amylase, lipase, and proteases. These enzymes are essential for the breakdown of macronutrients, facilitating nutrient absorption and overall growth performance. Recent studies have highlighted the role of bifidobacteria in modulating the activity of these enzymes, thereby enhancing digestive processes in poultry. The dietary inclusion of a probiotic mix including bifidobacteria significantly elevated the activities of amylase, lipase, trypsin, and chymotrypsin in the duodenum of broilers[31]. This enhancement in enzyme activities was associated with improved nutrient digestibility and growth performance, suggesting that bifidobacteria play a role in stimulating endogenous enzyme secretion or directly contributing exogenous enzymes to the digestive process.
Immune system regulation
The immunomodulatory properties of Bifidobacterium spp. have been increasingly recognized in poultry production systems, where the enhancement of immune competence is a critical component of disease prevention and improved performance under intensive conditions. Supplementation with bifidobacteria has been shown to modulate both innate and adaptive immune responses, contributing to improved resistance to enteric infections and inflammatory challenges[17]. One of the primary mechanisms by which bifidobacteria exert their immunomodulatory effects is through the regulation of cytokine production. In a study by Yang et al. (2022), dietary administration of B. lactis JYBR-190 to chicks challenged with
Another important immunological effect of bifidobacteria pertains to the modulation of pattern recognition receptor pathways, particularly those involving Toll-like receptors (TLRs). In broiler models, probiotic supplementation has been associated with altered expression of TLR2 and TLR4, which are key in microbial recognition and the orchestration of downstream immune signaling[33]. By modulating these pathways, bifidobacteria may help balance immune activation and tolerance, mitigating excessive inflammatory damage. Furthermore, metabolites produced by bifidobacteria, particularly short-chain fatty acids (SCFAs) such as acetate and butyrate, play a significant role in modulating the host immune system in poultry. SCFAs have been shown to influence the differentiation and function of regulatory T cells (Tregs) through epigenetic mechanisms, including the inhibition of histone deacetylases and activation of G protein-coupled receptors such as GPR4 and GPR109A[39]. These metabolites also modulate cytokine production, enhancing anti-inflammatory mediators, such as IL-10, while suppressing pro-inflammatory cytokines, like TNF-α and IFN-γ[40].
EFFECT OF BIFIDOBACTERIA ON POULTRY PRODUCTIVITY
The administration of Bifidobacterium spp. has demonstrated promising effects not only on poultry gut health and immune modulation but also on key productivity parameters, such as body weight gain (BWG), feed conversion ratio (FCR), and carcass quality. These outcomes are especially relevant in the current trend of antibiotic-free production systems, where sustainable strategies to enhance performance are critically needed. Numerous studies have demonstrated that dietary inclusion of bifidobacteria or bifidobacteria-containing probiotic blends enhances growth performance in broilers by modulating the intestinal microbiota, specifically, increasing beneficial bacterial populations while suppressing pathogens, which directly contributes to improved FCR. For example, Wang et al. (2024) observed that a compound probiotic supplement including a Bifidobacterium strain (DSM Singapore Industrial Pte. Ltd.) significantly increased average daily gain (ADG) and body weight (BW) and reduced FCR by 7.0%, 6.9%, and 7.9%, respectively, in broilers over 42 days. These improvements were associated with increased SCFA concentration in the cecum (acetic acid, energy-supplying butyric acid, and total SCFA), better nutrient absorption, enhanced gut morphology (villus height and villus/crypt ratio in duodenum and jejune), and increased activity of digestive enzymes such as amylase, lipase, trypsin, and chymotrypsin in ileum and duodenum[31]. Liu et al. (2023) found that broilers receiving a probiotic mixture (basal diet + 1, 5, or 10 g of probiotic/kg) including a Bifidobacterium strain, L. casei, and L. acidophilus (Shanxi Ruimao Biotechnology Co., Ltd.) exhibited improved FCR and ADG compared to controls, especially for the higher dose of probiotic, which was attributed to enhanced fiber digestion and enzyme activities[33]. In another study, the 35-day administration of a supplement containing B. animalis subsp. animalis DSM 16284, E. faecium DSM 21913, and
Recent work demonstrates that the gut microbial baseline and probiotic responsiveness vary significantly across poultry breeds and species. For instance, Ross 308 broilers, Cobb 500 broilers, and Hy-line layer hens respond differently to the same probiotic blend: increases in villus height, crypt depth, and villus/crypt ratio are observed in Cobb 500 and layers, while Ross broilers often exhibit more modest histomorphometric changes and microbial modulation[14]. Likewise, native Indian chicken breeds exhibit distinctly different cecal microbiota composition compared to commercial broilers, suggesting breed-specific core microbiomes that may influence probiotic colonization and function[49]. These findings underscore that broiler-derived efficacy data cannot be directly extrapolated to layers, heritage breeds, or other poultry species like quail.
Specifically, studies in Japanese quail supplemented with B. bifidum ATCC 29521 (alone or combined with B. toyonensis ATCC 55050) reported improvements in feed efficiency, egg weight, fertility, and hatchability, but with a smaller magnitude in FCR and weight gain than seen in broilers[50]. This difference may reflect the unique gut physiology and slower growth kinetics of quails. In laying hens, blends containing Bifidobacterium spp. and L. casei strains improved egg weight and yield, but these effects varied by strain and duration, a contrast to more robust broiler growth responses[15].
Overall, the integration of Bifidobacterium spp. into poultry nutrition programs can positively impact productivity through multiple mechanisms, including microbiota modulation, improved nutrient digestibility, enhanced intestinal structure, and reduced pathogen pressure. Bifidobacterium spp. modulate the intestinal microbiota of broilers, increasing the proportion of Firmicutes to Bacteroidota, which results in increased energy extraction from dietary fiber, positively affecting BW[51]. Amylase, lipase, trypsin, chymotrypsin, and other enzymes with increased activities in the gut of birds administered with Bifidobacterium spp. play a crucial role in the hydrolysis, digestion, and absorption of crude proteins, lipids, and carbohydrates, converting them into amino acids, triglycerides, and glucose in broilers. Consequently, the enhanced activity of digestive enzymes observed with the probiotic supplementation may account for improved nutrient digestibility and growth performance[31]. This elevation in enzymatic activity is thought to result either from direct secretion of digestive enzymes by probiotics, stimulation of host cell secretion, or a synergistic effect of both mechanisms, ultimately facilitating greater digestive enzyme production[52]. Bifidobacterium spp. have been shown to increase SCFA in the cecum, which supports the maintenance of intestinal mucosa integrity, while some SCFAs can also be directly absorbed as nutrients[53]. In addition, SCFAs lower the intestinal pH, inhibiting pathogens[31]. Moreover, broilers supplemented with Bifidobacterium spp. exhibited improved intestinal morphology, characterized by longer villi and a higher villus/crypt ratio. These structural enhancements are likely to facilitate more efficient nutrient absorption, thereby contributing to improved growth performance[36]. These effects are highly strain-specific and dose-dependent, necessitating careful selection and standardization for optimal performance outcomes[54]. Continued research under commercial-scale conditions is essential to validate these benefits and to fine-tune formulation strategies that maximize productivity while maintaining animal welfare and sustainability.
To translate these benefits into practical outcomes, it is crucial to evaluate the different methods of administering Bifidobacterium spp. in poultry systems.
ADMINISTRATION ROUTES OF BIFIDOBACTERIA IN POULTRY
The successful application of Bifidobacterium strains as probiotics in poultry production relies not only on strain selection and viability but also on the method of administration. The route by which bifidobacteria are delivered influences their capacity to colonize the GIT, survive the host’s physiological barriers, and exert their intended immunological and metabolic effects. Effective colonization is a prerequisite for the modulation of host-microbe interactions, including competitive exclusion of pathogens, enhancement of mucosal immunity, and production of bioactive metabolites such as SCFAs[36]. Consequently, selecting an appropriate administration route is pivotal for optimizing the probiotic’s efficacy in poultry systems.
Several delivery strategies have been developed and tested to ensure that probiotic bifidobacteria reach their target sites in the gut in sufficient numbers and remain viable during the course of intestinal transit. These include in ovo injection, oral supplementation via feed or water, spray application onto feathers or litter, and more invasive routes such as cloacal or direct gastrointestinal delivery[47,55-57]. Each method offers distinct advantages in terms of early microbial programming, logistical feasibility, and compatibility with large-scale poultry operations. However, they also present unique limitations that may influence colonization kinetics, probiotic stability, and overall performance outcomes. On the other hand, authors suggested that combinations of administration routes are more efficient to prevent pathogen colonization[58,59]. The main advantages and disadvantages of each administration route are summarized in Table 1.
Comparative analysis of Bifidobacterium administration routes in poultry production
| Administration route | ||||
| Oral administration | In ovo injection | Spray and litter application | Cloacal and direct gastrointestinal administration | |
| Advantages | - Simple, non-invasive, and scalable - Easily incorporated into feed or water - Allows repeated dosing | - Enables colonization before hatch - Promotes early immune system development - Standardizable via automated systems | - Mass application without handling birds - Ingestion via preening or environment - Useful in hatcheries and brooding areas | - Bypasses acidic and oxygenated upper GIT - Directly delivers bacteria to their ecological niche - Promotes fast and stable colonization at the ceca |
| Disadvantages | - Exposure to oxygen during storage and handling - Harsh gastric conditions reduce viability - Often requires protective encapsulation | - Requires technical precision - Risk of embryonic mortality - Limited probiotic dosage volume - Exposure to oxygen during formulation and injection | - High oxygen exposure reduces anaerobe viability - Inconsistent individual exposure - Dependent on bird behavior and environment | - Impractical for mass application - Labor-intensive with high handling stress - Potential risk of cross-contamination between birds |
| Anaerobic suitability | Low | Low-Moderate | Low | High |
| Early microbial programming | Moderate-high | Very high | Moderate | High |
| Colonization efficiency | Moderate | High | Variable | Very high |
| Microencapsulation requirement | High | High | High | Low |
| Logistical feasibility | Very high | Moderate | Very high | Very low |
| Large-scale compatibility | Very high | High | Very high | Very low |
As our understanding of host-microbiota interactions deepens, it becomes increasingly clear that the timing, dosage, and route of administration must be tailored to specific production goals, whether to support early-life immune development, improve feed conversion, or enhance resistance to enteric pathogens[34]. Thus, optimizing delivery methods is not merely a technical issue but a strategic one that integrates microbiological, immunological, and zootechnical knowledge.
Oral administration
Oral administration represents the most prevalent and operationally feasible method for delivering Bifidobacterium spp. in commercial poultry systems, primarily through incorporation into feed or drinking water[60]. This route is applicable across diverse poultry categories, including broilers, layers, and breeders, with particular emphasis on neonatal and juvenile stages due to their heightened responsiveness to microbial modulation[61]. Delivery via water offers flexibility in dosage and timing, promoting consistent exposure during critical early-life periods, and is often more effective than feed-based delivery, especially in newly hatched chicks that may not readily access solid feed in the first 24-48 h[62,63]. Early oral supplementation, beginning immediately post-hatch, plays a pivotal role in microbial programming of the GIT. Establishing beneficial microbial populations early in life not only shapes a stable gut microbiota but also promotes intestinal maturation and primes the host immune system, thereby enhancing resilience to pathogens[64]. From a logistical standpoint, oral administration integrates seamlessly with existing infrastructure, such as automated feeding and watering systems, enabling uniform delivery to large flocks with minimal additional labor or cost. In broilers, whose production cycles are rapid and tightly synchronized, consistent and scalable delivery is particularly crucial. Similarly, in layer and breeder operations, the maintenance of long-term intestinal health and productivity benefits from periodic supplementation during stress-associated periods such as vaccination or peak laying phases[65]. However, several limitations must be acknowledged. The survivability of Bifidobacterium strains through the upper GIT and during feed processing (e.g., pelleting) is often compromised due to exposure to oxygen, heat, pressure, and moisture[66]. Furthermore, environmental factors such as water chlorination, pH, and storage conditions can negatively impact probiotic viability[67]. These stability issues can lead to inconsistent colonization kinetics, ultimately influencing the efficacy of the probiotic intervention. Although numerous studies have reported improvements in performance parameters, such as FCR, growth rate, and reduced morbidity, these outcomes are highly dependent on strain selection, dosage, and timing of administration[64]. Therefore, to fully leverage the benefits of oral administration, it is imperative to optimize delivery protocols through protective formulation strategies (e.g., microencapsulation), careful strain selection, and standardized dosing regimens tailored to the physiological and immunological needs of specific poultry types and developmental stages.
In ovo injection
In ovo injection of Bifidobacterium spp. presents a strategic and increasingly explored route for early microbial programming in poultry, whereby probiotics are delivered into the amniotic sac during late embryogenesis (typically day 17-18 of incubation)[68]. This approach is predominantly applied in broiler chickens due to the tight synchronization of their incubation and hatching processes, and the high scalability of commercial hatchery systems[69]. In ovo delivery enables the colonization of the GIT with beneficial bacteria before hatch, which can suppress early colonization by opportunistic and pathogenic microbes, stimulate mucosal immunity, and support intestinal maturation. Furthermore, in fast-growing broilers with a short production cycle, early-life microbial modulation can yield measurable improvements in performance and health outcomes within a few weeks[34]. Studies have demonstrated that in ovo application of B. saeculare B2-2 and B3-4, either alone or combined with LAB, reduces post-hatch colonization by gram-negative bacteria and Enterococcus spp., while enhancing early BWG and intestinal morphology[56]. While most evidence comes from broiler models, less is known about its utility in layer-type chickens, where embryonic development timelines and commercial incubation practices differ slightly and may require adjusted protocols[70]. The in ovo administration of probiotics in poultry species other than chickens remains poorly investigated and, to date, has yielded limited or inconsistent results[71]. From a practical standpoint, this technique is compatible with existing automated vaccine delivery systems in commercial hatcheries, offering a logistically feasible and scalable solution for mass administration[72]. However, challenges remain regarding probiotic strain stability, survival during in ovo delivery, and standardization of dosing without compromising hatchability[73]. Moreover, while early benefits are clear, sustained colonization and long-term functional outcomes may require subsequent reinforcement through post-hatch supplementation[74]. Despite these limitations, in ovo injection offers a promising route to establish health-promoting microbiota during a critical developmental window, aligning microbiological efficacy with the practical demands of modern poultry production systems.
Spray and litter application
Spray and litter application of probiotics has emerged as a non-invasive and operationally practical strategy for probiotic delivery in poultry, particularly during the early post-hatch period[58]. This method involves spraying probiotic suspensions directly onto eggshells, newly hatched chicks, hatchery trays, or litter substrates, thereby enabling rapid exposure and oral ingestion of beneficial microbes through preening and environmental contact[75]. It is especially suited for neonatal chicks in broiler and layer operations, when the immune system and GIT are still under development and most susceptible to microbial programming[76]. In broilers, early colonization via this method can promote intestinal barrier maturation and reduce early-life susceptibility to enteric infections[76]. In layer-type chicks, litter application during brooding can aid in establishing a stable gut microbiota, with potential long-term effects on productivity and health during the laying cycle. The appeal of this method lies in its simplicity, compatibility with existing hatchery and barn equipment, and potential to uniformly treat large bird populations without individual handling[77,78]. Furthermore, this method minimizes labor and stress associated with oral gavage or feed incorporation, making it logistically advantageous in commercial operations. Early exposure via this route may help shape the gut microbial ecosystem during critical developmental windows, promoting intestinal maturation and immune priming[58]. However, the application of strictly anaerobic species such as Bifidobacterium spp. via spray or litter raises specific challenges related to their oxygen sensitivity, which may be overcome through protective technologies such as microencapsulation. Bifidobacteria are highly susceptible to oxidative stress, which can significantly reduce their viability during preparation, storage, and environmental exposure[79]. Upon aerosolization or contact with ambient air, the metabolic activity and survival of these organisms may be compromised, undermining colonization efficiency and functional efficacy. Furthermore, the uneven distribution of probiotics in the litter or on chick surfaces can lead to inconsistent intake among individuals, reducing the uniformity of microbiota establishment. These issues are more pronounced in older birds, such as growers and adults, where behavioral patterns and immune status differ, and this route of administration is less commonly used due to reduced environmental susceptibility and lower interaction with litter surfaces.
Cloacal and direct gastrointestinal administration
Cloacal and direct gastrointestinal administration of Bifidobacterium spp., though less conventional than oral or in ovo routes, offer targeted delivery directly to the lower intestine, bypassing the hostile upper GIT conditions (i.e., low pH and proteolytic enzymes) and minimizing loss of probiotic viability through gastric digestion[80]. In this approach, probiotics are delivered either via cloacal inoculation or direct deposition into specific gut segments, ensuring immediate access to the ceca and colon, which are critical sites for microbial interaction and metabolic activity[81,82]. These routes are primarily applied in neonatal chicks or juvenile poultry under experimental or controlled production settings, where early colonization can influence the trajectory of microbiota establishment and immune development[80]. In the case of cloacal administration, the procedure typically involves the use of a sterile, blunt-end polyethylene or polypropylene pipette or cannula, carefully inserted approximately 1.5-2.0 cm into the cloaca of day-old chicks, depending on body size, to avoid injury or perforation of the rectal mucosa[83]. The volume of inoculum generally ranges from 50 to 100 µL per bird and must be delivered slowly to prevent expulsion. Aseptic conditions are critical throughout the process to avoid introducing opportunistic pathogens; thus, sterile gloves, disinfected equipment, and a clean environment are essential. Proper restraint of the bird is also important to minimize stress and ensure accurate deposition. Unlike oral or spray routes, which expose bacteria to atmospheric oxygen, this method delivers the probiotic directly into the lower GIT, which is more anaerobic, enhancing the survival and functional activity of strict anaerobes, such as Bifidobacterium spp. In day-old chicks, whose lower GIT is still maturing and relatively oxygen-rich, direct inoculation can facilitate the early dominance of beneficial anaerobes before competitive exclusion by endogenous flora is fully established[84]. However, the use of this method in adult birds is rare due to practical limitations associated with bird size, handling complexity, and reduced responsiveness of the mature GUT to microbial manipulation. While these routes excel in ensuring probiotic survival and immediate local effects on the mucosal immune system and microbial communities, practical constraints limit their scalability under commercial conditions. Individual handling of birds is labor-intensive and time-consuming, and it carries risks of cross-contamination and bird stress, which may impact welfare and performance outcomes[85]. Additionally, the long-term residence and functional impact of the introduced bacteria depend on continued competitive activity and may require subsequent supplementation to maintain colonization and performance benefits[85]. Therefore, although cloacal and direct gastrointestinal delivery methods provide precise and potent probiotic deployment and are valuable for research and targeted interventions, their integration into large-scale operations necessitates simplified administration techniques or automation to balance efficacy and practicality.
While the choice of administration route determines the extent of probiotic colonization and functional activity, the success of any method ultimately depends on the ability of bifidobacteria to survive processing, storage, and the physiological challenges of the GIT. This need for enhanced stability has driven increasing attention toward microencapsulation technologies, which provide protective barriers and controlled delivery systems.
MICROENCAPSULATION TECHNIQUES FOR BIFIDOBACTERIA
Microencapsulation has emerged as a pivotal strategy to protect oxygen-sensitive Bifidobacterium spp. during industrial processing, storage, and passage through the harsh environment of the GIT[86]. The encapsulation of probiotic cells within biopolymeric matrices forms a physical barrier that preserves cell viability amid adverse conditions, such as high temperatures, oxygen exposure, and acidic pH[87]. The addition of antioxidants within the encapsulation matrix can help mitigate this issue to some extent[88]. Literature in food biotechnology emphasizes that successful microencapsulation enhances functional probiotic performance in food and feed applications by stabilizing cell membrane integrity and facilitating controlled release at the target site within the host GIT[89-91]. Microencapsulation plays a crucial role not only in preserving the viability of bifidobacteria but also in maintaining their surface structures, which may undergo alterations prior to reaching their target site of action. The bioactive molecules mediating interactions between the microorganism and the host predominantly reside on the bacterial cell surface[92]. Key components include exopolysaccharides (EPSs), cell wall polysaccharides, (lipo)teichoic acids (LTAs), glycolipids, peptidoglycan, and surface proteins. Variations in EPS structure influence the adhesion capacity of Bifidobacterium strains to intestinal epithelial cell lines, as well as modulating peripheral blood mononuclear cell proliferation and cytokine secretion in vitro[93]. Moreover, evidence suggests that neutral EPSs and those with high molecular weight tend to suppress pro-inflammatory cytokine production, whereas low molecular weight or acidic EPSs exhibit immunostimulatory effects[93]. Cell wall polysaccharides contribute to pathogen control by obstructing potential binding sites on the intestinal epithelium[94], enhance bacterial resilience under environmental stress, and promote biofilm formation within the intestine[95]. Recognition of LTAs is mediated via the TLR2/TLR6 heterodimer complex, which involves co-receptors CD14 and CD36[96]. The glycolipids on the surface of bifidobacteria are detected by host pattern recognition receptors TLR2, TLR1, and TLR6. Notably, TLR2 is pivotal for recognition of several bacterial surface antigens such as lipids, LTAs, and potentially peptidoglycan; however, the recognition of lipoproteins and lipopeptides requires TLR2 to form heterodimers with either TLR1 or TLR6[97,98]. The peptidoglycan of B. breve YY induces differentiation of naïve T cells toward Th1 and promotes dendritic cell maturation[99]. Furthermore, studies employing the RAW 264.7 macrophage cell line have revealed that cell wall extracts from bifidobacteria stimulate production of TNF-α, IL-6, and nitric oxide[100-102]. Among surface proteins, serpin and pilin are notable; serpin functions primarily by inhibiting host or microbial proteases, thereby enhancing the survival and colonization capacity of bifidobacteria within the GIT[103]. Effective colonization of the gut by bifidobacteria is also dependent on pili formation, mediated by pilin proteins, which facilitate bacterial adhesion to the mucosal surfaces of the host intestine[104].
Advances in encapsulation technologies, including spray drying, freeze drying, spray chilling, extrusion, and emulsion-based methods, offer modular platforms to adjust capsule size, release kinetics, and carrier composition, thereby tailoring probiotic delivery to specific production needs[105]. Strategic selection of wall materials such as polysaccharides (e.g., alginate, gum Arabic, etc.), proteins (e.g., whey, soy, etc.), and prebiotic blends (defined as a substrate that is selectively utilized by host microorganisms to confer a health benefit[106]; e.g., inulin conjugates, among others) further strengthens bacterial survival under aerobic storage and digestive stress, ultimately improving population levels at the site of action[107]. As such, microencapsulation stands as a cornerstone technology that bridges microbiological efficacy with logistical practicality, enabling the effective use of Bifidobacterium spp. as functional feed additives in poultry production. Selected recent reports on the microencapsulation of Bifidobacterium spp. are detailed in Table 2.
Selected recent reports on the microencapsulation of Bifidobacterium spp.
| Method | Strain | Encapsulating matrix | Encapsulation efficiency (%) | Particle size (µm) | Viability during storage | Resistance to gastrointestinal digestion | Key parameters | Reference |
| Spray drying | B. lactis B94 | Polyvinylpyrrolidone polymer and lactose | 99.14* | ND | Decrease from 9.18 ± 0.04 to 8.19 ± 0.02 log CFU/g (33 days at 25 ºC) | ND | IAT: NI; OAT: NI | [108] |
| Spray drying | B. animalis subsp. lactis BB-12 | Goat’s milk | 96.97* | ND | ND | ND | IAT: 150 ºC; OAT: 50 ± 3 ºC | [109] |
| Spray drying | B. animalis subsp. lactis BB-12 | Goat’s milk and inulin | 92.58* | ND | ND | ND | IAT: 150 ºC; OAT: 50 ± 3 ºC | [109] |
| Spray drying | B. animalis subsp. lactis BB-12 | Goat’s milk and oligofructose | 90.49* | ND | ND | ND | IAT: 150 ºC; OAT: 50 ± 3 ºC | [109] |
| Spray drying | B. animalis subsp. lactis BB-12 | Goat’s milk, inulin, and oligofructose | 86.12* | ND | ND | ND | IAT: 150 ºC; OAT: 50 ± 3 ºC | [109] |
| Spray drying | B. bifidum | Gum arabic and β-cyclodextrin | ND | ND | Decrease from 5.7 ± 0.2 to < 1.0 log CFU/g (90 days) | ND | IAT: 120 ºC; OAT: 50 ºC | [110] |
| Spray drying/spray chilling | B. bifidum | Gum arabic, β-cyclodextrin, hydrogenated palm oil, and Tween 80 | ND | ND | Decrease from 3.5 ± 0.2 to < 1.0 log CFU/g (90 days) | ND | IAT: 120 ºC; OAT: 50 ºC (spray drying) Feeding temperature: 45 ºC; Nozzle temperature: 38 ºC (spray chilling) | [110] |
| Freeze drying/nanoemulsion | B. bifidum NRRL B-41410 | Clay hydrophilic bentonite, whey protein concentrate, sodium alginate, and maltodextrin | 88.84 | ND | Increase from 7.33 to 8.27 log CFU/g (20 days at 7 ºC) | 69.23% survival (sequential exposure to gastric and intestinal juices) | Vacuum degree: NI | [111] |
| Freeze drying/nanocomposite | B. bifidum NRRL B-41410 | Clay hydrophilic bentonite, whey protein concentrate, sodium alginate, and maltodextrin | 98.49 | ND | Increase from 7.48 to 8.40 log CFU/g (20 days at 7 ºC) | 68.84% survival (sequential exposure to gastric and intestinal juices) | Vacuum degree: NI | [111] |
| Spray chilling | B. bifidum | Hydrogenated palm oil and Tween 80 | ND | ND | Decrease from 6.1 ± 0.1 to 2.4 ± 0.1 log CFU/g (90 days) | ND | Feeding temperature: 45 ºC; Nozzle temperature: 38 ºC (spray chilling) | [110] |
| Spray chilling/spray drying | B. bifidum | Hydrogenated palm oil, tween 80, gum arabic, β-cyclodextrin, and lecithin | ND | ND | Decrease from 3.6 ± 0.1 to 2.3 ± 0.2 log CFU/g (90 days) | ND | Feeding temperature: 45 ºC; Nozzle temperature: 38 ºC (spray chilling) IAT: 120 ºC; OAT: 50 ºC (spray drying) | [110] |
| Extrusion | B. lactis | Alginate, hydroxypropyl methyl cellulose, gellan gum, carboxymethyl chitosan with polyethylenimine | ND | ND | Decrease of 1.64 ± 0.17 log CFU/g (12 weeks at 4 ºC) Decrease of 2.91 ± 0.23 log CFU/g (12 weeks at 30 ºC) | Decrease of 0.59 ± 0.02 (exposure to simulated gastric fluid) and 1.19 ± 0.05 log CFU/g (exposure to simulated intestinal fluid) | Frequency: 300 Hz; Voltage: 500 V; Nozzle aperture: 750 µm | [90] |
| Extrusion | B. pseudocatenulatum G7 | Sodium alginate | ND | 462 | Decrease from ~8.06 to ~5.74 log CFU/g (4 weeks at 4 ºC)** | Decrease from ~9.18 to ~4.58 log CFU/g (exposure to simulated gastric fluid); no detectable after complete gastrointestinal digestion** | Frequency: 800 Hz; Voltage: 800 V; Nozzle aperture: 200 µm | [112] |
| Extrusion | B. pseudocatenulatum G7 | Sodium alginate and CaCO3 | ND | 520 | Decrease from ~8.73 to ~6.93 log CFU/g (4 weeks at 4 ºC)** | Decrease from ~6.44 to ~8.70 (exposure to simulated gastric fluid), and to ~5.60 log CFU/g (complete gastrointestinal digestion)** | Frequency: 800 Hz; Voltage: 800 V; Nozzle aperture: 200 µm | [112] |
| Extrusion | B. animalis subsp. animalis ATCC 25527 | Ciceritol and sodium alginate | 95.23 ± 0.13 | 1.41 ± 0.07 | Decrease from ~9.67 to ~8.78 (30 days at 4 ºC)** | Decrease from ~9.67 to ~7.17 log CFU/g (gastric digestion)** | NI | [113] |
| Extrusion | B. infantis ATCC 15697 | Whey protein isolate and sodium alginate | 86.15 ± 2.51 | 1.27 ± 0.12 | Decrease from 9.02 ± 0.11 to 7.21 ± 0.04 log CFU/g (28 days at 4 ºC) | 33.2% survival (gastric digestion) | Frequency: 800 Hz; Voltage: 800 V; Nozzle aperture: 450 µm | [114] |
| Extrusion | B. infantis ATCC 15697 | Pea protein isolate and sodium alginate | 90.59 ± 0.46 | 1.55 ± 0.06 | Decrease from 9.81 ± 0.04 to 8.23 ± 0.09 log CFU/g (28 days at 4 ºC) | 40.2% survival (gastric digestion) 84.2% survival (intestinal digestion) | Frequency: 800 Hz; Voltage: 800 V; Nozzle aperture: 450 µm | [114] |
| Extrusion | B. infantis ATCC 15697 | Whey protein isolate, pea protein isolate, and sodium alginate | 94.09 ± 1.76 | 1.62 ± 0.14 | Decrease from 9.89 ± 0.07 to 8.68 ± 0.13 log CFU/g (28 days at 4 ºC) | 45.4% survival (gastric digestion) 89.4% survival (intestinal digestion) | Frequency: 800 Hz; Voltage: 800 V; Nozzle aperture: 450 µm | [114] |
| Nanoemulsion/extrusion | B. pseudocatenulatum G7 | Sodium alginate and CaCO3 | ND | 729 | Decrease from ~8.40 to ~4.51 log CFU/g (4 weeks at 4 ºC)** | Decrease from ~9.32 to ~8.70 (exposure to simulated gastric fluid), and to ~6.60 log CFU/g (complete gastrointestinal digestion)** | Frequency: 800 Hz; Voltage: 800 V; Nozzle aperture: 200 µm | [112] |
| Emulsion | B. bifidum F-35 | Whey protein and sodium alginate | ND | 280 | Decrease from 10.73 to 9.36 log CFU/g (14 days at 4 ºC) | ND | Organic phase: soybean oil; Cross-linking agent: 10 U of TGase/g | [115] |
| Emulsion | B. animalis BB-12 | Sodium alginate | ~96** | ~0.8** | Decrease from ~7.87 to ~7.00 log CFU/g (30 days at 4 ºC)** | Decrease from ~9.57 to ~8.50/~8.40 (60/120 min in gastric juice), and to ~8.05/~7.58 log CFU/g (50/150 min in intestinal juice)** | Organic phase: raspberry oil; Cross-linking agent: 0.1 M CaCl2 | [116] |
| Emulsion | B. animalis BB-12 | Pectin | ~95** | ~2.7** | Decrease from ~7.78 to ~7.47 log CFU/g (30 days at 4 ºC)** | Decrease from ~9.57 to ~8.41/~8.22 (60/120 min in gastric juice), and to ~7.78/~7.00 log CFU/g (50/150 min in intestinal juice)** | Organic phase: raspberry oil; Cross-linking agent: 0.1 M CaCl2 | [116] |
| Emulsion | B. bifidum, B. breve, and B. longum | Sodium alginate and chitosan | 40.21 ± 3.18 | 10 to 15 | ND | 78.28 ± 7.55% survival (sequential exposure to gastric and intestinal juices) | Organic phase: olive oil; Cross-linking agent: 0.2 M CaCl2 | [117] |
| Emulsion | B. bifidum R0071 | Pectin | ND | ND | ND | Decrease from 8.78 ± 0.05 to 8.78 ± 0.06/8.60 ± 0.13 log CFU/g (20/60 min of exposure to gastric juice) | Organic phase: soybean oil; Cross-linking agent: 0.05 M CaCl2.2H2O | [118] |
| Emulsion | B. bifidum R0071 | Sodium alginate | ND | ND | ND | Decrease from 8.74 ± 0.06 to 8.67 ± 0.22/8.53 ± 0.04 log CFU/g (20/60 min of exposure to gastric juice) | Organic phase: soybean oil; Cross-linking agent: 0.05 M CaCl2.2H2O | [118] |
| Emulsion | B. bifidum R0071 | Pectin and osteopontin | ND | ND | ND | Decrease from 8.77 ± 0.02 to 8.79 ± 0.02/8.74 ± 0.08 log CFU/g (20/60 min of exposure to gastric juice) | Organic phase: soybean oil; Cross-linking agent: 0.05 M CaCl2.2H2O | [118] |
| Emulsion | B. bifidum R0071 | Sodium alginate and osteopontin | ND | ND | ND | Decrease from 8.64 ± 0.02 to 8.57 ± 0.06/8.53 ± 0.01 log CFU/g (20/60 min of exposure to gastric juice) | Organic phase: soybean oil; Cross-linking agent: 0.05 M CaCl2.2H2O | [118] |
| Emulsion | B. animalis F1-7 | Sodium alginate | 90.67 ± 1.45 | 297.46 | 80.43% survival (28 days at 4 ºC) | 74.67% survival (gastric digestion) | Organic phase: soybean oil; Cross-linking agent: 0.05-0.8 M CaCl2 | [119] |
| Emulsion | B. animalis F1-7 | Sodium alginate and human milk oligosaccharides | 92.19 ± 1.80 | 308.07 | 89.50% survival (28 days at 4 ºC) | 86.97% survival (gastric digestion) | Organic phase: soybean oil; Cross-linking agent: 0.05-0.8 M CaCl2 | [119] |
| Fluid bed | B. animalis subsp. lactis NH019 | Lactose, stearic acid, sodium alginate, hydroxypropyl cellulose, and microcrystalline cellulose | 94.86* | 50 to 300 | ND | Decrease of 0.2/0.58 CFU/g (45/60 min in gastric juice) | First coating: 5% (w/w) stearic acid; second coating: 2% (w/w) sodium alginate; Third coating: 5% (w/w) hydroxypropyl cellulose and microcrystalline cellulose | [120] |
Spray drying
Spray drying is a widely adopted microencapsulation technique with high throughput and cost-effectiveness[121]. Fundamentally, the process consists of three stages: atomization of the feed into microscale droplets, rapid moisture removal through exposure to hot drying air, and powder collection via cyclones or separators[122]. The high temperatures and air exposure during spray drying impose thermal and oxidative stress on microorganisms. Studies have shown that while spray drying of B. bifidum PTCC 1644 can achieve survival rates above 28% with optimized inlet/outlet temperatures and protective excipients, much of the population is typically lost due to heat and oxygen exposure[123]. Research highlights that oxygen-sensitive strains may incur membrane and DNA damage when exposed to hot, aerated environments unless specialized formulations are used[79,124]. However, recent advances, such as electrostatic spray drying, have demonstrated improved encapsulation efficiencies (over 93%) and therefore survival rates to the drying step, in other Bifidobacterium species (i.e., B. lactis BL03 and BAL005, B. bifidum BB30, B. longum BLL2, and
From an industrialization perspective, spray drying of Bifidobacterium spp. offers substantial advantages in terms of scalability and cost-efficiency compared to other drying methodologies. The continuous nature of spray drying enables high-throughput production, reducing labor and energy costs per unit of product, which is critical for commercial viability[129]. However, the upfront capital investment for spray drying equipment and the optimization of process parameters can vary depending on strain-specific requirements and formulation complexity, influencing production costs. Economically, studies estimate production costs for spray-dried probiotic powders to be significantly lower than freeze-dried counterparts[130]. Furthermore, product stability during downstream feed processing is crucial for ensuring the practical application value of spray-dried Bifidobacterium spp. in animal nutrition. Stability tests to 60-100 ºC for different times, conditions that may be present during feed extrusion and pelleting, have shown that appropriately formulated spray-dried powders retain over 70% viability[131]. Additionally, the use of protective excipients and post-process coating can further enhance survival rates during storage and incorporation into feed matrices[132]. These findings underscore the feasibility of integrating spray-dried Bifidobacterium spp. powders into commercial feed production pipelines while maintaining functional probiotic properties. Nevertheless, continuous monitoring and strain-specific optimization remain essential to maximize industrial process efficiency and product stability.
Freeze drying
Freeze drying, also known as lyophilization, is widely regarded as the gold-standard preservation method for probiotic cultures, particularly for oxygen-sensitive Bifidobacterium spp. The process preserves cell viability by avoiding the thermal stress characteristic of other drying methods such as spray drying. Freeze drying involves three key phases: rapid freezing, sublimation, and desorption, which collectively remove water under vacuum at low temperatures (< -40 ºC)[133]. Critical to its success is the rapid formation of small, uniform ice crystals during freezing, which minimizes mechanical damage to cell membranes. At the same time, the vacuum conditions protect cells from oxidative damage. The inclusion of cryoprotectants, such as sorbitol, sucrose, trehalose, skim milk, and glycerol, further enhances survival by stabilizing membrane structures and intracellular proteins through hydrogen bonding and vitrification during drying[134]. For instance, B. crudilactis freeze-dried with sorbitol achieved over 80% initial viability and retained acceptable counts after six months at 4 ºC[134]. Similarly, B. longum subsp. longum Reuter 1963 subjected to optimized freeze drying protocols with trehalose supplementation maintained over 50% viability during storage at 4 ºC with maltodextrin carriers, and cell integrity was largely preserved[135]. Moreover, freeze-dried formulations have demonstrated exceptional resistance to simulated gastrointestinal conditions, maintaining over 90% viability after exposure to gastric fluid and bile salts[136].
Despite the superior viability retention offered by freeze drying, its industrial-scale application for Bifidobacterium spp. preservation presents challenges primarily related to production costs and process complexity. Freeze drying is inherently resource- and energy-intensive due to the requirement for ultra-low temperatures and extended processing times under vacuum conditions, which translates into higher operational costs compared to other drying methods like spray drying[137]. However, recent advances in process optimization and cryoprotectant use, such as trehalose and sorbitol, have enabled more cost-efficient protocols and improved shelf life, thereby reducing losses during storage and transport[134,135]. Moreover, economic feasibility studies indicate that although freeze drying has a higher upfront cost, the longer shelf life and improved viability reduce the need for over-formulation, which can offset initial expense in commercial feed applications[138]. Furthermore, freeze-dried Bifidobacterium formulations are resilient to typical feed manufacturing stresses, such as pelleting and extrusion, provided appropriate protective matrices are included. Oral administration, via incorporation into feed or drinking water, is the most practical and effective route for delivering freeze-dried Bifidobacterium spp. in poultry.
Spray chilling
Spray chilling (also known as spray cooling) is a lipid-based microencapsulation technique that solidifies molten fat, such as cocoa butter or hydrogenated oils, around probiotic cells by atomizing the mixture into a cooled chamber, thereby forming solid microparticles without applying thermal stress[139]. This process is particularly advantageous for oxygen-sensitive Bifidobacterium spp., such as B. animalis subsp. lactis, as it avoids the heat and oxidative exposure associated with spray drying, preserving higher viability both immediately after production (higher than 80%-90%) and during refrigerated storage (higher than 50% after 120 days at 4 ºC)[140]. Lipid matrices protect anaerobic probiotics by creating an oxygen-impermeable barrier; upon ingestion, these matrices are degraded by digestive lipases, facilitating the gradual release of viable cells into the gut[141]. For example, single- and double-layered capsules containing B. bifidum BB-12, obtained by using hydrogenated palm oil, presented an encapsulation efficiency of 92% and survival rates of 88% to 75% during gastric and intestinal digestion, respectively[142].
From an industrial perspective, spray chilling is recognized as one of the most cost-effective microencapsulation methods for large-scale production[143,144]. It employs relatively inexpensive, widely available lipid materials and simple cooling systems that require less energy compared to many drying methods[145]. The process supports continuous production lines with relatively short processing times, which further reduces operational costs[145,146]. In practical feed applications, spray-chilled Bifidobacterium spp. microcapsules have demonstrated stability against mechanical and thermal stresses typical of feed processing techniques such as pelleting and extrusion. The lipid coating effectively protects the probiotic cells, maintaining significant viability after exposure to such conditions[147]. Additionally, these microcapsules retain their functional properties during storage in feed matrices for extended periods, supporting shelf-life requirements necessary for commercial use[145]. These findings confirm that spray chilling is a promising method to produce probiotic feed additives compatible with existing industrial feed production workflows. Nonetheless, challenges remain in optimizing encapsulation parameters, such as lipid composition and particle size, to improve release mechanisms and maximize cost-efficiency. Regulatory considerations for lipid excipients in animal nutrition also need to be addressed to facilitate industrial adoption[143].
It is also noteworthy that while spray-chilled Bifidobacterium spp. are well-suited for oral delivery through feed or water, the lipid-coated particles are typically too large and may not be compatible with precise injection tools used for in ovo delivery[73,147]. In conclusion, spray chilling represents a promising, scalable, and economically viable method for formulating anaerobic probiotics like Bifidobacterium spp. for poultry use. Continued research and scale-up trials are essential to optimize microencapsulation parameters and fully unlock its industrial and practical application potential.
Extrusion
Extrusion encapsulation involves forcing a mixture of microbial cells and hydrophilic polymers, commonly sodium alginate alone or in combination with proteinaceous materials, through a fine nozzle into a Ca2+ solution in the form of droplets, inducing rapid gelation and formation of spherical microbeads[148]. This gentle, aqueous-based method is particularly advantageous for strictly anaerobic bacteria such as Bifidobacterium spp., as it avoids heat and oxygen exposure during processing[148,149]. In studies with
From an industrial perspective, extrusion microencapsulation is recognized as a cost-effective, scalable, and straightforward method for probiotic microencapsulation. It utilizes readily available, inexpensive materials, such as sodium alginate and Ca2+ salts, and employs simple equipment like nozzles and curing baths. This results in comparatively low capital investment and operational costs relative to drying or spray-coating techniques[152]. The aqueous and mild processing conditions also reduce energy consumption and eliminate the use of harmful solvents, enhancing cost efficiency and suitability for large-scale production[88].
Extrusion-encapsulated Bifidobacterium microbeads demonstrate substantial resilience to temperatures of 60-100 ºC, typical of feed processing, maintaining viability above 70%[131]. These microcapsules sustain probiotic functionality and viable counts over several weeks at ambient temperature, supporting satisfactory shelf life for practical commercial use[131]. Despite these advantages, further optimization is required to balance bead size, polymer composition, and processing parameters to fine-tune probiotic release profiles, and improve feed palatability with diverse feed formulations. Additionally, regulatory approval of feed-grade biopolymers remains an important step for broader industrial adoption.
In summary, extrusion microencapsulation combines physiological compatibility with industrial feasibility to maintain viability and deliver functional anaerobic Bifidobacterium spp. effectively in poultry feed. Ongoing research and scale-up validation will be essential to fully realize the practical and commercial benefits of this technology in the animal feed industry.
Emulsion
Emulsion encapsulation involves dispersing probiotic-laden aqueous phases into an immiscible oil phase (typically vegetable oil), forming water-in-oil droplets which are stabilized by emulsifiers and subsequently gelled or solidified to create microcapsules[153]. Given the inherent thermodynamic instability of conventional emulsions, advanced emulsion techniques, such as nanoemulsions, Pickering emulsions, and Pickering high internal phase emulsions, have been developed to enhance the encapsulation efficiency of probiotics[154]. Nanoemulsions are comparatively stable systems characterized by droplet sizes typically smaller than 100 nm[155]. In contrast, Pickering emulsions achieve stabilization without the use of traditional emulsifiers; instead, they are stabilized by solid particles, with hydrophobic particles demonstrating greater effectiveness[156]. Pickering high internal phase emulsions represent a subset of Pickering emulsions that contain a high fraction of the internal oil phase. By minimizing the exposure of probiotics to water and oxygen, these high internal phase systems exhibit superior encapsulation efficiency and hold significant potential as probiotic delivery vehicles[157]. Emulsion encapsulation is especially appropriate for anaerobic bifidobacteria because it enables encapsulation entirely in oxygen-free aqueous environments before emulsification. Moreover, anoxic regions created in the center of the beads protect cells from oxidative damage[158]. For instance, B. bifidum F-35 was subsequently encapsulated with whey protein and sodium alginate to obtain 280 µm double-layered beads. The microencapsulated bacteria retained significantly higher viability than free cells after 2 weeks at 4 ºC [Table 2], indicating effective protection by the double-layer barrier[115]. Similarly, B. animalis BB-12 encapsulated within pectin or sodium alginate droplets stabilized by Ca2+ and emulsified in rapeseed oil retained over 107 CFU/g of viable cells after 30 days at 4 ºC, and exhibited higher resistance to gastrointestinal conditions than free cells[116]. In another study, B. bifidum encapsulated by the emulsion technique within resistant starch beads showed better survivability than free and freeze-dried cells after 3-month storage. Moreover, emulsion-encapsulated bacteria presented better resistance to gastrointestinal conditions than free cells[159].
Emulsion parameters, such as water-to-oil ratio, stirring speed, emulsifier type (e.g., lecithin or Tween 80), and droplet size, can be precisely tuned to control bead diameter and release characteristics, facilitating targeted delivery within the host GIT[160]. Moreover, the method’s gentle processing, absence of heat, and aqueous formulation render it cost-effective and easily scalable[161].
Recent research highlights the suitability of emulsion microencapsulation for industrial-scale production due to its relative simplicity, scalability, and compatibility with continuous or semi-continuous processing, compared to other microencapsulation techniques[154,162]. For instance, millifluidic-assisted and emulsification-based systems can achieve high encapsulation efficiencies (up to 98%), producing microcapsules with optimal size and sphericity for food and feed applications[162]. Moreover, employing food-grade polysaccharides and vegetable oils is economically favorable, utilizing inexpensive and readily accessible materials that can be seamlessly incorporated into current production processes[154].
From a stability perspective, emulsion-encapsulated Bifidobacterium spp. maintain high viability under feed processing conditions. The use of double-layer encapsulating materials, such as chitosan and sodium alginate, better preserves probiotic viability during storage, ensuring sufficient viable counts for probiotic efficacy at the moment of administration[162].
However, the choice of oil phase and emulsifiers must be compatible with feed formulations and downstream processing, as residual oil may affect palatability or regulatory compliance in feed additives. Therefore, emulsion microencapsulation presents a promising commercial method, provided that process parameters and encapsulating agents are optimized for specific feed matrices and regulatory requirements.
CONCLUSION
In an era marked by growing restrictions on antibiotic use and increasing demand for sustainable poultry production, Bifidobacterium spp. have emerged as promising probiotic candidates capable of enhancing gut health, immunity, and overall performance in poultry. Despite their proven functional benefits, including modulation of the gut microbiota, improvement of intestinal architecture, regulation of immune responses, and inhibition of pathogens, their widespread application in the poultry industry remains limited due to their strict anaerobic nature and sensitivity to environmental stressors. To overcome these challenges, recent advances in administration strategies and microencapsulation technologies have opened new avenues for the practical integration of bifidobacteria into poultry systems. Routes such as in ovo injection and cloacal delivery offer high colonization efficiency, while oral and spray-based applications provide scalable and operationally feasible alternatives. Crucially, the use of microencapsulation techniques, ranging from extrusion and emulsion to spray chilling and freeze drying, enhances the survival, stability, and functional performance of bifidobacteria, enabling their deployment even under suboptimal conditions.
Moving forward, the strategic combination of strain selection, encapsulation optimization, and targeted delivery routes holds great potential for fully realizing the benefits of Bifidobacterium spp. in poultry health and productivity. Future research should focus on field-scale validations, long-term colonization dynamics, and host-microbe interactions under commercial settings to support the rational design of next-generation probiotic formulations. By aligning biotechnological innovation with practical application, bifidobacteria-based probiotics can become integral components of antibiotic-free and performance-driven poultry production systems.
DECLARATIONS
Acknowledgments
The authors are grateful to Lic. Mabel Taljuk for her help with the bibliography search.
Authors’ contributions
Data acquisition: Argañaraz-Martínez E, Apella MC, Perez Chaia A, Babot JD
Data analysis: Argañaraz-Martínez E, Apella MC, Perez Chaia A, Babot JD
Manuscript drafting: Argañaraz-Martínez E, Apella MC, Perez Chaia A, Babot JD
Manuscript revision: Babot JD
Final approval of the manuscript: Argañaraz-Martínez E, Apella MC, Perez Chaia A, Babot JD
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (No. PIBAA2022-2023 0766 and No. PIP2023 0535) and Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (No. PICT2021 0763).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Corrêa-Junior D, Parente CET, Frases S. Hazards associated with the combined application of fungicides and poultry litter in agricultural areas. J Xenobiot. 2024;14:110-34.
2. Fonseca A, Kenney S, Van Syoc E, et al. Investigating antibiotic free feed additives for growth promotion in poultry: effects on performance and microbiota. Poult Sci. 2024;103:103604.
3. Wickramasuriya SS, Ault J, Ritchie S, Gay CG, Lillehoj HS. Alternatives to antibiotic growth promoters for poultry: a bibliometric analysis of the research journals. Poult Sci. 2024;103:103987.
4. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-14.
5. Gioia D, Aloisio I, Mazzola G, Biavati B. Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol. 2014;98:563-77.
6. Abd El-Hack ME, El-Saadony MT, Shafi ME, et al. Probiotics in poultry feed: a comprehensive review. J Anim Physiol Anim Nutr. 2020;104:1835-50.
7. Grande SMM, Argañaraz Martı Nez E, Babot JD, et al. The species and physiological diversity of Bifidobacterium genus in Gallus gallus domesticus are influenced by feeding model and niche adaptations. Benef Microbes. 2024;15:19-38.
8. Lee JH, O’Sullivan DJ. Genomic insights into bifidobacteria. Microbiol Mol Biol Rev. 2010;74:378-416.
9. Sharma M, Wasan A, Sharma RK. Recent developments in probiotics: an emphasis on Bifidobacterium. Food Bioscience. 2021;41:100993.
10. Allende A, Alvarez-Ordóñez A, Bortolaia V, et al; EFSA Panel on Biological Hazards (BIOHAZ). Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 22: Suitability of taxonomic units notified to EFSA until March 2025. EFSA J. 2025;23:e9510.
11. Tripathy A, Dash J, Kancharla S, et al. Probiotics: a promising candidate for management of colorectal cancer. Cancers. 2021;13:3178.
12. Abou-Kassem DE, Elsadek MF, Abdel-Moneim AE, et al. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum). Poult Sci. 2021;100:84-93.
13. Feng Y, Wu X, Hu D, Wang C, Chen Q, Ni Y. Comparison of the effects of feeding compound probiotics and antibiotics on growth performance, gut microbiota, and small intestine morphology in yellow-feather broilers. Microorganisms. 2023;11:2308.
14. Galosi L, Desantis S, Roncarati A, et al. Positive influence of a probiotic mixture on the intestinal morphology and microbiota of farmed guinea fowls (Numida meleagris). Front Vet Sci. 2021;8:743899.
15. Lokapirnasari WP, Pribadi TB, Arif AA, et al. Potency of probiotics Bifidobacterium spp. and Lactobacillus casei to improve growth performance and business analysis in organic laying hens. Vet World. 2019;12:860-7.
16. Mnisi CM, Njeri FM, Maina AN, et al. A review on the potential use of eubiotics in non-chicken poultry species. Trop Anim Health Prod. 2025;57:4466.
17. Idowu PA, Mpofu TJ, Magoro AM, Modiba MC, Nephawe KA, Mtileni B. Impact of probiotics on chicken gut microbiota, immunity, behavior, and productive performance-a systematic review. Front Anim Sci. 2025;6:1562527.
18. Obianwuna UE, Agbai Kalu N, Wang J, et al. Recent trends on mitigative effect of probiotics on oxidative-stress-induced gut dysfunction in broilers under necrotic enteritis challenge: a review. Antioxidants. 2023;12:911.
19. Chen J, Chen X, Ho CL. Recent development of probiotic Bifidobacteria for treating human diseases. Front Bioeng Biotechnol. 2021;9:770248.
20. He BL, Xiong Y, Hu TG, Zong MH, Wu H.
21. Zhang X, Cao J, Han S, et al. Bacillus subtilis: applications in the livestock and poultry industry in recent years. Anim Biosci. 2025;Epub ahead of print.
22. Pang Y, Zhang H, Wen H, et al. Yeast probiotic and yeast products in enhancing livestock feeds utilization and performance: an overview. J Fungi. 2022;8:1191.
23. Sirisopapong M, Shimosato T, Okrathok S, Khempaka S. Assessment of lactic acid bacteria isolated from the chicken digestive tract for potential use as poultry probiotics. Anim Biosci. 2023;36:1209-20.
24. Jurić M, Goksen G, Donsì F, Jurić S. Innovative applications of electrospun nanofibers loaded with bacterial cells towards sustainable agri-food systems and regulatory compliance. Food Eng Rev. 2024;16:270-303.
25. Gutiérrez-álzate K, Beltrán-cotta LA, dos Santos Rekowsky BS, Cavalheiro CP, Pereira da Costa M. Micro- and nanoencapsulation of probiotics: exploring their impact on animal-origin foods. ACS Food Sci Technol. 2024;4:2799-812.
26. Dev K, Begum J, Biswas A, et al. Hepatic transcriptome analysis reveals altered lipid metabolism and consequent health indices in chicken supplemented with dietary Bifidobacterium bifidum and mannan-oligosaccharides. Sci Rep. 2021;11:17895.
27. Liu M, Uyanga VA, Cao X, Liu X, Lin H. Regulatory effects of the probiotic Clostridium butyricum on gut microbes, intestinal health, and growth performance of chickens. J Poult Sci. 2023;60:2023011.
28. Dixon B, Kilonzo-Nthenge A, Nzomo M, Bhogoju S, Nahashon S. Evaluation of selected bacteria and yeast for probiotic potential in poultry production. Microorganisms. 2022;10:676.
29. Igbafe J, Kilonzo-nthenge A, Nahashon SN, Mafiz AI, Nzomo M. Probiotics and antimicrobial effect of Lactiplantibacillus plantarum, Saccharomyces cerevisiae, and Bifidobacterium longum against common foodborne pathogens in poultry. Agriculture. 2020;10:368.
30. Kathayat D, Closs G Jr, Helmy YA, Deblais L, Srivastava V, Rajashekara G. In vitro and in vivo evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 against avian pathogenic escherichia coli and identification of novel probiotic-derived bioactive peptides. Probiotics Antimicrob Proteins. 2022;14:1012-28.
31. Wang W, Dang G, Hao W, et al. Dietary supplementation of compound probiotics improves intestinal health by modulated microbiota and its SCFA products as alternatives to in-feed antibiotics. Probiotics Antimicrob Proteins. 2024;Epub ahead of print.
32. Dittoe DK, Olson EG, Ricke SC. Impact of the gastrointestinal microbiome and fermentation metabolites on broiler performance. Poult Sci. 2022;101:101786.
33. Liu X, Ma Z, Wang Y, Li L, Jia H, Zhang L. Compound probiotics can improve intestinal health by affecting the gut microbiota of broilers. J Anim Sci. 2023;101:skad388.
34. Naeem M, Bourassa D. Probiotics in poultry: unlocking productivity through microbiome modulation and gut health. Microorganisms. 2025;13:257.
35. Babot JD, Argañaraz-Martínez E, Quiroga M, Grande SM, Apella MC, Perez Chaia A. Protection of the intestinal epithelium of poultry against deleterious effects of dietary lectins by a multi-strain bacterial supplement. Res Vet Sci. 2021;135:27-35.
36. Yang L, Chen Y, Bai Q, et al. Protective effect of Bifidobacterium lactis JYBR-190 on intestinal mucosal damage in chicks infected with Salmonella pullorum. Front Vet Sci. 2022;9:879805.
37. Hu D, Wu X, Song P, et al. Dietary supplementation with multi-strain probiotic formulation (Bifidobacterium B8101, Lactobacillus L8603, Saccharomyces bayanus S9308, and Enterococcus SF9301), betaine or their combination promotes growth performance via improving intestinal development in broilers. Probiotics Antimicrob Proteins. 2024;Epub ahead of print.
38. Wu Y, Yang F, Jiang W, et al. Effects of compound probiotics on intestinal barrier function and caecum microbiota composition of broilers. Avian Pathol. 2022;51:465-75.
39. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-50.
40. Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858-76.
41. Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific opinion on the safety and efficacy of biomin C3 (Enterococcus faecium, Bifidobacterium animalis and Lactobacillus salivarius) for chickens for fattening. EFS2. 2012;10:2965.
42. Dev K, Akbar Mir N, Biswas A, Kannoujia J, Begum J, Kant R. Dietary Mannan-oligosaccharides potentiate the beneficial effects of Bifidobacterium bifidum in broiler chicken. Lett Appl Microbiol. 2020;71:520-30.
43. Agustono B, Warsito SH, Yunita MN, et al. Influence of microbiota inoculum as a substitute for antibiotic growth promoter during the initial laying phase on productivity performance, egg quality, and the morphology of reproductive organs in laying hens. Vet World. 2023;16:1461-7.
44. Nour MA, El-Hindawy MM, Qattan SYA, et al. Effect of graded levels of dietary Bacillus toyonensis and Bifidobacterium bifidum supplementation on growth, carcass traits and ileal histomorphometry and microbiota of growing quails. Saudi J Biol Sci. 2021;28:4532-41.
45. Abdel-Moneim AE, Elbaz AM, Khidr RE, Badri FB. Effect of in ovo inoculation of Bifidobacterium spp. on growth performance, thyroid activity, ileum histomorphometry, and microbial enumeration of broilers. Probiotics Antimicrob Proteins. 2020;12:873-82.
46. El-Moneim AEEA, El-Wardany I, Abu-Taleb AM, Wakwak MM, Ebeid TA, Saleh AA. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob Proteins. 2020;12:439-50.
47. El-Sharkawy H, Tahoun A, Rizk AM, et al. Evaluation of Bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens. Animals. 2020;10:1023.
48. Khan A, Kango N, Srivastava R. Impact of dietary probiotics on the immune and reproductive physiology of pubertal male Japanese quail (Coturnix coturnix japonica) administered at the onset of pre-puberty. Probiotics Antimicrob Proteins. 2025;17:1399-417.
49. Paul SS, Chatterjee RN, Raju MVLN, et al. Gut microbial composition differs extensively among indian native chicken breeds originated in different geographical locations and a commercial broiler line, but breed-specific, as well as across-breed core microbiomes, are found. Microorganisms. 2021;9:391.
50. Nour MA, El-Hindawy MM, Abou-Kassem DE, et al. Productive performance, fertility and hatchability, blood indices and gut microbial load in laying quails as affected by two types of probiotic bacteria. Saudi J Biol Sci. 2021;28:6544-55.
51. Zou Y, Liang N, Zhang X, Han C, Nan X. Functional differentiation related to decomposing complex carbohydrates of intestinal microbes between two wild zokor species based on 16SrRNA sequences. BMC Vet Res. 2021;17:216.
52. Zuo ZH, Shang BJ, Shao YC, Li WY, Sun JS. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019;86:160-8.
53. Tsvetikova SA, Koshel EI. Microbiota and cancer: host cellular mechanisms activated by gut microbial metabolites. Int J Med Microbiol. 2020;310:151425.
54. Li Q, Wan G, Peng C, et al. Effect of probiotic supplementation on growth performance, intestinal morphology, barrier integrity, and inflammatory response in broilers subjected to cyclic heat stress. Anim Sci J. 2020;91:e13433.
55. van der Klein SAS, Arora SS, Haldar S, Dhara AK, Gibbs K. A dual strain probiotic administered via the waterline beneficially modulates the ileal and cecal microbiome, sIgA and acute phase protein levels, and growth performance of broilers during a dysbacteriosis challenge. Poult Sci. 2024;103:104462.
56. Rowland MC, Teague KD, Forga AJ, et al. Evaluation of the effect of in ovo applied bifidobacteria and lactic acid bacteria on enteric colonization by hatchery-associated opportunistic pathogens and early performance in broiler chickens. Poultry. 2025;4:15.
57. Stęczny K, Kokoszyński D. Effect of probiotic preparations (EM) on productive characteristics, carcass composition, and microbial contamination in a commercial broiler chicken farm. Anim Biotechnol. 2021;32:758-65.
58. Brugaletta G, De Cesare A, Zampiga M, et al. Effects of alternative administration programs of a synbiotic supplement on broiler performance, foot pad dermatitis, caecal microbiota, and blood metabolites. Animals. 2020;10:522.
59. Chen M, Stern NJ, Bailey JS, Cox NA. Administering mucosal competitive exclusion flora for control of salmonellae. J Appl Poult Res. 1998;7:384-91.
60. Yaqoob MU, Wang G, Wang M. An updated review on probiotics as an alternative of antibiotics in poultry - a review. Anim Biosci. 2022;35:1109-20.
61. Kabir SL. Dietary probiotics in poultry: a game-changer for growth, immunity, and microbiota balance. Asian J Med Biol Res. 2025;11:1-4.
62. Drauch V, Ghanbari M, Reisinger N, Mohnl M, Hess C, Hess M. Differential effects of synbiotic delivery route (feed, water, combined) in broilers challenged with Salmonella infantis. Poult Sci. 2025;104:104890.
63. Willemsen H, Debonne M, Swennen Q, et al. Delay in feed access and spread of hatch: importance of early nutrition. World's Poult Sci J. 2010;66:177-88.
64. Halder N, Sunder J, De AK, Bhattacharya D, Joardar SN. Probiotics in poultry: a comprehensive review. JoBAZ. 2024;85:379.
65. Ren Y, Muyyarikkandy MS, Gao M, et al. Sustained in-ovo and in-feed probiotic supplementation promotes embryo development and post-hatch performance in broilers. Poult Sci. 2025;104:105395.
66. Kiarie EG, Mills A. Role of feed processing on gut health and function in pigs and poultry: conundrum of optimal particle size and hydrothermal regimens. Front Vet Sci. 2019;6:19.
67. Gyawali I. A review on enhancing gut health in poultry: probiotic stability, stress management, and encapsulation strategies. Poult Sci J. 2024;12:145.
68. Siwek M, Slawinska A, Stadnicka K, Bogucka J, Dunislawska A, Bednarczyk M. Prebiotics and synbiotics - in ovo delivery for improved lifespan condition in chicken. BMC Vet Res. 2018;14:402.
69. Wishna-Kadawarage RN, Połtowicz K, Dankowiakowska A, Hickey RM, Siwek M. Prophybiotics for in-ovo stimulation; validation of effects on gut health and production of broiler chickens. Poult Sci. 2024;103:103512.
70. Muyyarikkandy MS, Mathew E, Kuttappan D, Amalaradjou MA. Research Note: in ovo and in-feed probiotic supplementation improves layer embryo and pullet growth. Poult Sci. 2023;102:103092.
71. Abdulqader AF, Aygun A, Maman AH, Olgun O. The effect of in-ovo injection of Lactobacilla Rhamnosus on hatching traits and growth parameters of quails. Selcuk J Agr Food Sci. 2018;32:174-8.
72. Gao M, Ren Y, Lu S, Reddyvari R, Venkitanarayanan K, Amalaradjou MA. In ovo probiotic supplementation supports hatchability and improves hatchling quality in broilers. Poult Sci. 2024;103:103624.
73. Triplett MD, Zhai W, Peebles ED, McDaniel CD, Kiess AS. Investigating commercial in ovo technology as a strategy for introducing probiotic bacteria to broiler embryos. Poult Sci. 2018;97:658-66.
74. White MB. In ovo and feed application of probiotics or synbiotics and response of broiler chicks to post-hatch necrotic enteritis. Available from: https://vtechworks.lib.vt.edu/items/9b83a740-d64f-4b86-894c-7600f7ee6f56. [Last accessed on 20 Aug 2025].
75. Villumsen KR, Sandvang D, Vestergård G, et al. Effects of a novel, non-invasive pre-hatch application of probiotic for broilers on development of cecum microbiota and production performance. Anim Microbiome. 2023;5:41.
76. Kayal A, Yu SJ, Van TTH, Bajagai YS, Stanley D, Barekatain R. Effect of early gut microbiota intervention using pre-designed poultry microbiota substitute on broiler health and performance. Anim Prod Sci. 2025;65:AN24354.
77. Olnood CG, Beski SSM, Iji PA, Choct M. Delivery routes for probiotics: effects on broiler performance, intestinal morphology and gut microflora. Anim Nutr. 2015;1:192-202.
78. Hernandez-Patlan D, Solis-Cruz B, Hargis BM, Tellez G. The use of probiotics in poultry production for the control of bacterial infections and aflatoxins. In: Franco-Robles E, Ramírez Emiliano J, Editors. Prebiotics and probiotics - potential benefits in nutrition and health. London: IntechOpen; 2019. pp. 1-21.
79. Simpson PJ, Stanton C, Fitzgerald GF, Ross RP. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J Appl Microbiol. 2005;99:493-501.
80. Arsi K, Donoghue AM, Woo-Ming A, Blore PJ, Donoghue DJ. Intracloacal inoculation, an effective screening method for determining the efficacy of probiotic bacterial isolates against Campylobacter colonization in broiler chickens. J Food Prot. 2015;78:209-13.
81. Keerqin C, Morgan N, Wu S, Svihus B, Choct M. Reintroduction of microflora from necrotic enteritis-resistant chickens reduces gross lesions and improves performance of necrotic enteritis-challenged broilers. J Appl Poult Res. 2017;26:449-57.
82. Alqazlan N, Astill J, Taha-Abdelaziz K, Nagy É, Bridle B, Sharif S. Probiotic Lactobacilli enhance immunogenicity of an inactivated H9N2 influenza virus vaccine in chickens. Viral Immunol. 2021;34:86-95.
83. Baxter M, Sylvester J, St-Pierre NR, Graham BDM. Methods of applying a product to poultry via cloacal drinking. US-2023190436-A1, 2023.
84. Papouskova A, Rychlik I, Harustiakova D, Cizek A. Research note: a mixture of Bacteroides spp. and other probiotic intestinal anaerobes reduces colonization by pathogenic E. coli strain O78:H4-ST117 in newly hatched chickens. Poult Sci. 2023;102:102529.
85. Wyszyńska AK, Godlewska R. Lactic acid bacteria - a promising tool for controlling chicken Campylobacter infection. Front Microbiol. 2021;12:703441.
86. D’Amico V, Cavaliere M, Ivone M, et al. Microencapsulation of probiotics for enhanced stability and health benefits in dairy functional foods: a focus on pasta filata cheese. Pharmaceutics. 2025;17:185.
87. Sibanda T, Marole TA, Thomashoff UL, Thantsha MS, Buys EM.
88. Frakolaki G, Giannou V, Kekos D, Tzia C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit Rev Food Sci Nutr. 2021;61:1515-36.
89. Kiprono S, Wambani J, Rono J, Langat V, Shi Z, et al. Microencapsulation of probiotics and their applications: a review of the literature. ES Food Agrofor. 2024;17:1106.
90. Schofield T, Kavanagh J, Li Z, et al. Microencapsulation of Bifidobacterium lactis and Lactobacillus plantarum within a novel polysaccharide-based core-shell formulation: improving probiotic viability and mucoadhesion. ACS Biomater Sci Eng. 2024;10:6903-14.
91. Vivek K, Mishra S, Pradhan RC, et al. A comprehensive review on microencapsulation of probiotics: technology, carriers and current trends. Appl Food Res. 2023;3:100248.
92. Pyclik M, Srutkova D, Schwarzer M, Górska S. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins - their chemical structure and biological attributes. Int J Biol Macromol. 2020;147:333-49.
93. López P, Monteserín DC, Gueimonde M, et al. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res Int. 2012;46:99-107.
94. Fanning S, Hall LJ, Cronin M, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci U S A. 2012;109:2108-13.
95. Kšonžeková P, Bystrický P, Vlčková S, et al. Exopolysaccharides of Lactobacillus reuteri: their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr Polym. 2016;141:10-9.
96. Nilsen NJ, Deininger S, Nonstad U, et al. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84:280-91.
97. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801.
98. Jang JH, Shin HW, Lee JM, Lee HW, Kim EC, Park SH. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediators Inflamm. 2015;2015:794143.
99. Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, et al. Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Med. 2010;53:37-45. Available from: . [Last accessed on 20 Aug 2025].
100. Zhu J, Zhao L, Guo H, Jiang L, Ren F. Immunomodulatory effects of novel bifidobacterium and lactobacillus strains on murine macrophage cells. Afr J Microbiol Res. 2011;5:8-15.
101. Wang LS, Zhu HM, Zhou DY, Wang YL, Zhang WD. Influence of whole peptidoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol. 2001;7:440-3.
102. Tejada-Simon MV, Pestka JJ. Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J Food Prot. 1999;62:1435-44.
103. Ivanov D, Emonet C, Foata F, et al. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J Biol Chem. 2006;281:17246-52.
104. Foroni E, Serafini F, Amidani D, et al. Genetic analysis and morphological identification of pilus-like structures in members of the genus Bifidobacterium. Microb Cell Fact. 2011;10 Suppl 1:S16.
105. Contreras-lópez G, Carrillo-lópez LM, Vargas-bello-pérez E, García-galicia IA. Microencapsulation of feed additives with potential in livestock and poultry production: a systematic review. Chil j agric anim sci. 2024;40:229-49.
106. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502.
107. Ntsefong GN, Lodygin A, Evdokimov I, et al. Polymer selection for microencapsulation of probiotics: impact on viability, stability, and delivery in functional foods for improved manufacturing and product development in the food industry. Potr S J F Sci. 2023;17:712-27.
108. Nogueira MB, Massaut KB, Vitola HRS, Siqueira MFF, da Silva WP, Fiorentini ÂM. Antagonistic activity of Lactobacillus spp. and Bifidobacterium spp. against cariogenic Streptococcus mutans in vitro and viability when added to chewing gum during storage. Braz J Microbiol. 2023;54:2197-204.
109. Verruck S, Silva KJ, de Oliveira Santeli H, et al. Bifidobacterium animalis ssp. lactis BB-12 enumeration by quantitative PCR assay in microcapsules with full-fat goat milk and inulin-type fructans. Food Res Int. 2020;133:109131.
110. Arslan-Tontul S, Erbas M, Gorgulu A. The use of probiotic-loaded single- and double-layered microcapsules in cake production. Probiotics Antimicrob Proteins. 2019;11:840-9.
111. El-Sayed HS, Youssef K, Hashim AF. Stirred yogurt as a delivery matrix for freeze-dried microcapsules of synbiotic EVOO nanoemulsion and nanocomposite. Front Microbiol. 2022;13:893053.
112. Zhang Z, Gu M, You X, Sela DA, Xiao H, Mcclements DJ. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocolloids. 2021;116:106634.
113. Azam M, Saeed M, Yasmin I, et al. Microencapsulation and invitro characterization of Bifidobacterium animalis for improved survival. Food Measure. 2021;15:2591-600.
114. Khan WA, Butt MS, Yasmin I, Wadood SA, Mahmood A, Gad HA. Protein-polysaccharide based double network microbeads improves stability of Bifidobacterium infantis ATCC 15697 in a gastro-Intestinal tract model (TIM-1). Int J Pharm. 2024;652:123804.
115. Mousa AH, Korma SA, Ali AH, et al. Microencapsulation of Bifidobacterium bifidum F-35 via modulation of emulsifying technique and its mechanical effects on the rheological stability of set-yogurt. J Food Sci Technol. 2023;60:2968-77.
116. Moghanjougi Z, Rezazadeh Bari M, Alizadeh Khaledabad M, Amiri S, Almasi H. Microencapsulation of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB-12 in pectin and sodium alginate: a comparative study on viability, stability, and structure. Food Sci Nutr. 2021;9:5103-11.
117. Lohrasbi V, Abdi M, Asadi A, et al. The effect of improved formulation of chitosan-alginate microcapsules of Bifidobacteria on serum lipid profiles in mice. Microb Pathog. 2020;149:104585.
118. Huang Y, Lu Z, Liu F, et al. Osteopontin associated Bifidobacterium bifidum microencapsulation modulates infant fecal fermentation and gut microbiota development. Food Res Int. 2024;197:115211.
119. Huang X, Liu R, Wang J, et al. Preparation and synbiotic interaction mechanism of microcapsules of Bifidobacterium animalis F1-7 and human milk oligosaccharides (HMO). Int J Biol Macromol. 2024;259:129152.
120. Penhasi A, Reuveni A, Baluashvili I. Microencapsulation may preserve the viability of probiotic bacteria during a baking process and digestion: a case study with Bifidobacterium animalis subsp. lactis in Bread. Curr Microbiol. 2021;78:576-89.
121. Babot JD, Lorenzo Pisarello MJ, Obregozo M, Argañaraz-martínez E, Apella MC, Perez Chaia A. Soy protein improves the shelf life of a spray-dried probiotic for poultry. Arab J Sci Eng..
122. Sin PY, Tan SH, Farida Asras MF, Karmawan LU. From preparation to product: factors influencing probiotic viability in spray drying. Curr Sci Technol. 2024;4:22-35.
123. Shokri Z, Fazeli MR, Ardjmand M, Mousavi SM, Gilani K. Factors affecting viability of Bifidobacterium bifidum during spray drying. Daru. 2015;23:7.
124. Lu Z, Imlay JA. When anaerobes encounter oxygen: mechanisms of oxygen toxicity, tolerance and defence. Nat Rev Microbiol. 2021;19:774-85.
125. Jiang T, Lu W, Cui S, Zhang H, Zhao J. Characteristic analysis of different microencapsulated Bifidobacterium. Sci Technol Food Industry. 2021;42:128-34.
126. Liu SL, Chen CY, Chen YS. Characteristic properties of spray-drying Bifidobacterium adolescentis microcapsules with biosurfactant. J Biosci Bioeng. 2022;133:250-7.
127. Burns P, Alard J, Hrdỳ J, et al. Spray-drying process preserves the protective capacity of a breast milk-derived Bifidobacterium lactis strain on acute and chronic colitis in mice. Sci Rep. 2017;7:43211.
128. Muthusany N, Natarajan A, Kumerasan G, et al. Viability of spray dried probiotics in crumble feed during storage. Int J Curr Microbiol App Sci. 2020;9:1389-96.
129. Kakuda L, Jaramillo Y, Niño-Arias FC, et al. Process development for the spray-drying of probiotic bacteria and evaluation of the product quality. J Vis Exp. 2023.
130. Archacka M, Celińska E, Białas W. Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends. Food Bioprod. Process. 2020;123:354-66.
131. Pupa P, Apiwatsiri P, Sirichokchatchawan W, et al. The efficacy of three double-microencapsulation methods for preservation of probiotic bacteria. Sci Rep. 2021;11:13753.
132. Gullifa G, Risoluti R, Mazzoni C, et al. Microencapsulation by a spray drying approach to produce innovative probiotics-based products extending the shelf-life in non-refrigerated conditions. Molecules. 2023;28:860.
133. Acosta-Piantini E, Villarán MC, Martínez Á, Lombraña JI. Examining the effect of freezing temperatures on the survival rate of micro-encapsulated probiotic Lactobacillus acidophilus LA5 using the flash freeze-drying (FFD) strategy. Microorganisms. 2024;12:506.
134. Tanimomo J, Delcenserie V, Taminiau B, Daube G, Saint-hubert C, Durieux A. Growth and freeze-drying optimization of Bifidobacterium crudilactis. Food Nutr Sci. 2016;07:616-26.
135. Haindl R, Neumayr A, Frey A, Kulozik U. Impact of cultivation strategy, freeze-drying process, and storage conditions on survival, membrane integrity, and inactivation kinetics of Bifidobacterium longum. Folia Microbiol. 2020;65:1039-50.
136. Buahom J, Siripornadulsil S, Sukon P, Sooksawat T, Siripornadulsil W. Survivability of freeze- and spray-dried probiotics and their effects on the growth and health performance of broilers. Vet World. 2023;16:1849-65.
137. Oxley JD, Castilla-gutierrez C, Collazos SR, et al. Comparison of energy consumption and probiotic stability with pilot scale drying processes. LWT. 2024;211:116937.
138. Kourkoutas Y, Sipsas V, Papavasiliou G, Koutinas AA. An economic evaluation of freeze-dried kefir starter culture production using whey. J Dairy Sci. 2007;90:2175-80.
139. Silva R, Pimentel TC, Eustáquio de Matos Junior F, et al. Microencapsulation with spray-chilling as an innovative strategy for probiotic low sodium requeijão cremoso processed cheese processing. Food Bioscience. 2022;46:101517.
140. Bampi GB, Backes GT, Cansian RL, et al. Spray chilling microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis. ;9:1422-8.
141. Favaro-Trindade CS, de Matos Junior FE, Okuro PK, et al. Encapsulation of active pharmaceutical ingredients in lipid micro/nanoparticles for oral administration by spray-cooling. Pharmaceutics. 2021;13:1186.
142. Arslan-tontul S, Erbas M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT. 2017;81:160-9.
143. Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol. 2007;18:184-90.
144. Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery--drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149-55.
145. Figueiredo JA, Silva CRP, Souza Oliveira MF, et al. Microencapsulation by spray chilling in the food industry: Opportunities, challenges, and innovations. Trends Food Sci Technol. 2022;120:274-87.
146. Mahapatra A, Patil S, Dhakane-Lad J. Spray chilling/cooling of nutraceutical ingredients. In: Rajakumari R, Thomas S, Editors. Handbook of Nutraceuticals. Cham: Springer; 2024. pp 1-21.
147. Pedroso DL, Dogenski M, Thomazini M, Heinemann RJ, Favaro-Trindade CS. Microencapsulation of Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus in cocoa butter using spray chilling technology. Braz J Microbiol. 2013;44:777-83.
148. Homayouni-Rad A, Mortazavian AM, Pourjafar H, Moghadam SK. Extrusion and co-extrusion: a technology in probiotic encapsulation with alternative materials. Curr Pharm Biotechnol. 2024;25:1986-2000.
149. Lee Y, Ji YR, Lee S, Choi MJ, Cho Y. Microencapsulation of probiotic Lactobacillus acidophilus KBL409 by extrusion technology to enhance survival under simulated intestinal and freeze-drying conditions. J Microbiol Biotechnol. 2019;29:721-30.
150. Frakolaki G, Kekes T, Lympaki F, Giannou V, Tzia C. Use of encapsulated Bifidobacterium animalis subsp. lactis. ;45:e13792.
151. Rojas-Muñoz YV, Santagapita PR, Quintanilla-Carvajal MX. Probiotic encapsulation: bead design improves bacterial performance during in vitro digestion. Polymers. 2023;15:4296.
152. Singh S, Gupta R, Chawla S, et al. Natural sources and encapsulating materials for probiotics delivery systems: recent applications and challenges in functional food development. Front Nutr. 2022;9:971784.
153. Shi Z, Wu J, Wang X, et al. Development of Pickering water-in-oil emulsions using a dual stabilization of candelilla wax and acylated EGCG derivatives to enhance the survival of probiotics (Lactobacillus plantarum) powder. Food Funct. 2024;15:11141-57.
154. Koh WY, Lim XX, Tan T, Kobun R, Rasti B. Encapsulated probiotics: potential techniques and coating materials for non-dairy food applications. Applied Sciences. 2022;12:10005.
155. de Oca-ávalos JMM, Candal RJ, Herrera ML. Nanoemulsions: stability and physical properties. Curr Opin Food Sci. 2017;16:1-6.
156. Chen L, Ao F, Ge X, Shen W. Food-grade pickering emulsions: preparation, stabilization and applications. Molecules. 2020;25:3202.
157. Haji F, Cheon J, Baek J, Wang Q, Tam KC. Application of pickering emulsions in probiotic encapsulation- a review. Curr Res Food Sci. 2022;5:1603-15.
158. Talwalkar A, Kailasapathy K. Effect of microencapsulation on oxygen toxicity in probiotic bacteria - ProQuest. Aust J Dairy Technol. 2003;58:36.
159. Gani A, Akther G, Ashwar BA, Jhan F, Shah A. Resistant starch as a novel carrier for delivery of probiotics exploring effectiveness of two different strategies of encapsulation. Starch Stärke. 2023;75:2100285.
160. Babot JD, Argañaraz-Martínez E, Apella MC, Perez Chaia A. Microencapsulation of probiotics with soy protein isolate and alginate for the poultry industry. Food Bioproc Tech. 2023;16:1478-87.
161. Dantanarayana SK, Bogahawatta LBGS, Jayabhanu APNE, et al. Encapsulation techniques for probiotic non-dairy products: a comprehensive review. Global Scientific Journals. 2024;12:518-63.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data







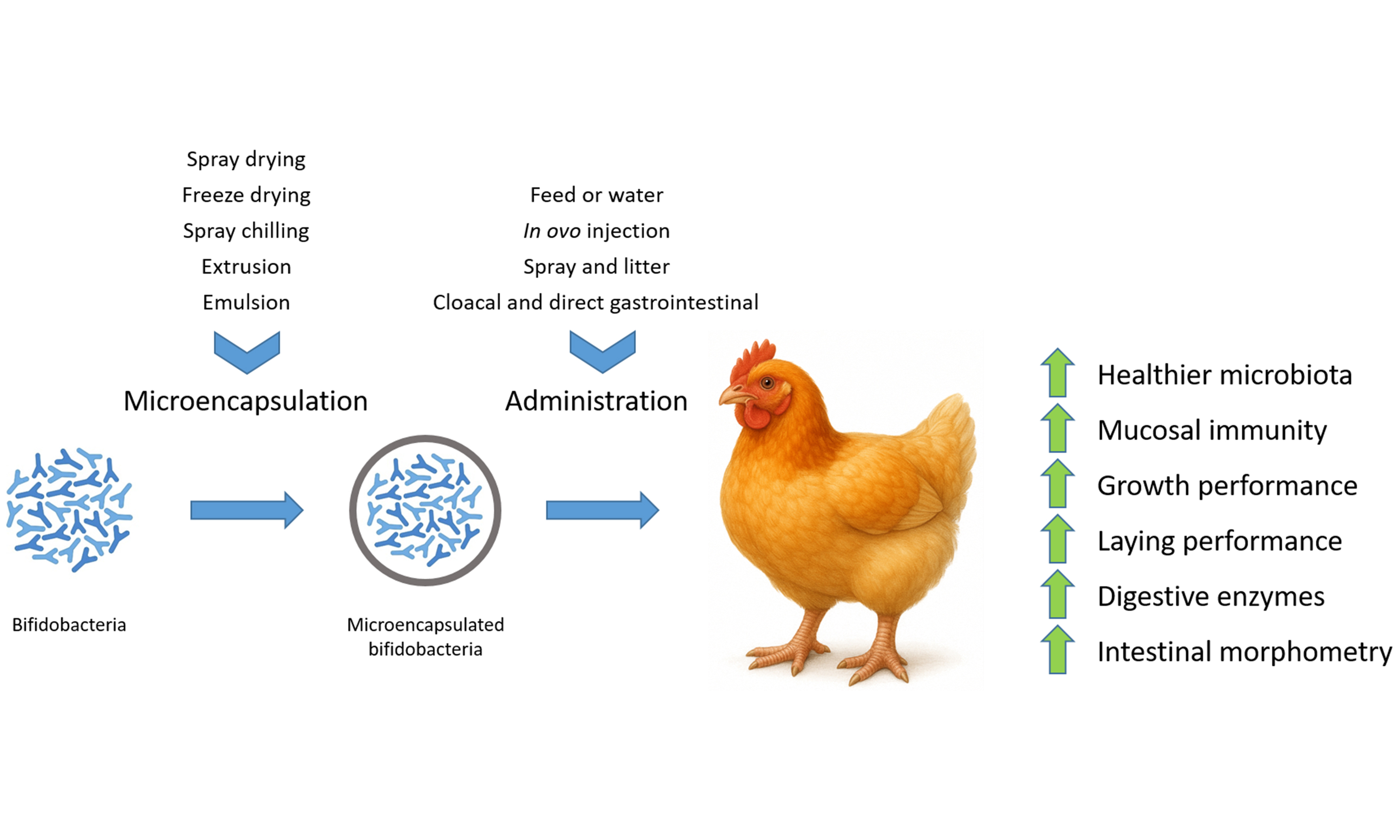
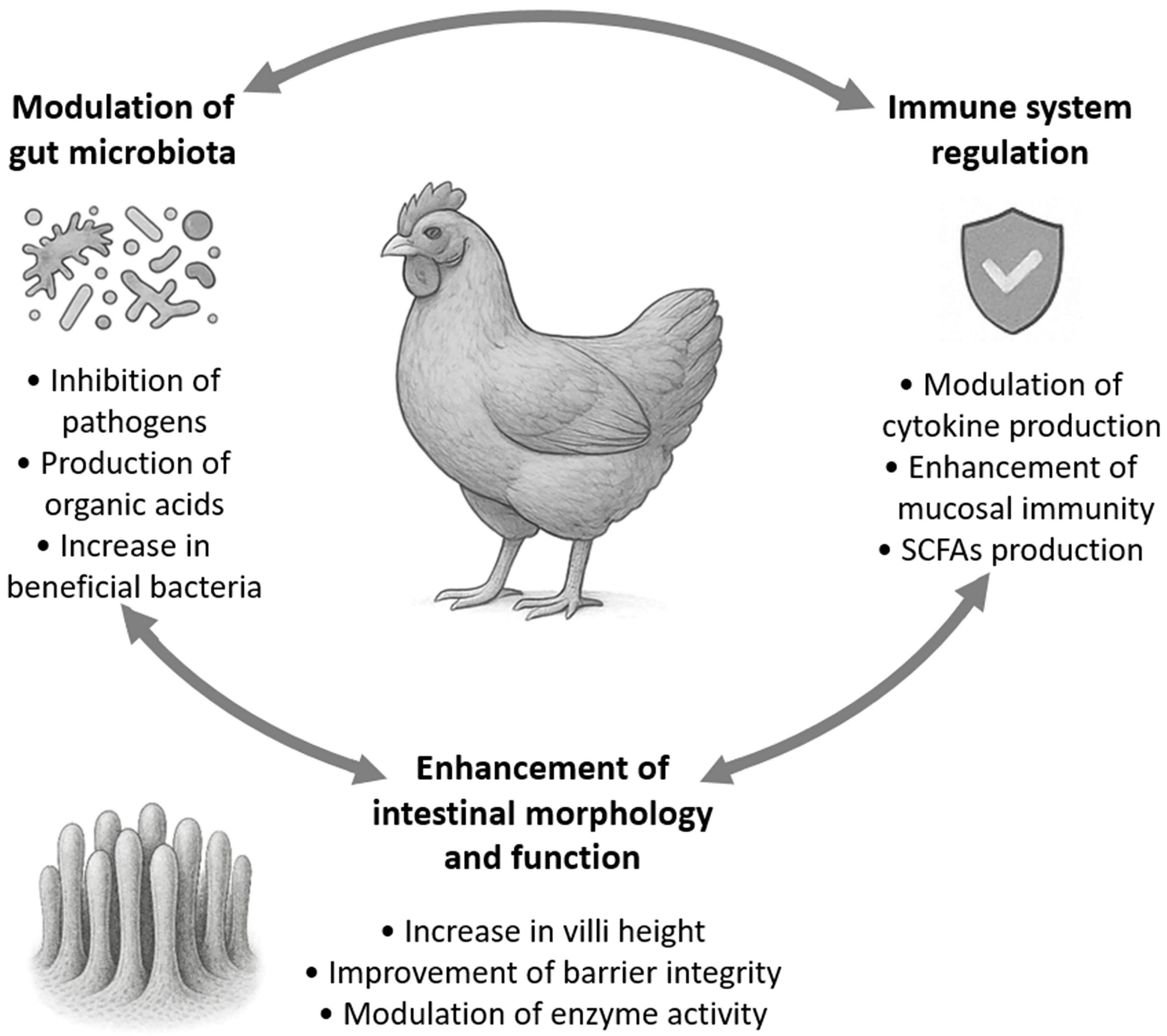











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].